Abstract
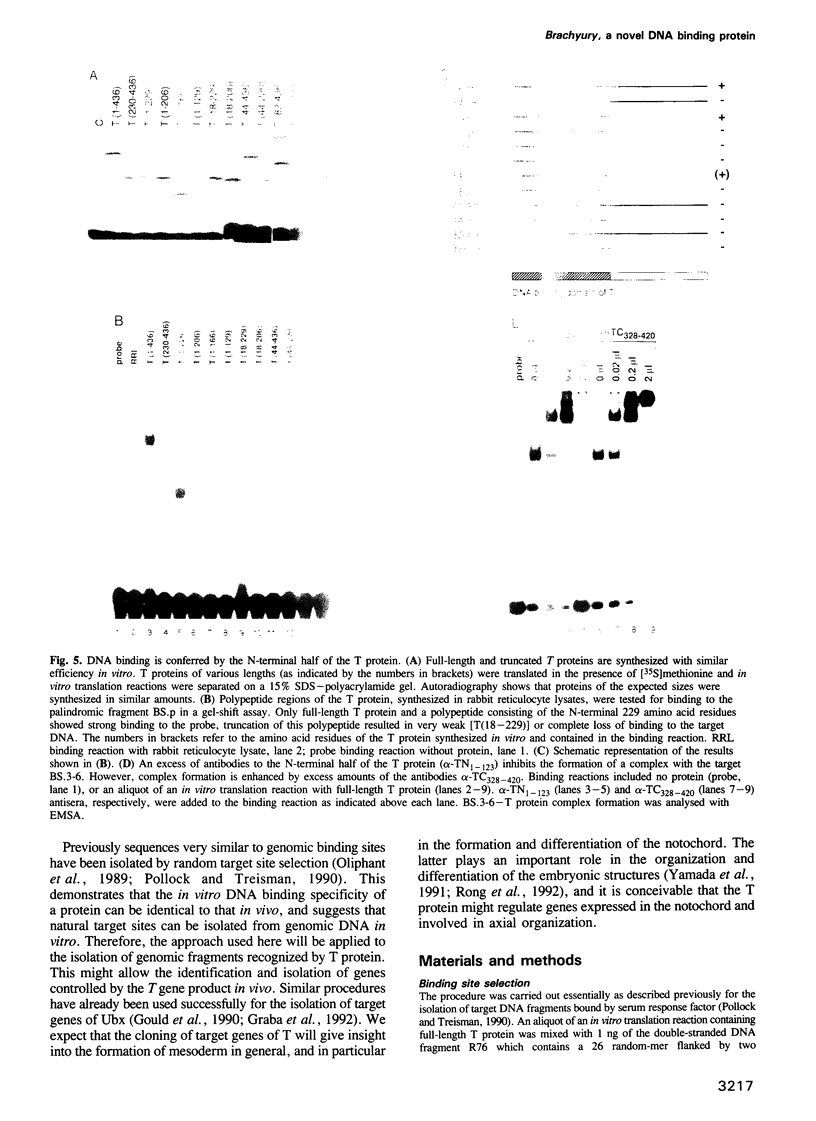
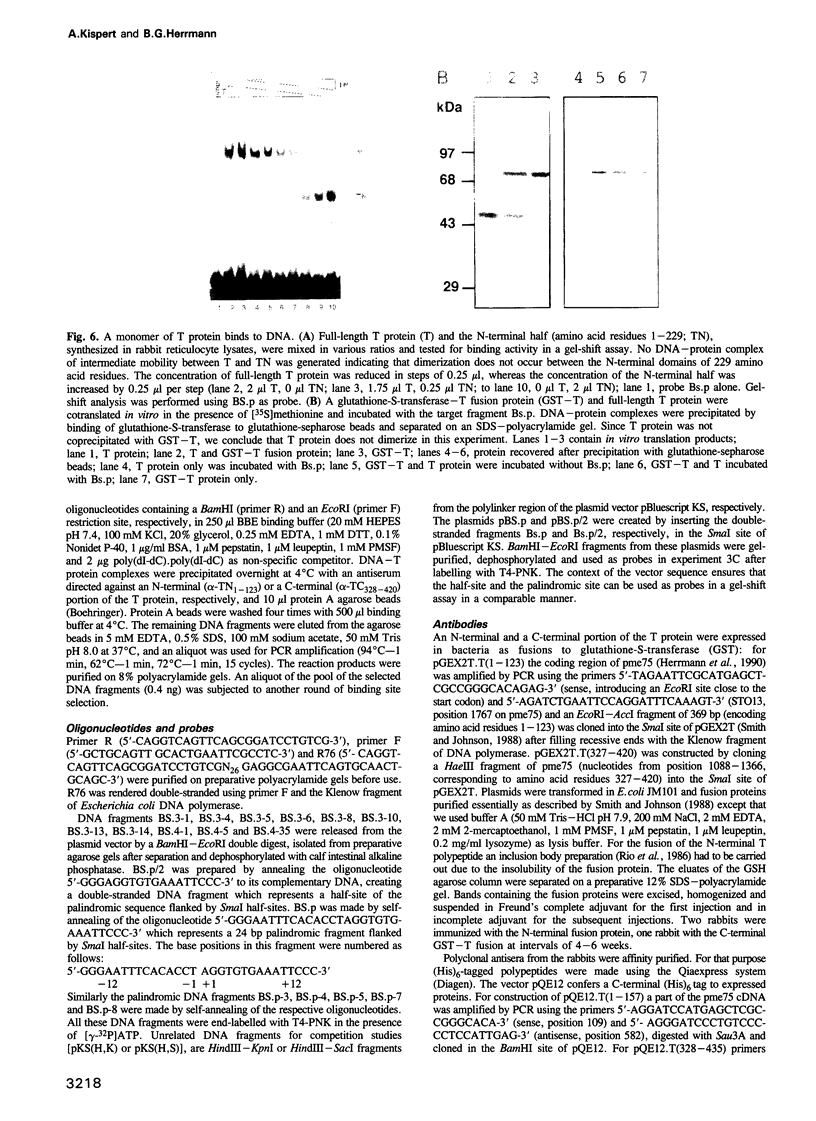
Brachyury (T) mutant embryos are deficient in mesoderm formation and do not complete axial development. The notochord is most strongly affected. The T gene is expressed transiently in primitive streak-derived nascent and migrating mesoderm cells and continuously in the notochord. Ectopic expression of T protein in the animal cap of Xenopus embryos results in ectopic mesoderm formation. The T protein is located in the nucleus. These and other data suggested that the T gene might be involved in the control of transcriptional regulation. In an attempt to demonstrate specific DNA binding of the T protein we have identified a consensus sequence among DNA fragments selected from a mixture of random oligomers. Under our experimental conditions T protein binds as a monomer to DNA. This property resides in the N-terminal domain of 229 amino acid residues which is strongly conserved between the mouse protein, and its Xenopus and zebrafish homologues. The latter proteins also recognize the consensus DNA binding site. We suggest that the T protein is involved in the control of genes required for mesoderm formation, and for the differentiation and function of chorda mesoderm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch S. J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990 Feb;6(2):36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Cunliffe V., Smith J. C. Ectopic mesoderm formation in Xenopus embryos caused by widespread expression of a Brachyury homologue. Nature. 1992 Jul 30;358(6385):427–430. doi: 10.1038/358427a0. [DOI] [PubMed] [Google Scholar]
- GRUNEBERG H. Genetical studies on the skeleton of the mouse. XXIII. The development of brachyury and anury. J Embryol Exp Morphol. 1958 Sep;6(3):424–443. [PubMed] [Google Scholar]
- Gogos J. A., Tzertzinis G., Kafatos F. C. Binding site selection analysis of protein-DNA interactions via solid phase sequencing of oligonucleotide mixtures. Nucleic Acids Res. 1991 Apr 11;19(7):1449–1453. doi: 10.1093/nar/19.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. P., Brookman J. J., Strutt D. I., White R. A. Targets of homeotic gene control in Drosophila. Nature. 1990 Nov 22;348(6299):308–312. doi: 10.1038/348308a0. [DOI] [PubMed] [Google Scholar]
- Graba Y., Aragnol D., Laurenti P., Garzino V., Charmot D., Berenger H., Pradel J. Homeotic control in Drosophila; the scabrous gene is an in vivo target of Ultrabithorax proteins. EMBO J. 1992 Sep;11(9):3375–3384. doi: 10.1002/j.1460-2075.1992.tb05416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. A structural taxonomy of DNA-binding domains. Nature. 1991 Oct 24;353(6346):715–719. doi: 10.1038/353715a0. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991 Nov;113(3):913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- Herrmann B. G., Labeit S., Poustka A., King T. R., Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990 Feb 15;343(6259):617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- Howe K. M., Watson R. J. Nucleotide preferences in sequence-specific recognition of DNA by c-myb protein. Nucleic Acids Res. 1991 Jul 25;19(14):3913–3919. doi: 10.1093/nar/19.14.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacMurray A., Shin H. S. The antimorphic nature of the Tc allele at the mouse T locus. Genetics. 1988 Oct;120(2):545–550. doi: 10.1093/genetics/120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Brandl C. J., Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol. 1989 Jul;9(7):2944–2949. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder G. O., Roth H., Poeck B. A homology domain shared between Drosophila optomotor-blind and mouse Brachyury is involved in DNA binding. Biochem Biophys Res Commun. 1992 Jul 31;186(2):918–925. doi: 10.1016/0006-291x(92)90833-7. [DOI] [PubMed] [Google Scholar]
- Pollock R., Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990 Nov 11;18(21):6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass P., Cooke L. A., Herrmann B. G., Beddington R. S. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature. 1991 Sep 26;353(6342):348–351. doi: 10.1038/353348a0. [DOI] [PubMed] [Google Scholar]
- Rong P. M., Teillet M. A., Ziller C., Le Douarin N. M. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992 Jul;115(3):657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., Ho R. K., Herrmann B. G., Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992 Dec;116(4):1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Johnson A. D. A molecular mechanism for combinatorial control in yeast: MCM1 protein sets the spacing and orientation of the homeodomains of an alpha 2 dimer. Cell. 1992 Jan 10;68(1):133–142. doi: 10.1016/0092-8674(92)90212-u. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Stott D., Kispert A., Herrmann B. G. Rescue of the tail defect of Brachyury mice. Genes Dev. 1993 Feb;7(2):197–203. doi: 10.1101/gad.7.2.197. [DOI] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Thiesen H. J., Bach C. Target Detection Assay (TDA): a versatile procedure to determine DNA binding sites as demonstrated on SP1 protein. Nucleic Acids Res. 1990 Jun 11;18(11):3203–3209. doi: 10.1093/nar/18.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Herrmann B. G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990 Feb 15;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Willison K. The mouse Brachyury gene and mesoderm formation. Trends Genet. 1990 Apr;6(4):104–105. doi: 10.1016/0168-9525(90)90106-g. [DOI] [PubMed] [Google Scholar]
- Yamada T., Placzek M., Tanaka H., Dodd J., Jessell T. M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991 Feb 8;64(3):635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K. O. Does the T gene determine the anteroposterior axis of a mouse embryo? Jpn J Genet. 1990 Oct;65(5):287–297. doi: 10.1266/jjg.65.287. [DOI] [PubMed] [Google Scholar]