Abstract
Animal and human data from various viral infections and vaccine studies suggest that nonneutralizing antibodies (nNAb) without neutralizing activity in vitro may play an important role in protection against viral infection in vivo. This was illustrated by the recent human immunodeficiency virus (HIV) RV144 vaccine efficacy trial, which demonstrated that HIV-specific IgG-mediated nNAb directed against the V2 loop of HIV type 1 envelope (Env) were inversely correlated with risk for HIV acquisition, while Env-specific plasma IgA-mediated antibodies were directly correlated with risk. However, tier 1 NAb in the subset of responders with a low level of plasma Env-specific IgA correlated with decreased risk. Nonhuman primate simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) challenge studies suggest that Env-mediated antibodies are essential and sufficient for protection. A comparison of immune responses generated in human efficacy trials reveals subtle differences in the fine specificities of the antibody responses, in particular in HIV-specific IgG subclasses. The underlying mechanisms that may have contributed to protection against HIV acquisition in humans, although not fully understood, are possibly mediated by antibody-dependent cell-mediated cytotoxicity (ADCC) and/or other nonneutralizing humoral effector functions, such as antibody-mediated phagocytosis. The presence of such functional nNAb in mucosal tissues and cervico-vaginal and rectal secretions challenges the paradigm that NAb are the predominant immune response conferring protection, although this does not negate the desirability of evoking neutralizing antibodies through vaccination. Instead, NAb and nNAb should be looked upon as complementary or synergistic humoral effector functions. Several HIV vaccine clinical trials to study these antibody responses in various prime-boost modalities in the systemic and mucosal compartments are ongoing. The induction of high-frequency HIV-specific functional nNAb at high titers may represent an attractive hypothesis-testing strategy in future HIV vaccine efficacy trials.
INTRODUCTION
The identification of immune markers that correlate with protection against infection or disease after vaccination (or natural infection) remains the “holy grail” for vaccine scientists, as these immune markers have the potential to expedite vaccine development and provide unique insights into the pathogenesis of infection. For most of the vaccines available today, a functional antibody response is the identified correlate of protection (1). In the cases of candidate preventive human immunodeficiency virus (HIV) vaccines and experimental vaccines against the closely related retrovirus simian immunodeficiency virus (SIV), recent studies have suggested an antibody correlate of risk or protection. However, these studies suggested an association with antibodies that did not mediate broad or potent neutralization (2–5).
Transmission of HIV type 1 (HIV-1) occurs through homosexual or heterosexual intercourse, through injection of blood or blood-derived products, and from mother to child during pregnancy, at delivery, or through breastfeeding. Although several nonexclusive mechanisms may contribute to protection from HIV-1 acquisition (6), given the confounding variables of risk, route, and intensity of exposure, thinking about the HIV-1 immune correlates of protection has been dominated by the paradigm that the induction of neutralizing antibodies (NAb), in particular broadly neutralizing antibodies (bNAb) covering 50 to 90% of transmitted viruses, should be the goal of vaccine development efforts (7, 8). With regard to HIV-1, the Thai RV144 trial showed that vaccine-induced IgG antibody against the first and second variable domains (V1V2) of the surface glycoprotein envelope (Env) was associated with lower risk of HIV infection but that IgA against Env was directly associated with risk of infection (2, 9, 10). While these V1V2 antibodies are not broadly neutralizing, they do appear to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) and limited, tier 1 NAb (11–14), and in the setting of a low level of Env-specific IgA, they are inversely correlated with the risk of infection. In addition, several groups have described correlates of protection against the acquisition of SIV (4, 5, 15, 16) exclusive of bNAb. These findings prompted this review of the potential functions of vaccine-induced antibody responses, aside from in vitro neutralization, that may contribute to protection against HIV-1 acquisition (17).
We review the vaccine-induced immune mechanisms of protection that are antibody mediated for viral diseases, along with the latest findings of the RV144 correlate-of-risk analysis suggesting that nonneutralizing antibodies (nNAb) can be functional and indeed neutralize pathogens in vivo, including HIV-1.
LESSONS LEARNED FROM NON-HIV VACCINES
Neutralization of viruses has long been considered the main mechanism of protection, and this may well be true, but it also must be admitted that neutralization is usually measured by prevention of infection of cultured cells (18). Thus, these in vitro measurements may or may not relate to the protection afforded by prior infection or vaccination.
A recent and comprehensive review on nNAb defines nNAb as functional antibodies with low or no neutralization activity in vitro (19). Functional nNAb have long been recognized as contributing to protection against non-HIV infections, such as, for example, alphaviruses (20–22), flaviviruses (23–27), influenza virus (28–32), respiratory syncytial virus (33, 34), vesicular stomatitis virus (35), Ebola virus (36, 37), mouse hepatitis virus (38), cytomegalovirus (39–41), lymphocytic choriomeningitis virus (42), and vaccinia virus (43). Vaccines against feline leukemia virus protect by a mechanism that is not neutralization and may be either nonneutralizing antibody or cytotoxic T lymphocytes (44). nNAb function in multiple different ways, including ADCC, complement-mediated cytotoxicity, steric inhibition of proteins important in viral replication, and antibody-dependent cell-mediated phagocytosis (ADCP) (19, 45, 46). Plasma samples obtained from convalescent H1N1-infected human subjects showed high ADCC activity against pandemic H1N1 influenza virus. E1 and E2 ADCC epitopes overlapped immunodominant epitopes of hemagglutinin (47). Although high titers and the avidity of nNAb for influenza vaccine antigen have also been associated with severe influenza (48), nNAb induced by influenza vaccines that fail to prevent influenza infection may nevertheless have collateral benefit, protecting against secondary bacterial diseases like Streptococcus pneumoniae and Klebsiella pneumoniae when a neutralizing-antibody-inducing vaccine is not available (49). Moreover, nNAb act together with CD8+ T cells to confer heterosubtypic immunity (31). Although nNAb may not prevent infection, they may modify disease. nNAb to Sindbis virus in mice lyse virus-infected cells and prevent encephalitis (20). nNAb also can reduce the neurovirulence of flavivirus infection (23) and play an alternative role either in protection or in enhancement of disease (26, 27). Although protection against rotavirus is usually mediated by neutralizing antibodies directed against the vp4 or vp7 attachment protein, nonneutralizing IgA antibodies directed against the vp6 capsid protein also protect by inducing a conformational change in the trimer (50). In the case of lymphocytic choriomeningitis virus, chronic infection can be prevented in mice if nonneutralizing, binding antibody that interacts with T cells is present at the time of challenge (40, 42). The ubiquity of these phenomena argue that functional nNAb are important in protection against viral infection without negating in any way the desirability of evoking neutralizing antibodies through vaccination (51).
NONNEUTRALIZING ANTIBODIES AND CANCER THERAPY
HIV and cancer share interesting similarities; in both, it is presumed that in order to be effective, all affected cells (productively infected and neoplastic, respectively) must be eliminated. Like cancer cells, HIV has evolved mechanisms to evade or suborn immune surveillance. Monoclonal antibody (MAb) treatment of solid-tumor malignancies is associated with the development of resistance due to a broad array of potential genetic alterations (52). In cancer, numerous proteins are expressed at the surfaces of tumor cells, representing potential targets for therapeutic and, by definition, nonneutralizing MAb. The killing of tumor cells is accomplished via several mechanisms, including direct action of the antibody through receptor blockade or agonist activity, induction of apoptosis, delivery of a drug or cytotoxic agent, complement-dependent cytotoxicity, ADCC, regulation of T-cell function, and specific effects of an antibody on tumor vasculature and stroma (reviewed in references 53 and 54). Several MAb, such as rituximab, an anti-CD20 antibody with good activity in patients with chronic lymphocytic leukemia (CLL), have now been licensed for clinical use. During clinical CLL, there are millions of transformed cells per milliliter of blood. In contrast, the target of an HIV-specific nNAb are the few cells infected (with a single transmitted/founder virus [9]) during inchoate HIV infection.
HIV-1 VACCINE EFFICACY TRIALS USING RECOMBINANT ENVELOPE PROTEINS
Data from three studies of nonhuman primate (NHPs) (4, 5, 55) have suggested that Env is a necessary component for successful protection from SIV acquisition. The failure of the Merck recombinant adenovirus type 5 (rAd5) Gag-Pol-Nef vaccine seems to support this finding (56, 57). Monomeric gp120 HIV-1 envelope proteins alone failed to protect high-risk volunteers in two efficacy trials, Vax003 (58) and Vax004 (59–61), while the RV144 prime-boost regimen using a recombinant canarypox vector prime and the same alum-adjuvanted envelope protein boost used in Vax003 conferred modest protection against HIV acquisition (62). Another efficacy trial (HVTN 505) tested a regimen with a DNA vaccine prime composed of DNA plasmids encoding Gag, Pol, and Nef from HIV-1 subtype B and Env from subtypes A, B, and C and a replication-defective-rAd5–HIV-1 vaccine boost containing a mixture of four rAd5 vectors encoding the HIV-1 subtype B Gag-Pol and Env matching the DNA Env components. The phase IIB trial was recently stopped for futility, showing no efficacy and no statistically significant effect on viral load and a nonsignificant excess of HIV infection in the vaccinated group (63). These findings allowed for a comparison of the vaccine-induced immune antibody responses between these three trials, which may shed some light on our still-limited understanding of the potential mechanisms of protection.
Vax003 AND Vax004 MONOMERIC HIV-1 ENVELOPE PROTEIN EFFICACY TRIALS
Vax004 tested AIDSVAX B/B, a bivalent recombinant HIV-1 subtype B (GNE8 and MN) gp120 envelope glycoprotein subunit vaccine in U.S. men who have sex with men (MSM) and women at high risk for heterosexual transmission of HIV-1 (59). The vaccine did not prevent HIV-1 acquisition, nor did it affect HIV-1 disease progression (61). High NAb levels against the easy-to-neutralize MN strain were, however, significantly inversely correlated with HIV-1 incidence, while low levels against more-difficult-to-neutralize viruses did not, suggesting that the level and breadth of elicited NAb were not sufficient for protection (60). Interestingly, the level of vaccine-induced antibody-dependent cellular virus inhibition (ADCVI) activity correlated inversely with the rate of acquiring HIV-1 infection following vaccination. Moreover, ADCVI activity correlated poorly with NAb or CD4-gp120-blocking antibody activity measured against laboratory strains and was modulated by Fc receptor (FcR) polymorphisms (64).
Vax003 tested AIDSVAX B/E, a bivalent recombinant HIV-1 subtype B/E (A244 CRF01_AE and MN subtype B) gp120 envelope glycoprotein subunit vaccine in injection drug users in Bangkok, Thailand. The vaccine did not prevent HIV-1 acquisition, nor did it affect HIV-1 disease progression. Prior to infection, the levels of (i) antibodies to gp120, A244 V2, and A244 V3, (ii) blocking of A244 binding to CD4, and (iii) MN neutralization were not significantly different between the HIV-infected and uninfected vaccine recipients and did not correlate with the rate of HIV infection (58).
RV144 PRIME-BOOST EFFICACY TRIAL
Vaccine efficacy.
RV144, consisting of an ALVAC HIV vaccine (vCP1521) prime and an AIDSVAX gp120 B/E boost, provided the first evidence that an HIV-1 vaccine can provide protective efficacy against HIV-1 acquisition. A modified intent-to-treat analysis showed estimated efficacies of 31.2% after 42 months (62, 65), 60% at 12 months, and 44% at 18 months after the first vaccination, suggesting an early, but nondurable, vaccine effect (66). Vaccination did not affect the clinical course of HIV-1 disease after infection, though there was evidence of reduction in seminal fluid viral load (67).
Vaccine-induced immune responses.
Gamma interferon (IFN-γ) enzyme-linked immunosorbent spot assay (ELISPOT)-positive responses were detected in 41% of the vaccinees and were predominantly CD4+ T-cell mediated. Responses were targeted within the HIV-1 Env region, with 60% of vaccinees recognizing peptides derived from the Env V2 region, which includes the α4β7 integrin binding site (68, 69). Intracellular cytokine staining confirmed that Env responses predominated and were mediated by polyfunctional effector memory CD4+ T cells. All Env-specific CD4+ T-cell lines displayed a cytolytic activity despite the absence of CD8+ T cells (70).
Binding antibody against the MN and A244 Env vaccine antigens was nearly uniformly present but dropped 15-fold after 6 months; p24 responses were less frequent. Antibody-dependent cell-mediated cytotoxicity (ADCC) in vaccine recipients and MAb from vaccine recipients mediating ADCC were described previously (13, 71). Whereas NAb to tier 1 viruses were detected in both RV144 and Vax003, the tier 1 NAb titers were higher after the RV144 regimen (ALVAC HIV vaccine prime and two-protein boosts) than after two gp120 protein administrations alone, confirming a priming effect for the ALVAC HIV vaccine (11). Further analysis suggested that the lack of a clade-specific HIV-1 NAb response was associated with the presence of certain HLA class II alleles and heterodimers in Southeast Asians (72).
Correlates of protection.
The RV144 trial provided a unique opportunity to perform a case-control study of correlates of risk for HIV acquisition. Plasma IgG binding antibody to scaffolded gp70 V1V2 CaseA2 envelope protein (HIV-1 subtype B) correlated inversely with risk, while Env plasma IgA correlated directly with risk, raising the hypotheses that IgA responses directed against Env and IgG responses directed against V1V2 may be mechanistically associated with protection but in opposite directions. Neither low levels of V1V2 antibodies nor high levels of Env-specific IgA antibodies were associated with higher rates of infection than in the placebo group. In vaccinees with low levels of Env-specific IgA antibodies, levels of IgG avidity, ADCC, NAb, and Env-specific CD4+ T cells were inversely correlated with risk of infection (2, 3, 73). A subsequent analysis showed that RV144 antibodies to subtype A, C, and CRF01_AE gp70 V1V2 scaffolded proteins also correlated inversely with risk (74), suggesting that the RV144 regimen might protect against heterosexual transmission of HIV strains heterologous (A and C) to the vaccine components. Two weeks after the last vaccination, 97% of RV144-studied plasma samples from vaccine recipients contained antibodies to V2 region synthetic peptides, falling to 19% at 48 weeks, suggesting that waning vaccine efficacy may be correlated to waning V2 antibody response.
Interestingly, gp70 V1V2 antibodies were lower in HVTN 505 than in RV144 (75). The response to V3 CRF01_AE also inversely correlated with the risk of HIV infection in vaccine recipients with lower levels of Env-specific plasma IgA and neutralizing antibodies. In Vax003 and Vax004 (no protection), serum IgG responses targeted the same epitopes as in RV144, with the exception of an additional C1 reactivity in Vax003 and infrequent V2 reactivity in Vax004. These results along with a recent sieve analysis (76) generate the hypothesis that IgG to linear epitopes in the V2 and V3 regions of gp120 are part of a complex interplay of immune responses that contributed to protection in RV144 (77).
Approximately 90% of the incident infections in RV144 were infections with CRF01_AE, the predominant circulating strain in much of Southeast Asia (78, 79). A sieve analysis identified two vaccine-associated genetic signatures in V2 corresponding to sites 169 and 181, further supporting the hypothesis that vaccination-induced immune responses directed against the V2 loop were associated with protection (9). Monoclonal antibodies from RV144 vaccine recipients contact the V2 K169 residue, providing additional evidence that vaccine-induced antibodies correspond to the observed sieve effect. These V2-specific antibodies can mediate ADCC, neutralization, and low-level virus capture (12, 80). These findings generate the hypothesis that V2 IgG plays a role in protection against HIV-1 acquisition but do not distinguish between a mechanistic or nonmechanistic means of protection (81).
In sera from HIV-1 CRF01_AE-infected blood donors, nine ADCC epitopes have been localized to the C1 and V2 region of gp120 (82). Sequences in gp70 V1V2 antigens other than V2, such as C1 and V1, may significantly contribute to the binding responses. It is suggested that nNAb mediating ADCC that can block HIV infection are directed against viral entry epitopes, including the CD4-inducible A32 epitope of a highly conserved region of gp120 at the gp120-gp41 interface (83), and there is evidence of cooperativity between the anti-C1 and anti-V2 activities, with increased anti-V1V2 binding in the presence of vaccine-induced anti-C1 antibody (84). This epitope was a target of ADCC-mediating MAb isolated from RV144 vaccinees (13). Some light has recently been shed on the role of plasma IgA in RV144. In the presence of low anti-Env IgA, both ADCC and NAb responses correlated with decreased risk of infection. ADCC responses were predominantly directed to the C1 conformational region of gp120 (13, 85, 86). These ADCC antibodies mediated cross-clade target cell killing. ADCC-mediating antibodies exhibited low levels (0.5% to 1.5%) of somatic mutations of the V heavy (VH) chain and disproportionately utilized VH1 family genes (74%) (13), similar to what occurred with CD4 binding site bNAb; however, these antibodies clearly differed from bNAb in both degree of somatic hypermutation and neutralization (87, 88). IgA antibodies elicited by RV144 block C1 region-specific IgG-mediated ADCC (10). Whether V2 antibodies might block the gp120-α4β7 interaction and contribute at least partially to the protective effect against HIV-1 sexual transmission remains to be demonstrated (89–91). In future trials, assessing IgG and IgA to V1V2 binding antibody immune responses in the mucosal compartments will be key.
In previous clinical studies, monomeric gp120 induced high levels of Env-specific IgG4 antibodies (92), while an ALVAC (vCP1452) prime and an MN gp120 in alum boost elicited lower levels of IgG4 than of IgG1 and IgG3 antibodies (93). Antigen-specific IgG3 antibodies are associated with long-term control of Plasmodium falciparum (94) and monocyte-mediated cellular inhibition of parasite growth in vitro (95). Similarly, an early appearance of chikungunya virus-specific neutralizing IgG3 antibodies is associated with clearance of the virus and long-term clinical protection (96). Conversely, an IgG4 response has been associated with progression to AIDS (97). IgG3 can fix the complement and has a high affinity for FcγR (98). In RV144, an Env IgG3 response was correlated with decreased risk of HIV infection, a response that declined rapidly compared to overall IgG responses (99). A recent comparison of RV144 and Vax003 showed that Env-specific IgG3 and V1/V2 IgG3 response rates were higher in RV144 vaccinees than in Vax003 vaccinees and, conversely, that IgG4 responses were considerably lower in RV144. V1/V2 IgG3 responses and IgG3 responses specific for V1/V2 169K correlated with decreased risk of HIV-1 infection after IgA adjustment (100). Chung et al. recently showed that the RV144 regimen elicited highly coordinated Fc-mediated effector responses, with the selective induction of highly functional IgG3 antibodies. In contrast, Vax003 elicited monofunctional antibody responses influenced by IgG4 selection. Moreover, only RV144 induced IgG1 and IgG3 antibodies targeting the crown of the HIV envelope V2 loop, although with low coverage of breakthrough viral sequences (101). It is speculated that ALVAC priming, due to its unique proinflammatory cytokine and chemokine response following vaccination in rhesus monkeys and infection in human peripheral blood mononuclear cells (PBMC), may shift the IgG subclass response to IgG3 in humans after vector priming and envelope protein boosting compared with envelope vaccination alone (102). The contribution of Fc-FcγR interaction-mediated functions through mechanisms such as ADCC, antibody-dependent cell-mediated viral inhibition (ADCVI), and ADCP antibodies remains to be explored (103, 104). A recent post hoc analysis of RV144 showed an association between the FcγRIIC polymorphism, vaccine efficacy, and correlates of risk, emphasizing the potential role of FcR genetics in predicting vaccine efficacy (105).
NONHUMAN PRIMATE CHALLENGE STUDIES
Several nonhuman primate (NHP) challenge studies support the RV144 findings. Vaccination with ALVAC SIV alone conferred protection from infection in neonate macaques exposed to repeated oral low-dose challenge. However, neither binding antibody, NAb, nor cell-mediated immune responses correlated with protection (106). Recently, an immunization regimen recapitulating the RV144 regimen protected against mucosal challenge of SIVmac251 acquisition in 30% of the vaccinated animals. Protected animals had high-avidity antibodies to gp120 that recognized the V2 variable envelope region and reduced SIVmac251 infectivity in cells expressing a high level of α4β7, suggesting a functional role for V2 antibodies (16). In another experiment where macaques were immunized with ALVAC SIV and SIV gp120 formulated either in alum or MF59, only the alum group showed a significantly reduced rate of SIV acquisition compared to that of unvaccinated controls. The frequency of plasmablasts expressing α4β7 and CXCR4 (hematopoietic homing marker) was higher in the alum group, while there was a trend for a higher frequency of plasmablasts expressing CXCR3 (inflammatory site homing marker) in the MF59 group (107). The significance of these findings remains unclear.
An Ad26 prime and modified vaccinia virus Ankara (MVA) boost regimen using vaccines expressing gag-pol and env from SIVsme543 conferred 80 to 83% reduction in the per-exposure probability of infection against repeated low-dose intrarectal inoculations of heterologous, neutralization-resistant SIVmac251. Protection against SIV acquisition correlated with Env- and V2-specific binding and tier 1-strain-neutralizing antibodies. Monkeys vaccinated with an Ad35/Ad26 prime-boost regimen expressing either Gag-Pol and Env or only Gag-Pol showed protection only when Env was present, suggesting that Env-specific responses are critical in preventing virus acquisition. Immunological correlates of protection were consistent with the prior experiment (4). A recent NHP intrarectal SIVsmE660 challenge study in which animals were vaccinated with a DNA prime and an Ad5 boost expressing either mosaic Gag, mosaic heterologous Env, or heterologous Env based on a natural SIVmac239 sequence confirmed that Env-elicited immune responses are necessary and sufficient to provide protection from acquisition (69%). Plasma IgG binding to gp120 Env at the time of challenge did not correlate significantly with time to infection, but excluding animals who were infected with neutralization-resistant viruses resulted in a strong correlation of protection with IgG binding to gp120. Correlation with a delayed time to infection was also observed for plasma antibody avidity, CD4 binding site activity, and neutralization of some viral strains. Antibody to SIV V1V2 predicted protection against infection. However, the sieve analysis showed that selection against V1V2 sequences by the SIV vaccines was limited to neutralization-sensitive viruses, suggesting that selection is vaccine sequence specific and not broad. As in the human RV144 trial, there was no association between protection from infection and protection from pathogenesis, suggesting that humoral responses that effectively block acquisition are not necessarily correlated with cellular responses that control pathogenesis. The authors suggest that the primary mechanism of protection is by lowering the effective infectious dose, that is, in vivo neutralization (55), and that the moderate RV144 efficacy was due to antibodies that could neutralize some viruses circulating in that cohort; these sensitive viruses were susceptible to vaccine-matched V1V2, leading to sieving being an alternative hypothesis with regard to the possible role of nNAb in protection against HIV acquisition in RV144.
An emerging understanding of the early events in mucosal SIV, simian-human immunodeficiency virus (SHIV), and HIV-1 infections was recently presented (108), justifying the need to develop vaccines inducing mucosal immune responses. A relatively small number of immune effectors at the mucosal site of entry might be at the right place at the right time to be “enough and soon enough” to clear infection. Interestingly, human dimeric IgA1 MAb-treated rhesus macaques remained free of virus after intrarectal SHIV challenge, while treatment with dimeric IgA2 was much less effective. Protection was correlated with virus capture and inhibition of transcytosis of cell-free virus (109). Another approach using gp41 protein and derived peptide administered by mixed intramuscular and intranasal modalities was capable of protecting immunized monkeys against SHIV challenge (110) and of eliciting systemic and mucosal antibodies that inhibited HIV transcytosis in the absence of neutralizing antibodies in humans (111).
Recently, it was demonstrated that Ad26-MVA and Ad26-Ad35 vector-based vaccines expressing HIV-1 mosaic Env, Gag, and Pol delivered in a prime-boost regimen resulted in a significant reduction in the per-exposure acquisition risk following repetitive, intrarectal SHIV-SF162P3 challenge, a difficult-to-neutralize virus. Protection was most strongly correlated with enzyme-linked immunosorbent assay (ELISA) binding antibody titers against the homologous Mos1 Env immunogen and to a lesser extent with ELISA binding antibody titers against other Env immunogens. In addition, protection was significantly correlated with NAb titers against SF162, which is a neutralization-sensitive virus related to the challenge virus SHIV-SF162P3. NAb titers against other tier 1 viruses also showed trends toward correlation with protection. Interestingly, protection was also correlated with a functional nNAb phagocytic score (ADCP), and a trend was observed with antibody-dependent complement deposition (ADCD) involving C3b complement. However, no significant correlation of protection with surface plasmon resonance binding to cyclic V2 peptides or with gp70-V1V2 scaffolds nor any measure of CD8+ T lymphocyte responses was detected. These key findings suggest that multiple antibody functions may contribute to protection against acquisition of difficult-to-neutralize viruses (5).
A panel of bNAb and nNAb screened for their high ability to block HIV acquisition and replication in vitro in an independent or FcγR-dependent manner were formulated for topical vaginal application in a microbicide gel and tested for their antiviral activity against SHIV-SF162P3 vaginal challenge in nonhuman primates. While a combination of 2G12, 2F5, and 4E10 fully prevented vaginal transmission in 10 of 15 treated monkeys, a combination of 246-D and 4B3 nNAb monoclonal antibodies (112) had no impact on HIV acquisition but reduced plasma viral load. The results of this challenge study did not recapitulate the in vitro findings with 246-D and 4B3, which recognize the principal immuno-dominant (PID) domain of gp41, a relatively conserved epitope among HIV-1 subtypes and easily accessible on virions (113), and can efficiently capture a broad range of HIV strains (114). A similar observation was made with another anti-PID antibody, F240, whose vaginal application was able to protect two macaques against neutralization-sensitive SHIV-SF162P4 and decreased viral load in two of the five animals (115). These results indicate that antibodies with distinct neutralization and inhibitory functions differentially affect in vivo HIV acquisition and replication by interfering with early viral replication and dissemination (116).
Two V2-specific MAb, CH58 and CH59, were isolated from B cells of RV144 vaccine recipients (12). The epitope mapping suggested that CH58 and CH59, although not bNAb, bind at or near the site in V2 to which the HIV-1 V1V2 bNAb (e.g., PG9 and CH01) bind (117, 118). These MAb inhibit the interaction of V2 peptide with α4β7 and capture infectious CRF01_AE 92TH023 virions (12). The availability of pathogenic SHIV constructs with HIV-1 E, C, or B envelopes are critically needed for assessing the efficacy of the infusion of these two MAb as well as of passive HIV-1-specific immunoglobulins isolated from vaccine recipients.
PLAUSIBLE NONNEUTRALIZING ANTIBODY MECHANISMS OF PROTECTION
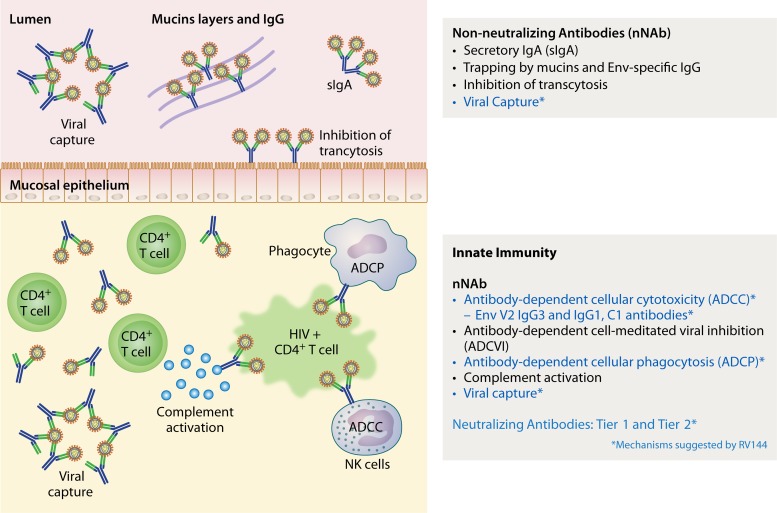
Figure 1 displays the possible vaccine-induced immune mechanisms mediated by antibodies that might be involved in protection against HIV acquisition. It remains difficult to attribute a predominant role to one given mechanism over the others, as it is likely that several mechanisms are involved simultaneously, synergistically, or even perhaps antagonistically. Mechanistic and nonmechanistic (81) functions involved in protection against acquisition may differ by body compartment and be redundant.
FIG 1.
Possible vaccine-induced immune mechanisms of protection against HIV-1 acquisition in humans. To date, viral capture has been demonstrated in plasma but not in mucosal secretions, as mucosal secretions were not collected in RV144. Whether this potential mechanism occurs in mucosal secretions remains to be demonstrated in ongoing clinical trials where mucosal secretions are collected.
HIV-infected women with gp120-specific IgG-mediated ADCC activity in their cervico-vaginal secretions had lower genital viral loads than did women with serum ADCC activity only (119). In another study, HIV-infected women had ADCC activity in their cervico-vaginal secretions without serum ADCC, suggesting that ADCC antibodies were produced locally (120). Breast milk IgG ADCC responses to gp120 but not to virus neutralization correlated with reduced perinatal transmission of HIV-1 (121). In the cervico-vaginal and rectal secretions present in the lumen, one could speculate that nNAb act by inhibiting transcytosis, mostly mediated by secretory IgA (sIgA), as suggested by several animal (109, 110, 122, 123) and human (111, 124) studies. It must be stressed, however, that IgA may be a two-faced antibody. In RV144, levels of Env-specific plasma (monomeric) IgA antibodies were inversely correlated with risk of HIV infection (2) by blocking C1 region-specific IgG-mediated ADCC (10). Conversely, secretory IgA antibodies play a crucial role in protection in the mucosal compartment. Whether HIV vaccines can induce plasma IgG antibodies without IgA antibodies (“excision of the spine on the rose”) or only sIgA (preferentially IgA1) in mucosal tissues remains speculative. The specific mechanisms of inhibition of transcytosis involved have recently been reviewed (104). Whether IgG can also contribute to this inhibition is not entirely clear. Other mechanisms evoked include the possible hindrance of HIV mobility trapping of viruses linked to IgG and IgA within mucin layers outside the cervico-vaginal epithelium (125, 126) and viral capture by sIgA and IgG.
It is speculated that the nNAb detected in the lumen are also present in the mucosal tissues that contain cells capable of effector functions. ADCC and ADCVI have been less explored in rectal secretions and gut tissues in both animal and human studies. These mechanisms may play a critical role for protection against HIV acquisition in MSM populations, with rectal transmission, the highest-risk mode of sexual HIV transmission, at 1:20 to 1:300 infections per exposure (127). Two distinct subsets of NK cells exist in the gut, one localized to intraepithelial spaces (intraepithelial lymphocytes [IEL]) and the other to the lamina propria (LP). The frequency of both subsets of NK cells is reduced in chronic infection, whereas IEL NK cells remained stable in spontaneous controllers with protective killer immunoglobulin-like receptor/human leukocyte antigen genotypes (128). The main mechanism is represented by NK cell-mediated ADCC (reviewed in references 103 and 104), while monocytes might also play a significant role (129, 130). Antibody-dependent cell phagocytosis (ADCP) is generally mediated by the expression of FcγRII on the surfaces of macrophages, immature dendritic cells, and neutrophils, leading to opsonized virus being taken up into endosomes and degradation (131). Mostly described in HIV-infected subjects, the protective role of vaccine-induced ADCP remains to be explored in future vaccine studies (103). Antibody-dependent cell-mediated virus inhibition (ADCVI) is the measure of the net antiviral activity resulting from ADCC, production of β chemokines, and phagocytosis (132). Vaccine-elicited ADCVI-inducing antibodies correlated with protection in SIV or SHIV challenge studies (122, 123, 133, 134). In Vax004, ADCVI activity was associated with lower rates of infection (64). The inhibition of virus replication in mucosal tissues ex vivo along with HIV-specific nNAb in mucosal secretions is currently being explored in RV144 follow-up studies (RV305, RV306, RV328) (135–137).
However, ADCC may be modulated by several factors, including antibody Fc glycosylation, fucosylation of antibody glycans, and IgG subclasses (138). Similarly, acidity and Env-specific IgG were found to enhance virus transcytosis across epithelial cells via FcRn and may facilitate translocation of virus to susceptible target cells following sexual exposure (139). In HIV-infected individuals, ADCC has been shown to exert immune pressure on the virus, leading to escape mutants (140). Whether this phenomenon may apply in the context of vaccine-induced ADCC remains to be shown.
While the main function of HIV-specific NAb is to block viral entry into host cells, nNAb and NAb functions are not exclusive of each other and may act as allies (141, 142). ADCC activity has been described for several bNAb, including b12 (143–145), 2G12 (146), and 2F5 (147). The ability of bNAb to protect macaques against SHIV challenge was mediated by the Fc receptor but not by complement binding alone, suggesting that interaction of Fc-receptor-bearing effector cells with antibody-complexed infected cells is important in reducing virus yield from infected cells (134).
IMPROVING THE INDUCTION OF HIV-SPECIFIC NONNEUTRALIZING ANTIBODIES
The RV144 correlates-of-risk analysis suggests that an increase in the magnitude, affinity, breadth, and, importantly, frequency and durability of V2- and V3-specific antibodies of IgG3 and IgG1 subclasses may confer a higher and more durable rate of protection against HIV-1 infection than other antibodies. Perhaps the first approach to this end is to improve the antigen design of the HIV-1 envelope based on the RV144 results.
ANTIGEN DESIGN
The analysis of A244 gp120 used in RV144 showed that the antigenicity of the gp120 C1 region and the V2 conformational epitopes may be enhanced by the deletion of 11 N terminus amino acids of gp120 (Δ11). This enhanced antigenicity is specific to the CRF01_AE gp120 A244 antigen and may not necessarily apply to other gp120 proteins from the same strain or from different HIV-1 strains. Conformational V1/V2 MAbs gave significantly higher levels of blocking of plasma IgG from A244 Δ11 gp120-immunized animals than that of IgG from animals immunized with unmodified A244 gp120 (148). This improved envelope design is now proposed for the next generation of CRF01_AE- and B-based HIV vaccines in Thailand. The priming agent may also significantly contribute to the shaping of the antibody response to the envelope protein boost, as suggested earlier (100, 102, 149). Head-to-head comparisons of different pox vectors and DNA primings with the same adjuvant-formulated envelope protein boost might help to elucidate the quality of the antibody response, in particular the IgG and IgA subclasses and cytokine profiles induced by the protein boost and the early gene activations that lead to these responses.
MAGNITUDE, FREQUENCY, AND DURABILITY OF ANTIBODIES
The vaccine-induced HIV-1 Env-specific antibody response was not durable in vaccinated HIV-uninfected (66) or HIV-infected (61) humans or NHP (150). Several causes have been evoked, including poorly elicited Tfh responses (151–153), poor Env B-cell receptor signaling strength (154), poor B-cell memory (155), Env-antibody complex killing of FcγRIIb plasma cells before they reach bone marrow (156), and altered catabolism of Ig in plasma cells (157).
HIV vaccine developers have included more-potent adjuvants than the universally used aluminum hydroxide in vaccine candidates to enhance the efficacy of envelope protein antigens, to induce appropriate immune responses not sufficiently generated in the absence of adjuvant, to enhance and/or to shape antigen-specific immune responses, to expand B-cell diversity, and to contribute to dose sparing of the antigen (158, 159). Several adjuvants have been tested in mice (149), NHP, and humans (160), showing a significant benefit of envelope proteins formulated with either MF59 (161), AS02 (162), AS01 (163, 164), or virosomes (111). In AVEG 015, a trial comparing an HIV-1 envelope protein formulated with different adjuvants, the formulation of HIV-1 gp120 with L(MPLA) and alum induced significantly higher levels of neutralizing antibodies and T-cell lymphoproliferation than alum, MF59, or MPLA alone (165). A recent post hoc analysis of the AVEG 015 plasma samples revealed very high titers of cyclic V2 and gp70 V1V2 CaseA2 (subtype B) antibodies as early as after the second protein administration. The frequency of the antibody response to gp70 V1V2 CaseA2 was 100% as early as 2 weeks after the second vaccination and 10 months after the fourth vaccination in the L(MPLA) adjuvant-plus-alum group, while the frequencies were 85% and 100%, respectively, in the alum group. These antibody titers were 5- to 10-fold higher than those observed in the group who received the antigen formulated with alum, were detected at high levels 40 weeks after the fourth vaccination, and were higher than those observed in RV144. Moreover, antibodies were cross-reactive with gp70 scaffolds AE and C (166).
Experiments with nanoparticle malaria vaccines suggest that an increased antigen deposition/retention locally in the tissue drives B-cell responses, enhancing dendritic cell antigen presentation over that obtained with soluble protein immunizations (167). Prolonged antigen presentation mediated by nanoparticle vaccines (168) may also have contributed to the formation of germinal centers (169) with enhanced development of CD4+ Tfh cells (170), which provide critical cytokines and signals required to initiate somatic hypermutation and affinity maturation for effective B-cell memory (171).
The RV144 regimen administered ALVAC HIV and AIDSVAX BE in two different arms. Coimmunization of a DNA prime and an SIV Env protein boost administered in the same muscle was able to significantly augment the magnitude of the Env-specific antibody titers in NHP (172, 173) and rabbits (174). One hypothesis might be that the coadministerion in the same muscle targets antigen-presenting cells (in particular dendritic cells and macrophages) that would reach the same draining lymph nodes, acting synergistically.
CONCLUSIONS
Animal and human data from various viral infections and vaccine studies suggest that functional nNAb that are not neutralizing in vitro are important in protection against viral infection in vivo, without negating in any way the desirability of evoking neutralizing antibodies through vaccination. This was illustrated by the recent RV144 HIV vaccine efficacy trial, which demonstrated that IgG-mediated nNAb were correlated inversely with risk for HIV acquisition while IgA-mediated antibodies were directly correlated with risk. The underlying mechanisms that may have contributed to protection, although not fully understood, are likely mediated by ADCC, tier 1-strain neutralization, viral capture, and ADCP. Whether ADCVI and inhibition of transcytosis may have played a role remains to be explored. Taken together, correlated data from RV144, sieve analysis of RV144 breakthrough viruses, and low-dose intrarectal challenge studies of NHP combined with the presence of these nNAb in mucosal tissues, cervico-vaginal secretions, and rectal secretions challenges the hypothesis that bNAb are the only immune response that might confer protection. The induction of a high frequency and high titers of HIV-specific functional nNAb may represent an attractive hypothesis-testing strategy in future HIV vaccine efficacy trials.
ACKNOWLEDGMENTS
The preparation of the manuscript was supported in part by interagency agreement Y1-AI-2642-12 between the U.S. Army Medical Research and Materiel Command (USAMRMC) and the National Institutes of Allergy and Infectious Diseases. In addition, this work was supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF), and the U.S. Department of Defense (DOD).
We are grateful to Stephanie Stevens, U.S. Military HIV Research Program, for help with designing the figure.
The opinions herein are those of the authors and should not be construed as official or representing the views of the U.S. Department of Defense or the Department of the Army.
Biographies

Jean-Louis Excler is Board certified in pediatrics and tropical medicine (Lyon, France). He worked for several years in Africa as a pediatrician and public health officer for UNICEF. Since 1991, he has been working extensively on HIV vaccine clinical development, first as chief of HIV vaccine clinical development for Sanofi Pasteur and then as scientific director of the Henry M. Jackson Foundation for Advancement of Military Medicine for the U.S. Military HIV Research Program in the United States and in Bangkok, Thailand. He joined the International AIDS Vaccine Initiative in 2002 as the senior medical director and was based in New Delhi, India, and Switzerland (from November 2007 to December 2012). He was responsible for the clinical development of several vaccine candidates in India, the United States, Europe, and Africa. He has been senior consultant for the U.S. Military HIV Research Program since 2010 and collaborates to advance clinical development of lead HIV vaccine candidates in Thailand and Africa.

Julie Ake, Major (P), M.D., M.Sc., is the associate director for vaccine research of the U.S. Military HIV Research Program (MHRP) at the Walter Reed Army Institute of Research. Major (P) Ake attended university at Stanford and then obtained Master's training in health policy at the London School of Hygiene and Tropical Medicine. She attended the University of Washington School of Medicine and completed internal medicine residency at the Madigan Army Medical Center prior to completing infectious disease fellowship training at the Walter Reed Army Medical Center. She served as the Iraq theater consultant for infectious diseases in a 2010 deployment to Baghdad. At MHRP, she leads multiple international HIV-focused cohort studies and a phase I DNA/MVA HIV vaccine trial. Dr. Ake also serves as the product manager for the global HIV vaccine program of U.S. Army Medical Materiel Development Activity.

Merlin Robb is the deputy director for clinical research for the U.S. Military HIV Research Program (MHRP). He is a board-certified pediatric infectious disease specialist with research experience in molecular biology, neutralizing antibody assay development, perinatal and pediatric HIV research, HIV correlates of protection research, acute-infection studies, HIV immuno-therapeutic trials, design and conduct of phase I to III clinical trials, and strategic planning and organization of vaccine development programs for East Africa. He was instrumental in developing MHRP's global research infrastructure in Uganda, Kenya, Tanzania, Nigeria, Mozambique, and Thailand. Dr. Robb has contributed to HIV vaccine prevention and therapy research since 1992, including to the first phase III HIV vaccine trial in Thailand (RV144) to show protection from infection. He joined MHRP in 1990 while on active duty in the U.S. Army and is currently employed by the Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) as the HJF director for MHRP.

Jerome H. Kim, Colonel, M.D., is currently principal deputy of the U.S. Military HIV Research Program (MHRP) and the chief of the Laboratory of Molecular Virology and Pathogenesis, MHRP, at the Walter Reed Army Institute of Research. He is the project manager for HIV Vaccines, U.S. Army Medical Materiel Development Activity, and runs the Army's HIV vaccine advanced-development program. MHRP is an international research program encompassing vaccine research and development, HIV prevention research, and clinical research. Dr. Kim's current research interests include HIV molecular epidemiology, host genetics, and HIV vaccine development. He is a professor in the Department of Medicine, Uniformed Services University of the Health Sciences.

Stanley A. Plotkin is emeritus professor of the University of Pennsylvania. Until 1991, he was professor of pediatrics and microbiology at the University of Pennsylvania, professor of virology at the Wistar Institute, and, at the same time, director of infectious diseases and senior physician at the Children's Hospital of Philadelphia. For 7 years, he was the medical and scientific director of Sanofi Pasteur, Marnes-la-Coquette, France, outside Paris. He is now consultant to vaccine manufacturers and nonprofit research organizations. He is a member of the Institute of Medicine of the National Academy of Sciences and the French Academy of Medicine. His bibliography includes 700 articles, and he has edited several books, including a textbook on vaccines. He developed the rubella vaccine now in standard use throughout the world, is codeveloper of the newly licensed pentavalent rotavirus vaccine, and has worked extensively on the development and application of other vaccines, including vaccines for anthrax, oral polio, rabies, varicella, and cytomegalovirus.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, Berman P, Shen X, Andrews C, O'Connell RJ, Ngauy V, Nitayaphan S, de Souza M, Korber B, Koup R, Bailer RT, Mascola JR, Pinter A, Montefiori D, Haynes BF, Robb ML, Rerks-Ngarm S, Michael NL, Gilbert PB, Kim JH. 2013. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 8:e53629. 10.1371/journal.pone.0053629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. 10.1038/nature10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous shiv challenges in rhesus monkeys. Cell 155:531–539. 10.1016/j.cell.2013.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin SA. 2013. Complex correlates of protection after vaccination. Clin. Infect. Dis. 56:1458–1465. 10.1093/cid/cit048 [DOI] [PubMed] [Google Scholar]
- 7.Koff WC, Russell ND, Walport M, Feinberg MB, Shiver JW, Karim SA, Walker BD, McGlynn MG, Nweneka CV, Nabel GJ. 2013. Accelerating the development of a safe and effective HIV vaccine: HIV vaccine case study for the decade of vaccines. Vaccine 31(Suppl 2):B204–B208. 10.1016/j.vaccine.2012.10.115 [DOI] [PubMed] [Google Scholar]
- 8.Burton DR, Desrosiers RC, Doms RW, Feinberg MB, Gallo RC, Hahn B, Hoxie JA, Hunter E, Korber B, Landay A, Lederman MM, Lieberman J, McCune JM, Moore JP, Nathanson N, Picker L, Richman D, Rinaldo C, Stevenson M, Watkins DI, Wolinsky SM, Zack JA. 2004. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science 303:316. 10.1126/science.1094620 [DOI] [PubMed] [Google Scholar]
- 9.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. 10.1038/nature11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl. Acad. Sci. U. S. A. 110:9019–9024. 10.1073/pnas.1301456110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441. 10.1093/infdis/jis367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 86:11521–11532. 10.1128/JVI.01023-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. 10.1002/cyto.a.21084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3:81ra36. 10.1126/scitranslmed.3002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. 2013. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J. Virol. 87:1708–1719. 10.1128/JVI.02544-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hope TJ. 2011. Moving ahead an HIV vaccine: to neutralize or not, a key HIV vaccine question. Nat. Med. 17:1195–1197. 10.1038/nm.2528 [DOI] [PubMed] [Google Scholar]
- 18.Dimmock NJ. 1984. Mechanisms of neutralization of animal viruses. J. Gen. Virol. 65:1015–1022. 10.1099/0022-1317-65-6-1015 [DOI] [PubMed] [Google Scholar]
- 19.Schmaljohn AL. 2013. Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. Curr. HIV Res. 11:345–353. 10.2174/1570162X113116660057 [DOI] [PubMed] [Google Scholar]
- 20.Schmaljohn AL, Johnson ED, Dalrymple JM, Cole GA. 1982. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature 297:70–72. 10.1038/297070a0 [DOI] [PubMed] [Google Scholar]
- 21.Schmaljohn AL, Kokubun KM, Cole GA. 1983. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology 130:144–154. 10.1016/0042-6822(83)90124-1 [DOI] [PubMed] [Google Scholar]
- 22.Parker MD, Buckley MJ, Melanson VR, Glass PJ, Norwood D, Hart MK. 2010. Antibody to the E3 glycoprotein protects mice against lethal Venezuelan equine encephalitis virus infection. J. Virol. 84:12683–12690. 10.1128/JVI.01345-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould EA, Buckley A, Barrett AD, Cammack N. 1986. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J. Gen. Virol. 67:591–595. 10.1099/0022-1317-67-3-591 [DOI] [PubMed] [Google Scholar]
- 24.Schlesinger JJ, Brandriss MW, Cropp CB, Monath TP. 1986. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 60:1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung KM, Thompson BS, Fremont DH, Diamond MS. 2007. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile virus-infected cells. J. Virol. 81:9551–9555. 10.1128/JVI.00879-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. 2010. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol. J. 7:28. 10.1186/1743-422X-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green S, Rothman A. 2006. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19:429–436. 10.1097/01.qco.0000244047.31135.fa [DOI] [PubMed] [Google Scholar]
- 28.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. 2013. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J. Virol. 87:5512–5522. 10.1128/JVI.03030-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. 2008. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J. Immunol. 181:4168–4176. 10.4049/jimmunol.181.6.4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, Barr IG, Brooks AG, Reading PC, Kent SJ. 2013. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J. Infect. Dis. 208:1051–1061. 10.1093/infdis/jit294 [DOI] [PubMed] [Google Scholar]
- 31.Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, Wherry EJ. 2013. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. 9:e1003207. 10.1371/journal.ppat.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLain L, Dimmock NJ. 1989. Protection of mice from lethal influenza by adoptive transfer of non-neutralizing haemagglutination-inhibiting IgG obtained from the lungs of infected animals treated with defective interfering virus. J. Gen. Virol. 70:2615–2624. 10.1099/0022-1317-70-10-2615 [DOI] [PubMed] [Google Scholar]
- 33.Gupta N, LeGoff J, Chamat S, Mercier-Delarue S, Touzelet O, Power UF, Kazatchkine MD, Simon F, Lacroix-Desmazes S, Bayry J, Kaveri SV. 2013. Affinity-purified respiratory syncytial virus antibodies from intravenous immunoglobulin exert potent antibody-dependent cellular cytotoxicity. PLoS One 8:e69390. 10.1371/journal.pone.0069390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harker JA, Yamaguchi Y, Culley FJ, Tregoning JS, Openshaw PJ. 2014. Delayed sequelae of neonatal respiratory syncytial virus infection are dependent on cells of the innate immune system. J. Virol. 88:604–611. 10.1128/JVI.02620-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefrancois L. 1984. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J. Virol. 51:208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664–1666. 10.1126/science.287.5458.1664 [DOI] [PubMed] [Google Scholar]
- 37.Shedlock DJ, Bailey MA, Popernack PM, Cunningham JM, Burton DR, Sullivan NJ. 2010. Antibody-mediated neutralization of Ebola virus can occur by two distinct mechanisms. Virology 401:228–235. 10.1016/j.virol.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokomori K, Baker SC, Stohlman SA, Lai MM. 1992. Hemagglutinin-esterase-specific monoclonal antibodies alter the neuropathogenicity of mouse hepatitis virus. J. Virol. 66:2865–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aicheler RJ, Wang ECY, Tomasec P, Wilkinson GWG, Stanton RJ. 2013. Potential for natural killer cell-mediated antibody-dependent cellular cytotoxicity for control of human cytomegalovirus. Antibodies 2:617–635. 10.3390/antib2040617 [DOI] [Google Scholar]
- 40.Farrell HE, Shellam GR. 1991. Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J. Gen. Virol. 72:149–156. 10.1099/0022-1317-72-1-149 [DOI] [PubMed] [Google Scholar]
- 41.Inada T, Mims CA. 1985. Association of virulence of murine cytomegalovirus with macrophage susceptibility and with virion-bound non-neutralizing antibody. J. Gen. Virol. 66:879–882. 10.1099/0022-1317-66-4-879 [DOI] [PubMed] [Google Scholar]
- 42.Richter K, Oxenius A. 2013. Non-neutralizing antibodies protect from chronic LCMV infection independently of activating FcgammaR or complement. Eur. J. Immunol. 43:2349–2360. 10.1002/eji.201343566 [DOI] [PubMed] [Google Scholar]
- 43.Moss B. 2011. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 239:8–26. 10.1111/j.1600-065X.2010.00975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres AN, O'Halloran KP, Larson LJ, Schultz RD, Hoover EA. 2010. Feline leukemia virus immunity induced by whole inactivated virus vaccination. Vet. Immunol. Immunopathol. 134:122–131. 10.1016/j.vetimm.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson HL. 2013. Non-neutralizing antibodies in prevention of HIV infection. Expert Opin. Biol. Ther. 13:197–207. 10.1517/14712598.2012.743527 [DOI] [PubMed] [Google Scholar]
- 46.Wren L, Kent SJ. 2011. HIV vaccine efficacy trial: glimmers of hope and the potential role of antibody-dependent cellular cytotoxicity. Hum. Vaccin. 7:466–473. 10.4161/hv.7.4.14123 [DOI] [PubMed] [Google Scholar]
- 47.Srivastava V, Yang Z, Hung IF, Xu J, Zheng B, Zhang MY. 2013. Identification of dominant antibody-dependent cell-mediated cytotoxicity epitopes on the hemagglutinin antigen of pandemic H1N1 influenza virus. J. Virol. 87:5831–5840. 10.1128/JVI.00273-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, Ng JC, Chan JF, Chan KH, Yuen KY. 2012. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin. Vaccine Immunol. 19:1012–1018. 10.1128/CVI.00081-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haynes L, Szaba FM, Eaton SM, Kummer LW, Lanthier PA, Petell AH, Duso DK, Luo D, Lin JS, Lefebvre JS, Randall TD, Johnson LL, Kohlmeier JE, Woodland DL, Smiley ST. 2012. Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections. J. Immunol. 189:4921–4929. 10.4049/jimmunol.1201916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng N, Lawton JA, Gilbert J, Kuklin N, Vo P, Prasad BV, Greenberg HB. 2002. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J. Clin. Invest. 109:1203–1213. 10.1172/JCI14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCoy LE, Weiss RA. 2013. Neutralizing antibodies to HIV-1 induced by immunization. J. Exp. Med. 210:209–223. 10.1084/jem.20121827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pillay V, Gan HK, Scott AM. 2011. Antibodies in oncology. N. Biotechnol. 28:518–529. 10.1016/j.nbt.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 53.Scott AM, Wolchok JD, Old LJ. 2012. Antibody therapy of cancer. Nat. Rev. Cancer. 12:278–287. 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 54.Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. 2012. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget 3:744–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. 2014. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505:502–508. 10.1038/nature12893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894–1905. 10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671. 10.1086/508748 [DOI] [PubMed] [Google Scholar]
- 59.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654–665. 10.1086/428404 [DOI] [PubMed] [Google Scholar]
- 60.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, Sinangil F, Burke D, Berman PW. 2005. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 191:666–677. 10.1086/428405 [DOI] [PubMed] [Google Scholar]
- 61.Gilbert PB, Ackers ML, Berman PW, Francis DP, Popovic V, Hu DJ, Heyward WL, Sinangil F, Shepherd BE, Gurwith M. 2005. HIV-1 virologic and immunologic progression and initiation of antiretroviral therapy among HIV-1-infected subjects in a trial of the efficacy of recombinant glycoprotein 120 vaccine. J. Infect. Dis. 192:974–983. 10.1086/432734 [DOI] [PubMed] [Google Scholar]
- 62.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 63.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 369:2083–2092. 10.1056/NEJMoa1310566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forthal DN, Gilbert PB, Landucci G, Phan T. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J. Immunol. 178:6596–6603. 10.4049/jimmunol.178.10.6596 [DOI] [PubMed] [Google Scholar]
- 65.Gilbert PB, Berger JO, Stablein D, Becker S, Essex M, Hammer SM, Kim JH, Degruttola VG. 2011. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: a case study for statistical issues in efficacy trials. J. Infect. Dis. 203:969–975. 10.1093/infdis/jiq152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, Paris RM, Chiu J, Adams E, Francis D, Gurunathan S, Tartaglia J, Gilbert P, Stablein D, Michael NL, Kim JH. 2012. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect. Dis. 12:531–537. 10.1016/S1473-3099(12)70088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rerks-Ngarm S, Paris RM, Chunsutthiwat S, Premsri N, Namwat C, Bowonwatanuwong C, Li SS, Kaewkungkal J, Trichavaroj R, Churikanont N, de Souza MS, Andrews C, Francis D, Adams E, Flores J, Gurunathan S, Tartaglia J, O'Connell RJ, Eamsila C, Nitayaphan S, Ngauy V, Thongcharoen P, Kunasol P, Michael NL, Robb ML, Gilbert PB, Kim JH. 2013. Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquired HIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX B/E. J. Infect. Dis. 207:1195–1205. 10.1093/infdis/jis478 [DOI] [PubMed] [Google Scholar]
- 68.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877–20882. 10.1073/pnas.0911796106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301–309. 10.1038/ni1566 [DOI] [PubMed] [Google Scholar]
- 70.de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, Nuntapinit B, Valencia-Micolta A, Thelian D, Nitayaphan S, Pitisuttithum P, Paris RM, Kaewkungwal J, Michael NL, Rerks-Ngarm S, Mathieson B, Marovich M, Currier JR, Kim JH. 2012. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J. Immunol. 188:5166–5176. 10.4049/jimmunol.1102756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, Brown AE, Gurunathan S, Tartaglia J, Heyward WL, McNeil JG, Birx DL, de Souza MS. 2005. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine 23:2522–2529. 10.1016/j.vaccine.2004.10.028 [DOI] [PubMed] [Google Scholar]
- 72.Paris R, Bejrachandra S, Thongcharoen P, Nitayaphan S, Pitisuttithum P, Sambor A, Gurunathan S, Francis D, Ratto-Kim S, Karnasuta C, de Souza MS, Polonis VR, Brown AE, Kim JH, Stephens HA. 2012. HLA class II restriction of HIV-1 clade-specific neutralizing antibody responses in ethnic Thai recipients of the RV144 prime-boost vaccine combination of ALVAC-HIV and AIDSVAX((R)) B/E. Vaccine 30:832–836. 10.1016/j.vaccine.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 73.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. 2012. The Thai phase III HIV type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res. Hum. Retroviruses 28:1444–1457. 10.1089/aid.2012.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zolla-Pazner S, Decamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O'Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 9:e87572. 10.1371/journal.pone.0087572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomaras G, Shen X, Seaton K, Janes H, Grove D, deCamp A, Fong Y, Liao H, Yang Z, Xu T, Kim JH, Michael NL, Bailer RT, Ferrari G, Mascola J, Koup RA, Nabel G, Corey R, Karuna S, Montefiori DC, McElrath MJ, Haynes BF, Golbert P, Graham BS, Sobieszczyk M, Hammer S. 2013. Vaccine induced antibody responses in HVTN 505, a phase IIb HIV-1 efficacy trial, abstr PL04.04. AIDS Vaccine 2013, Barcelona, Spain [Google Scholar]
- 76.Rolland M, Edlefsen PT, Gottardo R, Montefiori DC, Zolla-Pazner S, Moody A, Liao LH, Liu P, Tomaras GD, Haynes BF, Bailer RT, Koup RA, Mascola JR, Shen X, Korber BT, Tovanabutra S, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Robb ML, Michael NL, Mullins JI, Gilbert PB, Kim JH. 2013. Genetic and immunological evidence for a role of Env-V3 antibodies in the RV144 trial, abstr P03.73 LB. AIDS Vaccine 2013, Barcelona, Spain [Google Scholar]
- 77.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. 10.1371/journal.pone.0075665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kijak GH, Tovanabutra S, Rerks-Ngarm S, Nitayaphan S, Eamsila C, Kunasol P, Khamboonruang C, Thongcharoen P, Namwat C, Premsri N, Benenson M, Morgan P, Bose M, Sanders-Buell E, Paris R, Robb ML, Birx DL, De Souza MS, McCutchan FE, Michael NL, Kim JH. 2013. Molecular evolution of the HIV-1 Thai epidemic between the time of RV144 immunogen selection to the execution of the vaccine efficacy trial. J. Virol. 87:7265–7281. 10.1128/JVI.03070-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitisuttithum P, Supachai R-N, O'Connell RJ, Kim JH, Excler J-L. 2013. An HIV vaccine for South-East Asia—opportunities and challenges. Vaccines 1:348–366. 10.3390/vaccines1030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu P, Yates NL, Shen X, Bonsignori M, Moody MA, Liao HX, Fong Y, Alam SM, Overman RG, Denny T, Ferrari G, Ochsenbauer C, Kappes JC, Polonis VR, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Montefiori DC, Gilbert P, Michael NL, Kim JH, Haynes BF, Tomaras GD. 2013. Infectious virion capture by HIV-1 gp120-specific IgG from RV144 vaccinees. J. Virol. 87:7828–7836. 10.1128/JVI.02737-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plotkin SA, Gilbert PB. 2012. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 54:1615–1617. 10.1093/cid/cis238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ampol S, Pattanapanyasat K, Sutthent R, Permpikul P, Kantakamalakul W. 2012. Comprehensive investigation of common antibody-dependent cell-mediated cytotoxicity antibody epitopes of HIV-1 CRF01_AE gp120. AIDS Res. Hum. Retroviruses 28:1250–1258. 10.1089/aid.2011.0346 [DOI] [PubMed] [Google Scholar]
- 83.Lewis GK, Guan Y, Kamin-Lewis R, Sajadi M, Pazgier M, Devico AL. 2014. Epitope target structures of Fc-mediated effector function during HIV-1 acquisition. Curr. Opin. HIV AIDS 9:263–270. 10.1097/COH.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. 2014. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J. Virol. 88:7715–7726. 10.1128/JVI.00156-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 85:7029–7036. 10.1128/JVI.00171-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moody MA, Yates NL, Amos JD, Drinker MS, Eudailey JA, Gurley TC, Marshall DJ, Whitesides JF, Chen X, Foulger A, Yu JS, Zhang R, Meyerhoff RR, Parks R, Scull JC, Wang L, Vandergrift NA, Pickeral J, Pollara J, Kelsoe G, Alam SM, Ferrari G, Montefiori DC, Voss G, Liao HX, Tomaras GD, Haynes BF. 2012. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J. Virol. 86:7496–7507. 10.1128/JVI.00426-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. 10.1126/science.1207227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang ZY, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. 10.1126/science.1207532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura GR, Fonseca DP, O'Rourke SM, Vollrath AL, Berman PW. 2012. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PLoS One 7:e39045. 10.1371/journal.pone.0039045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mayr LM, Cohen S, Spurrier B, Kong XP, Zolla-Pazner S. 2013. Epitope mapping of conformational V2-specific anti-HIV human monoclonal antibodies reveals an immunodominant site in V2. PLoS One 8:e70859. 10.1371/journal.pone.0070859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao M, Peachman KK, Kim J, Gao G, Alving CR, Michael NL, Rao VB. 2013. HIV-1 variable loop 2 and its importance in HIV-1 infection and vaccine development. Curr. HIV Res. 11:427–438. 10.2174/1570162X113116660064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorse GJ, Patel GB, Mandava M, Berman PW, Belshe RB. 1999. MN and IIIB recombinant glycoprotein 120 vaccine-induced binding antibodies to native envelope glycoprotein of human immunodeficiency virus type 1 primary isolates. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. AIDS Res. Hum. Retroviruses 15:921–930 [DOI] [PubMed] [Google Scholar]
- 93.Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, Walker BD, Moore JP. 2010. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res. Hum. Retroviruses 26:445–458. 10.1089/aid.2009.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Perignon JL, Druilhe P. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4:e320. 10.1371/journal.pmed.0040320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tebo AE, Kremsner PG, Luty AJ. 2001. Plasmodium falciparum: a major role for IgG3 in antibody-dependent monocyte-mediated cellular inhibition of parasite growth in vitro. Exp. Parasitol. 98:20–28. 10.1006/expr.2001.4619 [DOI] [PubMed] [Google Scholar]
- 96.Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, Renia L, Leo YS, Ng LF. 2012. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 205:1147–1154. 10.1093/infdis/jis033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ljunggren K, Broliden PA, Morfeldt-Manson L, Jondal M, Wahren B. 1988. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin. Exp. Immunol. 73:343–347 [PMC free article] [PubMed] [Google Scholar]
- 98.Cavacini LA, Kuhrt D, Duval M, Mayer K, Posner MR. 2003. Binding and neutralization activity of human IgG1 and IgG3 from serum of HIV-infected individuals. AIDS Res. Hum. Retroviruses 19:785–792. 10.1089/088922203769232584 [DOI] [PubMed] [Google Scholar]
- 99.Seaton K, Yates N, Williams W, Liao L, A. decamp A, Fong Y, Montefiori D, Spearman P, Elizaga M, Barnett S, Koutsoukos M, Bourguignon P, Protocol Team V. McElrath J, Corey L, Michael NL, Pitisuttithum P, Rerks-Ngarm S, Kim J, Voss G, Gilbert G, Haynes B, Tomaras G. 2013. Human HIV-1 vaccine induced antibody durability and Env IgG3 responses, abstr P13.71 LB. AIDS Vaccine 2013, Barecelona, Spain [Google Scholar]
- 100.Yates NL, Liao HX, Fong Y, Decamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 6:228ra239. 10.1126/scitranslmed.3007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 6:228ra238. 10.1126/scitranslmed.3007736 [DOI] [PubMed] [Google Scholar]
- 102.Teigler JE, Phogat S, Franchini G, Hirsch VM, Michael NL, Barouch DH. 2014. The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys. J. Virol. 88:1809–1814. 10.1128/JVI.02386-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Forthal D, Hope TJ, Alter G. 2013. New paradigms for functional HIV-specific nonneutralizing antibodies. Curr. Opin. HIV AIDS 8:393–401. 10.1097/COH.0b013e328363d486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vargas-Inchaustegui DA, Robert-Guroff M. 2013. Fc receptor-mediated immune responses: new tools but increased complexity in HIV prevention. Curr. HIV Res. 11:407–420. 10.2174/1570162X113116660063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Zolla-Pazner S, Evans DT, Li Y, Gottardo R, Dai JY, Janes H, Morris D, Fong Y, Edlefsen PT, Li F, Magaret CA, Frahm N, Alpert MD, Rerks-Ngarm S, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Robb ML, O'Connell RJ, Michael NL, Kim JH, McElrath MJ, Geraghty DE. 2013. Association of FcγRIIC polymorphism with vaccine efficacy and correlates of HIV-1 infection risk in RV144, abstr P06.20 LB. AIDS Vaccine 2013, Barcelona, Spain [Google Scholar]
- 106.Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. 2005. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J. Acquir. Immune Defic. Syndr. 38:124–134. 10.1097/00126334-200502010-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schifanella L, Gordon S, Vaccari M, Binello N, Caccuri F, Blackburn M, Fenizia C, Doster M, Pegu P, Liynage N, Shen X, Tomaras G, Rao M, Ferrari G, Venzon D, Stablein D, Barnett S, Tartaglia J, Franchini G. 2013. MF59 and ALUM, in combination with an ALVAC-SIV/gp120 vaccine, induce plasmablasts that differ in the expression of homing markers, abstr OA05.05. AIDS Vaccine 2013, Barcelona, Spain [Google Scholar]
- 108.Belyakov IM, Ahlers JD. 2012. Mucosal immunity and HIV-1 infection: applications for mucosal AIDS vaccine development. Curr. Top. Microbiol. Immunol. 354:157–179. 10.1007/82_2010_119 [DOI] [PubMed] [Google Scholar]
- 109.Watkins JD, Sholukh AM, Mukhtar MM, Siddappa NB, Lakhashe SK, Kim M, Reinherz EL, Gupta S, Forthal DN, Sattentau QJ, Villinger F, Corti D, Ruprecht RM. 2013. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 27:F13–F20. 10.1097/QAD.0b013e328360eac6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. 2011. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280. 10.1016/j.immuni.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 111.Leroux-Roels G, Maes C, Clement F, van Engelenburg F, van den Dobbelsteen M, Adler M, Amacker M, Lopalco L, Bomsel M, Chalifour A, Fleury S. 2013. Randomized phase I: safety, immunogenicity and mucosal antiviral activity in young healthy women vaccinated with HIV-1 Gp41 P1 peptide on virosomes. PLoS One 8:e55438. 10.1371/journal.pone.0055438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, Moog C. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177–6181. 10.1128/JVI.02625-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moore PL, Crooks ET, Porter L, Zhu P, Cayanan CS, Grise H, Corcoran P, Zwick MB, Franti M, Morris L, Roux KH, Burton DR, Binley JM. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80:2515–2528. 10.1128/JVI.80.5.2515-2528.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burrer R, Haessig-Einius S, Aubertin AM, Moog C. 2005. Neutralizing as well as non-neutralizing polyclonal immunoglobulin (Ig)G from infected patients capture HIV-1 via antibodies directed against the principal immunodominant domain of gp41. Virology 333:102–113. 10.1016/j.virol.2004.12.034 [DOI] [PubMed] [Google Scholar]
- 115.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186. 10.1073/pnas.1103012108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G, Heyndrickx L, Van Dorsselaer A, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C, Shattock RJ, Le Grand R. 2014. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 7:46–56. 10.1038/mi.2013.23 [DOI] [PubMed] [Google Scholar]
- 117.Doria-Rose NA, Georgiev I, O'Dell S, Chuang GY, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, Burton DR, Koff WC, Kwong PD, Mascola JR. 2012. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J. Virol. 86:8319–8323. 10.1128/JVI.00696-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. 10.1038/nature10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nag F, De A, Banerjee K, Chatterjee G. 2013. Non healing leg ulcer infected with Stenotrophomonas maltophilia: first reported case from India. Int. Wound J. 10:356–358. 10.1111/j.1742-481X.2012.00938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Battle-Miller K, Eby CA, Landay AL, Cohen MH, Sha BE, Baum LL. 2002. Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1-infected women. J. Infect. Dis. 185:439–447. 10.1086/338828 [DOI] [PubMed] [Google Scholar]
- 121.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 8:e1002739. 10.1371/journal.ppat.1002739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, Robert-Guroff M. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J. Virol. 83:791–801. 10.1128/JVI.01672-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 84:7161–7173. 10.1128/JVI.00410-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, Kimani J, Lopalco L, Piconi S, Bwayo JJ, Plummer F, Clerici M, Hinkula J. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 165:5170–5176. 10.4049/jimmunol.165.9.5170 [DOI] [PubMed] [Google Scholar]
- 125.Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, Gioia CJ, Spongberg EJ, Kauffman SM, McRaven MD, Lakougna HY, Hammond C, Kiser PF, Hope TJ. 2013. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 6:427–434. 10.1038/mi.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. 2013. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS One 8:e76176. 10.1371/journal.pone.0076176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shaw GM, Hunter E. 2012. HIV transmission. Cold Spring Harb. Perspect. Med. 2:a006965. 10.1101/cshperspect.a006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, Martin JN, Deeks SG, Hunt PW, Alter G. 2012. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 5:30–40. 10.1038/mi.2011.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smalls-Mantey A, Connors M, Sattentau QJ. 2013. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PLoS One 8:e74858. 10.1371/journal.pone.0074858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kramski M, Parsons MS, Stratov I, Kent SJ. 2013. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr. HIV Res. 11:388–406. 10.2174/1570162X113116660061 [DOI] [PubMed] [Google Scholar]
- 131.Holl V, Peressin M, Moog C. 2009. Antibody-mediated Fcgamma receptor-based mechanisms of HIV inhibition: recent findings and new vaccination strategies. Viruses 1:1265–1294. 10.3390/v1031265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Forthal DN, Landucci G, Daar ES. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953–6961. 10.1128/JVI.75.15.6953-6961.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 182:3718–3727. 10.4049/jimmunol.0803115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- 135.Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065–1075. 10.1084/jem.20022212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fletcher PS, Elliott J, Grivel JC, Margolis L, Anton P, McGowan I, Shattock RJ. 2006. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 20:1237–1245. 10.1097/01.aids.0000232230.96134.80 [DOI] [PubMed] [Google Scholar]
- 137.Herrera C, Schuetz A, Olejniczak N, Assawadarachai V, Karasavvas N, Nitayaphan S, Kaewkungwal J, Ngauy V, Pitisuttithum P, Rerks-Ngarm S, Michael NL, O'Connell RJ, Excler JL, Shattock RJ, Kim JH. 2013. Preliminary evaluation of mucosal immune responses with mucosal tissue explants in humans vaccinated with ALVAC/AIDSVAX B/E during the ongoing RV305 trial, abstr P08.26 B. AIDS Vaccine 2013, Barcelona, Spain [Google Scholar]
- 138.Wren LH, Stratov I, Kent SJ, Parsons MS. 2013. Obstacles to ideal anti-HIV antibody-dependent cellular cytotoxicity responses. Vaccine 31:5506–5517. 10.1016/j.vaccine.2013.08.035 [DOI] [PubMed] [Google Scholar]
- 139.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. 2013. The neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 9:e1003776. 10.1371/journal.ppat.1003776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. 2011. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc. Natl. Acad. Sci. U. S. A. 108:7505–7510. 10.1073/pnas.1016048108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mascola JR. 2007. HIV/AIDS: allied responses. Nature 449:29–30. 10.1038/449029a [DOI] [PubMed] [Google Scholar]
- 142.Corti D, Lanzavecchia A. 2013. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 31:705–742. 10.1146/annurev-immunol-032712-095916 [DOI] [PubMed] [Google Scholar]
- 143.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. 2001. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 75:12161–12168. 10.1128/JVI.75.24.12161-12168.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. 2011. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcgamma receptors to define the role of effector functions in protection against HIV. J. Virol. 85:10572–10581. 10.1128/JVI.05541-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. 2012. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 86:6189–6196. 10.1128/JVI.00491-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Forthal DN, Gach JS, Landucci G, Jez J, Strasser R, Kunert R, Steinkellner H. 2010. Fc-glycosylation influences Fcgamma receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J. Immunol. 185:6876–6882. 10.4049/jimmunol.1002600 [DOI] [PubMed] [Google Scholar]
- 147.Tudor D, Bomsel M. 2011. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRI-dependent manner. AIDS 25:751–759. 10.1097/QAD.0b013e32834507bd [DOI] [PubMed] [Google Scholar]
- 148.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J. Virol. 87:1554–1568. 10.1128/JVI.00718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buglione-Corbett R, Pouliot K, Marty-Roix R, West K, Wang S, Lien E, Lu S. 2013. Serum cytokine profiles associated with specific adjuvants used in a DNA prime-protein boost vaccination strategy. PLoS One 8:e74820. 10.1371/journal.pone.0074820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sundling C, Martinez P, Soldemo M, Spangberg M, Bengtsson KL, Stertman L, Forsell MN, Karlsson Hedestam GB. 2013. Immunization of macaques with soluble HIV type 1 and influenza virus envelope glycoproteins results in a similarly rapid contraction of peripheral B-cell responses after boosting. J. Infect. Dis. 207:426–431. 10.1093/infdis/jis696 [DOI] [PubMed] [Google Scholar]
- 151.Pillai S. 2013. Love the one you're with: the HIV, B cell and TFH cell triangle. Nat. Med. 19:401–402. 10.1038/nm.3141 [DOI] [PubMed] [Google Scholar]
- 152.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. 2012. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 122:3281–3294. 10.1172/JCI63039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 19:494–499. 10.1038/nm.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amanna IJ, Slifka MK. 2010. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 236:125–138. 10.1111/j.1600-065X.2010.00912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, Redfield RR, DeVico AL, Gallo RC, Lewis GK. 2009. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 106:3952–3957. 10.1073/pnas.0813392106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. 2007. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8:419–429. 10.1038/ni1440 [DOI] [PubMed] [Google Scholar]
- 157.Bumbaca D, Boswell CA, Fielder PJ, Khawli LA. 2012. Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J. 14:554–558. 10.1208/s12248-012-9369-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat. Med. 19:1597–1608. 10.1038/nm.3409 [DOI] [PubMed] [Google Scholar]
- 159.Alving CR, Peachman KK, Rao M, Reed SG. 2012. Adjuvants for human vaccines. Curr. Opin. Immunol. 24:310–315. 10.1016/j.coi.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mastelic B, Garcon N, Del Giudice G, Golding H, Gruber M, Neels P, Fritzell B. 2013. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biologicals 41:458–468. 10.1016/j.biologicals.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 161.O'Hagan DT, Ott GS, De Gregorio E, Seubert A. 2012. The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine 30:4341–4348. 10.1016/j.vaccine.2011.09.061 [DOI] [PubMed] [Google Scholar]
- 162.Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, Voss G, Koutsoukos M, Pedneault L, Vandepapeliere P, McElrath MJ, Spearman P, Fuchs JD, Koblin BA, Blattner WA, Frey S, Baden LR, Harro C, Evans T. 2007. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 25:510–518. 10.1016/j.vaccine.2006.07.050 [DOI] [PubMed] [Google Scholar]
- 163.Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, Janssens M, Bourguignon P, Cohen K, Altfeld M, Vandepapeliere P, Pedneault L, McNally L, Leroux-Roels G, Voss G. 2010. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three adjuvant systems. Vaccine 28:7016–7024. 10.1016/j.vaccine.2010.08.035 [DOI] [PubMed] [Google Scholar]
- 164.Garcon N, Van Mechelen M. 2011. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 10:471–486. 10.1586/erv.11.29 [DOI] [PubMed] [Google Scholar]
- 165.McElrath MJ. 1995. Selection of potent immunological adjuvants for vaccine construction. Semin. Cancer Biol. 6:375–385. 10.1016/1044-579X(95)90007-1 [DOI] [PubMed] [Google Scholar]
- 166.Rao MOS, Peachman K, Padilla-Sanchez V, Yamini G, Jobe O, Matyas G, Zolla-Pazner S, Cardozo T, Pinter A, Kim J, Rao V, Alving A. 2014. Potent V2-specific antibodies induced in humans using liposome-encapsulated HIV-1 gp120 recognize a well-exposed V2 epitope on envelope trimer, abstr 2049. Keystone Symp. Mol. Cell. Biol. HIV Vaccin. Adapt. Immun. Beyond, Banff, Alberta, Canada [Google Scholar]
- 167.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ. 2011. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 10:243–251. 10.1038/nmat2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. 2014. Nanoparticle vaccines. Vaccine 32:327–337. 10.1016/j.vaccine.2013.11.069 [DOI] [PubMed] [Google Scholar]
- 169.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. 2012. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. U. S. A. 109:1080–1085. 10.1073/pnas.1112648109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. 2010. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33:241–253. 10.1016/j.immuni.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nutt SL, Tarlinton DM. 2011. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 12:472–477 [DOI] [PubMed] [Google Scholar]
- 172.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Hannaman D, Reed SG, Felber BK, Pavlakis GN. 2013. HIV/SIV DNA vaccine combined with protein in aimmunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine 31:3747–3755. 10.1016/j.vaccine.2013.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, Bess JW, Jr, Piatak M, Jr, Lifson JD, Williams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon DJ, Hirsch VM, Felber BK, Pavlakis GN. 2013. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc. Natl. Acad. Sci. U. S. A. 110:2975–2980. 10.1073/pnas.1215393110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pissani F, Malherbe DC, Schuman JT, Robins H, Park BS, Krebs SJ, Barnett SW, Haigwood NL. 2014. Improvement of antibody responses by HIV envelope DNA and protein co-immunization. Vaccine 32:507–513. 10.1016/j.vaccine.2013.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]