Abstract
Burkholderia cepacia complex (Bcc) pulmonary infections in people living with cystic fibrosis (CF) are difficult to treat because of the extreme intrinsic resistance of most isolates to a broad range of antimicrobials. Fosmidomycin is an antibacterial and antiparasitic agent that disrupts the isoprenoid biosynthesis pathway, a precursor to hopanoid biosynthesis. Hopanoids are involved in membrane stability and contribute to polymyxin resistance in Bcc bacteria. Checkerboard MIC assays determined that although isolates of the Bcc species B. multivorans were highly resistant to treatment with fosmidomycin or colistin (polymyxin E), antimicrobial synergy was observed in certain isolates when the antimicrobials were used in combination. Treatment with fosmidomycin decreased the MIC of colistin for isolates as much as 64-fold to as low as 8 μg/ml, a concentration achievable with colistin inhalation therapy. A liquid chromatography-tandem mass spectrometry technique was developed for the accurate quantitative determination of underivatized hopanoids in total lipid extracts, and bacteriohopanetetrol cyclitol ether (BHT-CE) was found to be the dominant hopanoid made by B. multivorans. The amount of BHT-CE made was significantly reduced upon fosmidomycin treatment of the bacteria. Uptake assays with 1-N-phenylnaphthylamine were used to determine that dual treatment with fosmidomycin and colistin increases membrane permeability, while binding assays with boron-dipyrromethene-conjugated polymyxin B illustrated that the addition of fosmidomycin had no impact on polymyxin binding. This work indicates that pharmacological suppression of membrane hopanoids with fosmidomycin treatment can increase the susceptibility of certain clinical B. multivorans isolates to colistin, an agent currently in use to treat pulmonary infections in CF patients.
INTRODUCTION
Lung damage due to recurrent and chronic pulmonary infections is the major cause of morbidity and mortality in people living with cystic fibrosis (CF). The Burkholderia cepacia complex (Bcc) is composed of at least 18 closely related Gram-negative species with remarkable metabolic versatility (1). This group of bacteria emerged in the 1980s as opportunistic pulmonary pathogens of particular importance to patients with CF (2–4). Bcc bacteria are ominous CF pathogens because of their potential to cause rapid clinical deterioration and death (3). The major challenge to clinical therapy of Bcc pulmonary infections is their innate resistance to a broad range of antimicrobials, including polycationic agents that are typically used to treat other CF pulmonary pathogens (5). Although Burkholderia cenocepacia has widely been considered the most prevalent and virulent Bcc species in CF infections, the proportion of B. multivorans infections is increasing (6), with its incidence in CF patients now exceeding that of B. cenocepacia in the United States and Canada (7, 8). Epidemic outbreaks of B. multivorans causing severe morbidity and mortality in CF patients were described in the United Kingdom (9) and France (10). B. multivorans was selected as the model Bcc species for this study.
To combat increasing antimicrobial resistance, physicians are increasingly turning to polymyxins, especially inhaled colistin (polymyxin E), for therapy of the major CF respiratory pathogen Pseudomonas aeruginosa (11). Polymyxins are rapid-acting bactericidal cationic peptides with detergent-like properties. These agents accumulate in the bacterial membrane and affect selective permeability. Polymyxin therapy is not considered an option for CF patients with Bcc infections because of the high constitutive polymyxin resistance of these bacteria. The intrinsic resistance of Bcc bacteria to polymyxins is multifaceted (12) but is due primarily to unique features of the cell membrane (12, 13). Polymyxin B binds poorly to its lipopolysaccharide (LPS) target sites on intact Bcc cells (14). There is a constitutive presence of 4-amino-4-deoxy-l-arabinose linked to the lipid A phosphate groups in the LPS of Bcc bacteria, eliminating the negative charge required for polymyxin binding (15). The composition of the LPS core oligosaccharide is also responsible in part for the resistance of B. cenocepacia to polymyxin B (16, 17).
Beyond these issues of binding, the contribution of membrane hopanoids to polymyxin resistance in the Bcc has been revealed recently (18–20). Hopanoids are pentacyclic triterpenoid lipids that are analogues of cholesterol in prokaryotic membranes. They are involved in membrane stability and barrier function (21) and contribute to the maintenance of membrane fluidity and permeability (21, 22). Not all bacteria produce hopanoids, and few bacteria associated with human infections possess the genetic machinery necessary for their biosynthesis (18). Yet the key hopanoid biosynthesis gene shc, which encodes the squalene-hopene cyclase, is distributed throughout the sequenced Bcc species (18). Hopanoids have been isolated from a variety of Burkholderia spp. but not from the closely related and typically polymyxin B-sensitive Pseudomonas spp. and Ralstonia spp. (23).
B. cenocepacia hopanoid biosynthesis mutants are more susceptible than their wild-type parents to antimicrobials, including polymyxin B (20), as well as membrane-disrupting agents, including chlorhexidine (19) and sodium dodecyl sulfate (SDS) (20). In B. multivorans, hopanoid biosynthesis specifically contributes to resistance to polymyxins (polymyxin B and colistin) (18). The hopanoid biosynthesis mutants exhibited an up-to-8-fold increase in polymyxin B susceptibility and a 16-fold increase in colistin susceptibility compared to the parent B. multivorans strain (18). This increased susceptibility to polymyxins was demonstrated to be due to altered membrane permeability in the hopanoid biosynthesis mutants and not to an alteration of polymyxin binding capacity (18).
Fosmidomycin is a phosphonic acid derivative with antibacterial and antiparasitic activities. This agent acts exclusively on enzymes of the nonmevalonate pathway of isoprenoid biosynthesis by directly inhibiting 1-deoxy-d-xylulose 5-phosphate reductoisomerase and indirectly inhibiting a downstream enzyme, methylerythritol phosphate cytidyltransferase (IspD) (24). Bacteria synthesize isoprenoids to serve as the prenyl chains of the ubiquinones and menaquinones of the electron transport chains, as carbohydrate carriers for the biosynthesis of peptidoglycan, or as precursors to hopanoid biosynthesis (25). Bcc bacteria are highly resistant to fosmidomycin because of insufficient uptake of the agent into the bacteria and the presence of an efflux pump that efficiently transports any internalized fosmidomycin out of the cell (26).
Although Bcc species are highly resistant to monotherapy with either fosmidomycin or polymyxins, here we sought to test the hypothesis that inhibition of isoprenoid biosynthesis by fosmidomycin would reduce the concentration of hopanoids in the bacterial membrane and potentiate the inhibitory effects of polymyxins on B. multivorans growth. This work serves as proof of the principle that suppressing the concentration of hopanoids in the bacterial membrane can increase the susceptibility of particular clinical B. multivorans strains to antimicrobials that are currently used to treat P. aeruginosa infections, thereby providing physicians with a new therapeutic option to consider for B. multivorans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
B. multivorans ATCC 17616 and P. aeruginosa ATCC 27853 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The B. multivorans hopanoid biosynthesis mutants 26D7 (Bmul_2133::Tn5) and RMI17 (ΔBmul_2134) were constructed for our previous study (18). B. multivorans clinical isolates are part of the Canadian Bcc Research and Referral Repository (CBCCRRR), housed in D. P. Speert's laboratory (27). B. cenocepacia K56-2 (28) was a gift from P. A. Sokol of the University of Calgary. All strains were stored at −70°C in cation-adjusted Mueller-Hinton II (MH) broth with 8.0% (vol/vol) dimethyl sulfoxide. For routine bacterial growth, Luria-Bertani or MH broth was used. Unless otherwise stated, bacteria were incubated at 37°C and liquid cultures were grown with aeration. Bacteriological medium components were purchased from Becton, Dickinson and Company (Sparks, MD), and all chemicals and antibiotics were from Sigma-Aldrich Canada Ltd. (Oakville, ON, Canada) unless otherwise stated. Fosmidomycin was purchased from Life Technologies (Burlington, ON, Canada). MH broth was used for antimicrobial susceptibility testing, polymyxin B binding, membrane permeability assays, and quantitative hopanoid analysis.
Antimicrobial sensitivity testing.
The MICs of all single antibiotics were determined by the standard microtiter broth dilution method in accordance with the protocols outlined by the Clinical and Laboratory Standards Institute (29). MICs of polycationic antimicrobials were determined in 96-well polypropylene plates (Corning Inc., Corning, NY). Interactions between fosmidomycin and colistin were evaluated by using checkerboard broth titrations in 96-well plates. The fractional inhibitory concentration (FIC) was calculated as the MIC of the antimicrobial in combination with 256 μg/ml fosmidomycin divided by the MIC of the antimicrobial alone. FIC indices (FICIs) cannot be determined for Bcc species, as they are highly resistant to fosmidomycin and a MIC cannot be determined. Thus, definitions of synergy were based on the FICs of colistin; synergy was defined as a FIC of ≤0.25, and additive activity was defined as a MIC of >0.25 and <1, i.e., one-half of the typical FICI cutoffs for synergy and addition (30). Colistin Etests were purchased from bioMérieux (St. Laurent, QC, Canada) and performed in accordance with the manufacturer's instructions.
Clinical data.
B. multivorans clinical isolates were collected as part of routine surveillance. Patient data, including colistin treatment information, was collected with Research Ethics Board approval during a previous retrospective clinical chart review of patients attending the child and adult CF clinics in Vancouver, BC, Canada, between 1981 and 2008 (31).
Total lipid extraction.
B. multivorans isolates were grown in MH broth overnight and subcultured into 15 ml of MH with or without 256 μg/ml of fosmidomycin and grown at 37°C with shaking at 200 rpm until the early stationary phase (optical density at 600 nm [OD600] of approximately 1.4). The bacteria were collected by centrifugation at 4,000 × g for 10 min and washed twice with 10 ml phosphate-buffered saline (PBS). The cells were resuspended in 10 ml of PBS and then heat killed at 80°C for 30 min. The heat-killed bacteria were frozen and lyophilized until dry. Lyophilized cells (11 to 19 mg) were transferred into 20-ml glass vials and suspended in 2 ml water–5 ml methanol–2.5 ml dichloromethane (DCM). The suspended cells were sonicated in a bath sonicator (VWR B2500A-DTH; 42-kHz radio frequency power, 85 W) for 1 h at room temperature (RT), followed by the addition of 7.5 ml of DCM. The samples were vortexed and allowed to separate for 1 h before the lower DCM layer was collected and dried at RT overnight. Samples were stored at −20°C. The yield of total lipids was 7.3% ± 1.2% of the dry cells.
UPLC-MS analysis.
Protocols for total-lipid analysis by ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) were based on a Waters application note (32) with modifications. The total lipids were dissolved in isopropanol-acetonitrile-water (2:1:1) at a final concentration of 1 mg/ml. Bath sonication was used to facilitate the dissolution of the lipids. The samples (5 μl/injection, three replicates per sample, all in randomized order) were separated by a charged surface hybrid (CSH) C18 column (Waters Acquity UPLC CSH C18, 2.1 by 100 mm, 1.7 μm) and analyzed with a Waters LC-tandem MS (LC-MS/MS) system (Acquity I class UPLC with a Xevo G2-S time-of-flight [TOF] mass spectrometer). The column was maintained at 55°C and eluted with a binary solvent system containing solvent A (acetonitrile-water, 60:40) and solvent B (isopropanol-acetonitrile, 90:10), both with 10 mM ammonium formate and 0.1% formic acid. The flow rate was set at 400 μl/min, and the elution program started at 40% B, increased linearly to 43% B in 2 min, increased to 50% B in 0.1 min, increased linearly to 54% B over 9.9 min, jumped to 70% B in 0.1 min, increased linearly to 99% B over 5.9 min, decreased immediately to 40% B in 0.1 min, and was then maintained at the same level for 1.9 min. The column eluent was ionized by electrospray ionization. MS and high-energy fragmented lipid (MSE) data (interleaved low-energy and collisionally activated scans with argon as the collision gas) were collected in either positive- or negative-ion mode with m/z 100 to 1,500. MSE consists of both low- and high-energy scans obtained simultaneously. The collision energy is changed in concert with alternate pushes in the TOF mass spectrometer. During data analysis, product ions can be associated with parent ions if they are coincident in chromatographic time. Electrospray conditions were a capillary voltage of 2.0 kV, a cone voltage of 30 V, a source offset of 60 V, a source temperature of 120°C, a desolvation temperature of 550°C, a cone gas flow rate of 20 liters/h, and a desolvation gas flow rate of 900 liters/h. TOF MS was done in resolution mode, typically 32,000 m/Δm. The mass axis was calibrated with sodium formate clusters. Leucine enkephalin was used as a mass reference during acquisition. The data were collected in continuum mode and then converted to centroid mode for quantitative analysis with the Quanlynx program (Waters Corporation, Milford, MA).
Membrane permeability to NPN.
Membrane permeability in the presence of increasing concentrations of colistin was assayed by using the hydrophobic fluorescent probe 1-N-phenylnaphthylamine (NPN) as previously described for polymyxin B (18). The excitation and emission wavelengths were set at 350 and 420 nm, respectively, and fluorescence was measured with a Tecan Infinite M200 (Tecan, Durham, NC).
Polymyxin binding assay.
The binding of fluorescent polymyxin to whole bacteria was examined as previously described (18), with the following modifications. BODIPY FL-conjugated polymyxin B (Life Technologies) was used in place of the fluorescent dansyl derivative. BODIPY FL-conjugated polymyxin decreases in fluorescence as it transitions from a soluble to an aggregated or bound form. The assay was read in clear 96-well plates (Corning Inc.) with a Tecan Infinite M200 (Tecan) with an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The data are reported as quenched fluorescence (fluorescence emitted from the negative control minus fluorescence emitted from the bacterial sample).
Statistical analysis.
Prism v5.0 (GraphPad Software) was used to perform statistical analysis. An unpaired Student t test was used to determine the difference between the means of independent samples, and multiple-group comparisons were made by two-way analysis of variance (ANOVA), followed by a Bonferroni posttest. In all cases, a P value of <0.05 was considered significant.
RESULTS AND DISCUSSION
Fosmidomycin potentiates polymyxin inhibition of B. multivorans growth.
B. multivorans is highly resistant to both fosmidomycin and polymyxins (5, 26). To determine if the growth of B. multivorans can be synergistically inhibited by treatment with a combination of colistin and fosmidomycin, initial MIC checkerboard titrations testing a range of fosmidomycin concentrations (64 to 256 μg/ml) with a range of colistin concentrations (8 to 512 μg/ml) were performed with B. multivorans isolate ATCC 17616 (data not shown). Fosmidomycin at a concentration of 256 μg/ml decreased the MIC of colistin 4-fold, from 256 to 64 μg/ml. The FIC was 0.25 (Table 1), indicating synergistic inhibition of the growth of B. multivorans 17616 by these two antimicrobial agents in vitro.
TABLE 1.
Antimicrobial activities of antibiotics, alone and in combination with fosmidomycin, against B. multivorans ATCC 17616
| Class and antimicrobial | MIC (μg/ml) of antimicrobial |
FIC | |
|---|---|---|---|
| Alone | With 256 μg/ml fosmidomycin | ||
| Polymyxins | |||
| Fosmidomycin | >256 | ||
| Colistin (polymyxin E) | 256 | 64 | 0.25 |
| Polymyxin B | 256 | 64 | 0.25 |
| Aminoglycosides | |||
| Gentamicin | 64 | 64 | 1 |
| Tobramycin | 8 | 8 | 1 |
| β-Lactam ampicillin | >256 | >256 | 1 |
To determine if the potentiating effect of fosmidomycin was specific for colistin, other antimicrobial classes that perturb the bacterial membrane were tested in concert with fosmidomycin for inhibition of B. multivorans growth (Table 1). The antimicrobial activities of polymyxin B, the aminoglycosides gentamicin and tobramycin, and the β-lactam ampicillin were evaluated in the presence of 256 μg/ml of fosmidomycin (Table 1). The only classes of antimicrobials that exhibited synergy with fosmidomycin were the polymyxins.
Because the isoprenoid biosynthesis gene BCAL2710ispH in B. cenocepacia and the homologue lytB in B. pseudomallei contribute to polymyxin resistance (12, 33), we wanted to confirm that the mechanism by which fosmidomycin potentiates the effects of colistin is a change in hopanoids rather than other effects from the inhibition of isoprenoid biosynthesis. Our previous work demonstrated that the B. multivorans 17616 hopanoid biosynthesis mutants 26D7 and RMI17 are specifically more susceptible to polymyxin B and colistin (18). The genes impacted in these mutants are hpnJ, which encodes a putative hopanoid biosynthesis-associated radical S-adenosylmethionine protein, and hpnI, which encodes a putative hopanoid biosynthesis-associated glycosyltransferase; both are downstream of isoprenoids in the hopanoid biosynthesis pathway and permit modifications of the hopanoid backbone structure (18). Fosmidomycin treatment of these two mutants did not change their colistin MICs (Table 2), suggesting that fosmidomycin potentiates the susceptibility of B. multivorans to colistin because of the impact of fosmidomycin on the biosynthesis of particular hopanoids (e.g., glycosylated hopanoids).
TABLE 2.
Antimicrobial activities of fosmidomycin and colistin, alone and in combination, against a panel of bacterial isolates and strains
| Strain or isolate | MIC (μg/ml) |
FIC | ||
|---|---|---|---|---|
| Fosmidomycin | Colistin | Colistin with 256 μg/ml fosmidomycin | ||
| B. multivorans | ||||
| ATCC 17616 | >256 | 256 | 64 | 0.25 |
| 26D7 | >256 | 32 | 32 | 1 |
| RMI17 | >256 | 64 | 64 | 1 |
| C6398 | >256 | >512 | >512 | 1 |
| D2433 | >256 | >512 | 8 | <0.016 |
| D2434 | >256 | 64 | 8 | 0.125 |
| D1285 | >256 | >512 | >512 | 1 |
| D2408 | >256 | 64 | 16 | 0.25 |
| B. cenocepacia K56-2 | >256 | >512 | >512 | 1 |
| P. aeruginosa ATCC 27853 | 8 | ≤1 | ≤1 | NAa |
NA, not applicable.
Clinical B. multivorans isolates from CF patients with chronic pulmonary infections have increased susceptibility to colistin that is further potentiated by fosmidomycin.
To investigate if combination therapy with fosmidomycin and inhalation colistin would have clinical utility, we evaluated the synergistic susceptibility of B. multivorans pulmonary isolates from CF patients. We first tested colistin susceptibility in archived sequential clinical isolates of B. multivorans from 10 patients who attended CF clinics in Vancouver, BC, Canada, between 1995 and 2007. The MICs for colistin were determined for 10 genetically related sequential pairs of B. multivorans isolates, as determined by random amplified polymorphic DNA (RAPD) analysis (34), and representing early and late stages of chronic infection. For all but two of the isolates tested, the MICs of colistin were >512 μg/ml (Table 3). Notably, the late-infection isolates from patients A and B had a colistin MIC of 64 μg/ml. To determine if these isolates were globally more susceptible to antimicrobials, the early and late isolates from patients A and B were tested for susceptibility to a panel of antimicrobials of various classes (Table 4). For the isolates from patient A, late isolate D2434 was at least 16- and 8-fold more susceptible to polymyxin B and colistin, respectively, and 2-fold more susceptible to chlorhexidine, than early isolate C6398. Polymyxins and chlorhexidine are cationic agents that act by binding to and disturbing the bacterial membrane. These data suggest that the late isolate from patient A has undergone changes to its membrane during the course of pulmonary infection that led to increased susceptibility to these agents. Isolate D2434 was more resistant to all of the other antimicrobials than C6398 was, except for ampicillin where no change was observed. Late isolate D2408 from patient B was more susceptible to most of the antimicrobials than early isolate D1285 was.
TABLE 3.
MIC of colistin against B. multivorans sequential, genetically related clinical isolates
| Patient and isolate | Isolation date | RAPD type | Colistin MIC (μg/ml)a |
|---|---|---|---|
| A | |||
| C6398 | February 1995 | BM-049 | >512 |
| D2434 | April 2007 | BM-049 | 64 |
| B | |||
| D1285 | September 2004 | BM-044 | >512 |
| D2408 | March 2007 | BM-044 | 64 |
| C | |||
| C8298 | September 1998 | BM-029 | >512 |
| D2156 | August 2006 | BM-029 | >512 |
| D | |||
| C8814 | October 1999 | BM-055 | >512 |
| D0999 | January 2004 | BM-055 | >512 |
| E | |||
| D0400 | November 2002 | BM-055 | >512 |
| D2420 | April 2007 | BM-055 | >512 |
| F | |||
| C7062 | April 1996 | BM-020 | >512 |
| C9496 | January 2001 | BM-020 | >512 |
| G | |||
| C6396 | February 1995 | BM-049 | >512 |
| D0913 | November 2003 | BM-049 | >512 |
| H | |||
| C9861 | September 2001 | BM-032 | >512 |
| D1268 | August 2004 | BM-032 | >512 |
| I | |||
| D1407 | December 2004 | BM-042 | >512 |
| D2324 | January 2007 | BM-042 | >512 |
| J | |||
| C9876 | October 2005 | BM-055 | >512 |
| D2181 | September 2006 | BM-055 | >512 |
MIC determined by broth microdilution.
TABLE 4.
Antimicrobial susceptibilities of early and late B. multivorans isolates from two CF patients
| Class and antimicrobial | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| P. aeruginosa ATCC 27853 | B. multivorans ATCC 17616 | Patient A |
Patient B |
|||
| Early isolate C6398 | Late isolate D2434 | Early isolate D1285 | Late isolate D2408 | |||
| Polymyxins | ||||||
| Colistin | ≤1 | 256 | >512 | 64 | >512 | 64 |
| Polymyxin B | ≤1 | 256 | >512 | 32 | >512 | 32 |
| Aminoglycosides | ||||||
| Gentamicin | ≤1 | 64 | 256 | >512 | >512 | 4 |
| Tobramycin | ≤1 | 4 | 64 | >512 | 64 | 1 |
| Macrolides | ||||||
| Erythromycin | 64 | 64 | 64 | >256 | 64 | 64 |
| Azithromycin | 4 | 4 | 128 | >512 | 128 | 64 |
| Cephalosporin ceftazidime | 1 | 1 | 1 | 64 | 4 | ≤1 |
| Fluoroquinolone ciprofloxacin | ≤1 | ≤1 | ≤1 | 4 | 1 | ≤1 |
| β-Lactam ampicillin | >256 | >256 | >256 | >256 | >256 | >256 |
| Tetracycline | 16 | 4 | 4 | 8 | 8 | 8 |
| Antiseptic chlorhexidine | NDa | 16 | 16 | 8 | 32 | 2 |
ND, not determined.
Temporal changes in the antimicrobial resistance phenotypes of CF Bcc infections have been described, although not yet for polymyxins (35, 36). To further understand the dynamics of colistin resistance in bacteria from these two patients, the colistin MICs for all of the archived B. multivorans isolates from each of the patients were determined (Table 5). After >9.5 years of infection, B. multivorans isolates with increased colistin susceptibility were cultured from this patient. During long-term pulmonary colonization, multiple phenotypic variants of an underlying clonal bacterial population can emerge and become established in the patient's airways (37). The more susceptible isolates were a subpopulation in the lungs of patient A, as isolates with colistin MICs of >256 μg/ml continued to be isolated late in the infection (Table 5). The archived collection from patient B only had four B. multivorans isolates. These isolates were recovered over 3 years of chronic infection. The latest isolate, D2408, was the only one with increased sensitivity to polymyxins (Table 5).
TABLE 5.
Summary of B. multivorans clinical isolates from patients A and B
| Patient and isolate | Isolation date | RAPD type | Colistin MIC (μg/ml)a |
|---|---|---|---|
| A | |||
| C6398 | 21 February 1995 | BM-049 | >256 |
| C7263 | 04 September 1996 | BM-049 | >256 |
| C8610 | 04 May 1999 | BM-049 | >256 |
| C9294 | 15 September 2000 | BM-049 | >256 |
| D0004 | 10 January 2002 | BM-049 | >256 |
| D0496 | 10 January 2003 | BM-049 | >256 |
| D0745 | 27 June 2003 | BM-049 | >256 |
| D0746 | 27 June 2003 | BM-049 | >256 |
| D1375 | 03 November 2004 | BM-049 | 32 |
| D1441 | 18 January 2005 | BM-049 | >256 |
| D1607 | 30 May 2005 | BM-049 | 64 |
| D2122 | 28 June 2006 | BM-049 | >256 |
| D2356 | 28 February 2007 | BM-049 | >256 |
| D2357 | 28 February 2007 | BM-049 | >256 |
| D2433 | 17 April 2007 | BM-049 | >256 |
| D2434 | 17 April 2007 | BM-049 | 64 |
| B | |||
| D1285 | 23 September 2004 | BM-044 | >256 |
| D1446 | 31 January 2005 | BM-044 | >256 |
| D2179 | 15 September 2006 | BM-044 | >256 |
| D2408 | 28 March 2007 | BM-044 | 16 |
MIC determined by Etest.
After establishing that the MICs of colistin for chronic B. multivorans pulmonary isolates can decrease over the course of infection, we assessed if fosmidomycin could further enhance the colistin susceptibility of these isolates. The early and late clinical isolates from patients A and B were tested for fosmidomycin susceptibility alone and for antimicrobial synergy with colistin. All of the clinical isolates had fosmidomycin MICs of >256 μg/ml. With the addition of 256 μg/ml fosmidomycin, the MICs of colistin for D2434 and D2408 were reduced 8- and 4-fold, respectively, over that of colistin alone (Table 2). The FICs of 0.125 and 0.25, respectively, indicate antimicrobial synergy. Isolate D2433 was obtained from patient A on the same day as isolate D2434. This isolate was highly resistant to colistin and fosmidomycin, but treatment of the isolate with 256 μg/ml fosmidomycin decreased the colistin MIC for the isolate >64-fold (FIC of <0.016). This is evidence that even isolates from chronic infections with extreme colistin resistance can become more susceptible to colistin with the addition of fosmidomycin.
There are no available clinical breakpoints for colistin and B. multivorans. As a point of reference for a clinical context, the breakpoint for P. aeruginosa is ≥8 μg/ml (29). A strategy to further enhance the antibacterial effect of antibiotics is to deliver the agents directly to the site of infection by the aerosol route. Routine application of nebulized colistin, alone or in combination with other inhaled or parenteral antibiotics, is often used in CF patients (38). Sputum colistin concentrations of 20 μg/ml can be achieved and maintained for >4 h of treatment with a well-tolerated dose of 200 U of colistin administered by nebulizer (39). The concentrations of fosmidomycin used in our in vitro studies could not be achieved in serum by using well-tolerated doses (40). Aerosolized fosmidomycin or antimicrobials that specifically target hopanoids may prove effective at a physiologically relevant dose.
Mechanism of fosmidomycin and colistin synergy in B. multivorans clinical isolates.
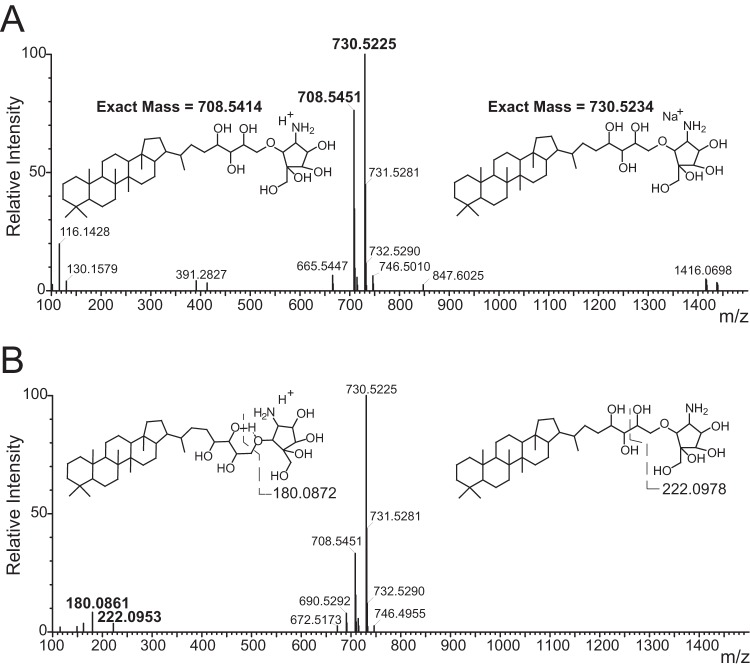
To determine whether the molecular mechanism of the synergy between fosmidomycin and colistin in B. multivorans correlates with the abundance of particular hopanoids, we measured the impact of fosmidomycin on hopanoid production and membrane permeability. As a first step in hopanoid quantification, the hopanoids of B. multivorans were identified. Lipid extracts from B. multivorans clinical isolates C6398 and D2434 were analyzed by a novel LC-MS/MS approach to directly detect nonderivatized hopanoids. One-step total lipid extraction was performed, and the native lipids were analyzed directly without any common derivatization such as acetylation for hopanoids or saponification for phospholipids to minimize artifacts associated with these modifications that could affect lipid quantification. The resulting MS data were then matched against the database in the LIPID MAPS Lipidomics Gateway (http://www.lipidmaps.org) (41). A close hit to a specific hopanoid, bacteriohopanetetrol cyclitol ether (BHT-CE), also known as bacteriohopanetetrol carbapseudopentose ether, was found in the sample (Fig. 1A). This hopanoid has also been identified in other Burkholderia species (23).
FIG 1.
Mass spectra of underivatized BHT-CE identified in B. multivorans. (A) Spectra taken in a low-energy channel in the positive-ion mode showing the protonated (m/z 708.5451) and sodiated hopanoid (m/z 730.5225) molecular ions. The calculated mass and structure are shown. (B) Spectrum taken in a high-energy channel in the positive-ion mode showing the decrease in the intensity of the protonated BHT-CE concomitant with the appearance of its fragmented daughter ions of m/z 180.0861 and 222.0953. The fragmented structures and their calculated masses are shown.
To confirm the identity of this hopanoid, structural information was obtained by fragmentation of the molecular ion under higher collisional energy to form daughter ions. Daughter ions corresponding to breaking of the ether bond and the carbon-carbon bond at the hydroxyl tails were observed (Fig. 1B). A similar fragmentation pattern of BHT-CE in its acetylated form has been observed previously, further supporting our structural assignment (42). While we looked for the presence of other common hopanoids, such as diplopterol, adenosylhopane, bacteriohopanetetrol, and bacteriohopaneaminotriol, in our B. multivorans samples, we could detect only a trace amount of diplopterol (∼1% of the peak intensity of BHT-CE). We therefore focused on the quantification of BHT-CE in B. multivorans.
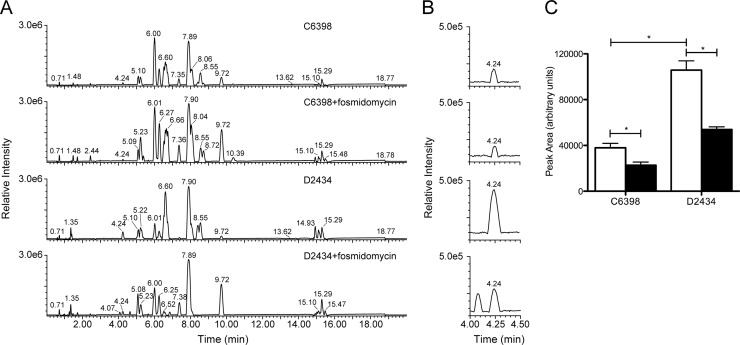
To determine if fosmidomycin alters the amount of BHT-CE in the membrane of B. multivorans isolates, we performed quantitative LC-MS/MS on total lipid extracts in the presence or absence of 256 μg/ml fosmidomycin (Fig. 2). BHT-CE eluted at 4.24 min (Fig. 2B). We verified that there were no analytes coeluting with hopanoids, ensuring that variable ion suppression effects in the electrospray process are similar among all of the data sets. Therefore, the quantitation reported is accurate to the levels claimed. MS signals from the protonated and sodiated hopanoid were integrated, and the results are shown in Fig. 2C. The total lipid extract of D2434 contained significantly more BHT-CE than that of C6398 (P < 0.05; Student's t test). The identification of hopanoid biosynthesis as a dynamic phenotype during the course of infection implies that this phenotype has clinical relevance. Intuitively, the isolate expressing the most hopanoids would be more resistant to membrane-disrupting agents. However, polymyxin resistance in Bcc bacteria is complex, with multiple means of resistance working in concert. Although hopanoids certainly contribute to polymyxin resistance in B. multivorans, they are not essential (18). At any time during the course of an infection, diverse mechanisms could contribute to the polymyxin resistance of any isolate.
FIG 2.
Effect of fosmidomycin on the dominant hopanoid forms (BHT-CE) in B. multivorans clinical isolates. LC-MS base peak chromatogram of total lipids from B. multivorans clinical isolates. Five micrograms of total lipids from each clinical isolate was separated in a hybrid C18 column and analyzed by TOF MS. Representative chromatograms (recorded in the positive-ion mode) of three biological replicates, each containing three technical replicates, are shown. (A) Clinical isolates C6398 and D2434 in the absence or presence of 256 μg/ml of fosmidomycin. (B) Six-fold vertical expansion of the region of panel A where BHT-CE elutes. (C) Graphic representation of the sum of the area of peak integration from the protonated and sodiated hopanoid chromatogram (panel B). Bacteria grown without fosmidomycin (white bars) or with 256 μg/ml of fosmidomycin (black bars). The data are mean values of three technical replicates each of three biological replicate samples (i.e., n = 9) ± the standard errors of the means. Asterisks denote significant differences (P < 0.05; unpaired t test).
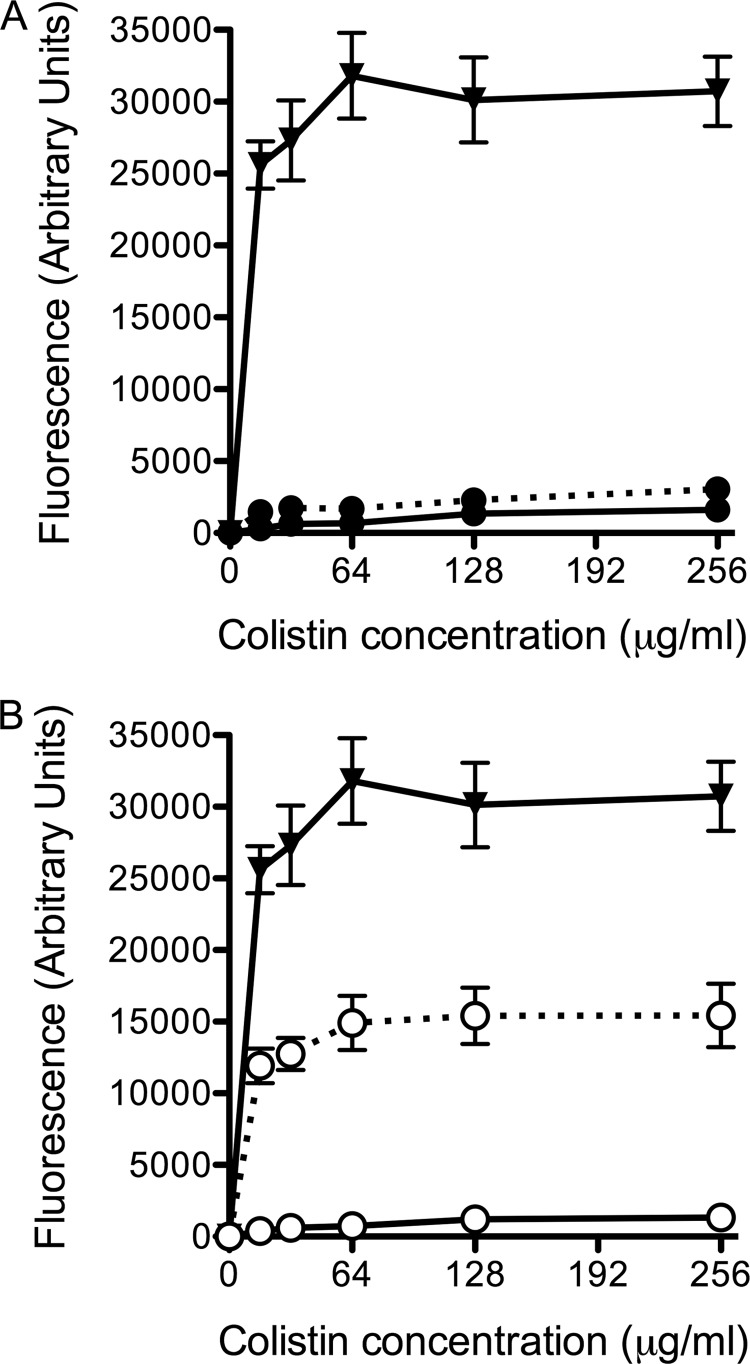
As hypothesized, the addition of fosmidomycin significantly decreased the amounts of BHT-CE in the total lipid extracts of isolates C6398 and D2434 by 40 and 49%, respectively (Fig. 2C). The change in lipids due to fosmidomycin treatment translated into antimicrobial synergy for D2434 but not C6398. To determine if fosmidomycin treatment impacts membrane permeability in both clinical isolates, NPN uptake assays were performed. NPN is a hydrophobic compound that fluoresces weakly in a hydrophilic environment, as in the periplasm of Gram-negative bacteria. An increase in membrane permeability and uptake of NPN is measured as an increase in fluorescence (43). As expected from the antimicrobial synergy data, isolate C6398 remained highly impermeable in the presence of colistin and fosmidomycin (Fig. 3A). Isolate D2434 was remarkably impermeable when treated with colistin alone but was significantly more permeable when treated with 256 μg/ml fosmidomycin at all of the concentrations of colistin tested (P < 0.05; ANOVA, Bonferroni posttest) (Fig. 3B). Colistin-sensitive P. aeruginosa ATCC 27853 was used as a positive control in the assay.
FIG 3.
Effects of increasing concentrations of colistin on the outer membrane permeability of B. multivorans clinical isolates from patient A. Colistin was titrated into suspensions of whole bacteria at an OD600 of 0.5 and 10 μM NPN. An increase in fluorescence corresponded to an increase in membrane permeability and uptake of NPN into the hydrophobic inner and outer membranes. Data were calculated as the fluorescence with colistin minus the fluorescence without colistin and represent the mean values of three biological replicates ± the standard errors of the means. Solid lines; medium alone, dashed lines, 256 μg/ml fosmidomycin. (A) Symbols: ●, B. multivorans C6398; ▼, P. aeruginosa ATCC 27853. (B) Symbols: ○, B. multivorans D2434; ▼, P. aeruginosa ATCC 27853. B. multivorans D2434 was significantly more permeable to NPN in the presence of 256 μg/ml fosmidomycin at all of the concentrations of colistin tested (P < 0.05; ANOVA, Bonferroni posttest).
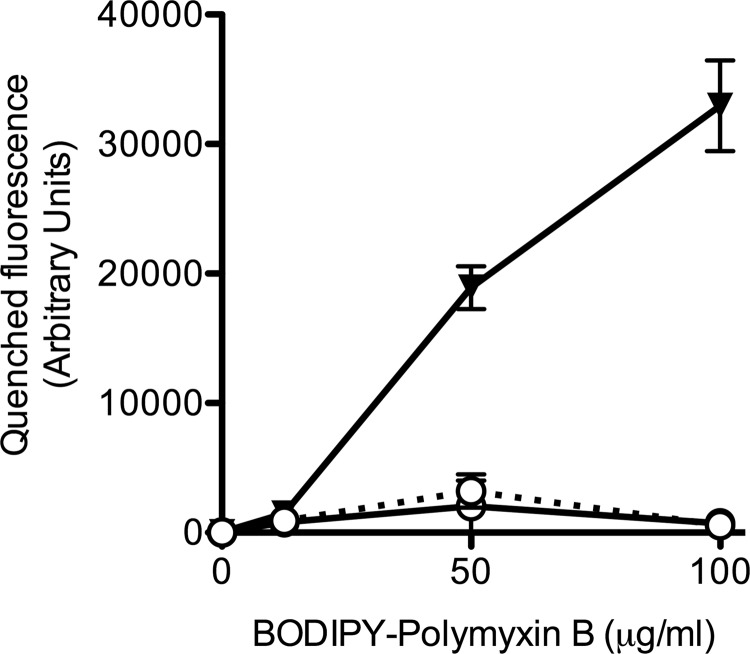
To determine if fosmidomycin treatment has an impact on the binding of polymyxin, the level of antimicrobial binding was evaluated by using BODIPY-conjugated polymyxin B (B-PMX) (Fig. 4). Binding of the conjugated antimicrobial to the bacterial membrane quenches the fluorescence (44). There was no difference in fluorescence when whole cells of D2434 were exposed to B-PMX in the presence or absence of 256 μg/ml of fosmidomycin, suggesting that under such conditions, fosmidomycin does not affect the binding of B-PMX to the outer membrane of D2434. This result is expected, as the increased colistin susceptibility exhibited by the B. multivorans hopanoid biosynthesis mutants is due to altered membrane permeability in the hopanoid biosynthesis mutants but not to an alteration of polymyxin binding capacity (18).
FIG 4.
Binding of fluorescent polymyxin B to whole B. multivorans D2434. Bacterial cells were treated with increasing concentrations of B-PMX, and fluorescence emission at 485 nm upon excitation at 340 nm was determined. Symbols: ○, B. multivorans D2434; ▼, P. aeruginosa ATCC 27853; solid lines, medium alone; dotted line, 256 μg/ml fosmidomycin. The data are the fluorescence of the negative control minus the fluorescence emitted from bacteria with conjugated polymyxin B. Shown are the mean values of three biological replicates ± the standard errors of the means.
The data herein reveal BHT-CE production and polymyxin resistance as heterogeneous phenotypes of Bcc bacteria that are dynamic during the course of infection in CF patients. Our work shows that the pharmacological suppression of membrane hopanoids in certain clinical B. multivorans isolates can potentiate the effects of colistin, an antimicrobial agent currently used to treat P. aeruginosa infections in CF patients. There are few antimicrobial options for the therapy of Bcc infections, and eradication of the bacteria from the lungs of people living with CF is a major challenge (45). Further investigations are necessary to determine if our in vitro data have the potential to translate into a clinical application.
ACKNOWLEDGMENTS
We thank S. D. Gray-Owen of the University of Toronto for generously providing laboratory space and reagents.
R.J.M. received fellowship funding from Cystic Fibrosis Canada (CFC) and the Canadian Institutes of Health Research (CIHR). J.E.A.Z. received fellowship funding from CFC. This work was supported by operating funds from CFC. The UPLC-TOF MS equipment in the California Institute of Technology's Environmental Analysis Center was used in the work described here. This work was also supported by grants from NASA (NNX12AD93G), the National Science Foundation (1224158), and the Howard Hughes Medical Institute (HHMI) to D.K.N. D.K.N. is an HHMI Investigator.
Footnotes
Published ahead of print 23 June 2014
REFERENCES
- 1.Mahenthiralingam E, Baldwin A, Dowson CG. 2008. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J. Appl. Microbiol. 104:1539–1551. 10.1111/j.1365-2672.2007.03706.x [DOI] [PubMed] [Google Scholar]
- 2.Drevinek P, Mahenthiralingam E. 2010. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 16:821–830. 10.1111/j.1469-0691.2010.03237.x [DOI] [PubMed] [Google Scholar]
- 3.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206–210. 10.1016/S0022-3476(84)80993-2 [DOI] [PubMed] [Google Scholar]
- 4.Mahenthiralingam E, Urban TA, Goldberg JB. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144–156. 10.1038/nrmicro1085 [DOI] [PubMed] [Google Scholar]
- 5.Loutet SA, Valvano MA. 2011. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front. Microbiol. 2:159. 10.3389/fmicb.2011.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govan JR, Brown AR, Jones AM. 2007. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2:153–164. 10.2217/17460913.2.2.153 [DOI] [PubMed] [Google Scholar]
- 7.Bumford AA, Spilker T, LiPuma J. 2010. Epidemiology of Burkholderia infection in U.S. CF patients, abstr 1A, p 1. Abstracts of the International Burkholderia cepacia Working Group Meeting, 21 to 24 April 2010, Seattle, WA [Google Scholar]
- 8.Zlosnik JE, Henry DA, Speert DP. 2011. Burkholderia cepacia complex infections in Vancouver and British Columbia from 1981 to 2010, abstr A1, p 1, Abstracts of the International Burkholderia cepacia Working Group Meeting, 14 to 16 April 2011, Prague, Czech Republic [Google Scholar]
- 9.Whiteford ML, Wilkinson JD, McColl JH, Conlon FM, Michie JR, Evans TJ, Paton JY. 1995. Outcome of Burkholderia (Pseudomonas) cepacia colonisation in children with cystic fibrosis following a hospital outbreak. Thorax 50:1194–1198. 10.1136/thx.50.11.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segonds C, Heulin T, Marty N, Chabanon G. 1999. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 37:2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beringer P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 7:434–440. 10.1097/00063198-200111000-00013 [DOI] [PubMed] [Google Scholar]
- 12.Loutet SA, Mussen LE, Flannagan RS, Valvano MA. 2011. A two-tier model of polymyxin B resistance in Burkholderia cenocepacia. Environ. Microbiol. Rep. 3:278–285. 10.1111/j.1758-2229.2010.00222.x [DOI] [PubMed] [Google Scholar]
- 13.Manniello JM, Heymann H, Adair FW. 1978. Resistance of spheroplasts and whole cells of Pseudomonas cepacia to polymyxin B. Antimicrob. Agents Chemother. 14:500–504. 10.1128/AAC.14.3.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore RA, Chan L, Hancock RE. 1984. Evidence for two distinct mechanisms of resistance to polymyxin B in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:539–545. 10.1128/AAC.26.4.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox AD, Wilkinson SG. 1991. Ionizing groups in lipopolysaccharides of Pseudomonas cepacia in relation to antibiotic resistance. Mol. Microbiol. 5:641–646. 10.1111/j.1365-2958.1991.tb00735.x [DOI] [PubMed] [Google Scholar]
- 16.Loutet SA, Flannagan RS, Kooi C, Sokol PA, Valvano MA. 2006. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J. Bacteriol. 188:2073–2080. 10.1128/JB.188.6.2073-2080.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega X, Silipo A, Saldias MS, Bates CC, Molinaro A, Valvano MA. 2009. Biosynthesis and structure of the Burkholderia cenocepacia K56-2 lipopolysaccharide core oligosaccharide: truncation of the core oligosaccharide leads to increased binding and sensitivity to polymyxin B. J. Biol. Chem. 284:21738–21751. 10.1074/jbc.M109.008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malott RJ, Steen-Kinnaird BR, Lee TD, Speert DP. 2012. Identification of hopanoid biosynthesis genes involved in polymyxin resistance in Burkholderia multivorans. Antimicrob. Agents Chemother. 56:464–471. 10.1128/AAC.00602-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sass A, Marchbank A, Tullis E, Lipuma JJ, Mahenthiralingam E. 2011. Spontaneous and evolutionary changes in the antibiotic resistance of Burkholderia cenocepacia observed by global gene expression analysis. BMC Genomics 12:373. 10.1186/1471-2164-12-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmerk CL, Bernards MA, Valvano MA. 2011. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J. Bacteriol. 193:6712–6723. 10.1128/JB.05979-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannenberg EL, Poralla K. 1999. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 86:168–176. 10.1007/s001140050592 [DOI] [Google Scholar]
- 22.Rezanka T, Siristova L, Melzoch K, Sigler K. 2010. Hopanoids in bacteria and cyanobacteria—their role in cellular biochemistry and physiology, analysis and occurrence. Mini Rev. Org. Chem. 7:300–313. 10.2174/157019310792246436 [DOI] [Google Scholar]
- 23.Cvejic JH, Putra SR, El-Beltagy A, Hattori R, Hattori T, Rohmer M. 2000. Bacterial triterpenoids of the hopane series as biomarkers for the chemotaxonomy of Burkholderia, Pseudomonas and Ralstonia spp. FEMS Microbiol. Lett. 183:295–299. 10.1111/j.1574-6968.2000.tb08974.x [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. 2011. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry 50:3570–3577. 10.1021/bi200113y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohmer M, Grosdemange-Billiard C, Seemann M, Tritsch D. 2004. Isoprenoid biosynthesis as a novel target for antibacterial and antiparasitic drugs. Curr. Opin. Investig. Drugs 5:154–162 [PubMed] [Google Scholar]
- 26.Messiaen AS, Verbrugghen T, Declerck C, Ortmann R, Schlitzer M, Nelis H, Van Calenbergh S, Coenye T. 2011. Resistance of the Burkholderia cepacia complex to fosmidomycin and fosmidomycin derivatives. Int. J. Antimicrob. Agents 38:261–264. 10.1016/j.ijantimicag.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 27.Speert DP, Henry D, Vandamme P, Corey M, Mahenthiralingam E. 2002. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8:181–187. 10.3201/eid0802.010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahenthiralingam E, Coenye T, Chung JW, Speert DP, Govan JR, Taylor P, Vandamme P. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard—ninth edition. M07–A9, vol 32 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 30.Yokota Y, Murakawa T, Nishida M. 1981. In vitro synergism of FR-31564, a new phosphonic acid antibiotic. J. Antibiot. 34:876–883. 10.7164/antibiotics.34.876 [DOI] [PubMed] [Google Scholar]
- 31.Zlosnik JE, Costa PS, Brant R, Mori PY, Hird TJ, Fraenkel MC, Wilcox PG, Davidson AG, Speert DP. 2011. Mucoid and nonmucoid Burkholderia cepacia complex bacteria in cystic fibrosis infections. Am. J. Respir. Crit. Care Med. 183:67–72. 10.1164/rccm.201002-0203OC [DOI] [PubMed] [Google Scholar]
- 32.Isaac G, McDonald S, Astarita G. 2011. Lipid separation using UPLC with charged surface hybrid technology. Waters Corporation, Milford, MA [Google Scholar]
- 33.Burtnick MN, Woods DE. 1999. Isolation of polymyxin B-susceptible mutants of Burkholderia pseudomallei and molecular characterization of genetic loci involved in polymyxin B resistance. Antimicrob. Agents Chemother. 43:2648–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeon SA, Nguyen DT, Viteri DF, Zlosnik JE, Sokol PA. 2011. Functional quorum sensing systems are maintained during chronic Burkholderia cepacia complex infections in patients with cystic fibrosis. J. Infect. Dis. 203:383–392. 10.1093/infdis/jiq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coutinho CP, de Carvalho CC, Madeira A, Pinto-de-Oliveira A, Sa-Correia I. 2011. Burkholderia cenocepacia phenotypic clonal variation during a 3.5-year colonization in the lungs of a cystic fibrosis patient. Infect. Immun. 79:2950–2960. 10.1128/IAI.01366-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jassem AN, Zlosnik JE, Henry DA, Hancock RE, Ernst RK, Speert DP. 2011. In vitro susceptibility of Burkholderia vietnamiensis to aminoglycosides. Antimicrob. Agents Chemother. 55:2256–2264. 10.1128/AAC.01434-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahenthiralingam E, Baldwin A, Vandamme P. 2002. Burkholderia cepacia complex infection in patients with cystic fibrosis. J. Med. Microbiol. 51:533–538 [DOI] [PubMed] [Google Scholar]
- 38.Hancock RE, Speert DP. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat. 3:247–255. 10.1054/drup.2000.0152 [DOI] [PubMed] [Google Scholar]
- 39.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 57:306–311. 10.1093/jac/dki461 [DOI] [PubMed] [Google Scholar]
- 40.Na-Bangchang K, Ruengweerayut R, Karbwang J, Chauemung A, Hutchinson D. 2007. Pharmacokinetics and pharmacodynamics of fosmidomycin monotherapy and combination therapy with clindamycin in the treatment of multidrug resistant falciparum malaria. Malar. J. 6:70. 10.1186/1475-2875-6-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sud M, Fahy E, Cotter D, Brown A, Dennis EA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CR, Russell DW, Subramaniam S. 2007. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35:D527–D532. 10.1093/nar/gkl838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talbot HM, Rohmer M, Farrimond P. 2007. Rapid structural elucidation of composite bacterial hopanoids by atmospheric pressure chemical ionisation liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 21:880–892. 10.1002/rcm.2911 [DOI] [PubMed] [Google Scholar]
- 43.Loh B, Grant C, Hancock RE. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 26:546–551. 10.1128/AAC.26.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benincasa M, Pacor S, Gennaro R, Scocchi M. 2009. Rapid and reliable detection of antimicrobial peptide penetration into gram-negative bacteria based on fluorescence quenching. Antimicrob. Agents Chemother. 53:3501–3504. 10.1128/AAC.01620-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avgeri SG, Matthaiou DK, Dimopoulos G, Grammatikos AP, Falagas ME. 2009. Therapeutic options for Burkholderia cepacia infections beyond co-trimoxazole: a systematic review of the clinical evidence. Int. J. Antimicrob. Agents 33:394–404. 10.1016/j.ijantimicag.2008.09.010 [DOI] [PubMed] [Google Scholar]