Abstract
In the last decade, peanut allergy has increased substantially. Significant differences in the prevalence among different countries are attributed to the type of thermal processing. In spite of the high prevalence and the severe reaction induced by peanuts, there is no immunotherapy available. The aim of this work was to evaluate the potential application of poly(anhydride) nanoparticles (NPs) as immunoadjuvants for peanut oral immunotherapy. NPs loaded with raw or roasted peanut proteins were prepared by a solvent displacement method and dried by either lyophilization or spray-drying. After physicochemical characterization, their adjuvant capacity was evaluated after oral immunization of C57BL/6 mice. All nanoparticle formulations induced a balanced TH1 and TH2 antibody response, accompanied by low specific IgE induction. In addition, oral immunization with spray-dried NPs loaded with peanut proteins was associated with a significant decrease in splenic TH2 cytokines (interleukin 4 [IL-4], IL-5, and IL-6) and enhancement of both TH1 (gamma interferon [IFN-γ]) and regulatory (IL-10) cytokines. In conclusion, oral immunization with poly(anhydride) NPs, particularly spray-dried formulations, led to a pro-TH1 immune response.
INTRODUCTION
Among food allergies, peanut allergy represents a major health issue for many reasons. Peanuts and nuts are responsible for the majority of anaphylactic reactions among children and, unlike allergies to cow milk, very few children outgrow this allergy (1). In addition, there has been an alarming increase in peanut sensitization in countries where it used to be a rarity. The geographical differences in peanut allergy prevalences are attributed to the types of processing which might affect the peanut allergenicity (2). Thermal processing may affect food in a manner that may induce allergen expression and/or the contrary, with the loss of epitopes altering both the immunogenicity and the allergenicity of the food proteins. Peanuts are consumed after roasting or boiling, as peanut butter, and as ingredients in a wide range of food products. Although the protein composition seems to be very consistent among different peanut species, there are geographical differences in peanut allergy prevalence. Furthermore, it is known that roasting increases the allergenicity of peanut proteins due to the Maillard reaction, which leads to the formation of advanced glycation end (AGE) products. Further studies have demonstrated a correlation between these products and increased IgE binding. Also, in vitro studies have demonstrated higher IgE binding of allergens in roasted peanut extract than of those in boiled or fried peanuts (3, 4).
Until recent years, the only treatment option for peanut allergy was strict avoidance and an emergency plan in case of accidental exposures (5). In this context, oral induced immunotherapy is emerging as one of the most promising approaches to treat this disease. However, in spite of its efficacy, it produces side effects and systemic reactions. At the same time, when available, it should be offered to the community through standardized doses and protocols (6–8). Thus, research on increasing the efficacy and safety of immunotherapy is clearly needed. Several strategies are currently under study to decrease these problems (9). Among these, particular attention has been focused on nanoparticle-based allergen-delivery systems (10–12). The synergistic value of the polymeric nanoparticles includes the protection of allergenic proteins from degradation in the gastrointestinal tract (13, 14) and the efficient antigen uptake by M cells, improving vaccine efficacy after oral administration.
Poly(anhydride) nanoparticles have been successfully associated with several proteins, including allergens (15–17) and bacterial antigens (18, 19), increasing their ability to induce protective immune responses after mucosal immunization. Likewise, previous studies had described the bioadhesive properties of poly(anhydride) nanoparticles (20). Thus, these polymeric systems present an enhanced interaction with the gut mucosa, a key factor for the induction of strong mucosal immune responses (20–22). Moreover, it has been demonstrated that the decoration of the surface of poly(anhydride) nanoparticles with specific ligands (i.e., mannosamine or thiamine) increased their recognition and/or their capture by antigen-presenting cells (APCs) (18, 19, 23), allowing an effective immune response associated with an increased TH1 profile (24). Accordingly, previous studies of our research group demonstrated that the incorporation of raw peanut proteins into poly(anhydride) nanoparticles enhances their immunogenic properties after intradermal immunization (12). However, oral delivery offers an alternative means of treatment to the subcutaneous or intradermic (i.d.) routes. Thus, the aim of the present work was to evaluate the potential application of these nanoparticles for oral immunotherapy. For this purpose, poly(anhydride) nanoparticles loaded with either raw or roasted peanut proteins were developed in order to study the immunologic and allergenic profiles induced after oral immunization in a murine animal model (C57BL/6 mice). Results indicated that oral immunization with poly(anhydride) nanoparticles, particularly spray-dried formulations, led to a pro-TH1 immune response.
MATERIALS AND METHODS
Preparation of poly(anhydride) nanoparticles.
Poly(methyl vinyl ether-co-maleic anhydride) (PVM/MA) or poly(anhydride) nanoparticles (NPs) were prepared by a solvent displacement method. After purification of nanoparticle suspensions, the formulations were dried by freeze-drying (12) or spray-drying (25). These polymeric nanoparticles were loaded with the peanut extracts (either raw or roasted [Ro]) (Diater Laboratories, Madrid, Spain).
(i) Lyophilized nanoparticles.
Briefly, in order to prepare the lyophilized nanoparticles (NP-L), peanut extract (3 mg of raw or Ro) was dispersed in purified water (pH 3.0). After ultrasonication (Microson), the peanut extract was resuspended in acetone. The resulting dispersion was incorporated in PVM/MA-acetone. After incubation (magnetic stirring for 45 min at room temperature [RT]), the nanoparticles were obtained by the addition of 20 ml of a mixture of ethanol and water (1:1 by volume). The organic solvents were eliminated under reduced pressure (Büchi R-144; Switzerland). Finally, the nanoparticle suspensions were purified twice by centrifugal filtration (3,000 × g for 20 min) and freeze-drying using an aqueous solution containing sucrose (5%, wt/vol) as a cryoprotector (Genesis 12EL; Virtis, USA). The lyophilized formulations used in this study were named NP-Raw-L (lyophilized nanoparticles loaded with raw peanut proteins [PE]) and NP-Ro-L (lyophilized nanoparticles loaded with roasted peanut proteins). Empty lyophilized nanoparticles (NP-L) were prepared in the same way, in the absence of peanut extract proteins (PE), and included as negative controls.
(ii) Spray-dried nanoparticles.
Spray-dried PE-loaded nanoparticles were prepared as previously described (12) and dried in a mini spray-dryer (Büchi B191; Büchi Labortechnik AG, Switzerland). Control spray-dried nanoparticles (NP-SD) were prepared in the same way, in the absence of PE. Spray-dried nanoparticle formulations were identified as NP-Raw-SD (spray-dried nanoparticles loaded with raw peanut proteins) and NP-Ro-SD (spray-dried nanoparticles loaded with roasted peanut proteins).
Characterization of nanoparticles.
(i) Size, zeta potential, and morphology. The size and zeta potential of nanoparticles were determined by photon correlation spectroscopy (PCS) and electrophoretic laser Doppler anemometry, respectively, using a Zetamaster analyzer system (Malvern Instruments, United Kingdom). The average particle size is expressed as the volume mean diameter (Vmd) in nanometers (nm), and the average surface charge as mV. The morphological characteristics of nanoparticles were assessed by scanning electron microscopy (SEM) (Emitech K550 equipment; United Kingdom).
(ii) Yield.
The amount of polymer transformed into nanoparticles was determined by gravimetry, as previously described (12, 26). The yield was calculated from the difference between the initial amount of the polymer used to prepare the nanoparticles and the weight of the either lyophilized or spray-dried samples.
(iii) Protein loading and encapsulation efficiency.
The amount of peanut protein associated with the nanoparticles was measured using the bicinchoninic acid method (MicroBCA), as previously described (27). Each sample was assayed in triplicate and results of PE loading were expressed as the amount of protein (in μg) per mg NP. Similarly, the encapsulation efficiency (EE) was calculated as previously described (12): EE(%) = (Q associated/Q initial) × 100, where Qinitial was the initial amount of PE added per mg of polymer that forms the nanoparticles and Qassociated was the amount of loaded PE per mg of nanoparticles, which was calculated by MicroBCA.
(iv) Structural integrity and antigenicity of the loaded peanut proteins.
To evaluate the effects of the manufacturing process on the integrity and antigenicity of peanut proteins, SDS-PAGE and immunoblotting were performed, as previously described (12). The apparent molecular masses of peanut proteins were determined by comparison with standard molecular weight markers. Pooled sera from four patients allergic to peanut were used as quality controls of immunoblotting. Patients included had a history of anaphylaxis upon peanut ingestion, positive skin prick test against peanut extract (Bial-Aristegui, Bilbao, Spain), and specific IgE against peanut (Thermo Fisher Scientific, Uppsala, Sweden).
In vivo studies. (i) Animals.
Experiments were performed in compliance with the regulations of the Ethics Committee of the University of Navarra in line with the European legislation on animal experiments (approved protocol 048/09).
(ii) Oral immunization.
Female C57BL/6 mice (Harlan Interfauna Ibérica, Spain), 8 weeks old (weight, 20 ± 1 g), were randomly divided into nine experimental groups (n = 6 per each experimental group). Oral immunization was performed by oral gavage of animals with a single dose of free peanut proteins (1 mg) or incorporated into one of the nanoparticle formulations. Thus, animals were immunized with 200 μl (125 mg/ml) of different nanoparticle suspensions: (i) lyophilized nanoparticles loaded with raw peanut proteins (NP-Raw-L); (ii) spray-dried nanoparticles loaded with raw peanut proteins (NP-Raw-SD); (iii) lyophilized nanoparticles loaded with roasted peanut proteins (NP-Ro-L); or (iv) spray-dried nanoparticles loaded with roasted peanut proteins (NP-Ro-SD). Empty nanoparticles (NP-L and NP-SD) which were free of peanut extract proteins (PE) were administered as controls. A group of nonimmunized mice (control [C]) were also included in the study.
Quantification of anti-PE antibodies by indirect ELISA.
The presence of PE-specific antibodies was determined in sera (IgG1, IgG2a, IgA, and IgE) by indirect enzyme-linked immunosorbent assay (ELISA), as previously described (12). Measurements were taken in triplicate and included negative controls (no mouse serum sample background and no antigen coating control).
Cytokine production.
Five weeks after immunization, mice were sacrificed and spleens were extracted. Spleen cell suspensions were obtained and stimulated with peanut proteins (150 μg/ml), as previously described (12). Culture supernatants were analyzed for the presence of cytokines by ELISA, using recombinant cytokines as standard controls (BD Pharmingen).
Statistical analysis.
Analysis of variance (ANOVA) was employed to analyze the data. When ANOVA indicated a significant difference, the Tukey post hoc test was used to assess the difference between groups. Differences were regarded as statistically significant at P values of <0.05. Data are expressed as the mean ± standard deviation (SD) from independent experiments.
RESULTS
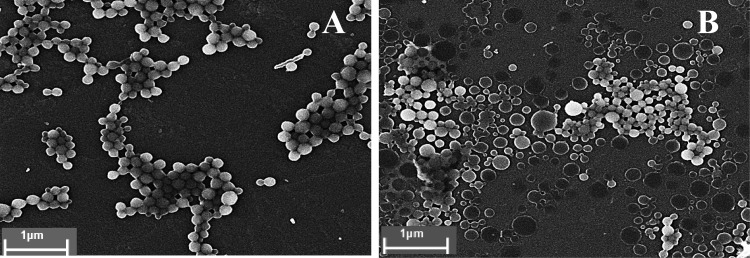
The main physicochemical characteristics of poly(anhydride) nanoparticles are summarized in Table 1. The PE content was about 30 to 40 μg/mg NP, with a high encapsulation efficiency (>60%). Moreover, it is interesting to note that both the yield of the manufacturing process and the encapsulation efficiency (EE) were higher for lyophilized NPs than for spray-dried ones. In addition, the analysis of the morphology by scanning electron microscopy (SEM) (Fig. 1) showed that all nanoparticle formulations were spherically shaped, with a homogeneous size, confirming the results of the polydispersity index (PdI) obtained by laser diffractometry.
TABLE 1.
Physicochemical characterization of poly(anhydride) nanoparticles prepared by lyophilization or spray-drying
| Nanoparticle formulation | Result (mean ± SD) (n = 6) for: |
||||
|---|---|---|---|---|---|
| Size (nm) | Zeta potential (mV) | PE content (μg/mg NP) | Encapsulation efficiency (%) | Yield (%) | |
| NP-La | 201 ± 3 | −49 ± 2 | 80 ± 2 | ||
| NP-SDb | 172 ± 4 | −42 ± 4 | 63 ± 1 | ||
| NP-Raw-Lc | 161 ± 3 | −46 ± 2 | 31 ± 2 | 75 ± 5 | 73 ± 5 |
| NP-Ro-Ld | 155 ± 2 | −45 ± 5 | 30 ± 3 | 72 ± 6 | 76 ± 4 |
| NP-Raw-SDe | 150 ± 5 | −47 ± 2 | 35 ± 5 | 62 ± 3 | 60 ± 4 |
| NP-Ro-SDf | 143 ± 6 | −48 ± 3 | 32 ± 3 | 60 ± 4 | 61 ± 3 |
NP-L, unloaded lyophilized nanoparticles.
NP-SD, unloaded spray-dried nanoparticles.
NP-Raw-L, lyophilized nanoparticles loaded with raw peanut proteins.
NP-Ro-L, lyophilized nanoparticles loaded with roasted peanut proteins.
NP-Raw-SD, spray-dried nanoparticles loaded with raw peanut proteins.
NP-Ro-SD, spray-dried nanoparticles loaded with roasted peanut proteins.
FIG 1.
Scanning electron microscopy of lyophilized (A) and spray-dried (B) nanoparticles.
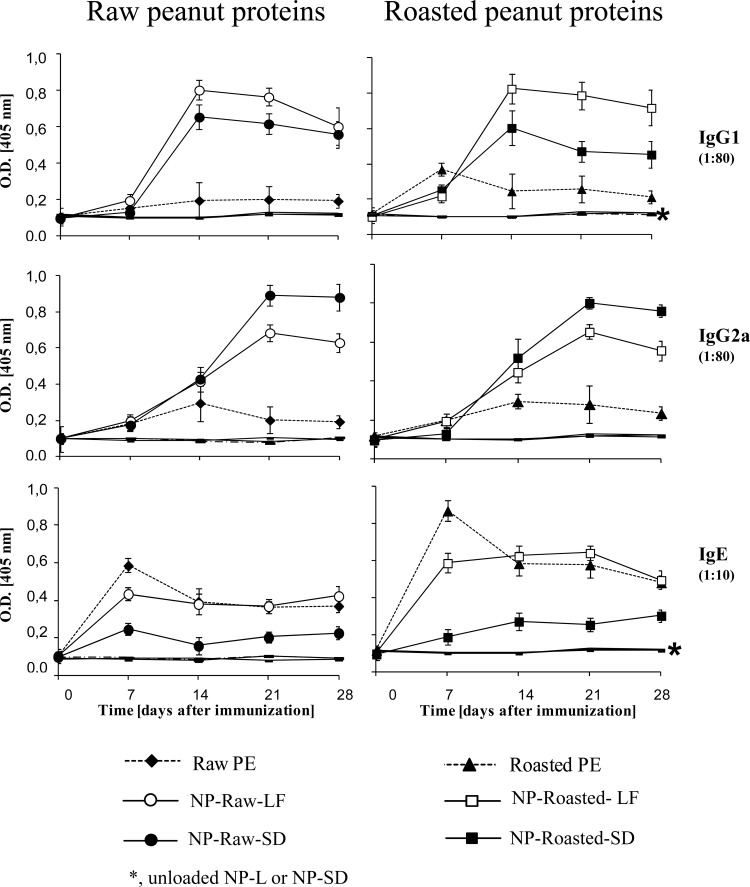
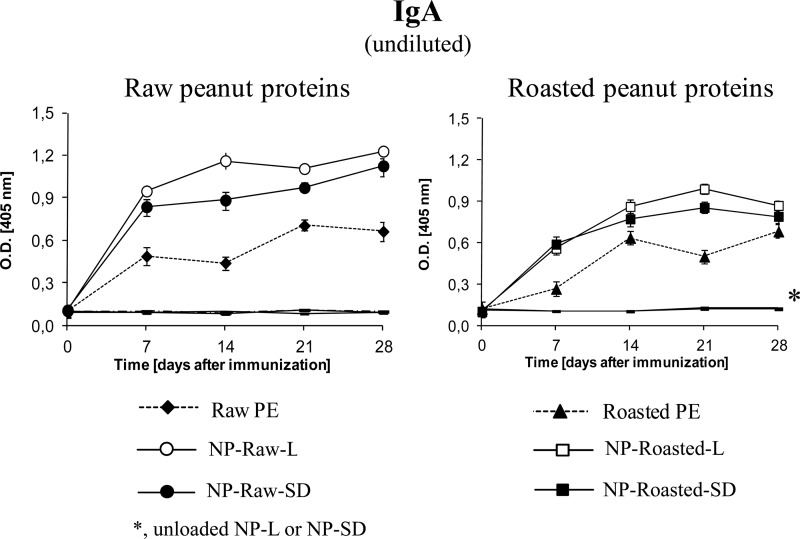
All the animals immunized with the peanut proteins encapsulated into nanoparticles (NP-Raw-L, NP-Raw-SD, NP-Ro-L, or NP-Ro-SD) showed higher levels of IgG1 and IgG2a antibodies (TH2 and TH1 markers, respectively) than the groups that received the proteins in their free forms (raw or roasted) (Fig. 2). The isotopic response profiles elicited by lyophilized or spray-dried nanoparticles revealed different patterns precluding any conclusion on the correlation of these physical parameters with the immunogenicity. Overall, lyophilized PE-loaded nanoparticles (NP-Raw-L and NP-Ro-L) induced higher levels of IgG1 than spray-dried nanoparticles (NP-Raw-SD and NP-Ro-SD). In contrast, spray-dried PE-loaded nanoparticles displayed higher IgG2a levels than lyophilized formulations (NP-Raw-L and NP-Ro-L). Concerning IgE, animals immunized with spray-dried nanoparticles (NP-Raw-SD and NP-Ro-SD) showed lower specific levels of IgE than the groups that received the proteins in their free forms (raw and roasted). The potential of the nanoparticle formulations to induce mucosal response was evaluated by the specific IgA in fecal content (Fig. 3). All nanoparticle formulations (NP-Raw-L, NP-Raw-SD, NP-Ro-L, and NP-Ro-SD) elicited higher levels of intestinal IgA than did free PE, especially for nanoparticles loaded with raw peanut proteins (NP-Raw-L versus raw PE and NP-Raw-SD versus Raw PE). Specific antibodies were undetectable in the groups immunized with unloaded NPs (NP-L and NP-SD) and in the nonimmunized control group.
FIG 2.
Specific antibody response (IgG1, IgG2a, and IgE) elicited after oral immunization. C57BL/6 mice (n = 6 per experimental group) were immunized with a single dose (1 mg) of peanut extract proteins (PE) incorporated into one of the following formulations: lyophilized raw PE-loaded nanoparticles (NP-Raw-L), lyophilized roasted PE-loaded nanoparticles (NP-Ro-L), spray-dried raw PE-loaded nanoparticles (NP-Ra-SD), or spray-dried roasted PE-loaded nanoparticles (NP-Ro-SD). Control groups received one of the following: empty lyophilized NPs (NP-L), empty spray-dried NPs (NP-SD), or the same dose of raw (Ra) or roasted (Ro) peanut proteins. Data are expressed as the mean ± SD. O.D. [405 nm], optical density at 405 nm.
FIG 3.
Specific fecal IgA levels induced after oral immunization. C57BL/6 mice (n = 6 per experimental group) were immunized on day 0 with a single dose (1 mg) of peanut extract proteins (PE) incorporated in one of the following formulations: lyophilized raw PE-loaded nanoparticles (NP-Ra-L), lyophilized roasted PE-loaded nanoparticles (NP-Ro-L), spray-dried raw PE-loaded nanoparticles (NP-Ra-SD), or spray-dried roasted PE-loaded nanoparticles (NP-Ro-SD). Control groups received one of the following: empty lyophilized NPs (NP-L), empty spray-dried NPs (NP-SD), or the same dose of raw (Ra) or roasted (Ro) peanut extract proteins (PE). Data are expressed as means ± SD.
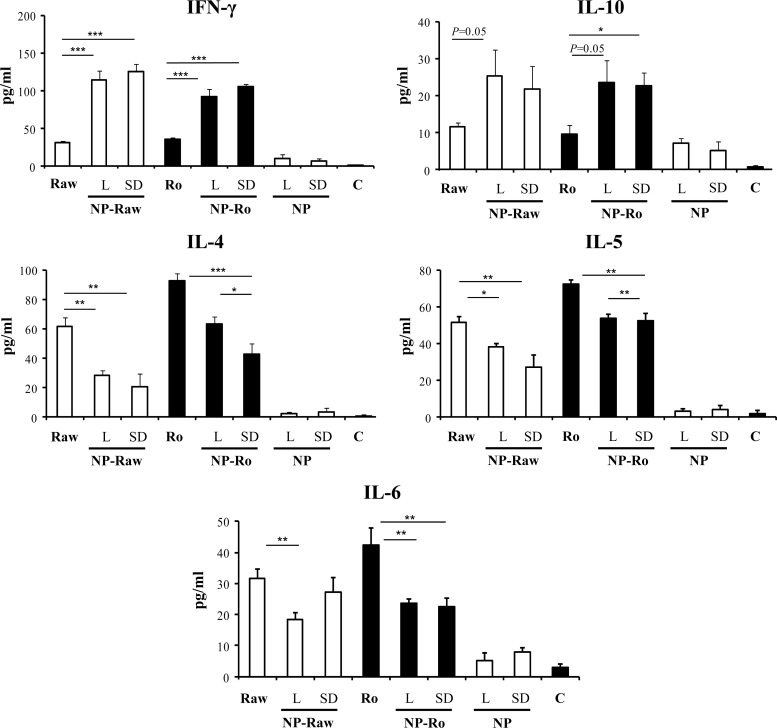
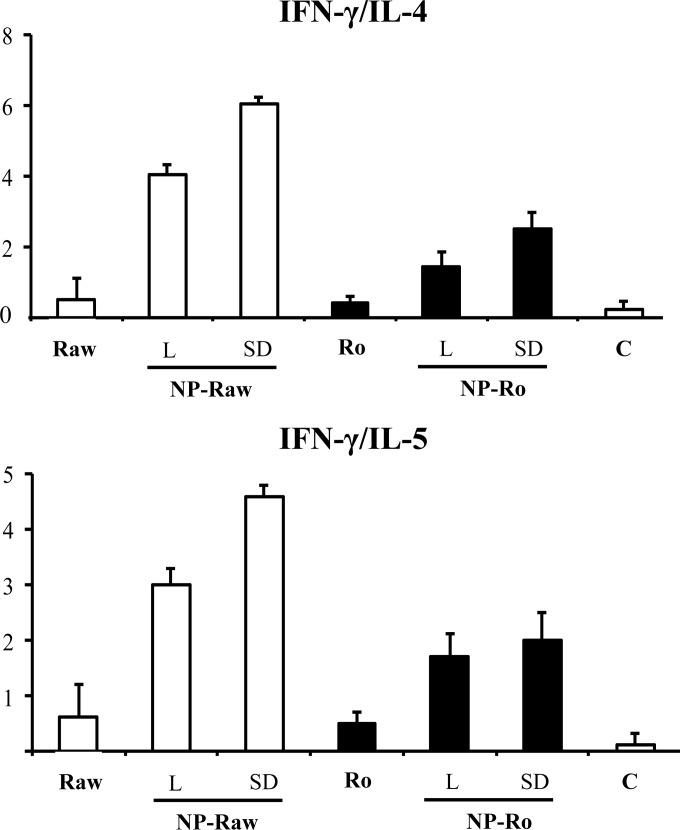
The cytokine secretion profiles were determined after in vitro restimulation of splenocytes isolated from orally immunized mice with the nanoparticle formulations (Fig. 4). The levels of pro-TH2 cytokines (interleukin 4 [IL-4], IL-5, and IL-6) detected in the animals immunized with the nanoparticle formulations were lower than those in the mice that received free peanut proteins. In addition, all nanoparticle formulations induced significantly higher production of gamma interferon [IFN-γ] than did free peanut extract. Likewise, mice immunized with nanoparticle formulations showed significant increases in the secretion of IL-10, an important regulatory cytokine, compared with the animals that received the peanut proteins in their free form. As a result, oral immunization with peanut proteins incorporated into nanoparticles led to a high increase in the TH1/TH2 ratio (Fig. 5), based on the IFN-γ/IL-4 and IFN-γ/IL-5 ratios. Taken together, these results demonstrate that the adjuvant effect of poly(anhydride) nanoparticles is characterized by a pro-TH1 response.
FIG 4.
Specific cytokine secretion after oral immunization with nanoparticle formulations. C57BL/6 mice (n = 6 per experimental group) were immunized on day 0 with a single dose (1 mg) of peanut extract proteins (PE) incorporated in one of the following formulations: lyophilized raw PE-loaded nanoparticles (NP-Ra-L), lyophilized roasted PE-loaded nanoparticles (NP-Ro-L), spray-dried raw PE-loaded nanoparticles (NP-Ra-SD), or spray-dried roasted PE-loaded nanoparticles (NP-Ro-SD). Control groups received one of the following: empty lyophilized NPs (NP-L), empty spray-dried NPs (NP-SD), or the same dose of raw (Ra) or roasted (Ro) peanut proteins. A group of nonimmunized mice (C) was used to establish the basal levels of cytokine. Spleen cells were obtained from immunized and nonimmunized mice. After in vitro restimulation with peanut proteins (150 μg/ml), cytokine levels were measured by ELISA. Data are expressed as means ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 5.
TH1/TH2 ratio elicited after oral immunization, represented by the ratio IFN-γ/IL-4 and the ratio IFN-γ/IL-5. Immunization was performed with a single dose of the following formulations: lyophilized raw PE-loaded nanoparticles (NP-Ra-L), lyophilized roasted PE-loaded nanoparticles (NP-Ro-L), spray-dried raw PE-loaded nanoparticles (NP-Ra-SD), or spray-dried roasted PE-loaded nanoparticles (NP-Ro-SD). Animals received the same dose of raw (Ra) or roasted (Ro) peanut proteins. A group of nonimmunized mice (C) was used to establish the basal levels of cytokine. Data are expressed as means ± SD.
DISCUSSION
In the last decade, novel therapeutic interventions have been explored for treatment of food allergy (28). Among them, oral immunotherapy (OIT) is increasingly being investigated as a potential treatment for peanut and other food allergies (6, 29). OIT results in induction of clinical tolerance to a variety of food proteins. Unfortunately, in spite of its efficacy, OIT shows limitations in long-term efficacy and safety due to local side effects and risk of anaphylaxis (30). In a multicenter study with 28 children, three subjects receiving OIT against their peanut allergy were excluded from the study because of allergic side effects (8). An additional major issue concerning OIT is the lack of power-controlled studies with standardized protocols (9). In general, OIT may offer some advantages over parenteral routes of desensitization, such as intravenous, intradermal, and subcutaneous. However, the clinical efficacy associated with OIT is achieved with high doses of allergens, decreasing the safety of the treatment. Nevertheless, mucosal immunotherapy is hampered by the absence of adequate adjuvants capable of inducing strong humoral and cellular immune responses. Thus, effective and safe vaccines with reduced doses of allergen have been developed using adjuvants. In this context, allergen immunotherapy using polymeric NPs as mucosal adjuvants may represent an attractive alternative to the conventional OIT with native whole extracts.
Pharmaceutical technology is rendering numerous particle systems acceptable for applications to allergen oral delivery to the host. Specifically, NPs appear to have a sound scientific rationale based on the protection of the antigen from exposure to extreme pH conditions, bile, and pancreatic secretions (26, 31). Also, NPs offer a controlled release platform that provides an adequate supply of the loaded compound to its site of absorption or action (32). Simultaneously, advantage is taken of the inherent inclination of particles to be naturally captured by mucosal antigen-presenting cells as part of their duty as sentinels in triggering of mucosal immunity against pathogens (33–35). In particular, nanoparticulate systems made by the copolymer of methyl vinyl ether and maleic anhydride (PVM/MA) have demonstrated their efficacy as adjuvants to induce TH1 immune responses (15, 16, 24). Actually, these poly(anhydride) NPs may induce innate immune responses mediated in a Toll-like receptor 2 (TLR2)- and TLR4-dependent manner (24, 36). This is an important finding since it has been recently shown that the use of multiple TLR agonists carried by NPs influence the induction of long-term memory cells, and the ultimate goal for any vaccine is the stimulation of long-lasting protective immunological memory (37, 38). Several studies have demonstrated that these poly(anhydride) NPs are able to successfully encapsulate allergens (10, 15, 16). Thus, these NPs containing a protein extract from Lolium perenne induced a significant TH1 response which protected the sensitized mice against severe anaphylactic symptoms induced by challenge (17). Furthermore, the encapsulation of peanut proteins into poly(anhydride) NPs enhanced their immunogenic properties by intradermal immunization in mice (12).
On the other hand, processing may affect the peanut protein immunogenicity and allergenicity profile. Thus, it is known that roasting increases the allergenicity of peanut proteins (39–41). Therefore, this study was also designed to evaluate the effects of encapsulation of both thermally treated (roasted) and untreated peanut proteins (raw) into polymeric NPs. In the current study, both raw and roasted peanut extracts were successfully encapsulated into poly(anhydride) NPs, and all NP formulations induced a balanced TH1 and TH2 antibody response after oral immunization characterized by a high TH1/TH2 ratio. These results are in line with previous results obtained by Gómez and coworkers, who loaded a Lolium perenne extract in a similar NP formulation (17). The mechanism underlying such pro-TH1 adjuvant potency might be due to their ability to activate dendritic cells though TLR2 and TLR4 interaction (24, 36). In addition, the OIT NP-based formulations used in this study induced high levels of IL-10. IL-10, a multifunctional modulatory cytokine that plays an important role in immune homeostasis, downregulates the expression of the IgE receptor and promotes the generation of IgG-blocking antibodies (42). In fact, severe food allergy symptoms are associated with lower serum IL-10 levels (43). Interestingly, spray-dried NPs simultaneously demonstrated lower IgE and higher IgG1 induction than did lyophilized NPs. With regard to mucosal response, peanut proteins encapsulated into poly(anhydride) NPs elicited higher IgA than free proteins. These results are in accordance with previous studies that have shown specific bioadhesive properties of these NPs, which enhance mucosal adhesion (20–23).
Finally, the immunogenic properties of the poly(anhydride) NPs were also influenced by the preparative method, which might be explained by the stability and kinetic release from these poly(anhydride) NPs. Spray-dried NPs showed higher stability over time, leading to an increase of their adjuvant effects. Furthermore, this higher stability led to a slower release of the allergens entrapped in the polymeric matrix, which might explain the lower induction of IgE by spray-dried NPs (44).
In summary, results obtained with poly(anhydride) nanoparticles suggest that they may be an alternative for OIT due to their pro-TH1 adjuvant properties. Nevertheless, studies on immunization of sensitized animals are required to determine if the elicited TH1 response is high enough to dampen a TH2-biased immune response elicited in the allergic mice. Finally, poly(anhydride) nanoparticles might facilitate encapsulating exact amounts of whole-peanut extract or allergens, allowing the standardization of food oral immunotherapy.
ACKNOWLEDGMENTS
We are grateful to Fernando Pineda from Diater Laboratorios, S.A. (Madrid, Spain) for kindly supplying peanut extracts.
This research was financially supported by the Health Department of “Gobierno de Navarra” (grant 28/2007), the “Instituto de Salud Carlos III” (grant PS09/01083), and the “Fundación Ramón Areces.” J. de S. Rebouças was also financially supported by the “Asociación de Amigos,” University of Navarra, Spain. M. Ferrer, M. L. Sanz, and Gabriel Gastaminza are supported by grant RD07/0064 from the Spanish Research Network on Adverse Reactions to Allergens and Drugs (RIRAAF) (Red de Investigación de Reacciones Adversas a Alérgenos y Fármacos) of the Carlos III Health Institute.
Footnotes
Published ahead of print 4 June 2014
REFERENCES
- 1.Finkelman FD. 2010. Peanut allergy and anaphylaxis. Curr. Opin. Immunol. 22:783–788. 10.1016/j.coi.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulay A, Houghton J, Gancheva V, Sterk Y, Strada A, Schlegel-Zawadzka M, Sora B, Sala R, van Ree R, Rowe G. 2008. EuroPrevall review of factors affecting incidence of peanut allergy: priorities for research. Allergy 63:797–809. 10.1111/j.1398-9995.2008.01776.x [DOI] [PubMed] [Google Scholar]
- 3.Mondoulet L, Paty E, Drumare MF, Ah-leung S, Scheinmann P, Willemot RM, Wal JM, Bernard H. 2005. Influence of thermal processing on the allergenicity of peanut proteins. J. Agric. Food Chem. 53:4547–4553. 10.1021/jf050091p [DOI] [PubMed] [Google Scholar]
- 4.Mueller GA, Maleki SJ, Pedersen LC. 2014. The molecular basis of peanut allergy. Curr. Allergy Asthma Rep. 14:429–438. 10.1007/s11882-014-0429-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vetander M, Helander D, Flodström C, Ostblom E, Alfvén T, Ly DH, Hedlin G, Lilja G, Nilsson C, Wickman M. 2012. Anaphylaxis and reactions to foods in children—a population-based case study of emergency department visits. Clin. Exp. Allergy 42:568–577. 10.1111/j.1365-2222.2011.03954.x [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA. 2013. Peanut oral immunotherapy: is it ready for clinical practice? J. Allergy Clin. Immunol. Pract. 1:15–21. 10.1016/j.jaip.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. 2011. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin. Exp. Allergy 41:1273–1281. 10.1111/j.1365-2222.2011.03699.x [DOI] [PubMed] [Google Scholar]
- 8.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, Kulis M, Pons L, Vickery B, Burks AW. 2011. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J. Allergy Clin. Immunol. 127:654–660. 10.1016/j.jaci.2010.12.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer K. 2012. A European perspective on immunotherapy for food allergies. J. Allergy Clin. Immunol. 129:1179–1184. 10.1016/j.jaci.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 10.Scholl I, Boltz-Nitulescu G, Jensen-Jarolim E. 2005. Review of novel particulate antigen delivery systems with special focus on treatment of type I allergy. J. Control. Release 104:1–27. 10.1016/j.jconrel.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 11.Broos S, Lundberg K, Akagi T, Kadowaki K, Akashi M, Greiff L, Borrebaeck CA, Lindstedt M. 2010. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: Implications for specific immunotherapy. Vaccine 28, 5075–5085. 10.1016/j.vaccine.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 12.De Souza Rebouças J, Esparza I, Ferrer M, Sanz ML, Irache JM, Gamazo C. 26 February 2012. Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J. Biomed. Biotechnol. 474605. 10.1155/2012/474605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. 2006. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control. Release. 116:1–27. 10.1016/j.jconrel.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 14.Scholl I, Kopp T, Bohle B, Jensen-Jarolim E. 2006. Biodegradable PLGA particles for improved systemic and mucosal treatment of type I allergy. Immunol. Allergy Clin. North. Am. 26:349–364. 10.1016/j.iac.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Gómez S, Gamazo C, San Roman B, Vauthier C, Ferrer M, Irache JM. 2006. Development of a novel vaccine delivery system based on Gantrez nanoparticles. J. Nanosci. Nanotechnol. 10:3283–3289 [DOI] [PubMed] [Google Scholar]
- 16.Gómez S, Gamazo C, San Roman B, Ferrer M, Sanz ML, Irache JM. 2007. Gantrez AN nanoparticles as an adjuvant for oral immunotherapy with allergens. Vaccine 25:5263–5271. 10.1016/j.vaccine.2007.05.020 [DOI] [PubMed] [Google Scholar]
- 17.Gómez S, Gamazo C, San Roman B, Grau A, Espuelas S, Ferrer M, Sanz ML, Irache JM. 2009. A novel nanoparticulate adjuvant for immunotherapy with Lolium perenne. J. Immunol. Methods 348:1–8. 10.1016/j.jim.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Salman HH, Irache JM, Gamazo C. 2009. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine 27:4784–4790. 10.1016/j.vaccine.2009.05.091 [DOI] [PubMed] [Google Scholar]
- 19.Da Costa Martins R, Gamazo C, Sánchez-Martínez M, Barberán M, Peñuelas I, Irache JM. 2012. Conjunctival vaccination against Brucella ovis in mice with mannosylated nanoparticles. J. Control. Release 162:553–560. 10.1016/j.jconrel.2012.07.030 [DOI] [PubMed] [Google Scholar]
- 20.Irache JM, Huici M, Konecny M, Espuelas S, Campanero MA, Arbos P. 2005. Bioadhesive properties of Gantrez nanoparticles. Molecules 10:126–145. 10.3390/10010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salman HH, Gamazo C, Campanero MA, Irache JM. 2005. Salmonella-like bioadhesive nanoparticles. J. Control. Release 106:1–13. 10.1016/j.jconrel.2005.03.033 [DOI] [PubMed] [Google Scholar]
- 22.Salman HH, Gamazo C, de Smidt PC, Russell-Jones G, Irache JM. 2008. Evaluation of bioadhesive capacity and immunoadjuvant properties of vitamin B(12)-Gantrez nanoparticles. Pharm. Res. 25:2859–2868. 10.1007/s11095-008-9657-5 [DOI] [PubMed] [Google Scholar]
- 23.Salman HH, Gamazo C, Agueros M, Irache JM. 2007. Bioadhesive capacity and immunoadjuvant properties of thiamine-coated nanoparticles. Vaccine 25:8123–8132. 10.1016/j.vaccine.2007.09.044 [DOI] [PubMed] [Google Scholar]
- 24.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. 2010. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 17:1356–1362. 10.1128/CVI.00164-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojer P, Salman H, Martins RD, Ceráin AL, Irache JM. 2010. Spray-drying of poly(anhydride) nanoparticles for drug/antigen delivery. J. Drug Del. Sci. Tech. 20:353–359 [Google Scholar]
- 26.Arbós P, Campanero MA, Arnangoa MA, Renedo MJ, Irache JM. 2003. Influence of the surface characteristics of PVM/MA nanoparticles on their bioadhesive properties. J. Control. Release 89:19–30. 10.1016/S0168-3659(03)00066-X [DOI] [PubMed] [Google Scholar]
- 27.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. 10.1016/0003-2697(85)90442-7 [DOI] [PubMed] [Google Scholar]
- 28.Jones SM, Burks AW, Dupont C. 2014. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J. Allergy Clin. Immunol. 133:318–323. 10.1016/j.jaci.2013.12.1040 [DOI] [PubMed] [Google Scholar]
- 29.Vickery BP, Burks W. 2010. Oral immunotherapy for food allergy. Curr. Opin. Pediatr. 22:765–770. 10.1097/MOP.0b013e32833f5fc0 [DOI] [PubMed] [Google Scholar]
- 30.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, Sampson H, Burks AW. 2010. Peanut oral immunotherapy is not ready for clinical use. J. Allergy Clin. Immunol. 126:31–32. 10.1016/j.jaci.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olbrich C, Müller RH, Tabatt K, Kayser O, Schulze C, Schade R. 2002. Stable biocompatible adjuvants—a new type of adjuvant based on solid lipid nanoparticles: a study on cytotoxicity, compatibility and efficacy in chicken. Altern. Lab. Anim. 30:443–458 [DOI] [PubMed] [Google Scholar]
- 32.Storni T, Kündig TM, Senti G, Johansen P. 2005. Immunity in response to particulate antigen-delivery systems. Adv. Drug Deliv. Rev. 57:333–355. 10.1016/j.addr.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 33.O'Hagan DT, Jeffery H, Maloy KJ, Mowat AM, Rahman D, Challacombe SJ. 1995. Biodegradable microparticles as oral vaccines. Adv. Exp. Med. Biol. 371B:1463–1467 [PubMed] [Google Scholar]
- 34.Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14:141–153. 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- 35.Vila A, Sánchez A, Tobío M, Calvo P, Alonso MJ. 2002. Design of biodegradable particles for protein delivery. J. Control. Release. 78:15–24. 10.1016/S0168-3659(01)00486-2 [DOI] [PubMed] [Google Scholar]
- 36.Camacho AI, Da Costa Martins R, Tamayo I, de Souza J, Lasarte JJ, Mansilla C, Esparza I, Irache JM, Gamazo C. 2011. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine 22:7130–7135. 10.1016/j.vaccine.2011.05.072 [DOI] [PubMed] [Google Scholar]
- 37.Lycke N, Bemark M. 2010. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 3:556–566. 10.1038/mi.2010.54 [DOI] [PubMed] [Google Scholar]
- 38.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. 10.1038/nature09737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, Sampson HA. 2001. Effects of cooking methods on peanut allergenicity. J. Allergy Clin. Immunol. 107:1077–1081. 10.1067/mai.2001.115480 [DOI] [PubMed] [Google Scholar]
- 40.Pomés A, Butts CL, Chapman MD. 2006. Quantification of Ara h 1 in peanuts: why roasting makes a difference. Clin. Exp. Allergy 36:824–830. 10.1111/j.1365-2222.2006.02490.x [DOI] [PubMed] [Google Scholar]
- 41.Maleki SJ, Hulrburt BK. 2004. Structural and functional alterations in major peanut allergens caused by thermal processing. J. AOAC Int. 87:1475–1479 [PubMed] [Google Scholar]
- 42.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. 1998. IgE versus IgG4 production can be differentially regulated by IL-10. J. Immunol. 160:3555–3561 [PubMed] [Google Scholar]
- 43.Kosaka S, Tamauchib H, Terashimac M, Maruyamad H, Habue S, Kitasatoa H. 2011. IL-10 controlsTh2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology. 216:811–820. 10.1016/j.imbio.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 44.Rebouças Jde S, Irache JM, Camacho AI, Esparza I, Del Pozo V, Sanz ML, Ferrer M, Gamazo C. 2012. Development of poly(anhydride) nanoparticles loaded with peanut proteins: the influence of preparation method on the immunogenic properties. Eur. J. Pharm. Biopharm. 82:241–249. 10.1016/j.ejpb.2012.06.014 [DOI] [PubMed] [Google Scholar]