Abstract
Encapsulation of antibiotics may improve treatment of intracellular infections by prolonging antibiotic release and improving antibiotic uptake into cells. In this study, liposome-encapsulated ciprofloxacin for inhalation (CFI) was evaluated as a postexposure therapeutic for the treatment of Coxiella burnetii, the causative agent of Q fever. Intranasal treatment of male A/Jola (A/J) mice with CFI (50 mg/kg of body weight) once daily for 7 days protected mice against weight loss and clinical signs following an aerosol challenge with C. burnetii. In comparison, mice treated twice daily with oral ciprofloxacin or doxycycline (50 mg/kg) or phosphate-buffered saline (PBS) lost 15 to 20% body weight and exhibited ruffled fur, arched backs, and dehydration. Mice were culled at day 14 postchallenge. The weights and bacterial burdens of organs were determined. Mice treated with CFI exhibited reduced splenomegaly and reduced bacterial numbers in the lungs and spleen compared to mice treated with oral ciprofloxacin or doxycycline. When a single dose of CFI was administered, it provided better protection against body weight loss than 7 days of treatment with oral doxycycline, the current antibiotic of choice to treat Q fever. These data suggest that CFI has potential as a superior antibiotic to treat Q fever.
INTRODUCTION
Coxiella burnetii is a Gram-negative intracellular bacterium and the etiological agent of the disease Q fever. Q fever is prevalent throughout the world, with the exception of New Zealand, and is a zoonosis that typically presents as an acute, self-limiting febrile disease or atypical pneumonia in humans (1). In addition, there are several recent reports of outbreaks among military personnel serving in Iraq and Afghanistan, and C. burnetii is listed as a category B agent by the Centers for Disease Control and Prevention (CDC) (2–4). Recovery from acute disease usually occurs within 2 weeks and can be accelerated by treatment twice daily with oral doxycycline. However, delays in diagnosis of acute Q fever or an incorrect diagnosis often result in ineffective or inappropriate antibiotic treatment (5). C. burnetii can also cause a chronic form of disease which is a severe, and sometimes life-threatening, manifestation, often characterized by endocarditis or hepatitis (6). Chronic infections are treated with a combination of doxycycline and chloroquine for at least 18 months and up to 4 years (7). A recent fatal case of Q fever was caused by a doxycycline-resistant strain, and cases presenting with endocarditis are associated with frequent relapses, despite antibiotic treatment (8, 9).
Encapsulation of antibiotics in delivery vesicles, such as liposomes, has been shown to enhance drug availability and activity and reduce toxicity in general (10), and antibiotics delivered by inhalation particularly display these characteristics (11). Ciprofloxacin is a broad-spectrum fluoroquinolone that demonstrates in vitro bactericidal effects against several respiratory pathogens, including C. burnetii, Mycobacterium tuberculosis, and Neisseria meningitidis (12–14). However, oral or intravenous delivery of ciprofloxacin results in a suboptimal pharmacokinetic profile in the lower respiratory tract, and emergence of antibiotic resistance makes treatment problematic (15–17). Formulations of liposome-encapsulated ciprofloxacin have been developed and shown to improve antibiotic efficacy in Francisella tularensis, Mycobacterium avium, and Streptococcus pneumoniae in vivo models (18–21).
The liposome-encapsulated ciprofloxacin tested in this study, ciprofloxacin for inhalation (CFI), is an advanced formulation specifically designed for inhalation that has already been shown to be highly efficacious in vivo against several intracellular pathogens, specifically F. tularensis LVS and Schu S4 strains (22) and Yersinia pestis CO92 strain (K. A. Hamblin, J. D. Blanchard, C. Davies, and S. V. Harding, presented at the 19th Congress of the International Society of Aerosols in Medicine, April 2013, Chapel Hill, NC) in mouse models of lung infection.
In this study, we report for the first time the evaluation of antibiotic efficacy in an aerosol murine model of Q fever. The efficacy of CFI, ciprofloxacin, and doxycycline as postexposure therapeutics were compared, and single-dose pharmacokinetic profiles were determined.
MATERIALS AND METHODS
Bacteria.
C. burnetii strain Nine Mile was grown axenically in ACCM-2 in 250-ml Erlenmeyer flasks containing 100 ml medium (23). Cultures were incubated at 37°C, shaking at 75 rpm for 6 days, with a GENbox microaer atmosphere generator (bioMérieux, France) to displace oxygen. Bacteria were harvested by centrifugation at 10,000 × g at 21°C for 20 min and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of approximately 1 × 109 genome equivalents (GE)/ml. All manipulations of C. burnetii were carried out in a class III microbiological safety cabinet complying with the 2000 European standard EN 12469 (24). Using the criteria within the “Proposed Framework for the Oversight of Dual Use Life Sciences Research: Strategies for Minimizing the Potential Misuse of Research Information” (25) by the National Science Advisory Board for Biosecurity (NSABB), this research does not fall into any of the categories for dual-use research of concern.
Bacterial enumeration.
C. burnetii was enumerated using real-time PCR (RT-PCR) targeting the sod gene using the forward primer TCTTCAACAATGCAGCACAACAT and reverse primer TGAAGCCAATTCGCCAGAA. The probe sequence was CATTTTATTGGCACTGCATGAGCCCTG, and the probe was covalently labeled at the 5′ end with the reporter dye 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher dye black hole quencher 1 (BHQ-1). Chromosomal DNA was extracted by addition using Qiagen QIAmp DNA minikit/blood mini/tissue depending on the sample type. Real-time PCR mixtures comprised 2 μl template DNA, forward primer (900 nM), reverse primer (900 nM), probe (250 nM) and ABI Fast TaqMan mastermix. PCR cycling conditions comprised 3 min at 95°C, 30 s at 60°C, followed by 50 two-step cycles, with 1 cycle consisting of 15 s at 95°C and 30 s at 60°C. For each PCR, a control of linearized synthetic plasmid that contains a single copy of the target was included. The synthetic plasmid was quantitated after linearization and purification using a ND-2500 NanoDrop spectrophotometer. For each reaction, a standard curve of the synthetic plasmid was run in duplicate in the range 1 × 107 GE/ml to 1 × 102 GE/ml. Plasmid concentration of 1 × 101 GE/ml in triplicate is used as a lower-limit check in every assay. The lower limit of detection for this assay is 2.5 × 103 GE/ml culture or 4.4 × 101 GE/mg spleen or 2.2 × 101 GE/mg lung/liver/testes.
Mice.
Groups of age-matched male A/Jola (A/J) mice (Charles River) were housed on a 12-h day-night light cycle, with food and water available ad libitum in an Advisory Committee on Dangerous Pathogens (ACDP) (United Kingdom) level 3 flexible-film isolator, complying with the 2000 European Standard EN 12469 (24), and allowed to acclimatize before challenge. All procedures were conducted under a project license approved by internal ethical review, and in accordance with both the Animal (Scientific Procedures) Act (1986) (26) and the 1989 Codes of Practice for the Housing and Care of Animals used in Scientific Procedures (27).
Antimicrobial agents.
Ciprofloxacin (Bayer, Newbury, United Kingdom) or doxycycline hyclate (Sigma-Aldrich, United Kingdom) were dissolved in distilled water to a working concentration of 25 mg/ml or 40 mg/ml, respectively. Ciprofloxacin for inhalation (CFI) was provided by Aradigm Corporation (Hayward, CA, USA) and diluted in PBS to a working concentration of 25 mg/ml. All antibiotics were prepared freshly each day prior to dosing.
Infection of mice and antibiotic efficacy studies.
Mice were challenged with an aerosol produced from 10-ml suspension of C. burnetii at a concentration of 2.3 × 109 GE/ml (study 1), 6 × 109 GE/ml (study 2), or 1 × 1010 GE/ml (study 3) using the AeroMP-Henderson apparatus. The challenge aerosol was generated using a six-jet Collison nebulizer (BGI, Waltham, MA) operating at 15 liters/min. The aerosol was mixed with conditioned air in the spray tube and delivered via a head-only exposure chamber. Samples of the aerosol were taken using an AGI-30 (Ace Glass Inc., USA) at 6 liters/min containing PBS and an aerodynamic particle sizer (TSI Instruments, Ltd., Bucks, United Kingdom); these processes were controlled and monitored using the AeroMP management platform (Biaera Technologies, LLC, Frederick, MD). A back titration of the aerosol samples taken at the time of challenge was performed using RT-PCR as described above. Direct bacterial enumeration was used to calculate the presented dose using a derived respiratory minute volume of 19.9 ml estimated using the average weight of the animals (28).
For study 1, groups of six infected mice were observed, and clinical signs (piloerection, arched back, dehydration, eyes shut, wasp-waisted appearance, immobile, and death) and weight were evaluated twice daily. Mice were culled on days 3, 4, 5, 6, 7, 8, 9, 11, and 13 postchallenge (p.c.), and the lungs, spleen, liver, testes, and blood were aseptically removed. Organs were weighed and homogenized using a Precellys24 homogenizer, and bacteria were enumerated using RT-PCR as described above. Antibody titers were determined using Q fever phase I IgG and phase II IgM Novalisa kits (Novatec, Germany).
For study 2, groups of 10 infected mice were treated from 24 h p.c. for 7 consecutive days with 40 μl of a 25-mg/ml ciprofloxacin (50 mg/kg of body weight) solution administered orally via pipette twice daily (12-h intervals) or as 40 μl of a 25-mg/ml solution of CFI (50 mg/kg) delivered once daily (24-h intervals) by intranasal (i.n.) instillation using a pipette. Control mice received PBS orally twice daily for 7 days postchallenge. At day 14 p.c., mice were culled, and the lungs and spleens were aseptically removed. The lungs and spleens were weighed, and half the organ was placed in 10% neutral buffered formalin for histological analysis. The remaining organ was homogenized using a Precellys24 instrument, and bacteria were enumerated using RT-PCR as described above. Mice were monitored as discussed above for study 1.
For study 3, groups of 20 infected mice were treated from 24 h p.c. for 1, 3, or 7 consecutive days with 25 μl of a 40-mg/ml (50-mg/kg) doxycycline solution administered orally via pipette twice daily (12-h intervals) to mimic treatment of human Q fever or as CFI delivered once daily (24-h intervals) by i.n. instillation (as described above for study 2). Control mice received PBS orally twice daily for the same number of days postchallenge as the respective treatment groups. At day 7 or 14 postchallenge, 10 mice from each treatment group were culled, and the lungs and spleens were aseptically removed and weighed. Mice were monitored as described above for study 1, and the organs were processed as described above for study 2.
Pharmacokinetic studies.
Ciprofloxacin was administered to mice (n = 30) by the oral route at a dose of 50 mg/kg. Groups of three mice were culled at 10 min, 20 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 4 h, 8 h, and 24 h after dosing.
Doxycycline was administered to mice (n = 30) by the oral route at a dose of 50 mg/kg. Groups of three mice were culled at 10 min, 20 min, 30 min, 45 min, 1 h, 2 h, 4 h, 8 h, 16 h, and 24 h after dosing.
CFI was administered to mice (n = 30) by the i.n. route at a dose of 50 mg/kg. Groups of three mice were culled at 1 min, 15 min, 30 min, 2 h, 4 h, 8 h, 12 h, 16 h, 20 h, and 24 h after dosing.
Lungs and blood samples were collected from all animals immediately postmortem. All blood samples were heparinized and centrifuged to separate the plasma fraction from the whole blood. Samples were immediately frozen and stored at −80°C until further analysis. Three untreated control mice were included with each antibiotic group.
Histopathology.
Samples of lung and spleen were processed and embedded in paraffin wax. Sections were cut at 5 to 6 μm and stained with hematoxylin and eosin. Microscopic lesions in lung and spleen were assessed and recorded as within normal limits (where C. burnetii-associated lesions were not detected) or minimal, mild, moderate, and marked where C. burnetii-associated lesions were detected. The slides were assessed independently by two pathologists in a blind manner (unaware of the treatment).
Determination of antibiotic concentrations in plasma and lung tissues by liquid chromatography-mass spectrometry (LC-MS).
Lung samples were weighed and mixed with 3 × the volume of 0.1% (vol/vol) aqueous formic acid. Tissue was homogenized in a Precellys24-Dual bead homogenizer using 2.8-mm beads for two 20-s cycles at 5,000 rpm. The lung homogenate was mixed with an equal volume of 1-μg/ml internal standard solution (ciprofloxacin-d8 hydrochloride hydrate or demeclocycline hydrochloride hydrate; Fluka, United Kingdom), and 2 volumes of dichloromethane were added. The plasma samples were mixed with an equal volume of internal standard solution and 3 volumes of acetonitrile. Following centrifugation, the supernatant was removed and reduced in volume to remove the acetonitrile using a centrifugal evaporator (Genevac Ltd., Ipswich, United Kingdom) and injected onto the LC-MS system consisting of an Agilent 1100 binary pump (Agilent Technologies UK Ltd., Wokingham, United Kingdom), CTC PAL injector (Presearch Ltd., Basingstoke, United Kingdom), Sciex API3000 LC-MS (AB Sciex, Warrington, United Kingdom) using an ACE-3–C18HL 20- by 2.1-mm column (Hichrom, Theale, United Kingdom) and a gradient mobile phase. Mobile-phase solvent A consisted of 0.1% (vol/vol) aqueous formic acid in water, and mobile-phase solvent B consisted of 0.1% (vol/vol) aqueous formic acid in acetonitrile. The initial mobile-phase composition was 95% solvent A and 5% solvent B. For ciprofloxacin analysis, the gradient was increased from 1 to 4 min to 95% solvent B, maintained for 1 min, then decreased back to 5% solvent B, and maintained for 3 min. For doxycycline analysis, the gradient was increased from 1 to 8 min to 55% solvent B, maintained for 1 min, then decreased back to 5% solvent B, and maintained for 3 min. The solvents were pumped at a flow rate of 250 μl/min. The LC-MS system was controlled by Analyst software (AB Sciex, United Kingdom). Calibration curves were prepared in naive mouse plasma or lung tissue samples, using reference standards ciprofloxacin (>98%; Fluka, United Kingdom) and doxycycline hyclate (>98%; Sigma-Aldrich) and consisted of 11 points from 1 ng/ml to 20,000 ng/ml. All calibration curves had r of ≥0.99 and were fitted using linear regression with 1/x2 weighting.
Pharmacokinetic calculations.
The pharmacokinetic parameters terminal half-life (t1/2), rate of clearance (CL), maximum concentration (Cmax), time of maximum concentration (Tmax), and area under the concentration-time curve (AUC) were determined by noncompartmental pharmacokinetic analysis using WinNonlin Phoenix (v6.1; Pharsight Corp., USA) on mean concentration data. The relative bioavailability of oral ciprofloxacin and i.n. ciprofloxacin quantified in lung homogenate was calculated using an equation adapted from Rowland and Tozer (29), with the error associated with the relative bioavailability equation adapted from Taylor (30).
Statistical analysis.
All statistical analysis was performed using SPSS v18.0 (IBM). All graphs were produced using Graphpad Prism v5. Analysis of mouse body weight loss compared to controls was carried out using a parametric univariate model of variance and Bonferroni's post test. Bacterial burden data were first transformed by log10 and then analyzed using a univariate linear model and Bonferroni's post test. Organ weight (as a percentage of body weight) was first transformed by square root function and then analyzed using a univariate linear model and Bonferroni's post test. The validity of the univariate model approach (general linear model [GLM]) was tested by Levene's test for unequal variance. Statistical significance was indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Study 1 (development of a murine model of Q fever).
In order to establish a model suitable for antibiotic efficacy studies, mice were challenged by the aerosol route with a mean presented dose of 5 × 106 GE of C. burnetii. Infected mice showed clinical signs between 5 and 9 days after challenge, exhibiting ruffled coats, arched backs, and a wasp-waisted appearance. In addition, weight loss was apparent in all animals from day 5 postchallenge, with peak weight loss observed between days 7 and 8. In order to characterize the pathogenesis of disease over time, groups of six mice were culled at multiple time points after challenge, and the organs were harvested. During the 13-day course of this experiment, no significant changes in organ weight in uninfected mice were observed (sampled at 0, 7, and 13 days; P = 0.981 for the spleen and P = 0.795 for the lung). In contrast, the weights of both the spleen and the lung (but not liver and testes) from infected mice increased over time (P < 0.001) (Table 1), with the difference in the spleen weight becoming statistically significant at day 8 postinfection (P < 0.001) and the difference in the lung weight becoming statistically significant at 5 days (P = 0.033). C. burnetii was isolated from all tissues postchallenge, with consistently high levels of bacteria (105 to 106 GE/ml) in the lungs. The bacterial burden in the spleen, liver, testes, and blood increased throughout the course of infection (Table 1). In addition, all infected mice culled at day 13 had developed antibodies to C. burnetii phase I and II antigens (phase I IgG titers of <1:200; phase II IgM titers from 1:200 to 1:1,600).
TABLE 1.
Natural history of C. burnetii infection in A/J micea
| Day postchallenge | Organ wt (mg) (mean ±SD) |
Bacterial burden (GE/mg or GE/ml)b (mean ±SD) in: |
|||||
|---|---|---|---|---|---|---|---|
| Lung | Spleen | Lung | Spleen | Liver | Testesc | Blood | |
| 3 | 146.10 ± 14.50 | 59.87 ± 4.10 | 2.98 × 105 ± 5 × 104 | 5.67 × 102 ± 1.39 × 102 | 0 | 0 | 3.60 × 104 ± 7.05 × 104 |
| 4 | 175.95 ± 26.96 | 64.42 ± 16.02 | 1.43 × 106 ± 9.31 × 105 | 9.64 × 102 ± 1.75 × 103 | 2.9 × 101 ± 2.71 × 101 | 0 | 1.10 × 104 ± 4.30 × 103 |
| 5 | 250.58 ± 55.58 | 75.60 ± 8.30 | 1.07 × 106 ± 3.54 × 105 | 3.53 × 103 ± 8.89 × 102 | 1.98 × 102 ± 9.06 × 101 | 0 | 1.70 × 104 ± 0.00 |
| 6 | 230.38 ± 23.65 | 94.13 ± 10.99 | 9.50 × 105 ± 4.80 × 104 | 2.67 × 104 ± 1.55 × 104 | 3.36 × 103 ± 2.12 × 103 | 2.93 × 101 ± 4.29 × 101 | 6.00 × 105 ± 1.01 × 106 |
| 7 | 266.85 ± 29.88 | 86.80 ± 19.54 | 6.95 × 105 ± 2.02 × 105 | 1.15 × 105 ± 5.82 × 104 | 6.95 × 105 ± 5.37 × 103 | <LOD | 3.50 × 105 ± 4.02 × 105 |
| 8 | 273.40 ± 35.02 | 164.13 ± 14.53 | 6.38 × 105 ± 3.22 × 105 | 1.01 × 105 ± 7.40 × 104 | 2.58 × 104 ± 1.31 × 104 | 7.62 × 101 ± 9.92 × 101 | 1.70 × 104 ± 0.00 |
| 9 | 252.92 ± 24.36 | 176.53 ± 20.92 | 4.83 × 105 ± 1.44 × 105 | 4.6 × 104 ± 4.85 × 104 | 1.99 × 104 ± 1.81 × 104 | 8.57 × 101 ± 1.04 × 102 | 4.40 × 105 ± 1.06 × 106 |
| 11 | 260.15 ± 36.69 | 141.93 ± 25.98 | 8.95 × 105 ± 2.18 × 105 | 1.13 × 105 ± 9.48 × 104 | 2.60 × 104 ± 1.90 × 104 | 2.49 × 102 ± 2.27 × 102 | 2.80 × 105 ± 2.72 × 105 |
| 13 | 303.02 ± 70.36 | 156.20 ± 12.48 | 6.03 × 105 ± 4.91 × 105 | 4.07 × 104 ± 2.78 × 104 | 2.61 × 104 ± 1.55 × 104 | 1.05 × 102 ± 1.16 × 102 | 2.10 × 105 ± 4.56 × 103 |
In uninfected animals, no changes in organ weight were observed, and no bacteria were detected.
Bacterial burden shown in genome equivalents (GE) per milligram for the solid organs (lung, spleen, liver, and testes) or GE per milliliter for blood.
<LOD, less than the limit of detection.
Study 2 (in vivo efficacy of free ciprofloxacin and CFI).
The efficacy of ciprofloxacin or CFI for the treatment of mice infected with C. burnetii was determined (Fig. 1). Mice were challenged with a mean presented dose of 2.8 × 106 GE and treated with free ciprofloxacin or PBS lost almost 20% body weight by day 7 postchallenge (p.c.) and exhibited clinical signs such as piloerection and arched backs from days 4 to 9 p.c. In contrast, mice treated with CFI were significantly protected against weight loss from day 4 p.c. (P < 0.001) and showed no clinical signs of disease.
FIG 1.
Efficacy of ciprofloxacin or CFI against C. burnetii in vivo (n = 10). Mean percentage change in body weight of mice infected with 2.8 × 106 GE of C. burnetii per ml and treated with free ciprofloxacin (by oral administration) or CFI (by i.n. administration) for 7 days postchallenge.
Infection of mice with C. burnetii significantly increased the weight of the spleen and lungs (P < 0.001; data not shown). Treatment with CFI significantly moderated lung and spleen weight increases compared to treatment with ciprofloxacin or PBS (controls) at day 14 p.c. (P < 0.05 for the lung; P < 0.001 for the spleen; Fig. 2A). In addition, while mice treated with PBS were colonized with approximately 105 to 106 GE/ml or 104 to 105 GE/ml in the lungs or spleen, respectively, treatment with CFI reduced bacterial numbers by 10- to 100-fold, which was significant in the lungs (P < 0.001; Fig. 2B).
FIG 2.
Weight and bacterial colonization of lungs and spleen at day 14 p.c. (n = 10). (A) Weight of lungs and spleens from mice challenged with 2.8 × 106 GE of C. burnetii per ml and treated with PBS, free ciprofloxacin, or CFI for 7 days. (B) Bacterial colonization of lungs and spleens from mice challenged with 2.8 × 106 GE of C. burnetii/ml and treated with PBS, free ciprofloxacin, or CFI for 7 days. Values are means ± standard errors (error bars). Values that are significantly different from the value for the ciprofloxacin control are indicated by asterisks as follows: *, P < 0.05; ***, P < 0.001.
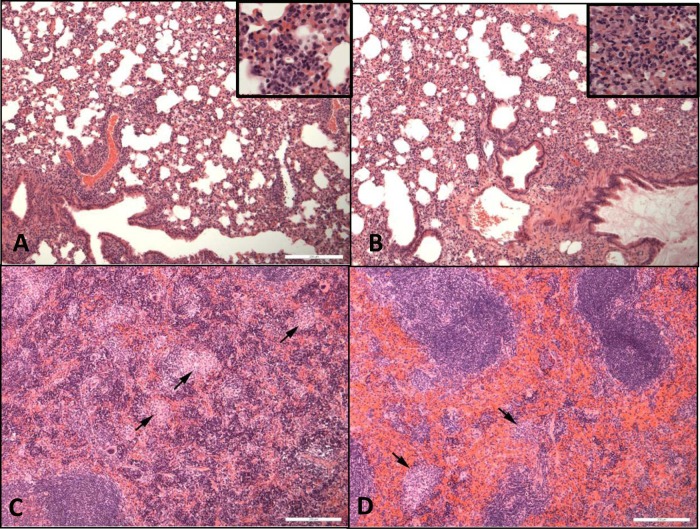
On histological examination, in the lungs of all challenged animals, focal or diffuse granulomatous alveolitis was observed, and the severity of alveolitis was similar in treated and PBS control groups (Fig. 3A and B). In the spleen, multifocal granulomatous foci were observed in mice treated with free ciprofloxacin or in PBS control groups (Fig. 3C), but reduced severity or absence of lesions was observed following treatment with CFI (Fig. 3D).
FIG 3.
Hematoxylin-and-eosin-stained spleen and lung tissues from mice at day 14 p.c. (A) Lung from a mouse treated with PBS for 7 days. Moderate granulomatous alveolitis is present. (B) Lung from a mouse treated with CFI for 7 days. Moderate granulomatous alveolitis is present. (C) Spleen from a mouse treated with PBS for 7 days. The sites of moderate, multifocal, granulomatous lesions are indicated by black arrows. (D) Spleen from a mouse treated with CFI for 7 days. The sites of minimal granulomatous lesions are indicated by black arrows. Bar, 200 µm.
Study 3 (in vivo efficacy of doxycycline and CFI).
Doxycycline is the standard antibiotic of choice to treat Q fever. Thus, the efficacy of 1 day, 3 days, and 7 days of doxycycline or CFI treatment postchallenge of mice challenged with a mean presented dose of 7 × 106 GE of C. burnetii was determined (Fig. 4). Mice treated with PBS lost 15% body weight by day 7 p.c., and 80% of the mice exhibited clinical signs such as piloerection at least once during the postchallenge period. Mice treated with doxycycline for 1 day, 3 days, or 7 days also lost 13 to 15% body weight; however, there was a delay in initiation of weight loss of 1, 3, or 7 days, respectively, compared to PBS-treated control mice. In all doxycycline treatment groups, 70% of mice exhibited clinical signs that correlated with peak weight loss. In contrast, mice treated with CFI had significantly reduced weight loss from day 8 p.c. compared to the corresponding doxycycline treatment regime (Fig. 4A and B) (P < 0.001). In addition, from day 12 to day 14 p.c., mice treated with a single dose of CFI had significantly reduced weight loss compared to mice treated with doxycycline for 1, 3, or 7 days (P < 0.01). The proportion of mice treated with CFI presenting with clinical signs ranged from 50% (1 day of CFI treatment) to 0% (7 days of CFI treatment).
FIG 4.
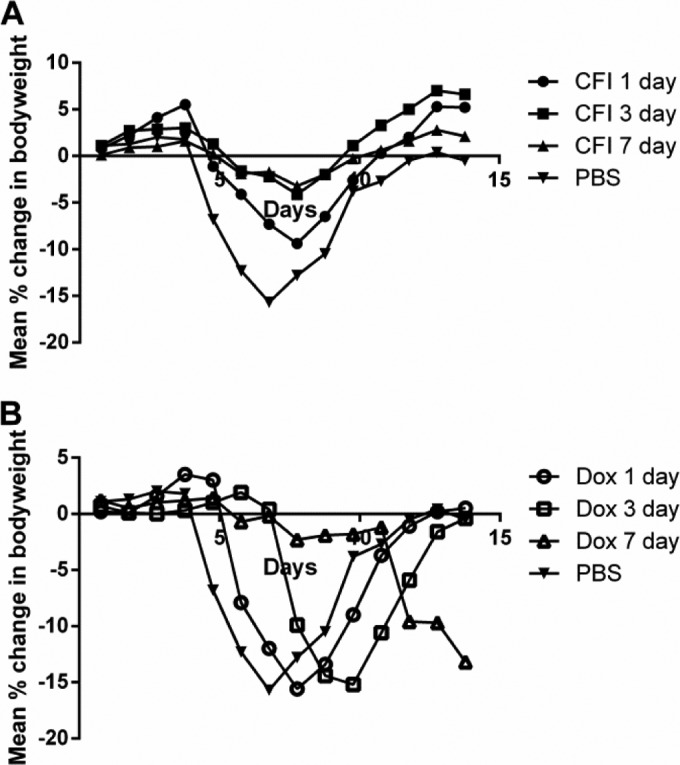
Efficacy of doxycycline or CFI against C. burnetii in vivo (n = 10) versus PBS-treated control mice. Mean percentage change in body weight of mice infected with 7 × 107 GE of C. burnetii/ml and treated with CFI (A) (by i.n. administration) or free doxycycline (Dox) (B) (by oral administration) for 1 day, 3 days, or 7 days postchallenge.
Treatment with CFI for 7 days significantly moderated lung weight increases compared to treatment with doxycycline for 7 days at day 14 p.c. (P < 0.01; Fig. 5A). Spleen weight gain of CFI-treated mice was significantly moderated compared to PBS-treated control mice (P < 0.05), but not doxycycline-treated mice. In addition, mice treated with CFI for 7 days had significantly reduced bacterial numbers in the lungs and spleen compared to mice treated with doxycycline for 7 days (P < 0.001; Fig. 5B).
FIG 5.
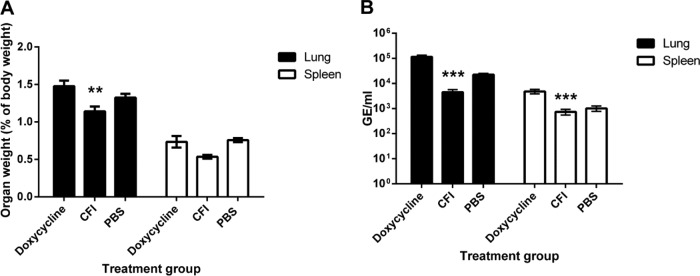
Weight and bacterial colonization of lungs and spleen at day 14 p.c. (n = 10). (A) Weight of lungs and spleens from mice challenged with 7 × 106 GE of C. burnetii/ml and treated with doxycycline or CFI for 7 days. (B) Bacterial colonization of lungs and spleen from mice challenged with 9.68 × 107 GE of C. burnetii/ml and treated with doxycycline or CFI for 7 days. Values are means ± standard errors. Values that are significantly different from the value for the doxycycline control are indicated by asterisks as follows: **, P < 0.01; ***, P < 0.001.
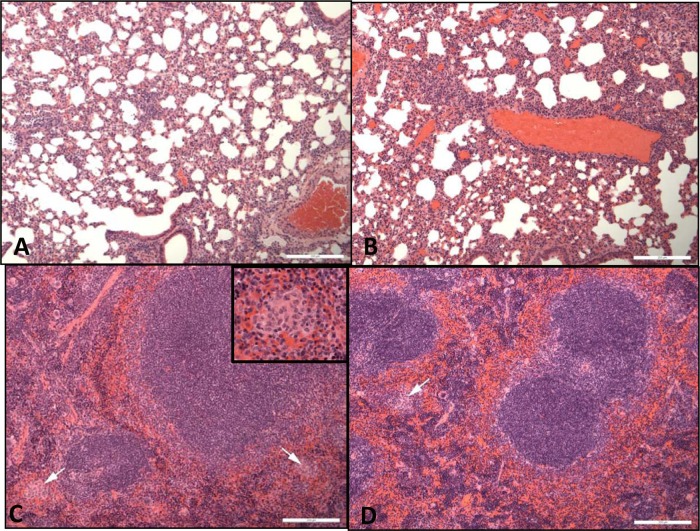
On histopathological examination, in the lungs of all challenged animals, focal or diffuse granulomatous alveolitis was observed, with no differences observed between control and treated animals (Fig. 6A and B). In the spleen, multifocal granulomatous foci were observed in mice in the PBS control group, and lesion severity was reduced in mice receiving CFI, but not in mice receiving doxycycline (Fig. 6C and D).
FIG 6.
Hematoxylin-and-eosin-stained spleen and lung tissues from mice at day 14 p.c. (A) Lung from a mouse treated with PBS for 7 days. Mild granulomatous alveolitis is present. (B) Lung from a mouse treated with CFI for 7 days. Mild granulomatous alveolitis is present. (C) Spleen from a mouse treated with PBS for 7 days. Mild granulomatous lesions are present (indicated by white arrows). (D) Spleen from a mouse treated with CFI for 7 days. Mild granulomatous lesions are present (indicated by white arrows). Bars, 200 µm.
Pharmacokinetic profile.
In order to determine drug concentrations in treated mice, ciprofloxacin, doxycycline, and CFI were administered to naive animals. Animals were culled at set time points over a 24-h period, and lungs and plasma samples were collected postmortem. The concentration of ciprofloxacin after oral administration decreased rapidly over time in both the lungs and plasma, with terminal half-lives of 1.3 and 0.8 h, respectively (Fig. 7). Pharmacokinetic parameters are shown in Table 2. In contrast, mice receiving i.n. CFI had 100-fold-greater concentration of drug in the lung, with an extended terminal half-life of 4.1 h. In addition, the Cmax of CFI in the lung was higher than that of oral doxycycline. Importantly, the AUC in the lung following CFI administration was almost 3 orders of magnitude greater than the AUC following the same dose of oral ciprofloxacin, resulting both from the superior Cmax and the duration in the lung. The relative bioavailability of oral ciprofloxacin in the lung compared to i.n. CFI is 0.0014; in contrast, the relative bioavailability of oral ciprofloxacin in the plasma compared to i.n. CFI is 0.363.
FIG 7.
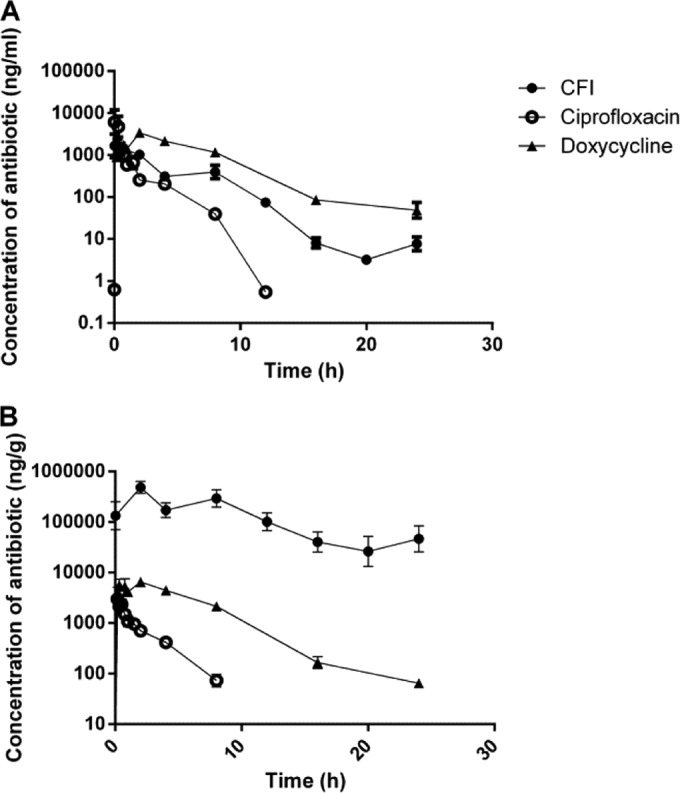
Profiles of the drug concentration in the plasma (A) or lungs (B) of mice (n = 3 per time point) after i.n. administration of CFI or oral administration of ciprofloxacin or doxycycline. All compounds were given at a dose of 50 mg/kg. Values are means ± standard errors.
TABLE 2.
Pharmacokinetic parameters in mice for intranasal CFI or oral ciprofloxacin or doxycyclinea
| Antibiotic | Formulation | Route | Sampling site | Terminal half-life (h) | Clearance (g/h/kg or liter/h/kg)b | Cmax (µg/ml) | Tmax (h) | AUC0–t (µg · h/ml)c |
|---|---|---|---|---|---|---|---|---|
| Doxycycline | PBS vehicle | Oral | Lung | 3.6 | 1,015 | 6.4 | 2 | 43.6 |
| Plasma | 3.5 | 2.1 | 3.4 | 2 | 21.3 | |||
| Ciprofloxacin | PBS vehicle | Oral | Lung | 0.8 | 14,500 | 3.0 | 0.17 | 4.9 |
| Plasma | 1.3 | 25.4 | 1.4 | 0.5 | 2.4 | |||
| CFI | i.n. | Lung | 4.1 | 131 | 483 | 2 | 3,461 | |
| Plasma | 2.2 | 8.5 | 1.7 | 0.017 | 6.5 |
All antibiotic doses were 50 mg/kg. Values are means for three individual animals per time point.
Clearance is shown in grams/hour/kilogram for the lung and in liters/hour/kilogram for plasma.
AUC0–t, area under the concentration-time curve from 0 h to the last measurable concentration.
DISCUSSION
The recommended antibiotic used in the treatment of Q fever is doxycycline, a bacteriostatic tetracycline used to treat several respiratory diseases, including pneumonic plague and anthrax (31). Antibiotic activity against C. burnetii can be determined in embryonated eggs, tissue culture, animal models, and more recently in axenic media (23, 32). The susceptibility of C. burnetii, in vitro, to doxycycline has been determined with a MIC ranging from 0.05 to 2 μg/ml (14, 33). Treatment of chronic Q fever endocarditis with doxycycline alone resulted in >50% mortality and frequent relapse and requires the addition of alkalinization agents, such as hydroxychloroquine (7, 34). In addition, at least one doxycycline-resistant C. burnetii strain has been isolated from a patient who died following unsuccessful antibiotic treatment (6). Although ciprofloxacin is not commonly used to treat acute Q fever, it has been used to successfully treat Q fever endocarditis (35, 36). In vitro, the MIC of ciprofloxacin is slightly higher than the MIC of doxycycline (1 to 4 μg/ml), and resistance to fluoroquinolones has been observed in some clinical isolates of C. burnetii (14, 33, 36). To date, there is only one report evaluating antibiotic treatment in an animal model of Q fever (37). Therefore, the development of novel methods to overcome resistance and improve treatment of Q fever is desirable.
In order to assess the efficacy of novel antibiotics against C. burnetii in vivo, an aerosol model of Q fever was established. There are several reported animal models of C. burnetii infection, using invertebrates, mice, guinea pigs, and nonhuman primates (38, 39). A/J mice have been previously shown to be the most susceptible immunocompetent mouse strain, and the aerosol route of infection is of relevance to the majority of human Q fever cases (40). The model is nonlethal, and therefore, five alternative parameters were used in order to monitor progression of disease, namely, weight loss, clinical signs, organ weight, bacterial burden, and histopathology. Weight loss and clinical signs were used as key indicators of disease severity, as all infected mice showed significant weight loss and reproducible clinical signs, such as piloerection and arched backs. These signs resolve, in the absence of treatment, within 14 days of infection, which is a similar length of time to resolution of acute disease in human Q fever cases (32). The lungs and spleen were selected for monitoring, as both exhibited a significant increase in weight and high levels of bacteria throughout the course of C. burnetii infection. In addition, these organs are of particular relevance, as the lungs are the foci of infection following an aerosol challenge, and the spleen may provide an indication of the extent of dissemination of the bacteria during a systemic infection.
Using this murine model, we report for the first time that doxycycline acts to delay the onset of body weight loss, reduce clinical signs, and reduce pathology associated with Q fever. However, following the cessation of doxycycline treatment, infected mice still succumb to infection. This is unlikely to be due to toxicity of oral doxycycline and instead may be due to relapse of infection due to suboptimal antibiotic treatment. A possible reason for this is that A/J mice are deficient in C5 complement and subsequently may not be able to clear the infection. Alternatively, this could be attributed to the antibiotic mechanism of action, with doxycycline having a bacteriostatic mode of action that may also explain why relapse is often observed in Q fever patients, in particular associated with cessation of the antibiotic regime (7). We have also confirmed that, when given by the oral route, ciprofloxacin is not an appropriate treatment for acute Q fever. However, we have identified a novel, encapsulated formulation of ciprofloxacin, specifically designed for inhalation that enables pulmonary delivery into the lung, which significantly improves efficacy in a mouse model of C. burnetii infection. Mice treated with CFI exhibit significantly reduced, rather than delayed, body weight loss throughout the course of the study compared to mice treated with doxycycline or with PBS (control).
Other in vivo studies have demonstrated the broad-spectrum antibiotic activity of CFI. It is highly efficacious against several intracellular pathogens, specifically F. tularensis LVS and Schu S4 strains (22) and Y. pestis CO92 strain (K. A. Hamblin, J. D. Blanchard, C. Davies, and S. V. Harding, presented at the 19th Congress of the International Society of Aerosols in Medicine, April 2013, Chapel Hill, NC), with only a single dose being needed to provide full protection against all three pathogens in mouse models of lung infection. This formulation is also very efficacious against the extracellular pathogen Pseudomonas aeruginosa and has completed multiple phase 2 clinical trials for infection in cystic fibrosis and non-cystic fibrosis bronchiectasis patients (41, 42).
Encapsulation of drugs in liposomes has been shown to be effective for the pulmonary delivery of various therapeutic agents, including antibiotics (11, 19, 43, 44). Liposomes are taken up by phagocytic cells, improving delivery of antibiotics into the primary site of infection for facultative intracellular pathogens, such as C. burnetii (45). In vivo, C. burnetii multiplies in monocytes within an acidic vacuole, and most antibiotics, including doxycycline and ciprofloxacin, exhibit reduced activity in such a low-pH environment (46, 47). Encapsulation of antibiotics enhances intracellular uptake and subsequent accumulation to higher therapeutic levels within the intracellular site of infection. Inhaled liposomally encapsulated antibiotics can be a particularly attractive means to provide high sustained concentrations at the sites of action within the respiratory tract, reducing local side effects and systemic exposure and providing more convenient therapy such as once-daily dosing (11) and smaller number of total doses administered for prophylaxis or treatment as shown in this paper and previous studies with CFI (22).
As shown in this study, in addition to improving uptake into cells, encapsulation of ciprofloxacin allows effective delivery by the i.n. route, resulting in high initial concentrations of antibiotic in the lungs. This is demonstrated by the Cmax for CFI in the lungs, which is 100-fold higher than for free ciprofloxacin or doxycycline. Previous studies have also shown that free ciprofloxacin is ineffective when delivered by the i.n. or aerosol route, whereas liposome-encapsulated ciprofloxacin, which is specifically developed for inhalational delivery, has been shown to be efficacious at 50 mg/kg (19, 21). This is because unencapsulated ciprofloxacin is rapidly absorbed and cleared from the lungs (19, 21). The enhanced therapeutic efficacy of CFI can therefore be partly attributed to the increased initial retained dose of antibiotic in the lungs (21). This property is particularly important for the treatment of diseases such as Q fever, where the initial site of infection is the lung. We have shown that treatment with CFI also improves the overall exposure within the lungs over a 24-h period, as demonstrated by the increased area under the curve (AUC) (~1,000-fold) compared to free ciprofloxacin. Delivery of ciprofloxacin in a liposome results in a sustained release of antibiotics, indicated by a prolonged half-life both in the lungs and in plasma. In addition, the bioavailability of ciprofloxacin in the lungs was improved by 99.9% when delivered in the encapsulated formulation compared to oral ciprofloxacin.
We also evaluated the effect of length of antibiotic treatment on outcome of disease. The length of treatment with doxycycline correlates with a delay in body weight loss and clinical signs. However, the length of treatment with CFI correlates with overall prognosis of disease, with mice receiving 7 days treatment exhibiting minimal weight loss and no clinical signs. This result, taken together with the use of a single daily dose of CFI compared to a twice-daily dose of doxycycline, indicates that fewer doses of CFI may be required to treat Q fever. The CDC currently recommends 100 mg of doxycycline, given orally, twice daily for 14 days for treating acute Q fever. The efficacy of doxycycline has been correlated with the AUC pharmacokinetic parameter (48). Published average human AUCs for plasma following 100-mg oral dose of doxycycline range from 13 to 40 mg · h/liter (49). The selected doxycycline dose in the AJ mouse model, 50 mg/kg, results in an average plasma AUC within this range (21.3 mg · h/liter). The differences observed in organ weight and bacterial burden between doxycycline- and CFI-treated mice were less marked. Both antibiotics did not significantly reduce lung or spleen organ weight compared to the PBS-treated control mice; in fact, treatment with doxycycline appeared to increase bacterial numbers within organs compared to the PBS-treated control mice. This finding may be due to using RT-PCR to detect residual bacterial DNA in the organs. An alternative method to enumerate viable bacteria would be to plate organ homogenates onto ACCM-2 agarose (23).
In conclusion, our study demonstrates that liposome encapsulation of ciprofloxacin allows effective pulmonary delivery and improves efficacy of an alternative antibiotic against C. burnetii infection. Furthermore, we report that treatment of infected mice with encapsulated ciprofloxacin shows significant improvements over doxycycline, the current treatment for Q fever. We have developed an animal model suitable for testing novel antibiotic therapies, which will allow evaluation and progression of next-generation antimicrobial treatments against Q fever.
ACKNOWLEDGMENTS
We acknowledge Tom Laws for assistance with statistical analysis and Geoff Pearson and Igor Gonda for critical reviewing the manuscript. We thank the Biological Investigations Group at Public Health England (PHE) for conducting the animal procedures and also Melanie Davison, Simon Bate, and Gemma McLuckie for technical assistance.
This work was funded by the United Kingdom Ministry of Defense.
J.D.B. is an employee of and owns stock in Aradigm Corporation, which produces the liposome-encapsulated ciprofloxacin for inhalation (CFI) formulation that was tested. He is also a coinventor on Aradigm patents on inhaled formulations involving CFI, though not CFI alone.
Footnotes
Published ahead of print 7 July 2014
REFERENCES
- 1.Hilbink F, Penrose M, Kovacova E, Kazar J. 1993. Q fever is absent from New Zealand. Int. J. Epidemiol. 22:945–949. 10.1093/ije/22.5.945 [DOI] [PubMed] [Google Scholar]
- 2.Moodie CE, Thompson HA, Meltzer MI, Swerdlow DL. 2008. Prophylaxis after exposure to Coxiella burnetii. Emerg. Infect. Dis. 14:1558–1566. 10.3201/eid1410.080576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faix DJ, Harrison DJ, Riddle MS, Vaughn AF, Yingst SL, Earhart K, Thibault G. 2008. Outbreak of Q fever among US military in western Iraq, June-July 2005. Clin. Infect. Dis. 46:e65–e68. 10.1086/528866 [DOI] [PubMed] [Google Scholar]
- 4.Bailey MS, Trinick TR, Dunbar JA, Hatch R, Osborne JC, Brooks TJ, Green AD. 2011. Undifferentiated febrile illnesses amongst British troops in Helmand, Afghanistan. J. R. Army Med. Corps 157:150–155. 10.1136/jramc-157-02-05 [DOI] [PubMed] [Google Scholar]
- 5.Fournier PE, Marrie TJ, Raoult D. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouqui P, Dupont HT, Drancourt M, Berland Y, Etienne J, Leport C, Goldstein F, Massip P, Micoud M, Bertrand A, Raoult D. 1993. Chronic Q fever. Ninety-two cases from France, including 27 cases without endocarditis. Arch. Intern. Med. 153:642–648 [DOI] [PubMed] [Google Scholar]
- 7.Raoult D, Houpikian P, Dupont H, Riss J, Arditi-Djiane JJ, Brouqui P. 1999. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch. Intern. Med. 159:167–173. 10.1001/archinte.159.2.167 [DOI] [PubMed] [Google Scholar]
- 8.Million M, Thuny F, Richet H, Raoult D. 2010. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect. Dis. 10:527–535. 10.1016/S1473-3099(10)70135-3 [DOI] [PubMed] [Google Scholar]
- 9.Rouli L, Rolain JM, El Filali A, Robert C, Raoult D. 2012. Genome sequence of Coxiella burnetii 109, a doxycycline-resistant clinical isolate. J. Bacteriol. 194:6939. 10.1128/JB.01856-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allison SD. 2007. Liposomal drug delivery. J. Infus. Nurs. 30:89–120. 10.1097/01.NAN.0000264712.26219.67 [DOI] [PubMed] [Google Scholar]
- 11.Cipolla D, Gonda I, Chan HK. 2013. Liposomal formulations for inhalation. Ther. Deliv. 4:1047–1072. 10.4155/tde.13.71 [DOI] [PubMed] [Google Scholar]
- 12.Gay JD, Deyoung DR, Roberts GD. 1984. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob. Agents Chemother. 26:94–96. 10.1128/AAC.26.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaunt PN. 1988. Ciprofloxacin vs ceftriaxone for eradication of meningococcal carriage. Lancet 2:218–219 [DOI] [PubMed] [Google Scholar]
- 14.Gikas A, Spyridaki I, Psaroulaki A, Kofterithis D, Tselentis Y. 1998. In vitro susceptibility of Coxiella burnetii to trovafloxacin in comparison with susceptibilities to pefloxacin, ciprofloxacin, ofloxacin, doxycycline, and clarithromycin. Antimicrob. Agents Chemother. 42:2747–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergogne-Berezin E. 1993. Interpretation of pharmacologic data in respiratory tract infections. Int. J. Antimicrob. Agents 3(Suppl 1):S3–S14. 10.1016/0924-8579(93)90030-9 [DOI] [PubMed] [Google Scholar]
- 16.Campos J, Roman F, Georgiou M, Garcia C, Gomez-Lus R, Canton R, Escobar H, Baquero F. 1996. Long-term persistence of ciprofloxacin-resistant Haemophilus influenzae in patients with cystic fibrosis. J. Infect. Dis. 174:1345–1347. 10.1093/infdis/174.6.1345 [DOI] [PubMed] [Google Scholar]
- 17.Grimaldo ER, Tupasi TE, Rivera AB, Quelapio MID, Cardano RC, Derilo JO, Belen VA. 2001. Increased resistance to ciprofloxacin and ofloxacin in multidrug-resistant Mycobacterium tuberculosis isolates from patients seen at a tertiary hospital in the Philippines. Int. J. Tuberc. Lung Dis. 5:546–550 [PubMed] [Google Scholar]
- 18.Oh YK, Nix DE, Straubinger RM. 1995. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection. Antimicrob. Agents Chemother. 39:2104–2111. 10.1128/AAC.39.9.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley J, Yang HM, Wilson T, Blasetti K, DiNinno V, Schnell G, Wong JP. 1997. Aerosol delivery of liposome-encapsulated ciprofloxacin: aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob. Agents Chemother. 41:1288–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellbogen MH, Olsen KM, Gentry-Nielsen MJ, Preheim LC. 2003. Efficacy of liposome-encapsulated ciprofloxacin compared with ciprofloxacin and ceftriaxone in a rat model of pneumococcal pneumonia. J. Antimicrob. Chemother. 51:83–91. 10.1093/jac/dkg024 [DOI] [PubMed] [Google Scholar]
- 21.Wong JP, Yang HM, Blasetti KL, Schnell G, Conley J, Schofield LN. 2003. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J. Control. Release 92:265–273. 10.1016/S0168-3659(03)00358-4 [DOI] [PubMed] [Google Scholar]
- 22.Hamblin KA, Armstrong SJ, Barnes KB, Davies C, Wong JP, Blanchard JD, Harding SV, Simpson AJH, Atkins HS. 17 March 2014. Liposome encapsulation of ciprofloxacin improves protection against highly virulent Francisella tularensis Schu S4 strain. Antimicrob. Agents Chemother. 10.1128/AAC.02555-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol. 77:3720–3725. 10.1128/AEM.02826-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Committee for Standardization. 2000. Biotechnology - performance criteria for microbiological safety cabinets. European standard EN 12469. European Committee for Standardization, Brussels, Belgium [Google Scholar]
- 25.National Science Advisory Board for Biosecurity. 2007. Proposed framework for the oversight of dual use life sciences research: strategies for minimizing the potential misuse of research information. A report of the National Science Advisory Board for Biosecurity (NSABB). National Science Advisory Board for Biosecurity, Office of Biotechnology Activities, Office of Science Policy, Office of the Director, National Institutes of Health, Bethesda, MD [Google Scholar]
- 26.United Kingdom Act of Parliament. 1986. Animals (Scientific Procedures) Act 1986. Her Majesty's Stationery Office, London, United Kingdom [Google Scholar]
- 27.United Kingdom Home Office. 1989. Code of practice for the housing and care of animals used in scientific procedures. Home Office Animals (Scientific Procedures) Act 1986. Her Majesty's Stationery Office, London, United Kingdom [Google Scholar]
- 28.Guyton AC. 1947. Measurement of the respiratory minute volume of laboratory animals. Am. J. Physiol. 150:70–77 [DOI] [PubMed] [Google Scholar]
- 29.Rowland M, Tozer TM. 2010. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 30.Taylor JR. 1997. An introduction to error analysis: the study of uncertainties in physical measurements. University Science Books, Sausalito, CA [Google Scholar]
- 31.Whitby M, Ruff TA, Street AC, Fenner FJ. 2002. Biological agents as weapons 2: anthrax and plague. Med. J. Aust. 176:605–608 [DOI] [PubMed] [Google Scholar]
- 32.Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lever MS, Bewley KR, Dowsett B, Lloyd G. 2004. In vitro susceptibility of Coxiella burnetti to azithromycin, doxycycline, ciprofloxacin and a range of newer fluoroquinolones. Int. J. Antimicrob. Agents 24:194–196. 10.1016/j.ijantimicag.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 34.Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, Vene S, Jarnestrom V, Raoult D. 2005. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin. Infect. Dis. 40:82–88. 10.1086/426440 [DOI] [PubMed] [Google Scholar]
- 35.Yebra M, Ortigosa J, Albarran F, Crespo MG. 1990. Ciprofloxacin in a case of Q fever endocarditis. N. Engl. J. Med. 323:614. 10.1056/NEJM199008303230916 [DOI] [PubMed] [Google Scholar]
- 36.Zekanovic D, Morovic M, Borcilo MN, Rode OD. 2010. First case of Q fever endocarditis in Croatia and a short review. Coll. Antropol. 34:1135–1137 [PubMed] [Google Scholar]
- 37.Huebner RJ, Hottle GA, Robinson EB. 1948. Action of streptomycin in experimental infection with Q fever. Public Health Rep. 63:357–362. 10.2307/4586483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bewley KR. 2013. Animal models of Q fever (Coxiella burnetii). Comp. Med. 63:469–476 [PMC free article] [PubMed] [Google Scholar]
- 39.Norville IH, Hartley MG, Martinez E, Cantet F, Bonazzi M, Atkins TP. 27 March 2014. Galleria mellonella as an alternative model of Coxiella burnetii infection. Microbiology 10.1099/mic.0.077230-0 [DOI] [PubMed] [Google Scholar]
- 40.Scott GH, Williams JC, Stephenson EH. 1987. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J. Gen. Microbiol. 133:691–700 [DOI] [PubMed] [Google Scholar]
- 41.Bruinenberg P, Blanchard DJ, Cipolla D, Dayton F, Mudumba S, Gonda I. 2010. Inhaled liposomal ciprofloxacin: once a day management of respiratory infections. Proc. Respir. Drug Deliv. 2010 1:73–82 [Google Scholar]
- 42.Serisier D. 2012. Inhaled antibiotics for lower respiratory tract infections: focus on ciprofloxacin. Drugs Today 48:339–351. 10.1358/dot.2012.48.5.1789474 [DOI] [PubMed] [Google Scholar]
- 43.Fountain MW, Weiss SJ, Fountain AG, Shen A, Lenk RP. 1985. Treatment of Brucella canis and Brucella abortus in vitro and in vivo by stable plurilamellar vesicle-encapsulated aminoglycosides. J. Infect. Dis. 152:529–535. 10.1093/infdis/152.3.529 [DOI] [PubMed] [Google Scholar]
- 44.Nightingale SD, Saletan SL, Swenson CE, Lawrence AJ, Watson DA, Pilkiewicz FG, Silverman EG, Cal SX. 1993. Liposome-encapsulated gentamicin treatment of Mycobacterium avium-Mycobacterium intracellulare complex bacteremia in AIDS patients. Antimicrob. Agents Chemother. 37:1869–1872. 10.1128/AAC.37.9.1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto-Alphandary H, Andremont A, Couvreur P. 2000. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int. J. Antimicrob. Agents 13:155–168. 10.1016/S0924-8579(99)00121-1 [DOI] [PubMed] [Google Scholar]
- 46.Maurin M, Benoliel AM, Bongrand P, Raoult D. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60:5013–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM. 2003. Intracellular pharmacodynamics of antibiotics. Infect. Dis. Clin. North Am. 17:615–634. 10.1016/S0891-5520(03)00066-7 [DOI] [PubMed] [Google Scholar]
- 48.Agwuh KN, Macgowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58:256–265. 10.1093/jac/dkl224 [DOI] [PubMed] [Google Scholar]
- 49.Craig WA. 2002. Pharmacodynamics of antimicrobials: general concepts and applications, p 1–22 In Nightingale CH, Murakawa T, Ambrose PG. (ed), Antimicrobial pharmacodynamics in theory and clinical practice. Marcel Dekker, Oxford, United Kingdom [Google Scholar]