Abstract
SUMMARY
Among the melanized fungi, the so-called “black yeasts” and their filamentous relatives are particularly significant as agents of severe phaeohyphomycosis, chromoblastomycosis, and mycetoma in humans and animals. The pathogenicity and virulence of these fungi may differ significantly between closely related species. The factors which probably are of significance for pathogenicity include the presence of melanin and carotene, formation of thick cell walls and meristematic growth, presence of yeast-like phases, thermo- and perhaps also osmotolerance, adhesion, hydrophobicity, assimilation of aromatic hydrocarbons, and production of siderophores. Host defense has been shown to rely mainly on the ingestion and elimination of fungal cells by cells of the innate immune system, especially neutrophils and macrophages. However, there is increasing evidence supporting a role of T-cell-mediated immune responses, with increased interleukin-10 (IL-10) and low levels of gamma interferon (IFN-γ) being deleterious during the infection. There are no standardized therapies for treatment. It is therefore important to obtain in vitro susceptibilities of individual patients' fungal isolates in order to provide useful information for selection of appropriate treatment protocols. This article discusses the pathogenesis and host defense factors for these fungi and their severity, chronicity, and subsequent impact on treatment and prevention of diseases in human or animal hosts.
INTRODUCTION
Black yeasts and their filamentous relatives, while rather uncommon in human pathology, are unique in causing a wide range of recalcitrant infections in both immunocompetent and immunocompromised humans, among which are fatal cerebral or disseminated disorders in otherwise apparently healthy individuals (1–3). They also have been reported in cold-blooded vertebrates, with a wide variety of clinical features (4). Black yeasts multiply by budding (i.e., produce yeast-like cells) in certain stages of their development or under certain environmental conditions, and their colonies are pasty with some shade of black, while their filamentous relatives are strictly hyphal (3). However, these fungi are united on the basis of phylogenetic relationships. They belong to a limited but phylogenetically dispersed number of orders of ascomycetes (5) and show divergent adaptations to extreme conditions (6), among which are the bodies of human and animal hosts (7, 8). Most clinically relevant species are located in the family Herpotrichiellaceae, within the order Chaetothyriales, in genera such as Exophiala, Cladophialophora, Coniosporium, Cyphellophora, Fonsecaea, Phialophora, and Rhinocladiella (2, 4). Numerous members of the genus Exophiala are potential agents of human and animal mycoses (8–13) worldwide (14–16). In addition, during the last few decades, the list of black yeasts and their filamentous relatives implicated in human infections has continued to evolve (17, 18), and it will expand further, in line with increases in the numbers of susceptible patients (19, 20) and the employment of better diagnostic tools.
All members of the Chaetothyriales group are obligatorily melanized. Diseases caused by these organisms comprise mycetoma, chromoblastomycosis, and various types of phaeohyphomycosis (21, 22). In addition, asymptomatic colonization of the skin and lungs can also occur. Mycetoma is a deep tissue infection, usually of the lower extremities, characterized by the presence of mycotic granules (23, 24). McGinnis concisely defined the term “mycetoma” as an infection of humans and animals caused by one of a number of different fungi and actinomycetes and classically characterized by draining sinuses, granules, and tumefaction (25). In chromoblastomycosis, the fungi basically occur as large, muriform, thick-walled dematiaceous cells. In phaeohyphomycosis, however, the fungi characteristically occur as dark, septate hyphal elements or catenulate cells (toruloid hyphae). Mycetoma and phaeohyphomycosis result in tissue necrosis, whereas chromoblastomycosis infections lead to excessive proliferation of host tissue (3).
The knowledge of the host response against these fungi is still limited. The host defense against chronic forms of disease in experimental black yeast models of infection has been shown to rely mainly on the ingestion and elimination of fungal cells by cells of the innate immune system, especially neutrophils and macrophages. However, there is also increasing evidence supporting a role of T-cell-mediated immune responses, with increased interleukin-10 (IL-10) and low levels of gamma interferon (IFN-γ) being deleterious during the infection (26, 27).
Given the fact that severe infections may occur in immunocompetent individuals, immunological aspects of the host must play a role in the development of disease and in the severity and chronic nature of the infection. Here we review the current understanding of the pathogenesis of infection and the interface between black yeasts and host defense mechanisms.
CHARACTERISTICS OF BLACK YEASTS AND THEIR FILAMENTOUS RELATIVES
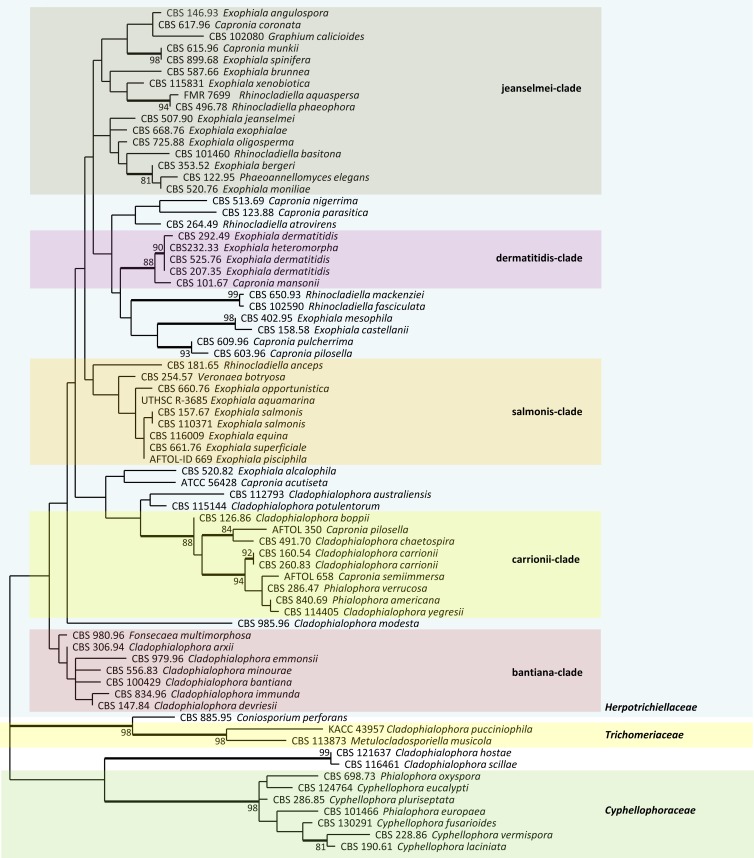
Black yeasts have been known since the end of the 19th century. The term “black yeasts” indicates those melanized fungi that are able to reproduce in culture by unicellular budding (yeast-like cells) (3, 28). Not all members of the group causing infection have this ability, and therefore the fungi are united on the basis of phylogenetic relationships. The species causing disease belong to a single order of ascomycetes, the Chaetothyriales, and show divergent adaptations to extreme, nutritionally poor, or toxic environments (6, 29). A general taxonomic overview including a large set of representative black yeasts of the Chaetothyriales is shown in Fig. 1, which was constructed using partial large-subunit ribosomal DNA (LSU rDNA) data, with Phaeococcomyces catenatus CBS 65076 as an outgroup.
FIG 1.
Phylogenetic tree of the order Chaetothyriales based on the D1–D2 region of the large-subunit ribosomal DNA, constructed by the maximum likelihood method implemented in MEGA, applying the Tamura 3-parameter model and midpoint rooting, with 1,000 bootstrap replications. Most clinically relevant species are located in the family Herpotrichiellaceae, in which approximate groups with different pathologies are recognized. A group with superficial colonizers of human skin was recently separated as the Cyphellophoraceae, while another family, the Trichomeriaceae, nearly exclusively contains environmental fungi.
The great majority of clinically relevant species belong to a single family, the Herpotrichiellaceae. To date, genera are still defined on the basis of morphology of their clonal reproduction phase, which is performed by dry sympodial conidia, by slimy phialoconidia, by budding cells, or by isodiametric enlargement and passive disarticulation of cell clumps. Elaborate sexual fruiting bodies are known only for strictly environmental species.
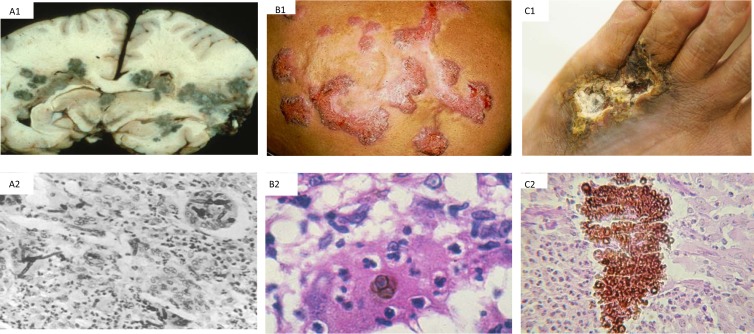
Chaetothyriales is the only order in the fungal kingdom that is associated with a wide and heterogeneous spectrum of diseases. Phaeohyphomycosis is an umbrella term covering superficial (cutaneous, corneal, and ungual), subcutaneous, and systemic infections, in which the fungal pathogens are present in host tissue as brownish to olivaceous hyphal elements due to the presence of melanin in the cell wall (30, 31). Fungal cells may also be unicellular and yeast-like, which makes the term somewhat confusing. Clinically, phaeohyphomycoses range from superficial colonization to systemic abscess formation and dissemination (32). Two more histopathological types are recognized, viz., chromoblastomycosis and black-grain mycetoma, characterized by spherical, cruciately septate “muriform cells” (33) and multicellular grains (33), respectively, and being invariably cutaneous and subcutaneous (Fig. 2).
FIG 2.
Clinical and histopathological features of fungal diseases. (A) Phaeohyphomycosis. (A1) Coronal section of the brain showing multiple abscesses caused by Cladophialophora modesta. (A2) Section of an abscess stained with hematoxylin and eosin (H&E), showing numerous darkly pigmented hyphae and a prominent neutrophil infiltration. Magnification, ×400. (Reprinted from reference 180.) (B) Chromoblastomycosis. (B1) Cutaneous plaque-form lesion caused by black yeasts. (B2) Cruciately septate muriform cells in tissue. (Courtesy of A. Bonifaz. Reproduced with permission.) (C) Eumycetoma. (C1) Mycetoma caused by Exophiala jeanselmei. The image shows a deformed tumorous area of the foot, with nodules, draining sinuses with discharging, and ulcers. (C2) Section of biopsy material stained with H&E, exhibiting pigmented granules; some were sickle-shaped and surrounded by a dense inflammatory infiltrate consisting of numerous neutrophils. Magnification, ×400. (Reprinted from reference 181 with permission.)
ECOLOGY AND ROUTE OF INFECTION
The potential pathogenicity of a species is determined partly by its natural habitat (28). Apparently, oligotrophism is an ecological mainstay. Most agents are found in the domestic and man-made environment as saprobes colonizing inert surfaces, or in hydrocarbon- or heavy-metal-polluted habitats. Despite the opportunistic nature of most species, several taxa, such as the agent of cerebritis, Cladophialophora bantiana, are not known outside the vertebrate host. A large number of species cause superficial traumatic human infections, such as keratitis (2, 34, 35). Cutaneous and subcutaneous infections also take place traumatically, but the route of infection of systemic and disseminated cases is still a mystery for many of the black yeasts and their filamentous relatives.
PATHOGENICITY AND VIRULENCE
The pathogenicity and virulence of chaetothyrialean black yeasts may differ significantly between closely related species (36). A striking example is the species pair Cladophialophora carrionii and Cladophialophora yegresii, which are nearly exclusively found as agents of human disease and on cactus thorns, respectively (37).
The Chaetothyriales can be divided roughly into the following three ecological groups: (i) saprobes not known from vertebrate disorders or that are asymptomatic colonizers, at most; (ii) agents of chromoblastomycosis that can be isolated from the environment but seem to have an advantage of human infection; and (iii) highly virulent systemic pathogens that possibly require a living animal as bait to be isolated from the environment or are known exclusively from infections of the human host (38).
Virulence Factors
Presence of melanin and carotene.
Pigments such as melanin and carotenoids are deposited in the cell walls of melanized fungi and play an important role in virulence and pathogenicity (39–43) but are not essential for growth and development (44, 45). Melanins are composed of various types of phenolic monomers and are often complexed with proteins and, less often, carbohydrates (46). The complex polymers enhance the survival and competitive abilities of fungi in hostile environments (42). Melanins in fungi are named according to their composition and synthesis pathway and include dihydroxyphenylalanine melanin, catechol melanin, γ-glutaminyl-4-hydroxybenzene melanin, and 1,8-dihydroxynaphthalene (DHN) melanin (47). The melanin pigment is believed to contribute to the organism's ability to elude host immune responses through blocking of the effects of hydrolytic enzymes on the cell wall and scavenging of free radicals liberated by phagocytic cells during the oxidative burst (2, 48). The presence of melanin has an inhibitory effect on receptor-mediated phagocytosis, interfering with nitric oxide production (49), and protects black yeast agents from destruction by host cells in vitro (50). Zoopathogenic fungi with an ascomycete affiliation produce melanin endogenously from acetate via the pentaketide pathway, with polymerization of endogenous DHN as the last step of synthesis (39). Notably, this melanin is different in structure from that of Cryptococcus neoformans, which is synthesized from 3,4-dihydroxyphenylalanine via tyrosinase, with polymerization of dopachrome as the last step of synthesis (51, 52). The redox functions of these melanins have been shown to be identical (53). Besides melanin, black yeasts and their filamentous relatives also synthesize the carotenoids 3,4-didehydro-γ-carotene (torulene) and 3,4-didehydro-γ-carotene-16-oic acid (torularhodin) (39). The mechanism of carotenoid action is more likely to consist of shielding sensitive molecules or organelles than of neutralization of harmful oxidants (39).
Chitin synthases as cell wall-associated virulence factors.
The cell walls of fungi act as initial protective barriers that contact potential hostile environments (54). By using a variety of synthetic and hydrolytic enzymes, fungi constantly remodel their cell walls during growth and sporulation (55). Chitin, an unbranched beta-1,4-linked homopolymer of N-acetylglucosamine (GlcNAc), is a structural component of the fungal cell wall. Together with beta-1,3-linked glucan, chitin plays important roles in cellular development, structural morphogenesis, spore formation, and the maintenance of cell wall integrity (43). Among fungi, filamentous molds incorporate significantly larger amounts of chitin into their cell walls than do fungi with yeast-like propagation (56). The class V chitin synthase purified from the black yeast Exophiala dermatitidis is the only chitin synthase required for sustained cell growth at elevated temperatures and, consequently, for virulence (57).
Formation of muriform cells.
Cellular plasticity is an important virulence factor for black yeasts and their filamentous relatives (45). Some of these fungi produce hyphal forms in nature but exist almost exclusively as meristematic bodies (muriform cells) in infected tissue, whereas others are hyphal both in nature and in tissue. Meristematic growth is characterized by the production of swollen, isodiametrically enlarging cell clumps with thick cell walls, in which melanin is deposited. Meristematic cells have an optimal surface/volume ratio (58). The presence of this form of reproduction is thought to be an adaptation to harsh environmental conditions (28), such as low temperature, low water availability (59), acidity, nutrient deficiency (60), high UV exposure (61), high salt concentrations (62), or high temperature (63). By manipulation of nutritional and environmental conditions, each of the varied morphotypes of E. dermatitidis was produced in vitro in a controlled fashion (64). For example, in most rich media, a polarized budding yeast morphotype is most common, whereas hyphal and erroneously called sclerotic morphotypes are produced under starvation conditions. In humans, the invasive form in chromoblastomycosis almost invariably exhibits meristematic growth (muriform cells). In some fungi, meristematic growth can be induced by acidification of the culture medium (65). In addition, in clinical practice, asymptomatic colonization with meristematic forms of black yeasts is observed in protected body sites, e.g., in the mucus of lungs in 2 to 8% of patients with cystic fibrosis (CF), a disease characterized by an elevated salt content of tissues (66).
In addition to identification of a number of environmental factors (as mentioned above) that control the transition of the yeast morphotypes of black yeasts to one of their alternate forms, the molecular mechanisms controlling this transition have been investigated. Wang and Szaniszlo reported that the APSES transcription factor, encoded by the WdSTUA gene, is an important regulator of yeast-hypha transitions (both a positive and a negative morphotype regulator) in E. dermatitidis (64), indicating its important role in cellular development and differentiation. In another study, Ye and Szaniszlo demonstrated that the WdCdc42p gene plays a unique regulatory role in cellular morphogenesis in E. dermatitidis (67). Activation of this protein in response to extracellular or intracellular signals committed yeast-like cells to a phenotype transition and produced sclerotic bodies, whereas it repressed hyphal development.
Presence of yeast-like phases.
A yeast-to-hypha transition has been shown for black yeasts and their filamentous relatives (64). The ability to reproduce unicellular growth in black yeasts is found exclusively in the genus Exophiala. However, yeast-like growth is not a prerequisite for membership in the group in a phylogenetic sense (28). Species may lack yeast-like phases depending on their environmental ecological niches. For example, certain species that occupy dynamic habitats, such as intermittently drying pools of water, reproduce as yeasts as long as submersed growth is possible. When conditions change, a more hydrophobic, hyphal stage may be formed, with erect conidiophores holding the conidia up and away from the substrate for other types of dispersal. Other related species, which occupy nonaqueous ecological niches, possess a thallus that is hydrophobic in all stages of development. As a consequence, closely related members of “black yeast species” may be highly dissimilar morphologically (28). In the human host, the ability to bud might enable a fungus to disseminate hematogenously; with hyphal growth alone, the fungus usually causes a localized infection. The majority of disseminated black yeast infections are caused by Exophiala species. Infections due to dematiaceous fungi are usually restricted to the skin and soft tissues. The fungus usually causes a localized infection in which mainly different hyphal growth morphotypes are produced. However, hematogenous dissemination may occur in different settings of infection. The majority of hematogenously disseminated black yeast infections are caused by Exophiala species, which are able to produce yeast-like cells (68–70).
Thermotolerance.
Black yeast species that are able to grow at temperatures of 37°C or higher (the bantiana, dermatitidis, and jeanselmei clades) (71) may cause systemic or disseminated infections in mammals, while those with maximum growth temperatures of around 35 to 37°C (the carrionii and europaea clades) cause (sub)cutaneous and superficial infections in humans and systemic infections in cold-blooded animals. Black yeast species with maximum growth temperatures of 42 to 45°C (Exophiala dermatitidis) may have a natural habitat in association with birds and bats, which have a body temperature well above that of humans (72, 73). Mesophilic species with maximum growth temperatures of 27 to 33°C are restricted to cold-blooded animals (71) and, rarely, occur in invertebrates (4, 74).
Adhesion and hydrophobicity.
Cell wall hydrophobicity and adhesion may be factors for pathogenesis of some black yeasts and their filamentous relatives. Inside the host, infectious propagules adhere to epithelial cells and differentiate into muriform cells, which effectively resist destruction by host effector cells and allow the establishment of chronic disease (75). This phenomenon may be enhanced by relative cellular hydrophobicity (76) due to the presence of hydrophilic extracellular polysaccharides.
Most Exophiala species show strong morphological plasticity determined by environmental conditions. The fungal “giant cell” is a central cell in dimorphism that enables rapid change between waterborne yeast and hydrophobic filamentous growth, and vice versa. Hydrophobic, catenate conidia (anamorph genus Cladophialophora) may serve for airborne dispersal (37). In the human host, hydrophobicity may explain the neurotropic character of some severe pathogens, such as Cladophialophora bantiana. Several species, including Fonsecaea pedrosoi, Cladophialophora carrionii, Exophiala dermatitidis, and Exophiala spinifera, produce additional phialides, with sticky balls of small conidia, suggesting adherence to an arthropod or invertebrate vector.
Assimilation of aromatic hydrocarbons.
Numerous species of black yeasts of the Chaetothyriales can be isolated from odd environments, such as those polluted with toxic hydrocarbons (29). These species show a remarkable association with sites containing monoaromatic hydrocarbons and may have a competitive advantage when these compounds are present (77). Some species are isolated preferentially with the use of enrichment with toluene (78). A potential role in virulence of this assimilative ability has been surmised on the basis of the structural similarity of these compounds and neurotransmitters, explaining the fungal predilection for nervous system tissue (77, 78).
Production of extracellular, acidic or alkaline secondary metabolites and siderophores.
Black yeasts are able to produce extracellular, acidic or alkaline metabolites and siderophores (28), which may act as virulence factors. Low concentrations of salt stimulate production of muriform cells in various pathogenic agents of chromoblastomycosis (79). Slightly raised salt levels determine the occurrence of Exophiala dermatitidis in the lungs of patients with cystic fibrosis (80, 81). On the other hand, high concentrations of iron increase siderophore production in tissues. Notably, species able to cause disseminated mycoses are more active producers than species which remain superficial (79). Exophiala dermatitidis, when disseminated, shows marked neurotropism (33). This may be caused by a slightly higher level of free iron in the central nervous system than in serum.
Comparative Pathogenesis in Humans and Animals
Potential pathogenicities of black yeast infections have been recognized in molluscs and crustaceans (4, 82), but particularly in captive and farmed fish, amphibians, aquarium animals, and other cold-blooded vertebrates (2, 4, 83–85). Birds and mammals are rarely affected. In invertebrates, cold-blooded vertebrates with moist or mucous membranes, or those having a water-associated lifestyle, black yeast infections are prevalent. For these animals, the skin is a major organ, and microbial disintegration frequently leads to death. This also holds true for waterborne reptiles, such as turtles (4), whereas terrestrial reptiles have a thick, dry, water-repellent skin and are much more often subject to infection by phylogenetically ancestral dermatophyte species (genus Chrysosporium, family Onygenaceae, order Onygenales) (86). Birds and mammals have a protected, water-repellent coverage, and black yeast infections are highly unusual (87, 88) and mostly neurological. Similar to infections in humans, systemic phaeohyphomycoses frequently occur in otherwise apparently healthy hosts. However, susceptibility to these infections was recently found to be associated with mutations in the CARD9 gene, encoding a critical adaptor mediating C-type lectin receptors and impairing cytokine production of innate immune cells (89). In contrast to expectations, black yeasts are not observed as emerging opportunists in immunocompromised or otherwise debilitated vertebrates. In animals, they may take zoonotic proportions, as frequently observed in farmed fish (71) and in crabs (74). In humans, infections by members of the order Chaetothyriales mostly occur in healthy individuals. Although they are rare, invasive infections may take a fatal course (90).
IMMUNE RESPONSES TO BLACK YEASTS
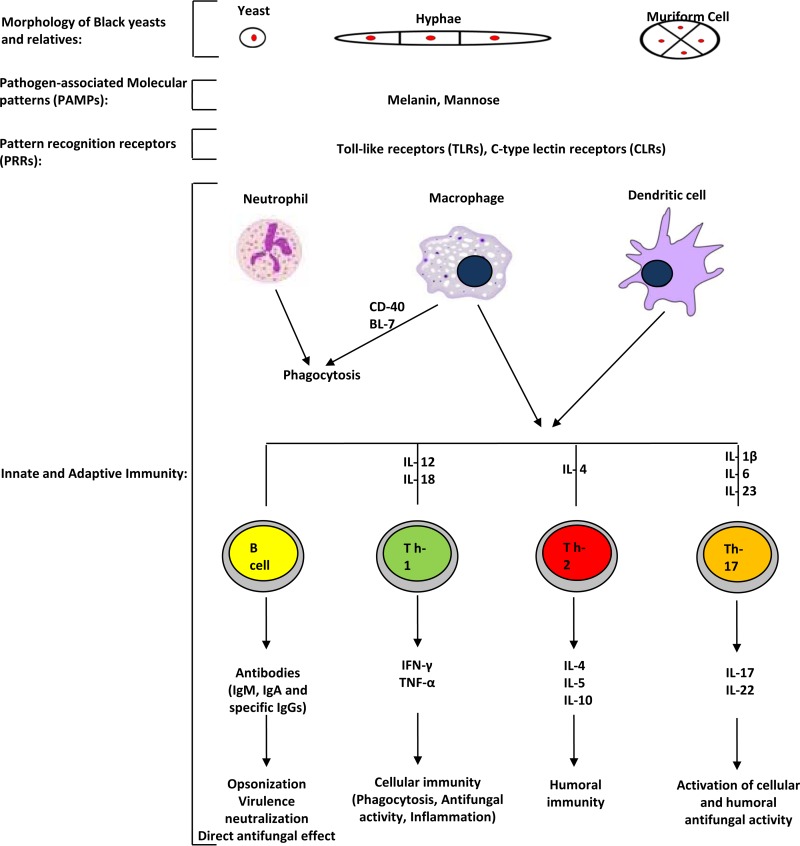
The host immune responses to black yeasts and their filamentous relatives have not been studied in detail and are still poorly understood. However, several studies have demonstrated that both innate and adaptive immune responses are required for effective containment of infections (27, 91). A schematic representation of known mechanisms of host defenses against black yeasts is shown in Fig. 3.
FIG 3.
General overview of innate and adaptive immune responses to and regulation of black yeasts. The innate immune response consists of complement proteins and diverse cellular components, including neutrophils, macrophages, and dendritic cells. The adaptive immune response consists of antibodies, B cells, and CD4+ and CD8+ T-helper lymphocytes. CD4+ T-helper cells can differentiate into Th1 cells, Th2 cells, and Th17 cells. Only known activating and regulating mechanisms are indicated. Innate immune cells recognize black yeasts through pattern recognition receptors (PRRs) and other cell surface molecules, such as Toll-like and C-type lectin receptors. The PRRs recognize pathogen-associated molecular patterns (PAMPs), such as mannoproteins expressed on the surfaces of black yeasts. Neutrophils and macrophages lyse black yeasts through the production of nitric oxide and reactive oxygen species. Macrophages phagocytose black yeasts through CD40 and BL-7. Mature dendritic cells and macrophages secrete IL-12 and IL-18 to promote the generation of Th1 cells secreting IFN-γ; IL-4 to induce differentiation of Th2 cells secreting IL-4, IL-5, and IL-10; and IL-1β, IL-6, and IL-23 to promote Th17 responses releasing IL-17 and IL-22. Adaptive immune cells are initially stimulated by MHC-II to recognize fungi and produce effective antibodies.
Host Specialization
The complexity of the immune system determines the type of tissue response against black yeasts and their filamentous relatives (4). Crustaceans have poorly developed innate immunity and no adaptive immunity. An absence of advanced immune responses, such as granuloma formation or a significant inflammatory response, is observed in systemic infections in crabs and primitive fishes, such as seahorses. In the case of invasive infection, hyphae grow densely and regularly. Primitive fishes such as seahorses, with poorly developed adaptive immunity, show similar histopathology. Granulomas with lymphocytes, granulocytes, and giant cells are observed only in higher vertebrates, which are equipped with fully developed innate and adaptive cellular immunity (4).
Innate Immunity
The innate immune response functions as the first line of defense against black yeasts. It comprises the barrier functions of skin and mucosal epithelial surfaces, microbial antagonism by the normal flora, complement activation and opsonization of invading pathogens, and participation of a variety of cells with antigen-presenting and phagocytic activities, such as neutrophils, mononuclear leukocytes (macrophages and monocytes), and dendritic cells (DCs). Although natural killer T cells (NK cells) have been shown to influence host defenses against several human-pathogenic fungi (92), practically nothing is known about their potential role in the pathogenesis of infections with black yeasts.
Macrophages.
Among the cells of the immune system, macrophages (sometimes identified as epithelioid or giant cells) play the most important role in controlling fungal growth (92).
Sotto et al. investigated the distribution and pathways of the fungal antigens and the possible role of the different immunocompetent cells in antigen processing in skin biopsy specimens from chromoblastomycosis patients (93). Notably, the majority of fungal antigens accumulated as homogenous or granular materials in the skin macrophages, which might suggest the function of these cells in the initiation of local innate immune defenses, as well as that of antigen-presenting cells together with dendritic cells. However, we emphasize that chronic and granulomatous inflammation is generally characterized by the dominating presence of macrophages in the injured tissue, independent of causative agents.
Rozental et al. (94) showed that activated macrophages were fungistatic to F. pedrosoi, delaying germ tube and hypha formation. However, Hayakawa et al. (75) demonstrated that the fungicidal ability of macrophages is dependent on the fungal species that causes the infection. The phagocytic index was higher for Fonsecaea pedrosoi, C. carrionii, and Rhinocladiella aquaspersa than for other fungi, suggesting that the phagocytic processes of macrophages may differ between black yeast species. Complement-mediated phagocytosis was more important with Phialophora verrucosa and R. aquaspersa than with F. pedrosoi and was inhibited by mannan (75). In this study, killing by macrophages was significant for R. aquaspersa only, while the remaining fungi were able to survive within macrophages. This indicates that resident macrophages have little or no cytotoxic effect on F. pedrosoi, C. carrionii, and P. verrucosa but a larger effect on R. aquaspersa. On the other hand, Bocca et al. showed that during infection with F. pedrosoi, macrophages were unable to produce NO, even after stimulation with lipopolysaccharide (LPS) and gamma interferon (IFN-γ) (95). Moreover, major histocompatibility complex class II (MHC-II) and CD80 expression was downregulated. Similarly, in vitro studies demonstrated that the expression of MHC-II and CD80 molecules in macrophages was downmodulated in the presence of black yeasts (75). In addition, phagocytosis of black yeast cells or conidia by Langerhans cells inhibited the expression of CD40 and CD86 (96).
In another study, by Gimenes et al. (97), the production of proinflammatory cytokines by macrophages was analyzed. Peripheral blood mononuclear cells (PBMC) from patients with chromoblastomycosis secreted high levels of tumor necrosis factor alpha (TNF-α) independently of the clinical form of disease. Interleukin-1 (IL-1) was induced only by F. pedrosoi and R. aquaspersa, and IL-6 production by macrophages was induced when the cells were in contact with C. carrionii, indicating that the production of IL-6 or IL-1 by macrophages was dependent on the fungal species. In another study, the cytokine response to F. pedrosoi was defective due to a lack of fungus recognition by Toll-like receptors (TLRs), while addition of TLR ligands restored cytokine production in vitro and resulted in protection against the fungus in an in vivo experimental model (91).
Neutrophils.
Polymorphonuclear neutrophils represent another component of the first line of defense. Neutrophils are one of the predominant phagocytic cell types in lesions caused by black yeasts and their filamentous relatives, and their successful killing of yeast cells helps to prevent further invasive growth of infecting fungi (98). Therefore, low killing rates for a given species can be taken as a predictor of potential invasiveness and higher overall pathogenicity for humans and animals. Notably, the characteristic granulomatous reaction associated with neutrophil-rich purulent abscesses shows an expressive frustrated phagocytosis of brown thick-walled fungal cells, whose in situ persistence is considered the main factor explaining this chronic and highly organized inflammatory reaction (99).
Dendritic cells.
Dendritic cells are an important line of defense against black yeasts and their filamentous relatives when the infection starts with cutaneous or subcutaneous inoculation into tissues. DCs play an important role in phagocytosis and serve as major antigen-presenting cells and inducers of adaptive T-cell responses (100). DCs have been described as initiators and modulators of the immune response (101). Mature DCs are able to prime naive lymphocytes and polarize them toward a T-helper type 1 (Th1) response, whereas immature DCs have been shown to induce tolerance (102). Immature DCs, which reside in most tissues and organs, actively capture and process antigens. Mature DCs decrease antigen uptake, undergo a change in chemokine receptor expression that regulates MHC, costimulatory, and adhesion molecules, and secrete chemokines and IL-12. IL-12 plays a key role in inducing cell-mediated immunity by triggering the production of IFN-γ by natural killer and T cells (101). On the other hand, the ability of DCs to produce IL-12 and to prime Th1 responses can be modulated by hormones, cytokines, or microbial products (103, 104). Sousa et al. reported that dendritic cells are the first line of defense against inoculation of black yeasts and their filamentous relatives into subcutaneous tissues (100). In their study, the interaction between F. pedrosoi and DCs obtained from patients with chromoblastomycosis was investigated. Monocyte-derived dendritic cells from patients with severe forms of chromoblastomycosis induced CD4+ T-cell activation (100) and increased the expression of human leukocyte antigen D-related (HLA-DR) and costimulatory molecules (such as CD86, TNF-α, IL-10, and IL-12) in vitro. Notably, in the presence of conidia, the expression of HLA-DR and CD86 was upregulated by DCs from both patients and controls.
Host receptors.
Although the availability of melanin in fungal cell walls has been proposed to evade host defense mechanisms, there is also evidence that melanin plays a major role in activating the host immune system (46). From the point of antigenicity, melanin is an immunologically active fungal molecule that activates both humoral and cellular responses that might help to control the infection (105). Melanin is potentially involved in the activation of TLR4, with consequent production of the proinflammatory chemokine IL-8 (106). In a study by Alviano et al., the interaction of Fonsecaea pedrosoi with phagocytes in the presence of melanin resulted in higher levels of fungal internalization and destruction by host cells, accompanied by greater degrees of oxidative burst (105). This is in line with the cytokine responses to classical fungal molecules such as beta-glucans (107), indicating that such immune mechanisms may be involved in the innate response to black yeasts. However, another study reported that the cytokine response to F. pedrosoi was defective due to a lack of recognition of the fungus by TLRs (91). Cytokine stimulation can be considered for the control of severity of corresponding infection (108), as beta-glucans are also ligands involved in the activation of the fungicidal activity of neutrophils through binding to Dectin-1 (109). In addition to melanin, mannose-bearing structures have also been described as surface components of black yeasts that activate mannose receptors and help the host to control infections (110). In a study by Alviano et al., mannose was detected as one of the principal sugar constituents in the cells of black yeasts, with the ability to activate platelet-activating factor (PAF) and consequently induce the differentiation of several immune cell types (110).
Adaptive Immunity
The adaptive immune response to black yeasts develops more slowly but manifests as increased antigenic specificity and memory. It consists of antibodies, B cells, and CD4+ and CD8+ T-helper lymphocytes.
Cell-mediated immunity.
Both in vitro and preclinical studies focusing on fungus-host interaction indicate that cell-mediated immunity, and in particular activation of helper T cells by macrophages involved in fungal phagocytosis, plays a crucial role in prevention of disease caused by black yeasts (111, 112).
It has been demonstrated that cell-mediated immunity in patients with a chronic form of black yeast infection is impaired and that patients are unable to develop an efficient immune reaction to fungal antigens, which corresponds to the chronicity and persistence of the fungus in tissue (113). In experimental infection models using athymic mice infected with black yeasts, it was shown that during the course of infection, granulomas became diffuse and confluent, with a random fungal distribution, suggesting a role for a T-cell-mediated immune response (114).
In exploring the immunophenotypes of the cellular elements related to cell-mediated immunity, it was demonstrated that CD4+ T-helper cells and B lymphocytes have a major role in defense against black yeasts (115). Two recent studies showed that CD4+ lymphocytes are potential key cells for the control of chromoblastomycosis (97, 116). Notably, immunization of mice with living black yeast conidia reduced a large influx of CD4+ cells into draining lymph nodes (116). In addition, the use of mice lacking CD4+ or CD8+ cells in an experimental chromoblastomycosis model revealed that fungal burdens in the livers and spleens of intraperitoneally infected mice were higher in the absence of CD4+ cells but not influenced by CD8+ lymphocytes. Moreover, mice lacking CD4+ cells, but not CD8+ cells, presented decreased delayed-type hypersensitivity. These animals also produced smaller amounts of IFN-γ than those in wild-type mice, whereas the levels of this cytokine in mice lacking CD8+ cells were not altered (116). On the other hand, Sotto et al. demonstrated that skin macrophages accumulate antigens from black yeasts in their cytoplasm, as homogenous or granular material (93).
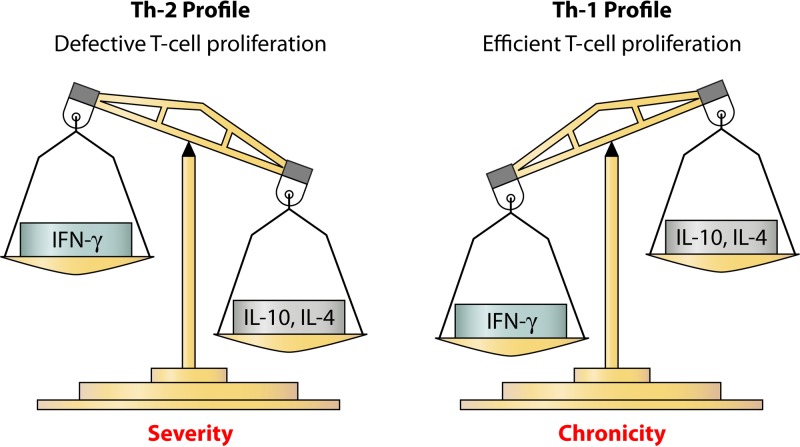
The severity of diseases caused by chaetothyrialean black yeasts is dependent on Th1/Th2 activation. Patients with chronic forms of infection exhibit increased IL-10 and low levels of IFN-γ (26). The T-helper 2 profile has been linked to extensive, severe verrucous forms (97, 115). However, patients with erythematous atrophic plaques presented with a T-helper 1 profile (97, 117), as shown in Fig. 4. Notably, one should consider that a relative imbalance between Th1 and Th2 subsets and this type of cytokine profile might appear in the course of several infectious diseases, such as chronic mucocutaneous candidiasis (118, 119), chronic hyperkeratotic dermatophytosis (120), chronic leishmaniasis (121, 122), and lepromatous leprosy (123).
FIG 4.
Cytokine profiles in severe versus chronic (mild) forms of infection caused by chaetothyrialean black yeasts, in which the Th1/Th2 imbalance plays a major role in host immune responses and inflammations. It has been shown that the severity of diseases caused is linked to a Th2 profile associated with defective T-cell proliferation, increased IL-10, and low levels of IFN-γ production, while the mild form of infections is linked to a Th1 profile associated with efficient T-cell proliferation, low levels of IL-10, and predominant production of IFN-γ.
In a study by Gimenes et al. (97), the lymphoproliferative response and cytokine production induced by black yeast antigens were investigated in relation to disease severity (97). Lymphocytes obtained from patients with the severe form of disease and stimulated with chromo-antigen exhibited defective lymphoproliferation and secreted predominantly IL-10 and TNF-α, while IFN-γ or IL-4 was not detected. In contrast, in patients with a mild form of disease, predominant production of IFN-γ, low levels of IL-10, and efficient T-cell proliferation were observed. This is in accordance with studies in which the proliferation of PBMC was documented following curative treatment (93, 94, 124), suggesting that healing of infection requires a robust T-cell-mediated immune response and IFN-γ production.
A further argument for a role of CD4+ T-helper cells in susceptibility to black yeasts is an increased susceptibility to phaeohyphomycosis in patients with HIV infection (125). As indicated earlier, CD4+ T-helper cells play an important role in controlling infections caused by black yeasts and their filamentous relatives (115). On the other hand, the principal cellular target of HIV infection is CD4+ T-helper lymphocytes (126, 127). HIV infects and kills CD4+ T lymphocytes, which function as regulators and amplifiers of the immune response, thus impairing helper cell responses, in this case, against infections caused by black yeasts and their filamentous relatives.
Very little is known about the role of Th17 lymphocytes in the host defense against infections with black yeasts. Th17 responses, including the release of the activation cytokines IL-17 and IL-22, are a crucial component of mucosal and skin antifungal host defenses (128). It has been shown that host genetic variation may play an important role in host susceptibility against fungal infections (92, 129). Recently, Wang et al. reported that T-cell deficiencies may be linked to infections caused by black yeasts and their filamentous relatives (89). In a preliminary study of 4 patients, they showed that CARD9 mutation was linked to subcutaneous phaeohyphomycosis and Th17-cell deficiencies. In all patients, CARD9 mutation was linked to decreased proportions of cytokines of Th17 cells and to impaired immune responses against Phialophora verrucosa. CARD9 is a critical adaptor that can mediate Dectin-1-, Dectin-2-, and Mincle-induced NF-κB activation by forming the CARD9-BCL10-MALT1 complex in response to fungal infection (44). CARD9 deficiencies may impair pivotal cytokine production in innate immune cells, as well as the differentiation of Th17 cells (42). It was reported that CARD9-deficient mice are highly susceptible to Candida albicans infections, and a homozygous loss-of-function nonsense mutation in CARD9 (Q295X) has been found to cause chronic mucocutaneous candidiasis in a large consanguineous family, with reduced Th17 cells and impaired immune responses to the Dectin-1 agonist (129). Apparently, further studies are warranted to evaluate the roles of host genetic susceptibility and immunologic defects in the pathophysiology of black yeast infections.
Humoral immunity.
Like the case for many fungal pathogens, the humoral immune response does not seem to be protective against infections caused by black yeasts and their filamentous relatives. The presence of immunoglobulin M (IgM), IgA, and IgG antibodies has been identified in some studies by use of enzyme-linked and immunoblotting techniques (130, 131). In contrast, in vitro studies indicated that interactions between fungi and antimelanin antibodies inhibited fungal growth (130). Likewise, individuals from areas of endemicity with previous exposure to a fungus develop a specific humoral response, although this has not been correlated clearly with the course of the disease (132). Furthermore, antibodies with direct antimicrobial action have been detected in sera of infected individuals (105, 133).
The period required for serology to become negative is variable, and some patients may have persistent positive results for more than 1 year after the end of antifungal treatment. In a longitudinal study by Esterre et al., high levels of antibodies were identified in the sera of patients with chromoblastomycosis in Madagascar (131). In another study, levels of IgG also correlated with the chronicity of disease, similar to antineutrophil antibody levels (134). In addition, the status of the host response against black yeasts may be dependent on many predisposing factors, such as gamma globulin levels (135). Notably, hypogammaglobulinemia occurs in one- to two-thirds of patients with lymphocytic immunosuppression (136), which is associated with low levels of antigen-specific antifungal IgG and IgA antibodies and corresponds with chronicity of fungal infections (137). Therefore, such factors may also be considered important prerequisites for susceptibility to infections caused by black yeasts (135). Therefore, one additional aspect that should be considered is the fact that patients with defects in humoral immunity, such as agammaglobulinemia or hypogammaglobulinemia, might have relatively normal resistance to black yeasts and their filamentous relatives. However, exogenous induction of humoral immunity, e.g., through vaccination and induction of protective antibodies, may induce additional protection in specific groups of patients (138).
RECOMMENDED DIAGNOSTICS AND TREATMENT APPROACHES
For diagnosis, direct microscopy, culture, histopathological examination of clinical samples, or, mostly, molecular analysis is required (14, 31, 139–141). A small number of black yeasts, e.g., Exophiala spinifera, possess sufficient features for phenotypic characterization. For most species of the Chaetothyriales, sequencing of the rDNA internal transcribed spacer (ITS) is necessary for reliable identification to the species level (142). In addition, mitochondrial DNA (mtDNA) can be used for rough estimations of the geographical origins and molecular epidemiology of strains (143–145). In view of epidemiological surveys, Corbellini et al. showed that a delayed-type skin test using antigens produced in synthetic media may be useful for the assessment of primary exposure to chromoblastomycosis (146). Delayed-type hypersensitivity reactions can be used to indicate T-cell-mediated inflammatory responses to either exogenous antigens or autoantigens (147) and are important determinants of previous exposure to fungal elements. This parameter is often used to evaluate the host cellular immune response to a wide range of pathogens, including chaetothyrialean black yeasts (146). However, in recent years, there has been a decline in the use of this test method, mainly because of the unpredictability of reactions and the lack of specificity of the antigens used.
For treatment of systemic phaeohyphomycosis, there are no standardized therapies (148, 149); however, itraconazole (ITC), voriconazole (VRC), and posaconazole (POS) demonstrate the most consistent in vitro activities against the relevant group of fungi (150). Importantly, due to the large variability in the spectrum of dematiaceous fungi, it is important to obtain in vitro susceptibilities of individual patients' fungal isolates. Culture and in vitro antifungal susceptibility testing provide useful information for selecting appropriate treatment protocols.
VRC has been shown to have good in vitro activity against dematiaceous fungi (151), and due to its ability to achieve an adequate therapeutic level in cerebrospinal fluid, it may be superior for use in cases of cerebral phaeohyphomycosis (152, 153).
POS has also been used successfully in cases of cerebral and disseminated phaeohyphomycosis (154, 155). Preclinical studies demonstrated that POS was active against both disseminated phaeohyphomycosis (156–159) and chromoblastomycosis caused by dematiaceous fungi (160, 161). Amphotericin B (AmB) alone or in combination with flucytosine (5FC), VRC, or ITC was not completely satisfactory (14, 149). However, the triple combination of AmB, 5FC, and ITC was associated with improved survival in a few cases of systemic phaeohyphomycosis (90). Currently, there are few data to support the use of echinocandins (caspofungin [CAS], micafungin [MFG], and anidulafungin [AFG]) for the treatment of infections caused by the black yeasts and their filamentous relatives (162–164). Notably, combination antifungal therapy is recommended for cerebral abscesses when surgery is not possible and for disseminated infections in immunocompromised patients (149).
The management of chromoblastomycosis is complicated and requires long-term antifungal therapy, surgery, thermotherapy, chemotherapy, or combinations of these (26, 148, 165). Given extensive clinical experience, ITC and terbinafine (TRB), alone or in combination, are the currently recommended treatments for chromoblastomycosis (166). However, infections by F. pedrosoi strains resistant to ITC have been reported (167). TRB, however, is still considered one of the best options for the treatment of chromoblastomycosis, due to its high degree of effectiveness and tolerability (168). In a study by Calvo et al., using an athymic murine model of chromoblastomycosis caused by Fonsecaea pedrosoi, TRB, especially at the highest dose, was able to reduce the inflammatory response to the infection to levels similar to those with POS and ITC (161). The clinical experience with POS and VRC is limited for chromoblastomycosis, but the good in vitro and in vivo efficacies of POS against dematiaceous fungi (14, 150, 169), together with the tolerance of the drug in long-term therapies, suggest this drug as a promising, well-tolerated alternative for the treatment of chromoblastomycosis (168, 170–172). In comparison, the efficacy of VRC against chromoblastomycosis has been poorly studied (173), and further studies are warranted to evaluate the potential use of VRC for treatment of such infections.
Importantly, most infections are chronic and, in humans, have been observed to reside in tissues for decades (174). The chronic nature of infections seems to be due to inadequate innate recognition and subsequent failure to mount protective inflammatory responses (91). In an experimental chromoblastomycosis model, the restoration of pattern recognition receptors was demonstrated to be a costimulatory factor in treating the chronic form of infections (91). While C-type lectin receptors (CLRs) were able to recognize fungi, there was a lack of sufficient costimulation of the TLRs, which resulted in defective inflammatory responses. However, the cytokine responses were costimulated by exogenous administration of TLR agonists (91). Collectively, the above-described data suggest that CD4+ T-helper cells secreting IFN-γ may be a useful option for inducing protective immunity against black yeast infections (97, 116), and these can be provided by adjunct cytokine immunotherapy (175, 176). Specific proinflammatory cytokines, such as IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and G-CSF, are critical components of host defense which promote the upregulation of chemotaxis, phagocytosis, respiratory burst, and/or degranulation of neutrophils, monocytes, and macrophages (177). These recombinant cytokines have been used in both experimental models of fungal infections and small clinical trials with patients with fungal infections, and investigations of their potential roles in treatment of infections with black yeasts are warranted.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
In conclusion, factors which probably are significant for the pathogenicity of black yeasts and their filamentous relatives include the presence of melanin and carotene, formation of thick cell walls and meristematic growth, presence of yeast-like phases, thermo- and perhaps also osmotolerance, adhesion, hydrophobicity, assimilation of aromatic hydrocarbons, and production of siderophores. All black yeasts and their filamentous relatives in the Chaetothyriales are obligatorily melanized, and thus the presence of melanin alone does not explain the apparently species-specific intrinsic virulence. Thermotolerance is not a decisive factor either, since many hosts of the Chaetothyriales are cold-blooded. Thermotolerance determines the choice of host: species with maximum growth temperatures of 27 to 33°C may cause diseases in cold-blooded animals, whereas those growing at temperatures above 37°C may cause systemic infections in mammals.
Host defense against black yeasts and their filamentous relatives has been shown to rely mainly on the ingestion and elimination of fungal cells by cells of the innate immune system, especially neutrophils and macrophages. Notably, a failure in innate recognition can result in chronic features that highlight the lack of pattern recognition receptor signaling. Cell-mediated immunity is more critical than a humoral immune response for host defense against diseases caused by black yeasts and their filamentous relatives. In addition, the discovery of genetic susceptibility to and immunologic defects against infection caused by these fungi can lead to a better understanding of the pathogenesis of the disease, as well as to the design of novel immunotherapeutic strategies.
The severe form of disease is characterized by elevated production of IL-4 and IL-10. These mediators, associated with low IFN-γ and lymphocyte proliferation levels, may result in a depressed cellular immune response and in severe manifestations of the disease. In contrast, patients with the mild form represent the opposite pole, in which high-level IFN-γ production parallels lymphocyte proliferation, in addition to very low levels of IL-10. This Th1 profile could lead to the development of an efficient immune response that is able to prevent the development of the disease. In this context, the moderate form of disease would present an intermediate immune response between Th2 and Th1 activation.
There is also enough evidence to support a role for CD4+ T-cell-mediated immune responses (26, 27). Both CD4+ T-helper subsets could be activated during infection. Among factors which are important in determining T-helper-cell selection are the cytokine subsets with which T-helper differentiation takes place. The recovery of immune function, especially that of phagocytic cells, is also critically important in disseminated cases.
These findings are important for the understanding of the pathophysiology of the diseases and for designing novel strategies for the treatment and control of the severe form of infection. Notably, increasing amounts of evidence from animal models of fungal infections suggest that recombinant human IFN-γ, GM-CSF, or G-CSF immunotherapies have utility in the treatment of invasive fungal infections, particularly in patients with impaired T-cell immunity (178, 179). Given the fact that a failure to restore host immunity leads to worse outcomes of black yeast infections (91), further studies are required to evaluate the restoration of endogenous immunological responses through adjuvant immunotherapy for humans and animals in various immunosuppressive states.
ACKNOWLEDGMENTS
We thank A. Bonifaz for kindly preparing the original version of the chromoblastomycosis figure.
This publication was prepared as a collaborative study between the Department of Medical Microbiology, Radboud UMC, Nijmegen, Netherlands, and the Veterinary Mycology and Black Yeast Working Groups of the International Society for Human and Animal Mycology (ISHAM).
M.G.N. was supported by a Vici grant from the Netherlands Organization for Scientific Research.
S.S., M.G.N., W.J.G.M., and G.S.D.H. have no conflicts of interest. J.W.M. and P.E.V. have served as consultants to and have received research grants from Astellas, Basilea, Gilead Sciences, Merck, and Pfizer.
Biographies

Seyedmojtaba Seyedmousavi, Ph.D., is a postdoctoral scientist at the Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Netherlands. He was previously appointed an Academic Lecturer in Medical Microbiology and Assistant Professor of Medical Mycology in Iran (2002 to 2009). From 2009 to 2010, he did postdoctoral research in molecular mycology in the lab of G. Sybren de Hoog at the CBS-KNAW Fungal Biodiversity Centre, Netherlands. He completed his Ph.D. and postdoctoral research programs in medical mycology and the pharmacokinetics and pharmacodynamics (PK/PD) of antifungals, focusing on Aspergillus fumigatus, at the Department of Medical Microbiology, Radboud UMC, Nijmegen, Netherlands. His current research focuses on fungal infection models and on the PK/PD of anti-infectives in the context of invasive fungal and bacterial infections. He also investigates the principles of pathogenesis and host defense against Aspergillus spp., black yeasts, and their filamentous relatives in the setting of chronic infections.

Mihai G. Netea, M.D., Ph.D., is Professor of Immunology at the Department of Medicine, Radboud UMC, Netherlands. Dr. Netea's research focuses on pathogen recognition by pattern recognition receptors, with a main focus on fungal pathogens. His main interest is to elucidate the pathways responsible for the recognition of Candida albicans. His research interests also encompass the understanding of the complex functional networks between pattern recognition receptor families responsible for the activation of the innate immune system. In addition, his research has also focused on the identification of novel immunodeficiencies leading to specific susceptibilities to fungal infections, leading to the first identification of a C-type lectin receptor deficiency (Dectin-1 deficiency) and the discovery of the genetic defect in chronic mucocutaneous candidiasis.

Johan W. Mouton, M.D., Ph.D., received degrees in biology (M.Sc., 1983) as well as medicine (M.D., 1988) at the University of Utrecht. He completed his Ph.D. at Erasmus University in Rotterdam, Netherlands, studying PK/PD, in 1993, and his training as a Consultant Medical Microbiologist in 1994. He is a Professor in Pharmacokinetics and Pharmacodynamics at the Radboudumc and also Unit Head at the Department of Microbiology and Infectious Diseases at Erasmus MC in Rotterdam. His main research interest is optimization of antimicrobial therapy and preventing resistance emergence through application of pharmacokinetic and pharmacodynamic principles. Major areas of interest include translation of preclinical studies (in vitro and animals) to therapeutic regimens in humans by using modeling techniques and dosing in special populations

Willem J. G. Melchers, Ph.D., is Associate Professor in Molecular Microbiology at the Department of Medical Microbiology, Radboud UMC, Netherlands. His research interests are in molecular microbiology, with a specific focus on the molecular mechanisms of fungal resistance. His research activities are the molecular diagnosis and pathogenesis of infectious diseases. Microbial infections are the primary cause of death in humans worldwide. Improvements in advanced diagnosis will be a main focus for preventing, diagnosing, and treating infectious diseases. Developments in molecular diagnostics have grown explosively in the last decade. The impact of molecular diagnosis of infectious diseases is related both to a laboratory change from artisanal to technology-based diagnostics and to patient management (monitoring, therapy, etc.). This part of his work involves technology development and clinical applications for the benefit of public health. He investigates the pathogenesis of infectious diseases and the impact of molecular diagnostics, as well as resistance development in Aspergillus.

Paul E. Verweij, M.D., Ph.D., is Professor of Medical Microbiology and Consultant Microbiologist at the Department of Medical Microbiology, Radboudumc, Netherlands. His main research interest is the management of invasive fungal diseases. Topics of interest for him include the diagnosis of invasive mycoses by non-culture-based methods such as biological markers and fungal DNA. The emergence of azole resistance in Aspergillus is another topic of interest of his which addresses the origin and epidemiology of resistance and clinical management. His third topic of interest is the efficacy of antifungal agents in relation to pharmacodynamic and pharmacokinetic parameters, using experimental models of fungal infection.

G. Sybren de Hoog, Ph.D., studied biology at the State University of Utrecht. From 1971 onwards, he has been a Researcher in Phylogenetic and Ecological Mycology at the Centraalbureau voor Schimmelcultures-KNAW Fungal Biodiversity Centre at Baarn, Netherlands (in Utrecht since 2000). He has also been appointed Professor of Mycology at the Institute of Biodiversity and Ecosystem Dynamics of the University of Amsterdam, at the Research Center for Medical Mycology, Peking University Health Science Center, Beijing, China, at the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China, at the Shanghai Institute of Medical Mycology, Changzheng Hospital, Second Military Medical University, Shanghai, China, at the Basic Pathology Department, Federal University of Paraná State, Curitiba, Paraná, Brazil, and at the King Abdulassiz University, Jeddah, Saudi Arabia. His areas of interest are the taxonomy, ecology, and evolution of black yeast-like fungi, with an emphasis on possible lines of adaptation to the human host.
REFERENCES
- 1.Matsumoto T, Ajello L, Matsuda T, Szaniszlo PJ, Walsh TJ. 1994. Developments in hyalohyphomycosis and phaeohyphomycosis. J. Med. Vet. Mycol. 32(Suppl 1):S329–S349 [DOI] [PubMed] [Google Scholar]
- 2.Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin. Microbiol. Rev. 23:884–928. 10.1128/CMR.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto T, Padhye AA, Ajello L. 1987. Medical significance of the so-called black yeasts. Eur. J. Epidemiol. 3:87–95 [DOI] [PubMed] [Google Scholar]
- 4.Seyedmousavi S, Guillot J, de Hoog GS. 2013. Phaeohyphomycoses, emerging opportunistic diseases in animals. Clin. Microbiol. Rev. 26:19–35. 10.1128/CMR.00065-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Hoog GS, McGinnis MR. 1987. Ascomycetous black yeasts, p 187–199 In de Hoog GS, Smith MT, Weijman ACM. (ed), The expanding realm of yeast-like fungi. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 6.Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS. 2009. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud. Mycol. 64:123-S7–133-S7. 10.3114/sim.2009.64.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto T, Cooper CR, Jr, Szaniszlo PJ. 2011. Chromoblastomycosis and phaeohyphomycosis, p 569–572 In Guerrant RL, Walker DH, Weller PF. (ed), Tropical infectious diseases: principles, pathogens, and practice, 3rd ed. Elsevier, Edinburgh, United Kingdom [Google Scholar]
- 8.Matsumoto T, Matsuda T, McGinnis MR, Ajello L. 1993. Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36:145–155 [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Nishimoto K, Kimura K, Padhye AA, Ajello L, McGinnis MR. 1984. Phaeohyphomycosis caused by Exophiala moniliae. Sabouraudia 22:17–26. 10.1080/00362178485380051 [DOI] [PubMed] [Google Scholar]
- 10.Hachisuka H, Matsumoto T, Kusuhara M, Nomura H, Nakano S, Sasai Y. 1990. Cutaneous phaeohyphomycosis caused by Exophiala jeanselmei after renal transplantation. Int. J. Dermatol. 29:198–200. 10.1111/j.1365-4362.1990.tb03799.x [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Matsuda T, McGinnis MR. 1990. A previously undescribed synanamorph of Wangiella dermatitidis. J. Med. Vet. Mycol. 28:437–444. 10.1080/02681219080000551 [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Matsuda T, Padhye AA, Standard PG, Ajello L. 1992. Fungal melanonychia: ungual phaeohyphomycosis caused by Wangiella dermatitidis. Clin. Exp. Dermatol. 17:83–86. 10.1111/j.1365-2230.1992.tb00170.x [DOI] [PubMed] [Google Scholar]
- 13.De Hoog GS, Matsumoto T, Matsuda T, Uijthof JM. 1994. Exophiala jeanselmei var. lecanii-corni, an aetiologic agent of human phaeohyphomycosis, with report of a case. J. Med. Vet. Mycol. 32:373–380. 10.1080/02681219480000491 [DOI] [PubMed] [Google Scholar]
- 14.Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. 2007. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 45:3713–3720. 10.1128/JCM.02012-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto T. 2000. Wangiella dermatitidis infections: a paradigm of the opportunistic mycoses caused by black yeasts and moulds. Kor. J. Med. Mycol. 5:45–50 [Google Scholar]
- 16.Suh MK. 2005. Phaeohyphomycosis in Korea. Jpn. J. Med. Mycol. 46:67–70. 10.3314/jjmm.46.67 [DOI] [PubMed] [Google Scholar]
- 17.Matsushita A, Jilong L, Hiruma M, Kobayashi M, Matsumoto T, Ogawa H, Padhye AA. 2003. Subcutaneous phaeohyphomycosis caused by Veronaea botryosa in the People's Republic of China. J. Clin. Microbiol. 41:2219–2222. 10.1128/JCM.41.5.2219-2222.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Padhye AA, Ajello L, McGinnis MR. 1986. Sarcinomyces phaeomuriformis: a new dematiaceous hyphomycete. J. Med. Vet. Mycol. 24:395–400. 10.1080/02681218680000601 [DOI] [PubMed] [Google Scholar]
- 19.Takahara M, Imafuku S, Matsuda T, Uenotsuchi T, Matsumoto T, Padhye AA, Furue M. 2005. Concurrent double infections of the skin: phaeohyphomycosis and nocardiosis in a patient with idiopathic thrombocytopenic purpura. J. Am. Acad. Dermatol. 53:S277–S280. 10.1016/j.jaad.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 20.Kim HU, Kang SH, Matsumoto T. 1998. Subcutaneous phaeohyphomycosis caused by Exophiala jeanselmei in a patient with advanced tuberculosis. Br. J. Dermatol. 138:351–353. 10.1046/j.1365-2133.1998.02090.x [DOI] [PubMed] [Google Scholar]
- 21.Pappagianis D, Ajello L. 1994. Dematiaceous—a mycologic misnomer? J. Med. Vet. Mycol. 32:319–321. 10.1080/02681219480000401 [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Ajello L. 1991. Dematiaceous fungi potentially pathogenic to humans and lower animals, p 117–162 In Arora DK, Ajello L, Mukerji KG. (ed), Handbook of applied mycology. Marcel Dekker, New York, NY [Google Scholar]
- 23.Pang KR, Wu JJ, Huang DB, Tyring SK. 2004. Subcutaneous fungal infections. Dermatol. Ther. 17:523–531. 10.1111/j.1396-0296.2004.04056.x [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto T, Ajello L. 1986. No granules, no mycetomas. Chest 90:151–152 [PubMed] [Google Scholar]
- 25.McGinnis MR. 1980. Laboratory handbook of medical mycology. Academic Press, New York, NY [Google Scholar]
- 26.Ameen M. 2009. Chromoblastomycosis: clinical presentation and management. Clin. Exp. Dermatol. 34:849–854. 10.1111/j.1365-2230.2009.03415.x [DOI] [PubMed] [Google Scholar]
- 27.Santos AL, Palmeira VF, Rozental S, Kneipp LF, Nimrichter L, Alviano DS, Rodrigues ML, Alviano CS. 2007. Biology and pathogenesis of Fonsecaea pedrosoi, the major etiologic agent of chromoblastomycosis. FEMS Microbiol. Rev. 31:570–591. 10.1111/j.1574-6976.2007.00077.x [DOI] [PubMed] [Google Scholar]
- 28.de Hoog GS. 1993. Evolution of black yeasts: possible adaptation to the human host. Antonie Van Leeuwenhoek 63:105–109. 10.1007/BF00872386 [DOI] [PubMed] [Google Scholar]
- 29.Seyedmousavi S, Badali H, Chlebicki A, Zhao J, Prenafeta-Boldu FX, De Hoog GS. 2011. Exophiala sideris, a novel black yeast isolated from environments polluted with toxic alkyl benzenes and arsenic. Fungal Biol. 115:1030–1037. 10.1016/j.funbio.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 30.McGinnis MR. 1983. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J. Am. Acad. Dermatol. 8:1–16. 10.1016/S0190-9622(83)70001-0 [DOI] [PubMed] [Google Scholar]
- 31.Ajello L. 1986. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur. J. Epidemiol. 2:243–251. 10.1007/BF00419488 [DOI] [PubMed] [Google Scholar]
- 32.Salfelder K. 1990. Atlas of fungal pathology. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 33.Matsumoto T, Padhye AA, Ajello L, Standard P, McGinnis MR. 1984. Critical review of human isolates of Wangiella dermatitidis. Mycologia 76:232–239. 10.2307/3793099 [DOI] [Google Scholar]
- 34.Benaoudia F, Assouline M, Pouliquen Y, Bouvet A, Gueho E. 1999. Exophiala (Wangiella) dermatitidis keratitis after keratoplasty. Med. Mycol. 37:53–56. 10.1080/02681219980000071 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto N, Matsumoto T, Ishibashi Y. 2001. Fungal keratitis caused by Colletotrichum gloeosporioides. Cornea 20:902–903. 10.1097/00003226-200111000-00027 [DOI] [PubMed] [Google Scholar]
- 36.Vicente VA, Attili-Angelis D, Pie MR, Queiroz-Telles F, Cruz LM, Najafzadeh MJ, de Hoog GS, Zhao J, Pizzirani-Kleiner A. 2008. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 61:137–144. 10.3114/sim.2008.61.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Hoog GS, Nishikaku AS, Fernandez-Zeppenfeldt G, Padin-Gonzalez C, Burger E, Badali H, Richard-Yegres N, van den Ende AH. 2007. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud. Mycol. 58:219–234. 10.3114/sim.2007.58.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Hoog GS, Queiroz-Telles F, Haase G, Fernandez-Zeppenfeldt G, Attili Angelis D, Gerrits Van Den Ende AH, Matos T, Peltroche-Llacsahuanga H, Pizzirani-Kleiner AA, Rainer J, Richard-Yegres N, Vicente V, Yegres F. 2000. Black fungi: clinical and pathogenic approaches. Med. Mycol. 38(Suppl 1):S243–S250 [PubMed] [Google Scholar]
- 39.Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zundorf J, Lutticken R, Haase G. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 67:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polak A. 1990. Melanin as a virulence factor in pathogenic fungi. Mycoses 33:215–224 [DOI] [PubMed] [Google Scholar]
- 41.Dixon DM, Migliozzi J, Cooper CR, Jr, Solis O, Breslin B, Szaniszlo PJ. 1992. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidis in mice: mortality and histopathology studies. Mycoses 35:17–21 [DOI] [PubMed] [Google Scholar]
- 42.Dixon DM, Szaniszlo PJ, Polak A. 1991. Dihydroxynaphthalene (DHN) melanin and its relationship with virulence in the early stages of phaeohyphomycosis, p 297–318 In Cole GT, Hoch HC. (ed), Fungal spore and disease initiation in plants. Plenum Press, New York, NY [Google Scholar]
- 43.Szaniszlo PJ. 2006. Virulence factors in black molds with emphasis on melanin, chitin, and Wangiella as a molecularly tractable model, p 407–428 In Heitman J, Filler SG, Edwards JE, Mitchell AP. (ed), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC [Google Scholar]
- 44.Wheeler MH, Abramczyk D, Puckhaber LS, Naruse M, Ebizuka Y, Fujii I, Szaniszlo PJ. 2008. New biosynthetic step in the melanin pathway of Wangiella (Exophiala) dermatitidis: evidence for 2-acetyl-1,3,6,8-tetrahydroxynaphthalene as a novel precursor. Eukaryot. Cell 7:1699–1711. 10.1128/EC.00179-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szaniszlo PJ. 2002. Molecular genetic studies of the model dematiaceous pathogen Wangiella dermatitidis. Int. J. Med. Microbiol. 292:381–390. 10.1078/1438-4221-00221 [DOI] [PubMed] [Google Scholar]
- 46.Nosanchuk JD, Casadevall A. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 50:3519–3528. 10.1128/AAC.00545-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143–158. 10.1016/S1087-1845(02)00526-1 [DOI] [PubMed] [Google Scholar]
- 48.Nosanchuk JD, Casadevall A. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203–223. 10.1046/j.1462-5814.2003.00268.x [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Wang L, Xi L, Huang H, Hu Y, Li X, Huang X, Lu S, Sun J. 2013. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia 175:515–522. 10.1007/s11046-012-9588-x [DOI] [PubMed] [Google Scholar]
- 50.Farbiarz SR, de Carvalho TU, Alviano C, de Souza W. 1992. Inhibitory effect of melanin on the interaction of Fonsecaea pedrosoi with mammalian cells in vitro. J. Med. Vet. Mycol. 30:265–273. 10.1080/02681219280000351 [DOI] [PubMed] [Google Scholar]
- 51.Wheeler MH, Bell AA. 1988. Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 2:338–387. 10.1007/978-1-4612-3730-3_10 [DOI] [PubMed] [Google Scholar]
- 52.Polacheck I, Kwon-Chung KJ. 1988. Melanogenesis in Cryptococcus neoformans. J. Gen. Microbiol. 134:1037–1041 [DOI] [PubMed] [Google Scholar]
- 53.Jacobson ES, Hove E, Emery HS. 1995. Antioxidant function of melanin in black fungi. Infect. Immun. 63:4944–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latge JP. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279–290. 10.1111/j.1365-2958.2007.05872.x [DOI] [PubMed] [Google Scholar]
- 55.Abramczyk D, Park C, Szaniszlo PJ. 2009. Cytolocalization of the class V chitin synthase in the yeast, hyphal and sclerotic morphotypes of Wangiella (Exophiala) dermatitidis. Fungal Genet. Biol. 46:28–41. 10.1016/j.fgb.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Nobel H, van Den Ende H, Klis FM. 2000. Cell wall maintenance in fungi. Trends Microbiol. 8:344–345. 10.1016/S0966-842X(00)01805-9 [DOI] [PubMed] [Google Scholar]
- 57.Abramczyk D, Szaniszlo PJ. 2009. Immunoaffinity purification of the class V chitin synthase of Wangiella (Exophiala) dermatitidis. Prep. Biochem. Biotechnol. 39:277–288. 10.1080/10826060902953244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wollenzien U, de Hoog GS, Krumbein W, Uijthof JM. 1997. Sarcinomyces petricola, a new microcolonial fungus from marble in the Mediterranean basin. Antonie Van Leeuwenhoek 71:281–288. 10.1023/A:1000157803954 [DOI] [PubMed] [Google Scholar]
- 59.Wollenzien U, de Hoog GS, Krumbein WE, Urzì C. 1995. On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Sci. Total Environ. 167:287–294. 10.1016/0048-9697(95)04589-S [DOI] [Google Scholar]
- 60.Sterflinger K, Krumbein WE. 1997. Dematiaceous fungi as a major agent for biopitting on Mediterranean marbles and limestones. Geomicrobiol. J. 14:219–230. 10.1080/01490459709378045 [DOI] [Google Scholar]
- 61.Urzì C, Wollenzien U, Criseo G, Krumbein WE. 1995. Biodiversity of the rock inhabiting microflora with special reference to black fungi and black yeasts, p 289–302 In Allsopp D, Colwell RR, Hawksworth DL. (ed), Diversity and ecosystem function. CAB International, Wallington, United Kingdom [Google Scholar]
- 62.Selbmann L, de Hoog GS, Mazzaglia A, Friedmann EI, Onofri S. 2005. Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud. Mycol. 51:1–32 [Google Scholar]
- 63.Sterflinger K. 1998. Temperature and NaCl-tolerance of rock-inhabiting meristematic fungi. Antonie Van Leeuwenhoek 74:271–281. 10.1023/A:1001753131034 [DOI] [PubMed] [Google Scholar]
- 64.Wang Q, Szaniszlo PJ. 2007. WdStuAp, an APSES transcription factor, is a regulator of yeast-hyphal transitions in Wangiella (Exophiala) dermatitidis. Eukaryot. Cell 6:1595–1605. 10.1128/EC.00037-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendoza L, Karuppayil SM, Szaniszlo PJ. 1993. Calcium regulates in vitro dimorphism in chromoblastomycotic fungi. Mycoses 36:157–164 [DOI] [PubMed] [Google Scholar]
- 66.Haase G, Skopnik H, Groten T, Kusenbach G, Posselt HG. 1991. Long-term fungal cultures from sputum of patients with cystic fibrosis. Mycoses 34:373–376 [DOI] [PubMed] [Google Scholar]
- 67.Ye X, Szaniszlo PJ. 2000. Expression of a constitutively active Cdc42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. J. Bacteriol. 182:4941–4950. 10.1128/JB.182.17.4941-4950.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nachman S, Alpan O, Malowitz R, Spitzer ED. 1996. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 34:1011–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Obaid I, Ahmad S, Khan ZU, Dinesh B, Hejab HM. 2006. Catheter-associated fungemia due to Exophiala oligosperma in a leukemic child and review of fungemia cases caused by Exophiala species. Eur. J. Clin. Microbiol. Infect. Dis. 25:729–732. 10.1007/s10096-006-0205-0 [DOI] [PubMed] [Google Scholar]
- 70.Nucci M, Akiti T, Barreiros G, Silveira F, Revankar SG, Wickes BL, Sutton DA, Patterson TF. 2002. Nosocomial outbreak of Exophiala jeanselmei fungemia associated with contamination of hospital water. Clin. Infect. Dis. 34:1475–1480. 10.1086/340344 [DOI] [PubMed] [Google Scholar]
- 71.de Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S. 2011. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27:46–72. 10.3767/003158511X614258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sudhadham M, Prakitsin S, Sivichai S, Chaiyarat R, Dorrestein GM, Menken SB, de Hoog GS. 2008. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 61:145–155. 10.3114/sim.2008.61.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horré R, de Hoog GS. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176–193 [Google Scholar]
- 74.Vicente VA, Orelis-Ribeiro R, Najafzadeh MJ, Sun J, Guerra RS, Miesch S, Ostrensky A, Meis JF, Klaassen CH, de Hoog GS, Boeger WA. 2012. Black yeast-like fungi associated with lethargic crab disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae). Vet. Microbiol. 158:109–122. 10.1016/j.vetmic.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 75.Hayakawa M, Ghosn EE, da Gloria Teixeria de Sousa M, Ferreira KS, Almeida SR. 2006. Phagocytosis, production of nitric oxide and pro-inflammatory cytokines by macrophages in the presence of dematiaceous [correction of dematiaceus] fungi that cause chromoblastomycosis. Scand. J. Immunol. 64:382–387. 10.1111/j.1365-3083.2006.01804.x [DOI] [PubMed] [Google Scholar]
- 76.Satow MM, Attili-Angelis D, de Hoog GS, Angelis DF, Vicente VA. 2008. Selective factors involved in oil flotation isolation of black yeasts from the environment. Stud. Mycol. 61:157–163. 10.3114/sim.2008.61.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prenafeta-Boldu FX, Summerbell R, de Hoog GS. 2006. Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiol. Rev. 30:109–130. 10.1111/j.1574-6976.2005.00007.x [DOI] [PubMed] [Google Scholar]
- 78.Zhao J, Zeng J, de Hoog GS, Attili-Angelis D, Prenafeta-Boldu FX. 2010. Isolation and identification of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microb. Ecol. 60:149–156. 10.1007/s00248-010-9651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Hoog GS, Haase G. 1993. Nutritional physiology and selective isolation of Exophiala dermatitidis. Antonie Van Leeuwenhoek 64:17–26. 10.1007/BF00870917 [DOI] [PubMed] [Google Scholar]
- 80.Kondori N, Gilljam M, Lindblad A, Jonsson B, Moore ER, Wenneras C. 2011. High rate of Exophiala dermatitidis recovery in the airways of patients with cystic fibrosis is associated with pancreatic insufficiency. J. Clin. Microbiol. 49:1004–1009. 10.1128/JCM.01899-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kusenbach G, Skopnik H, Haase G, Friedrichs F, Dohmen H. 1992. Exophiala dermatitidis pneumonia in cystic fibrosis. Eur. J. Pediatr. 151:344–346. 10.1007/BF02113255 [DOI] [PubMed] [Google Scholar]
- 82.Van Dover CL, Ward ME, Scott JL, Underdown J, Anderson B, Gustafson C, Whalen M, Carnegie RB. 2007. A fungal epizootic in mussels at a deep-sea hydrothermal vent. Mar. Ecol. 28:54–62. 10.1111/j.1439-0485.2006.00121.x [DOI] [Google Scholar]
- 83.Yanong RP. 2003. Fungal diseases of fish. Vet. Clin. N. Am. 6:377–400 [DOI] [PubMed] [Google Scholar]
- 84.Densmore CL, Green DE. 2007. Diseases of amphibians. ILAR J. 48:235–254. 10.1093/ilar.48.3.235 [DOI] [PubMed] [Google Scholar]
- 85.Boeger WA, Pie MR, Ostrensky A, Patella L. 2005. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Mem. Inst. Oswaldo Cruz 100:161–167. 10.1590/S0074-02762005000200009 [DOI] [PubMed] [Google Scholar]
- 86.Paré JA, Sigler L, Rosenthal KL, Mader DR. 2006. Microbiology: fungal and bacterial diseases of reptiles, p 217–239 In Mader DR. (ed), Reptile medicine and surgery, vol 2 Saunders Elsevier, St Louis, MO [Google Scholar]
- 87.Frank C, Vemulapalli R, Lin T. 2011. Cerebral phaeohyphomycosis due to Cladophialophora bantiana in a Huacaya alpaca (Vicugna pacos). J. Comp. Pathol. 145:410–413. 10.1016/j.jcpa.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 88.Haligur M, Ozmen O, Dorrestein GM. 2010. Fatal systemic cladosporiosis in a merino sheep flock. Mycopathologia 170:411–415. 10.1007/s11046-010-9331-4 [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Wang W, Lin Z, Wang X, Li T, Yu J, Liu W, Tong Z, Xu Y, Zhang J, Guan L, Dai L, Yang Y, Han W, Li R. 2014. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J. Allergy Clin. Immunol. 133:905–908.e3. 10.1016/j.jaci.2013.09.033 [DOI] [PubMed] [Google Scholar]
- 90.Revankar SG, Sutton DA, Rinaldi MG. 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 38:206–216. 10.1086/380635 [DOI] [PubMed] [Google Scholar]
- 91.Sousa MG, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD. 2011. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 9:436–443. 10.1016/j.chom.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romani L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11:275–288. 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 93.Sotto MN, De Brito T, Silva AM, Vidal M, Castro LG. 2004. Antigen distribution and antigen-presenting cells in skin biopsies of human chromoblastomycosis. J. Cutan. Pathol. 31:14–18. 10.1046/j.0303-6987.2004.0131.x [DOI] [PubMed] [Google Scholar]
- 94.Rozental S, Alviano CS, de Souza W. 1994. The in vitro susceptibility of Fonsecaea pedrosoi to activated macrophages. Mycopathologia 126:85–91. 10.1007/BF01146200 [DOI] [PubMed] [Google Scholar]
- 95.Bocca AL, Brito PP, Figueiredo F, Tosta CE. 2006. Inhibition of nitric oxide production by macrophages in chromoblastomycosis: a role for Fonsecaea pedrosoi melanin. Mycopathologia 161:195–203. 10.1007/s11046-005-0228-6 [DOI] [PubMed] [Google Scholar]
- 96.da Silva JP, da Silva MB, Salgado UI, Diniz JA, Rozental S, Salgado CG. 2007. Phagocytosis of Fonsecaea pedrosoi conidia, but not sclerotic cells caused by Langerhans cells, inhibits CD40 and B7-2 expression. FEMS Immunol. Med. Microbiol. 50:104–111. 10.1111/j.1574-695X.2007.00239.x [DOI] [PubMed] [Google Scholar]
- 97.Gimenes VMF, Sousa MG, Ferreira KS, Marques SG, Gonçalves AG, Santos DVCL, de Silva Azevedo CMP, Almeida SR. 2005. Cytokine and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 7:708–713. 10.1016/j.micinf.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 98.Peltroche-Llacsahuanga H, Schnitzler N, Jentsch S, Platz A, De Hoog S, Schweizer KG, Haase G. 2003. Analyses of phagocytosis, evoked oxidative burst, and killing of black yeasts by human neutrophils: a tool for estimating their pathogenicity? Med. Mycol. 41:7–14. 10.1080/mmy.41.1.7.14 [DOI] [PubMed] [Google Scholar]
- 99.Esterre P, Queiroz-Telles F. 2006. Management of chromoblastomycosis: novel perspectives. Curr. Opin. Infect. Dis. 19:148–152. 10.1097/01.qco.0000216625.28692.67 [DOI] [PubMed] [Google Scholar]
- 100.Sousa MG, Ghosn EE, Nascimento RC, Bomfim GF, Noal V, Santiago K, de Maria Pedrozo ESAC, Marques SG, Goncalves AG, de Castro Lima Santos DW, Criado PR, Costa Martins JE, Almeida SR. 2009. Monocyte-derived dendritic cells from patients with severe forms of chromoblastomycosis induce CD4+ T cell activation in vitro. Clin. Exp. Immunol. 156:117–125. 10.1111/j.1365-2249.2008.03870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reis e Sousa C, Sher A, Kaye P. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392–399. 10.1016/S0952-7915(99)80066-1 [DOI] [PubMed] [Google Scholar]
- 102.Cella M, Sallusto F, Lanzavecchia A. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10–16. 10.1016/S0952-7915(97)80153-7 [DOI] [PubMed] [Google Scholar]
- 103.Hochrein H, O'Keeffe M, Luft T, Vandenabeele S, Grumont RJ, Maraskovsky E, Shortman K. 2000. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192:823–833. 10.1084/jem.192.6.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kalinski P, Smits HH, Schuitemaker JH, Vieira PL, van Eijk M, de Jong EC, Wierenga EA, Kapsenberg ML. 2000. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J. Immunol. 165:1877–1881. 10.4049/jimmunol.165.4.1877 [DOI] [PubMed] [Google Scholar]
- 105.Alviano DS, Franzen AJ, Travassos LR, Holandino C, Rozental S, Ejzemberg R, Alviano CS, Rodrigues ML. 2004. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 72:229–237. 10.1128/IAI.72.1.229-237.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El-Obeid A, Hassib A, Ponten F, Westermark B. 2006. Effect of herbal melanin on IL-8: a possible role of Toll-like receptor 4 (TLR4). Biochem. Biophys. Res. Commun. 344:1200–1206. 10.1016/j.bbrc.2006.04.035 [DOI] [PubMed] [Google Scholar]
- 107.Levitz SM. 2004. Interactions of Toll-like receptors with fungi. Microbes Infect. 6:1351–1355. 10.1016/j.micinf.2004.08.014 [DOI] [PubMed] [Google Scholar]
- 108.Dennehy KM, Brown GD. 2007. The role of the beta-glucan receptor Dectin-1 in control of fungal infection. J. Leukoc. Biol. 82:253–258. 10.1189/jlb.1206753 [DOI] [PubMed] [Google Scholar]
- 109.Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. 2007. Dectin-1 promotes fungicidal activity of human neutrophils. Eur. J. Immunol. 37:467–478. 10.1002/eji.200636653 [DOI] [PubMed] [Google Scholar]
- 110.Alviano DS, Kneipp LF, Lopes AH, Travassos LR, Meyer-Fernandes JR, Rodrigues ML, Alviano CS. 2003. Differentiation of Fonsecaea pedrosoi mycelial forms into sclerotic cells is induced by platelet-activating factor. Res. Microbiol. 154:689–695. 10.1016/j.resmic.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 111.Kurita N. 1979. Cell-mediated immune responses in mice infected with Fonsecaea pedrosoi. Mycopathologia 68:9–15. 10.1007/BF00490385 [DOI] [PubMed] [Google Scholar]
- 112.Whitcomb MP, Jeffries CD, Weise RW. 1981. Curvularia lunata in experimental phaeohyphomycosis. Mycopathologia 75:81–88. 10.1007/BF00505782 [DOI] [PubMed] [Google Scholar]
- 113.Fuchs J, Pecher S. 1992. Partial suppression of cell mediated immunity in chromoblastomycosis. Mycopathologia 119:73–76. 10.1007/BF00443936 [DOI] [PubMed] [Google Scholar]
- 114.Ahrens J, Graybill JR, Abishawl A, Tio FO, Rinaldi MG. 1989. Experimental murine chromomycosis mimicking chronic progressive human disease. Am. J. Trop. Med. Hyg. 40:651–658 [DOI] [PubMed] [Google Scholar]
- 115.d'Avila SC, Pagliari C, Duarte MI. 2003. The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia 156:51–60 [DOI] [PubMed] [Google Scholar]
- 116.Teixeira de Sousa MG, Ghosn EE, Almeida SR. 2006. Absence of CD4+ T cells impairs host defence of mice infected with Fonsecaea pedrosoi. Scand. J. Immunol. 64:595–600. 10.1111/j.1365-3083.2006.01846.x [DOI] [PubMed] [Google Scholar]
- 117.Esterre P, Peyrol S, Sainte-Marie D, Pradinaud R, Grimaud JA. 1993. Granulomatous reaction and tissue remodelling in the cutaneous lesion of chromomycosis. Virchows Arch. A Pathol. Anat. Histopathol. 422:285–291. 10.1007/BF01608337 [DOI] [PubMed] [Google Scholar]
- 118.van de Veerdonk FL, Netea MG. 2010. T-cell subsets and antifungal host defenses. Curr. Fungal Infect. Rep. 4:238–243. 10.1007/s12281-010-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takeya K, Nomoto K, Matsumoto T, Miyake T, Himeno K. 1976. Chronic mucocutaneous candidiasis accompanied by enhanced antibody production. Clin. Exp. Immunol. 25:497–500 [PMC free article] [PubMed] [Google Scholar]
- 120.Woodfolk JA. 2005. Allergy and dermatophytes. Clin. Microbiol. Rev. 18:30–43. 10.1128/CMR.18.1.30-43.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts MT. 2005. Current understandings on the immunology of leishmaniasis and recent developments in prevention and treatment. Br. Med. Bull. 75–76:115–130. 10.1093/bmb/ldl003 [DOI] [PubMed] [Google Scholar]
- 123.Spellberg B, Edwards JE., Jr 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76–102. 10.1086/317537 [DOI] [PubMed] [Google Scholar]
- 124.Gimenes VM, Criado PR, Martins JE, Almeida SR. 2006. Cellular immune response of patients with chromoblastomycosis undergoing antifungal therapy. Mycopathologia 162:97–101. 10.1007/s11046-006-0041-x [DOI] [PubMed] [Google Scholar]
- 125.Parente JN, Talhari C, Ginter-Hanselmayer G, Schettini AP, Eiras JC, de Souza JV, Tavares R, Buzina W, Brunasso AM, Massone C. 2011. Subcutaneous phaeohyphomycosis in immunocompetent patients: two new cases caused by Exophiala jeanselmei and Cladophialophora carrionii. Mycoses 54:265–269. 10.1111/j.1439-0507.2009.01795.x [DOI] [PubMed] [Google Scholar]
- 126.Roy SM, Wodarz D. 2012. Infection of HIV-specific CD4 T helper cells and the clonal composition of the response. J. Theor. Biol. 304:143–151. 10.1016/j.jtbi.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alimonti JB, Ball TB, Fowke KR. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649–1661. 10.1099/vir.0.19110-0 [DOI] [PubMed] [Google Scholar]
- 128.van de Veerdonk FL, Gresnigt MS, Kullberg BJ, van der Meer JW, Joosten LA, Netea MG. 2009. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 42:776–787. 10.5483/BMBRep.2009.42.12.776 [DOI] [PubMed] [Google Scholar]
- 129.Smeekens SP, van de Veerdonk FL, Kullberg BJ, Netea MG. 2013. Genetic susceptibility to Candida infections. EMBO Mol. Med. 5:805–813. 10.1002/emmm.201201678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vidal MS, Castro LG, Cavalcante SC, Lacaz CS. 2004. Highly specific and sensitive, immunoblot-detected 54 kDa antigen from Fonsecaea pedrosoi. Med. Mycol. 42:511–515. 10.1080/13693780310001654337 [DOI] [PubMed] [Google Scholar]
- 131.Esterre P, Jahevitra M, Andriantsimahavandy A. 2000. Humoral immune response in chromoblastomycosis during and after therapy. Clin. Diagn. Lab. Immunol. 7:497–500. 10.1128/CDLI.7.3.497-500.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esterre P, Jahevitra M, Ramarcel E, Andriantsimahavandy A. 1997. Evaluation of the ELISA technique for the diagnosis and the seroepidemiology of chromoblastomycosis. J. Mycol. Med. 7:137–141 [Google Scholar]
- 133.Nimrichter L, Cerqueira MD, Leitao EA, Miranda K, Nakayasu ES, Almeida SR, Almeida IC, Alviano CS, Barreto-Bergter E, Rodrigues ML. 2005. Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect. Immun. 73:7860–7868. 10.1128/IAI.73.12.7860-7868.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galperin C, Shoenfeld Y, Gilburd B, Esterre P, Meroni PL, Del Papa N, Halpern GM, Andriantsimahavandy A, Gershwin ME. 1996. Anti-neutrophil cytoplasmic antibodies in patients with chromomycosis. Clin. Exp. Rheumatol. 14:479–483 [PubMed] [Google Scholar]
- 135.Basilio FM, Hammerschmidt M, Mukai MM, Werner B, Pinheiro RL, Moritz S. 2012. Mucormycosis and chromoblastomycosis occurring in a patient with leprosy type 2 reaction under prolonged corticosteroid and thalidomide therapy. An. Bras. Dermatol. 87:767–771. 10.1590/S0365-05962012000500017 [DOI] [PubMed] [Google Scholar]
- 136.Rothberg MS, Eisenbud L, Griboff S. 1989. Chronic mucocutaneous candidiasis-thymoma syndrome. A case report. Oral Surg. Oral Med. Oral Pathol. 68:411–413. 10.1016/0030-4220(89)90138-2 [DOI] [PubMed] [Google Scholar]
- 137.Mok CC, Que TL, Tsui EY, Lam WY. 2003. Mucormycosis in systemic lupus erythematosus. Semin. Arthritis Rheum. 33:115–124. 10.1016/S0049-0172(03)00081-7 [DOI] [PubMed] [Google Scholar]
- 138.Netea MG. 2013. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur. J. Clin. Invest. 43:881–884. 10.1111/eci.12132 [DOI] [PubMed] [Google Scholar]
- 139.Matsumoto T. 1989. Mycoses, p 116–117 In Ueki H, Yaoita H. (ed), A colour atlas of dermato-immunohistocytology. Wolfe Medical Publications, London, United Kingdom [Google Scholar]
- 140.Balajee SA, Sigler L, Brandt ME. 2007. DNA and the classical way: identification of medically important molds in the 21st century. Med. Mycol. 45:475–490. 10.1080/13693780701449425 [DOI] [PubMed] [Google Scholar]
- 141.Ferrer C, Colom F, Frases S, Mulet E, Abad JL, Alio JL. 2001. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 39:2873–2879. 10.1128/JCM.39.8.2873-2879.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zeng JS, De Hoog GS. 2008. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med. Mycol. 46:193–208. 10.1080/13693780701799217 [DOI] [PubMed] [Google Scholar]
- 143.Kawasaki M, Aoki M, Ishizaki H, Miyaji M, Nishimura K, Nishimoto K, Matsumoto T, De Vroey C, Negroni R, Mendonca M, Andriantsimahavandy A, Esterre P. 1999. Molecular epidemiology of Fonsecaea pedrosoi using mitochondrial DNA analysis. Med. Mycol. 37:435–440. 10.1080/mmy.37.6.429.440 [DOI] [PubMed] [Google Scholar]
- 144.Kawasaki M, Ishizaki H, Miyaji M, Nishimura K, Matsumoto T, Hombo S, Muir D. 1993. Molecular epidemiology of Cladosporium carrionii. Mycopathologia 124:149–152. 10.1007/BF01103731 [DOI] [PubMed] [Google Scholar]
- 145.Kawasaki M, Ishizaki H, Matsumoto T, Matsuda T, Nishimura K, Miyaji M. 1999. Mitochondrial DNA analysis of Exophiala jeanselmei var. lecanii-corni and Exophiala castellanii. Mycopathologia 146:75–77. 10.1023/A:1007057713782 [DOI] [PubMed] [Google Scholar]
- 146.Corbellini VA, Scroferneker ML, Carissimi M, Santolin LD. 2006. Delayed-type hypersensitivity response to crude and fractionated antigens from Fonsecaea pedrosoi CMMI 1 grown in different culture media. Mycopathologia 162:51–55. 10.1007/s11046-006-0034-9 [DOI] [PubMed] [Google Scholar]
- 147.Brown DL. 1993. The interpretation of tests of T-cell and natural killer cell function in man, p 843–858 In Lachmann PJ, Peters SDK, Rosen FS, Walport MJ. (ed), Clinical aspects of immunology. Blackwell Scientific, Boston, MA [Google Scholar]
- 148.Matsumoto T, Matsuda T. 1997. Sporotrichosis, chromoblastomycosis and phaeohyphomycosis: diagnosis and treatment. Dermatol. Ther. 3:91–96 [Google Scholar]
- 149.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Florl C, Lortholary O, Meletiadis J, Munoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin. Microbiol. Infect. 20(Suppl 3):47–75. 10.1111/1469-0691.12515 [DOI] [PubMed] [Google Scholar]
- 150.Fothergill AW, Rinaldi MG, Sutton DA. 2009. Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazole. Med. Mycol. 47:41–43. 10.1080/13693780802512451 [DOI] [PubMed] [Google Scholar]
- 151.Espinel-Ingroff A, Boyle K, Sheehan DJ. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101–115. 10.1023/A:1010954803886 [DOI] [PubMed] [Google Scholar]
- 152.Koo S, Klompas M, Marty FM. 2010. Fonsecaea monophora cerebral phaeohyphomycosis: case report of successful surgical excision and voriconazole treatment and review. Med. Mycol. 48:769–774. 10.3109/13693780903471081 [DOI] [PubMed] [Google Scholar]
- 153.Takei H, Goodman JC, Powell SZ. 2007. Cerebral phaeohyphomycosis caused by Ladophialophora bantiana and Fonsecaea monophora: report of three cases. Clin. Neuropathol. 26:21–27. 10.5414/NPP26021 [DOI] [PubMed] [Google Scholar]
- 154.Al-Abdely HM, Alkhunaizi AM, Al-Tawfiq JA, Hassounah M, Rinaldi MG, Sutton DA. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91–95. 10.1080/13693780400011104 [DOI] [PubMed] [Google Scholar]
- 155.Negroni R, Helou SH, Petri N, Robles AM, Arechavala A, Bianchi MH. 2004. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38:e15–e20. 10.1086/380840 [DOI] [PubMed] [Google Scholar]
- 156.Calvo E, Pastor FJ, Rodriguez MM, Mayayo E, Salas V, Guarro J. 2010. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 54:919–923. 10.1128/AAC.01284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Calvo E, Pastor FJ, Guarro J. 2010. Antifungal therapies in murine disseminated phaeohyphomycoses caused by Exophiala species. J. Antimicrob. Chemother. 65:1455–1459. 10.1093/jac/dkq171 [DOI] [PubMed] [Google Scholar]
- 158.Graybill JR, Najvar LK, Johnson E, Bocanegra R, Loebenberg D. 2004. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob. Agents Chemother. 48:2288–2291. 10.1128/AAC.48.6.2288-2291.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marine M, Pastor FJ, Guarro J. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 47:45–49. 10.1080/13693780802526840 [DOI] [PubMed] [Google Scholar]
- 160.Defaveri J, Graybill JR. 1990. Treatment of chronic murine chromoblastomycosis with the triazole SCH39304. Am. J. Trop. Med. Hyg. 42:601–606 [DOI] [PubMed] [Google Scholar]
- 161.Calvo E, Pastor FJ, Mayayo E, Hernandez P, Guarro J. 2011. Antifungal therapy in an athymic murine model of chromoblastomycosis by Fonsecaea pedrosoi. Antimicrob. Agents Chemother. 55:3709–3713. 10.1128/AAC.01662-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rivard RG, McCall S, Griffith ME, Hawley JS, Ressner RA, Borra H, Moon JE, Beckius ML, Murray CK, Hospenthal DR. 2007. Efficacy of caspofungin and posaconazole in a murine model of disseminated Exophiala infection. Med. Mycol. 45:685–689. 10.1080/13693780701390157 [DOI] [PubMed] [Google Scholar]
- 163.Trinh JV, Steinbach WJ, Schell WA, Kurtzberg J, Giles SS, Perfect JR. 2003. Cerebral phaeohyphomycosis in an immunodeficient child treated medically with combination antifungal therapy. Med. Mycol. 41:339–345. 10.1080/369378031000137369 [DOI] [PubMed] [Google Scholar]
- 164.Najafzadeh MJ, Suh MK, Lee MH, Ha GY, Kim JR, Kim TH, Lee HJ, Choi JS, Meis JF, De Hoog GS. 2013. Subcutaneous phaeohyphomycosis caused by Exophiala equina, with susceptibility to eight antifungal drugs. J. Med. Microbiol. 62:797–800. 10.1099/jmm.0.057406-0 [DOI] [PubMed] [Google Scholar]
- 165.Koga T, Matsuda T, Matsumoto T, Furue M. 2003. Therapeutic approaches to subcutaneous mycoses. Am. J. Clin. Dermatol. 4:537–543. 10.2165/00128071-200304080-00003 [DOI] [PubMed] [Google Scholar]
- 166.Bonifaz A, Carrasco-Gerard E, Saul A. 2001. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses 44:1–7. 10.1046/j.1439-0507.2001.00613.x [DOI] [PubMed] [Google Scholar]
- 167.Andrade TS, Castro LG, Nunes RS, Gimenes VM, Cury AE. 2004. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses 47:216–221. 10.1111/j.1439-0507.2004.00984.x [DOI] [PubMed] [Google Scholar]
- 168.Queiroz-Telles F, Esterre P, Perez-Blanco M, Vitale RG, Salgado CG, Bonifaz A. 2009. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med. Mycol. 47:3–15. 10.1080/13693780802538001 [DOI] [PubMed] [Google Scholar]
- 169.Calvo E, Pastor FJ, Salas V, Mayayo E, Capilla J, Guarro J. 2012. Histopathology and antifungal treatment of experimental murine chromoblastomycosis caused by Cladophialophora carrionii. J. Antimicrob. Chemother. 67:666–670. 10.1093/jac/dkr537 [DOI] [PubMed] [Google Scholar]
- 170.Negroni R, Tobon A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. 2005. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev. Inst. Med. Trop. Sao Paulo 47:339–346. 10.1590/S0036-46652005000600006 [DOI] [PubMed] [Google Scholar]
- 171.Bonifaz A, Paredes-Solis V, Saul A. 2004. Treating chromoblastomycosis with systemic antifungals. Expert Opin. Pharmacother. 5:247–254. 10.1517/14656566.5.2.247 [DOI] [PubMed] [Google Scholar]
- 172.Gupta AK, Taborda PR, Sanzovo AD. 2002. Alternate week and combination itraconazole and terbinafine therapy for chromoblastomycosis caused by Fonsecaea pedrosoi in Brazil. Med. Mycol. 40:529–534. 10.1080/mmy.40.5.529.534 [DOI] [PubMed] [Google Scholar]
- 173.Hamza SH, Mercado PJ, Skelton HG, Smith KJ. 2003. An unusual dematiaceous fungal infection of the skin caused by Fonsecaea pedrosoi: a case report and review of the literature. J. Cutan. Pathol. 30:340–343. 10.1034/j.1600-0560.2003.00067.x [DOI] [PubMed] [Google Scholar]
- 174.Najafzadeh MJ, Rezusta A, Cameo MI, Zubiri ML, Yus MC, Badali H, Revillo MJ, De Hoog GS. 2010. Successful treatment of chromoblastomycosis of 36 years duration caused by Fonsecaea monophora. Med. Mycol. 48:390–393. 10.3109/13693780903008813 [DOI] [PubMed] [Google Scholar]
- 175.Casadevall A, Pirofski LA. 2001. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 33:1048–1056. 10.1086/322710 [DOI] [PubMed] [Google Scholar]
- 176.Pappas PG. 2004. Immunotherapy for invasive fungal infections: from bench to bedside. Drug Resist. Updat. 7:3–10. 10.1016/j.drup.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 177.Safdar A. 2007. Antifungal immunity and adjuvant cytokine immune enhancement in cancer patients with invasive fungal infections. Clin. Microbiol. Infect. 13:1–4. 10.1111/j.1469-0691.2006.01571.x [DOI] [PubMed] [Google Scholar]
- 178.Armstrong-James D, Teo IA, Shrivastava S, Petrou MA, Taube D, Dorling A, Shaunak S. 2010. Exogenous interferon-gamma immunotherapy for invasive fungal infections in kidney transplant patients. Am. J. Transplant. 10:1796–1803. 10.1111/j.1600-6143.2010.03094.x [DOI] [PubMed] [Google Scholar]
- 179.Armstrong-James D, Harrison TS. 2012. Immunotherapy for fungal infections. Curr. Opin. Microbiol. 15:434–439. 10.1016/j.mib.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 180.McGinnis MR, Lemon SM, Walker DH, de Hoog GS, Haase G. 1999. Fatal cerebritis caused by a new species of Cladophialophora. Stud. Mycol. 43:166–171 [Google Scholar]
- 181.Badali H, Najafzadeh MJ, van Esbroeck M, van den Enden E, Tarazooie B, Meis JF, de Hoog GS. 2010. The clinical spectrum of Exophiala jeanselmei, with a case report and in vitro antifungal susceptibility of the species. Med. Mycol. 48:318–327. 10.1080/13693780903148353 [DOI] [PubMed] [Google Scholar]