Abstract
Hexon modification of adenovirus type 5 (Ad5) vectors with the hypervariable regions (HVRs) of Ad48 has been shown to allow Ad5HVR48 vectors to circumvent the majority of the preexisting Ad5-neutralizing antibodies. However, it remains unclear whether modifying hexon HVRs impacts innate or adaptive immune responses elicited by this vector. In this study, we investigated the influence of the HVR substitution of Ad5 on innate and adaptive immune responses following vaccination. Ad5HVR48 displayed an intermediate level of innate immune cytokines and chemokines relative to those of Ad5 and Ad48, consistent with its chimeric nature. Hepatotoxicity was observed after Ad5 immunization but not after Ad5HVR48 or Ad48 immunization. However, the CD8+ T-cell responses elicited by Ad5HVR48 vectors displayed a partially exhausted phenotype, as evidenced by the sustained expression of programmed death 1 (PD-1), decreased effector-to-central memory conversion, and reduced memory recall responses, similar to those elicited by Ad5 vectors and in contrast to those induced by Ad48 vectors. Taken together, these results indicate that although Ad5HVR48 largely bypasses preexisting Ad5 neutralizing antibodies and shows reduced hepatotoxicity compared to that of Ad5, it induces adaptive immune phenotypes that are functionally exhausted similar to those elicited by Ad5.
INTRODUCTION
Adenovirus (Ad) vectors are widely utilized as platforms for vaccines and gene therapy (1–3). The utility of certain common Ad serotypes, however, is hampered by prevalent preexisting vector-specific neutralizing antibodies in the global population (4, 5). In particular, adenovirus type 5 (Ad5)-based vectors have been shown to exhibit decreased immunogenicity in the presence of baseline Ad5 antibodies (6). Ad5 vectors at high doses also exhibit hepatotoxicity as a result of liver tropism due to the binding of blood coagulation factor X (FX) in the systemic circulation (7–10). Both of these properties appear to be mediated primarily by the hexon hypervariable regions (HVRs) (8, 11–16). HVRs are highly variable among Ad serotypes and represent the primary determinant of neutralization specificity (17–20).
The role of the HVRs in influencing the immunogenicity and biological properties of adenoviral vectors has led to the development of strategies to either circumvent preexisting immunity or decrease liver tropism and associated hepatotoxicity. These strategies include HVR chemical modifications, such as PEGylation, HVR sequence modification by poly-His sequence insertion, or replacement of HVRs with those from less prevalent Ad serotypes (12, 21–26). Our laboratory has described an Ad5 vector with its surface HVRs replaced with those of Ad48, a chimeric vector referred to as Ad5HVR48 (19–21). This vector evades the majority of preexisting Ad5 neutralizing antibodies and has been evaluated as a human immunodeficiency virus (HIV) vaccine vector in mice, nonhuman primates, and humans in a recent phase I clinical trial (Integrated Preclinical/Clinical AIDS Vaccine Development program [IPCAVD] 002; D. H. Barouch, unpublished data) (19, 21). Furthermore, studies from our laboratory and others have described marked differences in vaccine-elicited innate and adaptive immune responses following Ad5 or Ad48 immunization (4, 27, 28). While Ad5HVR48 has shown a lack of vector-induced toxicity in both nonhuman primates (NHP) and humans, a previous report suggested that Ad5HVR48 may exhibit unexpected hepatotoxic and inflammatory responses in mice that are greater than those observed with Ad5 or Ad48 vectors (29). Understanding the impact of HVR modifications on the toxicity and innate and adaptive immune phenotypes elicited by Ad vectors is important for future clinical development of HVR-chimeric Ad vectors.
In this study, we investigated the innate and adaptive immune responses elicited by Ad5HVR48 vectors compared with those of the parental vectors Ad5 and Ad48 in mice. We found that the serum cytokine and chemokine responses elicited by Ad5HVR48 were higher than those elicited by Ad5 but lower than those elicited by Ad48, consistent with its chimeric nature. Neither Ad5HVR48 nor Ad48 vectors induced hepatotoxicity, as measured by liver enzymes and histopathology. The CD8+ T lymphocytes elicited by Ad5HVR48 exhibited a partially exhausted phenotype similar to those induced by Ad5 vectors and characterized by a high surface expression of programmed death 1 (PD-1) and low production of gamma interferon (IFN-γ) and interleukin 2 (IL-2). Taken together, these data show that hexon modification of Ad5, while enabling the chimeric Ad5HVR48 vector to circumvent the majority of preexisting neutralizing antibodies, resulted in innate immune profiles intermediate between Ad5 and Ad48 vectors but did not detectably improve the cellular adaptive immune phenotype compared with those from Ad5 vectors.
MATERIALS AND METHODS
Animals and viruses.
Replication-incompetent E1/E3-deleted Ad5, Ad5HVR48, and Ad48 were generated as previously described (4, 21). Viruses were grown in E1-complementing PER.55K cells and purified on a CsCl gradient, and they exhibited similar viral particle-to-PFU (vp/PFU) ratios. C57BL/6 and MF1 mice were purchased from Charles River Laboratories (Wilmington, MA). For measuring the adaptive immune responses vectors expressing a simian immunodeficiency virus (SIVmac239), the Gag transgene were used. The mice were injected intravenously with 3 × 1010 or 1 × 1011 viral particles of the vectors indicated. All studies involving animals were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC).
Serum cytokine and transaminase level determination.
Serum samples were collected 6 h and 48 h after vaccination. For the cytokine and chemokine assays, the sera were thawed on ice prior to inactivation with 0.05% Tween for 15 min at room temperature and then utilized on a Milliplex MAP mouse magnetic cytokine and chemokine panel, according to the manufacturer's instructions (Millipore, Billerica, MA) (27). The Luminex data were analyzed on the Bio-Plex 200 instrument running Bio-Plex Manager 4.1 (Bio-Rad, Hercules, CA) with an 80% to 120% standard acceptance range. For serum aminotransferase level determination, the sera were thawed on ice and then measured for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity by standard methods at the Beth Israel Deaconess Medical Center Small Animal Imaging Facility (SAIF).
Microscopy.
The livers were harvested from mice 48 h following vaccination and processed for paraffin embedding and hematoxylin and eosin staining according to standard methods developed by the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core at Harvard Medical School. The slides were imaged on a Zeiss AxioImager M1 running AxioVision version 4.6.3.0 (Carl Zeiss GmbH, Jena, Germany) at the Beth Israel Deaconess Medical Center Imaging Core.
Antibodies and flow cytometry.
Single-cell suspensions were stained at 4°C for 30 min. The following antibody clones were used: CD8α (53-6.7), CD127 (A7R34), CD62L (MEL-14), PD-1 (RMP1-30), gamma interferon (IFN-γ) (XMG1.2), and interleukin 2 (IL-2) (JES6-5H4). All antibodies were purchased from BD Pharmingen, except for PD-1 (BioLegend). Biotinylated major histocompatibility complex I (MHC-I) H-2Db monomers loaded with the previously described immunodominant AL11 peptide (AAVKNWMTQTL) (30) were obtained from the NIH Tetramer Core Facility from Emory University. Intracellular cytokine staining was used for measuring the functionality of Gag-specific CD8+ T-cell responses using the AL11 peptide. Intracellular cytokine staining was performed with the Cytofix/Cytoperm kit (BD Biosciences) (31). The fixed cells were acquired using an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo (TreeStar).
Data analysis and statistical methods.
The concentrations of cytokines and chemokines were obtained from Luminex assays using a 5-parameter logistic model. For all statistics shown, P values were calculated using unpaired two-tailed Student's t tests with no multiple-comparison corrections. All statistics were calculated in GraphPad Prism v5.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Ad5HVR48 displays an intermediate innate stimulatory phenotype compared to those of parental Ad5 and Ad48 vectors.
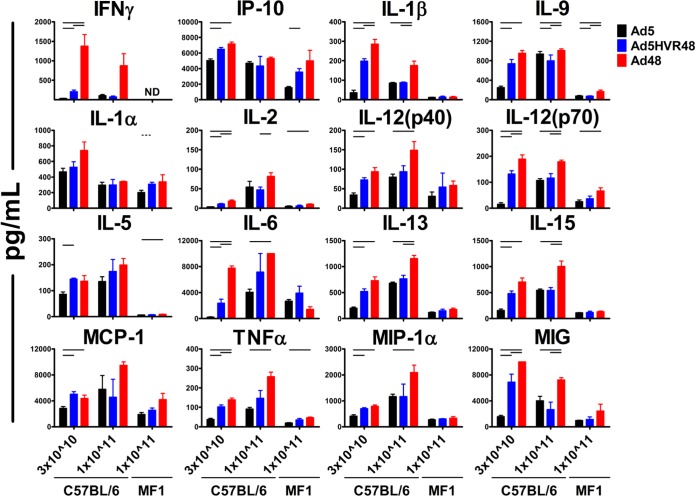
We initiated studies by assessing the systemic cytokine and chemokine responses in two strains of mice after vaccination, inbred C57BL/6 mice that had been extensively utilized for characterizing Ad vector immunology, and outbred MF1 mice that had been used in previous studies assessing Ad vector toxicity (4, 8, 12, 14, 19, 21). The mice (C57BL/6 or MF1; n = 3 to 4/group) were inoculated intravenously (i.v.) with 3 × 1010 or 1 × 1011 viral particles (vp) of Ad5, Ad5HVR48, or Ad48 vectors, consistent with procedures in prior studies (29). Serum samples were collected 6 h after vaccination, and systemic levels of cytokines and chemokines were assessed by Luminex analyses. A comparison of the systemic cytokine and chemokine levels revealed a greater induction of many cytokines and chemokines in mice vaccinated with Ad48 than in those vaccinated with Ad5, including gamma interferon-induced protein 10 (IP-10), interleukin 1 beta (IL-1β), IL-6, IL-12(p70), tumor necrosis factor alpha (TNF-α), and monokine induced by gamma interferon (MIG) (Fig. 1). These differences were observed in both C57BL/6 strain and MF1 mice, although an overall lower cytokine induction was observed in MF1 mice. Ad5HVR48 displayed an intermediate cytokine and chemokine elicitation profile (e.g., IP-10, IL-1β, IL-13, IL-12[p70], and TNF-α) compared with those of Ad5 and Ad48 vectors in both strains of mice (Fig. 1), although it more closely resembled the profile elicited by Ad5 vectors.
FIG 1.
Serum cytokines and chemokines of mice after Ad5, Ad5HVR48, or Ad48 vector administration. Mice (C57BL/6 or MF1) were injected intravenously with 3 × 1010 (n = 4/group) or 1 × 1011 (n = 3/group) vp Ad vectors. Serum samples were collected 6 h postvaccination, and systemic cytokine and chemokine levels were measured by Luminex analyses. Data are shown as means ± standard errors of the mean (SEM). The horizontal lines at the top indicate P values of <0.05 using Student's t tests.
Ad5HVR48 vectors display reduced hepatotoxicity relative to those of Ad5 vectors.
We next sought to assess the potential impact of HVR modification on Ad5-induced hepatotoxicity. Mice (C57BL/6 or MF1; n = 3 to 4/group) were injected i.v. with 3 × 1010 or 1 × 1011 vp Ad5, Ad5HVR48, or Ad48 vectors, and serum samples and livers were harvested 48 h after immunization. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were determined, and liver sections were assessed by histopathology. The C57BL/6 mice exhibited negligible hepatotoxicity with all vectors, but MF1 mice immunized with Ad5 vectors demonstrated a dramatic increase in systemic levels of AST and ALT (Fig. 2). Ad48 and Ad5HVR48 vectors did not result in increased AST or ALT levels in either mouse strain, indicating that Ad5HVR48 was less hepatotoxic than Ad5. None of the vectors at the doses utilized led to histopathological changes (data not shown).
FIG 2.
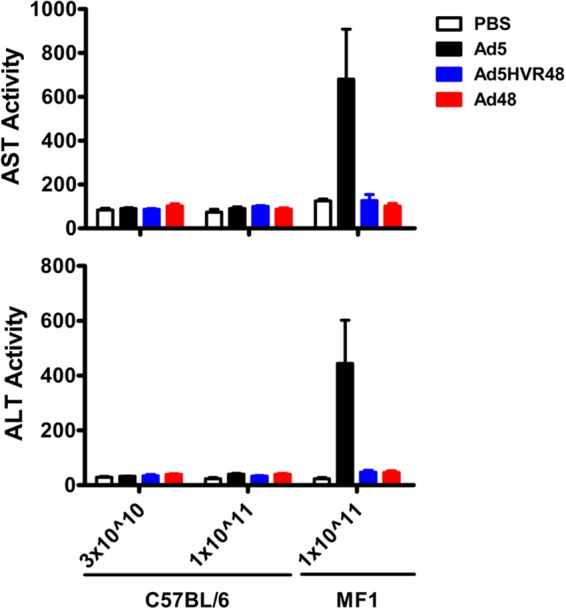
Serum AST and ALT levels in mice after Ad5, Ad5HVR48, or Ad48 vector administration. Mice (C57BL/6 or MF1) were injected intravenously with 3 × 1010 (n = 4/group) or 1 × 1011 (n = 3/group) vp Ad vectors. Serum samples were collected 48 h postvaccination, and systemic AST and ALT levels were measured. Data are shown as means ± SEM.
Both Ad5 and Ad5HVR48 induce partially exhausted CD8+ T-cell responses.
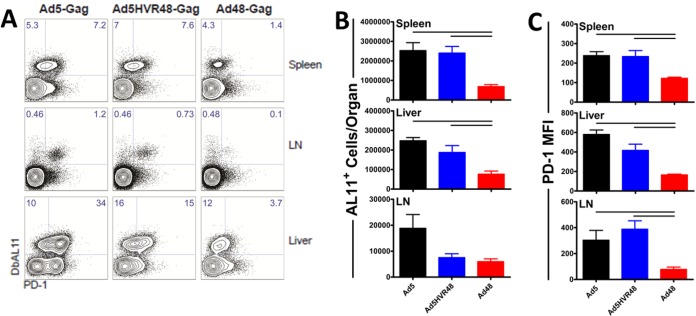
We next assessed the potential influence of HVR modification on the phenotype and function of Ad-elicited CD8+ T-cell responses. Mice (C57BL/6; n = 8/group) were vaccinated intramuscularly with 3 × 1010 vp Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. Each mouse was sacrificed 60 days after vaccination, and Gag-specific CD8+ T-cell responses were assessed in the liver, spleen, and lymph nodes. Analysis of Gag-specific CD8+ T-cell responses by Db/AL11 tetramer staining showed that both Ad5 and Ad5HVR48 vectors elicited potent and comparable antigen-specific immune responses in the spleen and liver (Fig. 3A). In contrast, the magnitude of antigen-specific CD8+ T cells elicited by Ad48 vectors was lower than that elicited by Ad5 and Ad5HVR48 in the spleen (3.7- and 3.5-fold-lower induction and P = 0.0045 and 0.0031, respectively; Student's t tests) and liver (3.2- and 2.5-fold-lower induction and P = 0.0003 and 0.0273, respectively) (Fig. 3B). However, while Ad5 and Ad5HVR48 elicited greater numbers of antigen-specific CD8+ T cells than those elicited by Ad48, the CD8+ T cells elicited by Ad5 and Ad5HVR48 vectors displayed a partially exhausted phenotype, with greater surface expression of the inhibitory receptor PD-1 (Fig. 3C). The CD8+ T cells elicited by Ad5 and Ad5HVR48 vaccination displayed greater mean fluorescence intensity (MFI) of PD-1 relative to Ad48 in the spleen (2.0× and 1.9× greater MFI than that of Ad48 and P = 0.0014 and 0.014, respectively), liver (3.5× and 2.5× greater MFI, and P = 0.0001 and 0.0082, respectively), and draining lymph nodes (LN) (3.9× and 5.0× greater MFI and P = 0.0284 and 0.0037, respectively) (Fig. 3C).
FIG 3.
Ad5 and Ad5HVR48 are highly immunogenic but result in generation of partially exhausted CD8+ T cells. Mice (C57BL/6; n = 8/group) were injected intramuscularly with 3 × 1010 vp of Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. Each mouse was sacrificed 60 days postimmunization, and memory CD8+ T-cell responses were assessed by flow cytometry. (A) Representative fluorescence-activated cell sorter (FACS) plots showing the percentage of PD-1 expressing AL11-specific CD8+ T cells in tissues. (B) Absolute numbers of Gag-specific CD8+ T cells (DbAL11+) in tissues. (C) Mean fluorescence intensity indicating PD-1 expression by AL11-specific CD8+ T cells in tissues. Data are shown as means ± SEM. The horizontal lines at the top indicate P values of <0.05 using Student's t tests.
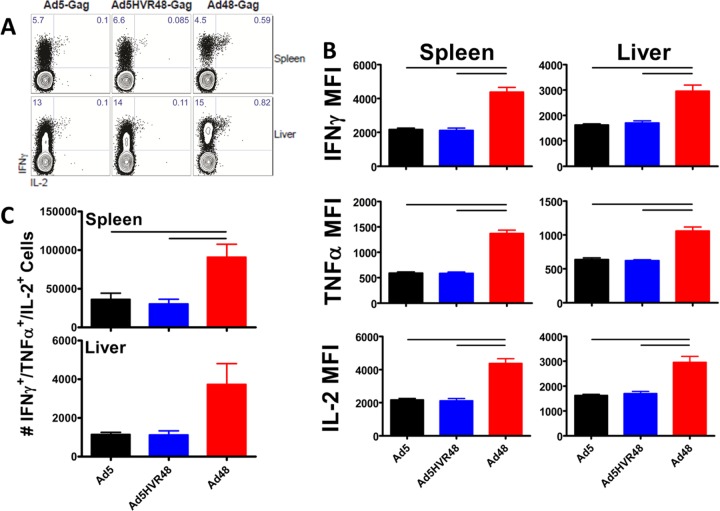
The vaccination of mice with Ad5, Ad5HVR48, and Ad48 elicited comparable levels of CD8+ T cells able to produce IFN-γ, but Gag-specific CD8+ T cells elicited by Ad5 and Ad5HVR48 vaccination had a reduced ability to coproduce IFN-γ and IL-2 compared to CD8+ T cells elicited by Ad48 (Fig. 4A). Moreover, on a per-cell basis, the Gag-specific CD8+ T cells elicited by Ad5 and Ad5HVR48 vaccination expressed significantly less IFN-γ, TNF-α, and IL-2 than cells elicited by Ad48 vaccination in both the spleen and liver (Fig. 4B). Furthermore, vaccination with either Ad5 or Ad5HVR48 elicited lower levels than Ad48 of polyfunctional CD8+ T cells able to coproduce IFN-γ, TNF-α, and IL-2 (Fig. 4C). Together, these results indicate that while Ad5 and Ad5HVR48 are highly immunogenic, both vectors elicited CD8+ T cells with an exhausted phenotype characterized by a greater expression of PD-1 and lower expression of IFN-γ, TNF-α, and IL-2, whereas Ad48 elicited a lower magnitude of but more functional CD8+ T cells.
FIG 4.
Ad5 and Ad5HVR48 induce lower levels of polyfunctional cytokine-secreting CD8+ T cells than Ad48. (A) Representative FACS plots showing the percentage of IFN-γ- and IL-2-expressing CD8+ T cells following AL11 peptide stimulation. (B) Mean fluorescence intensity indicating IFN-γ, TNF-α, and IL-2 expression following AL11 peptide in spleen and liver. (C) Absolute numbers of Gag-specific CD8+ T cells (DbAL11+) expressing IFN-γ, TNF-α, and IL-2 in the spleen and liver. Data are shown as means ± SEM. The horizontal lines at the top indicate P values of <0.05 using Student's t tests.
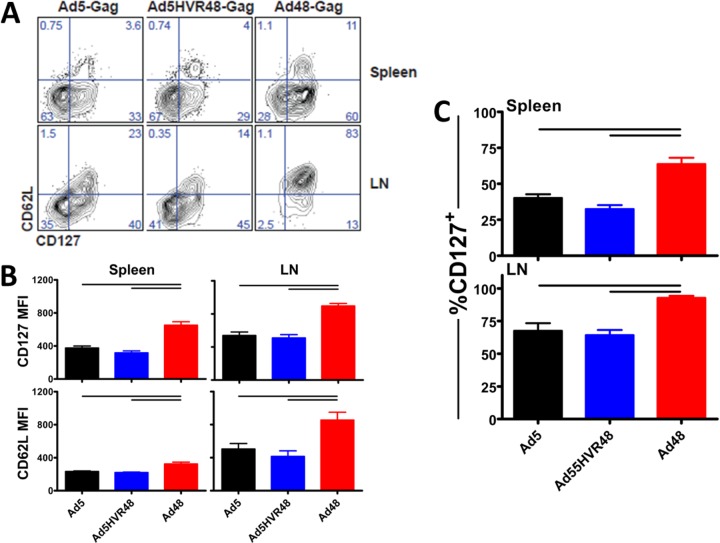
Immunization with Ad48 but not Ad5 or Ad5HVR48 results in accelerated effector-to-central memory CD8+ T-cell conversion.
We next probed the potential influence of HVR modification on memory CD8+ T-cell differentiation. Mice (C57BL/6; n = 8/group) were injected intramuscularly (i.m.) with 3 × 1010 vp of Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. The spleens and lymph nodes of the mice were harvested 49 days after vaccination, and effector-to-central memory CD8+ T-cell differentiation was assessed by analysis of CD127 and CD62L. The vaccination of mice with an Ad48 vector resulted in markedly higher levels of CD127+ CD62L+ CD8+ T cells in both the spleen and lymph nodes, whereas vaccination with Ad5 or Ad5HVR48 resulted in decreased expression of these memory markers (Fig. 5A). Analysis of CD127 and CD62L expression on SIV Gag-specific CD8+ T cells on a per-cell basis in both the spleen and LN revealed significantly higher levels of CD127 and CD62L in mice vaccinated with Ad48 than in those vaccinated with Ad5 and Ad5HVR48 (Fig. 5B). Furthermore, the vaccination of mice with Ad48 elicited significantly higher levels than either Ad5 or Ad5HVR48 of CD127+ SIV Gag-specific CD8+ T cells (Fig. 5C). Together, these results indicate that Ad48 induces accelerated effector-to-central memory CD8+ T-cell conversion after vaccination relative to that induced by Ad5, which is consistent with our prior results, and that Ad5HVR48 shares a similar immunologic profile with Ad5 (28, 31).
FIG 5.
Memory conversion of Ad5 and Ad5HVR48 is reduced compared to that in Ad48-immunized mice. Mice (C57BL/6; n = 8/group) were injected intramuscularly with 3 × 1010 vp of Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. Forty-nine days following vaccination, CD127 and CD62L expression of Db/AL11+ Gag-specific CD8+ T cells was measured by flow cytometry. (A) Representative flow plots of CD127 and CD62L expression on Db/AL11+ CD8+ T cells at day 49 after vaccination with Ad vectors expressing the SIV Gag transgene. (B) Comparison of mean fluorescence intensity (MFI) for the memory markers CD127 and CD62L 49 days after Ad vector vaccination. (C) Percentage of CD127+ T cells at day 49 after vaccination. Data are shown as means ± SEM. The horizontal lines at the top indicate P values of <0.05 using Student's t tests.
Hexon modification of Ad5 does not alter the recall potential of memory CD8+ T cells.
We lastly sought to assess the impact of HVR modification on the recall potential of vaccine-elicited memory CD8+ T-cell responses. Mice (C57BL/6; n = 4/group) were immunized i.m. with 3 × 1010 vp of Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. More than 30 days following the prime, the animals were boosted with 1 × 106 PFU modified Vaccinia virus Ankara (MVA) expressing SIV Gag. Both preboost and d7 postboost frequencies of transgene-specific CD8+ T cells were assessed by Db/AL11 tetramer binding assays. Following MVA boosting, the Ad48-primed mice showed a greater increase in the frequencies and numbers of AL11-specific CD8+ T cells relative to those primed with Ad5 or Ad5HVR48 (Fig. 6A and B). Taken together, these results show that the memory CD8+ T-cell responses elicited by Ad5HVR48 vectors were similar to Ad5 vectors in terms of their exhausted cellular phenotypes, impaired memory conversion, and poor recall potential. Thus, HVR modification of Ad5 vectors evaded Ad5-neutralizing antibodies and reduced hepatotoxicity but did not improve the immunologic phenotype of vaccine-elicited CD8+ T-cell responses.
FIG 6.
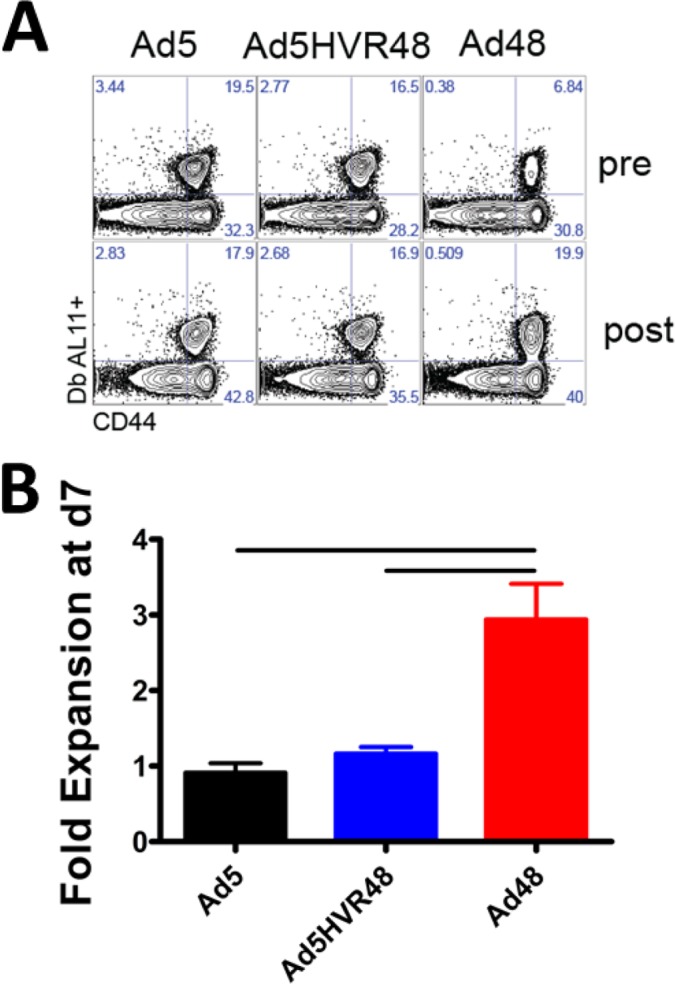
Memory recall potential of Ad5 and Ad5HVR48 is markedly reduced compared to that in Ad48-immunized mice. Mice (C57BL/6; n = 4/group) were injected intramuscularly with 3 × 1010 vp of Ad5, Ad5HVR48, or Ad48 expressing SIV Gag. (A) Representative FACS plots showing the percentage of AL11-specific CD8+ T cells in blood from the same mouse before and after boosting. (B) Summary of fold expansion of AL11-specific CD8+ T cells in blood. The animals were boosted >30 days postprime with 1 × 106 PFU MVA-Gag. Data are shown as means ± SEM. The horizontal lines at the top indicate P values of <0.01 using Student's t tests.
DISCUSSION
In this study, we show that hexon modification of Ad5 vectors with the HVRs of Ad48 (Ad5HVR48) resulted in a vector that induced innate cytokine and chemokine responses that were intermediate between Ad5 and Ad48 and exhibited reduced hepatotoxicity compared with that of Ad5. However, Ad5HVR48 did not significantly improve the immunologic phenotype of vaccine-elicited CD8+ T-cell responses compared with Ad5. These data may reflect the fact that HVRs determine the hepatic tropism of Ad5 vectors, whereas both HVRs and other capsid components contribute to innate immunity (8, 11, 27, 32).
Ad5 and Ad5HVR48 elicited similar innate and adaptive immune responses, suggesting that the hexon HVRs had a minimal impact on these immunologic properties. The CD8+ T-cell responses elicited by Ad5 or Ad5HVR48 vectors but not Ad48 vectors displayed a partially exhausted and dysfunctional phenotype (28, 31). The CD8+ T cells elicited by Ad5 and Ad5HVR48 displayed low levels of CD127 and CD62L memory markers. The expression of CD127 is high in long-lived memory T cells, and CD62L is highly expressed in central memory CD8+ T cells that provide robust immune protection following various immune challenges (33, 34). As expected, CD127 and CD62L expression correlated with the immunological boosting capacity of the Ad vectors. Collectively, these data indicate that the replacement of the HVRs of Ad5 with those of Ad48 did not prevent the induction of partially exhausted CD8+ T-cell responses.
The hexon HVRs not only serve as the primary determinant for neutralizing antibodies, but they also mediate interactions with serum coagulation factors (8, 35). While the replacement of the Ad5 HVRs with those of Ad48 led to a relative increase in innate immune cytokines elicited postvaccination, this increase in apparent innate immunity was not accompanied by an increase in adaptive immunity. Previous studies from our lab and others indicated that Ad vectors can differ markedly in their innate and adaptive immunological properties and that both the Ad fiber and capsid proteins influence these properties (4, 8, 21, 27, 36–38). The present study expands on these previous findings by analyzing the innate and adaptive immune responses elicited by Ad vectors. Our data suggest that Ad5HVR48 vectors may exhibit immunologic limitations similar to those of Ad5 vectors in terms of inducing high levels of PD-1 on CD8+ T cells with slow effector-to-central memory T-cell differentiation and low levels of IFN-γ and IL-2 secretion (28, 31).
Notably, our results contrast with those of a prior study that reported unexpected hepatotoxicity of Ad5HVR48 compared with that of Ad5 and Ad48 (27, 29). Our data show substantial hepatotoxicity with Ad5 vectors in MF1 mice but no detectable hepatotoxicity with Ad5HVR48 and Ad48 vectors, likely reflecting the lack of liver tropism of Ad5HVR48 and Ad48 (29). Moreover, innate cytokine profiles with Ad5HVR48 were intermediate between Ad5 and Ad48. The differences between our data and those of the prior study may reflect the high vp-to-PFU ratios of Ad48 in the prior study that likely dampened the observed Ad48 innate responses, leading to the apparently higher cytokine levels with Ad5HVR48 (29). Recent advances in Ad48 production methods have led to improved vector batches that were used in the present study with lower vp-to-PFU ratios.
In summary, our data demonstrate the chimeric nature of Ad5HVR48 vectors in terms of the phenotypes of the innate and adaptive immune responses induced. Ad5HVR48 possesses a serologic profile similar to that of Ad48, as well as a similar lack of hepatotoxicity, presumably as a direct consequence of HVR exchange (8, 19–21). Similarly, Ad5HVR48 displays an innate immune cytokine profile intermediate between Ad5 and Ad48, in agreement with prior publications showing that both Ad fiber and capsid proteins influence innate cytokine and chemokine profiles (27). Adaptive T-cell phenotypes elicited by Ad5HVR48, however, closely resemble those elicited by Ad5 (28, 31). These findings have substantial implications for the clinical development of Ad5HVR48 and other HVR-modified vectors.
ACKNOWLEDGMENTS
We thank L. Coughlan and A. Baker for helpful discussions.
This work was supported by NIH grants AI007245 and T32AI07387 (to P.P.-M.) and AI078526 and AI096040 (to D.H.B.), the Bill and Melinda Gates Foundation OPP1033091 (to D.H.B.), the Ragon Institute of MGH, MIT, and Harvard (to D.H.B.), the U.S. Department of Defense, National Defense Science and Engineering Graduate Fellowship (to J.E.T.), and Harvard University Herchel Smith Graduate Fellowships (to J.E.T. and N.M.P.).
We declare no financial conflicts of interest.
Footnotes
Published ahead of print 18 June 2014
REFERENCES
- 1.Chengalvala MV, Lubeck MD, Selling BJ, Natuk RJ, Hsu KH, Mason BB, Chanda PK, Bhat RA, Bhat BM, Mizutani S. 1991. Adenovirus vectors for gene expression. Curr. Opin. Biotechnol. 2:718–722. 10.1016/0958-1669(91)90041-3 [DOI] [PubMed] [Google Scholar]
- 2.Boyer JL, Kobinger G, Wilson JM, Crystal RG. 2005. Adenovirus-based genetic vaccines for biodefense. Hum. Gene Ther. 16:157–168. 10.1089/hum.2005.16.157 [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH. 2010. Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV AIDS 5:386–390. 10.1097/COH.0b013e32833cfe4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663. 10.1128/JVI.02696-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng'ang'a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. 2011. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 29:5203–5209. 10.1016/j.vaccine.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayo-Puxan N, Cascallo M, Gros A, Huch M, Fillat C, Alemany R. 2006. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 87:2487–2495. 10.1099/vir.0.81889-0 [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409. 10.1016/j.cell.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 9.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594–6604. 10.1128/JVI.02497-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemany R, Suzuki K, Curiel DT. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 81:2605–2609 [DOI] [PubMed] [Google Scholar]
- 11.Baker AH, Mcvey JH, Waddington SN, Di Paolo NC, Shayakhmetov DM. 2007. The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol. Ther. 15:1410–1416. 10.1038/sj.mt.6300206 [DOI] [PubMed] [Google Scholar]
- 12.Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, Nicklin SA, van Rooijen N, Custers J, Goudsmit J, Barouch DH, McVey JH, Baker AH. 2009. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood 114:965–971. 10.1182/blood-2009-03-208835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald R a, Waddington SN, Buckley SM, Greig JA, Parker AL, Miller AM, Wang H, Lieber A, van Rooijen N, McVey JH, Nicklin SA, Baker AH. 2010. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 116:2656–2664. 10.1182/blood-2009-12-260026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradshaw AC, Coughlan L, Miller AM, Alba R, van Rooijen N, Nicklin SA, Baker AH. 2012. Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses. J. Control. Release 164:394–402. 10.1016/j.jconrel.2012.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradshaw AC, Parker AL, Duffy MR, Coughlan L, van Rooijen N, Kähäri VM, Nicklin SA, Baker AH. 2010. Requirements for receptor engagement during infection by adenovirus complexed with blood coagulation factor X. PLoS Pathog. 6:e1001142. 10.1371/journal.ppat.1001142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corjon S, Gonzalez G, Henning P, Grichine A, Lindholm L, Boulanger P, Fender P, Hong SS. 2011. Cell entry and trafficking of human adenovirus bound to blood factor X is determined by the fiber serotype and not hexon:heparan sulfate interaction. PLoS One 6:e18205. 10.1371/journal.pone.0018205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson CM, Singh G, Lee JY, Dehghan S, Rajaiya J, Liu EB, Yousuf MA, Betensky RA, Jones MS, Dyer DW, Seto D, Chodosh J. 2013. Molecular evolution of human adenoviruses. Sci. Rep. 3:1812. 10.1038/srep01812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford-Miksza L, Schnurr DP. 1996. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 70:1836–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley RR, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. 2012. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J. Virol. 86:625–629. 10.1128/JVI.06254-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley RR, Maxfield LF, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. 2012. Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J. Virol. 86:1267–1272. 10.1128/JVI.06165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AAC, Holterman CL, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239–243. 10.1038/nature04721 [DOI] [PubMed] [Google Scholar]
- 22.Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, Tordjmann T, Vigne E, Perricaudet M, Benihoud K. 2008. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol. Ther. 16:1474–1480. 10.1038/mt.2008.132 [DOI] [PubMed] [Google Scholar]
- 23.Abe S, Ohuda K, Kondo A, Yoshida A, Yoshizaki S, Mizuguchi H, Klinman D, Shimada M. 2009. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5:570–579. 10.1002/jgm.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prill JM, Espenlaub S, Samen U, Engler T, Schmidt E, Vetrini F, Rosewell A, Grove N, Palmer D, Ng P, Kochanek S, Kreppel F. 2011. Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol. Ther. 19:83–92. 10.1038/mt.2010.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicol CG, Graham D, Miller WH, White SJ, Smith TA, Nicklin SA, Stevenson SC, Baker AH. 2004. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 10:344–354. 10.1016/j.ymthe.2004.05.020 [DOI] [PubMed] [Google Scholar]
- 26.Prill J, Espenlaub S, Samen U, Engler T, Schmidt E, Vetrini F, Rosewell A, Grove N, Palmer D, Ng P, Kochanek S, Kreppel F. 2009. Modifications of adenovirus hexon allow for either hepatocyte detargeting or targeting with potential evasion from Kupffer cells. Mol. Ther. 19:83–92. 10.1038/mt.2010.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teigler JE, Iampietro MJ, Barouch DH. 2012. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J. Virol. 86:9590–9598. 10.1128/JVI.00740-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penaloza-MacMaster P, Provine NM, Ra J, Borducchi EN, McNally A, Simmons NL, Iampietro MJ, Barouch DH. 2013. Alternative serotype adenovirus vaccine vectors elicit memory T cells with enhanced anamnestic capacity compared to Ad5 vectors. J. Virol. 87:1373–1384. 10.1128/JVI.02058-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlan L, Bradshaw AC, Parker AL, Robinson H, White K, Custers J, Goudsmit J, Van Roijen N, Barouch DH, Nicklin SA, Baker AH. 2012. Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol. Ther. 20:2268–2281. 10.1038/mt.2012.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290–6297. 10.4049/jimmunol.172.10.6290 [DOI] [PubMed] [Google Scholar]
- 31.Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. 2013. Comparative analysis of simian immunodeficiency virus Gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J. Virol. 87:1359–1372. 10.1128/JVI.02055-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greig JA, Buckley SM, Waddington SN, Parker AL, Bhella D, Pink R, Rahim AA, Morita T, Nicklin SA, McVey JH, Baker AH. 2009. Influence of coagulation factor X on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol. Ther. 17:1683–1691. 10.1038/mt.2009.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wherry EJ, Teichgräber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- 34.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- 35.Baker AH, Nicklin SA, Shayakhmetov DM. 2013. FX and host defense evasion tactics by adenovirus. Mol. Ther. 21:1109–1111. 10.1038/mt.2013.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91. 10.1038/nature07469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Loré K, Gall JG, Gostick E, Lefebvre F, Cameron MJ, Price DA, Haddad E, Sekaly RP, Seder RA, Koup RA. 2012. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J. Immunol. 188:6109–6118. 10.4049/jimmunol.1103717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Farin FM, Stewart PL, Shayakhmetov DM. 2012. Coagulation factor X activates innate immunity to human species C adenovirus. Science 338:795–798. 10.1126/science.1226625 [DOI] [PMC free article] [PubMed] [Google Scholar]