Abstract
Previous investigations demonstrated that saponins isolated from the root of Panax ginseng C. A. Meyer (i.e., ginseng root saponin [GS-R]) had adjuvant activity. In the present study, the combined effects of rapeseed oil (RO) and GS-R on the immune responses elicited by foot-and-mouth disease (FMD) vaccine were investigated by measuring FMD virus (FMDV)-specific antibody levels, cytokine levels, lymphocyte proliferation, and long-lived IgG-secreting plasma cells from bone marrow in a mouse model. The results indicated that RO in combination with GS-R significantly enhanced serum IgG and isotype concentrations, gamma interferon (IFN-γ) and interleukin 5 (IL-5) levels, splenocyte proliferative responses to stimulations with concanavalin A (ConA), lipopolysaccharide (LPS), and FMDV antigen, and the numbers of IgG-secreting plasma cells in the bone marrow, suggesting that RO/GS-R enhanced both Th1 and Th2 immune responses. In addition, no significant difference was found between RO/GS-R and the commercial adjuvant oil ISA 206 in the promotion of FMD vaccine-induced immune responses. Considering the vegetable origin of RO and GS-R and the potent adjuvant activity, RO/GS-R should be studied further for the development of veterinary vaccines, especially for use in food animals in order to promote food safety.
INTRODUCTION
Foot-and-mouth disease (FMD) is an infectious disease of cloven-hoofed animals such as swine, cattle, sheep, and goats. The disease spreads quickly through farm animals and causes large-scale epidemics, resulting in serious economic loss (1). FMD virus (FMDV) is the causal agent and belongs to the genus Aphthovirus in the family Picornaviridae. The virus appears as seven serotypes worldwide, namely, A, C, O, South African Territories (SAT) 1 to 3, and Asia 1 (2–4). Vaccination remains an important approach to FMD control in many countries. To induce effective immune responses, oil adjuvant is usually used in vaccines. Montanide ISA 206, a mineral oil-based adjuvant, is the most commonly used commercial adjuvant in China. The oil-emulsified vaccine forms depots at the site of injection, from which antigens are slowly released to stimulate the lymphatic system to produce a durable immune response (2). However, mineral oil-based adjuvant is not welcomed by food consumers, because it is reported to cause undesirable tissue reactions, it persists for long periods of time in animal tissues, and it is potentially carcinogenic to consumers (5, 6). In China, a mandatory vaccination program is enforced, and every commercially maintained pig receives 2 or 3 injections of FMD vaccine. Statistics show that 710 million pigs are slaughtered annually (http://livestock.feedtrade.com.cn). Therefore, for the control of FMD alone, approximately 1,770 tons of mineral oil-based adjuvant is estimated to be consumed at dining tables annually. Although current commercially available oil adjuvants are highly refined, searching for safer adjuvants for use in food animals remains an important topic, attracting many researchers (6–8).
Previous investigations showed that ginseng stem-leaf saponin (GSLS) and mineral oil synergistically activated the immune responses to the FMD vaccine in mice and pigs and to the Newcastle disease vaccine in birds (9, 10). We recently tested edible vegetable oils extracted from soybeans, corn, sunflower seeds, sesame seeds, olives, camellia seeds, and rapeseeds for their ability to form water-in-oil emulsions, and we found that rapeseed oil (RO) could form a stable oil emulsion with a relatively low cost (11). In the present study, first we compared two ginseng saponins, GSLS and ginseng root saponin (GS-R), for their synergistic adjuvant activities with RO, and then we evaluated the combined effects of ginseng saponins and RO on the immune responses elicited by the FMD vaccine in a mouse model, by measuring FMDV-specific antibodies, cytokines, and lymphocyte proliferation, as well as IgG-secreting plasma cells from bone marrow.
MATERIALS AND METHODS
Animals.
ICR mice (female, 5 weeks of age) weighing 18 to 22 g were purchased from the Shanghai Laboratory Animal Center Co. Ltd. (Shanghai, China) and were kept in polypropylene cages with sawdust bedding. The temperature was maintained at 24 ± 1°C, with humidity of 50% ± 10% and a 12-h/12-h light/dark cycle. Food and water were available ad libitum. The procedures used for the animals and their care followed the internationally accepted Guidelines for Keeping Experimental Animals, issued by the government of China. The researchers received ethical training from the Zhejiang University ethics committee.
Rapeseed oil, adjuvant, ginseng saponins, and antigen.
Rapeseed oil (RO) was obtained from a local food store and was manufactured according to standard GB1536 (12) by the Shanghai Jiali Food Industry Co. Ltd. (Shanghai, China). One liter of RO contained 491 kilocalories of energy and 56 g of fat. Standardized ginseng stem-leaf saponin (GSLS) (13) and ginseng root saponin (GS-R) (14) were purchased from Hongjiu Ginseng Industry Co. Ltd. (Jilin, China). Based on analysis by high-performance liquid chromatography (HPLC), GSLS contained Rb1 (1.4%), Rb2 (3.0%), Rc (2.5%), Rd (8.0%), Re (12.0%), and Rg1 (6.0%) and GS-R contained Rb1 (18.0%), Rb2 (9.5%), Rc (10.0%), Rd (7.6%), Re (8.7%), and Rg1 (3.5%). Montanide ISA 206 adjuvant and inactivated FMDV type Asia 1 antigen (28.19 μg of 146s protein per ml) were kindly provided by Jin Yu Bao Ling Bio-Pharmaceutical Co. Ltd. (Huhhot, China).
Vaccines.
The FMDV vaccine adjuvanted with ISA 206 was prepared according to the protocol of Jin Yu Bao Ling Bio-Pharmaceutical Co. Ltd. (Huhhot, China). To produce experimental FMDV vaccines, oil phases with different amounts of GSLS or GS-R were prepared by mixing GSLS or GS-R with RO containing 6% Span-80, using dimethyl sulfoxide (DMSO) as a cosolvent; the aqueous phase was prepared by adding Tween 80 to the FMDV antigen solution to produce an antigen solution with 3% Tween 80. Then the oil phase was emulsified in the aqueous phase at 1:1 (vol/vol) with a dispersing device (Polytron System PT 1200 E; Kinematica AG, Switzerland), to produce FMD vaccines in RO containing 0, 10, 20, or 30 μg of GSLS or GS-R per ml. The amounts of FMDV antigen were the same in the different vaccine formulations throughout the study. The vaccines were prepared 1 day before immunization.
Viscosity.
The viscosities of the vaccines were measured according to the work of Stone (6, 15). The vaccines were removed from storage at 4°C and allowed to equilibrate to room temperature. One milliliter of the sample was drawn into a 1-ml pipette, and then the time required for 0.4 ml of the sample to flow out of the vertically positioned pipette was recorded.
Experiment 1.
To compare the adjuvant effects of GSLS and GS-R in the RO-emulsified FMD vaccine, 64 mice were randomly allocated into eight groups, with eight mice per group. The mice were subcutaneously (s.c.) injected twice with FMDV antigen mixed with physiological saline solution, RO, or RO containing GSLS (2, 4, or 6 μg) or GS-R (2, 4, or 6 μg), at 3-week intervals. Blood samples were collected 3 and 7 days after the boost immunization. The samples were stored at 4°C overnight and centrifuged at 4,000 rpm for 10 min to isolate the serum, which was then used for the detection of FMDV-specific IgG. The injection volume of the different vaccines was 200 μl throughout the study.
Experiment 2.
To investigate the synergistic effects of RO and GS-R on immune responses, 32 mice were randomly allocated into four groups, with eight mice per group. The animals were s.c. injected twice with FMDV antigen mixed with physiological saline solution, GS-R (4 μg), RO, or RO containing GS-R (4 μg), at 3-week intervals. Blood samples were collected 1 and 2 weeks after the boost immunization. Sera were prepared in the same way as for experiment 1, for the detection of FMDV-specific IgG.
Experiment 3.
To analyze the effects of RO containing GS-R on IgG and isotype responses, 48 mice were randomly allocated into six groups, with eight mice per group. The animals were s.c. injected twice with FMDV antigen mixed with physiological saline solution, RO containing GS-R (2, 4, or 6 μg), or ISA 206, at 3-week intervals. Blood samples were collected 1 week after the primary immunization and 1 to 6 weeks after the boost immunization, for the detection of FMDV-specific IgG and IgG isotypes.
Experiment 4.
To test the effects of RO containing GS-R on cellular immune responses, 48 mice were randomly allocated into six groups, with eight mice per group. The animals were s.c. injected twice with FMDV antigen mixed with physiological saline solution, RO containing GS-R (2, 4, or 6 μg), or ISA 206, at 3-week intervals. Two weeks after the boost immunization, the serum was sampled for detection of cytokines; the spleens were collected and splenocytes were prepared for detection of splenocyte proliferation in response to concanavalin A (ConA), lipopolysaccharide (LPS), and FMDV antigen.
Experiment 5.
To investigate the effects of RO/GS-R on long-lived, antibody-secreting plasma cells, 24 mice were randomly allocated into three groups, with eight mice per group. The animals were s.c. injected twice with FMDV antigen mixed with physiological saline, RO, or RO containing GS-R (4 μg), at 3-week intervals. Bone marrow cells were prepared 8 weeks after the boosting, to measure the long-lived, antibody-secreting plasma cells.
Determination of IgG and isotype levels.
Serum samples from experiments 1, 2, and 3 were analyzed for FMDV-specific IgG and isotypes mainly as described by Song et al. (9). In brief, 96-well microtiter plates were coated with 50 μl of rabbit FMDV serotype Asia 1-specific antibody (LVRI, China) in 0.05 M carbonate buffer (pH 9.6), at a dilution of 1:800, and were stored overnight at 4°C. After five washes with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST), the wells were blocked with skim milk (5%) and incubated for 2 h at 37°C. Subsequently, 50 μl of FMDV Asia 1 antigen at a dilution of 1:8 was added, and the wells were incubated for 2 h at 4°C. After five washes, 50 μl of serum (serial dilutions for analysis of IgG or dilution of 1:200 for analysis of IgG isotypes, in PBS) was added to each well, and the wells were incubated for 1 h at 37°C. The plates were then washed five times with PBST. To determine the IgG titer, 50 μl of goat anti-mouse IgG-horseradish peroxidase (HRP) conjugate (1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was added to each well, and the wells were incubated for 1 h at 37°C. Following washes with PBST, 50 μl of 3,3′,5,5′-tetramethylbenzidine solution containing 0.1 M citrate phosphate (100 μg/ml, pH 5.0) was added to each well, and the wells were incubated for 15 min at 37°C. Fifty microliters of 2 M H2SO4 was added to each well to stop the reaction. The optical density (OD) of the wells was evaluated at 450 nm with an enzyme-linked immunosorbent assay (ELISA) plate reader. Values over the cutoff background value (the mean of serum samples isolated from unimmunized mice multiplied by 2.1) were considered positive, and titers were expressed as reciprocal end dilutions. To determine IgG subclasses, 50 μl of HRP-labeled goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (1:1,000; Santa Cruz Biotechnology Inc.) was added to each well, and the wells were then incubated for 1 h at 37°C. Subsequently, the same procedures as described above were performed to determine the specific IgG isotypes, by reading the OD values at 450 nm.
Splenocyte proliferation assay.
Splenocytes were prepared mainly as described by Song et al. (9). Briefly, the spleen was removed 2 weeks after the second immunization, placed in PBS, minced, and passed through steel mesh to yield homogeneous cells. To remove contaminated red blood cells (RBCs), RBC lysis buffer (Beijing Solarbio Science & Technology Co.) was added. After 10 min of centrifugation at 380 × g at 4°C, the sedimented cells were washed twice with PBS and resuspended in RPMI 1640 medium with 100 μg/ml streptomycin, 100 IU/ml penicillin, 0.05 mM 2-mercaptoethanol, and 10% heat-inactivated fetal calf serum (FCS). The viability of the cells was measured by trypan blue exclusion, and 95% of the cells were viable.
The splenocyte proliferation assay was performed as described previously (16). Briefly, spleen cells were added to a 96-well flat-bottom plate (Nunc) at a concentration of 5.0 × 106 cells/ml. After that, ConA (5 μg/ml), LPS (8 μg/ml), FMDV antigen (10 μg/ml), or medium was added to provide a final volume of 200 μl. Plates were incubated for 48 h at 37°C in a humidified atmosphere with 5% CO2. To each well, 50 μl of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] (Sigma-Aldrich, St. Louis, MO) solution (2 mg/ml) was added 4 h before the end of the incubation. The plates were then centrifuged at 1,400 × g for 5 min, and untransformed MTT was carefully removed. To each well, 150 μl of a DMSO solution (192 μl of DMSO plus 8 μl of 1 M HCl) was added. After incubation for 15 min, the OD values at 570 nm were measured using an automatic ELISA reader with a 630-nm reference. The stimulation index (SI) was estimated based on the following formula: SI = OD value from stimulated cells/OD value from unstimulated cells.
Determination of serum cytokine levels.
Serum gamma interferon (IFN-γ) levels were determined using an ELISA kit (MultiSciences Biotech Co., Ltd., Hangzhou, China) (17), and serum interleukin 5 (IL-5) levels were measured using an ELISA kit (eBioscience Inc., San Diego, CA, USA). Serum cytokine levels were evaluated by interpolation of cytokine standard curves.
Counting of long-lived, IgG-secreting plasma cells by ELISPOT assay.
Cell suspensions prepared from the bone marrow of killed mice were diluted to 2.4 × 107 cells/ml and added to MultiScreen HA 96-well plates (Millipore, Bedford, MA) that had been coated with 7 μg/ml FMDV antigen (18, 19). Subsequently, the plates were incubated for 5 h at 37°C and washed three more times. After overnight incubation at 4°C with HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), the plates were washed five times with PBST followed by three washes with PBS, and filters were then developed with the Vectastain 3-amino-9-ethylcarbazole (AEC) (Sigma) peroxidase substrate kit. The reaction was finished by washing the plates in deionized water (20). After the plates dried, the spots were counted with an enzyme-linked immunosorbent spot assay (ELISPOT) reader (CTL S5 UV analyzer; Cellular Technology, Cleveland, OH), and the data were evaluated using ImmunoSpot 5.0.9 software.
Statistical analysis.
The data were analyzed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). Values were expressed as means ± the standard deviation (SD). Analysis of variance (ANOVA) with Fisher's least significant difference post hoc test was used for multiple comparisons between groups. P values of <0.05 were considered statistically significant.
RESULTS
Viscosity of rapeseed oil-emulsified vaccines.
The viscosity of the rapeseed oil-emulsified vaccine supplemented with GS-R (4 μg/200 μl) was 1.9 ± 0.1 s (n = 4), which was significantly less than that of the ISA 206-emulsified vaccine (2.8 ± 0.1 s [n = 4]; P < 0.05).
Comparison of GSLS and GS-R adjuvant activities in RO-emulsified vaccines.
To compare the adjuvant activities of GSLS and GS-R in RO-emulsified vaccines, FMDV vaccines in RO containing different amounts of GSLS or GS-R were prepared and injected into mice. Blood was sampled from the mice for the measurement of FMDV-specific IgG levels. The order of the groups based on their IgG levels was as follows: RO/GS-R > RO/GSLS > RO only > no adjuvant (Fig. 1). The highest IgG response was induced by the RO-emulsified vaccine supplemented with 4 μg of GS-R. Thus, GS-R was used as an additive in the oil-emulsified vaccines in the subsequent investigations.
FIG 1.
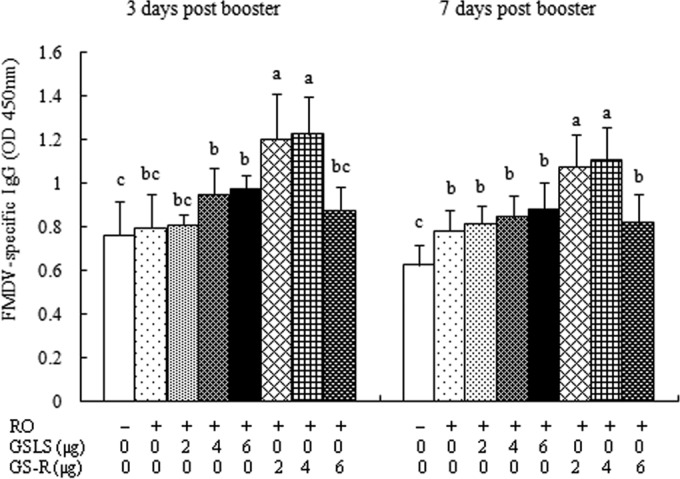
Serum FMDV-specific IgG levels. Mice (n = 8/group) were s.c. injected with FMDV antigen plus physiological saline, RO, or RO containing GSLS (2, 4, or 6 μg) or GS-R (2, 4, or 6 μg) on days 1 and 21. Sera were collected 3 and 7 days after boosting for analysis of FMDV-specific IgG levels by an indirect ELISA. The values are presented as means ± SD. Bars with different letters in the same time point have statistically significant differences (P < 0.05).
Synergistic effects of RO and GS-R.
To investigate whether RO and GS-R had synergistic adjuvant activities, FMDV vaccines containing GS-R, RO, or RO/GS-R were prepared and injected into mice. Blood was sampled from the mice for the measurement of FMDV-specific IgG levels. The results showed that the vaccine containing both RO and GS-R induced significantly higher IgG levels than did RO and GS-R used alone (Fig. 2).
FIG 2.
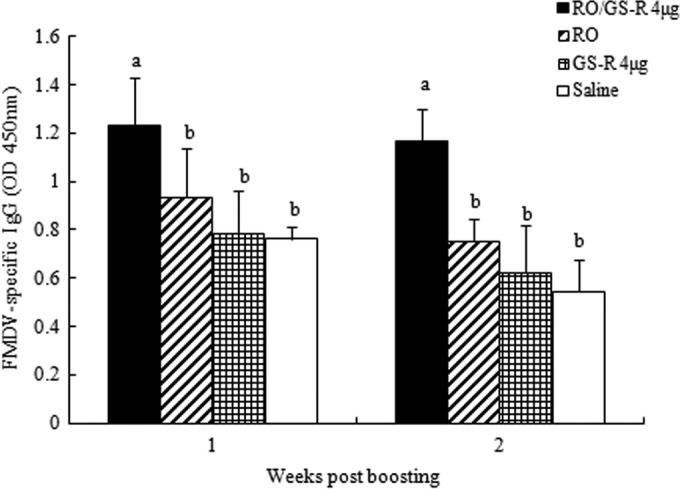
Synergistic effects of RO and GS-R. Mice (n = 8/group) were s.c. injected with FMDV antigen plus physiological saline, GS-R, RO, or RO containing GS-R (4 μg) on days 1 and 21. Sera were collected 1 and 2 weeks after boosting for analysis of FMDV-specific IgG levels by an indirect ELISA. The values are presented as means ± SD. Bars with different letters, P < 0.05.
FMDV-specific IgG and isotype levels.
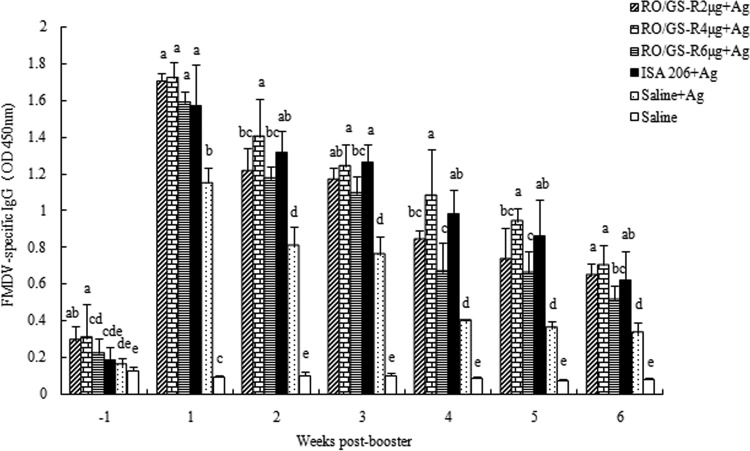
To investigate the adjuvant effects of RO supplemented with GS-R on serum FMDV-specific IgG and isotypes, FMDV vaccines containing GS-R, RO, RO/GS-R, or ISA 206 were prepared and injected into mice. Blood was sampled from the mice for determination of FMDV-specific IgG and isotypes. The results showed that the vaccine in RO supplemented with GS-R (2, 4, or 6 μg) induced significantly higher serum IgG levels than did the antigen in physiological saline during the period from 1 week before to 6 weeks after the second immunization. There was no significant difference in IgG responses between the mice vaccinated with FMDV vaccine in RO/GS-R (4 μg) and the mice treated with vaccine in ISA 206 (Fig. 3).
FIG 3.
Duration of serum FMDV-specific IgG responses. Mice (n = 8/group) were s.c. immunized with FMDV antigen (Ag) plus physiological saline, RO containing GS-R (2, 4, or 6 μg), or ISA 206 on days 1 and 21. Control mice were injected with physiological saline solution in the same manner. Sera were collected 1 week before and 1 to 6 weeks after boosting for analysis of FMDV-specific IgG levels by an indirect ELISA. The values are presented as means ± SD. Bars with different letters in the same time point have statistically significant differences (P < 0.05).
Figure 4 presents the FMDV-specific titers of the sera collected 2 weeks after the booster immunization. The IgG titer in the serum of mice injected with the vaccine in RO/GS-R (1:2,152) was 16-fold higher than that of mice injected with the vaccine in saline solution (1:134), which was not significantly different from the titer in the serum of mice injected with the vaccine adjuvanted with ISA 206 (1:2,228). Except for the IgG3 levels in the RO/GS-R (6 μg) group, RO/GS-R and ISA 206 enhanced all IgG1, IgG2a, IgG2b, and IgG3 responses, with no significant difference between the RO/GS-R (4 μg) and ISA 206 groups (Fig. 5).
FIG 4.
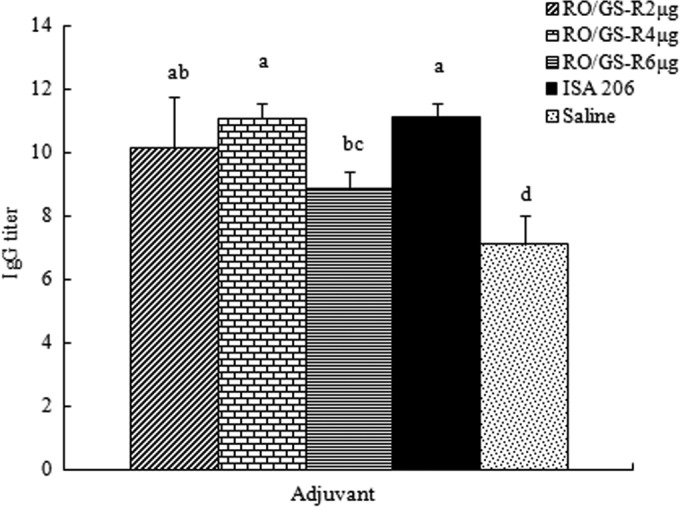
Serum FMDV-specific IgG titers. Mice (n = 8/group) were s.c. immunized with FMDV antigen plus physiological saline, RO containing GS-R (2, 4, or 6 μg), or ISA 206 on days 1 and 21. Sera were collected 2 weeks after boosting, and serum FMDV-specific IgG levels were measured by an indirect ELISA. Values above the cutoff background level (the mean from unimmunized mice as negative controls multiplied by a factor of 2.1) were considered positive. Titers are depicted as reciprocal end dilutions. Bars with different letters have statistically significant differences (P < 0.05).
FIG 5.
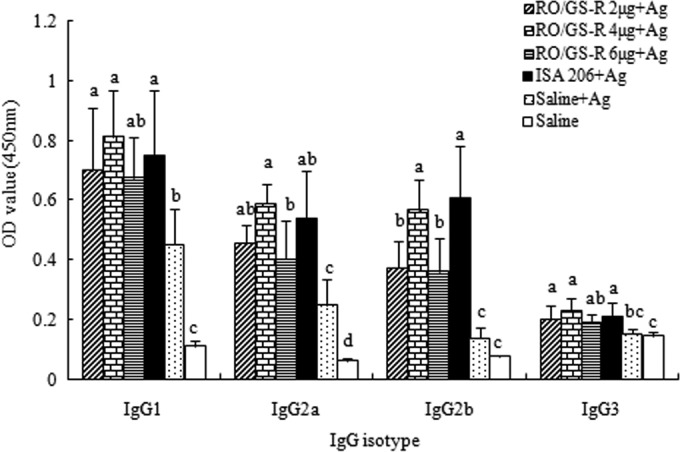
Serum FMDV-specific IgG isotype levels. Mice (n = 8/group) were s.c. immunized with FMDV antigen (Ag) plus physiological saline, RO containing GS-R (2, 4, or 6 μg), or ISA 206 on days 1 and 21. Control mice were injected with physiological saline solution in the same manner. Sera were collected 2 weeks after boosting, and serum FMDV-specific isotype levels were measured by an indirect ELISA. The values are presented as means ± SD. Bars with different letters have statistically significant differences (P < 0.05).
Lymphocyte proliferative response.
The effects of RO and GS-R on splenocyte proliferation in response to ConA, LPS, and FMDV stimulations are shown in Fig. 6. Compared with the control (saline plus antigen), RO supplemented with GS-R significantly enhanced the proliferative responses (P < 0.05). The greatest proliferation was detected in the group treated with RO plus 4 μg of GS-R, which was not significantly different from findings for the ISA 206 group.
FIG 6.
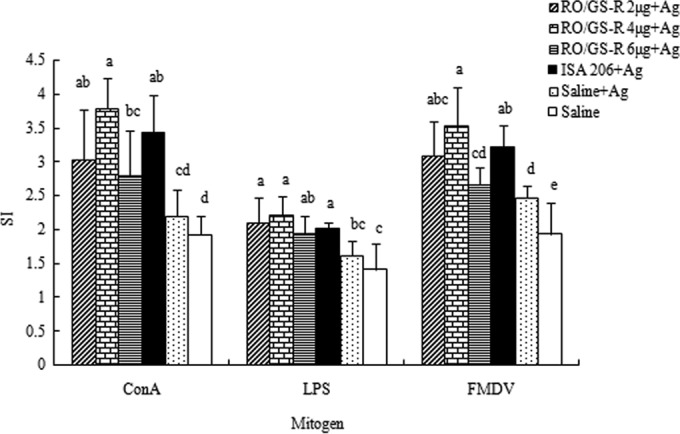
Splenocyte proliferative responses to ConA, LPS, and FMDV antigen. Mice (n = 8/group) were s.c. immunized with FMDV antigen (Ag) plus physiological saline, RO containing GS-R (2, 4, or 6 μg), or ISA 206 on days 1 and 21. The control mice were injected with physiological saline in the same manner. Splenocytes were prepared 2 weeks after boosting and cultured with ConA (5 μg/ml), LPS (8 μg/ml), FMDV antigen (1 μg/ml), or RPMI 1640 medium. Splenocyte proliferation was measured by the MTT method, as described in the text, and is shown as a stimulation index (SI). The values are presented as means ± SD. Bars with different letters have statistically significant differences (P < 0.05).
Cytokines.
The effects of RO and GS-R on serum IFN-γ and IL-5 levels are shown in Fig. 7. IFN-γ and IL-5 levels significantly higher than the control (saline plus antigen) values were found in the sera of mice immunized with vaccine in RO supplemented with 4 μg of GS-R or in ISA 206 (P < 0.05). In mice that received the vaccine in RO in combination with 2 μg or 6 μg of GS-R, only IFN-γ and not IL-5 levels were found to be significantly higher than the control (saline plus antigen) values.
FIG 7.
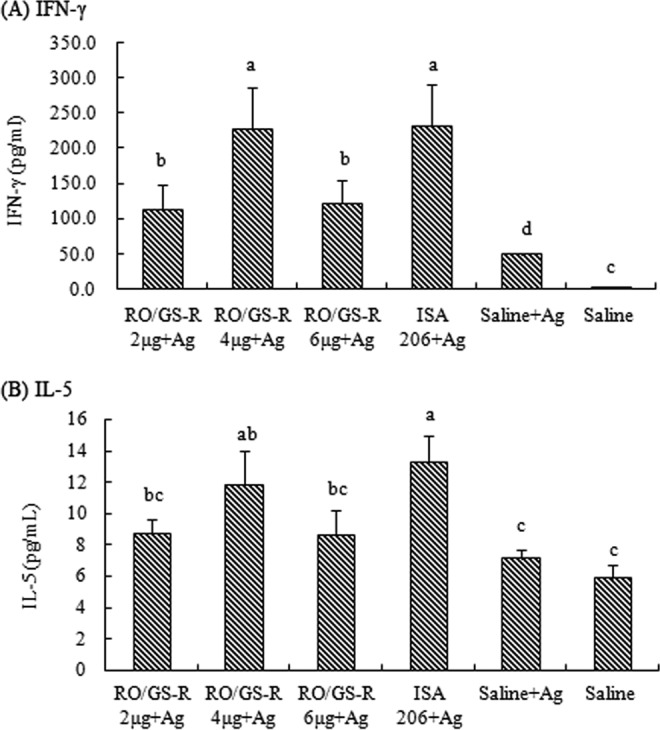
Serum IFN-γ (A) and IL-5 (B) levels. Mice (n = 8/group) were s.c. immunized with FMDV antigen (Ag) plus physiological saline, RO containing GS-R (2, 4, or 6 μg), or ISA 206 on days 1 and 21. Control mice were injected with physiological saline in the same manner. Sera were collected 2 weeks after boosting for analysis of IFN-γ and IL-5 levels by indirect ELISAs. The values are presented as means ± SD. Bars with different letters have statistically significant differences (P < 0.05).
IgG-secreting plasma cells in bone marrow.
FMDV-specific IgG-secreting plasma cells were measured in the bone marrow of mice immunized as shown in Fig. 8. The order of the groups with respect to the number of IgG-secreting plasma cells in bone marrow was as follows: RO/GS-R > RO > saline.
FIG 8.
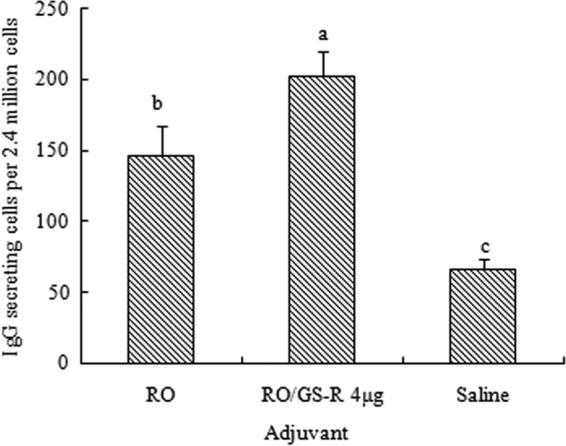
Numbers of FMDV-specific IgG-secreting cells in bone marrow. Mice (n = 8/group) were s.c. immunized with FMDV antigen plus physiological saline, RO, or RO containing GS-R (4 μg) on days 1 and 21. Bone marrow cells were prepared 8 weeks after boosting, and IgG-secreting cells were counted by an ELISPOT assay. The data are presented as means ± SD of FMDV-specific IgG-secreting cells standardized to responses per 2.4 million cells. Bars with different letters have statistically significant differences (P < 0.05).
DISCUSSION
The present study demonstrated that rapeseed oil and GS-R synergistically promoted the Th1/Th2 immune responses induced by an FMD vaccine in a mouse model. When the mice were immunized with FMDV antigen emulsified in an RO/GS-R formulation, the animals produced significantly increased levels of serum antibodies (IgG and isotypes) and cytokines (IFN-γ and IL-5), higher splenocyte proliferative responses to ConA, LPS, and FMDV antigen, and more antibody-secreting plasma cells in the bone marrow than when FMDV antigen was used alone.
Saponins used with different oils may generate different effects on immune responses. We previously reported the synergistic adjuvant effects of GSLS and mineral oil in mice and pigs (9, 10). In this study, the synergistic adjuvant effects were found only for the combination of RO with GS-R and not for the combination of RO with GSLS (Fig. 1). This discrepancy may result from the different oils used (mineral and vegetable oils) and the different fractions of the saponins, as GSLS contained Rb1 (1.4%), Rb2 (3.0%), Rc (2.5%), Rd (8.0%), Re (12.0%), and Rg1 (6.0%) and GS-R contained Rb1 (18.0%), Rb2 (9.5%), Rc (10.0%), Rd (7.6%), Re (8.7%), and Rg1 (3.5%). Rb1 has been reported to be a potent adjuvant (21, 22). Among the fractions, Rb1 may be a crucial component that may create higher GS-R adjuvant activity than GSLS.
Specific antibody responses are important in defense against FMD (23–26). Protection against FMDV infection is considered to be closely related to serum antibody levels (27). High FMDV-specific IgG isotype levels were related to the protection of swine challenged with FMD (28). The present study showed that RO in combination with GS-R (4 μg) promoted a significantly greater serum IgG response induced by FMD vaccine than did RO or GS-R alone (Fig. 2), indicating the synergistic adjuvant activities of RO and GS-R. In comparison with the antigen injected alone, RO/GS-R significantly increased IgG (Fig. 3) and isotype (IgG1, IgG2a, IgG2b, and IgG3) (Fig. 5) levels in experiment 3. The IgG titer induced by the FMD vaccine formulated in the RO/GS-R emulsion (1:2,152) was 16-fold higher than the titer induced by the vaccine in a saline solution (1:134) (Fig. 4). Although we did not carry out neutralizing studies, the serum antibody levels detected by the method described in this study parallel indirect hemagglutination titers, which were reported to be closely correlated with protection against FMDV infection (29). In comparison with 4 μg of GS-R, 6 μg of GS-R showed little adjuvant effect (Fig. 3, 4, 5, 6, and 7). The exact reasons for the reduced adjuvant effect of GS-R at a higher dose are unclear. It may be caused by the antagonistic effects of the fractions in GS-R, because we found that Rg1 could negatively influence the Rb1-generated adjuvant effect, resulting in a diminished antibody response when Rg1 and Rb1 were used together (21).
Different IgG subclasses, such as IgG1, IgG2a, IgG2b, and IgG3, supply animals with the bulk of immunity against the majority of infectious agents. During immune responses depending on T lymphocytes, the principal IgG class of the specific antibodies progressively changes. T lymphocytes and their cytokines influence these changes. In mice, the cytokines IL-4 and IL-5 are favored to switch activated B cells to produce the IgG1 isotype (Th2 type), IFN-γ boosts IgG2a and IgG3 responses (Th1 type), and transforming growth factor β (TGF-β) provokes changes to IgA or IgG2b (30). The data in Fig. 5 show that RO/GS-R promoted significantly higher levels of all IgG subclasses. Increased levels of IgG subclasses may be attributed to increased levels of both IL-5 and IFN-γ, as shown in Fig. 7. All of these results indicated that RO/GS-R activated both Th1 and Th2 responses.
Lymphocyte proliferation varies based on the mitogens used. To induce antibody production, activated B lymphocytes are required for clonal expansion (31). In the RO/GS-R group, significantly increased splenocyte proliferation was stimulated by ConA, LPS, and FMDV antigen (Fig. 6), indicating that T cells as well as B cells were provoked. The improved lymphocyte responses to ConA and LPS match the increased serum IgG levels found in the animals injected with the FMD vaccine adjuvanted with RO/GS-R.
Previous investigations showed that GS-R possesses adjuvant properties. Rivera et al. reported increased antibody titers with immunization against porcine parvovirus (PPV) in guinea pigs when GS-R and the PPV antigen were administered together (21). Hu et al. observed enhanced antibody responses elicited by Staphylococcus aureus antigen in dairy cattle when the antigen was coadministered with ginsenoside Rb1 or GS-R (22). There are two major saponin types in GS-R, namely, protopanaxadiols and protopanaxatriols. After comparing protopanaxadiols (Rb1, Rb2, Rc, Rd, and Rg3) and protopanaxatriols (Rg1, Rg2, and Re), we found that Rg1, Rg2, Rg3, Re, and Rb1 had strong adjuvant properties (32). The components Rb1, Rg1, and Re contained in GS-R might contribute to the adjuvant effects described in this report.
Adjuvant combinations have been described in previous studies (33). Freund's complete adjuvant is a well-known adjuvant combination that fuses the immunostimulating properties of Mycobacterium tuberculosis with the depot effect of water-in-oil emulsion (21). This adjuvant induces potent Th1 and Th2 responses. The mixture of saponin with oil adjuvant is not new, and the synergistic effects of this combination on immunization have been reported previously. For example, Gerber reported that Quil A, in combination with oil as an adjuvant, promoted greater immune responses in pigs immunized against pseudorabies virus, in guinea pigs immunized against canine parvovirus, and in cats immunized against feline infectious virus (33), Martinez-Fernandez et al. found that an oil emulsion containing Quil A increased the immune responses induced by Fh12 fatty acid-binding protein of Fasciola hepatica in sheep (34), and Xiao et al. observed that supplements of saponins such as Quil A and extract from the seeds of Momordica cochinchinensis (Lour.) Spreng in FMD vaccines significantly enhanced immune responses in pigs (29, 35). The synergism between RO and GS-R may be explained by the depot effect of oil emulsion, which slowly releases antigens at the injection site in combination with the immunostimulating effect created by GS-R.
Figure 3 showed that serum IgG levels progressively declined but RO/GS-R reduced the rate of the decline. The IgG levels in the RO/GS-R group at 6 weeks after the boost were similar to those in the group without adjuvant at week 3 but higher than those at week 4 after the boost. The long-lasting IgG response may be attributed to the enhanced FMDV-specific antibody-secreting plasma cells of the bone marrow with RO/GS-R (Fig. 8). Plasma cells resulting from activated B lymphocytes secrete antigen-specific antibodies. The cells that home to and persist in the bone marrow continue secreting antigen-specific antibodies and act as important controllers in the long-term protection responses against pathogenic agents (19, 36). In experiment 5, increased numbers of FMDV-specific antibody-secreting plasma cells in the bone marrow were found even 8 weeks after the second immunization (Fig. 8), suggesting that RO/GS-R stimulated the production of long-lived IgG plasma cells.
In experiments 3 and 4, ISA 206 exhibited potent adjuvant activities (Fig. 3, 4, 5, 6, and 7). Although ISA 206 is widely used in many veterinary vaccines, searching for safer adjuvants for use in food animals remains a priority for many researchers, as ISA 206 is derived from mineral oil and has the potential to retain harmful impurities. In the present study, FMD vaccine formulated in RO/GS-R emulsion induced immune responses similar to those observed with the vaccine adjuvanted with ISA 206, had lower viscosity, and was easier to inject. Therefore, the important finding of this study is the discovery of an adjuvant effect created by rapeseed oil combined with ginseng root saponin (RO/GS-R). This combination should be studied further for the development of veterinary vaccines, especially for use in food animals in order to improve food safety. Work on the use of RO/GS-R as an adjuvant in the Newcastle disease vaccine is ongoing in our laboratory.
ACKNOWLEDGMENTS
This study was supported by the National Natural Scientific Foundation of China (grant 31372471) and the Special Fund for Agro-Scientific Research in the Public Interest (grant 201303040-03).
We thank senior researcher Jinshui Chen for valuable discussions and Ye Xu for assistance in the preparation of figures.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Cao Y, Lu Z, Li D, Fan P, Sun P, Bao H, Fu Y, Li P, Bai X, Chen Y, Xie B, Liu Z. 2014. Evaluation of cross-protection against three topotypes of serotype O foot-and-mouth disease virus in pigs vaccinated with multi-epitope protein vaccine incorporated with poly(I:C). Vet. Microbiol. 168:294–301. 10.1016/j.vetmic.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 2.Li D, Zhou C, She D, Li P, Sun P, Bai X, Chen Y, Xie B, Liu Z. 2013. The comparison of the efficacy of swine FMD vaccine emulsified with oil adjuvant of ISA 201 VG or ISA 206 VG. J. Biosci. Med. 1:22–25. 10.4236/jbm.2013.13005 [DOI] [Google Scholar]
- 3.Rweyemamu M, Roeder P, Mackay D, Sumption K, Brownlie J, Leforban Y, Valarcher JF, Knowles NJ, Saraiva V. 2008. Epidemiological patterns of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 55:57–72. 10.1111/j.1865-1682.2007.01013.x [DOI] [PubMed] [Google Scholar]
- 4.Ward MP, Laffan SW, Highfield LD. 2007. The potential role of wild and feral animals as reservoirs of foot-and-mouth disease. Prev. Vet. Med. 80:9–23. 10.1016/j.prevetmed.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka M, Okabe T, Nakai M, Goto N. 1993. Local pathological reactions and immune response of chickens to ISA-70 and other adjuvants containing Newcastle disease virus antigen. Avian Dis. 37:459–466. 10.2307/1591673 [DOI] [PubMed] [Google Scholar]
- 6.Stone HD. 1997. Newcastle disease oil emulsion vaccines prepared with animal, vegetable, and synthetic oils. Avian Dis. 41:591–597. 10.2307/1592149 [DOI] [PubMed] [Google Scholar]
- 7.Vajdy M. 2011. Immunomodulatory properties of vitamins, flavonoids and plant oils and their potential as vaccine adjuvants and delivery systems. Expert Opin. Biol. Ther. 11:1501–1513. 10.1517/14712598.2011.623695 [DOI] [PubMed] [Google Scholar]
- 8.Roy P, Venugopalan AT, Koteeswaran A. 1999. Efficacy of live adjuvanted mesogenic Newcastle disease vaccine in chickens. Vaccine 17:2674–2676. 10.1016/S0264-410X(99)00024-9 [DOI] [PubMed] [Google Scholar]
- 9.Song X, Bao S, Wu L, Hu S. 2009. Ginseng stem-leaf saponins (GSLS) and mineral oil act synergistically to enhance the immune responses to vaccination against foot-and-mouth disease in mice. Vaccine 27:51–55. 10.1016/j.vaccine.2008.10.030 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Yan X, Hu S. 2012. Ginseng stem-leaf saponins and oil adjuvant synergistically promote the immune responses to Newcastle disease in chickens. J. Anim. Vet. Adv. 11:2423–2428. 10.3923/javaa.2012.2423.2428 [DOI] [Google Scholar]
- 11.Xu WP. 2008. Analysis and outlook of the current market of edible vegetable oil. Agric. Outlook 3:10–13 [Google Scholar]
- 12.China State Bureau of Standards. 2004. National standard of the People's Republic of China (GB 1536): rapeseed oil. China Standards Press, Beijing, China [Google Scholar]
- 13.Chinese Veterinary Pharmacopoeia Commission. 2010. Total ginsenoside of ginseng stems and leaves, p 529–530 In Veterinary pharmacopoeia of People's Republic of China, part II. China Agricultural Press, Beijing, China [Google Scholar]
- 14.Chinese Pharmacopoeia Commission. 2010. Total ginsenoside ginseng root, p 367–368 In Pharmacopoeia of People's Republic of China, part I. China Medical Science and Technology Press, Beijing, China [Google Scholar]
- 15.Stone HD. 1993. Efficacy of experimental animal and vegetable oil-emulsion vaccines for Newcastle disease and avian influenza. Avian Dis. 37:399–405. 10.2307/1591665 [DOI] [PubMed] [Google Scholar]
- 16.Xie F, Li Y, Su F, Hu S. 2012. Adjuvant effect of Atractylodis macrocephalae Koidz. polysaccharides on the immune response to foot-and-mouth disease vaccine. Carbohydr. Polym. 87:1713–1719. 10.1016/j.carbpol.2011.09.080 [DOI] [Google Scholar]
- 17.Zhu J, Liu Y, Pi Y, Jia L, Wang L, Huang Y. 2014. Systemic application of sphingosine 1-phosphate receptor 1 immunomodulator inhibits corneal allograft rejection in mice. Acta Ophthalmol. 92:e12–e21. 10.1111/aos.12237 [DOI] [PubMed] [Google Scholar]
- 18.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, Mompoint F, Vedvick TS, Bertholet S, Coler RN, Reed SG. 2009. Enhanced humoral and type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine 27:5956–5963. 10.1016/j.vaccine.2009.07.081 [DOI] [PubMed] [Google Scholar]
- 19.Slifka MK, Matloubian M, Ahmed R. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor BP, Cascalho M, Noelle RJ. 2002. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195:737–745. 10.1084/jem.20011626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera E, Hu S, Concha C. 2003. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine 21:1149–1157. 10.1016/S0264-410X(02)00518-2 [DOI] [PubMed] [Google Scholar]
- 22.Hu S, Concha C, Lin F, Persson WK. 2003. Adjuvant effect of ginseng extracts on the immune responses to immunisation against Staphylococcus aureus in dairy cattle. Vet. Immunol. Immunopathol. 91:29–37. 10.1016/S0165-2427(02)00264-7 [DOI] [PubMed] [Google Scholar]
- 23.Meloen RH, Rowlands DJ, Brown F. 1979. Comparison of the antibodies elicited by the individual structural polypeptides of foot-and-mouth disease and polio viruses. J. Gen. Virol. 45:761–763. 10.1099/0022-1317-45-3-761 [DOI] [PubMed] [Google Scholar]
- 24.McCullough KC, De Simone F, Brocchi E, Capucci L, Crowther JR, Kihm U. 1992. Protective immune response against foot-and-mouth disease. J. Virol. 66:1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough KC, Crowther JR, Butcher RN, Carpenter WC, Brocchi E, Capucci L, De Simone F. 1986. Immune protection against foot-and-mouth disease virus studied using virus-neutralizing and non-neutralizing concentrations of monoclonal antibodies. Immunology 58:421–428 [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough KC, Parkinson D, Crowther JR. 1988. Opsonization-enhanced phagocytosis of foot-and-mouth disease virus. Immunology 65:187–191 [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Q, Li Y, Zhang M. 2005. Detection of immune response to swine fever and FMD on a pig farm in Ningxia Province. Gansu J. Anim. Husb. Vet. 35:8–10 [Google Scholar]
- 28.Mayr GA, O'Donnell V, Chinsangaram J, Mason PW, Grubman MJ. 2001. Immune responses and protection against foot-and-mouth disease virus (FMDV) challenge in swine vaccinated with adenovirus-FMDV constructs. Vaccine 19:2152–2162. 10.1016/S0264-410X(00)00384-4 [DOI] [PubMed] [Google Scholar]
- 29.Xiao C, Rajput ZI, Hu S. 2007. Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine 25:4795–4800. 10.1016/j.vaccine.2007.04.027 [DOI] [PubMed] [Google Scholar]
- 30.Male D, Roitt I, Brostoff J, Roteth D. 2012. Immunology, 8th ed. Saunders, Philadelphia, PA [Google Scholar]
- 31.Tizard IR. 2009. Veterinary immunology: an introduction. Saunders, Philadelphia, PA [Google Scholar]
- 32.Sun J, Hu S, Song X. 2007. Adjuvant effects of protopanaxadiol and protopanaxatriol saponins from ginseng roots on the immune responses to ovalbumin in mice. Vaccine 25:1114–1120. 10.1016/j.vaccine.2006.09.054 [DOI] [PubMed] [Google Scholar]
- 33.Gerber JD. February 1989. Vaccine formation. US patent 4,806,350
- 34.Martinez-Fernandez AR, Nogal-Ruiz JJ, Lopez-Aban J, Ramajo V, Oleaga A, Manga-Gonzalez Y, Hillyer GV, Muro A. 2004. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet. Parasitol. 126:287–298. 10.1016/j.vetpar.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 35.Xiao C, Rajput ZI, Liu D, Hu S. 2007. Enhancement of serological immune responses to foot-and-mouth disease vaccine by a supplement made of extract of cochinchina momordica seeds. Clin. Vaccine Immunol. 14:1634–1639. 10.1128/CVI.00339-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manz RA, Radbruch A. 2002. Plasma cells for a lifetime? Eur. J. Immunol. 32:923–927. [DOI] [PubMed] [Google Scholar]