Abstract
SUMMARY
In 2008, a previously unknown Escherichia coli clonal group, sequence type 131 (ST131), was identified on three continents. Today, ST131 is the predominant E. coli lineage among extraintestinal pathogenic E. coli (ExPEC) isolates worldwide. Retrospective studies have suggested that it may originally have risen to prominence as early as 2003. Unlike other classical group B2 ExPEC isolates, ST131 isolates are commonly reported to produce extended-spectrum β-lactamases, such as CTX-M-15, and almost all are resistant to fluoroquinolones. Moreover, ST131 E. coli isolates are considered to be truly pathogenic, due to the spectrum of infections they cause in both community and hospital settings and the large number of virulence-associated genes they contain. ST131 isolates therefore seem to contradict the widely held view that high levels of antimicrobial resistance are necessarily associated with a fitness cost leading to a decrease in pathogenesis. Six years after the first description of E. coli ST131, this review outlines the principal traits of ST131 clonal group isolates, based on the growing body of published data, and highlights what is currently known and what we need to find out to provide public health authorities with better information to help combat ST131.
INTRODUCTION
Escherichia coli is a common, diverse microorganism that lives as a commensal organism of the gastrointestinal tracts of humans and many animals. This relationship between the bacterium and its host is symbiotic, providing both with a number of advantages. However, E. coli has developed into a pathogen well adapted to its host through the loss and gain of genes. Some pathogenic E. coli strains cause diarrheal illness (intraintestinal pathogenic E. coli), whereas others cause extraintestinal infections (extraintestinal pathogenic E. coli [ExPEC]) (1). E. coli is the leading cause of urinary tract infections (UTI), whether nosocomial or acquired in the community. It also frequently causes soft tissue (e.g., peritonitis) and central nervous system (e.g., neonatal meningitis) infections. The worldwide burden of these extraintestinal infections is staggering, with hundreds of millions of people affected annually and considerable morbidity and mortality in cases of complication with bacteremia or sepsis syndrome (2).
Moreover, E. coli pathogens, particularly those causing extraintestinal infections, have developed resistance to every class of antibiotics introduced to treat human and animal infections. The prevalence of resistance to first-line oral antibiotics, such as trimethoprim-sulfamethoxazole, amoxicillin and amoxicillin plus clavulanic acid, which are widely used to treat community-acquired E. coli infections, has increased steadily over time (3). The release onto the market of fluoroquinolones (FQ) and extended-spectrum cephalosporins (ESC) in the 1980s increased expectations of treatment efficacy, but these hopes have been dashed. Indeed, resistance to fluoroquinolones and ESC due to the production of extended-spectrum β-lactamases (ESBL) by E. coli isolates has increased steadily over the last 20 years. There is also evidence to suggest that this increase in resistance is linked to the worldwide spread, since 2008, of a specific clone of E. coli, E. coli sequence type 131 (ST131) (4–10).
In this context of a clear global spread of E. coli ST131, many investigations have been carried out, some of which have focused on the intrinsic bacterial traits of E. coli ST131, with others trying to determine possible epidemiological reasons for the success of this clone.
It is now 6 years since the first description of E. coli ST131. This timely review has been structured and illustrated so as to provide readers with a practical overview, a documented summary of the most important microbiological and epidemiological data published to date, and an indication of what remains to be discovered.
BIOLOGICAL AND PATHOGENIC CHARACTERISTICS OF E. COLI ST131
Following on from the initial detection of E. coli O25:H4, ST131 on three continents, this global clone of phylogenetic group B2 was shown, by pulsed-field gel electrophoresis (PFGE) and multiple virulence factor (VF) gene profile analyses, to consist of multiple subclones. The survival of this clone was also improved by its acquisition of various genes encoding resistance to antibiotics, including several borne on plasmids (8, 9). Many studies since 2008 have focused on these traits, in an attempt to determine the precise nature of E. coli ST131.
Bacterial Characteristics
Phylogenetic group and serotypes.
E. coli ST131 belongs to phylogenetic group B2, which includes both strains responsible for extraintestinal infections (11) and the strains most frequently isolated from the feces of asymptomatic humans (12, 13). The phylogeny of E. coli reported by Le Gall et al. grouped all B2 strains together, with group B2 as the basal group and a more recent divergence of groups A and B1 (14). Le Gall et al. identified nine subgroups within group B2. Clermont et al. placed E. coli ST131 in subgroup I, which was suggested to be the basal subgroup of B2 strains (15), suggesting that the characteristics of non-ST131 B2 strains may have evolved after the divergence of ST131 and related genotypes (ST1680, ST1982, and ST1461), (16, 17). E. coli ST131 strains are mostly of serotype O25:H4, with a specific O25 type, O25b. However, ST131 E. coli isolates of serotype O16:H5 have recently been identified in Japan, Denmark, Australia, Spain, Pakistan, and France (18–24). Moreover, some E. coli ST131 isolates have been shown to be nontypeable for O or H antigens (18, 25). Systematic serotyping is therefore necessary for E. coli ST131 isolates.
VF-encoding genes and virotypes.
Group B2 strains are known to harbor many more virulence factor (VF)-encoding genes than the other E. coli groups (26). Several studies have therefore investigated the VF gene composition of E. coli ST131 isolates. Key initial findings included an absence of adhesin-encoding P fimbria pap genes and classical group B2 cytotoxic necrotizing factor (cnf1) genes in intercontinental E. coli ST131 isolates (8). In contrast, the following VF genes have been found to be uniformly or frequently present in E. coli ST131 isolates: sat (secreted autotransporter toxin), fimH (type 1 fimbriae), fyuA (yersiniabactin receptor), kpsM II (group 2 capsule synthesis), usp (uropathogen-specific protein), malX (pathogenicity island marker), iha (adhesin siderophore receptor), ompT (outer membrane receptor) iucD (aerobactin), iutA (aerobactin receptor), and tratT (serum resistance associated) (8, 10, 27–32). All of the ST131 E. coli isolates investigated in these studies may be considered to be extraintestinal pathogenic E. coli (ExPEC), due to the presence of two (kpsM II and iutA) of the five molecular factors used to define ExPEC status (33). However, the number of VF-encoding gene profiles identified is increasing with the number of studies carried out to identify VF-encoding genes in E. coli ST131 isolates. These studies identified several VF-encoding genes characterizing distinct VF profiles in E. coli ST131 isolates, most of which can be grouped into specific PFGE clusters (18). Blanco et al. (32) referred to these VF profiles as “virotypes” and identified a number of VF-encoding genes identifying them (Table 1). Some of these genes are VF genes classically identified in non-ST131 group B2 ExPEC: afa/draBC (encoding Afa/Dr adhesins), papG (P fimbrial adhesins), and toxin genes, such as cnf1 and hlyA (alpha hemolysin). The ibeA (invasion of brain endothelium) gene, which can be used to identify virotype D and its subvirotypes, was previously reported in recently emerging avian O25b:H4 ST131 isolates (35). With the exception of afa/draBC, none of these virotype-distinguishing VF genes were found in the first strains described as ST131, even though they originated from different countries (8). This may reflect the large proportion of isolates from the recently recognized virotype C, which seems to be the most prevalent E. coli ST131 virotype (32), in this first collection of E. coli ST131 isolates.
TABLE 1.
Virulence factor-encoding gene scheme for discriminating virotypes among Escherichia coli ST131a
| Virotype | Virulence factor-encoding gene statusb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| afa/draBC | afa operon | iroN | sat | ibeA | papGII | cnf1 | hlyA | papGIII | cdtB | neuC-K1 | |
| A | + | + | − | +/−c | − | − | − | − | − | − | − |
| B | − | − | + | +/−c | − | − | − | − | − | − | − |
| C | − | − | − | + | − | − | − | − | − | − | − |
| D | +/− | +/− | +/− | +/− | + | − | +/− | +/− | +/− | +/− | +/− |
| E | − | − | − | + | − | + | + | + | − | − | − |
+, positive PCR result; −, negative PCR result. afa/draBC, Afa/Dr adhesins; afa operon, FM955459; iroN, catecholate siderophore receptor; sat, secreted autotransporter toxin; ibeA, invasion of brain endothelium; papGII, allele II of papG gene; cnf1, cytotoxic necrotizing factor type 1; hlyA, alpha-hemolysin; papGIII, allele III of papG gene; cdtB, cytolethal distending toxin; neuC-K1, K1 variant of group II capsule.
Most isolates of virotypes A and B are sat positive.
MLST and PFGE typing.
E. coli ST131 isolates have been shown to have uniform housekeeping gene sequences across the seven multilocus sequence typing (MLST) loci (adk, fumC, gyrB, icd, mdh, purA, and recA) defined by Achtman (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli), but some diversity has been found within the E. coli ST131 lineage in analyses of the eight MLST loci (dinB, icdA, pabB, polB, putB, trpA, trpB, and uidA) defined by the Pasteur Institute (http://www.pasteur.fr/recherche/genopole/PF8/mlst/EColi.html). Matsumura et al. reported three different “Pasteur” sequence types (PST) among O25b ST131 isolates (PST43, PST527, and PST568) and three among O16 ST131 isolates (PST506, PST566, and PST567) (19). Mora Gutierrez et al. found seven different PSTs in 23 E. coli ST131 isolates: PST43 corresponded to O25b isolates of virotypes A, B, and C; PST9, PST43, and PST527 corresponded to O25b isolates of virotype D; PST621 corresponded to O25b isolates of virotype E; and PST506, PST567, and PST625 corresponded to O16 isolates (34). However, PFGE profiles displayed much higher levels of within-lineage genetic variation. This variation was noted during the initial description of clone ST131, together with the presence of ST131 isolates with similar PFGE profiles at distant locations and the presence of isolates with different profiles at the same site (8). Johnson et al. carried out PFGE profiling on a collection of 579 E. coli ST131 isolates obtained between 1967 and 2009 from diverse sources (humans, animals, and environmental samples) from different countries (36). This study identified 170 distinct pulsotypes accounting for between one (105 pulsotypes) and 136 (1 pulsotype, called “968”) isolates. There were 65 pulsotypes containing multiple isolates (multiple-isolate pulsotypes), 12 of which contained at least six isolates, leading to their recognition as high-prevalence pulsotypes. Temporal occurrence profiles differed significantly between pulsotypes. Both multiple-isolate pulsotypes and high-prevalence pulsotypes were found to be associated with more recent isolation. The 12 high-prevalence pulsotypes included three (968, 800, and 812) appearing sequentially in 1990 to 1999, 2000 to 2002, and 2005, respectively, identified as the top three most prevalent species overall and within each interval considered, from 1990 onwards. The prevalence of pulsotype 968 remained high after its initial emergence, whereas peaks in prevalence followed by a steep decline were observed for pulsotypes 800 and 812. Thus, although ST131 is highly diverse at the pulsotype level, this clonal lineage is dominated by a small number of highly prevalent pulsotypes. Spatial analysis showed that the broad geographic distribution of pulsotypes prevailed over local specific segregation patterns, indicating a pattern of widespread dispersal (pandemicity) rather than localized endemicity.
fimH subtyping.
All E. coli ST131 isolates harbor the fimH gene, like most other isolates of E. coli (37), which displays a remarkably high level of allelic diversity (38–40). The fimH typing region (fimHTR) carries a highly diverse set of alleles that may be considered to be phylogenetically restricted (41). This typing method was applied to clone ST131 isolates (25, 42–46). Adams-Sapper et al. typed 246 E. coli bacteremia isolates by MLST and fimH subtyping methods and showed that the three most frequent fimH types accounted for 96% of E. coli ST131 isolates (42). Johnson et al. explored the subclonal structure of 352 historical and recent ST131 isolates (1967 to 2011). They identified seven fimH types, with fimH30 the most frequent (n = 236; 67%), followed by fimH22 (n = 73; 21%), with fimH35 and fimH41 in joint third place (19 isolates each; 5%) (43). The diversity of fimH alleles in clone ST131 isolates sheds light on the molecular mechanisms underlying clonal diversification.
Screening methods for detecting the E. coli ST131 clone and subclones.
The proposed screening methods are based on the molecular diversity observed within E. coli and within E. coli ST131isolates. Once an E. coli isolate has been assigned to group B2 by the multiplex PCR methods developed by Clermont et al. (47, 48), other single-nucleotide polymorphism (SNP)-based methods can be used to determine whether the isolate concerned belongs to the ST131 lineage. Specific-allele PCR of the 5′ portion of the rfb locus can detect the most common O serogroups, including the allele specific for O25b (49). Other methods make use of so-called “ST131-specific” alleles of genes used in the MLST methods designed by Achtman and the Pasteur Institute. For example, Clermont et al. described a specific-allele ST131 PCR based on SNPs of the pabB gene included in the Pasteur Institute MLST method (15). Johnson et al. suggested a method for E. coli ST131 screening based on ST131-associated SNPs (sequencing method) of the mdh and gyrB genes included in Achtman's MLST method (50). Weismann et al. proposed the use of an ST131-associated SNP of the fumC gene (Achtman's MLST method) for detecting clone ST131 and the use of fimH sequencing for the detection of subclones of ST131 E. coli isolates (41). This method, known as CH clonotyping, was successfully used for the direct testing of urine samples (44). Blanco et al. proposed two triplex PCRs. The first was based on the detection of O25b (O25b rfb allele) E. coli producing CTX-M-15 (encoded by the 3′ end of the blaCTX-M-15 gene) and harboring the afa/draBC gene, a VF gene specific to virotype A (32). The second was based on the detection of VF genes specific for virotypes B, C, and D (iroN, sat, and ibeA, respectively) (32). The CH clonotyping method appears to be the most relevant of the methods described, as it can detect E. coli ST131 and distinguish between the two serogroups of this lineage identified to date: O25b (fimH30) and O16 (fimH41).
In conclusion, various bacteriological analyses have revealed the existence of diversity within the E. coli ST131 lineage and have shown that subclones are characterized by combinations of bacterial traits.
Antibiotic Resistance
Phenotypic antibiotic resistance in clinical isolates of E. coli ST131.
Two traits were identified as common to all the intercontinental E. coli ST131 isolates initially described: resistance to ESC, due to the production of ESBL CTX-M-15, and resistance to fluoroquinolones (8, 9). Subsequent studies aiming to detect E. coli ST131 isolates in other countries frequently identified these associated traits. However, some studies also showed a particularly high prevalence of clone ST131 among non-ESBL-producing, fluoroquinolone-resistant E. coli isolates (50, 51). This suggests that CTX-M enzymes may have been acquired by E. coli ST131 isolates that were already resistant to fluoroquinolones. One study carried out in remote northern Saskatchewan communities (Canada) showed that clone ST131 was the second most prevalent clone, after ST95, which was the most prevalent clone among the ESC- and fluoroquinolone-susceptible urine isolates studied (52). Such antibiotic-susceptible E. coli ST131 isolates have also been identified in the dominant fecal E. coli populations of healthy subjects living in the Paris area (53). It is, therefore, clear that antibiotic-susceptible E. coli ST131 isolates exist.
A very small number of studies (16, 54) have provided information about resistance to various antibiotic families in E. coli ST131 isolates, comparing the results obtained with those for non-ST131 E. coli isolates producing or not producing ESBL (Tables 2 and 3). For ESBL-producing isolates, these studies showed that E. coli ST131 isolates were consistently more frequently resistant to amikacin than non-ST131 isolates and potentially more frequently resistant to amoxicillin-clavulanic acid, piperacillin-tazobactam, or ciprofloxacin; they were also more frequently susceptible to gentamicin or co-trimoxazole than non-ST131 isolates, depending on the country considered (Table 2). For isolates that did not produce ESBL (Table 3), E. coli ST131 isolates were systematically found to be more frequently resistant to quinolones/fluoroquinolones and to ampicillin/amoxicillin than non-ST131 isolates. Resistance to ampicillin/amoxicillin in E. coli isolates is widely known to be mediated principally by the production of plasmid-encoded TEM-1/-2, SHV-1, or OXA-1 enzyme. The blaTEM-1 and blaOXA-1 genes were commonly found associated with the blaCTX-M gene on plasmids of the IncF type (Table 4). This suggests that IncF plasmids encoding TEM-1 and/or OXA-1 may have gone on to acquire the genes encoding the CTX-M enzymes. The E. coli ST131 isolates resistant to fluoroquinolones and producing IncF-mediated TEM-1 and/or OXA-1 may be the ancestors of the current CTX-M-producing E. coli ST131 isolates found worldwide.
TABLE 2.
Antimicrobial resistance among ESBL-producing ST131 and non-ST131 Escherichia coli isolates
| Antibiotic | Study 1, France (16) |
Study 2, Spain (54)b |
||||
|---|---|---|---|---|---|---|
| % Resistant isolates |
P valuea | % Resistant isolates |
P value | |||
| ST131 (n = 55) | Non-ST131 (n = 97) | ST131 (n = 34) | Non-ST131 (n = 112) | |||
| Amoxicillin + clavulanic acid | 93 | 89 | 80 | 60 | 0.04 | |
| Piperacillin + tazobactam | 67 | 44 | 0.015 | 9 | 6 | |
| Cefotaxime/ceftriaxone | 100 | 100 | 100 | 99 | ||
| Ceftazidime | 100 | 100 | 97 | 97 | ||
| Nalidixic acid | 96 | 68 | <10−4 | ND | ND | |
| Ciprofloxacin | 94 | 57 | <10−4 | 91 | 86 | |
| Gentamicin | 31 | 38 | 3 | 26 | 0.002 | |
| Amikacin | 34 | 15 | 0.01 | 20 | 5 | 0.006 |
| Co-trimoxazole | 43 | 77 | <10−4 | 74 | 56 | |
| Fosfomycin | 0 | 0 | 0 | 0 | ||
Only significant P values are shown.
ND, no data.
TABLE 3.
Antimicrobial resistance among non-ESBL-producing ST131 and non-ST131 Escherichia coli isolates from any sample
| Antibiotic | Study 1, France (16) |
Study 2, Spain (54)b |
||||
|---|---|---|---|---|---|---|
| % Resistant isolates |
P valuea | % Resistant isolates |
P value | |||
| ST131 (n = 15) | Non-ST131 (n = 137) | ST131 (n = 110) | Non-ST131 (n = 288) | |||
| Amoxicillin/ampicillin | 80 | 47 | 0.03 | 79 | 59 | <0.001 |
| Amoxicillin + clavulanic acid | 33 | 22 | 32 | 24 | ||
| Piperacillin + tazobactam | 10 | 10 | 6 | 6 | ||
| Cefotaxime/ceftriaxone | 0 | 2 | 0 | 5 | 0.01 | |
| Ceftazidime | 0 | 2 | 0 | 6 | 0.005 | |
| Nalidixic acid | 73 | 24 | <10−4 | ND | ND | |
| Ciprofloxacin | 67 | 13 | <10−4 | 70 | 44 | <0.001 |
| Gentamicin | 20 | 4 | 0.03 | 14 | 10 | |
| Amikacin | 0 | 1 | 3 | 1 | ||
| Co-trimoxazole | 29 | 34 | 29 | 28 | ||
| Fosfomycin | 14 | 1 | 0 | 1 | ||
Only significant P values are shown.
ND, no data.
TABLE 4.
Characteristics of plasmids encoding antibiotic resistance in Escherichia coli ST131
| Country(ies) | Strain or plasmid | Plasmid familya | Conjugative transferb | Size (kb) | Replicon(s) | bla gene(s) | Reference |
|---|---|---|---|---|---|---|---|
| France, Portugal, India | IncF | + | 85 | FII | CTX-M-15 | 9 | |
| India, Kuwait, Canada, Portugal | IncF | + | 120 | FII-FIA | CTX-M-15 | ||
| France | IncF | + | 150 | FII-FIA-FIB | CTX-M-15 | ||
| Kuwait | IncF | + | 100 | FII | CTX-M-15 | ||
| Switzerland | IncF | + | 160 | FII-FIA | CTX-M-15 | ||
| UK | pEK499 | IncF | − | 117.5 | FII-FIA | CTX-M-15, OXA-1, TEM-1 | 55 |
| pEK516 | IncF | + | 64 | FII | CTX-M-15, OXA-1, TEM-1 | ||
| pEK204 | IncI1 | + | 95 | N | CTX-M-3, TEM-1 | ||
| Norway | K5–56 | IncF | + | 150 | FII-FIA | CTX-M-15 | 56 |
| K4–55 | IncN | NA | 50 | N | CTX-M-1 | ||
| K2–70 | IncF | NA | >200 | FII-FIA | CTX-M-15 | ||
| K5–09 | IncF | − | 200 | FII-FIA | CTX-M-15 | ||
| K2–63 | IncF | NA | 150 | FII-FIA-FIB | CTX-M-15 | ||
| K45–37 | IncI1, IncF | NA | 80c | I1, FII-FIB | CMY-2 | 57 | |
| K46–65 | IncI1, IncF | NA | 140c | I1, FII-FIA-FIB | CMY-2 | ||
| Belgium | 920415 | IncF | NA | NA | FII-FIA | CTX-M-15, OXA-1, TEM-1 | 58 |
| 211114 | IncF | NA | NA | FII-FIA | CTX-M-15, TEM-1 | ||
| 520811 | IncF | NA | NA | FI | CTX-M-15, OXA-1, TEM-1 | ||
| Germany | 404-405 | IncF | + | NA | FII | CTX-M-15 | 59 |
| 406-409-394 | IncN | + | NA | N | CTX-M-1, TEM-1 | ||
| 396 | IncN | + | NA | N | CTX-M-65, TEM-1 | ||
| 385 | IncI1 | + | NA | I1 | CTX-M-15 | ||
| China | WCE035 | IncF | + | NA | FII | CTX-M-14 | 60 |
| WCE307 | IncN | + | NA | N | CTX-M-65 | ||
| South Korea | WKE0506 | IncF | + | 140 | FIB | CTX-M-14 | 61 |
| HYE0515 | IncF | + | 120 | FII-FIA | CTX-M-14, TEM-1, SHV-2, CTX-M-15 | ||
| Spain | IncF | + | 70–85 | FII | CTX-M-15, OXA-1, TEM-1 | 62 | |
| IncF, IncN | + | 85, 45 | FII, N | CTX-M-15, OXA-1, TEM-1 | |||
| IncF | − | 90 | FII-FIA | CTX-M-15, OXA-1 | |||
| IncF, IncNd | +, + | 70–85, 30–35 | FII, N | CTX-M-15, OXA-1, TEM-1 | |||
| IncF | + | 80 | FII | CTX-M-15, OXA-1, TEM-1 | |||
| IncF, IncA/C | +, + | 80, 140 | FII, A/C | CTX-M-15, OXA-1, TEM-1 | |||
| IncF, IncN | +, + | 75, 30 | FII, N | CTX-M-15, OXA-1, TEM-1, SHV-12 | |||
| IncF, IncA/Cd | +/+ | 80, 150 | FII, A/C | CTX-M-15, OXA-1, TEM-1 | |||
| Australia | pJIE143 | pir-type plasmid | + | 35 | CTX-M-15 | 63 | |
| India | pGUE-NDM | IncF | + | 87 | FII | NDM-1, OXA-1 | 64 |
| France | C3, C13 | IncF | + | 145 | FII-FIA | CTX-M-15 | 24 |
| C5, C20 | IncF | + | 145 | FII-FIA-F1B | CTX-M-27 | ||
| C10 | IncI1 | + | 110 | I1 | CTX-M-1 |
β-Lactamase-encoding plasmids are in bold.
+, positive; −, negative; NA, not available.
Size of IncI1 plasmid.
A second copy of the blaCTX-M-15 gene was identified in this plasmid.
Characterization of plasmids harbored by clinical isolates of E. coli ST131.
Various plasmids, differing in incompatibility groups (Inc), conjugative transfer, size, replicon types, and bla genes, have been characterized in E. coli ST131 strains of different origins. These plasmids include IncF plasmids, which have a host range limited to Enterobacteriaceae and are known to contribute to bacterial fitness through their virulence and antimicrobial resistance determinants (65, 66). IncF plasmids were the most common. Three IncF plasmids harbored by three epidemic E. coli ST131 strains (strains A, C, and D) from the United Kingdom were completely sequenced (55, 67).
Plasmid pEK499, from a United Kingdom representative of strain A, presented a fusion of two replicons of types FII and FIA (Table 4). It harbored 185 predicted genes, including 10 conferring resistance to eight different classes of antibiotics. All the antibiotic resistance genes, other than blaTEM-1, were clustered in a 25-kb region. This region included blaCTX-M-15 and blaOXA-1, together with genes conferring resistance to both aminoglycosides (amikacin and tobramycin) and ciprofloxacin [aac(6′)-Ib-cr], macrolides [mph(A)], chloramphenicol (catB4), and tetracycline (tetA). A 1.8-kb class I integron was present within this multiresistance region and carried dfrA7 and aadA5, conferring resistance to trimethoprim and streptomycin, respectively, and sulI, conferring sulfonamide resistance. Plasmid pEK499 was found to encode four systems for postsegregation killing and stable plasmid inheritance: (i) the postsegregation killing protein Hok and its modulator Mok, (ii) the pemI-pemK toxin-antitoxin system, (iii) two copies of the vagC-vagD virulence-associated genes, and (iv) one copy of the ccdA-ccdB toxin-antitoxin system. All these systems would ensure the persistence of pEK499 in the absence of antibiotic selection pressure. However, pEK499 has an incomplete transfer region, containing the traC but not the traX gene. It therefore has no functional conjugation machinery.
Plasmid pEK516, from a strain D isolate obtained in the United Kingdom, belongs to incompatibility group IncFII (Table 4) and harbors 103 predicted genes, seven of which are clustered in a 22-kb region and confer antibiotic resistance: blaCTX-M-15, blaOXA-1 blaTEM-1, aac(6′)-Ib-cr, aac (3)-II (conferring resistance to gentamicin), catB4, and tetA. There is an ISEcp1 element 48 bp upstream from blaCTX-M-15 in pEK516, whereas pEK499 contains a 24-bp remnant of ISEcp1 flanked by IS26. The pEK516 and pEK499 plasmids display 75% DNA sequence identity, albeit with considerable rearrangement, despite pEK516 being 53 kb (45%) smaller than pEK499. The pEK516 plasmid carries the two addiction systems, pemI-pemK and hok-mok, and, unlike pEK499, it also carries the type I partitioning locus parM and the stbB gene, responsible for ensuring stable plasmid inheritance. It lacks the macrolide resistance [mph(A)] gene and the class 1 integron, which carries genes conferring resistance to trimethoprim, streptomycin, and sulfonamides, both of which are present in pEK499. On the basis of these features, pEK516 may be considered similar to pC15-1A, which is harbored by a widespread Canadian strain of E. coli that produces CTX-M-15 and probably also belongs to ST131 (68). The deletion of the transfer region is more extensive in pEK516 than in pEK499. However, pEK516 was transferred by conjugation in the in vitro experiments performed by Karisik et al. (69). It therefore appears plausible that the tra deletion occurred during subsequent storage and before plasmid sequencing.
Plasmid pEK204 from a Belfast representative of strain C belongs to incompatibility group IncI1 (Table 4), harbors 112 predicted genes, and can be transferred between strains by in vitro conjugation. Its structure is very similar to that of the IncI1 plasmid pCOLIb-P9. Unlike the multiple-resistance plasmids pEK499 and pEK516, pEK204 carries only two resistance genes, blaCTX-M-3 and blaTEM-1. An ISEcp1 element was identified 128 bp upstream from the blaCTX-M-3 gene. This element is flanked by 5-bp direct repeats of TATTG, consistent with ISEscp1-mediated transposition (70). None of the known systems for ensuring stable plasmid inheritance and postsegregation killing has been identified in pEK204.
This thorough analysis of plasmids in E. coli ST131 made it possible to use amplification of replicon system typing (65), various resistance and virulence determinants, and the regions surrounding the blaCTX-M gene to characterize in more detail the plasmids harbored by E. coli ST131 isolates from different countries: Belgium (58), China (60, 71), the Czech Republic (72), Germany (59), India (64, 73), Japan (74), South Korea (61), Pakistan (23), Spain (62), Switzerland (75), Tunisia (76), and the United Kingdom (77). This procedure was also used by Dhanji et al. (78) to characterize the plasmids harbored by the fecal CTX-M-15-producing E. coli ST131 isolates detected in stool samples from travelers returning to the United Kingdom from abroad. They showed that 62% of isolates harbored the blaCTX-M-15 genetic environment found in pEK516 and 4.6% harbored the genetic environment found in pEK499, whereas 33% harbored five blaCTX-M-15 genetic environments that had not previously been seen in the United Kingdom. Some of these isolates were obtained from individuals returning to the United Kingdom from Afghanistan, whereas those with an environment similar to that harbored by pEK499 were all obtained from individuals returning from India.
These findings indicate that plasmids of the IncF family, which has a complex structure, have clearly played a major role in the dissemination of the blaCTX-M-15 gene expressed by E. coli ST131 strains. However, E. coli ST131 strains can harbor IncF plasmids encoding ESBL other than CTX-M-15 (Table 4), such as CTX-M-14, SHV-2, and SHV-12 in particular, and they can even harbor CTX-M-15-encoding plasmids from families other than the IncF family. In particular, they can carry resistance genes on plasmids from the IncI1, IncN, and IncA/C families or on pir-type plasmids. The pir-type plasmid pJIE143, first identified in a community-acquired Australian E. coli ST131 isolate in 2006, has been fully sequenced and shown to be organized similarly to plasmids in the narrow-host-range IncX groups found in Enterobacteriaceae (63). No resistance-associated gene other than blaCTX-M-15 has been identified on pJIE143. CTX-M-1 enzymes may also be encoded by IncI1 (France) and IncN (Norway and Germany) plasmids, whereas CTX-M-3 (the United Kingdom) and CTX-M-65 (Germany and China) enzymes may be encoded by IncN plasmids (Table 4). CMY-2, the plasmid-encoded cephalosporinase most frequently identified in E. coli ST131 to date, is carried by an IncI1 plasmid. Finally, although the blaNDM-1 gene has most frequently been detected on broad-host-range plasmids, such as IncA/C plasmids, particularly in clinical or environmental isolates from the New Delhi area (79), it has also been found on an IncFII plasmid (pGUE-NDM) in an E. coli ST131 isolate. This isolate was obtained in France, from a patient returning home from Darjeeling (India), where she had lived for several years without hospitalization (80). Peirano et al. reported similar findings for a patient admitted to a hospital in Chicago after hospitalization in New Delhi. However, in the New Delhi E. coli ST131 strain, NDM-1 was harbored by a larger IncF plasmid carrying the FIA replicon (81). The complete genome sequence of pGUE-NDM showed that blaNDM-1 was acquired by a plasmid resembling those previously reported to harbor blaCTX-M-15. These findings are particularly alarming given the success with which E. coli ST131 has disseminated blaCTX-M-15 on IncF plasmids. Finally, one E. coli ST131 isolate has been found to contain a plasmid (pJIE186-2) harboring only VF genes classically carried by the chromosome (82) and a second plasmid of the same incompatibility group (IncF) harboring the classical resistance-associated genes identified in E. coli ST131 (blaCTX-M-15, blaOXA-1, blaTEM-1, aac6′-Ib-cr, and aac3-II). This result and those reported in Table 4 clearly indicate that E. coli ST131 strains commonly harbor multiple plasmids.
Molecular epidemiology of resistance in clinical isolates of E. coli.
An extensive review of studies of resistance mechanisms in E. coli ST131 isolates revealed that CTX-M enzymes were by far the most frequent ESBL. The most prevalent of these enzymes was CTX-M-15, which currently has a worldwide distribution. The production of other CTX-M-type enzymes in ST131 E. coli isolates has been documented for CTX-M-1, -2, -3, -9, -10, -14, -18, -24, -27, -28, -32, -39, -52, -55, -65, and 103 (15, 16, 18–20, 22, 29, 32, 45, 60, 71, 74, 83–93). E. coli ST131 isolates producing a number of these enzymes seem to be more frequent in particular countries: ST131 producing CTX-M-14 is particularly common in Canada, China, Japan, and Spain; ST131 producing CTX-M-3 is particularly frequent in the United Kingdom, and ST131 producing CTX-M-27 is common in France, Japan, and Switzerland (18, 24, 71, 74, 75, 78, 84, 94). Other non-CTX-M ESBL Ambler class A enzymes have also been described in E. coli ST131 isolates. These enzymes include derivatives of the SHV family (mostly SHV-2 and SHV-12) (32, 84, 87, 95, 96) and, based on anecdotal evidence, derivatives of the TEM family (TEM-24 and -52) (15, 88). The class A carbapenemase KPC-2 has been found in E. coli ST131 isolates from the United States (seven isolates), France (one isolate), Ireland (one isolate), and China, where they recently caused outbreaks (97–100). Ambler class B enzymes from E. coli ST131 isolates have been reported in only a few studies: NDM-1 (two cases from India) (64, 81), VIM-1 (one case in Italy) (101), and IMP-8 (one case in Taiwan) (102). Ambler class C enzymes have been detected more frequently than class B enzymes in ST131 isolates. The class C enzymes detected include CMY-2 (19, 103) and CMY-4 (87), with DHA-1 described only rarely (19). Finally, Ambler class D β-lactamases, such as OXA-48, have only rarely been found in ST131 isolates (104, 105). Two narrow-spectrum β-lactamases, OXA-1 and TEM-1, have frequently been found in ST131 isolates, generally in association with CTX-M-15 enzymes, following plasmid transfer (see “Characterization of plasmids harbored by clinical isolates of E. coli ST131” above) (9, 55, 58, 62, 94, 106). Another resistance gene, aac(6′)-Ibcr, which confers resistance to both aminoglycosides (amikacin and tobramycin) and ciprofloxacin, has frequently been detected in association with CTX-M-15 enzymes (9, 19, 107–110). Only a few studies in Spain and Portugal have reported the detection of qnr determinants, another plasmid-mediated mechanism of quinolone resistance in ST131 isolates (111–113). The main mechanism reported to confer resistance to fluoroquinolones in E. coli ST131 isolates is amino acid substitutions within the quinolone resistance-determining regions (QRDR) of GyrA and ParC, the targets of quinolones/fluoroquinolones (8, 43, 51, 114).
These reports clearly indicate that all the resistance mechanisms acquired by E. coli ST131 isolates to date, with the exception of fluoroquinolone resistance, are plasmid mediated. However, one or several copies of the blaCTX-M-15 gene have been detected on the chromosome (9, 115–117). Some strains with chromosomally encoded CTX-M-15 harbor an IncFII-type plasmid, but without the blaCTX-M-15 gene. This suggests that the blaCTX-M-15 gene may have been transferred from the plasmid to the chromosome (115).
Phylogeny and Dynamics of Clinical Isolates of E. coli ST131
The subclonal structure of ST131 and the clonality of fluoroquinolone resistance have been explored by subjecting historical (1967 to 2009) and international E. coli ST131 isolates (n = 236) and recent American ST131 E. coli isolates (n = 116) to PFGE profiling and the sequencing of fimH, gyrA, and parC (43). The events affecting the gyrA and parC genes of the historical and recent E. coli ST131 isolates were compared with those affecting the gyrA and parC genes of 737 recent non-ST131 isolates. This analysis identified 185 unique pulsotypes among the 352 ST131 isolates, together with seven distinct fimH-based putative clonal lineages (H15, H22, H27, H30, H35, H41, and H94). Allele H30, the most frequent fimH allele, was present in 70% of the isolates with pulsotypes displaying at least 75% similarity and in 29% of those with pulsotypes displaying less than 75% similarity. The results of fimH-based typing corresponded broadly to the PFGE profiles obtained. The association of H subclones with fluoroquinolone resistance and prevalence during the study period (1967 to 2009) was therefore investigated. Only fluoroquinolone-susceptible ST131 subclones, mostly H22 and H35, were encountered at the start of the study period, from 1967 to 1999. Fluoroquinolone-resistant ST131 isolates first emerged between 2000 and 2005, and almost all these isolates belonged to subclone H30. Thereafter, the H30 subclone continued to account for more than 97% of fluoroquinolone-resistant ST131 isolates and an increasing proportion of total ST131 isolates. The H30 subclone was closely associated with fluoroquinolone resistance in the 236 historical isolates, regardless of location and source, and with ESBL production and blaCTX-M-15. The history of SNPs and mutations of the gyrA and parC genes leading to amino acid substitutions in the ST131 and non-ST131 isolates demonstrated an association between subclone H30 and a distinctive gyrA and parC allele combination (GyrA, S83L and D87N; ParC, S80I and E84V) as arising from a single strain as little as 10 years ago. In genome-wide SNP studies of 105 historical E. coli ST131 isolates from five countries and 23 states and provinces in Canada and the United States, including 22 CTX-M-15-producing isolates, Price et al. resolved the phylogeny of ST131 strains with a higher degree of accuracy (115). This led to the identification of four large recombinant regions acquired by horizontal gene transfer and accounting for 31% of the genome. The exclusion of SNPs from these four regions led to the detection of distinct clusters of O-type strains carrying specific fimH alleles and gyrA and parC alleles. In particular, strains (n = 64) carrying the fimH30 allele clustered as a single low-diversity clade, designated H30. This clade included 95% of the 61 fluoroquinolone-resistant isolates. Almost all the CTX-M-15-producing isolates could be condensed into a single distinct subclade within the H30 clade. The evolutionary history of the H30 subclone was resolved further by constructing a new phylogenetic tree on the basis of genomic analyses of the H30 strains and the NA114 reference genome. This new tree suggested that the fimH30 allele was acquired before fluoroquinolone resistance, by a single ancestor within the H30 lineage, and that this acquisition was followed by the extensive clonal expansion of fluoroquinolone-resistant H30 strains. The fluoroquinolone-resistant (R) subclone within the H30 lineage was designated H30-R. Moreover, 91% of the CTX-M-15 (x)-producing isolates formed a distinct, single-ancestor subclone within H30-R. This subclone was designated H30-Rx and could be distinguished from the rest of H30-R on the basis of three canonical SNPs. Together, these results provide support for the hypothesis that the spread of both CTX-M-15 production and fluoroquinolone resistance in E. coli ST131 strains was dependent principally on clonal expansion.
Pathogenic Characteristics
Infection spectrum.
E. coli ST131 strains cause community- and hospital-acquired UTI (cystitis and pyelonephritis) and bacteremia worldwide (42, 109, 118). They have also been reported to cause other types of infection: intra-abdominal and soft tissue infections (54), meningitis (119), osteoarticular infection (120), myositis (121), epididymo-orchitis (119), and septic shock (122, 123). This spectrum of infections, typical of ExPEC, has increased the degree of concern about ST131, which is already considered a major potential problem due to its multidrug resistance.
Transmissibility.
ST131 transmission has essentially been documented between members of the same household and between family members and pets (dogs and cats in particular) (124). Ender et al. provided strong evidence of the transmission of a CTX-M-15-producing ST131 isolate resistant to gentamicin, trimethoprim-sulfamethoxazole, and fluoroquinolones between a father and his daughter (122). The father was admitted to a hospital for pyelonephritis due to a clone ST131 strain, where he was visited by his adult daughter. She used his bathroom during the visit and subsequently developed emphysematous pyelonephritis, renal abscess, bacteremia, and septic shock due to the same ST131 strain, which appeared to be particularly virulent. However, the VF gene profile of this strain identified it as a classical virotype A strain. Johnson et al. provided novel evidence of the within-household transmission of an ST131 strain between an infected patient (an 8-month-old girl with an osteoarticular infection) and another previously healthy member of the same family (the girl's mother). The same ST131 strain was detected in the digestive tracts of both patients (120), but it remains unclear in which direction the infection was transmitted. In this case, the ST131 strain was a fluoroquinolone-resistant strain that did not produce an ESBL. Owens et al. reported a fatal case of urosepsis involving the community-associated intrafamilial spread of a CTX-M-15-producing, fluoroquinolone-resistant ST131 strain (123). The index case was a middle-aged female patient with chronic, recurrent symptomatic urinary tract infections due to E. coli. This patient and her younger sister had alpha-1-antitrypsin deficiency, which was particularly severe in the younger sister. The younger sister had stayed with the index patient, who had taken care of her, for several months before the onset of infection in the younger sister. The younger sister developed pyelonephritis and septic shock and died shortly after her admission to hospital. The molecular and epidemiological evidence reported in these studies suggests that infection can be transmitted between family members of the same household. Hilty et al. designed a specific study assessing the transmission of ESBL-producing E. coli between patients and their contacts at the hospital and between patients and household contacts (91). Any patient (inpatients, outpatients, children, and adults) newly identified as colonized or infected with an ESBL-producing E. coli isolate between May 2008 and September 2009 was classified as an index patient. A prospective survey of the hospital and household contacts of these patients was performed over a period of 12 months following contact to check for possible ESBL-producing E. coli isolate carriage in these individuals. In cases of positive carriage, transmission was assumed to have occurred if the index patient and contact had clonally related ESBL-producing E. coli isolates with identical blaESBL genes. This approach identified 72 index patients (40 inpatients and 32 outpatients) colonized or infected with an ESBL-producing E. coli isolate during the study period, 13% of whom were children. E. coli ST131 accounted for 28% of the ESBL-producing E. coli isolates obtained from the index patients. Transmission from the index patient was considered to have occurred in four (4.5%) of the 88 hospital contacts exposed to the 40 index patients with ESBL-producing isolates. In two cases (50%), the strain transmitted was an E. coli ST131 strain. Transmission was considered to have occurred in 20 of the 88 (22.7%) household contacts. Transmission was detected between six mother-to-child and two child-to-child pairs, suggesting that children may make an important contribution to the epidemiological characteristics of ESBL-producing E. coli. E. coli ST131 strains accounted for 35% (7/20) of the strains transmitted. E. coli ST131 strains were commonly identified as transmitted within the hospital and between members of the same household. Transmission rates were significantly higher in households than at the hospital. The authors suggested that this difference was due to the infection control measures applied at their hospital to prevent the patient-to-patient transmission of multidrug-resistant isolates in particular.
In conclusion, the transmission of E. coli strains, including ST131 strains, between human and animal members of the same household, probably by direct host-to-host transmission, may make a significant contribution to the community-wide dissemination of emerging antimicrobial-resistant ExPEC strains, such as E. coli ST131.
Pathogenesis.
Various studies have investigated the virulence potential of E. coli ST131.
(i) Biofilm production and metabolic potential.
Very few studies have investigated the biofilm production and metabolic potential of E. coli ST131 isolates, but the results of these studies are concordant. Clermont et al. found that the two ST131 strains studied produced a biofilm after 48 h, and Kudinha et al. found that the prevalence of isolates producing biofilms was greater among ST131 E. coli isolates than among non-ST131 clinical isolates (22, 125).
Using the 47 tests set up by Vitek AES, Gibreel et al. showed that ST131 isolates were significantly more likely to have a higher Bio score than the isolates of any other ST (126). Vimont et al. investigated the capacity of the tested ST131 isolate to colonize the digestive tract by measuring its maximal growth rate (MGR) under three sets of culture conditions: a rich medium and two minimal media containing either gluconate or glucose. They compared the results obtained with those for the commensal E. coli strain K-12 MG1655 and two ExPEC strains, CFT073 and HT7 (127). The ST131 strain had a significantly higher MGR than the other three strains.
These data suggest that E. coli ST131 has a high metabolic potential, probably enhancing its ability to establish and maintain intestinal colonization, the first step toward uropathogenicity.
(ii) Adhesion and colonization abilities.
Martinez-Medina et al., who characterized the similarity and divergence of adherent invasive E. coli (AIEC) and ExPEC strains, found that one of the 12 ExPEC ST131 strains tested had an AIEC phenotype and displayed 50% similarity to two other ST131 AEIC strains (128). The VF genes harbored by these three strains were different, suggesting that ST131 AIEC strains, like other AIEC strains, have virulence-specific features that can currently be detected only phenotypically. These features include an ability to adhere to and invade intestinal epithelial cells and an ability to survive and replicate within macrophages.
Vimont et al. investigated the ability of a virotype C E. coli ST131 strain to colonize the intestine and infect the kidney in experimental mouse models (127). The ST131 strain was mixed (1:1 ratio) with commensal E. coli K-12 MG1655 (phylogenetic group A), commensal E. coli IAI1 (group B1), or commensal E. coli ED1a (group B2), and 106 CFU of each bacterial mixture was administered per os to the mice. A competitive index analysis showed that the ST131 strain outcompeted all the commensal non-ST131 strains. The ST131 strain outcompeted E. coli K-12 MG1655 or E. coli ED1a more easily than E. coli IAI1 in the digestive tracts of the mice. The ability of the ST131 strain to colonize bladder and kidney was compared with those of E. coli strains CFT073 (group B2 ST73) and HT7 (group B2 ST95). Seven days after retrograde inoculation, the median number of CFU in the bladder was greater for the ST131 strain than for strain HT7 (P = 0.01). A similar pattern, but with a smaller difference, was observed for the comparison with strain CFT073. In the kidney, the median number of CFU on day 7 was significantly higher for strain ST131 than for strain HT7 or strain CFT073. However, strain ST131 triggered an inflammatory response in the kidneys similar to that induced by strain CFT073.
Type 1 fimbriae, encoded by the fim genes, are a major virulence factor in uropathogenic E. coli. Totsika et al. analyzed the fim operon sequence of strain ST131 EC958, a representative of the ST131 CTX-M-15-producing lineage circulating in the northwest of England (67, 129). They found that there was an ISL3-like transposase in the fimB gene of this strain. This transposase was also found in the recombinase-encoding fimB genes of 60% of the other 54 English ST131 E. coli isolates studied and in 71% of the Australian ST131 E. coli isolates studied. Blanco et al. found that all virotype A, B, and C ST131 strains harbored this transposase in their fimB genes (32). Totsika et al. demonstrated, by means of in vitro functional assays (shaking and static cultures in LB broth), that this insertion resulted in the expression of type 1 fimbriae being switched on less frequently. This suggested the involvement of a second recombinase in the switching on of type 1 fimbrial expression observed in most ST131 isolates. Totsika et al. showed that only the ST131 E. coli isolates able to switch on expression adhered strongly to and invaded bladder epithelial cells. Croxall et al. studied 55 E. coli ST131 isolates from urine samples of elderly patients. They found that 56% had a strong ability, 30% had an intermediate ability, and 14% had a limited ability to invade bladder epithelium cells. They found no correlation between invasive potential and the carriage of virulence genes (17). Totsika et al. used the mouse model of ascending urinary tract infection and showed that the E. coli ST131isolates able to switch on the expression of type 1 fimbriae colonized the mouse bladder and urine, whereas those unable to switch on the expression of type 1 fimbriae colonized only the urine. This study clearly indicated that type 1 fimbriae were a key virulence factor underlying the pathogenicity of most E. coli ST131 isolates.
Peirano et al. assessed the adhesion of two clinical isolates of E. coli ST131 (one producing the carbapenemase NDM-1 and another producing the ESBL CTX-M-15) to human intestinal cell lines (130). They found that these two isolates adhered less strongly than two other isolates, from groups B1 and D. Their experiment was carried out in the presence of mannose, which inhibits adhesion mediated by type 1 fimbriae, potentially accounting for the weaker adhesion of the two ST131 isolates.
(iii) Animal models.
Clermont et al. used a mouse model of systemic infection to assess the intrinsic virulence of three clinical isolates of E. coli ST131 with a VF profile corresponding to virotype C and one isolate with a VF profile corresponding to virotype B (Table 1) (125). These four E. coli ST131 isolates grouped together on an MLST-based phylogenetic tree, along with the pyelonephritic diffusely adhering EC7372 reference strain. They were also located close to two diarrhea-associated diffusely adhering strains (virotype C) and slightly further from an ECOR66 strain harboring three VF genes (papC, papG, and hlyC) absent from the isolates studied and the reference strains. All of these strains killed all 10 mice inoculated in tests, despite their different VF profiles.
In a study on the same animal model, involving comparison with the reference strain CFT073, Johnson et al. (131) tested 61 E. coli isolates: (i) 44 clinical isolates classified into four sets of 11 isolates matched on the basis of their characteristics (ST131 and non-ST131 isolates resistant to fluoroquinolones and ST131 and non-ST131 isolates susceptible to fluoroquinolones), (ii) 12 urine and blood isolates corresponding to nine classical ExPEC clonal groups, and (iii) five fluoroquinolone-resistant E. coli ST131 isolates from case reports (distinctive clinical behavior or within-household transmission) (120, 122, 123). They found a broad range of virulence potential within the study population. Neither ST131 status nor fluoroquinolone phenotype was significantly associated with virulence. In contrast, some ExPEC virulence genes (adhesins, papAH and papGIII; toxin, vat; protectins, kpsM II and K1 capsule; invasin, ibeA; and miscellaneous, clbB/N), ExPEC status, and aggregate virulence score were found to be significantly correlated with virulence. Thus, ST131 isolates are neither uniformly virulent nor, as a group, markedly more virulent than other extraintestinal E. coli isolates.
In the same murine model of sepsis, Mora Gutierrez et al. found a wide range of virulence patterns associated with certain virotypes for the 23 E. coli ST131 isolates tested (34). Fourteen of the 23 isolates killed 90 to 100% of the mice challenged. The virotype A, B, and C isolates had high final lethality scores (≥80% of the mice challenged), whereas a pattern of slow, low-level lethality was observed for some of the virotype D isolates and the virotype E isolates. The O16:H4 ST131 isolates included in this study had a high virulence potential.
Lavigne et al. used two other animal models: Caenorhabditis elegans and zebrafish embryos (31). They tested three E. coli ST131isolates, two of which were obtained from urine and produced CTX-M-15 enzymes and the third being a fluoroquinolone-susceptible fecal isolate without ESBL production. The two CTX-M-15-producing isolates harbored the same VF genes, except that one had afra/draBC whereas the other did not. The two CTX-M-15-producing isolates were of the C and A virotypes. The fecal isolate lacked the genes required for classification as ExPEC (33). These three strains were tested in comparison with group B2 pyelonephritic reference strains (CFT073, 536) and the group B2 commensal reference strain ED1A, together with one group B2 (ST73) clinical isolate and one group A urine non-ST131 clinical isolate. The three ST131 strains had a virulence potential in C. elegans similar to that of the group B2 commensal reference strain. This potential was significantly lower than that of the group B2 non-ST131 strains but significantly higher than that of the group A clinical isolate. The virulence patterns observed in zebrafish embryos differed markedly from those in C. elegans. The fecal ST131 strain was more virulent than the two urine ST131 strains, whereas the commensal reference strain was not virulent and the group A strain was highly virulent. The urine ST131 strains were less virulent than the reference strains CFT073 and 536. These two experiments suggest that the urine ST131 strains were of intermediate virulence. The virulence of the fecal ST131 strain was intermediate in C. elegans but high in zebrafish, despite the lack of VF genes known to contribute to intrinsic virulence in E. coli. The transparent nature of zebrafish embryos makes it possible to follow the infection in real time. Studies in this model revealed a tropism of the fecal and virotype C E. coli ST131 isolates for the central nervous system.
All these studies indicate that E. coli ST131 has a high metabolic potential, is able to colonize intestine, bladder, and kidney, and is virulent, just like other group B2 ExPEC strains. However, none of these studies found a correlation between pathogenic properties and VF gene carriage in E. coli ST131 strains. This suggests that as-yet-unknown underlying mechanisms are probably involved in the virulence of E. coli ST131 strains. Whole-genome comparisons might identify genetic differences corresponding to the different virulence patterns observed in E. coli ST131 strains, which are otherwise highly similar.
GENOMICS OF E. COLI ST131
The entire genome sequences of 13 E. coli ST131 strains are currently available (117, 129, 132, 133). In 2011, Avasthi et al. (132) published the chromosome sequence of strain NA114, a typical uropathogenic E. coli ST131 isolate from the city of Pune in Western India (134), and the sequence of the single 3.5-kb plasmid harbored by this strain. The NA114 chromosome was 4,935,666 bp long, with a GC content of 51.16% and a coding percentage of 88.4%. It had 4,875 protein-encoding sequences, 67 tRNAs, and three rRNA genes. The authors limited their comments on this genome essentially to confirmation of the presence of the virulence-associated genes classically identified by PCR in E. coli ST131 and the detection of several genes rarely identified in E. coli ST131 isolates (cnf1, sfa, and aer) and an intact polyketide synthetase island. Andersen et al. (117) recently reported the complete genome of strain JJ1886, a uropathogenic strain (123) considered representative of the epidemic and highly virulent CTX-M-15-producing H30-Rx subclone of E. coli ST131. This complete genome corresponds to a 5,129,938-bp chromosome with a GC content of 50.8%, 5,086 protein-encoding sequences, 88 tRNAs, and 22 rRNA genes plus five plasmids of 1.6, 5.2, 5.6, 56, and 110 kb in size. Again, few analytical data have been reported for this genome sequence. However, the authors indicated that only the largest plasmid (110 kb) carried genes for antibiotic resistance. The blaCTX-M-15 gene was found to have been integrated into the strain JJ1886 chromosome by the insertion of an incomplete Tn3 element into a lambda-like prophage. Further analyses are required to clarify the differences and similarities between strains NA114 and JJ1886, but the data already available indicate some differences between these strains. Totsika et al. determined the genome sequence of strain EC958, a representative example of the CTX-M-15-producing ST131 isolates circulating in the northwest of England (67, 129). The complete genome of this strain comprises a 5,070,614-bp chromosome plus two plasmids of 134 kb and 4 kb in size. The smallest plasmid is 97% identical to pSE11-6 from the commensal group B1 E. coli strain SE11, whereas the largest, which harbors blaCTX-M-15, displays a high level of identity to pEK499 and pC15-a (see “Characterization of plasmids harbored by clinical isolates of E. coli ST131” above). The chromosome of strain EC958 contains several regions different from previously sequenced E. coli chromosomes, and most of these regions can be identified as prophage elements. This comparison also showed the presence of four genomic islands in chromosomal integration hot spots (GI-pheV, GI-selC, GI-leu, and GI-thrW) containing virulence-associated genes and a 31-kb tRNA-asnT-associated island 99% identical to the high-pathogenicity island (HPI) of Yersinia pestis, which contains the fyuA and irp2 VF genes. The fyuA gene has been identified in all ST131 isolates tested by PCR, whereas the irp2 gene has not (see “VF-encoding genes and virotypes” above). The tRNA-thrW chromosomal integration hot spot in strain EC958 contains a 10.8-kb genomic island encoding a type I restriction/modification system almost identical to the equivalent region in the group B2 commensal E. coli strain SE15 but different from other published uropathogenic strain genomes. Strain EC958 also contains three large regions of difference displaying a high level of identity to syntenic regions in E. coli strain SE15. These regions contain genes with a predicted sugar transport/metabolism function, two tandemly arranged autotransporter homologs, a chaperone-usher gene pair, and a putative fimbrial subunit gene. The presence of these genes in the commensal group B2 strain SE15 led the authors to speculate on their possible contribution to the fitness of EC958 for gastrointestinal colonization. Strain EC958 was also investigated by Phan et al., who looked at its serum resistome (135). This strain was found to contain 56 genes, 82% of which were implicated in its serum resistance. Clark et al. sequenced the genome of strain UTI18, a representative ESBL-producing urinary tract isolate from elderly people from the East Midlands in the United Kingdom (17, 133, 136). The UTI18 genome is very similar to that of strain NA114. However, it differs from that of NA114 in having neither the pathogenicity island containing cnf and the intact pap operon nor the sfa fimbrial operon. An ISL3-like transposase is present within the fimB gene of strain UTI18, which also has a truncated HPI. Comparative analyses of regions outside the accessory virulome of ExPEC have highlighted differences in metabolic pathway-encoding genes between strain UTI18 and other ExPEC strains for which genome sequences have been published. Clark et al. sequenced the genomes of nine additional urine ST131 strains from other elderly patients in the East Midlands and compared them with the genome of strain UTI18. They found no strain-specific insertion or deletion of accessory mobile islands in the set of strains from elderly patients and identified two regions that were different from the equivalent regions in the Indian strain NA114 genome, which seemed to correspond to plasmid fragments. They also compared the SNP profiles of strains from elderly patients with those of the uropathogenic reference strain UTI89 and an ST12 isolate from their strain collection. They found low levels of SNP variation within the genomes of their ST131 strains, similar to that commonly observed in monomorphic, highly pathogenic, and host-restricted species, such as Salmonella enterica serotype Typhi. The mapping of ST131-specific SNPs against the UTI89 genome showed the distribution of these SNPs to be nonrandom, suggesting a significant role for recombination in the emergence and stability of clone ST131 among the urine isolates causing infection in unrelated elderly patients from the East Midlands in the United Kingdom. McNally et al. applied algorithms designed for the estimation of recombination and population structure to large genome data sets for E. coli, including the available genome sequences for ST131 strains, to investigate the correlation between recombination and pathogenesis (137). A detailed analysis of core genome recombination suggested the evolution from a ubiquitous intestinal commensal organism displaying relatively frequent core genome recombination into a highly specialized ExPEC with much lower levels of core genome recombination and, in clone ST131, an almost stable core genome sexually isolated from the rest of the species, including the most closely related group B2 ExPEC strains. Using the MLST database, they showed that E. coli ST131 formed a monophyletic clade with the other group B2 E. coli isolates and that the CTX-M-15-producing E. coli ST131 isolates displayed significantly less recombination than the other group B2 isolates, such as ST73 and ST95. ST131 is a classical group B2 strain containing classical ExPEC-specific virulence factors. As the main difference between ST131 and its phylogenetic near neighbors is ESBL production, it would appear likely that ST131 has specialized, becoming a multidrug-resistant group B2 ExPEC strain, through horizontal gene transfer and subsequently displaying lower levels of recombination, due to ecological or genetic factors. Evidence for lower levels of recombination linked to the ecological separation of E. coli ST131 from other E. coli strains is provided by the presence of E. coli O157 in the cluster containing the E. coli strains with the lowest level of detectable core recombination. This E. coli lineage, which has become a globally successful human pathogen, colonizes the recto-anal junctions of livestock but not the intestinal lumens. This suggests that lineage O157, which is ecologically distinct, encounters fewer opportunities to recombine with distantly related E. coli lineages, because it is not present in the mammalian intestinal tract, where such interactions are likely to occur. Interestingly, Shigella, a subset of E. coli with a niche restricted to the human digestive tract, has an extreme monomorphic structure, with little recombination.
In conclusion, the most pertinent information provided by genome analysis concerns the high degree of specialization of E. coli ST131, which displays very low levels of core genome recombination. This genomic property seems to be linked to its ecological characteristics. Thus, further studies are required, not only to explore the genetic factors potentially accounting for the global success of E. coli ST131 but also to characterize the specific ecological features of this lineage.
EPIDEMIOLOGY OF E. COLI STI131
Global Dissemination of E. coli ST131
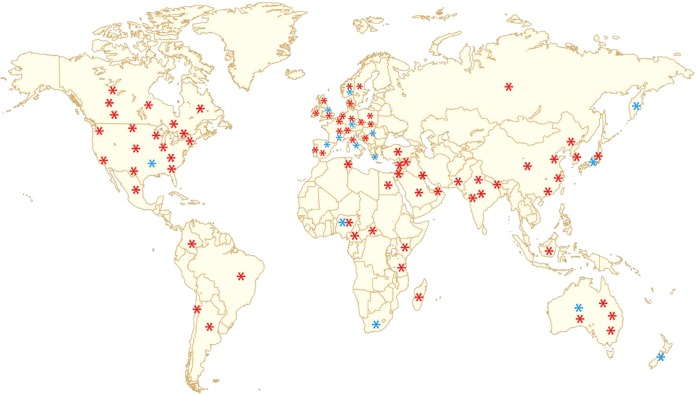
Following the initial identification of E. coli ST131 in 2008 in a limited number of countries on three continents—North America (Canada) Europe (France, Portugal, Spain, Switzerland), and Asia (India, South Korea, Kuwait, and Lebanon) (8, 9)—this clone was successfully detected in many other countries on these three continents and on the two remaining continents, Africa and Oceania (Fig. 1).
FIG 1.
Global dissemination of Escherichia coli ST131 clone (2013). Articles which mentioned for the first time the presence of clone ST131 in a given country or a given location are cited. Red stars indicate isolates producing ESBL enzymes, and blue stars indicate fluoroquinolone-resistant, non-ESBL-producing isolates. For Europe, references are for Belgium (58, 83), Croatia (220), the Czech Republic (206), Denmark (221), France (8), Germany (166, 170), Italia (222), Netherland (223), Norway (57), Portugal (8), Spain (8), Sweden (224), Switzerland (8), and the United Kingdom (67, 78, 108, 109). For Asia, references are for China (71, 95, 164, 225, 226), India (73, 227), Israel (228), Japan (25, 95, 171, 229), South Korea (8), Kuwait (8), Lebanon (8), Pakistan (23), Russia (192), Turkey (230), and United Arab Emirates (231). For the Americas, references are for Canada (8, 50, 84, 85, 140, 147), the United States (10, 42, 43, 46, 156, 232), Argentina (233), Brazil (234), Colombia (235), and Mexico (236). For Africa, references are for Cameroon (237), Central African Republic (125), Egypt (238), Kenya (177), Madagascar (239), Nigeria (240), South Africa (241), Tanzania (242) and Tunisia (243). For Oceania, references are for Australia (150, 172, 173, 244, 245) and New Zealand (246).
Prevalence and Epidemiology of E. coli ST131 among Human Clinical Isolates of E. coli
The first clinical isolates of E. coli ST131 identified around the world were characterized by three major epidemiological traits. They all produced CTX-M-15 ESBL, were resistant to fluoroquinolones, and caused infections in both the hospital and community settings. These traits were therefore often used as the population background (denominator) for assessing the prevalence of E. coli ST131 among clinical isolates of E. coli.
Prevalence of E. coli ST131 among ESBL-producing or fluoroquinolone-resistant isolates.
Table 5 shows data on the prevalence of E. coli ST131 among ESBL-producing E. coli strains from different countries in Europe (the Czech Republic, Denmark, France, Spain, Sweden, Switzerland, and the United Kingdom), the Americas (Canada, the United States, and South America) and Asia (South Korea and Japan) for the period from 2000 to 2011.
TABLE 5.
Prevalence of Escherichia coli ST131 among ESBL-producing and fluoroquinolone-resistant E. coli isolatesa
| Isolate type, sample type, and country(ies) | Study period | Study and population characteristics | % of E. coli ST131 among E. coli isolates |
Reference | |
|---|---|---|---|---|---|
| ESBL+ | FQr | ||||
| ESBL+ isolates | |||||
| Any sample | |||||
| Czech Republic | 2006 | Prospective study (one hospital), any sample, 9 ESBL+ E. coli isolates | 44 | 72 | |
| Spain | 2006-2007 | Prospective study (one hospital, Lugo), any sample, outpatients, 105 ESBL+ E. coli isolates | 22 | 138 | |
| Spain | 2008 | Prospective study (one hospital, Barcelona), any sample, 94 ESBL+ E. coli isolates | 32 | 96 | |
| UK | 2006 | West Midlands region (13 hospitals), CA and HA infections, any sample, 232 ESBL+ E. coli isolates | 66 | 139 | |
| Canada | 2007-2009 | National study (hospital), any sample, 155 ESBL+ E. coli isolates | 50 | 140 | |
| Denmark | 2008-2009 | Retrospective study (three hospitals and 100 general practitioners, Copenhagen), 115 ESBL+ E. coli isolates | 38 | 20 | |
| Switzerland | 2011 | Prospective study (one hospital), CA and HA infections, any sample, 30 ESBL+ E. coli isolates | 33 | 75 | |
| US | 2009-2010 | Prospective, multicenter study, CA infections, any sample, 292 ESBL+ E. coli isolates | 54 | 93 | |
| US | 2010-2011 | Prospective study among children (one hospital), any sample, 68 ESBL+ E. coli isolates | 10 | 141 | |
| US (18 states) | 2010-2011 | 24 laboratories serving the Department of Veterans Affairs, any sample, each laboratory provided 10 FQr and 10 FQsb E. coli isolates (2011) and10 ESBL+ E. coli isolates (2010-2011) | 64 | 78 | 45 |
| Japan | 2008-2011 | Retrospective study (one hospital), any sample, 71 ESBL+ E. coli isolates | 52 | 142 | |
| UTI | |||||
| France | 2006 | Prospective national survey from private laboratories, UTI ESBL+ Enterobacteriaceae among which were 48 ESBL+ E. coli isolates | 21 | 86 | |
| Sweden | 2007-2011 | National survey, 851 UTI ESBL+ E. coli isolates | 34 | 143 | |
| India | 2009 | Retrospective study (one hospital, Pune), 100 UTI isolates, 23 ESBL+ | 70 | 73 | |
| South America | 2006-2007 | CA UTI isolates (one center, Mexico), 500 ESBL+ E. coli isolates of which 56 were molecularly studied | 25 (of 56 isolates) | 144 | |
| Bacteremia | |||||
| France | 2005 | Multicenter study, bacteremia, 19 ESBL+ E. coli isolates | 32 | 94 | |
| Canada | 2000-2010 | Retrospective study, bacteremia, 197 ESBL+ E. coli isolates | 60 | 145 | |
| South Korea | 2006-2011 | Retrospective study, CA bacteremia, 103 ESBL+ E. coli isolates of which 76 were molecularly studied | 20 (of 76 isolates) | 146 | |
| Travel | |||||
| Canada | 2004-2006 | Prospective study, travel-related infections, any sample, 105 ESBL+ isolates of which 68 produced CTX-M-15 or CTX-M-14 | 53 (of 68 isolates) | 147 | |
| UK | 2006-2008 | Prospective study, travel-acquired diarrhea, stool samples, 174 CTX-M-15-producing isolates | 11.5 | 78 | |
| Canada | 2009 | Prospective case-control study, diarrheal stool samples, 26 ESBL+ isolates form travelers and 5 ESBL+ isolates from nontravelers | 23 (of 26 isolates), 40 (of 5 isolates) | 148 | |
| FQr isolates | |||||
| Europe (8 countries) | 2003-2006 | Uncomplicated CA UTI, 148 FQr E. coli isolates from womenc | 24 | 51 | |
| Canada | 2002-2004 | Multicenter study, ambulatory patients,d 199 UTI isolates randomly selected among E. coli isolates susceptible and/or resistant to TS and/or FQ | 44 | 50 | |
| Japan, South Korea, China | 1998-2007 | Multicenter study, any sample, 219 FQr E. coli isolates | 34 | 95 | |
| South Korea | 2006-2007 | CA UTI from 7 regions, 154 FQr E. coli isolates | 25 | 149 | |
Abbreviations: CA, community acquired; HA, hospital acquired; UTI, urinary tract infection; ESBL, extended-spectrum β-lactamase; TS, trimethoprim-sulfamethoxazole; FQ, fluoroquinolone; ESBL+, ESBL producing; ICU, intensive care unit.
Among FQs isolates, 7% were ST131.
ARES study.
NAUTICA survey.
Considering all sample types, the prevalence of E. coli ST131 among ESBL-producing E. coli isolates ranged from 22% (Spain) to 66% (United Kingdom) in Europe (mean value, 39% for five European countries) between 2006 and 2011 (20, 72, 75, 96, 139). The two studies conducted in Spain, 2 years apart at two hospitals in Galicia and Catalonia, indicated a marked increase in prevalence over this period: 22% in 2006 and 32% in 2008. In the United States (2009 to 2011), E. coli ST131 accounted for 54%, 10%, and 64% of the ESBL-producing E. coli isolates in the general population, children, and veterans, respectively (45, 93, 141). It accounted for 50% of such isolates in Canada between 2007 and 2009 and 52% in Japan between 2008 and 2011 (140, 142).
E. coli ST131 accounted for 21 and 25% of urinary tract infection (UTI) ESBL-producing E. coli isolates acquired in the community in France (2006) and in Mexico (2006 to 2007), respectively, and for 34% and 70% of those acquired in the community and in hospitals in Sweden (2007 to 2011) and India (2009), respectively (73, 86, 143, 144).
Three studies investigated bloodstream isolates. A prevalence of 60% of the ESBL-producing E. coli isolates causing bacteremia was reported in Canada for the period from 2000 to 2010, versus 32% of isolates causing bacteremia in France in 2005 and only 20% of isolates causing community-acquired bacteremia in South Korea between 2006 and 2011 (78, 145, 146).
Three other studies assessed the prevalence of E. coli ST131 among the ESBL-producing E. coli isolates causing travel-related infections of any type (Canada) or identified in the diarrheal stool samples (Canada and the United Kingdom) of travelers returning from abroad. Pitout et al. and Dhanji et al. sought E. coli ST131 only among CTX-M-15- and CTX-M-14-producing isolates and among CTX-M-15-producing isolates, respectively (Table 5), because these isolates account for most ESBL producers. E. coli ST131 accounted for 53% of the CTX-M-15- and CTX-M-14-producing isolates responsible for infections in travelers returning to Canada and 11.5% of the CTX-M-15-producing isolates detected in diarrheal stools from travelers returning to the United Kingdom (78, 147). A third study, also performed in Canada, was a case-control study comparing diarrheal stool samples from travelers (cases) and subjects who had not recently traveled abroad (controls) (148). Travelers were found to be significantly more likely to be colonized with ESBL-producing isolates than nontravelers, but the proportion of E. coli ST131 was higher among the ESBL-producing isolates obtained from nontravelers (40%) than among those obtained from travelers (23%). Rogers et al., who studied the potential risks and dynamics of the prolonged carriage of resistant E. coli in returned travelers, found that E. coli ST131 was infrequent, accounting for only 3% of the ESBL-producing E. coli isolates identified in samples from the participants (150). Studies other than those cited in Table 5 have reported that travel abroad, notably in India and Africa, increases the risk of being colonized (151) or presenting a community-acquired infection (152, 153) with an ESBL-producing E. coli strain. However, as none of these studies determined the ST of the ESBL-producing isolates identified, it was not possible to assess the role played by E. coli ST131. Nonetheless, these studies suggest that traveling abroad increases the risk of ESBL-producing-E. coli isolate carriage, specifically for non-ST131 isolates.
The available data for the prevalence of E. coli ST131 among fluoroquinolone-resistant E. coli isolates essentially concern UTI isolates from European (24%), East Asian (25 to 34%), and American (44 to 60%) patients (Table 5). The prevalence was found to be higher (60%) among UTI isolates from U.S. patients who had undergone renal transplantation (154). However, the highest prevalence, 78%, was that among fluoroquinolone-resistant E. coli isolates from U.S. veterans from 18 states (45).
Prevalence of E. coli ST131 among all E. coli isolates.
Recently published studies carried out in Europe (France, Poland, Spain, and United Kingdom), Oceania (Australia and New Zealand), and the United States determined the overall prevalence of E. coli ST131, because they included unselected, nonrepeated, and consecutive E. coli isolates (Table 6).
TABLE 6.
Overall prevalence of Escherichia coli ST131 among E. coli isolatesa
| Sample type and country | Study period | Study and population characteristics | % of E. coli ST131 among E. coli isolates |
Reference | ||
|---|---|---|---|---|---|---|
| ESBL+ | ESBL− | Overall | ||||
| Any sample | ||||||
| France | 2008-2009 | Multicenter (Paris) prospective case-control study, any sample, 152 ESBL+ and 152 ESBL− E. coli isolates | 36 | 10 | 16 | |
| Spain | 2010 | Multicenter (Seville) case-control study, any sample, 1,077 E. coli isolates of which 149 were ESBL+ and 928 ESBL− | 23 | 12 | 14 | 54 |
| US | 2007-2010 | Multicenter prospective case-control study, 94 ESBL+ and 158 ESBL− E. coli isolates | 50 | 13 | 110 | |
| US | 2007-2011 | Prospective case-control study (Chicago), outpatients > inpatients, 100 ESBL+ and 107 ESBL− E. coli isolates | 49 | 13 | 46 | |
| Spain | 2009 | Multicenter study, CA and HA infections, any sample, 500 E. coli isolates | 12 | 155 | ||
| US | 2007c | National study from hospitalized patients, 127 non-UTI E. coli isolates | 17 | 10 | ||
| US | 2011 | Retrospective study (Olmsted County medical centers), any sample, 299 E. coli isolates | 27 | 156 | ||
| Bacteremia | ||||||
| France | 1997-2006 | Retrospective study (one hospital), spontaneous bacteremia or bacterial peritonitis from cirrhotic patients, 110 E. coli isolates | 3b | 157 | ||
| UK | 2010-2012 | Multicenter study, bacteremia, 770 E. coli isolates | 62 | 13 | 17 | 158 |
| US | 2007-2012 | Prospective study (one hospital, San Francisco), bacteremia, 194 E. coli isolates | 72 | 25 | 26 | 42 |
| New Zealand | 2007-2010 | Case-control study (one hospital), CA bacteremia after prostate biopsy (47 E. coli isolates), CA bacteremia (54 E. coli isolates) | 38 (of 47 isolates), 17 (of 54 isolates) | 118 | ||
| UTI | ||||||
| UK | 2008-2009 | Prospective study of CA and HA UTI (two hospitals), 300 E. coli isolates | 54 | 9 | 12 | 109 |
| UK | 2008-2009 | Regional cohort of elderly patients, CA and HA UTI, 121 E. coli isolates | 22 | 17 | ||
| Australia | 2009-2011 | Prospective study, children (clinics and hospitals), 212 (cystitis/pyelonephritis) E. coli isolates, | 6 (cystitis), 11 (pyelonephritis) | 159 | ||
| Australia | 2009-2011 | Multicenter prospective study, reproductive-age women, 623 (cystitis/pyelonephritis) E. coli isolates, | 13 (cystitis), 30 (pyelonephritis) | 22 | ||
| Other | ||||||
| Poland | 2009-2012 | Multicenter study, neonatal ICUs, 90 E. coli isolates of which 25 were ESBL+ | 64 | 36 | 160 | |
Abbreviations: CA, community acquired; HA, hospital acquired; UTI, urinary tract infection; ESBL, extended-spectrum β-lactamase; ESBL+, ESBL producing; ESBL−, non-ESBL producing.
Three non-ESBL-producing isolates.
MYSTIC study.
Four of the studies on the general population were case-control studies in which ESBL-producing and non-ESBL-producing isolates from any type of sample were compared. These studies reported similar prevalences of E. coli ST131 among the non-ESBL-producing isolates: 10% in France (2008 to 2009), 12% in Spain (2010), and 13% in each of the other two studies, carried out in the United States (2007 to 2012). However, the reported prevalence among ESBL-producing isolates was higher in the United States (49 to 50%) than in France (36%) or Spain (23%) (16, 54, 156, 161).
Other study designs (prospective or retrospective cohort studies) have been used to evaluate the prevalence of E. coli ST131 isolates among total E. coli isolates. In 2009, Blanco et al. (155) carried out a study at several different centers and showed that E. coli ST131 accounted for 12% of all E. coli isolates. In contrast, the American studies conducted by Johnson et al. in 2007 on non-UTI isolates and by Banerjee et al. in 2011 on E. coli isolates of all types showed that E. coli ST131 clone accounted for 17 and 27% of the isolates considered, respectively (10, 110). It is not currently possible to exclude the possibility that the lower prevalence observed in the United States in 2007 was partly linked to the absence of UTI isolates in this study.
The other nine studies were performed on isolates from specific infections and/or subpopulations. E. coli ST131 accounted for 3% of the E. coli isolates causing spontaneous bacterial peritonitis and/or bacteremia in cirrhotic patients in France between 1997 and 2006 (157), none of which produced ESBL. This low prevalence is consistent with the results obtained by Chung et al. (162), who found a negative association between liver cirrhosis and bacteremia due to an E. coli isolate of clone ST131. A higher prevalence, 13%, was found among the non-ESBL-producing E. coli ST131 isolates causing bacteremia in the United Kingdom (158). This prevalence increased to 62% if the United Kingdom bacteremia isolates producing ESBL were included. Higher values were obtained for the bacteremia isolates characterized at San Francisco General Hospital between 2007 and 2010: 25% among the non-ESBL-producing isolates and 72% among the ESBL producers (42). However, if all the E. coli isolates causing bacteremia collected in San Francisco were taken into account, the prevalence of E. coli ST131 was 26%, similar to the 27% reported for the United States general population in 2011 (110). This raises questions about the suitability of the prevalence of E. coli ST131 among bacteremia E. coli isolates as an indicator of the actual prevalence of E. coli ST131 infection in in- and outpatients infected with E. coli. In New Zealand, E. coli ST131 was found to account for 17% of the E. coli isolates causing community-acquired bacteremia but for 38% of the isolates obtained when the community-acquired bacteremia was after prostate biopsy (118).
Four studies focused on UTI in the general population or subpopulations. In the United Kingdom, an analysis of 300 consecutive UTI E. coli isolates showed the prevalence of E. coli ST131 to be 9% among the non-ESBL-producing isolates and 54% among those producing ESBL (109). The lower of these two figures (9%) is similar to that reported in France for all non-ESBL-producing isolates (10%), whereas the higher figure (54%) is closer to that reported in the United States among all ESBL-producing isolates (49 to 50%) than to that reported in France (36%) (16, 110). A study by Croxall et al. focused on elderly patients in the United Kingdom, in whom E. coli ST131 accounted for 22% of the E. coli isolates causing community- and hospital-acquired UTI (17). Kudinha et al., who investigated UTI in Australian children and women of reproductive age showed, for both these subpopulations, that the prevalence of E. coli ST131 was higher among the E. coli isolates causing pyelonephritis than among those causing cystitis: 11 and 30% versus 6 and 13%, respectively (22, 159). The last study, carried out in neonatal intensive care units in Poland, showed that E. coli ST131 accounted for 36% of all E. coli isolates and that 64% of these ST131 isolates produced ESBL (160).
The most robust of the studies designed to determine the actual prevalence of clone ST131 were those in which the ST was determined for all unselected isolates (16, 17, 42, 109, 156, 157). This approach made it possible to describe the polyclonal structure of E. coli populations, evaluating the proportions of unique and clonal group strains and the proportion of ST131 strains among both the clonal group strains and in the predominant clonal group (Table 7). Clonal groups accounted for 45 to 90% of the strains in the six studies cited, and the major clonal groups identified were the same in all these studies: ST131, ST73, ST95, ST69, ST127, and ST10. This clearly indicates that people from different countries, with no apparent epidemiological link, can be infected with the clonal groups identified. However, several differences between these studies should be highlighted. The study by Brisse et al., which analyzed the clonal composition of the ESBL-producing and non-ESBL-producing E. coli populations separately, showed that clonal groups other than clone ST131 among ESBL-producing isolates differed from those identified among the non-ESBL-producing isolates or the E. coli isolates analyzed in other studies (16). The proportion of ESBL-producing isolates among the E. coli isolates analyzed in these other studies was either negligible (157) or low, at 11% (156), 9% (109), 17% (17), and 10% (42). This may explain the similarity between the clonal group compositions of the E. coli isolates investigated in these five studies and those of the non-ESBL-producing E. coli isolates characterized separately in the study by Brisse et al. (16). It therefore appears to be essential to analyze ESBL- and non-ESBL-producing isolates separately when E. coli populations are investigated at the level of population structure, because, as shown by Brisse et al., these two kinds of isolates seem to have different population structures, suggesting epidemiological differences (16).
TABLE 7.
Population structure of Escherichia coli and percentage of E. coli ST131 strains among clonal group strainsa
| Country (reference) | Sample | No. of isolates | No. of distinct STs | % of clonal group strains | % of E. coli ST131 among clonal group strains | Predominant ST | Other major ST clonal groups |
|---|---|---|---|---|---|---|---|
| France (157) | Blood | 110 | 67 | 49 | 5 | 95 | 73, 69, 14, 10, 23 |
| UK (17) | Urine | 121 | 52 | 45 | 22 | 131 | 73, 69, 127, 95 |
| UK (109) | Urine | 300 | 102 | 52 | 21 | 73 | 131, 69, 95, 10, 127 |
| France (16) | All | 304 (ESBL+, 152; ESBL−, 152) | 105 (ESBL+, 49; ESBL−, 73) | 70 (ESBL+, 77; ESBL−, 60) | 23 (ESBL+, 47; ESBL−, 17) | 131 (ESBL+, 131; ESBL−: 73) | ESBL+, 10, 167, 648, 117, 345; ESBL−, 131, 10, 95, 141, 69 |
| US (156) | All | 299 | 47 | 50 | 40 | 131 | 95, 73, 127, 69 |
| US (42) | Blood | 194 | 41 | 90 | 29 | 131 | 95, 73, 69, 12, 10 |
ESBL+, ESBL-producing isolate; ESBL−, non-ESBL-producing isolate.
Another difference (Table 7) concerned the proportion of clonal group strains belonging to the ST131 clonal group (from 5 to 47%) and the predominant ST. ST131 was the predominant clone in five instances (including one instance in which ESBL-producing isolates were analyzed separately), ST95 was the predominant clone once, and ST73 was the predominant clone twice (including once for the separate analysis of non-ESBL-producing isolates). All the predominant clones belong to phylogenetic group B2, which is known to include the most virulent extraintestinal pathogenic E. coli strains. However, ST95 and ST73 have rarely been identified among strains producing ESBL, whereas ST131 was frequently identified among strains producing ESBL (16, 42, 46). This strongly suggests that E. coli ST131 is unusual among group B2 clones.
Overall, depending on the country considered, E. coli ST131 has accounted for 12 to 27% of all E. coli isolates causing infections in the general population over the last 10 years. Among UTI E. coli isolates, the prevalence of clone ST131 varied with age: <10% in children, 13% in women of reproductive age, and >20% in elderly patients. Banerjee et al. showed, with unselected isolates, that E. coli ST131 predominated in adults over the age of 50 years and that the prevalence of infections with this clonal group increased with age (110). If only ESBL-producing or fluoroquinolone-resistant E. coli isolates were considered, the prevalence of clone ST131 was generally much higher, varying from 20 to 66% among ESBL producers and from 10 to 72% among fluoroquinolone-resistant isolates, depending on the country, type of infection, and subpopulation considered.
Longitudinal epidemiology of clinical isolates of E. coli ST131.
As indicated in Tables 5 and 6, some studies covered prolonged time periods, making it possible to obtain data relating to the temporal evolution of clinical isolates of clone ST131. In an analysis of 351 ESBL-producing E. coli isolates collected from 15 widely dispersed centers in the United States between 2000 and 2009, Johnson et al. (30) noted a significantly higher prevalence of CTX-M-15 ESBL production and clone ST131 among isolates collected between 2004 and 2009 than among those collected before this period (for CTX-M-15, 77% versus 23% [P = 0.019]; for ST131, 49% versus 8% [P = 0.004]). E. coli ST131 isolates were significantly more likely to be have been obtained in the period from 2004 to 2009 than before 2004, regardless of CTX-M-15 status.
In Calgary (84), the number of E. coli isolates causing bacteremia increased from 334 in 2000 to 459 in 2010 (increase of 37%), whereas the number of the ESBL-producing isolates increased gradually until 2007 and then abruptly over the next 3 years (Fig. 2). E. coli ST131 accounted for 0 to 44% of ESBL-producing isolates between 2000 and 2007, with an exponential increase in this proportion from 2007 to 2010 (44 to 77%). Thus, E. coli ST131 has made a major contribution to the significant increase in bloodstream infections caused by ESBL-producing E. coli since 2007 in Calgary. The dynamics of seven different pulsotypes (arbitrarily called A-1 to A-6 and B) of E. coli ST131 isolates were characterized. Pulsotype A-3 appeared in 2000. It persisted throughout the study and became one of the three major pulsotypes present since 2009. Pulsotype A-1 appeared in 2003 and persisted throughout the study. Pulsotypes A-4 and A-5 appeared in 2005 and persisted throughout the study, but pulsotype A-5 became the predominant pulsotype in 2009 and 2010. Pulsotype A-6 appeared in 2007, and its proportion has increased annually, reaching the same levels as pulsotype A-3 in 2010. Finally, pulsotype B, which includes E. coli ST131 isolates and was not detected with the PCR for the pabB allele (see “Screening methods for detecting the E. coli ST131 clone and subclones” above), appeared in 2008 and was present in 2009 but was not detected in 2010. The authors checked that the ESBL-producing E. coli isolates were not clustered around a particular acute-care center or part of Calgary, thereby excluding patient-to-patient transmission in a hospital environment. This Canadian description is reminiscent of that reported by Johnson et al. for international E. coli ST131 isolates collected between 1997 and 2011 (36).
FIG 2.
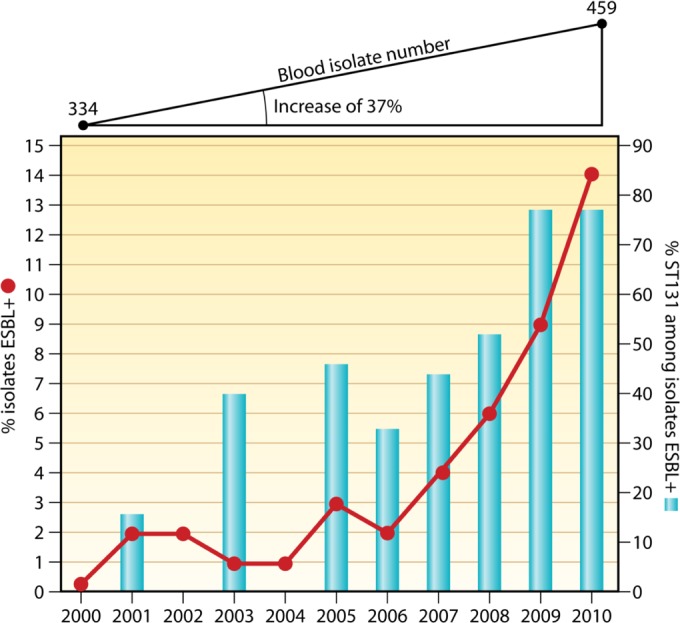
Dynamics of bacteremia ESBL-producing Escherichia coli isolates and proportion of ST131 E. coli isolates between 2000 and 2011 in Calgary (Canada) (84). The curve represents the percentage of ESBL-producing E. coli isolates and the histogram the proportion of E. coli ST131 among these isolates.
A 7-year retrospective study in Israel yielded results similar to those obtained in Calgary: an increase in the attack rate (per 10,000 admissions) of ESBL-producing E. coli isolates among cases of community-acquired bloodstream infections, an increase in ESBL production among E. coli isolates causing community-acquired bloodstream infections, and an increase in the proportion of E. coli ST131 isolates among these ESBL-producing isolates (25% in 2003, 61% in 2007, and 85% in 2009) (163). These findings suggest that E. coli ST131 made a large contribution to the relative increase in community-acquired bacteremia due to ESBL-producing E. coli isolates in Israel since 2003. This conclusion is supported by the absence of detection of this clone in a collection of nosocomial ESBL-producing E. coli isolates obtained before 2003.
Based on the findings of these three studies, E. coli ST131 seems to have appeared around 2003, subsequently accounting for an increasing proportion of ESBL-producing E. coli isolates.
Prevalence of E. coli ST131 among human fecal isolates.
The digestive tract is the principal reservoir of extraintestinal pathogenic E. coli, but only a few studies (Table 8) have investigated the colonization of the digestive tract with E. coli ST131 isolates, regardless of their ESBL production and fluoroquinolone resistance status. Kudinha et al. showed that 1% of healthy children and 4% of women of reproductive age carried E. coli ST131 in their intestinal tracts in 2010 to 2011 in Australia (22, 159). A higher prevalence, 7% in 2006 and 14% in 2011, was observed for the predominant fecal E. coli of healthy subjects living in the Paris area and visiting the same check-up center for the two time points considered (53, 165). The difference in prevalence between Australian and Parisian subjects may reflect a difference in age, with the Parisian population consisting of adults with a mean age of 55 years (±12). The higher prevalence in 2011 than in 2006 for Parisian subjects probably reflected the predominant fecal E. coli population of a single subject, consisting of ESBL-producing E. coli ST131.
TABLE 8.
Prevalence of subjects carrying Escherichia coli ST131 in the digestive tracta
| Country(ies) | Study period | Study and population characteristics | % of E. coli ST131 among fecal E. coli isolates |
Reference(s) | ||
|---|---|---|---|---|---|---|
| FQr | ESBL+ | Overall | ||||
| UK | 2005-2006 | Prospective study, 16 private nursing homes (Belfast), fecal samples, 294 residents, 119 with fecal ESBL+ E. coli isolates | 98 | 78, 92 | ||
| France | 2006 | Prospective study, stools, 2 and 51/332 healthy subjects (Paris area) with fecal ESBL+ and FQr E. coli isolates, respectively | 8 | 0 | 7 | 53 |
| France, Italy, Spain, Israel | 2008-2009 | Prospective study,b rehabilitation units, rectal swabs, 356 ESBL+ E. coli isolates | 41 | 87 | ||
| China | 2009 | Prospective study, stools, 55 of 109 healthy subjects with fecal ESBL+ E. coli isolates | 7 | 164 | ||
| France | 2011 | Prospective study, stools, 21/345 healthy subjects (Paris area) with fecal ESBL+ E. coli isolates whose ST was compared with the ST of E. coli dominant population | 9.5 | 14 | 165 | |
| Australia | 2009-2011 | Prospective study, children (clinics and hospitals), 115 fecal isolates | 1 | 159 | ||
| Australia | 2009-2011 | Prospective study, reproductive-age women, 330 fecal E. coli isolates | 4 | 22 | ||
| Germany | 2010-2011 | Nursing home, 240 residents, fecal samples, 25 ESBL+ E. coli isolates | 44 | 166 | ||
| Switzerland | 2012 | Prospective study, primary care, rectal swabs, 291 patients, 15 with ESBL+ fecal E. coli isolates | 26 | 167 | ||
| France | 2012 | Prospective study, day care center children, stools, 419 children, 27 ESBL+ E. coli isolates | 44 | 24 | ||
Abbreviations: ESBL+, ESBL producing; FQr, Fluoroquinolone resistance.
European Union-funded project MOSAR.
Five studies have assessed the prevalence of E. coli ST131 among ESBL-producing or fluoroquinolone-resistant E. coli isolates colonizing the digestive tracts of different subjects. This prevalence was found to be 7 and 9.5% in the general populations of China (2009) and France (2011), respectively (164, 165). It was 26% among patients managed by a primary care center in Switzerland and about 40% in European patients from rehabilitation units (2008 to 2009) (87, 167). The prevalence was very high in nursing home residents, i.e., 44% in Germany (2010 to 2011) and 98% in the United Kingdom (2005 to 2006) (87, 166), and in children from day care centers (44%) in France (24). In 2001 to 2002, Severin et al. investigated the rectal carriage of ESC-resistant Enterobacteriaceae in 3,995 people from two cities in Java (Indonesia), comprising three groups of patients and one group of healthy subjects (168). However, none of the CTX-M-15-positive E. coli isolates (corresponding to 50% of the ESBL-producing isolates) belonged to clone ST131 in any of the population groups studied. Thus, the prevalence of fecal ESBL-producing E. coli ST131 carriage in healthy subjects seems to vary considerably with the population and study year.
The two ESBL-producing E. coli isolates detected in the digestive tracts of the Parisian healthy subjects in 2006 did not include ST131 strains, but such strains accounted for 8% of the fecal fluoroquinolone-resistant E. coli isolates detected in this population (53). This strongly suggests that the fecal population of E. coli ST131 acquired resistance to fluoroquinolones well before it became resistant to ESC through ESBL acquisition, as suggested by Johnson et al. for clinical isolates of E. coli ST131 (43).
Further studies are therefore required to evaluate the worldwide prevalence of E. coli ST131 as the dominant E. coli population in healthy subjects. Indeed, if clone ST131 is the predominant E. coli population in a large proportion of human beings worldwide, the successful elimination of ESBL-producing bacteria will require a worldwide public health care strategy.
Molecular epidemiology of E. coli ST131 and E. coli ST131 subclones.
The relationships between virotypes and phenotypic, genotypic, epidemiological, or clinical traits have been established (32). All isolates of virotypes A and B and 63% of those of virotype C produced CTX-M-15, whereas none of the virotype D isolates were found to produce this enzyme (P < 0.001 for all comparisons with virotype D). In contrast, virotype D isolates produced group CTX-M-9 enzymes, SHV-12, and CTX-M-32. Ciprofloxacin resistance was significantly associated with virotype A, B, and C isolates. A cross analysis of pulsotypes and virotypes revealed four major clusters, which were largely virotype specific. All virotype A, B, and C isolates considered contained fimH30 and an ISL3-like transposase in the fimB gene, whereas all the virotype D isolates studied had a fimH22 gene and no ISL3-like transposase in the fimB gene. The associations of the four virotypes with demographic data and with the acquisition and type of infection have also been explored. Virotype B has been shown to be significantly associated with older patients and a lower likelihood of symptomatic infections, specifically for urinary tract infection, whereas virotype C was significantly associated with a higher likelihood of symptomatic infection. Virotype D was significantly associated with younger patients and community-acquired infections. Virotypes A and B displayed a significantly stronger association with nursing home residents than did virotypes C and D. Various teams have applied similar epidemiological approaches to ST131 subclones H30, H30-R and H30-Rx. All of these studies used the CH clonotyping method to identify the ST131 clone and its subclones (41). Banerjee et al. (46) found, in an investigation of 299 consecutive nonduplicate extraintestinal E. coli isolates collected in Olmsted County (MN, USA), that the H30 ST131 subclone was particularly common in very young children, older people, and health care-associated infections and among antimicrobial drug-resistant E. coli isolates, whereas non-H30 ST131 and other well-known pathogenic lineages (ST95, ST73, ST127, and ST69) were common in older children, young adults, and community-acquired infections and among antimicrobial drug-susceptible isolates. Blanc et al. found that the H30 ST131 subclone predominated among fecal ESBL-producing E. coli ST131isolates from children attending day care centers in France (24). In another study by Banerjee et al. (161) in Chicago, the H30 ST131 subclone was found to have expanded more widely in the study region than non-H30 ST131 subclones, and CTX-M-15-producing ST131 isolates were found to be confined almost exclusively to the H30-Rx subclone. These isolates had higher VF scores than non-ST131 isolates. In addition, three VF genes (the iha, sat, and iutA genes) were found to be more prevalent among H30 than among non-H30 ST131 isolates. Colpan et al., who screened for E. coli ST131 in veterans from 24 veteran medical centers in the United States, also observed a high prevalence of clone ST131 and subclone H30-R (45). Price et al. assessed the relative prevalences of H30-R and H30-Rx subclones among 261 urine E. coli isolates from different patient sources and locations. They found that H30-Rx had the highest prevalence among the German hospital isolates (H30-Rx > H30-R), an intermediate prevalence among United States-based hospital isolates, and the lowest prevalence among United States-based outpatient isolates (115). For the 162 U.S. isolates included in the study by Price et al., information about the presence or absence of clinically diagnosed sepsis was available. The rate of sepsis diagnosis was significantly higher for patients infected with H30-Rx strains than for those infected with non-H30-Rx, H30-R, non-H30 ST131, or non-ST131 strains.
These molecular analyses thus suggest that the distribution of E. coli ST131 subclones depends on the location at which the infection began (community/hospital), patient age, and antibiotic susceptibility of the isolate. These findings, with considerable implications for medical practice, require confirmation from studies on other populations in different countries.
Animal- and Food-Borne E. coli ST131
There have been very few reports on E. coli ST131 from either animals (healthy or sick) or foods (Table 9).
TABLE 9.
Escherichia coli ST131 isolates in animal and food sourcesa
| Source and country(ies) | Study period | Study design | No. of ST131 isolates/total | FQr ST131 | ESBL/AmpC gene(s) in ST131 | Reference(s) |
|---|---|---|---|---|---|---|
| Companion animals | ||||||
| Portugal | 2004-2006 | 61 UTI E. coli isolates, 41 dogs and 20 cats | 1/61 | Yes | CTX-M-15 | 89, 113 |
| United States | 2008 | Dogs and cats in the same household (index case plus colonization) | 3/6 | Yes | No | 169 |
| France | 2006-2010 | 19 ESBL-producing E. coli isolates among 518 clinical dogs and cats | 1/19 | Yes | CTX-M-14 | 196 |
| Europe (8 countries, mainly Germany) | 2008-2009 | 177 ESBL-producing E. coli isolates (mostly cats, dogs, horses) | 10/177 | Mostly yes | Mostly CTX-M-15 | 170 |
| Japan | 2011 | 33 cefazolin-resistant ExPEC isolates, dogs and cats | 4/33 | ND | CTX-M-27 | 171 |
| Australia | 2007-2009 | 125 clinical FQr ExPEC isolates, 120 dogs and 5 cats | 9/125 | Yes | ND | 21, 172 |
| Australia | 2009 | 123 hospitalized dogs, 23 carrying FQr E. coli isolates | 2/123 | Yes | ND | 173 |
| Switzerland | 2010-2011 | 107 UTI E. coli isolates, 59 dogs and 40 cats, 4 with ESBL/AmpC-producing E. coli isolates | 0/107 | NA | NA | 174 |
| Korea | 2006-2007 | 628 E. coli, 422 stray and 206 hospitalized dogs, 34 carrying ESBL/AmpC-producing E. coli isolates. | 0/34 | NA | NA | 175 |
| The Netherlands | 2007-2009 | 2,700 clinical Enterobacteriaceae isolates from mostly dogs, cats, and horses, 65 carrying ESBL/AmpC-producing E. coli isolates | 1/65 | Yes | CTX-M-15 | 176 |
| Kenya | 2009 | 49 ESBL-producing E. coli isolates, dog and cat carriers | 3/49 | Yes | CTX-M-15 | 177 |
| Food-producing animals and foodstuffs | ||||||
| Germany | 2006-2007 | 1,378 clinical E. coli isolates, mostly swine, poultry, cattle (1,167), 22 with ESBL/AmpC-producing E. coli isolates | 1/22 | No | CTX-M-1 | 178 |
| England and Wales | 1999-2008 | 39 clinical ExPEC isolates, mostly cattle | 0 | NA | NA | 179 |
| England, Wales, Scotland | 2008-2009 | 388 broilers and 442 turkeys (carriage) | 0 | NA | NA | 180 |
| Spain | 2003 | 86 ESBL/AmpC-producing E. coli isolates, 59 from floors of poultry farms, 27 from pig farms | 1 (poultry) | ND | CTX-M-9 | 181 |
| Italy | 2009 | 101 E. coli, 91 from healthy chickens and 10 from healthy turkeys | 1 | No | ND | 182 |
| Spain | 2007-2009 | 463 chicken E. coli clinical isolates | 7/463 | No | CTX-M-9 (n = 2) | 35 |
| Spain, France, Belgium | 1991-2001 | 1,601 avian E. coli clinical isolates | 3/1,601 | Yes (n = 2) | No | |
| Spain | 2003 | 57 healthy chicken E. coli isolates | 1/57 | No | CTX-M-9 | |
| France | 2009-2011 | 1,745 E. coli clinical isolates from cattle mastitis screened for ESBL/AmpC-producing E. coli isolates | 0 | NA | NA | 183 |
| The Netherlands | 2009 | 399 calves, 3 veal calf farms with fecal ESBL-producing isolates | 0 | NA | NA | 184 |
| The Netherlands | 2006 | 27 ESBL-producing E. coli isolates from poultry | 0 | NA | NA | 185 |
| The Netherlands | 2009 | 158 ESBL-producing E. coli isolates from chicken, other meat and human samples | In human samples only | NA | NA | 186 |
| France | 2007-2009 | 77 clinical ESBL-producing E. coli isolates from calves of which were 9 CTX-M-15 producers | 0 | NA | NA | 187 |
| China | 2007-2009 | 896 commensal E. coli isolates from pigs, chickens, and others | 0 | NA | NA | 188 |
| Canada | 2005-2007 | 417 E. coli isolates from retail meat | 1 | ND | ND | 189 |
| South America | 2008 | 141 ESBL/AmpC-producing E. coli isolates (raw chicken meat) | 0 | NA | NA | 190 |
| Spain | 2010 | 33 chicken and turkey meat samples | 0 | NA | NA | 191 |
| Spain | 2009-2010 | 100 retail chicken meat samples | 7/100 | Yes (n = 4) | CTX-M-9 (n = 4) | 35 |
| Animals living on the wild | ||||||
| Russia | 2008 | 532 E. coli isolates from wild birds screened for ESBL-producing isolates | 1 | ND | CTX-M-15 | 192 |
| Portugal | 2007-2008 | 45 ESBL-producing E. coli isolates from seagulls | 4/45 | ND | CTX-M-32, CTX-M-15, CTX-M-1 | 193 |
| Czech Republic | 2006-2008 | 499 E. coli isolates from cormorants screened, among which 8 ESBL producers | 2/8 | Yes | CTX-M-27 | 194 |
| Germany | 2009 | 66 urban rats (carriage) | 1/66 | Yes | CTX-M-9 | 195 |
Abbreviations: UTI, urinary tract infection; ESBL, extended-spectrum β-lactamase; FQr, fluoroquinolone resistant; AmpC, cephalosporinase; ExPEC. extraintestinal pathogenic E. coli; NA, not available; ND, not determined.
Prospective, targeted cohort studies are rare in veterinary medicine, so many of the published data were obtained in passive surveys or with opportunistic, nonrepresentative sampling methods. The prevalence of animal colonization (intestinal carriage) or infection with ST131 E. coli isolates, regardless of their susceptibility/resistance status for ESC and/or fluoroquinolones (FQ), therefore remains unclear. In such a context, it is difficult to assess the contribution of animal E. coli ST131 to the global expansion of E. coli ST131 in humans (including the differential expansion of certain ST131 subclones).
E. coli ST131 in pets.
The first report of an animal source of E. coli ST131 was published in 2009 and concerned a dog suffering from a UTI in Portugal. The isolate obtained from the dog was resistant to FQ and harbored the blaCTX-M-15, qnrB2, and aac(6′)-Ib-cr genes on an IncFII plasmid (113). This finding was interpreted as indicating the possible entry of the emerging, virulent human ST131 clone into the animal population, due to the proximity between pets and their owners, facilitating human-to-animal transfer. Other E. coli ST131 isolates have subsequently been reported in other pets. Some of these isolates produced ESBL, whereas others did not, and some reports were sporadic, whereas others emerged from large-scale studies. In the United States, non ESBL-producing but FQ-resistant ST131 isolates were recovered from a dog and two cats with UTI from the same household (124). All three animals were infected with the same ST131 strain, indicating a probable exchange between the animals.
In a large international study, E. coli ST131 accounted for 5.6% of the 177 ESBL-producing E. coli clinical isolates obtained from various animal species (mostly cats, dogs, and horses with UTI, wound infections, and diarrhea) from eight European countries (170). With the exception of an E. coli ST131 isolate producing the SHV-12 enzyme, all these isolates produced CTX-M-15, and most clustered with a human CTX-M-15-producing E. coli ST131 isolate included in the analysis for the purposes of comparison. In Australia, E. coli ST131 accounted for 7.2% of 125 FQ-resistant E. coli clinical isolates from companion animals (essentially dogs) (172). These FQ-resistant ST131 isolates were genetically closely related to non-FQ-resistant ST131 isolates recovered from the fecal flora of hospitalized dogs in Australia (173). In Japan, ST131 strains were recently found to account for 12% of 33 cefazolin-resistant E. coli clinical isolates from dogs and cats, and all the ST131 isolates produced CTX-M-27 (171). In France, a single ST131 isolate was found among 14 ESBL-producing E. coli isolates from pets, even though many of the non-ST131 E. coli isolates carried the blaCTX-M-15 gene on IncFII plasmids abundant in human ST131 E. coli isolates (196). The low prevalence of ST131 in France is consistent with data from the Netherlands, Switzerland, and South Korea (174–176). Albrechtova et al. recently showed that the dogs of nomadic pastoralists in northern Kenya harbor CTX-M-15-producing ST131 E. coli isolates, indicating possible human-to-animal transfer (177).
E. coli ST131 in food animals and foodstuffs.
Only a few studies have investigated the presence of E. coli ST131 in food animals. One FQ-susceptible, non-ESBL-producing E. coli ST131 isolate was identified among 101 (0.9%) E. coli isolates from healthy chickens and turkeys in Italy (197). Schink et al. (178) identified one E. coli ST131 isolate among 22 ESBL producers in a collection of 1,378 E. coli isolates from various animals (mostly pigs, poultry, and cattle). A single E. coli ST131 isolate was found in a pig with a gastrointestinal infection. This E. coli ST131 isolate harbored an IncN plasmid encoding CTX-M-1 (178). E. coli ST131 isolates producing CTX-M-9 have occasionally been recovered from poultry feces. In some of these instances, the animal isolates have presented a certain similarity to human ST131 isolates (181). Mora et al. (35) reported on CTX-M-9-producing E. coli ST131 among poultry E. coli isolates collected in different countries during different time periods. Three (0.2%) of the 1,601 E. coli isolates collected from diseased poultry in Spain, France, and Belgium between 1991 and 2001 belonged to clonal group ST131, none produced ESBL, and two were resistant to quinolones. One (1.8%) of the 57 fecal E. coli isolates collected from healthy chickens in Spain in 2003 was an FQ-susceptible, CTX-M-9-producing E. coli ST131 isolate. Finally, seven (1.5%) of the 463 E. coli isolates collected from diseased chickens in Spain between 2007 and 2009 were E. coli ST131, and two isolates produced CTX-M-9 and were susceptible to FQ (35). E. coli ST131 was also found in a pig in Denmark (198). In contrast, no ST131 isolates were identified by Wu et al. among 39 clinical isolates of E. coli from cattle, sheep, chicken, and pigs (179). Randall et al. also identified no such isolates among 388 broiler chicken cecal samples and 442 turkey rectal swab samples (180). E. coli ST131 was not found in cases of mastitis in cattle (183) or in veal calves in the Netherlands (184) and France (187). The O25b PCR excluded the presence of E. coli ST131 from 896 commensal E. coli isolates from 326 pigs, 316 chickens, 88 cattle, 58 ducks, 22 geese, 61 pigeons, and 25 partridges in China (188).
E. coli ST131 also appears to be very rare in foodstuffs of animal origin. Vincent et al. reported a single non-ESBL-producing ST131 isolate from 417 retail chicken samples analyzed. This isolate was indistinguishable from a human ST131 isolate responsible for UTI (189). None of the 141 ESBL/AmpC-producing E. coli isolates from raw chickens imported into the United Kingdom from South America were identified as ST131 (190). Similarly, Egea et al. did not detect the ST131 clone among ESBL-producing E. coli isolates from 33 raw retail meat samples (191). In contrast, E. coli ST131 was isolated from seven retail chicken meat samples from the 100 analyzed in Spain (prevalence, 7%). Three of these isolates both were resistant to FQ and produced CTX-M-9; one produced CTX-M-9, and one was resistant to FQ (35).
E. coli ST131 in animals living in the wild.
Current worldwide antimicrobial drug resistance data suggest that environmental reservoirs may play a major role in the selection and dissemination of resistance genes and multidrug-resistant bacteria (199). The principal pathways via which drug resistance circulates are unclear. However, animals living in the wild are almost certainly an underestimated vector of resistance. In a series of 532 isolates screened in Russia (192), one E. coli ST131 isolate was found among four ESBL-producing E coli isolates from glaucous winged gulls (Larus glaucescens). E. coli ST131 isolates producing ESBL have also been obtained from seagulls (4/45 isolates) in Portugal (193) and from the feces of great cormorants (Phalacrocorax carbo) in Central Europe (194). The single ESBL-producing E. coli isolate obtained from 220 Norway rats (Rattus norvegicus) in Germany was an E. coli ST131 isolate harboring CTX-M-9 (195).
Comparison of E. coli ST131 isolates from human and nonhuman sources.
With the identification of E. coli ST131 in animals and foods of animal origin, the possible contribution of nonhuman sources to the current expansion of this clonal group of E. coli in humans has become a major concern (200). Assessment of the possible interspecies transmission of E. coli ST131 requires specific studies, which might also generate hypotheses about the primary reservoir of E. coli ST131.
Several large-scale studies including retail meat and human samples (rectal swabs and blood cultures) based on MLST have yielded clusters including both human and poultry isolates, but with ST131 found exclusively in human samples (185, 186). A human rather than animal reservoir of ST131 was also strongly suggested by the findings of a broad survey carried out in the United Kingdom, the Netherlands, and Germany (179). These data are consistent with a human primary reservoir of E. coli ST131.
Other studies of possible interspecies transmission have focused on molecular comparisons of ST131 E. coli isolates collected from human and nonhuman sources. The most accurate tool for such purposes seems to be the determination of PFGE profiles for the isolates. In two studies based on a large PFGE library of human ST131 isolates, specific pulsotypes were found to be common to humans and pets (21, 173). In particular, the 968 pulsotype, the most prevalent ST131 pulsotype in North America, was found to predominant in human (7/20) ST131 isolates and companion animal (6/9) ST131 isolates in Australia (21). However, in a survey carried out in Spain and Portugal, human and dog ST131 isolates were of 95 pulsotypes, but the 968 pulsotype was restricted exclusively to humans, and only 3% of the other pulsotypes were common to human and pet E. coli ST131 isolates in Portugal (89). In another study (170), 6 of 10 companion animal ST131 isolates and a reference human ST131 strain clustered together but were not identical. This reference human ST131 strain was also compared with the single ST131 isolate identified among E. coli isolates from urban brown rats in Germany. However, the similarity between these two isolates was not entirely convincing (195). In their comparison of 33 human ST131 and 19 poultry ST131 isolates possessing the ibeA gene, Mora et al. found that some human and poultry isolates had PFGE profiles that were more than 85% similar. However, these isolates had different virulence genotypes (35).
As demonstrated by the conclusions of a broad international comparison of ST131 isolates (36), the data available provide little firm evidence to suggest that animals or foods of animal origin are likely to constitute the primary reservoir of E. coli ST131. Moreover, as far as the possible interspecies transmission of the ST131 E. coli pandemic clone is concerned, it should be borne in mind that genetic similarities between E. coli isolates of different origins (animals and humans, for instance) provide no indication as to the direction of the transfer.
Environmental E. coli ST131
Recent studies have shown that the intestinal carriage of ESBL-producing E. coli is frequent in human patients in both hospital and community settings. The digestive tracts of livestock also constitute a large reservoir of these bacteria. These intestinal ESBL-producing E. coli isolates are released into the environment either directly or after treatment at wastewater treatment plants (WWTP). Quantitative data for ESBL-producing E. coli in hospital and community effluent discharge and outflow from WWTP are scarce (201–203). The available data suggest that large quantities of ESBL-producing E. coli are present in the wastewater network and that wastewater treatment significantly decreases both the total E. coli load and the load of ESBL-producing E. coli. However, it has been shown that ESBL-producing E. coli may have an advantage over E. coli not producing such enzymes in water treatment plants, due to the selective pressure exerted by antibiotics in treatment plants (202). Wastewater treatment plants release large quantities of ESBL-producing E. coli into the environment in the outflow water (released into the river) and sludge (used as fertilizer) (202, 204).
No overall data are available about the proportions of ST131 isolates (with or without ESBL production) in the E. coli populations released into the environment. This clone has recently been detected in antimicrobial drug-resistant populations of E. coli in the Thames (London) (78) and Llobregat (Barcelona) (205) rivers, in WWTP outflow in Brno (the Czech Republic) (206), and on Adriatic Sea beaches (Italy) (207). The available prevalence data for ST131 as a proportion of the total ESBL-producing E. coli population show this prevalence to be high for the Thames (67%) and treated outflow water in Brno (73%) but low for Adriatic beaches (3.5%).
The scarcity and the heterogeneity of the studies published on this topic make it impossible to draw any firm conclusions about the amount of E. coli ST131 released into the environment.
FACTORS ASSOCIATED WITH CLINICAL ISOLATES OF E. COLI ST131
Four studies to date have investigated the factors associated with clinical isolates of E. coli ST131 (54, 156, 162, 208). These studies were performed in different countries (Taiwan, one hospital between 2005 and 2010; France, 10 hospitals from the Paris area between 2008 and 2009; Spain, all health care centers in the Seville area in 2010; and the United States, the Mayo Clinic and Olmsted Medical Center, the two centers serving Olmsted County, MN, in 2011). These studies differed in terms of the methodologies used. The study designs were different, with a case-control design used in France (case, a patient with a CTX-M-producing ST131 E. coli isolate from any clinical sample; control, a patient with an ESBL-producing non-ST131 E. coli clinical isolate from any sample) and in Spain (case 1, a patient with a non-ESBL-producing ST131 E. coli clinical isolate; control 1, a patient with a non-ESBL-producing non-ST131 E. coli clinical isolate; case 2, a patient with an ESBL-producing ST131 E. coli isolate from any clinical sample; and control 2, a patient with an ESBL-producing non-ST131 E. coli clinical isolate from any sample) (54, 208). In contrast, cohort studies were carried out in Taiwan (cases, patients with an ESBL-producing ST131 E. coli isolate causing bacteremia; controls, patients with an ESBL-producing non-ST131 E. coli isolate causing bacteremia) and the United States (cases, patients with an E. coli ST131 isolate from any sample; controls, patients with a non-ST131 E. coli isolate from any sample) (156, 162). The number of E. coli isolates producing ESBL was not mentioned in the American study. However, according to the ceftriaxone resistance data provided, it is possible to estimate the percentages of isolates producing ESBL among ST131 and non-ST131 E. coli isolates at maximums of 11% and 4%, respectively. These studies also differed in terms of the methods used to identify the ST131 clonal group among E. coli isolates. The MLST method was applied to all E. coli isolates included in the Taiwanese and French studies (162, 208). Screening methods were carried out in both the Spanish study (PCR for 025b rfb and allele 3 of the pabB gene in B2 group isolates) and in the American study (detection of single-nucleotide polymorphisms of the mdh and gyrB genes associated with the two-locus clonal typing strategy based on the sequencing of the fumC and fimH genes in B2 group E. coli isolates) (54, 156). A third difference concerned the populations included: adult patients in the Taiwanese, French, and Spanish studies and both adults and children in the American study. The number and type of variables also differed between studies. The Taiwanese, Spanish, American, and French studies investigated 29, 34, 43, and, 64 variables, respectively. All studies included standard demographic data and medical data: community or hospital acquisition of infection, comorbid conditions, and health care-associated variables before and during current hospitalization, including antibiotic treatment, surgery, and invasive devices. Only the French study took into account variables linked to the lifestyle of the patient, such as type of housing, professional status, diet, and travel abroad.
The factors found to be independently associated with clinical isolates of E. coli ST131 in the four studies are shown in Table 10. Two factors were associated with bacteremia due to an E. coli ST131 isolate in the Taiwanese study: secondary bacteremia and the absence of a urinary tract catheter. This suggests that E. coli ST131 causes bacteremia from a spontaneous focal infection. Despite their use of different methodologies on epidemiologically different populations, the other three studies consistently identified several independent factors positively or negatively associated with clinical isolates of E. coli ST131. Living in a long-term-care facility (LTCF) was positively associated with clinical isolates of E. coli ST131 in both the French and American studies. In France, this factor was associated with ESBL-producing clinical isolates of E. coli ST131, suggesting that ESBL producers, which made up a larger proportion of E. coli ST131 isolates (11%) than of non-ST131 isolates (4%) in the American study, were involved in identifying the risk factor in this study, in which ESBL- and non-ESBL-producing E. coli ST131 isolates were not separated for the analysis. Unfortunately, LTCF residence was not included as a variable in the Spanish study. All three studies investigated fluoroquinolone use. This factor was associated with non-ESBL-producing E. coli ST131 isolates in Spain and with E. coli ST131 isolates in the United States but was not associated with ESBL-producing E. coli ST131 isolates in either France or Spain. This finding highlights the importance of analyzing risk factors separately for non-ESBL- and ESBL-producing E. coli ST131 isolates. The use of amoxicillin-clavulanate or ESC was associated with E. coli ST131 isolates in the American study and with non-ESBL-producing E. coli ST131 isolates in the Spanish study. Antibiotic consumption, regardless of the drug concerned, was not associated with ESBL-producing E. coli ST131 isolates in the Spanish and French studies. This result again highlights the need to study E. coli ST131 isolates with and without ESBL production separately.
TABLE 10.
Factors independently associated with an Escherichia coli ST131 isolate
| Country (reference) | E. coli ST131 isolate type | Variable | Odds ratio (95% confidence interval) | P value |
|---|---|---|---|---|
| Taiwan (162) | Bacteremia, ESBL producers | Secondary bacteremia | 5.05 (1.05–23.56) | 0.0.4 |
| Without urinary catheter | 3.77 (1.17–12.18) | 0.03 | ||
| Chronic hepatitis/liver cirrhosis | 0.34 (0.07–0.88) | 0.05 | ||
| France (208) | CTX-M producers | Consumption of poultry ≥2 times per wk | 0.2 (0.1–0.6) | 0.002 |
| At last one invasive device in preceding 6 mo | 0.3 (0.1–0.7) | 0.01 | ||
| Stay between hospital admission and study inclusion in: | ||||
| LTCF | 4.4 (1.3–14.7) | 0.02 | ||
| ICU | 0.2 (0.05–0.8) | 0.02 | ||
| Spain (54) | Non-ESBL producers | Female gender | 1.94 (1.07–3.51) | 0.02 |
| Diabetes mellitus | 2.17 (1.29–3.67) | 0.003 | ||
| Amoxicillin/clavulanate use | 2.07 (1.05–3.96) | 0.02 | ||
| Fluoroquinolone use | 2.48 (1.41–4.34) | 0.001 | ||
| ESBL producers | Male gender | 2.20 (0.94–5.11) | 0.06 | |
| Health care-related acquisition | 0.30 (0.13–0.71) | 0.006 | ||
| Previous antibiotics | 0.43 (0.19–1.00) | 0.05 | ||
| US (156) | All | Age (per 10-yr increase) | 1.18 (1.01–1.36) | 0.03 |
| LTCF residence | 10.00 (3.75–26.68) | <0.001 | ||
| UTI 30 days prior | 3.93 (1.77–8.71) | <0.001 | ||
| Complex infectiona | 2.43 (1.13–5.21) | 0.02 | ||
| Extended-spectrum cephalosporin | 3.34 (1.24–8.97) | 0.02 | ||
| Fluoroquinolones | 2.42 (1.19–4.94) | 0.02 |
Colonization and noncomplex infection were combined to serve as the reference group.
Health care-associated infection was negatively associated with ESBL-producing E. coli ST131 isolates in the Spanish study. In the French study, health care-associated procedures, such as having had at least one invasive device in the preceding 6 months and having spent time in an intensive care unit between hospital admission and inclusion in the study, were also negatively associated with CTX-M-producing E. coli ST131 isolates. In other words, patients with health care-associated infections or procedures were more likely to become infected with non-ST131 than with ST131 strains in cases of infection with an ESBL-producing E. coli isolate.
One striking finding was the negative association between the consumption of poultry at least twice per week and CTX-M-producing clinical isolates of E. coli ST131 in France. This result is not particularly surprising, given that attempts to detect E. coli ST131 among ESBL-producing E. coli isolates from retail chicken meat have rarely been successful (35, 186, 190, 191, 209). In contrast, two STs commonly identified among ESBL-producing E. coli isolates from chicken meat, ST117 and ST354 (189, 209, 210), were identified among the dominant clonal groups for French CTX-M-producing non-ST131 clinical isolates (Table 7) (16). Interestingly, ST117 and ST354 (like ST131) were identified as clonal groups among ESBL-producing E. coli strains carried in the digestive tracts of healthy subjects living in the Paris area (165). These features together suggest that the source of the ESBL-producing E. coli clinical isolates is the E. coli ST131 human commensal organism that acquired ESBL or ESBL-producing E. coli strains (food-borne and health care-associated ESBL-producing isolates) transiently present in the human digestive tract. However, E. coli ST131 seems to be the more successful of the two types of ESBL-producing E. coli, as this clone was highly represented among clinical isolates of ESBL-producing E. coli (Table 5). Further studies are clearly required to determine the balance between the different non-ESBL- and ESBL-producing E. coli clonal groups in the human digestive tract, which is the major reservoir of E. coli strains causing human infections.
The last notable finding was the lack of association of travel abroad, which was investigated only in the French study, with CTX-M-producing E. coli ST131 isolates. In a previous study published by the French team that explored the factors associated with CTX-M-producing E. coli isolates carried out in the same population as was used for characterization of the factors associated with CTX-M-producing E. coli ST131 isolates, travel abroad was also found not to be associated with CTX-M-producing E. coli isolates. This team found that another foreign country-related factor was associated with CTX-M-producing E. coli isolates: being born outside Europe (211). However, this factor was not found to be associated with CTX-M-producing E. coli ST131 isolates. Finally, although Banerjee et al. identified travel to India, but not to other countries, as a factor associated with ESBL-producing E. coli isolates in a population-based study performed in Chicago (110), they did not include this variable in a subsequent study aiming to identify factors associated with E. coli ST131 isolates in the Olmsted County (MN) population (156).
Two important conclusions can be drawn from these studies. First, future investigations of the factors associated with clone ST131 should involve separate analyses of isolates with and without ESBL production. Second, it appears to be highly relevant to take into account factors both positively and negatively associated with ESBL-producing and non-ESBL-producing clinical isolates of E. coli ST131. Indeed, investigations of the factors positively associated with non-ST131 E. coli isolates (i.e., negatively associated with E. coli ST131 isolates) should improve the characterization of E. coli ST131 and provide insight into the reasons for its global success.
TREATMENT AND PREVENTION
Treatment
In this context of worldwide multidrug resistance in E. coli isolates and with only a few new antimicrobial molecules in the pipeline, empirical treatment guidelines, particularly for community-acquired urinary tract infections (UTI), require regular, locally updated surveys on the true prevalence of these isolates. In addition, antibiotic prescribers should be aware of the risk factors for infection with ESBL-producing E. coli isolates, including E. coli ST131, and should adapt their prescriptions accordingly (110, 156, 208, 211–213).
This review, focusing on E. coli ST131, shows that this clonal group predominates among multidrug-resistant E. coli isolates (i.e., isolates producing the ESBL CTX-M-15 and resistant to fluoroquinolones) and among fluoroquinolone-resistant isolates. As the ST131 clone has now spread worldwide and non-ST131 E. coli isolates are displaying marked resistance to fluoroquinolones (Tables 2 and 3), alternative treatments are now required to treat community-acquired urinary tract infections, particularly in elderly patients, the group most likely to carry E. coli ST131.
If the recommendations of the Infectious Diseases Society of America and the European Society of Microbiology and Infectious Diseases (214) for the treatment of acute uncomplicated cystitis in women (nitrofurantoin, trimethoprim-sulfamethoxazole, fosfomycin trometamol, or pivmecillinam) are followed, high rates of cure could be expected for cystitis due to ST131 for all molecules other than trimethoprim-sulfamethoxazole. Indeed, a significant or high proportion of ST131 isolates, depending on the country (Tables 2 and 3), have already been shown to be resistant to trimethoprim-sulfamethoxazole. The evolution of resistance to the other recommended molecules should be monitored continually. Oteo et al. showed that the increase in fosfomycin resistance observed in Spain from 2004 to 2008 in ESBL-producing urinary isolates was essentially due to the acquisition of fosfomycin resistance by E. coli ST131 isolates (215).
No study has yet prospectively or retrospectively collected data on the treatments used to cure different types of infections due to E. coli ST131isolates or provided information about the success or failure rates for a given treatment. One way of determining which molecules are commonly used to cure severe infections due to E. coli ST131 isolates would be to look at published case reports, such as those cited in this review. Most of these cases were infected with non-ESBL-producing E. coli ST131, so ESC treatment was possible. Carbapenems were used in cases in which the E. coli ST131 isolate produced CTX-M-15 (121–123). An increase in the number of infections with ESBL-producing Enterobacteriaceae has led to an increase in carbapenem consumption. Carbapenem-resistant Enterobacteriaceae, including those producing carbapenemases, have emerged in recent years. E. coli ST131 isolates harboring carbapenemases have already been reported (64, 81, 98–102). This finding highlights the need to find new treatments for infections with E. coli ST131 isolates. Pouillot et al. assessed the efficacy of bacteriophage therapy against the CTX-M-15-producing, fluoroquinolone-resistant E. coli ST131 strain S242 in experimental sepsis and meningitis models (216). In the sepsis model, they found that 100% of the rats survived when the bacteriophage was injected at 7 h postinfection, whereas only 50% survived when the bacteriophage was injected at 24 h postinfection. They identified a mutant of strain S242 that was phage resistant. In the meningitis model, they found that bacteriophage injection at 1 h postinfection resulted in sterile cerebrospinal fluid (CSF) samples 24 h later for all rats, with 100% of the rats still alive on day 5. The initiation of phage treatment at 7 h postinfection resulted in the survival of 100% of the infected rats on day 5, but 60% of the surviving rats were CSF positive for S242 on days 1 and 5, with a phage-resistant mutant present together with the initial strain on day 5. This therapeutic strategy is far from optimal at the moment but could provide an alternative approach to treatment to cure severe infections due to multidrug-resistant E. coli ST131. Totsika et al. explored another new treatment directed against the uropathogenic strain EC958 in mouse models of acute and chronic urinary tract infections (UTI). After showing that the E. coli ST131 strain EC958 could invade bladder epithelial cells, persist in the bladder, and induce chronic UTI due to FimH, the type 1 fimbrial adhesion (129), they used a low-molecular-weight compound to inhibit FimH (217). They showed that a single oral dose of this molecule significantly decreased bacterial colonization of the bladder and prevented acute UTI in a mouse model. However, the use of this molecule in chronically infected mice decreased the bacterial load in the bladder by only three orders of magnitude. The use of a FimH inhibitor seems to be an effective method of preventing UTI. It would be interesting to assess its efficacy in combination with antibiotics administered to inhibit or kill the bacteria released from urine. It would also be of interest to evaluate the impact of FimH inhibitors on gut colonization. Indeed, gut colonization is the first stage in E. coli ST131 pathogenesis, as in all infections with uropathogenic E. coli.
Prevention
Gut colonization is also the primary condition favoring the transmission of E. coli ST131 between hosts. The similar frequencies of E. coli ST131 isolations from patients with community- and hospital-acquired infections since the first decade of this century have led to suggestions that the dissemination of E. coli ST131 in the community has largely contributed to its global success (218, 219). Hilty et al. clearly showed that E. coli ST131 could be transmitted between hospitalized patients and other members of their households (91). In particular, they found that ST131 was more frequently transmitted to household than to hospital contacts. The development of measures to prevent E. coli ST131 transmission in the community thus presents a new, major challenge in the domain of public health. These measures will undoubtedly require improvements in basic hygiene practices within households.
CONCLUSION
After 50 years of the widespread use of huge amounts of antibiotics (an unprecedented occurrence in the world of bacteria) to treat human and animal infections and to enhance livestock growth, we are now seeing the global emergence of E. coli clone ST131, which is resistant to both fluoroquinolones and ESC due to the production of the ESBL CTX-M-15. The expansion of this clone has led to a worldwide increase in ESBL-producing E. coli isolates in both community and hospital settings since early 2000.
The success of this clone could therefore be attributed to its virulence and adaptation to humans. Indeed, it is rarely isolated from the environment, foodstuffs, or animals, with the exception of pets, probably due to their proximity to household members, which may therefore be involved in the transmission of E. coli ST131 within households. ST131 is the most competitive of the group B2 E. coli clones known to colonize the human digestive tract and cause human urinary tract infections, in terms of its ability to adhere to intestinal, bladder, and kidney epithelial cells. However, the bacterial traits underlying these virulence properties remain unknown because no study has yet established a correlation between virulence potential and VF gene carriage. One of the more remarkable features of E. coli ST131 is its low level of core genome recombination. This feature is also found in Shigella spp., a subset of E. coli with a niche restricted to the human digestive tract, and E. coli O157, a globally successful human pathogen with a niche restricted to the recto-anal junction in livestock. A more detailed understanding of the specific ecological niche of E. coli ST131 would be of considerable interest, particularly as E. coli ST131 has been identified as the predominant population of E. coli in the digestive tracts of healthy subjects. Despite its low core genome recombination capacity, clone ST131 remains the only group B2 E. coli clone to have been shown to evolve under antibiotic pressure by acquiring chromosomal fluoroquinolone resistance and plasmids encoding CTX-M enzymes. It remains unclear why B2 ST131 clones differ from other group B2 clones (e.g., ST73 and ST95) in their carriage of genes encoding CTX-M enzymes. The possibility of this clone being able to acquire plasmids encoding carbapenemases at some point in the future is, clearly, a matter of major concern.
ACKNOWLEDGMENTS
We thank Christel Boucher for technical assistance and Deirdre Edwards Lucey for her editing contribution.
Biographies

Marie-Hélène Nicolas-Chanoine, M.D., Ph.D., is Professor of Microbiology at the University Paris VII and head of the Microbiology Department at AP-HP Hospital Beaujon (Clichy). Her field of research has been focused primarily on β-lactamases, either chromosomally mediated (Enterobacter cloacae, Klebsiella oxytoca, and Raoultella spp.) or plasmid mediated (TEM, IRT, and especially the extended-spectrum β-lactamases), as well as on the molecular epidemiology of multidrug-resistant Enterobacteriacae. In this context, she provided the first description of E. coli ST131. She is currently the President of the Observatoire National de l'Epidemiologie de la Resistance Bactérienne aux Antibiotiques (ONERBA), which provides the European network with nationwide data on antibiotic resistance in France.

Xavier Bertrand, Pharm.D., Ph.D., is currently a Full Professor of Microbiology at the University of Franche Comté and Head of the Infection Control Unit of Besançon University Hospital (France). His main fields of research focus on the epidemiology of antimicrobial-resistant bacteria and their spread in the environment. He is also involved in projects aimed at reducing antibiotic consumption.

Jean-Yves Madec, D.V.M., Ph.D., is currently Research Director at the French Agency for Food, Environmental and Health Safety (Anses) in Lyon, France. He is the Head of the Antimicrobial Resistance and Virulence Unit, which is involved in the surveillance of antimicrobial resistance in animals in France. His research focuses on the molecular genetics and epidemiology of resistance in Gram-negative and Gram-positive bacteria of animal origin as well as issues regarding the animal-human transfer of antimicrobial resistance.
REFERENCES
- 1.Croxen MA, Finlay BB. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8:26–38. 10.1038/nrmicro2265 [DOI] [PubMed] [Google Scholar]
- 2.Russo TA, Johnson JR. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlook epidemic. Microbes Infect. 5:449–456. 10.1016/S1286-4579(03)00049-2 [DOI] [PubMed] [Google Scholar]
- 3.Gupta K, Scholes D, Stamm WE. 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736–738. 10.1001/jama.281.8.736 [DOI] [PubMed] [Google Scholar]
- 4.Jones RN, Kehrberg EN, Erwin ME, Anderson SC. 1994. Prevalence of important pathogens and antimicrobial activity of parenteral drugs at numerous medical centers in the United States, I. Study on the threat of emerging resistances: real or perceived? Fluoroquinolone Resistance Surveillance Group Diagn. Microbiol. Infect. Dis. 19:203–215 [DOI] [PubMed] [Google Scholar]
- 5.Turnidge J. 1995. Epidemiology of quinolone resistance. Eastern hemisphere. Drugs 49:43–47 [DOI] [PubMed] [Google Scholar]
- 6.Hummers-Pradier E, Koch M, Ohse AM, Heizmann WR, Kochen MM. 2005. Antibiotic resistance of urinary pathogens in female general practice patients. Scand. J. Infect. Dis. 37:256–261. 10.1080/00365540410021009 [DOI] [PubMed] [Google Scholar]
- 7.Coque TM, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 13(47):pii=19044 [PubMed] [Google Scholar]
- 8.Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Canica MM, Park Y-J, Lavigne J-P, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 61:273–281 doi: 10.1093/jac/dkm464 [DOI] [PubMed] [Google Scholar]
- 9.Coque TM, Novais A, Carattoli A, Poirel L, Pitout J, Peixe L, Baquero F, Canton R, Nordmann P. 2008. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum β-lactamase CTX-M-15. Emerg. Infect. Dis. 14:195–200. 10.3201/eid1402.070350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, Brian J, Connie C, Kuskowski MA, Mariana C. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 51:286–294. 10.1086/653932 [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg M. 2002. Escherichia coli: virulence mechanism of a versatile pathogen. Academic Press, New York, NY [Google Scholar]
- 12.Escobar-Paramo P, Grenet K, Le Menac'h A, Rode L, Salgado E, Amorin C, Gouriou S, Picard B, Rahimy MC, Andremont A, Denamur E, Raymond R. 2004. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 70:5698–5700. 10.1128/AEM.70.9.5698-5700.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escobar-Paramo P, Le Menac'h A, Le Gall T, Amorin C, Gouriou S, Picard B, Skurnik D, Denamur E. 2006. Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ. Microbiol. Rep. 8:1975–1984. 10.1111/j.1462-2920.2006.01077.x [DOI] [PubMed] [Google Scholar]
- 14.Le Gall T, Clermont O, Gouriou S, Picard B, Nassif X, Denamur E, Tenaillon O. 2007. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol. Biol. Evol. 24:2373–2384. 10.1093/molbev/msm172 [DOI] [PubMed] [Google Scholar]
- 15.Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, Boyd D, Mulvey MR, Nordmann P, Ruppe E, Sarthou JL, Frank T, Vimont S, Arlet G, Branger C, Woodford N, Denamur E. 2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 64:274–277. 10.1093/jac/dkp194 [DOI] [PubMed] [Google Scholar]
- 16.Brisse S, Diancourt L, Laouenan C, Vigan M, Caro V, Arlet G, Drieux L, Leflon-Guibout V, Mentre F, Jarlier V, Nicolas-Chanoine M-H. 2012. Phylogenetic distribution of CTX-M- and non-extended-spectrum β-lactamase-producing Escherichia coli isolates: group B2 isolates, except clone ST131, rarely produce CTX-M enzymes. J. Clin. Microbiol. 50:2974–2981. 10.1128/JCM.00919-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croxall G, Hale J, Weston V, Manning G, Cheetham P, Achtman M, McNally A. 2011. Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. J. Antimicrob. Chemother. 66:2501–2508. 10.1093/jac/dkr349 [DOI] [PubMed] [Google Scholar]
- 18.Dahbi G, Mora A, Lopez C, Alonso MP, Mamani R, Marzoa J, Coira A, Garicia-Garrote F, Pita J-M, Velasco D, Herrera A, Viso S, Blanco JE, Blanco M, Blanco J. 2013. Emergence of new variants of ST131 clonal group among extraintestinal pathogenic Escherichia coli producing extended-spectrum β-lactamases. Int. J. Antimicrob. Agents. 42:347–351. 10.1016/j.ijantimicag.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y, Yamamoto M, Nagao M, Hotta G, Matsushima A, Ito Y, Takakura S, Ichiyama S. 2012. Emergence and spread of B2-ST131-O25b, B2-ST131-O16 and D-ST405 clonal groups among extended-spectrum β-lactamase-producing Escherichia coli in Japan. J. Antimicrob. Chemother. 67:2612–2620. 10.1093/jac/dks278 [DOI] [PubMed] [Google Scholar]
- 20.Olesen B, Hansen DS, Nilsson F, Frimodt-Moller J, Leihof RF, Struve C, Scheutz F, Johnston B, Krogfelt KA, Johnson JR. 2013. Prevalence and characteristics of the epidemic multiresistant Escherichia coli ST131 clonal group among extended-spectrum β-lactamase-producing E. coli isolates in Copenhagen, Denmark. J. Clin. Microbiol. 51:1779–1785. 10.1128/JCM.00346-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platell JL, Cobbold RN, Johnson JR, Heisig A, Heisig P, Clabots C, Kuskowski MA, Trott DJ. 2011. Commonality among fluoroquinolone-resistant sequence type ST131 extraintestinal Escherichia coli isolates from humans and companion animals in Australia. Antimicrob. Agents Chemother. 55:3782–3787. 10.1128/AAC.00306-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudinha T, Johnson JR, Andrew S, Kong F, Anderson P, Gilbert GL. 2013. Escherichia coli sequence type 131 (ST131) as a prominent cause of antibiotic resistance among urinary Escherichia coli isolates from reproductive-age women. J. Clin. Microbiol. 51:3270–3276. 10.1128/JCM.01315-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habeeb MA, Haque A, Iversen A, Giske CG. 14 September 2013. Occurrence of virulence genes, 16S rRNA methylases, and plasmid-mediated quinolone resistance genes in CTX-M-producing Escherichia coli from Pakistan. Eur. J. Clin. Microbiol. Infect. Dis. 10.1007/s10096-013-1970-1 [DOI] [PubMed] [Google Scholar]
- 24.Blanc V, Leflon-Guibout V, Blanco J, Haenni M, Madec J-Y, Rafignon G, Bruno P, Mora A, Lopez C, Dahbi G, Dunais B, Anastasy M, Branger C, Moreau R, Pradier C, Nicolas-Chanoine MH. 8 January 2014. Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising O25b:H4 and O16:H5 ST131 strains. J. Antimicrob. Chemother. 10.1093/jac/dkt519 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Shibata N, Yamane K, Wachino J, Ito K, Arakawa Y. 2009. Change in the prevalence of extended-spectrum-β-lactamase-producing Escherichia coli in Japan by clonal spread. J. Antimicrob. Chemother. 63:72–79. 10.1093/jac/dkn463 [DOI] [PubMed] [Google Scholar]
- 26.Russo TA, Johnson JR. 2000. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181:1753–1754. 10.1086/315418 [DOI] [PubMed] [Google Scholar]
- 27.van der Bij AK, Peirano G, Pitondo-Silva A, Pitout JDD. 2012. The presence of genes encoding for different virulence factors in clonally related Escherichia coli that produce CTX-Ms. Diagn. Microbiol. Infect. Dis. 72:297–302. 10.1016/j.diagmicrobio.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Karisik E, Ellington MJ, Livermore DM, Woodford N. 2008. Virulence factors in Escherichia coli with CTX-M-15 and other extended-spectrum β-lactamases in the UK. J. Antimicrob. Chemother. 61:54–58. 10.1093/jac/dkm401 [DOI] [PubMed] [Google Scholar]
- 29.Mora A, Blanco M, Lopez C, Mamani R, Blanco JE, Alonso MP, Garcia-Garrote F, Dahbi G, Herrera A, Fernandez A, Fernandez B, Agulla A, Bou G, Blanco J. 2011. Emergence of clonal groups O1:HNM-D-ST59, O15:H1-D-ST393, O20:H34/HNM-D-ST354, O25b:H4-B2-ST131 and ONT:H21,42-B1-ST101 among CTX-M-14-producing Escherichia coli clinical isolates in Galicia, northwest Spain. Int. J. Antimicrob. Agents. 37:16–21. 10.1016/j.ijantimicag.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 30.Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS, II, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, Paterson D, Quinn J, Hanson ND, Johnston BD, Clabots C, Kuskowski MA. 2012. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob. Agents Chemother. 56:2364–2370. 10.1128/AAC.05824-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavigne JP, Vergunst AC, Goret L, Sotto A, Combescure C, Blanco J, O'Callaghan D, Nicolas-Chanoine M-H. 2012. Virulence potential and genomic mapping of the worldwide clone Escherichia coli ST131. PLoS One 7:e34294. 10.1371/journal.pone.0034294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco J, Mora A, Mamani R, Lopez C, Blanco M, Dahbi G, Herrera A, Marzoa J, Fernandez V, de la Cruz F, Martinez-Martinez L, Alonso MP, Nicolas-Chanoine M-H, Johnson JR, Johnston B, Lopez-Cerero L, Pascual A, Rodriguez-Bano J. 2013. Four main virotypes among extended-spectrum-β-lactamase-producing isolates of Escherichia coli O25b:H4-B2-ST131: bacterial, epidemiological, and clinical characteristics. J. Clin. Microbiol. 51:3358–3367. 10.1128/JCM.01555-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JR, Murray AC, Gajewski A, Sullivan M, Snippes P, Kuskowski MA. 2003. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 47:2161–2168. 10.1128/AAC.47.7.2161-2168.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora Gutierrez A, Dahbi G, Mamani R, Marzoa J, Dion S, Picard B, Blanco M, Alonso PM, Denamur E, Blanco J. 2014. Virulence patterns in a murine sepsis model of ST131 Escherichia coli clinical isolates belonging to serotypes O25b:H4 and O16:H5 are associated to specific virotypes. PLoS One 9:e87025. 10.1371/journal.pone.0087025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mora A, Herrera A, Mamani R, Lopez C, Alonso MP, Blanco JE, Blanco M, Dahbi G, Garcia-Garrote F, Pita JM, Coira A, Bernardez MI, Blanco J. 2010. Recent emergence of clonal group O25b:K1:H4-B2-ST131 ibeA strains among Escherichia coli poultry isolates, including CTX-M-9-producing strains, and comparison with clinical human isolates. Appl. Environ. Microbiol. 76:6991–6997. 10.1128/AEM.01112-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JR, Nicolas-Chanoine M-H, DebRoy C, Castanheira M, Robiscek A, Hansen G, Weissman S, Urban C, Platell J, Trott D, Zhanel G, Clabots C, Johnston BD, Kuskowski MA, The MASTER Investigators 2012. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967-2009. Emerg. Infect. Dis. 18:598–607. 10.3201/eid1804.111627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JR, O'Bryan TT, Delavari P, Kuskowski M, Stapleton A, Carlino U, Russo TA. 2001. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J. Infect. Dis. 183:1508–1517. 10.1086/320198 [DOI] [PubMed] [Google Scholar]
- 38.Diaz MA, Hernandez-Bello JR, Rodriguez-Bano J, Martinez-Martinez L, Calvo J, Blanco J, Pascual A. 2010. Diversity of Escherichia coli strains producing extended-spectrum beta-lactamases in Spain: second nationwide study. J. Clin. Microbiol. 48:2840–2845. 10.1128/JCM.02147-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tartof SY, Solberg OD, Riley LW. 2007. Genotypic analyses of uropathogenic Escherichia coli based on fimH single nucleotide polymorphisms (SNPs). J. Med. Microbiol. 56:1363–1369. 10.1099/jmm.0.47262-0 [DOI] [PubMed] [Google Scholar]
- 40.Weissman S, Chattopadhyay S, Aprikian P, Obata-Yasuoka M, Yarova-Yarovaya Y, Stapleton A, Ba-Theim W, Dydhuizen D, Johnson J, Sokurenko E. 2006. Clonal analysis reveals high rate of structural mutations in fimbrial adhesins of extraintestinal pathogenic Escherichia coli. Mol. Microbiol. 59:975–988. 10.1111/j.1365-2958.2005.04985.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman SJ, Johnson JR, Tchesnokova V, Billig M, Dykhuizen D, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Scholes D, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 78:1353–1360. 10.1128/AEM.06663-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams-Sapper S, Diep BA, Perdreau-Remington F, Riley LW. 2013. Clonal composition and community clustering of drug-susceptible and -resistant Escherichia coli isolates from bloodstream infections. Antimicrob. Agents Chemother. 57:490–497. 10.1128/AAC.01025-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine M-H, Debroy C, Robicsek A, Hansen G, Urban C, Platell J, Trott DJ, Zhanel G, Weissman SJ, Cookson BT, Fang FC, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko EV. 2013. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J. Infect. Dis. 207:919–928. 10.1093/infdis/jis933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tchesnokova V, Billig M, Chattopadhyay S, Linardopoulou E, Aprikian P, Roberts PL, Skrivankova V, Johnston B, Gileva A, Igusheva I, Toland A, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Kahl B, Price LB, Weissman SJ, Limaye A, Scholes D, Johnson JR, Sokurenko EV. 2013. Predictive diagnostics for infections based on the clonal association of antimicrobial resistance and clinical outcome. J. Clin. Microbiol. 51:2991–2999. 10.1128/JCM.00984-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colpan A, Johnston B, Porter S, Clabots C, Anway R, Thao L, Kuskowski MA, Tchesnokova V, Sokurenko EV, Johnson JR. 2013. Escherichia coli sequence type 131 (ST131) subclone H30 as an emergent multidrug-resistant pathogen among US veterans. Clin. Infect. Dis. 57:1256–1265. 10.1093/cid/cit503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banerjee R, Johnston B, Lohse C, Chattopadhyay S, Tchesnokova V, Sokurenko EV, Johnson JR. 2013. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob. Agents Chemother. 57:5912–5917. 10.1128/AAC.01065-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558. 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clermont O, Christenson JK, Denamur E, Gordon DM. 2013. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 5:58–65. 10.1111/1758-2229.12019 [DOI] [PubMed] [Google Scholar]
- 49.Clermont O, Johnson JR, Menard M, Denamur E. 2007. Determination of Escherichia coli O types by allele-specific polymerase chain reaction: application to the O types involved in human septicemia. Diagn. Microbiol. Infect. Dis. 57:214–223 [DOI] [PubMed] [Google Scholar]
- 50.Johnson JR, Menard E, Johnston B, Kuskowski MA, Nichol KA, Zhanel GG. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002 to 2004. Antimicrob. Agents Chemother. 53:2733–2739. 10.1128/AAC.00297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagnacci S, Gualco L, Debbia E, Schito GC, Marchese A. 2008. European emergence of ciprofloxacin-resistant Escherichia coli clonal groups O25:H4-ST131 and O15:K52:H1 causing community-acquired uncomplicated cystitis. J. Clin. Microbiol. 46:2605–2612. 10.1128/JCM.00640-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golding GR, Persaud N, Levett PN, McDonald RR, Irvine J, Nsungu M, Woods S, Khan M, Mataseje LF, Mulvey MR. 2012. Characterization of Escherichia coli urinary tract infection isolates in remote northern Saskatchewan communities: the Northern Antibiotic Resistance Partnership. Diagn. Microbiol. Infect. Dis. 74:242–247. 10.1016/j.diagmicrobio.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 53.Leflon-Guibout V, Blanco J, Amaqdouf K, Mora A, Guize L, Nicolas-Chanoine MH. 2008. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J. Clin. Microbiol. 46:3900–3905. 10.1128/JCM.00734-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Cerero L, Navarro MD, Bellido M, Martin-Pena A, Vinas L, Cisneros JM, Gomez-Langley SL, Sanchez-Monteseirin H, Morales I, Pascual A, Rodriguez-Bano J. 11 October 2013. Escherichia coli belonging to the worldwide emerging epidemic clonal group O25b/ST131: risk factors and clinical implications. J. Antimicrob. Chemother. 10.1093/jac/dkt405 [DOI] [PubMed] [Google Scholar]
- 55.Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, Livermore DM. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob. Agents Chemother. 53:4472–4482. 10.1128/AAC.00688-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naseer U, Haldorsen B, Tofteland S, Hegstad K, Scheutz F, Simonsen GS, Sundsfjord A. 2009. Molecular characterization of CTX-M-15-producing clinical isolates of Escherichia coli reveals the spread of multidrug-resistant ST131 (O25:H4) and ST964 (O102:H6) strains in Norway. APMIS 117:526–536. 10.1111/j.1600-0463.2009.02465.x [DOI] [PubMed] [Google Scholar]
- 57.Naseer U, Haldorsen B, Simonsen GS, Sundsfjord A. 2010. Sporadic occurrence of CMY-2-producing multidrug-resistant Escherichia coli of ST-complexes 38 and 448, and ST131 in Norway. Clin. Microbiol. Infect. 16:171–178. 10.1111/j.1469-0691.2009.02861.x [DOI] [PubMed] [Google Scholar]
- 58.Montesinos I, Rodriguez-Villalobos H, De Mendonca R, Bogaerts P, Deplano A, Glupczynski Y. 2010. Molecular characterization of plasmids encoding CTX-M-15 extended-spectrum β-lactamase associated with the ST131 Escherichia coli clone in Belgium. J. Antimicrob. Chemother. 65:1828–1830. 10.1093/jac/dkq208 [DOI] [PubMed] [Google Scholar]
- 59.Cullik A, Pfeifer Y, Prager R, von Baum H, Witte W. 2010. A novel IS26 structure surrounds blaCTX-M genes in different plasmids from German clinical Escherichia coli isolates. J. Med. Microbiol. 59:580–587. 10.1099/jmm.0.016188-0 [DOI] [PubMed] [Google Scholar]
- 60.Zong Z, Yu R, Wang X, Lu X. 2011. blaCTX-M-65 is carried by a Tn1722-like element on an IncN conjugative plasmid of ST131 Escherichia coli. J. Med. Microbiol. 60:435–441. 10.1099/jmm.0.026997-0 [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Bae IK, Jeong SH, Chang CL, Lee CH, Lee K. 2011. Characterization of IncF plasmids carrying the blaCTX-M-14 gene in clinical isolates of Escherichia coli from Korea. J. Antimicrob. Chemother. 66:1263–1268. 10.1093/jac/dkr106 [DOI] [PubMed] [Google Scholar]
- 62.Novais A, Viana D, Baquero F, Martinez-Botas J, Canton R, Coque TM. 2012. Contribution of IncFII and broad-host IncA/C and IncN plasmids to the local expansion and diversification of phylogroup B2 Escherichia coli ST131 clones carrying blaCTX-M-15 and qnrS1 genes. Antimicrob. Agents Chemother. 56:2763–2766. 10.1128/AAC.06001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Partridge SR, Ellem JA, Tetu SG, Zong Z, Paulsen IT, Iredell JR. 2011. Complete sequence of pJIE143, a pir-type plasmid carrying ISEcp1-blaCTX-M-15 from an Escherichia coli ST131 isolate. Antimicrob. Agents Chemother. 55:5933–5935. 10.1128/AAC.00639-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:34752. 10.1371/journal.pone.0034752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65:2518–2529. 10.1093/jac/dkq347 [DOI] [PubMed] [Google Scholar]
- 66.Johnson TJ, Nolan LK. 2009. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 73:750–774. 10.1128/MMBR.00015-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau SH, Kaufmann ME, Livermore DM, Woodford N, Willshaw GA, Cheasty T, Stamper K, Reddy S, Cheesbrough J, Bolton FJ, Fox AJ, Upton M. 2008. UK epidemic Escherichia coli strains A-E, with CTX-M-15 β-lactamase, all belong to the international O25:H4-ST131 clone. J. Antimicrob. Chemother. 62:1241–1244. 10.1093/jac/dkn380 [DOI] [PubMed] [Google Scholar]
- 68.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum β-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758–3764. 10.1128/AAC.48.10.3758-3764.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karisik E, Ellington MJ, Pike R, Warren RE, Livermore DM, Woodford N. 2006. Molecular characterization of plasmids encoding CTX-M-15 β-lactamases from Escherichia coli strains in the United Kingdom. J. Antimicrob. Chemother. 58:665–668. 10.1093/jac/dkl309 [DOI] [PubMed] [Google Scholar]
- 70.Poirel L, Lartigue M, Decousser J, Nordmann P. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 49:447–450. 10.1128/AAC.49.1.447-450.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao X, Cavaco LM, Lv Y, Li Y, Zheng B, Wang P, Hasman H, Liu Y, Aarestrup FM. 2011. Molecular characterization and antimicrobial susceptibility testing of Escherichia coli isolates from patients with urinary tract infections in 20 Chinese hospitals. J. Clin. Microbiol. 49:2496–2501. 10.1128/JCM.02503-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hrabak J, Empel J, Bergerova T, Fajfrlik K, Urbaskova P, Kern-Zdanowicz I, Hryniewicz W, Gniadkowski M. 2009. International clones of Klebsiella pneumoniae and Escherichia coli with extended-spectrum β-lactamases in a Czech hospital. J. Clin. Microbiol. 47:3353–3357. 10.1128/JCM.00901-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussain A, Ewers C, Nandanwar N, Guenther S, Jadhav S, Wieler LH, Ahmed N. 2012. Multiresistant uropathogenic Escherichia coli from a region in India where urinary tract infections are endemic: genotypic and phenotypic characteristics of sequence type 131 isolates of the CTX-M-15 extended-spectrum-β-lactamase-producing lineage. Antimicrob. Agents Chemother. 56:6358–6365. 10.1128/AAC.01099-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumura Y, Yamamoto M, Nagao M, Ito Y, Takakura S, Ichiyama S. 2013. Association of fluoroquinolone resistance, virulence genes, and IncF plasmids with extended-spectrum β-lactamase-producing Escherichia coli ST131 and ST405 clonal groups. Antimicrob. Agents Chemother. 57:4736–4742. 10.1128/AAC.00641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seiffert SN, Hilty M, Kronenberg A, Droz S, Perreten V, Endimiani A. 2013. Extended-spectrum cephalosporin-resistant Escherichia coli in community, specialized outpatient clinic and hospital settings in Switzerland. J. Antimicrob. Chemother. 68:2249–2254. 10.1093/jac/dkt208 [DOI] [PubMed] [Google Scholar]
- 76.Mnif B, Harhour H, Jdidi J, Mahjoubi F, Genel N, Arlet G, Hammami A. 2013. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in Tunisia and characterization of their virulence factors and plasmid addiction systems. BMC Microbiol. 13:147. 10.1186/1471-2180-13-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dimude JU, Amyes SG. 2013. Molecular diversity associated with the dissemination of CTX-M-15 β-lactamase gene in blood culture isolates of Escherichia coli from Edinburgh. Scand. J. Infect. Dis. 45:32–37. 10.3109/00365548.2012.708781 [DOI] [PubMed] [Google Scholar]
- 78.Dhanji H, Doumith M, Rooney PJ, O'Leary MC, Loughrey AC, Hope R, Woodford N, Livermore DM. 2011. Molecular epidemiology of fluoroquinolone-resistant ST131 Escherichia coli producing CTX-M extended-spectrum β-lactamases in nursing homes in Belfast, UK. J. Antimicrob. Chemother. 66:297–303. 10.1093/jac/dkq463 [DOI] [PubMed] [Google Scholar]
- 79.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11:355–362. 10.1016/S1473-3099(11)70059-7 [DOI] [PubMed] [Google Scholar]
- 80.Poirel L, Hombrouck-Alet C, Freneaux C, Bernabeu S, Nordmann P. 2010. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect. Dis. 10:832. 10.1016/S1473-3099(10)70279-6 [DOI] [PubMed] [Google Scholar]
- 81.Peirano G, Schreckenberger PC, Pitout JDD. 2011. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob. Agents Chemother. 55:2986–2988. 10.1128/AAC.01763-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zong Z. 2013. Complete sequence of pJIE186-2, a plasmid carrying multiple virulence factors from a sequence type 131 Escherichia coli O25 strain. Antimicrob. Agents Chemother. 57:597–600. 10.1128/AAC.01081-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodriguez-Villalobos H, Bogaerts P, Berhin C, Bauraing C, Deplano A, Montesinos I, de Mendonca R, Jans B, Glupczynski Y. 2011. Trends in production of extended-spectrum β-lactamases among Enterobacteriaceae of clinical interest: results of a nationwide survey in Belgian hospitals. J. Antimicrob. Chemother. 66:37–47. 10.1093/jac/dkq388 [DOI] [PubMed] [Google Scholar]
- 84.Peirano G, van der Bij AK, Gregson DB, Pitout JD. 2012. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum β-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J. Clin. Microbiol. 50:294–299. 10.1128/JCM.06025-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peirano G, Richardson D, Nigrin J, McGeer A, Loo V, Toye B, Alfa M, Pienaar C, Kibsey P, Pitout JD. 2010. High prevalence of ST131 producing CTX-M-15 and CTX-M-14 among extended-spectrum β-lactamase producing Escherichia coli across Canada. Antimicrob. Agents Chemother. 54:1327–1330. 10.1128/AAC.01338-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arpin C, Quentin C, Grobost F, Cambau E, Robert J, Dubois V, Coulange L, Andre C. 2009. Nationwide survey of extended-spectrum β-lactamase-producing Enterobacteriaceae in the French community setting. J. Antimicrob. Chemother. 63:1205–1214. 10.1093/jac/dkp108 [DOI] [PubMed] [Google Scholar]
- 87.Izdebski R, Baraniak A, Fiett J, Adler A, Kazma M, Salomon J, Lawrence C, Rossini A, Salvia A, Vidal Samso J, Fierro J, Paul M, Lerman Y, Malhotra-Kumar S, Lammens C, Goossens H, Hryniewicz W, Brun-Buisson C, Carmeli Y, Gniadkowski M. 2013. Clonal structure, extended-spectrum β-lactamases, and acquired AmpC-type cephalosporinases of Escherichia coli populations colonizing patients in rehabilitation centers in four countries. Antimicrob. Agents Chemother. 57:309–316. 10.1128/AAC.01656-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Voets GM, Platteel TN, Fluit AC, Scharringa J, Schapendonk CM, Stuart JC, Bonten MJ, Hall MA, National ESBL Surveillance Working Group 2012. Population distribution of β-lactamase conferring resistance to third-generation cephalosporins in human clinical Enterobacteriaceae in the Netherlands. PLoS One 7:52102. 10.1371/journal.pone.0052102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pomba C, Lopez-Cerero L, Bellido M, Serrano L, Belas A, Couto N, Cavaco-Silva P, Rodriguez-Bano J, Pascual A. 2014. Within-lineage variability of ST131 Escherichia coli isolates from humans and companion animals in the south of Europe. J. Antimicrob. Chemother. 69:271–273. 10.1093/jac/dkt343 [DOI] [PubMed] [Google Scholar]
- 90.Oteo J, Diestra K, Juan C, Bautista V, Novais A, Perez-Vazquez M, Moya B, Miro E, Coque TM, Oliver A, Canton R, Navarro F, Campos J. 2009. Extended-spectrum β-lactamase-producing Escherichia coli in Spain belong to a large variety of multilocus sequence typing types, including ST10 complex/A, ST23 complex/A and ST131/B2. Int. J. Antimicrob. Agents. 34:173–176. 10.1016/j.ijantimicag.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 91.Hilty M, Betsch BY, Bogli-Stuber K, Heiniger N, Stadler M, Kuffer M, Kronenberg A, Rohrer C, Aebi S, Endimiani A, Droz S, Muhlemann K. 2012. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin. Infect. Dis. 55:967–975. 10.1093/cid/cis581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rooney PJ, O'Leary MC, Loughrey AC, McCalmont M, Smyth B, Donaghy P, Badri M, Woodford N, Karisik E, Livermore DM. 2009. Nursing homes as a reservoir of extended-spectrum β-lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J. Antimicrob. Chemother. 64:635–641. 10.1093/jac/dkp220 [DOI] [PubMed] [Google Scholar]
- 93.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, II, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin. Infect. Dis. 56:641–648. 10.1093/cid/cis942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Courpon-Claudinon A, Lefort A, Panhard X, Clermont O, Dornic Q, Fantin B, Mentre F, Wolff M, Denamur E, Branger C. 2011. Bacteraemia caused by third-generation cephalosporin-resistant Escherichia coli in France: prevalence, molecular epidemiology and clinical features. Clin. Microbiol. Infect. 17:557–565. 10.1111/j.1469-0691.2010.03298.x [DOI] [PubMed] [Google Scholar]
- 95.Uchida Y, Mochimaru T, Morokuma Y, Kiyosuke M, Fujise M, Eto F, Eriguchi Y, Nagasaki Y, Shimono N, Kang D. 2010. Clonal spread in Eastern Asia of ciprofloxacin-resistant Escherichia coli serogroup O25 strains, and associated virulence factors. Int. J. Antimicrob. Agents. 35:444–450. 10.1016/j.ijantimicag.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 96.Coelho A, Mora A, Mamani R, Lopez C, Gonzalez-Lopez JJ, Larrosa MN, Quintero-Zarate JN, Dahbi G, Herrera A, Blanco JE, Blanco M, Alonso MP, Prats G, Blanco J. 2011. Spread of Escherichia coli O25b:H4-B2-ST131 producing CTX-M-15 and SHV-12 with high virulence gene content in Barcelona (Spain). J. Antimicrob. Chemother. 66:517–526. 10.1093/jac/dkq491 [DOI] [PubMed] [Google Scholar]
- 97.Kim YA, Qureshi ZA, Adams-Haduch JM, Park YS, Shutt KA, Doi Y. 2012. Features of infections due to Klebsiella pneumoniae carbapenemase-producing Escherichia coli: emergence of sequence type 131. Clin. Infect. Dis. 55:224-231. 10.1093/cid/cis387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naas T, Cuzon G, Gaillot O, Courcol R, Nordmann P. 2011. When carbapenem-hydrolyzing β-lactamase KPC meets Escherichia coli ST131 in France. Antimicrob. Agents Chemother. 55:4933–4934. 10.1128/AAC.00719-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morris D, Boyle F, Ludden C, Condon I, Hale J, O'Connell N, Power L, Boo TW, Dhanji H, Lavallee C, Woodford N, Cormican M. 2011. Production of KPC-2 carbapenemase by an Escherichia coli clinical isolate belonging to the international ST131 clone. Antimicrob. Agents Chemother. 55:4935–4936. 10.1128/AAC.05127-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cai JC, Zhang R, Hu YY, Zhou HW, Chen GX. 2014. Emergence of Escherichia coli sequence type 131 isolates producing KPC-2 carbapenemase in China. Antimicrob. Agents Chemother. 58:1146–1152. 10.1128/AAC.00912-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mantengoli E, Luzzaro F, Pecile P, Cecconi D, Cavallo A, Attala L, Bartoloni A, Rossolini GM. 2011. Escherichia coli ST131 producing extended-spectrum β-lactamases plus VIM-1 carbapenemase: further narrowing of treatment options. Clin. Infect. Dis. 52:690–691. 10.1093/cid/ciq194 [DOI] [PubMed] [Google Scholar]
- 102.Yan J-J, Tsai L-H, Wu J-J. 2012. Emergence of the IMP-8 metallo-β-lactamase in the Escherichia coli ST131 clone in Taiwan. Int. J. Antimicrob. Agents. 40:281–282. 10.1016/j.ijantimicag.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 103.Naseer U, Olsson-Liljequist BE, Woodford N, Dhanji H, Canton R, Sundsfjord A, Lindstedt BA. 2012. Multi-locus variable number of tandem repeat analysis for rapid and accurate typing of virulent multidrug resistant Escherichia coli clones. PLoS One 7:e41232. 10.1371/journal.pone.0041232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morris D, McGarry E, Cotter M, Passet V, Lynch M, Ludden C, Hannan MM, Brisse S, Cormican M. 2012. Detection of OXA-48 carbapenemase in the pandemic clone Escherichia coli O25b:H4-ST131 in the course of investigation of an outbreak of OXA-48-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 56:4030–4031. 10.1128/AAC.00638-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. 2012. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J. Antimicrob. Chemother. 67:1660–1665. 10.1093/jac/dks124 [DOI] [PubMed] [Google Scholar]
- 106.Birgy A, Mariani-Kurkdjian P, Bidet P, Doit C, Genel N, Courroux C, Arlet G, Bingen E. 2013. Characterization of extended-spectrum β-lactamase-producing Escherichia coli strains involved in maternal-fetal colonization: prevalence of E. coli ST131. J. Clin. Microbiol. 51:1727-1732. 10.1128/JCM.03255-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pitout JD, Gregson DB, Campbell L, Laupland KB. 2009. Molecular characteristics of extended-spectrum-β-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007: emergence of clone ST131 as a cause of community-acquired infections. Antimicrob. Agents Chemother. 53:2846–2851. 10.1128/AAC.00247-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones GL, Warren RE, Skidmore SJ, Davies VA, Gibreel T, Upton M. 2008. Prevalence and distribution of plasmid-mediated quinolone resistance genes in clinical isolates of Escherichia coli lacking extended-spectrum β-lactamases. J. Antimicrob. Chemother. 62:1245–1251. 10.1093/jac/dkn406 [DOI] [PubMed] [Google Scholar]
- 109.Gibreel TM, Dodgson AR, Cheesbrough J, Fox AJ, Bolton FJ, Upton M. 2012. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. J. Antimicrob. Chemother. 67:346–356. 10.1093/jac/dkr451 [DOI] [PubMed] [Google Scholar]
- 110.Banerjee R, Strahilevitz J, Johnson JR, Nagwekar PP, Schora DM, Shevrin I, Du H, Peterson LR, Robicsek A. 2013. Predictors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli infection in a midwestern community. Infect. Control. Hosp. Epidemiol. 34:947–953. 10.1086/671725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vredenburg J, Varela AR, Hasan B, Bertilsson S, Olsen B, Narciso-da-Rocha C, Bonnedahl J, Stedt J, Da Costa PM, Manaia CM. 27 August 2013. Quinolone-resistant Escherichia coli isolated from birds of prey in Portugal are genetically distinct from those isolated from water environments and gulls in Portugal, Spain and Sweden. Environ. Microbiol. Rep. 10.1111/1462-2920.12231 [DOI] [PubMed] [Google Scholar]
- 112.Novais A, Rodrigues C, Branquinho R, Antunes P, Grosso F, Boaventura L, Ribeiro G, Peixe L. 2012. Spread of an OmpK36-modified ST15 Klebsiella pneumoniae variant during an outbreak involving multiple carbapenem-resistant Enterobacteriaceae species and clones. Eur. J. Clin. Microbiol. Infect. Dis. 31:3057–3063. 10.1007/s10096-012-1665-z [DOI] [PubMed] [Google Scholar]
- 113.Pomba C, da Fonseca JD, Baptista BC, Correia JD, Martinez-Martinez L. 2009. Detection of the pandemic O25-ST131 human virulent Escherichia coli CTX-M-15-producing clone harboring the qnrB2 and aac(6′)-Ib-cr genes in a dog. Antimicrob. Agents Chemother. 53:327–328. 10.1128/AAC.00896-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leflon-Guibout V, Jurand C, Bonacorsi S, Espinasse F, Guelfi MC, Duportail F, Heym B, Bingen E, Nicolas-Chanoine M-H. 2004. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrob. Agents Chemother. 48:3736–3742. 10.1128/AAC.48.10.3736-3742.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemice of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4(6):e00377-13. 10.1128/mBio.00377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hirai I, Fukui N, Taguchi M, Yamauchi K, Nakamura T, Okano S, Yamamoto Y. 2013. Detection of chromosomal bla in Escherichia coli O25b-B2-ST131 isolates from the Kinki region of Japan. Int. J. Antimicrob. Agents. 42:500–506. 10.1016/j.ijantimicag.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 117.Andersen PS, Stegger M, Aziz M, Contente-Cuomo T, Gibbons HS, Keim P, Sokurenko EV, Johnson JR, Price LB. 2013. Complete genome sequence of the epidemic and highly virulent CTX-M-15-producing H30-Rx subclone of Escherichia coli ST131. Genome Announc. 1(6):e00988-13. 10.1128/genomeA.00988-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williamson DA, Freeman JT, Porter S, Roberts S, Wiles S, Paterson DL, Johnson JR. 2013. Clinical and molecular correlates of virulence in Escherichia coli causing bloodstream infection following transrectal ultrasound-guided (TRUS) prostate biopsy. J. Antimicrob. Chemother. 68:2898–2906. 10.1093/jac/dkt276 [DOI] [PubMed] [Google Scholar]
- 119.Assimacopoulos A, Johnston B, Clabots C, Johnson JR. 2012. Post-prostate biopsy infection with Escherichia coli ST131 leading to epididymo-orchitis and meningitis caused by Gram-negative bacilli. J. Clin. Microbiol. 50:4157–4159. 10.1128/JCM.02026-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson JR, Anderson JT, Clabots C, Johnston B, Cooperstock M. 2010. Within-household sharing of a fluoroquinolone-resistant Escherichia coli sequence type ST131 strain causing pediatric osteoarticular infection. Pediatr. Infect. Dis. J. 29:473–475. 10.1097/INF.0b013e3181c89bd7 [DOI] [PubMed] [Google Scholar]
- 121.Vigil KJ, Johnson JR, Johnston BD, Kontoyiannis DP, Mulanovich VE, Raad II, Dupont HL, Adachi JA. 2010. Escherichia coli pyomyositis: an emerging infectious disease among patients with hematologic malignancies. Clin. Infect. Dis. 50:374–380. 10.1086/649866 [DOI] [PubMed] [Google Scholar]
- 122.Ender PT, Gajanana D, Johnston B, Clabots C, Tamarkin FJ, Johnson JR. 2009. Transmission of an extended-spectrum-β-lactamase-producing Escherichia coli (sequence type ST131) strain between a father and daughter resulting in septic shock and emphysematous pyelonephritis. J. Clin. Microbiol. 47:3780–3782. 10.1128/JCM.01361-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Owens RCJ, Johnson JR, Stogsdill P, Yarmus L, Lolans K, Quinn J. 2011. Community transmission in the United States of a CTX-M-15-producing sequence type ST131 Escherichia coli strain resulting in death. J. Clin. Microbiol. 49:3406–3408. 10.1128/JCM.00993-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson JR, Miller S, Johnston B, Clabots C, Debroy C. 2009. Sharing of Escherichia coli sequence type ST131 and other multidrug-resistant and urovirulent E. coli strains among dogs and cats within a household. J. Clin. Microbiol. 47:3721–3725. 10.1128/JCM.01581-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J. Antimicrob. Chemother. 61:1024–1028. 10.1093/jac/dkn084 [DOI] [PubMed] [Google Scholar]
- 126.Gibreel TM, Dodgson AR, Cheesbrough J, Bolton FJ, Fox AJ, Upton M. 2012. High metabolic potential may contribute to the success of ST131 uropathogenic Escherichia coli. J. Clin. Microbiol. 50:3202–3207. 10.1128/JCM.01423-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vimont S, Boyd A, Bleibtreu A, Bens M, Goujon JM, Garry L, Clermont O, Denamur E, Arlet G, Vandewalle A. 2012. The CTX-M-15-producing Escherichia coli clone O25b: H4-ST131 has high intestine colonization and urinary tract infection abilities. PLoS One 7:e46547. 10.1371/journal.pone.0046547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Martinez-Medina M, Mora A, Blanco M, Lopez C, Alonso MP, Bonacorsi S, Nicolas-Chanoine MH, Darfeuille-Michaud A, Garcia-Gil J, Blanco J. 2009. Similarity and divergence among adherent-invasive Escherichia coli and extraintestinal pathogenic E. coli strains. J. Clin. Microbiol. 47:3968–3979. 10.1128/JCM.01484-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, Szubert M, Sidjabat HE, Paterson DL, Upton M, Schrembi MA. 2011. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One 6:e26578. 10.1371/journal.pone.0026578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peirano G, Mulvey GL, Armstrong GD, Pitout JD. 2013. Virulence potential and adherence properties of Escherichia coli that produce CTX-M and NDM beta-lactamases. J. Med. Microbiol. 62:525–530. 10.1099/jmm.0.048983-0 [DOI] [PubMed] [Google Scholar]
- 131.Johnson JR, Porter SB, Zhanel G, Kuskowski MA, Denamur E. 2012. Virulence of Escherichia coli clinical isolates in a murine sepsis model in relation to sequence type ST131 status, fluoroquinolone resistance, and virulence genotype. Infect. Immun. 80:1554–1562. 10.1128/IAI.06388-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Avasthi TS, Kumar N, Baddam R, Hussain A, Nandanwar N, Jadhav S, Ahmed N. 2011. Genome of multidrug-resistant uropathogenic Escherichia coli strain NA114 from India. J. Bacteriol. 193:4272–4273. 10.1128/JB.05413-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Clark G, Paszkiewicz K, Hale J, Weston V, Constantinidou C, Penn C, Achtman M, McNally A. 2012. Genomic analysis uncovers a phenotypically diverse but genetically homogeneous Escherichia coli ST131 clone circulating in unrelated urinary tract infections. J. Antimicrob. Chemother. 67:868–877. 10.1093/jac/dkr585 [DOI] [PubMed] [Google Scholar]
- 134.Jadhav S, Hussain A, Devi S, Kumar A, Parveen S, Gandham N, Wieler LH, Ewers C, Ahmed N. 2011. Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from a semi urban locality in India. PLoS One 6:e18063. 10.1371/journal.pone.0018063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Phan M-D, Peters KM, Sarkar S, Lukowski SW, Allsopp LP, Gomes Moriel D, Achard MES, Totsika M, Marshall VM, Upton M, Beatson SA, Schembri MA. 2013. The serum resistome of a globally disseminated multidrug resistant uropathogenic Escherichia coli clone. PLoS Genet. 9:e1003834. 10.1371/journal.pgen.1003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Croxall G, Weston V, Joseph S, Manning G, Cheetham P, McNally A. 2011. Increased human pathogenic potential of Escherichia coli from polymicrobial urinary tract infections in comparison to isolates from monomicrobial culture samples. J. Med. Microbiol. 60:102–109. 10.1099/jmm.0.020602-0 [DOI] [PubMed] [Google Scholar]
- 137.McNally A, Cheng L, Harris SR, Corander J. 2013. The evolutionary path to extraintestinal pathogenic, drug-resistant Escherichia coli is marked by drastic reduction in detectable recombination within the core genome. Genome Biol. Evol. 5:699–710. 10.1093/gbe/evt038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blanco M, Alonso MP, Nicolas-Chanoine MH, Dahbi G, Mora A, Blanco JE, Lopez C, Cortes P, Llagostera M, Leflon-Guibout V, Puentes B, Mamani R, Herrera A, Coira MA, Garcia-Garrote F, Pita JM, Blanco J. 2009. Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases in Lugo (Spain): dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 63:1135–1141. 10.1093/jac/dkp122 [DOI] [PubMed] [Google Scholar]
- 139.Xu L, Shabir S, Bodah T, McMurray C, Hardy K, Hawkey P, Nye K. 2011. Regional survey of CTX-M-type extended-spectrum β-lactamases among Enterobacteriaceae reveals marked heterogeneity in the distribution of the ST131 clone. J. Antimicrob. Chemother. 66:505–511. 10.1093/jac/dkq482 [DOI] [PubMed] [Google Scholar]
- 140.Simner PJ, Zhanel GG, Pitout J, Tailor F, McCracken M, Mulvey MR, Lagace-Wiens PR, Adam HJ, Hoban DJ. 2011. Prevalence and characterization of extended-spectrum β-lactamase- and AmpC β-lactamase-producing Escherichia coli: results of the CANWARD 2007-2009 study. Diagn. Microbiol. Infect. Dis. 69:326–334. 10.1016/j.diagmicrobio.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 141.Chandramohan L, Revell PA. 2012. Prevalence and molecular characterization of extended-spectrum β-lactamase-producing Enterobacteriaceae in a pediatric patient population. Antimicrob. Agents Chemother. 56:4765–4770. 10.1128/AAC.00666-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yano H, Uemura M, Endo S, Kanamori H, Inomata S, Kakuta R, Ichimura S, Ogawa M, Shimojima M, Ishibashi N, Aoyagi T, Hatta M, Gu Y, Yamada M, Tokuda K, Kunishima H, Kitagawa M, Hirakata Y, Kaku M. 2013. Molecular characteristics of extended-spectrum β-Lactamases in clinical isolates from Escherichia coli at a Japanese tertiary hospital. PLoS One 8:e64359. 10.1371/journal.pone.0064359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brolund A, Edquist PJ, Makitalo B, Olsson-Liljequist B, Soderblom T, Tegmark Wisell K, Giske CG. 3 October 2013. Epidemiology of ESBL-producing Escherichia coli in Sweden 2007-2011. Clin. Microbiol. Infect. 10.1111/1469-0691.12413 [DOI] [PubMed] [Google Scholar]
- 144.Reyna-Flores F, Barrios H, Garza-Ramos U, Sanchez-Perez A, Rojas-Moreno T, Uribe-Salas FJ, Fagundo-Sierra R, Silva-Sanchez J. 2013. Molecular epidemiology of Escherichia coli O25b-ST131 isolates causing community-acquired UTIs in Mexico. Diagn. Microbiol. Infect. Dis. 76:396–398. 10.1016/j.diagmicrobio.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 145.Peirano G, Hung King Sang J, Pitondo-Silva A, Laupland KB, Pitout JDD. 2012. Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae over a 10 year period in Calgary, Canada. J. Antimicrob. Chemother. 67:1114–1120. 10.1093/jac/dks026 [DOI] [PubMed] [Google Scholar]
- 146.Kang CI, Cha MK, Kim SH, Ko KS, Wi YM, Chung DR, Peck KR, Lee NY, Song J-H. 2013. Clinical and molecular epidemiology of community-onset bacteremia caused by extended-spectrum β-lactamase-producing Escherichia coli over a 6-year period. J. Korean Med. Sci. 28:998–1004. 10.3346/jkms.2013.28.7.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pitout JD, Campbell L, Church DL, Gregson DB, Laupland KB. 2009. Molecular characteristics of travel-related extended-spectrum-β-lactamase-producing Escherichia coli isolates from the Calgary Health Region. Antimicrob. Agents Chemother. 53:2539–2543. 10.1128/AAC.00061-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peirano G, Laupland KB, Gregson DB, Pitout JD. 2011. Colonization of returning travelers with CTX-M-producing Escherichia coli. J. Travel Med. 18:299–303. 10.1111/j.1708-8305.2011.00548.x [DOI] [PubMed] [Google Scholar]
- 149.Lee MY, Choi HJ, Choi JY, Song M, Song Y, Kim SW, Chang HH, Jung SI, Kim YS, Ki HK, Son JS, Kwon KT, Heo ST, Yeom JS, Shin SY, Chung DR, Peck KR, Song JH, Ko KS. 2010. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J. Infect. 60:146–153. 10.1016/j.jinf.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 150.Rogers BA, Kennedy KJ, Sidjabat HE, Jones M, Collignon P, Paterson DL. 2012. Prolonged carriage of resistant E. coli by returned travellers: clonality, risk factors and bacterial characteristics. Eur. J. Clin. Microbiol. Infect. Dis. 31:2413–2420. 10.1007/s10096-012-1584-z [DOI] [PubMed] [Google Scholar]
- 151.Tangden T, Cars O, Melhus A, Lowdin E. 2010. Foreign travel is a major risk factor for colonization with Escherichia coli producing extended-spectrum β-lactamases of the CTX-M type: a prospective study on Swedish volunteers. Antimicrob. Agents Chemother. 54:3564–3568. 10.1128/AAC.00220-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Freeman JT, McBride SJ, Hefferman H, Bathgate T, Pope C, Ellis-Pegler R. 2008. Community-onset genitourinary tract infection due to CTX-M-15-producing Escherichia coli travelers to the Indian Subcontinent in New Zeland. Clin. Infect. Dis. 47:689–692. 10.1086/590941 [DOI] [PubMed] [Google Scholar]
- 153.Laupland KB, Church DL, Vidakovich J, Mucenski M, Pitout JD. 2008. Community-onset extended-spectrum β-lactamase (ESBL) producing Escherichia coli: importance of international travel. J. Infect. 57:441–448. 10.1016/j.jinf.2008.09.034 [DOI] [PubMed] [Google Scholar]
- 154.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Pendyala S, Debroy C, Nowicki B, Rice J. 2010. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients. Antimicrob. Agents Chemother. 54:546–550. 10.1128/AAC.01089-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blanco J, Mora A, Mamani R, Lopez C, Blanco M, Dahbi G, Herrera A, Blanco JE, Alonso MP, Garcia-Garrote F, Chaves F, Orellana MA, Martinez-Martinez L, Calvo J, Prats G, Larrosa MN, Gonzalez-Lopez JJ, Lopez-Cerero L, Rodriguez-Bano J, Pascual A. 2011. National survey of Escherichia coli causing extraintestinal infections reveals the spread of drug-resistant clonal groups O25b:H4-B2-ST131, O15:H1-D-ST393 and CGA-D-ST69 with high virulence gene content in Spain. J. Antimicrob. Chemother. 66:2011–2021. 10.1093/jac/dkr235 [DOI] [PubMed] [Google Scholar]
- 156.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect. Control. Hosp. Epidemiol. 34:361–369. 10.1086/669865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bert F, Johnson JR, Ouattara B, Leflon-Guibout V, Johnston B, Marcon E, Valla D, Moreau R, Nicolas-Chanoine MH. 2010. Genetic diversity and virulence profiles of Escherichia coli isolates causing spontaneous bacterial peritonitis and bacteremia in patients with cirrhosis. J. Clin. Microbiol. 48:2709–2714. 10.1128/JCM.00516-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Horner C, Fawley W, Morris K, Parnell P, Denton M, Wilcox M. 2014. Escherichia coli bacteraemia: 2 years of prospective regional surveillance (2010-12). J. Antimicrob. Chemother. 69:91–100. 10.1093/jac/dkt333 [DOI] [PubMed] [Google Scholar]
- 159.Kudinha T, Johnson JR, Andrew SD, Kong F, Anderson P, Gilbert GL. 2013. Genotypic and phenotypic characterization of Escherichia coli isolates from children with urinary tract infection and from healthy carriers. Pediatr. Infect. Dis. J. 32:543–548. 10.1097/INF.0b013e31828ba3f1 [DOI] [PubMed] [Google Scholar]
- 160.Chmielarczyk A, Pobiega M, Wojkowska-Mach J, Romaniszyn D, Adamski P, Heczko PB, Bulanda M. 2013. Molecular epidemiology, plasmid analysis, virulence, and resistance of Escherichia coli isolated from neonatal intensive care units in Poland. Diagn. Microbiol. Infect. Dis. 76:542–545. 10.1016/j.diagmicrobio.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 161.Banerjee R, Robicsek A, Kuskowski MA, Porter S, Johnston BD, Sokurenko E, Tchesnokova V, Price LB, Johnson JR. 2013. Molecular epidemiology of Escherichia coli sequence type 131 and its H30 and H30-Rx subclones among extended-spectrum-β-lactamase-positive and -negative E. coli clinical isolates from the Chicago region, 2007 to 2010. Antimicrob. Agents Chemother. 57:6385–6388. 10.1128/AAC.01604-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chung H-C, Lai C-H, Lin J-N, Huang C-K, Liang S-H, Chen W-F, Shih Y-C, Lin H-H, Wang J-L. 2012. Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob. Agents Chemother. 56:618–622. 10.1128/AAC.05753-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Karfunkel D, Carmeli Y, Chmelnitsky I, Kotlovsky T, Navon-Venezia S. 2013. The emergence and dissemination of CTX-M-producing Escherichia coli sequence type 131 causing community-onset bacteremia in Israel. Eur. J. Clin. Microbiol. Infect. Dis. 32:513–521. 10.1007/s10096-012-1765-9 [DOI] [PubMed] [Google Scholar]
- 164.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. 2011. High prevalence of CTX-M β-lactamases in feacal Escherichia coli strains from healthy humans in Fuzhou, China. Scand. J. Infect. Dis. 43:170–174. 10.3109/00365548.2010.538856 [DOI] [PubMed] [Google Scholar]
- 165.Nicolas-Chanoine M-H, Gruson C, Bialek-Davenet S, Bertrand X, Thomas-Jean F, Bert F, Moyat M, Meiller E, Marcon E, Danchin N, Noussair L, Moreau R, Leflon-Guibout V. 2013. 10-Fold increase (2006-11) in the rate of healthy subjects with extended-spectrum β-lactamase-producing Escherichia coli faecal carriage in a Parisian check-up centre. J. Antimicrob. Chemother. 68:562–568. 10.1093/jac/dks429 [DOI] [PubMed] [Google Scholar]
- 166.Arvand M, Moser V, Pfeifer Y. 2013. Prevalence of extended-spectrum-β-lactamase-producing Escherichia coli and spread of the epidemic clonal lineage ST131 in nursing homes in Hesse, Germany. J. Antimicrob. Chemother. 68:2686–2688. 10.1093/jac/dkt226 [DOI] [PubMed] [Google Scholar]
- 167.Nuesch-Inderbinen MT, Abgottspon H, Zurfluh K, Nuesch HJ, Stephan R, Hachler H. 2013. Cross-sectional study on fecal carriage of Enterobacteriaceae with resistance to extended-spectrum cephalosporins in primary care patients. Microb. Drug Resist. 19:362–369. 10.1089/mdr.2013.0013 [DOI] [PubMed] [Google Scholar]
- 168.Severin JA, Lestari ES, Kloezen W, Lemmens-den Toom N, Mertaniasih NM, Kuntaman K, Purwanta M, Offra Duerink D, Hadi U, van Belkum A, Verbrugh HA, Goessens WH. 2012. Faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae among humans in Java, Indonesia, in 2001-2002. Trop. Med. Int. Health 17:455–461. 10.1111/j.1365-3156.2011.02949.x [DOI] [PubMed] [Google Scholar]
- 169.Johnson JR, Clabots C. 2006. Sharing of virulent Escherichia coli clones among household members of a woman with acute cystitis. Clin. Infect. Dis. 43:101–108. 10.1086/508541 [DOI] [PubMed] [Google Scholar]
- 170.Ewers C, Grobbel M, Stamm I, Kopp PA, Diehl I, Semmler T, Fruth A, Beutlich J, Guerra B, Wieler LH, Guenther S. 2010. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-β-lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 65:651–660. 10.1093/jac/dkq004 [DOI] [PubMed] [Google Scholar]
- 171.Harada K, Nakai Y, Kataoka Y. 2012. Mechanisms of resistance to cephalosporin and emergence of O25b-ST131 clone harboring CTX-M-27 β-lactamase in extraintestinal pathogenic Escherichia coli from dogs and cats in Japan. Microbiol. Immunol. 56:480–485. 10.1111/j.1348-0421.2012.00463.x [DOI] [PubMed] [Google Scholar]
- 172.Platell JL, Cobbold RN, Johnson JR, Trott DJ. 2010. Clonal group distribution of fluoroquinolone-resistant Escherichia coli among humans and companion animals in Australia. J. Antimicrob. Chemother. 65:1936–1938. 10.1093/jac/dkq236 [DOI] [PubMed] [Google Scholar]
- 173.Guo S, Brouwers HJM, Cobbold RN, Platell JL, Chapman TA, Barrs VR, Johnson JR, Trott DJ. 2013. Fluoroquinolone-resistant extraintestinal pathogenic Escherichia coli, including O25b-ST131, isolated from faeces of hospitalized dogs in an Australian veterinary referral centre. J. Antimicrob. Chemother. 68:1025–1031. 10.1093/jac/dks515 [DOI] [PubMed] [Google Scholar]
- 174.Huber H, Zweifel C, Wittenbrink MM, Stephan R. 2013. ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 162:992–996. 10.1016/j.vetmic.2012.10.029 [DOI] [PubMed] [Google Scholar]
- 175.Tamang MD, Nam HM, Jang GC, Kim SR, Chae MH, Jung SC, Byun JW, Park YH, Lim SK. 2012. Molecular characterization of extended-spectrum-β-lactamase-producing and plasmid-mediated AmpC β-lactamase-producing Escherichia coli isolated from stray dogs in South Korea. Antimicrob. Agents Chemother. 56:2705–2712. 10.1128/AAC.05598-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dierikx CM, van Duijkeren E, Schoormans AH, van Essen-Zandbergen A, Veldman K, Kant A, Huijsdens XW, van der Zwaluw K, Wagenaar JA, Mevius DJ. 2012. Occurrence and characteristics of extended-spectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 67:1368–1374. 10.1093/jac/dks049 [DOI] [PubMed] [Google Scholar]
- 177.Albrechtova K, Dolejska M, Cizek A, Tausova D, Klimes J, Bebora L, Literak I. 2012. Dogs of nomadic pastoralists in northern Kenya are reservoirs of plasmid-mediated cephalosporin- and quinolone-resistant Escherichia coli, including pandemic clone B2-O25-ST131. Antimicrob. Agents Chemother. 56:4013–4017. 10.1128/AAC.05859-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schink AK, Kadlec K, Kaspar H, Mankertz J, Schwarz S. 2013. Analysis of extended-spectrum-β-lactamase-producing Escherichia coli isolates collected in the GERM-Vet. monitoring programme. J. Antimicrob. Chemother. 68:1741–1749. 10.1093/jac/dkt123 [DOI] [PubMed] [Google Scholar]
- 179.Wu G, Day MJ, Mafura MT, Nunez-Garcia J, Fenner JJ, Sharma M, van Essen-Zandbergen A, Rodriguez I, Dierikx C, Kadlec K, Schink AK, Wain J, Helmuth R, Guerra B, Schwarz S, Threlfall J, Woodward MJ, Woodford N, Coldham N, Mevius D. 2013. Comparative analysis of ESBL-positive Escherichia coli isolates from animals and humans from the UK, the Netherlands and Germany. PLoS One 8:75392. 10.1371/journal.pone.0075392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Randall LP, Clouting C, Horton RA, Coldham NG, Wu G, Clifton-Hadley FA, Davies RH, Teale CJ. 2011. Prevalence of Escherichia coli carrying extended-spectrum β-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J. Antimicrob. Chemother. 66:86–95. 10.1093/jac/dkq396 [DOI] [PubMed] [Google Scholar]
- 181.Cortes P, Blanc V, Mora A, Dahbi G, Blanco JE, Blanco M, Lopez C, Andreu A, Navarro F, Alonso MP, Bou G, Blanco J, Llagostera M. 2010. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol. 76:2799–2805. 10.1128/AEM.02421-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Giuffre M, Cipolla D, Bonura C, Geraci DM, Aleo A, Di Noto S, Nociforo F, Corsello G, Mammina C. 2013. Outbreak of colonizations by extended-spectrum b-lactamase-producing Escherichia coli sequence type 131 in a neonatal intensive care unit, Italy. Antimicrob. Resist. Infect. Control. 2:8. 10.1186/2047-2994-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dahmen S, Metayer V, Gay E, Madec JY, Haenni M. 2013. Characterization of extended-spectrum β-lactamase (ESBL)-carrying plasmids and clones of Enterobacteriaceae causing cattle mastitis in France. Vet. Microbiol. 162:793–799. 10.1016/j.vetmic.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 184.Hordijk J, Wagenaar JA, van de Giessen A, Dierikx C, van Essen-Zandbergen A, Veldman K, Kant A, Mevius DJ. 2013. Increasing prevalence and diversity of ESBL/AmpC-type β-lactamase genes in Escherichia coli isolated from veal calves from 1997 to 2010. J. Antimicrob. Chemother. 68:1970–1973. 10.1093/jac/dkt132 [DOI] [PubMed] [Google Scholar]
- 185.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJ, Mevius DJ. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 17:873–880. 10.1111/j.1469-0691.2011.03497.x [DOI] [PubMed] [Google Scholar]
- 186.Overdevest I, Willemsen I, Rijnsburger M, Eustace A, Xu L, Hawkey P, Heck M, Savelkoul P, Vandenbroucke-Grauls C, Van der Zwaluw K, Huijsdens X, Kluytmans J. 2011. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg. Infect. Dis. 17:1216–1222. 10.3201/eid1707.110209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Madec J-Y, Poirel L, Saras E, Gourguechon A, Girlich D, Nordmann P, Haenni M. 2012. Non-ST131 Escherichia coli from cattle harbouring human-like blaCTX-M-15-carrying plasmids. J. Antimicrob. Chemother. 67:578–581. 10.1093/jac/dkr542 [DOI] [PubMed] [Google Scholar]
- 188.Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, Chen X, Lv L, Zhuo C, Chen Z, Liu J-H. 2012. Prevalence and characterisation of CTX-M β-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int. J. Antimicrob. Agents. 39:305–310. 10.1016/j.ijantimicag.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 189.Vincent C, Boerlin P, Daignault D, Dozois CM, Dutil L, Galanakis C, Reid-Smith RJ, Tellier PP, Tellis PA, Ziebell K, Manges AR. 2010. Food reservoir for Escherichia coli causing urinary tract infections. Emerg. Infect. Dis. 16:88–95. 10.3201/eid1601.091118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dhanji H, Murphy NM, Doumith M, Durmus S, Lee SS, Hope R, Woodford N, Livermore DM. 2010. Cephalosporin resistance mechanisms in Escherichia coli isolated from raw chicken imported into the UK. J. Antimicrob. Chemother. 65:2534–2537. 10.1093/jac/dkq376 [DOI] [PubMed] [Google Scholar]
- 191.Egea P, Lopez-Cerero L, Torres E, Gomez-Sanchez Mdel C, Serrano L, Navarro Sanchez-Ortiz MD, Rodriguez-Bano J, Pascual A. 2012. Increased raw poultry meat colonization by extended spectrum β-lactamase-producing Escherichia coli in the South of Spain. Int. J. Food Microbiol. 159:69–73. 10.1016/j.ijfoodmicro.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 192.Hernandez J, Bonnedahl J, Eliasson I, Wallensten A, Comstedt P, Johansson A, Granholm S, Melhus A, Olsen B, Drobni M. 2010. Globally disseminated human pathogenic Escherichia coli of O25b-ST131 clone, harbouring blaCTX-M-15, found in Glaucous-winged gull at remote Commander Islands, Russia. Environ. Microbiol. Rep. 2:329–332. 10.1111/j.1758-2229.2010.00142.x [DOI] [PubMed] [Google Scholar]
- 193.Simoes RR, Poirel L, Da Costa PM, Nordmann P. 2010. Seagulls and beaches as reservoirs for multidrug-resistant Escherichia coli. Emerg. Infect. Dis. 16:110–112. 10.3201/eid1601.090896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tausova D, Dolejska M, Cizek A, Hanusova L, Hrusakova J, Svoboda O, Camlik G, Literak I. 2012. Escherichia coli with extended-spectrum β-lactamase and plasmid-mediated quinolone resistance genes in great cormorants and mallards in Central Europe. J. Antimicrob. Chemother. 67:1103–1107. 10.1093/jac/dks017 [DOI] [PubMed] [Google Scholar]
- 195.Guenther S, Grobbel M, Beutlich J, Guerra B, Ulrich RG, Wieler LH, Ewers C. 2010. Detection of pandemic B2-O25-ST131 Escherichia coli harbouring the CTX-M-9 extended-spectrum β-lactamase type in a feral urban brown rat (Rattus norvegicus). J. Antimicrob. Chemother. 65:582–584. 10.1093/jac/dkp496 [DOI] [PubMed] [Google Scholar]
- 196.Dahmen S, Haenni M, Châtre P, Madec J-Y. 2013. Characterization of blaCTX-M IncFII plasmids and clones of Escherichia coli from pets in France. J. Antimicrob. Chemother. 68:2797–2801. 10.1093/jac/dkt291 [DOI] [PubMed] [Google Scholar]
- 197.Giufre M, Graziani C, Accogli M, Luzzi I, Busani L, Cerquetti M. 2012. Escherichia coli of human and avian origin: detection of clonal groups associated with fluoroquinolone and multidrug resistance in Italy. J. Antimicrob. Chemother. 67:860–867. 10.1093/jac/dkr565 [DOI] [PubMed] [Google Scholar]
- 198.Trobos M, Christensen H, Sunde M, Nordentoft S, Agerso Y, Simonsen GS, Hammerum AM, Olsen JE. 2009. Characterization of sulphonamide-resistant Escherichia coli using comparison of sul2 gene sequences and multilocus sequence typing. Microbiology 155:831–836. 10.1099/mic.0.024190-0 [DOI] [PubMed] [Google Scholar]
- 199.Guenther S, Ewers C, Wieler LH. 2011. Extended-spectrum β-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Front. Microbiol. 2:246. 10.3389/fmicb.2011.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Platell JL, Johnson JR, Cobbold RN, Trott DJ. 2011. Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet. Microbiol. 153:99–108. 10.1016/j.vetmic.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 201.Jakobsen L, Sandvang D, Hansen LH, Bagger-Skjot L, Westh H, Jorgensen C, Hansen DS, Pedersen BM, Monnet DL, Frimodt-Moller N, Sorensen SJ, Hammerum AM. 2008. Characterisation, dissemination and persistence of gentamicin resistant Escherichia coli from a Danish university hospital to the waste water environment. Environ. Int. 34:108–115. 10.1016/j.envint.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 202.Galvin S, Boyle F, Hickey P, Vellinga A, Morris D, Cormican M. 2010. Enumeration and characterization of antimicrobial-resistant Escherichia coli bacteria in effluent from municipal, hospital, and secondary treatment facility sources. Appl. Environ. Microbiol. 76:4772–4779. 10.1128/AEM.02898-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mesa RJ, Blanc V, Blanch AR, Cortes P, Gonzalez JJ, Lavilla S, Miro E, Muniesa M, Saco M, Tortola MT, Mirelis B, Coll P, Llagostera M, Prats G, Navarro F. 2006. Extended-spectrum β-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J. Antimicrob. Chemother. 58:211–215. 10.1093/jac/dkl211 [DOI] [PubMed] [Google Scholar]
- 204.Zarfel G, Galler H, Feierl G, Haas D, Kittinger C, Leitner E, Grisold AJ, Mascher F, Posch J, Pertschy B, Marth E, Reinthaler FF. 2013. Comparison of extended-spectrum-β-lactamase (ESBL) carrying Escherichia coli from sewage sludge and human urinary tract infection. Environ. Pollut. 173:192–199. 10.1016/j.envpol.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 205.Colomer-Lluch M, Mora A, Lopez C, Mamani R, Dahbi G, Marzoa J, Herrera A, Viso S, Blanco JE, Blanco M, Alonso MP, Jofre J, Muniesa M, Blanco J. 2013. Detection of quinolone-resistant Escherichia coli isolates belonging to clonal groups O25b:H4-B2-ST131 and O25b:H4-D-ST69 in raw sewage and river water in Barcelona, Spain. J. Antimicrob. Chemother. 68:758–765. 10.1093/jac/dks477 [DOI] [PubMed] [Google Scholar]
- 206.Dolejska M, Frolkova P, Florek M, Jamborova I, Purgertova M, Kutilova I, Guenther ACS, Literak I. 2011. CTX-M-15-producing Escherichia coli clone B2-O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J. Antimicrob. Chemother. 66:2784–2786. 10.1093/jac/dkr363 [DOI] [PubMed] [Google Scholar]
- 207.Vignaroli C, Luna GM, Pasquaroli S, Di Cesare A, Petruzzella R, Paroncini P, Biavasco F. 2013. Epidemic Escherichia coli ST131 and Enterococcus faecium ST17 in coastal marine sediments from an Italian beach. Environ. Sci. Technol. 47:13772–13780. 10.1021/es4019139 [DOI] [PubMed] [Google Scholar]
- 208.Nicolas-Chanoine M-H, Robert J, Vigan M, Laouenan C, Brisse S, Mentre F, Jarlier V. 2013. Different factors associated with CTX-M-producing ST131 and non-ST131 Escherichia coli clinical isolates. PLoS One 8:e72191. 10.1371/journal.pone.0072191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kluytmans JA, Overdevest ITM, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CM, Savelkoul PHM, Johnston BD, Gordon D, Johnson JR. 2013. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 56:478–487. 10.1093/cid/cis929 [DOI] [PubMed] [Google Scholar]
- 210.Cohen Stuart J, van den Munckhof T, Voets G, Scharringa J, Fluit A, Hall ML. 2012. Comparison of ESBL contamination in organic and conventional retail chicken meat. Int. J. Food Microbiol. 154:212–214. 10.1016/j.ijfoodmicro.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 211.Nicolas-Chanoine M-H, Jarlier V, Robert J, Arlet G, Drieux L, Leflon-Guibout V, Laouénan C, Larroque B, Caro V, Mentré F, Study Group Coli β 2012. Patient's origin and lifestyle associated with CTX-M-producing Escherichia coli: a case-control-control study. PLoS One 7:e30498. 10.1371/journal.pone.0030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Cisneros JM, Pena C, Almela M, Almirante B, Grill F, Colomina J, Molinos S, Oliver A, Fernandez-Mazarrasa C, Navarro G, Coloma A, Lopez-Cerero L, Pascual A. 2010. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli. J. Clin. Microbiol. 48:1726–1731. 10.1128/JCM.02353-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Ruiz M, Pena C, Almela M, Almirante B, Grill F, Colomina J, Gimenez M, Oliver A, Horcajada JP, Navarro G, Coloma A, Pascual A. 2010. Community-onset bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: risk factors and prognosis. Clin. Infect. Dis. 50:40–48. 10.1086/649537 [DOI] [PubMed] [Google Scholar]
- 214.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:103–120. 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 215.Oteo J, Orden B, Bautista V, Cuevas O, Arroyo M, Martinez-Ruiz R, Perez-Vazquez M, Alcaraz M, Garcia-Cobos S, Campos J. 2009. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. J. Antimicrob. Chemother. 64:712–717. 10.1093/jac/dkp288 [DOI] [PubMed] [Google Scholar]
- 216.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, Bingen E, Bonacorsi S. 2012. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b:H4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob. Agents Chemother. 56:3568–3575. 10.1128/AAC.06330-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Totsika M, Kostakioti M, Hannan TJ, Upton M, Beatson SA, Janetka JW, Hultgren SJ, Schembri MA. 2013. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J. Infect. Dis. 208:921–928. 10.1093/infdis/jit245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pitout JD. 2010. Infections with ESBL-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs 70:313–333. 10.2165/11533040-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 219.Peirano G, Pitout JD. 2010. Molecular epidemiology of Escherichia coli producing CTX-M β-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents. 35:316–321. 10.1016/j.ijantimicag.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 220.Literacka E, Bedenic B, Baraniak A, Fiett J, Tonkic M, Jajic-Bencic I, Gniadkowski M. 2009. blaCTX-M genes in Escherichia coli strains from Croatian hospitals are located in new (blaCTX-M-3a) and widely spread (blaCTX-M-3a and blaCTX-M-15) genetic structures. Antimicrob. Agents Chemother. 53:1630–1635. 10.1128/AAC.01431-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nielsen JB, Albayati A, Jorgensen RL, Hansen KH, Lundgren B, Schonning K. 2013. An abbreviated MLVA identifies Escherichia coli ST131 as the major extended-spectrum β-lactamase-producing lineage in the Copenhagen area. Eur. J. Clin. Microbiol. Infect. Dis. 32:431–436. 10.1007/s10096-012-1764-x [DOI] [PubMed] [Google Scholar]
- 222.Cerquetti M, Giufre M, Garcia-Fernandez A, Accogli M, Fortini D, Luzzi I, Carattoli A. 2010. Ciprofloxacin-resistant, CTX-M-15-producing Escherichia coli ST131 clone in extraintestinal infections in Italy. Clin. Microbiol. Infect. 16:1555–1558. 10.1111/j.1469-0691.2010.03162.x [DOI] [PubMed] [Google Scholar]
- 223.van der Bij AK, Peirano G, Goessens WH, van der Vorm ER, van Westreenen M, Pitout JD. 2011. Clinical and molecular characteristics of extended-spectrum-β-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam area, Netherlands. Antimicrob. Agents Chemother. 55:3576–3578. 10.1128/AAC.00074-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Woksepp H, Jernberg C, Tarnberg M, Ryberg A, Brolund A, Nordvall M, Olsson-Liljequist B, Wisell KT, Monstein H-J, Nilsson L, Schon T. 2011. High-resolution melting-curve analysis of ligation-mediated real-time PCR for rapid evaluation of an epidemiological outbreak of extended-spectrum-β-lactamase-producing Escherichia coli. J. Clin. Microbiol. 49:4032–4039. 10.1128/JCM.01042-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zong Z, Yu R. 2011. blaCTX-M-carrying Escherichia coli of the O25b ST131 clonal group have emerged in China. Diagn. Microbiol. Infect. Dis. 69:228–231. 10.1016/j.diagmicrobio.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 226.Ho P-L, Lo WU, Lai EL, Chow K-H, Yam W-C. 2012. Escherichia coli O25b-ST131 is an important cause of antimicrobial-resistant infections in women with uncomplicated cystitis. J. Antimicrob. Chemother. 67:2534–2535. 10.1093/jac/dks248 [DOI] [PubMed] [Google Scholar]
- 227.Roy S, Krishnan R, Mukherjee S, Schneiders T, Niyogi SK, Basu S. 2013. Prevalence of ST131 virulence-associated strains among CTX-M-producing Escherichia coli in the gut of hospitalized neonates in India. Diagn. Microbiol. Infect. Dis. 77:158–159. 10.1016/j.diagmicrobio.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 228.Adler A, Gniadkowski M, Baraniak A, Izdebski R, Fiett J, Hryniewicz W, Malhotra-Kumar S, Goossens H, Lammens C, Lerman Y, Kazma M, Kotlovsky T, Carmeli Y, groups MWaWs 2012. Transmission dynamics of ESBL-producing Escherichia coli clones in rehabilitation wards at a tertiary care centre. Clin. Microbiol. Infect. 18:E497–E505 [DOI] [PubMed] [Google Scholar]
- 229.Yokota S-i, Sato T, Okubo T, Ohkoshi Y, Okabayashi T, Kuwahara O, Tamura Y, Fujii N. 2012. Prevalence of fluoroquinolone-resistant Escherichia coli O25:H4-ST131 (CTX-M-15-nonproducing) strains isolated in Japan. Chemotherapy 58:52–59. 10.1159/000336129 [DOI] [PubMed] [Google Scholar]
- 230.Yumuk Z, Afacan G, Nicolas-Chanoine M-H, Sotto A, Lavigne JP. 2008. Turkey: a further country concerned by community acquired Escherichia coli clone O25-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 62:284–288. 10.1093/jac/dkn181 [DOI] [PubMed] [Google Scholar]
- 231.Szijarto V, Pal T, Nagy G, Nagy E, Ghazawi A, al-Haj M, El Kurdi S, Sonnevend A. 2012. The rapidly emerging ESBL-producing Escherichia coli O25-ST131 clone carries LPS core synthesis genes of the K-12 type. FEMS Microbiol. Lett. 332:131–136. 10.1111/j.1574-6968.2012.02585.x [DOI] [PubMed] [Google Scholar]
- 232.Tiruvury H, Johnson JR, Mariano N, Grenner L, Colon-Urban R, Erritouni M, Wehbeh W, Segal-Maurer S, Rahal JJ, Johnston B, Urban C. 2012. Identification of CTX-M β-lactamases among Escherichia coli from the community in New York City. Diagn. Microbiol. Infect. Dis. 72:248–252. 10.1016/j.diagmicrobio.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 233.Sennati S, Santella G, Di Conza J, Pallecchi L, Pino M, Ghiglione B, Rossolini GM, Radice M, Gutkind G. 2012. Changing epidemiology of extended-spectrum β-lactamases in Argentina: emergence of CTX-M-15. Antimicrob. Agents Chemother. 56:6003–6005. 10.1128/AAC.00745-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peirano G, Asensi MD, Pitondo-Silva A, Pitout JD. 2011. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli from Rio de Janeiro, Brazil. Clin. Microbiol. Infect. 17:1039–1043. 10.1111/j.1469-0691.2010.03440.x [DOI] [PubMed] [Google Scholar]
- 235.Ruiz SJ, Montealegre MC, Ruiz-Garbajosa P, Correa A, Briceno DF, Martinez E, Rosso F, Munoz M, Quinn JP, Canton R, Villegas MV. 2011. First characterization of CTX-M-15-producing Escherichia coli ST131 and ST405 clones causing community-onset infections in South America. J. Clin. Microbiol. 49:1993–1996. 10.1128/JCM.00045-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Molina-Lopez J, Aparicio-Ozores G, Ribas-Aparicio RM, Gavilanes-Parra S, Chavez-Berrocal ME, Hernandez-Castro R, Manjarrez-Hernandez HA. 2011. Drug resistance, serotypes, and phylogenetic groups among uropathogenic Escherichia coli including O25-ST131 in Mexico City. J. Infect. Dev. Ctries. 5:840–849. 10.3855/jidc.1703 [DOI] [PubMed] [Google Scholar]
- 237.Magoue CL, Melin P, Gangoue-Pieboji J, Okomo Assoumou MC, Boreux R, De Mol P. 2013. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Ngaoundere, Cameroon. Clin. Microbiol. Infect. 19:E416–E420. 10.1111/1469-0691.12239 [DOI] [PubMed] [Google Scholar]
- 238.Fam N, Leflon-Guibout V, Fouad S, Aboul-Fadl L, Marcon E, Desouky D, El-Defrawy I, Abou-Aitta A, Klena J, Nicolas-Chanoine M-H. 2011. CTX-M-15-producing Escherichia coli clinical isolates in Cairo (Egypt), including isolates of clonal complex ST10 and clones ST131, ST73, and ST405 in both community and hospital settings. Microb. Drug Resist. 17:67–73. 10.1089/mdr.2010.0063 [DOI] [PubMed] [Google Scholar]
- 239.Rakotonirina HC, Garin B, Randrianirina F, Richard V, Talarmin A, Arlet G. 2013. Molecular characterization of multidrug-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae isolated in Antananarivo, Madagascar. BMC Microbiol. 13:85. 10.1186/1471-2180-13-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Aibinu I, Odugbemi T, Koenig W, Ghebremedhin B. 2012. Sequence type ST131 and ST10 complex (ST617) predominant among CTX-M-15-producing Escherichia coli isolates from Nigeria. Clin. Microbiol. Infect. 18:E49–E51. 10.1111/j.1469-0691.2011.03730.x [DOI] [PubMed] [Google Scholar]
- 241.Peirano G, van Greune CHJ, Pitout JDD. 2011. Characteristics of infections caused by extended-spectrum β-lactamase-producing Escherichia coli from community hospitals in South Africa. Diagn. Microbiol. Infect. Dis. 69:449–453. 10.1016/j.diagmicrobio.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 242.Mshana SE, Imirzalioglu C, Hain T, Domann E, Lyamuya EF, Chakraborty T. 2011. Multiple ST clonal complexes, with a predominance of ST131, of Escherichia coli harbouring blaCTX-M-15 in a tertiary hospital in Tanzania. Clin. Microbiol. Infect. 17:1279–1282. 10.1111/j.1469-0691.2011.03518.x [DOI] [PubMed] [Google Scholar]
- 243.Dahmen S, Bettaieb D, Mansour W, Boujaafar N, Bouallegue O, Arlet G. 2010. Characterization and molecular epidemiology of extended-spectrum β-lactamases in clinical isolates of Enterobacteriaceae in a Tunisian university hospital. Microb. Drug Resist. 16:163–170. 10.1089/mdr.2009.0108 [DOI] [PubMed] [Google Scholar]
- 244.Kudinha T, Johnson JR, Andrew SD, Kong F, Anderson P, Gilbert GL. 2013. Distribution of phylogenetic groups, sequence type ST131, and virulence-associated traits among Escherichia coli isolates from men with pyelonephritis or cystitis and healthy controls. Clin. Microbiol. Infect. 19:E173–E180. 10.1111/1469-0691.12123 [DOI] [PubMed] [Google Scholar]
- 245.Sidjabat HE, Derrington P, Nimmo GR, Paterson DL. 2010. Escherichia coli ST131 producing CTX-M-15 in Australia. J. Antimicrob. Chemother. 65:1301–1303. 10.1093/jac/dkq098 [DOI] [PubMed] [Google Scholar]
- 246.Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, Rice M, Freeman JT. 2012. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin. Infect. Dis. 54:1406–1412. 10.1093/cid/cis194 [DOI] [PubMed] [Google Scholar]