Abstract
In this study, our objective was to determine whether a synergistic antimicrobial combination in vitro would be beneficial in the downregulation of pneumococcal virulence genes and whether the associated inflammation of the lung tissue induced by multidrug-resistant Streptococcus pneumoniae infection in vivo needs to be elucidated in order to consider this mode of therapy in case of severe pneumococcal infection. We investigated in vivo changes in the expression of these virulence determinants using an efficacious combination determined in previous studies. BALB/c mice were infected with 106 CFU of bacteria. Intravenous levofloxacin at 150 mg/kg and/or ceftriaxone at 50 mg/kg were initiated 18 h postinfection; the animals were sacrificed 0 to 24 h after the initiation of treatment. The levels of cytokines, chemokines, and C-reactive protein (CRP) in the serum and lungs, along with the levels of myeloperoxidase and nitric oxide the inflammatory cell count in bronchoalveolar lavage fluid (BALF), changes in pneumolysin and autolysin gene expression and COX-2 and inducible nitric oxide synthase (iNOS) protein expression in the lungs were estimated. Combination therapy downregulated inflammation and promoted bacterial clearance. Pneumolysin and autolysin expression was downregulated, with a concomitant decrease in the expression of COX-2 and iNOS in lung tissue. Thus, the combination of levofloxacin and ceftriaxone can be considered for therapeutic use even in cases of pneumonia caused by drug-resistant isolates.
INTRODUCTION
Streptococcus pneumoniae is the pathogen most frequently isolated from clinical samples of respiratory tract infection, including acute exacerbation of chronic bronchitis, bacteremic pneumococcal pneumonia, and community-acquired pneumonia (CAP) (1, 2). Despite advances in infection prevention by vaccination, microbiological diagnostics, and antibiotic therapy, pneumonia is still characterized by high mortality and morbidity and is associated with a significant health cost (3). The treatment of CAP continues to be a challenge in the 21st century. Issues involved in treatment recommendations include the emergence of antibiotic resistance among S. pneumoniae and monotherapy versus combination antibiotic therapy. Combination therapy is defined as the use of two of the antibiotics of the β-lactam, macrolide, and fluoroquinolone classes.
A series of recent studies have cast serious doubt on whether monotherapy is appropriate for treating severely ill patients. Despite similar aspects of activity and favorable resistance patterns of CAP pathogens, emerging evidence suggests the superiority of dual therapy over monotherapy for certain populations, particularly patients with severe CAP or bacteremic pneumococcal CAP (4, 5). Combination therapy using antimicrobials with different mechanisms of action has been used for a decade to treat infections, with the goals of producing a wider spectrum of action, preventing the emergence of multidrug-resistant (MDR) populations, reducing the dose of a single agent, or achieving a synergistic effect (6). Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia (7, 8). Data from retrospective analyses of patients with bacteremic pneumococcal pneumonia suggest that combination antibiotic therapy is associated with reduced mortality compared to that seen in those who receive monotherapy only (9). Combinations of antibiotics are usually adopted with the double aim of widening coverage and increasing antibacterial activity, which limits the occurrence and spread of resistant strains (10). A highly bactericidal antibiotic combination with excellent tissue penetration that does not lead to the emergence of resistance would be a major advantage in the treatment of pneumococcal diseases, particularly for the most severe forms.
Antimicrobial therapy remains a controversial issue for the treatment of CAP. Treatment with a single antibiotic, such as a β-lactam, a third-generation cephalosporin, or an antipneumococcal fluoroquinolone, was considered to be effective previously (11), but with the emergence of MDR S. pneumoniae strains, the use of a β-lactam or macrolide as empirical therapy for such infections is of great concern. Emerging evidence shows beneficial effects on outcome with combination therapies, especially with that of a β-lactam agent and a macrolide given together to hospitalized patients with pneumococcal pneumonia (12). Fluoroquinolones with increased activity against S. pneumoniae, such as levofloxacin, moxifloxacin, and gemifloxacin, are now being recommended. However, there is growing concern about the emergence of fluoroquinolone-resistant pneumococci (13).
If the benefit of combination therapy in patients with severe bacteremic pneumococcal pneumonia is real, something that remains to be definitively established is its mechanism in relation to lung inflammation. Until now, β-lactam antibiotics have remained the drugs of choice for pneumococcal diseases, except when their penetration to the infected tissues is limited. However, broad-spectrum cephalosporins, like cefotaxime (CTX) and ceftriaxone (CRO), which are characterized by higher levels in serum and lower MICs, have been suggested for the treatment of pneumonia since 1992 (14, 15). In contrast, more consistent data have been obtained in studies assessing the activities of combinations involving fluoroquinolones and β-lactams, particularly cephalosporins, both in vitro and in vivo. However, most of the cited studies limited their observations to a few pneumococcal strains and did not compare different combinations between them (16, 17). Among respiratory fluoroquinolones, levofloxacin is recommended in combination with a β-lactam (a third-generation cephalosporin or a carbapenem) as an alternative to standard combinations, according to Italian guidelines (18). The Canadian Thoracic Society, the American Thoracic Society, and the British Thoracic Society guidelines recommend either combined empirical therapy (β-lactam agent plus a macrolide) or a fluoroquinolone for the treatment of severe pneumonia (19).
Pulmonary phagocytic cell recruitment and inflammatory mediator release also play a pivotal role in the effective killing of respiratory pathogens. There has been increasing evidence indicating that during acute bacterial pneumonia, the combination of bacterial virulence factors and excessive inflammatory reactions from the host contribute collectively to the induction of severe lung injury, shock, and death (20).
In this study, we investigated the antibacterial activity of combination therapy with levofloxacin and ceftriaxone in a murine model of pneumococcal pneumonia and the concomitant evolution of the inflammatory response, including phagocytic cell recruitment in bronchoalveolar lavage fluid (BALF), release of cytokines and C-reactive protein (CRP) in blood, and myeloperoxidase (MPO) activity and vascular permeability in lung tissue. Accumulating evidence indicates that the excessive production of nitric oxide (NO) plays a pathogenic role in both acute and chronic models of inflammation (21). This has led many investigators to examine the roles of the inducible form of nitric oxide synthase (iNOS) and NO under pathophysiological conditions. Prostaglandins (PG) produced by cyclooxygenase (COX) are also considered to be biological mediators in all stages of inflammation (22). Out of the two isoforms of COX, the expression of COX-2 is rapidly induced by proinflammatory stimuli, like tumor necrosis factor alpha (TNF-α), and is thought to be involved in inflammation. Moreover, during the inflammatory response to infection, NO and PG may work cooperatively and synergistically under both physiological and pathological conditions (23).
The high death rate associated with pneumococcal disease, the emergence of antibiotic resistance, and the problems associated with current vaccines (24) have stimulated interest in the mechanisms of virulence. Two proteins that have been implicated are pneumolysin and autolysin. Pneumolysin is a multifunctional toxin that lyses cells with cholesterol in their membranes and interferes with the function of cells in the immune system (25). Autolysin causes cell autolysis typical of pneumococci and may be involved in cell division and competence for genetic transformation (26). Several pieces of evidence suggest that both proteins have a role in disease (27), but the strongest has come from insertion-duplication mutagenesis experiments using the cloned genes to make pneumolysin-negative (28) and autolysin-negative (29) mutants of the pneumococci that have reduced virulence in mice. Alterations in the expression of these two most important virulence factors of S. pneumoniae postcompletion of combination therapy will be crucial in the understanding of the effect of combination therapy on the mechanism of bacterial pathogenesis in the host.
The aim of this study was to investigate the potential synergy between antibiotics of therapeutic use against S. pneumoniae, as they have broad-spectrum activity and excellent tissue penetration and are less toxic to host tissue with some secondary anti-inflammatory properties against multidrug-resistant pneumococci in vitro and in experimental pneumonia. We demonstrated that a combination of ceftriaxone and levofloxacin was very efficacious in experimental pneumonia, particularly by downregulating pneumolysin and autolysin expression and lung inflammation. Therefore, the use of such antibiotics in combination against S. pneumoniae may result in synergism and should be considered for therapy.
MATERIALS AND METHODS
Antimicrobial agents, media, and challenge organisms.
The study drugs, which included penicillin (PEN), ampicillin (AMP), azithromycin (AZM), amoxicillin-potassium clavulanate (AMC), oxacillin (OXA), ceftazidime (CAZ), cefotaxime (CTX), cefuroxime (CXM), ceftriaxone (CRO), clindamycin (CLI), imipenem (IPM), meropenem (MEM), levofloxacin (LVX), ciprofloxacin (CIP), rifampin (RIF), vancomycin (VAN), trimethoprim-sulfamethoxazole (TMP-SXT), cefepime (FEP), and gentamicin (GEN) (HiMedia, Mumbai, India), were used for all in vitro testing, and the same LVX and CRO were used for intravenous injection in mice. The clinical isolate of S. pneumoniae used for the experiment, AMRI-SP1, was obtained from a sputum sample from a patient with a lower respiratory tract infection admitted to the Advanced Medicare and Research Institute (AMRI) hospital in Kolkata, West Bengal, India. A quality control strain of S. pneumoniae, ATCC 49619, was obtained as a kind gift from Indranil Roy, the Kolkata Medical Research Institute (CMRI), West Bengal, India. The strains were stored in skim milk-tryptone-glucose-glycerol (STGG) medium (HiMedia) at −80°C and subcultured twice onto Columbia blood agar plates (BAP) supplemented with 5% sheep blood (bioMérieux, Lyon, France) overnight at 37°C in a 10% CO2 air incubator before use in all in vitro and in vivo experiments. All in vitro experiments were carried out in Mueller-Hinton broth (MHB) (HiMedia). Brain heart infusion broth (BHI) (HiMedia) was used as the medium for pneumococcal cultures prior to the experiments with mice. All experimental samples were placed on Columbia BAP supplemented with 5% sheep blood.
In vitro susceptibility tests.
The in vitro susceptibilities of the isolates were compared with that of S. pneumoniae ATCC 49619, in accordance with the National Committee for Clinical Laboratory Standards (NCCLS) guidelines. The MICs and minimal bactericidal concentrations (MBCs) were determined by the tube dilution method in MHB (HiMedia, Mumbai, India) supplemented with 5% sheep blood, and the disk agar diffusion (DAD) test was performed using Mueller-Hinton agar supplemented with 5% sheep blood (30).
Test for synergism.
A checkerboard assay was performed according to the method described earlier (31). For each combination, a synergy test was performed in a 96-well microtiter plate containing two antimicrobial agents in 2-fold dilutions (4× to 1/32× MIC) dispensed in a checkerboard fashion on the day of the assay. The summation of the fractional inhibitory concentrations (Σ FICs) was calculated according to the method described earlier (32). The Σ FICs in each well was used to classify the effects of combinations of antimicrobial agents as synergistic for FIC indexes of ≤0.5, no interaction for FIC indexes of >0.5 to 4, and antagonistic for FIC indexes of >4.
Time-kill assay.
A time-kill assay was performed according to the method described earlier (33), with a few modifications. Each antibiotic was tested alone and in combination at concentrations at which they showed synergy in the checkerboard assay. Bacterial counts were performed at 0, 1, 2, 4, 6, 8, 12, and 24 h of incubation at 37°C by plating aliquots of 10 and 100 μl after dilution in sterile saline (0.9%) onto Columbia BAP supplemented with 5% sheep blood. Synergy, indifference, and antagonism between the combining antibiotics were concluded according to methods described earlier (33).
Lung infection model.
All experiments involving animals were conducted according to the protocols that had been approved by the Institutional Animal Ethics Committee (IAEC), Department of Physiology, University of Kolkata, under the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (approval no. 820/04/ac/CPCSEA, dated 6 August 2004), Ministry of Environment and Forest, Government of India. This study did not involve any invasive study using human subjects. Male BALB/c mice (weighing 25 ± 2 g) were obtained from registered animal suppliers to the Department of Physiology. All animals were maintained and utilized in accordance with recommendations from the IAEC and were provided with food and water ad libitum. Before infection, bacteria were grown statically at 37°C in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY broth) to an optical density at 600 nm (OD600) of 0.25 (equivalent to approximately 1 × 108 CFU ml−1). Experimental pneumonia was induced in the animals by infecting them with a penicillin-resistant (MIC, >64 mg/liter) strain of S. pneumoniae, AMRI-SP1, that is also resistant to macrolides (AZM MIC, 8 mg/liter) and fluoroquinolones (LVX MIC, 16 mg/liter). Each mouse was anesthetized lightly by intravenous injection of ketamine hydrochloride (Sigma Life Science) at 1 mg/kg of body weight through the tail vein, and 100 μl of a bacterial suspension (containing approximately 107 CFU) was inoculated through the nares into the lungs of each mouse (50 μl per nostril). The advantage of intranasal inoculation is that it mimics oropharyngeal aspiration, effectively infects the upper and lower respiratory tract, and is very simple. The challenge dose was confirmed by serial dilution and plating of the inoculum on blood agar. Previously, we established that mice infected intranasally with 1 × 107 bacteria developed pneumonia within 18 h from the time of infection, and thus, the 18-h postinfection time point was chosen for initiating antibiotic therapy (34).
Treatment regimens.
Eighteen hours after bacterial inoculation, groups of mice were treated with multiple intravenous (through the tail vein) doses of either LVX (150 mg/kg of body weight) or CRO (50 mg/kg of body weight) as monotherapy only or administered together as combination therapy in a 0.1-ml volume, with an interval of 6 h in between successive doses, such that at the end of 24 h, each group of animals received 4 doses of the drugs either alone (monotherapy) or in combination. Each animal was sacrificed for sample collection at the previously stated time point, starting at 18 h (0 h after antibiotic treatment) and continuing until 42 h (24 h after antibiotic treatment), with an interval of ≥2 h in between successive sampling time points. Mice receiving combination therapy received 0.1 ml of LVX, immediately followed by 0.1 ml of CRO. These dosing intervals were chosen so as to simulate the in vivo efficacy of short-term high-dose treatment with the drugs in humans. Untreated S. pneumoniae-infected animals were used as controls and were administered the same volume of isotonic saline.
Collection of lung and blood samples.
Each animal was sacrificed under ether anesthesia, starting at 18 h postinfection (0 h after antibiotic treatment) and continuing until 24 h posttherapy with either one or a combination of antibiotics. Blood (0.5 ml) samples were obtained at 0 h (immediately after administration of the first dose of the drug) and also at 2, 4, 8, 10, and 24 h (18 to 42 h postinfection) after antibiotic treatment by cardiac puncture under ether anesthesia, and each animal was exsanguinated at those selected intervals. Before perfusion, a collected blood sample was placed on ice until further use in heparinized tubes, and the rest was collected in anticoagulant-free tubes kept at 37°C for half an hour before centrifugation at 3,000 rpm for 15 min to obtain the serum. The serum thus obtained was stored in separate tubes at −80°C until further use. The mice were subsequently perfused with sterile phosphate-buffered saline (PBS) to remove blood-borne bacteria from the lungs. The lungs were removed and homogenized on ice in 2 ml sterile PBS using a tissue homogenizer. To separate pneumococci from the host cells, the lung homogenates and blood samples were centrifuged at 855 × g for 6 min at 4°C, as described previously (35). The lung and blood supernatants were subsequently centrifuged at 15,500 × g for 2 min at 4°C, and the bacterial pellet was stored at −80°C until further processing. Prior to pelleting the harvested bacteria, 20 μl was removed, serially diluted in PBS, and plated onto blood agar in order to enumerate the pneumococci present in the sample and to determine the presence, if any, of contaminating microflora. The blood plates were incubated overnight at 37°C in 95% air–5% CO2. The numbers of CFU were determined by counting the single colonies that appeared on the plates showing alpha-hemolysis (a characteristic specific to S. pneumoniae).
Extraction of RNA from bacteria.
RNA was isolated from bacterial pellets with acid-phenol-chloroform-isoamyl alcohol (125:24:1 ratio [pH 4.5]; catalog no. AM9722; Ambion), essentially as described previously (35). The extract was then precipitated at −80°C overnight in the presence of 40 ng glycogen μl−1 (catalog no. G1767; Sigma). Subsequently, the preparation was treated with 10 U RNase-free DNase (Roche) at 37°C for 30 min in the presence of 1 U μl−1 recombinant RNasin RNase inhibitor (catalog no. N2511; Promega), after which RQ1 DNase stop buffer (catalog no. M6101; Promega) was added to inactivate the DNase. The purity of the RNA preparation was confirmed by one-step reverse transcriptase PCR (RT-PCR) with or without reverse transcriptase, using 16S rRNA-specific primers, and the products were visualized after electrophoresis on a 2% Tris-borate-EDTA (TBE)-agarose gel. In all cases, a PCR product was seen only in the presence of reverse transcriptase. RNA samples from a specific group of four to five mice were pooled, based on the number of CFU recovered and also on the absence of contaminating bacteria, and then purified further using a Qiagen RNeasy minikit (catalog no. 74104). RNA obtained from the lung homogenates was further enriched for prokaryotic RNA using the MICROBEnrich kit (catalog no. AM1901; Ambion). The amount of RNA recovered following purification/enrichment was determined by OD260/280 measurements.
Linear amplification of mRNA.
Previously, in vivo RNA studies for bacteria, such as S. pneumoniae, have been restricted by the amount of bacteria harvested from the animal and therefore the yield of RNA obtained. This problem was circumvented by using an advanced RNA linear amplification kit, the MessageAmp Premier RNA amplification kit (catalog no. 4383452; Ambion, USA), which employs terminal transferase to synthesize a poly(T) tail onto prokaryotic cDNA. Linear amplification is then driven by a T7 phage promoter, which is incorporated at the end of a synthetic poly(A) primer. A second round of amplification was performed for all lung samples in order to obtain sufficient quantities of RNA for analysis by semiquantitative RT-PCR. Analysis of the data obtained indicated a high correlation coefficient, and no significant difference between the amplifications was revealed by Student's t test (data not shown).
cDNA synthesis using RT-PCR.
A sufficient quantity of RNA thus obtained was used to synthesize first-strand cDNA by using the RevertAid H Minus First Strand cDNA synthesis kit (Thermo Scientific) (lot no. 00110899, catalog no. K1632). The 20-μl reaction mixture, according to the manufacturer's instructions, contained 11 μl of RNA templates, 1 μl of primers (equivalent to 15 to 20 pmol), 4 μl 5× reaction buffer, 1 μl of RiboLock RNase inhibitor (20 U/μl), 2 μl of 10 mM deoxynucleoside triphosphate (dNTP) mixture, and 1 μl of reverse transcriptase enzyme (200 U/μl). The nucleotide sequence of the forward primer for the pneumolysin gene (ply) was 5′-TGC AGA GCG TCC TTT GGT CTAT-3′, and the sequence for the reverse primer was 5′-CTC TTA CTC GTG GTT TCC-3′ (36). The nucleotide sequence of the forward primer for the autolysin gene (LytA) was 5′-ACGCAATCTAGCAGATGAAGC-3′, and the sequence for the reverse primer was 5′-TGTTTGGTTGGTTATTCGTGC-3′ (37). The reaction was started by an initial incubation at 42°C for 30 min and denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 15 s, 55°C for 30 s, and 68°C for 1 min and a final extension at 68°C for 10 min (38). The products were stored at −80°C until further use. The PCR products were run on 2% agarose gels containing 0.01% ethidium bromide and viewed in a Bio-Rad Gel Doc Imager. The images were analyzed using the Image Lab software from Bio-Rad Laboratories.
Inflammatory cells.
Leukocyte recruitment to the alveoli was determined in the bronchoalveolar lavage fluid (BALF). Briefly, each animal was sacrificed under ether anesthesia, its trachea was exposed and intubated with a catheter before perfusion, and repeated 1-ml injections of PBS were made until a total of 3 ml of BALF was recovered. The BALF was centrifuged at 3,400 × g for 10 min, and the supernatant was frozen at −80°C until the analysis of inflammatory mediators. The cells in the pellet were resuspended in PBS for the quantification of leukocytes with a hemacytometer, and the cell populations were enumerated using the Diff-Quik stain kit (catalog no. NC9943455; Thermo Fisher Scientific, Inc.) cytospin preparation (39).
Pharmacokinetic and pharmacodynamic studies.
The pharmacokinetic profiles of LVX and CRO were examined in parallel in both infected untreated control and infected antibiotic-treated BALB/c mice.
Bioassay.
The concentrations in the lungs and serum were determined after single intravenous (i.v.) doses of 150 mg/kg of LVX and 50 mg/kg of CRO. In AMRI-SP1-infected mice, antibiotic therapy was initiated at 18 h postinfection (40, 41). At 0 (5 min after drug administration), 2, 4, 8, 10, and 24 h following drug administration, three animals per group were sacrificed under ether anesthesia, and blood was collected by cardiac puncture. The blood samples were centrifuged to isolate serum, which was pooled and frozen at −80°C until the assay. The blood-free lungs were isolated and harvested from exsanguinated mice, washed in sterile sodium chloride solution, and frozen. On the day of assay, the organs were weighed, pooled, and homogenized in 1 ml of potassium phosphate buffer (50 mM [pH 6]) at 4°C. The homogenates were centrifuged at 3,000 × g for 30 min, and the supernatants were used to quantify the LVX and CRO concentrations. The drug concentrations were determined by the agar well diffusion method, using Bacillus subtilis ATCC 6633 as a test strain for LVX (42) and Escherichia coli strain ATCC 25922 for ceftriaxone (43) as the bioassay organism. Standard curves were determined with solutions of LVX and CRO in phosphate buffer for serum and tissue in order to evaluate the active fractions of the drugs. The results were expressed as mg/liter of blood or mg/g of lung tissue. Standard curves were prepared in normal serum and the lung homogenates from the untreated mice. The standard curve was linear from 0.125 to 32 mg/liter. The sensitivity of the assay was about 0.1 mg/liter of sample, and the coefficients of between- and within-day variations (n = 3) were ≤7.5% at 0.5, 1, 7.5, and 20 mg/liter.
Protein binding in serum.
We assumed that unbound or free drug equilibrates with the extravascular space and that the total concentration of antibiotic in any given space is a combination of the free and protein-bound drug has been considered for the binding of protein in serum. Moreover, the actual levels of free drug change very little, with alterations in binding to serum proteins of as much as 80% or 90%. Thus, the total concentration of antibiotic in serum has been estimated for studying the in vivo efficacy of the therapy (44).
Analysis.
Noncompartmental analyses were performed by routine graphical methods for the pharmacokinetic parameters and were estimated by standard methods (45). The elimination rate constant (kel) was first estimated from the slope obtained by least-squares regression analysis for the terminal portion of the concentration-versus-time curve. The elimination half-life (t1/2) was then calculated according to the formula t1/2 = ln2/kel. Cmax (mg/liter), the peak plasma concentration of a drug after the administration of a dose, was extrapolated from the curve. The trapezoidal rule was used to determine the area under the concentration-time curve over 24 h (AUC0–24); this value was divided by the MIC for the study isolate to determine the AUC0–24-to-MIC (AUC/MIC) ratio. The peak concentration-to-MIC (peak/MIC) ratios were also calculated for the isolate. Total serum clearance (CL) was calculated as the dose/AUC. The degree of penetration into the lung tissue was determined by a comparison of the area under the concentration-time curve for the lung tissue with that for the serum. Among the pharmacodynamic (PD) parameters assessed were the AUC/MIC ratio, T>MIC (defined as the time period [in h] during which the serum antibiotic concentration remains above the MIC level), and Cmax/MIC (the ratio of maximum achievable concentration of the drug in serum to the MIC).
Survival rate study.
A determination of the efficacy of combination antibiotic therapy against pneumococcal pneumonia was first established in survival rate studies. Groups of mice (n = 21) were inoculated intranasally with S. pneumoniae strain AMRI-SP1, as described before. Treatments with LVX at 150 mg/kg of body weight and CRO at 50 mg/kg of body weight either alone or in combination and by the intravenous route (through the tail vein) were initiated 18 h postinfection (p.i.). The control mice received sterile saline. The survival rate was recorded every 6 h until day 3 (72 h) postinfection.
MPO activity as a marker of neutrophil infiltration.
Myeloperoxidase (MPO) enzyme activity was analyzed as an index of neutrophil infiltration in the lung tissue, because it is closely related with the number of neutrophils present in the tissue. Blood-free lung homogenates were homogenized and centrifuged at 3,000 × g for 30 min at 4°C. MPO activity was estimated against a standard curve made with commercially available MPO, using methods previously described (20). MPO activities in the noninfected and LVX-treated mice were also estimated from a separate set of experiments.
Estimation of nitric oxide production.
The concentration of nitrite in the lung tissues was measured as an index of NO production. Equal weights of lung samples of control (infected-only) mice and of mice infected and treated with antibiotics either alone or in combination were homogenized in sterile PBS (1 ml). The supernatants were collected and analyzed for NO production by a modified Griess method, as described earlier (46). Briefly, nitrate was converted to nitrites with β-NADP (NADPH; 1.25 mg/ml) and nitrate reductase, followed by the addition of the Griess reagent. The reaction mixture was incubated at room temperature for 20 min, followed by the addition of trichloroacetic acid (TCA). The samples were centrifuged, clear supernatants were collected, and the optical density at 550 nm was recorded. The amounts of NO produced were determined by calibrating a standard curve using sodium nitrite.
Lung vascular permeability.
The Evans blue permeability assay was used to quantify lung capillary permeability. Evans blue avidly binds to serum albumin and can therefore be used as a tracer for transcapillary flux of macromolecules. Evans blue (0.2 ml at a concentration of 25 mg/ml) was injected into the tail vein 30 min prior to the sacrifice of 3 mice from each group. The lungs were homogenized in 2 ml of potassium phosphate buffer. Evans blue was extracted by incubating samples in 4 ml of formamide at 60°C for 24 h, followed by centrifugation at 5,000 × g for 30 min. The concentration of Evans blue was estimated by dual-wavelength (620 and 740 nm) spectrophotometry, which allowed the correction of optical densities (E) for contaminating heme pigments. Thus, we used the formula E620 (corrected) = E620 − (1.426 × E740 + 0.03) (47).
Sample preparation for cytokine measurement from serum.
The blood samples were transferred into microcentrifuge tubes and allowed to clot at 4°C, followed by centrifugation at 3,000 × g for 5 min at 4°C. The supernatant pale yellow serum was pipetted out carefully with the help of micropipettes into fresh microcentrifuge tubes, labeled, and used for cytokine analysis. Sera from different groups were normalized to the protein content by the Bradford method before the assay, and levels of cytokines (interleukin-6 [IL-6], IL-10, TNF-α, and gamma interferon [IFN-γ]) were determined by sandwich enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (RayBiotech), in a Bio-Rad ELISA reader. The serum cytokine levels were expressed in pg/ml of serum analyzed. For each study, the cytokine levels were determined in duplicate in a single run to avoid interassay variability, and intra-assay variability was <10 to 12%. The minimum detectable doses of the cytokines for IL-6, IL-10, TNF-α, and IFN-γ were 2 pg/ml, 45 pg/ml, 60 pg/ml, and 5 pg/ml, respectively.
Cytokine levels in the lung.
For cytokine (IL-6, IL-10, TNF-α, and IFN-γ) measurements, lung homogenates were lysed in lysis buffer (pH 7.4) consisting of 300 mmol NaCl/liter, 15 mmol Tris/liter, 2 mmol MgCl2/liter, 2 mmol Triton X-100/liter, 20 ng pepstatin A/ml, 20 ng leupeptin/ml, and 20 ng aprotinin/ml, and they were centrifuged at 2,900 × g for 15 min at 4°C; the supernatant was frozen at −20°C until cytokine measurement by ELISA, in accordance with the manufacturer's protocol (RayBiotech). Normalization of the lung tissue homogenate before the cytokine assay was performed by determining the protein content of the homogenate using the Bradford method. Next, the samples were loaded in the well of the ELISA plate having equal protein content.
Serum C-reactive protein estimation.
Serum CRP concentrations were estimated using an ELISA kit manufactured by GenWay Biotech, Inc., San Diego, CA, USA, according to the manufacturer's instructions. The serum CRP levels from the noninfected and LVX-treated mice were also estimated from data from a separate set of experiments.
Expression of COX-2 and iNOS in lung tissue.
The expression of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in lung tissues was determined by immunoblotting by methods described elsewhere (48). Briefly, the protein levels in the tissue homogenates were estimated by the Bradford method. Twenty micrograms of each sample was electrophoresed on a polyacrylamide gel and transferred onto a nitrocellulose membrane. After blocking with 7% skim milk, the blots were incubated overnight at 4°C with primary antibodies against Cox-2 (1:1,000; Chemicon, USA) and iNOS (1:1,000; Santa Cruz Biotechnology, USA). After extensive washes with PBS-Tween, the blots were incubated with appropriate secondary antibodies conjugated with peroxidase (Vector Laboratories, USA). The blots were again washed in PBS-Tween and processed for development using a chemiluminescence reagent (Millipore, USA). The images were captured and analyzed using the Chemi Genius bioimaging system (Syngene, Cambridge, United Kingdom). The blots were then stripped (30 min at 50°C in 62.5 mmol/liter Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, and 100 mM β-mercaptoethanol) and reprobed with anti-β-tubulin (Santa Cruz Biotechnology) to determine the equivalent loading of samples.
Statistical methods.
The observers involved in data collection and analysis were not completely blind to the treatment conditions. However, the methodology used for sample identification prevented subjective bias in the experiments. On the other hand, the doses and animals were randomized to the treatment conditions. The data were expressed as the mean ± standard deviation (SD). The survival rate data were analyzed by the unpaired t test. The mean values were compared between groups using a one-way analysis of variance (ANOVA). A P value of <0.05 was considered significant for the two tests.
RESULTS
In vitro data.
The median MIC values for different antibiotics against the test isolates AMRI-SP1 and S. pneumoniae quality control strain ATCC 49619 were determined in sets of three, according to the NCCLS broth microdilution technique. The MIC, MBC, and DAD values of the pneumococcal isolate and the reference strain are listed in Table 1.
TABLE 1.
In vitro susceptibilities of S. pneumoniae isolates ATCC 49619 and AMRI-SP1 to different antimicrobial agents
| Antibiotica | MIC (mg/liter) for: |
MBC (mg/liter) for: |
Zone diam (mm) of: |
|||
|---|---|---|---|---|---|---|
| ATCC 49619 | AMRI-SP1 | ATCC 49619 | AMRI-SP1 | ATCC 49619 | AMRI-SP1 | |
| PEN | 0.06b | >64c | 0.06 | —d | 26 | NZe |
| AMP | 0.25b | >32c | 0.5 | — | 20 | NZ |
| AMC | 0.06b | 2b | 0.12 | 8 | 24 | 22 |
| OXA | 0.06b | 0.25b | 0.25 | 0.5 | 23 | 22 |
| CAZ | 8f | 16c | 16 | >64 | 23 | 15 |
| CRO | 1b | 2c | 4 | 4 | 22 | 16 |
| CTX | 4b | 0.5b | 8 | 1 | 23 | 22 |
| CXM | 0.5b | 2b | 1 | 64 | 25 | 23 |
| FEP | 0.06b | 0.5b | 0.06 | 1 | 25 | 24 |
| IPM | 0.06b | 0.12b | 0.12 | 1 | 23 | 25 |
| MEM | 0.06b | 0.06b | 0.12 | 1 | 24 | 24 |
| AZM | 0.12b | >8c | 2 | 16 | 23 | 10 |
| CLI | 0.5b | 4c | 0.5 | 16 | 17 | 13 |
| LVX | 0.12b | 16c | 0.12 | 32 | 19 | 12 |
| CIP | 0.06b | 1b | 0.12 | 2 | 23 | 25 |
| RIF | 0.06b | 0.12b | 0.12 | 1 | 20 | 21 |
| VAN | 1b | >64c | 4 | — | 19 | NZ |
| GEN | 0.5b | 2 | 1 | 4 | 23 | 20 |
| TMP-SXT | 1b | >64c | 2 | — | 18 | NZ |
PEN, penicillin; AMP, ampicillin; AMC, amoxicillin-potassium clavulanate; OXA, oxacillin; CAZ, ceftazidime; CRO, ceftriaxone; CTX, cefotaxime; CXM, cefuroxime; FEP, cefepime; IPM, imipenem; MEM, meropenem; AZM, azithromycin; CLI, clindamycin; LVX, levofloxacin; CIP, ciprofloxacin; RIF, rifampin; VAN, vancomycin; GEN, gentamicin; TMP-SXT, trimethoprim-sulfamethoxazole.
Susceptible to the indicated antibiotic.
Resistant to the indicated antibiotic.
—, not within the detectable limit.
NZ, no zone of inhibition detected.
Intermediate to the indicated antibiotic.
Test for synergism.
The checkerboard method was performed in duplicate for the multidrug-resistant (MDR) isolate AMRI-SP1 for all possible drug combinations that would be of therapeutic interest. Out of the listed antibiotics tested (listed in Table 1) to determine the MICs, MBCs, and zone of inhibition, a combination study using a checkerboard assay was performed, and the data obtained are represented in Table 2. The combinations that showed synergy (Σ FIC < 0.5) with the pneumococcal isolate are, in descending order, ciprofloxacin and cefepime, gentamicin and ceftriaxone, levofloxacin and ceftriaxone, ampicillin and azithromycin, and ciprofloxacin and ceftriaxone. Antimicrobial combinations, like ampicillin with levofloxacin or gentamicin, levofloxacin plus cefepime, azithromycin plus ciprofloxacin, and amoxicillin-potassium clavulanate plus rifampin showed indifference, with an Σ FIC between 0.5 and 4. Combinations of vancomycin with rifampin or imipenem were found to be antagonistic in their action. Combinations of levofloxacin with cefotaxime or azithromycin or rifampin were ineffective in inhibiting the growth of the tested MDR strain, AMRI-SP1 (Σ FIC > 4).
TABLE 2.
Evaluation of synergy by checkerboard assay
| Antibiotic combination (A-B) | Concn (mg/liter) of antibiotic: |
FICa: |
Interaction result | |||
|---|---|---|---|---|---|---|
| A | B | Antibiotic A | Antibiotic B | Σ FIC | ||
| Ampicillin-azithromycin | 1 | 2 | 0.093 | 0.037 | 0.468 | Synergy |
| Ampicillin-levofloxacin | 1 | 8 | 0.281 | 0.562 | 0.843 | Indifference |
| Ampicillin-gentamicin | 1 | 0.25 | 0.039 | 0.620 | 0.659 | Indifference |
| Levofloxacin-ceftriaxone | 0.5 | 0.25 | 0.046 | 0.375 | 0.421 | Synergy |
| Levofloxacin-cefotaxime | —b | — | Rc | R | R | R |
| Levofloxacin-cefepime | 0.03 | 0.06 | 0.035 | 1.12 | 1.159 | Indifference |
| Levofloxacin-azithromycin | — | — | R | R | R | R |
| Levofloxacin-rifampin | — | — | R | R | R | R |
| Vancomycin-rifampin | 2 | 0.06 | 0.064 | 17.16 | 17.22 | Antagonism |
| Vancomycin-imipenem | 16 | 0.12 | 0.251 | 134.33 | 134.58 | Antagonism |
| Azithromycin-ciprofloxacin | 0.25 | 4 | 0.132 | 1.062 | 1.194 | Indifference |
| Ciprofloxacin-ceftriaxone | 0.06 | 0.25 | 0.312 | 0.156 | 0.468 | Synergy |
| Ciprofloxacin-cefepime | 0.06 | 0.06 | 0.124 | 0.248 | 0.372 | Synergy |
| Amoxicillin and potassium clavulanate-rifampin | 0.06 | 0.03 | 0.032 | 0.531 | 0.562 | Indifference |
| Gentamicin-ceftriaxone | 0.06 | 0.12 | 0.187 | 0.187 | 0.374 | Synergy |
FIC, fractional inhibitory concentration. Σ FIC (FIC A + FIC B) values: <0.5, synergy; 0.5 to 4, indifference; >4, antagonism.
—, no effective inhibition in growth after 24 h of incubation at 37°C compared to OD at 550 nm observed at 0 h.
R, resistant to the combination.
Time-kill studies.
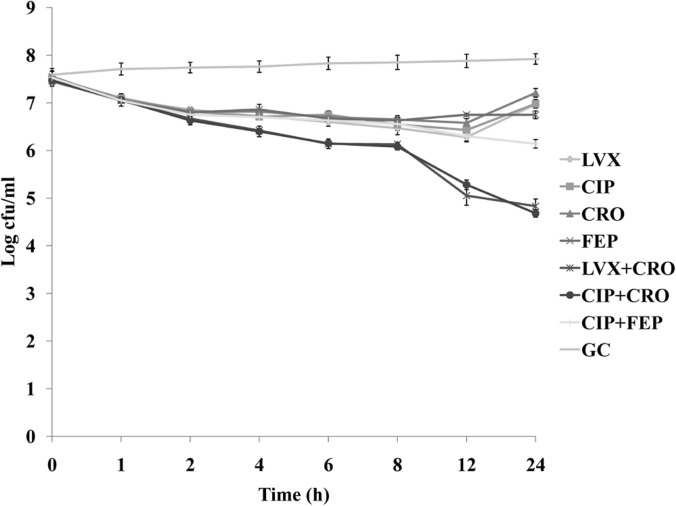
Those combinations which showed synergistic action in the checkerboard assay were used to observe their bactericidal activities using that particular antimicrobial agent(s) either alone or in combination, at a concentration from which their Σ FIC was evaluated. Although the killing activities of all antimicrobials showed remarkable improvement when they were tested in combination, synergy was observed in the cases of levofloxacin plus ceftriaxone and ciprofloxacin plus ceftriaxone. Representative time-kill curves that showed the most effective combinations, as determined from the viable cell count (CFU), are demonstrated graphically for the isolate in Fig. 1. The combination of a fluoroquinolone (LVX or CIP) with a third-generation cephalosporin (CRO) showed synergy, as the combinations resulted in a >2-log10 decrease in viable count at 24 h. The time-kill curve also clearly showed a large decrease in viable titer at 12 h after antibiotic exposure for these two combinations. The combination of CIP with FEP was able to decrease the viable titer by <1 log10 CFU and was thus defined as indifferent in action. No antagonism was observed for the chosen combinations tested in the time-kill assay.
FIG 1.
Time-kill assay. The killing rates of levofloxacin (LVX) at a concentration of 0.5 μg/ml (1/32× MIC), ciprofloxacin (CIP) at a concentration of 0.06 μg/ml (1/16× MIC), ceftriaxone (CRO) at a concentration of 0.25 μg/ml (1/8× MIC), and cefepime (FEP) at a concentration of 0.06 μg/ml (1/8× MIC) either alone or in combination (LVX + CRO, CIP + CRO, or CIP + FEP) for the multidrug-resistant S. pneumoniae strain AMRI-SP1. GC, growth control. The experiments were performed in triplicate, and the results are expressed as mean ± SD. P < 0.05 was considered to be statistically significant.
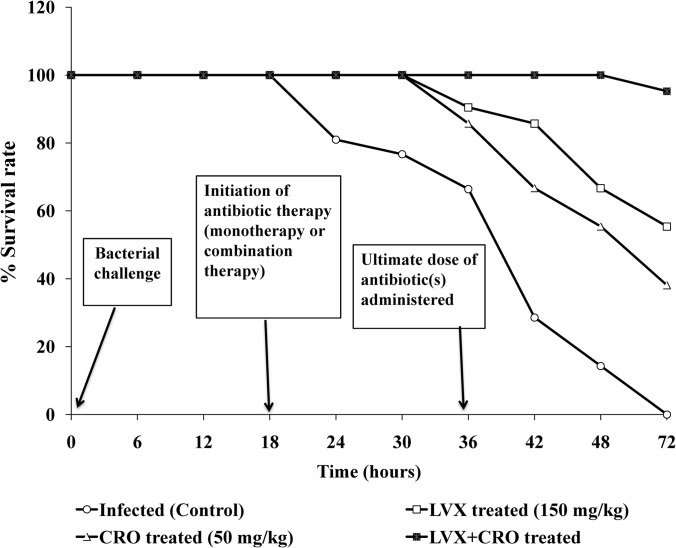
Therapeutic efficacy of the combination therapy in experimental pneumonia.
The inoculation of mice with 106 CFU of S. pneumoniae (AMRI-SP1) resulted in 100% mortality in the untreated animals within 3 days postinfection. (Fig. 2) LVX administered at 150 mg/kg of body weight starting at 18 h postinfection, followed by 3 more similar doses at an interval of 6 h in between successive doses, was associated with ∼55% survival rates, whereas therapy with CRO alone at 50 mg/kg of body weight initiated at the same time, followed by a similar pattern of dosing, resulted in an ∼38% survival rate. Furthermore, treatment with both antibiotics was associated with survival rates of >95% (P < 0.05). Thus, combination therapy with LVX and CRO proved to be effective in increasing the survival rate.
FIG 2.
Cumulative survival of group of mice (n = 21) infected by intranasal challenge with multidrug-resistant S. pneumoniae isolate AMRI-SP1 and treated with antibiotics levofloxacin (LVX) and ceftriaxone (CRO) either alone or in combination, as well as the untreated control. As of 18 h postinfection (p.i.), the mice received 4 i.v. administrations of LVX at 150 mg/kg and/or CRO at 50 mg/kg of body weight with a 6-h interval between successive doses.
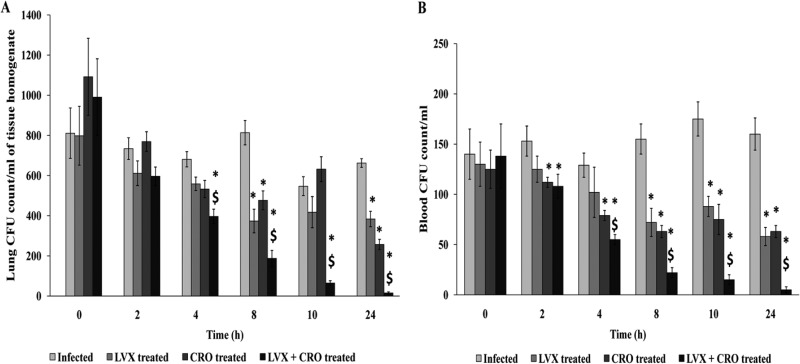
Bacterial clearance from lungs.
The results with the AMRI-SP1 strain are represented in Fig. 3A. Four hours after the first intravenous dose (150 mg/kg LVX or 50 mg/kg CRO), the intrapulmonary decrease in bacterial colony count was significant in the combined antibiotic-treated group compared to monotherapy with LVX or CRO alone and also in the infected control group (P < 0.05). Moreover, subsequent doses at an interval of 6 h showed a prolonged antibacterial effect with combination therapy, whereas in the case of monotherapy, a decrease in CFU count showed no significant difference with respect to the controls (P < 0.05).
FIG 3.
Bacterial burdens in lungs (A) and blood (B) of mice infected with S. pneumoniae and receiving either single or combined antibiotic treatment. Monotherapy or combination therapy was done with 4 simultaneous doses of levofloxacin (LVX) at 150 mg/kg or ceftriaxone (CRO) at 50 mg/kg of body weight, or both, at an interval of 6 h between two successive doses, respectively. The mean bacterial counts (±SD) in the lungs and blood at 18 h postinfection and at several time points after the initiation of antimicrobial therapy are shown. *, significant difference with respect to infected (control) but untreated group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant.
Clearance of bacteremia among survivors.
Combination therapy was able to clear bacteria from the blood significantly compared to in the control (infected but nontreated) and monotherapy groups after 4 h from the initiation of antibiotic treatment (P < 0.05). Multiple doses of the combination eradicated almost all pathogens in circulation more effectively than did monotherapy with either LVX or CRO, and the effect was significant until the end of the experiment (Fig. 3B).
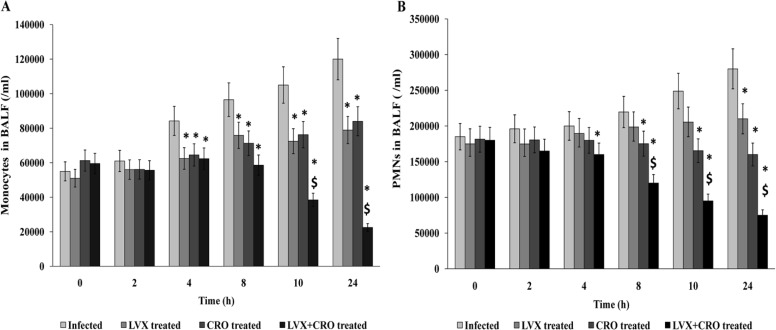
Analysis of bronchoalveolar lavage fluid.
Leukocyte recruitment to the alveoli was determined in the BALF. Antibiotic therapy either alone or in combination exhibited a steady drop in polymorphonuclear leukocyte (PMN) counts in BALF at every time point of the experiment compared to those in the S. pneumoniae-infected untreated control group. Furthermore, combination therapy was more effective than monotherapy at decelerating PMN counts. A significant decrease in PMN recruitment occurred from 4 h after the initiation of therapy, which corresponds to a gradual cure from bacterial invasion. As for the monocyte/macrophage recruitment in the alveoli (BALF), a gradual increase was noted in the untreated infected mice. A significant reduction in those cell counts was observed at 4 h to 24 h after the initiation of treatment compared to either of the antibiotics alone (Fig. 4A and B).
FIG 4.
Analysis of bronchoalveolar lavage fluid (BALF) samples. Mean (±SD) neutrophil (A) and monocyte (B) counts in BALF samples from infected mice treated with levofloxacin (LVX) at 150 mg/kg and/or ceftriaxone (CRO) at 50 mg/kg of body weight. *, significant difference with respect to infected (control) but untreated group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant.
Pharmacokinetic and pharmacodynamic studies of serum and lungs.
The concentration-time curves for LVX and CRO in the serum and lungs were determined (data not shown). Following a single intravenous bolus administration of 150 mg/kg of body weight LVX or 50 mg/kg of body weight CRO, the pharmacokinetic (PK) and pharmacodynamic (PD) values obtained from analysis of the serum and lungs of mice infected with S. pneumoniae AMRI-SP1 are shown in Table 3. A single intravenous administration of high-dose LVX resulted in a peak drug concentration in the lung tissue of 40.9 ± 5.36 mg/liter, which was above the high MIC value for the MDR strain AMRI-SP1, but our regimen failed to achieve a greater Cmax in the serum. This fact might be attributed to the high tissue penetration ability of LVX. CRO concentrations achieved in the lung tissue and serum were above the MIC value for the clinical isolate tested (P < 0.05).
TABLE 3.
Pharmacokinetic and pharmacodynamic parameters for levofloxacin and ceftriaxone following a single intravenous dose 18 h postinfection
| Parametera | Data (mean ± SD) for the indicated antibiotic in the indicated tissue |
|||
|---|---|---|---|---|
| 150 mg/kg levofloxacin |
50 mg/kg ceftriaxone |
|||
| Serum | Lungs | Serum | Lungs | |
| Pharmacokinetics | ||||
| Cmax (mg/liter) | 9.8 ± 2.03 | 40.9 ± 5.36 | 44.5 ± 3.5 | 38.7 ± 7.9 |
| AUC0–24 (mg · h/liter) | 92.64 ± 9.08 | 146.26 ± 23.37 | 97.6 ± 11.23 | 79.5 ± 8.36 |
| t1/2 (h) | 12.37 ± 3.21 | 12.06 ± 2.67 | 1.51 ± 0.67 | 1.12 ± 0.47 |
| CL | 1.62 ± 0.16 | 1.02 ± 0.07 | 0.51 ± 0.05 | 1.29 ± 0.19 |
| Pharmacodynamics | ||||
| AUC/MIC (h) | 5.79 ± 1.42 | 9.14 ± 1.33 | 48.8 ± 5.61 | 39.75 ± 4.18 |
| T>MIC (h) | —b | 16.47 ± 2.48 | 9.19 ± 2.21 | 7.64 ± 1.12 |
| Cmax/MIC | 0.619 ± 0.12 | 2.56 ± 0.29 | 22.25 ± 0.25 | 19.35 ± 3.95 |
Cmax, maximum concentration of drug in serum; t1/2, time taken to reach half of the maximum concentration; AUC, area under the concentration-time curve from time zero to 24 h after antibiotic treatment; T>MIC, time during which the drug concentration remains above the MIC in serum; AUC/MIC, ratio of the area under the curve during 24 h of antibiotic treatment to the MIC for the S. pneumoniae strain AMRI-SP1; Cmax/MIC, ratio of maximum concentration of the drug achieved in serum to the MIC of the drug.
—, the peak drug concentration achieved in serum was below the MIC of levofloxacin (16 mg/liter) for the MDR strain AMRI-SP1 but was quite above the sub-MIC level of 0.5 mg/liter (1/32× MIC) used for the time-kill assay in vitro using the same isolate.
Neutrophil infiltration determined via lung tissue myeloperoxidase enzyme activity.
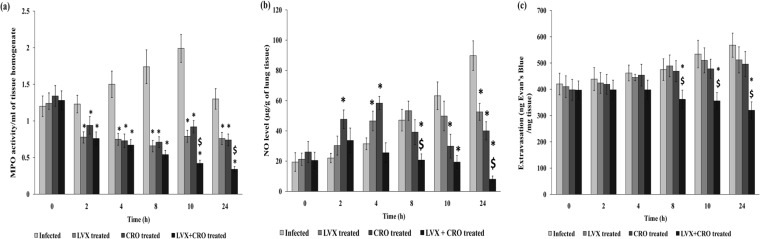
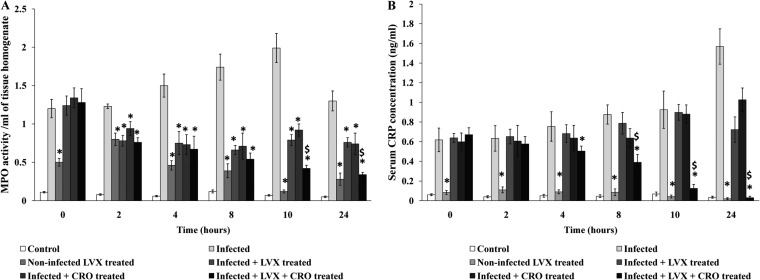
The activity of the MPO enzyme, which is an indicator of neutrophil infiltration, was found to be highest in the lungs of infected animals at 10 h posttherapy (the infected controls received sterile saline). When LVX or CRO was administered alone or in combination, it caused a significant (P < 0.05) time-dependent reduction in tissue MPO enzyme activity compared to that in nontreated AMRI-SP1-infected mice. Combination therapy was able to lower the activity significantly after 8 h after antibiotic administration compared to that achieved by monotherapy (Fig. 5a).
FIG 5.
(a) MPO activity in lung tissue of mice after intranasal administration of S. pneumoniae (AMRI-SP1), followed by treatment with levofloxacin or ceftriaxone alone or in combination. MPO activity was analyzed as the index of neutrophil infiltration in the lung tissue. The rate of change in absorbance was measured spectrophotometrically at 405 nm. MPO activity was defined as the concentration of enzyme degrading 1 μmol peroxide/min at 37°C and is expressed as the change in absorbance/min · mg of tissue protein. The results were reproduced in three repeated experiments. The data are expressed as mean ± SD results of 4 mice per group. A P value of <0.05 was considered significant. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests. (b) NO production. The data are expressed as mean ± SD of the results of 4 mice per group. Values are expressed as μg NO released per g of tissue. A P value of <0.05 was considered statistically significant. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests. (c) Lung vascular permeability measurement. Shown is the pulmonary vascular permeability in S. pneumoniae-infected groups (mean ± SD of results from 4 mice). The results were reproduced in three repeated experiments. The data are expressed as mean ± SD. A P value of <0.05 was considered significant. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests.
NO estimation from lung tissues.
The amount of NO released into the lung tissue was measured and is represented in Fig. 5b. NO production in the lungs of the control (untreated) mice was accelerated with time until the end of the experiment, acting as a biological signal molecule that regulates the release of other proinflammatory mediators. In our study, monotherapy with LVX or CRO failed to downregulate the production of NO until 10 h, but a significant decrease was observed at 24 h after antibiotic therapy compared to the untreated control group (P < 0.05). Combination therapy with these drugs resulted in a significant decrease in the NO level at 8 h postinitiation of antibiotic therapy and was successful in suppressing the release of this molecule until the end at 24 h posttherapy compared to the NO level in the control or monotherapy with either drug (P < 0.05).
Vascular permeability.
The pulmonary vascular permeability (as evaluated by Evans blue extravasations) showed higher values (P < 0.05) in S. pneumoniae-infected untreated mice (control), which was decreased gradually after treatment with LVX in combination with CRO at 8, 10, and 24 h after antibiotic treatment. Monotherapy with LVX or CRO was unable to decrease vascular permeability in the lungs, which represented persistent inflammation at that infected site (Fig. 5c).
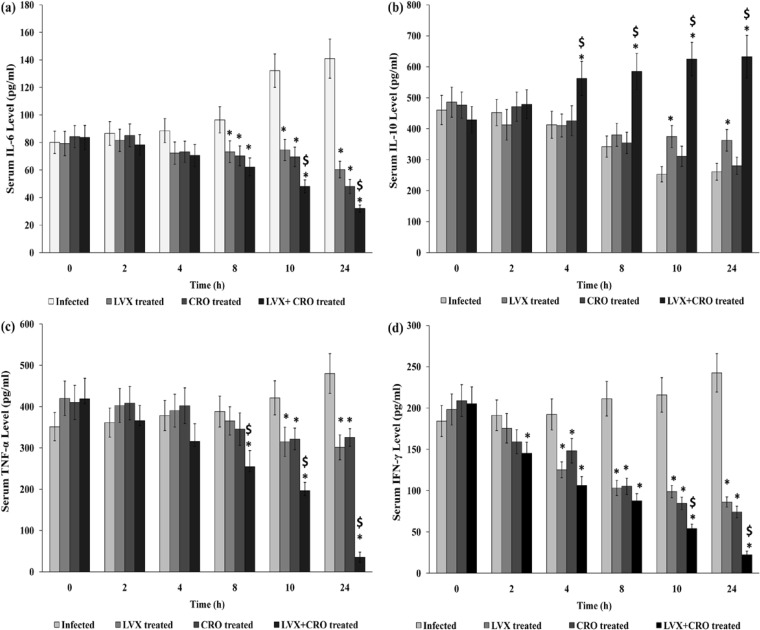
Cytokine levels in serum after treatment with combined antibiotics in AMRI-SP1-induced experimental pneumonia.
The serum IL-6 and IFN-γ levels decreased significantly, whereas the IL-10 level increased significantly 4 h after antibiotic therapy in S. pneumoniae-infected mice compared to the IL-10 level in the untreated group (P < 0.05). The serum TNF-α levels remained steady until 10 h posttherapy and then decreased steeply in the combination treatment group compared to the TNF-α levels in the untreated controls or the monotherapy group. IL-10 levels increased significantly after 4 h of antibiotic therapy and remained substantially high until the end of the experiment at 24 h (P < 0.05) (Fig. 6a to d).
FIG 6.
Serum levels of IL-6 (a), IL-10 (b), TNF-α (c), and IFN-γ (d) in different groups of mice at 0 to 24 h after antibiotic treatment. The levels of IL-6 (a), IL-10 (b), TNF-α (c), and IFN-γ (d) in serum from S. pneumoniae-infected mice untreated or treated with levofloxacin (LVX) at 150 mg/kg and/or ceftriaxone (CRO) at 50 mg/kg 18 h postinfection were determined by utilizing ELISA, according to the manufacturer's instruction, and the values obtained are expressed in pg/ml as mean ± SD (n = 3/group). AMRI-SP1-infected animals that were left untreated were considered a control for comparison with those treated with LVX or CRO or both. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered to be significant for all tests.
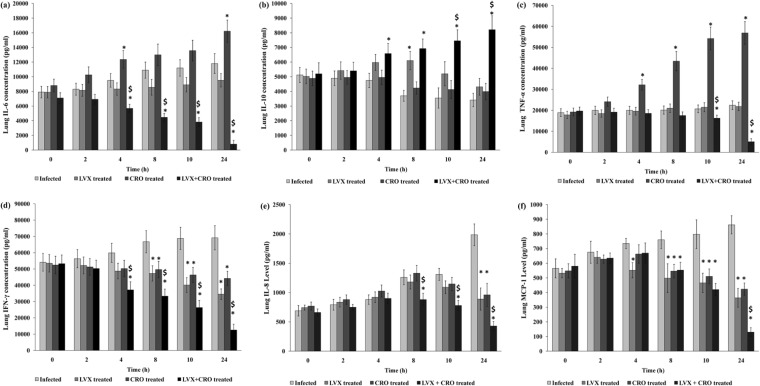
Cytokine (IL-6, IL-10, IFN-γ, and TNF-α) and chemokine (IL-8 and MCP-1) levels in lungs after treatment with combined antibiotics in AMRI-SP1-induced experimental pneumonia.
The release of proinflammatory and anti-inflammatory cytokines and chemokines was measured from 18 h postinitiation of therapy until 24 h after antibiotic treatment to assess the level of inflammation in the lungs of S. pneumoniae-infected mouse groups (Fig. 7a to f). Combination therapy was able to reduce the release of IL-6, TNF-α, IFN-γ, IL-8, and monocyte chemotactic protein 1 (MCP-1) in the lungs ≥4 to 8 h after the initiation of therapy compared to the levels in the untreated controls or single-antibiotic-treated group (P < 0.05). The IL-10 level also significantly increased in the combined-antibiotic-treated group 4 h after the initiation of treatment compared to the IL-10 levels in the untreated and single-antibiotic-treated groups, and the level was maintained at a high level until the end of the observation period (P < 0.05).
FIG 7.
Lung tissue cytokines and chemokines. A group of mice (n = 21) was infected with AMRI-SP1 and was monitored for the development of pneumonia. Eighteen hours postinfection, treatment with either levofloxacin (LVX) at 150 mg/kg or ceftriaxone (CRO) at 50 mg/kg body weight in 4 doses of antibiotics via tail vein at an interval of 6 h between two successive doses was initiated. Considering the 18th h to be 0 h of antibiotic treatment, the animals were sacrificed at 0, 2, 4, 8, 10, and 24 h after antibiotic treatment. After the administration of the antibiotics either alone (monotherapy) or in combination, the lungs were homogenized and assayed for an estimation of cytokine and chemokine levels. The levels of IL-6 (a), IL-10 (b), TNF-α (c), IFN-γ (d), IL-8 (e), and MCP-1 (f) were determined, and the mean ± SD of the values obtained are expressed in pg/ml from triplicate experiments. Infected, S. pneumoniae infected and saline treated; LVX treated, S. pneumoniae infected and treated with levofloxacin; CRO treated, S. pneumoniae infected and treated with ceftriaxone; LVX + CRO treated, S. pneumoniae infected and treated with both antibiotics. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests.
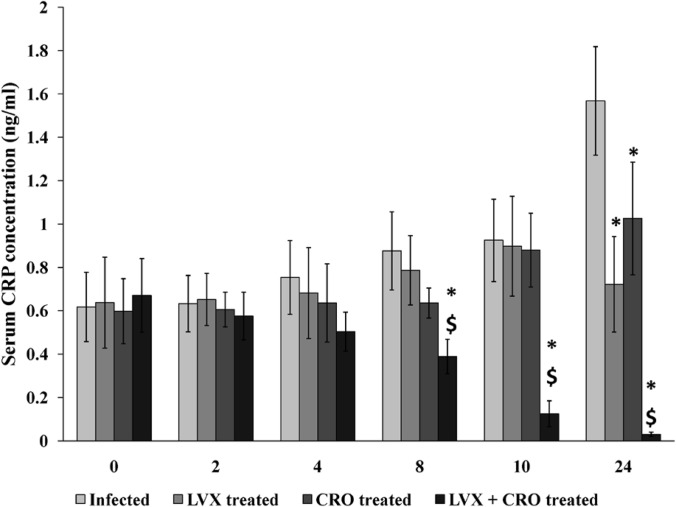
Estimation of serum C-reactive protein level.
The serum CRP level increased in the infected untreated group 18 h postinfection and remained elevated at the end of 24 h of observation. Treatment with LVX or CRO alone was not able to downregulate the CRP release compared to that in the control untreated animals (P < 0.05). However, combination therapy was able to significantly reduce this acute-phase inflammatory protein level 8 h after antibiotic therapy compared to the levels in the untreated control and monotherapy groups (P < 0.05) (Fig. 8).
FIG 8.
Serum C-reactive protein (CRP) concentrations. The serum CRP concentration is expressed as mean ± SD. *, significant difference with respect to infected control group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests.
Expression of pneumolysin (ply) and autolysin (lytA) mRNAs.
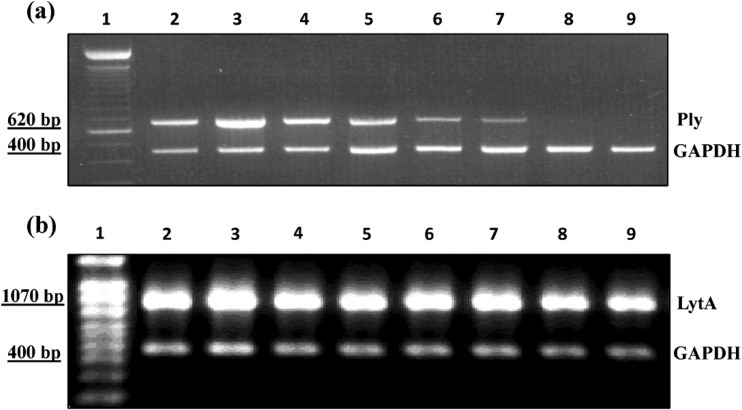
To study the expression of S. pneumoniae genes in vivo, we first sought to assess whether the expression of the ply and lytA genes could be detected and amplified by RT-PCR and conventional PCR, respectively. Total RNA extracted from lung samples of the AMRI-SP1-infected and monotherapy or combination therapy groups were utilized as a template in RT-PCRs that allowed for the detection of ply and lytA messages (Fig. 9a and b). The current study showed a higher level of ply mRNA in the lungs of infected animals 18 h postinfection. Combination therapy with LVX and CRO resulted in a diminished expression of ply and lytA after 8 h of antibiotic treatment. ply expression was not detected after 10 h postinitiation of combination antibiotic therapy.
FIG 9.
Expression of pneumolysin (ply) and autolysin (lytA). Lane 1, the 100-bp ladder; lane 2, effect of AMRI-SP1 infection (107 CFU per mouse) on untreated control group at 18 h postinfection; lanes 3 and 4, effect of monotherapy with either CRO (50 mg/kg) or LVX (150 mg/kg), respectively, at 24 h postinitiation of antibiotic treatment; lanes 5 to 8, effect of combination therapy on the expression of ply gene (a) and lytA gene (b) mRNAs at 2, 4, 8, 10, and 24 h posttherapy. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effect of LVX and CRO treatment on cyclooxygenase-2 and inducible nitric oxide synthase levels in S. pneumoniae-infected mouse lungs.
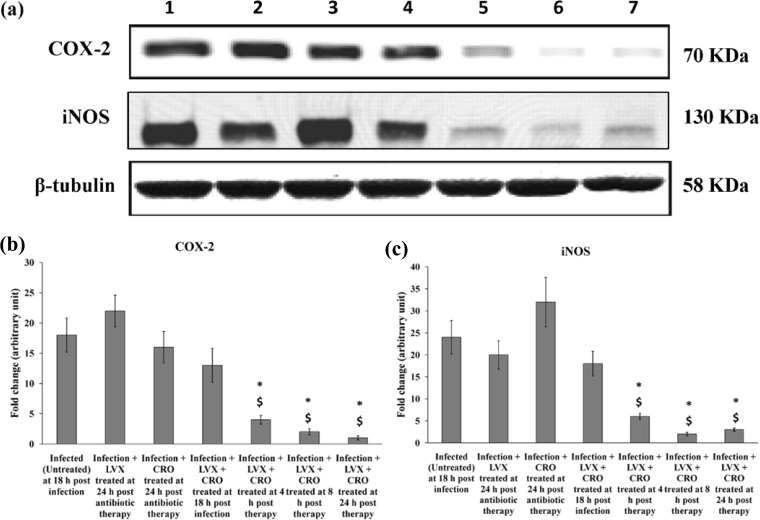
Immunoblot analysis of lung tissue homogenate showed that the COX-2 level was significantly increased at 18 h postinfection in the case of the S. pneumoniae AMRI-SP1-infected mouse group, which gradually decreased after 8 h of antibiotic treatment with the drugs in combination compared to that in the untreated control and single-antibiotic-treated groups (P < 0.05) (Fig. 10).
FIG 10.
Expression of COX-2 and iNOS in the lungs. (a) Representative results of Western blot analysis of the inhibitory effects of levofloxacin (LVX) at 150 mg/kg and ceftriaxone (CRO) at 50 mg/kg four times in 24 h with an interval of 6 h between successive doses on the production of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) by a multidrug-resistant clinical isolate of S. pneumoniae AMRI-SP1 in murine lungs. Lane 1, effect of AMRI-SP1 infection (107 CFU per mice) on untreated control group at 18 h postinfection; lanes 2 and 3, effect of monotherapy with either LVX (150 mg/kg) or CRO (50 mg/kg) at 24 h, respectively, postinitiation of antibiotic treatment; lanes 4 to 7, effect of combination therapy on the expression of COX-2 and iNOS at 0, 4, 8, and 24 h after antibiotic therapy. (b and c) Signal intensity of each band and control expressed in arbitrary units. The data are presented as mean ± SD of the results for 4 mice. *, significant difference with respect to control (untreated) group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests.
Determination of neutrophil infiltration and estimation of serum CRP levels in noninfected and levofloxacin-treated mice.
To delineate that LVX has some secondary anti-inflammatory role, we estimated lung tissue neutrophil infiltration by measuring the MPO activity and serum CRP concentrations, as CRP is an acute-phase inflammatory protein. The results showed decreased MPO activity and serum CRP levels, indicating reduced PMN infiltration into the lungs concomitant with a lower acute-phase response (Fig. 11A and B).
FIG 11.
(A) MPO activity of lung tissue of mice after intranasal administration of S. pneumoniae (AMRI-SP1), followed by treatment with levofloxacin or ceftriaxone alone or in combination. MPO activity was analyzed as the index of neutrophil infiltration in the lung tissue. The rate of change in absorbance was measured spectrophotometrically at 405 nm. MPO activity was defined as the concentration of enzyme degrading 1 μmol peroxide/min at 37°C and is expressed as the change in absorbance/min · mg of tissue protein. The data are expressed as mean ± SD of the results from 4 mice per group. *, significant difference with respect to untreated infected group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests. (B) Serum C-reactive protein (CRP) concentration is expressed as mean ± SD. *, significant difference with respect to untreated infected group; $, significant difference compared to monotherapy with LVX or CRO. A P value of <0.05 was considered significant for all tests.
DISCUSSION
In the era of emerging antibiotic resistance, pneumococci have developed several strategies to survive the pressure of numerous therapeutic modalities. They are now able to resist β-lactams, macrolides, tetracyclines, fluoroquinolones, vancomycins, and trimethoprims by modifying the structure of bacterial cell wall-synthesizing enzymes, by point mutations in genes, or by activating efflux pumps to prevent intracellular accumulation of the drug (49). The emergence of macrolide- and cephalosporin-tolerant strains, leading to treatment failures in cases of bacteremic pneumonia, has jeopardized the efficacy of this antibiotic combination, usually recommended by the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) as empirical treatment for community-acquired and bacteremic pneumonia. These organizations now also recommend a combination of a fluoroquinolone and a β-lactam for the treatment of severe pneumonia (18). Furthermore, the emergence of quinolone resistance during therapy might undermine the use of quinolones as monotherapy for pneumococcal diseases (50). The aim of the present study was to investigate the efficacy of administering a highly bactericidal combination regimen in an experimental model of pneumococcal pneumonia in mice, which was a prerequisite for the treatment of pneumococcal pneumonia induced by a multidrug-resistant clinical isolate of S. pneumoniae.
This study revealed that several antimicrobial combinations act synergistically in vitro against a multidrug-resistant S. pneumoniae isolate. In some in vitro studies, combinations of fluoroquinolones with β-lactams showed synergy against pneumococci, as well as a wider spectrum of activity than those of the standard combinations (i.e., a macrolide plus a β-lactam) (51). More consistent data have been obtained in studies assessing the activities of combinations involving fluoroquinolones and β-lactams, particularly cephalosporins, both in vitro and in vivo (4, 15, 52). In our study, we observed that the combination of ceftriaxone with levofloxacin at sub-MIC levels showed synergistic interaction against the multidrug-resistant strain, which was consistent with previous findings.
Furthermore, to support our observation, we determined time-kill curves, which are recognized means of detecting in vitro synergy or antagonism between antimicrobial combinations (53). Levofloxacin at a concentration of 0.5 mg/liter (1/32× MIC) in combination with ceftriaxone at 0.25 mg/liter (1/8× MIC) was more effective, producing >2-log10 killing of CFU within 12 h postexposure to the antibiotics in vitro. Since the multidrug-resistant isolate showed a high level of penicillin and levofloxacin resistance but synergistic interaction when levofloxacin and ceftriaxone were present together and at sub-MIC levels, we decided to treat infections due to this pathogen with an in vivo dose at which the ratio of levofloxacin would be approximately two to three times greater than that of ceftriaxone to mimic our findings of the in vitro time-killing assays and checkerboard experiments. Moreover, it has been reported that a high-dose short-course levofloxacin regimen maximizes its concentration-dependent antibacterial activity, decreases the potential for drug resistance, and results in better patient compliance (54). Hence, we hypothesized that if the combination of levofloxacin and ceftriaxone was more effective in vitro than when each was present alone (levofloxacin MIC of 16 mg/liter and ceftriaxone MIC of 2 mg/liter for AMRI-SP1), this antimicrobial combination may be useful in the treatment of infection due to this multidrug-resistant isolate. Moreover, how this combination regimen would be beneficial for the host in increasing the survival rate and downregulating inflammation warranted an in vivo experimental pneumonia model with this pathogen.
In our study, the administration of levofloxacin at 150 mg/kg at a 6-h interval for 24 h (12 mg/mouse/day) closely relates to the administration of 500 mg levofloxacin every 6 h (total dose, 2,000 mg/day) in adult individuals with normal renal function, as approximately 80% of this drug is eliminated as unchanged drug in the urine through glomerular filtration and tubular secretion, with minimal metabolism taking place (55). Ceftriaxone at 50 mg/kg at a 6-h interval for 24 h (4 mg/mouse/day) was equivalent to approximately 180 mg ceftriaxone every 6 h for 24 h (total dose, 720 mg/day) in adult individuals. Thus, the unusually high dose of levofloxacin and low dose of ceftriaxone studied in our in vivo experiment are not like as those recommended to date in the treatment of infection due to multidrug-resistant or penicillin-resistant S. pneumoniae, and they need further in vivo experiments and PK/PD simulations before considering the dosages for therapeutic use.
We have studied the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of serum and tissue drug concentrations over time and in the area under the concentration curve and integrated these with the MIC for the microorganism. This might predict the likelihood of clinical success and pathogen eradication and may help in the proper evaluation of the outcome of a therapy (56). It has been reported that the PK of levofloxacin during a multiple-dose regimen and with a single dose are similar, and the Cmax and AUC increase linearly in a dose-dependent fashion (55). Since levofloxacin exhibits concentration-dependent killing and prolonged persistent effects, our aim was to maximize the concentration of the drug at the site of infection. We achieved high peak level/MIC and AUC/MIC ratios for levofloxacin in lung tissue, which were required for extensive and faster killing (57). On the other hand, ceftriaxone exhibits time-dependent killing, and the only way to achieve greater killing is to maintain adequate drug levels at the site of infection for a longer period of time, as these drugs have minimally to moderately persistent effects. We achieved a T>MIC of ceftriaxone of approximately 8 h with a single dose, and hence, multiple doses of ceftriaxone were required to maintain the drug concentration above the MIC level for the strain during the 24-h period after antibiotic therapy.
The PK and PD parameters studied using a single intravenous dose of both drugs suggested that the drugs easily penetrate lung tissue, thereby mediating its effect, which resulted in synergistic interactions in combination therapy. Since the drugs are from different classes with different modes of action, we have not compared their PK and PD parameters, but our study showed a considerable amount of drug penetration at the site of infection from the bloodstream, with a longer half-life, and the concentrations of both levofloxacin and ceftriaxone were well above the MIC level in the lung tissue. Higher doses of levofloxacin resulted in a slightly longer half-life, which may be due to a larger volume of distribution of the drug.
Fluoroquinolones have been proposed to possess secondary anti-inflammatory properties, targeting the production of proinflammatory cytokines both in vitro and in vivo (58). Neutrophil infiltration into the inflammatory site is the hallmark of acute inflammation. Locally produced chemotactic factors are presumed to mediate the sequence of events leading to infiltration at the inflammation site. Our findings are also consistent with the existing literature because we have observed that monotherapy with either of the antibiotics was unable to downregulate the production and release of these immune mediators at the inflammation site (lungs) and blood (cytokines measured in serum). In contrast, combination therapy successfully downregulated the levels of IL-6, TNF-α, IFN-γ, IL-8, and MCP-1 in the lung tissue and increased the release of the anti-inflammatory cytokine IL-10. Combination therapy also reduced the infiltration of inflammatory cells in the lungs more than monotherapy did.
It has been reported that the influx of neutrophils and increase in vascular permeability during acute inflammation caused by stimulation with pneumococci or pneumococcal cell wall leads to the formation of edema, resulting in impaired air exchange from the lungs (59). In our study, we observed that combination therapy was effective in reducing the pulmonary vascular leakage and edema compared to these effects in the groups treated with levofloxacin or ceftriaxone alone.
C-reactive protein (CRP) is the prototypic acute-phase protein considered to be a multifunctional component of the acute-phase response and innate host defense machinery (60, 61). CRP displays calcium-dependent binding specificity for phosphocholine (PCh) residues present on C-polysaccharide (PnC) of the cell wall of S. pneumoniae (62). Once CRP is complexed with a ligand, such as PnC, it activates mouse complement, although not through the classical pathway (63). Complement activation protects mice from S. pneumoniae infection. The protection involves a combined but independent effect of complement and CRP. Thus, we can conclude that reduced bacterial loads in the lungs and blood during combination therapy led to the downregulation of the serum CRP level.
The present study has shown the effects of combination therapy with levofloxacin and ceftriaxone on the expression of pneumolysin (ply) and autolysin (lytA) in vivo. Several studies have confirmed that pneumolysin and autolysin contribute to pneumococcal virulence and are key proteins for pathogenesis in humans and animals (64, 65). Pneumolysin is a multifunctional pneumococcal virulence factor that appears to augment intrapulmonary growth and dissemination during the early pathogenesis of S. pneumoniae infection. Inactivation of the ply gene of pneumococci results in an avirulent mutant (65). Murine monoclonal antibodies to pneumolysin might prolong the survival of mice with pneumococcal pneumonia (66). The inhibition of pneumolysin in the mouse lung can therefore lead to improvements in the clinical outcomes of pneumococcal pneumonia patients. Pneumolysin is not actively secreted but is stored inside the bacterial cell. It is released during bacterial lysis, from antibiotic treatment, or by the action of the pneumococcal virulence factor lytA, which in turn can be triggered by the immune system, antibiotic treatment (67), or other bacterial virulence factors (68). Moreover, pneumolysin has many immunomodulatory effects, like inhibiting migration, respiratory burst, degranulation, and other bactericidal effects in PMNs and monocytes (69). Autolysin degrades the pneumococcal cell wall, resulting in lysis and the release of intracellular and cell wall molecules, and it is known to be crucial in pneumococcal virulence during pneumonia infection (70). The present study shows that combination therapy was effective in downregulating the expression of ply and lytA genes compared to monotherapy with levofloxacin or ceftriaxone alone. The expression levels of these virulence genes were exaggerated in the AMRI-SP1-infected group left untreated until the end of the observation period. However, the treatment of S. pneumoniae-infected mice with CRO led to a slightly increased production of pneumolysin compared to that in the infected-only group. Antibiotic-mediated anti-inflammatory activity is achieved directly via antimicrobial activity and, in a few cases, by the secondary anti-inflammatory properties of levofloxacin, which target immune and inflammatory cells. With respect to immune cells, levofloxacin (LVX) due to its secondary anti-inflammatory activities may prevent the release of proinflammatory protein toxins from Gram-positive bacteria and the production of other virulence factors. Second, levofloxacin may act directly on the cells of the host defense system to inhibit the excessive production of several potent mediators of inflammation. Since the bacterial strain AMRI-SP1 was found to be resistant to both ceftriaxone and levofloxacin, treatment with these two antibiotics separately was not successful in eradicating bacteria from the blood and lungs. Moreover, in the presence of these antibiotics, i.e., the groups treated with LVX or CRO alone, the release of pneumolysin, a proinflammatory intracellular toxin, was increased by the disintegrating bacterial cells compared to the release observed in the absence of antibiotic treatment, i.e., the group infected by S. pneumoniae only. Thus, the reduced expression of these genes was responsible for downregulation of the proinflammatory cytokine release, respiratory burst, degranulation, and other bactericidal effects in PMNs and monocytes.
In our recent previous study, we reported that 18 h postinfection, the initiation of combination antibiotic therapy with β-lactam and macrolide-like agents was able to downregulate infection and inflammation in an experimental pneumococcal pneumonia model with the isolate AMRI-SP1 (34). In this study, we have used ceftriaxone, which participates in the lysis of the bacterial cell wall and release of those cell wall products to increase inflammation at the site of infection. Second, the levofloxacin used in this study has some secondary anti-inflammatory properties, though the macrolides and macrolide-like agents possess greater anti-inflammatory properties than those of the fluoroquinolones. Thus, the way in which combination therapy downregulated inflammation in the lungs and proved to be bactericidal, thereby increasing the survival rate by >90%, still remains to be studied in detail, with more experiments needed on animals using this model. To delineate the secondary anti-inflammatory role of LVX, we have estimated lung tissue neutrophil infiltration by measuring MPO activity and serum CRP concentration, an acute-phase inflammatory protein from the noninfected and LVX-treated mice. Animals treated with LVX showed decreased MPO activity and serum CRP levels, indicating reduced PMN infiltration into the lungs concomitant with a lower acute-phase response.
To study these above-mentioned mechanisms, we estimated the presence of the proteins COX-2 and inducible isoform of nitric oxide synthase (iNOS) at the site of infection and site of inflammation (i.e., the lungs). It has been reported that the expression of COX-2 mRNA was significantly suppressed by aminoguanidine, a selective iNOS inhibitor, in infected rats with group B streptococcus-induced inflammation of lung tissue (71). From our study, we observed that infection with multidrug-resistant S. pneumoniae AMRI-SP1 exaggerated the expression of iNOS and COX-2 at 18 h postinfection in the lungs. Combination therapy with levofloxacin and ceftriaxone downregulated the expression of these proteins in the lungs, while monotherapy with these antibiotics failed to do so. The expression of COX-2 is also partly regulated by the nitric oxide (NO) pathway, because it has been reported that infection with S. pneumoniae exacerbates the inflammatory response, leading to the production of several inflammatory mediators, like TNF, interleukins, prostaglandins (PG), and NO in the lungs of rats (72).
Decreased NO production in the lungs of mice adds evidence to the fact that the combination therapy was efficacious for the treatment of pneumococcal infection due to a multidrug-resistant strain. Thus, elucidating these complex interactions may allow for the development of more rational pharmacological approaches to the treatment and prevention of the disease in which they play a role.
Since it is usually recommended that combination therapy be continued for ≥3 days, though it has been reported that the potential benefit of combination therapy was present for both 1 and 3 days (7), changing the time of infection or duration of drug administration with considerations of the PK/PD parameters still remains to be elucidated before considering this regimen for therapeutic use.
ACKNOWLEDGMENTS
Biswadev Bishayi thanks Sunil Kumar Manna, scientist and head of the Immunology Division, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India, for providing us with the primers for pneumolysin and autolysin.
We thank the University Grants Commission, Government of India, New Delhi, India, for providing a fellowship to Arnab Majhi (sanction UGC/561/Jr. Fellow. SC. dated 22 July 2010) and also the Department of Science and Technology, Government of India, for additional support under the DST-PURSE program to the Department of Physiology, University of Kolkata. However, the funding agency had no involvement with the design, execution, or data analysis of this study, manuscript preparation, or the decision to submit the paper for publication.
We declare no conflicts of interest for this article in regard to submission to this journal. We also declare no direct financial relationship with the commercial identities mentioned in this article that might lead to a conflict of interest.
Footnotes
Published ahead of print 23 June 2014
REFERENCES
- 1.Aspa J, Rajas O, de Castro FR. 2008. Pneumococcal antimicrobial resistance: therapeutic strategy and management in community-acquired pneumonia. Expert Opin. Pharmacother. 9:229–241. 10.1517/14656566.9.2.229 [DOI] [PubMed] [Google Scholar]
- 2.Neill DR, Fernandes VE, Wisby L, Haynes AR, Ferreira DM, Laher A, Strickland N, Gordon SB, Denny P, Kadioglu A, Andrew PW. 2012. T regulatory cells control susceptibility to invasive pneumococcal pneumonia in mice. PLoS Pathog. 8:1–12. 10.1371/journal.ppat.1002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meijvis SC, Grutters JC, Thijsen SF, Rijkers GT, Biesma DH, Endeman H. 2011. Therapy in pneumonia: what is beyond antibiotics? Neth. J. Med. 69:21–26 [PubMed] [Google Scholar]
- 4.Waterer GW, Somes GW, Wunderink RG. 2001. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch. Intern. Med. 161:1837–1842. 10.1001/archinte.161.15.1837 [DOI] [PubMed] [Google Scholar]
- 5.Weiss K, Low DE, Cortes L, Beaupre A, Gauthier R, Gregoire P, Legare M, Nepveu F, Thibert D, Tremblay C, Tremblay J. 2004. Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults. Can. Respir. J. 11:589–593 [DOI] [PubMed] [Google Scholar]
- 6.Waterer GW. 2005. Optimal antibiotic treatment in severe pneumococcal pneumonia–time for real answers. Eur. J. Clin. Microbiol. Infect. Dis. 24:691–692. 10.1007/s10096-005-0019-5 [DOI] [PubMed] [Google Scholar]
- 7.Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, Chedid MB, Hui DS, Andremont A, Chiou CC, International Pneumococcal Study Group 2004. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am. J. Respir. Crit. Care Med. 170:440–444. 10.1164/rccm.200311-1578OC [DOI] [PubMed] [Google Scholar]
- 8.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C, Infectious Diseases Society of America 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405–1433. 10.1086/380488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterer GW, Rello J. 2006. Choosing the right combination therapy in severe community-acquired pneumonia. Crit. Care 10:115. 10.1186/cc3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. 2007. Comparison of β-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob. Agents Chemother. 51:3977–3982. 10.1128/AAC.00006-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touyama M, Higa F, Nakasone C, Shinzato T, Akamine M, Haranaga S, Tateyama M, Nakasone I, Yamane N, Fujita J. 2006. In vitro activity of sitafloxacin against clinical strains of Streptococcus pneumoniae with defined amino acids substitutions in QRDRs of gyrase A and topoisomerase IV. J. Antimicrob. Chemother. 58:1279–1282. 10.1093/jac/dkl427 [DOI] [PubMed] [Google Scholar]
- 12.Feldman C. 2004. Appropriate management of lower respiratory tract infections in primary care. Prim. Care Respir. J. 13:159–166. 10.1016/j.pcrj.2004.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrose PG, Bast D, Doern GV, Iannini PB, Jones RN, Klugman KP, Low DE. 2004. Fluoroquinolone-resistant Streptococcus pneumoniae, an emerging but unrecognized public health concern: is it time to resight the goalposts? Clin. Infect. Dis. 39:1554–1556. 10.1086/425508 [DOI] [PubMed] [Google Scholar]
- 14.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bédos JP. 1996. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flatz L, Cottagnoud M, Kühn F, Entenza J, Stucki A, Cottagnoud P. 2004. Ceftriaxone acts synergistically with levofloxacin in experimental meningitis and reduces levofloxacin-induced resistance in penicillin-resistant pneumococci. J. Antimicrob. Chemother. 53:305–310. 10.1093/jac/dkh082 [DOI] [PubMed] [Google Scholar]
- 16.Drago L, Nicola L, Rodighiero V, Larosa M, Mattina R, De Vecchi E. 2011. Comparative evaluation of synergy of combinations of β-lactams with fluoroquinolones or a macrolide in Streptococcus pneumoniae. J. Antimicrob. Chemother. 66:845–849. 10.1093/jac/dkr016 [DOI] [PubMed] [Google Scholar]
- 17.Gross ME, Giron KP, Septimus JD, Mason EO, Jr, Musher DM. 1995. Antimicrobial activities of β-lactam antibiotics and gentamicin against penicillin-susceptible and penicillin-resistant pneumococci. Antimicrob. Agents Chemother. 39:1166–1168. 10.1128/AAC.39.5.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society 2007. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, Dean N, File T, Fine MJ, Gross PA, Martinez F, Marrie TJ, Plouffe JF, Ramirez J, Sarosi GA, Torres A, Wilson R, Yu VL, American Thoracic Society 2001. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Res. Crit. Care Med. 163:1730–1754. 10.1164/ajrccm.163.7.at1010 [DOI] [PubMed] [Google Scholar]
- 20.Bergeron Y, Ouellet N, Deslauriers AM, Simard M, Olivier M, Bergeron MG. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moncada S, Higgs EA. 1995. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 9:1319–1330 [PubMed] [Google Scholar]
- 22.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. 1994. Inducible isoforms of cyclooxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. U. S. A. 91:2046–2050. 10.1073/pnas.91.6.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvemini D, Seibert K, Marino MH. 1996. New concepts in inflammation and therapy. Drug News Perspect. 9:204–219 [Google Scholar]
- 24.Andrew PW, Boulnois GJ, Mitchell TJ, Lee CJ, Paton JC, Poolman JT, Peeters CCAM. 1994. Pneumococcal vaccines. Zentralbl. Bakteriol. Suppl. 24:453–466 [Google Scholar]
- 25.Marriott HM, Mitchell TJ, Dockrell DH. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8:497–509. 10.2174/156652408785747924 [DOI] [PubMed] [Google Scholar]
- 26.Nombela C. 1984. Microbial cell wall synthesis and autolysis. Elsevier Press, Amsterdam, The Netherlands [Google Scholar]
- 27.Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89–115. 10.1146/annurev.mi.47.100193.000513 [DOI] [PubMed] [Google Scholar]
- 28.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry AM, Lock RA, Hansman D, Paton JC. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 57:2324–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard—6th ed. NCCLS document M7-A6. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 31.Jain SN, Vishwanatha T, Reena V, Divyashree BC, Aishwarya S, Siddhalingeshwara KG, Venugopal N, Ramesh I. 2011. Antibiotic synergy test: checkerboard method on multidrug resistant Pseudomonas aeruginosa. Int. Res. J. Pharm. 2:196–198 [Google Scholar]
- 32.Odds FC. 2003. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemother. 52:1. 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 33.Eliopoulos GM, Moellering R. 1996. Antimicrobial combinations, p 330–336 In Lorian V. (ed), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD [Google Scholar]
- 34.Majhi A, Kundu K, Adhikary R, Banerjee M, Mahanti S, Basu A, Bishayi B. 2014. Combination therapy with ampicillin and azithromycin in an experimental pneumococcal pneumonia is bactericidal and effective in down regulating inflammation in mice. J. Inflamm. 11:5. 10.1186/1476-9255-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogunniyi AD, Giammarinaro P, Paton JC. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045–2053 [DOI] [PubMed] [Google Scholar]
- 36.Gisselsson-Solén M, Bylander A, Wilhelmsson C, Hermansson A, Melhus A. 2007. The Binax NOW test as a tool for diagnosis of severe acute otitis media and associated complications. J. Clin. Microbiol. 45:3003–3007. 10.1128/JCM.00299-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAvin JC, Reilly PA, Roudabush RM, Barnes WJ, Salmen A, Jackson GW, Beninga KK, Astorga A, McCleskey FK, Huff WB, Niemeyer D, Lohman KL. 2001. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. J. Clin. Microbiol. 39:3446–3451. 10.1128/JCM.39.10.3446-3451.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai F, Talekar SJ, Klugman KP, Vidal JE, Investigators Group 2013. Expression of Streptococcus pneumoniae virulence-related genes in the nasopharynx of healthy children. PLoS One 8:e67147. 10.1371/journal.pone.0067147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duong M, Simard M, Bergeron Y, Bergeron MG. 2001. Kinetic study of the inflammatory response in Streptococcus pneumoniae experimental pneumonia treated with the ketolide HMR 3004. Antimicrob. Agents Chemother. 45:252–262. 10.1128/AAC.45.1.252-262.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azoulay-Dupuis E, Bédos JP, Mohler J, Peytavin G, Isturiz R, Moine P, Rieux V, Cherbuliez C, Péchère JC, Fantin B, Köhler T. 2004. Activities of garenoxacin against quinolone-resistant Streptococcus pneumoniae strains in vitro and in a mouse pneumonia model. Antimicrob. Agents Chemother. 48:765–773. 10.1128/AAC.48.3.765-773.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang E, Bergeron Y, Bergeron MG. 2005. Ceftriaxone pharmacokinetics in interleukin-10 treated murine pneumococcal pneumonia. J. Antimicrob. Chemother. 55:721–726. 10.1093/jac/dki085 [DOI] [PubMed] [Google Scholar]
- 42.Simon HJ, Yin EJ. 1970. Microbioassay antimicrobial agents. Appl. Microbiol. 19:573–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Täuber MG, Hackbarth CJ, Scott KG, Rusnak MG, Sande MA. 1985. New cephalosporins cefotaxime, cefpimizole, BMY 28142, and HR 810 in experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 27:340–342. 10.1128/AAC.27.3.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson LR, Gerding DN. 1980. Influence of protein binding of antibiotics on serum pharmacokinetics and extravascular penetration: clinically useful concepts. Rev. Infect. Dis. 2:340–348. 10.1093/clinids/2.3.340 [DOI] [PubMed] [Google Scholar]
- 45.Greenblatt DJ, Koch-Weser J. 1975. Clinical pharmacokinetics. N. Engl. J. Med. 297:702–705 [DOI] [PubMed] [Google Scholar]
- 46.Nandi D, Mishra MK, Basu A, Bishayi B. 2010. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology 215:443–451. 10.1016/j.imbio.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 47.Green TP, Johnson DE, Marchessault RP, Gatto CW. 1988. 1988. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J. Lab. Clin. Med. 111:73–83 [PubMed] [Google Scholar]
- 48.Mahanti S, Majhi A, Chongder S, Dutta K, Basu A, Bishayi B. 2013. Increased resistance of immobilized-stressed mice to infection: correlation with behavioral alterations. Brain Behav. Immun. 28:115–127. 10.1016/j.bbi.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 49.van der Poll T, Opal SM. 2009. Pathogenesis, treatment and prevention of pneumococcal pneumonia. Lancet 374:1543–1556. 10.1016/S0140-6736(09)61114-4 [DOI] [PubMed] [Google Scholar]
- 50.Davidson R, Cavalcanti R, Brunton JL, Bast DJ, de Azavedo JC, Kibsey P, Fleming C, Low DE. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747–750. 10.1056/NEJMoa012122 [DOI] [PubMed] [Google Scholar]
- 51.Blasi F, Bulfoni A, Concia E, Costantino S, Giusti M, Iori I, Mazzei T, Schito GC. 2010. Attualità nella gestione delle infezioni delle basse vie respiratorie in medicina interna. Ital. J. Med. 4:1–78 (In Italian.) [Google Scholar]
- 52.Cottagnoud P, Acosta F, Cottagnoud M, Neftel K, Täuber MG. 2000. Synergy between trovafloxacin and ceftriaxone against penicillin-resistant pneumococci in the rabbit meningitis model and in vitro. Antimicrob. Agents Chemother. 44:2179–2181. 10.1128/AAC.44.8.2179-2181.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacGowan AP, Wootton M, Hedges AJ, Bowker KE, Holt HA, Reeves DS. 1996. A new time-kill method of assessing the relative efficacy of antimicrobial agents alone and in combination developed using a representative β-lactam, aminoglycoside and fluoroquinolone. J. Antimicrob. Chemother. 38:193–203. 10.1093/jac/38.2.193 [DOI] [PubMed] [Google Scholar]
- 54.Noreddin AM, Elkhatib WF, Cunnion KM, Zhanel GG. 2011. Cumulative clinical experience from over a decade of use of levofloxacin in community-acquired pneumonia: critical appraisal and role in therapy. Drug Healthc. Patient Saf. 3:59–68. 10.2147/DHPS.S15599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fish DN, Chow AT. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101–119. 10.2165/00003088-199732020-00002 [DOI] [PubMed] [Google Scholar]
- 56.Smith HJ, Nichol KA, Hoban DJ, Zhanel GG. 2003. Stretching the mutant prevention concentration (MPC) beyond its limits. J. Antimicrob. Chemother. 51:1323–1325. 10.1093/jac/dkg255 [DOI] [PubMed] [Google Scholar]
- 57.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 58.Anderson R, Tintinger G, Cockeran R, Potjo M, Feldman C. 2010. Beneficial and harmful interactions of antibiotics with microbial pathogens and the host innate immune system. Pharmaceuticals 3:1694–1710. 10.3390/ph3051694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Flier M, Coenjaerts F, Kimpen JLL, Hoepelman AM, Geelen SP. 2000. Streptococcus pneumoniae induces secretion of vascular endothelial growth factor by human neutrophils. Infect. Immun. 68:4792–4794. 10.1128/IAI.68.8.4792-4794.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volanakis JE. 2001. Human C-reactive protein: expression, structure, and function. Mol. Immunol. 38:189–197. 10.1016/S0161-5890(01)00042-6 [DOI] [PubMed] [Google Scholar]
- 61.Agrawal A. 2005. CRP after 2004. Mol. Immunol. 42:927–930. 10.1016/j.molimm.2004.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volanakis JE, Kaplan MH. 1971. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc. Soc. Exp. Biol. Med. 136:612–614. 10.3181/00379727-136-35323 [DOI] [PubMed] [Google Scholar]
- 63.Suresh MV, Singh SK, Ferguson DA, Jr, Agrawal A. 2006. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from Streptococcus pneumoniae infection. J. Immunol. 176:4369–4374. 10.4049/jimmunol.176.7.4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119–123. 10.1093/infdis/172.1.119 [DOI] [PubMed] [Google Scholar]
- 65.Berry AM, Alexander JE, Mitchell TJ, Andrew PW, Hansman D, Paton JC. 1995. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect. Immun. 63:1969–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.del Mar Garcia-Suarez M, Cima-Cabal MD, Flórez N, García P, Cernuda-Cernuda R, Astudillo A, Vázquez F, De los Toyos JR, Méndez FJ. 2004. Protection against pneumococcal pneumonia in mice by monoclonal antibodies to pneumolysin. Infect. Immun. 72:4534–4540. 10.1128/IAI.72.8.4534-4540.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson R, Steel HC, Cockeran R, von Gottberg A, de Gouveia L, Klugman KP, Mitchell TJ, Feldman C. 2007. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J. Antimicrob. Chemother. 60:1155–1158. 10.1093/jac/dkm338 [DOI] [PubMed] [Google Scholar]
- 68.Berry AM, Paton JC, Hansman D. 1992. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb. Pathog. 12:87–93. 10.1016/0882-4010(92)90111-Z [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Puelles JM, Ronda C, Garcia JL, Garcia P, Lopez R, Garcia E. 1986. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur. J. Biochem. 158:289–293 [DOI] [PubMed] [Google Scholar]
- 70.López R, García E, García P, García JL. 1997. The pneumococcal cell wall degrading enzymes: a modular design to create new lysins? Microb. Drug Resist. 3:199–211. 10.1089/mdr.1997.3.199 [DOI] [PubMed] [Google Scholar]
- 71.Natarajan G, Glibetic M, Raykova V, Ofenstein JP, Thomas RL, Aranda JV. 2007. Nitric oxide and prostaglandin response to group B streptococcal infection in the lung. Ann. Clin. Lab. Sci. 37:170–176 [PubMed] [Google Scholar]
- 72.Raykova VD, Glibetic M, Ofenstein JP, Aranda JV. 2003. Nitric oxide-dependent regulation of pro-inflammatory cytokines in group B streptococcal inflammation of rat lung. Ann. Clin. Lab. Sci. 33:62–67 [PubMed] [Google Scholar]