ABSTRACT
Increasing data suggest that NK cells can mediate antiviral activity in HIV-1-infected humans, and as such, novel approaches harnessing the anti-HIV-1 function of both T cells and NK cells represent attractive options to improve future HIV-1 immunotherapies. Chronic progressive HIV-1 infection has been associated with a loss of CD4+ T helper cell function and with the accumulation of anergic NK cells. As several studies have suggested that cytokines produced by CD4+ T cells are required to enhance NK cell function in various infection models, we hypothesized that reconstitution of HIV-1-specific CD4+ T-cell responses by therapeutic immunization would restore NK cell activity in infected individuals. Using flow cytometry, we examined the function of CD4+ T cells and NK cells in response to HIV-1 in subjects with treated chronic HIV-1 infection before and after immunization with an adjuvanted HIV-1 Gp120/NefTat subunit protein vaccine candidate provided by GlaxoSmithKline. Vaccination induced an increased expression of interleukin-2 (IL-2) by Gp120-specific CD4+ T cells in response to HIV-1 peptides ex vivo, which was associated with enhanced production of gamma interferon (IFN-γ) by NK cells. Our data show that reconstitution of HIV-1-specific CD4+ T-cell function by therapeutic immunization can enhance NK cell activity in HIV-1-infected individuals.
IMPORTANCE NK cells are effector cells of the innate immune system and are important in the control of viral infection. Recent studies have demonstrated the crucial role played by NK cells in controlling and/or limiting acquisition of HIV-1 infection. However, NK cell function is impaired during progressive HIV-1 infection. We recently showed that therapeutic immunization of treated HIV-1-infected individuals reconstituted strong T-cell responses, measured notably by their production of IL-2, a cytokine that can activate NK cells. The current study suggests that reconstitution of T-cell function by therapeutic vaccination can enhance NK cell activity in individuals with chronic HIV-1 infection. Our findings provide new insights into the interplay between adaptive and innate immune mechanisms involved in HIV-1 immunity and unveil opportunities to harness NK cell function in future therapeutic vaccine strategies to target HIV-1.
INTRODUCTION
Recent studies in mice and nonhuman primates have reported that antigen-specific CD4+ T cells can provide immunological help not only to CD8+ T cells and B cells but also to NK cells (1, 2). In humans, the potential of T cell-derived interleukin-2 (IL-2) to enhance NK cell activation has been suggested in vitro in response to influenza virus (3) and to Plasmodium falciparum infections (4) and in vivo in subjects receiving rabies virus vaccination (5) or exposed to malaria (6, 7). Chronic HIV-1 infection is associated with a loss of HIV-1-specific CD4+ T helper cell proliferative responses and IL-2 production (8–11), and T helper cell function is only marginally restored in individuals with chronic infection on suppressive antiretroviral therapy (ART) (12–14). Furthermore, HIV-1 infection can significantly affect NK cell function, leading to reduced ability to lyse target cells and to produce cytokines and chemokines (reviewed in reference 15). However, it remains unknown how the loss of antigen-specific CD4+ T-cell function affects NK cell responses in individuals with HIV-1 infection and whether NK cell function can be reconstituted in chronically infected persons. As human NK cells can mediate anti-HIV-1 activity (16–24), novel approaches harnessing the function of both HIV-1-specific T cells and NK cells represent an attractive option to improve future vaccines or immunotherapeutic interventions. Virus-specific T-cell proliferation can be restored by therapeutic immunizations in individuals with ART-treated chronic HIV-1 infection, and this functional recovery primarily relies on increased HIV-1-specific T helper cell responses (10, 11, 25). Therefore, we assessed whether enhancing HIV-1-specific CD4+ T-cell function through therapeutic immunization could restore NK cell activity in chronically HIV-1-infected individuals on antiretroviral treatment. Here, we show that IL-2-secreting HIV-1-specific CD4+ T cells play a critical role in activating NK cells in HIV-1-infected subjects receiving an adjuvanted HIV-1 Gp120/NefTat subunit protein vaccine candidate, highlighting an underappreciated cooperative mechanism at play in HIV-1 infection.
MATERIALS AND METHODS
Study participants.
We used cryopreserved peripheral blood mononuclear cells (PBMCs) derived from 17 subjects with chronic HIV-1 infection, including 6 subjects who were ART treated for >1 year, 5 with untreated progressive disease, and 6 elite controllers (HIV-1 RNA below the limit of detection of standard viral load assays [<50 copies/ml] for at least 1 year) (Table 1) and from 13 ART-treated HIV-1-infected volunteers participating in a double-blinded, randomized, placebo-controlled clinical trial of GlaxoSmithKline (GSK) Vaccines' HIV-1 Gp120/NefTat subunit protein vaccine formulated with the AS02A adjuvant system (ClinicalTrials.gov identifier NCT00117429) (8 received the vaccine, 3 the placebo, and 2 the adjuvant alone) (Table 2) (25). A total of 13 subjects out of the 20 (10 vaccine recipients and 10 placebo or adjuvant-alone recipients) enrolled in the original vaccine trial had samples available and were included in this study. Among the 8 vaccinated individuals, postvaccination samples were from week 6 postimmunization for 7 vaccinees and from week 14 for one subject. All postinjection samples from subjects receiving the placebo were from week 6. The 13 subjects did not differ in age, sex and CD4+ T-cell counts and viral loads from the 20 individuals originally enrolled in the study. The study was approved by the Massachusetts General Hospital (MGH) Institutional Review Board, and written informed consent was obtained from each individual.
TABLE 1.
Characteristics of HIV-1-infected subjects
| Characteristic | Value for: |
|||
|---|---|---|---|---|
| Entire study population (n = 17) | Study population by cohorta |
|||
| CT (n = 6) | CU (n = 5) | EC (n = 6) | ||
| Median age (yr) | 48 | 51 | 40 | 48 |
| Gender (% male) | 94 | 83 | 100 | 100 |
| Median CD4 cell count (range) (cells/mm3) | 533 (219-1,359) | 373 (219–818) | 458 (382–549) | 1,076 (686-1,359) |
| Median viral load (range) (RNA cop/ml) | 75 (48-30,000) | 57 (48–81) | 5,600 (2,057-30,000) | 70 (48–271) |
CT, chronic treated HIV-1 infection; CU, chronic progressive untreated HIV-1 infection; EC, elite controllers.
TABLE 2.
Characteristics of HIV-1-infected subjects enrolled in the GSK vaccine trial
| Characteristic | Value for: |
||
|---|---|---|---|
| Entire study population (n = 13) | Study population by treatment group |
||
| Vaccine (n = 8) | Placebo/adjuvant (n = 5) | ||
| Age (median, yr) | 48 | 49 | 41 |
| Gender (% male) | 69 | 50 | 100 |
| Median duration of ARTa (yr) | 11 | 11 | 11 |
| Median baseline CD4 cell counts (/mm3) | 760 | 768 | 731 |
Before study entry.
Analysis of CD4+ T-cell and NK cell function.
Cryopreserved PBMCs were thawed and used directly or after CD4+ T-cell depletion using the EasySep human CD4+ T-cell enrichment kit and the human cell depletion procedure provided by the manufacturer (Stemcell Technologies). Whole or CD4+ T cell-depleted PBMCs were incubated overnight with 2 μg/ml of pools of 18-amino-acid (aa) peptides overlapping by 10 aa and spanning either Gp120, Gag, or Nef HIV-1 proteins or with 2 μg/ml of a pool of 15-aa peptides with an 11-aa overlap covering the complete sequence of the pp65 protein of human cytomegalovirus (CMV) (PepTivator; Miltenyi) in the presence of 1 ng/ml of recombinant human IL-15 (rhIL-15), 1 μg/ml of CD28/CD49d costimulatory reagent, and 10 μg/ml of isotype control, IL-2, or IL-12 neutralizing antibody. After 14 h, 0.9 nM monensin and 5 μg/ml of brefeldin A were added for the last 6 h of incubation. Incubation in the presence of 5 μg/ml of phytohemagglutinin (PHA) or 200 U/ml of rhIL-2 alone or in combination with 1 ng/ml rhIL-12 was used as the positive control. PBMCs were stained first with the LIVE/DEAD fixable blue dead cell stain kit and then with CD3-Alexa Fluor 700, CD4-Pacific Blue, CD16-allophycocyanin (APC)-Cy7, and CD56-phycoerythrin (PE)-Cy7 to discriminate CD4+ T cells and NK cells. Finally, they were fixed, permeabilized, and stained with IL-2-PE-CF594 and IFN-γ-fluorescein isothiocyanate (FITC) antibodies to detect intracellular cytokines. A fluorescence-minus-one (FMO) control and PHA-stimulated PBMCs were used to set the gates for positive cytokine responses. Acquisition of data was performed on a BD Fortessa. Data were analyzed using Flow Jo v.7.6.5.
Quantification of cytokines.
The concentration of IL-2 in the supernatant was measured in duplicate using a Milliplex human cytokine/chemokine 4-plex magnetic bead panel according to the manufacturer's instructions (Millipore). Samples were read on a Bio-Plex 3D suspension array system (Bio-Rad) and xPonent software (Luminex) and analyzed using Bio-Plex Manager software, version 6.0 (Bio-Rad). Extrapolated values below the range of the standard curve were set at the assay detection minimum (3.2pg/ml).
Statistical analysis.
All bars in scatter dot plots represent median values. Column bar graphs represent means ± standard errors of the means (SEM). Statistical analyses were performed using GraphPad Prism software, version 5.04. The nonparametric Wilcoxon signed-rank test was used to assess longitudinal differences in immune functions in vaccinated individuals and differences in cytokine production in response to various peptides in HIV-1-infected subjects. The nonparametric Mann-Whitney test was used to compare immune functions between vaccinated individuals and control subjects. P values of <0.05 were considered significant. Spearman's rank correlation was used for correlation analysis.
RESULTS
NK cell responses to HIV-1 Gag correlate with IL-2 production.
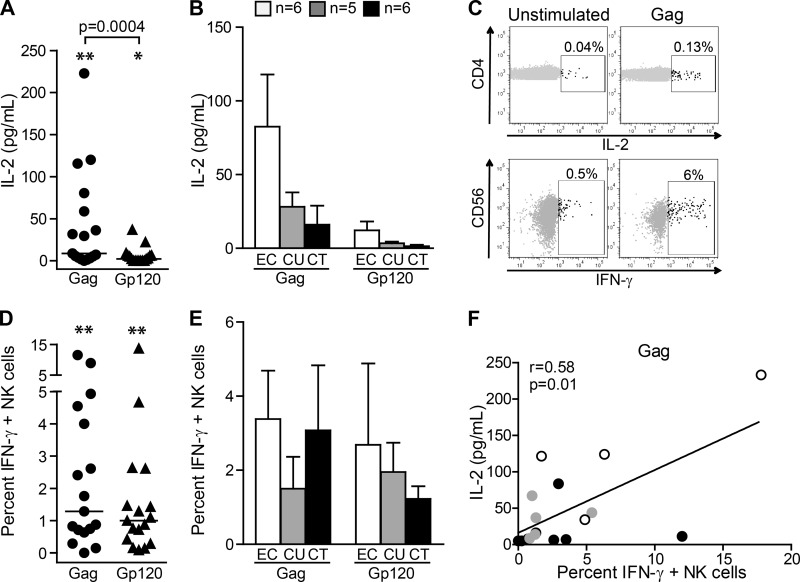
To determine the significance of T-cell help for NK cell function in HIV-1 infection, we initially investigated the impact of IL-2 production by virus-specific CD4+ T cells on NK cell activity in 17 HIV-1-infected individuals, including 6 subjects with ART-treated chronic infection, 5 with progressive untreated infection, and 6 elite controllers (Table 1). Stimulation of PBMCs with HIV-1 Gag-derived and, to a lesser extent, with Gp120-derived peptides led to significant production of IL-2 compared to that of the unstimulated control (P = 0.002 and P = 0.02, respectively) (Fig. 1A). As previously reported, elite controllers tended to have a higher IL-2 response than individuals with chronic progressive HIV-1 infection (9, 26–30) (Fig. 1B). Intracellular cytokine staining confirmed that IL-2 was released mainly by HIV-1-specific CD4+ T cells (Fig. 1C, upper row). However, a significant increase in percentages of IL-2+ CD4+ T cells was observed in a subset of 8 individuals upon stimulation with Gp120 (P = 0.008) or Gag (P = 0.008) but not in the remaining 9 subjects, who also displayed a low concentration (less than 3 times above the background) of IL-2 in the supernatant in response to HIV-1 peptides. Importantly, stimulation of PBMCs with HIV-1-derived peptides also translated into an overall significant increase in IFN-γ production by NK cells (P = 0.005 with Gag and P = 0.003 with Gp120 compared to the unstimulated control), notably in 5 out of those 8 individuals with documented IL-2+ CD4+ T-cell responses (Fig. 1C, lower row, and D). In 3 subjects, percentages of IFN-γ+ NK cells in response to Gag peptides were also more than three times above the background, although no increased production of IL-2 could be detected. In those 3 individuals, additional factors, including production of other key cytokines, might account for the observed enhanced NK cell function. Among the 8 HIV-1-infected individuals with increased NK cell responses, 3 were elite controllers and 5 were subjects with chronic progressive infection, including 4 who were ART treated. Accordingly, there were no significant differences in IFN-γ production between elite controllers and subjects with chronic HIV-1 infection (Fig. 1E). NK cell responses to Gag stimulation correlated with the concentration of IL-2 in the supernatant (Fig. 1F). In contrast, there was no correlation between the percentages of IFN-γ+ NK cells and IL-2 production in response to Gp120 peptides (P = 0.86, r = 0.005). Thus, our data suggest that IL-2 production by HIV-1-specific CD4+ T cells is associated with increased NK cell activation and is restricted to a subset of HIV-1-infected individuals.
FIG 1.
Increased production of IFN-γ by NK cells correlates with IL-2 production in response to HIV-1 Gag. (A) Quantification of IL-2 in the supernatant after 20 h of incubation of PBMCs in the presence of HIV-1 Gp120- or Gag-derived peptides in 17 HIV-infected individuals, including 6 with chronic ART-treated infection, 5 with untreated progressive infection, and 6 elite controllers. P < 0.05 (*) and P < 0.005 (**) compared to unstimulated PBMCs. (B) Each bar represents the mean ± SEM concentration of IL-2 in the supernatant for each cohort after stimulation with the indicated peptides. (C) Flow cytometry panels show representative examples of IL-2 production by HIV-1-specific CD4+ T cells (upper row) and IFN-γ production by NK cells (lower row) after intracellular staining. (D) Percentages of IFN-γ+ NK cells after 20 h of incubation of PBMCs in the presence of the indicated HIV-1-derived peptides. **, P < 0.005 compared with unstimulated PBMCs. (E) Each bar represents the mean ± SEM IFN-γ+ NK cell percentages for each cohort after stimulation with the indicated peptides. (F) Positive correlation between the concentration of IL-2 in the supernatant and the percentages of IFN-γ+ NK cells after 20 h of incubation of PBMCs in the presence of Gag peptides (Spearman's rank correlation test). Data are reported after background subtraction. Horizontal lines indicate the medians. Statistical differences with P < 0.05 are indicated and were determined using the nonparametric Wilcoxon signed-rank test.
Vaccine-induced IL-2 production by HIV-1-specific CD4+ T cells in response to HIV-1-derived peptides enhances NK cell function.
The lack of detectable HIV-1-specific CD4+ T-cell responses observed in more than half of the HIV-1-infected subjects studied here is in line with previously published work (8–11). Therapeutic vaccine trials have demonstrated that HIV-1-specific T helper cell responses can be enhanced by therapeutic immunizations in individuals with ART-treated chronic HIV-1 infection, resulting in the restoration of virus-specific T-cell proliferation (10, 11, 25). In those studies, rescued proliferative activity of HIV-1-specific T cells critically depended on the recovery of IL-2-secreting, HIV-1-specific CD4+ T cells (11, 25). As accumulating evidence suggests a role for IL-2-producing CD4+ T cells in supporting NK cell function (1–4, 6), we hypothesized that a loss of CD4+ T-cell help accounts for the impairment of NK cell responses in chronic HIV-1 infection.
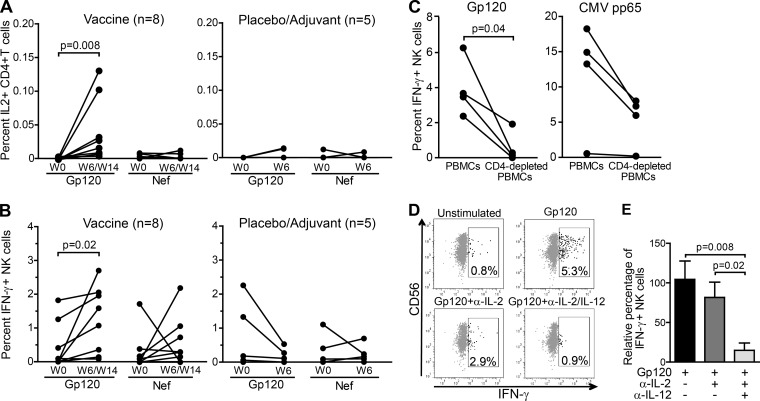
To test this hypothesis, we investigated whether NK cell function could be restored in vivo by enhancing CD4+ T-cell help in ART-treated HIV-1-infected patients immunized with GlaxoSmithKline Vaccines' HIV-1 Gp120/NefTat subunit protein candidate vaccine formulated with the AS02A adjuvant system (25) (Table 2). PBMC samples collected before and after vaccination were stimulated with HIV-1 Gp120- and Nef-derived peptides, and CD4+ T-cell and NK cell responses were quantified. For each subject, we analyzed the postvaccination sample corresponding to the time point with the highest CD4+ T-cell response to HIV-1 vaccine-matched peptides according to our previously published data, which occurred either at 6 or 14 weeks postimmunization (25). In line with the previous data (25), CD4+ T cells from individuals receiving the therapeutic vaccine produced significantly higher levels of IL-2 in response to Gp120 at 6 or 14 weeks postvaccination compared to the baseline, while there was no difference between those time points in response to Nef or in control subjects receiving either adjuvant alone or placebo (Fig. 2A). Remarkably, enhanced HIV-1-specific CD4+ T-cell responses to Gp120-derived peptides postvaccination were associated with a more than one log increase in IFN-γ production by NK cells (median percentages of IFN-γ+ NK cells were 0.05 at baseline versus 1.33 postvaccination; P = 0.02), whereas stimulation with Nef-derived peptides did not lead to a significant rise in the percentages of IFN-γ+ NK cells (median percentages of IFN-γ+ NK cells were 0.05 at baseline and 0.3 postvaccination; P = 0.3) (Fig. 2B).
FIG 2.
Reconstitution of IL-2 production by therapeutic vaccination leads to enhanced NK cell function. Proportions of CD4+ T cells that produce IL-2 (A) and of NK cells that produce IFN-γ (B) quantified by intracellular cytokine staining in response to Gp120- and Nef-derived HIV-1 peptides before (W0) and after (W6/W14) vaccination. The plots on the left represent the vaccine group, while the plots on the right represent the control group. Values are represented before and after vaccination for each subject. Statistical differences with P < 0.05 are indicated and were determined using the nonparametric Wilcoxon signed-rank test. Data are reported after background subtraction. (C) Percentages of IFN-γ+ NK cells in PBMCs and CD4+ T-cell-depleted PBMCs from 4 vaccinated subjects postvaccination, stimulated with either HIV-1 Gp120 peptides (left) or CMV pp65 peptides (right). Statistical differences with P < 0.05 are indicated and were determined using the nonparametric Wilcoxon signed-rank test. Data are reported after background subtraction. (D) Flow cytometry panels show representative examples of IFN-γ production by NK cells after intracellular cytokine staining of PBMCs that were left unstimulated or were stimulated with Gp120 in the presence of either isotype antibody control or IL-2 and IL-12 blocking antibodies, as indicated, in a vaccinated individual at 6 weeks postimmunization. (E) Relative percentages of IFN-γ+ NK cells after stimulation of PBMCs from 8 study subjects postvaccination with Gp120-derived peptides in the presence of isotype control antibody, IL-2 neutralizing antibody, or IL-2 and IL-12 neutralizing antibodies, as indicated. Values are expressed relative to the average percentage of IFN-γ+ NK cells in the absence of blocking antibodies after background subtraction, which was given the arbitrary value of 100%. Displayed values are the means ± SEM. Statistical differences with P < 0.05 are indicated and were determined using the nonparametric Wilcoxon signed-rank test.
In order to confirm that, as previously described for human NK cell responses to other pathogens (3, 5–7, 31), NK cells require signals, including IL-2, from HIV-1-specific CD4+ T cells to respond optimally to HIV-1-derived peptides, we stimulated CD4+ T cell-depleted PBMCs from 4 vaccinated subjects at 6 weeks postvaccination with Gp120 peptides. In accordance with a crucial role for HIV-1-specific CD4+ T cells in activating NK cells during recall responses, the NK cell IFN-γ response was either completely abrogated or significantly decreased when CD4+ T cells were removed (Fig. 2C). As mentioned above, the ability of IL-2 derived from pathogen-specific CD4+ T cells to sustain NK cell function is not restricted to HIV; therefore, stimulation with CMV peptides also promoted IFN-γ production by NK cells (Fig. 2C). As expected, percentages of IL-2-producing CD4+ T cells in response to CMV pp65 did not significantly differ between pre- and post-HIV-1 therapeutic vaccination samples (median value at baseline [n = 3], 0.005; range, 0 to 0.012; median value at week 6 postvaccination [n = 7], 0.006; range, 0 to 0.025). One subject did not show any CD4+ T-cell or NK cell response to CMV pp65 peptides and most likely was never exposed to CMV. Depletion of CD4+ T cells was associated with decreased percentages of IFN-γ+ NK cells yet did not entirely abrogate the NK cell IFN-γ response, probably due to the presence of IL-2-secreting CMV-specific CD8+ T cells.
Furthermore, increased IFN-γ production by NK cells in vaccinated subjects was dependent not only on IL-2 but also on IL-12, a central regulator of IFN-γ production by NK cells (reviewed in reference 32) required for optimal restoration of NK cell function in response to IL-2 (Fig. 2D and E). Indeed, combined blockade of both cytokines led to almost complete abrogation of NK cell responses to HIV-1 Gp120 at 6 or 14 weeks postvaccination (the average decrease in relative percentages of IFN-γ+ NK cells was 34% in the presence of IL-2 neutralizing antibodies and 83% in the presence of both IL-2 and IL-12 neutralizing antibodies, respectively) (Fig. 2D and E). Accordingly, incubation of postvaccination PBMCs from vaccinated individuals in the absence of peptides and in the presence of a combination of 1 ng/ml rhIL-15, 200 U/ml rhIL-2, and 1 ng/ml rhIL-12 led to a significant production of IFN-γ by NK cells and did not differ in samples from the baseline and postvaccination (median value at baseline [n = 3], median, 29.13; range, 22.79 to 48.68; median value at week 6 postvaccination [n = 7], 28.05; range, 23.05 to 50.41). However, incubation with rhIL-15 and rhIL-2 without addition of rhIL-12 led to the production of IFN-γ by NK cells that tended to be lower than that in the presence of HIV-1 Gp120 peptides (median value for rhIL-15 plus rhIL-2 [n = 5], 0.7560; range, 0.22 to 6; median value for rhIL-15 plus HIV-1-Gp120 peptides [n = 8], 1.328; range, 0.081 to 2.7).
Taken together, the data from this therapeutic vaccine trial show that rescued release of IL-2 following HIV-1 therapeutic vaccination synergizes with IL-12 to significantly increase NK cell production of IFN-γ in response to HIV-1-derived peptides.
DISCUSSION
HIV-1-specific T helper cell responses can be successfully enhanced by therapeutic immunization in individuals with chronic HIV-1 infection on suppressive ART (10, 11, 25). Here, we show that vaccine-elicited HIV-1-specific T-cell responses lead to increased NK cell function, suggesting that NK cells significantly contribute to the cellular effector response to vaccination by cooperating with the adaptive arm of HIV-1 immunity.
During chronic HIV-1 infection, production of IL-2 by CD4+ T cells in response to HIV-1-derived peptides is limited and can be detected in only a subset of HIV-1-infected subjects (Fig. 1). HIV-1-specific CD4+ T cells from infected individuals who control viremia in the absence of antiviral therapy were shown to produce more IL-2 and to be more polyfunctional than those from subjects with chronic progressive HIV-1 infection (9, 26–30), and cooperative NK and CD4+ T-cell responses correlate with control of disease progression in SIV-infected rhesus macaques (2). In our study, increased production of IL-2 by HIV-1-specific CD4+ T cells in response to Gag or Gp120 was not associated with the presence of protective HLA-B alleles or lower viral load. However, the investigation of CD4+ T-cell and NK cell function in a larger cohort of individuals is warranted to establish whether IL-2-dependent NK cell responses to HIV-1-derived peptides are more potent in HIV-1 elite controllers and associated with better control of HIV-1 viral replication.
Induction of HIV-1-specific T helper cell responses by therapeutic immunization was associated with a significant enhancement of IFN-γ production by NK cells in response to Gp120. Blockade of both IL-2 and IL-12 was required to abrogate the enhanced NK cell response in HIV-1-infected individuals 6 weeks postimmunization with the therapeutic vaccine (Fig. 2C and D). While production of IL-2 was elevated after stimulation of PBMCs from vaccinated individuals with HIV-1-derived peptides, no significant increase in IL-12 levels in the supernatant from stimulated cells was detected. However, the combination of IL-2 and IL-12 has a synergistic effect in promoting IFN-γ release by NK cells (33, 34), and very low doses (picomolar) of IL-2 have been shown to be sufficient to dramatically enhance IL-12-induced IFN-γ production by NK cells (31). Effective NK cell responses to rabies virus in vaccinated individuals were shown to depend not only on IL-2-secreting antigen-specific CD4+ T cells but also on the production of IL-12 and IL-18 from accessory cells (5). In our study, we cannot exclude that other NK cell activatory cytokines, such as IL-18 and IL-15, synergize with IL-2 and IL-12 to enhance NK cell responses to HIV-1-derived peptides. In addition, further investigations are warranted to explore the in vivo importance of the observed enhanced NK cell IFN-γ response to HIV-1-derived peptides in individuals receiving therapeutic immunization.
Overall, these data suggest that the progressive loss of NK cell function in HIV-1 infection is closely associated with the loss of HIV-1-specific, IL-2-secreting CD4+ T cells, and that therapeutic immunization in HIV-1-infected individuals rescues NK cell activity in vivo through the reconstitution of HIV-1-specific T-cell help. To our knowledge, these results provide the first clinical evidence for a direct functional linkage between virus-specific CD4+ T-cell help and NK cell function in HIV-1 infection and have important implications for the design of future immunotherapeutic interventions aimed at enhancing HIV-1 immunity in infected individuals.
ACKNOWLEDGMENTS
We thank GlaxoSmithKline Biologicals SA, Rixensart, Belgium, for provision of vaccine and collaboration on the clinical trial. This work was further supported by the National Institutes of Health (MA R01 AI50429), the Doris Duke Charitable Foundation (M.A. and M.L.), the Terry and Susan Ragon Foundation (M.A. and S.J.), and the Harvard University Center for AIDS Research (CFAR) (S.J.).
We are grateful to Mike Waring and Adam Chicoine for excellent assistance with flow cytometry through the Harvard University Center for AIDS Research (HU CFAR) Immunology Core at the Ragon Institute. The thoughtful and critical review of the manuscript by Marylyn M. Addo is greatly appreciated.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Kelly MN, Zheng M, Ruan S, Kolls J, D'Souza A, Shellito JE. 2013. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. 190:285–295. 10.4049/jimmunol.1200861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargas-Inchaustegui DA, Xiao P, Tuero I, Patterson LJ, Robert-Guroff M. 2012. NK and CD4+ T cell cooperative immune responses correlate with control of disease in a macaque simian immunodeficiency virus infection model. J. Immunol. 189:1878–1885. 10.4049/jimmunol.1201026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM, Parham P, Greenberg HB. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812–1819. 10.1172/JCI22797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. 2010. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J. Immunol. 184:6043–6052. 10.4049/jimmunol.1000106 [DOI] [PubMed] [Google Scholar]
- 5.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol. 185:2808–2818. 10.4049/jimmunol.1000844 [DOI] [PubMed] [Google Scholar]
- 6.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM. 2012. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J. Immunol. 188:5054–5062. 10.4049/jimmunol.1102710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCall MB, Roestenberg M, Ploemen I, Teirlinck A, Hopman J, de Mast Q, Dolo A, Doumbo OK, Luty A, van der Ven AJ, Hermsen CC, Sauerwein RW. 2010. Memory-like IFN-gamma response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur. J. Immunol. 40:3472–3477. 10.1002/eji.201040587 [DOI] [PubMed] [Google Scholar]
- 8.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, Hallahan CW, Davey RT, Jr, Dybul M, Vogel S, Metcalf J, Connors M. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900–10909. 10.1128/JVI.77.20.10900-10909.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909–1922. 10.1084/jem.20031598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins GK, Addo MM, Troung H, Rathod A, Habeeb K, Davis B, Heller H, Basgoz N, Walker BD, Rosenberg ES. 2003. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS 17:1121–1126. 10.1097/00002030-200305230-00002 [DOI] [PubMed] [Google Scholar]
- 11.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD, Altfeld M. 2004. Loss of HIV-1-specific CD8+ T-cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701–712. 10.1084/jem.20041270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angel JB, Kumar A, Parato K, Filion LG, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard JM, Cameron DW. 1998. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J. Infect. Dis. 177:898–904. 10.1086/515244 [DOI] [PubMed] [Google Scholar]
- 13.Blankson JN, Gallant JE, Siliciano RF. 2001. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657–661. 10.1086/318545 [DOI] [PubMed] [Google Scholar]
- 14.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. 1999. Control of HIV despite the discontinuation of antiretroviral therapy. N. Engl. J. Med. 340:1683–1684. 10.1056/NEJM199905273402114 [DOI] [PubMed] [Google Scholar]
- 15.Iannello A, Debbeche O, Samarani S, Ahmad A. 2008. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J. Leukoc. Biol. 84:1–26 [DOI] [PubMed] [Google Scholar]
- 16.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31:429–434. 10.1038/ng934 [DOI] [PubMed] [Google Scholar]
- 17.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, Goedert JJ, Buchbinder S, Kirk GD, Telenti A, Connors M, O'Brien SJ, Walker BD, Parham P, Deeks SG, McVicar DW, Carrington M. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39:733–740. 10.1038/ng2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 476:96–100. 10.1038/nature10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, Lifson JD, Allen TM, Carrington M, Altfeld M. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204:3027–3036. 10.1084/jem.20070695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alter G, Rihn S, Walter K, Nolting A, Martin M, Rosenberg ES, Miller JS, Carrington M, Altfeld M. 2009. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J. Virol. 83:6798–6805. 10.1128/JVI.00256-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. 2008. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS 22:1487–1491. 10.1097/QAD.0b013e3282ffde7e [DOI] [PubMed] [Google Scholar]
- 22.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. 2008. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22:595–599. 10.1097/QAD.0b013e3282f56b23 [DOI] [PubMed] [Google Scholar]
- 23.O'Connell KA, Han Y, Williams TM, Siliciano RF, Blankson JN. 2009. Role of natural killer cells in a cohort of elite suppressors: low frequency of the protective KIR3DS1 allele and limited inhibition of human immunodeficiency virus type 1 replication in vitro. J. Virol. 83:5028–5034. 10.1128/JVI.02551-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons MS, Boulet S, Song R, Bruneau J, Shoukry NH, Routy JP, Tsoukas CM, Bernard NF. 2010. Mind the gap: lack of association between KIR3DL1*004/HLA-Bw4-induced natural killer cell function and protection from HIV infection. J. Infect. Dis. 202(Suppl 3):S356–S360. 10.1086/655966 [DOI] [PubMed] [Google Scholar]
- 25.Lichterfeld M, Gandhi RT, Simmons RP, Flynn T, Sbrolla A, Yu XG, Basgoz N, Mui S, Williams K, Streeck H, Burgett-Yandow N, Roy G, Janssens M, Pedneault L, Vandepapeliere P, Koutsoukos M, Demoitie MA, Bourguignon P, McNally L, Voss G, Altfeld M. 2012. Induction of strong HIV-1-specific CD4+ T-cell responses using an HIV-1 gp120/NefTat vaccine adjuvanted with AS02A in antiretroviral-treated HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 59:1–9. 10.1097/QAI.0b013e3182373b77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571. 10.1086/526786 [DOI] [PubMed] [Google Scholar]
- 27.Harari A, Petitpierre S, Vallelian F, Pantaleo G. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966–972. 10.1182/blood-2003-04-1203 [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991. 10.1128/JVI.75.24.11983-11991.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J. Virol. 81:13904–13915. 10.1128/JVI.01401-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450. 10.1126/science.278.5342.1447 [DOI] [PubMed] [Google Scholar]
- 31.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. 2003. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101:3052–3057. 10.1182/blood-2002-09-2876 [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269–278. 10.1007/BF02786451 [DOI] [PubMed] [Google Scholar]
- 33.Aste-Amezaga M, D'Andrea A, Kubin M, Trinchieri G. 1994. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell. Immunol. 156:480–492. 10.1006/cimm.1994.1192 [DOI] [PubMed] [Google Scholar]
- 34.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. 1991. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J. Exp. Med. 173:869–879. 10.1084/jem.173.4.869 [DOI] [PMC free article] [PubMed] [Google Scholar]