Abstract
SUMMARY
Neisseria gonorrhoeae is evolving into a superbug with resistance to previously and currently recommended antimicrobials for treatment of gonorrhea, which is a major public health concern globally. Given the global nature of gonorrhea, the high rate of usage of antimicrobials, suboptimal control and monitoring of antimicrobial resistance (AMR) and treatment failures, slow update of treatment guidelines in most geographical settings, and the extraordinary capacity of the gonococci to develop and retain AMR, it is likely that the global problem of gonococcal AMR will worsen in the foreseeable future and that the severe complications of gonorrhea will emerge as a silent epidemic. By understanding the evolution, emergence, and spread of AMR in N. gonorrhoeae, including its molecular and phenotypic mechanisms, resistance to antimicrobials used clinically can be anticipated, future methods for genetic testing for AMR might permit region-specific and tailor-made antimicrobial therapy, and the design of novel antimicrobials to circumvent the resistance problems can be undertaken more rationally. This review focuses on the history and evolution of gonorrhea treatment regimens and emerging resistance to them, on genetic and phenotypic determinants of gonococcal resistance to previously and currently recommended antimicrobials, including biological costs or benefits; and on crucial actions and future advances necessary to detect and treat resistant gonococcal strains and, ultimately, retain gonorrhea as a treatable infection.
INTRODUCTION
The sexually transmitted infection (STI) gonorrhea remains a significant global public health concern. This requires immediate international attention and resources because the global burden of infection is increasing (1), and Neisseria gonorrhoeae (gonococcus), the etiological agent of gonorrhea, is evolving into a superbug and may become untreatable due to its resistance to all classes of antimicrobials available for treating infections. Gonorrhea has been treated successfully by use of antimicrobials for the past 70 to 80 years. However, internationally, there is now a high prevalence of N. gonorrhoeae strains with resistance to most antimicrobials previously and currently widely available for treatment (e.g., sulfonamides, penicillins, earlier cephalosporins, tetracyclines, macrolides, and fluoroquinolones). The recent occurrence of failures to treat gonorrhea with the extended-spectrum cephalosporins (ESCs) cefixime and ceftriaxone and the emergence of gonococcal strains exhibiting high-level clinical resistance to all ESCs (2–5), combined with resistance to nearly all other available therapeutic antimicrobials, have caused great concern, as evidenced by publications in the medical literature (5–9) and the lay press (10) and by development of global, regional, and national action/response plans (11–14). In most settings worldwide, ceftriaxone is the last remaining option for empirical first-line antimicrobial monotherapy. Due to this fact, there is fear that gonorrhea might become untreatable using antimicrobial monotherapy. In response to this concern, recommendations to use dual-antimicrobial therapy, i.e., mainly ceftriaxone and azithromycin, have been introduced in the United States (15), the United Kingdom (16), and all of Europe (17). Unfortunately, the susceptibility of gonococcal isolates to ceftriaxone has been decreasing globally, and resistance to azithromycin is easily selected and already prevalent in many settings. Accordingly, these dual-antimicrobial regimens might not be effective long-term solutions and, additionally, are not affordable in many resource-poor settings (5, 8). Furthermore, more expensive antimicrobials, such as high-quality ceftriaxone, are frequently not available even for monotherapy in low-resource settings.
The looming public health crisis of antimicrobial-resistant gonococci cannot be understated, as treatment regimens will most certainly become more expensive, and as treatment failures occur, medical costs will increase substantially as a result of severe complications that compromise the general and reproductive health of infected individuals (11–14). Given the global nature and large burden of gonorrhea, the high-level and frequently uncontrolled usage of antimicrobials, suboptimal control and monitoring of antimicrobial resistance (AMR) and treatment failures, slow update of treatment guidelines in most geographical settings, and the extraordinary capacity of the gonococci to develop and retain AMR, the global problem of gonococcal AMR will likely worsen in the foreseeable future, and the severe complications of gonorrhea will emerge as a silent epidemic.
By understanding the evolution, emergence, and spread of AMR in N. gonorrhoeae, including its molecular and phenotypic mechanisms, resistance to antimicrobials used clinically can be anticipated, future methods for genetic testing for AMR might permit region-specific and tailor-made antimicrobial therapy, and the design of novel antimicrobials to circumvent the problem of resistance can be undertaken more rationally. This review focuses on the history and evolution of gonorrhea treatment regimens and emerging resistance to them, on genetic and phenotypic mechanisms of gonococcal resistance to previously and currently recommended antimicrobials, including biological costs or benefits, and on crucial actions and advances necessary to detect and treat resistant gonococcal strains and, ultimately, retain gonorrhea as a treatable infection.
GONORRHEA
Gonorrhea (“the clap”; the term was introduced in 1378 and likely descended from the name of the old Parisian district where prostitutes were housed, i.e., Les Clapiers) is an ancient disease with biblical references (Old Testament; Leviticus 15:1–3). The obligate pathogen N. gonorrhoeae infects only humans in nature and causes urethritis in men and cervicitis in women. A minority of men (≤10%) but a large proportion of women (≥50%) can have asymptomatic urogenital infections. Rectal and pharyngeal gonorrhea, which is commonly asymptomatic, is mostly identified in men who have sex with men (MSM); however, depending on sexual practice, it can be found in both sexes. If the urogenital infection remains undetected or not appropriately treated, it might ascend to the upper genital tract and result in many severe reproductive complications (especially, but not exclusively, in women), such as endometritis, pelvic inflammatory disease, penile edema, and epididymitis, resulting in infertility or involuntary loss of life through ectopic pregnancy. The failure to curb the transmission of gonorrhea also promotes the transmission of other STIs, including HIV infection (14, 17–20). Conjunctivitis can occur in adults, but most commonly, infection of the eye presents as ophthalmia neonatorum in the newborn, which can result in blindness. Disseminated gonococcal infection can occur in both sexes but is nowadays rarely encountered (14, 17, 20).
In 2008, the World Health Organization (WHO) estimated 106 million new cases of gonorrhea among adults globally. This was a 21% increase compared to the number in 2005. The highest estimates were in the WHO Western Pacific Region (42.0 million cases), WHO South-East Asia Region (25.4 million cases), and WHO Africa Region (21.1 million cases) (1). However, the numbers of reported cases, especially from low-resource settings, are substantially smaller. This is due to suboptimal diagnostics (lack of appropriate methods or access to testing and use of syndromic management) and/or incomplete case reporting and epidemiological surveillance. These problems can result in substantial, unrecognized morbidity and hidden health care costs for countries. Accordingly, gonorrhea, including its severe complications, causes substantial morbidity and socioeconomic consequences.
Public health control of gonorrhea relies totally on appropriate antimicrobial treatment, together with generalized and targeted prevention efforts, use of effective diagnostics, partner notification processes, and epidemiological surveillance. Therapy should cure individual cases to reduce the risk of complications and prevent further transmission of the infection. Considering the large number of annual estimated cases (106 million) worldwide (1), AMR in N. gonorrhoeae has considerable implications for control of gonorrhea, including its severe complications, in communities globally. The prospect of gonococcal strains exhibiting multidrug resistance (MDR) and extensive drug resistance (XDR), including resistance to ceftriaxone, the last remaining option for first-line empirical monotherapy in many settings, is cause for great concern. XDR gonococcal strains are those resistant to ≥2 (MDR strains are resistant to 1) of the antibiotic classes currently generally recommended for treatment (ESCs [oral and injectable ones are considered separately] and spectinomycin) and ≥3 (MDR strains are resistant to ≥2) of the classes now less frequently used or proposed for use (e.g., penicillins, fluoroquinolones, azithromycin, aminoglycosides, and carbapenems) (19).
DIAGNOSIS OF GONORRHEA AND DETECTION OF ANTIMICROBIAL RESISTANCE IN NEISSERIA GONORRHOEAE
Diagnosis of Gonorrhea
Guidelines for the diagnosis of gonorrhea have been described previously (17, 20). As aforementioned, gonorrhea is frequently asymptomatic, and if symptoms are present, they are commonly nonspecific. Accordingly, appropriate laboratory diagnostics are crucial for confirmed diagnosis, case finding, and test of cure. The diagnosis of gonorrhea is established by detection of N. gonorrhoeae or its genetic material in genital or extragenital specimens by microscopy of stained smears, culture, or nucleic acid amplification tests (NAATs). Only evaluated and quality-assured methods should be used. Ideally, AMR testing of gonococcal isolates should be an integral part of the laboratory diagnosis.
Gonococci can be identified as intracellular diplococci in polymorphonuclear leukocytes by microscopy (magnification, ×1,000) of Gram- or methylene blue-stained smears. This method is cheap, provides rapid results, and has a high sensitivity and specificity for the diagnosis of symptomatic men with urethral discharge. However, microscopy is not recommended as the only method for diagnosis of cervical, pharyngeal, or rectal gonorrhea, or for asymptomatic patients, because negative results do not exclude infection, due to the low sensitivity of the method. Furthermore, the performance characteristics highly depend on the experience of the microscopist. Importantly, this method does not provide any AMR data.
Culture, the old “gold standard,” offers high sensitivity and up to 100% specificity (if appropriate species-verifying assays are applied) and is the only established method that enables complete AMR testing. Nevertheless, the method is relatively slow, and to obtain high sensitivity and specificity, it is crucial to strictly optimize the conditions for sample collection, transport, and storage and the culture methodology, as gonococci are exceedingly sensitive to external environmental factors.
In settings with more resources, NAATs have rapidly replaced culture for detection of gonococci. NAATs have many advantages, e.g., they detect nonviable gonococci; have a sensitivity superior to those of all other diagnostic methods, particularly for pharyngeal and rectal specimens; and are less demanding regarding specimen collection (noninvasive, self-collected samples, such as urine [males] and vaginal swabs [females], can effectively be used), transportation, and storage. NAATs also are rapid, allow automation, and enable simultaneous detection of several pathogens. However, NAATs also have disadvantages, e.g., they do not allow AMR testing, the appropriate time for test of cure is still debated, and the commercially available and in-house NAATs show different sensitivities and particular specificities in their detection of N. gonorrhoeae. Commensal Neisseria species, frequently present in the pharynx and rectum but also, more rarely, in the urogenital tract, have genetic homology with N. gonorrhoeae and might cross-react in gonococcal NAATs, resulting in false-positive reports. The suboptimal specificities of gonococcal NAATs result in low positive predictive values (PPV), particularly in low-prevalence populations (17, 20–24). In Europe and Australia, if the PPV obtained using NAAT is ≤90% in the local setting, it is recommended that a supplementary NAAT (with a different target sequence) be used for verification of all NAAT-screening-positive samples (17, 25). Importantly, in settings using only NAATs for detection of gonococci, it is essential for the laboratories, epidemiologists, and clinicians to be involved in and aware of an adequate local, national, and/or international gonococcal antimicrobial surveillance program (GASP). It is important to note that immunofluorescence assays, enzyme immunoassays, and rapid point-of-care tests for antigen or antibody detection with sufficient specificity and, particularly, sensitivity for diagnosis are not available for clinical diagnostic purposes.
Detection of Antimicrobial Resistance in N. gonorrhoeae
The quantitative agar dilution method determines the MICs (μg/ml) of antimicrobials and is the “gold standard” method. Nevertheless, particularly for testing small numbers of isolates, this method is laborious and not ideal for routine AMR testing. Consequently, the quantitative Etest method for MIC determination, which is comparable to the agar dilution method, is frequently utilized. Furthermore, a qualitative AMR determination can be obtained with a disc diffusion assay. However, for adequate reproducibility and interpretation to appropriately reflect the MIC values of given antimicrobials, these methods require considerable quality assurance and appropriate quality controls. Disc diffusion methods are recommended for use only when MIC determination cannot be performed, due to limited resources or other reasons, and any new, emerging, or rare AMR should be confirmed by MIC determination (20).
For AMR testing, culture and phenotypic AMR testing remain crucial. However, in many lower-resource settings, diagnosis of gonorrhea depends upon syndromic management of patients, and no specimens are taken. In many higher-resource settings, NAATs have rapidly replaced culture for detection of N. gonorrhoeae. For enhanced AMR surveillance, it is essential to strengthen the culture capacity globally. However, it is also imperative to develop rapid genetic AMR testing (5, 14, 26–28). These methods should ideally be used at point of care simultaneously with a rapid, sensitive, and specific genetic test for gonococcal detection. These methods could directly provide a diagnosis and guide individually tailored treatments, ensuring rational antimicrobial use and affecting the control of both gonorrhea and AMR. Mathematical modeling can then explore the impact of NAAT-based AMR tests on the spread of resistance and on clinical outcomes. Dynamic transmission models can capture the net effects of competing factors, such as increased detection and treatment of gonorrhea, increased reinfection risk, and reduced or delayed detection of AMR upon gonorrhea transmission and of AMR-resistant strains (26). Unfortunately, no commercially available gonococcal NAATs detect any AMR determinants. However, in-house molecular assays exist for detection of one or more genetic AMR resistance determinants involved in plasmid-mediated penicillin resistance (29–31), chromosomally mediated penicillin resistance (26, 32–35), plasmid- and chromosomally mediated tetracycline resistance (36), resistance to macrolides (32, 37–42), fluoroquinolone resistance (43–49), ESC resistance (50–53), and multidrug resistance (54–56). Unfortunately, for most AMR determinants, the sensitivity and specificity of these molecular AMR assays for determination of AMR are often low. For example, for the currently recommended antimicrobials, the ESCs, the correlates between most described resistance determinants, the ESC MICs of the gonococcal strains, and the treatment outcome are highly suboptimal (5). The ongoing evolution of ESC resistance, involving many different mutations in several divergent genes, is a major challenge for the development of a genetic AMR test for ESCs. Tests that need continual updating with new target sequences are unlikely to be profitable for companies manufacturing NAATs in the short term. Some “strain-specific” molecular assays that detect mutations involved in ESC resistance in the described XDR gonococcal strains were also recently developed (57, 58).
HISTORY OF TREATMENT REGIMENS AND RESISTANCE EVOLUTION
The Preantimicrobial Era
During the preantimicrobial era, treatment of gonorrhea consisted mainly of living a healthier lifestyle, with fresh air, appropriate food, and rest, abstaining from alcohol and sexual activity, and receiving systemic treatment with different types of balsams, urethral irrigations, chemical compounds, and hyperthermia (59). During the second half of the 19th century, gonorrhea was frequently treated with an Indonesian type of pepper (cubebs) and with balsam extracted from a tree in South America (copaiba) (60). To mask the taste and reduce toxicity, copaiba was frequently mixed with licorice, magnesium hydroxide, or ammonium carbonate or incorporated into gelatin capsules (61). Antiphlogistic regimens, avoiding irritation, maintaining the body cool (using salts), and dilution of the urine could also be used until inflammatory symptoms diminished, and then cubebs or copaiba balsam was used three times a day (62). Soap and water enemas, oral laudanum (opium tincture), and warm baths were used to ease retention of urine, and if needed, catheterization was performed. Acute urethritis could also be treated by anterior urethral irrigation with dilutions of warm potassium permanganate for several weeks (59, 63).
In the late 1800s, the search for more specific antibacterial compounds was initiated, and many metallic compounds, e.g., compounds of arsenic, antimony, bismuth, gold, silver, and mercury, were investigated. During World War I, soldiers were provided prophylactic packages comprising condoms, calomel (mercuryl chloride) ointment, and Argyrol/Protargol (silver compounds), and postcoital treatment centers, including urethral irrigation facilities, were also used (59, 63). Mercury compounds were later frequently used; for instance, Mercurochrome-220 was used as a urinary tract antiseptic (64). By mixing 1% mercurochrome in a 50% glucose solution, the injection became safer and more efficacious (65). Subsequently, in addition to intravenous mercurochrome, a silver-protein complex or mercurochrome was instilled into the urethra, or the seminal vesicles were irrigated with potassium permanganate (66).
Diathermy or hyperthermia was also used early in several settings. Initially, only inflamed joints in patients with gonococcal arthritis were heated. However, when some arthritis cases responded only with the addition of genital hyperthermia, genitalia also began to be treated (67, 68). A fever cabinet was used, with only the head outside, and temperatures above 41°C were maintained for 4 to 6 h. For cure, usually 5 or 6 treatments, provided every third day, were required (69). Up to 80 to 90% of gonococcal arthritis cases could be cured (70). Pretreatment with mercurochrome in hypertonic glucose was later shown to increase the efficacy of hyperthermia (71). Finally, fewer hyperthermia treatments were usually required with the addition of pelvic heating, i.e., insertion of heating elements for about 2 h into the rectum in men and the vagina in women (sometimes also the rectum), resulting in local temperatures of up to 44°C (72, 73). Hyperthermia was considered the best treatment for gonococcal arthritis and, most commonly, also resolved any genital symptoms (73).
The Antimicrobial Era
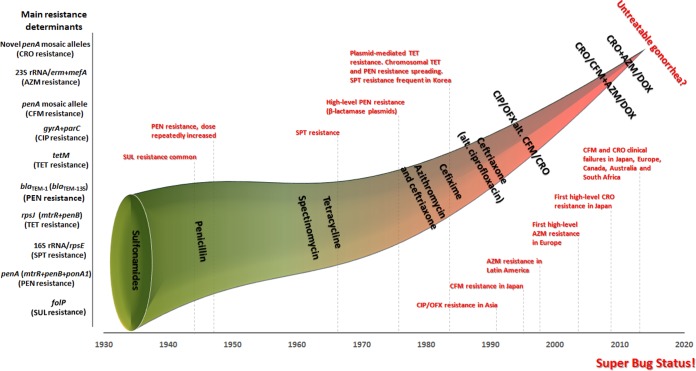
The history of discovered and introduced antimicrobials and the evolution of resistance, including genetic resistance determinants, as well as changes in the recommended first-line antimicrobial(s), are summarized in Fig. 1.
FIG 1.
History of discovered and recommended antimicrobials and evolution of resistance in Neisseria gonorrhoeae, including the emergence of genetic resistance determinants, internationally. During the preantimicrobial era (before the 1930s), treatment consisted of, e.g., a healthier lifestyle, copaiba, cubebs, urethral irrigations, potassium permanganate, silver compounds, mercury compounds, and hyperthermia. SUL, sulfonamides; PEN, penicillin; SPT, spectinomycin; TET, tetracycline; CIP, ciprofloxacin; OFX, ofloxacin; CFM, cefixime; CRO, ceftriaxone; AZM, azithromycin; DOX, doxycycline.
Sulfonamides.
In 1935, Gerhard Domagk discovered sulfanilamide (59, 63). The sulfonamides were the first antimicrobials used for treatment of gonorrhea; sulfanilamide initially cured 80 to 90% of gonorrhoea cases (74–76). Sulfapyridine became available in 1940 to 1941, and a 1-week course of sulfapyridine could cure many cases where sulfanilamide had failed (77). The subsequent drug sulfathiazol was as effective as sulfapyridine but was more tolerable (76, 78). Unfortunately, by 1944, many gonococcal strains showed clinical resistance, and by the late 1940s, >90% of gonococcal isolates were resistant to sulfonamides in vitro (75, 79). Sulfonamides (e.g., sulfamethoxazole) continued to be used, particularly in combination with trimethoprim and in low-resource settings, for many decades (19, 63, 80, 81).
Penicillin.
In 1928, Alexander Fleming accidentally discovered that a compound produced by a fungus could lyse staphylococci and other bacteria causing many infectious diseases. He identified that the fungus belonged to the Penicillium genus, and after initially being called “mold juice,” the compound was named penicillin in early 1929. In 1930, Cecil Paine used a crude extract from the penicillin-producing fungus Penicillium notatum to cure gonococcal ophthalmia in an infant (82). However, it took until 1943 before this “wonder drug” was appropriately documented to be therapeutically effective for gonococcal urethritis, and penicillin subsequently marked a new era in the treatment of gonorrhea as well as other infectious diseases (83, 84). Penicillin quickly supplanted the sulfonamides as the first-line treatment of gonorrhea (83, 85). Penicillin cured more than 95% of cases, with total doses as low as 45 mg being used (85). However, over time, the MICs of penicillin against gonococcal strains increased due to an accumulation of chromosomal resistance determinants, and the prescribed doses were progressively increased to obtain appropriate cure rates (8, 63, 86–89). Thus, by 1946, four gonorrhea cases resistant to “high” doses of penicillin (0.6 to 1.6 million units) were reported, and this resistance was also verified by in vitro testing. A gradual increase in the proportion of gonococcal strains with increasing resistance to penicillin was observed during the two subsequent decades (90, 91). Despite this developing situation, penicillin remained an effective antimicrobial for treatment of gonorrhea for many decades. Nevertheless, after the “epidemic” of gonorrhea in the United States and many other countries associated with the “sexual revolution” of the 1960s, the level of penicillin required to treat uncomplicated gonorrhea had substantially increased, and treatment failures were reported (8, 89, 92). The emergence in 1976 of two types of β-lactamase-encoding plasmids which caused high-level resistance to penicillin, originating in Southeast Asia and sub-Saharan West Africa, in certain gonococcal strains from the United States and the United Kingdom (93–95) reinforced the fear that the decades-long use of penicillin might soon end. The rapid international spread of these strains was of great concern. However, when penicillin was abandoned as a first-line antimicrobial in the United States and several other countries about a decade later, the primary reason was the emergence of chromosomally mediated clinical resistance to penicillin. An outbreak of chromosomally mediated penicillin-resistant gonorrhea in Durham, NC (96, 97), was the first major blow to the continued use of penicillin. Currently, gonococcal strains with plasmid- and/or chromosomally mediated resistance to penicillin are common globally (5, 8, 19, 81, 98–106).
Tetracycline.
The first tetracycline, chlortetracycline (aureomycin), was discovered in an allotment soil bacterium in 1945 by Benjamin Minge Duggar. Tetracyclines were used early to treat gonorrhea, especially in patients with penicillin allergy. However, the MICs of tetracycline against gonococcal strains increased over time, due to chromosomal resistance determinants (88). The emergence of the tetM determinant (causing high-level tetracycline resistance) on the conjugative plasmid in the mid-1980s (107) resulted in the exclusion of tetracycline from treatment guidelines in the United States and many countries worldwide. These gonococcal strains with plasmid-mediated high-level resistance to tetracyclines were first reported in 1986 in the United States and soon thereafter in the Netherlands (108) and are now widespread internationally (8, 19, 81, 98–104).
Spectinomycin.
In the early 1960s, spectinomycin was synthesized and commercialized as a specific gonorrhea treatment. Spectinomycin is an aminocyclitol that is closely related to the aminoglycosides produced by Streptomyces spectabilis. Spectinomycin is produced in nature by many organisms, including cyanobacteria. After the emergence of plasmid-mediated high-level resistance to penicillin, spectinomycin was frequently used for treatment of these cases (109, 110). Nevertheless, in 1967, spectinomycin resistance was reported for a penicillin-susceptible gonococcal strain in the Netherlands (111), and in 1981, a spectinomycin-resistant gonococcal isolate with plasmid-mediated high-level resistance to penicillin was reported in the Philippines (112). In 1981 in South Korea, spectinomycin was introduced as a first-line gonorrhea treatment in U.S. military personnel. However, after only 4 years, a clinical failure rate of 8.2% was described (113). Furthermore, in 1983, many spectinomycin-resistant gonococcal isolates were reported from London, United Kingdom (114). Subsequently, spectinomycin was abandoned as a first-line empirical monotherapy for gonorrhea internationally. Currently, spectinomycin resistance, particularly high-level resistance, is exceedingly rare in gonococcal strains worldwide. However, spectinomycin is currently not available and used in many countries, and it is feared that resistance will be selected rapidly if spectinomycin is introduced for first-line treatment. Furthermore, spectinomycin is suboptimal for treatment of pharyngeal gonorrhea, i.e., its efficacy rate is around 80% (115–117).
Quinolones.
Synthetic quinolone antimicrobials were discovered by George Lesher and colleagues as a by-product of the manufacture of chloroquine in the 1960s, and the quinolone nalidixic acid was introduced for treatment of urinary tract infections in humans. Nalidixic acid is the predecessor of all quinolones, and subsequent, broader-spectrum quinolones are known as fluoroquinolones. The fluoroquinolones ciprofloxacin and ofloxacin were previously recommended for gonorrhea treatment, and ciprofloxacin in particular was widely used to treat gonorrhea from the mid- to late 1980s onwards. Initially, low doses, e.g., 250 mg, of ciprofloxacin were used, but clinical failures were already reported by 1990 (118). The recommended ciprofloxacin dose was raised to 500 mg, but resistance developed and spread quickly, initially in the Asian Western Pacific Region (119, 120). In some Asian Western Pacific countries, fluoroquinolones were abandoned as first-line empirical treatments of gonorrhea by the mid- to late 1990s (8). Ciprofloxacin-resistant gonococcal strains were subsequently rapidly exported internationally or emerged independently (121–123). In regard to the United States, in 2000, fluoroquinolone-resistant strains initially imported from Asia were prevalent in Hawaii (124), and subsequently, these strains spread first to the West Coast and then to the rest of the United States, predominantly among MSM (125). In 2007, the fluoroquinolones were abandoned from the CDC-recommended treatment regimens for gonorrhea, with no exceptions (126). Due to high levels of fluoroquinolone resistance, many Asian and European countries removed ciprofloxacin as a first-line treatment in the early to mid-2000s (8). Currently, the prevalences of fluoroquinolone-resistant gonococcal strains are high worldwide (5, 8, 19, 63, 81, 98–106).
Macrolides.
In 1952, the macrolides were discovered when erythromycin was isolated from the soil microorganism Streptomyces erythraeus, currently known as Saccharopolyspora erythraea. In 1980, azithromycin, a synthetic derivative of erythromycin, was developed. Clinical and in vitro AMR data showed early that erythromycin is not sufficiently effective for the treatment of gonorrhea (63, 127). Compared to erythromycin, azithromycin has a substantially higher activity against N. gonorrhoeae. However, by the mid- to late 1990s, decreased susceptibility and resistance to azithromycin were reported from Latin America, where azithromycin was frequently used early on for treatment of bacterial STIs, including gonorrhea (99, 128, 129). Subsequently, azithromycin resistance emerged in many countries, particularly where there was a high level of azithromycin usage for treatment of gonorrhea and also, e.g., for Chlamydia trachomatis infections (99, 102, 130, 131). Worryingly, gonococcal isolates with high-level resistance to azithromycin (MICs of ≥256 μg/ml) were identified in Scotland (132), England (37), Argentina (38), Italy (133), the United States (39), and Sweden (42). Despite being used in several countries, azithromycin is not recommended for empirical monotherapy of gonorrhea. This is due particularly to the concerns of a rapid selection of resistance but also to the possible adverse events from taking the 2-g azithromycin oral dose (37, 134, 135). Nevertheless, azithromycin is one of the two antimicrobials in all the introduced dual-antimicrobial therapeutic regimens for gonorrhea (15–17).
Cephalosporins.
The first cephalosporin compounds were isolated from cultures of the fungus Cephalosporium acremonium, first discovered by Giuseppe Brotzu in 1948. Chemical modifications of these and similar compounds resulted in the first useful antimicrobial agent, i.e., cefalotin, launched in 1964. The cephalosporins most commonly recommended internationally for treatment of gonorrhea following the demise of fluoroquinolones are the third-generation ESCs ceftriaxone (injectable) and cefixime (oral). No other injectable or oral ESCs have any evident advantages over ceftriaxone and cefixime (17, 63, 136). Nevertheless, other oral cephalosporins have been used when cefixime has not been available, e.g., cefditoren and celdinir in Japan, cefuroxime in several European countries, cefpodoxime in the United States, and ceftibuten in Hong Kong (63, 136–138). During the last 2 decades, gonococcal strains exhibiting resistance to ESCs seem to have initially emerged in Japan and then spread worldwide. In Japan, ceftriaxone was not endorsed for gonorrhea treatment from the 1990s to the early 2000s. Consequently, many oral cephalosporins and dose regimens, including some with suboptimal efficacies, were prescribed for monotherapy, but if resistance was identified, cefodizime or spectinomycin was administered (139, 140). Multiple low-dose regimens of oral cephalosporins were frequently used, which could have resulted in subinhibitory cephalosporin concentrations and, accordingly, may have selected for cephalosporin resistance (5, 140–143). Furthermore, when single-dose cefixime (the most potent oral ESC) therapy was applied in Japan, it commonly included only 300 mg of cefixime, in contrast to the 400-mg dose used internationally (5, 144). Thus, between 1995 and 2000, in Fukuoka, Japan, the MIC peaks for cefixime and ceftriaxone against gonococcal isolates reached 0.25 μg/ml and 0.064 μg/ml, respectively (140). Furthermore, between 1999 and 2002, in six hospitals in central Japan, the proportions of gonococcal isolates with in vitro resistance to cefixime (MICs of ≥0.5 μg/ml) and ceftriaxone (MICs of ≥0.5 μg/ml) reached 30.2% and 0.9%, respectively (143). This also translated into treatment failures with cefixime. Accordingly, from 1999 to 2001, eight treatment failures with cefixime (200 mg orally twice, 6 h apart) were reported (142), and in 2002 to 2003, four treatment failures with an extended cefixime regimen (200 mg orally twice a day for 3 days) were documented (145). In 2006, all oral ESCs were excluded from the treatment guidelines in Japan, and since then, ceftriaxone (1 g intravenously), which is mostly used, cefodizime (1 g intravenously), and spectinomycin (2 g intramuscularly) have been recommended as first-line empirical treatments for uncomplicated anogenital and pharyngeal gonorrhoea (146). During the last decade, strains with decreased susceptibility or resistance to ESCs spread internationally, and their presence has been documented basically globally (5, 8, 98–100, 102, 104–106, 131, 147–151). Worryingly, gonococcal AMR surveillance remains highly limited in many regions worldwide (104, 106, 152), and accordingly, the global burden of decreased susceptibility and resistance to ESCs is largely unknown. Currently, cefixime treatment failures have been verified in Japan, several European countries, Canada, and South America (4, 142, 145, 153–157), and a few ceftriaxone treatment failures for pharyngeal gonorrhea have been identified in Japan, some European countries, and Australia (3, 158–161).
It is of great concern that the first gonococcal XDR strains, exhibiting high-level clinical resistance to all ESCs combined with resistance to nearly all other available therapeutic antimicrobials, were recently identified in Kyoto, Japan (3), Quimper, France (4), and Catalonia, Spain (2). All these XDR strains were also identified in high-risk, frequently transmitting populations, i.e., commercial sex workers (CSWs) or MSM. Since ceftriaxone is the last option for first-line empirical monotherapy of gonorrhea, the emergence of XDR gonococci might initiate an era of gonorrhea that is untreatable using antimicrobial monotherapy. Fortunately for now, however, based on the intensified surveillance undertaken in Kyoto and Osaka (2010 to 2012) after identification of the first XDR strain (H041), this strain has not spread further within the local community (162), which might indicate a lowered biological fitness.
MECHANISMS OF ANTIMICROBIAL RESISTANCE IN N. GONORRHOEAE
Resistance Emergence and Spread
N. gonorrhoeae has an extraordinary capacity to alter its genetic material, given that it is naturally competent for transformation (transfer of partial or whole genes) during its entire life cycle and because it can effectively change its genome through all types of mutations. N. gonorrhoeae uses these mechanisms to rapidly adapt to and survive in the often hostile environments at different sites in the human host, and accordingly, the bacterium is a great example of “survival of the fittest.” The gonococcus has in this way evolved and acquired or developed nearly all known physiological mechanisms of AMR to all antimicrobials recommended and/or used for treatment, e.g., (i) antimicrobial destruction or modification by enzymatic means, (ii) target modification or protection that reduces affinity for the antimicrobials, (iii) decreased influx of antimicrobials, and (iv) increased efflux of antimicrobials. Most genetic AMR determinants in N. gonorrhoeae are situated chromosomally, and only the blaTEM gene (93, 95) and the tetM gene (107), which result in high-level resistance to penicillin and tetracycline, respectively, are known to be plasmid borne in gonococci. Certain AMR determinants alone can result in high-level resistance in vitro and in vivo, i.e., treatment failure, for the antimicrobial in question. However, in other instances, acquisition of a single AMR determinant confers only an incremental increase in AMR that is of less clinical significance; nevertheless, the cumulative effects of several AMR determinants and their complex interactions and interplay can ultimately result in clinical levels of AMR. For example, this is the scenario resulting in the demise of penicillin as an effective treatment for gonorrhea, due to several chromosomally mediated resistance determinants. The described determinants and mechanisms of AMR in N. gonorrhoeae are summarized in Table 1.
TABLE 1.
Resistance determinants and mechanisms in Neisseria gonorrhoeae for antimicrobials previously or currently recommended for treatment of gonorrhea
| Antimicrobial class | Resistance determinants/mechanisms |
|---|---|
| Sulfonamides | Oversynthesis of p-aminobenzoic acid, which dilutes the sulfonamide. |
| Mutations in folP (encoding the sulfonamide target DHPS) reduce target affinity. The folP mutations comprise SNPs or a mosaic folP gene containing sequences from commensal Neisseria spp. | |
| Penicillins (e.g., penicillin G and ampicillin) | Mutations in penA (encoding the main lethal target PBP2). Traditionally, the mutations were the single amino acid insertion D345 in PBP2 and 4 to 8 concomitant mutations in the PBP2 carboxyl-terminal region, decreasing the PBP2 acylation rate and reducing susceptibility ∼6- to 8-fold. In the last decade, many mosaic penA alleles with up to 70 amino acid alterations, also reducing PBP2 acylation, were described. |
| Mutations in mtrR, in the promoter (mainly a single nucleotide [A] deletion in the 13-bp inverted repeat sequence) or coding sequence (commonly a G45D substitution), result in overexpression of and increased efflux from the MtrCDE efflux pump. See the text for rarer mutations resulting in increased MtrCDE efflux. | |
| porB1b SNPs, e.g., encoding G120K and G120D/A121D mutations in loop 3 of PorB1b, reduce influx (penB resistance determinants). Interestingly, the penB phenotype is apparent only in strains with the mtrR resistance determinant. | |
| A SNP in pilQ (encoding the pore-forming secretin PilQ of the type IV pili), i.e., E666K, reduces influx. Note that this SNP has been found only in the laboratory and is unlikely to be present in clinical isolates, because it disrupts type IV pilus formation, which is essential for pathogenesis. | |
| A SNP in ponA (encoding the second penicillin target, PBP1), i.e., “ponA1 determinant” (L421P), reduces penicillin acylation of PBP1 ∼2- to 4-fold. | |
| “Factor X,” an unknown, nontransformable determinant, increases penicillin MICs ∼3- to 6-fold. | |
| Penicillinase (TEM-1 or TEM-135)-encoding plasmids, i.e., Asian, African, Toronto, Rio, Nîmes, New Zealand, and Johannesburg plasmids, hydrolyze the cyclic amide bond of the β-lactam ring and render the penicillin inactive. | |
| Tetracyclines (e.g., tetracycline and doxycycline) | A SNP in rpsJ (encoding ribosomal protein S10), i.e., V57M, reduces the affinity of tetracycline for the 30S ribosomal target. |
| mtrR mutations (see above). | |
| penB mutations (see above). | |
| A SNP in pilQ (see above). | |
| TetM-encoding plasmids, i.e., American and Dutch plasmids. Evolved derivatives have been described in Uruguay and South Africa. TetM, resembling elongation factor G, binds to the 30S ribosomal subunit and blocks tetracycline target binding. | |
| Spectinomycin | A 16S rRNA SNP, i.e., C1192U, in the spectinomycin-binding region of helix 34, reduces the affinity of the drug for the ribosomal target. |
| Mutations in rpsE (encoding the 30S ribosomal protein S5), i.e., the T24P mutation and deletions of V25 and K26E, disrupt the binding of spectinomycin to the ribosomal target. | |
| Quinolones (e.g., ciprofloxacin and ofloxacin) | gyrA SNPs, e.g., S91F, D95N, and D95G, in the QRDR, reduce quinolone binding to DNA gyrase. |
| parC SNPs, e.g., D86N, S88P, and E91K, in the QRDR, reduce quinolone binding to topoisomerase IV. | |
| Many additional mutations in the QRDR of gyrA and parC have been described. An overexpressed NorM efflux pump also slightly enhances quinolone MICs. | |
| Macrolides (e.g., erythromycin and azithromycin) | 23S rRNA SNPs, i.e., C2611T and A2059G (in 1 to 4 alleles), result in a 23S rRNA target (peptidyltransferase loop of domain V) with a reduced affinity for the 50S ribosomal macrolide target. |
| mtrR mutations (see above). | |
| erm genes (ermB, ermC, and ermF), encoding rRNA methylases that methylate nucleotides in the 23S rRNA target, block the binding of macrolides. | |
| MacAB efflux pump; its overexpression increases the MICs of macrolides. | |
| mef-encoded efflux pump exports macrolides out of the bacterial cell and increases the MICs of macrolides. | |
| Cephalosporins (e.g., ceftibuten, cefpodoxime, cefixime, cefotaxime, and ceftriaxone) | Mosaic penA alleles encoding mosaic PBP2s with a decreased PBP2 acylation rate. These proteins have up to 70 amino acid alterations and are derived from horizontal transfer of partial penA genes from mainly commensal Neisseria spp. Mutations in mosaic PBP2s verified to contribute to resistance are A311V, I312M, V316T, V316P, T483S, A501P, A501V, N512Y, and G545S. The resistance mutations need other epistatic mutations in the mosaic penA allele. |
| penA SNPs, i.e., A501V and A501T, in nonmosaic alleles can also enhance cephalosporin MICs. Some additional SNPs (G542S, P551S, and P551L) were statistically associated with enhanced cephalosporin MICs, but their effects remain to be proven with, e.g., site-directed penA mutants in isogenic backgrounds. | |
| mtrR mutations (see above). | |
| penB mutations (see above). | |
| “Factor X,” an unknown, nontransformable determinant (see above). |
Gonococci develop AMR through gene transfer (transformation and subsequent recombination into the genome) or by specific mutations. Exposure of gonococci or other Neisseria spp. to antimicrobials given for treatment of gonorrhea or other infections can select for resistant strains. The commensal Neisseria spp. frequently inhabit human anatomical sites, particularly the pharynx, and are often exposed to antimicrobials. Accordingly, resistance can initially emerge in commensal Neisseria spp. that act as a reservoir of AMR genes, which can readily be transferred to gonococci through transformation. Likely, the mostly asymptomatic pharyngeal gonorrhea, where gonococci and commensal Neisseria spp. can coexist for extended periods, provides the means for this gene transfer (5, 8, 19, 163–166). Horizontal gene transfer most probably played a pivotal role in the spread of mosaic penA alleles (see “Cephalosporin Resistance”), resulting in decreased susceptibility or resistance to ESCs (3, 4, 166). After their emergence, AMR gonococcal strains can spread quickly, first within a geographical region and then later establishing an international presence. Furthermore, AMR genes can be spread between gonococcal strains by transformation (of chromosomal or plasmid DNA) or conjugal transfer of plasmid AMR genes. Gonococci use a sequence-specific DNA uptake system to recognize DNA from themselves or closely related species (167, 168). Chromosomal DNA transformation frequencies in gonococci can be quite high (10−2/μg DNA/108 CFU). However, plasmid transformation frequencies are substantially lower (10−6), and deletions occur frequently (169). Thus, even though the rate of spontaneous missense mutations that result in AMR can be low, horizontal transfer of these alleles by transformation is very efficient in disseminating AMR within the community.
Antimicrobials most frequently initiate their activity through binding to a specific target that is critical for viability of a bacterium. By this binding, the antimicrobials block the bacterium's function and the microbe succumbs. However, bacteria can develop low- to high-level resistance through mutations that reduce or abrogate antimicrobial binding to the specific target. Because these targets are critical for cell viability, the changes that occur must remodel the active site of the target to lower its affinity for the antimicrobial without greatly affecting the normal function of the enzyme and having a detrimental impact on bacterial physiology and fitness. In N. gonorrhoeae, most of the acquired or developed AMR mechanisms do not appear to cause significantly lower biological fitness (possibly mainly due to compensatory mutations), which results in the persistence of AMR and MDR/XDR strains even in the absence of obvious antimicrobial selection. In fact, some AMR determinants can enhance the biological fitness of specific gonococcal strains (170–172). Consequently, the prospect of being able to use previously withdrawn antimicrobials for gonorrhea treatment appears bleak (5, 8).
Sulfonamide Resistance
Sulfonamides target the bacterial dihydropteroate synthase (DHPS) enzymes, thereby inhibiting the synthesis of folic acid in the bacterium. Sulfonamide resistance can be mediated by oversynthesis of p-aminobenzoic acid, which dilutes the antimicrobial agent, or by alterations in the folP gene (point mutations or the presence of a mosaic gene containing DNA sequences from commensal Neisseria spp.), encoding the drug target DHPS. The alterations of DHPS result in a significantly lowered affinity for the sulfonamide agents and a bacteriostatic activity (173–175).
Penicillin Resistance
Plasmid-mediated penicillin resistance.
β-Lactam antimicrobials, such as penicillins and cephalosporins, inhibit the formation of peptidoglycan cross-links in the bacterial cell wall through binding of the β-lactam ring to transpeptidase enzymes (penicillin-binding proteins [PBPs]), which results in bactericidal activity.
Gonococcal strains with plasmid-mediated high-level resistance to penicillin traditionally contain plasmids with a blaTEM-1 gene, encoding a TEM-1-type β-lactamase. This enzyme hydrolyzes the cyclic amide bond of β-lactamase-susceptible penicillins, opening the β-lactam ring and rendering the penicillin inactive. The gonococcal β-lactamase plasmids were likely acquired by conjugal transfer from Haemophilus parainfluenzae (176, 177), which can carry a closely related R plasmid, RSF0885. After the first descriptions of gonococcal strains with β-lactamase-producing plasmids in 1976 (93, 95), these strains and the plasmids themselves (between gonococcal strains) spread rapidly internationally. Currently, gonococcal strains possessing the Asian (7,426 bp) and African (5,599 bp) plasmids (named after their epidemiological origins) are globally widespread (19, 81). However, other types of β-lactamase-producing plasmids have been described for gonococci, some of which are also prevalent, including the Toronto (5,153 bp), Rio (5,153 bp; possibly identical to Toronto), Nîmes, New Zealand, and Johannesburg plasmids. The Asian plasmid appears to be the ancestral plasmid from which the other plasmids evolved, through deletions and/or insertions. Accordingly, these β-lactamase-producing plasmids may be characterized as either deletion derivatives of the Asian plasmid (Africa, Toronto, Rio, and Johannesburg plasmids) or insertion derivatives of either the Asian (New Zealand plasmid) or African (Nîmes plasmid) plasmid (30, 178–182). All these plasmids likely contain a TnA (Tn2) transposable element carrying the blaTEM-1 gene that encodes TEM-1 β-lactamase. No extended-spectrum β-lactamase (ESBL) has yet been acquired or developed in gonococci. Nevertheless, in many currently circulating strains, the blaTEM-135 gene, which differs by one single nucleotide polymorphism (SNP) from blaTEM-1, has been found, and only one additional SNP could result in an ESBL capable of hydrolyzing and degrading ESCs (183–185).
Chromosomally mediated penicillin resistance.
Chromosomally mediated penicillin resistance in gonococci is due to mutations that modify the target proteins (PBPs), in complex interactions and in an interplay with resistance determinants that increase the efflux and decrease the influx of penicillin (see below).
The target molecules for β-lactam antimicrobials, i.e., transpeptidases (PBPs), contain three conserved motifs in their active sites: the SxxK, SxN, and KTG motifs. In penicillin-resistant gonococci, traditionally there have been 5 to 9 mutations in the penA gene (encoding PBP2, the main lethal target for β-lactam antimicrobials), and together, these decrease the acylation rates of PBP2 and, accordingly, decrease the susceptibility to penicillin 6- to 8-fold (186–188). These penA mutations were acquired by gonococci through transformation of penA sequences from commensal Neisseria spp. that possess a PBP2 with a reduced rate of acylation by penicillin (189–192). The most common PBP2 mutation in penicillin-resistant gonococcal strains has traditionally been insertion of an aspartate (named Asp345a), and the remaining mutations lie in the carboxyl-terminal region of PBP2 (193). The structures of wild-type PBP2 and PBP2 containing four C-terminal mutations found in the penicillin-resistant strain FA6140 were recently published (186). Asp345a is located on a β-hairpin loop (β2a to β2d) close to the active site, and the C-terminal mutations are also relatively close to the active site (186, 194). Although the C-terminal mutations significantly affect rates of acylation by penicillin, the crystal structure of PBP2 is not altered (186), which is consistent with the necessity for the mutated PBP2 enzyme to retain activity with its natural substrate. In the absence of a crystal structure of PBP2 containing the Asp345a insertion, the impact of this mutation is not totally evident, but most likely it is more significant than the C-terminal substitutions. Notably, only an aspartate insertion confers resistance (195), and consistent with this, only an aspartate insertion is observed in clinical gonococcal strains (190). This might suggest that only an insertion of aspartate, not closely related amino acids, such as glutamate or asparagine, can discriminate against β-lactam antimicrobials without abolishing the PBP2 transpeptidase activity essential for viability (196). During the latest decade, many mosaic penA genes have also been described. These mosaic genes contain up to 60 to 70 amino acid changes compared to a wild-type penA gene and can result in resistance to both penicillins and ESCs (2–5). For details regarding these genes, see “Cephalosporin Resistance.”
Although PBP2 alteration is the primary mechanism for chromosomally mediated penicillin resistance in gonococci, strains exhibiting high-level penicillin resistance also harbor a single missense mutation in the ponA gene (termed the ponA1 allele) that encodes PBP1, which has an approximately 16-fold lower penicillin acylation rate than that of wild-type PBP2 (187, 196). The ponA1 allele encodes a Leu421Pro alteration in PBP1, which reduces the rate of penicillin acylation of PBP1 3- to 4-fold (187). The structural consequences of this mutation are unknown because of the lack of any crystal structure for PBP1. Interstingly, in a penicillin-resistant strain, reversion of ponA1 to wild-type ponA decreases the penicillin MIC 2- to 4-fold, but introduction of ponA1 into a strain with penA, mtrR, and penB resistance determinants (see below) does not affect the penicillin MIC (187). This may indicate epistasis or the presence of some unknown resistance determinant, such as “factor X” (see below).
Furthermore, penicillin MICs can be increased further by specific mutations resulting in increased efflux by overexpression of the MtrCDE efflux pump system, which exports the penicillin out of the cell (mtrR resistance determinant) (3–5, 197–199), and by mutations resulting in a decreased influx (intake) of penicillin, through a decreased permeability of the outer membrane channel porin PorB1b (penB resistance determinants) (3–5, 199–201). In laboratory isolates, specific mutations in pilQ (encoding loss-of-function alterations, e.g., E666K, in the pore-forming secretin PilQ of type IV pili) are also found in strains with high-level resistance to penicillin that contain alterations of penA, mtrR, and penB (5, 187, 199, 202). However, these pilQ mutations are unlikely to be found in clinical isolates because they disrupt proper formation of the type IV pili, which are essential for pathogenesis of gonococci (203). Details regarding increased efflux and decreased influx of antimicrobials are described below. Finally, there remains at least one unknown, nontransformable penicillin resistance determinant, “factor X,” which can increase the MICs of penicillin approximately 3- to 6-fold (3–5, 204).
Tetracycline Resistance
Plasmid-mediated tetracycline resistance.
Tetracyclines inhibit the binding of aminoacyl-tRNA to the mRNA-ribosome complex, mainly by binding to the 30S ribosomal subunit, and accordingly inhibit the protein synthesis that results in a bacteriostatic effect.
High-level plasmid-mediated resistance to tetracycline (MICs of ≥16 μg/ml) in gonococci is due to the tetM gene, initially described for the Streptococcus genus (107). TetM confers high-level resistance to tetracycline by binding to the ribosomes and causing the release of the tetracycline molecule, thereby permitting protein synthesis to proceed. TetM achieves this by its resemblance to elongation factor G (EF-G), involved in protein synthesis, and TetM also has ribosome-dependent GTPase activity (205–207). tetM initially established itself in gonococci by integrating into the 24.5-MDa conjugative plasmid to produce a 25.2-MDa (40.6 kb) plasmid (208, 209). Once established, it was stably maintained and could be transferred to other gonococci by conjugation. The first N. gonorrhoeae conjugative plasmid was identified in 1974 (210), and this plasmid can also transfer β-lactamase-producing plasmids between different gonococcal strains, and to Neisseria meningitidis (211–213), Haemophilus influenzae, and Escherichia coli (214). The tetM-possessing conjugative plasmid was first described in 1985 in the United States and was designated the American tetM plasmid (107). In 1991, the highly homologous (215) Dutch tetM plasmid was described (216). Subsequently, evolved derivatives of these plasmids have been identified, e.g., the Uruguay (217) and South Africa (218) plasmids.
Chromosomally mediated tetracycline resistance.
Chromosomally mediated tetracycline resistance in gonococci is due to mutations that modify the ribosomal protein (target) structure, in an interplay with resistance determinants that increase the efflux and decrease the influx of tetracycline.
It was early shown that tetracycline-resistant gonococcal strains had the so-called tet-2 mutation in addition to mtrR and penB mutations (188). tet-2 was subsequently shown to be a mutated rpsJ allele, which encoded an altered form of ribosomal protein S10 (219). The mutated rpsJ allele contained a SNP that altered Val57 to Met57 in S10, and Leu57 and Gln57 substitutions conferred identical levels of resistance. Val57 in S10 is located at the vertex of a small loop near the aminoacyl-tRNA region that forms the tetracycline-binding site, and it has been suggested that replacement of the native Val57 with large uncharged amino acids alter the rRNA structure, thereby reducing the affinity of tetracycline for the ribosome (219).
In addition to these target modifications, as for penicillin (see above), an increased efflux and decreased influx of tetracycline, due to the mtrR resistance determinant (197–199) and penB resistance determinants (199–201), respectively, result in an enhanced resistance to tetracycline.
Spectinomycin Resistance
Spectinomycin binds to the 30S ribosomal subunit of the bacterium and inhibits protein translation, resulting in a bacteriostatic effect. Specifically, spectinomycin interacts with 16S rRNA and, during polypeptide elongation, blocks the EF-G-catalyzed translocation of the peptidyl-tRNA from the A site to the P site. This 16S rRNA interaction is close to the base-paired nucleotides G1064–C1192 (E. coli numbering) in helix 34 (194, 220).
For gonococci, high-level spectinomycin resistance (MICs of >1,024 μg/ml) was early shown to be caused by a C1192U SNP in the spectinomycin-binding region of helix 34 in 16S rRNA, including the cross-linked positions 1063 to 1066 and 1190 to 1193 (221, 222). Recently, a deletion of Val25 and a K26E alteration in the 30S ribosomal protein S5, encoded by the rpsE gene, were also verified to result in high-level spectinomycin resistance in gonococci (223). A T24P mutation in S5 resulted in low-level spectinomycin resistance (MIC of 128 μg/ml) (223, 224). The N terminus of S5, specifically amino acids 21 to 35, forms a loop that can bind to helix 34 of 16S rRNA and is also involved in spectinomycin binding to the ribosome (225). The alterations in ribosomal protein S5 likely disrupt its binding to 16S rRNA, which results in spectinomycin resistance.
Quinolone Resistance
Bacterial DNA gyrase and topoisomerase IV are highly conserved type II topoisomerases that are essential for DNA metabolism. They act by breaking and rejoining double-stranded DNA in a reaction that is coupled with ATP hydrolysis. Quinolones act by inhibition of DNA gyrase and topoisomerase IV, resulting in bactericidal activity.
Bacteria develop quinolone resistance through mutations that alter quinolone recognition of these target enzymes. DNA gyrase consists of a heterotetramer of two GyrA subunits and two GyrB subunits; in gonococci, initial mutations in the primary target gene, gyrA, are associated with resistance. The gyrA mutations reduce quinolone binding affinity, rendering the enzyme (and the bacteria) resistant to its inhibitory effect. Topoisomerase IV is a tetramer of two ParC and two ParE subunits, encoded by the parC and parE genes, respectively. The enzymatic activity of topoisomerase IV can also be inhibited by quinolones, although higher concentrations than those needed to inhibit DNA gyrase in vitro are required. Specific SNPs in gyrA alone provide low- to intermediate-level resistance, but high-level resistance requires one or several specific concomitant mutations in parC. These mutations can easily be selected by exposure to subinhibitory ciprofloxacin concentrations and also transferred to other gonococci by transformation (226). Thus, a missense mutation at codon 91 in gyrA (S91F), which is located within the so-called quinolone resistance-determining region (QRDR) in E. coli GyrA, was shown to confer a 100-fold increase in resistance to ciprofloxacin. Subsequent mutation at codon 95 (D95N) further increased ciprofloxacin resistance, by 2-fold. Higher levels of quinolone resistance required mutations in parC in addition to those in gyrA. These parC mutations mapped to codons 88 (S88P) and 91 (E91K) (226). Subsequently, additional GyrA/ParC amino acid alteration patterns were identified in ciprofloxacin-resistant strains isolated internationally (227–229). Mutations in gyrB and parE do not appear to have any significant impact on ciprofloxacin resistance (227, 228, 230).
Macrolide Resistance
Macrolides inhibit protein synthesis by binding to the 50S ribosomal subunit, preventing translocation of the peptidyl-tRNA, blocking the peptide exit channel in 50S subunits by interacting with 23S rRNA, and causing ribosomes to release incomplete polypeptides (231). This results in a bacteriostatic effect.
Bacterial resistance to macrolides may result from modification of the ribosomal target by either rRNA methylase-associated modification of 23S rRNA or specific mutations in 23S rRNA and/or from an overexpressed efflux pump system. rRNA methylases can cause macrolide resistance through blocking of macrolide binding to 23S rRNA by methylating an adenosine residue at position 2058 (E. coli numbering system), which is located in peptidyl transferase domain V. Genes encoding rRNA methylase (referred to as macrolide-lincosamide-streptogramin B resistance genes, or erm genes) can be carried by conjugative transposons, and certain erm genes were identified in gonococcal strains in the early 1990s (41). In gonococci, erm genes can confer high-level resistance to erythromycin (MICs of 4 to 16 μg/ml) and decreased susceptibility or low-level resistance to azithromycin (MICs of 1 to 4 μg/ml) in the absence of other resistance determinants, such as mtrR mutations or a mef-encoded efflux pump (see below) (41, 232). Clinical gonococcal isolates from the United States and Uruguay were reported by Roberts et al. (41) to contain the rRNA methylase gene ermF or ermB/ermF. ermF was part of a complete conjugative element, and its nucleotide sequence was 97% identical over 374 bp to ermF of Bacteroides fragilis. ermF could also be transferred conjugally to other gonococci, meningococci, and Enterococcus faecalis. Nevertheless, during recent years, erm genes have been very rare among macrolide-resistant gonococcal strains (37, 233). Specific mutations of the macrolide target, 23S rRNA, can also result in both low-level resistance (C2611T mutation) (40) and high-level resistance (A2059G mutation) (37–39, 42) to erythromycin and azithromycin. The MICs of macrolides against these resistant gonococcal isolates depend on how many of the four 23S rRNA gene alleles contain the specific mutation. For example, A2059G mutations in three or all four of the 23S rRNA gene alleles result in high-level azithromycin resistance (MICs of ≥256 μg/ml and up to 4,096 μg/ml) (42), while strains with only one A2059G mutant allele can have an MIC of azithromycin similar to that for wild-type strains. Strains with a single A2059G mutation, while susceptible to azithromycin, quickly develop high-level azithromycin resistance (MICs of ≥256 μg/ml) upon serial passage with subinhibitory concentrations of macrolides (37).
Gonococcal resistance to macrolides because of overexpressed efflux systems, particularly the MtrCDE efflux pump (234–240), but also the MacAB (241) and mef-encoded (41, 232, 242) efflux pumps, can affect the MICs of macrolides; this issue is discussed below.
Cephalosporin Resistance
Cephalosporins, like other β-lactam antimicrobials, inhibit the cross-links of peptidoglycan within the bacterial cell wall by binding of the β-lactam ring to PBPs (transpeptidases), which results in bactericidal activity. Cephalosporin resistance in gonococci is due primarily to mutations that modify the target proteins (PBPs), but also to an increased efflux and decreased influx of cephalosporin.
Like the case for chromosomally mediated penicillin resistance, the primary ESC resistance determinants in N. gonorrhoeae are specific alterations of the penA gene encoding PBP2, which is also the main lethal target for cephalosporins (2–5, 199, 204). However, in contrast to the penA-Asp345a gene found in penicillin-resistant strains, the penA gene from intermediate-level or fully ESC-resistant strains is most frequently a mosaic gene that contains up to 60 to 70 amino acid alterations, and notably, these mosaic penA alleles do not contain the Asp345a insertion (3, 5, 243). These mosaic penA alleles are considered to have emerged by DNA transformation followed by recombination with partial penA genes, particularly those from commensal Neisseria species commonly residing in the oropharynx, such as Neisseria perflava, Neisseria sicca, Neisseria polysaccharea, Neisseria cinerea, and/or Neisseria flavescens (5, 244–246). This in vivo intrageneric horizontal transfer of entire or partial penA genes most likely occurred during pharyngeal gonococcal infections (5, 19, 163, 164). The acquisition of a penA mosaic allele appears to increase the MICs of cefixime more than those of ceftriaxone (3, 4, 199, 204), which might be caused by structure-function relationships due to the longer C-3 side chain of the cephem skeleton of ceftriaxone (5, 243, 246–248).
In gonococcal isolates with decreased susceptibility or resistance to cefixime, three mutations in the mosica penA allele, resulting in the amino acid alterations G545S, I312M, and V316T in PBP2, were early suggested as important for the decreased ESC susceptibility, particularly to cefixime (246, 248). However, while reversion of these three amino acids in PBP2 of a strain with decreased susceptibility or resistance to ESCs to those in wild-type PBP2 dramatically decreased ESC resistance (MICs of ceftriaxone and cefixime decreased 16- and 25-fold, respectively), introduction of only these three amino acids into a wild-type PBP2 sequence had little to no effect on resistance (MICs of ceftriaxone and cefixime increased only 2- to 3-fold). Accordingly, these three mutations increase resistance only in the presence of additional mutations in the mosaic penA alleles that have limited effect on the ESC MICs on their own, i.e., using a mechanism of epistasis (5, 204). These additional mutations might act as “compensatory” or “stabilizing” mutations to restore transpeptidase activity essential for the viability of the gonococcal strains. In the crystal structure of PBP2, all three mutations are located near the β-lactam active site, with two residing on the same α-helix as the serine nucleophile, Ser310, of the SxxK active site motif. The G545S mutation is present at the beginning of the α11 helix. The G545 and G546 main chain amides can bind (by hydrogen bonding) the side chain hydroxyls of Thr498 and Thr500, respectively, located within the KTG(T) active site motif. The main chain amide of Thr500 likely forms the oxyanion hole that stabilizes the transition state. Accordingly, the G545S mutation might decrease acylation by interfering with the structure of the transition state/tetrahedral intermediate. Optionally, the Thr498 and Thr500 hydroxyl side chains might interact with the carboxylate of the β-lactam in the covalent complex, and the acylation might be decreased by changes of these contacts (204). Ile312 and Val316 are situated opposite Ser310 and Lys313 in the SxxK active site motif on the α2 helix, and they pack into a hydrophobic pocket. These interactions may be disrupted and the location of the SxxK active site motif altered by mutations to larger (I312M) or more hydrophilic (V316T) side chains, resulting in lowered acylation (204). Furthermore, the N512Y alteration in mosaic PBP2 has also been shown to be involved in decreasing the susceptibility to ESCs, without affecting the susceptibility to penicillin (204). This amino acid residue is located relatively distant from the active site, on the same disordered β3-β4 loop that harbors mutations associated with penicillin resistance, and its reversion in mosaic PBP2 of a gonococcal strain with decreased ESC susceptibility to the wild type decreased the MICs of ceftriaxone and cefixime 2-fold. Possibly, the N512Y alteration perturbs the architecture of the KTG active site motif of the β3 strand (204). Finally, introduction of an A501V mutation, which has been found mainly in gonococcal isolates with decreased ESC susceptibility that lack a mosaic penA gene (246, 249–251), into a mosaic PBP2 increased the MICs of ceftriaxone and cefixime 2- to 4-fold, i.e., resulted in in vitro resistance (204), which further emphasizes the importance of the β3-β4 loop of PBP2 for ESC resistance.
Some additional PBP2 alterations, such as the G542S, P551S, and P551L mutations, have also been associated statistically with elevated MICs of ceftriaxone in gonococci (252). However, their effects on the ceftriaxone MIC have not yet been proven with, e.g., site-directed penA mutations in isogenic strain backgrounds.
In regard to N. gonorrhoeae strains displaying high-level resistance to all ESCs, the new mosaic penA allele in the first reported XDR strain (from Kyoto, Japan), showing high-level resistance to ceftriaxone and cefixime, contained 12 amino acid changes compared to mosaic penA allele X, which was associated with most of the early cefixime resistance and treatment failures in Japan (3). Recently, it was verified that three of these mutations (A311V, V316P, and T483S) resulted in the significant increases in the MICs of ceftriaxone and cefixime (253). Ala311 and Val316 are located on the same α2 helix of PBP2 as the Ser310 nucleophile. Alterations in the hydrophobic packing of α2 caused by the A311V and V316P mutations might modify the dynamics of transition state formation and result in decreased ESC acylation. Furthermore, the bulky amino acid proline, known to promote helix kinking, at position 316 might substantially influence the α2 helix structure. The T483S mutation is highly conservative, but the loss of the methyl group of Thr can have a substantial impact on ESC acylation. Thr483 is located on a loop preceding the β3 strand that comprises the KTG motif and is situated near the active site. Thus, the T483S alteration may disturb the interaction with ESCs that can increase the energy for activating the formation of the transition state and, accordingly, result in a lowered acylation rate, and/or Thr483 might be important for binding ESCs, and the T483S mutation increases binding and accordingly decreases the second-order acylation (253). XDR and ESC-resistant gonococcal strains have now been isolated in France (4) and Spain (2), and both isolated strains belong to the multilocus sequence type ST1901 and the N. gonorrhoeae multiantigen sequence type ST1407 (254), which has been stated as a multidrug-resistant clone accounting for a large proportion of the decreased susceptibility and resistance to ESCs in many countries worldwide (5). Both strains also contained a mosaic penA allele type XXXIV gene (3) with an additional A501P alteration and displayed high-level resistance to ceftriaxone and cefixime (2, 4). Transformation verified that the new mosaic penA allele resulted in the ESC resistance. It was also proposed that replacement of the methyl side chain of Ala501, situated on the β3-β4 loop, very close to the PBP2 KTG active site motif (4, 204), with the more bulky side chain of proline (A501P) results in secondary structure changes and inhibits the binding of ceftriaxone and cefixime to PBP2 by clashing with their R1 substituents (4). In general, the full cause of ESC resistance in all XDR gonococcal strains with high-level resistance to all ESCs needs to be appropriately elucidated and verified, and this is in progress. Site-directed mutagenesis experiments and crystal structures of the altered forms of PBP2 in these strains are crucial for detailing the molecular mechanisms underlying the reduced acylation by ESCs, all mutations involved in influencing the MICs of ESCs (mutations causing resistance and involved in epistasis), and how the essential transpeptidase function is concomitantly preserved. Furthermore, it is crucial to investigate the in vitro and, particularly, in vivo biological fitness, e.g., in appropriate mouse models, of these XDR gonococcal strains with high-level resistance to all ESCs, and this is also in progress.
Finally, despite the fact that specific alteration of the lethal target PBP2 is the primary resistance mechanism, as for penicillin (see above), an increased efflux and decreased influx of ESCs, due to the mtrR resistance determinant (3–5, 197–199) and penB resistance determinants (3–5, 199–201, 249), respectively, result in increased MICs of ESCs. Interestingly, both mtrR and penB have greater effects on the MICs of ceftriaxone than on those of cefixime, suggesting that cefixime is not an ideal substrate for either the MtrCDE efflux pump or the PorB1b porin (199). The stepwise transfer and interplay of the chromosomally mediated resistance determinants have been elucidated for both penicillin (187) and ESCs (199).
ponA (L421P) and pilQ (e.g., E666K) mutations (resistance determinants for penicillin) do not seem to affect the MICs of ESCs in presently circulating clinical gonococcal strains (5, 187, 199, 202). Most strains with decreased susceptibility or resistance to ESCs contain the ponA1 allele. Nevertheless, this most likely reflects only that these strains evolved by horizontal transfer of mosaic penA alleles into preexisting chromosomally mediated penicillin-resistant strains, which remain highly prevalent in the N. gonorrhoeae population (5, 199). pilQ2 (or any other pilQ loss-of-function mutation) has never been reported in clinical isolates, likely because it disrupts type IV pilus formation, and thus pathogenic potential (5, 203, 255). Nevertheless, contributions to enhanced resistance of ponA and/or pilQ polymorphisms in future ESC-resistant strains cannot be excluded. Finally, as with penicillin resistance, the nontransformable “factor X” can influence the MICs of ESCs (3–5, 199, 204).
Increased Efflux and Decreased Influx of Antimicrobials
Increased efflux.
The capacity of cells to export drugs from their interior was first described in the field of oncology, with the observation that the P-glycoprotein could export antitumor agents (256). Since then, many bacterial efflux pumps have been described, and they can be grouped into the following main categories based on their overall composition and the structures of their transporter protein and the entire pump system: (i) the major facilitator (MF) family, (ii) the small multidrug resistance (SMR) family, (iii) the resistance-nodulation-cell division (RND) family, (iv) the multidrug and toxic compound extrusion (MATE) family, and (v) the ATP-binding cassette (ABC) family (89).
In gonococci, four efflux pump systems (MtrCDE, MacAB, NorM, and FarAB) produced by all strains have been identified (235, 241, 257, 258). The MtrCDE, MacAB, NorM, and FarAB systems belong to the RND, ABC, MATE, and MF families, respectively, and the MtrCDE, MacAB, and NorM efflux pump systems have been shown to recognize antimicrobials previously or currently recommended for gonorrhea treatment. Furthermore, the mef-encoded efflux pump protein, which recognizes macrolides, has been identified in a few gonococcal strains, harbored on a conjugative transposon (41, 242, 259). Based on decreases in MICs of antimicrobials that result from insertional inactivation of the gene encoding the cytoplasmic membrane transporter component of the relevant pump, it has been shown that from the periplasmic space, the MtrCDE efflux pump can export structurally diverse hydrophobic antimicrobials, such as macrolides, β-lactam antimicrobials such as penicillin and ESCs, ciprofloxacin, and tetracycline (198, 234, 235, 237–240, 260). The NorM efflux pump exports fluoroquinolones (258), while the MacAB efflux pump can export macrolides, and its loss has been linked to increased susceptibility of gonococci to penicillin G and ESCs (241, 260).
The MtrCDE and FarAB efflux pumps also export host-derived antimicrobials, including cationic antimicrobial peptides (261) and long-chain fatty acids (257). Possession of an active MtrCDE efflux pump is critical for gonococcal survival during experimental infection of the lower genital tract of female mice (262), and its capacity to export host antimicrobials has been suggested to be important for virulence (263) and gonococcal fitness in this mouse infection model (171, 172).
The MtrCDE pump is the efflux system that is studied most in regard to gonococcal AMR. Expression of the mtrCDE efflux pump operon is under the negative and positive control of both cis- and trans-acting factors (Fig. 2). Gonococcal strains showing intermediate-level resistance to substrates (hydrophobic drugs, dyes, detergents, and host-derived antimicrobials [cationic peptides and progesterone]) of the MtrCDE efflux pump (89) typically have missense mutations in a DNA-binding-domain coding region of the mtrR gene (commonly a G45D mutation in the helix-turn-helix [HTH] domain of amino acid residues 32 to 53 [264]), which encodes the MtrR repressor that binds to the mtrCDE promoter (265). However, strains expressing high-level resistance have mutations (most frequently a single nucleotide [A] deletion in the 13-bp inverted repeat sequence between the −10 and −35 hexamer sequences) in the overlapping mtrR promoter (237, 239) or, substantially more rarely, have a C-to-T transition 120 bp upstream of the mtrC translational start codon that generates a consensus −10 hexamer sequence (TATAAT) and a novel promoter for high-level transcription of mtrCDE outside the control of DNA-binding proteins, such as MtrR (Fig. 2), which modulates expression from the wild-type promoter (172, 198). Other rare alterations that increase expression of the MtrCDE efflux pump have been described, e.g., a 153-bp Correia element (CE) (266) insertion sequence located between the mtrR/mtrC promoter and the mtrC gene (267, 268). The emergence of this alteration suggests that either a gonococcal CE element located elsewhere on the chromosome was repositioned to the mtr locus or meningococcal CE DNA sequences containing flanking mtr DNA were imported and recombined at this site.
FIG 2.
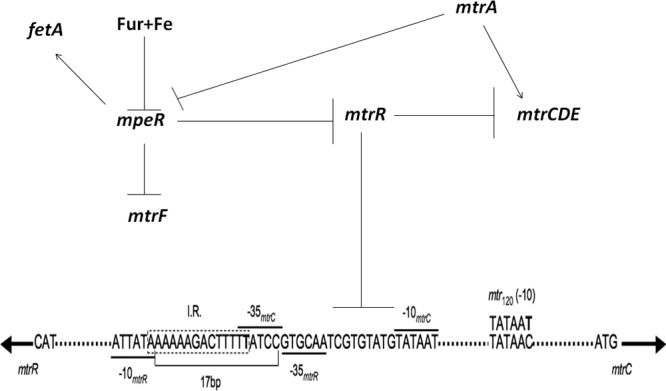
Trans- and cis-acting regulatory elements that control expression of the mtrCDE efflux pump operon in Neisseria gonorrhoeae. Trans-acting elements behaving as repressors or genes that encode them are shown as barred lines (⊥), while those encoding activators are shown by arrows. The promoters responsible for transcription of mtrR and mtrCDE are shown with their respective −10 and −35 hexamer sequences (see bars over hexamers). Note that mtrR and mtrCDE are transcriptionally divergent, and only the mtrCDE coding strand is shown. The position of the 13-bp inverted repeat sequence (I.R.; AAAAAGACTTTTT) between the −10 and −35 hexamers of the mtrR promoter is shown within the 14 nucleotides in the dotted box, and a T nucleotide that is frequently deleted in strains that overexpress mtrCDE and exhibit a high level of resistance to pump substrates is shown in bold (329). The position of the new −10 hexamer sequence generated by a point mutation (C to T) that acts as a new promoter (mtr120) for mtrCDE transcription (198) is shown. MtrR repression of mtrCDE is due to binding of two homodimers to the mtrCDE promoter, as shown by the barred line that extends to the region shown in the nucleotide sequence. MtrA binds upstream of the mtrCDE promoter (265). (Adapted from reference 348 with permission.)
In regard to the MacAB and NorM efflux pump systems, mutations upstream of the macAB and norM loci have been shown to modulate levels of gonococcal susceptibility to antimicrobials by altering gene expression (241, 258). The macA and macB genes in N. gonorrhoeae are organized as an operon, and the start point of macAB transcription is located 37 nucleotides upstream of the translational start codon. A SNP in the putative −10 hexamer sequence (TAGAAT → TATAAT) has been shown to increase transcription of macAB and to result in an increased resistance to macrolides (241). Similarly, a SNP in the −35 hexamer sequence (CTGACG → TTGACG) in the gonococcal norM promoter can enhance transcription of norM, resulting in decreased susceptibility to norfloxacin and ciprofloxacin (258). Resistance levels could be increased further by a second mutation that mapped to the ribosome-binding site (TGAA → TGGA). While the levels of resistance were insufficient to provide clinical resistance by themselves, they could be significant in strains expressing a level of ciprofloxacin resistance near the MIC breakpoint.
Decreased influx.
The Gram-negative outer membrane is an important permeability barrier to different compounds, including antimicrobials (269). Certain antimicrobials, such as penicillin, tetracycline, and ESCs, diffuse into the periplasmic space of Gram-negative bacteria through outer membrane channels formed by porin proteins. A gonococcal strain produces one of two mutually exclusive forms of porin, namely, PorB1a and PorB1b (previously named major outer membrane protein, protein I, or Por), which are universally present as trimeric pore-forming transmembrane porins (270). Strains expressing PorB1a were early shown to be slightly more susceptible to penicillin and tetracycline than strains expressing PorB1b (188, 201, 271). Moreover, amino acid alterations in loop 3 of PorB1b, which folds into the barrel of the porin and constricts the pore, confer decreased susceptibility of gonococci to penicillin, tetracycline, and ESCs (199, 201, 202, 271). Interestingly, penicillin and ceftriaxone are more affected than cefixime, suggesting that either cefixime does not readily diffuse into the periplasm through PorB1b or such diffusion is not altered by the penB determinant. The net charge of cefixime (−2) also differs from the net charge of penicillin (−1) and ceftriaxone (−1), which may affect the permeation properties (202). The most-studied mutations (collectively named penB), which result in amino acid replacements at position 120 alone (G120K) or positions 120 and 121 (G120D/A121D), decrease antimicrobial entry (201). Interestingly, the phenotypic impact of penB is only apparent in an mtrR mutant strain that overexpresses the MtrCDE efflux pump (200, 272).
PilQ is a doughnut-like multimeric secretin in the outer membrane, composed of 12 identical 75-kDa subunits, that is used by gonococci to secrete the growing pilus molecule (273, 274). PilQ also appears to be involved in controlling the influx of antimicrobials by gonococci, as specific mutations in pilQ have a significant impact on levels of gonococcal susceptibility to structurally distinct antimicrobials (202, 275). A spontaneously arising missense mutation (penC) that decreased gonococcal susceptibility to penicillin was earlier mapped to pilQ (202). Interestingly, penC (E666K mutant; later renamed the pilQ2 mutation) had no effect on the MICs of penicillins (or tetracycline and ciprofloxacin) in the absence of the mtrR and penB resistance determinants, suggesting that the rate of antimicrobial influx through the PilQ complex in cells lacking mtrR and penB is only a small fraction of the rate through porins (202). Only when mtrR and penB mutations, which together substantially decrease antimicrobial levels in the periplasm, are present is the influx through PilQ a significant proportion of the total antimicrobial influx. Although PilQ has a role in permeation of antimicrobials, it is unlikely that pilQ2-like mutations or pilQ deletions have any role in clinical resistance, since these mutations also decrease the stability of the PilQ dodecamer and disrupt the proper piliation that is critical for the pathogenesis of gonococci (203). In accordance with this, sequencing of pilQ genes from a collection of geographically and temporally diverse isolates (n = 63) with a range of ESC susceptibilities revealed nine different PilQ amino acid sequences, but none of these showed a significant association with increased MICs of ESCs (255).
ANTIMICROBIAL RESISTANCE AND GONOCOCCAL FITNESS
Bacterial strains with resistance to an antimicrobial have an advantage both in vitro and in vivo over antimicrobial-susceptible strains in the presence of the given antimicrobial; nevertheless, they are commonly less fit in the absence of the antimicrobial (276–278). However, compensatory/stabilizing/repairing mutations that restore fitness while retaining the AMR occur frequently in vitro, and likely also in vivo. Interestingly, in gonococci, AMR mechanisms do not necessarily cause significantly lower biological fitness, which results in the persistence of AMR and MDR/XDR strains even in the absence of obvious antimicrobial pressure (the antimicrobial is not used in the gonorrhea treatment anymore). Nevertheless, selection due to the general antimicrobial pressure in society remains. In fact, some AMR determinants (e.g., mtrR and gyrA mutations) appear to even enhance the biological fitness of at least some gonococcal strains. Consequently, many years after penicillin, tetracycline, and fluoroquinolones were abandoned from the recommended treatment of gonorrhea, resistant strains continue to represent a significant percentage of isolates globally (5, 8, 19, 81).
AMR and AMR determinants enhancing the fitness of bacteria in vivo are exceedingly rare, but derepression of the gonococcal mtrCDE efflux pump operon is one example. In a female mouse (BALB/c) model of lower genital tract infections, null mutations in mtrD or mtrE, resulting in loss of the MtrCDE efflux pump, decreased the capacity of gonococcal strains to cause a sustained (12-day) infection (262). This was likely caused by the inability of the strain to export host-derived hydrophobic agents (e.g., antimicrobial peptides and/or progesterone) by the MtrCDE efflux pump (235, 261). Furthermore, an mtrR null mutation that resulted in loss of MtrR, a moderate overexpression of mtrCDE, and enhanced AMR greatly increased the gonococcal fitness in vivo. In contrast, loss of MtrA, which would abrogate the inducible overexpression of the efflux pump (Fig. 2), decreased fitness in vivo. However, this decrease in fitness could be reversed by compensatory mutations that mapped to mtrR (171). Interestingly, clinically isolated mutations in the mtr locus differ in the degree to which they derepress the mtrCDE operon, and the resultant gradient in erythromycin MICs parallels the degrees of resistance to host-derived substrates and in vivo fitness conferred by these mutations (172). It is important to remember that other MtrR- and/or MtrA-regulated genes may also be important for the altered fitness. In addition to regulating mtrCDE, MtrR can positively or negatively regulate >70 other genes in the gonococcal genome, including glnA, glnE, ponA, pilMNOPQ, and rpoH (197, 279, 280). There is also suggestive clinical evidence that the MtrCDE efflux pump enhances the ability of gonococci to survive during human infection. For instance, gonococcal mtrR mutants have for decades been isolated frequently from cases of rectal gonorrhea (281–283), presumably because this environment is rich in hydrophobic agents, such as long-chain fatty acids and bile salts. The MtrCDE efflux pump probably also helps gonococci to evade innate immune responses involving antimicrobial peptides, since the pump can recognize the human cathelicidin LL-37 (261).
It was also recently shown that S91F and D95N mutations in gyrA, resulting in intermediate resistance to ciprofloxacin, enhanced gonococcal fitness in vivo as assessed by competition with the parental (wild type or mtrR negative) strain in the lower genital tract of female mice (170). Interestingly, this fitness benefit was not observed when the strains were cultured competitively in vitro, and in fact, strains with both gyrA and mtrR mutations were attenuated for growth relative to the parent strain. Addition of a D86N mutation in parC, resulting in high-level ciprofloxacin resistance, caused a substantial fitness cost in vivo. Nevertheless, compensatory mutations that increased fitness while maintaining ciprofloxacin resistance were selected in some mice. Subsequent studies have shown that gyrA together with parC mutations in other strain backgrounds have a fitness benefit similar to that conferred by gyrA mutations alone, which suggests that ciprofloxacin-resistant strains may spread even without acquiring compensatory mutations.
ENVIRONMENTAL CONDITIONS AND GONOCOCCAL RESISTANCE TO ANTIMICROBIALS
N. gonorrhoeae can respond to stresses imposed by its local environment and alter the expression of genes involved in metabolism and cell envelope structure. With respect to AMR, Rouquette et al. (284) showed that expression of the mtrCDE-encoded efflux pump is enhanced when gonococci are grown in the presence of sublethal levels of pump substrates. This induction required the presence of the MtrA protein (265, 284), a member of the AraC family of transcriptional activators (Fig. 2). In addition, expression of mtrCDE is linked to the availability of free iron (285). Such iron-mediated regulation of mtrCDE involves the capacity of the MpeR regulator to transcriptionally repress mtrR, encoding the master repressor of mtrCDE, as well as mtrF (286) (Fig. 2). In turn, mpeR expression is repressed by Fur in the context of iron (Fig. 2). Thus, under conditions of low iron, mtrCDE expression could be enhanced, allowing gonococci to increase their resistance to substrates of the MtrCDE efflux pump, including conventional antimicrobials and host-derived antimicrobials. Based on the work of Warner et al. (171), resistance to the latter would increase the fitness of gonococci during infection. It is important that this model suggests that during infection, gonococcal resistance to antimicrobials recognized by this efflux pump may be elevated compared to the resistance (in regard to MIC values) obtained under laboratory conditions, which typically employ media rich in iron. Interestingly, MpeR also transcriptionally activates expression of fetA, which encodes a receptor for enterobactin-like siderophores produced by other bacteria (287). Recent work (288) has shown that both fetA and mpeR are upregulated during infection of human macrophages, suggesting that iron limitation in phagocytes may enhance bacterial metabolism and AMR. Taken together, conditions of iron limitation may support the ability of gonococci to resist antimicrobials and proliferate during infection.
Gonococci also use host-derived compounds to resist antimicrobials. For instance, physiologic levels of polyamines (spermine and spermidine) found in the male genital tract can coat the surfaces of bacteria, making them more resistant to complement-mediated killing by normal human serum and cationic antimicrobial peptides (289). The importance of gonococcal resistance to cationic antimicrobial peptides mediated by phosphoethanolamine (PEA) decoration of lipid A during infection was recently documented (290). In this work, loss of the gene (lptA) encoding the enzyme that adds PEA to the 4′ position of lipid A decreased gonococcal fitness during experimental genital tract infections of female mice and human male volunteers.
Gonococci, like other bacteria (291), have the ability to form biofilms under laboratory conditions, and this community lifestyle also likely exists during infection (292, 293). Bacteria growing in biofilms are often more resistant to antimicrobials than their planktonic counterparts, and this property should be considered in evaluating the possible efficacy of newly developed antimicrobials to treat gonorrhea. Interestingly, recent work by Goytia et al. (294) showed that gonococcal biofilm formation can be blocked by the presence of spermine and spermidine, an observation that should be considered in testing the efficacy of new antimicrobials.
INTERNATIONAL RESPONSES TO RESISTANCE EVOLUTION AND POSSIBLE EMERGENCE OF UNTREATABLE GONORRHEA
The evolution of AMR in gonococci, and particularly the emergence of resistance to ceftriaxone, is of great concern internationally. As a response to this serious situation, recommendations to use dual-antimicrobial treatment regimens for uncomplicated anogenital and pharyngeal gonorrhea were introduced in all of Europe (17), the United States (15), the United Kingdom (16), and some regions within countries (e.g., Ontario, Canada [295]). These treatment guidelines recommend intramuscular ceftriaxone (250 mg [15] or 500 mg [16, 17, 295], in a single dose) together with either oral azithromycin (1 g [15, 16, 295] or 2 g [17]) or doxycycline (100 mg twice daily for 7 days) (15), which will also eradicate concomitant C. trachomatis infections. These dual-antimicrobial treatment regimens currently appear to be highly effective and can be recommended.
Furthermore, the WHO has published a global action plan (13, 14), and the U.S. CDC (11) and the European Centre for Disease Prevention and Control (ECDC) (12) have published regional response plans, to control and mitigate the spread of multidrug-resistant N. gonorrhoeae. A key component of these action/response plans is to substantially enhance the quality-assured surveillance of gonococcal AMR and gonorrhea treatment failures (using recommended treatments), locally, nationally, and internationally. The WHO Global GASP was established in the early 1990s but was relaunched in 2009 (13). The WHO GASP aims to act in close liaison with other existing GASPs; for example, in the European Union/European Economic Area (EU/EEA), the ECDC is responsible for the regional Euro-GASP (105, 106, 130, 296), and national GASPs exist in the United States (297; http://www.cdc.gov/std/gisp), the United Kingdom (298; http://www.hpa.org.uk/Publications/InfectiousDiseases/HIVAndSTIs/GRASPReports/), and several additional countries. This enhanced gonococcal AMR surveillance should monitor trends in resistance (including regional differences), identify newly emerging AMR, and inform treatment recommendations in a timely manner. Worryingly, longitudinal quality-assured GASPs have been incomplete in some regions (such as Latin America and the Caribbean [99, 129]) or have been sporadic or completely lacking in large parts of the world, including Eastern Europe, Central Asia, and Africa (104, 106, 152). The latter regions also suffer from a high burden of gonorrhea, creating the prerequisites for rapid emergence and spread of gonococcal AMR. Accordingly, it is crucial to establish and maintain gonococcal culture and AMR surveillance in these regions and others worldwide. This requires significant political will and funding as well as an investment in laboratory infrastructure and staff training. However, all the action/response plans also strongly emphasize the importance of more holistic actions. These include, for example, to also combat the global burden of gonorrhea and substantially improve the early prevention, diagnosis, contact tracing, treatment, and epidemiological surveillance of gonorrhea cases. These efforts need to be combined with wider strategies for general antimicrobial control (guidelines for appropriate use, selection, supplies, quality, etc.) and an increased awareness among microbiologists, epidemiologists, and clinicians and on political levels; the latter is especially important for financing efforts in gonorrhea control. In general, there is an urgent need for an enhanced focus on reducing gonorrhea burdens in high-risk, frequently transmitting populations (such as CSWs and MSM), as well as appropriate diagnosis and treatment of pharyngeal gonorrhea, which is harder to eradicate and is an asymptomatic reservoir for gonorrhea-causing bacteria and emergence of AMR. Finally, many crucial research needs should be a priority and should be stressed, including substantially intensifying research efforts to identify or develop alternative therapeutic strategies and, particularly, compounds for treatment of gonorrhea (14).
FUTURE PERSPECTIVES FOR TREATMENT
The dual-antimicrobial treatment regimens with ceftriaxone and azithromycin introduced in the United States (15) and Europe (17) appear to currently be highly effective and should be considered in all settings where comprehensive, quality-assured local AMR surveillance data are lacking or not clearly supporting any other treatment regimen (298, 299). However, the susceptibility to ceftriaxone has been decreasing globally in recent years, resistance to azithromycin is prevalent in many settings and emerges rapidly in settings where this drug is frequently used, and gonococcal strains with decreased susceptibility or resistance to ceftriaxone and concomitant azithromycin resistance are already circulating globally. Gonococcal strains with high-level resistance to azithromycin (MICs of ≥256 μg/ml) are also reported from an increasing number of countries (37–39, 42, 132, 133). Furthermore, dual-antimicrobial therapy might not be affordable in lower-resource settings, many of which suffer from the highest gonorrhea burdens, and consequently may not significantly mitigate AMR emergence and spread globally (5, 8, 299). This suggests that the currently recommended dual-antimicrobial regimens will not be effective long-term solutions. From a global public health perspective, novel antimicrobials or other therapeutic compounds for effective monotherapy, or at least for inclusion in a new dual-therapy regimen, are essential. A randomized clinical multicenter trial was recently conducted to evaluate gentamicin (240 mg given once intramuscularly) plus azithromycin (2 g given once orally) and gemifloxacin (320 mg given once orally) plus azithromycin (2 g given once orally) as potential alternative treatment options for uncomplicated gonorrhea, that is, as a potential salvage therapy if widespread ceftriaxone resistance emerges. Microbiological cure was accomplished by 100% of the gentamicin-azithromycin patients and by 99.5% of gemifloxacin-azithromycin patients. Unfortunately, adverse events were relatively frequent in both dual-antimicrobial arms (300).
The parenteral aminoglycoside gentamicin has been used as a first-line treatment for 20 years in Malawi, without any obvious in vitro resistance (301, 302), and in Europe the in vitro susceptibility is also high (303). Nevertheless, in Malawi, gentamicin has mainly not been used for gentamicin monotherapy but instead for syndromic management of urogenital infections, together with doxycycline, and no data regarding treatment efficacy against pharyngeal or anorectal gonorrhea exist. Furthermore, correlates between MICs of gentamicin, pharmacokinetic/pharmacodynamic parameters, and treatment outcomes and, consequently, evidence-based microbiological resistance breakpoints are lacking. Finally, single-dose gentamicin treatment had a pooled cure rate of only 91.5% in a recent meta-analysis (304). The novel oral fluoroketolide solithromycin was recently shown to have high in vitro activity against gonococci, including ESC-resistant XDR and MDR isolates (305). A recent minor phase 2 single-center, open-label study also showed that a single oral dose of solithromycin (1.2 g) successfully treated all 22 evaluable patients with uncomplicated gonococcal infection (306). Nevertheless, gonococcal isolates with high-level resistance to azithromycin (MICs of ≥256 μg/ml) have solithromycin MICs of 4 to 32 μg/ml, which indicates resistance (305). The in vitro activity of ertapenem (parenteral 1-β-methyl-carbapenem) against gonococci, including MDR and XDR isolates, is also high (307). However, ESC resistance determinants, i.e., mosaic penA alleles, mtrR, and penB, also increase the ertapenem MIC (307). Accordingly, future remodeling of PBP2, further decreasing acylation by ertapenem and working synergistically with the existing resistance determinants, appears likely. Gentamicin, solithromycin, and possibly ertapenem might be options for future treatment of gonorrhea, but probably not for first-line empirical monotherapy and instead for salvage therapy for ceftriaxone-resistant cases and/or as one of the antimicrobials in a dual-antimicrobial treatment regimen.
Regarding the pipeline of derivatives of previously developed antimicrobials, some novel drugs have been evaluated in vitro against N. gonorrhoeae isolates. The broad-spectrum parenteral glycylcycline tigecycline, a tetracycline derivative, showed potent in vitro activity against gonococci, including tetracycline-resistant strains (308, 309). Worryingly, tigecycline is mostly eliminated through the bile, and a limited proportion of the given antimicrobial is excreted unchanged in the urine, which calls into question the use of tigecycline to treat urinary tract infections (310–312). Another novel and fully synthetic tetracycline analog (fluorocycline), eravacycline (TP-434), was recently shown to have a high in vitro activity against tetracycline-, penicillin-, and ciprofloxacin-resistant gonococcal isolates (313). The semisynthetic lipoglycopeptide dalbavancin recently showed potent activity against a small number of gonococcal isolates (314). Two new broad-spectrum parenteral 2-acyl carbapenems, SM-295291 and SM-369926, showed high in vitro antimicrobial activities against gonococci, including ciprofloxacin-resistant isolates (315). Two novel broad-spectrum fluoroquinolones, avarofloxacin (JNJ-Q2) (316) and delafloxacin (317), also displayed high in vitro activities against gonococcal isolates, including ciprofloxacin-resistant isolates. A phase 3 clinical trial has also been designed to compare delafloxacin (2 450-mg tablets administered once) to ceftriaxone (250 mg given once intramuscularly) for treatment of uncomplicated gonorrhea (http://clinicaltrials.gov/show/NCT02015637).
Nevertheless, it is important to emphasize that N. gonorrhoeae has developed resistance to all antimicrobials introduced for first-line therapy during the last 70 to 80 years, and for more sustainable future treatment, it might be essential to “think outside the box.” Thus, it is imperative to focus not only on derivatives of previously developed antimicrobials but also on the development and investigation of new targets, compounds, and approaches for treatment. This could be a novel single target; however, ideally, multiple targets, compounds, and/or approaches to also suppress resistance development will retain the antimicrobials for longer use. Notably, several antimicrobials or other compounds, using new targets or mechanisms, were recently developed, and several have shown high in vitro activities against gonococcal isolates. These include novel inhibitors of protein synthesis, e.g., pleuromutilin BC-3781 (318, 319) and the boron-containing inhibitor AN3365 (320, 321); novel inhibitors of bacterial topoisomerases that target regions different from the fluoroquinolone-binding sites (322, 323), such as VT12-008911 (324) and AZD0914 (323, 325, 326); FabI inhibitors, such as MUT056399 (327, 328); noncytotoxic nanomaterials (329); inhibitors of efflux pumps, particularly coadministered with appropriate antimicrobials, that increase the susceptibility to certain antimicrobials, the innate host defense, and toxic metabolites (234, 260, 330); LpxC inhibitors (331); molecules mimicking host defensins; host defense peptides, such as LL-37 (multifunctional cathelicidin peptide) (332); and IL-12 NanoCap, which is a biodegradable sustained-release formulation of human interleukin 12 that aims to be a therapeutic vaccine against N. gonorrhoeae (TherapyX Inc.). Several of these novel antimicrobials or other types of compounds deserve further attention for their potential use as future treatments for gonorrhea. For example, the activities of many of these have not been examined against XDR or MDR gonococcal isolates, particularly those with ESC resistance or high-level azithromycin resistance. Furthermore, despite the fact that a breakthrough is lacking, as part of “thinking outside the box,” it can be important to continuously evaluate appropriate plant extracts for in vitro activity against gonococci and, subsequently, for treatment of gonorrhea (136, 333–345). All novel potential treatment regimens for gonorrhea require up-to-date and comprehensive in vitro and, subsequently, in vivo evaluations, including appropriately designed, randomized, and controlled clinical trials to evaluate parameters such as efficacy, safety, toxicity, cost, optimal dose, and pharmacokinetic/pharmacodynamic data for genital and extragenital (especially pharyngeal) gonorrhea. Furthermore, knowledge regarding current and future genetic resistance determinants (in vitro selected and in vivo emerged) for these antimicrobials (both in gonococci and in bystander organisms during treatment of gonorrhea), clear correlates between genetic and phenotypic laboratory parameters, and clinical treatment outcomes would be exceedingly valuable.
Additional knowledge regarding the structure and evolution of bacterial targets for antimicrobials or those that participate in resistance, prediction of the evolution of these targets and, accordingly, emergence of resistance, and whether the current or soon-to-emerge resistance mechanisms have a fitness cost or benefit will provide new opportunities for development of effective and sustainable antimicrobials. Genomic, transcriptomic, and proteomic studies coupled with additional advances in drug chemistry and high-throughput screening of compound libraries and insights gained from physiological experiments will identify novel bacterial targets and provide opportunities for rational design of new drugs. High-throughput genome sequencing and other novel molecular technologies, combined with appropriate epidemiological metadata and phylogenomic and phylogeographic analyses, will also provide an enhanced understanding of the dynamics of the national and international emergence, transmission, and evolution of antimicrobial-resistant gonococcal strains and might additionally revolutionize molecular AMR testing for both gonococcal isolates and NAAT-positive samples (346, 347). It might even be elucidated how, when, and where successfully transmitted gonococcal strains and their possible AMR emerge, further evolve, and spread (including mechanisms and time scales) in communities, nationally and internationally. This knowledge is imperative to mitigate and, ideally, predict the emergence and spread of current and newly evolved antimicrobial-resistant gonococcal strains (348). “Necessity is the mother of invention,” and the prospect of returning to the preantimicrobial era is hopefully reason enough for governments, industry, and academia to marshal their forces, and for sufficient political will and funding to be available, to combat this important problem in public health.
ACKNOWLEDGMENTS
Work in the laboratory of M.U. was supported by grants from the Research Committee of Örebro County and the Örebro University Hospital Foundation, Örebro, Sweden. Work in the laboratory of W.M.S. was supported by NIH grants R37 AI021150-29, U19 AI031496 (to P. F. Sparling, principal investigator, with project funding to W.M.S.), and R21 AI103270-01 and by a VA Merit Award from the Medical Research Service of the Department of Veterans Affairs. W.M.S. is the recipient of a Senior Research Career Scientist Award from the Medical Research Service of the Department of Veterans Affairs.
We are grateful to Daniel Golparian for support with Fig. 1.
W.M.S. serves as a consultant for Cubist Pharamaceuticals, which had no involvement with the preparation of the manuscript and did not provide research funds for work perfomed in his laboratory.
Biographies

Magnus Unemo received a Ph.D. in microbiology in 2003 at Linköping University, Sweden. In 2006, he became an Associate Professor in Medical Microbiology and Molecular Biology at Örebro University, Sweden. He currently directs the WHO Collaborating Centre for Gonorrhoea and Other STIs (linked to the WHO Headquarters in Geneva, Switzerland), Swedish Reference Laboratory for Pathogenic Neisseria, Department of Laboratory Medicine, Örebro University Hospital. His main research focuses on Neisseria gonorrhoeae and other bacterial STIs, and he has published >190 peer-reviewed papers and many chapters in international scientific books. His research involves, for example, diagnostics, molecular epidemiology, antimicrobial resistance, and mechanisms of antimicrobial resistance. Dr. Unemo is Editor of the Swedish STI reference methodology and Editor-in-Chief of the 2013 WHO manual for laboratory diagnosis of STIs and HIV, is on the Editorial Board for the European STI guidelines, and, together with the UK STI Reference Laboratory, supports the European CDC with STI reference functions.

William Shafer received a Ph.D. in microbiology in 1979 from Kansas State University, under the mentorship of J. Iandolo. From 1979 to 1982, he performed postdoctoral work with P. F. Sparling at the University of North Carolina-Chapel Hill, where he studied systems of gonococcal resistance to antimicrobials. In 1982, Dr. Shafer moved to Emory University School of Medicine in Atlanta, GA, where he is now a tenured Professor. In 1986, he moved his laboratory to the adjacent Atlanta VA Medical Center, where he established and since expanded, with continued funding from the NIH and Veterans Affairs (VA), a research program dealing with the molecular mechanisms of antimicrobial resistance. Dr. Shafer is a Fellow of the American Academy of Microbiology and a Senior Research Career Scientist of the VA Medical Research Service and has published over 120 manuscripts dealing with his continued interests in the areas of the mechanisms of pathogenesis and antibiotic resistance of Neisseria gonorrhoeae.
REFERENCES
- 1.World Health Organization (WHO). 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 67:1858–1860. 10.1093/jac/dks162 [DOI] [PubMed] [Google Scholar]
- 3.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545. 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56:1273–1280. 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Nicholas RA. 2012. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 7:1401–1422. 10.2217/fmb.12.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolan GA, Sparling PF, Wasserheit JN. 2012. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 366:485–487. 10.1056/NEJMp1112456 [DOI] [PubMed] [Google Scholar]
- 7.Ison CA. 2012. Antimicrobial resistance in sexually transmitted infections in the developed world: implications for rational treatment. Curr. Opin. Infect. Dis. 25:73–78. 10.1097/QCO.0b013e32834e9a6a [DOI] [PubMed] [Google Scholar]
- 8.Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 1230:E19–E28. 10.1111/j.1749-6632.2011.06215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiley DM, Goire N, Lahra MM, Donovan B, Limnios AE, Nissen MD, Sloots TP. 2012. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J. Antimicrob. Chemother. 67:2059–2061. 10.1093/jac/dks188 [DOI] [PubMed] [Google Scholar]
- 10.Groopman J. 1 October 2012. Sex and the superbug—the rise of drug-resistant gonorrhea. New Yorker 2012:26–30 [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). 2012. Cephalosporin-resistant Neisseria gonorrhoeae public health response plan, p 1–43 CDC, Atlanta, GA [Google Scholar]
- 12.European Centre for Disease Prevention and Control (ECDC). 2012. Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe, p 1–23 ECDC, Stockholm, Sweden [Google Scholar]
- 13.Ndowa F, Lusti-Narasimhan M, Unemo M. 2012. The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex. Transm. Infect. 88:317–318. 10.1136/sextrans-2012-050674 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO), Department of Reproductive Health and Research. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae, p 1–36 WHO, Geneva, Switzerland [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 61:590–594 [PubMed] [Google Scholar]
- 16.Bignell C, Fitzgerald M. 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int. J. STD AIDS 22:541–547. 10.1258/ijsa.2011.011267 [DOI] [PubMed] [Google Scholar]
- 17.Bignell C, Unemo M. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 24:85–92. 10.1177/0956462412472837 [DOI] [PubMed] [Google Scholar]
- 18.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, Zimba D, Vernazza PL, Maida M, Fiscus SA, Eron JJ., Jr 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868–1873. 10.1016/S0140-6736(97)02190-9 [DOI] [PubMed] [Google Scholar]
- 19.Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev. Anti Infect. Ther. 7:821–834. 10.1586/eri.09.63 [DOI] [PubMed] [Google Scholar]
- 20.Unemo M, Ison C. 2013. Gonorrhoea, p 21–54 In Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. World Health Organization (WHO), Geneva, Switzerland [Google Scholar]
- 21.Katz AR, Effler PV, Ohye RG, Brouillet B, Lee MV, Whiticar PM. 2004. False-positive gonorrhea test results with a nucleic acid amplification test: the impact of low prevalence on positive predictive value. Clin. Infect. Dis. 38:814–819. 10.1086/381895 [DOI] [PubMed] [Google Scholar]
- 22.Palmer HM, Mallinson H, Wood RL, Herring AJ. 2003. Evaluation of the specificities of five DNA amplification methods for the detection of Neisseria gonorrhoeae. J. Clin. Microbiol. 41:835–837. 10.1128/JCM.41.2.835-837.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll SO, Garland SM, Tapsall J. 2011. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J. Clin. Microbiol. 49:3610–3615. 10.1128/JCM.01217-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiley DM, Tapsall JW, Sloots TP. 2006. Nucleic acid amplification testing for Neisseria gonorrhoeae: an ongoing challenge. J. Mol. Diagn. 8:3–15. 10.2353/jmoldx.2006.050045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DW, Tapsall JW, Lum G. 2005. Guidelines for the use and interpretation of nucleic acid detection tests for Neisseria gonorrhoeae in Australia: a position paper on behalf of the Public Health Laboratory Network. Commun. Dis. Intell. 29:358–365 [DOI] [PubMed] [Google Scholar]
- 26.Goire N, Sloots TP, Nissen MD, Whiley DM. 2012. Protocol for the molecular detection of antibiotic resistance mechanisms in Neisseria gonorrhoeae. Methods Mol. Biol. 903:319–328. 10.1007/978-1-61779-937-2_22 [DOI] [PubMed] [Google Scholar]
- 27.Low N, Unemo M, Jensen JS, Breuer J, Stephenson JM. 2014. Molecular diagnostics for gonorrhoea: implications for antimicrobial resistance and the threat of untreatable gonorrhoea. PLoS Med. 11:e1001598. 10.1371/journal.pmed.1001598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadiq ST, Dave J, Butcher PD. 2010. Point-of-care antibiotic susceptibility testing for gonorrhoea: improving therapeutic options and sparing the use of cephalosporins. Sex. Transm. Infect. 86:445–446. 10.1136/sti.2010.044230 [DOI] [PubMed] [Google Scholar]
- 29.Dillon JAR, Li H, Yeung K, Aman TA. 1999. A PCR assay for discriminating Neisseria gonorrhoeae beta-lactamase-producing plasmids. Mol. Cell. Probes 13:89–92. 10.1006/mcpr.1998.0216 [DOI] [PubMed] [Google Scholar]
- 30.Palmer HM, Leeming LP, Turner A. 2000. A multiplex polymerase chain reaction to differentiate β-lactamase plasmids of Neisseria gonorrhoeae. J. Antimicrob. Chemother. 45:777–782. 10.1093/jac/45.6.777 [DOI] [PubMed] [Google Scholar]
- 31.Goire N, Freeman K, Tapsall JW, Lambert SB, Nissen MD, Sloots TP, Whiley DM. 2011. Enhancing gonococcal antimicrobial resistance surveillance: a real-time PCR assay for detection of penicillinase-producing Neisseria gonorrhoeae by use of noncultured clinical samples. J. Clin. Microbiol. 49:513–518. 10.1128/JCM.02024-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kugelman G, Tapsall JW, Goire N, Syrmis MW, Limnios A, Lambert SB, Nissen MD, Sloots TP, Whiley DM. 2009. Simple, rapid, and inexpensive detection of Neisseria gonorrhoeae resistance mechanisms using heat-denatured isolates and SYBR green-based real-time PCR. Antimicrob. Agents Chemother. 53:4211–4216. 10.1128/AAC.00385-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernel-Pauillac F, Merien F. 2006. A novel real-time duplex PCR assay for detecting penA and ponA genotypes in Neisseria gonorrhoeae: comparison with phenotypes determined by the E-test. Clin. Chem. 52:2294–2296. 10.1373/clinchem.2006.075309 [DOI] [PubMed] [Google Scholar]
- 34.Vernel-Pauillac F, Nandi S, Nicholas RA, Goarant C. 2008. Genotyping as a tool for antibiotic resistance surveillance of Neisseria gonorrhoeae in New Caledonia: evidence of a novel genotype associated with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 52:3293–3300. 10.1128/AAC.00020-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernel-Pauillac F, Falcot V, Whiley D, Merien F. 2006. Rapid detection of a chromosomally mediated penicillin resistance-associated ponA mutation in Neisseria gonorrhoeae using a real-time PCR assay. FEMS Microbiol. Lett. 255:66–74. 10.1111/j.1574-6968.2005.00053.x [DOI] [PubMed] [Google Scholar]
- 36.Starnino S, Neri A, Stefanelli P, Neisseria gonorrhoeae Italian Study Group 2008. Molecular analysis of tetracycline-resistant gonococci: rapid detection of resistant genotypes using a real-time PCR assay. FEMS Microbiol. Lett. 286:16–23. 10.1111/j.1574-6968.2008.01244.x [DOI] [PubMed] [Google Scholar]
- 37.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob. Agents Chemother. 54:3812–3816. 10.1128/AAC.00309-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galarza PG, Abad R, Canigia LF, Buscemi L, Pagano I, Oviedo C, Vázquez JA. 2010. New mutation in 23S rRNA gene associated with high level of azithromycin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 54:1652–1653. 10.1128/AAC.01506-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz AR, Komeya AY, Soge OO, Kiaha MI, Lee MV, Wasserman GM, Maningas EV, Whelen AC, Kirkcaldy RD, Shapiro SJ, Bolan GA, Holmes KK. 2012. Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin. Infect. Dis. 54:841–843. 10.1093/cid/cir929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng LK, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:3020–3025. 10.1128/AAC.46.9.3020-3025.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts MC, Chung WO, Roe D, Xia M, Marquez C, Borthagaray G, Whittington WL, Holmes KK. 1999. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob. Agents Chemother. 43:1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unemo M, Golparian D, Hellmark B. 2014. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: a threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob. Agents Chemother. 58:624–625. 10.1128/AAC.02093-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Booth SA, Drebot MA, Martin IE, Ng LK. 2003. Design of oligonucleotide arrays to detect point mutations: molecular typing of antibiotic resistant strains of Neisseria gonorrhoeae and hantavirus infected deer mice. Mol. Cell. Probes 17:77–84. 10.1016/S0890-8508(03)00005-7 [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Yokoi S, Kawamura Y, Maeda S, Ezaki T, Deguchi T. 2002. Rapid detection of quinolone resistance-associated gyrA mutations in Neisseria gonorrhoeae with a LightCycler. J. Infect. Chemother. 8:145–150. 10.1007/s101560200025 [DOI] [PubMed] [Google Scholar]
- 45.Lindbäck E, Unemo M, Akhras M, Gharizadeh B, Fredlund H, Pourmand N, Wretlind B. 2006. Pyrosequencing of the DNA gyrase gene in Neisseria species: effective indicator of ciprofloxacin resistance in Neisseria gonorrhoeae. APMIS 114:837–841. 10.1111/j.1600-0463.2006.apm_495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magooa MP, Muller EE, Gumede L, Lewis DA. 2013. Determination of Neisseria gonorrhoeae susceptibility to ciprofloxacin in clinical specimens from men using a real-time PCR assay. Int. J. Antimicrob. Agents 42:63–67. 10.1016/j.ijantimicag.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 47.Siedner MJ, Pandori M, Castro L, Barry P, Whittington WL, Liska S, Klausner JD. 2007. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J. Clin. Microbiol. 45:1250–1254. 10.1128/JCM.01909-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siedner MJ, Pandori M, Leon SR, Barry PM, Espinosa BJ, Hall ER, Coates TJ, Klausner JD. 2008. Detection of quinolone-resistant Neisseria gonorrhoeae in urogenital specimens with the use of real-time polymerase chain reaction. Int. J. STD AIDS 19:69–71. 10.1258/ijsa.2007.007206 [DOI] [PubMed] [Google Scholar]
- 49.Vernel-Pauillac F, Hogan TR, Tapsall JW, Goarant C. 2009. Quinolone resistance in Neisseria gonorrhoeae: rapid genotyping of quinolone resistance-determining regions in gyrA and parC genes by melting curve analysis predicts susceptibility. Antimicrob. Agents Chemother. 53:1264–1267. 10.1128/AAC.01104-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochiai S, Ishiko H, Yasuda M, Deguchi T. 2008. Rapid detection of the mosaic structure of the Neisseria gonorrhoeae penA gene, which is associated with decreased susceptibilities to oral cephalosporins. J. Clin. Microbiol. 46:1804–1810. 10.1128/JCM.01800-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandori M, Barry PM, Wu A, Ren A, Whittington WL, Liska S, Klausner JD. 2009. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 53:4032–4034. 10.1128/AAC.00406-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unemo M, Olcén P, Fredlund H, Thulin S. 2008. Real-time PCR and subsequent pyrosequencing for screening of penA mosaic alleles and prediction of reduced susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae. APMIS 116:1004–1008. 10.1111/j.1600-0463.2008.01062.x [DOI] [PubMed] [Google Scholar]
- 53.Whiley SM, Bates J, Limnios A, Nissen MD, Tapsall J, Sloots TP. 2007. Use of a novel screening PCR indicates presence of Neisseria gonorrhoeae with a mosaic penA gene sequence in Australia. Pathology 39:445–446. 10.1080/00313020701444515 [DOI] [PubMed] [Google Scholar]
- 54.Balashov S, Mordechai E, Adelson ME, Gygax SE. 2013. Multiplex bead suspension array for screening Neisseria gonorrhoeae antibiotic resistance genetic determinants in noncultured clinical samples. J. Mol. Diagn. 15:116–129. 10.1016/j.jmoldx.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 55.Ilina EN, Vereshchagin VA, Borovskaya AD, Malakhova MV, Sidorenko SV, Al-Khafaji NC, Kubanova AA, Govorun VM. 2008. Relation between genetic markers of drug resistance and susceptibility profile of clinical Neisseria gonorrhoeae strains. Antimicrob. Agents Chemother. 52:2175–2182. 10.1128/AAC.01420-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawung R, Cherdtrakulkiat R, Charoenwatanachokchai A, Nabu S, Suksaluk W, Prachayasittikul V. 2009. One-step PCR for the identification of multiple antimicrobial resistance in Neisseria gonorrhoeae. J. Microbiol. Methods 77:323–325. 10.1016/j.mimet.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 57.Goire N, Ohnishi M, Limnios AE, Lahra MM, Lambert SB, Nimmo GR, Nissen MD, Sloots TP, Whiley DM. 2012. Enhanced gonococcal antimicrobial surveillance in the era of ceftriaxone resistance: a real-time PCR assay for direct detection of the Neisseria gonorrhoeae H041 strain. J. Antimicrob. Chemother. 67:902–905. 10.1093/jac/dkr549 [DOI] [PubMed] [Google Scholar]
- 58.Goire N, Lahra MM, Ohnishi M, Hogan T, Liminios AE, Nissen MD, Sloots TP, Whiley DM. 2013. Polymerase chain reaction-based screening for the ceftriaxone-resistant Neisseria gonorrhoeae F89 strain. Euro Surveill. 18:20444 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20444 [DOI] [PubMed] [Google Scholar]
- 59.Oriel JD. 1994. The scars of Venus: a history of venereology. Springer-Verlag, London, United Kingdom [Google Scholar]
- 60.Milton JL. 1884. On the pathology and treatment of gonorrhoea, p 73–82 W Wood & Co, New York, NY [Google Scholar]
- 61.Bumstead FJ. 1864. The pathology and treatment of venereal diseases, revised ed, p 89–96 Blanchard & Lea, Philadelphia, PA [Google Scholar]
- 62.Dunglison RJ. 1874. A dictionary of medical science, revised ed, p 466 HC Lea, Philadelphia, PA [Google Scholar]
- 63.Lewis DA. 2010. The gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86:415–421. 10.1136/sti.2010.042648 [DOI] [PubMed] [Google Scholar]
- 64.Young HH, Hill JH, Scott WW. 1925. The treatment of infections and infectious diseases with Mercurochrome-220 soluble. Arch. Surg. 10:885–924 [Google Scholar]
- 65.Redewill FH, Potter JE, Garrison HA. 1926. Mercurochrome 220-soluble and sugar in the treatment of 1200 cases of gonorrhea urethritis and complications (with animal experimentation). J. Urol. 16:397–410 [Google Scholar]
- 66.Young HH, Colston JA, Hill JS. 1932. Infections in the genito-urinary tract, and complications. JAMA 98:715–722. 10.1001/jama.1932.02730350029007 [DOI] [Google Scholar]
- 67.Cumberbatch EP, Robinson CA. 1923. Treatment of gonococcal infection with diathermy. Br. Med. J. 2:54–56. 10.1136/bmj.2.3263.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warren SL, Wilson KM. 1932. The treatment of gonorrheal infections by artificial (general) hyperthermia. Am. J. Obstet. Gynecol. 24:592–598 [Google Scholar]
- 69.Desjardins AU, Stuhler LG, Popp WC. 1935. Fever therapy for gonococcic infections. JAMA 104:873–878. 10.1001/jama.1935.02760110001001 [DOI] [Google Scholar]
- 70.Hench PS, Slocumb CH, Popp WC. 1935. Fever therapy. Results for gonorrhea arthritis, chronic infectious (atrophic) arthritis and other forms of “rheumatism.” JAMA 104:1779–1790 [Google Scholar]
- 71.Potter JE, Redewill FH, Longley EG. 1937. Hyperpyrexia as an adjunct in the treatment of nonsurgical urologic conditions. J. Urol. 37:214–225 [Google Scholar]
- 72.Bierman W, Levenson CL. 1936. The treatment of gonorrhea arthritis by means of systemic and additional focal heating. Am. J. Med. Sci. 191:55–65. 10.1097/00000441-193601000-00006 [DOI] [Google Scholar]
- 73.Simmons EE. 1937. Value of fever therapy in the arthritides. Am. J. Med. Sci. 194:170–178. 10.1097/00000441-193708000-00003 [DOI] [Google Scholar]
- 74.Cokkinis AJ, McElligott GL. 1938. Sulfanilamide in gonorrhea. An analysis of 633 cases. Lancet ii:355–362 [Google Scholar]
- 75.Kampmeier RH. 1983. Introduction of sulfonamide therapy for gonorrhea. Sex. Transm. Dis. 10:81–84. 10.1097/00007435-198304000-00007 [DOI] [PubMed] [Google Scholar]
- 76.Van Slyke CJ, Wolcott RR, Mahoney JF. 1941. The chemotherapy of gonococcal infections. JAMA 116:276–280. 10.1001/jama.1941.02820040010004 [DOI] [Google Scholar]
- 77.Uhle CA, Latowsky LW, Knight F. 1941. Gonorrhea urethritis in the male. Treatment with sulfapyridine and sulfathiazole. JAMA 117:247–249 [Google Scholar]
- 78.Mahoney JF, Van Slyke CJ, Wolcott RR. 1941. Sulfathiazole treatment of gonococcal infections in men and women. Results in 360 patients. Vener. Dis. Inf. 22:425–431 [Google Scholar]
- 79.Dunlop EMC. 1949. Gonorrhoea and the sulphonamides. Br. J. Vener. Dis. 25:81–83 [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrence A, Phillips I, Nicol C. 1973. Various regimens of trimethoprim-sulfamethoxazole used in the treatment of gonorrhea. J. Infect. Dis. 128(Suppl):673–678 [DOI] [PubMed] [Google Scholar]
- 81.Tapsall JW. 2001. Antibiotic resistance in Neisseria gonorrhoeae. WHO document WHO/CDS/DRS/2001.3. World Health Organization, Geneva, Switzerland [Google Scholar]
- 82.Wainwright M, Swan HT. 1986. C.G. Paine and the earliest surviving clinical records of penicillin therapy. Med. Hist. 30:42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahoney JF, Ferguson C, Buchholtz M, van Slyke CJ. 1943. The use of penicillin sodium in the treatment of sulfonamide-resistant gonorrhea in men. A preliminary report. Am. J. Gonorr. Vener. Dis. 27:525–528 [Google Scholar]
- 84.Sternberg TH, Turner TB. 1944. The treatment of sulfonamide resistant gonorrhea with penicillin sodium. Results in 1686 cases. JAMA 126:157–163 [Google Scholar]
- 85.Van Slyke CJ, Arnold RC, Buchholtz M. 1943. Penicillin therapy in sulfonamide-resistant gonorrhea in men. Am. J. Public Health Nations Health 33:1392–1394. 10.2105/AJPH.33.12.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaffe HW, Biddle JW, Thornsberry C, Johnson RE, Kaufman RE, Reynolds GH, Wiesner PJ. 1976. National gonorrhea therapy monitoring study: in vitro antibiotic susceptibility and its correlation with treatment results. N. Engl. J. Med. 294:5–9. 10.1056/NEJM197601012940102 [DOI] [PubMed] [Google Scholar]
- 87.Martin JE, Jr, Lester A, Price EV, Schmale JD. 1970. Comparative study of gonococcal susceptibility to penicillin in the United States, 1955–1969. J. Infect. Dis. 122:459–461. 10.1093/infdis/122.5.459 [DOI] [PubMed] [Google Scholar]
- 88.Reyn A, Korner B, Bentzon MW. 1958. Effects of penicillin, streptomycin, and tetracycline on N. gonorrhoeae isolated in 1944 and in 1957. Br. J. Vener. Dis. 34:227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafer WM, Folster JP, Nicholas RA. 2010. Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria, p 245–268 In Genco C, Wetzler L. (ed), Neisseria—molecular mechanisms of pathogenic Neisseria. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 90.Amies CR. 1967. Development of resistance of gonococci to penicillin. An eight-year study. Can. Med. Assoc. J. 96:33–35 [PMC free article] [PubMed] [Google Scholar]
- 91.Franks AG. 1946. Successful combined treatment of penicillin-resistant gonorrhea. Am. J. Med. Sci. 211:553–555 [PubMed] [Google Scholar]
- 92.Willcox RR. 1970. A survey of problems in the antibiotic treatment of gonorrhoea. With special reference to South-East Asia. Br. J. Vener. Dis. 46:217–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ashford WA, Golash RG, Henning VG. 1976. Penicillinase producing Neisseria gonorrhoeae. Lancet ii:657–658 [DOI] [PubMed] [Google Scholar]
- 94.Percival A, Rowlands J, Corkill JE, Alergant CD, Arya OP, Rees E, Annels AE. 1976. Penicillinase-producing gonococci in Liverpool. Lancet ii:1379–1382 [DOI] [PubMed] [Google Scholar]
- 95.Phillips I. 1976. Beta-lactamase producing penicillin-resistant gonococcus. Lancet ii:656–657 [DOI] [PubMed] [Google Scholar]
- 96.Faruki H, Kohmescher RN, McKinney WP, Sparling PF. 1985. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally mediated resistance). N. Engl. J. Med. 313:607–611. 10.1056/NEJM198509053131004 [DOI] [PubMed] [Google Scholar]
- 97.Faruki H, Sparling PF. 1986. Genetics of resistance in a non-beta-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob. Agents Chemother. 30:856–860. 10.1128/AAC.30.6.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bala M, Kakran M, Singh V, Sood S, Ramesh V, Members of WHO GASP SEAR Network 2013. Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis. Sex. Transm. Infect. 89(Suppl 4):iv28–iv35. 10.1136/sextrans-2012-050904 [DOI] [PubMed] [Google Scholar]
- 99.Dillon JA, Trecker MA, Thakur SD, Gonococcal Antimicrobial Surveillance Program Network in Latin America and the Caribbean 1990–2011 2013. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex. Transm. Infect. 89(Suppl 4):iv36–iv41. 10.1136/sextrans-2012-050905 [DOI] [PubMed] [Google Scholar]
- 100.Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. 2013. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the U.S.A.: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex. Transm. Infect. 89(Suppl 4):iv5–iv10. 10.1136/sextrans-2013-051162 [DOI] [PubMed] [Google Scholar]
- 101.Kubanova A, Frigo N, Kubanov A, Sidorenko S, Priputnevich T, Vachnina T, Al-Khafaji N, Polevshikova S, Solomka V, Domeika M, Unemo M. 2008. National surveillance of antimicrobial susceptibility in Neisseria gonorrhoeae in 2005–2006 and recommendations of first-line antimicrobial drugs for gonorrhoea treatment in Russia. Sex. Transm. Infect. 84:285–289. 10.1136/sti.2007.029033 [DOI] [PubMed] [Google Scholar]
- 102.Lahra MM, Lo YR, Whiley DM. 2013. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex. Transm. Infect. 89(Suppl 4):iv19–iv23. 10.1136/sextrans-2012-050906 [DOI] [PubMed] [Google Scholar]
- 103.Martin IM, Hoffmann S, Ison CA, ESSTI Network 2006. European Surveillance of Sexually Transmitted Infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J. Antimicrob. Chemother. 58:587–593. 10.1093/jac/dkl265 [DOI] [PubMed] [Google Scholar]
- 104.Ndowa FJ, Francis JM, Machiha A, Faye-Kette H, Fonkoua MC. 2013. Gonococcal antimicrobial resistance: perspectives from the African region. Sex. Transm. Infect. 89(Suppl 4):iv11–iv15. 10.1136/sextrans-2012-050907 [DOI] [PubMed] [Google Scholar]
- 105.Spiteri G, Cole M, Unemo M, Hoffmann S, Ison C, van de Laar M. 2013. The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP)–a sentinel approach in the European Union (EU)/European Economic Area (EEA). Sex. Transm. Infect. 89(Suppl 4):iv16–iv18. 10.1136/sextrans-2013-051117 [DOI] [PubMed] [Google Scholar]
- 106.Unemo M, Ison CA, Cole M, Spiteri G, van de Laar M, Khotenashvili L. 2013. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex. Transm. Infect. 89(Suppl 4):iv42–iv46. 10.1136/sextrans-2012-050909 [DOI] [PubMed] [Google Scholar]
- 107.Morse SA, Johnson SR, Biddle JW, Roberts MC. 1986. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob. Agents Chemother. 30:664–670. 10.1128/AAC.30.5.664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roberts MC, Wagenvoort JH, van Klingeren B, Knapp JS. 1988. tetM- and beta-lactamase containing Neisseria gonorrhoeae (tetracycline resistant and penicillinase producing) in the Netherlands. Antimicrob. Agents Chemother. 32:158. 10.1128/AAC.32.1.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Easmon CS, Forster GE, Walker GD, Ison CA, Harris JR, Munday PE. 1984. Spectinomycin as initial treatment for gonorrhoea. Br. Med. J. 289:1032–1034. 10.1136/bmj.289.6451.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Judson FN, Ehret JM, Handsfield HH. 1985. Comparative study of ceftriaxone and spectinomycin for treatment of pharyngeal and anorectal gonorrhea. JAMA 253:1417–1419. 10.1001/jama.1985.03350340069019 [DOI] [PubMed] [Google Scholar]
- 111.Stolz E, Zwart HG, Michel MF. 1975. Activity of eight antimicrobial agents in vitro against N. gonorrhoeae. Br. J. Vener. Dis. 51:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ashford WA, Potts DW, Adams HJ, English JC, Johnson SR, Biddle JW, Thornsberry C, Jaffe HW. 1981. Spectinomycin-resistant penicillinase producing Neisseria gonorrhoeae. Lancet ii:1035–1037 [DOI] [PubMed] [Google Scholar]
- 113.Boslego JW, Tramont EC, Takafuji ET, Diniega BM, Mitchell BS, Small JW, Khan WN, Stein DC. 1987. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and penicillinase-producing Neisseria gonorrhoeae. N. Engl. J. Med. 317:272–278. 10.1056/NEJM198707303170504 [DOI] [PubMed] [Google Scholar]
- 114.Ison CA, Littleton K, Shannon KP, Easmon CS, Phillips I. 1983. Spectinomycin resistant gonococci. Br. Med. J. (Clin. Res. Ed.) 287:1827–1829. 10.1136/bmj.287.6408.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindberg M, Ringertz O, Sandström E. 1982. Treatment of pharyngeal gonorrhoea due to β-lactamase-producing gonococci. Br. J. Vener. Dis. 58:101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moran JS. 1995. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex. Transm. Dis. 22:39–47. 10.1097/00007435-199501000-00007 [DOI] [PubMed] [Google Scholar]
- 117.Moran JS, Levine WC. 1995. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin. Infect. Dis. 20(Suppl 1):S47–S65. 10.1093/clinids/20.Supplement_1.S47 [DOI] [PubMed] [Google Scholar]
- 118.Gransden WR, Warren CA, Phillips I, Hodges M, Barlow D. 1990. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. Lancet 335:51. 10.1016/0140-6736(90)90177-7 [DOI] [PubMed] [Google Scholar]
- 119.Tanaka M, Nakayama H, Haraoka M, Saika T. 2000. Antimicrobial resistance of Neisseria gonorrhoeae and high prevalence of ciprofloxacin-resistant isolates in Japan, 1993 to 1998. J. Clin. Microbiol. 38:521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanaka M, Kumazawa J, Matsumoto T, Kobayashi I. 1994. High prevalence of Neisseria gonorrhoeae strains with reduced susceptibility to fluoroquinolones in Japan. Genitourin. Med. 70:90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berglund T, Unemo M, Olcén P, Giesecke J, Fredlund H. 2002. One year of Neisseria gonorrhoeae isolates in Sweden: the prevalence study of antibiotic susceptibility shows relation to the geographic area of exposure. Int. J. STD AIDS 13:109–114. 10.1258/0956462021924730 [DOI] [PubMed] [Google Scholar]
- 122.Patrick D, Shaw C, Rekart ML. 1995. Neisseria gonorrhoeae with decreased susceptibility to ciprofloxacin in British Columbia: an imported phenomenon. Can. Common Dis. Rep. 21:137–139 [PubMed] [Google Scholar]
- 123.Su X, Lind I. 2001. Molecular basis of high-level ciprofloxacin resistance in Neisseria gonorrhoeae strains isolated from Denmark from 1995–1998. Antimicrob. Agents Chemother. 45:117–123. 10.1128/AAC.45.1.117-123.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iverson CJ, Wang SA, Lee MV, Ohye RG, Trees DL, Knapp JS, Effler PV, O'Connor NP, Levine WC. 2004. Fluoroquinolone-resistance among Neisseria gonorrhoeae isolates in Hawaii, 1990–2000: role of foreign importation and endemic spread. Sex. Transm. Dis. 31:702–708. 10.1097/01.olq.0000145846.45781.a4 [DOI] [PubMed] [Google Scholar]
- 125.Centers for Disease Control and Prevention (CDC). 2004. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb. Mortal. Wkly. Rep. 53:335–338 [PubMed] [Google Scholar]
- 126.Centers for Disease Control and Prevention (CDC). 2007. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 56:332–336 [PubMed] [Google Scholar]
- 127.Brown ST, Pedersen HB, Holmes KK. 1977. Comparison of erythromycin base and estolate in gonococcal urethritis. JAMA 238:1371–1373. 10.1001/jama.1977.03280140049015 [DOI] [PubMed] [Google Scholar]
- 128.Dillon JA, Ruben M, Li H, Borthagaray G, Márquez C, Fiorito S, Galarza P, Portilla JL, León L, Agudelo CI, Sanabria OM, Maldonado A, Prabhakar P. 2006. Challenges in the control of gonorrhea in South America and the Caribbean: monitoring the development of resistance to antibiotics. Sex. Transm. Dis. 33:87–95. 10.1097/01.olq.0000187231.28812.29 [DOI] [PubMed] [Google Scholar]
- 129.Starnino S, GASP-LAC Working Group. Galarza P, Carvallo ME, Benzaken AS, Ballesteros AM, Cruz OM, Hernandez AL, Carbajal JL, Borthagaray G, Payares D, Dillon JA. 2012. Retrospective analysis of antimicrobial susceptibility trends (2000–2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean show evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone. Sex. Transm. Dis. 39:813–821. 10.1097/OLQ.0b013e3182631c9f [DOI] [PubMed] [Google Scholar]
- 130.Cole M, Unemo M, Hoffmann S, Chisholm SA, Ison CA, van de Laar MJ. 2011. The European gonococcal antimicrobial surveillance programme, 2009. Euro Surveill. 16:19995 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19995 [PubMed] [Google Scholar]
- 131.Kubanova A, Frigo N, Kubanov A, Sidorenko S, Lesnaya I, Polevshikova S, Solomka V, Bukanov N, Domeika M, Unemo M. 2010. The Russian gonococcal antimicrobial susceptibility programme (RU-GASP)—national resistance prevalence in 2007 and 2008, and trends during 2005–2008. Euro Surveill. 15:19533 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19533 [PubMed] [Google Scholar]
- 132.Palmer HM, Young H, Winter A, Dave J. 2008. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J. Antimicrob. Chemother. 62:490–494. 10.1093/jac/dkn235 [DOI] [PubMed] [Google Scholar]
- 133.Starnino S, Stefanelli P, Neisseria gonorrhoeae Italian Study Group 2009. Azithromycin-resistant Neisseria gonorrhoeae strains recently isolated in Italy. J. Antimicrob. Chemother. 63:1200–1204. 10.1093/jac/dkp118 [DOI] [PubMed] [Google Scholar]
- 134.Young H, Moyes A, McMillan A. 1997. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. Int. J. STD AIDS 8:299–302. 10.1258/0956462971920127 [DOI] [PubMed] [Google Scholar]
- 135.Handsfield HH, Dalu ZA, Martin DH, Douglas JM, Jr, McCarty JM, Schlossberg D. 1994. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex. Transm. Dis. 21:107–111 [DOI] [PubMed] [Google Scholar]
- 136.Newman LM, Moran JS, Workowski KA. 2007. Update on the management of gonorrhea in adults in the United States. Clin. Infect. Dis. 44(Suppl 3):S84–S101. 10.1086/511422 [DOI] [PubMed] [Google Scholar]
- 137.Barry PM, Klausner JD. 2009. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin. Pharmacother. 10:555–577. 10.1517/14656560902731993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ison CA, Mouton JW, Jones K, Fenton KA, Livermore DM. 2004. Which cephalosporin for gonorrhoea? Sex. Transm. Infect. 80:386–388. 10.1136/sti.2004.012757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Akasaka S, Muratani T, Yamada Y, Inatomi H, Takahashi K, Matsumoto T. 2001. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J. Infect. Chemother. 7:49–50. 10.1007/s101560170034 [DOI] [PubMed] [Google Scholar]
- 140.Tanaka M, Nakayama H, Tunoe H, Egashira T, Kanayama A, Saika T, Kobayashi I, Naito S. 2002. A remarkable reduction in the susceptibility of Neisseria gonorrhoeae isolates to cephems and the selection of antibiotic regimens for the single-dose treatment of gonococcal infection in Japan. J. Infect. Chemother. 8:81–86. 10.1007/s101560200011 [DOI] [PubMed] [Google Scholar]
- 141.Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. 2010. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J. Antimicrob. Chemother. 65:2141–2148. 10.1093/jac/dkq289 [DOI] [PubMed] [Google Scholar]
- 142.Deguchi T, Yasuda M, Yokoi S, Ishida K, Ito M, Ishihara S, Minamidate K, Harada Y, Tei K, Kojima K, Tamaki M, Maeda S. 2003. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J. Infect. Chemother. 9:35–39. 10.1007/s10156-002-0204-8 [DOI] [PubMed] [Google Scholar]
- 143.Ito M, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Maeda S, Deguchi T. 2004. Remarkable increase in central Japan in 2001–2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob. Agents Chemother. 48:3185–3187. 10.1128/AAC.48.8.3185-3187.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takahashi S, Kurimura Y, Hashimoto J, Uehara T, Hiyama Y, Iwasawa A, Nishimura M, Sunaoshi K, Takeda K, Suzuki N, Tsukamoto T. 2013. Antimicrobial susceptibility and penicillin-binding protein 1 and 2 mutations in Neisseria gonorrhoeae isolated from male urethritis in Sapporo, Japan. J. Infect. Chemother. 19:50–56. 10.1007/s10156-012-0450-3 [DOI] [PubMed] [Google Scholar]
- 145.Yokoi S, Deguchi T, Ozawa T, Yasuda M, Ito S, Kubota Y, Tamaki M, Maeda S. 2007. Threat to cefixime treatment for gonorrhea. Emerg. Infect. Dis. 13:1275–1277. 10.3201/eid1308.060948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Japanese Society of Sexually Transmitted Infection. 2011. Gonococcal infection. Sexually transmitted infections, diagnosis and treatment guidelines 2011. Jpn. J. Sex. Transm. Dis. 22(Suppl 1):52–59 (In Japanese.) [Google Scholar]
- 147.Lo JY, Ho KM, Leung AO, Tiu FS, Tsang GK, Lo AC, Tapsall JW. 2008. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob. Agents Chemother. 52:3564–3567. 10.1128/AAC.00198-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hess D, Wu A, Golparian D, Esmaili S, Pandori W, Sena E, Klausner JD, Barry P, Unemo M, Pandori M. 2012. Genome sequencing of a Neisseria gonorrhoeae isolate of a successful international clone with decreased susceptibility and resistance to extended-spectrum cephalosporins. Antimicrob. Agents Chemother. 56:5633–5641. 10.1128/AAC.00636-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li SY. 2012. Global transmission of multiple-drug resistant Neisseria gonorrhoeae strains refractive to cephalosporin treatment. J. Formos. Med. Assoc. 111:463–464. 10.1016/j.jfma.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 150.Martin I, Sawatzky P, Allen V, Hoang L, Lefebvre B, Mina N, Wong T, Gilmour M. 2012. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001–2010. Sex. Transm. Dis. 39:316–323. 10.1097/OLQ.0b013e3182401b69 [DOI] [PubMed] [Google Scholar]
- 151.Su X, Jiang F, Qimuge Dai X, Sun H, Ye S. 2007. Surveillance of antimicrobial susceptibilities in Neisseria gonorrhoeae in Nanjing, China, 1999–2006. Sex. Transm. Dis. 34:995–999 [DOI] [PubMed] [Google Scholar]
- 152.Unemo M, Shipitsyna E, Domeika M. 2011. Gonorrhoea surveillance, laboratory diagnosis and antimicrobial susceptibility testing of Neisseria gonorrhoeae in 11 countries of the eastern part of the WHO European region. APMIS 119:643–649. 10.1111/j.1600-0463.2011.02780.x [DOI] [PubMed] [Google Scholar]
- 153.Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, Siebert H, Towns L, Melano RG, Low DE. 2013. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 309:163–170. 10.1001/jama.2012.176575 [DOI] [PubMed] [Google Scholar]
- 154.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. 2011. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 16:19833 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19833 [PubMed] [Google Scholar]
- 155.Lewis DA, Sriruttan C, Müller EE, Golparian D, Gumede L, Fick D, de Wet J, Maseko V, Coetzee J, Unemo M. 2013. Phenotypic and genetic characterization of the first two cases of extended-spectrum cephalosporin resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J. Antimicrob. Chemother. 68:1267–1270. 10.1093/jac/dkt034 [DOI] [PubMed] [Google Scholar]
- 156.Unemo M, Golparian D, Stary A, Eigentler A. 2011. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 16:19998 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19998 [PubMed] [Google Scholar]
- 157.Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. 2010. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 15:19721 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19721 [DOI] [PubMed] [Google Scholar]
- 158.Chen YM, Stevens K, Tideman R, Zaia A, Tomita T, Fairley CK, Lahra M, Whiley D, Hogg G. 2013. Failure of ceftriaxone 500 mg to eradicate pharyngeal gonorrhoea, Australia. J. Antimicrob. Chemother. 68:1445–1447. 10.1093/jac/dkt017 [DOI] [PubMed] [Google Scholar]
- 159.Read PJ, Limnios EA, McNulty A, Whiley D, Lahra LM. 2013. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500 mg ceftriaxone in Sydney, Australia. Sex. Health 10:460–462. 10.1071/SH13077 [DOI] [PubMed] [Google Scholar]
- 160.Unemo M, Golparian D, Hestner A. 2011. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 16:1–3 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19792 [PubMed] [Google Scholar]
- 161.Unemo M, Golparian D, Potočnik M, Jeverica S. 2012. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 17:1–4 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20200 [PubMed] [Google Scholar]
- 162.Shimuta K, Unemo M, Nakayama S, Morita-Ishihara T, Dorin M, Kawahata T, Ohnishi M. 2013. Antimicrobial resistance and molecular typing of Neisseria gonorrhoeae isolates in Kyoto and Osaka, Japan, 2010 to 2012: intensified surveillance after identification of the first strain (H041) with high-level ceftriaxone resistance. Antimicrob. Agents Chemother. 57:5225–5232. 10.1128/AAC.01295-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Furuya R, Onoye Y, Kanayama A, Saika T, Iyoda T, Tatewaki M, Matsuzaki K, Kobayashi I, Tanaka M. 2007. Antimicrobial resistance in clinical isolates of Neisseria subflava from the oral cavities of a Japanese population. J. Infect. Chemother. 13:302–304. 10.1007/s10156-007-0541-8 [DOI] [PubMed] [Google Scholar]
- 164.Saika T, Nishiyama T, Kanayama A, Kobayashi I, Nakayama H, Tanaka M, Naito S. 2001. Comparison of Neisseria gonorrhoeae isolates from the genital tract and pharynx of two gonorrhea patients. J. Infect. Chemother. 7:175–179. 10.1007/s101560100031 [DOI] [PubMed] [Google Scholar]
- 165.Tanaka M, Nakayama H, Huruya K, Konomi I, Irie S, Kanayama A, Saika T, Kobayashi I. 2006. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int. J. Antimicrob. Agents 27:20–26. 10.1016/j.ijantimicag.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 166.Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, Shimuta K, Okazaki N, Nakayama S, Watanabe H. 2010. Spreading of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob. Agents Chemother. 54:1060–1067. 10.1128/AAC.01010-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goodman SD, Scocca JJ. 1988. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 85:6982–6986. 10.1073/pnas.85.18.6982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59:376–385. 10.1111/j.1365-2958.2005.04964.x [DOI] [PubMed] [Google Scholar]
- 169.Sox TE, Mohammed W, Sparling PF. 1979. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J. Bacteriol. 138:510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kunz AN, Begum AA, Wu H, D'Ambrozio JA, Robinson JM, Shafer WM, Bash MC, Jerse AE. 2012. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J. Infect. Dis. 205:1821–1829. 10.1093/infdis/jis277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Warner DM, Folster JP, Shafer WM, Jerse AE. 2007. Regulation of the MtrC-MtrD-MtrE efflux pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J. Infect. Dis. 196:1804–1812. 10.1086/522964 [DOI] [PubMed] [Google Scholar]
- 172.Warner DM, Shafer WM, Jerse AE. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 70:462–478. 10.1111/j.1365-2958.2008.06424.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fermer C, Kristiansen BE, Sköld O, Swedberg G. 1995. Sulfonamide resistance in Neisseria meningitidis as defined by site-directed mutagenesis could have its origin in other species. J. Bacteriol. 177:4669–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Johnson SR, Morse SA. 1988. Antibiotic resistance in Neisseria gonorrhoeae: genetics and mechanisms of resistance. Sex. Transm. Dis. 15:217–224. 10.1097/00007435-198810000-00008 [DOI] [PubMed] [Google Scholar]
- 175.Swedberg G, Fermér C, Sköld O. 1993. Point mutations in the dihydropteroate synthase gene causing sulfonamide resistance. Adv. Exp. Med. Biol. 338:555–558. 10.1007/978-1-4615-2960-6_113 [DOI] [PubMed] [Google Scholar]
- 176.Elwell LP, Roberts M, Mayer LW, Falkow S. 1977. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 11:528–533. 10.1128/AAC.11.3.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roberts M, Elwell LP, Falkow S. 1977. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J. Bacteriol. 131:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brett M. 1989. A novel gonococcal beta-lactamase plasmid. J. Antimicrob. Chemother. 23:653–654. 10.1093/jac/23.4.653 [DOI] [PubMed] [Google Scholar]
- 179.Dillon JA, Yeung KH. 1989. Beta-lactamase plasmids and chromosomally mediated antibiotic resistance in pathogenic Neisseria species. Clin. Microbiol. Rev. 2(Suppl):S125–S133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gouby A, Bourg G, Ramuz M. 1986. Previously undescribed 6.6-kilobase R plasmid in penicillinase-producing Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 29:1095–1097. 10.1128/AAC.29.6.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Müller EE, Fayemiwo SA, Lewis DA. 2011. Characterization of a novel β-lactamase-producing plasmid in Neisseria gonorrhoeae: sequence analysis and molecular typing of host gonococci. J. Antimicrob. Chemother. 66:1514–1517. 10.1093/jac/dkr162 [DOI] [PubMed] [Google Scholar]
- 182.Pagotto F, Aman AT, Ng LK, Yeung KH, Brett M, Dillon JA. 2000. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae. Plasmid 43:24–34. 10.1006/plas.1999.1431 [DOI] [PubMed] [Google Scholar]
- 183.Chen SC, Yin YP, Dai XQ, Yu RX, Han Y, Sun HH, Ohnishi M, Unemo M, Chen XS. 2013. Prevalence and molecular epidemiological typing of penicillinase-producing Neisseria gonorrhoeae and their bla(TEM-135) gene variants in Nanjing, China. Sex. Transm. Dis. 40:872–876. 10.1097/OLQ.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 184.Nakayama SI, Tribuddharat C, Prombhul S, Shimuta K, Srifuengfung S, Unemo M, Ohnishi M. 2012. Molecular analyses of TEM genes and their corresponding penicillinase-producing Neisseria gonorrhoeae isolates in Bangkok, Thailand. Antimicrob. Agents Chemother. 56:916–920. 10.1128/AAC.05665-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ohnishi M, Ono E, Shimuta K, Watanabe H, Okamura N. 2010. Identification of TEM-135 beta-lactamase in penicillinase-producing Neisseria gonorrhoeae strains in Japan. Antimicrob. Agents Chemother. 54:3021–3023. 10.1128/AAC.00245-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Powell AJ, Tomberg J, Deacon AM, Nicholas RA, Davies C. 2009. Crystal structures of penicillin-binding protein 2 from penicillin-susceptible and -resistant strains of Neisseria gonorrhoeae reveal an unexpectedly subtle mechanism for antibiotic resistance. J. Biol. Chem. 284:1202–1212. 10.1074/jbc.M805761200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ropp PA, Hu M, Olesky M, Nicholas RA. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769–777. 10.1128/AAC.46.3.769-777.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sparling PF, Sarubbi FA, Blackman E. 1975. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J. Bacteriol. 124:740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bowler LD, Zhang QY, Riou JY, Spratt BG. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in Neisseria meningitidis: natural events and laboratory stimulation. J. Bacteriol. 176:333–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brannigan JA, Tirodimos IA, Zhang QY, Dowson CG, Spratt BG. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913–919. 10.1111/j.1365-2958.1990.tb00664.x [DOI] [PubMed] [Google Scholar]
- 191.Spratt BG. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173–176. 10.1038/332173a0 [DOI] [PubMed] [Google Scholar]
- 192.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J. Mol. Evol. 34:115–125 [DOI] [PubMed] [Google Scholar]
- 193.Dowson CG, Jephcott AE, Gough KR, Spratt BG. 1989. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 3:35–41. 10.1111/j.1365-2958.1989.tb00101.x [DOI] [PubMed] [Google Scholar]
- 194.Sigmund CD, Ettayebi M, Morgan EA. 1984. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 12:4653–4663. 10.1093/nar/12.11.4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tomberg J, Temple B, Fedarovich A, Davies C, Nicholas RA. 2012. A highly conserved interaction involving the middle residue of the SXN active-site motif is crucial for function of class B penicillin-binding proteins: mutational and computational analysis of PBP 2 from N. gonorrhoeae. Biochemistry 51:2775–2784. 10.1021/bi2017987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Barbour AG. 1981. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 19:316–322. 10.1128/AAC.19.2.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Folster JP, Johnson PJ, Jackson L, Dhulipali V, Dyer DW, Shafer WM. 2009. MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J. Bacteriol. 191:287–297. 10.1128/JB.01165-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ohneck EA, Zalucki YM, Johnson PJ, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187-11. 10.1128/mBio.00187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao S, Duncan M, Tomberg J, Davies C, Unemo M, Nicholas RA. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 53:3744–3751. 10.1128/AAC.00304-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Olesky M, Zhao S, Rosenberg RL, Nicholas RA. 2006. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 188:2300–2308. 10.1128/JB.188.7.2300-2308.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Olesky M, Hobbs M, Nicholas RA. 2002. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:2811–2820. 10.1128/AAC.46.9.2811-2820.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao S, Tobiason DM, Hu M, Seifert HS, Nicholas RA. 2005. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol. Microbiol. 57:1238–1251. 10.1111/j.1365-2958.2005.04752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Helm RA, Barnhart MM, Seifert HS. 2007. pilQ missense mutations have diverse effects on PilQ multimer formation, piliation, and pilus function in Neisseria gonorrhoeae. J. Bacteriol. 189:3198–3207. 10.1128/JB.01833-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tomberg J, Unemo M, Davies C, Nicholas RA. 2010. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49:8062–8070. 10.1021/bi101167x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Burdett V. 1986. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J. Bacteriol. 165:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Burdett V. 1991. Purification and characterization of Tet(M), a protein that renders ribosomes resistant to tetracycline. J. Biol. Chem. 266:2872–2877 [PubMed] [Google Scholar]
- 207.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232–260. 10.1128/MMBR.65.2.232-260.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Knapp JS, Johnson SR, Zenilman JM, Roberts MC, Morse SA. 1988. High-level tetracycline resistance resulting from TetM in strains of Neisseria spp., Kingella denitrificans, and Eikenella corrodens. Antimicrob. Agents Chemother. 32:765–767. 10.1128/AAC.32.5.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roberts MC, Knapp JS. 1988. Host range of the conjugative 25.2-megadalton tetracycline resistance plasmid from Neisseria gonorrhoeae and related species. Antimicrob. Agents Chemother. 32:488–491. 10.1128/AAC.32.4.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sox TE, Mohammed W, Blackman E, Biswas G, Sparling PF. 1978. Conjugative plasmids in Neisseria gonorrhoeae. J. Bacteriol. 134:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bäckman A, Orvelid P, Vazquez JA, Sköld O, Olcén P. 2000. Complete sequence of a beta-lactamase-encoding plasmid in Neisseria meningitidis. Antimicrob. Agents Chemother. 44:210–212. 10.1128/AAC.44.1.210-212.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dillon JAR, Pauzé M, Yeung KH. 1983. Spread of penicillinase-producing and transfer plasmids from the gonococcus to Neisseria meningitidis. Lancet i:779–781 [DOI] [PubMed] [Google Scholar]
- 213.Ikeda F, Tsuji A, Kaneko Y, Nishida M, Goto S. 1986. Conjugal transfer of beta-lactamase-producing plasmids of Neisseria gonorrhoeae to Neisseria meningitidis. Microbiol. Immunol. 30:737–742. 10.1111/j.1348-0421.1986.tb03000.x [DOI] [PubMed] [Google Scholar]
- 214.Flett F, Humphreys GO, Saunders JR. 1981. Intraspecific and intergeneric mobilization of non-conjugative resistance plasmids by a 24.5 megadalton conjugative plasmid of Neisseria gonorrhoeae. J. Gen. Microbiol. 125:123–129 [DOI] [PubMed] [Google Scholar]
- 215.Gascoyne-Binzi DM, Heritage J, Hawkey PM. 1993. Nucleotide sequences of the tet(M) genes from the American and Dutch type tetracycline resistance plasmids of Neisseria gonorrhoeae. J. Antimicrob. Chemother. 32:667–676. 10.1093/jac/32.5.667 [DOI] [PubMed] [Google Scholar]
- 216.Gascoyne DM, Heritage J, Hawkey PM, Turner A, van Klingeren B. 1991. Molecular evolution of tetracycline-resistance plasmids carrying TetM found in Neisseria gonorrhoeae from different countries. J. Antimicrob. Chemother. 28:173–183. 10.1093/jac/28.2.173 [DOI] [PubMed] [Google Scholar]
- 217.Marquez CM, Dillon JA, Rodriguez V, Borthagaray G. 2002. Detection of a novel Tet M determinant in tetracycline-resistant Neisseria gonorrhoeae from Uruguay, 1996–1999. Sex. Transm. Dis. 29:792–797. 10.1097/00007435-200212000-00010 [DOI] [PubMed] [Google Scholar]
- 218.Chalkley LJ, Janse van Rensburg MN, Matthee PC, Ison CA, Botha PL. 1997. Plasmid analysis of Neisseria gonorrhoeae isolates and dissemination of tetM genes in southern Africa 1993–1995. J. Antimicrob. Chemother. 40:817–822. 10.1093/jac/40.6.817 [DOI] [PubMed] [Google Scholar]
- 219.Hu M, Nandi S, Davies C, Nicholas RA. 2005. High-level chromosomally mediated tetracycline resistance in Neisseria gonorrhoeae results from a point mutation in the rpsJ gene encoding ribosomal protein S10 in combination with the mtrR and penB resistance determinants. Antimicrob. Agents Chemother. 49:4327–4334. 10.1128/AAC.49.10.4327-4334.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ramakrishnan V, White SW. 1992. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature 358:768–771. 10.1038/358768a0 [DOI] [PubMed] [Google Scholar]
- 221.Galimand M, Gerbaud G, Courvalin P. 2000. Spectinomycin resistance in Neisseria spp. due to mutations in 16S rRNA. Antimicrob. Agents Chemother. 44:1365–1366. 10.1128/AAC.44.5.1365-1366.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall JW. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63:1142–1151. 10.1093/jac/dkp098 [DOI] [PubMed] [Google Scholar]
- 223.Unemo M, Golparian D, Skogen V, Olsen AO, Moi H, Syversen G, Hjelmevoll SO. 2013. Neisseria gonorrhoeae strain with high-level resistance to spectinomycin due to a novel resistance mechanism (mutated ribosomal protein S5) verified in Norway. Antimicrob. Agents Chemother. 57:1057–1061. 10.1128/AAC.01775-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ilina EN, Malakhova MV, Bodoev IN, Oparina NY, Filimonova AV, Govorun VM. 2013. Mutation in ribosomal protein S5 leads to spectinomycin resistance in Neisseria gonorrhoeae. Front. Microbiol. 4:186. 10.3389/fmicb.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Davies C, Bussiere DE, Golden BL, Porter SJ, Ramakrishnan V, White SW. 1998. Ribosomal proteins S5 and L6: high resolution crystal structures and roles in protein biosynthesis and antibiotic resistance. J. Mol. Biol. 279:873–888. 10.1006/jmbi.1998.1780 [DOI] [PubMed] [Google Scholar]
- 226.Belland RJ, Morrison SG, Ison C, Huang WM. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluororoquinolone-resistant isolates. Mol. Microbiol. 14:371–380. 10.1111/j.1365-2958.1994.tb01297.x [DOI] [PubMed] [Google Scholar]
- 227.Alcalá B, Arreaza L, Salcedo C, Antolín I, Borrell N, Cacho J, De Las Cuevas C, Otero L, Sauca G, Vázquez F, Villar H, Vázquez JA. 2003. Molecular characterization of ciprofloxacin resistance of gonococcal strains in Spain. Sex. Transm. Dis. 30:395–398. 10.1097/00007435-200305000-00004 [DOI] [PubMed] [Google Scholar]
- 228.Lindbäck E, Rahman M, Jalal S, Wretlind B. 2002. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS 110:651–657. 10.1034/j.1600-0463.2002.1100909.x [DOI] [PubMed] [Google Scholar]
- 229.Trees DL, Sandul AL, Peto-Mesola V, Aplasca MR, Leng HB, Whittington WL, Knapp JS. 1999. Alterations within the quinolone resistance-determining regions of GyrA and ParC of Neisseria gonorrhoeae isolated in the Far East and the United States. Int. J. Antimicrob. Agents 12:325–332. 10.1016/S0924-8579(99)00081-3 [DOI] [PubMed] [Google Scholar]
- 230.Deguchi T, Yasuda M, Nakano M, Ozeki S, Kanematsu E, Kawada Y, Ezaki T, Saito I. 1996. Uncommon occurrence of mutations in the gyrB gene associated with quinolone resistance in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 40:2437–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Douthwaite S, Champney WS. 2001. Structures of ketolides and macrolides determine their mode of interaction with the ribosomal target site. J. Antimicrob. Chemother. 48(Suppl T1):1–8. 10.1093/jac/48.suppl_2.1 [DOI] [PubMed] [Google Scholar]
- 232.Cousin SL, Jr, Whittington WL, Roberts MC. 2003. Acquired macrolide resistance genes and the 1 bp deletion in the mtrR promoter in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 51:131–133. 10.1093/jac/dkg040 [DOI] [PubMed] [Google Scholar]
- 233.Allen VG, Farrell DJ, Rebbapragada A, Tan J, Tijet N, Perusini SJ, Towns L, Lo S, Low DE, Melano RG. 2011. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 55:703–712. 10.1128/AAC.00788-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Golparian D, Shafer WM, Ohnishi M, Unemo M. 2012. Inactivation of the MtrCDE, MacAB, and NorM efflux pumps in Neisseria gonorrhoeae strains with clinical resistance to extended-spectrum cephalosporins make them susceptible to several antimicrobials, abstr P171. 18th Int. Pathogenic Neisseria Conf., 9 to 14 September 2012, Wurzburg, Germany [Google Scholar]
- 235.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrCDE efflux system. Microbiology 141:611–622. 10.1099/13500872-141-3-611 [DOI] [PubMed] [Google Scholar]
- 236.Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC, Shafer WM. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117–2125. 10.1099/00221287-143-7-2117 [DOI] [PubMed] [Google Scholar]
- 237.Hagman KE, Shafer WM. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 171:4162–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Veal WL, Nicholas RA, Shafer WM. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184:5619–5624. 10.1128/JB.184.20.5619-5624.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zarantonelli L, Borthagaray G, Lee EH, Shafer WM. 1999. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob. Agents Chemother. 43:2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zarantonelli L, Borthagary G, Lee EH, Veal W, Shafer WM. 2001. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtrR promoter mutation in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 47:651–654. 10.1093/jac/47.5.651 [DOI] [PubMed] [Google Scholar]
- 241.Rouquette-Loughlin CE, Balthazar JT, Shafer WM. 2005. Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 56:856–860. 10.1093/jac/dki333 [DOI] [PubMed] [Google Scholar]
- 242.Luna VA, Cousin S, Jr, Whittington WLH, Roberts MC. 2000. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 44:2503–2506. 10.1128/AAC.44.9.2503-2506.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lindberg R, Fredlund H, Nicholas R, Unemo M. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 51:2117–2122. 10.1128/AAC.01604-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744–3749. 10.1128/AAC.46.12.3744-3749.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 49:137–143. 10.1128/AAC.49.1.137-143.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Osaka K, Takakura T, Narukawa K, Takahata M, Endo K, Kiyota H, Onodera S. 2008. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J. Infect. Chemother. 14:195–203. 10.1007/s10156-008-0610-7 [DOI] [PubMed] [Google Scholar]
- 247.Ochiai S, Sekiguchi S, Hayashi A, Shimadzu M, Ishiko H, Matsushima-Nishiwaki R, Kozawa O, Yasuda M, Deguchi T. 2007. Decreased affinity of mosaic-structure recombinant penicillin-binding protein 2 for oral cephalosporins in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 60:54–60. 10.1093/jac/dkm166 [DOI] [PubMed] [Google Scholar]
- 248.Takahata S, Senju N, Osaki Y, Yoshida T, Ida T. 2006. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 50:3638–3645. 10.1128/AAC.00626-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee SG, Lee H, Jeong SH, Yong D, Chung GT, Lee YS, Chong Y, Lee K. 2010. Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J. Antimicrob. Chemother. 65:669–675. 10.1093/jac/dkp505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. 2013. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect. Dis. 13:40. 10.1186/1471-2334-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Whiley DM, Limnios EA, Ray S, Sloots TP, Tapsall JW. 2007. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 51:3111–3116. 10.1128/AAC.00306-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Whiley DM, Goire N, Lambert SB, Ray S, Limnios EA, Nissen MD, Sloots TP, Tapsall JW. 2010. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J. Antimicrob. Chemother. 65:1615–1618. 10.1093/jac/dkq187 [DOI] [PubMed] [Google Scholar]
- 253.Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. 2013. Identification of the amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from the Neisseria gonorrhoeae strain H041. Antimicrob. Agents Chemother. 57:3029–3036. 10.1128/AAC.00093-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Unemo M, Dillon JA. 2011. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin. Microbiol. Rev. 24:447–458. 10.1128/CMR.00040-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Whiley DM, Jacobsson S, Tapsall JW, Nissen MD, Sloots TP, Unemo M. 2010. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J. Antimicrob. Chemother. 65:2543–2547. 10.1093/jac/dkq377 [DOI] [PubMed] [Google Scholar]
- 256.Gottesman MM, Ling V. 2006. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 580:998–1009. 10.1016/j.febslet.2005.12.060 [DOI] [PubMed] [Google Scholar]
- 257.Lee EH, Shafer WM. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839–845. 10.1046/j.1365-2958.1999.01530.x [DOI] [PubMed] [Google Scholar]
- 258.Rouquette-Loughlin C, Dunham SA, Kuhn M, Balthazar J, Shafer WM. 2003. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 185:1101–1106. 10.1128/JB.185.3.1101-1106.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Luna VA, Coates P, Eady EA, Cove JH, Nguyen TT, Roberts MC. 1999. A variety of Gram-positive bacteria carry mobile mef genes. J. Antimicrob. Chemother. 44:19–25. 10.1093/jac/44.1.19 [DOI] [PubMed] [Google Scholar]
- 260.Golparian D, Shafer WM, Ohnishi M, Unemo M. 2014. Importance of multidrug efflux pumps in the antimicrobial resistance property of clinical multidrug-resistant isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 58:3556–3559. 10.1128/AAC.00038-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shafer WM, Qu XD, Waring AJ, Lehrer RI. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. U. S. A. 95:1829–1833. 10.1073/pnas.95.4.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jerse AE, Sharma ND, Bodner ANB, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576–5582. 10.1128/IAI.71.10.5576-5582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rouquette-Loughlin C, Veal WL, Lee EJ, Zarantonelli L, Balthazar JT, Shafer WM. 2001. Antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis viewed as virulence factors, p 187–200 In Paulsen IT, Lewis K. (ed), Microbial drug efflux. Horizon Scientific Press, Wymonham, United Kingdom [Google Scholar]
- 264.Lucas CE, Balthazar JT, Hagman KE, Shafer WM. 1997. The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J. Bacteriol. 179:4123–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zalucki YM, Dhulipala V, Shafer WM. 2012. Dueling regulatory properties of a transcriptional activator (MtrA) and repressor (MtrR) that control efflux pump gene expression in Neisseria gonorrhoeae. mBio 3:e00446-12. 10.1128/mBio.00446-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Correia FF, Inouye S, Inouye M. 1988. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J. Biol. Chem. 264:12914–12916 [PubMed] [Google Scholar]
- 267.Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. 2003. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 21:414–419. 10.1016/S0924-8579(03)00039-6 [DOI] [PubMed] [Google Scholar]
- 268.Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM. 2004. Modulation of the mtrCDE-encoded efflux pump gene complex due to a Correia element insertion sequence. Mol. Microbiol. 54:731–741. 10.1111/j.1365-2958.2004.04299.x [DOI] [PubMed] [Google Scholar]
- 269.Nikaido H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382–388. 10.1126/science.8153625 [DOI] [PubMed] [Google Scholar]
- 270.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. 1999. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 67:2406–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shafer WM, Folster JP. 2006. Towards an understanding of chromosomally mediated penicillin resistance in Neisseria gonorrhoeae: evidence for a porin-efflux pump collaboration. J. Bacteriol. 188:2297–2299. 10.1128/JB.188.7.2297-2299.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Drake SL, Koomey M. 1995. The product of the pilQ gene is essential in the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975–986. 10.1111/j.1365-2958.1995.18050975.x [DOI] [PubMed] [Google Scholar]
- 274.Drake SL, Sandstedt SA, Koomey M. 1997. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol. Microbiol. 23:657–668. 10.1046/j.1365-2958.1997.2511618.x [DOI] [PubMed] [Google Scholar]
- 275.Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. 2004. A mutant form of the Neisseria gonorrhoeae secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730–739. 10.1128/JB.186.3.730-739.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nagaev I, Björkman J, Andersson DI, Hughes D. 2001. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 40:433–439. 10.1046/j.1365-2958.2001.02389.x [DOI] [PubMed] [Google Scholar]
- 277.Rozen DE, McGee L, Levin BR, Klugman KP. 2007. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 51:412–416. 10.1128/AAC.01161-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Trzcinski K, Thompson CM, Gilbey AM, Dowson CG, Lipsitch M. 2006. Incremental increase in fitness cost with increased beta-lactam resistance in pneumococci evaluated by competition in an infant rat nasal colonization model. J. Infect. Dis. 193:1296–1303. 10.1086/501367 [DOI] [PubMed] [Google Scholar]
- 279.Folster JP, Dhulipali V, Nicholas RA, Shafer WM. 2007. Differential regulation of ponA and pilMNOPQ expression by the Neisseria gonorrhoeae MtrR transcriptional regulatory protein. J. Bacteriol. 189:4569–4577. 10.1128/JB.00286-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Johnson PJ, Stringer VA, Shafer WM. 2011. Off-target gene regulation mediated by transcriptional repressors of antimicrobial efflux pump genes in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 55:2559–2565. 10.1128/AAC.00010-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Morse A, Lysko PG, McFarland L, Knapp JS, Sandstrom E, Critchlow C, Holmes KK. 1982. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect. Immun. 37:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shafer WM, Balthazar JT, Hagman KE, Morse SA. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 41:907–911 [DOI] [PubMed] [Google Scholar]
- 283.Xia M, Whittington WLH, Shafer WM, Holmes KK. 2000. Gonorrhea among men who have sex with men: outbreak caused by a single genotype of erythromycin-resistant Neisseria gonorrhoeae with a single base pair deletion in the mtrR promoter region. J. Infect. Dis. 181:2080–2082. 10.1086/315510 [DOI] [PubMed] [Google Scholar]
- 284.Rouquette C, Harmon JB, Shafer WM. 1999. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 33:651–658. 10.1046/j.1365-2958.1999.01517.x [DOI] [PubMed] [Google Scholar]
- 285.Mercante AD, Jackson L, Johnson PJ, Stringer VA, Dyer DW, Shafer WM. 2012. MpeR regulates the mtr efflux locus in Neisseria gonorrhoeae and modulates antimicrobial resistance by an iron-responsive mechanism. Antimicrob. Agents Chemother. 56:1491–1501. 10.1128/AAC.06112-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Veal WL, Shafer WM. 2003. Identification of a cell envelope protein (MtrF) involved in hydrophobic antimicrobial resistance in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 51:27–37. 10.1093/jac/dkg031 [DOI] [PubMed] [Google Scholar]
- 287.Hollander A, Mercante AD, Shafer WM, Cornelissen CN. 2011. The iron-repressed, AraC-like regulator, MpeR, activates expression of fetA in Neisseria gonorrhoeae. Infect. Immun. 79:4764–4776. 10.1128/IAI.05806-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zughaier SM, Kandler JL, Shafer WM. 2014. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PLoS One 9:e87688. 10.1371/journal.pone.0087688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Goytia M, Shafer WM. 2010. Polyamines can increase the resistance of Neisseria gonorrhoeae to mediators of innate host defense. Infect. Immun. 78:3187–3195. 10.1128/IAI.01301-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hobbs MM, Anderson JE, Balthazar JT, Kandler JL, Carlson RW, Ganguly J, Begum AA, Duncan JA, Lin JT, Sparling PF, Jerse AE, Shafer WM. 2013. Lipid A's structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 4:e00892-13. 10.1128/mBio.00892-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Falsetta ML, Steichen CT, McEwan AG, Cho C, Ketterer M, Shao J, Hunt J, Jennings MP, Apicella MA. 2011. The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front. Microbiol. 2:75. 10.3389/fmicb.2011.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, Apicella MA. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 73:1964–1970. 10.1128/IAI.73.4.1964-1970.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Goytia M, Dhulipala V, Shafer WM. 2013. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol. Lett. 343:64–69. 10.1111/1574-6968.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Public Health Ontario. 2013. Guidelines for testing and treatment of gonorrhea in Ontario, 2013. http://www.oahpp.ca/resources/gonorrhea-guideline.html.
- 296.Chisholm SA, Unemo M, Quaye N, Johansson E, Cole MJ, Ison CA, Van de Laar MJ. 2013. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrug-resistant clone. Euro Surveill. 18:20358 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20358 [PubMed] [Google Scholar]
- 297.Schwarcz SK, Zenilman JM, Schnell D, Knapp JS, Hook EW, 3rd, Thompson S, Judson FN, Holmes KK. 1990. National surveillance of antimicrobial resistance in Neisseria gonorrhoeae: the Gonococcal Isolate Surveillance Project. JAMA 264:1413–1417. 10.1001/jama.1990.03450110059027 [DOI] [PubMed] [Google Scholar]
- 298.Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, Lowndes CM, GRASP Collaborative Group 2013. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect. Dis. 13:762–768. 10.1016/S1473-3099(13)70143-9 [DOI] [PubMed] [Google Scholar]
- 299.Ison CA, Deal C, Unemo M. 2013. Current and future treatment options for gonorrhoea. Sex. Transm. Infect. 89(Suppl 4):iv52–iv56. 10.1136/sextrans-2012-050913 [DOI] [PubMed] [Google Scholar]
- 300.Kirkcaldy RD. 2013. Treatment of gonorrhoea in an era of emerging cephalosporin resistance and results of a randomized trial of new potential treatment options, abstr SO8.1. STI AIDS World Cong. 2013, 14 to 17 July 2013, Vienna, Austria [Google Scholar]
- 301.Brown LB, Krysiak R, Kamanga G, Mapanje C, Kanyamula H, Banda B, Mhango C, Hoffman M, Kamwendo D, Hobbs M, Hosseinipour MC, Martinson F, Cohen MS, Hoffman IF. 2010. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex. Transm. Dis. 37:169–172. 10.1097/OLQ.0b013e3181bf575c [DOI] [PubMed] [Google Scholar]
- 302.Ross JD, Lewis DA. 2012. Cephalosporin resistant Neisseria gonorrhoeae: time to consider gentamicin? Sex. Transm. Infect. 88:6–8. 10.1136/sextrans-2011-050362 [DOI] [PubMed] [Google Scholar]
- 303.Chisholm SA, Quaye N, Cole MJ, Fredlund H, Hoffmann S, Jensen JS, van de Laar MJ, Unemo M, Ison CA. 2011. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J. Antimicrob. Chemother. 66:592–595. 10.1093/jac/dkq476 [DOI] [PubMed] [Google Scholar]
- 304.Dowell D, Kirkcaldy RD. 2013. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex. Transm. Infect. 89:142–147. 10.1136/sextrans-2012-050531 [DOI] [PubMed] [Google Scholar]
- 305.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains including those with various high-level antimicrobial resistance—potential treatment option for gonorrhea? Antimicrob. Agents Chemother. 56:2739–2742. 10.1128/AAC.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hook E, III, Oldach D, Jamieson B, Clark K, Fernandes P. 2013. A phase 2 study to evaluate the efficacy and safety of single dose solithromycin (CEM-101) for the treatment of patients with uncomplicated urogenital gonorrhoea, abstr O274. 23rd Eur. Cong. Clin. Microbiol. Infect. Dis., 27 to 30 April 2013, Berlin, Germany [Google Scholar]
- 307.Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem vs. ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants—ertapenem for treatment of gonorrhea? Antimicrob. Agents Chemother. 56:3603–3609. 10.1128/AAC.00326-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Deshpande LM, Gales AC, Jones RN. 2001. GAR-936 (9-t-butylglycylamidominocycline) susceptibility test development for streptococci, Haemophilus influenzae and Neisseria gonorrhoeae: preliminary guidelines and interpretive criteria. Int. J. Antimicrob. Agents 18:29–35 [DOI] [PubMed] [Google Scholar]
- 309.Zhang YY, Zhou L, Zhu DM, Wu PC, Hu FP, Wu WH, Wang F. 2004. In vitro activities of tigecycline against clinical isolates from Shanghai, China. Diagn. Microbiol. Infect. Dis. 50:267–281. 10.1016/j.diagmicrobio.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 310.Alexander BT, Marschall J, Tibbetts RJ, Neuner EA, Dunne WM, Jr, Ritchie DJ. 2012. Treatment and clinical outcomes of urinary tract infections caused by KPC-producing Enterobacteriaceae in a retrospective cohort. Clin. Ther. 34:1314–1323. 10.1016/j.clinthera.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Falagas ME, Karageorgopoulos DE, Dimopoulos G. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of tigecycline. Curr. Drug Metab. 10:13–21. 10.2174/138920009787048356 [DOI] [PubMed] [Google Scholar]
- 312.Nix DE, Matthias KR. 2010. Should tigecycline be considered for urinary tract infections? A pharmacokinetic re-evaluation. J. Antimicrob. Chemother. 65:1311–1312. 10.1093/jac/dkq116 [DOI] [PubMed] [Google Scholar]
- 313.Kerstein K, Fyfe C, Sutcliffe JA, Grossman TH. 2013. Eravacycline (TP-434) is active against susceptible and multidrug-resistant Neisseria gonorrhoeae, poster E-1181. 53rd Annu. Intersci. Conf. Antimicrob. Agents Chemother., 10 to 13 September 2013, Denver, CO [Google Scholar]
- 314.Koeth LM, Fisher J. 2013. In vitro activity of dalbavancin against Neisseria gonorrhoeae and development of a broth microdilution method, poster 255. IDWeek 2013, 2 to 6 October 2013, San Francisco, CA [Google Scholar]
- 315.Fujimoto K, Takemoto K, Hatano K, Nakai T, Terashita S, Matsumoto M, Eriguchi Y, Eguchi K, Shimizudani T, Sato K, Kanazaw K, Sunagawa M, Ueda Y. 2013. Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals. Antimicrob. Agents Chemother. 57:697–707. 10.1128/AAC.01051-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Biedenbach DJ, Turner LL, Jones RN, Farell DJ. 2012. Activity of JNJ-Q2, a novel fluoroquinolone, tested against Neisseria gonorrhoeae, including ciprofloxacin-resistant strains. Diagn. Microbiol. Infect. Dis. 74:204–206. 10.1016/j.diagmicrobio.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 317.Roberts MC, Remy JM, Longcor JD, Marra A, Sun E, Duffy EM. 2013. In vitro activity of delafloxacin against Neisseria gonorrhoeae clinical isolates, poster P2.197. STI AIDS World Cong. 2013, 14 to 17 July 2013, Vienna, Austria [Google Scholar]
- 318.Novak R. 2011. Are pleuromutilin antibiotics finally fit for human use? Ann. N. Y. Acad. Sci. 1241:71–81. 10.1111/j.1749-6632.2011.06219.x [DOI] [PubMed] [Google Scholar]
- 319.Paukner S, Gruss A, Fritsche TR, Ivezic-Schoenfeld Z, Jones RN. 2013. In vitro activity of the novel pleuromutilin BC-3781 tested against bacterial pathogens causing sexually transmitted diseases (STD), poster E-1183. 53rd Annu. Intersci. Conf. Antimicrob. Agents Chemother., 10 to 13 September 2013, Denver, CO [Google Scholar]
- 320.Bouchillon SK, Hoban DJ, Hackel MA, Butler DL, Memarsh P, Alley MRK. 2010. In vitro activities of AN3365: a novel boron containing protein synthesis inhibitor, and other antimicrobial agents against anaerobes and Neisseria gonorrhoeae, poster F1-1640. 50th Annu. Intersci. Conf. Antimicrob. Agents Chemother., 12 to 15 September 2010, Boston, MA [Google Scholar]
- 321.Mendes RE, Alley MR, Sader HS, Biedenbach DJ, Jones RN. 2013. Potency and spectrum of activity of AN3365, a novel boron-containing protein synthesis inhibitor, tested against clinical isolates of Enterobacteriaceae and nonfermentative Gram-negative bacilli. Antimicrob. Agents Chemother. 57:2849–2857. 10.1128/AAC.00160-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Bradbury BJ, Pucci MJ. 2008. Recent advances in bacterial topoisomerase inhibitors. Curr. Opin. Pharmacol. 8:574–581. 10.1016/j.coph.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 323.Mayer C, Janin YL. 2014. Non-quinolone inhibitors of bacterial type IIA topoisomerases: a feat of bioisosterism. Chem. Rev. 114:2313–2342. 10.1021/cr4003984 [DOI] [PubMed] [Google Scholar]
- 324.Jeverica S, Golparian D, Hanzelka B, Fowlie AJ, Matičič M, Unemo M. 20 March 2014. High in vitro activity of a novel dual bacterial topoisomerase inhibitor of the ATPase activities of GyrB and ParE (VT12-008911) against Neisseria gonorrhoeae isolates with various high-level antimicrobial resistance and multidrug resistance. J. Antimicrob. Chemother. 10.1093/jac/dku073 [DOI] [PubMed] [Google Scholar]
- 325.Alm RA, Kutschke A, Otterson LG, Lahiri SD, McLaughlin RE, Lewis LA, Su X, Huband MD, Mueller JP, Gardner H. 2013. Novel DNA gyrase inhibitor AZD0914: low resistance potential and lack of cross-resistance in Neisseria gonorrhoeae (NG) and Staphylococcus aureus (SA), poster F-1225c. 53rd Annu. Intersci. Conf. Antimicrob. Agents Chemother., 10 to 13 September 2013, Denver, CO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Huband MD, Flamm RK, Rhomberg PR, Mueller JP, Jones RN. 2013. In vitro activity of AZD0914: a new benzisoxazole DNA gyrase inhibitor against Neisseria gonorrhoeae, poster F-1220. 53rd Annu. Intersci. Conf. Antimicrob. Agents Chemother., 10 to 13 September 2013, Denver, CO [Google Scholar]
- 327.Escaich S, Prouvensier L, Saccomani M, Durant L, Oxoby M, Gerusz V, Moreau F, Vongsouthi V, Maher K, Morrissey I, Soulama-Mouze C. 2011. The MUT056399 inhibitor of FabI is a new antistaphylococcal compound. Antimicrob. Agents Chemother. 55:4692–4697. 10.1128/AAC.01248-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Then RL, Sahl HG. 2010. Anti-infective strategies of the future: is there room for species-specific antibacterial agents? Curr. Pharm. Des. 16:555–566. 10.2174/138161210790361407 [DOI] [PubMed] [Google Scholar]
- 329.Li LH, Yen MY, Ho CC, Wu P, Wang CC, Maurya PK, Chen PS, Chen W, Hsieh WY, Chen HW. 2013. Non-cytotoxic nanomaterials enhance antimicrobial activities of cefmetazole against multidrug-resistant Neisseria gonorrhoeae. PLoS One 8:e64794. 10.1371/journal.pone.0064794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lomovskaya O, Watkins W. 2002. Inhibition of efflux pumps as a novel approach to combat drug resistance in bacteria, p 201–231 In Paulsen IT, Lewis K. (ed), Microbial multidrug efflux. Horizon Scientific Press, Wymondham, United Kingdom [Google Scholar]
- 331.Swanson S, Lee CJ, Liang X, Toone E, Zhou P, Nicholas R. 2012. LpxC inhibitors as novel therapeutics for treatment of antibiotic-resistant Neisseria gonorrhoeae, abstr P174. 18th Int. Pathog. Neisseria Conf., 9 to 14 September 2012, Wurzburg, Germany [Google Scholar]
- 332.Bucki R, Leszczyńska K, Namiot A, Sokołowski W. 2010. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch. Immunol. Ther. Exp. (Warsz.) 58:15–25. 10.1007/s00005-009-0057-2 [DOI] [PubMed] [Google Scholar]
- 333.Mbaveng AT, Kuete V, Mapunya BM, Beng VP, Nkengfack AE, Meyer JJ, Lall N. 2011. Evaluation of four Cameroonian medicinal plants for anticancer, antigonorrheal and antireverse transcriptase activities. Environ. Toxicol. Pharmacol. 32:162–167. 10.1016/j.etap.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 334.Cybulska P, Thakur SD, Foster BC, Scott IM, Leduc RI, Arnason JT, Dillon JA. 2011. Extracts of Canadian first nations medicinal plants, used as natural products, inhibit Neisseria gonorrhoeae isolates with different antibiotic resistance profiles. Sex. Transm. Dis. 38:667–671. 10.1097/OLQ.0b013e31820cb166 [DOI] [PubMed] [Google Scholar]
- 335.Ruddock PS, Charland M, Ramirez S, López A, Neil Towers GH, Arnason JT, Liao M, Dillon JA. 2011. Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 38:82–88. 10.1097/OLQ.0b013e3181f0bdbd [DOI] [PubMed] [Google Scholar]
- 336.Kuete V, Ngameni B, Mbaveng AT, Ngadjui B, Meyer JJ, Lall N. 2010. Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 116:100–104. 10.1016/j.actatropica.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 337.Kuete V, Tangmouo JG, Meyer JJ, Lall N. 2009. Diospyrone, crassiflorone and plumbagin: three antimycobacterial and antigonorrhoeal naphthoquinones from two Diospyros spp. Int. J. Antimicrob. Agents 34:322–325. 10.1016/j.ijantimicag.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 338.Chomnawang MT, Trinapakul C, Gritsanapan W. 2009. In vitro antigonococcal activity of Coscinium fenestratum stem extract. J. Ethnopharmacol. 122:445–449. 10.1016/j.jep.2009.01.036 [DOI] [PubMed] [Google Scholar]
- 339.Shokeen P, Bala M, Tandon V. 2009. Evaluation of the activity of 16 medicinal plants against Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 33:86–91. 10.1016/j.ijantimicag.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 340.Shokeen P, Bala M, Singh M, Tandon V. 2008. In vitro activity of eugenol, an active component from Ocimum sanctum, against multiresistant and susceptible strains of Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 32:174–179. 10.1016/j.ijantimicag.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 341.Ruddock PS, Liao M, Foster BC, Lawson L, Arnason JT, Dillon JA. 2005. Garlic natural health products exhibit variable constituent levels and antimicrobial activity against Neisseria gonorrhoeae, Staphylococcus aureus and Enterococcus faecalis. Phytother. Res. 19:327–334. 10.1002/ptr.1667 [DOI] [PubMed] [Google Scholar]
- 342.Shokeen P, Ray K, Bala M, Tandon V. 2005. Preliminary studies on activity of Ocimum sanctum, Drynaria quercifolia, and Annona squamosa against Neisseria gonorrhoeae. Sex. Transm. Dis. 32:106–111. 10.1097/01.olq.0000152821.23777.90 [DOI] [PubMed] [Google Scholar]
- 343.Silva O, Ferreira E, Vaz Pato M, Caniça M, Gomes ET. 2002. In vitro anti-Neisseria gonorrhoeae activity of Terminalia macroptera leaves. FEMS Microbiol. Lett. 217:271–274. 10.1111/j.1574-6968.2002.tb11487.x [DOI] [PubMed] [Google Scholar]
- 344.Swart H, Van Dyk S, Malan SF. 2002. The activity of p-methoxybenzylisothiocyanate against Neisseria gonorrhoeae, Haemophilus ducreyi, and other microorganisms. Bioorg. Med. Chem. Lett. 12:2435–2437. 10.1016/S0960-894X(02)00437-7 [DOI] [PubMed] [Google Scholar]
- 345.Cáceres A, Menéndez H, Méndez E, Cohobón E, Samayoa BE, Jauregui E, Peralta E, Carrillo G. 1995. Antigonorrhoeal activity of plants used in Guatemala for the treatment of sexually transmitted diseases. J. Ethnopharmacol. 48:85–88. 10.1016/0378-8741(95)01288-O [DOI] [PubMed] [Google Scholar]
- 346.Grad YH, Kirkcaldy RD, Trees D, Dordel J, Harris SR, Goldstein E, Weinstock H, Parkhill J, Hanage WP, Bentley S, Lipsitch M. 2014. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the U.S.A.: a retrospective observational study. Lancet Infect. Dis. 14:220–226. 10.1016/S1473-3099(13)70693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ohnishi M, Unemo M. 2014. Phylogenomics for drug-resistant Neisseria gonorrhoeae. Lancet Infect. Dis. 14:179–180. 10.1016/S1473-3099(13)70700-X [DOI] [PubMed] [Google Scholar]
- 348.Unemo M, Nicholas RA, Jerse AE, Davies C, Shafer WM. 2014. Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria, chapter 9 In Davies JK, Kahler CM. (ed), Pathogenic Neisseria: genomics, molecular biology and disease intervention. Caister Academic Press, London, United Kingdom [Google Scholar]