Abstract
SUMMARY
The protozoan parasite Cryptosporidium infects all major vertebrate groups and causes significant diarrhea in humans, with a spectrum of diseases ranging from asymptomatic to life-threatening. Children and immunodeficient individuals are disproportionately affected, especially in developing countries, where cryptosporidiosis contributes substantially to morbidity and mortality in preschool-age children. Despite the enormous disease burden from cryptosporidiosis, no antiprotozoal agent or vaccine exists for effective treatment or prevention. Cryptosporidiosis involving the respiratory tract has been described for avian species and mammals, including immunocompromised humans. Recent evidence indicates that respiratory cryptosporidiosis may occur commonly in immunocompetent children with cryptosporidial diarrhea and unexplained cough. Findings from animal models, human case reports, and a few epidemiological studies suggest that Cryptosporidium may be transmitted via respiratory secretions, in addition to the more recognized fecal-oral route. It is postulated that transmission of Cryptosporidium oocysts may occur by inhalation of aerosolized droplets or by contact with fomites contaminated by coughing. Delineating the role of the respiratory tract in disease transmission may provide necessary evidence to establish further guidelines for prevention of cryptosporidiosis.
INTRODUCTION
The genus Cryptosporidium comprises numerous protozoan parasites with a worldwide distribution, infecting all major vertebrate groups and causing significant diarrhea in most ruminant species and in humans. Large populations in sub-Saharan Africa, Asia, and Latin America experience cryptosporidial diarrhea, typically acquired by ingestion of fecally contaminated water or food. Cryptosporidium disproportionately affects preschool-age children and immunocompromised individuals, especially in developing countries. The recent Global Enteric Multicenter Study, investigating the etiology of diarrheal disease in children, found that Cryptosporidium was second only to rotavirus at seven sites in Africa and Asia (1, 2). Poor nutrition, HIV/AIDS and other immunodeficiencies, and immune system naiveté increase disease susceptibility. Children are particularly vulnerable to the effects of undernutrition, which is both a sequela of and a risk factor for cryptosporidiosis in low-income countries (3–11). Persistent diarrhea caused by Cryptosporidium leads to malnutrition, manifested by growth stunting and wasting, and poor nutrition decreases resistance to further bouts of infectious disease, perpetuating the cycle of chronic diarrhea and malnutrition. In sub-Saharan Africa, children with HIV carry a higher burden of illness from cryptosporidiosis (12–14). The spectrum of diseases caused by Cryptosporidium in humans ranges from asymptomatic to life-threatening. Compared to other causes of infectious diarrhea in children, cryptosporidiosis is associated with higher morbidity and mortality (1, 3, 11, 15, 16), and survivors often suffer persistent growth retardation and cognitive deficits (17, 18).
Despite the enormous toll of diarrheal disease and malnutrition exacted by cryptosporidiosis, no potent antiprotozoal agent or vaccine exists to effectively treat or prevent the disease. Prevention of fecal-oral transmission through clean water and sanitation (19), scrupulous hygiene in communal settings such as day care centers (20), and food safety (21, 22) are the few recognized effective ways to decrease the burden of clinical disease. In individuals coinfected with HIV, antiretroviral therapy (ART) has been successful in controlling chronic diarrhea and wasting due to cryptosporidiosis (23, 24).
Cryptosporidiosis involving the respiratory tract, with and without symptoms, has been described for several avian species and some mammals, including immunocompromised humans. Recent evidence also suggests that respiratory Cryptosporidium infections may commonly occur in immunocompetent children with cryptosporidial diarrhea and unexplained cough (25). It is postulated that involvement of the respiratory tract may result in person-to-person transmission of Cryptosporidium oocysts, the infectious and environmentally stable form of the parasite, by direct inhalation of aerosolized droplets or by fomites contaminated by coughing. Given the disease burden that Cryptosporidium places on children and the lack of an effective therapy or vaccine, elucidation of the role of the respiratory tract in cryptosporidiosis transmission is warranted. Further understanding of respiratory cryptosporidiosis is important to appreciate the full spectrum of diseases caused by Cryptosporidium, with a view to formulating preventative strategies to minimize the exposure of children.
A BRIEF OVERVIEW OF THE ORGANISM
History
More than 20 Cryptosporidium species infect birds, reptiles, fish, and mammals, including humans (26–28). Cryptosporidium was first named and reported in 1907, after its discovery in the stomachs of mice (29). After its original identification in animals, the first human Cryptosporidium infections were not reported until 1976 (30, 31). By 1980, only seven cases of human cryptosporidiosis had been confirmed (32–36); five involved immunocompromised patients, and three were fatal. With the exception of one hospital-based survey in Australia (37), cryptosporidiosis in humans remained largely unknown as a primary cause of acute diarrheal disease until the emergence of the global AIDS pandemic, when Cryptosporidium infection became one of the first defining entities of AIDS, before the discovery of the etiologic virus (38).
Life Cycle and Infectivity
Cryptosporidium spp. propagate via a direct life cycle involving a single host. Oocysts containing four naked infectious sporozoites are shed in feces and ingested by a new host, whereby both asexual and sexual stages develop in the intestinal epithelium, leading to the release of new oocysts (39). Cryptosporidium oocysts are also capable of autoinfection, a unique ability of this parasite that allows it to sporulate and persist within the same host indefinitely (40). Oocysts may be encountered in contaminated water or food, on environmental surfaces, via fomites, or directly when shed by infected animals or people (41).
Cryptosporidium spp. are highly infectious; in human volunteer studies, as few as 10 Cryptosporidium hominis oocysts produced disease in healthy adults (42). A quantitative risk assessment has estimated that ingestion of a single oocyst of the C. parvum IOWA isolate will result in clinical disease in 2.79% of immunologically normal persons (43). Given that the 50% infective dose (ID50) for C. hominis is less than 1/10 that of the IOWA isolate (44), ingestion of only one oocyst of a more infectious isolate may lead to a higher incidence of infection in the general immunocompetent population.
Cryptosporidiosis in industrialized nations is often associated with recreational water use (45, 46), animals on petting farms (47, 48), and day care centers (20, 49, 50). The small number of oocysts required to induce infection, susceptibility of young children, high risk of contamination of surfaces by diaper changing and inadequate hand washing, and the tendency of children to put nonfood items in their mouths make day care centers opportune sites for cryptosporidiosis outbreaks (51). While fecal-oral transmission is indisputably the major route of infection, transmission via coughing and fomites is also possible in situations of close contact (20).
Clinical Illness
While some infections go undetected, symptomatic cryptosporidiosis typically produces moderate to severe watery diarrhea, abdominal pain, vomiting, nausea, fever, anorexia, dehydration, and weight loss (39, 40). Host immune status dramatically alters the course of disease. Otherwise healthy individuals typically experience transient gastroenteritis lasting up to 2 weeks and recover without treatment (39). In contrast, persons with immunoglobulin deficiencies, renal failure, or inflammatory bowel disease, people receiving immunosuppressive treatment for other illnesses, and those with HIV/AIDS and not treated with ART often suffer fatal cryptosporidiosis following months of intractable diarrhea and wasting (40, 52). Immunocompromised individuals may experience disseminated cryptosporidiosis in other organ systems. In addition to the gastrointestinal and respiratory tracts, Cryptosporidium forms have been identified in the hepatobiliary system (53–58), pancreas (56, 59), and urinary bladder (60), highlighting the unusual ability of this parasite to colonize most, if not all, mucosal epithelial surfaces. These widespread infections have been observed mostly in hosts with dysfunctional immune systems, which are unable to control or eliminate the parasite.
Although respiratory cryptosporidiosis in humans has been reported to be uncommon, it produces symptoms that are indistinguishable from those associated with other common respiratory illnesses. Upper respiratory cryptosporidiosis may cause inflammation of the nasal mucosa, sinuses, larynx, and trachea, accompanied by nasal discharge and voice change (54, 61, 62). Cryptosporidiosis of the lower respiratory tract typically results in productive cough, dyspnea, fever, and hypoxemia (63–66).
RESPIRATORY CRYPTOSPORIDIOSIS IN ANIMALS
Natural Infection
The first cases of cryptosporidiosis of the respiratory tract were reported for flocks of commercially grown turkeys with cough, nasal and ocular discharge, poor weight gain, and absence of gastrointestinal signs (67). Cryptosporidial forms attached to the ciliated epithelial border were identified on histologic sections of the trachea, bronchi, and nasal turbinates. Deciliation of infected cells was frequently noted. The mucosa was thickened, with hyperplastic epithelial cells, dilated mucous glands, and a mixed inflammatory infiltrate of the lamina propria (67). Cryptosporidiosis has also been identified in the sinuses (68) and conjunctiva (69, 70) of birds, and histologic changes remain remarkably similar across affected tissues, as well as across species. Respiratory tract cryptosporidiosis has been confirmed for turkeys (68, 71) and other commercially raised birds, including chickens (72–74), partridges (75), peacocks (69), pheasants (76), and quail (77). Cases have also been documented for raptors (70, 78) and a jungle fowl (79). Cryptosporidium infections may occur in the respiratory tracts of avian species with or without gastrointestinal cryptosporidiosis. The paucity of other etiologic agents recovered from the respiratory tract and the frequent absence of gastrointestinal signs and pathology have helped to establish Cryptosporidium as a primary agent of avian respiratory disease that is capable of transmission via droplets and fomites (68).
Naturally occurring respiratory cryptosporidiosis is not limited to birds. Sporadic cases have been reported for a calf with confirmed cryptosporidial diarrhea (80) and several sheep from an abattoir (81). Four rhesus macaques, experimentally infected with simian immunodeficiency virus to simulate HIV infection in humans, subsequently developed disseminated cryptosporidiosis of the respiratory and gastrointestinal tracts and the bile and pancreatic ducts (82). In two of these primates, Cryptosporidium forms were found within alveoli (83), consistent with a well-established infection of the deep lung.
Experimental Infection
Respiratory cryptosporidiosis has been induced successfully in turkeys (84), chickens (85), and immunosuppressed rats (86, 87) by intratracheal inoculation, resulting in clinical signs and pathology mimicking those of natural infection. In turkeys, when C. baileyi oocysts were introduced directly into the trachea, respiratory signs and mortality ensued, while oral inoculation caused no clinical signs or loss of life (84). Pigs have also been explored as an animal model for respiratory cryptosporidiosis (88).
Preliminary findings from the gnotobiotic piglet model have shown that Cryptosporidium can readily be transmitted by inhalation. In our laboratory, five pairs of piglets were housed in sterile microbiological isolators. The pairs were physically separated but were allowed to share the same sterile air. They were fed from distinct food sources and were handled using separate sets of gloves. One piglet in each pair was intranasally inoculated with C. hominis oocysts. Consistent with serial infection, oocysts were shed first in the feces of each inoculated piglet, followed several days later by fecal shedding from each unchallenged piglet. Tracheal and pulmonary histologic sections taken 2 weeks after inoculation revealed no evidence of infection. Even if transient respiratory cryptosporidiosis was not substantiated, this work demonstrated that a patent intestinal infection with Cryptosporidium was established via airborne transmission between mammals (S. Tzipori, unpublished data).
RESPIRATORY CRYPTOSPORIDIOSIS IN HUMANS
Initial Descriptions
Cryptosporidium organisms in the respiratory tract of a human were first reported at autopsy in the trachea of a boy with congenital hypogammaglobulinemia and intestinal cryptosporidiosis (34) (Table 1). Cryptosporidium was subsequently identified at autopsy in the bronchial trees of two children with severe combined immunodeficiency (59, 89). Numerous oocysts were found in both endotracheal and fecal samples obtained from a newborn diagnosed with an immunoglobulin deficiency (90). Oocysts were found in sputum and maxillary sinus aspirates from an adolescent with congenital hypogammaglobulinemia and chronic intestinal cryptosporidiosis (54). More recently, a Turkish child with combined immunodeficiency harbored cryptosporidial oocysts in both tracheal and stool specimens (53). Each of these patients with congenital immune dysfunction manifested cryptosporidiosis in more than one organ system. In addition to intestinal and respiratory infections, two patients had evidence of hepatobiliary invasion (53, 54), and one had confirmed cryptosporidia in the pancreatic duct (59).
TABLE 1.
Case reports of respiratory cryptosporidiosis, 1980–2010
| Underlying disease category and authors (reference) | Yr | Country | Underlying disease | Diarrhea and positive fecal exam | Concurrent respiratory tract pathogen(s) |
|---|---|---|---|---|---|
| Congenital disease | |||||
| 34 | 1980 | UK | Congenital hypogammaglobulinemia | Yes | None |
| Kocoshis et al. (59) | 1984 | USA | Severe combined immunodeficiency | Yes | Mycoplasma, Pseudomonas |
| Manivel et al. (89) | 1985 | USA | Severe combined immunodeficiency/bone marrow transplant | Yes | Cytomegalovirus |
| O'Halloran et al. (90) | 1987 | UK | IgA, IgG2, IgG4 deficiency | Yes | Unspecified bacteria |
| Davis and Heyman (54) | 1988 | USA | Congenital hypogammaglobulinemia | Yes | Haemophilus influenzae |
| Kutukculer et al. (53) | 2003 | Turkey | CD40 deficiency/HIGM3 | Yes | Pseudomonas |
| Acquired disease | |||||
| Gentile et al. (91) | 1987 | Italy | Leukemia | Yes | Candida |
| Kibbler et al. (93) | 1987 | UK | Leukemia/bone marrow transplant | No diarrhea; fecal exam negative | Cytomegalovirus |
| Travis et al. (55) | 1990 | USA | Lymphoma/unspecified immunodeficiency | No diarrhea; fecal exam not performed | Aspergillus, Pseudomonas, Serratia |
| Shrikhande et al. (92) | 2009 | India | Nephrotic syndrome | Yes | None |
| AIDS | |||||
| Forgacs et al. (99) | 1983 | USA | AIDS | Yes | Cytomegalovirus |
| Brady et al. (101) | 1984 | USA | AIDS | Yes | Cytomegalovirus, Mycobacterium |
| Ma et al. (102) (3 patients) | 1984 | USA | AIDS | Yes | 1 patient with cytomegalovirus; 1 patient with cytomegalovirus and Mycobacterium; 1 patient with Legionella and Pneumocystis |
| Miller et al. (100) | 1984 | USA | AIDS | Yes | Candida, Pneumocystis |
| Gross et al. (56) | 1986 | USA | AIDS | Yes | Nocardia |
| Hojlyng and Jensen (105) (6 patients) | 1988 | Denmark | AIDS | 3/6 patients with diarrhea; fecal exams for 2/3 patients with diarrhea (both negative) | 1 patient with Pneumocystis; 1 patient with cytomegalovirus, Klebsiella, Pneumocystis, and Pseudomonas |
| Goodstein et al. (103) | 1989 | USA | AIDS | Yes | None |
| Jensen et al. (106) (8 patients) | 1990 | Denmark | AIDS | Unknown | 4 patients with Pneumocystis; 1 patient with unspecified bacteria |
| Ditrich et al. (60) | 1991 | Former Czechoslavakia | AIDS/renal transplant | Yes | None |
| Moore and Frenkel (98) | 1991 | USA | AIDS | Yes | Cytomegalovirus, Pneumocystis |
| Giang et al. (61) | 1994 | USA | AIDS | Yes | None |
| Mifsud et al. (114) | 1994 | UK | AIDS | No diarrhea; fecal exam positive | Pneumocystis |
| Lopez-Velez et al. (58) (7 patients) | 1995 | Spain | AIDS | Yes | 4 patients with Mycobacterium |
| Mohri et al. (115) | 1995 | Japan | AIDS | Yes | None |
| Clavel et al. (113) (5 patients) | 1996 | Spain | AIDS | Yes | 3 patients with Mycobacterium, 1 patient with cytomegalovirus and Pneumocystis |
| Dupont et al. (63) (2 patients) | 1996 | France | AIDS | Yes | None |
| Meynard et al. (112) | 1996 | France | AIDS | Yes | Cytomegalovirus, Pneumocystis, Streptococcus pneumoniae |
| Poirot et al. (57) (4 patients) | 1996 | France | AIDS | Yes | 1 patient with Aspergillus, 1 patient with Pneumocystis |
| Pellicelli et al. (64) | 1998 | Italy | AIDS | Yes | None |
| Palmieri et al. (66) | 2005 | Italy | AIDS | No diarrhea; fecal exam negative | None |
| Meamar et al. (65) | 2006 | Iran | AIDS | Yes | None |
| Mercado et al. (104) | 2007 | Chile | AIDS | Unknown | None |
| No underlying disease | |||||
| Harari et al. (62) | 1986 | Papua New Guinea | None known | Yes | None |
| Campayo Ibanez et al. (121) | 1994 | Spain | None known, HIV negative | No diarrhea; fecal exam negative | None |
| Mor et al. (25) (17 patients) | 2010 | Uganda | None known (1 patient HIV positive) | Yes | 2 patients with H. influenzae and S. pneumoniae; 2 patients with S. pneumoniae; 1 patient with Staphylococcus aureus |
Respiratory cryptosporidiosis has also been reported for individuals with induced immunosuppression. A patient undergoing intensive treatment for leukemia developed both pulmonary and intestinal cryptosporidiosis, and eventually succumbed (91) (Table 1). In India, a child receiving corticosteroid treatment for nephrotic syndrome developed respiratory cryptosporidiosis with intestinal involvement (92). A woman with an unspecified immunodeficiency associated with lymphoma acquired cryptosporidiosis in the bronchial tree; though she did not have diarrhea, cryptosporidial forms within the duodenum and gallbladder were discovered at autopsy (55). A bone marrow transplant recipient developed respiratory cryptosporidiosis diagnosed by bronchoalveolar lavage (BAL). Interestingly, diarrhea was absent, fecal examination for oocysts was negative, and there was no histologic evidence of Cryptosporidium infection in either the respiratory or intestinal tract at autopsy (93). Because they lacked gastrointestinal symptoms and oocyst excretion, the latter cases establish the possibility of primary respiratory infection with Cryptosporidium, which may have been acquired by inhalation of expectorated droplets or by contact with fomites.
Advent of HIV
As recently as 30 years ago, human cryptosporidiosis was still considered an uncommon parasitic infection. Fewer than 50 cases were reported by 1983, with two-thirds diagnosed in AIDS patients (52). As the AIDS epidemic unfolded, cryptosporidiosis became a marker for identifying people with AIDS (94–96). Predictably, as the human burden of AIDS mounted, so too did the incidence of Cryptosporidium infection in this subpopulation (97). HIV infection rapidly became a new risk factor for cryptosporidiosis, quickly outnumbering cases with other underlying causes.
In the absence of effective treatment for either Cryptosporidium or HIV infection, cryptosporidiosis, which typically causes self-limiting gastroenteritis, resulted in relentless diarrhea and wasting in individuals with HIV/AIDS. Cryptosporidiosis was most often limited to the gastrointestinal tract, but infections in other organ systems were identified. Respiratory infections involving the nasal mucosa (61), tracheobronchial tree (56, 60, 98–100) (Fig. 1), and deep lung (101–103) were reported (Table 1). While these early HIV/AIDS patients acquired disseminated cryptosporidiosis presumably originating in the intestinal tract, later cases lacking evidence of gastrointestinal involvement hinted at the possibility of respiratory transmission of cryptosporidiosis (66, 104–106). Regardless of the route of infection, all of these critically ill patients experienced fulminant disease, failed to respond to existing therapies, and succumbed.
FIG 1.
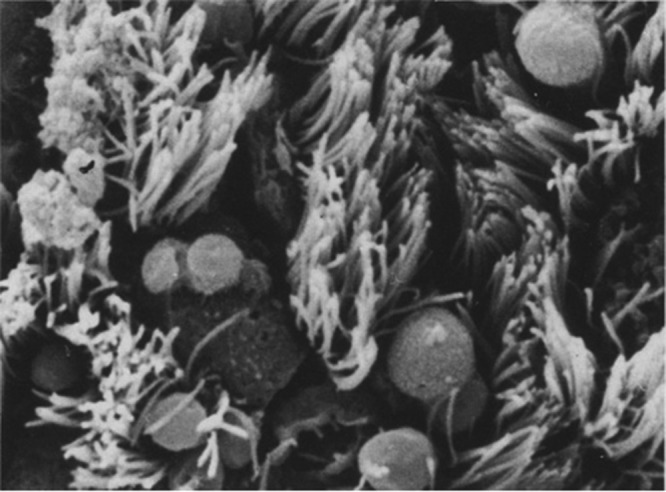
Scanning electron micrograph of cryptosporidia in the human bronchial mucosa. Magnification, ×1,000. (Reprinted from reference 60 with kind permission from Springer Science and Business Media.)
Antiretroviral Therapy
The introduction of combination ART enabled HIV-infected patients with access to these drugs to live longer (107), largely due to a decrease in frequency and severity of opportunistic infections, including cryptosporidiosis (108, 109). Flanigan et al. (110) found that individuals with CD4 counts of >180 cells/mm3 experienced only transient infection with Cryptosporidium, while those with fewer CD4 cells developed chronic, unremitting diarrhea. Additional studies demonstrated that combination ART including protease inhibitors dramatically resolved otherwise intractable cryptosporidial diarrhea in HIV-infected patients (23, 24, 111).
As ART substantially reduced the incidence of cryptosporidiosis in those with HIV, the number of reported cases of respiratory tract infections diminished drastically in the United States. Across Europe, respiratory cryptosporidiosis as a complication of HIV/AIDS has been reported sporadically in Denmark (105, 106), France (57, 63, 112), Italy (64, 66), Spain (58, 113), United Kingdom (114), and the former Czechoslovakia (60) (Table 1). Isolated cases have been documented in disparate countries, including Chile (104), Iran (65), and Japan (115).
Evidence of Respiratory Transmission
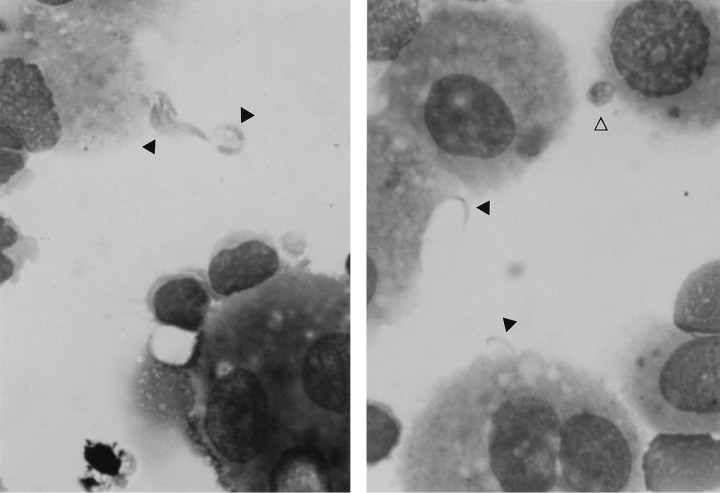
While Cryptosporidium infection of the respiratory tract has been well documented, particularly for immunocompromised individuals, disease transmission via this route has yet to be substantiated. The presence of various cryptosporidial forms lining the bronchial epithelium of lung sections suggests that the protozoan may be capable of propagating within the human respiratory tract in much the same way that it parasitizes the gastrointestinal epithelium (57, 98) (Fig. 2). Likewise, demonstration of intramacrophagic oocysts and extracellular invasive forms (sporozoites or merozoites) (57, 63, 90) (Fig. 3) in respiratory secretions implies an active life cycle within the respiratory tract, where transmission of oocysts may be facilitated by coughing or expectoration.
FIG 2.
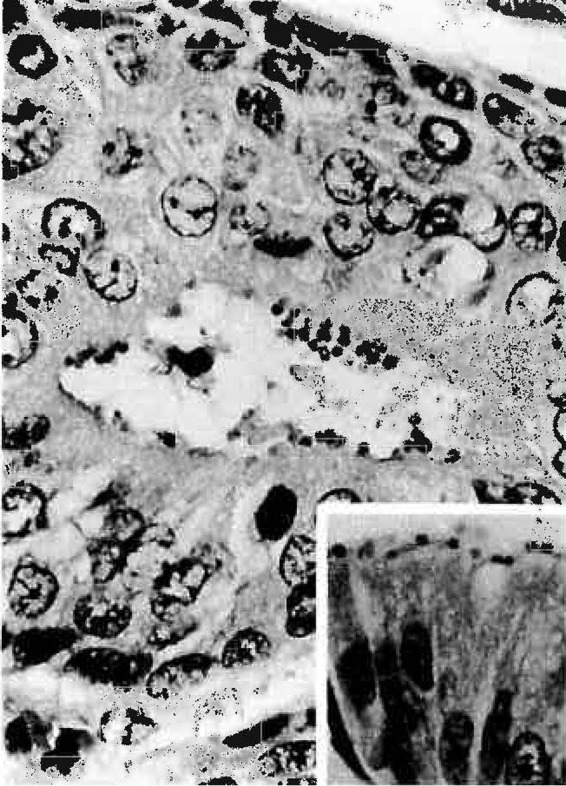
Similarity of cryptosporidial forms adherent to the luminal epithelium of a bronchial mucous gland and organisms lining the surface of the gastric epithelium (inset) (hematoxylin-eosin staining). Magnification, ×1,000. (Reprinted from reference 98 with permission of the publisher. Copyright 1991 American Medical Association. All rights reserved.)
FIG 3.
Extracellular (black arrows) and intracellular (white arrow) cryptosporidial forms in BAL fluid (Giemsa stain). Magnification, ×1,250. (Reprinted from reference 63 with permission.)
Hints of a respiratory dimension to parasite transmission have been promulgated by a few early epidemiological studies, mostly involving children presumed to be immunocompetent. In Switzerland, children diagnosed with diarrhea due to Cryptosporidium were more likely to have respiratory symptoms than children with diarrhea from other sources, suggesting that respiratory infection may be common but transient in healthy individuals (116). In rural Brazil, 50% of children with intestinal cryptosporidiosis had unexplained respiratory signs (117). In Bangladesh, one-third of children under the age of 2 years and diagnosed with cryptosporidial diarrhea also had an unexplained cough (118). In Gaza, half of children with gastrointestinal cryptosporidiosis also had respiratory symptoms; interestingly, 10% of children with primary respiratory disease (in the absence of diarrhea) were also shedding Cryptosporidium in feces (119). The latter finding leads to speculation that the respiratory system may serve as a viable alternative to the gut for parasite propagation, transmission, and diagnosis, with or without apparent respiratory symptoms.
While zoonotic fecal-oral transmission of cryptosporidiosis is well documented, to our knowledge, zoonotic airborne transmission of cryptosporidiosis has been reported only once. A veterinarian who administered oral fluids to a calf with confirmed cryptosporidial diarrhea subsequently developed intestinal cryptosporidiosis herself after sniffing the contents of the stomach tube while it was positioned in the animal (120). Infection presumably occurred by inhalation of oocysts, though she exhibited only gastrointestinal symptoms, and no respiratory samples were taken to further characterize the extent of the infection in the respiratory tract. Oocyst inhalation has been proposed by others (59, 102). This possible mechanism of infection is supported by a few cases of respiratory cryptosporidiosis that developed in immunodeficient patients without diarrhea or evidence of fecal oocyst shedding (55, 66, 93, 105).
To our knowledge, prior to 2010, there were only two known cases of respiratory cryptosporidiosis in immunocompetent individuals. The first was in an infant in Papua New Guinea who suffered from laryngotracheitis and diarrhea due to Cryptosporidium (62) (Table 1). The second was in a young Spanish woman with no evidence of diarrhea, Cryptosporidium in stool, or HIV infection (121). In the latter case, respiratory cryptosporidiosis without an apparent gastrointestinal component again suggests possible transmission via inhalation.
Currently, the strongest evidence for person-to-person respiratory transmission of cryptosporidiosis in immunocompetent children is the work of Mor et al. (25). Their study demonstrated that one-third (35%) of Ugandan children diagnosed with intestinal cryptosporidiosis and cough had sputum samples containing Cryptosporidium DNA, suggesting the presence of organisms in the respiratory tract. No parasite DNA was detected in saliva from any of the children with positive sputum, indicating little possibility of sputum contamination by gastrointestinal contents. The identification of numerous children with Cryptosporidium DNA (and possibly organisms) in their respiratory secretions further hints at transmission between persons. In addition, of the 17 children with both stool and sputum samples positive for Cryptosporidium spp., 16 were seronegative for HIV and 10 were normally nourished (25). This finding suggests that respiratory cryptosporidiosis may occur commonly in immunocompetent individuals.
Other Possible Routes of Infection
Other possible routes of infection for respiratory cryptosporidiosis have been proposed. In patients with both intestinal and respiratory involvement, transient respiratory infection resulting from aspiration of gastrointestinal contents during vomiting has been suggested (59, 91, 101). While this pathogenesis is certainly plausible, at least one case demonstrated the presence of Cryptosporidium organisms in bronchial samples for over 3 months, reducing the likelihood of a fleeting respiratory infection precipitated by emesis (99). There is some evidence to support the spread of cryptosporidiosis from the intestinal tract to the respiratory system via circulation. Histopathology samples from one patient revealed parasite forms within the colonic vasculature, hinting that dissemination of the organism to the respiratory tree may have occurred through a hematogenous route (91). In vitro, Cryptosporidium organisms have successfully replicated in murine macrophages, which may help to explain both the spread of extraintestinal infections and the inability of immunosuppressed individuals to eradicate the parasite (122). In addition, early experimental studies in mice were successful in establishing patent gastrointestinal infections by intravenous inoculation of oocysts (123).
Since immunocompromised hosts are often concurrently infected with more than one pathogen, some have considered Cryptosporidium only a secondary invader of the respiratory tract. Numerous cases of respiratory cryptosporidiosis have been characterized by coinfections with pathogens, including cytomegalovirus (89, 93, 99), Pneumocystis jirovecii (105, 114), and Mycobacterium (58, 113). Mixed infections involving multiple pathogens have also been identified (59, 98, 100–102, 112). Nonetheless, Cryptosporidium has been solely recovered from the respiratory tracts of a substantial number of patients with clinical disease (25, 58, 61, 62, 65, 66, 92, 104, 113, 115), suggesting a primary role for the organism as a respiratory pathogen. While two patients from whom only Cryptosporidium was recovered were asymptomatic and lacked radiographic evidence of lung disease (58), the overwhelming majority had a cough, with or without respiratory distress, and pulmonary infiltrates on thoracic radiographs. Furthermore, Cryptosporidium has been the only organism identified deep within the lung by BAL (57, 63, 64, 103, 105, 106, 121) and autopsy (60), implying an intimate association between the parasite and the respiratory tract.
DIAGNOSIS OF RESPIRATORY CRYPTOSPORIDIOSIS
Respiratory Samples
Respiratory samples obtained by sputum induction, tracheal aspiration, or BAL are examined for thick-walled Cryptosporidium oocysts and invasive forms of the parasite, including sporozoites and merozoites. Infective oocysts in respiratory secretions have been identified successfully by use of a variety of staining techniques, including acid-fast (57, 58, 113), auramine (58, 63, 100), and indirect immunofluorescence (58, 105, 106) staining. Sporozoites and merozoites have been recovered from BAL fluid specimens stained with Giemsa stain (57, 63) (Fig. 3), but these forms are reported less often, likely because their presence is not detectable by more widely used acid-fast and fluorescence staining methods. Ideally, both Giemsa and modified acid-fast or fluorescent stains would be utilized to identify all cryptosporidial forms present in respiratory specimens, which would more fully characterize the extent of infection and also reduce the likelihood of false-negative results. More recently, Mor et al. (25) successfully employed PCR-restriction fragment length polymorphism (PCR-RFLP) analysis to both diagnose infection and type Cryptosporidium species in respiratory samples.
Histopathology
Although this requires a more invasive evaluation, respiratory cryptosporidiosis may be diagnosed by detection of organisms in biopsy or autopsy specimens. Histopathologic sections stained with hematoxylin-eosin reveal multiple parasite forms lining the mucosal epithelium at the luminal surface of the trachea, bronchi, and bronchioles, with occasional organisms identified within bronchial mucous glands (60, 98, 102, 103) (Fig. 2). Bronchial and lung biopsy specimens have yielded intra- and extracellular cryptosporidia, including sporozoites or merozoites, as well as oocysts on the bronchoepithelial surface (57, 112).
Radiology
Radiographic findings are not pathognomonic for cryptosporidiosis, but evidence of pulmonary interstitial disease is often present (58, 63, 64, 66, 90, 112, 113).
TREATMENT OF RESPIRATORY CRYPTOSPORIDIOSIS
Individuals with respiratory cryptosporidiosis are often severely immunocompromised, resulting in a high fatality rate. The relatively small number of documented cases has produced only anecdotal therapies, many of which failed. A scarce few AIDS patients with respiratory cryptosporidiosis have been treated successfully with paromomycin (65, 66, 115), but it was no more effective than placebo in treating immunodeficient individuals when the drug was scrutinized in a clinical trial (124, 125). To our knowledge, nitazoxanide has not been used as a therapy against respiratory cryptosporidiosis, but its efficacy in the treatment of intestinal cryptosporidiosis in immunocompromised populations is generally poor (125). While nitazoxanide has modestly reduced the duration of diarrhea and oocyst shedding in immunocompetent individuals (126), the drug has not proved successful in eradicating the parasite from the guts of chronically infected children with HIV/AIDS (127).
Despite extensive testing of numerous pharmaceutical agents in animal models and directly in patients, to date, no treatment has been effective in eliminating Cryptosporidium infection in immunocompromised individuals (125, 128). Malnourished children with chronic diarrhea due to Cryptosporidium also bear a high disease burden as a consequence of the lack of effective therapy. Currently, supportive care and ART (for HIV/AIDS patients) form the basis for treatment of cryptosporidiosis (128). ART has produced parasitological clearance after treatment for several months, presumably by virtue of restoring immune function (24). Furthermore, protease inhibitors, including indinavir, have been shown to directly interfere with Cryptosporidium development (129, 130). With the success of ART in halting the progression of HIV/AIDS and reducing the incidence of opportunistic infections, the urgency to develop a viable therapy against Cryptosporidium has subsided considerably. In sub-Saharan Africa, South Asia, and Latin America, cryptosporidiosis remains a serious and neglected cause of diarrhea in children, who could benefit enormously from an effective treatment.
CRYPTOSPORIDIUM SPECIES IN RESPIRATORY INFECTIONS IN HUMANS
At least two species of Cryptosporidium have been identified in human respiratory samples, mirroring those commonly found in intestinal cryptosporidiosis: C. hominis (25, 104) and C. parvum (25, 53, 64–66). C. hominis is found almost exclusively in humans, while C. parvum, though it appears to be less prevalent in children examined so far in Africa (11, 12), is readily transmitted between humans and animals as well as between humans (28). Given the wider potential host range of C. parvum, its lower prevalence in both intestinal and respiratory infections is unexpected. We speculate that higher rates of C. hominis may be explained by the ability of the species to spread more readily via respiratory transmission, particularly in the pediatric population. In the largest study of respiratory cryptosporidiosis to date, 13 of 17 Ugandan children (76%) with Cryptosporidium-positive sputa and feces were infected with C. hominis, while the remaining 4 were infected with C. parvum (25), reflecting the species isolation rates from the gastrointestinal tract. C. meleagridis, another species producing diarrhea and suspected to cause respiratory disease in birds (75), has also been isolated from African children with diarrhea (11, 12, 131, 132). This parasite has been found in the intestinal tracts of HIV/AIDS patients (133, 134), but the role of C. meleagridis in human respiratory cryptosporidiosis has so far not been reported.
FUTURE DIRECTIONS
An effective therapy for cryptosporidiosis would indisputably benefit children with acute disease and immunodeficient individuals with chronic diarrhea and wasting. Cryptosporidium accounts for 50% of all diarrhea in patients with HIV/AIDS (135). ART has eliminated most cases of cryptosporidiosis and other opportunistic infections, as well as improved longevity, for those with ART access. Sadly, ART availability is scarce in resource-poor regions, and in 2012, HIV-related mortality in Africa alone totaled 1.2 million (136). Without viable treatment options, prevention of cryptosporidiosis is paramount.
Preliminary evidence suggests that Cryptosporidium may be transmitted via respiratory secretions as well as through the more recognized fecal-oral route. We currently have an observational study under way in Uganda to better characterize the clinical illness associated with respiratory cryptosporidiosis in children and to determine the prevalence and impact of the disease among children and their families. The effects of prior exposure to Cryptosporidium, parasite species, HIV/AIDS, and malnutrition on the frequency and severity of respiratory cryptosporidiosis are being examined. Cryptosporidium infection rates are being compared in families with children, with and without respiratory cryptosporidiosis.
While the gastrointestinal tract is undoubtedly the principal site of infection and disease caused by Cryptosporidium, this review presents ample evidence of respiratory tract involvement. The frequency, extent, nature, and role of the respiratory tract in Cryptosporidium infection and transmission, with or without respiratory symptoms, need further investigation. Potential other factors, such as age, physical proximity required for respiratory transmission, and Cryptosporidium species most frequently involved, may emerge as significant. Other unanswered questions are whether inhaled oocysts are immediately swallowed or infection begins in the respiratory tree and whether infection at either site may occur independently of the other. It is assumed, perhaps incorrectly, that Cryptosporidium invades the respiratory tree first, followed by infection of the gastrointestinal tract. Conversely, infection may begin in the gut and spread to the respiratory tract via circulation or aspiration of gastrointestinal contents, as vomiting is frequent in this disease. Future investigations should first address such questions in suitable animal models.
Numerous interventions to improve water, sanitation, nutrition, and health care, as well as the introduction of a vaccine and effective therapy, together comprise an integral strategy to minimize the deleterious impact of cryptosporidiosis on children in developing regions of the world. Delineating the role of the respiratory tract in disease transmission may provide necessary evidence to establish further guidelines for the prevention of cryptosporidiosis. The struggle not only to eliminate the ills of cryptosporidiosis but also to ameliorate the myriad other poverty-related maladies afflicting the world's poorest people must continue to be an international public health priority.
Biographies

Jerlyn K. Sponseller, D.V.M., M.P.H., is an instructor in the Department of Infectious Disease and Global Health, Tufts University Cummings School of Veterinary Medicine, North Grafton, MA, and in the Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA. She earned her veterinary degree at Purdue University, West Lafayette, IN, and completed her public health training at the University of Tennessee, Knoxville, TN. Dr. Sponseller is currently pursuing a Ph.D. by examining respiratory cryptosporidiosis and transmission in Ugandan children.

Jeffrey K. Griffiths, M.D., M.P.H., and T.M., is Director of Global Health and Professor of Public Health and of Medicine in the Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA. He is also an Adjunct Professor at both Tufts Friedman School of Nutrition Science and Policy and Tufts School of Engineering. Dr. Griffiths has a major interest in infectious diseases, especially the human, animal, and environmental epidemiologies of Cryptosporidium. His other research interests include linkages between human health, nutrition, and the environment. These include the relationships between malnutrition, aflatoxins, heavy metals, air pollution, diarrhea, and pneumonia. Dr. Griffiths is currently involved in several projects focused in East Africa. He is the Program Director for the USAID Nutrition Innovation Lab for Collaborative Research in Africa and the Principal Investigator for an NIH-funded study of respiratory cryptosporidiosis and transmission in Uganda.

Saul Tzipori, B.V.Sc., Ph.D., D.V.Sc., F.R.C.V.S., is Distinguished Professor and Chair of the Department of Infectious Disease and Global Health, Agnes Varis University Chair in Science and Society, and Director of the New England Regional Biosafety Laboratory at Tufts University Cummings School of Veterinary Medicine, North Grafton, MA, where he has been a faculty member since 1991. He has extensive research experience in infectious diseases of humans and domestic animals, with a particular interest in enteric pathogens (viruses, bacteria, fungi, and protozoa). His work includes investigating relative contributions of virulence attributes to pathogenesis and disease, screening and evaluation of chemical or immune-based therapeutic agents, and vaccine development. Dr. Tzipori is best known for his expertise on cryptosporidiosis, microsporidiosis, Escherichia coli O157:H7 diarrhea and hemolytic-uremic syndrome, shigellosis, and, more recently, Clostridium difficile. He is also the Tufts Principal Investigator of RESPOND, a USAID global health project within the Emerging Pandemic Threats program.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 2.Nasrin D, Wu Y, Blackwelder WC, Farag TH, Saha D, Sow SO, Alonso PL, Breiman RF, Sur D, Faruque AS, Zaidi AK, Biswas K, Van Eijk AM, Walker DG, Levine MM, Kotloff KL. 2013. Health care seeking for childhood diarrhea in developing countries: evidence from seven sites in Africa and Asia. Am. J. Trop. Med. Hyg. 89(Suppl 1):3–12. 10.4269/ajtmh.12-0749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallon S, el-Shawwa R, Khalil M, Ginsburg G, el Tayib J, el-Eila J, Green V, Hart CA. 1994. Diarrhoeal disease in children in Gaza. Ann. Trop. Med. Parasitol. 88:175–182 [DOI] [PubMed] [Google Scholar]
- 4.Adjei AA, Armah H, Rodrigues O, Renner L, Borketey P, Ayeh-Kumi P, Adiku T, Sifah E, Lartey M. 2004. Cryptosporidium spp., a frequent cause of diarrhea among children at the Korle-Bu Teaching Hospital, Accra, Ghana. Jpn. J. Infect. Dis. 57:216–219 http://www0.nih.go.jp/JJID/57/216.html [PubMed] [Google Scholar]
- 5.Sallon S, Deckelbaum RJ, Schmid II, Harlap S, Baras M, Spira DT. 1988. Cryptosporidium, malnutrition, and chronic diarrhea in children. Am. J. Dis. Child. 142:312–315 [DOI] [PubMed] [Google Scholar]
- 6.Macfarlane DE, Horner-Bryce J. 1987. Cryptosporidiosis in well-nourished and malnourished children. Acta Paediatr. Scand. 76:474–477. 10.1111/j.1651-2227.1987.tb10502.x [DOI] [PubMed] [Google Scholar]
- 7.Javier Enriquez F, Avila CR, Ignacio Santos J, Tanaka-Kido J, Vallejo O, Sterling CR. 1997. Cryptosporidium infections in Mexican children: clinical, nutritional, enteropathogenic, and diagnostic evaluations. Am. J. Trop. Med. Hyg. 56:254–257 [DOI] [PubMed] [Google Scholar]
- 8.Gay-Andrieu E, Adehossi E, Illa H, Garba Ben A, Kourna H, Boureima H. 2007. Prevalence of cryptosporidiosis in pediatric hospital patients in Niamey, Niger. Bull. Soc. Pathol. Exot. 100:193–196 [PubMed] [Google Scholar]
- 9.Sarabia-Arce S, Salazar-Lindo E, Gilman RH, Naranjo J, Miranda E. 1990. Case-control study of Cryptosporidium parvum infection in Peruvian children hospitalized for diarrhea: possible association with malnutrition and nosocomial infection. Pediatr. Infect. Dis. J. 9:627–631 [PubMed] [Google Scholar]
- 10.Cegielski JP, Ortega YR, McKee S, Madden JF, Gaido L, Schwartz DA, Manji K, Jorgensen AF, Miller SE, Pulipaka UP, Msengi AE, Mwakyusa DH, Sterling CR, Reller LB. 1999. Cryptosporidium, enterocytozoon, and cyclospora infections in pediatric and adult patients with diarrhea in Tanzania. Clin. Infect. Dis. 28:314–321. 10.1086/515131 [DOI] [PubMed] [Google Scholar]
- 11.Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Rich SM, Widmer G, Feng X, Tzipori S. 2003. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am. J. Trop. Med. Hyg. 68:710–715 http://www.ajtmh.org/content/68/6/710.long [PubMed] [Google Scholar]
- 12.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, Ndeezi G, Downing R, Feng X, Akiyoshi DE, Tzipori S. 2005. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am. J. Trop. Med. Hyg. 73:921–925 http://www.ajtmh.org/content/73/5/921.long [PubMed] [Google Scholar]
- 13.Nel ED, Rabie H, Goodway J, Cotton MF. 2011. A retrospective study of cryptosporidial diarrhea in a region with high HIV prevalence. J. Trop. Pediatr. 57:289–292. 10.1093/tropej/fmq094 [DOI] [PubMed] [Google Scholar]
- 14.Chintu C, Luo C, Bhat G, DuPont HL, Mwansa-Salamu P, Kabika M, Zumla A. 1995. Impact of the human immunodeficiency virus type-1 on common pediatric illnesses in Zambia. J. Trop. Pediatr. 41:348–353. 10.1093/tropej/41.6.348 [DOI] [PubMed] [Google Scholar]
- 15.Molbak K, Hojlyng N, Gottschau A, Sa JC, Ingholt L, da Silva AP, Aaby P. 1993. Cryptosporidiosis in infancy and childhood mortality in Guinea Bissau, west Africa. BMJ 307:417–420. 10.1136/bmj.307.6901.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amadi B, Kelly P, Mwiya M, Mulwazi E, Sianongo S, Changwe F, Thomson M, Hachungula J, Watuka A, Walker-Smith J, Chintu C. 2001. Intestinal and systemic infection, HIV, and mortality in Zambian children with persistent diarrhea and malnutrition. J. Pediatr. Gastroenterol. Nutr. 32:550–554. 10.1097/00005176-200105000-00011 [DOI] [PubMed] [Google Scholar]
- 17.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. 1999. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 61:707–713 [DOI] [PubMed] [Google Scholar]
- 18.Molbak K, Andersen M, Aaby P, Hojlyng N, Jakobsen M, Sodemann M, da Silva AP. 1997. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am. J. Clin. Nutr. 65:149–152 [DOI] [PubMed] [Google Scholar]
- 19.Checkley W, Gilman RH, Black RE, Epstein LD, Cabrera L, Sterling CR, Moulton LH. 2004. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet 363:112–118. 10.1016/S0140-6736(03)15261-0 [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg O, Robberecht F, Dauby N, Moens C, Talabani H, Dupont E, Menotti J, van Gool T, Levy J. 2012. Management of a Cryptosporidium hominis outbreak in a day-care center. Pediatr. Infect. Dis. J. 31:10–15. 10.1097/INF.0b013e318235ab64 [DOI] [PubMed] [Google Scholar]
- 21.Budu-Amoako E, Greenwood SJ, Dixon BR, Barkema HW, McClure JT. 2011. Foodborne illness associated with Cryptosporidium and Giardia from livestock. J. Food Prot. 74:1944–1955. 10.4315/0362-028X.JFP-11-107 [DOI] [PubMed] [Google Scholar]
- 22.Mota A, Mena KD, Soto-Beltran M, Tarwater PM, Chaidez C. 2009. Risk assessment of cryptosporidium and giardia in water irrigating fresh produce in Mexico. J. Food Prot. 72:2184–2188 [DOI] [PubMed] [Google Scholar]
- 23.Carr A, Marriott D, Field A, Vasak E, Cooper DA. 1998. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet 351:256–261. 10.1016/S0140-6736(97)07529-6 [DOI] [PubMed] [Google Scholar]
- 24.Miao YM, Awad-El-Kariem FM, Franzen C, Ellis DS, Muller A, Counihan HM, Hayes PJ, Gazzard BG. 2000. Eradication of cryptosporidia and microsporidia following successful antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 25:124–129. 10.1097/00126334-200010010-00006 [DOI] [PubMed] [Google Scholar]
- 25.Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, Griffiths JK. 2010. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin. Infect. Dis. 50:1366–1372. 10.1086/652140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fayer R, Ungar BL. 1986. Cryptosporidium spp. and cryptosporidiosis. Microbiol. Rev. 50:458–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzipori S. 1983. Cryptosporidiosis in animals and humans. Microbiol. Rev. 47:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao L, Fayer R, Ryan U, Upton SJ. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 17:72–97. 10.1128/CMR.17.1.72-97.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyzzer EE. 1907. A sporozoan found in the peptic glands of the common mouse. Proc. Soc. Exp. Biol. Med. 5:12–13. 10.3181/00379727-5-5 [DOI] [Google Scholar]
- 30.Nime FA, Burek JD, Page DL, Holscher MA, Yardley JH. 1976. Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterology 70:592–598 [PubMed] [Google Scholar]
- 31.Meisel JL, Perera DR, Meligro C, Rubin CE. 1976. Overwhelming watery diarrhea associated with a cryptosporidium in an immunosuppressed patient. Gastroenterology 70:1156–1160 [PubMed] [Google Scholar]
- 32.Weisburger WR, Hutcheon DF, Yardley JH, Roche JC, Hillis WD, Charache P. 1979. Cryptosporidiosis in an immunosuppressed renal-transplant recipient with IgA deficiency. Am. J. Clin. Pathol. 72:473–478 [DOI] [PubMed] [Google Scholar]
- 33.Lasser KH, Lewin KJ, Ryning FW. 1979. Cryptosporidial enteritis in a patient with congenital hypogammaglobulinemia. Hum. Pathol. 10:234–240. 10.1016/S0046-8177(79)80012-X [DOI] [PubMed] [Google Scholar]
- 34.BMJ Publishing Group. 1980. Immunodeficiency and cryptosporidiosis. Demonstration at the Royal College of Physicians of London. Br. Med. J. 281:1123–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stemmermann GN, Hayashi T, Glober GA, Oishi N, Frankel RI. 1980. Cryptosporidiosis. Report of a fatal case complicated by disseminated toxoplasmosis. Am. J. Med. 69:637–642 [DOI] [PubMed] [Google Scholar]
- 36.Tzipori S, Angus KW, Gray EW, Campbell I. 1980. Vomiting and diarrhea associated with cryptosporidial infection. N. Engl. J. Med. 303:818. [PubMed] [Google Scholar]
- 37.Tzipori S, Smith M, Birch C, Barnes G, Bishop R. 1983. Cryptosporidiosis in hospital patients with gastroenteritis. Am. J. Trop. Med. Hyg. 32:931–934 [DOI] [PubMed] [Google Scholar]
- 38.Tzipori S, Widmer G. 2008. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 24:184–189. 10.1016/j.pt.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzipori S. 1988. Cryptosporidiosis in perspective. Adv. Parasitol. 27:63–129. 10.1016/S0065-308X(08)60353-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Current WL, Garcia LS. 1991. Cryptosporidiosis. Clin. Microbiol. Rev. 4:325–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffiths JK. 1998. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv. Parasitol. 40:37–85. 10.1016/S0065-308X(08)60117-7 [DOI] [PubMed] [Google Scholar]
- 42.Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, Tzipori S. 2006. Cryptosporidium hominis: experimental challenge of healthy adults. Am. J. Trop. Med. Hyg. 75:851–857 http://www.ajtmh.org/content/75/5/851.long [PubMed] [Google Scholar]
- 43.Pouillot R, Beaudeau P, Denis JB, Derouin F. 2004. A quantitative risk assessment of waterborne cryptosporidiosis in France using second-order Monte Carlo simulation. Risk Anal. 24:1–17. 10.1111/j.0272-4332.2004.00407.x [DOI] [PubMed] [Google Scholar]
- 44.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855–859. 10.1056/NEJM199503303321304 [DOI] [PubMed] [Google Scholar]
- 45.Cantey PT, Kurian AK, Jefferson D, Moerbe MM, Marshall K, Blankenship WR, Rothbarth GR, Hwang J, Hall R, Yoder J, Brunkard J, Johnston S, Xiao L, Hill VR, Sarisky J, Zarate-Bermudez MA, Otto C, Hlavsa MC. 2012. Outbreak of cryptosporidiosis associated with a man-made chlorinated lake—Tarrant County, Texas, 2008. J. Environ. Health 75:14–19 http://www.cdc.gov/nceh/ehs/Docs/outbreak-crypto-man-made-chlorinated-lake-jeh.pdf [PubMed] [Google Scholar]
- 46.Hlavsa MC, Roberts VA, Kahler AM, Hilborn ED, Wade TJ, Backer LC, Yoder JS, Centers for Disease Control and Prevention 2014. Recreational water-associated disease outbreaks—United States, 2009–2010. MMWR Morb. Mortal. Wkly. Rep. 63:6–10 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6301a2.htm [PMC free article] [PubMed] [Google Scholar]
- 47.Gormley FJ, Little CL, Chalmers RM, Rawal N, Adak GK. 2011. Zoonotic cryptosporidiosis from petting farms, England and Wales, 1992–2009. Emerg. Infect. Dis. 17:151–152. 10.3201/eid1701.100902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lange H, Johansen OH, Vold L, Robertson LJ, Anthonisen IL, Nygard K. 2013. Second outbreak of infection with a rare Cryptosporidium parvum genotype in schoolchildren associated with contact with lambs/goat kids at a holiday farm in Norway. Epidemiol. Infect. 2013:1–9. 10.1017/S0950268813003002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. 1984. Cryptosporidiosis among children attending day-care centers—Georgia, Pennsylvania, Michigan, California, New Mexico. MMWR Morb. Mortal. Wkly. Rep. 33:599–601 [PubMed] [Google Scholar]
- 50.Artieda J, Basterrechea M, Arriola L, Yague M, Albisua E, Arostegui N, Astigarraga U, Botello R, Manterola JM. 2012. Outbreak of cryptosporidiosis in a child day-care centre in Gipuzkoa, Spain, October to December 2011. Euro Surveill. 17:20070 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20070 [DOI] [PubMed] [Google Scholar]
- 51.Brady MT. 2005. Infectious disease in pediatric out-of-home child care. Am. J. Infect. Control 33:276–285. 10.1016/j.ajic.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 52.Pitlik SD, Fainstein V, Garza D, Guarda L, Bolivar R, Rios A, Hopfer RL, Mansell PA. 1983. Human cryptosporidiosis: spectrum of disease. Report of six cases and review of the literature. Arch. Intern. Med. 143:2269–2275 [DOI] [PubMed] [Google Scholar]
- 53.Kutukculer N, Moratto D, Aydinok Y, Lougaris V, Aksoylar S, Plebani A, Genel F, Notarangelo LD. 2003. Disseminated cryptosporidium infection in an infant with hyper-IgM syndrome caused by CD40 deficiency. J. Pediatr. 142:194–196. 10.1067/mpd.2003.41 [DOI] [PubMed] [Google Scholar]
- 54.Davis JJ, Heyman MB. 1988. Cryptosporidiosis and sinusitis in an immunodeficient adolescent. J. Infect. Dis. 158:649. 10.1093/infdis/158.3.649 [DOI] [PubMed] [Google Scholar]
- 55.Travis WD, Schmidt K, MacLowry JD, Masur H, Condron KS, Fojo AT. 1990. Respiratory cryptosporidiosis in a patient with malignant lymphoma. Report of a case and review of the literature. Arch. Pathol. Lab. Med. 114:519–522 [PubMed] [Google Scholar]
- 56.Gross TL, Wheat J, Bartlett M, O'Connor KW. 1986. AIDS and multiple system involvement with cryptosporidium. Am. J. Gastroenterol. 81:456–458 [PubMed] [Google Scholar]
- 57.Poirot JL, Deluol AM, Antoine M, Heyer F, Cadranel J, Meynard JL, Meyohas MC, Girard PM, Roux P. 1996. Broncho-pulmonary cryptosporidiosis in four HIV-infected patients. J. Eukaryot. Microbiol. 43:78S–79S. 10.1111/j.1550-7408.1996.tb05007.x [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Velez R, Tarazona R, Garcia Camacho A, Gomez-Mampaso E, Guerrero A, Moreira V, Villanueva R. 1995. Intestinal and extraintestinal cryptosporidiosis in AIDS patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:677–681. 10.1007/BF01690873 [DOI] [PubMed] [Google Scholar]
- 59.Kocoshis SA, Cibull ML, Davis TE, Hinton JT, Seip M, Banwell JG. 1984. Intestinal and pulmonary cryptosporidiosis in an infant with severe combined immune deficiency. J. Pediatr. Gastroenterol. Nutr. 3:149–157. 10.1097/00005176-198401000-00028 [DOI] [PubMed] [Google Scholar]
- 60.Ditrich O, Palkovic L, Sterba J, Prokopic J, Loudova J, Giboda M. 1991. The first finding of Cryptosporidium baileyi in man. Parasitol. Res. 77:44–47. 10.1007/BF00934383 [DOI] [PubMed] [Google Scholar]
- 61.Giang TT, Pollack G, Kotler DP. 1994. Cryptosporidiosis of the nasal mucosa in a patient with AIDS. AIDS 8:555–556. 10.1097/00002030-199404000-00021 [DOI] [PubMed] [Google Scholar]
- 62.Harari MD, West B, Dwyer B. 1986. Cryptosporidium as cause of laryngotracheitis in an infant. Lancet i:1207. [DOI] [PubMed] [Google Scholar]
- 63.Dupont C, Bougnoux ME, Turner L, Rouveix E, Dorra M. 1996. Microbiological findings about pulmonary cryptosporidiosis in two AIDS patients. J. Clin. Microbiol. 34:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellicelli AM, Palmieri F, Spinazzola F, D'Ambrosio C, Causo T, De Mori P, Bordi E, D'Amato C. 1998. Pulmonary cryptosporidiosis in patients with acquired immunodeficiency syndrome. Minerva Med. 89:173–175 [PubMed] [Google Scholar]
- 65.Meamar AR, Rezaian M, Rezaie S, Mohraz M, Kia EB, Houpt ER, Solaymani-Mohammadi S. 2006. Cryptosporidium parvum bovine genotype oocysts in the respiratory samples of an AIDS patient: efficacy of treatment with a combination of azithromycin and paromomycin. Parasitol. Res. 98:593–595. 10.1007/s00436-005-0097-4 [DOI] [PubMed] [Google Scholar]
- 66.Palmieri F, Cicalini S, Froio N, Rizzi EB, Goletti D, Festa A, Macri G, Petrosillo N. 2005. Pulmonary cryptosporidiosis in an AIDS patient: successful treatment with paromomycin plus azithromycin. Int. J. STD AIDS 16:515–517. 10.1258/0956462054308332 [DOI] [PubMed] [Google Scholar]
- 67.Hoerr FJ, Ranck FM, Jr, Hastings TF. 1978. Respiratory cryptosporidiosis in turkeys. J. Am. Vet. Med. Assoc. 173:1591–1593 [PubMed] [Google Scholar]
- 68.Glisson JR, Brown TP, Brugh M, Page RK, Kleven SH, Davis RB. 1984. Sinusitis in turkeys associated with respiratory cryptosporidiosis. Avian Dis. 28:783–790. 10.2307/1590250 [DOI] [PubMed] [Google Scholar]
- 69.Mason RW, Hartley WJ. 1980. Respiratory cryptosporidiosis in a peacock chick. Avian Dis. 24:771–776. 10.2307/1589814 [DOI] [PubMed] [Google Scholar]
- 70.Molina-Lopez RA, Ramis A, Martin-Vazquez S, Gomez-Couso H, Ares-Mazas E, Caccio SM, Leiva M, Darwich L. 2010. Cryptosporidium baileyi infection associated with an outbreak of ocular and respiratory disease in otus owls (Otus scops) in a rehabilitation centre. Avian Pathol. 39:171–176. 10.1080/03079451003717589 [DOI] [PubMed] [Google Scholar]
- 71.Tarwid JN, Cawthorn RJ, Riddell C. 1985. Cryptosporidiosis in the respiratory tract of turkeys in Saskatchewan. Avian Dis. 29:528–532. 10.2307/1590516 [DOI] [PubMed] [Google Scholar]
- 72.Randall CJ. 1982. Cryptosporidiosis of the bursa of fabricius and trachea in broilers. Avian Pathol. 11:95–102. 10.1080/03079458208436084 [DOI] [PubMed] [Google Scholar]
- 73.Itakura C, Goryo M, Umemura T. 1984. Cryptosporidial infection in chickens. Avian Pathol. 13:487–499. 10.1080/03079458408418550 [DOI] [PubMed] [Google Scholar]
- 74.Dhillon AS, Thacker HL, Dietzel AV, Winterfield RW. 1981. Respiratory cryptosporidiosis in broiler chickens. Avian Dis. 25:747–751. 10.2307/1590007 [DOI] [PubMed] [Google Scholar]
- 75.Pages-Mante A, Pages-Bosch M, Majo-Masferrer N, Gomez-Couso H, Ares-Mazas E. 2007. An outbreak of disease associated with cryptosporidia on a red-legged partridge (Alectoris rufa) game farm. Avian Pathol. 36:275–278. 10.1080/03079450701439389 [DOI] [PubMed] [Google Scholar]
- 76.Whittington RJ, Wilson JM. 1985. Cryptosporidiosis of the respiratory tract in a pheasant. Aust. Vet. J. 62:284–285. 10.1111/j.1751-0813.1985.tb14255.x [DOI] [PubMed] [Google Scholar]
- 77.Tham VL, Kniesberg S, Dixon BR. 1982. Cryptosporidiosis in quails. Avian Pathol. 11:619–626. 10.1080/03079458208436138 [DOI] [PubMed] [Google Scholar]
- 78.van Zeeland YR, Schoemaker NJ, Kik MJ, van der Giessend JW. 2008. Upper respiratory tract infection caused by Cryptosporidium baileyi in three mixed-bred falcons (Falco rusticolus × Falco cherrug). Avian Dis. 52:357–363. 10.1637/8121-100207-Case.1 [DOI] [PubMed] [Google Scholar]
- 79.Randall CJ. 1986. Renal and nasal cryptosporidiosis in a junglefowl (Gallus sonneratii). Vet. Rec. 119:130–131. 10.1136/vr.119.6.130 [DOI] [PubMed] [Google Scholar]
- 80.Mascaro C, Arnedo T, Rosales MJ. 1994. Respiratory cryptosporidiosis in a bovine. J. Parasitol. 80:334–336. 10.2307/3283770 [DOI] [PubMed] [Google Scholar]
- 81.Fleta J, Sanchez-Acedo C, Clavel A, Quilez J. 1995. Detection of Cryptosporidium oocysts in extra-intestinal tissues of sheep and pigs. Vet. Parasitol. 59:201–205. 10.1016/0304-4017(94)00758-5 [DOI] [PubMed] [Google Scholar]
- 82.Blanchard JL, Baskin GB, Murphey-Corb M, Martin LN. 1987. Disseminated cryptosporidiosis in simian immunodeficiency virus/delta-infected rhesus monkeys. Vet. Pathol. 24:454–456 [DOI] [PubMed] [Google Scholar]
- 83.Yanai T, Chalifoux LV, Mansfield KG, Lackner AA, Simon MA. 2000. Pulmonary cryptosporidiosis in simian immunodeficiency virus-infected rhesus macaques. Vet. Pathol. 37:472–475. 10.1354/vp.37-5-472 [DOI] [PubMed] [Google Scholar]
- 84.Lindsay DS, Blagburn BL, Hoerr FJ. 1987. Experimentally induced infections in turkeys with Cryptosporidium baileyi isolated from chickens. Am. J. Vet. Res. 48:104–108 [PubMed] [Google Scholar]
- 85.Lindsay DS, Blagburn BL, Sundermann CA, Hoerr FJ, Ernest JA. 1986. Experimental Cryptosporidium infections in chickens: oocyst structure and tissue specificity. Am. J. Vet. Res. 47:876–879 [PubMed] [Google Scholar]
- 86.Lanzarini P, Gatti S, Bruno A, Corona S, Scaglia M. 1999. Experimental respiratory cryptosporidiosis in immunosuppressed rats: a light and electron microscopy study. Parasite 6:217–222 [DOI] [PubMed] [Google Scholar]
- 87.Meulbroek JA, Novilla MN, Current WL. 1991. An immunosuppressed rat model of respiratory cryptosporidiosis. J. Protozool. 38:113S–115S [PubMed] [Google Scholar]
- 88.Heine J, Moon HW, Woodmansee DB, Pohlenz JF. 1984. Experimental tracheal and conjunctival infections with Cryptosporidium sp. in pigs. Vet. Parasitol. 17:17–25. 10.1016/0304-4017(84)90061-X [DOI] [PubMed] [Google Scholar]
- 89.Manivel C, Filipovich A, Snover DC. 1985. Cryptosporidiosis as a cause of diarrhea following bone marrow transplantation. Dis. Colon Rectum 28:741–742. 10.1007/BF02560294 [DOI] [PubMed] [Google Scholar]
- 90.O'Halloran SM, Murphy NP, Hart CA, Baxby D, Smith CS. 1987. Fatal intestinal and respiratory cryptosporidiosis in early infancy. Pediatr. Rev. Commun. 2:81–84 [Google Scholar]
- 91.Gentile G, Baldassarri L, Caprioli A, Donelli G, Venditti M, Avvisati G, Martino P. 1987. Colonic vascular invasion as a possible route of extraintestinal cryptosporidiosis. Am. J. Med. 82:574–575 [DOI] [PubMed] [Google Scholar]
- 92.Shrikhande SN, Chande CA, Shegokar VR, Powar RM. 2009. Pulmonary cryptosporidiosis in HIV negative, immunocompromised host. Indian J. Pathol. Microbiol. 52:267–268. 10.4103/0377-4929.48942 [DOI] [PubMed] [Google Scholar]
- 93.Kibbler CC, Smith A, Hamilton-Dutoit SJ, Milburn H, Pattinson JK, Prentice HG. 1987. Pulmonary cryptosporidiosis occurring in a bone marrow transplant patient. Scand. J. Infect. Dis. 19:581–584. 10.3109/00365548709032426 [DOI] [PubMed] [Google Scholar]
- 94.Centers for Disease Control and Prevention. 1982. Cryptosporidiosis: assessment of chemotherapy of males with acquired immune deficiency syndrome (AIDS). MMWR Morb. Mortal. Wkly. Rep. 31:589–592 [PubMed] [Google Scholar]
- 95.Current WL, Reese NC, Ernst JV, Bailey WS, Heyman MB, Weinstein WM. 1983. Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N. Engl. J. Med. 308:1252–1257 [DOI] [PubMed] [Google Scholar]
- 96.Cooper DA, Wodak A, Marriot DJ, Harkness JL, Ralston M, Hill A, Penny R. 1984. Cryptosporidiosis in the acquired immune deficiency syndrome. Pathology 16:455–457. 10.3109/00313028409084739 [DOI] [PubMed] [Google Scholar]
- 97.Whiteside ME, Barkin JS, May RG, Weiss SD, Fischl MA, MacLeod CL. 1984. Enteric coccidiosis among patients with the acquired immunodeficiency syndrome. Am. J. Trop. Med. Hyg. 33:1065–1072 [DOI] [PubMed] [Google Scholar]
- 98.Moore JA, Frenkel JK. 1991. Respiratory and enteric cryptosporidiosis in humans. Arch. Pathol. Lab. Med. 115:1160–1162 [PubMed] [Google Scholar]
- 99.Forgacs P, Tarshis A, Ma P, Federman M, Mele L, Silverman ML, Shea JA. 1983. Intestinal and bronchial cryptosporidiosis in an immunodeficient homosexual man. Ann. Intern. Med. 99:793–794. 10.7326/0003-4819-99-6-793 [DOI] [PubMed] [Google Scholar]
- 100.Miller RA, Wasserheit JN, Kirihara J, Coyle MB. 1984. Detection of Cryptosporidium oocysts in sputum during screening for mycobacteria. J. Clin. Microbiol. 20:1192–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brady EM, Margolis ML, Korzeniowski OM. 1984. Pulmonary cryptosporidiosis in acquired immune deficiency syndrome. JAMA 252:89–90 [PubMed] [Google Scholar]
- 102.Ma P, Villanueva TG, Kaufman D, Gillooley JF. 1984. Respiratory cryptosporidiosis in the acquired immune deficiency syndrome. Use of modified cold Kinyoun and Hemacolor stains for rapid diagnoses. JAMA 252:1298–1301 [PubMed] [Google Scholar]
- 103.Goodstein RS, Colombo CS, Illfelder MA, Skaggs RE. 1989. Bronchial and gastrointestinal cryptosporidiosis in AIDS. J. Am. Osteopath. Assoc. 89:195–197 [PubMed] [Google Scholar]
- 104.Mercado R, Buck GA, Manque PA, Ozaki LS. 2007. Cryptosporidium hominis infection of the human respiratory tract. Emerg. Infect. Dis. 13:462–464. 10.3201/eid1303.060394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hojlyng N, Jensen BN. 1988. Respiratory cryptosporidiosis in HIV-positive patients. Lancet i:590–591 [DOI] [PubMed] [Google Scholar]
- 106.Jensen BN, Gerstoft J, Hojlyng N, Backer V, Paaske M, Gomme G, Skinhoj P. 1990. Pulmonary pathogens in HIV-infected patients. Scand. J. Infect. Dis. 22:413–420. 10.3109/00365549009027072 [DOI] [PubMed] [Google Scholar]
- 107.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 338:853–860 [DOI] [PubMed] [Google Scholar]
- 108.Michaels SH, Clark R, Kissinger P. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 339:405–406. 10.1056/NEJM199808063390612 [DOI] [PubMed] [Google Scholar]
- 109.Miller JR. 1998. Decreasing cryptosporidiosis among HIV-infected persons in New York City, 1995–1997. J. Urban Health 75:601–602. 10.1007/BF02427707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flanigan T, Whalen C, Turner J, Soave R, Toerner J, Havlir D, Kotler D. 1992. Cryptosporidium infection and CD4 counts. Ann. Intern. Med. 116:840–842. 10.7326/0003-4819-116-10-840 [DOI] [PubMed] [Google Scholar]
- 111.Maggi P, Larocca AM, Quarto M, Serio G, Brandonisio O, Angarano G, Pastore G. 2000. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur. J. Clin. Microbiol. Infect. Dis. 19:213–217. 10.1007/s100960050461 [DOI] [PubMed] [Google Scholar]
- 112.Meynard JL, Meyohas MC, Binet D, Chouaid C, Frottier J. 1996. Pulmonary cryptosporidiosis in the acquired immunodeficiency syndrome. Infection 24:328–331. 10.1007/BF01743372 [DOI] [PubMed] [Google Scholar]
- 113.Clavel A, Arnal AC, Sanchez EC, Cuesta J, Letona S, Amiguet JA, Castillo FJ, Varea M, Gomez-Lus R. 1996. Respiratory cryptosporidiosis: case series and review of the literature. Infection 24:341–346. 10.1007/BF01716076 [DOI] [PubMed] [Google Scholar]
- 114.Mifsud AJ, Bell D, Shafi MS. 1994. Respiratory cryptosporidiosis as a presenting feature of AIDS. J. Infect. 28:227–229. 10.1016/S0163-4453(94)95800-9 [DOI] [PubMed] [Google Scholar]
- 115.Mohri H, Fujita H, Asakura Y, Katoh K, Okamoto R, Tanabe J, Harano H, Noguchi T, Inayama Y, Amano T. 1995. Case report: inhalation therapy of paromomycin is effective for respiratory infection and hypoxia by cryptosporidium with AIDS. Am. J. Med. Sci. 309:60–62. 10.1097/00000441-199501000-00009 [DOI] [PubMed] [Google Scholar]
- 116.Egger M, Mausezahl D, Odermatt P, Marti HP, Tanner M. 1990. Symptoms and transmission of intestinal cryptosporidiosis. Arch. Dis. Child. 65:445–447. 10.1136/adc.65.4.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weikel CS, Johnston LI, De Sousa MA, Guerrant RL. 1985. Cryptosporidiosis in northeastern Brazil: association with sporadic diarrhea. J. Infect. Dis. 151:963–965. 10.1093/infdis/151.5.963 [DOI] [PubMed] [Google Scholar]
- 118.Shahid NS, Rahman AS, Anderson BC, Mata LJ, Sanyal SC. 1985. Cryptosporidiosis in Bangladesh. Br. Med. J. (Clin. Res. Ed.) 290:114–115. 10.1136/bmj.290.6462.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sallon S, el Showwa R, el Masri M, Khalil M, Blundell N, Hart CA. 1991. Cryptosporidiosis in children in Gaza. Ann. Trop. Paediatr. 11:277–281 [DOI] [PubMed] [Google Scholar]
- 120.Hojlyng N, Holten-Andersen W, Jepsen S. 1987. Cryptosporidiosis: a case of airborne transmission. Lancet ii:271–272 [DOI] [PubMed] [Google Scholar]
- 121.Campayo Ibanez A, Lacruz Rodrigo J, Valls Ferrer JM, Bonora Tamarit V. 1994. Pulmonary cryptosporidiosis in an immunocompetent female patient. Med. Clin. (Barc.) 103:237. [PubMed] [Google Scholar]
- 122.Martinez F, Mascaro C, Rosales MJ, Diaz J, Cifuentes J, Osuna A. 1992. In vitro multiplication of Cryptosporidium parvum in mouse peritoneal macrophages. Vet. Parasitol. 42:27–31. 10.1016/0304-4017(92)90099-U [DOI] [PubMed] [Google Scholar]
- 123.Yang S, Healey MC. 1994. Development of patent gut infections in immunosuppressed adult C57BL/6N mice following intravenous inoculations of Cryptosporidium parvum oocysts. J. Eukaryot. Microbiol. 41:67S. [PubMed] [Google Scholar]
- 124.Hewitt RG, Yiannoutsos CT, Higgs ES, Carey JT, Geiseler PJ, Soave R, Rosenberg R, Vazquez GJ, Wheat LJ, Fass RJ, Antoninievic Z, Walawander AL, Flanigan TP, Bender JF. 2000. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency virus infection. AIDS Clinical Trial Group. Clin. Infect. Dis. 31:1084–1092. 10.1086/318155 [DOI] [PubMed] [Google Scholar]
- 125.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. 2007. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 63:387–393. 10.1111/j.1365-2125.2007.02873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossignol JF, Ayoub A, Ayers MS. 2001. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:103–106. 10.1086/321008 [DOI] [PubMed] [Google Scholar]
- 127.Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. 2002. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 360:1375–1380. 10.1016/S0140-6736(02)11401-2 [DOI] [PubMed] [Google Scholar]
- 128.Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. 2004. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm. Rep. 53(RR-15):1–112 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5315a1.htm [PubMed] [Google Scholar]
- 129.Mele R, Gomez Morales MA, Tosini F, Pozio E. 2003. Indinavir reduces Cryptosporidium parvum infection in both in vitro and in vivo models. Int. J. Parasitol. 33:757–764. 10.1016/S0020-7519(03)00093-6 [DOI] [PubMed] [Google Scholar]
- 130.Hommer V, Eichholz J, Petry F. 2003. Effect of antiretroviral protease inhibitors alone, and in combination with paromomycin, on the excystation, invasion and in vitro development of Cryptosporidium parvum. J. Antimicrob. Chemother. 52:359–364. 10.1093/jac/dkg357 [DOI] [PubMed] [Google Scholar]
- 131.Abu Samra N, Thompson PN, Jori F, Frean J, Poonsamy B, du Plessis D, Mogoye B, Xiao L. 2013. Genetic characterization of Cryptosporidium spp. in diarrhoeic children from four provinces in South Africa. Zoonoses Public Health 60:154–159. 10.1111/j.1863-2378.2012.01507.x [DOI] [PubMed] [Google Scholar]
- 132.Molloy SF, Smith HV, Kirwan P, Nichols RA, Asaolu SO, Connelly L, Holland CV. 2010. Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am. J. Trop. Med. Hyg. 82:608–613. 10.4269/ajtmh.2010.09-0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kurniawan A, Dwintasari SW, Connelly L, Nichols RA, Yunihastuti E, Karyadi T, Djauzi S. 2013. Cryptosporidium species from human immunodeficiency-infected patients with chronic diarrhea in Jakarta, Indonesia. Ann. Epidemiol. 23:720–723. 10.1016/j.annepidem.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 134.Wang L, Zhang H, Zhao X, Zhang L, Zhang G, Guo M, Liu L, Feng Y, Xiao L. 2013. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 51:557–563. 10.1128/JCM.02758-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morgan D, Malamba SS, Orem J, Mayanja B, Okongo M, Whitworth JA. 2000. Survival by AIDS defining condition in rural Uganda. Sex. Transm. Infect. 76:193–197. 10.1136/sti.76.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.World Health Organization. 2013. Global health observatory (GHO): HIV/AIDS. World Health Organization, Geneva, Switzerland: http://www.who.int/gho/hiv/en/index.html Accessed 30 October 2013 [Google Scholar]