Abstract
Exchanging a central venous catheter (CVC) over a guide wire for a fresh uncoated CVC in the presence of bacteremia can result in cross-infection of the newly exchanged CVC. A recent retrospective clinical study showed that exchanging a catheter over a guide wire in the presence of bacteremia using an antimicrobial minocycline-rifampin (M/R) catheter may improve outcomes. To expand on this, we developed an in vitro cross-contamination model of exchange to evaluate the efficacy of different antimicrobial CVCs in preventing cross-contamination of multidrug-resistant organisms during exchange. Uncoated CVCs were allowed to form biofilm by methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans. After 24 h, the biofilm-colonized CVCs were placed in a glass tube containing bovine calf serum plus Mueller-Hinton broth, and each catheter was exchanged over a guide wire for a fresh uncoated or an M/R-, chlorhexidine-silver sulfadiazine (CHX/SS)-, or chlorhexidine-M/R (CHX-M/R)-coated CVC. Cross-contamination of exchanged catheters was enumerated by sonication and quantitative plating methods. The exchange of M/R CVCs completely prevented cross-contamination by MRSA biofilms compared to control exchanged CVCs (P < 0.0001). Exchange with CHX/SS CVCs reduced but did not completely prevent cross-contamination by MRSA (P = 0.005). Exchange with CHX-M/R CVCs completely prevented cross-contamination by MRSA, P. aeruginosa, and C. albicans biofilms (P < 0.0001). Furthermore, CHX-M/R CVCs were superior to M/R CVCs against P. aeruginosa and C. albicans (P = 0.003) and were superior to CHX/SS CVCs against MRSA and P. aeruginosa (P = 0.01). In conclusion, exchange with the novel CHX-M/R CVC was the only exchange effective in completely and concurrently preventing cross-contamination from bacteria and Candida.
INTRODUCTION
Central venous catheters (CVCs) are the lifelines of many patients with cancer, chronic diseases, and critical illnesses. CVCs provide direct vascular access for blood products, chemotherapy, fluids, nutrition, and other interventions (such as hemodialysis) or medications. While these catheters perform essential life-saving functions, they are also the leading cause of bloodstream infections (1). Infections on indwelling CVC surfaces are the direct consequences of microbial biofilm (2).
In critically ill patients, CVCs are the source of 87% of bloodstream infections occurring in the intensive care unit (ICU) (3), resulting in an increase in length of hospital stay ranging from 7 to 12 days (4). Cancer patients are also particularly vulnerable because radiation and chemotherapy frequently weaken the natural immune capabilities to combat opportunistic pathogens. Recent CDC guidelines recommended two antimicrobial CVCs (category 1A) coated with either minocycline-rifampin (M/R) or chlorhexidine-silver sulfadiazine (CHX/SS) to reduce the risk of central line-associated bloodstream infections (CLABSIs) (5).
The removal of CVCs has been recommended for the management of CLABSI (6). However, in critically ill patients or patients with cancer, CVC removal is not always possible because continued vascular access is required and alternative vascular access sites are often not available. Exchanging a CVC over a guide wire for a fresh noncoated CVC can result in cross-contamination of the newly exchanged CVC (7). Furthermore, the insertion of a new CVC at a different site may be undesirable and can be associated with serious mechanical complications, such as pneumothorax and bleeding, especially in cancer patients with thrombocytopenia or critically ill patients with intravascular coagulopathy (8–10). However, the benefit of exchanging CVCs with new the antimicrobial CVCs is relatively untested in an in vitro exchange model.
As methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans are common pathogens causing CLABSIs and have a propensity to form biofilms on catheter surfaces (11–14), we chose these pathogens to test in our study.
We developed an in vitro model for the exchange of a colonized catheter over a guide wire to evaluate the relative efficacy of different antimicrobial CVCs in preventing cross-contamination when exchanged for a CVC colonized with MRSA, S. epidermidis, E. coli, P. aeruginosa, or C. albicans biofilm.
MATERIALS AND METHODS
Catheters.
We used three different antimicrobial polyurethane CVCs and an uncoated polyurethane control CVC. All CVCs were 7-French triple-lumen catheters. Chlorhexidine M/R (CHX-M/R)-coated CVCs were prepared in our laboratory as described below. Commercially available nonantimicrobial polyurethane 7-French triple-lumen CVCs, M/R-coated CVCs (Cook Medical, Bloomington, IN), and 7-French triple-lumen chlorhexidine/silver sulfadiazine (CHX/SS)-coated CVCs (Arrowgard Blue Plus; Arrow International, Inc., Reading, PA) were also tested in the in vitro exchange model. Clinical isolates of multidrug-resistant (MDR) methicillin-resistant S. aureus (MRSA) strain 4798, E. coli strain 2131, P. aeruginosa strain 4689, C. albicans strain 009-3072 (15, 16), and S. epidermidis strain ATCC 12223 were tested in this model.
Preparation of CHX-M/R CVCs.
CHX-M/R CVCs were prepared by a proprietary sequential coating method. Briefly, full-length uncoated 7-French triple-lumen polyurethane CVC surfaces (Cook Medical, Bloomington, IN) were impregnated with minocycline-rifampin and then coated with CHX and dried (15).
In vitro exchange study model.
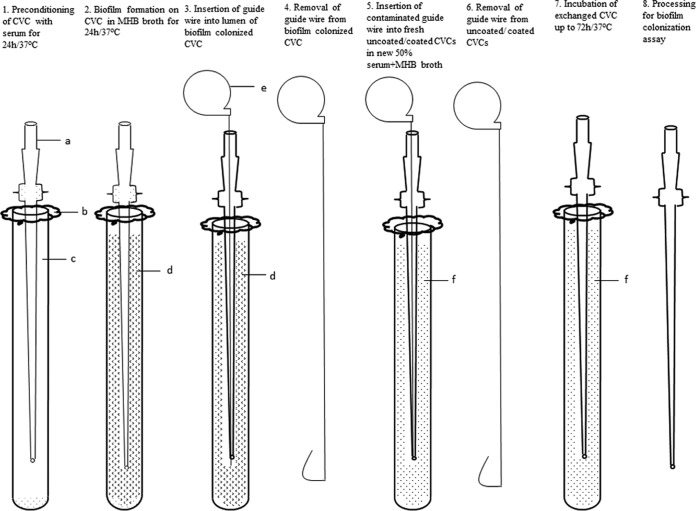
An in vitro exchange model was developed in our laboratory. A diagram of the exchange model is shown in Fig. 1. The model consisted of the following steps.
FIG 1.
In vitro exchange model showing sequence of steps (steps 1 through 8) performed. a, central venous catheter; b, foil cover; c, bovine calf serum; d, Mueller-Hinton broth; e, guide wire; f, fresh 50% serum plus Mueller-Hinton broth.
(i) Step 1.
Uncoated full-length CVCs were placed in a tall glass tube (2 by 20 cm; Kimble Chase Life Science and Research Products, Vineland, NJ) containing 75 ml of bovine calf serum.
(ii) Step 2.
Each catheter was transferred into Mueller-Hinton broth (MHB) inoculated with 5 × 105 CFU/ml of MRSA, S. epidermidis, E. coli, or P. aeruginosa for biofilm formation. The catheters colonized with 5 × 105 CFU/ml C. albicans were allowed to form biofilm for 48 h.
(iii) Step 3.
A guide wire was inserted into the lumen of each biofilm-colonized CVC.
(iv) Step 4.
Each catheter was removed by sliding it over the guide wire.
(v) Step 5.
A fresh uncoated or an M/R-, chlorhexidine-silver sulfadiazine (CHX/SS)-, or CHX-M/R-coated CVC was inserted over the guide wire into the tube containing fresh 50% MHB and 50% bovine calf serum for bacteria (MHB was used for Candida).
(vi) Step 6.
The guide wire was removed.
(vii) Step7.
Each exchanged CVC was incubated at 37°C up to 72 h.
(viii) Step 8.
Biofilm colonization was determined after 24 and 72 h for bacteria and after 48 and 72 h for Candida, as described below.
Quantification of colonization on the exchanged CVCs.
Biofilm colonization on the CVCs was enumerated using previously described methods (16, 17). Briefly, six segments (1 cm each) were cut after incubation of each exchanged CVC and sonicated in 5 ml of 0.9% sterile saline for 15 min at 40 kHz. After sonication, each sample was vortexed for 5 s, and 100 μl of liquid from each segment was serially diluted and spread onto Trypticase soy agar plus 5% sheep blood for quantitative culture of bacterial species or onto Sabouraud dextrose agar for culture of Candida species. Plates were incubated inverted at 37°C for 24 h, and counts for colony growth were performed. The experiments were repeated three times. The guide wires used for the exchanges were also cut into pieces and cultured.
Statistical methods.
The numbers of CFU were compared by a Kruskal-Wallis test for each organism. If a significant difference was detected for the test, we used Wilcoxon rank sum tests for the pairwise comparisons. The α levels of the post hoc pairwise comparisons were adjusted using a sequential Bonferroni adjustment to control type I error. All tests were two sided and had a significance level of 0.05. The statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
After exchange of the uncoated and antimicrobial-coated CVCs, biofilm cross-contamination by MRSA was completely prevented by the M/R and CHX-M/R CVCs. The difference compared to exchanging the uncoated CVCs was significant (P < 0.0001) (Fig. 2). CHX/SS CVCs did not show complete prevention of MRSA transfer (P = 0.01, compared to M/R CVCs). This difference was also significant compared to the exchange of uncoated CVCs (P = 0.005) (Fig. 2).
FIG 2.
Biofilm colonization of methicillin-resistant Staphylococcus aureus (MRSA) on different antimicrobial-coated CVCs after exchange over guide wires. M/R, minocycline-rifampin; CHX, chlorhexidine; SS, silver sulfadiazine.
The formation of P. aeruginosa biofilm was not completely inhibited by exchanging with M/R or CHX/SS CVCs compared to the uncoated control CVC (Fig. 3). The M/R CVCs reduced cross-contamination by only 2 log CFU/cm segment biofilm (median [range], 1.6 × 104 logs [1.1 × 104 to 2.1 × 104 logs]), and CHX/SS reduced it by only 3 log CFU/cm segment biofilm of P. aeruginosa (median [range], 1.5 ×102 logs [0 to 1.1 × 103 logs]), after 24 h of exchange. Compared to the uncoated CVCs, the difference was significant (P = 0.005). However, CHX-M/R CVCs completely prevented cross-contamination by P. aeruginosa. The differences between the CHX-M/R and M/R, the CHX/SS, and the uncoated control CVCs were significant (P < 0.0001) (Fig. 3).
FIG 3.
Biofilm colonization of Pseudomonas aeruginosa on different antimicrobial-coated CVCs after exchange over guide wires. M/R, minocycline-rifampin; CHX, chlorhexidine; SS, silver sulfadiazine.
The CHX-M/R and CHX/SS CVCs had greater antimicrobial activity against C. albicans than the control CVCs. Compared to M/R-coated and uncoated control CVCs, these differences were significant (P < 0.0001) (Fig. 4). The M/R CVCs showed a 1-log reduction in colonization, which was not significantly different from that of the uncoated control CVC (Fig. 4). We noticed 4 or 5 log CFU of bacteria or yeast isolated from guide wires used for exchanging, indicating that the guide wires were likely sources of cross-contamination. Only the CHX-M/R CVC completely prevented cross-contamination by all three MDR pathogens tested in this exchange study.
FIG 4.
Biofilm colonization of Candida albicans on different antimicrobial-coated CVCs after exchange over guide wires. M/R, minocycline-rifampin; CHX, chlorhexidine; SS, silver sulfadiazine.
Biofilm formation by S. epidermidis (median [range], 1.5 × 105 CFU [6.5 × 104 to 4.2 × 105 CFU]) and E. coli (median [range], 1.7 × 105 CFU [7.5 × 104 to 3.5 × 105 CFU]) was completely inhibited by exchanging with M/R, CHX/SS, and CHX-M/R CVCs (P < 0.0001 for all antimicrobial CVCs compared to the uncoated control CVCs).
DISCUSSION
Although CVCs are the lifelines for critically ill, cancer, and hemodialysis patients, CLABSIs continue to be the major source of morbidity and mortality. In 2011, the CDC issued guidelines in which M/R- and CHX/SS-coated antimicrobial CVCs are highly recommended at a level of category 1A for the prevention of CLABSI; hence, they were included in this study. In addition, a potential next-generation CHX-M/R-coated CVC was tested because of its promise of greater antimicrobial protection against resistant bacteria and fungi during catheter exchange (15, 16).
To our knowledge, this is the first in vitro model study to examine cross-contamination resulting from the exchange of contaminated CVCs using guide wires. We found that the exchange of biofilm-colonized CVCs (culprit CVCs) with fresh uncoated CVCs over guide wires resulted in cross-contamination by the three pathogens tested with 5- to 6-log recoverable colonies on the fresh uncoated catheter following 24 h of incubation. A separate assessment of the guide wires confirmed that they were a significant source of cross-contamination. Clinically, exchanging a CVC over a guide wire for a nonantimicrobial CVC often leads to cross-contamination of the newly exchanged CVC (7), and a significant increase in CLABSI associated with exchanged catheters has been noticed in adult (8) and pediatric (18) patients.
Although Infectious Diseases Society of America (IDSA) guidelines recommend the removal of CVCs for the management of CLABSI (6), removal is not always possible for critically ill cancer patients. Exchange over guide wires using antimicrobial catheters is recommended when removal is not an option.
CVCs impregnated with M/R have been associated with a lower rate of infection than CHX/SS CVCs and have broad-spectrum inhibitory activity both in vitro and in vivo against Gram-positive and -negative bacteria (15, 16, 19). Furthermore, the antimicrobial durability of M/R CVCs is longer than that of CHX/SS CVCs (19–22).
Our study suggests that the exchange of a culprit CVC for a CHX/SS CVC did not completely prevent microbial cross-contamination by MRSA in our model. In contrast, M/R CVCs prevented biofilm colonization by S. aureus upon exchange. A large multicenter study by the CDC determined that the majority (61%) of 4,088 S. aureus CLABSIs were caused by MRSA (23). The results of this exchange study are consistent with those of other studies, which have shown M/R CVCs to be significantly more effective than CHX/SS CVCs at preventing biofilm colonization by staphylococci (24).
A meta-analysis of 12 randomized controlled trials concluded that exchanging CVCs using guide wires is associated with a greater risk of catheter-related infections but fewer mechanical complications than new-site CVC replacement (25). In a recent retrospective study, patients with guide-wire-exchanged CVCs stayed in the ICU and in the hospital longer than controls (26). The catheter tips of the guide-wire-exchanged CVCs were found to have been colonized with Gram-negative bacteria, Staphylococcus, and Candida, whereas CVCs inserted at a new site were not contaminated (26), which is consistent with the cross-contamination of pathogens upon exchange in our study.
The results from the in vitro exchange model for M/R CVCs were consistent with those of a recent clinical study from our center showing that the exchange of culprit CVCs for new M/R CVCs in the setting of CLABSI was useful in improving the overall response rate, decreasing the risk of mechanical failure, and decreasing disease recurrence (27). Another clinical study from our group revealed that exchanging colonized CVCs over guide wires for M/R CVCs was an effective alternative to removing and inserting new CVCs at a new vascular site in the setting of S. aureus infection and CLABSI (28; A. M. Chaftari and A. El Zakhem, submitted for publication). Further, in vitro studies conducted in parallel with the clinical trial showed that M/R CVCs completely prevent the transfer of MRSA through the guide wire during exchange, but CHX/SS and uncoated CVCs do not (28).
Our study showed that cross-contamination was prevented by the exchange of culprit catheters colonized by C. albicans or P. aeruginosa for CHX-M/R catheters. The exchange of a culprit C. albicans-colonized catheter for an M/R CVC was unable to prevent cross-contamination by C. albicans. Catheter-related candidemia has been on the rise, and the attributable mortality rate of health care-associated candidemia has been reported to be in the range of 38% to 49% (29). Candida species contribute around 12% of all CLABSIs (12).
Exchanging culprit P. aeruginosa-colonized catheters for CHX/SS or M/R CVCs did not prevent cross-contamination by P. aeruginosa. Bacteremia caused by P. aeruginosa has been reported to be the leading cause of mortality in cancer patients. In addition, it is a bacterial organism frequently isolated from the bloodstream of patients who have died with infections (30). In critically ill patients, P. aeruginosa has been associated with a high mortality rate and is more likely to be part of polymicrobial bacteremia (31).
Polymicrobial infections account for roughly 15% of infections in immunocompromised patients with cancer. Catheter-related polymicrobial bloodstream infections have been reported in young and adult patients (32, 33). Our exchange model study clearly suggests that exchange for the novel CHX-M/R CVC, which has antimicrobial activity against MDR P. aeruginosa, may prove to be especially effective when polymicrobial infections are present. The performance of the CHX-M/R CVC during exchange was consistent with that described in a recent publication that demonstrated that the CHX-M/R CVC had superior antimicrobial effectiveness and prolonged antimicrobial durability over M/R and CHX/SS CVCs in inhibiting biofilm colonization by MDR Gram-positive bacteria, Gram-negative bacteria, and fungi in an in vitro biofilm model (15, 16).
In conclusion, our exchange model has demonstrated that the novel antimicrobial catheter containing chlorhexidine, minocycline, and rifampin (CHX-M/R) is uniquely effective in completely preventing cross-contamination by MRSA, P. aeruginosa, and C. albicans. Further testing is required to prove its effectiveness in a clinical setting.
ACKNOWLEDGMENTS
Issam I. Raad is a coinventor of the approved catheters coated with minocycline-rifampin. This technology is the property of the University of Texas M. D. Anderson Cancer Center and the Baylor College of Medicine and is licensed to Cook, Inc.
Footnotes
Published ahead of print 23 June 2014
REFERENCES
- 1.Edgeworth JD, Treacher DF, Eykyn SJ. 1999. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit. Care Med. 27:1421–1428. 10.1097/00003246-199908000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881–890. 10.3201/eid0809.020063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in medical intensive care units in the United States: National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887–892. 10.1097/00003246-199905000-00020 [DOI] [PubMed] [Google Scholar]
- 4.Pittet D, Tarara D, Wenzel RP. 1994. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601. 10.1001/jama.271.20.1598 [DOI] [PubMed] [Google Scholar]
- 5.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. 2002. Guidelines for the prevention of intravascular catheter-related infections: Centers for Disease Control and Prevention. MMWR Recomm. Rep. 51:1–29 [PubMed] [Google Scholar]
- 6.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45. 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettigrew RA, Lang SD, Haydock DA, Parry BR, Bremner DA, Hill GL. 1985. Catheter-related sepsis in patients on intravenous nutrition: a prospective study of quantitative catheter cultures and guidewire changes for suspected sepsis. Br. J. Surg. 72:52–55. 10.1002/bjs.1800720121 [DOI] [PubMed] [Google Scholar]
- 8.Cobb DK, High KP, Sawyer RG, Sable CA, Adams RB, Lindley DA, Pruett TL, Schwenzer KJ, Farr BM. 1992. A controlled trial of scheduled replacement of central venous and pulmonary-artery catheters. N. Engl. J. Med. 327:1062–1068. 10.1056/NEJM199210083271505 [DOI] [PubMed] [Google Scholar]
- 9.Eyer S, Brummitt C, Crossley K, Siegel R, Cerra F. 1990. Catheter-related sepsis: prospective, randomized study of three methods of long-term catheter maintenance. Crit. Care Med. 18:1073–1079. 10.1097/00003246-199010000-00005 [DOI] [PubMed] [Google Scholar]
- 10.Mansfield PF, Hohn DC, Fornage BD, Gregurich MA, Ota DM. 1994. Complications and failures of subclavian-vein catheterization. N. Engl. J. Med. 331:1735–1738. 10.1056/NEJM199412293312602 [DOI] [PubMed] [Google Scholar]
- 11.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sanchez-Ortega I, Duarte R, Calvo M, Carratala J. 2011. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J. Antimicrob. Chemother. 66:657–663. 10.1093/jac/dkq494 [DOI] [PubMed] [Google Scholar]
- 12.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Ko KS, Song JH, Peck KR. 2007. In vitro effectiveness of the antibiotic lock technique (ALT) for the treatment of catheter-related infections by Pseudomonas aeruginosa and Klebsiella pneumoniae. J. Antimicrob. Chemother. 60:782–787. 10.1093/jac/dkm295 [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos A, Falagas ME, Karatza DC, Alexandropoulou P, Papadakis E, Gregorakos L, Chalevelakis G, Pappas G. 2011. Epidemiologic, clinical characteristics, and risk factors for adverse outcome in multiresistant Gram-negative primary bacteremia of critically ill patients. Am. J. Infect. Control 39:396–400. 10.1016/j.ajic.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 15.Jamal MA, Rosenblatt JS, Hachem RY, Ying J, Pravinkumar E, Nates JL, Chaftari AM, Raad II. 2014. Prevention of Gram-negative bacterial biofilm on minocycline/rifampin impregnated catheters sequentially coated with chlorhexidine. Antimicrob. Agents Chemother. 58:1179–1182. 10.1128/AAC.01959-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raad I, Mohamed JA, Reitzel RA, Jiang Y, Raad S, Al Shuaibi M, Chaftari AM, Hachem RY. 2012. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob. Agents Chemother. 56:935–941. 10.1128/AAC.05836-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna H, Bahna P, Reitzel R, Dvorak T, Chaiban G, Hachem R, Raad I. 2006. Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrob. Agents Chemother. 50:3283–3288. 10.1128/AAC.01622-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Teresa MA, Casado-Flores J, Delgado Dominguez MA, Roqueta-Mas J, Cambra-Lasaosa F, Concha-Torre A, Fernandez-Perez C. 2007. Infectious complications of percutaneous central venous catheterization in pediatric patients: a Spanish multicenter study. Intensive Care Med. 33:466–476. 10.1007/s00134-006-0508-8 [DOI] [PubMed] [Google Scholar]
- 19.Raad I, Darouiche R, Hachem R, Mansouri M, Bodey GP. 1996. The broad-spectrum activity and efficacy of catheters coated with minocycline and rifampin. J. Infect. Dis. 173:418–424. 10.1093/infdis/173.2.418 [DOI] [PubMed] [Google Scholar]
- 20.Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G. 1999. A comparison of two antimicrobial-impregnated central venous catheters: Catheter Study Group. N. Engl. J. Med. 340:1–8. 10.1056/NEJM199901073400101 [DOI] [PubMed] [Google Scholar]
- 21.Raad II, Darouiche RO, Hachem R, Abi-Said D, Safar H, Darnule T, Mansouri M, Morck D. 1998. Antimicrobial durability and rare ultrastructural colonization of indwelling central catheters coated with minocycline and rifampin. Crit. Care Med. 26:219–224. 10.1097/00003246-199802000-00015 [DOI] [PubMed] [Google Scholar]
- 22.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericsson C. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections: a randomized, double-blind trial. Ann. Intern. Med. 127:267–274. 10.7326/0003-4819-127-4-199708150-00002 [DOI] [PubMed] [Google Scholar]
- 23.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. 2009. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA 301:727–736. 10.1001/jama.2009.153 [DOI] [PubMed] [Google Scholar]
- 24.Raad I, Reitzel R, Jiang Y, Chemaly RF, Dvorak T, Hachem R. 2008. Anti-adherence activity and antimicrobial durability of anti-infective-coated catheters against multidrug-resistant bacteria. J. Antimicrob. Chemother. 62:746–750. 10.1093/jac/dkn281 [DOI] [PubMed] [Google Scholar]
- 25.Cook D, Randolph A, Kernerman P, Cupido C, King D, Soukup C, Brun-Buisson C. 1997. Central venous catheter replacement strategies: a systematic review of the literature. Crit. Care Med. 25:1417–1424. 10.1097/00003246-199708000-00033 [DOI] [PubMed] [Google Scholar]
- 26.Parbat N, Sherry N, Bellomo R, Schneider AG, Glassford NJ, Johnson PD, Bailey M. 2013. The microbiological and clinical outcome of guide wire exchanged versus newly inserted antimicrobial surface treated central venous catheters. Crit. Care. 17:R184. 10.1186/cc12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaftari AM, Kassis C, El Issa H, Al Wohoush I, Jiang Y, Rangaraj G, Caillouet B, Pravinkumar SE, Hachem RY, Raad II. 2011. Novel approach using antimicrobial catheters to improve the management of central line-associated bloodstream infections in cancer patients. Cancer 117:2551–2558. 10.1002/cncr.25807 [DOI] [PubMed] [Google Scholar]
- 28.Jamal MA, Chaftari AM, Dvorak T, El Zakhem A, Rosenblatt J, Jiang Y, Hachem R, Raad II. 2013. Prevention of transmission of multidrug resistant organisms during catheter exchange using antimicrobial catheters, abstr E-622. Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother., Denver, CO, American Society for Microbiology, Washington, DC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177. 10.1086/378745 [DOI] [PubMed] [Google Scholar]
- 30.Samonis G, Vardakas KZ, Maraki S, Tansarli GS, Dimopoulou D, Kofteridis DP, Andrianaki AM, Falagas ME. 2013. A prospective study of characteristics and outcomes of bacteremia in patients with solid organ or hematologic malignancies. Support Care Cancer 21:2521–2526. 10.1007/s00520-013-1816-5 [DOI] [PubMed] [Google Scholar]
- 31.Ko HK, Yu WK, Lien TC, Wang JH, Slutsky AS, Zhang H, Kou YR. 2013. Intensive care unit-acquired bacteremia in mechanically ventilated patients: clinical features and outcomes. PLoS One 8:e83298. 10.1371/journal.pone.0083298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cairo J, Hachem R, Rangaraj G, Granwehr B, Raad I. 2011. Predictors of catheter-related Gram-negative bacilli bacteraemia among cancer patients. Clin. Microbiol. Infect. 17:1711–1716. 10.1111/j.1469-0691.2011.03504.x [DOI] [PubMed] [Google Scholar]
- 33.Downes KJ, Metlay JP, Bell LM, McGowan KL, Elliott MR, Shah SS. 2008. Polymicrobial bloodstream infections among children and adolescents with central venous catheters evaluated in ambulatory care. Clin. Infect. Dis. 46:387–394. 10.1086/525265 [DOI] [PubMed] [Google Scholar]