Abstract
Neuraminidase inhibitors (NAIs) play a major role for managing influenza virus infections. The widespread oseltamivir resistance among 2007-2008 seasonal A(H1N1) viruses and community outbreaks of oseltamivir-resistant A(H1N1)pdm09 strains highlights the need for additional anti-influenza virus agents. Laninamivir is a novel long-lasting NAI that has demonstrated in vitro activity against influenza A and B viruses, and its prodrug (laninamivir octanoate) is in phase II clinical trials in the United States and other countries. Currently, little information is available on the mechanisms of resistance to laninamivir. In this study, we first performed neuraminidase (NA) inhibition assays to determine the activity of laninamivir against a set of influenza A viruses containing NA mutations conferring resistance to one or many other NAIs. We also generated drug-resistant A(H1N1) and A(H3N2) viruses under in vitro laninamivir pressure. Laninamivir demonstrated a profile of susceptibility that was similar to that of zanamivir. More specifically, it retained activity against oseltamivir-resistant H275Y and N295S A(H1N1) variants and the E119V A(H3N2) variant. In vitro, laninamivir pressure selected the E119A NA substitution in the A/Solomon Islands/3/2006 A(H1N1) background, whereas E119K and G147E NA changes along with a K133E hemagglutinin (HA) substitution were selected in the A/Quebec/144147/2009 A(H1N1)pdm09 strain. In the A/Brisbane/10/2007 A(H3N2) background, a large NA deletion accompanied by S138A/P194L HA substitutions was selected. This H3N2 variant had altered receptor-binding properties and was highly resistant to laninamivir in plaque reduction assays. Overall, we confirmed the similarity between zanamivir and laninamivir susceptibility profiles and demonstrated that both NA and HA changes can contribute to laninamivir resistance in vitro.
INTRODUCTION
Influenza is a highly transmissible viral infection associated with serious public health and economic problems. Each year, various strains of influenza viruses circulate throughout the world and cause significant morbidity in the general population as well as substantial mortality among high-risk individuals. Among the 18 hemagglutinin (HA) and 11 neuraminidase (NA) subtypes, influenza A(H1N1) and A(H3N2) virus strains have been predominantly associated with epidemics during the last century. In addition to immunization programs, antiviral agents constitute an important means in the management of seasonal influenza and play a major role as a first-line defense in the case of pandemics. Two neuraminidase inhibitors (NAIs) are currently licensed worldwide for treatment and prophylaxis of influenza virus infections: oseltamivir phosphate (Tamiflu; Hoffmann-La Roche) and zanamivir (Relenza; GlaxoSmithKline).
Oseltamivir is the most widely used and stockpiled NAI due to its good bioavailability as an oral preparation (1–3). However, recent emergence of oseltamivir-resistant variants, especially among A(H1N1) viruses (4–6), is a matter of great concern. Oseltamivir has a large hydrophobic side chain requiring a conformational rearrangement in the viral NA that is essential to accommodate the drug. Any mutations that affect this rearrangement may reduce the binding affinity of oseltamivir, leading to viral resistance (7, 8). Notably, the H275Y substitution (N1 numbering), which is most commonly associated with oseltamivir resistance, has been shown to inhibit such rearrangement (9). In addition, influenza viruses of the N1 subtype containing the H275Y substitution, including A(H1N1) and A(H5N1) strains, are also resistant to peramivir, a cyclopentane NAI whose intravenous formulation is approved in a few countries (10). Fortunately, most oseltamivir-resistant strains exhibit susceptibility to zanamivir, a drug that is administered by an inhaler device (Diskhaler). On the other hand, zanamivir must be administered twice daily over 5 consecutive days for optimal benefits (11). Therefore, there is a need to develop new inhibitors that possess long-term half-lives and that are also effective against oseltamivir-resistant influenza viruses.
Laninamivir (R-125489) is a long-acting NAI which demonstrated a broad range of activity against influenza A (N1 to N9) and influenza B virus strains (12, 13). Laninamivir octanoate (CS-8958), the octanoyl prodrug of laninamivir, is commercially available in Japan under the name of Inavir (Daiichi Sankyo). As for zanamivir, laninamivir octanoate is formulated as a dry powder that has to be administered with a specific inhaler device. Importantly, a single nasal administration is associated with prolonged retention in the lungs, which confers a long-acting anti-neuraminidase effect (12) comparable to that of oseltamivir administered twice daily for 5 days (14, 15). Additionally, laninamivir was shown to be active against oseltamivir-resistant A(H5N1) and A(H1N1) variants containing the H275Y NA substitution (16). For these reasons, laninamivir octanoate offers advantages over both oseltamivir and zanamivir.
To date, no clinical cases of laninamivir-resistant influenza virus strains have been reported. Moreover, there are currently no data on the in vitro selection of viral mutations conferring resistance to laninamivir. Nevertheless, as with other NAIs, the emergence of laninamivir resistance should be considered. The objectives of the present study were, first, to evaluate the activity of laninamivir against a collection of NAI-resistant seasonal A(H1N1), A(H3N2), and 2009 pandemic A(H1N1)pdm09 viruses. Second, we aimed to generate and characterize laninamivir-resistant influenza A(H1N1) and A(H3N2) virus variants following in vitro passaging under laninamivir pressure.
MATERIALS AND METHODS
Cells culture.
ST6Gal1 Madin-Darby canine kidney cells, overexpressing the α2,6 sialic acid receptors (MDCK α2,6; kindly provided by Y. Kawaoka from the University of Wisconsin, Madison, WI), and human embryonic kidney 293T cells (ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Madin-Darby canine kidney (MDCK) cells were sourced from the European Collection of Cell Cultures (ECACC; Wiltshire, United Kingdom). These cells were maintained to generate cell bank stocks in minimal essential medium without l-glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Drug susceptibility testing.
A selection of seasonal A(H1N1), A(H3N2), and A(H1N1)pdm09 viruses harboring NAI-resistant NA mutations (Table 1) was used for assessing susceptibility to laninamivir (R-125489) (Biota Scientific Management, Notting Hill, Australia), oseltamivir carboxylate (Hoffmann-La Roche, Basel, Switzerland), zanamivir (GlaxoSmithKline, Stevenage, United Kingdom), and peramivir (BioCryst, Birmingham, United States) by NA inhibition assays, as previously described (17) with minor modifications. Briefly, viruses were standardized to an NA activity level 10-fold higher than that of the background, as measured by the production of a fluorescent product from the 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUNANA; Sigma, St-Louis, MO) substrate. Drug susceptibility profiles were determined by the extent of NA inhibition after incubation with 3-fold serial dilutions of NAIs at final concentrations ranging from 0 to 10,800 nM. The 50% inhibitory concentrations (IC50s) were determined from the dose-response curve.
TABLE 1.
Laninamivir susceptibility profiles of influenza A(H1N1)pdm09, A(H1N1), and A(H3N2) viruses harboring NA substitutions mediating resistance to other neuraminidase inhibitors
| Influenza virus strain | NA mutation(s)a | IC50 (nM [fold increase])b |
Phenotype in NA inhibition assaye |
Reference(s) | ||||
|---|---|---|---|---|---|---|---|---|
| Laninamivir | Zanamivir | Laninamivir | Zanamivir | Oseltamivir | Peramivir | |||
| A(H1N1)pdm09 (A/Quebec/144147/2009)c | WT | 1.41 ± 0.01 (1) | 0.15 ± 0.01 (1) | S | S | S | S | 18 |
| E119G | 151.20 ± 25.83 (108) | 124.9 ± 7.6 (832) | HRI | HRI | S | RI | 18, 28 | |
| E119V | 68.81 ± 8.80 (49) | 85.7 ± 10.6 (571) | RI | HRI | RI | RI | 18 | |
| I223R | 5.91 ± 2.20 (4) | 1.10 ± 0.1 (7) | S | S | RI | RI | 41 | |
| I223R-H275Y | 8.45 ± 3.06 (6) | 2.32 ± 0.1 (16) | S | RI | HRI | HRI | 41 | |
| I223V | 1.71 ± 0.01 (1) | 0.35 ± 0.02 (2) | S | S | S | S | 18, 41 | |
| I223V-H275Y | 2.01 ± 0.11 (1) | 0.32 ± 0.01 (2) | S | S | HRI | HRI | 18, 41 | |
| Q136K | 63.08 ± 14.00 (45) | 112.3 ± 15.8 (749) | RI | HRI | S | ND | 28 | |
| D199G | 2.46 ± 0.80 (2) | 0.90 ± 0.06 (6) | S | S | RI | S | 18 | |
| H275Y | 4.22 ± 0.11 (3) | 0.14 ± 0.01 (0.9) | S | S | HRI | HRI | 18 | |
| N295S | 3.70 ± 0.33 (3) | 0.49 ± 0.02 (3) | S | S | HRI | RI | 18 | |
| A(H1N1) (A/WSN/33)c | WT | 0.66 ± 0.01 (1) | 0.56 ± 0.07 (1) | S | S | S | S | 19 |
| H275Y | 1.35 ± 0.02 (2) | 0.62 ± 0.04 (1) | S | S | HRI | RI | 19 | |
| N295S | 1.28 ± 0.10 (2) | 3.17 ± 0.3 (5) | S | S | HRI | S | 42 | |
| A(H1N1) (A/Brisbane/59/07)c | WT | 4.50 ± 0.03 (1) | 0.25 ± 0 (1) | S | S | S | S | 43 |
| H275Y | 3.32 ± 0.17 (1) | 0.5 ± 0.15 (2) | S | S | HRI | HRI | 43 | |
| A(H3N2)d | WT | 4.61 ± 2.00 (1) | 3.6 ± 0.5 (1) | S | S | S | S | 44 |
| E119V | 22.69 ± 8.50 (5) | 4.7 ± 0.4 (1) | S | S | HRI | S | 44 | |
| E119V-I223V | 8.59 ± 0.95 (2) | 5.29 ± 2.43 (2) | S | S | HRI | S | 44 | |
| Del 245–248 | 8.88 ± 1.87 (2) | 10.1 ± 2.4 (2.8) | S | S | HRI | S | 45 | |
Residues are numbered according to N1 numbering for H1N1 variants and N2 numbering for H3N2 variants.
Values are means ± standard deviations from three independent experiments.
Recombinant.
Clinical isolate.
S, susceptibility or normal inhibition (<10-fold increase in IC50 over WT); RI, reduced inhibition (10- to 100-fold increase in IC50 over WT); HRI, highly reduced inhibition (>100-fold increase in IC50 over WT); ND, not determined.
Some drug-selected viruses were also assessed for their susceptibility to laninamivir and oseltamivir by using a plaque reduction assay. In method 1 (A/Quebec/144147/2009 and A/Brisbane/10/2007 influenza viruses), confluent monolayers of MDCK α2,6 cells were grown in 12-well dishes and infected with a dilution of virus required to obtain 15 to 30 plaques per well. After 1 h of incubation at 37°C with 5% CO2, unbound viruses were removed, and cells were overlaid with 0.8% agarose-containing maintenance medium in the presence of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin and different concentrations of NAIs. Three days later, the mean viral plaque areas were determined from a minimum of 15 plaques using ImageJ software (version 1.41; developed by Wayne Rasband of the National Institutes of Health). The IC50 was defined as the drug concentration resulting in the reduction of viral plaque areas by 50%.
In method 2 (A/Auckland/3/2009 and A/Solomon Islands/3/2006 influenza viruses), confluent monolayers of MDCK cells were grown in six-well plates and infected with a dilution of virus required to obtain 20 to 40 plaques per well. After 1 h of incubation at 37°C with 5% CO2, unbound viruses were removed, and cells were overlaid with 0.5% SeaKem agarose-containing maintenance medium in the presence of TPCK-treated trypsin and different concentrations of NAIs. Three days later, plaque diameter measurements were determined using Adobe Photoshop software CS3 (version 10.01). The IC50 was defined as the drug concentration that results in a 50% reduction in influenza virus-induced plaque diameter.
Selection of drug-resistant viruses in vitro.
Initial passages of A(H1N1)pdm09 (A/Quebec/144147/2009), which is an A/California/7/2009-like isolate, and A(H3N2) (A/Brisbane/10/2007-like) influenza viruses were performed by infecting MDCK α2,6 cells at a multiplicity of infection (MOI) of 0.01 in the presence of 50 nM laninamivir. In addition, passages of A(H1N1)pdm09 (A/Auckland/3/2009), another A/California/7/2009-like isolate, and A(H1N1) (A/Solomon Islands/3/2006-like) influenza viruses were performed by infecting MDCK cells at an MOI of 0.05 in the presence of 48 nM and 8 nM laninamivir, respectively. The drug concentration was then slowly increased, with some passages being performed at the same level to increase viral replication. Control viruses were also passaged concomitantly in the absence of drug. At passages 3, 6, 9, and 12 for A/Quebec/144147/2009, at passages 1 to 5 for A/Auckland/3/2009, at passages 1 to 11 for A/Solomon Islands/3/2006-like, and at passages 3, 6, and 9 for A/Brisbane/10/2007-like viruses, viral RNA was isolated from supernatants of infected cell cultures and reverse transcribed before PCR amplification of the entire HA and NA genes. PCR products were purified and sequenced using an Applied Biosystems 3730xl DNA Analyzer (Life Technologies Corporation, Carlsbad, CA).
Generation of recombinant viruses and proteins.
The pLLB plasmid containing the A/Quebec/144147/2009 NA gene was used for the introduction of three single substitutions (N1 numbering: E119A, E119K, and G147E) using appropriate primers and a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Similarly, the plasmid containing the HA gene was used for the introduction of three single substitutions (H3 numbering: K133E, K156E, and D225G). We used the plasmid pLLB-NAE119K (where NA carries the E119K substitution) to introduce the second substitution G147E and the plasmid pLLB-HAK156E to introduce the second substitution D225G. The eight plasmids were cotransfected into 293T cells by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) as previously described (18). Supernatants were collected at 72 h posttransfection and used to inoculate MDCK α2,6 cells. The resulting recombinant wild-type (WT) as well as mutant viruses were subsequently sequenced and titrated by plaque assays in MDCK α2,6 cells.
The pCAGGS-PA, -PB1, -PB2, and -NP expression plasmids and the pLLB plasmid containing a WT or variant (E119A, E119K, G147E, and E119K/G147E) NA were used to cotransfect 293T cells in order to express recombinant NA proteins (19). At 48 h after transfection, the cells were briefly treated with trypsin-EDTA and neutralized by the addition of serum, followed by centrifugation at 3,000 rpm for 5 min. After cells were washed twice with phosphate-buffered saline (PBS), they were resuspended in PBS containing 3.5 mM CaCl2 and used in an NA assay using the MUNANA substrate.
Hemagglutination and hemagglutination-elution (HAE) assays.
Serial 2-fold dilutions of viruses were prepared in 50 μl of PBS in round-bottomed 96-well plates to which 50 μl of a 0.7% suspension of erythrocytes (from guinea pigs and turkeys expressing predominantly α2,6 and α2,3 receptors, respectively) (Lampire Biologicals, Pipersville, PA) was added. Plates were incubated at 4°C for 1 h, and HA titers, given in hemagglutinating units (HAU), were determined. HA elution assays were performed as described elsewhere (20). Briefly, 4 HAU of viruses was preincubated for 30 min at room temperature with 1 μM NAIs. Guinea pig or turkey erythrocytes were then added, and the plates were incubated at 4°C for 1 h to allow agglutination to occur. The plates were then incubated at 37°C to allow virus elution. Elution was followed by the appearance of pelleted erythrocytes. One well contained the virus in the absence of drug as the elution control. The plates were monitored at 15, 30, 45, 60, 90, 120, and 240 min to determine the rate at which elution occurred.
Statistical analyses.
Amounts of NA activity of recombinant proteins were compared to those of the WT by the use of unpaired two-tailed t tests.
RESULTS
Laninamivir susceptibility profiles of influenza viruses harboring mutations of resistance to other NAIs.
The IC50s of laninamivir against various NAI-resistant influenza A virus variants as determined by NA inhibition assays are summarized in Table 1. All viruses that were susceptible to zanamivir also had a susceptible phenotype to laninamivir, including oseltamivir-resistant A(H1N1) variants containing H275Y and N295S substitutions as well as the A(H3N2) variant with the E119V change. Influenza A(H1N1)pdm09 variants containing the E119V/G and Q136K substitutions, which conferred resistance to zanamivir, exhibited reduced or highly reduced inhibition to laninamivir. Of note, a multidrug resistance phenotype to laninamivir, zanamivir, peramivir, and oseltamivir was observed for the E119V A(H1N1)pdm09 recombinant variant.
Selection of laninamivir-resistant variants in vitro.
Pandemic influenza A(H1N1)pdm09 (A/Quebec/144147/2009 and A/Auckland/3/2009) virus strains as well as seasonal A(H1N1) (A/Solomon Islands/3/2006-like) and A(H3N2) (A/Brisbane/10/2007-like) viruses were passaged under laninamivir pressure. Sequence changes in the NA and HA proteins were analyzed at different passages (Table 2). Sequence analysis of assay 1 with recombinant A/Quebec/144147/2009 virus passaged in the presence of laninamivir revealed two HA substitutions (V135A and G158E), but the latter was also found in control-passaged virus. In assay 2, two NA substitutions (E119K and G147E) and one HA change (K133E) were detected. The E119K NA substitution was also selected in the A/Auckland/3/2009 virus along with K156E and D225G HA substitutions after five passages under laninamivir pressure, whereas an A/Solomon Islands/3/2006 variant containing an E119A substitution in the NA protein emerged after 11 passages in the presence of laninamivir. The passage of the A/Brisbane/10/2007-like (H3N2) virus under laninamivir pressure resulted in the emergence of a large deletion (237 amino acids [aa]) in the NA protein (Fig. 1) which was observed after six passages, with no changes being detected in the NA protein of the control-passaged virus. On the other hand, the S138A and P194L HA changes were concomitantly found in the laninamivir-passaged virus, whereas the S138A and N144K HA substitutions were found in the control-passaged virus.
TABLE 2.
Sequence changes in the neuraminidase and hemagglutinin proteins of influenza viruses selected with laninamivir
| Influenza strain and/or assay | Passage no. | Laninamivir concn (μM)c | Amino acid change(s) in:d |
|
|---|---|---|---|---|
| NAa | HAb | |||
| A(H1N1)pdm09 (A/Quebec/144147/2009) | ||||
| Assay 1 | 3 | 0 | None | G158E |
| 1.00 | None | G158E; 135V, 135A | ||
| 6 | 0 | None | G158E | |
| 4.00 | None | V135A/G158E | ||
| 9 | 0 | None | G158E | |
| 15.00 | None | V135A/G158E | ||
| 12 | 0 | None | G158E | |
| 100.00 | None | V135A/G158E | ||
| Assay 2 | 3 | 0 | None | None |
| 1.00 | None | K133E | ||
| 6 | 0 | None | K122N | |
| 3.00 | 119E, 119K | K133E | ||
| 9 | 0 | None | K122N | |
| 10.00 | 119E, 119K; 147G, 147E | K133E | ||
| 12 | 0 | None | K122N | |
| 50.00 | E119K/G147E | K133E | ||
| A(H1N1)pdm09 (A/Auckland/3/2009) | 5 | 0 | None | K136E |
| 0.77 | E119K | K156E/D225G | ||
| A(H1N1) (A/Solomon Islands/3/2006) | 11 | 0 | None | None |
| 8.20 | E119A | None | ||
| A(H3N2) (A/Brisbane/10/2007) | 3 | 0 | None | 138S, 138A; 144N, 144K |
| 0.22 | None | 138S, 138A | ||
| 6 | 0 | None | 138S, 138A; 144N, 144K | |
| 1.80 | Del 106–342 | S138A; 194P, 194L | ||
| 9 | 0 | None | S138A/N144K | |
| 2.00 | Del 106–342 | S138A/P194L | ||
Based on N1 numbering for H1N1 variants and N2 numbering for H3N2 variants. Amino acid positions are given for deletions (Del).
Based on H3 numbering as in Nobusawa et al. (36).
A concentration of 0 μM represents the control.
At some positions, both WT and variant occurred (mixed population), and both are listed for the indicated residue.
FIG 1.
The A(H3N2) (A/Brisbane/10/2007) laninamivir-selected virus variant with a major deletion (aa 106 to 342) in the region encompassing the active site of the neuraminidase (NA) protein. (A) PCR products of the NA gene from the wild-type (WT) strain and the NA variant were separated on a 1% electrophoresis gel in the presence of a 1-kb molecular weight (MW) marker. (B) Schematic representation of the full-length (469 aa) and the deleted (Del; 232 aa) NA proteins.
Characterization of laninamivir-resistant influenza virus mutants selected in vitro.
Herein, we further characterized the A(H1N1)pdm09 (A/Quebec/144147/2009 and A/Auckland/3/2009), A(H1N1) (A/Solomon Islands/3/2006-like), and the A(H3N2) (A/Brisbane/10/2007-like) influenza virus variants selected with laninamivir. In plaque reduction assays, these variants exhibited a reduced or highly reduced susceptibility phenotype to laninamivir and oseltamivir as determined by criteria analogous to those reported for NA inhibition assays (Table 3 and Fig. 2). We further evaluated the laninamivir-passaged A(H3N2) virus (NA deletion, S138A and P194L HA changes), the control-passaged A(H3N2) virus (S138A and N144K HA substitutions), and the initial WT virus in a hemagglutination-elution (HAE) assay using either turkey or guinea pig red blood cells (RBCs) (data not shown). The HAE assay was done in the absence and presence of laninamivir, zanamivir, or oseltamivir. No difference in elution times was observed between the control-passaged virus and the initial virus in the absence or presence of NAIs; however, the elution time was faster with turkey RBCs than with guinea pig RBCs, equivalent to 30 and 90 min, respectively. On the other hand, the laninamivir-passaged virus containing the NA deletion and the two HA substitutions S138A/P194L did not agglutinate the two types of RBCs.
TABLE 3.
NAI susceptibility profiles of A(H3N2) and A(H1N1)pdm09 viruses selected with laninamivir as assessed by plaque reduction assay
| Influenza strain and/or assay | Passage no. | Amino acid change(s)a | IC50 (nM [fold increase])b |
|
|---|---|---|---|---|
| Laninamivir | Oseltamivir | |||
| A(H1N1)pdm09 (A/Quebec/144147/2009) | ||||
| Assay 1 | 0 | WT | 5.89 ± 0.84 (1.0) | 7.55 ± 0.37 (1.0) |
| 12 (control) | HA G158E | 19.37 ± 2.41 (3.3) | 4.65 ± 0.32 (0.6) | |
| 12 | HA V135A/G158E | 1,079.55 ± 49.58 (183.3) | 197.00 ± 23.27 (26.1) | |
| Assay 2 | 3 | HA K133E | 7.62 ± 1.01 (1.3) | 8.43 ± 0.08 (1.1) |
| 12 (control) | HA K122N | 4.90 ± 0.11 (0.8) | 4.03 ± 0.17 (0.5) | |
| 12 | NA E119K/G147E; HA K133E | >100,000 (>10,000) | >100,000 (>10,000) | |
| A(H3N2) (A/Brisbane/10/2007) | 0 | WT | 3.11 ± 0.83 (1.0) | 1.87 ± 0.04 (1.0) |
| 9 (control) | HA S138A/N144K | 3.71 ± 0.15 (1.2) | 3.12 ± 0.13 (1.7) | |
| 9 | NA Del 106–342; HA S138A/P194L | >100,000 (>10,000) | >100,000 (>10,000) | |
| A(H1N1)pdm09 (A/Auckland/3/2009) | 0 | WT | 253.45 ± 96.52 | 141.30 ± 2.28 |
| 5 (control) | HA K136E | 34.23 ± 19.04 | 67.96 ± 40.96 | |
| 5 | NA E119K; HA K156E/D225G | >100,000 (>394) | >100,000 (>708) | |
| A(H1N1) (A/Solomon Islands/3/2006) | 0 | WT | 10.34 ± 3.30 | 110.98 ± 5.26 |
| 11 (control) | WT | 14.04 ± 1.93 | 106.24 ± 88.90 | |
| 11 | NA E119 A | 3,364.50 ± 552.25 (325) | 3,448.55 ± 724.71 (31) | |
NA numbering based on N1 numbering for H1N1 variants and N2 numbering for H3N2 variants, and HA numbering is based on H3 numbering as in Nobusawa et al. (36). Amino acid positions are given with deletions (Del).
Values are means ± standard deviations from two independent experiments.
FIG 2.
Plaque sizes of the A(H1N1)pdm09 (A/Quebec/144147/2009) and A(H3N2) (A/Brisbane/10/2007) laninamivir-selected variants. Viruses selected with laninamivir were propagated in 12-well plates containing MDCK α2,6 cells under 0.8% agarose. Plaques were visualized after 48 h of incubation at 37°C in the absence or presence of laninamivir.
Generation and characterization of laninamivir-resistant recombinant A(H1N1)pdm09 viruses and proteins.
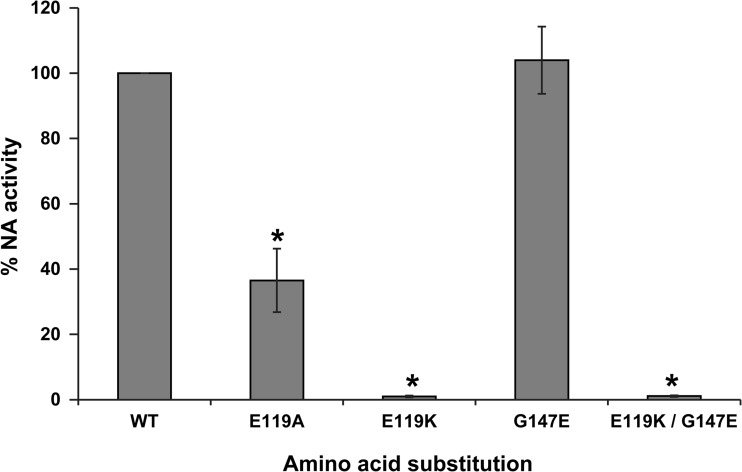
A recombinant A(H1N1)pdm09 (A/Quebec/144147/2009) E119A NA variant was successfully generated by reverse genetics. Susceptibility profiles of the WT and E119A recombinant viruses to laninamivir, zanamivir, oseltamivir, and peramivir, as determined by NA inhibition assays, are summarized in Table 4. The E119A NA substitution conferred reduced susceptibility to laninamivir, zanamivir, oseltamivir, and peramivir with 82-, 90-, 17-, and 12-fold increases in IC50s, respectively, compared with those of the WT virus. A recombinant A(H1N1)pdm09 (A/Quebec/144147/2009) virus harboring E119K NA and K133E HA substitutions was also generated by reverse genetics. Despite multiple attempts, we were unable to generate the NA E119K or E119K/G147E virus without the HA change. Consequently, we generated recombinant NA proteins expressed in 293T cells and assessed their NA enzymatic activity. As shown in Fig. 3, the E119A, E119K, and E119K/G147E variants were associated with a significant reduction of total NA activity, with relative total NA activities of 36.5% (P < 0.001), 1.0% (P < 0.001), and 1.1% (P < 0.001), respectively, compared to the WT protein. Of note, we cannot distinguish between decreased activity or expression based on the current assay. The G147E substitution alone did not significantly affect the relative total NA activity (104%) or susceptibility to laninamivir (7-fold decrease in IC50 compared to WT).
TABLE 4.
Susceptibility profiles to neuraminidase inhibitors of a recombinant A(H1N1)pdm09 virus harboring the E119A neuraminidase substitution
| A(H1N1)pdm09 (A/Québec/144147/09) strain | IC50 (nM [fold increase])b |
|||
|---|---|---|---|---|
| Laninamivir | Zanamivir | Oseltamivir | Peramivir | |
| WT | 1.41 ± 0.01 (1) | 1.53 ± 0.02 (1) | 3.20 ± 0.01 (1) | 0.61 ± 0.00 (1) |
| NA E119Aa | 115.80 ± 9.50 (82) | 137.90 ± 8.8 (90) | 53.06 ± 1.21 (17) | 7.22 ± 0.77 (12) |
Based on the N1 numbering system.
Values are means ± standard deviations from three independent experiments.
FIG 3.
Activity of recombinant A(H1N1)pdm09 (A/Quebec/144147/2009) neuraminidase proteins. 293T cells were transfected with pCAGGS-PA, -PB1, -PB2, and -NP plasmids in addition to plasmids expressing the WT or variant A/Quebec/144147/2009 neuraminidase (NA) proteins. At 48 h posttransfection, NA activity was measured by using the fluorogenic substrate MUNANA. Percent NA activities were determined in triplicate experiments ± standard deviations. *, P < 0.001, compared to the WT NA activity.
DISCUSSION
NAIs are expected to play a major role in the control of seasonal and eventual pandemic influenza virus infections. However, the emergence and spread of NAI-resistant variants is a serious concern. The identification of amino acid substitutions conferring resistance to NAIs from in vitro studies may help us to understand mechanisms of resistance and to predict clinical cases of resistance to this class of antivirals. In fact, the well-known NA changes conferring resistance to oseltamivir in humans, including the H1N1 H275Y variant and the H3N2 E119V and R292K variants, were previously predicted by in vitro studies (21–23). In the present study, we used an in vitro approach to investigate mechanisms of resistance to laninamivir, a novel NAI.
By testing several A(H1N1)pdm09 as well as seasonal A(H1N1) and A(H3N2) variants, previously found to be resistant to at least one NAI, we demonstrated a similar pattern of susceptibility between laninamivir and zanamivir. More specifically, laninamivir was shown to be active against oseltamivir-resistant H1N1-H275Y virus, as reported previously (24), and H3N2-E119V variants. Therefore, laninamivir could constitute an antiviral option for the treatment of severe oseltamivir-resistant cases. In contrast, the recombinant A(H1N1)pdm09 viruses containing E119A/G/V framework NA substitutions exhibited reduced or highly reduced susceptibility to zanamivir and laninamivir in our NA inhibition assays. Accordingly, passage of the seasonal A(H1N1) (A/Solomon/3/2006) as well as of the two A(H1N1)pdm09 (A/Quebec/144147/2009 and A/Auckland/3/2009) strains under laninamivir pressure induced an amino acid substitution at position 119 (E119A/K) of the viral NA. In previous reports, E119A/G/D NA changes were described in A(H3N2), A(H1N9), A(H5N1), and B variants that were selected through in vitro passages in the presence of zanamivir (25). The structural similarity between laninamivir and zanamivir confers to these two NAIs a common binding process with the enzyme (26). The latter process involves the 4-guanidino group which interacts with the carboxylate of the E119 side chain. The loss of this interaction in the E119A/K/G/V A(H1N1) variants described in this study may result in reduced binding to both zanamivir and laninamivir (27). Of note, as peramivir also contains a guanidine group, the zanamivir/laninamivir-resistant viruses also demonstrated resistance to peramivir (Tables 1 and 4). In contrast to the above three NAIs, oseltamivir contains an acetamide group instead of guanidine at that position, which explains the lower IC50s for the 119 A(H1N1) mutants.
In a previous work, we demonstrated that amino acid substitutions at residue 119 in recombinant A(H1N1)pdm09 viruses (i.e., E119A/G/V) were associated with altered NA activity, resulting in reduced viral titers in replicative capacity experiments (18). Moreover, in experimentally infected mice, the recombinant A/Québec/144747/2009 E119G variant was associated with a significant reduction of lung viral titers compared to the WT, and a reversion of this variant to the WT genotype was observed in the ferret model (28). In this study, the E119K change was shown to cause an even greater reduction in NA activity and/or cell surface expression, which may explain why we were unable to perform NA inhibition assays and to generate the NA variant without HA mutation by reverse genetics. Thus, we believe that the E119K substitution would alter the viral fitness of influenza A(H1N1)pdm09 viruses although this hypothesis remains to be confirmed.
The Q136K substitution is another NA change that was shown to confer resistance to laninamivir in our NA inhibition assays. This NA substitution was previously described in A(H3N2) and A(H1N1) cultured isolates (29, 30); the clinical relevance of this substitution remains unclear, however, as the Q136K change could be detected only in infected culture cells in some cases, whereas it was absent in clinical samples. Nonetheless, a recent report has described the emergence of this mutation under in vitro zanamivir pressure in A(H1N1)pdm09 viruses (31). The Q136 residue is located at the periphery of the NA binding site, and the Q136K change was suggested to alter interactions with D151 and R156, thereby disrupting hydrogen links between D151 and zanamivir (30, 32, 33). In one of the laninamivir-resistant A(H1N1)pdm09 variants described in our study, the E119K substitution was accompanied by an additional G147E NA change. However, the characterization of recombinant A(H1N1)pdm09 proteins in this study revealed no significant impact of the G147E change on the susceptibility to laninamivir and NA activity (Fig. 3) compared to the recombinant WT protein.
Following passages of the A(H3N2) (A/Brisbane/10/2007-like) virus under laninamivir pressure, we observed a large deletion in the NA protein. Of interest, almost the whole enzymatic head domain of the NA protein (residues 106 to 342), including the active site, was missing. Consequently, this A(H3N2) variant had undetectable NA enzymatic activity. We along with others previously observed a similar deletion in the seasonal A(H1N1) (A/WSN/33) virus exposed to zanamivir selective pressure (34, 35). In two of these cases, the NA deletion was accompanied by HA substitutions, with S138A/P194L for A(H3N2) and A200T for A(H1N1). The hemagglutination-elution (HAE) assay showed that our A(H3N2) mutant containing the NA deletion and the two HA substitutions did not agglutinate two RBC species. It is plausible that these HA substitutions reduce affinity or alter receptor specificity, resulting in a virus that is less dependent on the NA and allowing the replication of the NA-deficient viruses.
HA mutations were observed in all A(H1N1)pdm09 and A(H3N2) viruses passaged under laninamivir pressure. In the first series of passages of A(H1N1)pdm09 (A/Quebec/144147/2009) virus under laninamivir pressure, we did not observe any NA substitution but detected two HA substitutions. We further cultured this variant in the presence of a high laninamivir concentration (100 μM) and obtained good cytopathic effects. One of the HA substitutions (V135A) is located on the right edge of the receptor binding site (RBS) that comprises residues 134 to 138 (36). We also detected an HA substitution preceding the selection of the NA changes in the second series of passages of A(H1N1)pdm09 (A/Quebec/144147/2009) virus. This HA substitution at position 133 appeared after three passages under laninamivir pressure and was conserved throughout the selection process, even after the emergence of NA substitutions at positions 119 and 147, which occurred at passages 6 and 9, respectively. The residue 133 is located near the right edge of the RBS. An experiment with a different A(H1N1)pdm09 virus (A/Auckland/3/2009) in MDCK cells also resulted in the selection of the E119K NA variant along with two HA substitutions, K156E and D225G. Of note, the selected K156E substitution is located near the RBS, and it has been shown that the D225G change switches receptor binding specificity from α2,6 linkage binding to dual receptor binding (37). Similarly, the A(H3N2) (A/Brisbane/10/2007-like) virus containing an HA substitution at position 194 appeared after three passages under laninamivir pressure, concomitantly with the NA deletion. Residue 194 is located in the RBS, which probably explains the growth of the variant in cell culture, despite an NA deletion impairing its enzymatic activity.
Interestingly, we observed that control-passaged viruses, i.e., those not submitted to drug pressure, acquired some HA mutations during viral propagation in MDCK cells overexpressing the α2,6 sialic acid receptors. The A(H1N1)pdm09 (A/Quebec/144147/2009) virus acquired the G158E and K122N HA substitutions during passages in assays 1 and 2, respectively, while A(H3N2) (A/Brisbane/10/2007-like) acquired two HA substitutions, S138A and N144K. It has been previously reported that HA substitutions G158E and K122N could improve the growth of A(H1N1)pdm09 virus in MDCK cells and eggs and that the G158E change drastically reduced viral antigenicity (38). We selected another substitution of interest in the A(H3N2) virus antigenic site A, the N144K HA substitution. This amino acid change is present along with others (E62K, K158N, K173Q, and N189K) in a drifted strain that emerged in 2009 (39, 40).
In summary, our results demonstrate that the drug resistance patterns of laninamivir and zanamivir appear similar and that both NA and HA changes emerge during the in vitro selection of influenza A(H1N1)pdm09 and A(H3N2) virus variants with reduced susceptibility to laninamivir. Furthermore, the selection of HA mutations in or near the RBS in all A(H1N1)pdm09 and A(H3N2) viruses passaged under laninamivir pressure emphasizes the importance of HA binding affinity on the replicative capacity of laninamivir-resistant viruses. Overall, our results highlight the importance of the HA/NA balance in the resistance phenotype to NAIs such as laninamivir as well as the need to develop drugs with new viral targets.
ACKNOWLEDGMENTS
This study was supported by the Canadian Institutes of Health Research (grants 230187 and 229733 to G.B.), Biota Scientific Management and by the public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) (postdoctoral training award to M.S.). G.B. is the holder of the Canada research chair on influenza and other respiratory viruses.
Footnotes
Published ahead of print 23 June 2014
REFERENCES
- 1.Davies BE. 2010. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 65(Suppl 2):ii5–ii10. 10.1093/jac/dkq015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutkowski R, Thakrar B, Froehlich E, Suter P, Oo C, Ward P. 2003. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 26:787–801. 10.2165/00002018-200326110-00004 [DOI] [PubMed] [Google Scholar]
- 3.He G, Massarella J, Ward P. 1999. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin. Pharmacokinet. 37:471–484. 10.2165/00003088-199937060-00003 [DOI] [PubMed] [Google Scholar]
- 4.Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM. 2009. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301:1034–1041. 10.1001/jama.2009.294 [DOI] [PubMed] [Google Scholar]
- 5.Hauge SH, Dudman S, Borgen K, Lackenby A, Hungnes O. 2009. Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007– 08. Emerg. Infect. Dis. 15:155–162. 10.3201/eid1502.081031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijer A, Lackenby A, Hungnes O, Lina B, van der Werf S, Schweiger B, Opp M, Paget J, van de Kassteele J, Hay A, Zambon M. 2009. Oseltamivir-resistant influenza virus A (H1N1), Europe 2007-08 season. Emerg. Infect. Dis. 15:552–560. 10.3201/eid1504.081280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gamblin SJ. 2008. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 453:1258–1261. 10.1038/nature06956 [DOI] [PubMed] [Google Scholar]
- 8.Malaisree M, Rungrotmongkol T, Decha P, Intharathep P, Aruksakunwong O, Hannongbua S. 2008. Understanding of known drug-target interactions in the catalytic pocket of neuraminidase subtype N1. Proteins 71:1908–1918. 10.1002/prot.21897 [DOI] [PubMed] [Google Scholar]
- 9.Wang MZ, Tai CY, Mendel DB. 2002. Mechanism by which mutations at his274 alter sensitivity of influenza a virus n1 neuraminidase to oseltamivir carboxylate and zanamivir. Antimicrob. Agents Chemother. 46:3809–3816. 10.1128/AAC.46.12.3809-3816.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, Shimada J. 2011. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob. Agents Chemother. 55:2803–2812. 10.1128/AAC.01718-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. 2011. Relenza (zanamivir) information. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/drugs/drugsafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm183783.htm [Google Scholar]
- 12.Kubo S, Tomozawa T, Kakuta M, Tokumitsu A, Yamashita M. 2010. Laninamivir prodrug CS-8958, a long-acting neuraminidase inhibitor, shows superior anti-influenza virus activity after a single administration. Antimicrob. Agents Chemother. 54:1256–1264. 10.1128/AAC.01311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. 2009. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 53:186–192. 10.1128/AAC.00333-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugaya N, Ohashi Y. 2010. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob. Agents Chemother. 54:2575–2582. 10.1128/AAC.01755-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y. 2010. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: A double-blind, randomized, noninferiority clinical trial. Clin. Infect. Dis. 51:1167–1175. 10.1086/656802 [DOI] [PubMed] [Google Scholar]
- 16.Yamashita M. 2010. Laninamivir and its prodrug, CS-8958: long-acting neuraminidase inhibitors for the treatment of influenza. Antivir. Chem. Chemother. 21:71–84. 10.3851/IMP1688 [DOI] [PubMed] [Google Scholar]
- 17.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon S. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287–289. 10.1016/0003-2697(79)90362-2 [DOI] [PubMed] [Google Scholar]
- 18.Pizzorno A, Bouhy X, Abed Y, Boivin G. 2011. Generation and characterization of recombinant pandemic influenza A(H1N1) viruses resistant to neuraminidase inhibitors. J. Infect. Dis. 203:25–31. 10.1093/infdis/jiq010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abed Y, Goyette N, Boivin G. 2004. A reverse genetics study of resistance to neuraminidase inhibitors in an influenza A/H1N1 virus. Antivir. Ther. 9:577–581 [PubMed] [Google Scholar]
- 20.McKimm-Breschkin JL, Blick TJ, Sahasrabudhe A, Tiong T, Marshall D, Hart GJ, Bethell RC, Penn CR. 1996. Generation and characterization of variants of NWS/G70C influenza virus after in vitro passage in 4-amino-Neu5Ac2en and 4-guanidino-Neu5Ac2en. Antimicrob. Agents Chemother. 40:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubareva LV. 2004. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 103:199–203. 10.1016/j.virusres.2004.02.034 [DOI] [PubMed] [Google Scholar]
- 22.McKimm-Breschkin JL. 2000. Resistance of influenza viruses to neuraminidase inhibitors—a review. Antiviral Res. 47:1–17. 10.1016/S0166-3542(00)00103-0 [DOI] [PubMed] [Google Scholar]
- 23.Tai CY, Escarpe PA, Sidwell RW, Williams MA, Lew W, Wu H, Kim CU, Mendel DB. 1998. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiso M, Shinya K, Shimojima M, Takano R, Takahashi K, Katsura H, Kakugawa S, Le MT, Yamashita M, Furuta Y, Ozawa M, Kawaoka Y. 2010. Characterization of oseltamivir-resistant 2009 H1N1 pandemic influenza A viruses. PLoS pathogens 6:e1001079. 10.1371/journal.ppat.1001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson M, Pizzorno A, Abed Y, Boivin G. 2013. Influenza virus resistance to neuraminidase inhibitors. Antiviral Res. 98:174–185. 10.1016/j.antiviral.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 26.Vavricka CJ, Li Q, Wu Y, Qi J, Wang M, Liu Y, Gao F, Liu J, Feng E, He J, Wang J, Liu H, Jiang H, Gao GF. 2011. Structural and functional analysis of laninamivir and its octanoate prodrug reveals group specific mechanisms for influenza NA inhibition. PLoS Pathog. 7:e1002249. 10.1371/journal.ppat.1002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith BJ, McKimm-Breshkin JL, McDonald M, Fernley RT, Varghese JN, Colman PM. 2002. Structural studies of the resistance of influenza virus neuramindase to inhibitors. J. Med. Chem. 45:2207–2212. 10.1021/jm010528u [DOI] [PubMed] [Google Scholar]
- 28.Pizzorno A, Abed Y, Rhéaume C, Bouhy X, Boivin G. 2013. Evaluation of recombinant 2009 pandemic influenza A (H1N1) viruses harboring zanamivir resistance mutations in mice and ferrets. Antimicrob. Agents Chemother. 57:1784–1789. 10.1128/AAC.02269-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dapat C, Suzuki Y, Saito R, Kyaw Y, Myint YY, Lin N, Oo HN, Oo KY, Win N, Naito M, Hasegawa G, Dapat IC, Zaraket H, Baranovich T, Nishikawa M, Saito T, Suzuki H. 2010. Rare influenza A (H3N2) variants with reduced sensitivity to antiviral drugs. Emerg. Infect. Dis. 16:493–496. 10.3201/eid1603.091321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurt AC, Holien JK, Parker M, Kelso A, Barr IG. 2009. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J. Virol. 83:10366–10373. 10.1128/JVI.01200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaminski MM, Ohnemus A, Staeheli P, Rubbenstroth D. 2013. Pandemic 2009 H1N1 influenza A virus carrying a Q136K mutation in the neuraminidase gene is resistant to zanamivir but exhibits reduced fitness in the guinea pig transmission model. J. Virol. 87:1912–1915. 10.1128/JVI.02507-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han N, Liu X, Mu Y. 2012. Exploring the mechanism of zanamivir resistance in a neuraminidase mutant: a molecular dynamics study. PLoS One 7:e44057. 10.1371/journal.pone.0044057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ. 2006. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443:45–49. 10.1038/nature05114 [DOI] [PubMed] [Google Scholar]
- 34.Nedyalkova MS, Hayden FG, Webster RG, Gubareva LV. 2002. Accumulation of defective neuraminidase (NA) genes by influenza A viruses in the presence of NA inhibitors as a marker of reduced dependence on NA. J. Infect. Dis. 185:591–598. 10.1086/339358 [DOI] [PubMed] [Google Scholar]
- 35.Baz M, Abed Y, Boivin G. 2007. Characterization of drug-resistant recombinant influenza A/H1N1 viruses selected in vitro with peramivir and zanamivir. Antiviral Res. 74:159–162. 10.1016/j.antiviral.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 36.Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K. 1991. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182:475–485. 10.1016/0042-6822(91)90588-3 [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Shi Y, Qi J, Gao F, Li Q, Fan Z, Yan J, Gao GF. 2013. Molecular basis of the receptor binding specificity switch of the hemagglutinins from both the 1918 and 2009 pandemic influenza A viruses by a D225G substitution. J. Virol. 87:5949–5958. 10.1128/JVI.00545-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Wang W, Zhou H, Suguitan AL, Shambaugh C, Kim L, Zhao J, Kemble G, Jin H. 2010. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J. Virol. 84:44–51. 10.1128/JVI.02106-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ann J, Papenburg J, Bouhy X, Rhéaume C, Hamelin MÈ Boivin G. 2012. Molecular and antigenic evolution of human influenza A/H3N2 viruses in Quebec, Canada, 2009– 2011. J. Clin. Virol. 53:88–92. 10.1016/j.jcv.2011.09.016 [DOI] [PubMed] [Google Scholar]
- 40.Yang JR, Lin CH, Chen CJ, Liu JL, Huang YP, Kuo CY, Yao CY, Hsu LC, Lo J, Ho Y, Wu HS, Liu MT. 2010. A new antigenic variant of human influenza A (H3N2) virus isolated from airport and community surveillance in Taiwan in early 2009. Virus Res. 151:33–38. 10.1016/j.virusres.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 41.Pizzorno A, Abed Y, Bouhy X, Beaulieu E, Mallett C, Russell R, Boivin G. 2012. Impact of mutations at residue I223 of the neuraminidase protein on the resistance profile, replication level, and virulence of the 2009 pandemic influenza virus. Antimicrob. Agents Chemother. 56:1208–1214. 10.1128/AAC.05994-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abed Y, Baz M, Boivin G. 2006. Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir. Ther. 11:971–976 [PubMed] [Google Scholar]
- 43.Baz M, Abed Y, Simon P, Hamelin ME, Boivin G. 2010. Effect of the neuraminidase mutation H274Y conferring resistance to oseltamivir on the replicative capacity and virulence of old and recent human influenza A(H1N1) viruses. J. Infect. Dis. 201:740–745. 10.1086/650464 [DOI] [PubMed] [Google Scholar]
- 44.Baz M, Abed Y, McDonald J, Boivin G. 2006. Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin. Infect. Dis. 43:1555–1561. 10.1086/508777 [DOI] [PubMed] [Google Scholar]
- 45.Abed Y, Baz M, Boivin G. 2009. A novel neuraminidase deletion mutation conferring resistance to oseltamivir in clinical influenza A/H3N2 virus. J. Infect. Dis. 199:180–183. 10.1086/595736 [DOI] [PubMed] [Google Scholar]