Abstract
Patients with inflammatory bowel disease (IBD), namely ulcerative colitis (UC) and Crohn's disease (CD), have worse outcomes with Clostridium difficile infection (CDI), including increased readmissions, colectomy, and death. Oral vancomycin is recommended for the treatment of severe CDI, while metronidazole is the standard of care for nonsevere infection. We aimed to assess treatment outcomes of CDI in IBD. We conducted a retrospective observational study of inpatients with CDI and IBD from January 2006 through December 2010. CDI severity was assessed using published criteria. Outcomes included readmission for CDI within 30 days and 12 weeks, length of stay, colectomy, and death. A total of 114 patients met inclusion criteria (UC, 62; CD, 52). Thirty-day readmissions were more common among UC than CD patients (24.2% versus 9.6%; P = 0.04). Same-admission colectomy occurred in 27.4% of UC patients and 0% of CD patients (P < 0.01). Severe CDI was more common among UC than CD patients (32.2% versus 19.4%; P = 0.12) but not statistically significant. Two patients died from CDI-associated complications (UC, 1; CD, 1). Patients with UC and nonsevere CDI had fewer readmissions and shorter lengths of stay when treated with a vancomycin-containing regimen compared to those treated with metronidazole (30-day readmissions, 31.0% versus 0% [P = 0.04]; length of stay, 13.62 days versus 6.38 days [P = 0.02]). Patients with UC and nonsevere CDI have fewer readmissions and shorter lengths of stay when treated with a vancomycin-containing regimen relative to those treated with metronidazole alone. Patients with ulcerative colitis and CDI should be treated with vancomycin.
INTRODUCTION
Clostridium difficile causes roughly 20% of antibiotic-associated diarrhea and is responsible for significant morbidity. Its incidence has more than doubled over the past decade (1). Historically, C. difficile infection (CDI) has been associated with such risk factors as antibiotic use, immune suppression and dysfunction, prolonged hospital stays, and advanced age (1–3).
Inflammatory bowel disease (IBD) is generally classified as Crohn's disease (CD) or ulcerative colitis (UC), which manifest with intestinal symptoms, including diarrhea and abdominal pain. Patients with IBD are particularly susceptible to the effects of enterocolitic infections, due to underlying alterations in intestinal immunity coupled with often chronic exposure to immunosuppressive therapies. Recent studies have shown that patients with IBD have an increased incidence of developing CDI, with a 2- to 3-fold increase in patients with Crohn's disease and a 4- to 7-fold increase in patients with UC (4–7). Furthermore, patients with underlying IBD who develop CDI experience longer hospital stays and higher rates of colectomy and death than subjects without IBD (5, 8, 9). Consequently, CDI is estimated to have quadrupled the cost of hospitalizations to greater than $1 billion per year relative to matched hospitalizations (1, 10). Several agents have been evaluated for the treatment of CDI, including metronidazole and vancomycin (11).
A randomized, controlled trial by Zar et al. demonstrated that vancomycin for the treatment of CDI was associated with improved outcomes in patients meeting criteria for severe CDI, but it conferred no significant advantage over metronidazole for nonsevere disease (12). Notably, patients with IBD were excluded from this trial, and there are no prospective trials comparing antibiotic regimens among patients with CDI and underlying IBD. Largely on the basis of the study by Zar and colleagues, current treatment guidelines recommend metronidazole for nonsevere CDI and vancomycin for those meeting criteria for severe CDI (13). Several scoring systems exist for risk stratification of CDI (14), but none specifically lists concurrent underlying IBD as sufficient to warrant first-line vancomycin. We therefore aimed to assess treatment outcomes of hospitalized patients with CDI and concurrent IBD stratified by CDI severity in order to optimally inform antibiotic selection in this high-risk population.
(This work was presented in part as an oral presentation at Digestive Disease Week, San Diego, CA, 19 to 22 May 2012.)
MATERIALS AND METHODS
Study design and patient selection.
The study was a retrospective, observational study of patients with CDI and concurrent IBD who were hospitalized at Cedars-Sinai Medical Center between 1 January 2006 through 31 December 2010. Cases of CDI were identified from the microbiology laboratory database, and the study included only confirmed cases based on either an enzyme-linked immunoassay for toxins A and B (prior to 1 December 2009) or a two-step algorithm with initial glutamate dehydrogenase (GDH) antigen screening followed by a confirmatory PCR C. difficile toxin assay for positive screening tests (after 1 December 2009). IBD cases were identified using hospital discharge ICD9 codes 555.0 through 556.9. A positive C. difficile test was required within 7 days of admission. Subjects identified as having both CDI and IBD were included for record review to confirm both CDI and IBD through review of hospital notes, gastroenterology consultations, and prior endoscopy, pathology, laboratory, and imaging reports to establish a diagnosis of IBD for at least 6 months. In addition, subjects were required to have stool frequency of ≥3 loose stools in a 24-h period within 48 h of the diagnosis of CDI (9). Patients who had requested to be excluded from participation in research studies were not included. The study was approved by our institutional review board.
Data obtained from medical records included demographics, Montreal classification for IBD phenotype, comorbidities, antibiotic exposure up to 8 weeks prior to CDI, use of corticosteroids (prednisone, prednisolone, or budesonide at any dose) at the time of admission, history of malignancy, active and prior IBD treatments, including biologics, immune modulators, and 5-aminosalicylic acids (5-ASAs), the pertinent lab values, including complete cell count, white blood cell (WBC) differential, serum creatinine and albumin, IBD-associated serologies, colonoscopy or sigmoidoscopy findings within 1 week of CDI diagnosis, cross-sectional imaging, and whether ICU admission occurred during the index admission.
CDI treatment was documented and included metronidazole (oral and/or intravenous) and vancomycin (oral and/or rectal); concomitant antibiotics were also recorded. Of note, no subjects were treated with fidaxomicin, stool transplant, or investigational agents for CDI during the study period. For purposes of analysis, subjects were classified into two mutually exclusive treatment arms. These included (i) those treated with metronidazole only and (ii) those who received a vancomycin-containing regimen, which included receipt of vancomycin alone, concurrent vancomycin and metronidazole, or vancomycin after metronidazole.
Assessment of CDI severity.
To define CDI severity, we used criteria defined by Zar and colleagues, who utilize a point-based system in which “severe CDI” is defined as 2 or more points and “nonsevere CDI” as less than 2 points (12). For severity assessment, 2 points each are allocated for endoscopic evidence of pseudomembranes or intensive care unit (ICU) admission for CDI. One point is given for each of the following: age of >60 years, temperature above 38.3°C, serum albumin concentration below 2.5 mg/dl, and a peripheral WBC count of greater than 15,000 cells/mm within 48 h of diagnosis of CDI.
Assessment of efficacy.
The primary outcomes for this study included length of stay at index admission, CDI-related readmission at 30 days and 12 weeks, colectomy at index admission, and death during index admission. In addition, we assessed “sustained response” and “recurrence.” A sustained response was defined as a case not having an inpatient readmission, emergency room visit, or outpatient visit for CDI symptoms for 12 weeks after initial resolution of symptoms. A recurrence was defined as a readmission for CDI symptoms after an initial response and was measured at both 30 days and 12 weeks after discharge.
Statistical design and analysis.
The primary analyses included assessment of each of the 5 primary treatment outcomes (length of stay, 30-day and 12-week readmission, colectomy, and death) stratified by CDI severity (severe or nonsevere), with separate analyses performed for UC and CD. Missing data were not imputed. Continuous variables were compared using Student's t test, and categorical variables were compared using chi-square or Fisher's exact test. JMP version 9 (SAS Institute, Cary, NC), R statistical software (R Development Core Team, General Public License), and Stata SE (Stata Corp., College Station, TX) were used for the analysis.
RESULTS
Demographics.
We identified 5,120 subjects hospitalized with CDI during the study period; of these, 114 patients were identified as having underlying IBD and CDI as a primary reason for admission. Sixty-two patients carried a diagnosis of UC, and 52 had CD (Table 1). The average ages at the time of CDI diagnosis were 42.1 years for those patients with UC and 38.5 years for those with CD. Males comprised 36% of UC patients and 60% of CD patients (P = 0.02). Overall, 30 patients (26%) had severe disease, and 84 had nonsevere disease (Table 2). Fifty-five percent of UC patients and 38% of CD patients (P = 0.21) had antibiotic exposure within 8 weeks of developing CDI. Thirty-two percent of UC patients met criteria for severe CDI compared to 19% of CD patients (P = 0.12). Regarding IBD colonic involvement, as expected, 100% of UC patients had colonic involvement compared to 14% of CD patients. Fifty-two percent of UC patients were taking steroids (systemic and/or topical) at the time of admission for CDI compared to 27% of CD patients (P = 0.01). Similar percentages of patients with UC and CD were taking immune modulators (27.4% versus 26.9%; P = 0.42) and biologics (P = 0.38) at the time of admission. More patients with UC were using 5-ASA therapy than CD patients on admission (48.4% versus 25%; P = 0.02).
TABLE 1.
Patient characteristics at the time of index admission
| Condition (n) | Avg age (yr) | % (no.) of patients |
|||
|---|---|---|---|---|---|
| Male | Antibiotic exposure within 8 wk | Severe CDIa | Steroid exposurea | ||
| Ulcerative colitis (67) | 43.4 | 38.8 (26) | 55.2 (37) | 34.2 (23) | 50.7 (34) |
| Crohn's disease (58) | 40.4 | 60.3 (35) | 44.8 (26) | 19.0 (11) | 24.1 (14) |
P ≤ 0.05.
TABLE 2.
Criteria for classification of C. difficile severitya
| Disease classification (no. of patients) | No. (%) of patients with: |
|||||
|---|---|---|---|---|---|---|
| WBC count of >15,000 | Albumin concn of <2.5 mg/dl, | Temp of >38.3°C | Age of >60 yr | ICU admission | Pseudomembrane(s) | |
| Severe (30) | 10 (33.3) | 7 (23.3) | 16 (53.5) | 14 (46.7) | 7 (23.3) | 4 (13.3) |
| Nonsevere (84) | 6 (7.1) | 2 (2.4) | 8 (9.5) | 4 (4.8) | 0 (0) | 0 (0) |
Per reference 12. Severity was scored as follows (with 2 points or more indicating severe disease): 1 point for a WBC count of >15,000 cells/mm within 48 h of diagnosis, age of >60 years, temperature of >38.3°C (100.94°F), and albumin concentration of <2.5 mg/dl; 2 points for endoscopic evidence of pseudomembranes. In addition, 1 point was given for ICU admission due to CDI.
Overall outcomes of CDI for UC versus CD.
Patients with UC had significantly higher rates of readmission than those with CD at 30 days (24% versus 10%; P = 0.04) and at 12 weeks (29% versus 13%; P = 0.04) (Fig. 1). The lengths of stay at the index admission were 12.39 days for patients with UC and 9.44 days for those with CD (P = 0.44). Colectomy during index admission occurred in 27.4% of UC patients compared to 0% of CD patients (P < 0.01). Death occurred in 2.0% (n = 1) of UC patients and 2% (n = 1) of CD patients (P = 1.00).
FIG 1.
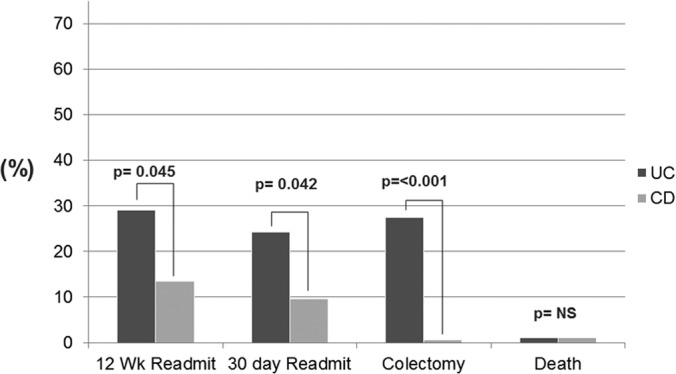
Outcomes of C. difficile infection for Crohn's disease versus ulcerative colitis. Readmit, readmission; NS, not significant.
Treatment.
Sixty-three percent of patients with UC and 64% of patients with CD who had CDI were treated with metronidazole alone, and 36% of those with UC and 29% of those with CD received a vancomycin-containing regimen (Table 3). Among these, only 8 patients with UC and 3 with CD received vancomycin alone. A switch in treatment from metronidazole to vancomycin occurred in 15% of UC patients and 15% of CD patients. No patients switched from vancomycin to metronidazole. A total of 1.6% (1) of UC patients and 7.7% (4) of CD patients received no treatment for a variety of reasons discussed below.
TABLE 3.
Treatment regimens for C. difficile infection among hospitalized patients with ulcerative colitis and Crohn's disease
| Treatment | % (no.) of patients witha: |
|
|---|---|---|
| Ulcerative colitis (n = 67) | Crohn's disease (n = 58) | |
| Metronidazole only | 64.2 (43) | 62.1 (36) |
| Vancomycin-containing regimen | 34.3 (23) | 29.3 (17) |
| Vancomycin only | 11.9 (8) | 7 (4) |
| Vancomycin with metronidazole | 7.4 (5) | 8.6 (5) |
| Initial metronidazole switched to vancomycin | 14.9 (10) | 13.8 (8) |
| None | 1.5 (1) | 8.6 (4) |
There were no significant differences between ulcerative colitis and Crohn's disease results.
Outcomes by severity and treatment regimen in CDI. (i) Crohn's disease with severe C. difficile infection.
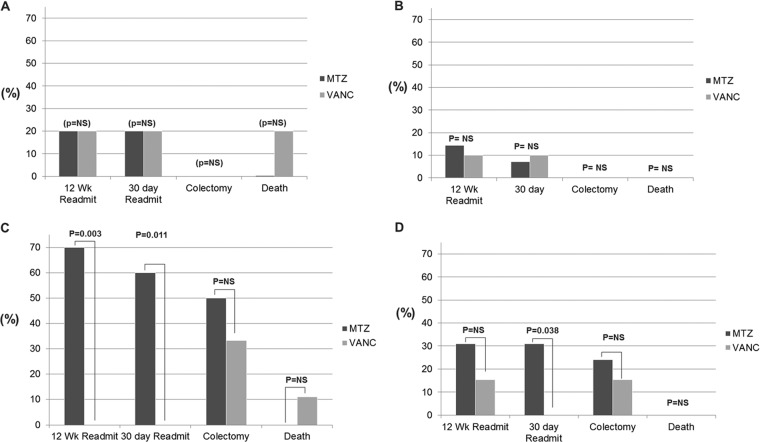
Ten of 52 patients with CD met criteria for severe CDI. Five patients were treated with metronidazole alone, and 5 were treated with a vancomycin-containing regimen. Treatment regimen was not associated with significant differences for readmission at 12 weeks and 30 days, colectomy, or death (Fig. 2A). The length of stay was 9.2 days for the metronidazole-only regimen group compared to 12.0 days for the vancomycin-containing regimen (P = 0.30).
FIG 2.
Outcomes by antibiotic grouping stratified by severity. (A) Severe CDI, Crohn's disease; (B) nonsevere CDI, Crohn's disease; (C) severe CDI, ulcerative colitis; (D) nonsevere CDI, ulcerative colitis. MTZ, metronidazole; VANC, vancomycin; NS, not significant.
(ii) Crohn's disease with nonsevere C. difficile infection.
There were 38 patients with CD and CDI who did not meet criteria for severe disease. Three patients were discharged or transferred prior to initiation of therapy, and one declined treatment. When stratified by treatment regimen, there were no significant differences for readmissions at 30 days or 12 weeks. There were no colectomies or deaths in this group (Fig. 2B). The lengths of stay were 9.4 days for those treated with metronidazole alone and 9.8 days for those treated with a vancomycin-containing regimen (P = 0.85).
(iii) Ulcerative colitis and severe C difficile infection.
A total of 20 out of 62 patients had UC and severe CDI. Ten were treated with metronidazole alone, while 9 received a vancomycin-containing regimen. One patient did not receive treatment. At 12 weeks, 7 of the 10 (70%) metronidazole-treated patients had been readmitted, and none of those treated with vancomycin were readmitted (P = 0.03). At 30 days, 6 of the 10 (60%) metronidazole-treated patients had been readmitted, and none of the vancomycin-treated patients was readmitted P = 0.1.
The mean hospital lengths of stay were 11.4 days for patients treated with metronidazole alone and 19.0 days for those who received a vancomycin-containing regimen (P = 0.06). Rates of colectomy were similar among treatment groups, comprising 5 of 10 (50%) patients who had received metronidazole alone and 3 of 9 (33%) patients who had received a vancomycin-containing regimen (P = 0.650) (Fig. 2C). There was one death in the vancomycin-treated group, and there were no deaths in the metronidazole-treated group.
(iv) Ulcerative colitis and nonsevere C. difficile infection.
There were 42 patients with UC and nonsevere CDI. Twenty-nine were treated with metronidazole alone, and 13 were treated with a vancomycin-containing regimen. Readmission at 30 days was 31% for patients treated with metronidazole alone; there was 0% readmission for those treated with a vancomycin-containing regimen. (P = 0.04) At 12 weeks, readmission for the metronidazole-alone group was 31% relative to 15.4%for the vancomycin-treated group (P = 0.45). The colectomy rates during the index admission were 24% in the metronidazole-only-treated group and 15% among those treated with vancomycin (P = 0.70) (Fig. 2D). There were no deaths among those with UC and nonsevere CDI. The lengths of stay were 13.62 days and.6.4 days for patients treated with metronidazole alone and vancomycin-treated patients, respectively (P = 0.02) (Fig. 3).
FIG 3.
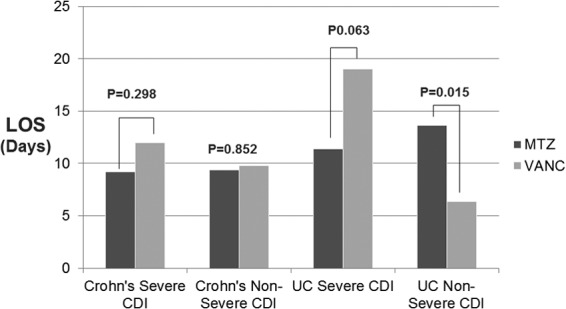
Antibiotic selection in C. difficile infection and effects on length of stay (LOS) in patients with Crohn's disease and ulcerative colitis. MTZ, metronidazole; VANC, vancomycin.
(v) Steroid use.
At the time of admission, 40.4% of all patients were receiving oral corticosteroids. Forty percent of patients using steroids met criteria for severe disease compared to 20.0% of those not using steroids at the time of admission (P = 0.04). Twenty-eight percent of patients on steroids at the time of index admission were readmitted at 12 weeks compared to 16% of those not on steroids (P = 0.09). Readmission rates at 30 days were 29% for patients on steroids compared to 8% for those not on steroids (P < 0.01). Colectomy during index admission occurred in 32% of patients on steroids at the time of admission compared to 6% of those not on steroids (P < 0.01). Of the 4 patients who died during the study period, 2 were on steroids at the time of admission. The lengths of stay were 10.2 days for patients using steroids and 11.0 days for those not on steroids at the time of admission (P = 0.63).
DISCUSSION
Infection with C difficile in IBD appears to be an increasingly important and expensive problem, with rising rates of infection and more severe outcomes than those in the non-IBD population (8). It is unclear whether this is due to altered immunity or genetic susceptibility (15) and whether treatment responses in patients with IBD might therefore warrant different considerations than those without IBD. There is a paucity of data on the most appropriate therapeutic regimens for patients with IBD (16), and guidelines thus lack evidence to support current recommendations based on CDI severity that do not separately consider the diagnosis of IBD in severity criteria. We aimed to retrospectively assess outcomes of hospitalized patients with IBD stratified by CDI disease severity who were treated with metronidazole versus vancomycin.
Our study has several important findings. First, we found that outcomes among vancomycin-treated patients with UC are better than those treated with metronidazole alone, regardless of whether criteria for severity are fulfilled. Specifically, vancomycin-treated subjects had significantly reduced rates of readmission and decreased length of stay with nonsevere infection. This suggests that patients with ulcerative colitis should be treated with a vancomycin-containing regimen, irrespective of whether criteria for severe disease are fulfilled. In contrast, treatment regimen does not appear to be clearly associated with differences in outcomes among patients with CD and nonsevere CDI, suggesting that treatment with metronidazole in this population may be appropriate. Second, we found that colectomy during the index admission was significantly greater among patients with UC than among those with Crohn's disease, further demonstrating the importance of more aggressive treatment of patients with UC. Third, we found that the use of corticosteroids at the time of admission in patients with ulcerative colitis and C. difficile was associated with double the risk for severe CDI, triple the risk of readmission at 30 days, and a 4-fold risk for colectomy compared to the risk in patients not on corticosteroids at the time of admission. Furthermore, risk of colectomy was higher among patients treated with corticosteroids regardless of CDI severity.
Finally, we found that the demographics, traditional risk factors, and outcomes for CDI in our hospitalized IBD population differ from published data for patients without IBD but are similar to those in other published IBD cohorts (5, 6, 12). Notably, our patients with IBD and concurrent CDI were on average younger (42 years) than published statistics for hospitalized patients without IBD (58 to 72 years) (6, 17), and only 45% of our subjects had recent antibiotic exposure, which is somewhat less than published rates of 60 to 80% in the non-IBD population (1, 4, 10). Importantly, our IBD population had increased rates of severe CDI relative to published rates for patients without IBD and associated increased rates of readmission and colectomy. The 30-day readmission rate for CDI was 24% among patients with UC, which is much higher than published rates of 3 to 9% reported for non-IBD patients (9, 10). Colectomy occurred in no patients with CD but in 27.4% of patients with UC during the index admission, which is much higher than rates reported in non-IBD patients (0.4 to 2%) (12, 17). Death occurred in 2% of patients overall in our study. This is higher than mortality rates in non-IBD patients with CDI (<1%); however, it is consistent with literature showing increased mortality in IBD patients with CDI as high as 5% (6, 8, 10).
Steroid use appears to be associated with worse outcomes, including significantly increased rates of severe CDI, 30-day readmissions, and colectomy compared to those in patients not on steroids at the time of admission. It is unclear why patients on steroids have worse outcomes when they develop CDI. Whether this is due to increased immune suppression and risk for systemic infection or is a marker of severe underlying IBD deserves further investigation.
Assessments of the relative efficacy of metronidazole versus vancomycin warrant discussion of antimicrobial resistance, cost, and tolerability. While C. difficile resistance to vancomycin is rare, the potential for the emergence of vancomycin-resistant enterococcus and other pathogens may be important (13). Furthermore, given that vancomycin is significantly more expensive than generically available metronidazole, it has typically been relegated to second-line therapy (for those who fail or are intolerant of metronidazole) or reserved for severe CDI infection in various CDI treatment guidelines and hospital formularies. However, cost differences may be less relevant when compounded oral formulations of parenteral vancomycin are used, which may be effective against CDI. These cost differences may be offset by significant reductions in length of stay that were identified among patients with ulcerative colitis and nonsevere CDI and thus may warrant further evaluation of cost-effectiveness.
Our colectomy rate of 27% is much higher than rates reported in population-based and multicenter studies, which range from 5 to 12% in patients with IBD (8, 18, 19). In contrast, studies performed at tertiary care centers have demonstrated much higher rates of colectomy, ranging from 36 to 44% at 1 year (20–22). Thus, our colectomy rate is consistent with these reports but may limit the generalizability of our findings to similar tertiary care hospitals.
There are several limitations to our study. First, few patients received vancomycin as a single, first-line agent due to existing guidelines and our hospital policy that limits the use of vancomycin. We therefore combined vancomycin-containing regimens, including for patients who failed metronidazole and were switched to vancomycin, a group for whom outcomes might be expected to be worse given failure of first-line therapy. Although we demonstrated improved length of stay and 30-day readmission among patients with nonsevere UC treated with a vancomycin-containing regimen, we did not find a statistically significant improvement in the harder endpoints of colectomy or death. Second, we were unable to clearly distinguish IBD flare from CDI, which raises uncertainty as to whether readmissions or even colectomies were due to refractory or relapsing CDI or underlying IBD. Our institutional policy (consistent with Infectious Diseases Society of America [IDSA] guidelines) (13) discourages rechecking for CDI infection within 30 days of a positive test, given the possibility of testing positive for a resolving infection even after a course of therapy. However, we required that readmissions without a toxin-positive confirmation of CDI be associated with symptoms of diarrhea, with at least 3 loose bowel movements daily. Third, given the open nature of our health care system, we were unable to assess outcomes (readmission, colectomy, and death) that occurred outside our institution. Fourth, the higher rates of colectomy among patients with UC relative to CD may intuitively reflect the presence of underlying colonic versus small bowel disease and potentially reflect the clinical recommendation for a “curative” colectomy in a patient with UC and concurrent CDI that might not apply to a patient with Crohn's disease. We were unable to discern outcomes among patients with Crohn's disease primarily involving the colon relative to those without significant colonic involvement, given the low numbers of subjects in the former group (n = 6). However, we would speculate that extensive colonic Crohn's is likely associated with worse CDI and might approach outcomes similar to UC. Interestingly, we found that patients with UC and severe CDI had a longer length of stay when treated with vancomycin compared to metronidazole. Although these results were not statistically significant (P = 0.06), they were intriguing and at a glance provide some contradiction of our main conclusions that vancomycin is superior to metronidazole in these patients. A likely explanation is that in our study, patients with severe CDI were more often initially treated with metronidazole and then switched to vancomycin, usually as a result of worsening disease. However, because we decided this switch was considered part of a “vancomycin-containing regimen,” similar to those for patients initially treated with vancomycin alone and those treated with both vancomycin and metronidazole, this may overestimate the illness and eventual length of stay for this patient population. Finally, we did not assess treatment with alternative therapies, including fidaxomicin, which was approved for CDI after the conclusion of our study period, rifaximin (23), or fecal microbacterial therapy (24), which is considered experimental.
Despite these limitations, our study represents one of the largest retrospective analyses of CDI treatment outcomes in IBD patients. We demonstrate that there may be an association between vancomycin treatment and reduced length of stay and readmission rates among patients with nonsevere CDI relative to those treated with metronidazole. On this basis, we believe a prospective trial is warranted to evaluate CDI therapy in patients with IBD, particularly those with UC. Until such a trial can be conducted, we recommend vancomycin as the treatment for CDI among patients with UC.
ACKNOWLEDGMENT
The authors of this article have no relevant financial disclosures.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Leffler DA, Lamont JT. 2009. Treatment of Clostridium difficile-associated disease. Gastroenterology 136:1899–1912. 10.1053/j.gastro.2008.12.070 [DOI] [PubMed] [Google Scholar]
- 2.Das R, Feuerstadt P, Brandt LJ. 2010. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with Clostridium difficile-associated disease. Am. J. Gastroenterol. 105:2040–2049. 10.1038/ajg.2010.142 [DOI] [PubMed] [Google Scholar]
- 3.Leung S, Metzger BS, Currie BP. 2010. Incidence of Clostridium difficile infection in patients with acute leukemia and lymphoma after allogeneic hematopoietic stem cell transplantation. Infect. Control Hosp. Epidemiol. 31:313–315. 10.1086/651066 [DOI] [PubMed] [Google Scholar]
- 4.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. 2007. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 5:339–344. 10.1016/j.cgh.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 5.Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, Skaros S, Weber LR, Komorowski RA, Knox JF, Emmons J, Bajaj JS, Binion DG. 2007. Impact of Clostridium difficile on inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 5:345–351. 10.1016/j.cgh.2006.12.028 [DOI] [PubMed] [Google Scholar]
- 6.Musa S, Thomson S, Cowan M, Rahman T. 2010. Clostridium difficile infection and inflammatory bowel disease. Scand. J. Gastroenterol. 45:261–272. 10.3109/00365520903497098 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen GC, Kaplan GG, Harris ML, Brant SR. 2008. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 103:1443–1450. 10.1111/j.1572-0241.2007.01780.x [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan AN, McGinley EL, Binion DG. 2008. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 57:205–210. 10.1136/gut.2007.128231 [DOI] [PubMed] [Google Scholar]
- 9.Jen MH, Saxena S, Bottle A, Aylin P, Pollok RC. 2011. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 33:1322–1331. 10.1111/j.1365-2036.2011.04661.x [DOI] [PubMed] [Google Scholar]
- 10.Kyne L, Hamel MB, Polavaram R, Kelly CP. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346–353. 10.1086/338260 [DOI] [PubMed] [Google Scholar]
- 11.Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, Rees R, Taylor B, Wood E, Malakun R. 2011. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst. Rev. 2011:CD004610. 10.1002/14651858.CD004610.pub3 [DOI] [PubMed] [Google Scholar]
- 12.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302–307. 10.1086/519265 [DOI] [PubMed] [Google Scholar]
- 13.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 31:431–455. 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 14.Fujitani S, George WL, Murthy AR. 2011. Comparison of clinical severity score indices for Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 32:220–228. 10.1086/658336 [DOI] [PubMed] [Google Scholar]
- 15.Ananthakrishnan AN, Oxford EC, Nguyen DD, Sauk J, Yajnik V, Xavier RJ. 2013. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment. Pharmacol. Ther. 38:522–530. 10.1111/apt.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodhand JR, Alazawi W, Rampton DS. 2011. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment. Pharmacol. Ther. 33:428–441. 10.1111/j.1365-2036.2010.04548.x [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishnan AN, Binion DG. 2010. Impact of Clostridium difficile on inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 4:589–600. 10.1586/egh.10.55 [DOI] [PubMed] [Google Scholar]
- 18.Ben-Horin S, Margalit M, Bossuyt P, Maul J, Shapira Y, Bojic D, Chermesh I, Al-Rifai A, Schoepfer A, Bosani M, Allez M, Lakatos PL, Bossa F, Eser A, Stefanelli T, Carbonnel F, Katsanos K, Checchin D, Miera IS, Chowers Y, Moran GW. 2009. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 7:981–987. 10.1016/j.cgh.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 19.Murthy SK, Steinhart AH, Tinmouth J, Austin PC, Daneman N, Nguyen GC. 2012. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 36:1032–1039. 10.1111/apt.12073 [DOI] [PubMed] [Google Scholar]
- 20.Navaneethan U, Mukewar S, Venkatesh PG, Lopez R, Shen B. 2012. Clostridium difficile infection is associated with worse long term outcome in patients with ulcerative colitis. J. Crohns Colitis 6:330–336. 10.1016/j.crohns.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 21.Jodorkovsky D, Young Y, Abreu MT. 2010. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig. Dis. Sci. 55:415–420. 10.1007/s10620-009-0749-9 [DOI] [PubMed] [Google Scholar]
- 22.Issa M, Weber LR, Skaros S, Borromeo Beaulieu DM, Emmons J, Knox JF, Lundeen S, Otterson MF, Binion DG. 2007. Decreasing rates of colectomy despite high rates of hospitalization in C. difficile infected IBD patients: a tertiary referral center experience. Gastroenterology 132:A663 [Google Scholar]
- 23.Mattila E, Arkkila P, Mattila PS, Tarkka E, Tissari P, Anttila VJ. 2013. Rifaximin in the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 37:122–128. 10.1111/apt.12111 [DOI] [PubMed] [Google Scholar]
- 24.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. 2012. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment. Pharmacol. Ther. 35:865–875. 10.1111/j.1365-2036.2012.05033.x [DOI] [PubMed] [Google Scholar]