ABSTRACT
Hepatitis C virus (HCV) causes not only severe liver problems but also extrahepatic manifestations, such as insulin resistance (IR). Wild-type peroxisome proliferator-activated receptor gamma coactivator 1 alpha (WT-PGC-1α) is essential in hepatic gluconeogenesis and has recently been demonstrated to link HCV infection to hepatic insulin resistance (IR). A recent study has characterized a novel human liver-specific PGC-1α (L-PGC-1α) transcript, which is proposed to reflect human adaption to more complex pathways. However, the effect of HCV infection on L-PGC-1α expression and the mechanism by which HCV modulates WT-PGC-1α/L-PGC-1α remain unclear. In this study, we showed that HCV infection upregulated both WT-PGC-1α and L-PGC-1α, which further promoted HCV production. The upregulation of both PGC-1α isoforms depended on HCV RNA replication. By using promoter-luciferase reporters, kinase inhibitors, and dominant negative mutants, we further observed that the HCV-induced upregulation of WT-PGC-1α was mediated by the phosphorylation of cyclic AMP (cAMP)-responsive element-binding protein (CREB), whereas that of L-PGC-1α was mediated by CREB phosphorylation and forkhead box O1 dephosphorylation. Moreover, HCV infection induced endoplasmic reticulum (ER) stress, and pharmacological induction of ER stress upregulated WT-PGC-1α/L-PGC-1α and phosphorylated CREB. In contrast, pharmacological inhibition of HCV-induced ER stress impaired WT-PGC-1α/L-PGC-1α upregulation along with decreased phosphorylated CREB. The correlation of hepatic mPGC-1α with ER stress was further confirmed in mice. Overall, HCV infection upregulates both WT-PGC-1α and L-PGC-1α through an ER stress-mediated, phosphorylated CREB-dependent pathway, and both PGC-1α isoforms promote HCV production in turn.
IMPORTANCE HCV causes not only severe liver problems but also extrahepatic manifestations, such as insulin resistance (IR). As a key regulator in energy metabolism, wild-type PGC-1α (WT-PGC-1α), has recently been demonstrated to link HCV infection to hepatic IR. A recent study has characterized a novel human liver-specific PGC-1α (L-PGC-1α), which reflects human adaption to more complex pathways. However, the effect of HCV infection on L-PGC-1α expression and the mechanism by which HCV regulates WT-PGC-1α/L-PGC-1α remain unclear. In this study, we showed that HCV infection upregulated both WT-PGC-1α and L-PGC-1α, which further promoted HCV production. WT-PGC-1α upregulation was mediated by CREB phosphorylation, whereas L-PGC-1α upregulation was mediated by CREB phosphorylation and FoxO1 dephosphorylation. HCV-induced ER stress mediated WT-PGC-1α/L-PGC-1α upregulation and CREB phosphorylation. Overall, this study provides new insights into the mechanism by which HCV upregulates WT-PGC-1α/L-PGC-1α and highlights the novel intervention of HCV-ER stress-PGC-1α signaling for HCV therapy and HCV-induced IR therapy.
INTRODUCTION
Hepatitis C virus (HCV) infection has become a serious health issue associated with substantial morbidity and mortality (1). The World Health Organization estimates that approximately 185 million people are or have been infected with HCV worldwide (2). HCV causes not only severe liver problems but also extrahepatic manifestations, such as insulin resistance (IR) and type 2 diabetes mellitus (T2DM). In patients with chronic HCV, the achievement of sustained virological response with drugs prevents the development of de novo IR (3). HCV-infected patients also have increased risk of T2DM compared with noninfected controls and hepatitis B virus-infected controls (4). These studies suggest that HCV directly contributes to the development of IR and T2DM.
Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) is an inducible transcription factor coactivator that controls cellular energy metabolism (5). Cumulative studies link altered PGC-1α signaling to IR and T2DM. PGC-1α induced in liver during fasting promotes hormone-stimulated gluconeogenesis (6). PGC-1α is robustly upregulated in diabetic liver (7), increasing hepatic glucose production. PGC-1α serves as a metabolic sensor; hence, PGC-1α expression is finely regulated to meet energy demands. Several levels of regulation have been implicated, including transcriptional regulation and posttranslational modifications (8). The transcriptional regulation of PGC-1α by the cyclic AMP (cAMP)-responsive element-binding protein (CREB) is a common pattern in the metabolic adaptions of the liver to gluconeogenic status in response to glucagon and glucocorticoids (7, 9) or in response to free fatty acids (10). Alternative splicing or transcription initiation represents another mode of regulation. A recent study has characterized a novel human liver-specific PGC-1α (L-PGC-1α) transcript (11), which results from alternative promoter usage. The previously described typical form of PGC-1α is referred to as wild-type PGC-1α (WT-PGC-1α).
Disparate promoter usage suggests different transcriptional regulation between L-PGC-1α and WT-PGC-1α. The promoter study by Felder et al. (11) suggests that forkhead box O1 (FoxO1) plays a greater role in L-PGC-1α transcription than in WT-PGC-1α transcription. Besides, like WT-PGC-1α mRNA, L-PGC-1α mRNA increases in response to glucocorticoids and CREB signaling. L-PGC-1α is located mainly in the nucleus and is identical to WT-PGC-1α except for a deletion of the N-terminal 127 amino acids (11). Therefore, L-PGC-1α shows overlapped coactivation properties compared with those of WT-PGC-1α (11). There are also functional differences between L-PGC-1α and WT-PGC-1α. For example, only WT-PGC-1α coactivates liver X receptor alpha (LXRα) at the promoter of sterol regulatory element-binding transcription factor 1c (SREBP-1c) (11). L-PGC-1α transcript homologues are not detected in livers of the rat, mouse, dog, and monkey (11), whereas all the main interacting domains of WT-PGC-1α are highly conserved in many chordate species (12). Thus, L-PGC-1α is proposed to reflect an adaption to more complex pathways in humans. Although substantial progress has been made in the study of WT-PGC-1α, the function and regulation of L-PGC-1α remain largely unknown.
Endoplasmic reticulum (ER) is the cellular organelle responsible for lipid metabolism, protein processing, and Ca2+ storage. Interference with ER functions by conditions such as high-fat feeding, virus infection, and glucose deprivation induces a stress response, known as ER stress. Upon sensing ER stress, cells activate a unique intracellular signaling pathway unfolded protein response (UPR) to cope with this insult. ER stress and the activated UPR have been associated with various diseases, including liver diseases (13). Replication of the HCV genome occurs at the so-called ER-associated membranous webs (14). Studies using in vitro and in vivo experimental models (15, 16) and clinical liver biopsy (17) have indicated that HCV induces ER stress. HCV further utilizes cellular responses to ER stress to promote HCV persistence and pathogenesis. For instance, HCV-induced ER stress has been implicated in the pathogenesis of HCV-related IR (18, 19).
The important function of WT-PGC-1α in the pathogenesis of IR and T2DM suggests that HCV infection may upregulate liver WT-PGC-1α expression and further promote hepatic IR. Shlomai et al. (20) showed that WT-PGC-1α is robustly upregulated by HCV infection and that elevated WT-PGC-1α links HCV infection to hepatic IR. However, the effect of HCV infection on L-PGC-1α expression remains unknown, and the mechanism by which HCV infection modulates WT-PGC-1α/L-PGC-1α remains unclear. In this study, we investigated whether L-PGC-1α is also regulated by HCV infection and whether the modulation of WT-PGC-1α/L-PGC-1α is associated with ER stress.
In the current study, we found that HCV infection upregulates both WT-PGC-1α and L-PGC-1α to promote HCV production. We also found that the HCV infection-induced upregulation of both WT-PGC-1α and L-PGC-1α depends on HCV RNA replication and CREB phosphorylation. Furthermore, HCV-induced ER stress has an intermediary function in the upregulation of both PGC-1α isoforms. Finally, the demonstrated efficacy of 4-phenylbutyric acid (PBA), an ER stress inhibitor, in blocking HCV provides a new perspective for HCV therapy.
MATERIALS AND METHODS
Cells.
Huh7, its derivative Huh7.5.1, and Hep3B cells were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS; HyClone, Beijing, China) and 1% penicillin-streptomycin. Huh7-sgHCV1b and Huh7.5.1-sgJFH2a cells, which harbor genotype 1b and 2a subgenomic replicons, respectively, were maintained in the medium described above and supplemented with 400 μg/ml of G418 and 2 μg/ml of puromycin. 3#, 4#, 6#, and 7# represent the respective subgenomic replicon clones of Huh7.5.1-sgJFH2a cells. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Plasmids.
Promoter-luciferase reporter vectors were generated in the pGL4.17 backbone (Promega). pL-PGC-1α-Prom-Luc and pWT-PGC-1α-Prom-Luc were constructed by cloning 3.518-kb and 2.614-kb upstream fragments into pGL4.17, respectively. Two mutants of the L-PGC-1α promoter region, namely, pL-PGC-1α-Prom-GRE-Mut and pL-PGC-1α-Prom-FoxO1-site-Mut, were created from pL-PGC-1α-Prom-Luc by using site-directed mutagenesis (11). Three mutants of the WT-PGC-1α promoter region were also constructed by using site-directed mutagenesis. pWT-PGC-1α-Prom-IRS-Mut was mutated with all the three insulin response sequences (IRSs) (21), and pWT-PGC-1α-Prom-CRE-Mut and pWT-PGC-1α-Prom-MEF2-site-Mut were constructed as previously described (22). pRL-SV40-Renilla reporter plasmid (Promega) served as a transfection control.
Plasmids expressing HCV proteins (except NS5B) were generated with the lentiviral vector (pRlenti) tagged with Flag sequence at the C terminus. Hemagglutinin (HA)-NS5B was constructed by cloning NS5B-encoding fragments into the plasmid pReceiver-M06 with 3 copies of the HA sequence at the N terminus. pRlenti was also used to generate the expression plasmids L-PGC-1α and WT-PGC-1α with HA tags at the N terminus. To express short hairpin RNA (shRNA) targeting WT-PGC-1α/L-PGC-1α, we used the pSuper retroviral plasmid that was created as described in reference 23 by using the following primers: for shPGC-1α 1#, 5′-GATCCCCCCAACACTCAGCTAAGTTATTCAAGAGATAACTTAGCTGAGTGTTGGTTTTTA-3′ and 5′-AGCTTAAAAACCAACACTCAGCTAAGTTATCTCTTGAATAACTTAGCTGAGTGTTGGGGG-3′ (20), and for shPGC-1α 2#, 5′-GATCCCCCCGTTATACCTGTGATGCTTTTTCAAGAGAAAAGCATCACAGGTATAACGGTTTTTA-3′ and 5′-AGCTTAAAAACCGTTATACCTGTGATGCTTTTCTCTTGAAAAAGCATCACAGGTATAACGGGGG-3′.
The expression plasmid pCREB was generated by C-terminal Flag-tagged CREB cloned in vector pCR3.1 (Invitrogen) in our laboratory (24). The pCREB133 vector, which contains a serine-to-alanine mutation corresponding to amino acid 133, was created from pCREB using site-directed mutagenesis.
Antibodies and reagents.
The following primary antibodies were used for Western blotting: monoclonal mouse anti-PGC-1α antibody (4C1.3; Calbiochem), polyclonal rabbit anti-PGC-1α antibody (ab54481; Abcam), monoclonal mouse anti-HCV core antibody (C7-50; Abcam), monoclonal mouse anti-HCV NS3 (H23; Abcam), monoclonal mouse phospho-CREB antibody (10E9; Santa Cruz), monoclonal mouse CREB antibody (X-12; Santa Cruz), polyclonal rabbit phospho-ATF2 (Thr71) antibody (no. 9221; Cell Signaling Technology), monoclonal rabbit phospho-FoxO1 (Thr24) antibody (4G6; Beyotime, China), monoclonal rabbit FoxO1 antibody (C29H4; Beyotime), rabbit Bip antibody (Beyotime), rabbit histone H3 antibody (Beyotime, China), polyclonal rabbit β-actin antibody (MultiSciences, China), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Kang Xiang, Shanghai, China). A goat anti-mouse antibody (Chemicon) and a goat anti-rabbit antibody (Chemicon), both conjugated with horseradish peroxidase, were used as secondary antibodies.
Naive Huh7.5.1 cells were treated with 1 μM thapsigargin (Tg) or 1.5 μg/ml of tunicamycin (Tm) for 8 h to induce ER stress. HCV-infected Huh7.5.1 cells were treated with 6 mM PBA for 24 h to inhibit ER stress. HCV-infected Huh7.5.1 cells were treated with H89 for 24 h, SB203580 for 24 h, or wortmannin for 12 h to inhibit kinase activities. PBA (Sigma) and H89 (Sigma) stocks were prepared in double-distilled water. Tg (Sigma), Tm (Fermentek), SB203580 (Sigma), and wortmannin (Sigma) were prepared in dimethyl sulfoxide.
RNA isolation, reverse transcription, XBP1 PCR, and real-time PCR.
Total RNA was extracted from cells using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Fermentas) in the presence of random hexamers (TaKaRa). The reaction mixture was incubated for 10 min at 25°C and for 1 h at 42°C and then the reaction was stopped by heating at 95°C for 5 min. The mRNA fragments of XBP1 and actin were amplified under the following PCR conditions with cDNA: 38 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min. Previously reported primers for XBP1 and actin were used (18). We analyzed three fragments representing spliced, unspliced, and hybrid XBP1 on a 2% agarose gel. The cDNA was used to perform SYBR-PCR using a SYBR Premix Ex Taq kit (TaKaRa). The primers used for real-time PCR are listed in Table 1. The housekeeping 18S rRNA or GAPDH gene served as an internal control. All reactions were run in triplicate using the CFX96 Touch real-time PCR detection system (Bio-Rad).
TABLE 1.
Sequences and positions of primers used in this study
| Gene (GenBank accession no.) | Primer | Position | PCR product size (bp) |
|---|---|---|---|
| HCV 5′ UTRa | TCTGCGGAACCGGTGAGTA | 147 + 19b | 150 |
| TCAGGCAGTACCACAAGGC | 296 − 19 | ||
| L-PGC-1α (HQ695733.1) | TCCCTCTGTTGCCTTTGTG | 144 + 19 | 93 |
| TAACCCCATGCCATCCAT | 236 − 18 | ||
| WT-PGC-1α (NM_013261.3) | TGAAAAAGCTTGACTGGCG | 87 + 19 | 133 |
| AAGATCTGGGCAAAGAGGC | 219 − 19 | ||
| PEPCK (NM_001018073.1) | TATTATGACCCGACTGGGGA | 699 + 20 | 143 |
| TCAGGGTTTTCTCTGGGTTG | 841 − 20 | ||
| CREB (NM_004379.3) | GTGTTACGTGGGGGAGAGAA | 163 + 20 | 110 |
| GGCTCCAGATTCCATGGTC | 272 − 19 | ||
| ATF2 (NM_001880.3) | GGTGCTTTGTAAACACGGCT | 85 + 20 | 104 |
| GCAGTCCTTTCTCAAGTTTCC | 188 − 21 | ||
| FoxO1 (NM_002915) | GAGGGTTAGTGAGCAGGTTAC | 2,352 + 21 | 217 |
| AGTCCTTATCTACAGCAGCAC | 2,568 − 21 | ||
| GAPDH (NM_002046.4) | GAAGGTGAAGGTCGGAGTC | 180 + 19 | 226 |
| GAA GATGGTGATGGGATTTC | 405 − 20 | ||
| 18S (NR_003286.2) | GGTGAAATTCTTGGACCGGC | 958 + 20 | 196 |
| GACTTTGGTTTCCCGGAAGC | 1,153 − 20 | ||
| mPGC-1α (NM_008904.2) | AATTGAAGAGCGCCGTGT | 2,152 + 18 | 127 |
| TCCATCATCCCGCAGATT | 2,278 − 18 | ||
| mBip (NM_001163434.1) | ACCAAGACATTTGCCCCA | 562 + 18 | 147 |
| TTTGGTTGCTTGTCGCTG | 708 − 18 | ||
| Murine actin (NM_007393.3) | CTCCTCCTGAGCGCAAGTACTC | 1,071 + 22 | 91 |
| TCCTGCTTGCTGATCCACATC | 1,161 − 21 |
UTR, untranslated region.
The first value is the nucleotide position of the 5′ terminus of the primer; the second represents the number of the nucleotide bases of the primer.
Preparation of cell extracts and Western blotting.
Proteins were extracted from cells using radioimmunoprecipitation assay buffer supplemented with protease inhibitors (Sigma), with GAPDH/β-actin as an internal control. Proteins were prepared using a nuclear and cytoplasmic protein extraction kit (Beyotime, China), with histone H3 as an internal control. For Western blotting, equal amounts of protein extracts boiled with a sodium dodecyl sulfate (SDS) loading buffer containing β-mercaptoethanol (140 mM) were subjected to SDS-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane (Portran; Whatman). The membrane was subsequently incubated with the respective primary antibodies. Horseradish peroxidase-conjugated secondary antibodies were used to visualize the respective proteins with an enhanced chemiluminescence detection system (Millipore).
Luciferase reporter assays.
Huh7.5.1 cells or HCV-infected Huh7.5.1 cells that were prepared in a 96-well tissue culture plate at a density of 1.5 × 104 cells per well were cotransfected with pWT-PGC-1α/L-PGC-1α-Prom-Luc reporter plasmid and pRL-SV40-Renilla. After 72 h, luciferase activity was determined using a Dual-Glo luciferase assay system kit (E2920; Promega). Firefly and Renilla luciferase activities were measured with luminometer (Turner Biosystems, USA). Firefly luciferase activity was normalized to Renilla luciferase activity for each sample.
In vitro transcription of HCV RNA, HCV preparation, and titration.
Plasmid pFL-J6/JFH/Jc1, used for the generation of infectious HCV-Jc1 (25), was linearized with XbaI and then purified by ethanol precipitation. Then, 1 μg of linearized plasmid was transcribed in vitro with a MEGAscript T7 kit (Ambion, Austin, TX) for the large-scale production of RNA. For RNA transfection, Huh7.5.1 cells were transfected with 24 μg of HCV-Jc1 RNA using Lipofectamine 2000 reagent (Invitrogen). Supernatants collected at 4 to 5 days posttransfection (dpt) were centrifuged at low speed to remove cell debris. Clarified viral supernatants were aliquoted and then stored at −80°C until use. HCV-Jc1 titers were determined as previously described (26). The EGFP gene was inserted into the C terminus of NS5A-encoding sequence within the HCV-Jc1 genome to generate an HCV reporter virus termed HCV-Jc1EGFP. The adapted HCV-Jc1EGFP was generated and prepared for stocks in our laboratory as previously described (27).
To determine the effect of PGC-1α overexpression on HCV production, Huh7.5.1 cells were infected with the lentivirus carrying the coding sequence of WT-PGC-1α or L-PGC-1α or the pRlenti control. After 12 h, Huh7.5.1 cells were infected with HCV-Jc1EGFP or HCV-Jc1 at a multiplicity of infection (MOI) of 0.02. The HCV-Jc1EGFP or HCV-Jc1 virus in the supernatant was collected at 72 h postinfection, and then the titer of the HCV-Jc1EGFP virus was determined by both limiting dilution assay and flow cytometry (FCM) and that of the HCV-Jc1 virus was determined only by limiting dilution assay. To determine the effect of PGC-1α knockdown on HCV production, Huh7.5.1 cells were infected with the retrovirus carrying the shRNA expression cassette against PGC-1α (shPGC-1α 1# or shPGC-1α 2#) or a nontarget control (shRNA-Ctrl). After 12 h, Huh7.5.1 cells were infected with HCV-Jc1EGFP or HCV-Jc1 at an MOI of 0.02. The HCV-Jc1EGFP or HCV-Jc1 in the supernatant was collected at 96 h postinfection, and the titer was determined as mentioned above. For the titration of supernatant virus by limiting dilution assay, viral supernatants collected at 72 h or 96 h postinfection were clarified and inoculated on Huh7.5.1 cells by endpoint dilution. At 72 h postinfection, Huh7.5.1 cells were fixed with 4% paraformaldehyde and then immunostained for HCV core antigen. Stained foci were counted and used to calculate the titer of focus-forming units (FFU)/ml. To determine the titer of HCV in the supernatant by flow cytometry, approximately 3 μl (PGC-1α knockdown experiment) or 5 μl (PGC-1α overexpression experiment) of viral supernatants was obtained to inoculate naive Huh7.5.1 cells in 24-well tissue culture plates; the infected cells were quantified for flow cytometry at 72 h postinfection. The percentage of NS5A-EGFP-positive cells was used to calculate the infectivity of HCV. For the intracellular infectivity of HCVcc, the pellets of HCV-infected cells were resuspended in DMEM containing 10% FBS and then subjected to three cycles of freezing and thawing to induce cell lysis. The samples were then centrifuged for 5 min at 3,000 rpm to remove cell debris; afterward, supernatants were collected to determine infectivity.
RNA extraction from mouse liver.
Male Leprdb/db mice were on a C57BL/6 genetic background, and they were maintained at the Animal Center of Guangzhou Institutes of Biomedicine and Health according to the guidelines of the Institutional Animal Committee. Livers isolated from 16-week-old mice were stored in liquid nitrogen until use. Total RNA was extracted from mouse liver using a total RNA isolation kit (Sangon Biotech, China) according to the manufacturer's instructions.
RESULTS
HCV infection upregulates WT-PGC-1α and L-PGC-1α to promote HCV production.
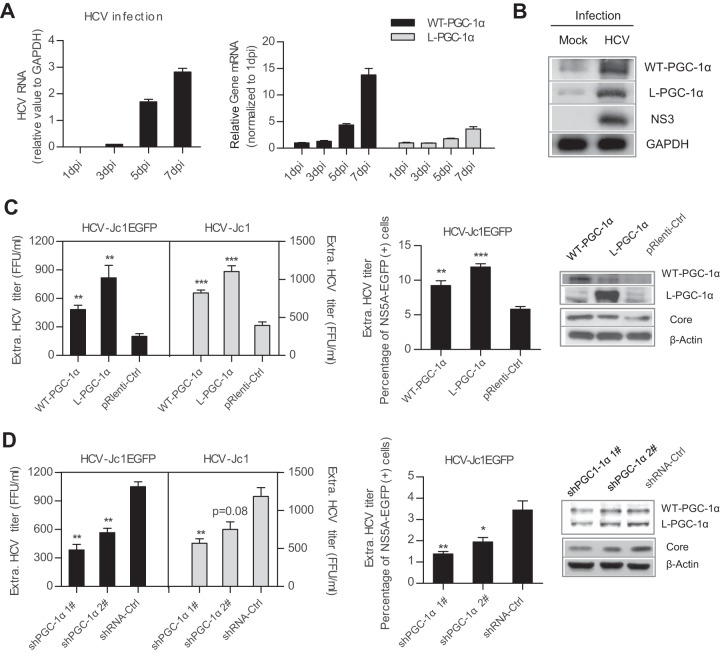
A previous study showed that WT-PGC-1α is upregulated following HCV infection (20). To investigate this phenomenon in more detail, gene expression level was analyzed by infecting Huh7.5.1 cells with HCV-Jc1. As the HCV RNA level continuously increased from 1 to 7 days postinfection (dpi) (Fig. 1A, left), the mRNA levels of both WT-PGC-1α and L-PGC-1α significantly increased (14-fold and 4-fold at 7 days, respectively) (Fig. 1A, right). Western blot analysis showed that HCV infection increased the protein levels of both WT-PGC-1α and L-PGC-1α (approximately 91 kDa and 77 kDa, respectively) (Fig. 1B). These results indicate that aside from WT-PGC-1α, HCV infection also induces L-PGC-1α elevation.
FIG 1.
WT-PGC-1α and L-PGC-1α are upregulated by HCV infection and promote HCV production. (A) Analysis of HCV RNA levels and WT-PGC-1α and L-PGC-1α mRNA levels after infection of Huh7.5.1 cells with HCV-Jc1 (MOI, 0.02) for different times. (B) Western blot analysis for detecting the expression of WT-PGC-1α and L-PGC-1α at 7 dpi in Huh7.5.1 cells. (C) Effect of WT-PGC-1α and L-PGC-1α overexpression on HCV production and on HCV core protein level. (D) Effect of WT-PGC-1α/L-PGC-1α knockdown on HCV production and on HCV core protein level. shRNA-Ctrl, nontargeting shRNA. (C and D) Huh7.5.1 cells were infected with HCV-Jc1EGFP or HCV-Jc1 at an MOI of 0.02. The extracellular HCV-Jc1EGFP titer was quantified by both endpoint dilution and flow cytometry (FCM), and the HCV-Jc1 titer was quantified by only endpoint dilution. In the FCM assay, the extracellular HCV-Jc1EGFP titer was determined as a percentage of NS5A-EGFP-positive cells by using viral supernatants to inoculate naive Huh7.5.1 cells. In all panels, data are presented as means ± SEMs (n ≥ 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (all determined by Student's t test).
We further investigated whether the elevated WT-PGC-1α/L-PGC-1α affects HCV infection in turn. Huh7.5.1 cells were transduced with PGC-1α-overexpressing lentiviruses or PGC-1α-silencing retroviruses, followed by inoculation with adapted HCV-Jc1EGFP or HCV-Jc1. The extracellular HCV-Jc1EGFP titer was quantified by both endpoint dilution and flow cytometry (FCM). The overexpression of both PGC-1α isoforms increased extracellular HCV-Jc1EGFP titer by limiting dilution assay (Fig. 1C, left) and by FCM (Fig. 1C, middle) and simultaneously increased HCV core expression (Fig. 1C, right). In contrast, knockdown of WT-PGC-1α/L-PGC-1α with specific shRNAs significantly impaired extracellular HCV-Jc1EGFP titer and HCV core expression (Fig. 1D). And herein both shPGC-1α 1# and shPGC-1α 2# did not discriminate between WT-PGC-1α and L-PGC-1α (Fig. 1D, right). In addition, overexpression of WT-PGC-1α/L-PGC-1α and knockdown of WT-PGC-1α and L-PGC-1α by shPGC-1α 1# have similar effects on the extracellular titer of HCV-Jc1 (Fig. 1C, left, and Fig. 1D, left).
Collectively, these results indicate that HCV infection upregulates both WT-PGC-1α and L-PGC-1α and then the upregulated WT-PGC-1α/L-PGC-1α enhances HCV production.
WT-PGC-1α/L-PGC-1α is involved in HCV RNA replication and assembly of infectious virions.
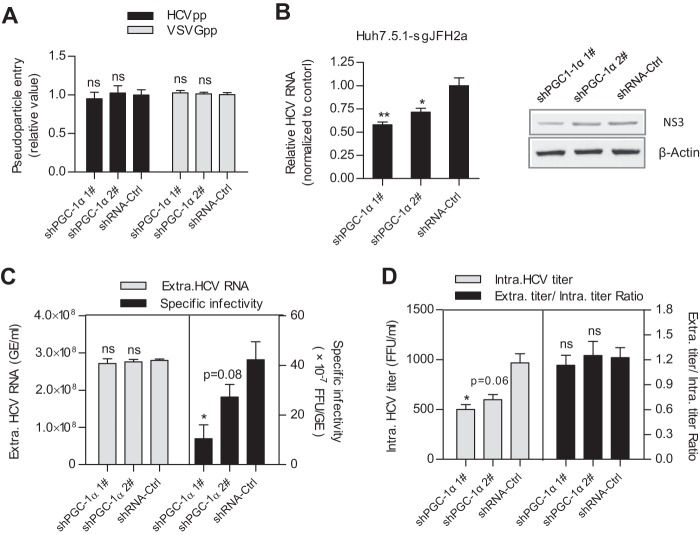
To identify in which step of the HCV life cycle WT-PGC-1α/L-PGC-1α is required, multiple virologic assays were performed. As shown in Fig. 2A, knockdown of WT-PGC-1α/L-PGC-1α with shPGC-1α 1# or shPGC-1α 2# did not affect HCV pseudovirus infection. This result suggests that WT-PGC-1α/L-PGC-1α is not required for HCVpp entry. In addition, shPGC-1α 1# significantly decreased both intracellular HCV RNA levels and NS3 protein levels in Huh7.5.1-sgJFH2a cells, which harbor HCV subgenomic replicons (Fig. 2B), and shPGC-1α 2# also significantly decreased intracellular HCV RNA levels. This result indicates that WT-PGC-1α/L-PGC-1α affects HCV RNA replication.
FIG 2.
WT-PGC-1α/L-PGC-1α is involved in HCV RNA replication and HCV assembly. (A) Effect of WT-PGC-1α/L-PGC-1α knockdown on pseudoparticle entry (means ± SEMs; n = 3). (B) Effect of WT-PGC-1α/L-PGC-1α knockdown on intracellular HCV RNA levels and NS3 protein levels in Huh7.5.1-sgJFH2a cells (means ± SEMs; n = 3). (C) Effect of WT-PGC-1α/L-PGC-1α knockdown on extracellular HCV RNA copies and specific infectivity. (D) Effect of WT-PGC-1α/L-PGC-1α silencing on intracellular HCV titer and the ratio of extracellular titer to intracellular titer. For panels C and D, Huh7.5.1 cells were infected with HCV-Jc1 at an MOI of 0.02. Results are means ± SEMs of three repetition measurements. For all panels, statistical analyses were performed by using Student's t test. ns, not significant. * and **, same as for Fig. 1.
To further investigate whether WT-PGC-1α/L-PGC-1α is involved in the late stage of the HCV life cycle, we calculated the specific infectivity (infectious titers divided by HCV RNA copy number) and the ratio of extracellular titer to intracellular titer as we previously described (27). Specific infectivity was calculated to evaluate the efficiency of HCV assembly (28), and the ratio of extracellular titer to intracellular titer was used to evaluate the secretion of viral particles (27). WT-PGC-1α/L-PGC-1α silencing by shPGC-1α 1# significantly reduced the extracellular HCV-Jc1 specific infectivity (Fig. 2C, right), with no effect on the extracellular HCV RNA (Fig. 2C, left). Moreover, similarly to the extracellular titer (Fig. 1D), the intracellular titer showed similar reduction upon shPGC-1α 1# treatment (Fig. 2D, left), whereas the ratio of extracellular titer to intracellular titer was not changed by shPGC-1α 1# (Fig. 2D, right). shPGC-1α 2# had a slight reduction effect on the extracellular specific infectivity and on the intracellular titer due to its moderate reduction of WT-PGC-1α/L-PGC-1α protein. Taken together, the reduced extracellular specific infectivity suggests that the assembly of HCV is regulated by WT-PGC-1α/L-PGC-1α, while the unchanged ratio of extracellular titer to intracellular titer suggests that viral secretion is not affected (Fig. 2F).
Collectively, these observations indicate that WT-PGC-1α/L-PGC-1α is involved in the HCV RNA replication and assembly of HCV.
Upregulation of WT-PGC-1α and L-PGC-1α depends on HCV RNA replication.
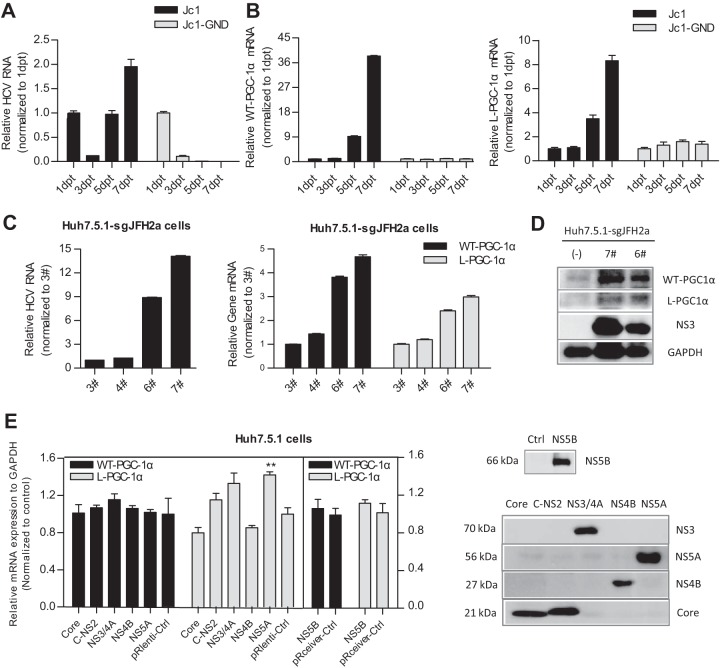
To investigate the mechanism by which HCV upregulates WT-PGC-1α/L-PGC-1α from the HCV point of view, Huh7.5.1 cells were transfected with the full-length HCV-Jc1 RNA for up to 7 days. Full-length HCV-Jc1 RNA with a lethal mutation in the RNA-dependent RNA polymerase served as a negative control (HCV-Jc1-GND) (29). As shown in Fig. 3A, HCV-Jc1 RNA increased sharply from 3 dpt and then peaked at 7 dpt. In contrast, HCV-Jc1-GND RNA declined continuously from 1 dpt to 7 dpt (Fig. 3A). The mRNA levels of both WT-PGC-1α and L-PGC-1α increased in a time-dependent manner in HCV-Jc1 RNA-transfected cells from 3 dpt to 7 dpt but were not elevated in HCV-Jc1-GND RNA-transfected cells (Fig. 3B). These results suggest that the expression of WT-PGC-1α and L-PGC-1α is upregulated in a HCV RNA replication-dependent manner. To further define the association between the expression of both PGC-1α isoforms and HCV RNA replication, we obtained several clones of Huh7.5.1-sgJFH2a cells that support HCV subgenomic RNA replication (clones 3#, 4#, 6#, and 7#). Different adaptive mutations in HCV replicon RNA may contribute to the different HCV RNA levels and NS3 protein levels among the above-listed clones (Fig. 3C and D). As shown in Fig. 3C, the mRNA levels of both PGC-1α isoforms were associated with the HCV RNA levels in these clones; as shown in Fig. 3D, the association with the protein level was further confirmed in the 6# and 7# clone cells. These results indicate that the upregulation of WT-PGC-1α/L-PGC-1α depends on HCV RNA replication.
FIG 3.
Upregulation of both WT-PGC-1α and L-PGC-1α depends on HCV RNA replication. HCV RNA levels (A) and WT-PGC-1α and L-PGC-1α mRNA levels (B) were determined after transfecting Huh7.5.1 cells with full-length HCV-Jc1 RNA for different times (means ± SEMs of triplicate measurements). (C) The association of the mRNA levels of both PGC-1α isoforms with HCV RNA levels in Huh7.5.1-sgJFH2a cells. 3#, 4#, 6#, and 7# represent the respective subgenomic replicon clones (means ± SEMs; n = 3). (D) Association of the protein levels of both PGC-1α isoforms with HCV NS3 protein levels in Huh7.5.1-sgJFH2a cells. (E) Effect of HCV proteins on WT-PGC-1α/L-PGC-1α mRNA expression. Left, effect of HCV protein overexpression on WT-PGC-1α and L-PGC-1α mRNA levels (means ± SEMs; n = 2); right, Western blot analysis to detect the expression of HCV protein at 3 days post-lentivirus transduction in Huh7.5.1 cells. The expressed HCV proteins were derived from HCV-Jc1 (25) (genotype 2a). (C-NS2 represents HCV proteins from core to NS2, namely, Core-E1-E2-p7-NS2; NS5B was expressed in the pReceiver plasmid, which was different from other HCV proteins.)
Considering that HCV RNA replication is accompanied by the abundant expression of HCV proteins, we determined whether any of the HCV proteins is responsible for the upregulation of WT-PGC-1α/L-PGC-1α. As indicated in Fig. 3E, none of the HCV proteins enhanced the expression of WT-PGC-1α or L-PGC-1α except that NS5A had an effect on L-PGC-1α elevation. These results indicate that a single HCV protein is not sufficient to upregulate WT-PGC-1α/L-PGC-1α except NS5A.
HCV-induced upregulation of WT-PGC-1α is mediated by CREB phosphorylation.
We subsequently explored the mechanism by which HCV upregulates WT-PGC-1α/L-PGC-1α from the PGC-1α point of view. We investigated WT-PGC-1α and L-PGC-1α separately because of their difference in transcriptional regulation.
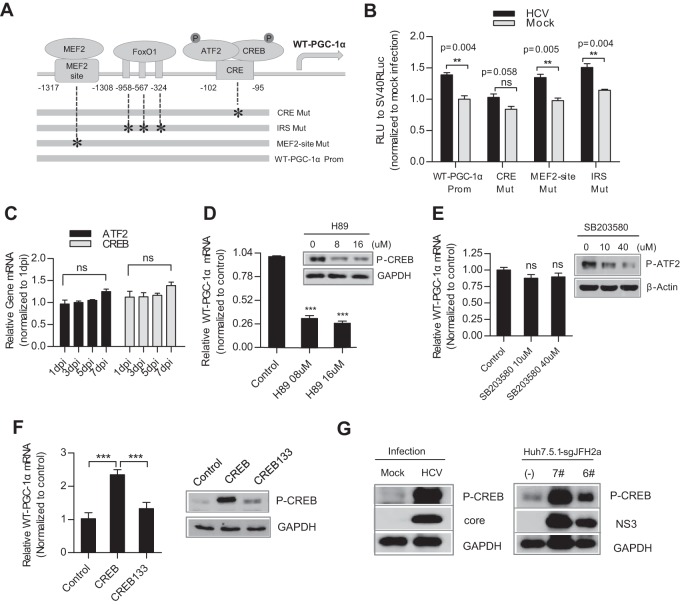
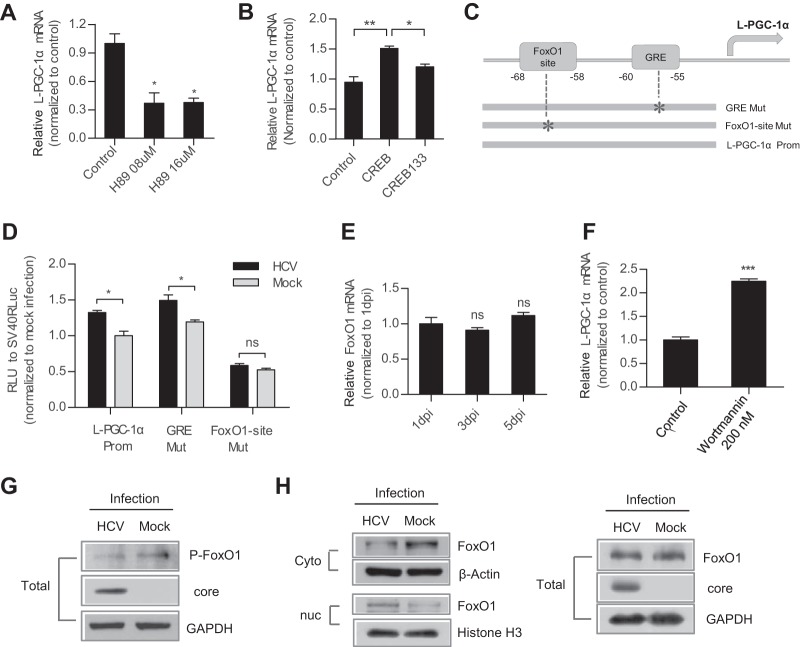
The regulation of WT-PGC-1α expression is linked to the status of energy stress. Transcription factors, including myocyte enhancer factor 2 (MEF2), FoxO1, activating transcription factor 2 (ATF2), and CREB, control WT-PGC-1α expression in response to physiologic stimuli (8). To determine the effect of these transcription factors on WT-PGC-1α elevation by HCV infection, three mutations (MEF2-site-Mut, IRS-Mut, and CRE-Mut) were introduced to the binding sites of these transcription factors within the WT-PGC-1α promoter (Fig. 4A; Table 2). CRE-Mut was not activated by HCV infection, although a slight but nonsignificant increase was detected (Fig. 4B). In contrast, MEF2-site-Mut and IRS-Mut were significantly activated by HCV infection, similar to the case with WT-PGC-1α-Prom (Fig. 4B). This result suggests that CRE is important for the HCV infection-induced upregulation of WT-PGC-1α.
FIG 4.
HCV-induced WT-PGC-1α upregulation is mediated by CREB phosphorylation. (A) Schematic of the three WT-PGC-1α promoter site-specific mutants. IRS, insulin response sequence; CRE, cAMP response element. (B) Different WT-PGC-1α promoter mutant activities by HCV infection using luciferase reporter assay (means ± SEMs; n = 3). (C) mRNA levels of CREB and ATF2 after HCV infection (means ± SEMs; n = 3). (D) Effect of H89 on WT-PGC-1α mRNA elevation after HCV infection. H89 is a PKA-specific inhibitor that inhibits CREB phosphorylation; the effect of H89 on CREB phosphorylation was shown by Western blotting (means ± SEMs; n = 3). (E) Effect of SB203580 on WT-PGC-1α/L-PGC-1α mRNA levels in HCV-infected Huh7.5.1 cells. SB203580 is a p38 MAPK inhibitor that inhibits the phosphorylation of ATF2; the effect of SB203580 on protein level of phospho-ATF2 (P-ATF2) was shown by Western blotting. Results are means ± SEMs of three repeat measurements. (F) Effect of CREB133 (a dominant negative mutant) on WT-PGC-1α mRNA expression (means ± SEMs; n = 2). (G) Western blot analysis for detecting the expression of phospho-CREB (P-CREB) in HCV-infected Huh7.5.1 cells (at 7 dpi) and in Huh7.5.1-sgJFH2a cells. For panels B to G, Huh7.5.1 cells were infected with HCV-Jc1 at an MOI of 0.02. For panels B to F, P values are as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (all determined by Student's t test). ns, not significant.
TABLE 2.
Mutant strategies of PGC-1α promotera
| Gene | TFBS | Position | Orientation | Length (bp) | Original sequence | Mutant sequence |
|---|---|---|---|---|---|---|
| WT-PGC-1α | MEF2-site | −1317 to 1308 | Direct | 10 | TTATATTTAG | TTCCGAGGAG |
| IRS1 | −961 to −955 | Direct | 7 | TATTTTT | TCAGGGT | |
| IRS2 | −571 to −564 | Direct | 8 | TATTTTGT | TCAGGGT | |
| IRS3 | −328 to −320 | Direct | 9 | TTGTTTTGG | TTCAGGGTGA | |
| CRE | −102 to −95 | Direct | 8 | TGACGTCA | TAGATCTA | |
| L-PGC-1α | FoxO1-site | −68 to −58 | Reverse | 11 | ACATTGTTTGC | ACATTCTTTGC |
| GRE | −60 to −55 | Direct | 6 | TGTTCT | TGACCT |
Abbreviations: TFBS, transcription factor binding site; CRE, cyclic AMP response element; GRE, glucocorticoid response element; IRS, insulin response sequence.
CRE is the binding site for phosphorylated CREB (phospho-CREB) and phosphorylated ATF2 (phospho-ATF2). Given that the mRNA levels of CREB and ATF2 were not altered following HCV infection (Fig. 4C), we investigated whether HCV infection enhances WT-PGC-1α expression through CREB and ATF2 phosphorylation. H89 is a phosphokinase A (PKA)-specific inhibitor that inhibits CREB phosphorylation by PKA (Fig. 4D), and SB203580 inhibits ATF2 phosphorylation by p38 mitogen-activated protein kinase (MAPK) (Fig. 4E). WT-PGC-1α upregulation was significantly inhibited when HCV-infected Huh7.5.1 cells were treated with H89 (Fig. 4D). However, no inhibition was observed after SB203580 treatment (Fig. 4E). These results suggest that phospho-CREB, not phospho-ATF2, is required for WT-PGC-1α upregulation. CREB133, which contains a serine-to-alanine mutation corresponding to amino acid 133 of the CREB protein, was constructed as a dominant negative mutant. As shown in Fig. 4F, CREB133 blocked the upregulation of WT-PGC-1α. Western blot analysis showed that phospho-CREB was dramatically increased in HCV-infected Huh7.5.1 (Fig. 4G, left) and Huh7.5.1-sgJFH2a (Fig. 4G, right) cells.
These results strongly suggest that the HCV-induced upregulation of WT-PGC-1α is mediated by CREB phosphorylation.
HCV-induced upregulation of L-PGC-1α is mediated by CREB phosphorylation and FoxO1 dephosphorylation.
To determine whether CREB phosphorylation is also important for the HCV infection-induced upregulation of L-PGC-1α, the inhibition activity of H89 was analyzed. As shown in Fig. 5A, H89 significantly decreased the upregulation of L-PGC-1α. CREB133 also blocked the upregulation of L-PGC-1α (Fig. 5B). These results suggest that the HCV-induced upregulation of L-PGC-1α is also mediated by CREB phosphorylation.
FIG 5.
L-PGC-1α upregulation by HCV is mediated by CREB phosphorylation and FoxO1 dephosphorylation. (A) Effect of H89 on L-PGC-1α mRNA upregulation after HCV infection. The effect of H89 on CREB phosphorylation was shown by Western blotting (means ± SEMs; n = 3). (B) Effect of CREB133 on L-PGC-1α mRNA expression (means ± SEMs; n = 2). (C) Schematic of the two L-PGC-1α promoter site-specific mutants. GRE, glucocorticoid response element. (D) Luciferase reporter assay for detecting different L-PGC-1α promoter mutant activities by HCV infection (means ± SEMs; n = 3). For panels A, B, and D, results were analyzed similarly to those for WT-PGC-1α as mentioned in the legend to Fig. 4. (E) mRNA levels of FoxO1 after HCV infection (mean ± SEM; n = 3) (F) Effect of wortmannin (a PI3K inhibitor that blocks FoxO1 phosphorylation) on L-PGC-1α mRNA upregulation after HCV infection (means ± SEMs; n = 2). (G) Western blot analysis for detecting the expression of phospho-FoxO1 (Thr 24) (P-FoxO1) at 7 days in HCV-infected Huh7.5.1 cells. (H) Western blot analysis to detect the expression of total, nuclear (nuc), and cytoplasmic (Cyto) FoxO1 at 7 days in HCV-infected Huh7.5.1 cells. For panels A, B, and D to H, Huh7.5.1 cells were infected with HCV-Jc1 at an MOI of 0.02. For pnels A, B, D, E, and F, P values are as follows: *, P < 0.05, and ***, P < 0.001 (determined by Student's t test). ns, not significant.
Aside from CREB phosphorylation, the expression of L-PGC-1α is also regulated by FoxO1 and glucocorticoid signaling (11). To verify whether the HCV-induced upregulation of L-PGC-1α is also mediated by FoxO1 or glucocorticoid signaling, FoxO1-site-Mut and glucocorticoid response element (GRE)-Mut in the L-PGC-1α promoter were constructed (Fig. 5C; Table 2). HCV infection significantly increased L-PGC-1α-Prom and GRE-Mut promoter-luciferase expression, whereas HCV infection had no effect on FoxO1-site-Mut promoter-luciferase expression (Fig. 5D). This result suggests that L-PGC-1α promoter activation is dependent on the FoxO1 site.
When phosphatidylinositol 3-kinase (PI3K), the kinase responsible for FoxO1 phosphorylation, is suppressed, FoxO1 is retained in the nucleus to activate downstream gene transcription. Given that the mRNA level of FoxO1 was not affected following HCV infection (Fig. 5E), we analyzed the effect of FoxO1 phosphorylation on L-PGC-1α upregulation. Wortmannin is a PI3K inhibitor. Treatment of HCV-infected cells with wortmannin increased the mRNA level of L-PGC-1α (Fig. 5F). This result suggests that HCV infection decreases the level of phosphorylated FoxO1 (phospho-FoxO1) and then upregulates L-PGC-1α. Furthermore, Western blot analysis showed that HCV infection indeed suppressed the level of phospho-FoxO1 at Thr24 (Fig. 5G), which is critical for FoxO1 nuclear exclusion (30). Accordingly, Western blotting of nuclear and cytoplasmic extracts showed that HCV infection upregulated nuclear FoxO1 expression and downregulated cytoplasmic FoxO1 expression compared with mock infection (Fig. 5H, left) but did not affect the total level of FoxO1 (Fig. 5H, right). Taken together, these results suggest that HCV infection results in decreased FoxO1 phosphorylation, FoxO1 nuclear accumulation, and, eventually, increased L-PGC-1α expression.
Collectively, these results indicate that the HCV infection-induced upregulation of L-PGC-1α is mediated by both CREB phosphorylation and FoxO1 dephosphorylation.
HCV upregulates WT-PGC-1α/L-PGC-1α through ER stress.
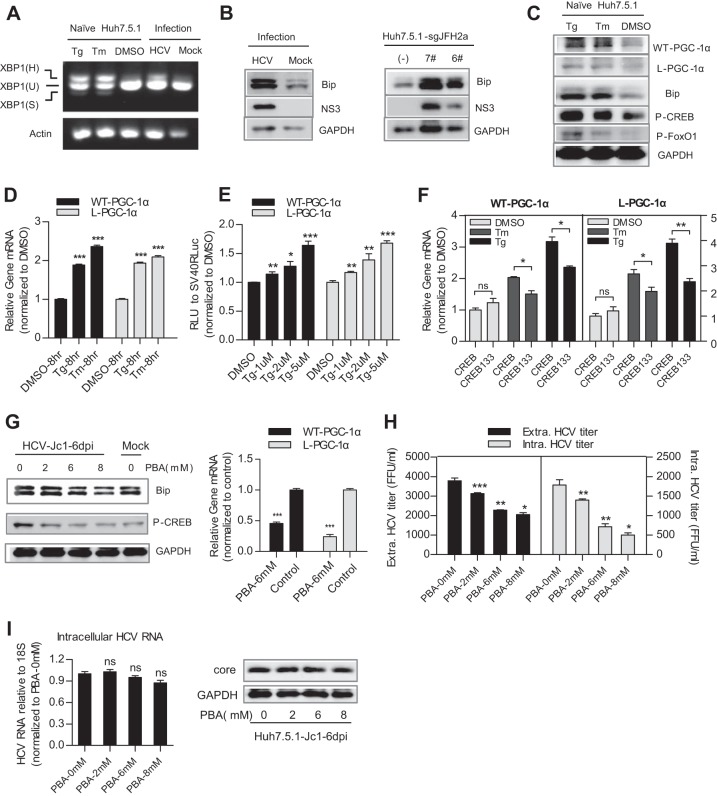
Previous studies have shown that ER stress can activate CREB (18) and that HCV RNA replication can result in ER stress (31). In light of our results, we hypothesized that ER stress mediates WT-PGC-1α/L-PGC-1α upregulation by HCV infection in a CREB-dependent manner.
We first tested whether HCV infection induces an ER stress response. ER stress can activate UPR via three different sensors: PERK, ATF6, and IRE1 (32). The UPR results in the upregulation of ER-resident molecular chaperones such as BiP/GRP78 and thereby augments the folding capacity of the ER (32). We accordingly examined the effect of HCV on IRE1-XBP1 activation and BiP upregulation to indicate ER stress. Once IRE1 is activated, it induces the splicing of the XBP1 mRNA to stimulate the expression of UPR target genes. Splicing of XBP1 mRNA was assayed by RT-PCR: the unspliced mRNA, XBP1(U), generates a 424-bp product, whereas the spliced mRNA, XBP1(S), generates a 398-bp product; XBP1(H) represents the hybrid DNA derived from XBP1(U) and XBP1(S). The appearance of XBP1(H) and/or XBP1(S) indicates XBP1 splicing (15). XBP1(H) was easily detected in HCV-infected Huh7.5.1 cells but not in mock-infected Huh7.5.1 cells (Fig. 6A). Both HCV infection in Huh7.5.1 cells and HCV RNA replication in Huh7.5.1-sgJFH2a cells upregulated the protein level of BiP (Fig. 6B). The splicing of XBP1 mRNA and the elevated levels of Bip protein demonstrate the activation of ER stress in these cells. Subsequently, we investigated whether pharmacological induction of ER stress can induce CREB phosphorylation and WT-PGC-1α/L-PGC-1α upregulation. Tg and Tm are ER stress inducers. Treatment of naive Huh7.5.1 cells with Tg and Tm induced a classical ER stress, as evidenced by XBP1 mRNA splicing and BiP upregulation (Fig. 6A and C). Phospho-CREB protein was simultaneously elevated by Tg and Tm treatment, along with the upregulation of the protein and mRNA levels of WT-PGC-1α and L-PGC-1α (Fig. 6C and D). However, Tg and Tm treatment resulted in FoxO1 phosphorylation (Fig. 6C), which is in contrast with the finding that HCV infection resulted in FoxO1 dephosphorylation (Fig. 5G); therefore, HCV-induced L-PGC-1α upregulation by FoxO1 dephosphorylation is not dependent on ER stress. Increasing concentrations of Tg led to a dose-dependent increase in luciferase expression under the control of both PGC-1α promoters (Fig. 6E). In addition, CREB133 blocked the pharmacological ER stress-induced mRNA upregulation of WT-PGC-1α and L-PGC-1α (Fig. 6F). Finally, we validated whether inhibition of ER stress can alleviate the HCV infection-induced upregulation of WT-PGC-1α/L-PGC-1α. PBA is a chemical chaperone that attenuates ER stress (33). Treatment of HCV-infected Huh7.5.1 cells with PBA markedly reduced the protein levels of Bip and phospho-CREB (Fig. 6G, left) and further impaired the mRNA levels of both WT-PGC-1α and L-PGC-1α (Fig. 6G, right). PBA treatment also inhibited the extracellular titer and intracellular titer of HCV-Jc1 (Fig. 6H). However, both the HCV core protein expression and the intracellular HCV RNA level were not changed after PBA treatment (Fig. 6I), which was consistent with the studies of Huang et al. (34). This result excludes the possibility that the observed inhibition of WT-PGC-1α/L-PGC-1α expression by PBA was due to the suppression of HCV RNA replication by PBA.
FIG 6.
HCV upregulates WT-PGC-1α/L-PGC-1α through ER stress in Huh7.5.1 cells. (A) The activation of ER stress is shown by the activation of the IRE1-XBP1 pathway. Splicing of XBP1 was shown by reverse transcription-PCR (RT-PCR). XBP1(U) and XBP1(S) represent DNA fragments were derived from unspliced and spliced XBP1 RNAs, respectively. XBP1(H) represents the hybrid DNA derived from XBP1(U) and XBP1(S). The actin RNA was also analyzed to serve as an internal control. Thapsigargin (Tg) and tunicamycin (Tm) are pharmacological inducers of ER stress. (B) Effect of HCV infection and HCV RNA replication on Bip protein levels. (C) Effect of Tg and Tm on WT-PGC-1α and L-PGC-1α protein levels, Bip protein levels, and phospho-CREB and phospho-FoxO1 protein levels. (D) Effect of Tg and Tm on WT-PGC-1α/L-PGC-1α mRNA levels. (E) Effect of Tg on WT-PGC-1α/L-PGC-1α promoter-luciferase activities in Huh7.5.1 cells. (F) Effect of CREB133 on the pharmacological ER stress-induced WT-PGC-1α and L-PGC-1α mRNA upregulation. (G) Effect of PBA on the protein levels of Bip and phospho-CREB (P-CREB) and on HCV-induced WT-PGC-1α/L-PGC-1α mRNA upregulation. (H) Effect of PBA on the extracellular titer and intracellular titer of HCV-Jc1. (I) Effect of PBA on the intracellular HCV RNA level and on the HCV core protein level in HCV-Jc1 infected Huh7.5.1 cells. For panels A, B, G, and H, Huh7.5.1 cells were infected with HCV-Jc1 at an MOI of 0.02. For panels D to I, data are presented as means ± SEMs (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (all determined by Student's t test). ns, not significant.
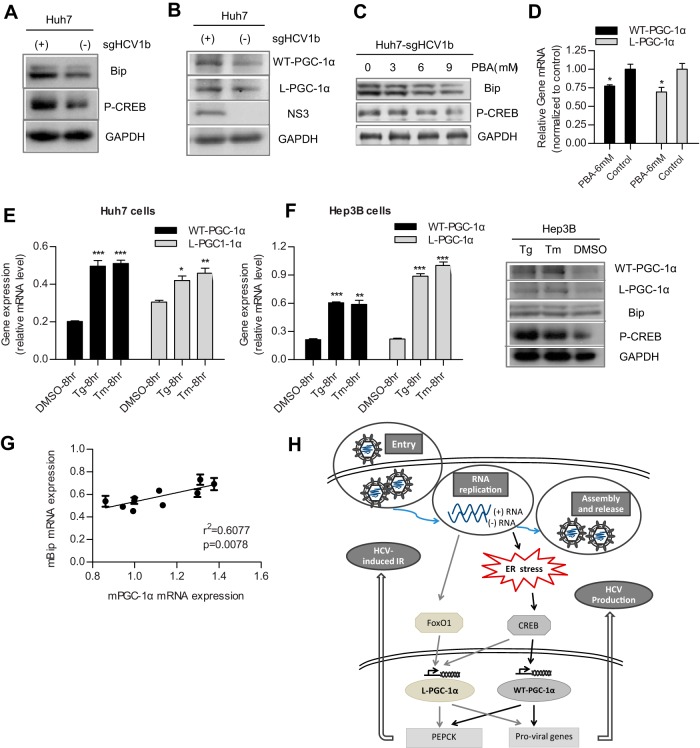
Huh7.5.1 cells are defective in innate immune response that involves retinoic acid-inducible gene I (RIG-I) (35). Robust HCV replication supported by Huh7.5.1 cells is more likely to cause severe ER stress. Therefore, we determined whether HCV replication in the parental Huh7 cells could also induce significant ER stress to upregulate WT-PGC-1α/L-PGC-1α expression. As shown in Fig. 7A, Huh7-sgHCV1b cells, which harbor genotype 1b subgenomic replicons, had higher levels of Bip protein than Huh7 cells, which indicated an ER stress by HCV replication in Huh7 cells. Moreover, phospho-CREB and WT-PGC-1α/L-PGC-1α proteins were simultaneously elevated in Huh7-sgHCV1b cells (Fig. 7A and B). The inhibition of ER stress by PBA in Huh7-sgHCV1b cells reduced the protein levels of Bip and phospho-CREB (Fig. 7C) and further impaired the mRNA levels of both PGC-1α isoforms (Fig. 7D). Treatment of Huh7 cells with Tg and Tm increased the mRNA levels of both WT-PGC-1α and L-PGC-1α (Fig. 7E). Taken together, these results suggest that ER stress mediates the HCV replication-induced upregulation of WT-PGC-1α/L-PGC-1α in Huh7 cells.
FIG 7.
HCV RNA replication upregulates WT-PGC-1α/L-PGC-1α through ER stress in Huh7 cells, and hepatic mPGC-1α correlates with ER stress marker mBip in mice. (A) The activation of ER stress and CREB is indicated by the high expression levels of Bip and phospho-CREB (P-CREB) in Huh7-sgJFH1b cells, respectively. (B) Western blot analysis for detecting the expression of WT-PGC-1α and L-PGC-1α in Huh7-sgJFH1b cells and in Huh7 cells. (C) Effect of PBA on the protein levels of Bip and phospho-CREB (P-CREB) in Huh7-sgJFH1b cells. (D) Effect of PBA on the HCV-induced WT-PGC-1α/L-PGC-1α mRNA upregulation in Huh7-sgJFH1b cells. (E) Effect of Tg and Tm on WT-PGC-1α/L-PGC-1α mRNA levels in Huh7 cells. (F) Effects of Tg and Tm on WT-PGC-1α/L-PGC-1α elevation in Hep3B cells. Left, effects of Tg and Tm on WT-PGC-1α/L-PGC-1α mRNA levels in Hep3B cells; right, Western blot analysis for detecting the protein levels of Bip, phospho-CREB (P-CREB), and both WT-PGC-1α and L-PGC-1α in Hep3B cells with Tg and Tm treatment. For panels D to F, results are presented as means ± SEMs of triplicate measurements. (G) Correlation of hepatic mPGC-1α and mBip transcript levels in Leprdb/db mice (individual dot represents one mouse; results are presented as means ± SEMs of triplicate measurements; n = 10). (H) A proposed model of the dual effects of WT-PGC-1α/L-PGC-1α in HCV-induced insulin resistance and HCV production, and the mechanism by which HCV upregulates WT-PGC-1α/L-PGC-1α. Gray and black solid arrows represent the signaling pathways by which HCV upregulates L-PGC-1α and WT-PGC-1α, respectively. HCV RNA replication induces ER stress, which further phosphorylates CREB to activate both WT-PGC-1α and L-PGC-1α transcription. L-PGC-1α transcription is also elevated by HCV infection-induced FoxO1 dephosphorylation, which is independent of ER stress. The increased levels of WT-PGC-1α and L-PGC-1α promote expression of PEPCK and proviral genes. The increased PEPCK expression could account for HCV-induced IR, and the increased proviral gene expression promotes HCV production. For panels D to F, P values are as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (all determined by Student's t test).
To confirm that WT-PGC-1α/L-PGC-1α was upregulated by pharmacological ER stress not restricted to Huh7 and Huh7-derived Huh7.5.1 cells, we used another hepatoma carcinoma cell, Hep3B cell, for Tg and Tm treatment. Results showed that the mRNA and protein levels of WT-PGC-1α and L-PGC-1α were significantly elevated when treated with Tg and Tm in Hep3B cells (Fig. 7F). To further validate the connection between PGC-1α expression and ER stress in vivo, the mRNA levels of hepatic murine Bip (mBip) and mPGC-1α were analyzed in Leprdb/db mice, a genetic model of T2DM. Leprdb/db mice were hyperphagic and obese (36), and Ozcan et al. (37) showed that obesity contributes to the development of type 2 diabetes because obesity causes ER stress. In the present study, results showed that the mRNA levels of mPGC-1α strongly correlated with the mRNA levels of ER stress marker mBip in Leprdb/db mice (Fig. 7G).
Collectively, these results strongly suggest that ER stress mediates the HCV-induced upregulation of WT-PGC-1α/L-PGC-1α.
DISCUSSION
In the current study, we demonstrated a previously unrecognized function of ER stress in mediating the HCV-induced upregulation of WT-PGC-1α/L-PGC-1α. This conclusion is supported by the following findings: (i) the expression levels of WT-PGC-1α/L-PGC-1α and ER stress markers increased in HCV-infected and HCV RNA-replicating cells; (ii) the upregulation of WT-PGC-1α/L-PGC-1α depended on CREB phosphorylation, and the pharmacological induction of ER stress induced CREB phosphorylation and WT-PGC-1α/L-PGC-1α upregulation; (iii) the pharmacological inhibition of ER stress reduced the HCV-induced WT-PGC-1α/L-PGC-1α upregulation; and (iv) the mRNA levels of mPGC-1α strongly correlated with that of mBip in vivo.
Our findings showed that WT-PGC-1α/L-PGC-1α was upregulated by ER stress in Huh7.5.1 cells infected by HCV or in hepatoma carcinoma cells (Huh7, Huh7.5.1, and Hep3B) treated with Tg or Tm and that the mRNA levels of mPGC-1α strongly correlated with that of mBip in diabetic mice. These studies indicate that an ER stress-PGC-1α signaling pathway exists in livers. A recent study has shown that tribbles 3 (TRB3) mediates ER stress-induced IR in skeletal muscles (38). Another study reported that the muscle-specific overexpression of PGC-1α in mice, which exacerbates IR, is associated with elevated TRB3 expression (39). These two studies suggest that an ER stress-PGC-1α-TRB3 signaling pathway exists in muscles. However, this hypothesis needs further investigation. Nevertheless, combined with our findings, these studies suggest that the ER stress-induced upregulation of WT-PGC-1α/L-PGC-1α is a universal mechanism in multiple tissues.
In search of the mechanism underlying HCV-associated WT-PGC-1α elevation, Shlomai et al. (20) speculated that HCV-induced oxidative stress promotes WT-PGC-1α upregulation from the result that treatment of HCV replicon cells with the antioxidant N-acetylcysteine resulted in reduction of WT-PGC-1α levels. ER stress and oxidative stress are intimately interrelated (40). ER stress can promote oxidative stress in cells that support HCV replication (32). Therefore, it is possibly that ER stress induces oxidative stress to upregulate WT-PGC-1α/L-PGC-1α in HCV-infected cells. In addition, the induction of PGC-1α expression was lower after 8 h of Tg or Tm treatment (Fig. 6C) than after 5 and 7 days of HCV infection (Fig. 1A). This result suggests that the HCV-mediated induction of PGC-1α expression involves other mechanisms aside from ER stress.
From the PGC-1α point of view, our results showed that the HCV-induced upregulation of WT-PGC-1α was mediated by CREB phosphorylation, whereas that of L-PGC-1α was mediated by both CREB phosphorylation and FoxO1 dephosphorylation. The activation of PGC-1α by CREB is a common pattern in the metabolic adaptations of the liver to gluconeogenic status, and HCV infection also exploits this CREB-PGC-1α signaling to elevate both WT-PGC-1α and L-PGC-1α, which are further hijacked by HCV for its production.
In the present study, L-PGC-1α upregulation was also dependent on FoxO1 dephosphorylation. This finding is consistent with previous results that FoxO1 has a more important function in L-PGC-1α transcription than in WT-PGC-1α transcription and that the mRNA level of L-PGC-1α strongly correlates with that of FoxO1 in the livers of obese subjects (11). We also observed an effect of NS5A on the elevation of L-PGC-1α. Deng et al. (41) previously reported that HCV promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway, and herein we demonstrated that the HCV-induced upregulation of L-PGC-1α depends on FoxO1 dephosphorylation. Taken together, the results show that there may be an NS5A-FoxO1-L-PGC-1α signaling pathway following HCV infection.
The HCV-induced upregulation of WT-PGC-1α/L-PGC-1α demonstrated dual effects. First, the HCV-induced upregulation of WT-PGC-1α/L-PGC-1α promoted the gluconeogenic response. Shlomai et al. (20) showed that WT-PGC-1α induction following HCV infection promotes hepatic gluconeogenesis by increasing glucose-6 phosphatase and glucose production. Phosphoenolpyruvate carboxykinase (PEPCK), another rate-limiting enzyme for hepatic gluconeogenesis, is also transcriptionally regulated by PGC-1α (6, 7). In the present study, the overexpression of both WT-PGC-1α and L-PGC-1α in Huh7.5.1 cells significantly increased the mRNA level of PEPCK (data not shown). Although the transcript levels of hepatic L-PGC-1α and WT-PGC-1α are similar in human liver, the mRNA level of hepatic PEPCK is more strongly associated with L-PGC-1α than with WT-PGC-1α (11). The interaction of the PGC-1α family with hepatocyte nuclear factor 4 alpha (HNF4α) is crucial in hepatic gluconeogenesis (42), and a direct physical interaction exists between L-PGC-1α and HNF4α at the PEPCK promoter (11). Therefore, similar to WT-PGC-1α, L-PGC-1α also has a vital function in the development of HCV-associated IR and T2DM. Daitoku et al. (21) showed that insulin activates the PI3K signaling pathway, which stimulates FoxO1 phosphorylation, followed by the nuclear exclusion of FoxO1 and the repression of PGC-1α transcription. In the present study, the demonstrated FoxO1 dephosphorylation and L-PGC-1α elevation by HCV infection may interrupt the insulin signaling pathway, and this interruption may result in IR from another perspective.
Second, WT-PGC-1α and L-PGC-1α demonstrated proviral functions in HCV production. HCV particle production relies on very-low-density lipoproteins (VLDLs) (43). PGC-1α is reportedly important in VLDL assembly (44). We also found that both WT-PGC-1α and L-PGC-1α can transcriptionally upregulate several factors involved in VLDL assembly and release, including apolipoprotein E and cell death-inducing DFFA-like effector B (data not shown). We have previously shown that HNF4α affects the late stages of HCV life cycle through the VLDL pathway (27). Given that HNF4α is a critical component of PGC-1α-mediated hepatic function, we speculate that WT-PGC-1α and L-PGC-1α promote HCV production through their downstream factors associated with the VLDL pathway by interacting with HNF4α. Studies on this issue are under way in our laboratory.
Emerging lines of evidence indicate that HCV-induced ER stress contributes to HCV persistence and HCV pathogenesis. HCV-induced ER stress and UPR can block innate immunity to promote HCV replication (45, 46). Aside from suppression of innate immune response, our results showed that ER stress induced WT-PGC-1α/L-PGC-1α upregulation to enhance HCV RNA replication and HCV assembly, which shall give new insight into the proviral effect of HCV-induced ER stress. Accumulating evidence implicates ER stress-induced cell death as a major contributor to many diseases (47); HCV-induced ER stress sensitizes infected cells to apoptosis (48) and accounts for HCV pathogenesis to some extent. The observed ER stress-PGC-1α pathway in the present study may account for the pathogenesis of HCV-induced IR.
Overall, we propose a model that illustrates the mechanism of WT-PGC-1α/L-PGC-1α upregulation by HCV infection and also illustrates the dual effects of WT-PGC-1α/L-PGC-1α (Fig. 7H). In this model, HCV infection upregulates both WT-PGC-1α and L-PGC-1α through an ER stress-mediated, phosphorylated CREB-dependent pathway, and both PGC-1α isoforms promote HCV-induced IR and HCV production in turn through their downstream factors; HCV-induced upregulation of L-PGC-1α also depends on FoxO1 dephosphorylation, which is independent of ER stress. A comprehensive understanding of the cross talk among HCV, ER stress, and WT-PGC-1α/L-PGC-1α is important to identify novel antiviral targets. The dual effects of WT-PGC-1α/L-PGC-1α suggest that their inhibitors would elicit therapeutic effects for both HCV infection and HCV-associated IR. The therapeutic value and promising safety profile of PGC-1α modulators have been demonstrated in animal models for different diseases (49); thus, the pharmacological regulation of WT-PGC-1α/L-PGC-1α may be a novel approach for HCV therapy. ER stress has also been considered another anti-HCV target because of its intermediary function. PBA, an ER stress inhibitor, can be orally administered and is currently used clinically (50). The demonstrated efficacy of PBA in attenuating WT-PGC-1α/L-PGC-1α upregulation and in blocking HCV production suggests that PBA may serve as another promising anti-HCV therapeutic choice.
ACKNOWLEDGMENTS
We thank Donghai Wu and Tao Nie for providing genetic Leprdb/db mouse samples.
This study was supported by the National Basic Research Program (973) (grant 2010CB530100) and the National Science Foundation (grant 31370204).
Footnotes
Published ahead of print 15 May 2014
REFERENCES
- 1.Thomas DL. 2013. Global control of hepatitis C: where challenge meets opportunity. Nat. Med. 19:850–858. 10.1038/nm.3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. 2013. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57:1333–1342. 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- 3.Aghemo A, Prati GM, Rumi MG, Soffredini R, D'Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. 2012. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology 56:1681–1687. 10.1002/hep.25867 [DOI] [PubMed] [Google Scholar]
- 4.White DL, Ratziu V, El-Serag HB. 2008. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J. Hepatol. 49:831–844. 10.1016/j.jhep.2008.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck BN, Kelly DP. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116:615–622. 10.1172/JCI27794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138. 10.1038/35093050 [DOI] [PubMed] [Google Scholar]
- 7.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183. 10.1038/35093131 [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Marcos PJ, Auwerx J. 2011. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93:884S–890S. 10.3945/ajcn.110.001917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Jr, Xiong Y, Daniel KW, Floering L, Collins S. 2005. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J. Biol. Chem. 280:42731–42737. 10.1074/jbc.M506223200 [DOI] [PubMed] [Google Scholar]
- 10.Collins QF, Xiong Y, Lupo EG, Jr, Liu HY, Cao W. 2006. p38 mitogen-activated protein kinase mediates free fatty acid-induced gluconeogenesis in hepatocytes. J. Biol. Chem. 281:24336–24344. 10.1074/jbc.M602177200 [DOI] [PubMed] [Google Scholar]
- 11.Felder TK, Soyal SM, Oberkofler H, Hahne P, Auer S, Weiss R, Gadermaier G, Miller K, Krempler F, Esterbauer H, Patsch W. 2011. Characterization of novel peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) isoform in human liver. J. Biol. Chem. 286:42923–42936. 10.1074/jbc.M111.227496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Handschin C, Spiegelman BM. 2005. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1:361–370. 10.1016/j.cmet.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 13.Malhi H, Kaufman RJ. 2011. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54:795–809. 10.1016/j.jhep.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moradpour D, Penin F, Rice CM. 2007. Replication of hepatitis C virus. Nat. Rev. Microbiol. 5:453–463. 10.1038/nrmicro1645 [DOI] [PubMed] [Google Scholar]
- 15.Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH. 2008. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 48:1054–1061. 10.1002/hep.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce MA, Walters KA, Lamb SE, Yeh MM, Zhu LF, Kneteman N, Doyle JS, Katze MG, Tyrrell DL. 2009. HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice. PLoS Pathog. 5:e1000291. 10.1371/journal.ppat.1000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asselah T, Bieche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, Bedossa P, Paradis V, Martinot-Peignoux M, Lebrec D, Guichard C, Ogier-Denis E, Vidaud M, Tellier Z, Soumelis V, Marcellin P, Moreau R. 2010. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J. Pathol. 221:264–274. 10.1002/path.2703 [DOI] [PubMed] [Google Scholar]
- 18.Christen V, Treves S, Duong FH, Heim MH. 2007. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology 46:558–565. 10.1002/hep.21611 [DOI] [PubMed] [Google Scholar]
- 19.Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. 2008. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J. Hepatol. 49:429–440. 10.1016/j.jhep.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 20.Shlomai A, Rechtman MM, Burdelova EO, Zilberberg A, Hoffman S, Solar I, Fishman S, Halpern Z, Sklan EH. 2012. The metabolic regulator PGC-1alpha links hepatitis C virus infection to hepatic insulin resistance. J. Hepatol. 57:867–873. 10.1016/j.jhep.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 21.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. 2003. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649. 10.2337/diabetes.52.3.642 [DOI] [PubMed] [Google Scholar]
- 22.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. 2003. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc. Natl. Acad. Sci. U. S. A. 100:7111–7116. 10.1073/pnas.1232352100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummelkamp TR, Bernards R, Agami R. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550–553. 10.1126/science.1068999 [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Li T, Zeng M, Peng T. 2012. Herpes simplex virus type 1 infection activates the Epstein-Barr virus replicative cycle via a CREB-dependent mechanism. Cell. Microbiol. 14:546–559. 10.1111/j.1462-5822.2011.01740.x [DOI] [PubMed] [Google Scholar]
- 25.Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. 2006. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. U. S. A. 103:7408–7413. 10.1073/pnas.0504877103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626. 10.1126/science.1114016 [DOI] [PubMed] [Google Scholar]
- 27.Li X, Jiang H, Qu L, Yao W, Cai H, Chen L, Peng T. 2014. Hepatocyte nuclear factor 4alpha and downstream secreted phospholipase A2 GXIIB regulate production of infectious hepatitis C virus. J. Virol. 88:612–627. 10.1128/JVI.02068-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pokrovskii MV, Bush CO, Beran RK, Robinson MF, Cheng G, Tirunagari N, Fenaux M, Greenstein AE, Zhong W, Delaney WE, IV, Paulson MS. 2011. Novel mutations in a tissue culture-adapted hepatitis C virus strain improve infectious-virus stability and markedly enhance infection kinetics. J. Virol. 85:3978–3985. 10.1128/JVI.01760-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blight KJ, Kolykhalov AA, Rice CM. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972–1974. 10.1126/science.290.5498.1972 [DOI] [PubMed] [Google Scholar]
- 30.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. 1999. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J. Biol. Chem. 274:17179–17183. 10.1074/jbc.274.24.17179 [DOI] [PubMed] [Google Scholar]
- 31.Tardif KD, Mori K, Siddiqui A. 2002. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 76:7453–7459. 10.1128/JVI.76.15.7453-7459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tardif KD, Waris G, Siddiqui A. 2005. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 13:159–163. 10.1016/j.tim.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 33.Welch WJ, Brown CR. 1996. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Kang R, Wang J, Luo G, Yang W, Zhao Z. 2013. Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy 9:175–195. 10.4161/auto.22791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumpter R, Jr, Loo YM, Foy E, Li K, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689–2699. 10.1128/JVI.79.5.2689-2699.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King AJ. 2012. The use of animal models in diabetes research. Br. J. Pharmacol. 166:877–894. 10.1111/j.1476-5381.2012.01911.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461. 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- 38.Koh HJ, Toyoda T, Didesch MM, Lee MY, Sleeman MW, Kulkarni RN, Musi N, Hirshman MF, Goodyear LJ. 2013. Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nat. Commun. 4:1871. 10.1038/ncomms2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. 2008. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl. Acad. Sci. U. S. A. 105:19926–19931. 10.1073/pnas.0810339105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra JD, Kaufman RJ. 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9:2277–2293. 10.1089/ars.2007.1782 [DOI] [PubMed] [Google Scholar]
- 41.Deng L, Shoji I, Ogawa W, Kaneda S, Soga T, Jiang DP, Ide YH, Hotta H. 2011. Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J. Virol. 85:8556–8568. 10.1128/JVI.00146-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. 2003. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 100:4012–4017. 10.1073/pnas.0730870100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartenschlager R, Penin F, Lohmann V, Andre P. 2011. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 19:95–103. 10.1016/j.tim.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Norris JY, Finck BN. 2010. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) stimulates VLDL assembly through activation of cell death-inducing DFFA-like effector B (CideB). J. Biol. Chem. 285:25996–26004. 10.1074/jbc.M110.141598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke PY, Chen SS. 2011. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 121:37–56. 10.1172/JCI41474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunduz F, Aboulnasr FM, Chandra PK, Hazari S, Poat B, Baker DP, Balart LA, Dash S. 2012. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol. J. 9:143. 10.1186/1743-422X-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano R, Reed JC. 2013. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 1833:3460–3470. 10.1016/j.bbamcr.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joyce MA, Walters KA, Lamb SE, Yeh MM, Zhu LF, Kneteman N, Doyle JS, Katze MG, Tyrrell DL. 2009. HCV induces oxidative and ER stress, and sensitizes infected cells to apoptosis in SCID/Alb-uPA mice. PLoS Pathog. 5(2):e1000291. 10.1371/journal.ppat.1000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Handschin C. 2009. The biology of PGC-1alpha and its therapeutic potential. Trends Pharmacol. Sci. 30:322–329. 10.1016/j.tips.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 50.Engin F, Hotamisligil GS. 2010. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12(Suppl 2):S108–S115. 10.1111/j.1463-1326.2010.01282.x [DOI] [PubMed] [Google Scholar]