Abstract
Gallium (Ga) is an iron mimetic that has successfully been repurposed for antibacterial chemotherapy. To improve the antibacterial potency of Ga on Pseudomonas aeruginosa, the effect of complexation with a variety of siderophores and synthetic chelators was tested. Ga complexed with the pyochelin siderophore (at a 1:2 ratio) was more efficient than Ga(NO3)3 in inhibiting P. aeruginosa growth, and its activity was dependent on increased Ga entrance into the cell through the pyochelin translocon.
TEXT
Iron (Fe) is an essential nutrient for nearly all forms of life, being the cofactor of many vital enzymes involved in DNA synthesis, metabolism, and the oxidative stress response (1). Pathogenic bacteria must counteract an Fe-poor environment during infection, since Fe is unavailable to invading pathogens due to sequestration by the Fe carrier and storage proteins of the host (2). Bacteria have evolved multiple strategies to acquire Fe from the host, the most common being through the production of siderophores. These compounds are secreted in the extracellular milieu, where they form stable complexes with Fe and other transition metals (depending on their coordination chemistry) and convey the metal to the bacterial cell via specific active transport systems (3). Given the importance of Fe in bacterial metabolism and the paucity of effective antibiotics for multidrug-resistant bacteria, Fe uptake and metabolism have recently been assessed as targets for the development of new antibacterials (4–7).
Gallium (GaIII) is a semimetal that shares a number of chemical similarities with the oxidized Fe form (FeIII). The most prominent FeIII mimetic features of GaIII are the nucleus radius and coordination chemistry, which enable GaIII to replace FeIII in Fe-containing enzymes. Being that GaIII is redox inactive, its incorporation in FeIII-containing enzymes results in the overall disruption of Fe metabolism (8). The antimicrobial properties of Ga(NO3)3, the active component of the FDA-approved formulation Ganite (Genta), have been investigated in a number of species (recently reviewed in references 9–12). In particular, GaIII inhibits both planktonic and biofilm growth of the opportunistic pathogen Pseudomonas aeruginosa and causes significant protection from P. aeruginosa infection in animal models (13, 14).
In the present study, we attempted to improve the antibacterial activity of GaIII on P. aeruginosa by complexation with suitable carriers (either synthetic chelators or siderophores) that are actively taken up by the bacterium and that stimulate, to a variable extent, its growth under conditions of extreme Fe deficiency (see Fig. S1 in the supplemental material). Both the P. aeruginosa reference strain PAO1 and the cystic fibrosis isolate TR1 (15) were used for growth promotion/inhibition assays. GaIII-chelator complexes were generated by mixing, in the appropriate ratios (Fig. 1), aqueous solutions of Ga(NO3)3 with ferrichrome (FER) (Sigma), sodium dicitrate (CIT) (Sigma), desferrioxamine (DFO) (Novartis), sodium salicylate (SAL) (Sigma), and the autologous siderophores pyoverdine (PVD) and pyochelin (PCH). PVD and PCH were purified from culture supernatants of a PCH-defective P. aeruginosa mutant (PAO1ΔpchD; see Table S1 and Supplemental Experimental Procedures in the supplemental material) and a PVD-defective P. aeruginosa mutant (PAO1ΔpvdA) (16), respectively, according to previously published procedures (17–19). The growth inhibitory activity of each GaIII complex was then assayed in the Fe-poor medium DCAA (18) and compared to the activity of each chelator or Ga(NO3)3 alone (Fig. 1; see also Fig. S2 in the supplemental material). In line with previous results (13), Ga(NO3)3 inhibited P. aeruginosa growth in a dose-dependent manner at concentrations of >3.13 μM. Consistent with previous findings (13), Ga(NO3)3 showed bacteriostatic activity at a growth inhibitory concentration (12.5 μM; data not shown).
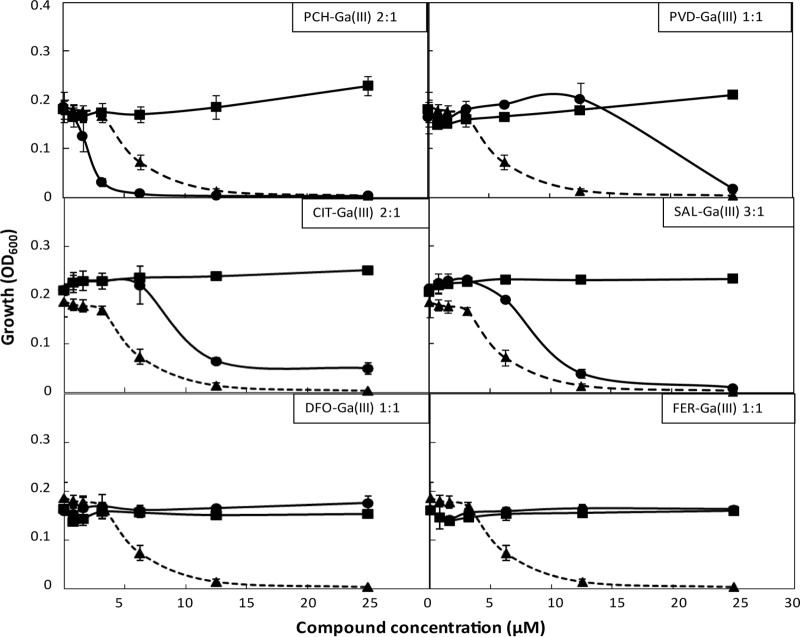
FIG 1.
Effect of Ga(NO3)3 and GaIII complexes on P. aeruginosa PAO1 growth. Growth (optical density at 600 nm [OD600]) of P. aeruginosa PAO1 in microtiter plates containing (per well) 200 μl DCAA supplemented with different concentrations of Ga(NO3)3 (triangles and dashed lines), the indicated GaIII-chelator complex (circles and solid lines), or the chelator alone as a control (squares and solid lines), after 24 h at 37°C. The stoichiometry (binding ratio) of each GaIII-chelator complex is indicated in the inset of each panel. The data are the mean ± standard deviation from at least two independent experiments.
The PCH-GaIII complex was the only combination endowed with higher inhibitory activity than that of Ga(NO3)3 alone. PVD-GaIII, SAL-GaIII, and CIT-GaIII complexes showed a moderate protective effect on P. aeruginosa, since they decreased GaIII-mediated growth inhibition in a dose-dependent manner. Notably, FER-GaIII and DFO-GaIII abrogated growth inhibition by GaIII, as one would expect for a compound endowed with strong GaIII-scavenging activity (Fig. 1; see also Fig. S2 in the supplemental material). A similar response to GaIII and its complexes was observed for both the P. aeruginosa PAO1 and TR1 strains (Fig. 1; see also Fig. S2 in the supplemental material).
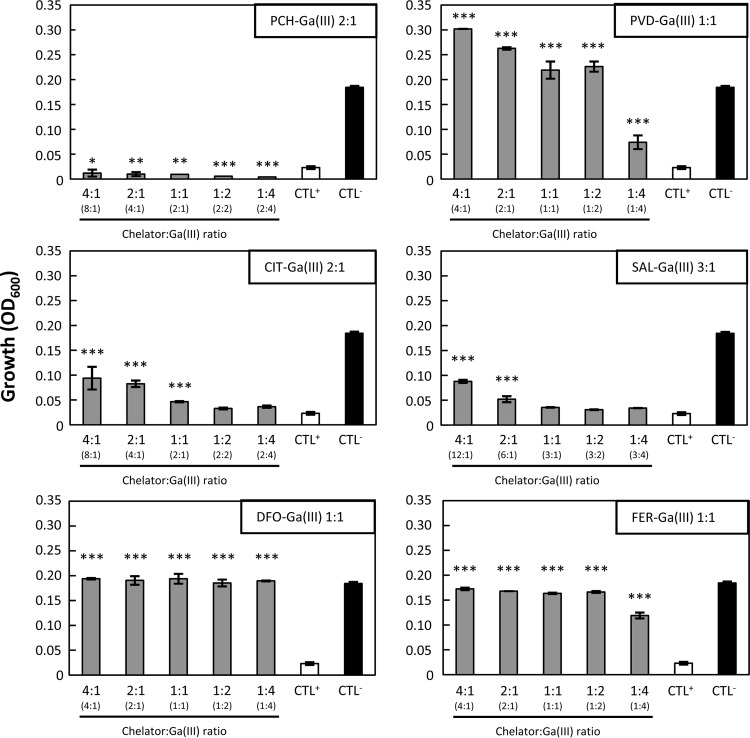
It appears, therefore, that some GaIII complexes alleviate GaIII inhibition rather than potentiate it, suggesting that they behave as GaIII scavengers rather than vehicles of GaIII to the cell. To gain further insight into the effect of the different chelators on GaIII activity, bacteria were grown in DCAA containing Ga(NO3)3 at a fixed inhibitory concentration (12.5 μM) and increasing concentrations of the different chelators in order to obtain different chelator-to-GaIII ratios (Fig. 2). DFO and FER protected P. aeruginosa from the growth inhibitory activity of Ga(NO3)3, even at 1:2 and 1:4 chelator-to-GaIII ratios, which is suggestive of a strong GaIII scavenging effect (Fig. 2). Similar results were obtained with PVD, though at higher PVD-to-GaIII ratios. SAL and CIT showed a poor scavenging effect, being unable to counteract the Ga(NO3)3 inhibitory effect even in the presence of an excess of chelator (chelator-to-GaIII ratio, 4:1). PCH never rescued P. aeruginosa growth in the presence of Ga(NO3)3 at all ratios tested (Fig. 2). Again, comparable results were obtained for the P. aeruginosa clinical strain TR1 (see Fig. S3 in the supplemental material). Taken together, these results indicate that DFO, FER, and PVD are strong inhibitors of GaIII activity, while PCH exerts an opposite effect, suggesting that PCH acts as a “Trojan horse” that conveys GaIII into the cell.
FIG 2.
Effect of different chelator-to-GaIII ratios on P. aeruginosa PAO1 growth. P. aeruginosa PAO1 was grown for 24 h at 37°C in microtiter plates containing (per well) 200 μl DCAA supplemented with 12.5 μM Ga(NO3)3 and various concentrations of each chelator to obtain different chelator-to-GaIII ratios. The chelator-to-GaIII ratio (x axis) takes into account the binding stoichiometry, as indicated in the inset of each panel. The actual chelator-to-GaIII ratio is given in parentheses on the x axis. CTL+, growth in the presence of 12.5 μM Ga(NO3)3; CTL−, growth without chelators or Ga(NO3)3. Growth was measured as the OD600 (y axis). Each value is the mean ± standard deviation from the results of three independent experiments. Statistically significant differences compared to CTL+ are indicated (analysis of variance [ANOVA]): *, P < 0.05; **, P < 0.01; ***, P < 0.001.
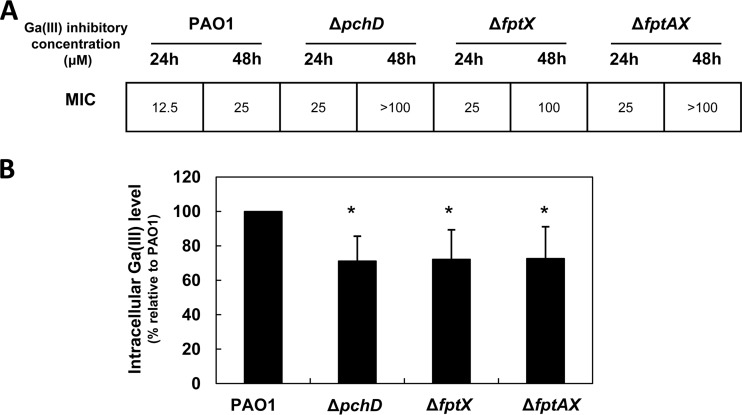
To shed more light on the mechanism by which PCH potentiates GaIII activity, we generated P. aeruginosa PAO1 mutants impaired in PCH biosynthesis (ΔpchD) or uptake (ΔfptAX and ΔfptX, with deletions of the whole PCH translocon or the inner membrane transporter only, respectively) (for details, see reference 20; see also Table S1 and Supplemental Experimental Procedures in the supplemental material). We reasoned that if the GaIII inhibitory effects were enhanced by PCH production and internalization, these PCH synthesis and uptake mutants would be more resistant to Ga(NO3)3. The effect of Ga(NO3)3 on these strains was thus assessed in DCAA supplemented with increasing Ga(NO3)3 concentrations (0 to 100 μM), and the data were expressed as the MICs at 24 h and 48 h (Fig. 3A). While all strains showed comparable growth levels in DCAA without Ga(NO3)3 (data not shown), the ΔpchD, ΔfptAX, and ΔfptX strains were more resistant to Ga(NO3)3 than the wild-type PAO1 (Fig. 3A), suggesting that PCH production and transport into the cell contribute to GaIII inhibitory activity. To confirm that the reduction of GaIII susceptibility in PCH-defective mutants was due to impaired internalization of GaIII into the cell, intracellular GaIII levels were measured in each strain grown in DCAA supplemented with a subinhibitory Ga(NO3)3 concentration (3 μM) by means of inductively coupled plasma optical emission spectrometry (ICP-OES) using an ICP-OES Varian 710 spectrometer (Agilent Technologies) (Fig. 3B). In line with the higher resistance shown by the ΔpchD, ΔfptAX, and ΔfptX mutants, the intracellular levels of GaIII were significantly lower in these mutants than those in wild-type PAO1 (Fig. 3B), although not nil, suggesting that GaIII may enter the cell via an alternative route(s). This is consistent with the recent finding that the HitAB Fe transport system is also involved in GaIII uptake by P. aeruginosa (21).
FIG 3.
GaIII inhibitory activity and intracellular GaIII levels in P. aeruginosa PAO1 and isogenic PCH-defective mutants. (A) MICs of Ga(NO3)3 (μM) for P. aeruginosa PAO1 and isogenic PCH-transport mutants grown for 24 h and 48 h in 200 μl DCAA supplemented with increasing Ga(NO3)3 concentrations (0 to 100 μM). (B) Intracellular concentrations of GaIII in the same P. aeruginosa strains grown in DCAA supplemented with 3 μM Ga(NO3)3 for 14 h, measured by the means of ICP-OES. The values are expressed as the percentage relative to PAO1 and represent the mean ± standard deviation from three independent experiments. Intracellular GaIII levels in wild-type PAO1 varied from 0.47 to 1.41 ng GaIII/mg total cell proteins, depending on the experiment. *, statistically significant differences relative to PAO1 (P < 0.05, ANOVA).
In conclusion, our data demonstrate that siderophores and synthetic chelators affect in different manners the in vitro activity of GaIII on P. aeruginosa. Among many GaIII complexes tested, only the endogenous siderophore PCH facilitates GaIII entrance into the cell through its specific uptake machinery, thereby potentiating the antipseudomonal activity of GaIII. These findings might be of guidance for the future design of more effective GaIII delivery systems to P. aeruginosa.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alessia Falsetti and Renato Baciocchi (Tor Vergata University of Rome) for performing the ICP-OES measurements.
This work was supported by the Italian Cystic Fibrosis Research Foundation (grant FFC 14/2010) and the Italian Ministry of University and Research-PRIN 2012 (project 2012WJSX8K) to P.V.
Footnotes
Published ahead of print 23 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03154-14.
REFERENCES
- 1.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237. 10.1016/S0168-6445(03)00055-X [DOI] [PubMed] [Google Scholar]
- 2.Weinberg ED. 2009. Iron availability and infection. Biochim. Biophys. Acta 1790:600–605. 10.1016/j.bbagen.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Krewulak KD, Vogel HJ. 2008. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta 1778:1781–1804. 10.1016/j.bbamem.2007.07.026 [DOI] [PubMed] [Google Scholar]
- 4.Ballouche M, Cornelis P, Baysse C. 2009. Iron metabolism: a promising target for antibacterial strategies. Recent Pat. Antiinfect. Drug Discov. 4:190–205. 10.2174/157489109789318514 [DOI] [PubMed] [Google Scholar]
- 5.Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D. 2009. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22:615–624. 10.1007/s10534-009-9219-2 [DOI] [PubMed] [Google Scholar]
- 6.Foley TL, Simeonov A. 2012. Targeting iron assimilation to develop new antibacterials. Expert Opin. Drug Discov. 7:831–847. 10.1517/17460441.2012.708335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imperi F, Massai F, Facchini M, Frangipani E, Visaggio D, Leoni L, Bragonzi A, Visca P. 2013. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc. Natl. Acad. Sci. U. S. A. 110:7458–7463. 10.1073/pnas.1222706110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein LR. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 50:665–682 [PubMed] [Google Scholar]
- 9.Bernstein LR. 2013. Gallium, therapeutic effects, p 823–835 In Kretsinger RH, Uversky VN, Permyakov EA. (ed), Encyclopedia of metalloproteins. Springer, New York, NY [Google Scholar]
- 10.Kelson AB, Carnevali M, Truong-Le V. 2013. Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 13:707–771. 10.1016/j.coph.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Bonchi C, Imperi F, Minandri F, Visca P, Frangipani E. 2014. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors 40:303–312. 10.1002/biof.1159 [DOI] [PubMed] [Google Scholar]
- 12.Minandri F, Bonchi C, Frangipani E, Imperi F, Visca P. 2014. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 9:379–397. 10.2217/fmb.14.3 [DOI] [PubMed] [Google Scholar]
- 13.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 117:877–888. 10.1172/JCI30783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E. 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. U. S. A. 105:16761–16766. 10.1073/pnas.0808608105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di Serio C, Döring G, Tümmler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am. J. Respir. Crit. Care Med. 180:138–145. 10.1164/rccm.200812-1943OC [DOI] [PubMed] [Google Scholar]
- 16.Imperi F, Putignani L, Tiburzi F, Ambrosi C, Cipollone R, Ascenzi P, Visca P. 2008. Membrane-association determinants of the omega-amino acid monooxygenase PvdA, a pyoverdine biosynthetic enzyme from Pseudomonas aeruginosa. Microbiology 154:2804–2813. 10.1099/mic.0.2008/018804-0 [DOI] [PubMed] [Google Scholar]
- 17.Cox CD, Graham R. 1979. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J. Bacteriol. 137:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visca P, Ciervo A, Sanfilippo V, Orsi N. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995–2001. 10.1099/00221287-139-9-1995 [DOI] [PubMed] [Google Scholar]
- 19.Meyer JM, Stintzi A, De Vos D, Cornelis P, Tappe R, Taraz K, Budzikiewicz H. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35–43. 10.1099/00221287-143-1-35 [DOI] [PubMed] [Google Scholar]
- 20.Reimmann C. 2012. Inner-membrane transporters for the siderophores pyochelin in Pseudomonas aeruginosa and enantio-pyochelin in Pseudomonas fluorescens display different enantioselectivities. Microbiology 158:1317–1324. 10.1099/mic.0.057430-0 [DOI] [PubMed] [Google Scholar]
- 21.García-Contreras R, Lira-Silva E, Jasso-Chávez R, Hernández-González IL, Maeda T, Hashimoto T, Boogerd FC, Sheng L, Wood TK, Moreno-Sánchez R. 2013. Isolation and characterization of gallium resistant Pseudomonas aeruginosa mutants. Int. J. Med. Microbiol. 303:574–582. 10.1016/j.ijmm.2013.07.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.