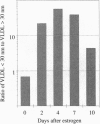
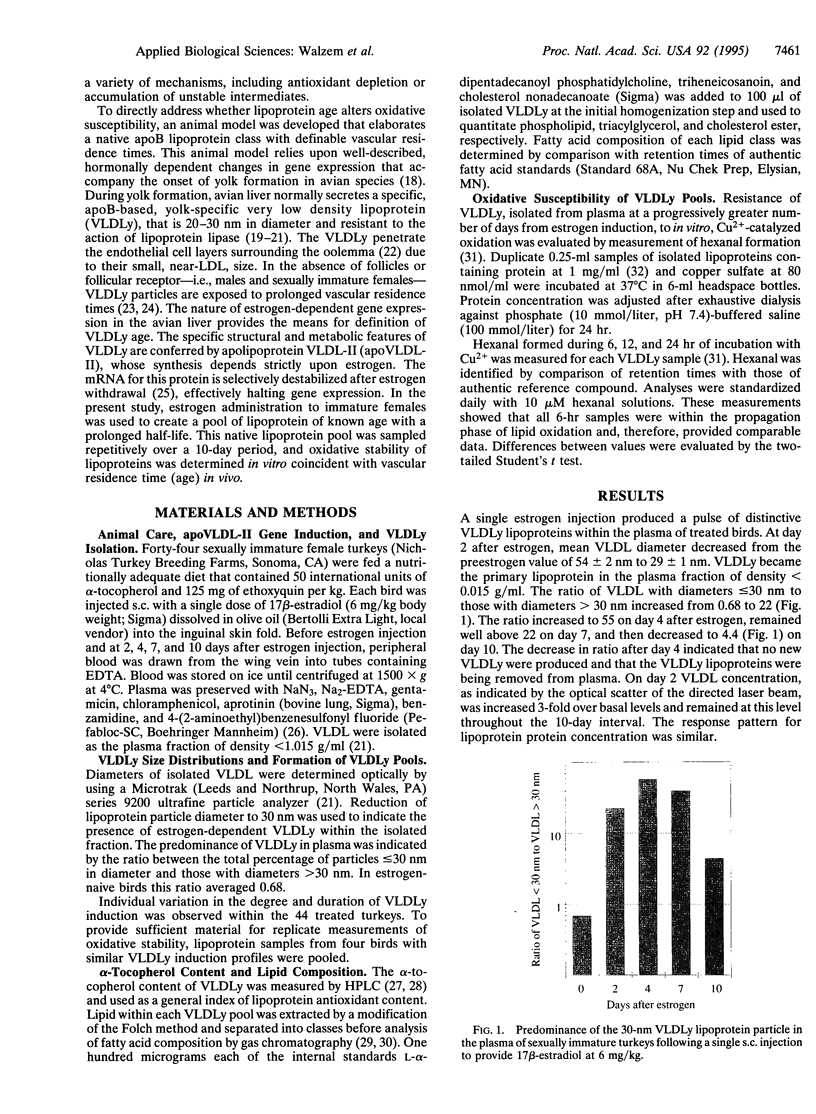
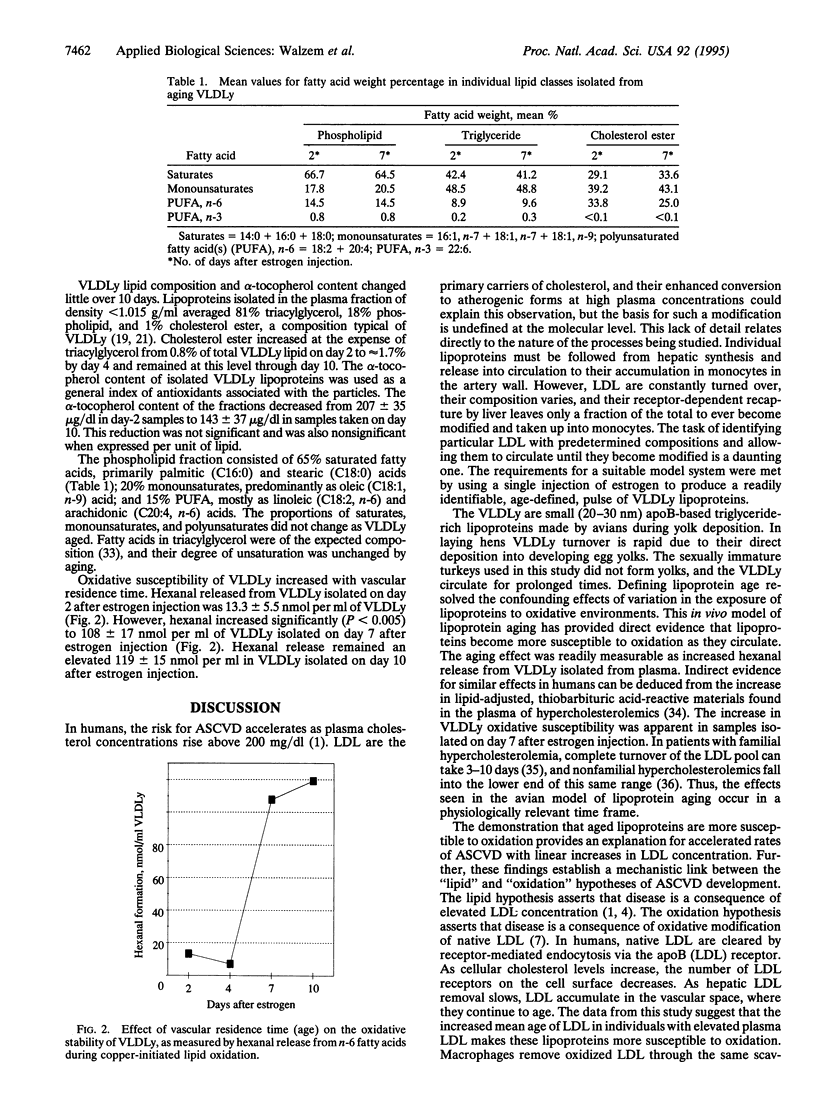
Abstract
Increases in plasma cholesterol are associated with progressive increases in the risk of atherosclerotic cardiovascular disease. In humans plasma cholesterol is contained primarily in apolipoprotein B-based low density lipoprotein (LDL). Cells stop making the high-affinity receptor responsible for LDL removal as they become cholesterol replete; this slows removal of LDL from plasma and elevates plasma LDL. As a result of this delayed uptake, hypercholesterolemic individuals not only have more LDL but have significantly older LDL. Oxidative modification of LDL enhances their atherogenicity. This study sought to determine whether increased time spent in circulation, or aging, by lipoprotein particles altered their susceptibility to oxidative modification. Controlled synchronous production of distinctive apolipoprotein B lipoproteins (yolk-specific very low density lipoproteins; VLDLy) with a single estrogen injection into young turkeys was used to model LDL aging in vivo. VLDLy remained in circulation for at least 10 days. Susceptibility to oxidation in vitro was highly dependent on lipoprotein age in vivo. Oxidation, measured as hexanal release from n-6 fatty acids in VLDLy, increased from 13.3 +/- 5.5 nmol of 2-day-old VLDLy per ml, to 108 +/- 17 nmol of 7-day-old VLDLy per ml. Oxidative instability was not due to tocopherol depletion or conversion to a more unsaturated fatty acid composition. These findings establish mathematically describable linkages between the variables of LDL concentration and LDL oxidation. The proposed mathematical models suggest a unified investigative approach to determine the mechanisms for acceleration of atherosclerotic cardiovascular disease risk as plasma cholesterol rises.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHRENS E. H., Jr, INSULL W., Jr, BLOMSTRAND R., HIRSCH J., TSALTAS T. T., PETERSON M. L. The influence of dietary fats on serum-lipid levels in man. Lancet. 1957 May 11;272(6976):943–953. doi: 10.1016/s0140-6736(57)91280-1. [DOI] [PubMed] [Google Scholar]
- Bacon W. L., Leclercq B., Blum J. C. Difference in metabolism of very low density lipoprotein from laying chicken hens in comparison to immature chicken hens. Poult Sci. 1978 Nov;57(6):1675–1686. doi: 10.3382/ps.0571675. [DOI] [PubMed] [Google Scholar]
- Barter P. J., Hopkins G. J., Calvert G. D. Pathways for the incorporation of esterified cholesterol into very low density and low density lipoproteins in plasma incubated in vitro. Biochim Biophys Acta. 1982 Oct 14;713(1):136–148. doi: 10.1016/0005-2760(82)90176-x. [DOI] [PubMed] [Google Scholar]
- Bowry V. W., Stanley K. K., Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Catignani G. L., Bieri J. G. Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983 Apr;29(4):708–712. [PubMed] [Google Scholar]
- Chirico S., Smith C., Marchant C., Mitchinson M. J., Halliwell B. Lipid peroxidation in hyperlipidaemic patients. A study of plasma using an HPLC-based thiobarbituric acid test. Free Radic Res Commun. 1993;19(1):51–57. doi: 10.3109/10715769309056498. [DOI] [PubMed] [Google Scholar]
- Cominacini L., Pastorino A. M., McCarthy A., Campagnola M., Garbin U., Davoli A., De Santis A., Lo Cascio V. Determination of lipid hydroperoxides in native low-density lipoprotein by a chemiluminescent flow-injection assay. Biochim Biophys Acta. 1993 Jan 10;1165(3):279–287. doi: 10.1016/0005-2760(93)90137-x. [DOI] [PubMed] [Google Scholar]
- Driskell W. J., Bashor M. M., Neese J. W. Beta-carotene determined in serum by liquid chromatography with an internal standard. Clin Chem. 1983 Jun;29(6):1042–1044. [PubMed] [Google Scholar]
- Edelstein C., Scanu A. M. Precautionary measures for collecting blood destined for lipoprotein isolation. Methods Enzymol. 1986;128:151–155. doi: 10.1016/0076-6879(86)28065-9. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Berglund L., Gabrielsson J., Lantz B., Angelin B. Non-steady-state kinetics of low density lipoproteins in man: studies after plasma exchange in healthy subjects and patients with familial hypercholesterolaemia. Eur J Clin Invest. 1993 Nov;23(11):746–752. doi: 10.1111/j.1365-2362.1993.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Waeg G., Puhl H., Dieber-Rotheneder M., Tatzber F. Inhibition of LDL oxidation by antioxidants. EXS. 1992;62:145–157. doi: 10.1007/978-3-0348-7460-1_15. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Wäg G., Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993 Jul;49(3):566–576. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- Finckh B., Niendorf A., Rath M., Beisiegel U. Antiatherosclerotic effect of probucol in WHHL rabbits: are there plasma parameters to evaluate this effect? Eur J Clin Pharmacol. 1991;40 (Suppl 1):S77–S80. [PubMed] [Google Scholar]
- Frankel E. N., German J. B., Davis P. A. Headspace gas chromatography to determine human low density lipoprotein oxidation. Lipids. 1992 Dec;27(12):1047–1051. doi: 10.1007/BF02535586. [DOI] [PubMed] [Google Scholar]
- Frei B., Gaziano J. M. Content of antioxidants, preformed lipid hydroperoxides, and cholesterol as predictors of the susceptibility of human LDL to metal ion-dependent and -independent oxidation. J Lipid Res. 1993 Dec;34(12):2135–2145. [PubMed] [Google Scholar]
- Fruebis J., Steinberg D., Dresel H. A., Carew T. E. A comparison of the antiatherogenic effects of probucol and of a structural analogue of probucol in low density lipoprotein receptor-deficient rabbits. J Clin Invest. 1994 Jul;94(1):392–398. doi: 10.1172/JCI117334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of low-density lipoprotein receptors: implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation. 1987 Sep;76(3):504–507. doi: 10.1161/01.cir.76.3.504. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Jr, Pownall H. J., Havel R. J. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- Griffin H., Grant G., Perry M. Hydrolysis of plasma triacylglycerol-rich lipoproteins from immature and laying hens (Gallus domesticus) by lipoprotein lipase in vitro. Biochem J. 1982 Sep 15;206(3):647–654. doi: 10.1042/bj2060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Vega G. L. Plasma cholesterol responsiveness to saturated fatty acids. Am J Clin Nutr. 1988 May;47(5):822–824. doi: 10.1093/ajcn/47.5.822. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992 Jul 27;307(1):108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- Hansen R. J., Walzem R. L. Avian fatty liver hemorrhagic syndrome: a comparative review. Adv Vet Sci Comp Med. 1993;37:451–468. [PubMed] [Google Scholar]
- Hegsted D. M., McGandy R. B., Myers M. L., Stare F. J. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965 Nov;17(5):281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- Kuksis A. Yolk lipids. Biochim Biophys Acta. 1992 Mar 25;1124(3):205–222. doi: 10.1016/0005-2760(92)90132-f. [DOI] [PubMed] [Google Scholar]
- Lynch S. M., Frei B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J Lipid Res. 1993 Oct;34(10):1745–1753. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mattson F. H., Grundy S. M. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985 Feb;26(2):194–202. [PubMed] [Google Scholar]
- Mensink R. P., Katan M. B. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med. 1989 Aug 17;321(7):436–441. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- Ohta A., Mayo M. C., Kramer N., Lands W. E. Rapid analysis of fatty acids in plasma lipids. Lipids. 1990 Nov;25(11):742–747. doi: 10.1007/BF02544044. [DOI] [PubMed] [Google Scholar]
- Park J. R., Cho B. H. Effects of estrogen on very-low-density lipoprotein triacylglycerol metabolism in chicks. Biochim Biophys Acta. 1990 Jul 16;1045(2):180–186. doi: 10.1016/0005-2760(90)90148-q. [DOI] [PubMed] [Google Scholar]
- Perry M. M., Gilbert A. B. Yolk transport in the ovarian follicle of the hen (Gallus domesticus): lipoprotein-like particles at the periphery of the oocyte in the rapid growth phase. J Cell Sci. 1979 Oct;39:257–272. doi: 10.1242/jcs.39.1.257. [DOI] [PubMed] [Google Scholar]
- Rutledge J. C. Temperature and hydrostatic pressure-dependent pathways of low-density lipoprotein transport across microvascular barrier. Am J Physiol. 1992 Jan;262(1 Pt 2):H234–H245. doi: 10.1152/ajpheart.1992.262.1.H234. [DOI] [PubMed] [Google Scholar]
- Sambrano G. R., Parthasarathy S., Steinberg D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3265–3269. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Valente A. J., Sprague E. A. A modern view of atherogenesis. Am J Cardiol. 1993 Feb 25;71(6):9B–14B. doi: 10.1016/0002-9149(93)90139-4. [DOI] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Grundy S. M., Yeshurun D., Gotto A. M., Jr, Taunton O. D. Effects of saturated and polyunsaturated fat diets on the chemical composition and metabolism of low density lipoproteins in man. J Lipid Res. 1980 Jan;21(1):91–99. [PubMed] [Google Scholar]
- Stacpoole P. W., von Bergmann K., Kilgore L. L., Zech L. A., Fisher W. R. Nutritional regulation of cholesterol synthesis and apolipoprotein B kinetics: studies in patients with familial hypercholesterolemia and normal subjects treated with a high carbohydrate, low fat diet. J Lipid Res. 1991 Nov;32(11):1837–1848. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Kalyanaraman B., Girotti A. W. Involvement of preexisting lipid hydroperoxides in Cu(2+)-stimulated oxidation of low-density lipoprotein. Arch Biochem Biophys. 1994 Dec;315(2):244–254. doi: 10.1006/abbi.1994.1496. [DOI] [PubMed] [Google Scholar]
- Vega G. L., Groszek E., Wolf R., Grundy S. M. Influence of polyunsaturated fats on composition of plasma lipoproteins and apolipoproteins. J Lipid Res. 1982 Aug;23(6):811–822. [PubMed] [Google Scholar]
- Walzem R. L., Davis P. A., Hansen R. J. Overfeeding increases very low density lipoprotein diameter and causes the appearance of a unique lipoprotein particle in association with failed yolk deposition. J Lipid Res. 1994 Aug;35(8):1354–1366. [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]