Abstract
A racemic mixture of R and S enantiomers of praziquantel (PZQ) is currently the treatment of choice for schistosomiasis. Though the S enantiomer and the metabolites are presumed to contribute only a little to the activity of the drug, in-depth side-by-side studies are lacking. The aim of this study was to investigate the in vitro activities of PZQ and its main metabolites, namely, R- and S-cis- and R- and S-trans-4′-hydroxypraziquantel, against adult worms and newly transformed schistosomula (NTS). Additionally, we explored the in vivo activity and hepatic shift (i.e., the migration of the worms to the liver) produced by each PZQ enantiomer in mice. Fifty percent inhibitory concentrations of R-PZQ, S-PZQ, and R-trans- and R-cis-4′-hydroxypraziquantel of 0.02, 5.85, 4.08, and 2.42 μg/ml, respectively, for adult S. mansoni were determined in vitro. S-trans- and S-cis-4′-hydroxypraziquantel were not active at 100 μg/ml. These results are consistent with microcalorimetry data and studies with NTS. In vivo, single 400-mg/kg oral doses of R-PZQ and S-PZQ achieved worm burden reductions of 100 and 19%, respectively. Moreover, worms treated in vivo with S-PZQ displayed an only transient hepatic shift and returned to the mesenteric veins within 24 h. Our data confirm that R-PZQ is the main effector molecule, while S-PZQ and the metabolites do not play a significant role in the antischistosomal properties of PZQ.
INTRODUCTION
Schistosomiasis or bilharzia is caused by blood flukes of the genus Schistosoma and is part of the group of neglected tropical diseases affecting more than 207 million people in tropical areas (1–3).
The exclusive treatment to date for schistosomiasis is praziquantel (PZQ), which was discovered in the 1970s by Merck and Bayer. PZQ is administered as a racemic mixture of R and S enantiomers in tablets of 600 mg. The recommended dosage to treat schistosomiasis is 20 mg/kg three times in 1 day, and since PZQ does not act on juvenile worms, follow-up treatment 4 to 6 weeks later is strongly advised (4). In preventive chemotherapy programs, PZQ is administered as a single 40-mg/kg dose to at-risk populations (5). PZQ undergoes significant first-pass metabolism through the liver enzyme cytochrome P450 (CYP) 3A4 and to a lesser extent through 1A2 and 2C19 (6). R-PZQ is metabolized at a much higher rate than S-PZQ. R-PZQ is transformed mainly into cis- and trans-hydroxypraziquantel (4-OH-PZQ), while S-PZQ is converted to other monohydroxylated metabolites. In rat liver microsomes, the main metabolite is cis-4-OH-PZQ (7, 8), while in humans it is trans-4-OH-PZQ (9).
The difference in the antischistosomal activity of each PZQ enantiomer has been known since 1983 (10), and several studies have observed greater activity of R-PZQ than of S-PZQ in vitro and in vivo (11–13). A clinical trial with Schistosoma japonicum-infected patients also recorded a higher efficacy of R-PZQ than of racemic PZQ at the same dosage (14, 15). Additionally, treatment with R-PZQ resulted in fewer adverse events than the standard treatment (14). However, since higher drug concentrations in plasma and slightly longer half-lives are achieved with the metabolites than with PZQ (16), it is possible that the metabolites contribute to the antischistosomal activity of PZQ. The efficacy of racemic trans-4-OH-PZQ was evaluated in vitro by Staudt et al. (11), who observed similar antischistosomal properties of the trans metabolite and R-PZQ against adult worms.
In this study, we comparatively assessed the in vitro activities of R-PZQ, S-PZQ, and the metabolites cis- and trans-4-OH-PZQ against adults and newly transformed schistosomula (NTS). Drug effects were evaluated by using both microscopic readouts and isothermal microcalorimetry. Since the metabolites are also chiral molecules, we evaluated for the first time the in vitro efficacy of the respective R and S enantiomers. We also studied the in vivo activity of each parent enantiomer in mice and estimated the hepatic shift of the worms after each treatment.
MATERIALS AND METHODS
Mice and infection.
All in vivo experiments were performed at the Swiss Tropical and Public Health Institute (Basel, Switzerland) and followed Swiss and cantonal animal welfare regulations (license no. 2070). Female NMRI mice (age, 3 weeks; weight, ca. 14 g) were purchased from Charles River (Sulzfeld, Germany) or Harlan Laboratories (Blackthorn, United Kingdom). The animals were allowed to adapt for 1 week under controlled conditions (22°C, 50% humidity, 12 h of light, and free access to water and a rodent diet) before experimental handling.
NMRI mice were infected subcutaneously with 80 to 100 cercariae, as previously described (17).
Drugs and media.
RPMI 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 5% heat-inactivated fetal calf serum (iFCS), penicillin (100 U/ml; Life Technologies), and streptomycin (100 μg/ml; Life Technologies) was used for adult schistosome in vitro and microcalorimetry experiments. For NTS in vitro culture, medium 199 (Life Technologies) supplemented with iFCS and antibiotics was used.
Racemic PZQ was purchased from Sigma-Aldrich (Buchs, Switzerland). Enantiomers of PZQ and cis- and trans-4-OH-PZQ were acquired from Merck Serono (Darmstadt, Germany) and synthesized by Matthew Todd (University of Sydney, Sydney, Australia) (18). Racemic cis- and trans-4-OH-PZQ were obtained from Gilles Gasser (University of Zurich, Zurich, Switzerland) (19). For in vitro studies, each compound was dissolved in dimethyl sulfoxide (DMSO; Fluka, Buchs, Switzerland) at a concentration of 10 mg/ml. For in vivo studies, the drugs were dissolved in 7% (vol/vol) Tween 80 and 3% (vol/vol) ethanol before oral treatment.
In vitro studies.
NTS were obtained from cercariae by mechanical transformation (17). Six to 12 h later, the schistosomula (100 NTS/well) were incubated in flat-bottom 96-well plates (BD Falcon) containing the drug solution in medium at 1.2, 3.7, 11.1, 33.3, and 100 μg/ml. Control NTS were incubated with the highest concentration of drug solvent used in the assays (2% DMSO). The plates were incubated at 37°C in 5% CO2 for 72 h, and compound activity was microscopically assessed by using a motility scale ranging from 3 (normal activity) to 0 (no activity and granularity present) (20).
To test the effect of each compound on adult worms, the drugs were diluted in medium in flat-bottom 24-well plates (BD Falcon) at concentrations ranging from 0.01 to 10 μg/ml for racemic PZQ and R-PZQ and from 0.4 to 100 μg/ml for S-PZQ and the metabolites. Control wells consisted of drug-free medium with 2% DMSO. At 7 to 8 weeks postinfection, S. mansoni-infected mice were euthanized with CO2 and dissected and adult worms were collected from the hepatic portal and mesenteric veins. Four to 6 worms of both sexes were deposited in each well and incubated at 37°C. After 4 and 72 h, the worm condition was microscopically evaluated on a scale of 3 (normal activity and no tegumental alteration) to 0 (dead, highly granulated) (20). To test the recovery of adult worms following a short exposure to S-PZQ, we incubated adult worms in medium with 100, 200, 300, or 400 μg/ml S-PZQ for 1 or 2 h and next transferred them to drug-free medium for up to 72 h. These motility values at 72 h were compared to the values of worms incubated in S-PZQ for 72 h and control worms incubated in drug-free medium. Fifty percent inhibitory concentrations (IC50s) and IC90s were determined with CompuSyn software by using the motility values obtained at different dosages. The eudysmic ratio (21) was calculated as follows: Eudysmic ratio = IC50distomer/IC50eutomer, where the eutomer, the active enantiomer, is R-PZQ and the distomer is S-PZQ.
Isothermal microcalorimetry.
The microcalorimetry experiments were performed in triplicate on a 48-channel isothermal microcalorimeter (TAM48; TA Instruments, New Castle, DE). First, glass ampoules were filled with 2,900 μl of medium and four worms of both sexes were added to each vial. Ampoules were then placed in the channels for the equilibration phase. Twelve hours later, 100 μl of a prewarmed drug solution prepared in medium was injected with a 1-ml syringe (BD Plastipak, Becton, Dickinson S.A., Madrid, Spain). End concentrations reached 0.04, 0.2, and 1 μg/ml for racemic and R-PZQ and 1, 5, and 50 μg/ml for S-PZQ and the metabolites. Ampoules containing schistosomes in the presence of DMSO alone (final concentration of 2%) served as negative controls, while ampoules containing dead worms, obtained by dipping them in 70% ethanol for 5 min and rinsing them in a medium solution, served as positive controls. Schistosome motility data derived from noise amplitudes were recorded for 5 days and analyzed with R software and Excel (22). The noise amplitudes produced by worm movements and metabolism decay exponentially as the worms die, until they reach the background noise level recorded in the dead-worm positive controls. The intersection of both curves determines the endpoint of worm motility (22).
In vivo studies.
At 49 days postinfection (chronic S. mansoni infection), groups of three to six mice were treated orally with 400 mg/kg racemic PZQ, 400 or 800 mg/kg S-PZQ, or 100, 200, or 400 mg/kg R-PZQ. At 14 days posttreatment, the mice were euthanized and dissected. The worms in the veins and liver were sexed and counted (23).
Mean worm burdens of treated mice were compared to those of untreated mice, and worm burden reductions (WBRs) were determined. IC50s and eudysmic ratios were calculated as described above.
The hepatic shift was investigated as follows. Groups of five mice infected with adult schistosomes were treated with 400 mg/kg S-PZQ, 400 mg/kg racemic PZQ, or 200 mg/kg R-PZQ. After 30 min, 1 h, 4 h, 24 h, and 7 days, one mouse in each group was euthanized and dissected and the worm burdens in its veins and liver were evaluated.
Statistical tests were performed with Stata (version 12.1; StataCorp LP, College Station, TX). Differences in worm burdens were assessed by using an unpaired t test and allowing for unequal variances by comparing the control groups with the treated groups. The significance threshold was set at a P value of 0.05.
RESULTS
In vitro studies.
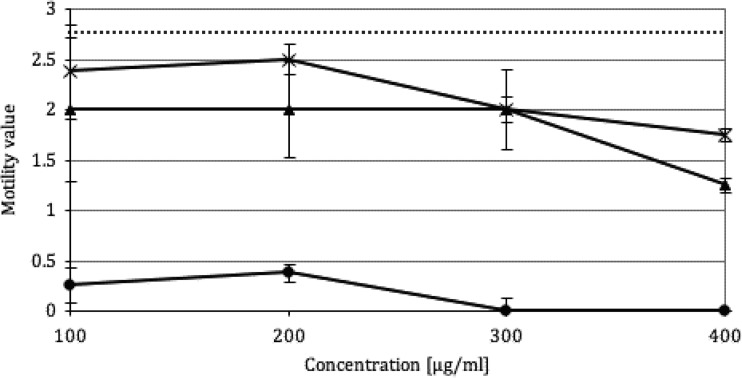
Table 1 summarizes the in vitro IC50 and IC90 of racemic and optically pure PZQ and 4-OH-PZQ metabolites against adult S. mansoni after 4 and 72 h of incubation. R-PZQ displayed the highest activity, with an IC50 of 0.04 μg/ml after 4 h of incubation. The IC50 of R-PZQ after 72 h was half of the value for the racemic mixture, while the IC50 of S-PZQ was higher by a factor 100. The IC50s of the metabolites at 72 h showed the same pattern; the R conformation was twice as active as the racemic form, while no activity of the S metabolites at 100 μg/ml was detected. When the activities of the cis and the trans configurations were compared, the cis metabolites displayed slightly better activity than trans metabolites but the IC50s of the metabolites were nevertheless much higher than that of racemic PZQ. The eudysmic ratio of PZQ in vitro at 72 h postexposure was estimated at 293. The antischistosomal activity of S-PZQ following short-term incubation is depicted in Fig. 1. Worms incubated for 1 or 2 h in high concentrations of S-PZQ recovered almost completely and displayed high motility values (1.25 to 2.5) after 3 days, in contrast to worms incubated for a full 72 h in S-PZQ, which did not score above 0.5.
TABLE 1.
Calculated in vitro IC50sand IC90s of racemic and enantiomeric PZQ and 4-OH metabolites against adult S. mansoni at 4 and 72 h postincubation
| Compound | IC50 (μg/ml) at |
IC90 (μg/ml) at 72 h | Eudysmic ratio | |
|---|---|---|---|---|
| 4 h | 72 h | |||
| Rac-PZQ | 0.1 | 0.05 | 0.4 | |
| R-PZQ | 0.04 | 0.02 | 0.04 | 293 |
| S-PZQ | 5.7 | 5.9 | 17.9 | |
| Rac-trans-4-OH-PZQ | 16.7 | 7.9 | 3,694a | |
| R-trans-4-OH-PZQ | 13.4 | 4.1 | 58.4 | |
| S-trans-4-OH-PZQ | NAb | NA | NA | |
| Rac-cis-4-OH-PZQ | 15.8 | 4.8 | 81.4 | |
| R-cis-4-OH-PZQ | 4.5 | 2.4 | 48.7 | |
| S-cis-4-OH-PZQ | NA | NA | NA | |
Extrapolated value determined by CompuSyn.
NA, not active at 100 μg/ml.
FIG 1.
Motility of adult worms (n = 4 to 6, in triplicate) after 1 h (*) or 2 h (▲) of incubation in S-PZQ, followed by incubation in drug-free medium until 72 h, compared with that of adults incubated for 72 h in S-PZQ (●) and controls incubated for 72 h in drug-free medium (dashed line).
The results of the in vitro assays against NTS are displayed in Table 2. The IC50 of R-PZQ was estimated at 0.03 μg/ml. S-PZQ showed markedly lower activity, with an IC50 of 40.0 μg/ml. The eudysmic ratio calculated against NTS was 1,196. The IC50s of the trans metabolites were determined as 133 and 28.5 μg/ml for the racemic and R derivatives, respectively, while the cis form of the R enantiomer showed moderate activity (IC50 of 34.3 μg/ml).
TABLE 2.
In vitro IC50s and IC90s of racemic and enantiomeric PZQ and 4-OH metabolites against NTS at 72 h postincubation
| Compound | IC50 (μg/ml) at 72 h | IC90 (μg/ml) at 72 h | Eudysmic ratio |
|---|---|---|---|
| Rac-PZQ | 1.5 | 34.5 | |
| R-PZQ | 0.03 | 18.8 | 1,196 |
| S-PZQ | 40.0 | 522a | |
| Rac-trans-4-OH-PZQ | 133a | 5,852a | |
| R-trans-4-OH-PZQ | 28.5 | 747 | |
| S-trans-4-OH-PZQ | NAb | NA | |
| Rac-cis-4-OH-PZQ | 911a | 2,448,837a | |
| R-cis-4-OH-PZQ | 34.3 | 1,161a | |
| S-cis-4-OH-PZQ | NA | NA |
Extrapolated value determined by CompuSyn.
NA, not active at 100 μg/ml.
Isothermal microcalorimetry.
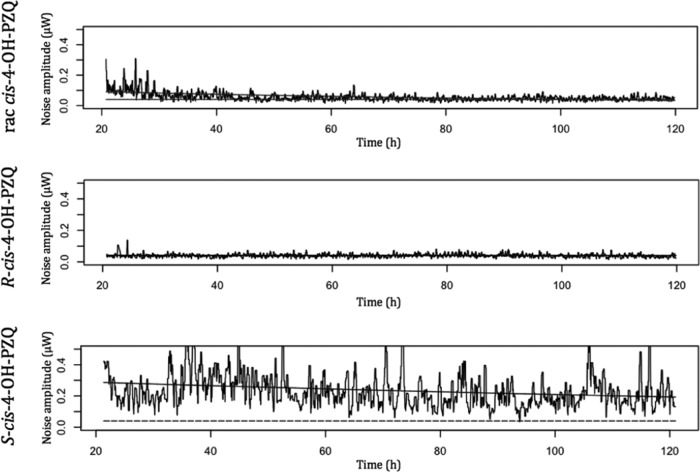
The worm motility endpoints after PZQ enantiomer and metabolite treatments are summarized in Table 3. With R-PZQ, worm motility ceased in the first 3 h postinjection at concentrations as low as 0.04 μg/ml, while the same effect was observed for racemic PZQ only at 0.2 μg/ml. Worms exposed to the racemic and R metabolites at a concentration of 1 μg/ml did not display a decrease in motility. For racemic and R-cis-4-OH-PZQ, the motility endpoints at 5 μg/ml were estimated, as depicted in Fig. 2, as 96.7 and <3 h postinjection, respectively. Racemic trans-4-OH-PZQ was not active at 5 μg/ml, but R-trans-4-OH-PZQ produced a motility endpoint of 75 h postinjection. At a very high concentration of the racemic and R metabolites of 50 μg/ml, the motility of worms stopped within 3 h. None of the S derivatives interfered with worm motility after incubation for 5 days at 50 μg/ml.
TABLE 3.
Endpoints of worm motility determined by noise amplitudes at different concentrations of racemic and enantiomeric PZQ and 4-OH metabolites
| Compound | Mean motility endpoint [h postinjection (SD)] at concn (μg/ml) of: |
|||||
|---|---|---|---|---|---|---|
| 0.04 | 1 | 0.2 | 5 | 1 | 50 | |
| Rac-PZQ | >120 | <3 | <3 | |||
| R-PZQ | <3 | <3 | <3 | |||
| S-PZQ | >120 | >120 | >120 | |||
| Rac-trans-4-OH-PZQ | >120 | >120 | <3 | |||
| R-trans-4-OH-PZQ | >120 | 75 (5) | <3 | |||
| S-trans-4-OH-PZQ | >120 | >120 | >120 | |||
| Rac-cis-4-OH-PZQ | >120 | 96.7 (16.1) | <3 | |||
| R-cis-4-OH-PZQ | >120 | <3 | <3 | |||
| S-cis-4-OH-PZQ | NTa | NT | >120 | |||
NT, not tested.
FIG 2.
Examples of heat production recorded by microcalorimetry of racemic cis-4-OH- and R-cis-4-OH-PZQ at 5 μg/ml and S-cis-4-OH-PZQ at 50 μg/ml.
In vivo studies.
In Table 4, the WBRs after different single oral doses of R- and S-PZQ are presented. Racemic PZQ produced a WBR of 94.1% at 400 mg/kg, while no significant effect was observed at 100 mg/kg. R-PZQ showed a WBR of 52% at 100 mg/kg and WBRs of >98% at 200 and 400 mg/kg. S-PZQ displayed a low WBR of 19.6% at 800 mg/kg. When the worm burdens at 400 mg/kg were compared, there were significant differences between racemic PZQ and R-PZQ and the control group (P values <0.001). There was no statistically significant difference between the worm burdens of controls and those of S-PZQ-treated mice (P = 0.68). The 50% effective doses (ED50s) of R- and S-PZQ were 95.4 and >1,000 mg/kg, respectively, and the corresponding eudysmic ratio was >10.
TABLE 4.
Total and female WBRs obtained with racemic PZQ, R-PZQ, and S-PZQ at different dosages in mice harboring adult S. mansoni
| Compound and dose (mg/kg) | No. of mice | Mean WBR [% (SD)] | ED50 (mg/kg) | Eudysmic ratio |
|---|---|---|---|---|
| Rac PZQ | ||||
| 400 | 4 | 94.1 (8.6) | 246.5 | |
| 100b | 6 | 15 (9.5) | ||
| R-PZQ | ||||
| 400 | 3 | 100.0 (0) | 95.4 | |
| 200 | 6 | 98.1 (2.3) | ||
| 100 | 6 | 52.0 (30.8) | ||
| S-PZQ | ||||
| 800 | 6 | 19.6 (22.2) | 3,066,777a | 32,136 |
| 400 | 4 | 18.0 (21.4) |
Extrapolated value determined by CompuSyn.
Data from reference 36.
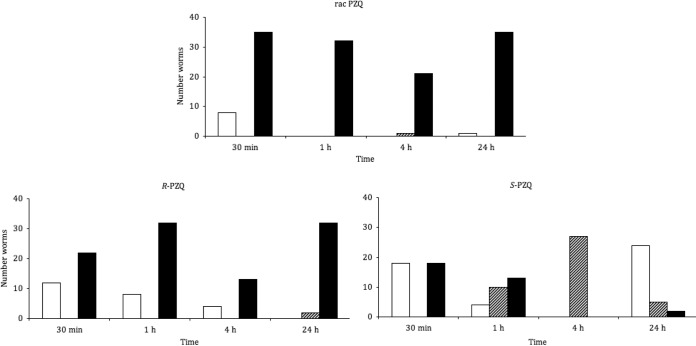
The observed hepatic shift obtained with PZQ enantiomers for a single mouse per time point is illustrated in Fig. 3. Racemic PZQ acted rapidly; at 30 min posttreatment, only a few living worms were still observed in the mesenteric veins, while from 1 h onward, all of the worms were found dead in the liver. Treatment with R-PZQ at half the dose of racemic PZQ produced fairly similar effects. Living worms in veins, however, were observed until 4 h posttreatment. In contrast, treatment with S-PZQ resulted in a high number of dead worms in the liver at 30 min posttreatment, after which the number of worms killed decreased over time, and after 4 h posttreatment only a small number of worms were found dead. At the 4-h examination point, all of the worms had migrated to the liver following treatment with S-PZQ. By 24 h posttreatment, the majority of the worms had returned to the mesenteric veins.
FIG 3.
In vivo hepatic shift after treatment with racemic PZQ at 400 mg/kg, R-PZQ at 200 mg/kg, or S-PZQ at 400 mg/kg. Shown are the numbers of worms alive in the mesenteric veins (white), alive in the liver (cross-hatched), and dead in the liver (black).
DISCUSSION
In the framework of a public-private partnership including Merck Serono, Astellas Pharma, the Swiss Tropical and Public Health Institute, and TI Pharma, efforts are ongoing to develop a pediatric formulation of PZQ. The project is currently in the preclinical phase, and in this work, we have for the first time conducted thorough side-by-side in vitro and in vivo studies with PZQ enantiomers and metabolites that will aid the development process.
Our data show that the antischistosomal activity is driven mainly by the R configuration. We observed that R-PZQ and the R-hydroxylated metabolites reveal 100- and 1,000-fold higher activities than their S counterparts in vitro. The racemic compounds display IC50s twice as high as those of their respective R configurations. Note that the IC50s observed against NTS were much higher than those against adults, which is in line with previous findings (24, 25). Nevertheless, R enantiomers are again more active than S conformations against NTS.
Microcalorimetry findings are consistent with our IC50s determined microscopically against adults in vitro. The loss of motility produced by R-PZQ at 0.04 and 0.2 μg/ml and by racemic PZQ between 0.04 and 0.2 μg/ml correlates nicely with the IC50s (0.02 and 0.05 μg/ml, respectively). As observed in the in vitro microscopic assays, S-PZQ is not active at 1 μg/ml. Microcalorimetric measurements confirmed that the R-cis and R-trans forms are the active metabolites (e.g., with the R-cis and R-trans forms at 5 μg/ml, a loss of motility was observed at 96.7 and 75 h postinjection, respectively). These observations are in agreement with our IC50 data (2.4 and 4.1 μg/ml for the R-cis and R-trans forms, respectively) based on microscopic viability scores.
A similar pattern was observed in vivo. A single oral dose of 400 mg/kg of racemic PZQ shows activity similar to that of R-PZQ at 200 mg/kg. In contrast, treatment with S-PZQ at 800 mg/kg did not result in a significant WBR and none of the treated mice were cured.
Dissection of mice at different time points after treatment allowed us to investigate the hepatic shift caused by PZQ and its enantiomers. The hepatic shift of worms into the liver had been characterized earlier for PZQ, as well as for several other drugs, including mefloquine (26), artemether (27), oxamniquine (28), or older antischistosomal drugs (29). Treatment with the racemate and R-PZQ efficiently immobilized or killed the majority of the worms, which were carried by blood flow to the liver, where they disintegrated over time. In contrast, treatment with S-PZQ killed only a few worms. Worms migrated to the liver and returned to the mesenteric veins 24 h posttreatment. The typical translocation of the worms into the liver might be explained by a loss of grip on the mesenteric vein wall due to the chemical action of the compound, and when the therapeutic effect ceases, they migrate back to the mesenteric veins (29). The return of worms to the mesenteric veins has been described for subtherapeutic doses or inefficient compounds (30). The transient hepatic shift observed in S-PZQ-treated mice is therefore strong additional evidence of its inefficacy.
In order to place our in vitro findings in context, we have summarized the pharmacokinetic (PK) parameters of R-PZQ, S-PZQ, and the R-trans and S-trans enantiomers obtained in humans (16) in Table 5. The maximal concentration (Cmax) of R-PZQ (0.16 μg/ml) is 8 and 4 times as high as its IC50 (0.02 μg/ml) and IC90 (0.04 μg/ml) at 72 h and still 4 times as high as its IC50 (0.04 μg/ml) at 4 h. Besides, the high ratio of the area under the curve (AUC) to the IC50 of 43.5 of R-PZQ might also describe its excellent antischistosomal activity. On the other hand, the concentrations of S-PZQ and the R-trans enantiomer in plasma do not exceed the calculated IC50 calculated in our work at any time (IC50s approximately 11 and 3 times as high as the Cmax, respectively). Though the AUC of the R-trans metabolite is much higher than those of R-PZQ and S-PZQ, the AUC/IC50 ratio is only 2.1. Furthermore, our in vitro recovery experiments with S-PZQ, even at concentrations up to 700 times its Cmax (16), demonstrated that the worms were still alive and recovered from 2 h of exposure. As mentioned before, the S-trans metabolite is not active at 100 μg/ml.
TABLE 5.
| Compound | Cmax (μg/ml) | tmax (h) | t1/2 (h) | AUC (μg ml−1 h) | Cmax/IC50c ratio | AUC/IC50c ratio |
|---|---|---|---|---|---|---|
| R-PZQ | 0.16 | 2.67 | 1.55 | 0.87 | 8 | 43.5 |
| S-PZQ | 0.52 | 2.55 | 1.46 | 2.99 | 0.09 | 0.5 |
| R-trans-4-OH-PZQ | 1.31 | 2.72 | 1.70 | 8.80 | 0.31 | 2.1 |
| S-trans-4-OH-PZQ | 0.78 | 3.05 | 1.91 | 5.60 | NAd | NAd |
tmax, time to Cmax; t1/2, half-life; AUC, area under the curve.
Adapted from reference 16 (oral dose of 23.3 mg/kg).
IC50s from adults after 72 h.
NA, not applicable (no IC50).
Published PK data for the cis metabolite are not yet available, but in light of its high IC50 compared to that of R-PZQ, it is also unlikely that it significantly contributes to the antischistosomal activity of PZQ.
Changes in the activity of CYP enzymes can dramatically change the PK parameters of PZQ and thereby its therapeutic activity. For example, coadministration of CYP 3A4 inducers such as dexamethasone dramatically reduces plasma PZQ levels in patients with neurocysticercosis (6, 31, 32). Albendazole is an inhibitor of CYP enzymes, and when it is administered concomitantly with PZQ, plasma R-PZQ levels are increased (16). The expression of CYP is also modulated during chronic schistosomiasis, with markedly lower activity in infected mice, probably resulting from the immune response to the infection (33). Interestingly, resistant isolates of S. mansoni do not inhibit host CYP as much as susceptible isolates do. This mechanism of resistance produces faster first-pass metabolism, hence a shorter time of exposure to the parent drug (34). These results support the evidence that R-PZQ is the active molecule and metabolites do not have a major role in the activity of PZQ.
We conclude that the activity of PZQ is based almost exclusively on R-PZQ and that neither S-PZQ nor the metabolites significantly contribute to the therapeutic effect. Our results favor the development of a child-friendly formulation of R-PZQ, since an enantiopure formulation displays two major advantages; first, it would allow clinicians to reduce the dosage by half, and second, it would ease administration to children, who are bothered by the bitter taste of S-PZQ (35).
ACKNOWLEDGMENTS
We are grateful to Mireille Vargas for assisting with the in vivo studies.
This work was supported by the European Research Council (ERC-2013-CoG 614739-A_HERO to J.K.), the Swiss National Science Foundation (professorship PPOOP2-133568 to G.G.), the University of Zurich (G.G.). and the Stiftung für Wissenschaftliche Forschung of the University of Zurich (G.G.).
Footnotes
Published ahead of print 30 June 2014
REFERENCES
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. 2014. Human schistosomiasis. Lancet 383:2253–2264. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 3.Utzinger J, N′Goran EK, Caffrey CR, Keiser J. 2011. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 120(Suppl 1):S121–S137. 10.1016/j.actatropica.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 4.Gryseels B, Polman K, Clerinx J, Kestens L. 2006. Human schistosomiasis. Lancet 368:1106–1118. 10.1016/S0140-6736(06)69440-3 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2006. Preventive chemotherapy in human helminthiasis. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf?ua=1 [Google Scholar]
- 6.Li XQ, Bjorkman A, Andersson T, Gustafsson L, Masimirembwa C. 2003. Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur. J. Clin. Pharmacol. 59:429–442. 10.1007/s00228-003-0636-9 [DOI] [PubMed] [Google Scholar]
- 7.Lerch C, Blaschke G. 1998. Investigation of the stereoselective metabolism of praziquantel after incubation with rat liver microsomes by capillary electrophoresis and liquid chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 708:267–275. 10.1016/S0378-4347(97)00638-5 [DOI] [PubMed] [Google Scholar]
- 8.Meier H, Blaschke G. 2001. Investigation of praziquantel metabolism in isolated rat hepatocytes. J. Pharm. Biomed. Anal. 26:409–415. 10.1016/S0731-7085(01)00417-4 [DOI] [PubMed] [Google Scholar]
- 9.Melo AJB, Iamamoto Y, Maestrin APJ, Smith JRL, Santos MD, Lopes NP, Bonato PS. 2005. Biomimetic oxidation of praziquantel catalysed by metalloporphyrins. J. Mol. Catal. A Chem. 226:23–31. 10.1016/j.molcata.2004.09.015 [DOI] [Google Scholar]
- 10.Andrews P, Thomas H, Pohlke R, Seubert J. 1983. Praziquantel. Med. Res. Rev. 3:147–200. 10.1002/med.2610030204 [DOI] [PubMed] [Google Scholar]
- 11.Staudt U, Schmahl G, Blaschke G, Mehlhorn H. 1992. Light and scanning electron microscopy studies on the effects of the enantiomers of praziquantel and its main metabolite on Schistosoma mansoni in vitro. Parasitol. Res. 78:392–397. 10.1007/BF00931694 [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Ohmae H, Utsunomiya H, Nara T, Irie Y, Yasuraoka K. 1989. A comparison of the antischistosomal effect of levo- and dextro-praziquantel on Schistosoma japonicum and S. mansoni in mice. Am. J. Trop. Med. Hyg. 41:198–203 [DOI] [PubMed] [Google Scholar]
- 13.Xiao SH, Catto BA. 1989. Comparative in vitro and in vivo activity of racemic praziquantel and its levorotated isomer on Schistosoma mansoni. J. Infect. Dis. 159:589–592. 10.1093/infdis/159.3.589 [DOI] [PubMed] [Google Scholar]
- 14.Wu MH, Wei CC, Xu ZY, Yuan HC, Lian WN, Yang QJ, Chen M, Jiang QW, Wang CZ, Zhang SJ, et al. 1991. Comparison of the therapeutic efficacy and side effects of a single dose of levo-praziquantel with mixed isomer praziquantel in 278 cases of schistosomiasis japonica. Am. J. Trop. Med. Hyg. 45:345–349 [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Zhou S, Lian W, Mao M, Yu Y. 1994. Electron microscopic observations of tegumental damage in adult Schistosoma japonicum after in vivo treatment with levo-praziquantel. Chin. Med. J. 107:771. [PubMed] [Google Scholar]
- 16.Lima RM, Ferreira MA, de Jesus Ponte Carvalho TM, Dumet Fernandes BJ, Takayanagui OM, Garcia HH, Coelho EB, Lanchote VL. 2011. Albendazole-praziquantel interaction in healthy volunteers: kinetic disposition, metabolism and enantioselectivity. Br. J. Clin. Pharmacol. 71:528–535. 10.1111/j.1365-2125.2010.03874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keiser J. 2010. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 137:589–603. 10.1017/S0031182009991739 [DOI] [PubMed] [Google Scholar]
- 18.Woelfle M, Seerden JP, de Gooijer J, Pouwer K, Olliaro P, Todd MH. 2011. Resolution of praziquantel. PLoS Negl. Trop. Dis. 5(9):e1260. 10.1371/journal.pntd.0001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patra M, Ingram K, Leonidova A, Pierroz V, Ferrari S, Robertson MN, Todd MH, Keiser J, Gasser G. 2013. In vitro metabolic profile and in vivo antischistosomal activity studies of (η(6)-praziquantel)Cr(CO)3 derivatives. J. Med. Chem. 56:9192–9198. 10.1021/jm401287m [DOI] [PubMed] [Google Scholar]
- 20.Manneck T, Haggenmüller Y, Keiser J. 2010. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137:85–98. 10.1017/S0031182009990965 [DOI] [PubMed] [Google Scholar]
- 21.Testa B, Trager WF. 1990. Racemates versus enantiomers in drug development: dogmatism or pragmatism? Chirality 2:129–133. 10.1002/chir.530020302 [DOI] [PubMed] [Google Scholar]
- 22.Manneck T, Braissant O, Haggenmüller Y, Keiser J. 2011. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J. Clin. Microbiol. 49:1217–1225. 10.1128/JCM.02382-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao SH, Keiser J, Chollet J, Utzinger J, Dong Y, Endriss Y, Vennerstrom JL, Tanner M. 2007. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 51:1440–1445. 10.1128/AAC.01537-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram K, Schiaffo CE, Sittiwong W, Benner E, Dussault PH, Keiser J. 2012. In vitro and in vivo activity of 3-alkoxy-1,2-dioxolanes against Schistosoma mansoni. J. Antimicrob. Chem. 67:1979–1986. 10.1093/jac/dks141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao SH, Catto BA, Webster LT. 1985. Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J. Infect. Dis. 151:1130–1137. 10.1093/infdis/151.6.1130 [DOI] [PubMed] [Google Scholar]
- 26.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3(1):e350. 10.1371/journal.pntd.0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao SH, Catto BA. 1989. In vitro and in vivo studies of the effect of artemether on Schistosoma mansoni. Antimicrob. Agents Chemother. 33:1557–1562. 10.1128/AAC.33.9.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster R, Cheetham BL. 1973. Studies with the schistosomicide oxamniquine (UK-4271). I. Activity in rodents and in vitro. Trans. R. Soc. Trop. Med. Hyg. 67:674–684. 10.1016/0035-9203(73)90038-2 [DOI] [PubMed] [Google Scholar]
- 29.Buttle GA, Khayyal MT. 1962. Rapid hepatic shift of worms in mice infected with Schistosoma mansoni after a single injection of tartar emetic. Nature 194:780–781. 10.1038/194780b0 [DOI] [PubMed] [Google Scholar]
- 30.Bueding E, Fischer J. 1970. Biochemical effects of niridazole on Schistosoma mansoni. Mol. Pharmacol. 6:532–539 [PubMed] [Google Scholar]
- 31.Na-Bangchang K, Vanijanonta S, Karbwang J. 1995. Plasma concentrations of praziquantel during the therapy of neurocysticercosis with praziquantel, in the presence of antiepileptics and dexamethasone. Southeast Asian J. Trop. Med. Public Health 26:120–123 [PubMed] [Google Scholar]
- 32.Vazquez ML, Jung H, Sotelo J. 1987. Plasma levels of praziquantel decrease when dexamethasone is given simultaneously. Neurology 37:1561–1561. 10.1212/WNL.37.9.1561 [DOI] [PubMed] [Google Scholar]
- 33.Gotardo MA, Hyssa JT, Carvalho RS, De-Carvalho RR, Gueiros LS, Siqueira CM, Sarpa M, De-Oliveira AC, Paumgartten F., Jr 2011. Modulation of expression and activity of cytochrome P450s and alteration of praziquantel kinetics during murine schistosomiasis. Mem. Inst. Oswaldo Cruz 106:212–219. 10.1590/S0074-02762011000200016 [DOI] [PubMed] [Google Scholar]
- 34.Botros SS, El-Din SH, El-Lakkany NM, Sabra AN, Ebeid FA. 2006. Drug-metabolizing enzymes and praziquantel bioavailability in mice harboring Schistosoma mansoni isolates of different drug susceptibilities. J. Parasitol. 92:1344–1349. 10.1645/GE-865R.1 [DOI] [PubMed] [Google Scholar]
- 35.Meyer T, Sekljic H, Fuchs S, Bothe H, Schollmeyer D, Miculka C. 2009. Taste, a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Negl. Trop. Dis. 3(1):e357. 10.1371/journal.pntd.0000357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keiser J, Manneck T, Vargas M. 2011. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J. Antimicrob. Chemother. 66:1791–1797. 10.1093/jac/dkr178 [DOI] [PubMed] [Google Scholar]