Abstract
SUMMARY
Invasive fungal infections constitute a serious threat to an ever-growing population of immunocompromised individuals and other individuals at risk. Traditional diagnostic methods, such as histopathology and culture, which are still considered the gold standards, have low sensitivity, which underscores the need for the development of new means of detecting fungal infectious agents. Indeed, novel serologic and molecular techniques have been developed and are currently under clinical evaluation. Tests like the galactomannan antigen test for aspergillosis and the β-glucan test for invasive Candida spp. and molds, as well as other antigen and antibody tests, for Cryptococcus spp., Pneumocystis spp., and dimorphic fungi, have already been established as important diagnostic approaches and are implemented in routine clinical practice. On the other hand, PCR and other molecular approaches, such as matrix-assisted laser desorption ionization (MALDI) and fluorescence in situ hybridization (FISH), have proved promising in clinical trials but still need to undergo standardization before their clinical use can become widespread. The purpose of this review is to highlight the different diagnostic approaches that are currently utilized or under development for invasive fungal infections and to identify their performance characteristics and the challenges associated with their use.
INTRODUCTION
Rapid advances in the fields of transplant medicine and cancer treatment, together with the ever-growing implementation of immunomodulatory regimens, have led to a significant increase in the prevalence and prolonged survival of people in immunocompromised states (1). This change in the epidemiologic trend has led to an increased incidence of opportunistic pathogens, which thrive under these circumstances in patients in transplant and cancer units and also in patients in general medical and surgical wards (2). Among the various opportunistic pathogens, fungi represent a serious and important threat.
Fungal microbes are abundant in nature and are frequent colonizers on various human mucosal surfaces, where they can live by evading host defenses (3). However, under conditions of impaired immune responses or a break in host barriers, fungi are able to invade normally sterile areas of the human body, where they can cause severe infections that are difficult to recognize and treat and are often ultimately lethal (3). Indeed, recent epidemiologic data from various studies show that invasive fungal infections (IFIs) are frequently encountered in clinical practice, with the most common offenders, by far, being Candida spp. and Aspergillus spp.
In order to effectively eliminate these infections, early diagnosis and species identification are of paramount importance. Unfortunately, the current standard diagnostic methods are far from adequate (4–6). To overcome this obstacle, many researchers have focused on the development of novel diagnostic approaches, with serologic and, especially, molecular methods currently in the spotlight of such investigations.
The purpose of our review is to provide the reader with comprehensive and up-to-date information on diagnostic methods for IFIs that are currently under development or under investigation, focusing especially on molecular approaches.
CHALLENGES OF VALIDATING DIAGNOSTIC TESTS FOR FUNGAL PATHOGENS
Before implementation into routine clinical practice, and before incorporation into guidelines, every new diagnostic test should go through a lengthy process of validation. Many different analytical aspects of a new test should be evaluated, including the limit of sensitivity, reproducibility, and accuracy and, for quantitative tests, the upper and lower limits of quantification and the linear range. Accuracy can be difficult to determine when there is not a gold standard test or standard material available, which is the case for most tests used in fungal diagnostics. Once the analytical validation is complete, a clinical validation is required to assess the clinical utility of the test. These studies can be challenging to perform due to the limited number of cases of fungal disease that may be seen at any given institution. The need to validate an array of specimen types (whole blood, serum, plasma, bronchoalveolar lavage [BAL] fluid, or urine) further complicates test validation. Other important factors that influence the uptake of a test in the clinical laboratory include the ease of use, cost, and the fact that several of the newer molecular tests are complex to perform, requiring multistep manual methods to purify nucleic acids. Taking these challenges together, it is not surprising that there are a limited number of FDA-cleared fungal diagnostics in routine clinical use.
Unfortunately, when it comes to IFIs, the gold standard tests are far from perfect, as already mentioned. Therefore, the direct comparison of a new diagnostic test to culture-based systems might fail to identify tests that may, in some aspects, perform better than the gold standard. In fact, direct comparison in that case may create a false impression of low specificity of the new test if truly positive IFIs are identified by the new method but fail to be identified by the gold standard. In an effort to overcome this obstacle, the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) issued definitions for invasive fungal infections to be used for research purposes in 2002 (7), and these were subsequently revised in 2008 (8). These definitions take into account results of histopathology reports and standard diagnostic tests, together with imaging findings, predisposing factors, and clinical findings, and separate the research cases into proven, probable, possible, and unlikely IFIs.
However, even after the publication of these definitions, there is still an ongoing debate about the most efficient way to use the criteria in reporting evaluation results. Some researchers argue that by disregarding possible cases and considering only proven and probable cases as truly positive (and unlikely cases as truly negative), one can reach valid conclusions about the performance of a novel diagnostic test (9). Others prefer to classify possible cases as truly positive, especially when clinical suspicion is high, attesting that by totally disregarding possible cases, it is very difficult to reach significant conclusions, since this considerably limits the number of true-positive cases (10). These inconsistencies in design between studies conducted by different researchers hinder the possibility of cross-comparisons and valid meta-analyses of different studies and thus create a significant obstacle in the process of widespread implementation of novel diagnostic tests in the field of clinical mycology. Finally, the decreasing rates of autopsies in modern hospitals make the diagnosis of proven IFIs impossible in some cases, thus further confounding the results of observational studies.
NONMOLECULAR METHODS
Nonmolecular diagnostic techniques remain the established practice for diagnosing invasive fungal infections. However, their relatively low sensitivity often leads to considerable delays in diagnosis and initiation of targeted treatment.
Invasive Candidiasis
The diagnosis of invasive candidiasis requires biopsy of the involved tissue, followed by staining, culture, and histopathology. Blood cultures remain the gold standard for the diagnosis of candidemia and should be the initial diagnostic test when candidemia is suspected. However, cultures take 1 to 3 days to grow and an additional 1 to 2 days for identification of the organism, which often leads to considerable delays in initiation of targeted treatment. The impact of such delays in the case of IFIs is vast, with studies showing significant daily increases in mortality and hospitalization costs for every day without appropriate antifungal agents (11–17). For example, a study by Morrell and colleagues evaluated the delay in treatment due to the time required for diagnosis as a mortality risk factor for invasive Candida sp. infection. Their analysis found that administration of antifungal treatment 12 h after the first blood culture sample that tested positive was drawn was an independent determinant of hospital mortality (odds ratio [OR] = 2.09; P = 0.018) (13). Bactec 9240 and Bac/T Alert are the most commonly used blood culture systems for the detection of Candida spp. (18). The average time to detection for these systems ranges from 14 to 38 h, may take up to 72 h, and varies depending on the culture conditions used (most Candida spp. grow better in aerobic than in anaerobic bottles) and on the number of circulating cells (19). Notably, it is still unclear whether the use of dedicated fungal cultures via an isolator collection system can improve the diagnostic yield in cases of fungemia (20).
The β-glucan assay is a useful adjunct, especially for patients with intra-abdominal infections, where the sensitivity of cultures is decreased. β-d-Glucan is a major component of the fungal cell wall that is found in sera of patients suffering from many different fungal infections, including invasive candidiasis, invasive aspergillosis (IA), invasive fusariosis, and Pneumocystis jirovecii infection, and thus is not specific. Also, dialysis filters made from cellulose are reported to significantly increase serum β-glucan concentrations, leading to false positivity of the test (21). A multicenter study which included 107 patients with proven candidiasis evaluated the positive predictive value (PPV) of the β-d-glucan assay in relation to the cutoff value used. The test's PPV was 83.8% when a cutoff value of 60 pg/ml was used, compared to 89% when a cutoff value of 80 pg/ml was used (22). Another study suggested the use of this assay for the diagnosis of catheter-associated candidemia by showing that β-d-glucan was 4 to 10 times more abundant in biofilm than in planktonic conditions (23). Several studies have investigated the performance of β-glucan assay for the diagnosis of IFIs in patients with hematologic malignancies. The β-glucan test has been associated with a large number of false-positive results in this population and is not considered appropriate for screening purposes and thus for the selection of patients that need preemptive antifungal therapy (24). The sensitivity of the assay is lower for this population than for noncancer patients, likely due to the higher rates of colonization of these patients with multiple fungi and bacteria that can affect the test results (25, 26). A recently published meta-analysis which included more than 1,770 patients with hematologic malignancies showed that the performance of 2 consecutive β-glucan tests has an excellent specificity (98.9%) but a low sensitivity (49.6%) for the diagnosis of invasive fungal infections (27).
The Candida albicans germ tube antibody (CAGTA) assay is based on the detection of antibodies against the surfaces of C. albicans germ tubes by indirect immunofluorescence (28–31) and has a sensitivity of 77 to 89% and a specificity of 91 to 100% (28, 32). A study published in 2009 by Zaragoza et al. showed that intensive care unit (ICU) patients with a CAGTA-positive assay had lower mortality than patients with a negative assay, likely due to the administration of the appropriate empirical treatment in this group of patients (33). A more recent study, published in 2011 by the same group, suggests that the CAGTA assay is not affected by Candida colonization or intake of antifungal agents, which makes it particularly useful in the ICU setting (34). In an effort to establish the position of this test in routine clinical practice, Leon et al. introduced a new diagnostic tool for invasive candidiasis in 2011, based on positive CAGTA and β-glucan assays (35). Finally, other serologic tests for candidemia are the mannan antigen and anti-mannan antibody tests. The combination of a positive mannan test and a positive anti-mannan antibody test has a sensitivity of 73% and a specificity of 80% for the diagnosis of invasive candidiasis in patients with neutropenic fever. The high negative predictive value of 95% suggests the use of the test for exclusion of the disease in this population (36).
In general, the sensitivity of antibody assays is limited for immunocompromised patients, who are at high risk for becoming infected by invasive candidiasis, since this population often cannot develop antibodies against Candida antigens. Also, the specificity of these assays is limited by the fact that Candida species are part of the normal flora.
Invasive Aspergillosis
The diagnosis of invasive aspergillosis is proven by demonstration of the fungal hyphae in tissue biopsy specimens. The sensitivity of culture for the diagnosis of aspergillosis is low and depends on the population tested. In two recently performed studies, among transplant recipients with a positive molecular test for invasive aspergillosis, only 25 to 50% had a positive culture result (37, 38). However, a recent study, published in 2005, suggested that incubation of cultures at 35°C leads to a 31% increase in sensitivity compared to incubation at 25°C, which suggests that attempting to mimic physiologic conditions may improve the yield of cultures (39). Also, the positive predictive value of culture depends on the prevalence of the infection, and thus it is higher among immunocompromised patients and in areas of endemicity. For example, in a study that assessed the positive predictive value of sputum or BAL fluid cultures for different patient populations, the positive predictive value was 72% for hematopoietic cell transplant (HCT) recipients, patients with hematological malignancies, and granulocytopenic patients, 58% for solid organ transplant recipients and patients receiving steroids, and only 14% for patients with HIV infection (40). In the same study, the PPV was highest for BAL fluid cultures, likely because patients are more likely to have an invasive fungal infection when there are radiographic findings that require bronchoscopy. As a general rule, isolation of Aspergillus spp. from sputum almost invariably represents colonization in immunocompetent patients, while it suggests invasive disease in the setting of a suppressed immune response (41). Therefore, repeated isolation of the same Aspergillus spp. or alternative diagnostic tests for invasive aspergillosis should be sought to accurately interpret a positive finding, especially in the absence of host factors (42). However, in the case of critically ill patients, an Aspergillus sp.-positive culture result portends a poor prognosis irrespective of colonization or active infection (43). Finally, we note that the yields of blood cultures for invasive aspergillosis are very low and thus have a low value even for individuals at high risk (44). Another disadvantage of cultures is the delay in identifying the species, especially those that are slow to sporulate, which may delay the selection of the appropriate antibiotic (45).
Histopathology has the advantage of detecting both the invasion of various tissues by fungi and the host response or tissue necrosis. It is almost always performed in conjunction with cultures and improves their positive predictive value by confirming positive culture results. Thus, direct tissue stains are often used to clarify if a positive culture is the result of infection, colonization, or contamination. Aspergillus species can be seen by Gomori methenamine silver or periodic acid-Schiff (PAS) staining. Most tissue stains are inexpensive and can be performed easily in various specimens, such as sputum, BAL fluid, aspirates from lesions, cerebrospinal fluid (CSF), and other tissues (46, 47). Fungi are identified based on size and morphological characteristics, which are generally nonspecific, thus allowing only for a descriptive diagnosis (48–50). Stains do not always allow for accurate identification; for example, Aspergillus spp., Fusarium spp., and Scedosporium spp. all appear as septate, narrow-angle-branching hyphae. Moreover, tissue stains do not allow for identification of the fungus to the species level, which is often needed for treatment. On the other hand, value can be added to the diagnosis by providing clinicians with information about the infecting cell morphology and the state of infected tissues. Furthermore, with the use of advanced microscopy techniques, direct tissue examination and visualization of the infection site have the potential to inform clinicians if a fungal biofilm has formed, a condition that is known for its resistance to commonly used antifungal regimens.
The β-d-glucan assay is often useful in combination with culture. Overall, the sensitivities of β-d-glucan testing in individual studies have ranged from 55% to 95%, and specificities from 77% to 96%, for patients with hematologic malignancies who are suffering from invasive aspergillosis (22, 25, 51–54). Note that the specificity of this test is lower among certain patient populations, such as dialysis recipients and individuals with concurrent Gram-negative bacterial infections (55–57). For example, when the reactivities of different bacterial cultures were tested using the Fungitell assay, bacterial β-d-glucan of Pseudomonas aeuroginosa cross-reacted with the assay, resulting in false-positive results for patients with bacteremia and no fungemia (58). Finally, although different β-glucan assays have different optimal cutoff values to define positivity, the data from existing clinical studies for the Fungitell assay (the most widely used assay) suggest that the use of a cutoff of 80 pg/ml is associated with better accuracy, while a result of 60 to 80 pg/ml is considered indeterminate, since higher cutoffs significantly decrease the test's sensitivity, although they increase the specificity of the assay (27, 53, 59).
The galactomannan (GM) assay is a fairly specific and sensitive test for the diagnosis of invasive aspergillosis, although galactomannan can also be found on the cell walls of Histoplasma capsulatum and Fusarium spp. (60, 61). It can be performed in serum, BAL fluid, CSF, or pleural fluid. Its specificity and sensitivity vary from 40 to 100% and are greatly dependent on the population tested (26, 62). Specifically, previous antibiotic treatment decreases the specificity of the test, while an antifungal regimen decreases its sensitivity. In addition, the type of antifungal agent affects the performance of the test, and caspofungin has been associated with a higher sensitivity than that with other antifungal agents (26), likely due to the increase in galactomannan levels after treatment with caspofungin (63). Note that the test has the highest sensitivity among patients with hematological malignancies or those who have undergone hematopoietic cell transplantation compared to those who have undergone solid organ transplantation or immunocompetent patients (64, 65). In addition, the sensitivity of the test varies depending on the species and is higher for patients with non-fumigatus aspergillosis than for patients with aspergillosis caused by Aspergillus fumigatus (26). Also, the performance of the test depends on the immune response of the host as well as the pathogenesis of the disease, with its sensitivity being lower for patients treated with steroids than for neutropenic patients (66). This might be explained by different progressions of the disease between these 2 types of patients, as shown by a study performed in rabbits. Specifically, neutropenic rabbits had more hyphae than steroid-treated rabbits (67). Thus, patients with invasive aspergillosis who have been treated with steroids are more likely than neutropenic patients to have a false-negative result (66).
The sensitivity of the galactomannan test is considered higher when performed with BAL fluid than when performed with serum, with a cutoff value of 1, and a relationship between serum galactomannan, but not BAL fluid galactomannan testing, and mortality of hematopoietic stem cell transplant patients has been described (68, 69). Other studies have also suggested the use of the galactomannan test as a predictor of all-cause mortality (69, 70). Since its sensitivity increases even more with sequential testing, it is often used in combination with culture for the definitive diagnosis of a fungal infection (71–74). False-positive results for BAL fluid may represent simple colonization of the airways by fungi, more often in lung transplant recipients, or contamination, but this does not significantly affect the specificity of the assay, which remains above 95% (71). Because performance may depend on the amount of BAL fluid tested, protocols for the application of the technique should be established. Although the galactomannan test has been used for a long time, the optimal cutoff for a positive result has yet to be determined, with some studies showing that a lower cutoff value of 0.5 versus 1 compromises specificity and thus should be avoided (73), while others suggest that the highest accuracy is achieved by selecting the lower cutoff value and testing consecutive samples (75, 76). This disagreement is reflected in the latest EORTC guidelines, which avoid recommending a specific cutoff and leave the onus to the manufacturer of the test (8). It is noteworthy that galactomannan assay is often used to monitor the response to treatment (64), as it is positive more frequently for patients who fail antifungal treatment (63). Galactomannan assay with serum but not BAL fluid may have prognostic value, according to a recent study by Fisher et al. which showed that higher serum galactomannan levels were associated with higher respiratory mortality in allogeneic HCT recipients (69). Note that the use of BAL as a diagnostic intervention in general has provoked some debate recently, due to the wide inconsistencies in the diagnostic yields reported by different operators, which is particularly problematic in the case of high-risk patients, who are frequently colonized or infected by multiple microbial species. A method that could help to reduce these inconsistencies without adversely affecting the diagnostic yield or the complications from the procedure would be the use of a standardized method to perform BAL, as shown by a large prospective study (77).
Lateral-flow devices (LFDs), which do not require any technical expertise, were recently shown to be more accurate than the standard serologic markers. Their excellent clinical performance and the fact that they can be performed easily and quickly suggest their use as point-of-care (POC) tests (78). In 2008, Thornton et al. introduced an LFD which detects a glucoprotein antigen in the sera and BAL fluid of patients with invasive aspergillosis in 15 min. This antigen, which is secreted during active growth of A. fumigatus, binds to a monoclonal antibody used to perform the assay and has increased specificity and sensitivity compared to the Fungitell and Platelia GM assays (79–81). However, since the interpretation of LFD test results is somewhat subjective, they are useful for the confirmation or exclusion of invasive aspergillosis in combination with other tests, such as PCR (78). Finally, a recent study published by Held et al. showed that an LFD has a better clinical performance than that of galactomannan assay when used as a screening test rather than a confirmatory test (82).
The use of electronic noses (E-noses), which assess volatile organic compounds (VOCs), was recently suggested for the diagnosis of invasive aspergillosis, as a diagnostic tool with a high accuracy (90.9%) (83). Recent studies have shown that patients with invasive aspergillosis exhale a specific VOC, which can be used as a biomarker for the development of fast and cheap diagnostic techniques (84, 85). Further studies are required to validate such easy-to-use techniques.
Other Fungal Infections
Pneumocystis jirovecii is an opportunistic fungal pathogen that causes severe lung disease to patients at risk. Various diagnostic methods performed on several different specimens have been proposed for the diagnosis of this infection (86). Older techniques rely on the visualization of Pneumocystis cysts or trophozoites by use of various stains (methenamine silver, toluidine blue, and calcofluor white) on expectorated or induced sputum specimens or on more invasive specimens, such as BAL fluid or lung tissue biopsy specimens. Alternatively, simple imaging with a chest X-ray has been proposed as a diagnostic method. Apart from chest X-ray, which has a questionable performance as a sole diagnostic test due to low specificity, all the other methods have high specificities, which often reach 100%. However, their sensitivities vary greatly, from 33% to 100%, depending on the stain and the specimen used. Because Pneumocystis cysts preferentially affect the alveolar space, expectorated sputum is the least accurate specimen, while BAL fluid is the best (87). Importantly, though, in choosing among different diagnostic practices, one should also consider the cost associated with their utilization, especially for evaluating diseases that preferentially affect populations of lower socioeconomic status, such as opportunistic infections. To address this issue, Harris et al. performed a cost-effectiveness analysis to compare the different diagnostic techniques for Pneumocystis pneumonia (PCP) (86). Their results indicate that toluidine blue staining of induced sputum samples is the most cost-effective among the staining methods, while the performance of BAL greatly increased the cost of each method, without significantly affecting the percentage of people successfully treated. A newer serologic method that was not involved in the previous analysis and that deserves to be mentioned is the β-glucan assay (88). A recent meta-analysis indicated that β-glucan assay performed on serum has a sensitivity and specificity of 94.8% and 86.3%, respectively, for the diagnosis of Pneumocystis pneumonia (89), while a large retrospective cohort showed that a positive β-glucan test correlates well with BAL fluid fungal loads (90). Therefore, β-glucan assay can be an excellent screening tool to rule out the disease in at-risk populations (88), while additional confirmatory tests are necessary because of the high rate of false-positive results (90). Furthermore, a prospective study involving 147 patients suspected of having Pneumocystis jirovecii infection showed that β-glucan assay is valuable for discriminating definite and probable infections from colonization (91). On the other hand, a recent study on the kinetics of the test showed that it is not useful as a predictor of a positive response to treatment, as decreases in β-glucan values lag significantly behind clinical improvement (92).
Cryptococcus spp. are known to affect primarily immunocompromised individuals, such as people with HIV infection, with the exception of Cryptococcus gattii, which is notorious for its ability to cause disease in immunocompetent patients (93). The main characteristic of all Cryptococcus spp., which is the basis for the majority of current diagnostic tests, is the polysaccharide capsule, which contains the glucuronoxylomannan antigen. Cryptococcal meningitis, the most common presentation of cryptococcal disease, is diagnosed primarily with CSF cultures, which grow cream-colored mucoid colonies within 3 to 7 days. However, this delay from suspicion to diagnosis is often unacceptable in the case of such a serious infection, and therefore, alternative screening tests that allow for timely treatment initiation are often performed as adjuncts to culture. Perhaps the oldest method that is still used in clinical laboratories involves staining of the CSF with India ink, which allows visualization of the cryptococcal cells under the microscope, as round, encapsulated yeast organisms, in more than 75% of patients (94). The most accurate screening method, however, is the cryptococcal antigen test. The test has a high sensitivity and specificity when performed with CSF (97% and 93 to 100%, respectively), while it also has the advantage that it can be performed on serum, with acceptable sensitivity (87%), when CSF is not available (95). False-positive findings have been reported in cases of Trichosporon sp., Capnocytophaga sp., or Stomatococcus sp. invasive infections (96). Thanks to its superior performance, the cryptococcal antigen test was included as a method of cryptococcal meningitis diagnosis in the latest EORTC/MSG guidelines. Latex agglutination testing and enzyme immunoassay (EIA) are both widely used methods for cryptococcal antigen detection, with a high concordance between them, although latex agglutination tends to give more false-positive results, especially at low titers (95). A newly developed method to detect cryptococcal antigen utilizes a lateral-flow immunoassay and has demonstrated a performance comparable to those of EIA and latex agglutination on both CSF and serum (97). Its low cost, ease of use, high accuracy, and ability to be performed on both serum and urine make it very promising as a point-of-care diagnostic method in settings with limited resources (98). This becomes particularly important when one considers that antiretroviral treatment alone is insufficient for the management of HIV-infected individuals with CD4 counts of <100 cells/μl who are positive for cryptococcal antigen (99). Therefore, for these populations, a rapid point-of-care screening test for Cryptococcus spp. can prove to be lifesaving (99). Finally, we note that while the β-glucan antigen test is useful as a screening method for most fungal infections, Cryptococcus spp. are among the exceptions (55). Therefore, this test is not recommended in this context.
The dimorphic fungi Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis share many similar characteristics in morphology and the clinical picture of the infections that they can cause. Microscopic examination followed by culture of the infected tissue is the primary diagnostic method. However, the sensitivity of histopathology is limited, and cultures can take up to 30 days to give a positive result, thus rendering them confirmatory. Therefore, there is a growing body of literature on alternative methods to diagnose these fungal infections, focusing primarily on antigen-antibody tests (Table 1). Indeed, antibody tests that use complement fixation (the most common) or immunodiffusion are available for Histoplasma spp., although their sensitivities are not ideal (75% for disseminated cases and 66.7% for acute pulmonary histoplasmosis) (100). Similarly, in the case of blastomycosis, antibody tests have low sensitivities, although immunodiffusion is more sensitive and specific than complement fixation (101). On the other hand, antigen tests are available for both of these fungal infections, can be performed on both urine and serum, and have superior sensitivities (83.3 to 91.8% for histoplasmosis and 92.9% for blastomycosis) (100, 101). Also, a recent study showed that H. capsulatum antigenemia, but not antigenuria, decreases rapidly after clearance of the infection in AIDS patients with disseminated disease, thus having the potential to be used to monitor the response to treatment (102). Note that the H. capsulatum antigen test has a high sensitivity for both AIDS patients and patients with other causes of immune deficiency, such as solid organ transplant recipients (103), while its sensitivity for nonimmunocompromised individuals is relatively lower in cases of disseminated histoplasmosis (100). Importantly, cross-reactivity of these antigen tests seems to be a problem, and although they are specific when tested against nonfungal pathogens, they cannot differentiate between H. capsulatum, B. dermatitidis, and C. immitis, despite the fact that the antigen level is generally higher in cases of disseminated histoplasmosis than in cases of other endemic mycoses (104). A novel antibody EIA for blastomycosis was recently developed and has a high sensitivity (87.8%), with the additional benefit of being highly specific for B. dermatitidis, showing 94% specificity in patients with histoplasmosis (105). Serologic tests for coccidioidomycosis include traditional complement fixation and tube precipitin antibody detection methods, which are both commonly employed as diagnostic tests due to their high levels of accuracy. A novel enzyme immunoassay for C. immitis antibody was recently developed and is promising (sensitivity of 95.5% and specificity of 98.5%) (106). However, the interpretation of a single positive IgM value can be different depending on the pretest probability, and false-positive results have been reported for asymptomatic individuals (107). Finally, we note that with the exception of blastomycosis, the β-glucan antigen test can also have value as a screening method for these fungal infections (108).
TABLE 1.
Techniques used for antigen-antibody detection
| Method | Description | Examples of tests or target detected |
|---|---|---|
| Latex agglutination testing | Latex beads coated with antibodies are mixed with the patient sample. If the antigen is present in the sample, the antibodies will attach to the antigen and agglutination will occur. Testing of serial dilutions of the sample can give a quantitative measure of the amount of antigen present. | Cryptococcal antigen detection. |
| Enzyme immunoassay | ||
| Direct | The patient's sample is spread on a plate, and time is allowed for the antigen to adhere to the plastic through charge interactions. An antibody with an enzyme conjugate that changes color after addition of a substrate is added to the patient's sample. If the antigen is present, the antibodies attach and a color change is detected through optical density measurement. | Coccidioides sp. and Blastomyces sp. antibody detection; Histoplasma sp., Blastomyces sp., and Cryptococcus sp. antigen detection. Also, the galactomannan antigen test uses sandwich immunoassay technology. |
| Indirect | The patient's sample is added to a mixture that contains a specific antigen. If antibodies are present in the sample, they will attach to the antigen. Subsequently, antibodies with an enzyme conjugate that bind the primary antibodies are added to the mixture. Color change is detected by optical density measurement. | |
| Sandwich | Same as direct EIA, with the exception that the plate already contains a capture antibody that binds the target antigen, so no charge interactions are necessary. | |
| Immunodiffusion | An agarose gel is prepared with wells cut into the gel. The patient's sample is placed in the center well, and the control antigens or antibodies are added to the outside wells. If the target antibodies or antigens are present in the tested sample, they will form precipitin lines by interacting with the control antigens or antibodies, respectively. | Histoplasma sp. and Blastomyces sp. antibody detection. |
| Complement fixation | The patient's sample is isolated and heated to destroy all existing complement proteins. Standardized complement proteins and a specific antigen or antibody are added to the sample. Sheep red blood cells (sRBCs) prebound with anti-sRBC antibodies are added to the mix. If the target antibodies or antigens are present in the sample, they will bind the added antigen or antibody and form complexes, which will react with and deplete the complement proteins and salvage the sRBCs. If not, the complement proteins will lyse the sRBCs, thus changing the color of the mix. | Histoplasma sp., Blastomyces sp., and Coccidioides sp. antibody detection. |
| Lateral-flow assay | The technology is based on a series of capillary beds on an element that can spontaneously transport fluid. The patient's sample is added to the first bed. This soaks up all the extra fluid. The remains are transferred to the second bed, which contains antibodies with an enzyme conjugate that bind antigens or antibodies in the tested sample. The antigen-antibody complexes then move to a third bed, which has capture antibodies that bind the complexes. An additional bed binds only the control antibodies without the antigen, thus serving as a control to ensure that the method worked properly. | Point-of-care diagnostic tests for Cryptococcus sp. and Aspergillus sp. antigen detection. |
| Immunofluorescence assay (IFA) | The methodology is very similar to that for enzyme immunoassay, but instead of antibodies with an enzyme conjugate, this assay utilizes fluorescein-labeled antibodies which can then be visualized under a fluorescence microscope. It can be performed as both direct and indirect assays. | CAGTAa assay is an indirect IFA. |
| G test | The G test is specific to beta-glucan detection. Factor G is a proclotting factor that is highly sensitive to beta-glucan. When a patient's sample containing beta-glucan is added to a mix containing factor G, it activates the factor, thus initiating an enzymatic cascade that results in a color or optical density change of the mixture, which can be detected with colorimetric or turbidimetric methods. | Beta-glucan detection. |
CAGTA, Candida albicans germ tube antibody.
It is evident that antigen-antibody detection methods are available for all the aforementioned fungal infections and have proven to be particularly useful as methods to either “rule in” or “rule out” the infection and to initiate targeted antifungal therapy in a timely manner. However, other infections, such as mucormycosis, fusariosis, and scedosporiosis, still do not have any available antigen-antibody methods that could be helpful. Specifically, mucormycosis is diagnosed mainly based on histopathology and cultures and requires a high index of suspicion (109). Interestingly, in a case series of a rare outbreak of cutaneous necrotizing mucormycosis, all cases were either culture or histopathology positive (110). Note that β-glucan testing has proven to be of little help in cases of Mucor sp. infections (55). Thankfully, in contrast to the case for invasive aspergillosis, blood cultures have proven to be useful in cases of other invasive mold infections, such as fusariosis (111) and scedosporiosis (112), but not for infections with rarer saprophytic molds (113). Also, Scedosporium sp. cultures tend to be more reliable for patients with hematologic malignancies than for patients with solid tumors (112).
Each of the nonmolecular assays (cultures, histopathology, and biomarker assays) provides a piece of information to aid clinicians with diagnosing fungal infections (Table 2). Taking into consideration that IFIs are difficult to diagnose and that any delay in treatment initiation could lead to a steep rise in mortality rates, newer diagnostic assays with high negative predictive values, such as β-glucan or galactomannan assay, should be evaluated in clinical decision algorithms for the ability to serve dual purposes. They could be used to rule out the disease and decrease empirical antifungal use in high-risk populations without other radiologic or microbiologic signs of an IFI, or they could have value as methods to justify stopping presumptive antifungal treatment already initiated in a patient who shows sequentially negative biomarkers for the disease. The combined use of multiple diagnostic assays may increase the accuracy of diagnosis, but at a higher cost; therefore, the cost-effectiveness of each diagnostic strategy should be evaluated further (114).
TABLE 2.
Nonmolecular tests used for diagnosis of the most common invasive fungal infectionsa
| Microorganism | Diagnostic test | Optimal specimen type | Sensitivity (%) | Specificity (%) | Reasons for false-positive results | Reasons for false-negative results | Comments |
|---|---|---|---|---|---|---|---|
| Candida spp. | Cultures | Blood | 50–60 | 95 | None | None | Gold standard. May take up to 3 days for a positive result. |
| Beta-glucan assay | Serum | 77.6–81.3 | 87.1–92.4 (for patients not infected with fungal pathogens) | Other fungal infection, dialysis filters made from cellulose, bacteremia? | Hemolyzed samples, higher cutoff values | A cutoff of 80 pg/ml is associated with higher accuracy; used as screening test for various fungal infections. | |
| CAGTA assay | Serum | 77–89 | 91–100 | Unknown | Unknown | CAGTA assay is not affected by Candida colonization or intake of antifungal. | |
| Aspergillus spp. | Histopathology | Various, depending on the infection site | 100 | 100 | Fusarium and Scedosporidium spp. have similar microscopic appearances | Formation of pseudoseptations by the organism | Most accurate test is tissue biopsy. Used as a last resort in undiagnosed cases. |
| Culture | Various, depending on the infection site | 30–68 | 72–100 | Aspergillus sp. colonization | Slow-sporulating organisms, hematopoietic stem transplant recipients | Gold standard, but with low sensitivity. PPV largely depends on the population tested. | |
| Galactomannan assay | Serum, BAL fluid, or CSF | 71 for serum, 90 for BAL fluid | 89 for serum, 94 for BAL | Histoplasma sp. and Fusarium sp. infections, fungal colonization | Steroid treatment | Optimal diagnostic cutoff is not yet established. GM levels could be used to monitor the response to treatment. | |
| Beta-glucan assay | Serum | 55–95 | 77–96 | Other fungal infection, Gram-negative bacteremia, dialysis | Hemolyzed samples, higher cutoff values | A cutoff of 80 pg/ml is associated with higher accuracy; screening test for various fungal infections. | |
| Lateral-flow device antigen detection | Serum and BAL fluid | 48–100 | 100 | Unknown | Unknown | Interpretation is subjective; perhaps has a better performance than that of GM assay. | |
| Pneumocystis spp. | Histopathology | Expectorated sputum, induced sputum, or BAL fluid | 33–100, depending on stain and specimen used | 100 | Uncommon | Varies by specimen type and stain | Methenamine silver stain on BAL fluid is the current gold standard. Toluidine blue stain on induced sputum may be the most cost-effective method. Pneumocystis does not grow easily in culture. |
| Beta-glucan assay | Serum | 94.8 | 86.3 | Other fungal infection, bacteremia, dialysis | Uncommon | Excellent screening test for high-risk patients, not useful for monitoring response to treatment. | |
| Cryptococcus spp. | Cultures | CSF | >95 | 100 | Uncommon | Uncommon | Gold standard, but takes 3–7 days for a positive result. |
| Histopathology | Mostly CSF | 75 | 100 | Uncommon | Low levels of microorganism | India ink stain often used as a screening test. | |
| Cryptococcal antigen test (LA, EIA, or LFD) | CSF or serum | 97 for CSF, 87 for serum | 93–100 | Trichosporon sp., Capnocytophaga sp., or Stomatococcus sp. invasive infections | Uncommon | Most accurate test when performed on CSF. The three methods are comparable, although LA gives more false-positive results. LFD is best for rapid point-of-care diagnosis. | |
| Histoplasma capsulatum | Culture | Tissue, BAL fluid, or other bodily fluids | 85 for disseminated and acute pulmonary infections | 100 | Uncommon | Low fungal levels on specimen | Gold standard, but takes 2–4 weeks to grow. |
| Histopathology | Tissue or BAL fluid | 76 for disseminated infection | 100 | Uncommon | Low fungal levels | Unacceptably low sensitivity, which is even lower for pulmonary infection. | |
| Antibody tests (CF or ID) | Serum | 75 for disseminated infection, 67 for acute pulmonary infection | 100 | Uncommon | Low fungal levels | Best performance with combination of the two methods. One study showed unacceptably low sensitivity for solid organ transplant patients. | |
| Antigen test | Urine and serum | 88–92 | 100 for patients without fungal infection | High cross-reactivity in cases of Blastomyces sp. or Coccidioides sp. infection | Uncommon | Most accurate test overall, but shows cross-reactivity with other dimorphic fungi. | |
| Blastomyces dermatitidis | Culture | Sputum, BAL fluid, tissue | 86 for sputum, 92 for BAL fluid | 100 | Uncommon | Low levels | Gold standard, grows better on fungal isolator cultures, takes a long time to grow. |
| Histopathology | Varies based on infection site | 46 for sputum, 90 for tissue | 100 | Uncommon | Incorrect specimen | Broad-based budding. | |
| Antibody test (CF and ID), new EIA | Serum | 57 for CF, 65–80 for ID, 88 for novel EIA | 37 for CF, 100 for ID, 100 for EIA | Cross-reactivity with other dimorphic fungi | Low levels of circulating antibodies | ID method is clearly preferable due to higher performance. EIA has high specificity even in cases of histoplasmosis. | |
| Antigen test | Mostly urine | 93 | 99 for patients free of fungal infection | High cross-reactivity with other dimorphic fungi (e.g., 96% with histoplasmosis) | Uncommon | Most accurate test, but with cross-reactivity issues | |
| Coccidioides spp. | Culture | Sputum or tissue | 90 | 100 | Uncommon | Low fungal levels | Grows better than all endemic fungi. Culture is used mainly for hospitalized patients. Can grow within a week, but identification can take longer. |
| Histopathology | Sputum or tissue | 31–42 | 100 | Uncommon | Low fungal levels on specimen | Spherule detection. | |
| Antibody assays (CF, TP, and novel EIA) | Serum | 95 | 99 | False-positive results have been reported for asymptomatic individuals | Uncommon | Most commonly used test. EIA seems to have the best accuracy but is still not widely tested. A single positive IgM result must be interpreted based on pretest probability. |
BAL, bronchoalveolar lavage; CAGTA, Candida albicans germ tube antibody; CF, complement fixation; CSF, cerebrospinal fluid; EIA, enzyme immunoassay; GM, galactomannan; ID, immunodiffusion; LA, latex agglutination; LFD, lateral-flow device; PPV, positive predictive value; TP, tube precipitin.
MOLECULAR METHODS
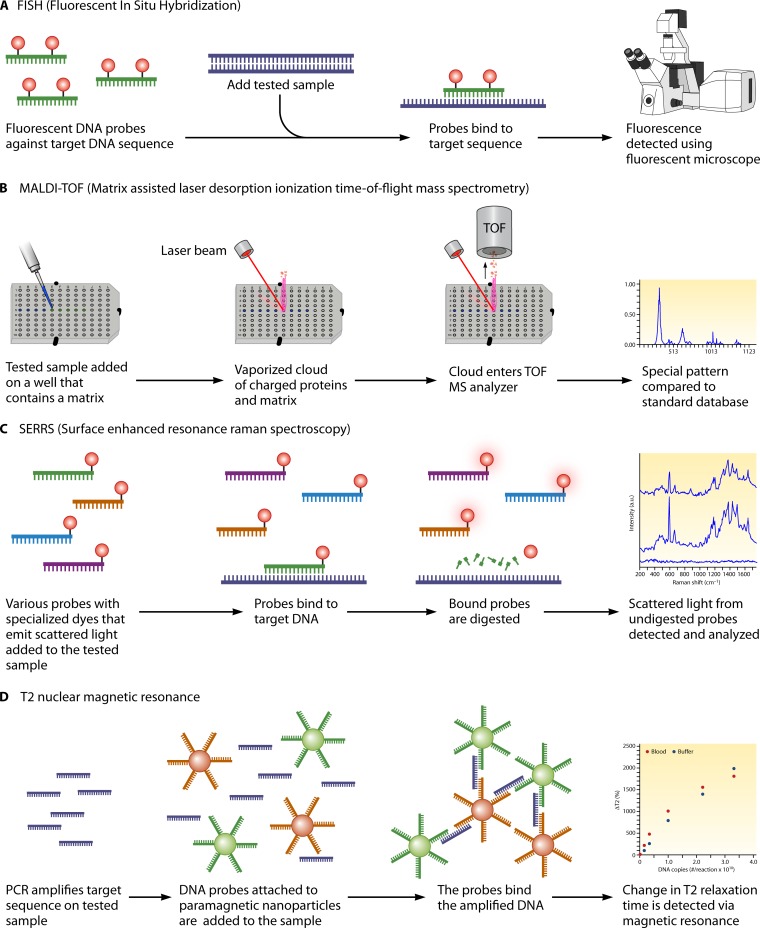
Given the rapid and significant advances in cell biology, a series of new diagnostic techniques aiming at identifying the unique molecular fingerprint of each pathogenic microorganism came into use in infectious disease diagnostics and soon became widely available and extremely efficient for diagnosis of certain diseases, such as viral infections. Indeed, molecular methods, the most important of which is PCR, are used every day in routine clinical practice and have replaced traditional diagnostic procedures for a variety of human infections (115). Their simplicity, ease of use, and short turnaround time are among their most important advantages over traditional techniques. Therefore, it is not surprising that these methods have for years been in the spotlight as a potential solution to the problem of IFI diagnosis (116).
PCR
The sensitivity of molecular methods raises the possibility of identifying an infection at a very early stage, when it is easier to treat or even completely prevent its clinical manifestation. PCR is one of the oldest and most widely used molecular methods in fungal diagnostics. A major drawback of all traditional PCR techniques initially developed as potential fungal diagnostic tests is that they do not quantify the amount of amplified DNA. Therefore, there is no reliable way to identify the microbial burden within the human body. When it comes to IFIs, this becomes very significant, as fungi are frequent colonizers of human surfaces, and this makes it impossible to determine if the identified fungal DNA is the result of the colonization or does in fact represent an active infection (117). A solution to the problem was given by the development of real-time PCR techniques. As the name suggests, real-time PCR is able to quantify the amount of amplified DNA in real time (118). As a result, real-time PCR techniques have largely replaced conventional PCR methods in clinical laboratories.
Despite the great potential of PCR methods, several technical issues associated with their use for fungal DNA isolation create significant discrepancies between different assays and impede efforts toward standardization. More specifically, fungal organisms, and especially molds, have strong cell walls that are particularly difficult to lyse, thus requiring complex and cumbersome methods for DNA isolation (119). Examples of lysis techniques utilized are enzymatic digestion processes that often rely on use of toxic chemicals, such as phenol-chloroform, mechanical disruption using glass beads, and sonication (120). In an effort to overcome this barrier, automated extraction methods have been developed that are able to decrease the time for sample processing and lessen the possibility of errors (121). However, it is still unclear whether these techniques alone adequately disrupt the fungal cell wall and significantly improve the sensitivity of fungal PCR assays (121, 122). Another problem associated with fungal PCR is the potential for contamination. Fungi are ubiquitous in the environment and can easily contaminate surfaces and materials used in all steps of fungal PCR, including commercially available reagents (123) and collection tubes (124). Therefore, careful precautions and highly experienced personnel are necessary to avoid false-positive findings associated with contaminants. Another challenge is the choice of the best sample to evaluate the new tests. For example, the significance of Aspergillus sp. isolation from sputum samples is difficult to ascertain for critically ill patients, as it can be hard to differentiate between colonization and chronic infection (41). Furthermore, without international standards, it is difficult to assess the agreement of quantitative data from different tests and thus to determine the clinical significance of various levels of fungal DNA. Finally, the choice of primers is another important factor that could alter the diagnostic performance of PCR tests for IFIs (Table 3).
TABLE 3.
Issues regarding the use of PCR for fungal diagnostics
| Factor | Potential solutions | Advantages and/or disadvantages |
|---|---|---|
| Choice of sample | Many different sample types have been proposed | The best sample varies depending on the target pathogen and the site where it is preferentially accumulated. Tissue samples can be ideal to evaluate a new test, as they can differentiate between colonization and infection. However, less invasive samples, such as BAL fluid, serum, and whole blood, are favorable because they can be utilized as screening tests. Serum also allows for multiple tests to be performed with the same sample. |
| DNA extraction | Use of larger sample volumes, lower elution volumes, and appropriate cell and fungal wall lysis methods | The general idea is to maximize and concentrate the amount of fungal cells or free fungal DNA in the tested sample. However, even with perfect DNA extraction, some fungal species may be found in the circulation only transiently when they establish deep-seated infections. |
| Primer selection | rDNA versus mitochondrial DNA versus other DNA | The target amplification sequence should be found in multiple repeats and should differ from the respective host sequence. rDNA seems to be superior to mitochondrial DNA for diagnosis of aspergillosis. |
| Type of PCR | Standard versus nested versus real-time PCR | Nested PCR requires additional time and might be more prone to contamination due to the additional amplification step. Real-time PCR allows for quantitation of the amplified DNA and thus could help to differentiate infection from colonization. |
| In vitro validation of a certain PCR | Use of reference strains or DNA calibrator materials | These methods are of paramount importance for the accurate evaluation of the sensitivity of any PCR and allow for interlaboratory comparisons of the results. |
| Coinfection by multiple microbial species | Broad-range PCR with postamplification identification methods | This technique allows for the simultaneous identification of multiple microbial pathogens from the same sample. The broadest method that has been proposed is the multiplex SeptiFast PCR. However, this has not yet been tested for use for patients at high risk for fungal infections. |
Due to the aforementioned issues, no single test has yet provided enough evidence of its accuracy to be incorporated into guidelines, and thus PCR is not yet widely used in the diagnosis of IFIs (95, 125). In order to understand the details behind this fact, and given the wide variety of fungal infections, with different characteristics and problems associated with each, it is best to study every disease separately.
IA.
Many different PCR assays have been developed over the years for invasive Aspergillus sp. infections. However, clinical reports of their sensitivities and specificities range considerably, from 43 to 100% and 64 to 100%, respectively (9, 10, 52, 72, 126–168). Table 4 lists clinical studies evaluating Aspergillus sp. PCR on various clinical specimens, with comments on special characteristics of each study. There are many reasons behind the obvious variability between different clinical trials, including but not limited to the choice of primers, the method for identification of the amplified DNA, the clinical specimen on which the PCR was performed, and the method of DNA isolation prior to the amplification process. Indeed, while most PCR methods studied so far use primers to amplify sequences within the rRNA genes of Aspergillus spp. (118, 130, 148, 169), there are some studies that have effectively described amplification of mitochondrial DNA of the fungus (127, 135, 146, 147). However, even within the rRNA genes selected for amplification, there are multiple different options, such as the 18S ribosomal DNA (rDNA) (72), the 28S rDNA (170), and the 5.8S rDNA (10), as well as internal transcriber regions between these genes (10, 145). Although a clinical study evaluating mitochondrial and rRNA gene PCRs on serum samples showed no significant difference between the methods and suggested that the use of both methods could increase the sensitivity of the test (160), a different multicenter evaluation showed that use of mitochondrial primers on serum samples can undermine PCR performance by decreasing its sensitivity (171).
TABLE 4.
Clinical studies evaluating Aspergillus sp. PCR methodsa
| Study (reference no.) | Date (yr) published | Study design | Patient population | Type of PCR | Type of specimen tested | Primer target | Method utilized to determine accuracy | Sensitivity (%) | Specificity (%) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| PCR studies | ||||||||||
| Bucheidt et al. (126) | 2001 | Retrospective | 67 febrile neutropenic patients and 33 immunocompetent individuals were tested with BAL fluid PCR, and 218 patients with hematologic malignancies and 60 immunocompetent individuals were tested with blood PCR | Nested PCR | BAL fluid and whole blood | 18S rDNA | Comparison to MSG criteria | 100 for BAL fluid, 91.7 for whole blood | 92.6 for BAL fluid, 83.6 for whole blood | |
| Raad et al. (127) | 2002 | Prospective | 54 patients with cancer and pulmonary infiltrates; 4 had definite infection | Traditional PCR with detection through ethidium bromide staining | Whole blood | Mitochondrial DNA and alkaline protease gene | Comparison to EORTC/MSG criteria | 100 for definite IA, 57 for probable and possible IA | 100 | |
| Bucheidt et al. (128) | 2002 | Retrospective | 176 patients, including 141 febrile neutropenic patients | Nested PCR | BAL fluid | 18S rDNA | Comparison to EORTC/MSG criteria | 93.9 | 94.4 | |
| Raad et al. (129) | 2002 | Prospective | 249 cancer patients with pulmonary infiltrates | Traditional PCR with ethidium bromide staining or Southern blotting | BAL fluid | Mitochondrial DNA and alkaline protease gene | Comparison to EORTC/MSG criteria | 80 for proven IA, 64 for probable IA | 93 | |
| Las-Flörl et al. (130) | 2004 | Prospective | 36 patients receiving antifungals due to suspicious pulmonary infiltrates | PCR-ELISA | 205 whole-blood specimens, 15 FNA or biopsy specimens, 21 BAL fluid or tracheal secretion specimens | 18S rRNA gene | Comparison to EORTC/MSG criteria | For proven IA, 100 for FNA/biopsy specimens and 40 for whole blood; for probable IA, 66 for lung fluid and 44 for whole blood | 100 (all possible IA patients were considered truly negative) | |
| Buchheidt et al. (131) | 2004 | Prospective | 165 patients with hematologic malignancies or HSCT from 6 centers | Nested PCR followed by ethidium bromide staining. Positive nested PCR specimens were also tested by qPCR with fluorescent probes | 1,522 samples of various types | 18S rRNA gene for nested PCR and mitochondrial cytochrome b gene for real-time PCR | Comparison to EORTC/MSG criteria | 63.6 for nested PCR | 63.5 for nested PCR | Possible IA cases were not included in the sensitivity and specificity determinations. Sensitivity dropped to 36.4% and specificity increased to 92.3% when only patients with at least 2 positive PCR results were considered “PCR positive.” |
| Las-Flörl et al. (133) | 2005 | Retrospective | 16 hematologic malignancy patients with proven or probable IA | PCR-ELISA | 108 whole-blood specimens, 9 FNA or tissue biopsy specimens, and 7 BAL fluid or tracheal secretion specimens | 18S rRNA gene | Comparison to EORTC/MSG criteria | For proven IA, 100 for FNA/tissue samples and 66 for whole blood; for probable IA, 85 for BAL fluid/tracheal secretions and 57 for whole blood | NA due to study design | Sensitivity of whole-blood PCR dropped to 54 and 42% for proven and probable IA, respectively, when tested during antifungal therapy. Consecutive positive PCR results were associated with fatal outcomes. |
| Halliday et al. (134) | 2005 | Prospective | 29 adults and 36 children with febrile neutropenia, undergoing intensive chemotherapy for hematologic malignancy or having received a hematopoietic stem cell transplant | Nested PCR followed by ethidium bromide staining | 998 whole-blood samples from 95 episodes of febrile neutropenia | 18S rRNA gene | Comparison to EORTC/MSG criteria; proven and probable cases were considered true-positive cases, cases with no evidence of IA were considered true-negative cases, and possible cases were examined differently | 100 for methods A and B, 70.6 for method C, 100 for method D | 75.4 for methods A and B, 75.4 for method C, 74.7 for method D | At least two positive PCR results were required for a case to be considered PCR positive. Positive PCR was the earliest indicator of IA, by a mean of 14 days. Antifungal therapy did not affect positive PCR results. |
| Scotter and Chambers (132) | 2005 | Retrospective | 25 patients with hematologic malignancies | PCR-ELISA | Blood | Comparison to EORTC/MSG criteria | 100 | 85 | Possible IA cases were considered truly negative. GM assay of the same samples resulted in a sensitivity and specificity of 60 and 95%, respectively. | |
| Florent et al. (135) | 2006 | Prospective | 201 patients with hematologic malignancies | PCR-ELISA | Serum | Mitochondrial DNA | Comparison to EORTC/MSG criteria | For proven cases, 100; for probable cases, 58.6–86.2*; for possible cases, 27.8–72.2 | 87.3–89.7 for consecutive positive results, 51.5–55.2 for single positive results | Combined use of PCR-ELISA and galactomannan assay increased the sensitivity to 83.3% |
| Hummel et al. (136) | 2006 | Retrospective | 6 patients with hematologic malignancies and probable, proven, or possible IA | Nested PCR | 35 CSF samples | 18S rRNA | Comparison to EORTC/MSG criteria | Each patient had at least one positive CSF sample | NA | |
| Badiee et al. (137) | 2008 | Prospective | 194 patients with hematologic malignancies | PCR-ELISA | Whole blood | rRNA | Comparison to EORTC/MSG criteria | 66 for proven and probable IA | 96 | |
| Shahid et al. (138) | 2008 | Retrospective | 69 patients with bronchogenic carcinoma and 18 healthy controls | Traditional PCR with ethidium bromide staining | BAL fluid | Comparison to EORTC/MSG criteria | 100 for proven and probable IA cases | 97 for non-IA cases, 100 for healthy controls | ||
| Hummel et al. (139) | 2009 | Prospective | 71 pediatric and adolescent immunocompromised patients | Nested PCR followed by ethidium bromide staining | Various | 18S rRNA | Comparison to EORTC/MSG criteria | 80 for proven/probable IA, 32.4 for possible IA | 81 (drops to 71 if cases with possible IA are considered truly negative) | Only 5 patients had proven/probable IA. Results were pooled for all different specimens tested. Patients with at least one positive PCR result were considered PCR positive |
| Lopes Da Silva et al. (140) | 2010 | Prospective | 172 patients who received high-dose chemotherapy | Traditional PCR followed by ethidium bromide staining | Serum and BAL fluid | 18S rRNA | Comparison to EORTC/MSG criteria | 75 (only proven and probable IA patients were considered truly positive) | 91.9 | The sensitivity and specificity of serum galactomannan assay were also tested (87.5% and 93%, respectively). The reported sensitivity and specificity refer to serum PCR. BAL fluid PCR was more sensitive (exact sensitivity not reported) |
| Hummel et al. (141) | 2010 | Prospective | 91 patients within the AmBiLoad trial | Nested PCR followed by ethidium bromide staining | 454 blood samples (not specified), 3 BAL fluid samples, 1 bronchial aspirate, 1 muscle biopsy specimen | 18S rRNA | Comparison to EORTC/MSG criteria | 43 for proven IA, 39 for probable IA | NA due to study design | Low sensitivity might be explained by the fact that all samples were received during antifungal treatment. Positive PCR results were associated with worse outcomes. |
| Badiee et al. (142) | 2012 | Prospective | 62 pediatric patients at increased risk for IA | Nested PCR followed by ethidium bromide staining | Serum | Comparison to EORTC/MSG criteria | 80 | 96.2 | Possible IA cases were excluded from the analysis. | |
| Reinwald et al. (143) | 2012 | Retrospective | 226 patients with hematologic malignancies | Nested PCR followed by ethidium bromide staining | BAL fluid | 18S rRNA | Comparison to EORTC/MSG | 58 for proven/probable IA | 87 (possible IA cases were considered truly negative) | Sensitivity dropped to 17% in considering only patients who were receiving at least two antifungals. Treatment with one antifungal agent during BAL sampling did not affect the PCR performance. |
| Reinwald et al. (144) | 2012 | Prospective | 87 patients at high risk for IA | Nested PCR followed by ethidium bromide staining | BAL fluid | 18S rRNA | Comparison to EORTC/MSG criteria | 59 | 87 (possible IA cases were considered truly negative) | For comparison, the sensitivity and specificity of BAL fluid GM testing on the same samples were 79% and 96%, respectively. |
| Buess et al. (145) | 2012 | Prospective | 191 immunocompromised patients undergoing bronchoscopy for suspected pulmonary infection | Nested PCR followed by ethidium bromide staining and sequencing | BAL fluid | 18S rRNA and 5.8S rRNA | Comparison to EORTC/MSG criteria | 0 for proven IA, 50 for probable IA, 24 for possible IA | 70 when only no-IA patients were considered truly negative | Only 3 patients had proven IA, and 8 had probable IA. |
| Reinwald et al. (9) | 2013 | Prospective | 55 immunocompromised patients for whom central nervous system aspergillosis was suspected | Nested PCR followed by ethidium bromide staining | CSF | 18S rRNA | Comparison to EORTC/MSG criteria | 100 for proven and probable IA | 93 | Possible IA cases were excluded from the analysis. |
| Real-time PCR studies | ||||||||||
| Costa et al. (146) | 2002 | Retrospective | 20 patients with hematologic malignancies who had proven or probable IA | Real-time PCR with fluorescein-labeled probes | Serum | Mitochondrial DNA | Comparison to EORTC/MSG criteria | 70 | NA | Plasma and white blood cell pellets were also tested by qPCR for some of the patients, yielding the same results as those obtained with the serum fraction. No frank increase in the DNA load during the course of disease was observed. |
| Spiess et al. (147) | 2003 | Retrospective | 18 patients with hematologic malignancies with positive nested PCR results for Aspergillus and 50 healthy controls | Real-time PCR with fluorescein-labeled probes | BAL fluid and whole blood | Mitochondrial cytochrome b DNA | Comparison to EORTC/MSG criteria | 100 for BAL fluid, 43 for blood | 100 | Only samples that tested positive with a previously validated nested PCR test were included in the study. |
| Sanguinetti et al. (148) | 2003 | Prospective | 44 patients undergoing bronchoscopy for suspicious pulmonary infiltrates | Real-time PCR with fluorescein-labeled probe | BAL fluid | 18S rRNA | Comparison to EORTC/MSG criteria | 90 for proven and probable IA | 100 (possible IA cases were considered truly negative) | Galactomannan testing of the same BAL fluid samples proved to have 100% sensitivity. Nested PCR testing of the same samples also had 90% sensitivity and 100% specificity. |
| Rantakokko-Jalava et al. (149) | 2003 | Retrospective | 66 patients at risk for IA and 33 immunocompetent controls | Real-time PCR with fluorescein-labeled probes | BAL fluid | Mitochondrial tRNA | Comparison to EORTC/MSG criteria | 86 for proven IA, 50 for probable IA, 80 for possible IA | 93 | Due to the primer and probe design, the assay only detected A. fumigatus infection. |
| Challier et al. (150) | 2004 | Retrospective | 41 immunocompromised patients at risk for IA and 29 controls | Real-time PCR with fluorescein-labeled probe | Serum | 28S rRNA | Comparison to EORTC/MSG criteria | 100 for proven cases, 78.9 for probable cases | All controls had negative qPCR results | ELISA galactomannan testing of the same samples showed 75.2% sensitivity for proven and probable IA. The combination of galactomannan assay and qPCR testing yielded a 100% sensitivity for proven and probable IA. |
| Kawazu et al. (52) | 2004 | Prospective | 96 patients at risk for IA | Real-time PCR with fluorescein-labeled probes | Plasma | 18S rRNA gene | Comparison to EORTC/MSG criteria | 55 | 93 | The cutoff for positive PCR was selected to achieve a 93% specificity. Possible IA cases were considered truly positive. Galactomannan ELISA achieved a sensitivity of 100% at a cutoff value that had the same specificity. |
| Musher et al. (72) | 2004 | Retrospective | 99 patients (49 cases of IA and 50 controls) | Real-time PCR with fluorescein-labeled probe | BAL fluid | 18S rRNA | Comparison to EORTC/MSG criteria | 67 | 100 | The sensitivity and specificity of the BAL fluid galactomannan assay for the same patients were 76% and 94%, respectively, with a cutoff of 0.5. The probe for the PCR assay was designed to detect most Aspergillus species as well as Penicillium species. |
| Millon et al. (151) | 2005 | Retrospective | 29 patients with at least one positive galactomannan test | Real-time PCR with fluorescein-labeled probes | Serum | Mitochondrial DNA | Comparison to EORTC/MSG criteria | 57.1 | 63.6 | Possible IA cases were disregarded. A PCR-positive result after the first GM-positive result was associated with a poor prognosis. |
| White et al. (152) | 2006 | Prospective | 203 patients at risk for IFI | Real-time nested PCR with hydrolysis (TaqMan) probes | Whole blood | 28S rRNA | Comparison to EORTC/MSG criteria | 92.3 | 94.6 | Possible IA cases were disregarded. Only patients with serial positive PCR results were considered “PCR positive.” |
| Cesaro et al. (153) | 2008 | Prospective | 62 pediatric patients at risk for IA | Real-time PCR with fluorescent probes | Whole blood | 18S rRNA gene | Comparison to EORTC/MSG criteria | 88 | 37 | When two PCR-positive results were required for a case to be considered PCR positive, the sensitivity and specificity changed to 63% and 81%, respectively. |
| Botterel et al. (154) | 2008 | Retrospective | 25 patients with at least 1 GM-positive serum sample | Real-time PCR with fluorescent probes | Serum | Mitochondrial DNA | Comparison to EORTC/MSG criteria | 61.5 for probable and possible IA cases | 100 | Possible IA cases were considered true-positive cases and were PCR positive. Sensitivity decreases to 54.5% if only probable cases are considered. |
| Suarez et al. (155) | 2008 | Prospective | 124 patients with hematologic malignancies undergoing chemotherapy or HSCT | Real-time PCR with fluorescent probes | Serum | 28S rRNA | Comparison to EORTC/MSG criteria | 100 when using large serum volumes for DNA extraction, 76.5 when using small serum volumes for DNA extraction | 96.7 | Two possible IA cases were considered truly positive. For comparison, GM test results for the same samples showed a sensitivity and specificity of 88.2% and 95.8%, respectively. |
| Khot et al. (156) | 2008 | Retrospective | 81 patients with pneumonia | Real-time PCR with fluorescein-labeled probes | BAL fluid | 18S rDNA | Comparison to EORTC/MSG criteria | 77 | 88 | |
| Ramirez et al. (157) | 2009 | Prospective | 127 patients at risk for IA | Real-time PCR with fluorescein-labeled probes; species were determined by melting curve analysis | Whole blood | 18S rRNA | Comparison to EORTC/MSG criteria | 100 for proven cases, 0 for probable cases | 100 if possible IA cases are disregarded. | Only 1% of the 948 tested samples were PCR positive. |
| Frealle et al. (158) | 2009 | Retrospective | 57 patients at risk for IA | Real-time PCR with fluorescein-labeled probes | BAL fluid | Mitochondrial DNA | Comparison to EORTC/MSG criteria | 50 for proven and probable IA cases | 100 | |
| Cuenca-Estrella et al. (159) | 2009 | Prospective | 83 patients with febrile neutropenia | Real-time PCR with hydrolysis probe | 1,122 whole-blood samples and 1,122 serum samples | ITS1 | Comparison to EORTC/MSG criteria | 91.6 | 94.4 | Cases with two consecutive positive PCR results were considered PCR positive. Combined with GM assay, the sensitivity increased to 100%. Possible IA cases were considered truly positive. |
| Springer et al. (10) | 2011 | Prospective | 46 patients receiving either allogeneic SCT or myeloablative chemotherapy | Real-time PCR with fluorescein-labeled probes | Whole blood | Multicopy ribosomal operon region from ITS1 to 5.8S region | Comparison to EORTC/MSG criteria | 55 for probable and possible IA (dropped to 27 when having more than one positive PCR result was considered “PCR positive”) | 75 (increased to 100 when having more than one positive PCR result was considered “PCR positive”) | Possible IA cases were considered truly positive. Selective pathogen DNA enrichment using affinity purification unexpectedly caused a decrease in the sensitivity of the assay. |
| Millon et al. (160) | 2011 | Retrospective | 44 patients with two sequential positive serum galactomannan results and a risk factor for IA | Two different real-time PCR assays with hybridization probes | Serum | Assay 1, mitochondrial DNA; assay 2, 18S rRNA | Comparison to EORTC/MSG criteria | For assay 1, 57.7; for assay 2, 50 (dropped to 53.8 and 46.2, respectively, when at least two positive results were needed for a PCR-positive outcome) | For assay 1, 94.4; for assay 2, 66.7 (increased to 100 for both when at least two positive results were needed for a PCR-positive outcome) | Due to the study design, no possible IA cases were included. The combination of the ribosomal and mitochondrial PCRs increased the sensitivity of IA diagnosis to 65.4%. Positive ribosomal PCR results were associated with a poor prognosis. |
| White et al. (161) | 2011 | Retrospective | 31 patients (10 with proven/probable IA and 21 with no IA) | Two different real-time PCR assays with fluorescently labeled probes | Serum | Assay 1, 28S rRNA; assay 2, 18S rRNA | Comparison to EORTC/MSG criteria | For assay 1, 80; for assay 2, 70 (dropped to 50 and 60, respectively, when at least two positive results were needed for a PCR-positive outcome) | For assay 1, 100; for assay 2, 90.5 (both reached 100 when at least two positive results were needed for a PCR-positive outcome) | Assay 2 is a commercially available PCR assay for the diagnosis of IA. |
| Bernal-Martinez et al. (162) | 2011 | Retrospective | 38 adult patients with a high clinical suspicion of IA | Real-time PCR with fluorescently labeled probes | Serum and whole blood | ITS1 | Comparison to EORTC/MSG criteria | 100 for serum and 94.4 for blood for proven/probable IA | NA | The aim of the study was to compare the sensitivities of the same PCR on serum and blood specimens. The results show that both specimens achieve similar sensitivities. One positive PCR result was necessary to classify a patient as PCR positive. |
| Luong et al. (163) | 2011 | Retrospective | 137 lung transplant recipients | Real-time PCR with fluorescently labeled probes | BAL fluid | Not specified | Comparison to EORTC/MSG criteria | 100 for proven/probable IA | 88 | For comparison, GM testing of the same BAL fluid samples resulted in a sensitivity and specificity of 93% and 89%, respectively, at a cutoff of 0.5 |
| Torelli et al. (164) | 2011 | Prospective | 158 patients from hematology and intensive care units | Real-time PCR with fluorescently labeled probes | BAL fluid | 18S rRNA gene | Comparison to EORTC/MSG criteria | 94.1 for proven and probable IA | 98.6 | |
| Springer et al. (165) | 2013 | Retrospective, multicenter | 47 patients with proven/probable IA and 31 controls | Various real-time PCR assays | Serum and whole blood | Various | Comparison to EORTC/MSG criteria | 85.1 for whole blood, 78.7 for serum (dropped to 46.8 and 51.1, respectively, when two positive PCR results were needed to consider a case “PCR positive”) | 64.5 for blood, 83.9 for serum (increased to 93.5 and 100, respectively, when two positive PCR results were needed to consider a case “PCR positive”) | Overall, no significant difference between the performances of the PCR assays on serum versus whole-blood specimens was found. |
| Rogers et al. (166) | 2013 | Prospective | 278 patients undergoing intensive chemotherapy or HSCT | Two different real-time PCR assays (a nested and a single run assay) | Whole blood | 28S rRNA (nested assay), ITS (single-run assay) | Comparison to EORTC/MSG criteria | 69–87 for nested assay and 55–80 for single-run PCR assay | 36–63 for nested assay and 57–84 for single-run assay | Possible IA cases were excluded from the analysis. Two centers were involved in the study, and the results were different between them, as evidenced by the ranges of sensitivity and specificity values. |
| Li et al. (167) | 2013 | Prospective | 72 patients with hematologic malignancies and suffering from fever, 4 with normal temperatures, and 10 healthy volunteers | Real-time PCR with hydrolysis probes | Whole blood and plasma | 28S-ITS2 rRNA genes | Comparison to EORTC/MSG criteria | 90.9 for proven and probable IA | 73.4 | Possible IA cases were considered truly negative. |
| Guinea et al. (168) | 2013 | Prospective | 175 patients with hematologic malignancies and at risk for IA | Real-time PCR with fluorescent probes | Lower respiratory tract samples | 18S rRNA | Comparison to EORTC/MSG criteria | 93.3 | 82.9 | No proven IA cases were included. |
BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; EORTC/MSG: European Organization for Research and Treatment of Cancer/Mycoses Study Group; FNA, fine-needle aspiration; GM, galactomannan; HSCT, hematopoietic stem cell transplant; IA, invasive aspergillosis; ITS, internal transcriber spacer; qPCR, quantitative PCR; NA, not applicable.
An additional reason for inconsistencies between results reported from various studies is the different ways used to define PCR positivity. Some investigators prefer to report sensitivity and specificity values, using only one positive result per patient tested (162, 167). However, others argue that this method significantly decreases the specificity of the results, thus decreasing their clinical value, while it is preferable to define cases that have at least two positive PCR results as “PCR positive” (152). A recent meta-analysis of 16 studies evaluating PCR assays for Aspergillus spp. in blood specimens found that two positive PCR results had the same sensitivity but superior specificity compared to a single positive result (172). Nevertheless, more head-to-head comparison studies between the two approaches are needed before a conclusion can safely be reached.
Another ongoing debate revolves around the sample type on which PCR is performed. Many different researchers have tried to determine whether whole-blood, serum, or plasma specimens are better for PCR testing for Aspergillus spp. In the oldest among those studies, Loeffler et al. found that PCR performed on whole blood has a higher sensitivity than PCR performed on plasma, although the two methods have the same lower detection limit (173). Note that the use of anticoagulants in plasma can significantly deteriorate the sensitivity of the PCR assay by inhibiting the enzymes used in the amplification process and that the greatest inhibition is observed by using heparin or sodium citrate as an anticoagulant (174). As a result of these differences, researchers who used plasma samples from 96 patients at risk for IA to prospectively test the performance of a real-time PCR assay reported a sensitivity of 55%, whereas the sensitivity of galactomannan testing on the same samples was 100% (52). A similar question that has been studied more thoroughly is the choice between serum and whole blood. The use of serum is expected to produce fewer false-negative results due to the smaller amount of host DNA that it contains, which is known to compete with the microbial target for amplification (156), while it will theoretically adversely affect the sensitivity due to the fact that it misses the fungal cell-associated DNA. However, two recent studies evaluating the performance of serum versus whole-blood real-time PCR assays for patients at risk for IA and negative controls failed to find significant differences between the specimens (162, 165). The reason might be that most of the circulating Aspergillus sp. DNA exists in the form of free DNA that is released after fungal cell breakdown and can be found in both serum and whole blood (175). Notably, though, in one of the two studies, there was a trend for PCR testing of whole blood to be more sensitive and to be positive earlier than that of serum (165). An important point to consider is that the volume of serum used to extract DNA might play a decisive role in the accuracy of subsequent PCR, as shown by a study evaluating spiked serum samples (171). This report was in agreement with a previous study by Suarez et al., who prospectively investigated serum samples from 124 patients with hematologic malignancies and found that the use of a larger serum volume (100 μl versus 1 ml) improved the sensitivity from 76.5% to 100%, without altering the specificity of the assay (155). Therefore, studies using smaller serum volumes to extract DNA might in fact underestimate the performance of a PCR assay on this sample. Furthermore, serum has the advantage of being easier to process, by avoiding cumbersome purification and DNA isolation procedures associated with the use of whole blood, which carry a high risk of contamination, and can also be used to run multiple different tests (PCR, galactomannan, and β-glucan assays). Thus, if the aforementioned reports are true and the differences among these specimens are minimal, serum has the potential to become the preferred specimen for PCR testing. Note that an important and often underestimated source of contamination of blood samples tested with Aspergillus sp. PCR that can undermine the test's performance by creating false-positive findings is the blood collection tube, as proved by a recent article which showed that up to 18% of blood collection tubes can be contaminated with Aspergillus sp. DNA (124). Thus, the use of specialized, contaminant-free means of collecting blood samples might be able to improve PCR performance.
BAL fluid has also been used for Aspergillus sp. PCR testing, with promising results. Indeed, results from experimental models of IA show that real-time PCR assays on BAL fluid have sensitivities of >80%, comparable to that of BAL fluid galactomannan assay (176). In clinical studies, the overall ranges of sensitivity and specificity varied widely and were 36 to 100% and 70 to 100%, respectively (72, 138, 145, 148). A recent meta-analysis evaluated the performance of BAL fluid PCR for IA and reported an average sensitivity and specificity of 91% and 92%, respectively (122). This tendency of average sensitivity to be skewed significantly toward higher values is a reflection of the distribution of the individual studies in the analysis. Indeed, among nine clinical studies published after 2002 and included in the meta-analysis, only three reported BAL PCR sensitivities of <70% (72, 129, 158), with one of <60% (158) and none of <50%. On the other hand, in four studies, the sensitivity was higher than 90%. Interestingly, the same analysis provided evidence that the use of a commercial DNA extraction protocol is the most important factor for improved performance (122), thus identifying a potential cause for discrepancies between results of different trials. Finally, two studies evaluated the performance of a nested PCR assay on CSF samples from patients with central nervous system (CNS) aspergillosis and reported a sensitivity of 100% and a specificity of 93% (9, 136), thus showing that this assay has the potential to contribute to the diagnosis of this rare disease entity. However, given the relatively small number of patients involved in these studies, more trials are needed before one can safely reach a conclusion.
An additional point of consideration is the optimal time for sampling and the effect of antifungal treatment on PCR performance. Indeed, due to the limits of nonmolecular diagnostic methods, many patients at risk for IA receive prophylactic antifungal therapy, and understanding how this can affect PCR results could help to determine whether the method could be used to monitor the response to therapy or can serve only as a screening procedure. However, none of these questions can be answered appropriately without knowing the kinetics of DNA release during fungal growth. In an effort to elucidate this aspect of Aspergillus infection pathogenesis, Mennink-Kersten et al. investigated the release of different biomarkers during the in vitro growth of Aspergillus spp. and found that DNA is released only after mycelium breakdown, during nutrient starvation, and not during hyphal growth, in contrast to galactomannan antigen, which is released mainly during the logarithmic growth phase (175). One could assume that antifungal treatment, by causing generalized mycelium breakdown, would lead to increased yields of the PCR assays, at least transiently, before fungal loads are significantly decreased. However, this hypothesis is undermined by reports from clinical studies and experimental models of IA, which show that PCR performance is negatively affected by antifungal treatment (132, 141, 143, 177). One explanation for this paradox could be the fact that most DNA extraction protocols focus on methods of fungal cell lysis which can destroy free-floating fungal DNA, and thus render it undetectable (175). Consequently, the use of an additional extraction setup to preserve free DNA could enhance PCR sensitivity. Indeed, in a recent study, Springer et al. used a new commercially available extraction protocol that includes both cellular and cell-free fungal DNAs and found significant improvements in sensitivity compared to an in-house extraction system, without affecting the specificity of the assay (178). Another intriguing question is whether all classes of antifungals affect PCR results in similar ways. Researchers studying these effects on a rat inhalation model of IA found that the measured reduction of PCR sensitivity with antifungal therapy could be attributed only to posaconazole and caspofungin, not to amphotericin B (179). However, other studies have failed to reach the same conclusion (141, 177). Interestingly, a recent study evaluating the performance of PCR to diagnose IA by use of BAL fluid after at least one full daily dose of antifungal therapy showed that its performance was not significantly affected unless two or more antifungals were used to treat the patient (143). This observation is very important when one considers that, in clinical practice, patients with prolonged fever and compromised immune responses often receive prophylactic antifungal regimens which consist of one antifungal agent, usually active against Candida spp., as they are the most common offenders. In such cases, the sensitivity of nonmolecular diagnostic methods is adversely affected, thus lowering the chance to start targeted treatment in patients with undiagnosed IA. Therefore, detection of Aspergillus spp. by BAL fluid PCR could be particularly useful for this population of patients.
Several studies in the recent literature have attempted to compare the diagnostic performances of PCR and galactomannan assays for Aspergillus spp. A recent meta-analysis compared the two diagnostic tests on BAL fluid samples and found that their performances were similar, without significant differences (180), which is in agreement with findings from previous studies (163, 164). Similarly, PCR and GM assays performed on serum samples have accuracies that are comparable to each other, although their accuracies are lower than the results obtained from BAL fluid (166). Interestingly, a diagnostic strategy that combines these two tests, requiring at least one positive result for the diagnosis, seems to have a high sensitivity without sacrificing specificity (97% sensitivity and 97.5% specificity) (180). Since invasive aspergillosis is a serious infection that is often treated empirically in high-risk groups to avoid detrimental outcomes, such a strategy could prove to be particularly useful and cost-effective for high-risk patients, as it could reduce unnecessary treatment without compromising patient safety (181).
Finally, it is tempting to investigate the implications of PCR results on treatment and outcomes of IA. Many clinical trials evaluating the use of PCR to detect Aspergillus spp. in high-risk populations have reached the conclusion that consecutive positive PCR results are associated with higher mortality in cases of suspected IA (133, 141), a phenomenon which is probably associated with the higher fungal burdens in this subset of patients, thus leading to larger amounts of circulating DNA. A more conclusive argument can be made for the potential of the method to guide empirical antifungal therapy. An early study suggested that the use of two consecutive positive PCR results to guide antifungal therapy in hematology patients could have led to a decrease in empirical antifungal agents of up to 37% (134). However, due to the noninterventional study design, the researchers were unable to determine how this could affect patient survival. On the other hand, a novel randomized controlled trial with 240 hematologic malignancy patients from six Australian centers compared traditional and biomarker (galactomannan and PCR) diagnostic strategies to guide the use of antifungal agents in patients with hematologic malignancies and concluded that the biomarker strategy led to a significant (17%) decrease in empirical antifungal therapy without affecting survival (181). Therefore, such a strategy could significantly reduce the inadvertent use of antifungal agents in patients who would not benefit from them.
Taking all the above into consideration, it becomes evident that PCR has the potential to play a decisive role in the diagnosis and management of Aspergillus sp. infections. However, due to the large number of PCR assays that, despite sharing the same core principles, differ in so many aspects of their performance, much effort should be given to implement a standardization that clinical practices can adopt. To this end, the European Aspergillus PCR Initiative (EAPCRI) studied the performances of different PCR assays and whole-blood preparation protocols for fungal DNA extraction and purification from spiked EDTA-anticoagulated blood samples (182). The study was performed by 24 centers using 13 different PCR assays and showed that PCR performances were similar regardless of the type of PCR assay used. The only reasons for significant discrepancies were the use of an internal control to avoid false-positive findings and the DNA extraction protocol, with results favoring bead beating, use of red and white blood cell lysis buffers, and use of elution volumes of <100 μl. Thus, the EAPCRI issued recommendations for optimal PCR performance from whole blood, with compliant centers being able to detect at least 50 conidia of Aspergillus spp. and achieving an average sensitivity and specificity of 88.7% and 91.6%, respectively. These results were corroborated by a follow-up study from the same group (183). Importantly, a further effort to evaluate and standardize different PCR methods with serum samples was recently completed (171). The panel investigated different PCR methods used by 23 centers on spiked serum samples and showed an overall sensitivity and specificity of 86.1% and 93.6%, respectively, and an ability to detect a threshold of 10 genomes/ml. Moreover, regression analysis of the results showed that larger serum volumes (>0.5 ml), elution volumes of <100 μl, the use of an internal control, and the use of PCR targeting the internal transcriber spacer region of rDNA (159, 162) were associated with higher accuracy, while the use of primers targeting mitochondrial DNA (151, 154) adversely affected sensitivity. Finally, based on the findings of the aforementioned studies, the Aspergillus Technology Consortium created and validated an Aspergillus sp. DNA calibrator material that can be used to standardize nucleic acid-based diagnostic assays, thus improving interlaboratory comparisons of qualitative and quantitative results between different techniques (184). Although the repercussions of these endeavors have yet to be realized in ensuing clinical trials, it is anticipated that they will reduce the inconsistencies observed in previous studies and will pave the way to large-scale implementation of Aspergillus sp. PCR.
Invasive candidiasis.
Multiple studies have evaluated the performance of PCR tests for the diagnosis of invasive Candida infections in patient populations (4, 118, 185–202) (studies are summarized in Table 5). The ranges of reported sensitivity and specificity values are 56.2 to 100% and 54 to 100%, respectively. However, most studies report higher sensitivity and specificity values, in the ranges of 80 to 100% and 90 to 100%, respectively. Importantly, the definitions of true-positive and true-negative results vary significantly among different reports. These differences should be considered fundamental, as accuracy values may change significantly depending on the way that each study handles cases of probable and possible invasive candidiasis. Interestingly, many studies show a tendency of PCR methods to detect Candida DNA in patients at high risk of invasive candidiasis with negative blood culture (187, 189, 202). In some cases of discordant results between blood cultures and PCR, Candida spp. were isolated from the same population of patients, from another sterile site, thus indicating that the PCR result was in fact positive and proving the superiority of the assay over the traditional gold standard diagnostic test (187). Indeed, a recent study analyzing PCR results from many clinical trials showed average sensitivity and specificity values of 95% and 92%, respectively, and indicated that PCR was able to detect 85% of cases of proven or probable invasive candidiasis, while blood cultures were positive in only 38% of the cases (203).
TABLE 5.
Clinical studies evaluating Candida PCRa
| Study (reference no.) | Study design | Patient population | Type of PCR | Type of specimen tested | Primer target | Method utilized to determine accuracy | Sensitivity (%) | Specificity (%) | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Ahmad et al., 2002 (185) | Retrospective | 28 culture-proven or suspected Candida-positive patients, 10 superficially colonized patients, 12 healthy controls | Seminested PCR | Serum | ITS2 | Comparison to blood culture results | 100 | 100 | The approach was able to identify Candida species in 9 culture-negative patients with suspected IC. All Candida-colonized patients had negative PCR results. |
| White et al., 2003 (186) | Prospective | 113 patients at risk for IC | Real-time PCR and nested PCR | Whole blood | 18S rDNA | NA | NA | NA | Only 3 of the 113 patients had blood culture-positive results, 2 of whom also had positive PCR results. PCR was positive in another 25 patients suspected to have IC. |
| Tirodker et al., 2003 (187) | Prospective | 70 pediatric and neonatal ICU patients with sepsis | Traditional PCR with gel electrophoresis | Whole blood | 18S rDNA | Comparison to blood culture results | 100 | 77.2 | Seven of 13 culture-negative and PCR-positive patients had other evidence of IFI. |
| Maaroufi et al., 2003 (118) | Retrospective | 61 hemato-oncology patients with proven or suspected IC | Real-time PCR with hydrolysis probes | Whole blood | 5.8S and 28S rDNA | Comparison to blood culture results | 100 | 97 | |
| Maaroufi et al., 2004 (188) | Retrospective | 39 patients with clinically proven or suspected Candida infection and 15 controls | Real-time PCR with fluorescent probes | Serum | 5.8S and 28S rDNA | Comparison to blood culture results | 100 | 97 | |
| Ahmad et al., 2004 (189) | Retrospective | 26 patients (6 proven cases, 10 suspected cases, 10 healthy controls) | Seminested PCR-ELISA | Serum | 5.8S and 28S rDNA and ITS2 | Comparison to blood culture results | 100 | 80 | PCR was positive in 4 of 10 patients with suspected IC and in none of the healthy controls. |
| White et al., 2005 (190) | Retrospective | 105 patients at high risk for IFI | Real-time PCR with fluorescent probe | Whole blood and serum | 18S rDNA | Comparison to EORTC/MSG criteria | 95 for proven and probable cases | 97 | Possible IC cases were not included in the sensitivity and specificity determinations. |
| Moreira-Oliveira et al., 2005 (191) | Prospective | 225 patients with hematologic malignancies and at risk for IC | Traditional PCR followed by sequencing | Whole blood | 5.8S rDNA | Comparison to blood culture results | 72.1 | 91.2 | |
| Alam et al., 2007 (192) | Retrospective | 27 patients with culture-proven Candida infection, 39 patients with suspected candidemia, 10 colonized patients, 16 controls | Seminested PCR | Serum | ITS2 | Comparison to EORTC/MSG criteria | 92.5 | 100 | Probable IC cases were excluded from analysis (53% of them were PCR positive). |
| McMullan et al., 2008 (193) | Prospective | 157 nonneutropenic patients in the ICU | Real-time PCR with hydrolysis probes | Serum | 18S and 5.8S rDNA, ITS1, ITS2 | Comparison to EORTC/MSG criteria, modified for nonneutropenic patients | 82 | 100 | Probable IC patients were excluded from the analysis. One of the 11 patients with proven IC was diagnosed with Candida famata infection, which was not possible to detect with the primers used. |
| Dunyach et al., 2008 (194) | Prospective | 23 ICU patients with cancer and 10 healthy controls | Real-time PCR with SYBR green fluorescence | Serum | ITS1 to ITS4, L18 | Comparison to blood culture results | 92 for L18 PCR, 76.9 for ITS PCR | 66 for L18 PCR, 100 for ITS PCR | Patients with probable IC were excluded from the analysis. Among the 10 patients with probable IC, 3 were positive with L18 PCR and 5 with ITS PCR. |
| Metwally et al., 2008 (195) | Retrospective | 104 patients included in a previous prospective study, from whom whole-blood specimens were obtained | Real-time PCR with hydrolysis probes | Whole blood and serum | 18S and 5.8S rDNA, ITS1, ITS2 | Comparison to EORTC/MSG criteria, modified for nonneutropenic patients | 100 for serum, 70 for whole blood | 100 | |
| Badiee et al., 2009 (196) | Prospective | 194 patients with hematologic malignancies | PCR-ELISA | Whole blood | 18S rDNA | Comparison to EORTC/MSG criteria | 100 (2 of 2 proven IC cases) | 95 | Patients with probable IC and patients with fever of unknown origin were excluded from the analysis. PCR remained positive until death when treatment failed. |
| Khlif et al., 2009 (197) | Prospective | 110 patients at risk for IC | Real-time and nested PCR assays | Blood cultures | 18S and 5.8S rDNA, ITS1, ITS2 | Comparison to blood cultures | 81 for real-time PCR, 86 for nested PCR | 96 for real-time PCR, 54 for nested PCR | |
| Wellinghausen et al., 2009 (198) | Prospective | 284 patients at risk for IC | Real-time PCR | Whole blood | 18S rDNA | Comparison to blood cultures | 87.5 | 93 | PCR also detected Candida DNA in 8 blood culture-negative patients with Candida isolated from culture-sterile sites. |
| Badiee et al., 2010 (199) | Prospective | 35 patients with bone marrow transplant | Real-time PCR with hydrolysis probes | Whole blood | 18S rDNA | Comparison to EORTC/MSG criteria | 100 (probable IC cases were considered truly positive) | 88.9 (increased to 100 when only patients with at least two positive PCR results were considered PCR positive) | |
| Lau et al., 2010 (200) | Retrospective | 109 patients with or at risk for candidemia | Multiplex tandem PCR | Whole blood | ITS1, ITS2, elongation factor 1a, β-tubulin | Comparison to blood cultures | 75 | 97 | Results were accelerated by an average of 2.2 days compared to culture. Serum and plasma PCRs were more sensitive with the few serum samples that were tested. |
| Schell et al., 2012 (201) | Retrospective | 16 patients with culture-proven Candida infection | Real-time and microfluidic PCR | Whole blood | ITS1, ITS2 | Comparison to blood cultures | 68.7 for real-time PCR, 56.2 for microfluidic PCR | NA due to study design | |
| Trovato et al., 2012 (202) | Retrospective | 86 neonatal ICU patients with suspected bloodstream infections | PCR followed by ethidium bromide staining | Blood cultures | 18S rDNA, ITS1, 28S rDNA | Comparison to EORTC/MSG criteria | 87.5 for proven and probable IC | 98.6 for no IC | For comparison, the sensitivity and specificity of the blood culture results were 50% and 100%, respectively. |
| Nguyen et al., 2012 (4) | Retrospective | 55 patients with IC and 73 hospitalized controls | Real-time PCR with fluorescently labeled probes | Whole blood, plasma, serum | ITS1, ITS2 | IC was defined as recovery of Candida from blood or a sterile site; controls were defined as those having no clinical or microbiological evidence of IC | 80 | 70 | “Positive PCR” was defined as one positive plasma or serum PCR result. In a preliminary run, whole-blood PCR was found to have a significantly lower sensitivity than that of plasma or serum PCR. The β-d-glucan sensitivity and specificity were 56% and 73%, respectively. |
EORTC/MSG, European Organization for Research and Treatment of Cancer/Mycoses Study Group; IC, invasive candidiasis; ICU, intensive care unit; IFI, invasive fungal infection; ITS, internal transcriber spacer; NA, not applicable.
Despite the promising reports of detection of Candida spp. by PCR, much effort should be made to standardize the method and decrease the inconsistencies between different tests. An important and ongoing debate is focused on the choice of specimen type on which to conduct the PCR test. Indeed, serum, whole blood, and plasma have all been used for Candida sp. DNA isolation (4, 185, 186). An in vitro study aiming to evaluate the kinetics of Candida sp. DNA release in blood fractions showed that most DNA is found in a cell-free form that remains in the plasma fraction after whole-blood centrifugation (204). Interestingly, clinical studies comparing PCR analysis of serum with that of whole blood or plasma found that PCR on whole blood is significantly less sensitive than PCR on the other two types of specimens (195, 200). This observation can be explained by the cumbersome purification and cell lysis steps associated with the use of whole blood for DNA extraction, which may decrease sensitivity by destroying some of the free circulating fungal DNA. To confound the ongoing argument, a recent meta-analysis of studies evaluating Candida sp. PCR on clinical specimens reached the exact opposite conclusion, that whole-blood PCR is associated with a better performance (203). Notably, though, since this is only an indirect comparison, with different clinical samples and different PCR methods, and since the number of studies using whole blood that were included in the analysis greatly exceeded the number of studies using serum (43 versus 12), the relevance of this comparison remains questionable. Importantly, the same analysis also showed that a higher performance was associated with the use of primers targeting the rRNA and P450 gene regions.
An alternative approach is to use PCR to identify Candida spp. directly from blood culture bottles (205–208). This method would significantly decrease the time for species identification from a positive blood culture, which can now reach 96 h (205). In fact, an early study showed that a multiplex real-time PCR assay was able to identify the isolated Candida spp. in less than 2 h, and the results were 100% concordant with results of nonmolecular methods (205). These results were supported by later studies using different PCR assays (207, 208). To understand the clinical significance of these findings, one should consider that some Candida spp., such as Candida glabrata, are associated with high rates of resistance to traditional antifungal azoles that are used as first-line agents in the treatment of invasive candidiasis (209). Thus, rapid identification of an azole-resistant strain would lead to earlier optimization of antifungal therapy. A recent retrospective study took this effort a step further by trying to detect fungal DNA from blood culture bottles of neonatal patients with suspected candidemia, irrespective of blood culture results (197). The study reported a sensitivity of 87.5% for the PCR test for proven and probable cases of invasive candidiasis, while the sensitivity of blood cultures was limited to 50%, thus proving the relevance of this method.
Importantly, both PCR and serum biomarkers, such as β-glucan, are valuable tools for the detection of not only candidemia but also deep-seated candidiasis, which is often missed by blood cultures. Indeed, in a recent prospective study, the sensitivity of blood cultures for patients with deep-seated Candida sp. infections was limited to 17%, whereas β-glucan assay and, even more so, PCR had superior performances, providing positive results for 62% and 88% of the patients, respectively (4). Similar to the case for invasive aspergillosis, the excellent negative predictive values of both these tests could prove to be particularly useful in the development of diagnostic algorithms for invasive candidiasis that could help to reduce the rate of unnecessary empirical antifungal therapy (210).
Finally, the identification of specific gene mutations that can confer resistance to known antifungal agents has allowed for the development of PCR methods that can rapidly detect these mutations. Specifically, PCR methods for the detection of mutations in the FKS1 gene that confer resistance to echinocandins (211) or of EGR11 mutations or overexpression of pump genes, such as CDR1, CDR2, and MDR1 (212), have recently been described. All these experimental techniques come with the promise of much more rapid identification of resistant Candida infections than the case with traditional broth microdilution methods, thus permitting timely initiation of appropriate treatment and decreasing the rates of treatment failure (213).
Other IFIs.
As is the case with Aspergillus spp. and Candida spp., detection of fungal DNAs from other fungal species is a tempting method for diagnosis of many invasive fungal infections. Therefore, PCR has been studied as a potential method to diagnose infections such as Pneumocystis jirovecii pneumonia, mucormycosis, and even rarer fungal infections, such as coccidioidomycosis and scedosporidiosis. Especially in the case of PCP, PCR seems to be an excellent alternative to traditional methods. Indeed, Pneumocystis jirovecii cannot be cultured, and its detection is based on staining methods using respiratory specimens, which suffer from low sensitivity (214). Consequently, many studies have evaluated the performance of PCR on respiratory specimens for PCP diagnosis, and the results seem promising, with sensitivity values as high as 100% (91, 215–224). However, in the case of PCP, colonization is a significant issue, with rates as high as 22% in high-risk populations (225), and more importantly, since DNA is fairly stable, it is difficult to distinguish between active and previous infections (226). Thus, the question arises about the clinical importance of identifying Pneumocystis jirovecii DNA in respiratory samples from such patients. Indeed, reports from traditional PCR assays indicate high rates of false positivity that can reach 46% (222). In order to avoid this situtation, two different methods have been employed. The first uses reverse transcription-PCR to identify mRNAs of the organism, which are easily degradable and thus signify a viable pathogen (227). An early pilot study evaluating the assay was disappointing, with a sensitivity and specificity of 67% and 100%, respectively, with BAL fluid samples (215). However, a later study with a larger population of patients reported more promising findings, with a sensitivity and specificity of 100% and 87%, respectively, with BAL fluid (218). This method, however promising, is still unable to discriminate colonization from infection. To overcome that obstacle, a second technique was developed that relies on the use of real-time PCR assays to quantify the number of DNA copies found in a specimen. Studies have shown that the mean concentration of DNA from BAL fluid samples can be manifold higher in infected individuals than in asymptomatic carriers (219). Thus, many different real-time PCR assays have been developed and evaluated, with studies showing sensitivity values comparable to those of conventional PCR techniques and consistently higher than 80% (91, 216, 217, 220, 222, 224). The real breakthrough, though, is found in the comparison of specificity values, with reports indicating a specificity of real-time PCR that can reach 98% and that is superior to those of conventional PCR techniques at cutoff points selected to not affect sensitivity (216). Indeed, a recent meta-analysis of studies evaluating PCR for PCP diagnosis found an average sensitivity and specificity of 99% and 90%, respectively, with real-time PCR associated with a significantly higher specificity value (93%), thus highlighting the great potential of the assay for the diagnosis of this disease (228).
Another fungal disease which might benefit from PCR diagnosis is the spectrum of mucormycosis. Four studies reported the development of PCR assays to diagnose the pathogens causing this disease (229–232). An early study focused on the identification of Rhizopus spp. from clinical specimens and reported promising results, although the number of specimens tested was too small to allow for a significant conclusion (230). A later study evaluated two real-time PCR assays in an experimental rabbit model of pulmonary mucormycosis, and both performed well on BAL fluid and plasma samples, with one of them achieving a higher sensitivity than that of quantitative culture with BAL fluid (100% versus 67%) (231). Other researchers investigated the performance of a different real-time PCR test for the detection of mucormycosis from culture isolates and concluded that the assay is useful for rapid and accurate detection of this infection (229). In a more recent article, investigators from different sites evaluated a PCR method in a murine model of disseminated mucormycosis and found a high performance of the assay on paraffin-embedded tissue specimens (93% sensitivity for 30 slide cuts) and a 100% interlaboratory reproducibility (232). Furthermore, a recent retrospective study of real-time PCR for mucormycosis concluded that this test, performed on serum, was able to accurately diagnose the infection in 9 of 10 patients at a time point that was 3 to 68 days earlier than diagnosis with histopathology or culture (109).
Finally, PCR methods have also been developed and evaluated for detection of other fungal species, such as Scedosporium spp. (233), Cryptococcus spp. (234), Coccidioides spp. (235), and Fusarium spp. (236), but the possibility for clinical implementation of these assays seems unlikely for the foreseeable future, either because serologic assays are already highly sensitive and specific, thus lowering the need for molecular techniques, as is the case for Cryptococcus spp. and Coccidioides spp. (95), or because clinical evaluation of their performance has not yet been accomplished (233).
Multiplex PCR.
A different method used in molecular diagnostics of fungal infections is the use of a PCR that can detect a wide variety of fungi at once in the same specimen. The technique is fairly simple and is based on the use of primers specifically designed to amplify a region that is conserved among different fungal genera. The identification method is slightly more complex and is based either on sequencing of the amplified fragments of DNA or on the design of probes that bind to amplified fragments and have different melting temperatures, so they can be detected by melting curve analysis. This method can be combined with either standard, nested, or real-time PCR, and depending on the primer and probe design, it can detect either some (237) or all (238) fungal species. The method has been tested thoroughly for the ability to detect fungal species in tissue samples, with most reports showing promising results and a high concordance with traditional histopathology methods (239–241). Indeed, the method appears to have great value in the case of culture-negative and histologically proven infection (242). In such cases, PCR facilitates species identification, which cannot be achieved through microscopy but can serve an important role in guiding antifungal therapy. Multiplex PCR has also been tested as a method to detect fungal species in whole-blood (236, 238, 243–245), serum (246), or BAL fluid (247) samples from patients at high risk for IFIs. The results are variable, but most studies report superior sensitivities and specificities of >80% (236, 238, 243, 248). Nonetheless, these methods suffer from the same inconsistencies as PCR analysis of Aspergillus spp. or Candida spp. and should undergo a thorough standardization process before clinical implementation. Moreover, an important problem in the case of patients who are at high risk for IFIs is that these patients are frequently colonized or infected by multiple microbial species at the same time, a fact that can confound the results of diagnostic assays and often leads to wrong diagnostic assumptions that can adversely affect care. Indeed, in a recent retrospective study of hematologic malignancy patients, 53% of patients were diagnosed as having probable IA, and 29% of those with proven IA were coinfected with other bacterial species (249). A potential solution to this problem could be the use of a broad-range multiplex PCR that can detect a wide variety of both bacteria and fungi, known as the SeptiFast assay, which has been developed commercially and is being used as a method to identify the pathogen in cases of sepsis (250, 251). Similarly, a multiplex assay that can rapidly identify >25 pathogens, including many Candida spp., and antibiotic resistance genes in positive blood cultures within 1 h, known as the FilmArray system, was recently developed and commercialized for use in the microbial laboratory (252). However, due to their use in sepsis cases, where fungal infections, although possible, are unlikely, studies evaluating these methods to date report small numbers of fungal cases. Therefore, further research is needed to prove whether these tests would be useful for individuals at high risk for IFIs. Finally, broad-range PCR methods have the potential to be used as methods of rapid identification of the pathogen in cases of outbreaks. An interesting example of this technique was recently used in the case of an Exserohilum sp. meningitis outbreak caused by contaminated methylprednisolone injections (253). Using a broad-range PCR targeting the rDNA fragment, with subsequent DNA sequencing and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), researchers were able to rapidly identify the species from the patient specimens and the methylprednisolone vials, thus finding both the pathogen and the source of the infection (254). Interestingly, in the same study, the investigators noted the importance of rapid freezing of the specimens in order to avoid destruction of free circulating fungal DNA by DNases.
Although still in need of standardization, PCR offers the promising potential of being able to identify the presence of fungal pathogens within human fluids, define the species, quantify the infection, and detect antimicrobial resistance markers. At the forefront of conditions that need to be optimized are nucleic acid isolation methods, primer selection, and fluid sampling. Eventual total automation will help with enhancing the reproducibility of this technique.
Novel Molecular Methods
A variety of other molecular methods have been designed and tested over the years, either as a supplement to improve the performance of PCR or as separate tests. For example, fluorescence in situ hybridization (FISH) is a technique that uses fluorescent probes to identify target areas on the genomes of microbial pathogens in human samples, which can then be detected by fluorescence microscopy (Fig. 1A). This method has been used as an adjunct to culture (255) or PCR (256) and has been proven to have high accuracy for the identification of Candida sp. infections from blood culture bottles (255). Furthermore, data from two studies, on coccidioidomycosis (257) and invasive fungal rhinosinusitis (258), show that the method has a promising performance on frozen tissue sections, even in cases where cultures are not available or have not been performed.
FIG 1.
Technology behind novel diagnostic methods for fungal infections. (A) Fluorescence in situ hybridization. Fluorescent probes against a specific target sequence are mixed with the tested sample and allowed to bind to their complementary DNA sequence, if present in the sample. The excess probes are then washed off, while the bound probes are detected via their fluorescence under a fluorescence microscope. (B) Matrix-assisted laser desorption ionization–time of flight mass spectrometry. The tested sample is added to a well that contains matrix material which has the ability to absorb UV light and transform it into heat. A laser beam is targeted to the mix. The laser beam is absorbed by the matrix, and part of the analyte-matrix mix is vaporized and ionized, creating a cloud of ionized proteins and matrix. This cloud subsequently is subjected to an electric field, which leads the particles to accelerate toward a detector. The mass and charge of each particle determine the time needed to reach the detector. This allows the mass spectrometer to determine the characteristics of the particles within the tested sample. Comparison of the produced spectral pattern against a standard database allows for identification of the microorganism in the sample. (C) Surface enhanced resonance Raman spectroscopy. The tested sample is placed on a rough surface, which helps to create scattered light. DNA probes coupled with specialized dyes that emit light are added to the sample. The probes then bind to the target DNA in the sample, and subsequently, a double-stranded DNA exonuclease is added to the mix and digests all bound probes, while the unbound probes, which are single stranded, are left indigested. Finally, the scattered light from the undigested probe is detected by a sensor and analyzed, thus identifying the DNA sequence of the digested probes. (D) T2 nuclear magnetic resonance. First, the target sequence of a microorganism that is found in the sample is amplified. Subsequently, DNA probes coupled with paramagnetic nanoparticles are added to the amplicon and are allowed to hybridize with the amplified sequence. This changes the T2 relaxation time of the nanoparticles, and the change is detected via magnetic resonance imaging, thus identifying the target.
Nucleic acid sequence-based amplification (NASBA) is a method very similar to PCR but differs in the sense that it amplifies mRNA by using an RNA polymerase instead of DNA, and it is isothermal (259). Arguably, its ability to detect mRNA gives it the advantage of detecting active disease instead of latent or previous infection (260), and its isothermal nature, coupled with the fact that RNA is less stable than DNA, could decrease the chance of contamination (261). Despite its availability since 1991, it was not until 10 years later that Loeffler et al. evaluated the potential of this method as a diagnostic tool for invasive aspergillosis (261). They reported a threshold for detection of 1 CFU per 100 μl of whole blood. An early clinical trial followed up by evaluating the method on blood samples from 128 hematology patients with neutropenic fever and found 100% sensitivity and 63% specificity for patients with proven or probable IA (260). In a later study, investigators developed and evaluated a real-time NASBA method on blood samples from 78 patients and reported a sensitivity and specificity of 100% and 43%, respectively (262). The high sensitivities reported by both groups increase hopes for the future use of the method as a screening test for high-risk populations to rule out IA. Finally, the same group of researchers reported later that this method could also be used to predict the clinical outcome, as negative conversion of a previously positive assay was associated with significantly more survival in patients with IA (263).
A different approach, MALDI-TOF MS, is based on mass spectrometry to identify the protein fingerprints of different microorganisms. By direct comparison of the spectral pattern of the organism in question with databases of known patterns from different microorganisms, it is possible to identify the detected microbe at the genus, species, and even strain levels (264) (Fig. 1B). Despite the fact that this technique was first described 30 years ago, it was not until recently that the scientific community started to realize its potential. Indeed, in recent years, this method has created a revolution for microbiological laboratories (265). Four commercial systems based on this method that are able to identify yeasts and mold species have been developed, and the first such system was recently cleared by the FDA for use in clinical microbiology laboratories. Studies evaluating their performances are promising, showing that this method is able to accurately and rapidly identify Candida spp. and Aspergillus spp. from positive cultures, with a high concordance (consistently >90%) in comparison to conventional methods (264, 266–278). Furthermore, in some reports, MALDI-TOF MS seems to outperform traditional identification techniques (274, 278). Notably, earlier MALDI systems used for fungal identification required a protein extraction step prior to the spectrometric analysis, whereas newer methods, such as the Vitek MS system, manage to overcome this by using a single deposit step that avoids formic acid lysis, thus making the technique faster and easier to use (274). Moreover, a MALDI method to rapidly assess the caspofungin susceptibility of isolated yeast and Aspergillus species was recently described (279) and subsequently modified to reduce turnaround times (280). This method, which is based on the identification of changes in the protein composition of fungal cells exposed to caspofungin, was designed as a much faster (3 h versus 24 h) alternative to the traditional CLSI method, with comparable results. Finally, a recent interventional study involving 501 patients with bacteremia or candidemia showed that MALDI-TOF combined with an antimicrobial stewardship team was able to decrease the time to organism identification, thus improving antimicrobial agent selection and patient outcomes (281). It is therefore evident that given the indisputable and ever-growing evidence of its superior, easier, and faster performance, MALDI-TOF MS has the potential to essentially replace conventional methods for identification of fungal pathogens in the next few years. Note that an alternative technique that has the same physical basis is the coupling of PCR amplification with electrospray ionization mass spectrometry (PCR-ESI MS) (282). However, due to the short period since its first development, data from clinical studies are still too immature to reach a valid conclusion about its clinical relevance (283).
A novel spectroscopic approach that could potentially help in diagnosis of fungal pathogens is the use of surface enhanced resonance Raman spectroscopy (SERRS) (284). This method employs specific sensors that can detect scattered light produced by DNA coupled with a specialized dye and placed against roughened surfaces consisting of metals such as gold or silver. In a pivotal study, researchers successfully combined a gold-nanowire SERRS sensor with a target recycling reaction to detect a variety of pathogenic fungi. In this method, the tested sample is combined with multiple DNA probes, each targeting a sequence specific to a different fungal pathogen. After binding of a probe to its complementary fungal DNA within the tested sample, an exonuclease with activity on double-stranded DNA (dsDNA) digests the probe. This step is repeated several times, until most or all probes from the fungal species found in the sample are digested. Finally, the scattered light from the remaining probes is measured by the SERRS sensor, and the missing probe reveals the fungal pathogen (Fig. 1C). The technique was tested on eight clinical blood culture samples that were positive for fungi and resulted in findings that were 100% concordant with results from culture (285). Although these results seem promising, prospective studies with larger patient populations are needed to establish the position of the method in clinical diagnosis.
Following a completely different idea, other investigators focused on the detection of human pathogens by using microscopic resonating cantilevers (286, 287). These are microchips with surfaces that are able to bind microorganisms from a fluid that is directed to pass through them. To achieve that, the surfaces are coated with antibodies or other proteins that can bind to microbial membranes. After binding of the pathogen, the mass of the cantilever increases, leading to a decrease in its resonance frequency, which can easily be detected. Researchers used cantilevers coated with concanavalin A, fibronectin, and IgG immunoglobulins to detect Aspergillus niger and Saccharomyces cerevisiae and reported detection limits of 103 to 106 CFU/ml (288). Although the approach seems intriguing, refinement of the technique to achieve lower detection thresholds and evaluation with clinical samples would be imperative before it can be considered useful.
Moreover, a different method that is based on a physical phenomenon that has been known for a long time but has only very recently been appreciated for its usefulness in pathogen identification is nuclear magnetic resonance (NMR) spectroscopy. The physical phenomenon on which it is based was described over 70 years ago (289), and its applications in different domains, such as chemistry (290) and radiology (291), are immense. However, it was not until 2001 that the method was first realized to be useful in the field of microbiology (292). Soon thereafter, investigators found that this method has the potential to identify Candida spp. from blood cultures by statistically comparing the magnetic resonance spectra of fungi in question to known databases (293). A novel approach that seems to be even more promising, though, is the combination of NMR spectroscopy with PCR to directly detect and identify Candida spp. from blood samples from patients at risk. In this technique, whole blood is subjected to PCR amplification of Candida sp. sequences, followed by hybridization to nanoparticles that elicit a T2 magnetic resonance (T2MR) signal (Fig. 1D). Results from a preliminary study of the T2MR method are exciting, showing that it is able to reduce the time to result to an average of 2 h, in contrast to the 48-h average of blood cultures (294). The T2MR method was able to accurately isolate yeast species from retrospectively collected, blinded clinical specimens. Based on these groundbreaking preliminary findings, a new large-scale prospective trial is under way to validate the assay and evaluate its clinical performance.
Finally, a novel exciting technique that was recently proposed and evaluated on a murine model of candidemia focuses on detecting the pathogen by measuring the induced host immune response (295). Specifically, based on previous reports that indicate that the host immune response differs in response to different pathogens (296), the researchers developed a method that was able to successfully differentiate between the gene expression signatures of mice with candidemia and mice with bacteremia or no infection, thus providing an alternative to all traditional methods that target the characteristics of the invading microorganism.
CONCLUSIONS
It is undoubtedly true that current gold standards for IFI diagnosis are lacking in both sensitivity and rapidity, thus delaying treatment and undermining survival of patients at risk. This underscores the need for the development of faster and more accurate diagnostic tests. Although novel serologic and molecular methods for detection and identification of fungal pathogens have been developed and are showing the potential to replace traditional diagnostic assays, inconsistencies between different approaches limit their reproducibility and prohibit large-scale clinical implementation. Thus, much effort should be made to standardize these techniques and ensure their reliability in order to significantly improve our ability to detect and treat fungal pathogens in an effective and timely manner. With continued emergence of new methods, we are reminded that fungal diagnostics is still in its infancy, with much room for improvement and refinement.
ACKNOWLEDGMENTS
E.M. has received grant support from Astellas Pharma and T2 Biosystems and has served in an advisory board for Astellas Pharma. A.M.C. has received grant support from T2 Biosystems. All other authors report no potential conflicts.
Biographies

Marios Arvanitis received his M.D. from the University of Athens and is currently a Research Fellow at the Warren Alpert Medical School of Brown University, working in the Division of Infectious Diseases. His research interests include the use of invertebrate model hosts to evaluate immune responses against fungal pathogens. He is also interested in quality improvement, cost-effectiveness, and comparative effectiveness research and is currently working on identifying the clinical performance of PCR on blood specimens for invasive aspergillosis.

Theodora Anagnostou, M.D., is an Internal Medicine Resident at Mount Auburn Hospital and a Clinical Fellow in Medicine at Harvard Medical School. She received her M.D. from the University of Athens, Greece, in 2008. She subsequently joined Massachusetts General Hospital as a Postdoctoral Research Fellow in Infectious Diseases. She joined the Alpert Medical School of Brown University in 2012, where she continued her research. Her research focuses on invasive fungal infections, specifically their pathogenesis, as well as pathogen-pathogen interactions. She is a member of the American College of Physicians and the American Medical Association.

Beth Burgwyn Fuchs received her Ph.D. from Saint Louis University and did her postdoctoral studies at the Donald Danforth Plant Science Center and Harvard Medical School. She is currently an Assistant Professor at Brown University and Rhode Island Hospital, working in the Division of Infectious Diseases. Dr. Fuchs studies fungal and bacterial pathogenesis and has a particular interest in the cell wall. She screens fungal mutant libraries to identify genes required for virulence and has also worked to identify antifungal and immunomodulatory compounds by using high-throughput automated technologies. Most recently, her research endeavors have focused on developing new technologies to aid in infectious disease diagnostics.

Angela M. Caliendo received a Ph.D. in biochemistry and an M.D. from Case Western Reserve University School of Medicine and completed an internship and residency in internal medicine at Brigham and Women's Hospital in Boston, MA, and an Infectious Diseases Fellowship at Massachusetts General Hospital. She is Professor and Vice Chair of the Department of Medicine at Alpert Medical School at Brown University. She is a member of numerous organizations and serves as Chair of the Microbiology Medical Devices Panel at the Food and Drug Administration. Her research interests include antiretroviral resistance in HIV-positive women and the development of molecular diagnostic tests for various infectious diseases.

Eleftherios Mylonakis completed medical training at the University of Athens and completed his medical residency training at Brown University and his Infectious Disease Fellowship at Massachusetts General Hospital. He is Chief of the Infectious Diseases Division and Dean's Professor of Medicine at the Warren Alpert Medical School of Brown University. He has authored over 200 peer-reviewed publications and has received a number of awards, and his research interests include microbial pathogenesis and immune responses in invertebrate model hosts. He is also actively involved in clinical research on fungal diagnostics and treatment, and his research is supported by the NIH and private foundations.
REFERENCES
- 1.Chen Y, Wang H, Kantarjian H, Cortes J. 2013. Trends in chronic myeloid leukemia incidence and survival in the United States from 1975 to 2009. Leuk. Lymphoma 54:1411–1417. 10.3109/10428194.2012.745525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111. 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 3.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci. Transl. Med. 4:165rv113. 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MH, Wissel MC, Shields RK, Salomoni MA, Hao B, Press EG, Shields RM, Cheng S, Mitsani D, Vadnerkar A, Silveira FP, Kleiboeker SB, Clancy CJ. 2012. Performance of Candida real-time polymerase chain reaction, beta-d-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin. Infect. Dis. 54:1240–1248. 10.1093/cid/cis200 [DOI] [PubMed] [Google Scholar]
- 5.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50:1559–1567. 10.1086/652768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt L, Ito JI, Kauffman CA, Lyon GM, Marr KA, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wingard JR, Walsh TJ, Kontoyiannis DP. 2011. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg. Infect. Dis. 17:1855–1864. 10.3201/eid1710.110087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ, Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer, Mycoses Study Group of the National Institute of Allergy and Infectious Diseases 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14. 10.1086/323335 [DOI] [PubMed] [Google Scholar]
- 8.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinwald M, Buchheidt D, Hummel M, Duerken M, Bertz H, Schwerdtfeger R, Reuter S, Kiehl MG, Barreto-Miranda M, Hofmann WK, Spiess B. 2013. Diagnostic performance of an Aspergillus-specific nested PCR assay in cerebrospinal fluid samples of immunocompromised patients for detection of central nervous system aspergillosis. PLoS One 8:e56706. 10.1371/journal.pone.0056706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer J, Loeffler J, Heinz W, Schlossnagel H, Lehmann M, Morton O, Rogers TR, Schmitt C, Frosch M, Einsele H, Kurzai O. 2011. Pathogen-specific DNA enrichment does not increase sensitivity of PCR for diagnosis of invasive aspergillosis in neutropenic patients. J. Clin. Microbiol. 49:1267–1273. 10.1128/JCM.01679-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarner J, Brandt ME. 2011. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 24:247–280. 10.1128/CMR.00053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran C, Grussemeyer CA, Spalding JR, Benjamin DK, Jr, Reed SD. 2010. Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. Am. J. Infect. Control 38:78–80. 10.1016/j.ajic.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640–3645. 10.1128/AAC.49.9.3640-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25–31. 10.1086/504810 [DOI] [PubMed] [Google Scholar]
- 15.Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. 2010. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect. Dis. 10:150. 10.1186/1471-2334-10-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold HM, Micek ST, Shorr AF, Zilberberg MD, Labelle AJ, Kothari S, Kollef MH. 2010. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacotherapy 30:361–368. 10.1592/phco.30.4.361 [DOI] [PubMed] [Google Scholar]
- 17.Chamilos G, Lewis RE, Kontoyiannis DP. 2008. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 47:503–509. 10.1086/590004 [DOI] [PubMed] [Google Scholar]
- 18.Ericson EL, Klingspor L, Ullberg M, Ozenci V. 2012. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn. Microbiol. Infect. Dis. 73:153–156. 10.1016/j.diagmicrobio.2012.02.020 [DOI] [PubMed] [Google Scholar]
- 19.Park BR, Kim TH, Kim HR, Lee MK. 2011. Comparative analysis of simulated candidemia using two different blood culture systems and the rapid identification of Candida albicans. Ann. Clin. Lab. Sci. 41:251–256 [PubMed] [Google Scholar]
- 20.Kosmin AR, Fekete T. 2008. Use of fungal blood cultures in an academic medical center. J. Clin. Microbiol. 46:3800–3801. 10.1128/JCM.00796-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato A, Takita T, Furuhashi M, Takahashi T, Maruyama Y, Hishida A. 2001. Elevation of blood (1→3)-beta-d-glucan concentrations in hemodialysis patients. Nephron 89:15–19. 10.1159/000046037 [DOI] [PubMed] [Google Scholar]
- 22.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, Ketchum PA, Wingard J, Schiff R, Tamura H, Finkelman MA, Rex JH. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654–659. 10.1086/432470 [DOI] [PubMed] [Google Scholar]
- 23.Nett J, Lincoln L, Marchillo K, Andes D. 2007. Beta-1,3 glucan as a test for central venous catheter biofilm infection. J. Infect. Dis. 195:1705–1712. 10.1086/517522 [DOI] [PubMed] [Google Scholar]
- 24.Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova L, Toskova M, Winterova J, Timilsina S, Rodriguez I, Mayer J. 2010. Difficulties in using 1,3-β-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies—high frequency of false-positive results and their analysis. J. Med. Microbiol. 59:1016–1022. 10.1099/jmm.0.019299-0 [DOI] [PubMed] [Google Scholar]
- 25.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650–1659. 10.1086/647942 [DOI] [PubMed] [Google Scholar]
- 26.Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. 2009. Utility of galactomannan enzyme immunoassay and (1,3) beta-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J. Clin. Microbiol. 47:129–133. 10.1128/JCM.00506-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O, Third European Conference on Infections in Leukemia 2012. Beta-glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin. Infect. Dis. 54:633–643. 10.1093/cid/cir897 [DOI] [PubMed] [Google Scholar]
- 28.Moragues MD, Ortiz N, Iruretagoyena JR, Garcia-Ruiz JC, Amutio E, Rojas A, Mendoza J, Quindos G, Ponton-San Emeterio J. 2004. Evaluation of a new commercial test (Candida albicans IFA IgG) for the serodiagnosis of invasive candidiasis. Enferm. Infecc. Microbiol. Clin. 22:83–88. 10.1016/S0213-005X(04)73039-6 [DOI] [PubMed] [Google Scholar]
- 29.Ponton J, Jones JM. 1986. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect. Immun. 54:864–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponton J, Quindos G, Arilla MC, Mackenzie DW. 1994. Simplified adsorption method for detection of antibodies to Candida albicans germ tubes. J. Clin. Microbiol. 32:217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quindos G, Ponton J, Cisterna R. 1987. Detection of antibodies to Candida albicans germ tube in the diagnosis of systemic candidiasis. Eur. J. Clin. Microbiol. 6:142–146. 10.1007/BF02018195 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Ruiz JC, del Carmen Arilla M, Regulez P, Quindos G, Alvarez A, Ponton J. 1997. Detection of antibodies to Candida albicans germ tubes for diagnosis and therapeutic monitoring of invasive candidiasis in patients with hematologic malignancies. J. Clin. Microbiol. 35:3284–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaragoza R, Peman J, Quindos G, Iruretagoyena JR, Cuetara MS, Ramirez P, Gomez MD, Camarena JJ, Viudes A, Ponton J, Candida albicans Germ Tube Antibody Detection in Critically Ill Patients 2009. Clinical significance of the detection of Candida albicans germ tube-specific antibodies in critically ill patients. Clin. Microbiol. Infect. 15:592–595. 10.1111/j.1469-0691.2009.02794.x [DOI] [PubMed] [Google Scholar]
- 34.Peman J, Zaragoza R, Quindos G, Alkorta M, Cuetara MS, Camarena JJ, Ramirez P, Gimenez MJ, Martin-Mazuelos E, Linares-Sicilia MJ, Ponton J, Candida albicans Germ Tube Antibody Detection in Critically Ill Patients 2011. Clinical factors associated with a Candida albicans germ tube antibody positive test in intensive care unit patients. BMC Infect. Dis. 11:60. 10.1186/1471-2334-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon C, Ruiz-Santana S, Saavedra P, Castro C, Ubeda A, Loza A, Martin-Mazuelos E, Blanco A, Jerez V, Ballus J, Alvarez-Rocha L, Utande-Vazquez A, Farinas O. 2012. Value of beta-d-glucan and Candida albicans germ tube antibody for discriminating between Candida colonization and invasive candidiasis in patients with severe abdominal conditions. Intensive Care Med. 38:1315–1325. 10.1007/s00134-012-2616-y [DOI] [PubMed] [Google Scholar]
- 36.Ellis M, Al-Ramadi B, Bernsen R, Kristensen J, Alizadeh H, Hedstrom U. 2009. Prospective evaluation of mannan and anti-mannan antibodies for diagnosis of invasive Candida infections in patients with neutropenic fever. J. Med. Microbiol. 58:606–615. 10.1099/jmm.0.006452-0 [DOI] [PubMed] [Google Scholar]
- 37.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265–273. 10.1086/595846 [DOI] [PubMed] [Google Scholar]
- 38.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50:1091–1100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 39.Tarrand JJ, Han XY, Kontoyiannis DP, May GS. 2005. Aspergillus hyphae in infected tissue: evidence of physiologic adaptation and effect on culture recovery. J. Clin. Microbiol. 43:382–386. 10.1128/JCM.43.1.382-386.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath JA, Dummer S. 1996. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am. J. Med. 100:171–178. 10.1016/S0002-9343(97)89455-7 [DOI] [PubMed] [Google Scholar]
- 41.Zmeili OS, Soubani AO. 2007. Pulmonary aspergillosis: a clinical update. QJM 100:317–334. 10.1093/qjmed/hcm035 [DOI] [PubMed] [Google Scholar]
- 42.Tashiro T, Izumikawa K, Tashiro M, Takazono T, Morinaga Y, Yamamoto K, Imamura Y, Miyazaki T, Seki M, Kakeya H, Yamamoto Y, Yanagihara K, Yasuoka A, Kohno S. 2011. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med. Mycol. 49:581–587. 10.3109/13693786.2010.548084 [DOI] [PubMed] [Google Scholar]
- 43.Khasawneh F, Mohamad T, Moughrabieh MK, Lai Z, Ager J, Soubani AO. 2006. Isolation of Aspergillus in critically ill patients: a potential marker of poor outcome. J. Crit. Care 21:322–327. 10.1016/j.jcrc.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 44.Simoneau E, Kelly M, Labbe AC, Roy J, Laverdiere M. 2005. What is the clinical significance of positive blood cultures with Aspergillus sp in hematopoietic stem cell transplant recipients? A 23 year experience. Bone Marrow Transplant. 35:303–306. 10.1038/sj.bmt.1704793 [DOI] [PubMed] [Google Scholar]
- 45.Balajee SA, Kano R, Baddley JW, Moser SA, Marr KA, Alexander BD, Andes D, Kontoyiannis DP, Perrone G, Peterson S, Brandt ME, Pappas PG, Chiller T. 2009. Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 47:3138–3141. 10.1128/JCM.01070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bariola JR, Perry P, Pappas PG, Proia L, Shealey W, Wright PW, Sizemore JM, Robinson M, Bradsher RW., Jr 2010. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin. Infect. Dis. 50:797–804. 10.1086/650579 [DOI] [PubMed] [Google Scholar]
- 47.Lemos LB, Guo M, Baliga M. 2000. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann. Diagn. Pathol. 4:391–406. 10.1053/adpa.2000.20755 [DOI] [PubMed] [Google Scholar]
- 48.Patel AJ, Gattuso P, Reddy VB. 2010. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am. J. Surg. Pathol. 34:256–261. 10.1097/PAS.0b013e3181ca48a5 [DOI] [PubMed] [Google Scholar]
- 49.Gazzoni AF, Severo CB, Salles EF, Severo LC. 2009. Histopathology, serology and cultures in the diagnosis of cryptococcosis. Rev. Inst. Med. Trop. Sao Paulo 51:255–259. 10.1590/S0036-46652009000500004 [DOI] [PubMed] [Google Scholar]
- 50.Saubolle MA. 2007. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann. N. Y. Acad. Sci. 1111:301–314. 10.1196/annals.1406.049 [DOI] [PubMed] [Google Scholar]
- 51.Pickering JW, Sant HW, Bowles CAP, Roberts WL, Woods GL. 2005. Evaluation of a (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957–5962. 10.1128/JCM.43.12.5957-5962.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, Motokura T, Hirai H, Ogawa S. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-beta-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733–2741. 10.1128/JCM.42.6.2733-2741.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obayashi T, Negishi K, Suzuki T, Funata N. 2008. Reappraisal of the serum (1→3)-beta-d-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin. Infect. Dis. 46:1864–1870. 10.1086/588295 [DOI] [PubMed] [Google Scholar]
- 54.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199–205. 10.1086/421944 [DOI] [PubMed] [Google Scholar]
- 55.Marty FM, Koo S. 2009. Role of (1→3)-beta-d-glucan in the diagnosis of invasive aspergillosis. Med. Mycol. 47(Suppl 1):S233–S240. 10.1080/13693780802308454 [DOI] [PubMed] [Google Scholar]
- 56.Mennink-Kersten MA, Ruegebrink D, Verweij PE. 2008. Pseudomonas aeruginosa as a cause of 1,3-beta-d-glucan assay reactivity. Clin. Infect. Dis. 46:1930–1931. 10.1086/588563 [DOI] [PubMed] [Google Scholar]
- 57.Mennink-Kersten MA, Verweij PE. 2006. Non-culture-based diagnostics for opportunistic fungi. Infect. Dis. Clin. North Am. 20:711–727. 10.1016/j.idc.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 58.Martins LM, de Andrade HM, Vainstein MH, Wanke B, Schrank A, Balaguez CB, dos Santos PR, Santi L, Pires Sda F, da Silva AS, de Castro JA, Brandao RM, do Monte SJ. 2013. Immunoproteomics and immunoinformatics analysis of Cryptococcus gattii: novel candidate antigens for diagnosis. Future Microbiol. 8:549–563. 10.2217/fmb.13.22 [DOI] [PubMed] [Google Scholar]
- 59.Metan G, Koc AN, Atalay A, Kaynar LG, Ozturk A, Alp E, Eser B. 2012. What should be the optimal cut-off of serum 1,3-beta-d-glucan for the detection of invasive pulmonary aspergillosis in patients with haematological malignancies? Scand. J. Infect. Dis. 44:330–336. 10.3109/00365548.2011.638319 [DOI] [PubMed] [Google Scholar]
- 60.Tortorano AM, Esposto MC, Prigitano A, Grancini A, Ossi C, Cavanna C, Cascio GL. 2012. Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J. Clin. Microbiol. 50:1051–1053. 10.1128/JCM.05946-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wheat LJ, Hackett E, Durkin M, Connolly P, Petraitiene R, Walsh TJ, Knox K, Hage C. 2007. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 14:638–640. 10.1128/CVI.00479-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maschmeyer G, Calandra T, Singh N, Wiley J, Perfect J. 2009. Invasive mould infections: a multi-disciplinary update. Med. Mycol. 47:571–583. 10.1080/13693780902946559 [DOI] [PubMed] [Google Scholar]
- 63.Klont RR, Mennink-Kersten MA, Ruegebrink D, Rijs AJ, Blijlevens NM, Donnelly JP, Verweij PE. 2006. Paradoxical increase in circulating Aspergillus antigen during treatment with caspofungin in a patient with pulmonary aspergillosis. Clin. Infect. Dis. 43:e23–e25. 10.1086/505603 [DOI] [PubMed] [Google Scholar]
- 64.Mennink-Kersten MA, Donnelly JP, Verweij PE. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349–357. 10.1016/S1473-3099(04)01045-X [DOI] [PubMed] [Google Scholar]
- 65.Marr KA, Laverdiere M, Gugel A, Leisenring W. 2005. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin. Infect. Dis. 40:1762–1769. 10.1086/429921 [DOI] [PubMed] [Google Scholar]
- 66.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 190:641–649. 10.1086/422009 [DOI] [PubMed] [Google Scholar]
- 67.Berenguer J, Allende MC, Lee JW, Garrett K, Lyman C, Ali NM, Bacher J, Pizzo PA, Walsh TJ. 1995. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 152:1079–1086 [DOI] [PubMed] [Google Scholar]
- 68.Zou M, Tang L, Zhao S, Zhao Z, Chen L, Chen P, Huang Z, Li J, Chen L, Fan X. 2012. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS One 7:e43347. 10.1371/journal.pone.0043347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher CE, Stevens AM, Leisenring W, Pergam SA, Boeckh M, Hohl TM. 2013. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin. Infect. Dis. 57:1001–1004. 10.1093/cid/cit393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mikulska M, Raiola AM, Signori A, Furfaro E, Del Bono V, Bacigalupo A, Viscoli C. 2013. Screening with serum galactomannan might be associated with better outcome than symptom-triggered galactomannan testing in allogeneic HSCT recipients with invasive aspergillosis. Clin. Infect. Dis. 57:1786–1787. 10.1093/cid/cit565 [DOI] [PubMed] [Google Scholar]
- 71.Husain S, Clancy CJ, Nguyen MH, Swartzentruber S, Leather H, LeMonte AM, Durkin MM, Knox KS, Hage CA, Bentsen C, Singh N, Wingard JR, Wheat LJ. 2008. Performance characteristics of the platelia Aspergillus enzyme immunoassay for detection of Aspergillus galactomannan antigen in bronchoalveolar lavage fluid. Clin. Vaccine Immunol. 15:1760–1763. 10.1128/CVI.00226-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musher B, Fredricks D, Leisenring W, Balajee SA, Smith C, Marr KA. 2004. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J. Clin. Microbiol. 42:5517–5522. 10.1128/JCM.42.12.5517-5522.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maertens J, Maertens V, Theunissen K, Meersseman W, Meersseman P, Meers S, Verbeken E, Verhoef G, Van Eldere J, Lagrou K. 2009. Bronchoalveolar lavage fluid galactomannan for the diagnosis of invasive pulmonary aspergillosis in patients with hematologic diseases. Clin. Infect. Dis. 49:1688–1693. 10.1086/647935 [DOI] [PubMed] [Google Scholar]
- 74.D'Haese J, Theunissen K, Vermeulen E, Schoemans H, De Vlieger G, Lammertijn L, Meersseman P, Meersseman W, Lagrou K, Maertens J. 2012. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J. Clin. Microbiol. 50:1258–1263. 10.1128/JCM.06423-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maertens J, Theunissen K, Verbeken E, Lagrou K, Verhaegen J, Boogaerts M, Eldere JV. 2004. Prospective clinical evaluation of lower cut-offs for galactomannan detection in adult neutropenic cancer patients and haematological stem cell transplant recipients. Br. J. Haematol. 126:852–860. 10.1111/j.1365-2141.2004.05140.x [DOI] [PubMed] [Google Scholar]
- 76.Maertens JA, Klont R, Masson C, Theunissen K, Meersseman W, Lagrou K, Heinen C, Crepin B, Van Eldere J, Tabouret M, Donnelly JP, Verweij PE. 2007. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin. Infect. Dis. 44:1329–1336. 10.1086/514349 [DOI] [PubMed] [Google Scholar]
- 77.Sampsonas F, Kontoyiannis DP, Dickey BF, Evans SE. 2011. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study. Cancer 117:3424–3433. 10.1002/cncr.25905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White PL, Parr C, Thornton C, Barnes RA. 2013. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 51:1510–1516. 10.1128/JCM.03189-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thornton CR. 2008. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vaccine Immunol. 15:1095–1105. 10.1128/CVI.00068-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thornton C, Johnson G, Agrawal S. 2012. Detection of invasive pulmonary aspergillosis in haematological malignancy patients by using lateral-flow technology. J. Vis. Exp. 22:3721. 10.3791/3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiederhold NP, Thornton CR, Najvar LK, Kirkpatrick WR, Bocanegra R, Patterson TF. 2009. Comparison of lateral flow technology and galactomannan and (1→3)-beta-d-glucan assays for detection of invasive pulmonary aspergillosis. Clin. Vaccine Immunol. 16:1844–1846. 10.1128/CVI.00268-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Held J, Schmidt T, Thornton CR, Kotter E, Bertz H. 2013. Comparison of a novel Aspergillus lateral-flow device and the Platelia(R) galactomannan assay for the diagnosis of invasive aspergillosis following haematopoietic stem cell transplantation. Infection 41:1163–1169. 10.1007/s15010-013-0472-5 [DOI] [PubMed] [Google Scholar]
- 83.de Heer K, van der Schee MP, Zwinderman K, van den Berk IA, Visser CE, van Oers R, Sterk PJ. 2013. Electronic nose technology for detection of invasive pulmonary aspergillosis in prolonged chemotherapy-induced neutropenia: a proof-of-principle study. J. Clin. Microbiol. 51:1490–1495. 10.1128/JCM.02838-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Syhre M, Scotter JM, Chambers ST. 2008. Investigation into the production of 2-pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 46:209–215. 10.1080/13693780701753800 [DOI] [PubMed] [Google Scholar]
- 85.Chambers ST, Syhre M, Murdoch DR, McCartin F, Epton MJ. 2009. Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med. Mycol. 47:468–476. 10.1080/13693780802475212 [DOI] [PubMed] [Google Scholar]
- 86.Harris JR, Marston BJ, Sangrujee N, DuPlessis D, Park B. 2011. Cost-effectiveness analysis of diagnostic options for pneumocystis pneumonia (PCP). PLoS One 6:e23158. 10.1371/journal.pone.0023158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaRocque RC, Katz JT, Perruzzi P, Baden LR. 2003. The utility of sputum induction for diagnosis of Pneumocystis pneumonia in immunocompromised patients without human immunodeficiency virus. Clin. Infect. Dis. 37:1380–1383. 10.1086/379071 [DOI] [PubMed] [Google Scholar]
- 88.Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, Morinobu A, Nishimura K, Kumagai S. 2012. Diagnostic accuracy of serum 1,3-beta-d-glucan for Pneumocystis jirovecii pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J. Clin. Microbiol. 50:7–15. 10.1128/JCM.05267-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME. 2013. Accuracy of beta-d-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin. Microbiol. Infect. 19:39–49. 10.1111/j.1469-0691.2011.03760.x [DOI] [PubMed] [Google Scholar]
- 90.Costa JM, Botterel F, Cabaret O, Foulet F, Cordonnier C, Bretagne S. 2012. Association between circulating DNA, serum (1→3)-beta-d-glucan, and pulmonary fungal burden in Pneumocystis pneumonia. Clin. Infect. Dis. 55:e5–e8. 10.1093/cid/cis412 [DOI] [PubMed] [Google Scholar]
- 91.Matsumura Y, Ito Y, Iinuma Y, Yasuma K, Yamamoto M, Matsushima A, Nagao M, Takakura S, Ichiyama S. 2012. Quantitative real-time PCR and the (1→3)-beta-d-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin. Microbiol. Infect. 18:591–597. 10.1111/j.1469-0691.2011.03605.x [DOI] [PubMed] [Google Scholar]
- 92.Koo S, Baden LR, Marty FM. 2012. Post-diagnostic kinetics of the (1→3)-beta-d-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin. Microbiol. Infect. 18:E122–E127. 10.1111/j.1469-0691.2012.03777.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D'Souza CA, Kronstad JW, Taylor G, Warren R, Yuen M, Hu G, Jung WH, Sham A, Kidd SE, Tangen K, Lee N, Zeilmaker T, Sawkins J, McVicker G, Shah S, Gnerre S, Griggs A, Zeng Q, Bartlett K, Li W, Wang X, Heitman J, Stajich JE, Fraser JA, Meyer W, Carter D, Schein J, Krzywinski M, Kwon-Chung KJ, Varma A, Wang J, Brunham R, Fyfe M, Ouellette BF, Siddiqui A, Marra M, Jones S, Holt R, Birren BW, Galagan JE, Cuomo CA. 2011. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2:e00342-10. 10.1128/mBio.00342-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dominic RS, Prashanth H, Shenoy S, Baliga S. 2009. Diagnostic value of latex agglutination in cryptococcal meningitis. J. Lab. Physicians 1:67–68. 10.4103/0974-2727.59702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S, European Conference on Infections in Leukemia Laboratory Working Group 2012. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 47:846–854. 10.1038/bmt.2011.178 [DOI] [PubMed] [Google Scholar]
- 96.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, ESCMID EFISG Study Group and ECMM 2014. ESCMID/ECMM joint clinical guideline for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 20(Suppl 3):76–98. 10.1111/1469-0691.12360 [DOI] [PubMed] [Google Scholar]
- 97.McMullan BJ, Halliday C, Sorrell TC, Judd D, Sleiman S, Marriott D, Olma T, Chen SC. 2012. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS One 7:e49541. 10.1371/journal.pone.0049541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawn SD, Wood R. 2012. Point-of-care urine antigen screening tests for tuberculosis and cryptococcosis: potential for mortality reduction in antiretroviral treatment programs in Africa. Clin. Infect. Dis. 54:739–740. 10.1093/cid/cir908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, Kamya MR, Bohjanen PR, Boulware DR. 2010. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin. Infect. Dis. 51:448–455. 10.1086/655143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, Alapat D, Babady NE, Parker M, Fuller D, Noor A, Davis TE, Rodgers M, Connolly PA, El Haddad B, Wheat LJ. 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 53:448–454. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- 101.Saccente M, Woods GL. 2010. Clinical and laboratory update on blastomycosis. Clin. Microbiol. Rev. 23:367–381. 10.1128/CMR.00056-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hage CA, Kirsch EJ, Stump TE, Kauffman CA, Goldman M, Connolly P, Johnson PC, Wheat LJ, Baddley JW. 2011. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clin. Vaccine Immunol. 18:661–666. 10.1128/CVI.00389-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assi M, Martin S, Wheat LJ, Hage C, Freifeld A, Avery R, Baddley JW, Vergidis P, Miller R, Andes D, Young JA, Hammoud K, Huprikar S, McKinsey D, Myint T, Garcia-Diaz J, Esguerra E, Kwak EJ, Morris M, Mullane KM, Prakash V, Burdette SD, Sandid M, Dickter J, Ostrander D, Antoun SA, Kaul DR. 2013. Histoplasmosis after solid organ transplant. Clin. Infect. Dis. 57:1542–1549. 10.1093/cid/cit593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hage CA, Wheat LJ. 2010. Diagnosis of pulmonary histoplasmosis using antigen detection in the bronchoalveolar lavage. Expert Rev. Respir. Med. 4:427–429. 10.1586/ers.10.36 [DOI] [PubMed] [Google Scholar]
- 105.Richer SM, Smedema ML, Durkin MM, Brandhorst TT, Hage CA, Connolly PA, Leland DS, Davis TE, Klein BS, Wheat LJ. 2014. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin. Vaccine Immunol. 21:143–146. 10.1128/CVI.00597-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ampel NM. 2010. The diagnosis of coccidioidomycosis. F1000 Med. Rep. 2:2. 10.3410/M2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blair JE, Mendoza N, Force S, Chang YH, Grys TE. 2013. Clinical specificity of the enzyme immunoassay test for coccidioidomycosis varies according to the reason for its performance. Clin. Vaccine Immunol. 20:95–98. 10.1128/CVI.00531-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thompson GR, 3rd, Bays DJ, Johnson SM, Cohen SH, Pappagianis D, Finkelman MA. 2012. Serum (1→3)-beta-d-glucan measurement in coccidioidomycosis. J. Clin. Microbiol. 50:3060–3062. 10.1128/JCM.00631-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Millon L, Larosa F, Lepiller Q, Legrand F, Rocchi S, Daguindau E, Scherer E, Bellanger AP, Leroy J, Grenouillet F. 2013. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin. Infect. Dis. 56:e95–e101. 10.1093/cid/cit094 [DOI] [PubMed] [Google Scholar]
- 110.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 367:2214–2225. 10.1056/NEJMoa1204781 [DOI] [PubMed] [Google Scholar]
- 111.Boutati EI, Anaissie EJ. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999–1008 [PubMed] [Google Scholar]
- 112.Lionakis MS, Kontoyiannis DP. 2004. The significance of isolation of saprophytic molds from the lower respiratory tract in patients with cancer. Cancer 100:165–172. 10.1002/cncr.11876 [DOI] [PubMed] [Google Scholar]
- 113.Lionakis MS, Bodey GP, Tarrand JJ, Raad II, Kontoyiannis DP. 2004. The significance of blood cultures positive for emerging saprophytic moulds in cancer patients. Clin. Microbiol. Infect. 10:922–925. 10.1111/j.1469-0691.2004.00933.x [DOI] [PubMed] [Google Scholar]
- 114.Norkin M, Wingard JR. 2013. Diagnostic strategies for invasive fungal infections in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J. Natl. Compr. Canc. Netw. 11:941–949 [DOI] [PubMed] [Google Scholar]
- 115.Strick LB, Wald A. 2006. Diagnostics for herpes simplex virus: is PCR the new gold standard? Mol. Diagn. Ther. 10:17–28. 10.1007/BF03256439 [DOI] [PubMed] [Google Scholar]
- 116.Ruchel R. 1993. Diagnosis of invasive mycoses in severely immunosuppressed patients. Ann. Hematol. 67:1–11. 10.1007/BF01709659 [DOI] [PubMed] [Google Scholar]
- 117.Stevens DA. 2002. Diagnosis of fungal infections: current status. J. Antimicrob. Chemother. 49(Suppl 1):11–19. 10.1093/jac/49.suppl_1.11 [DOI] [PubMed] [Google Scholar]
- 118.Maaroufi Y, Heymans C, De Bruyne JM, Duchateau V, Rodriguez-Villalobos H, Aoun M, Crokaert F. 2003. Rapid detection of Candida albicans in clinical blood samples by using a TaqMan-based PCR assay. J. Clin. Microbiol. 41:3293–3298. 10.1128/JCM.41.7.3293-3298.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muller FM, Werner KE, Kasai M, Francesconi A, Chanock SJ, Walsh TJ. 1998. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J. Clin. Microbiol. 36:1625–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gonzalez-Mendoza D, Argumedo-Delira R, Morales-Trejo A, Pulido-Herrera A, Cervantes-Diaz L, Grimaldo-Juarez O, Alarcon A. 2010. A rapid method for isolation of total DNA from pathogenic filamentous plant fungi. Genet. Mol. Res. 9:162–166. 10.4238/vol9-1gmr680 [DOI] [PubMed] [Google Scholar]
- 121.Francesconi A, Kasai M, Harrington SM, Beveridge MG, Petraitiene R, Petraitis V, Schaufele RL, Walsh TJ. 2008. Automated and manual methods of DNA extraction for Aspergillus fumigatus and Rhizopus oryzae analyzed by quantitative real-time PCR. J. Clin. Microbiol. 46:1978–1984. 10.1128/JCM.02246-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun W, Wang K, Gao W, Su X, Qian Q, Lu X, Song Y, Guo Y, Shi Y. 2011. Evaluation of PCR on bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis: a bivariate metaanalysis and systematic review. PLoS One 6:e28467. 10.1371/journal.pone.0028467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyajima Y, Satoh K, Umeda Y, Makimura K. 2009. Quantitation of fungal DNA contamination in commercial zymolyase and lyticase used in the preparation of fungi. Nihon Ishinkin Gakkai Zasshi 50:259–262. 10.3314/jjmm.50.259 [DOI] [PubMed] [Google Scholar]
- 124.Harrison E, Stalhberger T, Whelan R, Sugrue M, Wingard JR, Alexander BD, Follett SA, Bowyer P, Denning DW, Aspergillus Technology Consortium 2010. Aspergillus DNA contamination in blood collection tubes. Diagn. Microbiol. Infect. Dis. 67:392–394. 10.1016/j.diagmicrobio.2010.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kourkoumpetis TK, Fuchs BB, Coleman JJ, Desalermos A, Mylonakis E. 2012. Polymerase chain reaction-based assays for the diagnosis of invasive fungal infections. Clin. Infect. Dis. 54:1322–1331. 10.1093/cid/cis132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buchheidt D, Baust C, Skladny H, Ritter J, Suedhoff T, Baldus M, Seifarth W, Leib-Moesch C, Hehlmann R. 2001. Detection of Aspergillus species in blood and bronchoalveolar lavage samples from immunocompromised patients by means of 2-step polymerase chain reaction: clinical results. Clin. Infect. Dis. 33:428–435. 10.1086/321887 [DOI] [PubMed] [Google Scholar]
- 127.Raad I, Hanna H, Sumoza D, Albitar M. 2002. Polymerase chain reaction on blood for the diagnosis of invasive pulmonary aspergillosis in cancer patients. Cancer 94:1032–1036. 10.1002/cncr.10349 [DOI] [PubMed] [Google Scholar]
- 128.Buchheidt D, Baust C, Skladny H, Baldus M, Brauninger S, Hehlmann R. 2002. Clinical evaluation of a polymerase chain reaction assay to detect Aspergillus species in bronchoalveolar lavage samples of neutropenic patients. Br. J. Haematol. 116:803–811. 10.1046/j.0007-1048.2002.03337.x [DOI] [PubMed] [Google Scholar]
- 129.Raad I, Hanna H, Huaringa A, Sumoza D, Hachem R, Albitar M. 2002. Diagnosis of invasive pulmonary aspergillosis using polymerase chain reaction-based detection of aspergillus in BAL. Chest 121:1171–1176. 10.1378/chest.121.4.1171 [DOI] [PubMed] [Google Scholar]
- 130.Lass-Flörl C, Gunsilius E, Gastl G, Bonatti H, Freund MC, Gschwendtner A, Kropshofer G, Dierich MP, Petzer A. 2004. Diagnosing invasive aspergillosis during antifungal therapy by PCR analysis of blood samples. J. Clin. Microbiol. 42:4154–4157. 10.1128/JCM.42.9.4154-4157.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Buchheidt D, Hummel M, Schleiermacher D, Spiess B, Schwerdtfeger R, Cornely OA, Wilhelm S, Reuter S, Kern W, Sudhoff T, Morz H, Hehlmann R. 2004. Prospective clinical evaluation of a LightCycler-mediated polymerase chain reaction assay, a nested-PCR assay and a galactomannan enzyme-linked immunosorbent assay for detection of invasive aspergillosis in neutropenic cancer patients and haematological stem cell transplant recipients. Br. J. Haematol. 125:196–202. 10.1111/j.1365-2141.2004.04904.x [DOI] [PubMed] [Google Scholar]
- 132.Scotter JM, Chambers ST. 2005. Comparison of galactomannan detection, PCR-enzyme-linked immunosorbent assay, and real-time PCR for diagnosis of invasive aspergillosis in a neutropenic rat model and effect of caspofungin acetate. Clin. Vaccine Immunol. 12:1322–1327. 10.1128/CDLI.12.11.1322-1327.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lass-Flörl C, Gunsilius E, Gastl G, Freund M, Dierich MP, Petzer A. 2005. Clinical evaluation of Aspergillus-PCR for detection of invasive aspergillosis in immunosuppressed patients. Mycoses 48(Suppl 1):12–17. 10.1111/j.1439-0507.2005.01104.x [DOI] [PubMed] [Google Scholar]
- 134.Halliday C, Hoile R, Sorrell T, James G, Yadav S, Shaw P, Bleakley M, Bradstock K, Chen S. 2006. Role of prospective screening of blood for invasive aspergillosis by polymerase chain reaction in febrile neutropenic recipients of haematopoietic stem cell transplants and patients with acute leukaemia. Br. J. Haematol. 132:478–486. 10.1111/j.1365-2141.2005.05887.x [DOI] [PubMed] [Google Scholar]
- 135.Florent M, Katsahian S, Vekhoff A, Levy V, Rio B, Marie JP, Bouvet A, Cornet M. 2006. Prospective evaluation of a polymerase chain reaction-ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignancies. J. Infect. Dis. 193:741–747. 10.1086/500466 [DOI] [PubMed] [Google Scholar]
- 136.Hummel M, Spiess B, Kentouche K, Niggemann S, Bohm C, Reuter S, Kiehl M, Morz H, Hehlmann R, Buchheidt D. 2006. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by a nested PCR assay. J. Clin. Microbiol. 44:3989–3993. 10.1128/JCM.00466-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Badiee P, Kordbacheh P, Alborzi A, Ramzi M, Shakiba E. 2008. Molecular detection of invasive aspergillosis in hematologic malignancies. Infection 36:580–584. 10.1007/s15010-008-7385-8 [DOI] [PubMed] [Google Scholar]
- 138.Shahid M, Malik A, Bhargava R. 2008. Bronchogenic carcinoma and secondary aspergillosis—common yet unexplored: evaluation of the role of bronchoalveolar lavage-polymerase chain reaction and some nonvalidated serologic methods to establish early diagnosis. Cancer 113:547–558. 10.1002/cncr.23570 [DOI] [PubMed] [Google Scholar]
- 139.Hummel M, Spiess B, Roder J, von Komorowski G, Durken M, Kentouche K, Laws HJ, Morz H, Hehlmann R, Buchheidt D. 2009. Detection of Aspergillus DNA by a nested PCR assay is able to improve the diagnosis of invasive aspergillosis in paediatric patients. J. Med. Microbiol. 58:1291–1297. 10.1099/jmm.0.007393-0 [DOI] [PubMed] [Google Scholar]
- 140.Lopes da Silva R, Ribeiro P, Abreu N, Ferreira T, Fernandes T, Monteiro A, Costa F, Caldas J, Silva M, Carande L, Ferreira G, Conduto A, Cruz E, Sousa MH, Rodrigues AS, Costa I, Veiga J, de Sousa AB. 2010. Early diagnosis of invasive aspergillosis in neutropenic patients. Comparison between serum galactomannan and polymerase chain reaction. Clin. Med. Insights Oncol. 4:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hummel M, Spiess B, Cornely OA, Dittmer M, Morz H, Buchheidt D. 2010. Aspergillus PCR testing: results from a prospective PCR study within the AmBiLoad trial. Eur. J. Haematol. 85:164–169. 10.1111/j.1600-0609.2010.01452.x [DOI] [PubMed] [Google Scholar]
- 142.Badiee P, Alborzi A, Karimi M, Pourabbas B, Haddadi P, Mardaneh J, Moieni M. 2012. Diagnostic potential of nested PCR, galactomannan EIA, and beta-d-glucan for invasive aspergillosis in pediatric patients. J. Infect. Dev. Ctries. 6:352–357. 10.3855/jidc.2110 [DOI] [PubMed] [Google Scholar]
- 143.Reinwald M, Hummel M, Kovalevskaya E, Spiess B, Heinz WJ, Vehreschild JJ, Schultheis B, Krause SW, Claus B, Suedhoff T, Schwerdtfeger R, Reuter S, Kiehl MG, Hofmann WK, Buchheidt D. 2012. Therapy with antifungals decreases the diagnostic performance of PCR for diagnosing invasive aspergillosis in bronchoalveolar lavage samples of patients with haematological malignancies. J. Antimicrob. Chemother. 67:2260–2267. 10.1093/jac/dks208 [DOI] [PubMed] [Google Scholar]
- 144.Reinwald M, Spiess B, Heinz WJ, Vehreschild JJ, Lass-Flörl C, Kiehl M, Schultheis B, Krause SW, Wolf HH, Bertz H, Maschmeyer G, Hofmann WK, Buchheidt D. 2012. Diagnosing pulmonary aspergillosis in patients with hematological malignancies: a multicenter prospective evaluation of an Aspergillus PCR assay and a galactomannan ELISA in bronchoalveolar lavage samples. Eur. J. Haematol. 89:120–127. 10.1111/j.1600-0609.2012.01806.x [DOI] [PubMed] [Google Scholar]
- 145.Buess M, Cathomas G, Halter J, Junker L, Grendelmeier P, Tamm M, Stolz D. 2012. Aspergillus-PCR in bronchoalveolar lavage for detection of invasive pulmonary aspergillosis in immunocompromised patients. BMC Infect. Dis. 12:237. 10.1186/1471-2334-12-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Costa C, Costa JM, Desterke C, Botterel F, Cordonnier C, Bretagne S. 2002. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 40:2224–2227. 10.1128/JCM.40.6.2224-2227.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spiess B, Buchheidt D, Baust C, Skladny H, Seifarth W, Zeilfelder U, Leib-Mosch C, Morz H, Hehlmann R. 2003. Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNA in clinical samples from neutropenic patients. J. Clin. Microbiol. 41:1811–1818. 10.1128/JCM.41.5.1811-1818.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanguinetti M, Posteraro B, Pagano L, Pagliari G, Fianchi L, Mele L, La Sorda M, Franco A, Fadda G. 2003. Comparison of real-time PCR, conventional PCR, and galactomannan antigen detection by enzyme-linked immunosorbent assay using bronchoalveolar lavage fluid samples from hematology patients for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:3922–3925. 10.1128/JCM.41.8.3922-3925.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rantakokko-Jalava K, Laaksonen S, Issakainen J, Vauras J, Nikoskelainen J, Viljanen MK, Salonen J. 2003. Semiquantitative detection by real-time PCR of Aspergillus fumigatus in bronchoalveolar lavage fluids and tissue biopsy specimens from patients with invasive aspergillosis. J. Clin. Microbiol. 41:4304–4311. 10.1128/JCM.41.9.4304-4311.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Challier S, Boyer S, Abachin E, Berche P. 2004. Development of a serum-based Taqman real-time PCR assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 42:844–846. 10.1128/JCM.42.2.844-846.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Millon L, Piarroux R, Deconinck E, Bulabois CE, Grenouillet F, Rohrlich P, Costa JM, Bretagne S. 2005. Use of real-time PCR to process the first galactomannan-positive serum sample in diagnosing invasive aspergillosis. J. Clin. Microbiol. 43:5097–5101. 10.1128/JCM.43.10.5097-5101.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.White PL, Linton CJ, Perry MD, Johnson EM, Barnes RA. 2006. The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin. Infect. Dis. 42:479–486. 10.1086/499949 [DOI] [PubMed] [Google Scholar]
- 153.Cesaro S, Stenghele C, Calore E, Franchin E, Cerbaro I, Cusinato R, Tridello G, Manganelli R, Carli M, Palu G. 2008. Assessment of the Lightcycler PCR assay for diagnosis of invasive aspergillosis in paediatric patients with onco-haematological diseases. Mycoses 51:497–504. 10.1111/j.1439-0507.2008.01512.x [DOI] [PubMed] [Google Scholar]
- 154.Botterel F, Farrugia C, Ichai P, Costa JM, Saliba F, Bretagne S. 2008. Real-time PCR on the first galactomannan-positive serum sample for diagnosing invasive aspergillosis in liver transplant recipients. Transpl. Infect. Dis. 10:333–338. 10.1111/j.1399-3062.2008.00323.x [DOI] [PubMed] [Google Scholar]
- 155.Suarez F, Lortholary O, Buland S, Rubio MT, Ghez D, Mahe V, Quesne G, Poiree S, Buzyn A, Varet B, Berche P, Bougnoux ME. 2008. Detection of circulating Aspergillus fumigatus DNA by real-time PCR assay of large serum volumes improves early diagnosis of invasive aspergillosis in high-risk adult patients under hematologic surveillance. J. Clin. Microbiol. 46:3772–3777. 10.1128/JCM.01086-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khot PD, Ko DL, Hackman RC, Fredricks DN. 2008. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect. Dis. 8:73. 10.1186/1471-2334-8-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ramirez M, Castro C, Palomares JC, Torres MJ, Aller AI, Ruiz M, Aznar J, Martin-Mazuelos E. 2009. Molecular detection and identification of Aspergillus spp. from clinical samples using real-time PCR. Mycoses 52:129–134. 10.1111/j.1439-0507.2008.01548.x [DOI] [PubMed] [Google Scholar]
- 158.Frealle E, Decrucq K, Botterel F, Bouchindhomme B, Camus D, Dei-Cas E, Costa JM, Yakoub-Agha I, Bretagne S, Delhaes L. 2009. Diagnosis of invasive aspergillosis using bronchoalveolar lavage in haematology patients: influence of bronchoalveolar lavage human DNA content on real-time PCR performance. Eur. J. Clin. Microbiol. Infect. Dis. 28:223–232. 10.1007/s10096-008-0616-1 [DOI] [PubMed] [Google Scholar]
- 159.Cuenca-Estrella M, Meije Y, Diaz-Pedroche C, Gomez-Lopez A, Buitrago MJ, Bernal-Martinez L, Grande C, Juan RS, Lizasoain M, Rodriguez-Tudela JL, Aguado JM. 2009. Value of serial quantification of fungal DNA by a real-time PCR-based technique for early diagnosis of invasive aspergillosis in patients with febrile neutropenia. J. Clin. Microbiol. 47:379–384. 10.1128/JCM.01716-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Millon L, Grenouillet F, Legrand F, Loewert S, Bellanger AP, Gbaguidi-Haore H, Scherer E, Henon T, Rohrlich P, Deconinck E. 2011. Ribosomal and mitochondrial DNA target for real-time PCR diagnosis of invasive aspergillosis. J. Clin. Microbiol. 49:1058–1063. 10.1128/JCM.01904-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.White PL, Perry MD, Moody A, Follett SA, Morgan G, Barnes RA. 2011. Evaluation of analytical and preliminary clinical performance of Myconostica MycAssay Aspergillus when testing serum specimens for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 49:2169–2174. 10.1128/JCM.00101-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bernal-Martinez L, Gago S, Buitrago MJ, Gomez-Lopez A, Rodriguez-Tudela JL, Cuenca-Estrella M. 2011. Analysis of performance of a PCR-based assay to detect DNA of Aspergillus fumigatus in whole blood and serum: a comparative study with clinical samples. J. Clin. Microbiol. 49:3596–3599. 10.1128/JCM.00647-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luong ML, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Wissel MC, Grantham KJ, Shields RK, Crespo M, Pilewski J, Toyoda Y, Kleiboeker SB, Pakstis D, Reddy SK, Walsh TJ, Nguyen MH. 2011. Comparison of an Aspergillus real-time polymerase chain reaction assay with galactomannan testing of bronchoalvelolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in lung transplant recipients. Clin. Infect. Dis. 52:1218–1226. 10.1093/cid/cir185 [DOI] [PubMed] [Google Scholar]
- 164.Torelli R, Sanguinetti M, Moody A, Pagano L, Caira M, De Carolis E, Fuso L, De Pascale G, Bello G, Antonelli M, Fadda G, Posteraro B. 2011. Diagnosis of invasive aspergillosis by a commercial real-time PCR assay for Aspergillus DNA in bronchoalveolar lavage fluid samples from high-risk patients compared to a galactomannan enzyme immunoassay. J. Clin. Microbiol. 49:4273–4278. 10.1128/JCM.05026-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Springer J, Morton CO, Perry M, Heinz WJ, Paholcsek M, Alzheimer M, Rogers TR, Barnes RA, Einsele H, Loeffler J, White PL. 2013. Multicenter comparison of serum and whole-blood specimens for detection of Aspergillus DNA in high-risk hematological patients. J. Clin. Microbiol. 51:1445–1450. 10.1128/JCM.03322-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rogers TR, Morton CO, Springer J, Conneally E, Heinz W, Kenny C, Frost S, Einsele H, Loeffler J. 2013. Combined real-time PCR and galactomannan surveillance improves diagnosis of invasive aspergillosis in high risk patients with haematological malignancies. Br. J. Haematol. 161:517–524. 10.1111/bjh.12285 [DOI] [PubMed] [Google Scholar]
- 167.Li Y, Gao L, Ding Y, Xu Y, Zhou M, Huang W, Jing Y, Li H, Wang L, Yu L. 2013. Establishment and application of real-time quantitative PCR for diagnosing invasive aspergillosis via the blood in hematological patients: targeting a specific sequence of Aspergillus 28S-ITS2. BMC Infect. Dis. 13:255. 10.1186/1471-2334-13-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guinea J, Padilla C, Escribano P, Munoz P, Padilla B, Gijon P, Bouza E. 2013. Evaluation of MycAssay Aspergillus for diagnosis of invasive pulmonary aspergillosis in patients without hematological cancer. PLoS One 8:e61545. 10.1371/journal.pone.0061545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skladny H, Buchheidt D, Baust C, Krieg-Schneider F, Seifarth W, Leib-Mosch C, Hehlmann R. 1999. Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. J. Clin. Microbiol. 37:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.White PL, Barton R, Guiver M, Linton CJ, Wilson S, Smith M, Gomez BL, Carr MJ, Kimmitt PT, Seaton S, Rajakumar K, Holyoake T, Kibbler CC, Johnson E, Hobson RP, Jones B, Barnes RA. 2006. A consensus on fungal polymerase chain reaction diagnosis?: a United Kingdom-Ireland evaluation of polymerase chain reaction methods for detection of systemic fungal infections. J. Mol. Diagn. 8:376–384. 10.2353/jmoldx.2006.050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.White PL, Mengoli C, Bretagne S, Cuenca-Estrella M, Finnstrom N, Klingspor L, Melchers WJ, McCulloch E, Barnes RA, Donnelly JP, Loeffler J, European Aspergillus PCR Initiative 2011. Evaluation of Aspergillus PCR protocols for testing serum specimens. J. Clin. Microbiol. 49:3842–3848. 10.1128/JCM.05316-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. 2009. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect. Dis. 9:89–96. 10.1016/S1473-3099(09)70019-2 [DOI] [PubMed] [Google Scholar]
- 173.Loeffler J, Hebart H, Brauchle U, Schumacher U, Einsele H. 2000. Comparison between plasma and whole blood specimens for detection of Aspergillus DNA by PCR. J. Clin. Microbiol. 38:3830–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Garcia ME, Blanco JL, Caballero J, Gargallo-Viola D. 2002. Anticoagulants interfere with PCR used to diagnose invasive aspergillosis. J. Clin. Microbiol. 40:1567–1568. 10.1128/JCM.40.4.1567-1568.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mennink-Kersten MA, Ruegebrink D, Wasei N, Melchers WJ, Verweij PE. 2006. In vitro release by Aspergillus fumigatus of galactofuranose antigens, 1,3-beta-d-glucan, and DNA, surrogate markers used for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 44:1711–1718. 10.1128/JCM.44.5.1711-1718.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Francesconi A, Kasai M, Petraitiene R, Petraitis V, Kelaher AM, Schaufele R, Hope WW, Shea YR, Bacher J, Walsh TJ. 2006. Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 44:2475–2480. 10.1128/JCM.02693-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lass-Flörl C, Speth C, Mayr A, Wurzner R, Dierich MP, Ulmer H, Dietrich H. 2003. Diagnosing and monitoring of invasive aspergillosis during antifungal therapy by polymerase chain reaction: an experimental study in mice. Diagn. Microbiol. Infect. Dis. 47:569–572. 10.1016/S0732-8893(03)00168-8 [DOI] [PubMed] [Google Scholar]
- 178.Springer J, Schlossnagel H, Heinz W, Doedt T, Soeller R, Einsele H, Loeffler J. 2012. A novel extraction method combining plasma with a whole-blood fraction shows excellent sensitivity and reproducibility for patients at high risk for invasive aspergillosis. J. Clin. Microbiol. 50:2585–2591. 10.1128/JCM.00523-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McCulloch E, Ramage G, Rajendran R, Lappin DF, Jones B, Warn P, Shrief R, Kirkpatrick WR, Patterson TF, Williams C. 2012. Antifungal treatment affects the laboratory diagnosis of invasive aspergillosis. J. Clin. Pathol. 65:83–86. 10.1136/jcp.2011.090464 [DOI] [PubMed] [Google Scholar]
- 180.Avni T, Levy I, Sprecher H, Yahav D, Leibovici L, Paul M. 2012. Diagnostic accuracy of PCR alone compared to galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis: a systematic review. J. Clin. Microbiol. 50:3652–3658. 10.1128/JCM.00942-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Moore J, Schwarer AP, Guy S, Bajel A, Tramontana AR, Spelman T, Slavin MA, Australasian Leukaemia Lymphoma Group, New Zealand Mycology Interest Group 2013. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect. Dis. 13:519–528. 10.1016/S1473-3099(13)70076-8 [DOI] [PubMed] [Google Scholar]
- 182.White PL, Bretagne S, Klingspor L, Melchers WJ, McCulloch E, Schulz B, Finnstrom N, Mengoli C, Barnes RA, Donnelly JP, Loeffler J, European Aspergillus PCR Initiative 2010. Aspergillus PCR: one step closer to standardization. J. Clin. Microbiol. 48:1231–1240. 10.1128/JCM.01767-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.White PL, Perry MD, Loeffler J, Melchers W, Klingspor L, Bretagne S, McCulloch E, Cuenca-Estrella M, Finnstrom N, Donnelly JP, Barnes RA, European Aspergillus PCR Initiative 2010. Critical stages of extracting DNA from Aspergillus fumigatus in whole-blood specimens. J. Clin. Microbiol. 48:3753–3755. 10.1128/JCM.01466-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lyon GM, Abdul-Ali D, Loeffler J, White PL, Wickes B, Herrera ML, Alexander BD, Baden LR, Clancy C, Denning D, Nguyen MH, Sugrue M, Wheat LJ, Wingard JR, Donnelly JP, Barnes R, Patterson TF, Caliendo AM, aAsTeC, IAAM, EAPCRI Investigators 2013. Development and evaluation of a calibrator material for nucleic acid-based assays for diagnosing aspergillosis. J. Clin. Microbiol. 51:2403–2405. 10.1128/JCM.00744-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ahmad S, Khan Z, Mustafa AS, Khan ZU. 2002. Seminested PCR for diagnosis of candidemia: comparison with culture, antigen detection, and biochemical methods for species identification. J. Clin. Microbiol. 40:2483–2489. 10.1128/JCM.40.7.2483-2489.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.White PL, Shetty A, Barnes RA. 2003. Detection of seven Candida species using the Light-Cycler system. J. Med. Microbiol. 52:229–238. 10.1099/jmm.0.05049-0 [DOI] [PubMed] [Google Scholar]
- 187.Tirodker UH, Nataro JP, Smith S, LasCasas L, Fairchild KD. 2003. Detection of fungemia by polymerase chain reaction in critically ill neonates and children. J. Perinatol. 23:117–122. 10.1038/sj.jp.7210868 [DOI] [PubMed] [Google Scholar]
- 188.Maaroufi Y, Ahariz N, Husson M, Crokaert F. 2004. Comparison of different methods of isolation of DNA of commonly encountered Candida species and its quantitation by using a real-time PCR-based assay. J. Clin. Microbiol. 42:3159–3163. 10.1128/JCM.42.7.3159-3163.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ahmad S, Mustafa AS, Khan Z, Al-Rifaiy AI, Khan ZU. 2004. PCR-enzyme immunoassay of rDNA in the diagnosis of candidemia and comparison with amplicon detection by agarose gel electrophoresis. Int. J. Med. Microbiol. 294:45–51. 10.1016/j.ijmm.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 190.White PL, Archer AE, Barnes RA. 2005. Comparison of non-culture-based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. J. Clin. Microbiol. 43:2181–2187. 10.1128/JCM.43.5.2181-2187.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moreira-Oliveira MS, Mikami Y, Miyaji M, Imai T, Schreiber AZ, Moretti ML. 2005. Diagnosis of candidemia by polymerase chain reaction and blood culture: prospective study in a high-risk population and identification of variables associated with development of candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:721–726. 10.1007/s10096-005-0041-7 [DOI] [PubMed] [Google Scholar]
- 192.Alam FF, Mustafa AS, Khan ZU. 2007. Comparative evaluation of (1,3)-beta-d-glucan, mannan and anti-mannan antibodies, and Candida species-specific snPCR in patients with candidemia. BMC Infect. Dis. 7:103. 10.1186/1471-2334-7-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McMullan R, Metwally L, Coyle PV, Hedderwick S, McCloskey B, O'Neill HJ, Patterson CC, Thompson G, Webb CH, Hay RJ. 2008. A prospective clinical trial of a real-time polymerase chain reaction assay for the diagnosis of candidemia in nonneutropenic, critically ill adults. Clin. Infect. Dis. 46:890–896. 10.1086/528690 [DOI] [PubMed] [Google Scholar]
- 194.Dunyach C, Bertout S, Phelipeau C, Drakulovski P, Reynes J, Mallie M. 2008. Detection and identification of Candida spp. in human serum by LightCycler real-time polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 60:263–271. 10.1016/j.diagmicrobio.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 195.Metwally L, Fairley DJ, Coyle PV, Hay RJ, Hedderwick S, McCloskey B, O'Neill HJ, Webb CH, McMullan R. 2008. Comparison of serum and whole-blood specimens for the detection of Candida DNA in critically ill, non-neutropenic patients. J. Med. Microbiol. 57:1269–1272. 10.1099/jmm.0.2008/002444-0 [DOI] [PubMed] [Google Scholar]
- 196.Badiee P, Kordbacheh P, Alborzi A, Zakernia M, Haddadi P. 2009. Early detection of systemic candidiasis in the whole blood of patients with hematologic malignancies. Jpn. J. Infect. Dis. 62:1–5 [PubMed] [Google Scholar]
- 197.Khlif M, Mary C, Sellami H, Sellami A, Dumon H, Ayadi A, Ranque S. 2009. Evaluation of nested and real-time PCR assays in the diagnosis of candidaemia. Clin. Microbiol. Infect. 15:656–661. 10.1111/j.1469-0691.2009.02762.x [DOI] [PubMed] [Google Scholar]
- 198.Wellinghausen N, Siegel D, Winter J, Gebert S. 2009. Rapid diagnosis of candidaemia by real-time PCR detection of Candida DNA in blood samples. J. Med. Microbiol. 58:1106–1111. 10.1099/jmm.0.007906-0 [DOI] [PubMed] [Google Scholar]
- 199.Badiee P, Alborzi A, Vojdani R, Shakiba E, Rasouli M, Ravanfar P, Haddadi P. 2010. Early diagnosis of systemic candidiasis in bone marrow transplant recipients. Exp. Clin. Transplant. 8:98–103 [PubMed] [Google Scholar]
- 200.Lau A, Halliday C, Chen SC, Playford EG, Stanley K, Sorrell TC. 2010. Comparison of whole blood, serum, and plasma for early detection of candidemia by multiplex-tandem PCR. J. Clin. Microbiol. 48:811–816. 10.1128/JCM.01650-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schell WA, Benton JL, Smith PB, Poore M, Rouse JL, Boles DJ, Johnson MD, Alexander BD, Pamula VK, Eckhardt AE, Pollack MG, Benjamin DK, Jr, Perfect JR, Mitchell TG. 2012. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur. J. Clin. Microbiol. Infect. Dis. 31:2237–2245. 10.1007/s10096-012-1561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Trovato L, Betta P, Romeo MG, Oliveri S. 2012. Detection of fungal DNA in lysis-centrifugation blood culture for the diagnosis of invasive candidiasis in neonatal patients. Clin. Microbiol. Infect. 18:E63–E65. 10.1111/j.1469-0691.2011.03731.x [DOI] [PubMed] [Google Scholar]
- 203.Avni T, Leibovici L, Paul M. 2011. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J. Clin. Microbiol. 49:665–670. 10.1128/JCM.01602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kasai M, Francesconi A, Petraitiene R, Petraitis V, Kelaher AM, Kim HS, Meletiadis J, Sein T, Bacher J, Walsh TJ. 2006. Use of quantitative real-time PCR to study the kinetics of extracellular DNA released from Candida albicans, with implications for diagnosis of invasive candidiasis. J. Clin. Microbiol. 44:143–150. 10.1128/JCM.44.1.143-150.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Selvarangan R, Bui U, Limaye AP, Cookson BT. 2003. Rapid identification of commonly encountered Candida species directly from blood culture bottles. J. Clin. Microbiol. 41:5660–5664. 10.1128/JCM.41.12.5660-5664.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shin JH, Nolte FS, Morrison CJ. 1997. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J. Clin. Microbiol. 35:1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Metwally L, Hogg G, Coyle PV, Hay RJ, Hedderwick S, McCloskey B, O'Neill HJ, Ong GM, Thompson G, Webb CH, McMullan R. 2007. Rapid differentiation between fluconazole-sensitive and -resistant species of Candida directly from positive blood-culture bottles by real-time PCR. J. Med. Microbiol. 56:964–970. 10.1099/jmm.0.47149-0 [DOI] [PubMed] [Google Scholar]
- 208.Pryce TM, Palladino S, Price DM, Gardam DJ, Campbell PB, Christiansen KJ, Murray RJ. 2006. Rapid identification of fungal pathogens in BacT/ALERT, BACTEC, and BBL MGIT media using polymerase chain reaction and DNA sequencing of the internal transcribed spacer regions. Diagn. Microbiol. Infect. Dis. 54:289–297. 10.1016/j.diagmicrobio.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 209.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 29 May 2013. Echinocandin and triazole antifungal susceptibility profiles of opportunistic yeast and mould clinical isolates (2010–2011): application of new CLSI clinical breakpoints and epidemiological cutoff values to characterize geographic and temporal trends of antifungal resistance. J. Clin. Microbiol. 10.1128/JCM.00308-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Clancy CJ, Nguyen MH. 2013. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 56:1284–1292. 10.1093/cid/cit006 [DOI] [PubMed] [Google Scholar]
- 211.Balashov SV, Park S, Perlin DS. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 50:2058–2063. 10.1128/AAC.01653-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Frade JP, Warnock DW, Arthington-Skaggs BA. 2004. Rapid quantification of drug resistance gene expression in Candida albicans by reverse transcriptase LightCycler PCR and fluorescent probe hybridization. J. Clin. Microbiol. 42:2085–2093. 10.1128/JCM.42.5.2085-2093.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gygax SE, Vermitsky JP, Chadwick SG, Self MJ, Zimmerman JA, Mordechai E, Adelson ME, Trama JP. 2008. Antifungal resistance of Candida glabrata vaginal isolates and development of a quantitative reverse transcription-PCR-based azole susceptibility assay. Antimicrob. Agents Chemother. 52:3424–3426. 10.1128/AAC.00462-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morris A, Norris KA. 2012. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 25:297–317. 10.1128/CMR.00013-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Maher NH, Vermund SH, Welsh DA, Dillon HK, Awooda A, Unnasch TR. 2001. Development and characterization of a molecular viability assay for Pneumocystis carinii f sp hominis. J. Infect. Dis. 183:1825–1827. 10.1086/320738 [DOI] [PubMed] [Google Scholar]
- 216.Flori P, Bellete B, Durand F, Raberin H, Cazorla C, Hafid J, Lucht F, Sung RT. 2004. Comparison between real-time PCR, conventional PCR and different staining techniques for diagnosing Pneumocystis jirovecii pneumonia from bronchoalveolar lavage specimens. J. Med. Microbiol. 53:603–607. 10.1099/jmm.0.45528-0 [DOI] [PubMed] [Google Scholar]
- 217.Alvarez-Martinez MJ, Miro JM, Valls ME, Moreno A, Rivas PV, Sole M, Benito N, Domingo P, Munoz C, Rivera E, Zar HJ, Wissmann G, Diehl AR, Prolla JC, de Anta MT, Gatell JM, Wilson PE, Meshnick SR, Spanish PCP Working Group 2006. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jirovecii in clinical specimens. Diagn. Microbiol. Infect. Dis. 56:153–160. 10.1016/j.diagmicrobio.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 218.de Oliveira A, Unnasch TR, Crothers K, Eiser S, Zucchi P, Moir J, Beard CB, Lawrence GG, Huang L. 2007. Performance of a molecular viability assay for the diagnosis of Pneumocystis pneumonia in HIV-infected patients. Diagn. Microbiol. Infect. Dis. 57:169–176. 10.1016/j.diagmicrobio.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 219.Etoh K. 2008. Evaluation of a real-time PCR assay for the diagnosis of Pneumocystis pneumonia. Kurume Med. J. 55:55–62. 10.2739/kurumemedj.55.55 [DOI] [PubMed] [Google Scholar]
- 220.Huggett JF, Taylor MS, Kocjan G, Evans HE, Morris-Jones S, Gant V, Novak T, Costello AM, Zumla A, Miller RF. 2008. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveolar lavage fluid of HIV-infected patients. Thorax 63:154–159. 10.1136/thx.2007.081687 [DOI] [PubMed] [Google Scholar]
- 221.Gupta R, Mirdha BR, Guleria R, Kumar L, Samantaray JC, Agarwal SK, Kabra SK, Luthra K. 2009. Diagnostic significance of nested polymerase chain reaction for sensitive detection of Pneumocystis jirovecii in respiratory clinical specimens. Diagn. Microbiol. Infect. Dis. 64:381–388. 10.1016/j.diagmicrobio.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 222.Fujisawa T, Suda T, Matsuda H, Inui N, Nakamura Y, Sato J, Toyoshima M, Nakano Y, Yasuda K, Gemma H, Hayakawa H, Chida K. 2009. Real-time PCR is more specific than conventional PCR for induced sputum diagnosis of Pneumocystis pneumonia in immunocompromised patients without HIV infection. Respirology 14:203–209. 10.1111/j.1440-1843.2008.01457.x [DOI] [PubMed] [Google Scholar]
- 223.Tia T, Putaporntip C, Kosuwin R, Kongpolprom N, Kawkitinarong K, Jongwutiwes S. 2012. A highly sensitive novel PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveloar lavage specimens from immunocompromised patients. Clin. Microbiol. Infect. 18:598–603. 10.1111/j.1469-0691.2011.03656.x [DOI] [PubMed] [Google Scholar]
- 224.Muhlethaler K, Bogli-Stuber K, Wasmer S, von Garnier C, Dumont P, Rauch A, Muhlemann K, Garzoni C. 2012. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur. Respir. J. 39:971–978. 10.1183/09031936.00095811 [DOI] [PubMed] [Google Scholar]
- 225.Khodadadi H, Mirhendi H, Mohebali M, Kordbacheh P, Zarrinfar H, Makimura K. 2013. Pneumocystis jirovecii colonization in non-HIV-infected patients based on nested-PCR detection in bronchoalveolar lavage samples. Iran J. Public Health 42:298–305 [PMC free article] [PubMed] [Google Scholar]
- 226.Josephson KL, Gerba CP, Pepper IL. 1993. Polymerase chain reaction detection of nonviable bacterial pathogens. Appl. Environ. Microbiol. 59:3513–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Maher N, Vermund S, Lasbury M, Lee C, Bartlett M, Unnasch TR. 2000. Development and evaluation of a molecular viability assay for Pneumocystis carinii. J. Clin. Microbiol. 38:1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lu Y, Ling G, Qiang C, Ming Q, Wu C, Wang K, Ying Z. 2011. PCR diagnosis of Pneumocystis pneumonia: a bivariate meta-analysis. J. Clin. Microbiol. 49:4361–4363. 10.1128/JCM.06066-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hata DJ, Buckwalter SP, Pritt BS, Roberts GD, Wengenack NL. 2008. Real-time PCR method for detection of zygomycetes. J. Clin. Microbiol. 46:2353–2358. 10.1128/JCM.02331-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nagao K, Ota T, Tanikawa A, Takae Y, Mori T, Udagawa S, Nishikawa T. 2005. Genetic identification and detection of human pathogenic Rhizopus species, a major mucormycosis agent, by multiplex PCR based on internal transcribed spacer region of rRNA gene. J. Dermatol. Sci. 39:23–31. 10.1016/j.jdermsci.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 231.Kasai M, Harrington SM, Francesconi A, Petraitis V, Petraitiene R, Beveridge MG, Knudsen T, Milanovich J, Cotton MP, Hughes J, Schaufele RL, Sein T, Bacher J, Murray PR, Kontoyiannis DP, Walsh TJ. 2008. Detection of a molecular biomarker for zygomycetes by quantitative PCR assays of plasma, bronchoalveolar lavage, and lung tissue in a rabbit model of experimental pulmonary zygomycosis. J. Clin. Microbiol. 46:3690–3702. 10.1128/JCM.00917-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dannaoui E, Schwarz P, Slany M, Loeffler J, Jorde AT, Cuenca-Estrella M, Hauser PM, Shrief R, Huerre M, Freiberger T, Gaustad P, Rodriguez-Tudela JL, Bille J, Denning DW, Bretagne S, Lortholary O. 2010. Molecular detection and identification of Zygomycetes species from paraffin-embedded tissues in a murine model of disseminated zygomycosis: a collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) evaluation. J. Clin. Microbiol. 48:2043–2046. 10.1128/JCM.02319-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Castelli MV, Buitrago MJ, Bernal-Martinez L, Gomez-Lopez A, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Development and validation of a quantitative PCR assay for diagnosis of scedosporiosis. J. Clin. Microbiol. 46:3412–3416. 10.1128/JCM.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rappelli P, Are R, Casu G, Fiori PL, Cappuccinelli P, Aceti A. 1998. Development of a nested PCR for detection of Cryptococcus neoformans in cerebrospinal fluid. J. Clin. Microbiol. 36:3438–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Thompson GR, Sharma S, Bays DJ, Pruitt R, Engelthaler DM, Bowers J, Driebe EM, Davis M, Libke R, Cohen SH, Pappagianis D. 2013. Coccidioidomycosis: adenosine deaminase levels, serologic parameters, culture results, and polymerase chain reaction testing in pleural fluid. Chest 143:776–781. 10.1378/chest.12-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sugawara Y, Nakase K, Nakamura A, Ohishi K, Sugimoto Y, Fujieda A, Monma F, Suzuki K, Masuya M, Matsushima Y, Wada H, Nobori T, Katayama N. 2013. Clinical utility of a panfungal polymerase chain reaction assay for invasive fungal diseases in patients with haematologic disorders. Eur. J. Haematol. 90:331–339. 10.1111/ejh.12078 [DOI] [PubMed] [Google Scholar]
- 237.Paterson PJ, Seaton S, McHugh TD, McLaughlin J, Potter M, Prentice HG, Kibbler CC. 2006. Validation and clinical application of molecular methods for the identification of molds in tissue. Clin. Infect. Dis. 42:51–56. 10.1086/498111 [DOI] [PubMed] [Google Scholar]
- 238.Badiee P, Kordbacheh P, Alborzi A, Malekhoseini S, Zeini F, Mirhendi H, Mahmoodi M. 2007. Prospective screening in liver transplant recipients by panfungal PCR-ELISA for early diagnosis of invasive fungal infections. Liver Transpl. 13:1011–1016. 10.1002/lt.21175 [DOI] [PubMed] [Google Scholar]
- 239.Vollmer T, Stormer M, Kleesiek K, Dreier J. 2008. Evaluation of novel broad-range real-time PCR assay for rapid detection of human pathogenic fungi in various clinical specimens. J. Clin. Microbiol. 46:1919–1926. 10.1128/JCM.02178-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Imhof A, Schaer C, Schoedon G, Schaer DJ, Walter RB, Schaffner A, Schneemann M. 2003. Rapid detection of pathogenic fungi from clinical specimens using LightCycler real-time fluorescence PCR. Eur. J. Clin. Microbiol. Infect. Dis. 22:558–560. 10.1007/s10096-003-0989-0 [DOI] [PubMed] [Google Scholar]
- 241.Rickerts V, Just-Nubling G, Konrad F, Kern J, Lambrecht E, Bohme A, Jacobi V, Bialek R. 2006. Diagnosis of invasive aspergillosis and mucormycosis in immunocompromised patients by seminested PCR assay of tissue samples. Eur. J. Clin. Microbiol. Infect. Dis. 25:8–13. 10.1007/s10096-005-0078-7 [DOI] [PubMed] [Google Scholar]
- 242.Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, Jacobi V, Just-Nubling G, Bialek R. 2007. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin. Infect. Dis. 44:1078–1083. 10.1086/512812 [DOI] [PubMed] [Google Scholar]
- 243.Badiee P, Kordbacheh P, Alborzi A, Malekhoseini S, Ramzi M, Mirhendi H, Mahmoodi M, Shakiba E. 2009. Study on invasive fungal infections in immunocompromised patients to present a suitable early diagnostic procedure. Int. J. Infect. Dis. 13:97–102. 10.1016/j.ijid.2008.04.011 [DOI] [PubMed] [Google Scholar]
- 244.Ribeiro P, Costa F, Monteiro A, Caldas J, Silva M, Ferreira G, Veiga J, Sousa MO, Viegas MP, Santos E, Goncalves AJ, Sousa AB. 2006. Polymerase chain reaction screening for fungemia and/or invasive fungal infections in patients with hematologic malignancies. Support Care Cancer 14:469–474. 10.1007/s00520-005-0903-7 [DOI] [PubMed] [Google Scholar]
- 245.Pryce TM, Kay ID, Palladino S, Heath CH. 2003. Real-time automated polymerase chain reaction (PCR) to detect Candida albicans and Aspergillus fumigatus DNA in whole blood from high-risk patients. Diagn. Microbiol. Infect. Dis. 47:487–496. 10.1016/S0732-8893(03)00139-1 [DOI] [PubMed] [Google Scholar]
- 246.Pham AS, Tarrand JJ, May GS, Lee MS, Kontoyiannis DP, Han XY. 2003. Diagnosis of invasive mold infection by real-time quantitative PCR. Am. J. Clin. Pathol. 119:38–44. 10.1309/RQ05PP9NEG6DADXR [DOI] [PubMed] [Google Scholar]
- 247.Orsi CF, Gennari W, Venturelli C, La Regina A, Pecorari M, Righi E, Machetti M, Blasi E. 2012. Performance of 2 commercial real-time polymerase chain reaction assays for the detection of Aspergillus and Pneumocystis DNA in bronchoalveolar lavage fluid samples from critical care patients. Diagn. Microbiol. Infect. Dis. 73:138–143. 10.1016/j.diagmicrobio.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 248.Mauro MV, Cavalcanti P, Perugini D, Noto A, Sperli D, Giraldi C. 2012. Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients. Diagn. Microbiol. Infect. Dis. 73:308–311. 10.1016/j.diagmicrobio.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 249.Georgiadou SP, Kontoyiannis DP. 2012. Concurrent lung infections in patients with hematological malignancies and invasive pulmonary aspergillosis: how firm is the Aspergillus diagnosis? J. Infect. 65:262–268. 10.1016/j.jinf.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lamoth F, Jaton K, Prod'hom G, Senn L, Bille J, Calandra T, Marchetti O. 2010. Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J. Clin. Microbiol. 48:3510–3516. 10.1128/JCM.00147-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P, Menichella D. 2011. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J. Clin. Microbiol. 49:2252–2258. 10.1128/JCM.02460-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Blaschke AJ, Heyrend C, Byington CL, Fisher MA, Barker E, Garrone NF, Thatcher SA, Pavia AT, Barney T, Alger GD, Daly JA, Ririe KM, Ota I, Poritz MA. 2012. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn. Microbiol. Infect. Dis. 74:349–355. 10.1016/j.diagmicrobio.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J, Duwve J, Fontaine E, Chu A, Carothers B, Reilly A, Fiedler J, Wiese AD, Feaster C, Gibson L, Griese S, Purfield A, Cleveland AA, Benedict K, Harris JR, Brandt ME, Blau D, Jernigan J, Weber JT, Park BJ, Multistate Fungal Infection Outbreak Response Team 2013. Fungal infections associated with contaminated methylprednisolone injections. N. Engl. J. Med. 369:1598–1609. 10.1056/NEJMoa1213978 [DOI] [PubMed] [Google Scholar]
- 254.Lockhart SR, Pham CD, Gade L, Iqbal N, Scheel CM, Cleveland AA, Whitney AM, Noble-Wang J, Chiller TM, Park BJ, Litvintseva AP, Brandt ME. 2013. Preliminary laboratory report of fungal infections associated with contaminated methylprednisolone injections. J. Clin. Microbiol. 51:2654–2661. 10.1128/JCM.01000-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wilson DA, Joyce MJ, Hall LS, Reller LB, Roberts GD, Hall GS, Alexander BD, Procop GW. 2005. Multicenter evaluation of a Candida albicans peptide nucleic acid fluorescent in situ hybridization probe for characterization of yeast isolates from blood cultures. J. Clin. Microbiol. 43:2909–2912. 10.1128/JCM.43.6.2909-2912.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rickerts V, Khot PD, Myerson D, Ko DL, Lambrecht E, Fredricks DN. 2011. Comparison of quantitative real time PCR with sequencing and ribosomal RNA-FISH for the identification of fungi in formalin fixed, paraffin-embedded tissue specimens. BMC Infect. Dis. 11:202. 10.1186/1471-2334-11-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Montone KT, Litzky LA, Feldman MD, Peterman H, Mathis B, Baliff J, Kaiser LR, Kucharczuk J, Nachamkin I. 2010. In situ hybridization for Coccidioides immitis 5.8S ribosomal RNA sequences in formalin-fixed, paraffin-embedded pulmonary specimens using a locked nucleic acid probe: a rapid means for identification in tissue sections. Diagn. Mol. Pathol. 19:99–104. 10.1097/PDM.0b013e3181b3aa55 [DOI] [PubMed] [Google Scholar]
- 258.Montone KT, LiVolsi VA, Lanza DC, Kennedy DW, Palmer J, Chiu AG, Feldman MD, Loevner LA, Nachamkin I. 2011. In situ hybridization for specific fungal organisms in acute invasive fungal rhinosinusitis. Am. J. Clin. Pathol. 135:190–199. 10.1309/AJCPQLYZBDF30HTM [DOI] [PubMed] [Google Scholar]
- 259.Compton J. 1991. Nucleic acid sequence-based amplification. Nature 350:91–92. 10.1038/350091a0 [DOI] [PubMed] [Google Scholar]
- 260.Yoo JH, Choi JH, Choi SM, Lee DG, Shin WS, Min WS, Kim CC. 2005. Application of nucleic acid sequence-based amplification for diagnosis of and monitoring the clinical course of invasive aspergillosis in patients with hematologic diseases. Clin. Infect. Dis. 40:392–398. 10.1086/427284 [DOI] [PubMed] [Google Scholar]
- 261.Loeffler J, Hebart H, Cox P, Flues N, Schumacher U, Einsele H. 2001. Nucleic acid sequence-based amplification of Aspergillus RNA in blood samples. J. Clin. Microbiol. 39:1626–1629. 10.1128/JCM.39.4.1626-1629.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yoo JH, Choi SM, Lee DG, Park SH, Choi JH, Kwon EY, Shin WS. 2007. Comparison of the real-time nucleic acid sequence-based amplification (RTi-NASBA) with conventional NASBA, and galactomannan assay for the diagnosis of invasive aspergillosis. J. Korean Med. Sci. 22:672–676. 10.3346/jkms.2007.22.4.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kim SH, Park C, Kwon EY, Shin NY, Kwon JC, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH. 2012. Real-time nucleic acid sequence-based amplification to predict the clinical outcome of invasive aspergillosis. J. Korean Med. Sci. 27:10–15. 10.3346/jkms.2012.27.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hettick JM, Green BJ, Buskirk AD, Kashon ML, Slaven JE, Janotka E, Blachere FM, Schmechel D, Beezhold DH. 2008. Discrimination of Aspergillus isolates at the species and strain level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Anal. Biochem. 380:276–281. 10.1016/j.ab.2008.05.051 [DOI] [PubMed] [Google Scholar]
- 265.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 49:543–551. 10.1086/600885 [DOI] [PubMed] [Google Scholar]
- 266.Lacroix C, Gicquel A, Sendid B, Meyer J, Accoceberry I, Francois N, Morio F, Desoubeaux G, Chandenier J, Kauffmann-Lacroix C, Hennequin C, Guitard J, Nassif X, Bougnoux ME. 2014. Evaluation of two matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clin. Microbiol. Infect. 20:153–158. 10.1111/1469-0691.12210 [DOI] [PubMed] [Google Scholar]
- 267.De Carolis E, Posteraro B, Lass-Flörl C, Vella A, Florio AR, Torelli R, Girmenia C, Colozza C, Tortorano AM, Sanguinetti M, Fadda G. 2012. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 18:475–484. 10.1111/j.1469-0691.2011.03599.x [DOI] [PubMed] [Google Scholar]
- 268.Alanio A, Beretti JL, Dauphin B, Mellado E, Quesne G, Lacroix C, Amara A, Berche P, Nassif X, Bougnoux ME. 2011. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for fast and accurate identification of clinically relevant Aspergillus species. Clin. Microbiol. Infect. 17:750–755. 10.1111/j.1469-0691.2010.03323.x [DOI] [PubMed] [Google Scholar]
- 269.Ferroni A, Suarez S, Beretti JL, Dauphin B, Bille E, Meyer J, Bougnoux ME, Alanio A, Berche P, Nassif X. 2010. Real-time identification of bacteria and Candida species in positive blood culture broths by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 48:1542–1548. 10.1128/JCM.02485-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ferreira L, Sanchez-Juanes F, Porras-Guerra I, Garcia-Garcia MI, Garcia-Sanchez JE, Gonzalez-Buitrago JM, Munoz-Bellido JL. 2011. Microorganisms direct identification from blood culture by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 17:546–551. 10.1111/j.1469-0691.2010.03257.x [DOI] [PubMed] [Google Scholar]
- 271.Marklein G, Josten M, Klanke U, Muller E, Horre R, Maier T, Wenzel T, Kostrzewa M, Bierbaum G, Hoerauf A, Sahl HG. 2009. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J. Clin. Microbiol. 47:2912–2917. 10.1128/JCM.00389-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spanu T, Posteraro B, Fiori B, D'Inzeo T, Campoli S, Ruggeri A, Tumbarello M, Canu G, Trecarichi EM, Parisi G, Tronci M, Sanguinetti M, Fadda G. 2012. Direct matrix-assisted laser desorption ionization–time of flight mass spectrometry assay of blood culture broths for rapid identification of Candida species causing bloodstream infections: an observational study in two large microbiology laboratories. J. Clin. Microbiol. 50:176–179. 10.1128/JCM.05742-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yaman G, Akyar I, Can S. 2012. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn. Microbiol. Infect. Dis. 73:65–67. 10.1016/j.diagmicrobio.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 274.Iriart X, Lavergne RA, Fillaux J, Valentin A, Magnaval JF, Berry A, Cassaing S. 2012. Routine identification of medical fungi by the new Vitek MS matrix-assisted laser desorption ionization–time of flight system with a new time-effective strategy. J. Clin. Microbiol. 50:2107–2110. 10.1128/JCM.06713-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bille E, Dauphin B, Leto J, Bougnoux ME, Beretti JL, Lotz A, Suarez S, Meyer J, Join-Lambert O, Descamps P, Grall N, Mory F, Dubreuil L, Berche P, Nassif X, Ferroni A. 2012. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin. Microbiol. Infect. 18:1117–1125. 10.1111/j.1469-0691.2011.03688.x [DOI] [PubMed] [Google Scholar]
- 276.Lohmann C, Sabou M, Moussaoui W, Prevost G, Delarbre JM, Candolfi E, Gravet A, Letscher-Bru V. 2013. Comparison between the Biflex III-Biotyper and the Axima-SARAMIS systems for yeast identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 51:1231–1236. 10.1128/JCM.03268-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Rosenvinge FS, Dzajic E, Knudsen E, Malig S, Andersen LB, Lovig A, Arendrup MC, Jensen TG, Gahrn-Hansen B, Kemp M. 2013. Performance of matrix-assisted laser desorption–time of flight mass spectrometry for identification of clinical yeast isolates. Mycoses 56:229–235. 10.1111/myc.12000 [DOI] [PubMed] [Google Scholar]
- 278.Bader O, Weig M, Taverne-Ghadwal L, Lugert R, Gross U, Kuhns M. 2011. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 17:1359–1365. 10.1111/j.1469-0691.2010.03398.x [DOI] [PubMed] [Google Scholar]
- 279.De Carolis E, Vella A, Florio AR, Posteraro P, Perlin DS, Sanguinetti M, Posteraro B. 2012. Use of matrix-assisted laser desorption ionization–time of flight mass spectrometry for caspofungin susceptibility testing of Candida and Aspergillus species. J. Clin. Microbiol. 50:2479–2483. 10.1128/JCM.00224-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vella A, De Carolis E, Vaccaro L, Posteraro P, Perlin DS, Kostrzewa M, Posteraro B, Sanguinetti M. 2013. Rapid antifungal susceptibility testing by matrix-assisted laser desorption ionization–time of flight mass spectrometry analysis. J. Clin. Microbiol. 51:2964–2969. 10.1128/JCM.00903-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 57:1237–1245. 10.1093/cid/cit498 [DOI] [PubMed] [Google Scholar]
- 282.Massire C, Buelow DR, Zhang SX, Lovari R, Matthews HE, Toleno DM, Ranken RR, Hall TA, Metzgar D, Sampath R, Blyn LB, Ecker DJ, Gu Z, Walsh TJ, Hayden RT. 2013. PCR followed by electrospray ionization mass spectrometry for broad-range identification of fungal pathogens. J. Clin. Microbiol. 51:959–966. 10.1128/JCM.02621-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shin JH, Ranken R, Sefers SE, Lovari R, Quinn CD, Meng S, Carolan HE, Toleno D, Li H, Lee JN, Stratton CW, Massire C, Tang YW. 2013. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J. Clin. Microbiol. 51:136–141. 10.1128/JCM.01907-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Faulds K, Smith WE, Graham D. 2005. DNA detection by surface enhanced resonance Raman scattering (SERRS). Analyst 130:1125–1131. 10.1039/b500248f [DOI] [PubMed] [Google Scholar]
- 285.Yoo SM, Kang T, Kang H, Lee H, Kang M, Lee SY, Kim B. 2011. Combining a nanowire SERRS sensor and a target recycling reaction for ultrasensitive and multiplex identification of pathogenic fungi. Small 7:3371–3376. 10.1002/smll.201100633 [DOI] [PubMed] [Google Scholar]
- 286.Ilic B, Czaplewski D, Craighead HG, Neuzil P, Campagnolo C, Batt C. 2000. Mechanical resonant immunospecific biological detector. Appl. Phys. Lett. 77:450–452. 10.1063/1.127006 [DOI] [Google Scholar]
- 287.Gupta A, Akin D, Bashir R. 2004. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 84:1976–1978. 10.1063/1.1667011 [DOI] [Google Scholar]
- 288.Nugaeva N, Gfeller KY, Backmann N, Lang HP, Duggelin M, Hegner M. 2005. Micromechanical cantilever array sensors for selective fungal immobilization and fast growth detection. Biosens. Bioelectron. 21:849–856. 10.1016/j.bios.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 289.Bloch F. 1946. Nuclear induction. Phys. Rev. 70:460–474 [Google Scholar]
- 290.Ernst RR. 1966. Nuclear magnetic double resonance with an incoherent radio-frequency field. J. Chem. Phys. 45:3845. 10.1063/1.1727409 [DOI] [Google Scholar]
- 291.Lauterbur PC. 1973. Image formation by induced local interactions—examples employing nuclear magnetic-resonance. Nature 242:190–191. 10.1038/242190a0 [DOI] [PubMed] [Google Scholar]
- 292.Bourne R, Himmelreich U, Sharma A, Mountford C, Sorrell T. 2001. Identification of Enterococcus, Streptococcus, and Staphylococcus by multivariate analysis of proton magnetic resonance spectroscopic data from plate cultures. J. Clin. Microbiol. 39:2916–2923. 10.1128/JCM.39.8.2916-2923.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Himmelreich U, Somorjai RL, Dolenko B, Lee OC, Daniel HM, Murray R, Mountford CE, Sorrell TC. 2003. Rapid identification of Candida species by using nuclear magnetic resonance spectroscopy and a statistical classification strategy. Appl. Environ. Microbiol. 69:4566–4574. 10.1128/AEM.69.8.4566-4574.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Neely LA, Audeh M, Phung NA, Min M, Suchocki A, Plourde D, Blanco M, Demas V, Skewis LR, Anagnostou T, Coleman JJ, Wellman P, Mylonakis E, Lowery TJ. 2013. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci. Transl. Med. 5:182ra154. 10.1126/scitranslmed.3005377 [DOI] [PubMed] [Google Scholar]
- 295.Zaas AK, Aziz H, Lucas J, Perfect JR, Ginsburg GS. 2010. Blood gene expression signatures predict invasive candidiasis. Sci. Transl. Med. 2:21ra17. 10.1126/scitranslmed.3000715 [DOI] [PubMed] [Google Scholar]
- 296.Pukkila-Worley R, Ausubel FM, Mylonakis E. 2011. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 7:e1002074. 10.1371/journal.ppat.1002074 [DOI] [PMC free article] [PubMed] [Google Scholar]