Abstract
We observed that murine macrophages showed greater activation and increased interleukin 6 (IL-6), IL-12p40, and interferon gamma (IFN-γ) production during Neospora caninum infection. Many macrophages migrated to the site of infection. Furthermore, macrophage-depleted mice exhibited increased sensitivity to N. caninum infection. This study indicates that macrophages are required for achieving protective immunity against N. caninum.
TEXT
Neospora caninum was first identified as a Toxoplasma gondii-like parasite that caused encephalomyelitis and myositis in dogs in Norway in 1984 (1). It was classified as a distinct species in 1988 (2). N. caninum is a cause of neosporosis, which leads to abortion, neonatal mortality, and congenital infection in cattle and neuromuscular pathology in dogs (3). The ability to survive infection is dependent upon interferon gamma (IFN-γ), the major mediator of resistance against N. caninum (4, 5). While IFN-γ-producing CD4+ and CD8+ T cells are required for the acquisition of protective immunity against parasite infection (6), different types of innate cells may also exert distinct roles in achieving protective immunity. Generally, innate cells, such as natural killer (NK) cells and NKT cells, play essential roles as primary effector cells at the interface between the host and N. caninum (7, 8). Moreover, it is well known that the rapid recruitment of macrophages and dendritic cells (DCs) to sites of infection can enhance innate immune responses against N. caninum (9). However, these cells may also transport intracellular pathogens, such as N. caninum and T. gondii, away from the sites of primary infection and facilitate parasite propagation throughout the host (10, 11). However, the role of macrophages in generating protective immunity against N. caninum is not well characterized. Therefore, we investigated macrophage activation and the effects of macrophage depletion in N. caninum-infected mice.
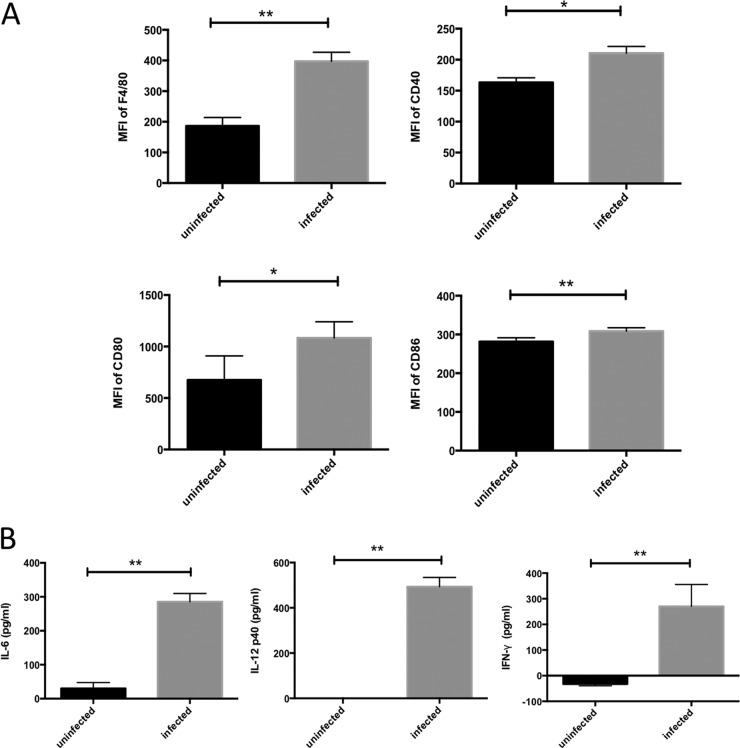
To evaluate the function of peritoneal macrophages, we infected these cells ex vivo with N. caninum and analyzed them by flow cytometry. C57BL/6J mice (Clea Japan, Tokyo, Japan) were injected by the intraperitoneal (i.p.) route with 1 ml 4.05% thioglycolate. Four days after injection, peritoneal exudate cells were harvested by lavage with 5 ml ice-cold phosphate-buffered saline (PBS). Cells were centrifuged at 800 ×g for 10 min and suspended in Dulbecco's modified Eagle medium (DMEM) (Sigma, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Macrophages were plated in 12-well plates at 1 × 106 cells/well. After incubation for 24 h, the cells were infected with 2 × 105 N. caninum (Nc-1 isolate) tachyzoites and incubated for an additional 24 h. The cells were treated with FcBlock (BD Pharmingen, San Diego, CA, USA) to avoid nonspecific adherence of monoclonal antibody (MAb) to Fc receptors and were then incubated with various MAbs (BD Pharmingen) for 30 min at 4°C. The stained cells were washed with cold PBS, fixed with 0.5% paraformaldehyde in PBS, and analyzed using an EPICS XL flow cytometer (Beckman Coulter, Brea, CA, USA). The purity of the peritoneal macrophage cultures quantified by flow cytometry using anti-CD11b was 97.4 ± 0.31% (n = 4). The mean fluorescence intensities (MFIs) of F4/80, CD40, CD80, and CD86 expression were determined. N. caninum infection significantly increased the expression levels of F4/80, CD40, CD80, and CD86 in macrophages (Fig. 1A). Next, cytokine levels in the macrophage culture supernatants were measured using the OptEIA mouse interleukin 6 (IL-6), IL-12(p40), and IFN-γ enzyme-linked immunosorbent assay (ELISA) (BD Bioscience, San Jose, CA, USA) kits according to the manufacturer's instructions. Production of IL-6, IL-12(p40), and IFN-γ in macrophages was triggered by N. caninum infection (Fig. 1B). These results indicated that macrophages were activated upon infection with N. caninum.
FIG 1.
Activation of macrophages exposed to N. caninum tachyzoites. (A) Macrophage activation was assessed by examining surface expression of F4/80, CD40, CD80, and CD86. The mean fluorescence intensity (MFI) of stained cells for each marker is shown. (B) IL-6, IL-12p40, and IFN-γ levels were measured by ELISA. Each bar represents the mean ± SD (n = 4), and significant differences were determined with Student's t test (*, P < 0.05; **, P < 0.01). Data are representative of results from three (A) or two (B) independent experiments.
To test the role of macrophages in protective immunity, mice were infected i.p. with 1 × 106 N. caninum tachyzoites. At 0, 5, and 10 days postinfection (dpi), mice were euthanized under anesthesia and peritoneal exudate cells were harvested by lavage with 5 ml ice-cold PBS. Peritoneal cells were stained with anti-CD11b MAb and examined by flow cytometry. The absolute number of CD11b+ cells was calculated as follows: absolute cell number = total host cell number × (% CD11b+ cells/100) × (% gated cells by flow cytometry/100). The number of peritoneal CD11b+ cells (monocytes and macrophages) significantly increased at 5 dpi (Fig. 2A), suggesting that migration of macrophages to the site of infection plays a crucial role in achieving protective immunity against N. caninum. To investigate whether macrophages play an important role during infection in vivo, macrophages were depleted by two i.p. injections of 300 μl clodronate or PBS liposomes (clodronate encapsulated liposome [Clodrosome]; Haarlem, the Netherlands), administered 3 days and immediately prior to infection of mice with 1 × 106 N. caninum tachyzoites. Liposomes were handled according to the manufacturer's instructions. The macrophage depletion was determined by flow cytometry by staining cells from peritoneal exudates of mice at 3 dpi with CD11b and F4/80 MAbs (Fig. 2B). All mice were monitored for survival and clinical signs of neosporosis, such as head tilting, limb paralysis, circling behavior, and febrile responses (e.g., a starchy stiff coat). In response to N. caninum infection, macrophage-depleted mice showed higher sensitivity, which was associated with early death (Fig. 2C). Neurological signs, including circling motion, head tilting, and leg paralysis, were observed in nine and three mice of PBS liposome-treated and clodronate liposome-treated animals, respectively. The mice did not show particular clinical signs during acute death. In addition, we detected the parasite DNA in the blood of mice treated with clodronate liposome at 1 dpi (clodronate treated, 4 positive in 13 mice; PBS treated, 0 positive in 12 mice).
FIG 2.
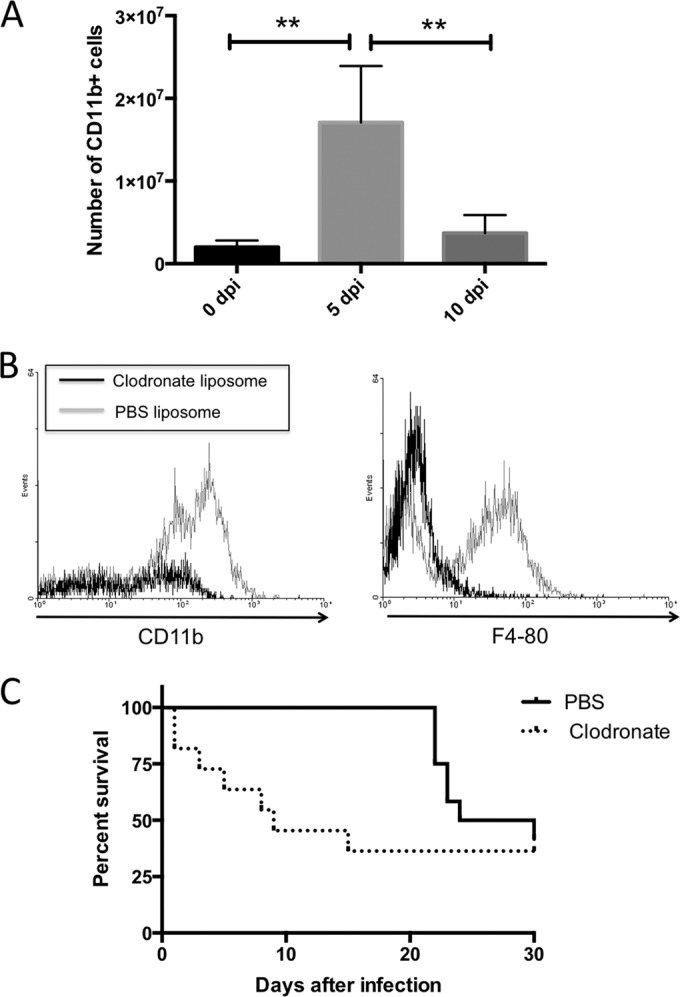
The recruitment of CD11b+ cells and the effects of macrophage depletion on survival in N. caninum-infected mice. (A) Mice were infected intraperitoneally with N. caninum tachyzoites. Peritoneal cells were harvested from uninfected or parasite-infected mice at 5 and 10 days postinfection (dpi). Cells were analyzed by flow cytometry to determine the absolute number of cells expressing CD11b. Bars indicate the average cell number for each experimental group (0 dpi, n = 3; 5 and 10 dpi, n = 5) and significant differences were determined using one-way analysis of variance (ANOVA) and Tukey's post hoc test (**, P < 0.01). Data are representative of results from two independent experiments. (B) Flow cytometry by staining cells from peritoneal exudates of mice at 3 dpi with CD11b and F4/80 MAbs. (C) Mice treated with clodronate (n = 13) or PBS liposomes (n = 12) were infected with N. caninum tachyzoites. Survival of mice was monitored for 30 dpi. Data from two independent experiments were combined.
This study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of the Obihiro University of Agriculture and Veterinary Medicine. The Committee on the Ethics of Animal Experiments of the Obihiro University of Agriculture and Veterinary Medicine approved the study protocol. All surgeries were performed while the mice were under isoflurane anesthesia, and all efforts were made to minimize suffering.
In this study, we determined that macrophages play a crucial role in generating protective immunity against N. caninum infection. N. caninum infection triggered IL-12 production and major histocompatibility complex (MHC) class II expression, resulting in the development of parasite-specific CD4+ cytotoxic T lymphocytes (5, 12). Cytotoxic T cells eliminate N. caninum-infected cells (12). On the other hand, IFN-γ produced from T cells and NK cells stimulates nitric oxide production in the macrophages associated with inhibition of parasite growth (5). Therefore, upon infection, macrophage activation and cytokine production might control parasites at the site of infection and allow the survival of the mice.
Innate immunity is also important to understanding of the host-parasite interactions. T. gondii possesses several unique molecules for stimulating immune responses and cell migration in the host (13). While profilins are actin-binding proteins that in T. gondii stimulate innate immunity in mice by binding Toll-like receptors on DCs, leading to release of inflammatory cytokines, N. caninum profilin also elicited strong IFN-γ and IL-12 responses (14). In addition, innate immune recognition of the excreted/secreted antigens of N. caninum triggered monocytic cell migration to the site of infection in a C-C chemokine receptor 5 (CCR5)-dependent manner (10). Moreover, N. caninum cyclophilin induces CCR5-dependent migration of murine and bovine cells (15). Although the ability of T. gondii to attract, invade, and survive inside immune cells (T cells, DCs, and macrophages), along with the migratory properties of DCs and macrophages that allow parasite dissemination around the host, have been reported (16), the role of immune cell migration is still unclear in N. caninum infection. Thus, study of parasite-derived ligands and host receptors may provide important information to understand host survival and parasitism. Further work will be required to dissect the mechanism of macrophage migration in response to host-factors and/or parasite molecules.
ACKNOWLEDGMENTS
We thank J. P. Dubey (U.S. Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, and Parasite Biology and Epidemiology Laboratory) for the N. caninum Nc-1 isolate.
The Japanese Society for the Promotion of Science supported this research through the “Funding Program for Next Generation World-Leading Researchers (NEXT Program)” that was initiated by the Council for Science and Technology Policy (2011/LS003).
Footnotes
Published ahead of print 28 May 2014
REFERENCES
- 1.Bjerkås I, Mohn SF, Presthus J. 1984. Unidentified cyst-forming sporozoon encephalomyelitis and myositis in dogs. Z Parasitenkd. 70:271–274. 10.1007/BF00942230 [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. 1988. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192:1269–1285 [PubMed] [Google Scholar]
- 3.Dubey JP, Schares G, Ortega-Mora LM. 2007. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20:323–367. 10.1128/CMR.00031-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baszler TV, Long MT, McElwain TF, Mathison BA. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 29:1635–1646. 10.1016/S0020-7519(99)00141-1 [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa Y, Tragoolpua K, Inoue N, Makala L, Nagasawa H, Otsuka H, Mikami T. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8:811–816. 10.1128/CDLI.8.4.811-817.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkers EY. 2003. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol. Med. Microbiol. 39:193–203. 10.1016/S0928-8244(03)00279-7 [DOI] [PubMed] [Google Scholar]
- 7.Boysen P, Klevar S, Olsen I, Storset AK. 2006. The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts. Infect. Immun. 74:953–960. 10.1128/IAI.74.2.953-960.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishikawa Y, Zhang H, Ibrahim HM, Yamada K, Nagasawa H, Xuan X. 2010. Roles of CD122+ cells in resistance against Neospora caninum infection in a murine model. J. Vet. Med. Sci. 72:1275–1282. 10.1292/jvms.10-0068 [DOI] [PubMed] [Google Scholar]
- 9.Dion S, Germon S, Guiton R, Ducournau C, Dimier-Poisson I. 2011. Functional activation of T cells by dendritic cells and macrophages exposed to the intracellular parasite Neospora caninum. Int. J. Parasitol. 41:685–695. 10.1016/j.ijpara.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 10.Mineo TW, Oliveira CJ, Silva DA, Oliveira LL, Abatepaulo AR, Ribeiro DP, Ferreira BR, Mineo JR, Silva JS. 2010. Neospora caninum excreted/secreted antigens trigger CC-chemokine receptor 5-dependent cell migration. Int. J. Parasitol. 40:797–805. 10.1016/j.ijpara.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 11.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gâtel D, Tardieux I. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316. 10.1182/blood-2005-02-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staska LM, McGuire TC, Davies CJ, Lewin HA, Baszler TV. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71:3272–3279. 10.1128/IAI.71.6.3272-3279.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter CA, Sibley LD. 2012. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10:766–778. 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins MC, Tuo W, Feng X, Cao L, Murphy C, Fetterer R. 2010. Neospora caninum: cloning and expression of a gene coding for cytokine-inducing profilin. Exp. Parasitol. 125:357–362. 10.1016/j.exppara.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Kameyama K, Nishimura M, Punsantsogvoo M, Ibrahim HM, Xuan X, Furuoka H, Nishikawa Y. 2012. Immunological characterization of Neospora caninum cyclophilin. Parasitology 139:294–301. 10.1017/S0031182011002022 [DOI] [PubMed] [Google Scholar]
- 16.Barragan A, Sibley LD. 2003. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 11:426–430. 10.1016/S0966-842X(03)00205-1 [DOI] [PubMed] [Google Scholar]