Abstract
Mycobacterium bovis causes animal tuberculosis (TB) in cattle, humans, and other mammalian species, including pigs. The goal of this study was to experimentally assess the responses of pigs with and without a history of tonsillectomy to oral vaccination with heat-inactivated M. bovis and challenge with a virulent M. bovis field strain, to compare pig and wild boar responses using the same vaccination model as previously used in the Eurasian wild boar (Sus scrofa), to evaluate the use of several enzyme-linked immunosorbent assays (ELISAs) and lateral flow tests for in vivo TB diagnosis in pigs, and to verify if these tests are influenced by oral vaccination with inactivated M. bovis. At necropsy, the lesion and culture scores were 20% to 43% higher in the controls than those in the vaccinated pigs. Massive M. bovis growth from thoracic tissue samples was observed in 4 out of 9 controls but in none of the 10 vaccinated pigs. No effect of the presence or absence of tonsils was observed on these scores, suggesting that tonsils are not involved in the protective response to this vaccine in pigs. The serum antibody levels increased significantly only after challenge. At necropsy, the estimated sensitivities of the ELISAs and dual path platform (DPP) assays ranged from 89% to 94%. In the oral mucosa, no differences in gene expression were observed in the control group between the pigs with and without tonsils. In the vaccinated group, the mRNA levels for chemokine (C-C motif) receptor 7 (CCR7), interferon beta (IFN-β), and methylmalonyl coenzyme A mutase (MUT) were higher in pigs with tonsils. Complement component 3 mRNA levels in peripheral blood mononuclear cells (PBMC) increased with vaccination and decreased after M. bovis challenge. This information is relevant for pig production in regions that are endemic for M. bovis and for TB vaccine research.
INTRODUCTION
Animal tuberculosis (TB) is caused by infection with Mycobacterium bovis and closely related members of the Mycobacterium tuberculosis complex (MTC). Although cattle are the main concern regarding animal TB in developed countries, several other species of mammals, including humans, can be infected (1, 2).
Globally, M. bovis is one of the 10 most important causes of pig losses (3). Pigs can come in contact with other MTC hosts if raised in free-range, open-air, or backyard systems with limited biosafety. Recent evidence from free-range domestic pigs on the Italian island of Sicily showed that naturally infected pigs develop lung lesions and can contribute to M. bovis maintenance in mixed farming systems (4). Evidence of M. bovis infection in domestic pigs is also available in other countries in Europe (5), Africa (6), and South America (7). Moreover, feral pigs are hosts of M. bovis in several regions worldwide (8–10). This, along with the well-established role of its ancestor, the native Eurasian wild boar (Sus scrofa), in MTC maintenance (11) makes pigs an important subject for study of the relationship of their response to MTC infection and the possibility of protecting them by vaccination.
The tonsils are known to play an important role in the detection and initiation of the immune response to pathogens entering through the mouth and nares, and also in maintaining a systemic immune response by producing lymphocytes, cytokines, and chemokines (12–14). The main pharyngeal mucosa-associated lymphoid tissues in swine are the tonsils of the soft palate, which constitute a point of entry for microorganisms (15).
Previous studies in wild boar analyzed the tonsils as a target organ for mycobacteria, demonstrating that (i) gene expression differs among tuberculous and nontuberculous animals and between tonsils and lymph nodes after natural exposure to M. bovis but also between vaccinated and nonvaccinated animals under experimental conditions, and (ii) differentially regulated molecules of the mandibular lymph nodes and the tonsils are involved in stress/inflammatory responses to mycobacterial infection and may be used as markers for diagnosing TB in wild boar (16–20).
In vivo detection of MTC infection in wild boar is possible through the detection of cell-mediated response (17, 21) and through the detection of specific antibodies by different enzyme-linked immunosorbent assay (ELISA) and animal side dual path platform (DPP) tests (e.g., references 17 and 22). In pigs, tests based on cell-mediated immunity are commonly used in some countries (e.g., Italy [23]). However, antibody detection by ELISA and/or immunochromatographic tests is not frequent in pigs.
Our aim was to experimentally assess the response of pigs with a history of tonsillectomy to oral vaccination with heat-inactivated M. bovis and challenge with a virulent M. bovis field strain, to compare pig and wild boar responses using the same vaccination model as previously used in wild boar (17), to evaluate the use of several ELISA and DPP tests for in vivo TB diagnosis in pigs, and verify if these serodiagnostic tests are influenced by oral vaccination with inactivated M. bovis.
MATERIALS AND METHODS
Ethics statement.
The handling and sampling frequency procedures were designed to reduce stress and health risks for the subjects, according to European (86/609) and Spanish legislation (R.D. 223/1988 and R.D. 1021/2005). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Regional Agriculture Authority (Comunidad de Madrid, permit CM180112-01).
Animals and experimental design.
Twenty 3- to 4-month-old piglets were purchased from an animal experiment facility (Centro de Cría y Suministro de Animales de Experimentación La Chimenea, IMIDRA, Madrid, Spain) known to be free of mycobacterial infections. All animals were tested by ELISA to diagnose M. bovis infection (22) on arrival, and all yielded a fully negative result.
Tonsillectomy took place 1 month prior to the start of the vaccination and challenge experiment. The soft palate tonsils were removed at the Hospital General Universitario Gregorio Marañón (Madrid, Spain). Premedication was performed using ketamine (Ketolar) (15 mg/kg of body weight intramuscularly [i.m.]; Pfizer Ireland Pharmaceuticals, Dublin, Ireland) and atropine (0.033 mg/kg i.m.; Braun Medical SA, Rubí, Barcelona, Spain). The animals were monitored using capnography, pulse oximetry, and electrocardiography. Anesthetic induction was performed with propofol (Propofol Lipomed) (1.66 mg/kg; Fresenius Kabi, Barcelona, Spain), fentanyl (Fentanest) (3 mg/kg; Kern Pharma, Terrassa, Barcelona, Spain), and atracurium besilate (0.60 mg/kg; Inibsa, Llisà de Vall, Barcelona, Spain) intravenously through the auricular dorsal vein. Next, the animals were connected to a Dräger ventilator (SA 1; Dräger Hispania S.A., Madrid, Spain). Anesthesia was maintained with continuous infusion of propofol (12 mg/kg/h), fentanyl (Fentanest) (0.30 mg/kg/h; Kern Pharma), and atracurium besilate (0.05 mg/kg/h). The animals received Ringer lactate solution at (5 to 6 ml/kg/h; Braun) as required. The oral cavity was opened and fixed with Tuffier rib spreaders (Aesculap; Braun). For the surgical approach, a cold light source connected to a column of endoscopy (Fiegert Endotech, Xenon, Tuttlingen, Germany) and laparoscopic equipment (Endoshears; Autosuture, Covidien, Dublin, Ireland) providing sterile cautery (ME 200; Martin Medizintechnik, Tuttlingen, Germany) was introduced into the oral cavity to perform a perimeter incision in the area of the soft palate. The postoperative procedure consisted of the administration of ceftriaxone GES (25 mg/kg; Laboratorios Torlan S.A, Cerdanyola del Vallès, Barcelona, Spain) and Dexketoprofen (2 mg/kg; Enantyum, Laboratorios Menarini, Badalona, Barcelona) intramuscularly for 3 days. One piglet died during anesthesia administration.
The animals were housed in level III biocontainment facilities (BSL3), where they had food and water ad libitum. The piglets were randomly assigned to one of four treatment groups: group 1, vaccinated and with tonsils (V) (n = 5); group 2, vaccinated but with a history of tonsillectomy (TE-V) (n = 5); group 3, unvaccinated but with tonsils (C) (n = 4); and group 4, unvaccinated with a history of tonsillectomy (TE-C) (n = 5).
After an acclimatization period in the BSL3 facility, the animals were handled, and blood was collected five times during the experiment, including at vaccination (T0, day 1), revaccination (T1, day 28), challenge (T2, day 57), one bleeding (T3, day 111 [54 days after challenge]), and necropsy (T4, day 195 [138 days after challenge]). For the challenge, 2 ml of a suspension containing 105 CFU of an M. bovis field strain (spoligotype SB0295) was administered by the oropharyngeal route, as described in previous experiments (17, 24).
Preparation of inactivated vaccine.
The M. bovis strain used (Neiker 1403, spoligotype SB0339) was a first-passage-level culture isolated from a naturally infected wild boar in Coletsos medium. The isolate was propagated in Middlebrook 7H9 broth enriched with oleic acid-albumin-dextrose-catalase (OADC) for 2 to 3 weeks. The cells were harvested by centrifugation at 2,500 × g for 20 min and washed twice in phosphate-buffered saline (PBS). The bacterial pellet was resuspended in PBS. The turbidity of this suspension was adjusted to an optical density of 1 McFarland standard. Before inactivation, 10-fold serial dilutions were prepared and plated in agar-solidified 7H9 medium with OADC in quadruplicate to assess the number of CFU in the inoculum. The inoculum was then inactivated in a water bath at 80°C for 30 min. The animals in groups 1 and 2 were orally administered approximately 107 bacteria, according to CFU counts, diluted in 2 ml of PBS. This inactivated vaccine was again cultured in duplicate to ensure the absence of viable M. bovis.
Necropsy, sample collection, and histopathology.
Each pig was anesthetized by intramuscular injection of Zoletil and euthanized by captive bolt. A thorough postmortem examination was done to detect the presence of macroscopic lesions. The samples for culture were immediately processed, and duplicates were frozen at −80°C for mRNA isolation. After the collection of samples for mRNA analysis, all main lymph nodes (LNs) and the tonsils were serially sliced into 1- to 2-mm-thick slices and carefully inspected for visible TB-compatible lesions. The organs were also carefully inspected, and each lung lobe was considered separately. TB-compatible lesions were classified based on lesion distribution and lesion intensity and scored as previously described (24). The samples of individual tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin stain using standard procedures. An additional section of those tissues with lesions indicative of tuberculosis was stained by the Ziehl-Neelsen (ZN) procedure to detect the presence of acid-fast organisms (AFO).
Microbiology.
The tissues collected were as follows: head lymphoid tissues, including the soft palate tonsil (groups 1 and 3 only), oral mucosa lateral to the soft palate tonsil, and mandibular, parotid, and retropharyngeal lymph nodes (LNs); lung (each lobe), tracheobronchial LNs, and mediastinal LN; and splenic, ileocecal valve, and mesenteric and hepatic LNs. When suspicious lesions were observed in the liver, the kidneys and LNs from other locations were also cultured. The samples were thoroughly homogenized in sterile distilled water (2 g in 10 ml or equivalent) and decontaminated with hexadecylpyridinium chloride at a final concentration of 0.75% (wt/vol) for 18 h. The samples were centrifuged at 1,500 × g for 30 min and the pellets cultured onto Coletsos and 0.20% (wt/vol) pyruvate-enriched Lowenstein-Jensen media (Difco FSM, Madrid, Spain) at 37°C. The culture media were checked weekly over 12 weeks for growth. All isolates were spoligotyped in order to confirm the strain identification (25).
We used a culture score for M. bovis infection, as defined in Garrido et al. (17) for wild boar. This score is the number of LNs or organ samples yielding an M. bovis isolate out of the total number of culture attempts (n ≥ 7 samples cultured per animal; score range, 0 to 7). Each culture sample with >50 CFU was considered massive growth.
Serum antibody detection.
The serum samples were tested for immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies by means of an in-house ELISA. Purified protein derivative (PPD) derived from M. bovis (bPPD) (CZ Veterinaria, Porriño, Spain) was used as an antigen and IgG and IgM antibodies (Bethyl, Inc., Montgomery, TX) as conjugates, and the protocol described by Boadella et al. (22) was applied. The sample results were expressed as an ELISA percentage (E%) that was calculated using the formula mean sample OD/(2 × mean negative control OD) × 100 (OD, optical density). Samples with an E% of >100 were considered positive.
The DPP technology was developed by Chembio Diagnostic Systems, Inc., using selected M. bovis antigens. The serum samples were tested with the DPP WildTB assay and DPP VetTB assay, as previously described (26), and the results were read 20 min after adding sample buffer for both tests. The presence and intensity of either the sole T band (a mixture of MPB70 and MPB83 proteins) of the DPP WildTB assay, or the 2 separate test bands (1, MPB83 antigen; 2, culture filtrate protein 10 [CFP-10]/early secreted antigenic target 6 [ESAT-6] fusion protein) of the DPP VetTB assay were evaluated by a DPP optical reader (in relative light units [RLU]). Reactivity above the cutoff value of 15.0 RLU in any of the test bands was considered a positive result for the presence of antibody (27).
Interferon gamma test.
At T2 and T3, blood samples were collected into tubes with lithium-heparin. Within 8 h of collection, stimulation of whole blood with PBS (nil control) and the avian and bovine purified protein derivative (PDD) (CZ Veterinaria, Porriño, Spain) was performed as described for other species (28, 29). In addition, we used ESAT-6/CFP-10 (EC) and Rv3615c antigens in a concentration of 55 μg/ml. The detection of interferon gamma (IFN-γ) in the supernatant was performed using a quantitative ELISA (Pierce Endogen, Rockford, IL, USA), according to the manufacturer's recommendations.
RNA isolation and real-time RT-PCR.
Total RNA was extracted from peripheral blood mononuclear cells (PBMC) (buffy coat) and oral mucosal samples using TRI reagent (Sigma, Madrid, Spain) and the RNeasy kit (Qiagen, Izasa, Madrid, Spain), respectively, according to the manufacturer's recommendations. RNA was used for real-time reverse transcription-PCR (RT-PCR) analysis of the mRNA levels of selected genes in individual samples. Real-time RT-PCR was performed with gene-specific primers (Table 1) using the One-Step RT-PCR kit with SYBR green and the CFX thermal cycler (Bio-Rad, Hercules, CA, USA), according to the manufacturer's recommendations. A dissociation curve was run at the end of RT-PCR to ensure that only one amplicon was formed and that the amplicon denatured consistently at the same temperature range for every sample (30). The mRNA levels were normalized against S. scrofa cyclophilin, β-actin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the genNorm −ΔΔCT method (31). Normalized threshold cycle (CT) values were compared between groups by Student's t test with unequal variance (P = 0.05).
TABLE 1.
Oligonucleotide primers and RT-PCR conditions for the analysis of mRNA levels for selected genes
| GenBank accession no. | Genea | Primers | PCR annealing conditions (temp [°C], time [s]) |
|---|---|---|---|
| NM_214009 | C3 | SsC3-L (acaaattgacccagcgtagg) and SsC3-R (gcacgtccttgctgtactga) | 55, 30 |
| NM_214405 | MUT | SsMUT-L (gtttgccaacggtgaaaagt) and SsMUT-R (aatgagcttcaaggcagcat) | 55, 30 |
| AY373815 | PRF1 | Ss-PerfF (gctccaccctgagttcaaga) and Ss-PerfR (agtcctccacctccttggat) | 57, 30 |
| NM_001001532 | CCR7 | Ss-CCR7F (tgtgcttcaagaaggacgtg) and Ss-CCR7R (aagggtcaggaggaagagga) | 57, 30 |
| NM_182919 | TRIF | Hs-TRIFF (caggagcctgaggagatgag) and Hs-TRIFR (ctgggtagttggtgctggtt) | 55, 30 |
| NM_001243133 | NLRP3 | Hs-NLRP3F (cttctctgatgaggcccaag) and Hs-NLRP3R (gcagcaaactggaaaggaag) | 55, 30 |
| JN391525 | IFN-β | Ss-IFNBF (tcagaagctcctgggacagt) and Ss-IFNBR (atctgcccatcaagttccac) | 55, 30 |
| DQ913893 | IFN-γ | SsIFNg-L (ttcagctttgcgtgactttg) and SsIFNg-R (tcctttgaatggcctggtta) | 55, 30 |
| NM_214055 | IL-1β | SsIL1beta-L (ccaaagagggacatggagaa) and SsIL1beta-R (ttatatcttggcggcctttg) | 55, 30 |
| NM_001206359 | GAPDH | Ss-GAPDHF (gtcggttgtggatctgacct) and Ss-GAPDHR (agcttgacgaagtggtcgtt) | 55, 30 |
| DQ452569 | β-Actin | Ss-BactinF (ggacctgaccgactacctca) and Ss-BactinR (ggcagctcgtagctcttcat) | 55, 30 |
| AY008846 | Cyclophilin | SsCyclophilin-L (agcactggggagaaaggatt) and SsCyclophilin-R (cttggcagtgcaaatgaaaa) | 55, 30 |
C3, complement component 3; MUT, methylmalonyl coenzyme A mutase; PRF1, perforin 1; CCR7, chemokine (C-C motif) receptor 7; TRIF, Toll-like receptor adaptor molecule 1; NLRP3, NLR family, pyrin domain containing 3; IFN-β, interferon beta; IFN-γ, interferon gamma; IL-1β, interleukin 1β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
We selected for mRNA analysis in oral mucosa at the T4 genes involved in innate immunity (complement component 3 [C3], NLR family, pyrin domain containing 3 [NLRP3], Toll-like receptor adaptor molecule 1 [TRIF 1], interleukin 1β [IL-1β], interferon gamma [IFN-γ], and interferon beta [IFN-β]), mucosal immunity (perforin [PERF] and chemokine [C-C motif] receptor 7 [CCR7]), and methylmalonyl coenzyme A (CoA) mutase (MUT) (Table 1), based on our previous results on differential gene expression in wild boar in response to M. bovis infection and vaccination and other studies (17–19, 24, 32–34).
The C3 mRNA levels of pigs were characterized from peripheral blood mononuclear cells (PBMC) (buffy coat) before vaccination (T0), after vaccination and before challenge (T2), and at the end of the experiment (T4) in vaccinated and control animals, with animals with and without a history of tonsillectomy grouped together after observing that tonsils had no effect on C3 mRNA levels in PBMC and oral mucosa.
Characterization of serum cytokine levels.
The cytokine concentrations in pooled pig sera were determined at T0, T2, and T4 using the Quantibody porcine cytokine array (RayBiotech, Inc., Norcross, GA, USA), an array-based multiplex ELISA system for the simultaneous quantitative measurement of multiple cytokines. Using this system, standard cytokines (IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, granulocyte macrophage colony-stimulating factor [GM-CSF], IFN-γ, transforming growth factor beta 1 [TGF-β1], and tumor necrosis factor alpha [TNF-α]) and samples were assayed in each array simultaneously through a sandwich ELISA procedure, according to the recommendations of the manufacturer (RayBiotech, Inc.). The signals were visualized using a Gene Pix 4100A laser scanner (Molecular Devices, Sunnyvale, CA, USA), and the data were extracted by GenePix Pro 6 software (Molecular Devices). Finally, the quantitative data analysis was performed using the Quantibody Q-Analyzer software (RayBiotech, Inc.). Two replicates were tested for each sample.
Statistical analyses.
The lesion scores among the groups were compared with the nonparametric Mann-Whitney U and median tests. Fisher's tests were used to compare the culture scores between the controls and vaccinated pigs. The differences in antibody levels over time and between the groups were analyzed by analysis of variance (ANOVA) and the median test. Correlations between the antigens and the lesion and culture scores were performed using Pearson (for parametric data) and Spearman (for nonparametric data) tests. The mRNA levels between the groups were compared by Student's t test, as described above.
RESULTS
Clinical signs and pathology.
No pig showed apparent clinical signs of infection, such as emaciation or coughing, during the experiment. At necropsy, visible TB-compatible lesions were recorded in all pigs from all groups, except in one vaccinated pig belonging to group 2. The total lesion and thorax lesion scores were highest in the two control groups (Fig. 1). Three controls and one vaccinated pig had severe visible lesions (score, >10) in the lung parenchyma. Histopathology (hematoxylin and eosin [H&E] staining) confirmed the presence of tuberculous granulomas in all samples with visible lesions. ZN staining revealed that only three vaccinated and two control pigs had no AFO in lung tissues. All controls and 8/10 vaccinated pigs had moderate visible abdominal lesions (score, <10). The mean lesion score was 33.67 (95% confidence interval [CI], 22.59 to 44.74) for the control pigs and 20.50 (95% CI, 11.48 to 29.52) for the vaccinated pigs. The observed reductions in the mean total and thorax lesion scores in vaccinated pigs was and control pigs were 39.11% (95% CI, 12.33 to 65.89%) and 43.28% (95% CI, 4.43 to 82.13%), respectively. The differences between the vaccinated and control pigs in their total and thorax lesion scores were not significant (U test, P > 0.05). No differences in the total and lung lesion scores were evidenced among the four treatment groups (vaccine/control and tonsillectomy/not; median test, chi-square = 3.75, P > 0.05). Also, no differences were found between the vaccinated pigs with or without a history of tonsillectomy (U test; total score, P = 0.69; thorax score, P = 0.55) and between control pigs with and without a history of tonsillectomy (U test; total score, P = 0.29; thorax score, P = 0.73).
FIG 1.

Lesion scores and massive culture growth produced by M. bovis in experimentally infected pigs. The total and thorax lesion scores are shown for the 4 different groups: vaccinated and tonsillectomized (TE-V), vaccinated but not tonsillectomized (V), unvaccinated but tonsillectomized (TE-C), and unvaccinated (C). Black squares indicate animals with massive M. bovis growth (≥50 CFU). The horizontal lines indicate mean scores.
M. bovis isolation.
All isolates belonged to the same spoligotype as the M. bovis strain used for challenge, indicating that it was the same strain. The total culture scores and thorax culture scores were highest in the control groups (5.78 [95% CI, 4.77 to 6.79] and 1.67 [95% CI, 1.36 to 1.97]) compared with the vaccinated groups (4.60 [95% CI, 3.15 to 6.04] and 1.30 [95% CI, 0.90 to 1.70]). We observed reductions in both the total mean culture score and thorax culture score compared to those of the controls (20.41% [95% CI, 0 to 45.42%] and 22.15% [95% CI, 0 to 45.92%], respectively). However, massive M. bovis growth on solid medium (>50 CFU) was observed among 6/9 controls and 2/10 vaccinated pigs (Fisher's test, P = 0.054). Additionally, when considering only the thoracic region tissues (tracheobronchial, mediastinal LNs, and lung tissue), >50 CFU were observed in 4 out of 9 controls but in none of the 10 vaccinated pigs (Fisher's test, P = 0.032). Again, no differences were found between the vaccinated pigs with and without a history of tonsillectomy (U test; total culture score, P = 0.55; thorax culture score, 0.84) and between control pigs with and without a history of tonsillectomy (U test; total culture score, P = 0.11; thorax culture score, P = 0.73).
Serum antibody response.
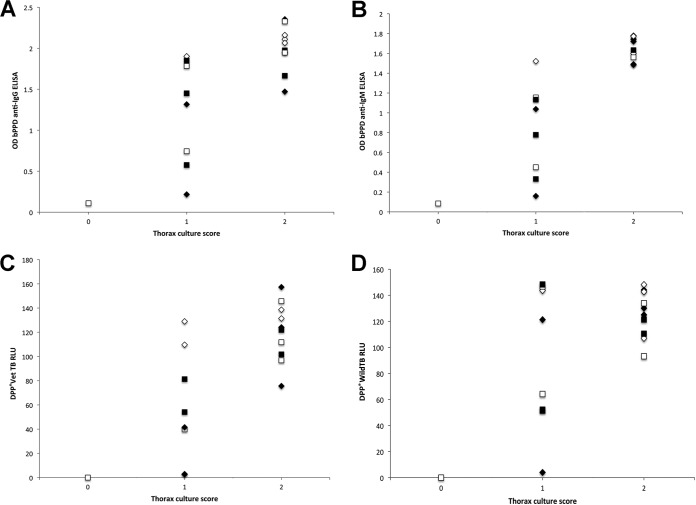
No detectable antibody response was evidenced at T1 and T2. With the exception of one pig belonging to group 2, all four groups consistently responded to challenge, producing detectable antibodies at bleeding (T3) and reaching the highest antibody levels at the time of necropsy (T4). No significant differences between the groups were found at T3 and T4. At T3 and T4, we found positive correlations between some of the antibodies and the lesion and culture scores (Table 2 and Fig. 2).
TABLE 2.
Correlations between serum antibody responses and lesion and culture scores in pigs at bleeding (T3) and necropsy time (T4) using bPPD ELISA (anti-pig IgG and anti-pig IgM), DPP WildTB, and DPP VetTB tests
| Antibody responses for test and time | Data type | Total lesion score | Thorax lesion score | Total culture score | Thorax culture scorea |
|---|---|---|---|---|---|
| OD for bPPD ELISA (time) | |||||
| Anti-IgM (T3) | Correlation coefficient | 0.689b | 0.545c | 0.573c | 0.652b |
| 2-tailed significance | 0.001 | 0.016 | 0.01 | 0.002 | |
| n | 19 | 19 | 19 | 19 | |
| Anti-IgM (T4) | Correlation coefficient | 0.484c | 0.356 | 0.523c | 0.848b |
| 2-tailed significance | 0.036 | 0.135 | 0.022 | 0 | |
| n | 19 | 19 | 19 | 19 | |
| Anti-IgG (T3) | Correlation coefficient | 0.657b | 0.537c | 0.585b | 0.670b |
| 2-tailed significance | 0.002 | 0.018 | 0.009 | 0.002 | |
| n | 19 | 19 | 19 | 19 | |
| Anti-IgG (T4) | Correlation coefficient | 0.394 | 0.29 | 0.449 | 0.777b |
| 2-tailed significance | 0.095 | 0.229 | 0.054 | 0 | |
| n | 19 | 19 | 19 | 19 | |
| DPP for DPP WildTB mixture of MPB70 and MPB83 proteins at time: | |||||
| T3 | Correlation coefficient | 0.436 | 0.272 | 0.630b | 0.479c |
| 2-tailed significance | 0.071 | 0.276 | 0.005 | 0.044 | |
| n | 18 | 18 | 18 | 18 | |
| T4 | Correlation coefficient | 0.382 | 0.211 | 0.537c | 0.348 |
| 2-tailed significance | 0.107 | 0.387 | 0.018 | 0.144 | |
| n | 19 | 19 | 19 | 19 | |
| DPP for DPP VetTB MPB83, CFP-10/ESAT-6 at time: | |||||
| T3 | Correlation coefficient | 0.305 | 0.141 | 0.506b | 0.304 |
| 2-tailed significance | 0.204 | 0.566 | 0.027 | 0.206 | |
| n | 19 | 19 | 19 | 19 | |
| T4 | Correlation coefficient | 0.34 | 0.2 | 0.496c | 0.705b |
| 2-tailed significance | 0.154 | 0.411 | 0.031 | 0.001 | |
| n | 19 | 19 | 19 | 19 |
Nonparametric Spearman correlations.
Significant at the 0.01 level.
Significant at the 0.05 level.
FIG 2.
Serum antibody response and thorax culture score in experimentally infected pigs. The thorax culture score ranged from 0 to 2. (A and B) Serum antibody responses in terms of optical density (OD) in the bovine PPD (bPPD) ELISA using anti-IgG and anti-IgM as conjugates. (C and D) Serum antibody responses in terms of relative light units (RLU) in the DPP VetTB and DPP WildTB tests. The diamonds represent control animals and the squares represent vaccinated animals. The open symbols represent tonsillectomized animals, and filled symbols represent nontonsillectomized pigs. High serum antibody levels were consistently detected in the 10 pigs with a high culture score.
From a diagnostic perspective, at T3 (54 days after challenge), the sensitivities of the IgG and IgM bPPD ELISAs were 94.44% (95% CI, 72.35 to 99.99%) and 77.78% (95% CI, 54.25 to 91.53%), respectively. Regarding the DPP tests, the sensitivity was 94.11% (95% CI, 71.08 to 99.99%) for the DPP WildTB assay and 77.8% (95% CI, 54.25 to 91.53%) for the DPP VetTB assay. The specificity was 100% (95% CI, 85.23 to 100%) for all tests at T0. At the time of necropsy (T4), 17 out of 18 confirmed-infected pigs were diagnosed both by bPPD ELISA (IgM and IgG) and the DPP WildTB assay, while 16 were diagnosed by the DPP VetTB assay. In the DPP VetTB assay, 16 pigs reacted to test 1 (MPB83), and only 3 also reacted to test 2 (ESAT-6/CFP-10 fusion protein). This means the sensitivity for both the bPPD ELISA and the DPP WildTB assay was 94.44% (72.35 to 99.99%), and it was 88.89% (65.95 to 98.14%) for the DPP VetTB assay.
IFN-γ response.
Similar responses regarding IFN-γ production were obtained regardless of whether blood was stimulated with bPPD, EC, or Rv3615c antigens. All pigs tested negative to IFN-γ at T2. All PBS controls also yielded consistently low results (mean OD, 0.10; 95% CI, 0.07 to 0.11). At T3, 54 days after challenge, all but one pig in group 2 showed a clear positive IFN-γ response. No significant correlations between IFN-γ responses and ZN-positive tissues, or culture or lesion scores were found. No differences in IFN-γ response were found between the vaccinated pigs with and without a history of tonsillectomy (U test, bPPD, EC, and Rv3615c, P = 0.55, 0.69, and 0.15, respectively) and between control pigs with and without a history of tonsillectomy (U test, bPPD, EC, and Rv3615c, P = 0.90, 0.69, and 0.20, respectively).
RNA isolation and real-time RT-PCR.
In the oral mucosa, no differences were observed in the control group between the animals with and without a history of tonsillectomy, but the mRNA levels for CCR7, IFN-β, and MUT were higher in vaccinated pigs with tonsils when animals with and without a history of tonsillectomy were compared (Fig. 3A and data not shown). However, the comparison between the vaccinated and control animals when pigs with and without a history of tonsillectomy were grouped together gave similar gene expression levels between the two groups, and only IL-1β expression in the oral mucosa was higher in vaccinated animals than in the controls (Fig. 3B). The C3 mRNA levels in PBMC increased with vaccination and decreased after challenge with M. bovis (Fig. 3C).
FIG 3.
Gene expression in vaccinated and control pigs. The mRNA levels of selected genes were analyzed by real-time RT-PCR in the oral mucosa of vaccinated and control pigs (A and B). Two independent comparisons were conducted between pigs with and without tonsillectomy (n = 5 each) (A) and vaccinated and control pigs when animals with and without a history of tonsillectomy were grouped together (n = 10 and 9, respectively) (B). The mRNA levels were normalized against S. scrofa cyclophilin, β-actin, and GAPDH, and normalized CT values were represented as the mean ± standard deviation (SD) and compared between groups by Student's t test with unequal variance (*, P ≤ 0.05). The samples were taken at the end of the experiment (T4). (C) C3 mRNA levels were analyzed by real-time RT-PCR in the PBMC of vaccinated and control pigs. (D) Cytokine protein levels were determined in pooled sera from vaccinated and control pigs at T0, T2, and T4 using the Quantibody porcine cytokine array. *, P ≤ 0.05.
Characterization of serum cytokine levels.
The serum IL-1β protein levels increased after vaccination and infection in vaccinated animals, while IL-6 levels increased after vaccination but decreased after infection (Fig. 3D). No significant differences were found for IL-4, IL-8, IL-10, IL-12, GM-CSF, IFN-γ, TGF-β1, or TNF-α.
DISCUSSION
This experiment produced three main results. First, the response of pigs to oral vaccination with inactivated M. bovis and challenge with an M. bovis field strain was similar to that recently described for its ancestor, the wild boar (17). Second, the consistent lack of differences between the experimental groups with and without a history of tonsillectomy implies that the role of the tonsils of the soft palate in the pig response to oral vaccination and to infection is smaller than expected. Third, serological tests were found to be highly sensitive for MTC infection diagnosis in pigs, and vaccination did not interfere with this sensitivity.
In general terms, the pigs showed more severe lesions after challenge with field M. bovis than wild boar challenged with similar doses in previous trials (17, 35, 36). Also, abdominal lesions were more evident in pigs. As in previous experiments in wild boar, the lung lesion and culture scores were higher in control pigs, and no massive growth of M. bovis in the thoracic tissues was recorded among the vaccinated pigs. The high challenge dose used might contribute to the severity of the observed lesions (17). However, these comparisons should be interpreted with caution, since the data were derived from different experiments.
The finding of no effect of tonsillectomy on the pig response to vaccination and challenge was unexpected, since it is known that gene expression differs between the tonsils and lymph nodes after natural exposure to M. bovis and after vaccination and challenge (16, 17, 19, 20). The tonsils of the soft palate have been recognized in many studies as the main pharyngeal mucosa-associated lymphoid tissues in swine (12, 14, 15, 37). However, this finding might indicate that other mucosa-associated lymphoid tissues located in the oropharyngeal area, including other tonsils, play a significant role in initiating immune responses against antigens in contact with mucosal surfaces (38).
As far as we know, this is the first time that bPPD ELISA and DPP TB tests have been used for TB diagnostics in pigs. The findings of 94% sensitivity with 100% specificity indicate that both the plate ELISA and the lateral flow DPP assay are as suitable for TB diagnosis in pigs as they are in wild boar (22). However, studies on uninfected free-range pigs and pigs known to be infected with nontuberculous mycobacteria are necessary to precisely define the specificity. The fact that the bPPD ELISA and DPP WildTB assay already detected >90% only 54 days after challenge shows that serology allows for early detection, at least under our experimental conditions. Although both vaccination and tonsillectomy potentially affect the natural antibody response of normal pigs to M. bovis infection, in this study, the vaccination effect and presence of tonsils did not influence the accuracy of the serological tests. The positive correlations between the pathology and serum antibody response are also in agreement with previous results in wild boar (17). The lack of antibody response prior to challenge suggests that oral vaccination with heat-inactivated M. bovis does not elicit serum antibody production in pigs. This is rather counterintuitive, since it is known that wild boar do develop detectable antibodies against orally administered antigens (39).
The levels of IFN-γ in response to the three different antigens increased in all confirmed-infected pigs at 54 days postchallenge (T3). Thus, the IFN-γ assay yielded a sensitivity of 100%. Despite that, this sensitivity was higher than the one reported for free-range domestic pigs in Italy, which was 79 to 85% (23); however, the conditions of the two studies are different, i.e., controlled laboratory versus field conditions. It is also important to note that all pigs tested negative to IFN-γ at T2 (i.e., prior to challenge). This implies that IFN-γ tests suffer no interference due to oral vaccination with heat-inactivated M. bovis.
The differences in the mRNA levels for CCR7, IFN-β, and MUT observed in vaccinated pigs but not in the controls when animals with and without a history of tonsillectomy were compared supports the role of the tonsils in the expression of some genes in response to vaccination (40). However, in agreement with the fact that tonsils did not affect the protective response in pigs, the expression of these genes was similar between the vaccinated and control animals when those with and without tonsils were grouped together.
The complement component C3 has been associated with resistance to TB in wild boar (18, 19, 33), and its gene expression increases after oral vaccination with M. bovis BCG or heat-inactivated M. bovis (17, 24). In this study, the C3 mRNA levels in PBMC increased with vaccination and decreased after challenge with M. bovis. These results agree with previous findings in wild boar (17, 24), in which C3 mRNA levels in PBMC also increased with vaccination and decreased after challenge with M. bovis.
When pigs with and without tonsils were grouped together, only IL-1β expression in the oral mucosa was higher in vaccinated animals than that in the controls. This result suggests a role for this molecule in the protection against MTC in pigs. It has been shown that IL-1β is an important mediator of innate immunity against mycobacterial infection, but it can also promote inflammatory tissue damage (41). The IL-1β produced by dendritic cells preserves and expands IL-22+ immature natural killer cells, potentially influencing mucosal innate immunity during infection (42). Furthermore, the expression of IL-22 was recently correlated with protection against bovine TB in cattle vaccinated with BCG (43). These results suggest that higher IL-1β expression in the oral mucosa and an increase in serum cytokine levels in vaccinated pigs may constitute a protective response to infection enhanced by the vaccine, likely through its effect on stimulating C3 production, which in turn has a role against mycobacterial infection (17–19, 24, 33, 44–47).
In conclusion, the reductions in TB total lesion, thorax lesion, and thorax culture scores in heat-inactivated M. bovis-vaccinated pigs (39%, 43%, and 22%, respectively) were lower than those in wild boar immunized in the same way (43%, 76%, and 33%, respectively), suggesting differences between the pig and wild boar models (17). However, the antibody and IFN-γ responses behaved in the same way in pigs and in wild boar. Also, the high sensitivities of the serological tests for diagnosis were similar to those previously found in wild boar (22). Regarding the gene expression studies, the results suggest that tonsils have a role in the expression of some genes in response to vaccination in pigs but are not associated with protection, which correlated with C3 expression, as previously shown in wild boar (17, 24). In summary, the results show that oral vaccination with heat-inactivated M. bovis does not interfere with serodiagnosis and that the response of pigs to oral vaccination with heat-inactivated M. bovis is similar to that in wild boar.
ACKNOWLEDGMENTS
We thank many colleagues at IREC, NEIKER, and VISAVET for technical support and helpful discussions. We also thank the authorities in the Spanish Ministry of Agriculture, Food, and Environment and the Gobierno de Castilla La Mancha for their continuous encouragement.
This study is a contribution to EU FP7 grant 613779 WildTBVac and to MINECO Plan Nacional grant AGL2011-30041 and FEDER.
Footnotes
Published ahead of print 11 June 2014
REFERENCES
- 1.Gortázar C, Delahay RJ, McDonald RA, Boadella M, Wilson GJ, Gavier-Widen D, Acevedo P. 2012. The status of tuberculosis in European wild mammals. Mammal Rev. 42:193–206. 10.1111/j.1365-2907.2011.00191.x [DOI] [Google Scholar]
- 2.Gortázar C, Vicente J, Boadella M, Ballesteros C, Galindo RC, Garrido J, Aranaz A, de la Fuente J. 2011. Progress in the control of bovine tuberculosis in Spanish wildlife. Vet. Microbiol. 151:170–178. 10.1016/j.vetmic.2011.02.041 [DOI] [PubMed] [Google Scholar]
- 3.International Bank for Reconstruction and Development, The World Bank, TAFS Forum. 2011. World livestock disease atlas: a quantitative analysis of global animal health data (2006–2009). International Bank for Reconstruction and Development, The World Bank, and the TAFS Forum, Washington, DC: http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2012/02/17/000356161_20120217030841/Rendered/PDF/668590WP00PUBL00Livestock0Atlas0web.pdf [Google Scholar]
- 4.Di Marco V, Mazzone P, Capucchio MT, Boniotti MB, Aronica V, Russo M, Fiasconaro M, Cifani N, Corneli S, Biasibetti E, Biagetti M, Pacciarini ML, Cagiola M, Pasquali P, Marianelli C. 2012. Epidemiological significance of the domestic black pig (Sus scrofa) in maintenance of bovine tuberculosis in Sicily. J. Clin. Microbiol. 50:1209–1218. 10.1128/JCM.06544-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richomme C, Boschiroli ML, Hars J, Casabianca F, Ducrot C. 2010. Bovine tuberculosis in livestock and wild boar on the Mediterranean Island, Corsica. J. Wildl. Dis. 46:627–631. 10.7589/0090-3558-46.2.627 [DOI] [PubMed] [Google Scholar]
- 6.Muwonge A, Johansen TB, Vigdis E, Godfroid J, Olea-Popelka F, Biffa D, Skjerve E, Djønne B. 2012. Mycobacterium bovis infections in slaughter pigs in Mubende district, Uganda: a public health concern. BMC Vet. Res. 8:168. 10.1186/1746-6148-8-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumárraga MJ, Arriaga C, Barandiaran S, Cobos-Marín L, de Waard J, Estrada-Garcia I, Figueiredo T, Figueroa A, Giménez F, Gomes HM, Gonzalez-Y-Merchand JA, Macías A, Milián-Suazo F, Rodríguez CA, Santillán MA, Suffys PN, Trangoni MD, Zárraga AM, Cataldi A. 2013. Understanding the relationship between Mycobacterium bovis spoligotypes from cattle in Latin American countries. Res. Vet. Sci. 94:9–21. 10.1016/j.rvsc.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Essey MA, Payne RL, Himes EM, Luchsinger D. 1981. Bovine tuberculosis surveys of axis deer and feral swine on the Hawaiian island of Molokai, p 538–549 Proc. 85th Ann. Meet. U.S. Animal Health Association, St. Louis, MO, 11 to 16 October 1981 [Google Scholar]
- 9.Meikle V, Bianco MV, Blanco FC, Gioffré A, Garbaccio S, Vagnoni L, Di Rienzo J, Canal A, Bigi F, Cataldi A. 2011. Evaluation of pathogenesis caused in cattle and guinea pig by a Mycobacterium bovis strain isolated from wild boar. BMC Vet. Res. 7:37. 10.1186/1746-6148-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan TJ, Livingstone PG, Ramsey DS, de Lisle GW, Nugent G, Collins DM, Buddle BM. 2006. Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: the experience from New Zealand. Vet. Microbiol. 112:211–219. 10.1016/j.vetmic.2005.11.025 [DOI] [PubMed] [Google Scholar]
- 11.Naranjo V, Gortazar C, Vicente J, de la Fuente J. 2008. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 127:1–9. 10.1016/j.vetmic.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Belz GT. 1998. Intercellular and lymphatic pathways associated with tonsils of the soft palate in young pigs. Anat. Embryol. (Berl.) 197:331–340. 10.1007/s004290050143 [DOI] [PubMed] [Google Scholar]
- 13.Cesta MF. 2006. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol. Pathol. 34:599–608. 10.1080/01926230600865531 [DOI] [PubMed] [Google Scholar]
- 14.Horter DC, Yoon KJ, Zimmerman JJ. 2003. A review of porcine tonsils in immunity and disease. Anim. Health Res. Rev. 4:143–155. 10.1079/AHRR200358 [DOI] [PubMed] [Google Scholar]
- 15.Belz GT, Heath TJ. 1996. Tonsils of the soft palate of young pigs: crypt structure and lymphoepithelium. Anat. Rec. 245:102–113 [DOI] [PubMed] [Google Scholar]
- 16.Galindo RC, Ayoubi P, Naranjo V, Gortazar C, Kocan KM, de la Fuente J. 2009. Gene expression profiles of European wild boar naturally infected with Mycobacterium bovis. Vet. Immunol. Immunopathol. 129:119–125. 10.1016/j.vetimm.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 17.Garrido JM, Sevilla IA, Beltrán-Beck B, Minguijón E, Ballesteros C, Galindo RC, Boadella M, Lyashchenko KP, Romero B, Geijo MV, Ruiz-Fons F, Aranaz A, Juste RA, Vicente J, de la Fuente J, Gortázar C. 2011. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS One 6:e24905. 10.1371/journal.pone.0024905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lastra JM, Galindo RC, Gortázar C, Ruiz-Fons F, Aranaz A, de la Fuente J. 2009. Expression of immunoregulatory genes in peripheral blood mononuclear cells of European wild boar immunized with BCG. Vet. Microbiol. 134:334–339. 10.1016/j.vetmic.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 19.Naranjo V, Höfle U, Vicente J, Martín MP, Ruiz-Fons F, Gortazar C, Kocan KM, de la Fuente J. 2006. Genes differentially expressed in oropharyngeal tonsils and mandibular lymph nodes of tuberculous and nontuberculous European wild boars naturally exposed to Mycobacterium bovis. FEMS Immunol. Med. Microbiol. 46:298–312. 10.1111/j.1574-695X.2005.00035.x [DOI] [PubMed] [Google Scholar]
- 20.Naranjo V, Villar M, Martín-Hernando MP, Vidal D, Höfle U, Gortazar C, Kocan KM, Vázquez J, de la Fuente J. 2007. Proteomic and transcriptomic analyses of differential stress/inflammatory responses in mandibular lymph nodes and oropharyngeal tonsils of European wild boars naturally infected with Mycobacterium bovis. Proteomics 7:220–231. 10.1002/pmic.200600527 [DOI] [PubMed] [Google Scholar]
- 21.Jaroso R, Vicente J, Fernandez-de-Mera IG, Aranaz A, Gortazar C. 2010. Eurasian wild boar response to skin-testing with mycobacterial and non-mycobacterial antigens. Prev. Vet. Med. 96:211–217. 10.1016/j.prevetmed.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 22.Boadella M, Lyashchenko K, Greenwald R, Esfandiari J, Jaroso R, Carta T, Garrido JM, Vicente J, de la Fuente J, Gortázar C. 2011. Serologic tests for detecting antibodies against Mycobacterium bovis and Mycobacterium avium subspecies paratuberculosis in Eurasian wild boar (Sus scrofa scrofa). J. Vet. Diagn. Invest. 23:77–83. 10.1177/104063871102300111 [DOI] [PubMed] [Google Scholar]
- 23.Pesciaroli M, Russo M, Mazzone P, Aronica V, Fiasconaro M, Boniotti MB, Corneli S, Cagiola M, Pacciarini M, Di Marco V, Pasquali P. 2012. Evaluation of the interferon-gamma (IFN-γ) assay to diagnose Mycobacterium bovis infection in pigs. Vet. Immunol. Immunopathol. 148:369–372. 10.1016/j.vetimm.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 24.Ballesteros C, Garrido JM, Vicente J, Romero B, Galindo RC, Minguijón E, Villar M, Martín-Hernando MP, Sevilla I, Juste R, Aranaz A, de la Fuente J, Gortázar C. 2009. First data on Eurasian wild boar response to oral immunization with BCG and challenge with a Mycobacterium bovis field strain. Vaccine 27:6662–6668. 10.1016/j.vaccine.2009.08.095 [DOI] [PubMed] [Google Scholar]
- 25.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyashchenko KP, Greenwald R, Esfandiari J, Chambers MA, Vicente J, Gortazar C, Santos N, Correia-Neves M, Buddle BM, Jackson R, O'Brien DJ, Schmitt S, Palmer MV, Delahay RJ, Waters WR. 2008. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Vet. Microbiol. 132:283–292. 10.1016/j.vetmic.2008.05.029 [DOI] [PubMed] [Google Scholar]
- 27.Greenwald R, Lyashchenko O, Esfandiari J, Miller M, Mikota S, Olsen JH, Ball R, Dumonceaux G, Schmitt D, Moller T, Payeur JB, Harris B, Sofranko D, Waters WR, Lyashchenko KP. 2009. Highly accurate antibody assays for early and rapid detection of tuberculosis in African and Asian elephants. Clin. Vaccine Immunol. 16:605–612. 10.1128/CVI.00038-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Álvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Reviriego Gordejo FJ, Briones V, Moreno MA, Mateos A, Domínguez L, Aranaz A. 2008. Interference of paratuberculosis with the diagnosis of tuberculosis in a goat flock with a natural mixed infection. Vet. Microbiol. 128:72–80. 10.1016/j.vetmic.2007.08.034 [DOI] [PubMed] [Google Scholar]
- 29.Gormley E, Doyle MB, Fitzsimons T, McGill K, Collins JD. 2006. Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam) assay. Vet. Microbiol. 112:171–179. 10.1016/j.vetmic.2005.11.029 [DOI] [PubMed] [Google Scholar]
- 30.Ririe KM, Rasmussen RP, Wittwer CT. 1997. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochem. 245:154–160. 10.1006/abio.1996.9916 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32.Johnson RP. 2011. Vaccinology: persistence pays off. Nature 473:456–457. 10.1038/473456a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naranjo V, Ayoubi P, Vicente J, Ruiz-Fons F, Gortazar C, Kocan KM, de la Fuente J. 2006. Characterization of selected genes upregulated in non-tuberculous European wild boar as possible correlates of resistance to Mycobacterium bovis infection. Vet. Microbiol. 116:224–231. 10.1016/j.vetmic.2006.03.013 [DOI] [PubMed] [Google Scholar]
- 34.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M, Blander JM. 2011. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474:385–389. 10.1038/nature10072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gortazar C, Beltrán-Beck B, Garrido JM, Aranaz A, Sevilla IA, Boadella M, Lyashchenko KP, Galindo RC, Montoro V, Domínguez L, Juste R, de la Fuente J. 2014. Oral re-vaccination of Eurasian wild boar with Mycobacterium bovis BCG yields a strong protective response against challenge with a field strain. BMC Vet. Res. 10:96. 10.1186/1746-6148-10-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beltrán-Beck B, de la Fuente J, Garrido JM, Aranaz A, Sevilla I, Villar M, Boadella M, Galindo RC, Pérez de la Lastra JM, Moreno-Cid JA, Fernández de Mera IG, Alberdi P, Santos G, Ballesteros C, Lyashchenko KP, Minguijón E, Romero B, de Juan L, Domínguez L, Juste R, Gortazar C. Oral vaccination with heat inactivated Mycobacterium bovis activates the complement system to protect against tuberculosis. PLoS One 9:e98048. 10.1371/journal.pone.0098048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kernaghan S, Bujold AR, MacInnes JI. 2012. The microbiome of the soft palate of swine. Anim. Health Res. Rev. 13:110–120. 10.1017/S1466252312000102 [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Yu Q, Li P, Yang Q. 2012. Histological and ultrastructural examinations of porcine tonsils. Anat. Rec. (Hoboken) 295:686–690. 10.1002/ar.21534 [DOI] [PubMed] [Google Scholar]
- 39.Ballesteros C, Gortázar C, Canales M, Vicente J, Lasagna A, Gamarra JA, Carrasco-García R, de la Fuente J. 2009. Evaluation of baits for oral vaccination of European wild boar piglets. Res. Vet. Sci. 86:388–393. 10.1016/j.rvsc.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 40.Van Kempen MJ, Rijkers GT, Van Cauwenberge PB. 2000. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 122:8–19. 10.1159/000024354 [DOI] [PubMed] [Google Scholar]
- 41.Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 14:52–60. 10.1038/ni.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, Zhang X, Gavrilin MA, Wewers MD, Caligiuri MA. 2010. Interleukin-1β selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 32:803–814. 10.1016/j.immuni.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhuju S, Aranday-Cortes E, Villarreal-Ramos B, Xing Z, Singh M, Martin Vordermeier H. 2012. Global gene transcriptome analysis in vaccinated cattle revealed a dominant role of IL-22 for protection against bovine tuberculosis. PLoS Pathog. 8:e1003077. 10.1371/journal.ppat.1003077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Aït-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. 1998. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J. Immunol. 160:1666–1676 [PubMed] [Google Scholar]
- 45.Moon MR, Parikh AA, Pritts TA, Fischer JE, Cottongim S, Szabo C, Salzman AL, Hasselgren PO. 1999. Complement component C3 production in IL-1β-stimulated human intestinal epithelial cells is blocked by NF-κB inhibitors and by transfection with Ser 32/36 mutant IκBα. J. Surg. Res. 82:48–55. 10.1006/jsre.1998.5503 [DOI] [PubMed] [Google Scholar]
- 46.Moon MR, Parikh AA, Pritts TA, Kane C, Fischer JE, Salzman AL, Hasselgren PO. 2000. Interleukin-1β induces complement component C3 and IL-6 production at the basolateral and apical membranes in a human intestinal epithelial cell line. Shock 13:374–378 [DOI] [PubMed] [Google Scholar]
- 47.van Kooten C, Fiore N, Trouw LA, Csomor E, Xu W, Castellano G, Daha MH, Gelderman KA. 2008. Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Mol. Immunol. 45:4064–4072. 10.1016/j.molimm.2008.07.015 [DOI] [PubMed] [Google Scholar]