Abstract
Clostridium difficile infections (CDI) in hospitalized patients are known to be closely related to antibiotic exposure. Although several substances can cause CDI, the risk differs between individual agents. In Vienna and other eastern parts of Austria, CDI ribotype 027 is currently highly prevalent. This ribotype has the characteristic of intrinsic moxifloxacin resistance. Therefore, we hypothesized that moxifloxacin restriction can decrease the number of CDI cases in hospitalized patients. Our antibiotic stewardship (ABS) group applied an information campaign on CDI and formal restriction of moxifloxacin in Wilhelminenspital (Vienna, Austria), a 1,000- bed tertiary care hospital. The preintervention period (period 1) was January through May 2013, and the intervention period (period 2) was June through December 2013. We recorded the defined daily doses (DDD) of moxifloxacin and the number of CDI patients/month. Moxifloxacin use was reduced from a mean (± standard error of the mean [SEM]) of 1,038 ± 109 DDD per month (period 1) to 42 ± 10 DDD per month (period 2) (P = 0.0045). Total antibiotic use was not affected. The mean (±SEM) numbers of CDI cases in period 1 were 59 ± 3 per month and in period 2 were 32 ± 3 per month (46% reduction; P = 0.0044). Reducing moxifloxacin use in combination with providing structured information on CDI was associated with an immediate decrease in CDI rates in this large community teaching hospital.
INTRODUCTION
Clostridium difficile infection (CDI) is a common side effect of antimicrobial therapy, and C. difficile is endemic in hospitals due to health care-associated transmissions. CDI is associated with increased mortality rates and economic burden (1, 2). Although several substances can cause CDI, the risk differs between individual antibiotics. A recent meta-analysis of community-associated CDI comprising 30,184 patients (3) showed that the risk for CDI was greatest with clindamycin (odds ratio [OR], 20.43; 95% confidence interval [CI], 8.50 to 49.09), followed by fluoroquinolones (OR, 5.65; 95% CI, 4.38 to 7.28).
In Austria, a recent shift in endemic ribotypes was observed. In 2008 and 2009, ribotype 053 was the most prevalent. Since 2010, ribotype 027 has been even more common, driven by a high prevalence in the eastern parts of Austria, as reported by the national reference center for CDI. Compared with other ribotypes, ribotype 027 is moxifloxacin resistant, while in one recent study, clindamycin resistance was seen in only one-third of the cases (A. S. D. Indra, S. Huhulescu, K. Stickler, M. Hell, F. Allerberger, submitted for publication). As shown by von Baum et al. (4), C. difficile selection differs among the types of quinolones. In that study, a switch in long-term antibiotic prophylaxis from levofloxacin to moxifloxacin increased the CDI episode rate dramatically.
In our hospital, the numbers of CDI patients are continuously recorded and reported. While the numbers were stable at <200 patients per year from 2009 to 2011 (0.56, 0.51, and 0.50 per 1,000 patient days, respectively), an increase to 313 patients was observed in 2012 (0.88/1,000 patient days). In the first quarter of 2013, a further increase in CDI patients was detected, and an antibiotic stewardship (ABS) team was established to develop measures for confining CDI.
Since other Viennese hospitals did not record CDI or similar increases in the number of CDI, hospital-specific causes were analyzed. The use of moxifloxacin was about twice as high in our hospital as in 7 other Viennese hospitals; the rate of use was high but stable from 2009 to 2011 (0.037, 0.035, and 0.036 defined daily doses [DDD]/patient day) and increased slightly in 2012 (0.038 DDD/patient day).
Since the numbers of CDI cases and ribotype 027 isolates seemed to be related to moxifloxacin use, we studied whether moxifloxacin restriction would result in a reduction in the number of CDI cases.
MATERIALS AND METHODS
Setting.
The Wilhelminenspital (Vienna, Austria) is a large tertiary care community hospital with 1,081 beds and 357,892 patient days in 2013. Areas of focus include adult medical and surgical specialties, pediatrics, and obstetrics.
CDI case definition.
A patient was diagnosed with CDI when he or she had diarrhea (i.e., ≥3 loose stools per day) and tested positive in a two-step diagnostic approach, as proposed by the European Society of Clinical Microbiology and Infectious Diseases (5). This approach included a glutamate dehydrogenase (GDH) test (ImmunoCard C. difficile GDH; Meridian Bioscience, Inc., Europe, Villa Cortese, Milan, Italy) and confirmation by PCR in cases of a positive result. Ribotype 027 detection was also accomplished by PCR, combining results for binary toxin and tcdC deletion. Results revealing presumptive positive toxigenic C. difficile 027/NAP1/BI were reported as positive for ribotype 027 without further sequencing (for PCR, we used the GeneXpert platform with the Xpert C. difficile assay; Cepheid Europe, Maurens-Scopont, France).
Further, histopathological diagnosis of CDI after endoscopy or colectomy was accepted. CDI was classified as nosocomial when the diagnosis was made later than in the first 72 h of admission. Severe disease was defined by patient readmittance to the hospital due to recurrent symptomatic CDI, transference to the intensive care unit, surgical intervention due to CDI, or death within 30 days after diagnosis. Recurrence of disease was defined as a new episode of CDI within 8 weeks after a symptom-free interval.
Measures proposed by the ABS team.
The ABS team was appointed by the hospital management and organized by the head of the Department of Pathology and Microbiology, who was also the quality assurance representative. The team consisted of a clinical pharmacist, a pathologist, and infection control professionals.
The team agreed on the hypothesis that the rising numbers of CDI and moxifloxacin-resistant ribotype 027 isolates were the result of the high use of moxifloxacin. Therefore, measures taken against CDI should primarily include a reduction of this specific antibiotic. The measures consisted of a bundle of information on CDI and a moxifloxacin formulary restriction. The medical director facilitated the implementation of these measures. Antibiotic contact persons from the clinical departments were informed in a conference organized by the ABS team.
(i) CDI information.
Information on CDI was distributed via lectures on pathogenesis, epidemiology, prevention, diagnostics, and treatment held by local personnel and invited experts. The content of the talks was made available via the hospital's intranet.
(ii) Formulary restriction.
Physicians who prescribed moxifloxacin had to fill in a form describing the amount of the drug, the medical diagnosis, the planned route of administration, any combined antibiotic treatment, and pretreatment, if applicable. The hospital pharmacy informed the Department of Hospital Hygiene about the request. Whenever possible, the indication, contraindications, and possible alternative treatment options were reviewed with the prescribing physician. If a consultation was not possible, the requested antibiotic was delivered. Further, the antibiotic was delivered when the treating physician did not agree with the alternative proposal, and treatment strategies sparing moxifloxacin were proposed by antibiotic guidelines that were available via the hospital's intranet.
Statistics.
Defined daily doses (DDD) of moxifloxacin, the number of CDI patients, and costs (in euros) are reported in absolute numbers. The means and standard errors of the mean (SEM) of absolute numbers per month, calculated to compare the periods before (period 1) and after (period 2) the start of the intervention, are presented here. Kruskal-Wallis analysis of variance (ANOVA) and chi-square and Mann-Whitney U tests were applied where appropriate using Statistica 6.0 (StatSoft, Tulsa, OK, USA), and the significance level was set at a P value of <0.05. Graphs were designed with Microsoft Excel for Windows and Statistica 6.0.
RESULTS
The absolute number of CDI was 515 cases in 2013, which was an incidence of 1.44/1,000 patient days. The mean age of the patients was 79 years (males, 75 years; females, 81 years). Presumptive ribotype 027 was detected in 172 (33%) of the cases. CDI was classified as nosocomial in 75% of the cases.
Severe disease was recorded for 31% of the patients, and in 131 (25%) of the cases, the patient died within 30 days of diagnosis. Of the cases in which the patient died, 57 (43.4%) involved ribotype 027 (chi-square test, mortality with ribotype 027 versus non-027 ribotype, P = 0.03).
Recurrent disease was seen in 63 (12%) of the cases, of which 51 (81%) involved ribotype 027 (chi-square test, recurrent disease with ribotype 027 versus non-027 ribotype, P = 0.02).
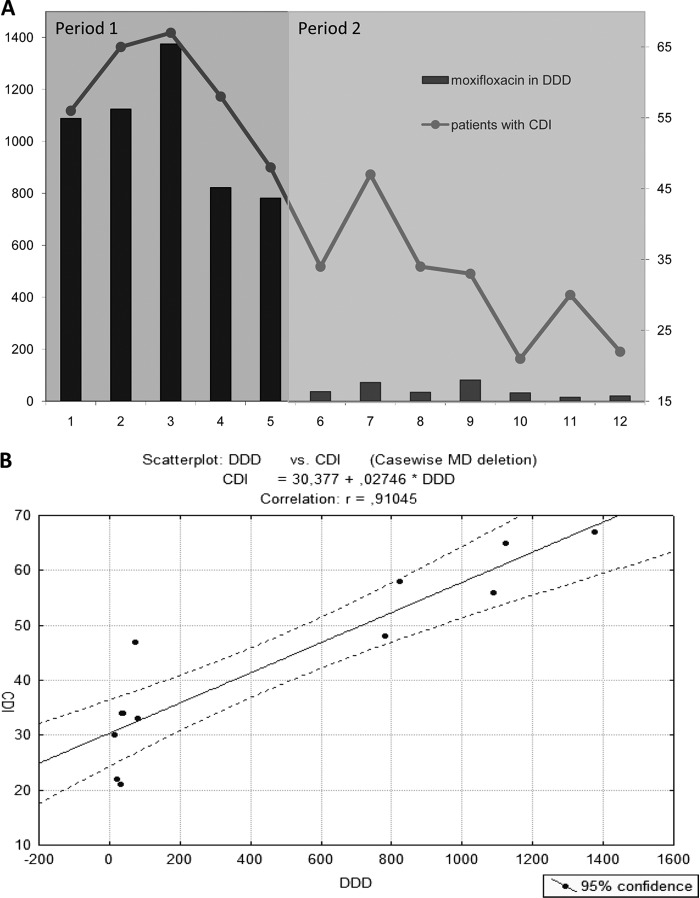
Moxifloxacin use was reduced from 1,038 ± 109 DDD per month (January to May, period 1) to 42 ± 10 DDD per month (June to December, period 2) (P = 0.0045) (Fig. 1A). Moxifloxacin was partly replaced by levofloxacin, while ciprofloxacin use was stable. In total, quinolone use decreased by about 37% in period 2 compared to that in period 1. In contrast, overall antibiotic use was not altered. Moxifloxacin costs were reduced from 15,681 ± 1,790 euros per month (total, 78,409 euros in period 1) to 713 ± 256 per month (total, 4,989 euros in period 2). In 2013, 94% of moxifloxacin use and costs were incurred in the first 5 months. In absolute numbers, a total of 5,189 DDD were prescribed in period 1, while 291 DDD were prescribed in period 2.
FIG 1.
Moxifloxacin use and Clostridium difficile infections (CDI). (A) Monthly moxifloxacin use and CDI during the year 2013 before (period 1) and after (period 2) antimicrobial stewardship interventions. Moxifloxacin use is depicted in DDD (left y axis), and CDI are depicted in absolute numbers (right y axis). (B) Monthly recorded number of CDI correlated with moxifloxacin use in DDD. MD, missing data.
The maximum number of CDI (67) was seen in March, accompanied by the highest use of moxifloxacin in 2013 (Fig. 1A). Then, moxifloxacin use and the number of CDI began to trend downward. Concrete moxifloxacin-reducing measures started in June, and the number of CDI reached prior observed levels by October (Fig. 1A). The mean (±SEM) numbers of CDI were 59 ± 3 per month in period 1 and 32 ± 3 per month in period 2 (46% reduction; P = 0.0044). Mean ribotype 027 levels trended downward from 15.2 ± 2 per month in period 1 to 13.7 ± 2 per month in period 2 (P > 0.05). The monthly recorded total number of CDI correlated with moxifloxacin use in DDD (r = 0.91) (Fig. 1B).
DISCUSSION
Our finding that enhanced antibiotic stewardship, including the restriction of moxifloxacin, is associated with a reduction in the number of CDI is in line with the findings of Aldeyab et al. and Talpaert et al. (6, 7), who showed a similar effect due to the reduction in use of high-risk broad-spectrum antibiotics, including fluoroquinolones, clindamycin, and cephalosporins. Further, an outbreak of a hospital-acquired epidemic CDI strain at a community hospital was controlled after an exclusive restriction of fluoroquinolones (8). However, we were the first to describe the restriction of moxifloxacin as an individual antibiotic having such an effect. As mentioned above, the specific focus on moxifloxacin was chosen due to the local circumstances of a rising rate of ribotype 027, which is moxifloxacin resistant, and the high use of moxifloxacin in our hospital. Although levofloxacin and ciprofloxacin have also been reported to be high-risk antibiotics, moxifloxacin seems to pose an even higher risk for C. difficile selection. As shown by von Baum et al. (4), a switch in long-term antibiotic prophylaxis in neutropenic patients from levofloxacin to moxifloxacin increased the CDI episode rate from 6% to 33%. This effect was reversible when moxifloxacin prophylaxis was switched back to levofloxacin, which has a different activity against anaerobes. This might explain why the partial replacement of moxifloxacin by levofloxacin did not seem to diminish the effect of moxifloxacin restriction on CDI rates in our hospital.
We report a 25% 30-day case fatality rate for patients with CDI. This is within the range of previous Viennese data, revealing treatment-dependent 30-day mortality rates between 7.4% and 38.1% (2, 9, 10). A 7-year study in the United Kingdom in a CDI patient population of comparable age (mean age, 80 years) found a 30-day case fatality rate of 32% (11). In Quebec, Loo et al. (12) found a similar crude 30-day mortality rate of 24.8% in patients with a median age of 76 years from 12 hospitals, with the attributable mortality fraction increasing with age. Interestingly, all isolates of the predominant pulsovar in this study were resistant to quinolones.
During the last 4 years, we observed an increasing rate of ribotype 027 and a parallel increase in recurrence and mortality rates in our hospital. Also, in 2013, the percentage of recurrent and fatal cases was significantly higher in ribotype 027 patients than in the total CDI collective. In the last decade, the incidence, complication, and mortality rates of CDI have increased globally due to the emergence of so-called hypervirulent strains (13–16). In addition to the production of toxin A (TcdA) and toxin B (TcdB) and the presence of binary toxins (CdtA and CdtB), altered resistance patterns and increased sporulation capacity were described as contributing to increased mortality and higher rates of recurrence with ribotype 027 (17–21). After implementation of antibiotic stewardship, the absolute numbers of ribotype 027 showed a nonsignificant downward trend in our hospital.
Restriction of moxifloxacin was only part of the program that also included the distribution of information on CDI. It has to be emphasized that concrete hygiene measures had already been implemented years earlier and were not changed during the program. Routine hygiene measures included a visit from an infection control specialist in every case of CDI to augment hand hygiene, i.e., hand-washing and disinfection in the appropriate indications, protective clothing, change to a sporicidal surface disinfectant, and strict isolation of the patient whenever possible.
The development of evidence-based practice guidelines incorporating local microbiology and resistance patterns is strongly recommended in antimicrobial stewardship programs (ASP) (22). A key aim of ASP is the improvement of anti-infective prescription behavior to reach the best clinical treatment response of the patients with a minimum of toxicity, side effects, microbiological resistance development, and costs (23). The combination of formulary restriction and direct feedback to the prescriber was highly effective in producing immediate and significant reductions in moxifloxacin use and costs. The challenge was to find alternatives to the highly promoted moxifloxacin therapy, generously prescribed to treat community-acquired pneumonia (CAP) and other respiratory infections, chosen for patients with renal insufficiency due to the practicability of no need for dose adjustments, used for skin and soft tissue infections, and given to patients with suspected or proven penicillin allergy. In clinical practice, antibiotic therapy was not necessary in every case in which moxifloxacin was requested. However, in the majority of cases, antibiotic treatment was inevitable. In these cases, a β-lactam treatment was advised. If this was not possible, doxycycline was sometimes a good alternative to the otherwise often-necessary quinolone or macrolide therapy. There is some evidence that doxycycline is not associated with CDI and may even be protective against it. For example, in a large meta-analysis (3), tetracyclines were not associated with an increased risk of CDI (OR, 0.91; 95% CI, 0.57 to 1.45). Further, in a recent study by Doernberg et al. (24), doxycycline was protective against the development of CDI in a cohort of hospitalized patients receiving ceftriaxone. The guidelines of the American Thoracic Society and Infectious Diseases Society of America recommend doxycycline plus a β-lactam antibiotic as an alternative to a macrolide or fluoroquinolone-containing regimen for the treatment of CAP (25).
This study had several limitations. First, we did not type all strains of C. difficile and were therefore unable to detect changes in strains other than the presumed ribotype 027. Further, we cannot entirely exclude the possibility that confounding factors were at least partly responsible for the reduction in CDI in addition to the applied measures, since even the high correlation of moxifloxacin use and CDI is not proof of causality. However, the fact that total antibiotic use was stable and no significant changes in other relevant substance classes were detected might allow the conclusion that a decrease in other high-risk antibiotics was not interfering. Further, no change in routine measures to control infection took place.
The observation period after the intervention was only 7 months. However, until the current time point, a clear and reportable success was noticed, and we will continue to record and evaluate the numbers of CDI in the following years.
Antibiotic exposure is the major risk factor for the development of CDI (26–28). In Europe and the United States, 35% to 63% of inpatients receive at least 1 dose of an antibiotic, and in Europe, 7.1% of patients develop health care-associated infections, including CDI (29, 30). Therefore, the aim of future antibiotic stewardship programs should include organizing detailed documentation of the indications for antibiotic treatment, allowing for review, audit, and feedback. In addition to the avoidance of high-risk antibiotics, this should result in a reduction in total antibiotic use and CDI. We conclude that antimicrobial stewardship, including the restriction of moxifloxacin, is effective in reducing the incidence of CDI in a population with a high rate of ribotype 027.
ACKNOWLEDGMENTS
We thank the management and the staff of the Wilhelminenspital for their continuous cooperation in fighting CDI. In addition, we thank the clinical microbiology, hospital pharmacy, and hospital hygiene teams for their special efforts. Finally, we thank Christoph Wenisch for critical review of the manuscript.
Footnotes
Published ahead of print 16 June 2014
REFERENCES
- 1.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. 2012. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J. Hosp. Infect. 81:1–14. 10.1016/j.jhin.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 2.Wenisch JM, Schmid D, Tucek G, Kuo HW, Allerberger F, Michl V, Tesik P, Laferl H, Wenisch C. 2012. A prospective cohort study on hospital mortality due to Clostridium difficile infection. Infection 40:479–484. 10.1007/s15010-012-0258-1 [DOI] [PubMed] [Google Scholar]
- 3.Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. 2013. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J. Antimicrob. Chemother. 68:1951–1961. 10.1093/jac/dkt129 [DOI] [PubMed] [Google Scholar]
- 4.von Baum H, Sigge A, Bommer M, Kern WV, Marre R, Dohner H, Kern P, Reuter S. 2006. Moxifloxacin prophylaxis in neutropenic patients. J. Antimicrob. Chemother. 58:891–894. 10.1093/jac/dkl320 [DOI] [PubMed] [Google Scholar]
- 5.Debast SB, Bauer MP, Kuijper EJ. 2014. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 20(Suppl 2):S1–S26. 10.1111/1469-0691.12418 [DOI] [PubMed] [Google Scholar]
- 6.Aldeyab MA, Kearney MP, Scott MG, Aldiab MA, Alahmadi YM, Darwish Elhajji FW, Magee FA, McElnay JC. 2012. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J. Antimicrob. Chemother. 67:2988–2996. 10.1093/jac/dks330 [DOI] [PubMed] [Google Scholar]
- 7.Talpaert MJ, Gopal Rao G, Cooper BS, Wade P. 2011. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J. Antimicrob. Chemother. 66:2168–2174. 10.1093/jac/dkr253 [DOI] [PubMed] [Google Scholar]
- 8.Kallen AJ, Thompson A, Ristaino P, Chapman L, Nicholson A, Sim BT, Lessa F, Sharapov U, Fadden E, Boehler R, Gould C, Limbago B, Blythe D, McDonald LC. 2009. Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect. Control Hosp. Epidemiol. 30:264–272. 10.1086/595694 [DOI] [PubMed] [Google Scholar]
- 9.Wenisch JM, Schmid D, Kuo HW, Allerberger F, Michl V, Tesik P, Tucek G, Laferl H, Wenisch C. 2012. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob. Agents Chemother. 56:1974–1978. 10.1128/AAC.05647-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenisch JM, Schmid D, Kuo HW, Simons E, Allerberger F, Michl V, Tesik P, Tucek G, Wenisch C. 2012. Hospital-acquired Clostridium difficile infection: determinants for severe disease. Eur. J. Clin. Microbiol. Infect. Dis. 31:1923–1930. 10.1007/s10096-011-1522-5 [DOI] [PubMed] [Google Scholar]
- 11.McGowan AP, Lalayiannis LC, Sarma JB, Marshall B, Martin KE, Welfare MR. 2011. Thirty-day mortality of Clostridium difficile infection in a UK National Health Service Foundation Trust between 2002 and 2008. J. Hosp. Infect. 77:11–15. 10.1016/j.jhin.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 12.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449. 10.1056/NEJMoa051639 [DOI] [PubMed] [Google Scholar]
- 13.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441. 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 14.Goorhuis A, Bakker D, Corver J, Debast SB, Harmanus C, Notermans DW, Bergwerff AA, Dekker FW, Kuijper EJ. 2008. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin. Infect. Dis. 47:1162–1170. 10.1086/592257 [DOI] [PubMed] [Google Scholar]
- 15.Pepin J, Valiquette L, Cossette B. 2005. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 173:1037–1042. 10.1503/cmaj.050978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pepin K, Chouinard D. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171:466–472. 10.1503/cmaj.1041104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, Burman LG. 2008. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J. Clin. Microbiol. 46:1530–1533. 10.1128/JCM.01964-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179. 10.1038/nature07822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, Gerding DN, Vedantam G. 2010. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J. Bacteriol. 192:4904–4911. 10.1128/JB.00445-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263. 10.1128/CMR.18.2.247-263.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knetsch CW, Hensgens MP, Harmanus C, van der Bijl MW, Savelkoul PH, Kuijper EJ, Corver J, van Leeuwen HC. 2011. Genetic markers for Clostridium difficile lineages linked to hypervirulence. Microbiology 157:3113–3123. 10.1099/mic.0.051953-0 [DOI] [PubMed] [Google Scholar]
- 22.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 44:159–177. 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 23.MacDougall C, Polk RE. 2005. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 18:638–656. 10.1128/CMR.18.4.638-656.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doernberg SB, Winston LG, Deck DH, Chambers HF. 2012. Does doxycycline protect against development of Clostridium difficile infection? Clin. Infect. Dis. 55:615–620. 10.1093/cid/cis457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas C, Stevenson M, Riley TV. 2003. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J. Antimicrob. Chemother. 51:1339–1350. 10.1093/jac/dkg254 [DOI] [PubMed] [Google Scholar]
- 27.Baxter R, Ray GT, Fireman BH. 2008. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect. Control Hosp. Epidemiol. 29:44–50. 10.1086/524320 [DOI] [PubMed] [Google Scholar]
- 28.Johnson S, Gerding DN. 1998. Clostridium difficile–associated diarrhea. Clin. Infect. Dis. 26:1027–1036. 10.1086/520276 [DOI] [PubMed] [Google Scholar]
- 29.Pakyz AL, MacDougall C, Oinonen M, Polk RE. 2008. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch. Intern. Med. 168:2254–2260. 10.1001/archinte.168.20.2254 [DOI] [PubMed] [Google Scholar]
- 30.Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens M, Vaerenberg S, Hopkins S, Catry B, Monnet D, Goossens H, Suetens C. 2012. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill. 17:pii=20316 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20316 [DOI] [PubMed] [Google Scholar]