ABSTRACT
Hantavirus cardiopulmonary syndrome (HCPS) is a rodent-borne disease with a high case-fatality rate that is caused by several New World hantaviruses. Each pathogenic hantavirus is naturally hosted by a principal rodent species without conspicuous disease and infection is persistent, perhaps for life. Deer mice (Peromyscus maniculatus) are the natural reservoirs of Sin Nombre virus (SNV), the etiologic agent of most HCPS cases in North America. Deer mice remain infected despite a helper T cell response that leads to high-titer neutralizing antibodies. Deer mice are also susceptible to Andes hantavirus (ANDV), which causes most HCPS cases in South America; however, deer mice clear ANDV. We infected deer mice with SNV or ANDV to identify differences in host responses that might account for this differential outcome. SNV RNA levels were higher in the lungs but not different in the heart, spleen, or kidneys. Most ANDV-infected deer mice had seroconverted 14 days after inoculation, but none of the SNV-infected deer mice had. Examination of lymph node cell antigen recall responses identified elevated immune gene expression in deer mice infected with ANDV and suggested maturation toward a Th2 or T follicular helper phenotype in some ANDV-infected deer mice, including activation of the interleukin 4 (IL-4) pathway in T cells and B cells. These data suggest that the rate of maturation of the immune response is substantially higher and of greater magnitude during ANDV infection, and these differences may account for clearance of ANDV and persistence of SNV.
IMPORTANCE Hantaviruses persistently infect their reservoir rodent hosts without pathology. It is unknown how these viruses evade sterilizing immune responses in the reservoirs. We have determined that infection of the deer mouse with its homologous hantavirus, Sin Nombre virus, results in low levels of immune gene expression in antigen-stimulated lymph node cells and a poor antibody response. However, infection of deer mice with a heterologous hantavirus, Andes virus, results in a robust lymph node cell response, signatures of T and B cell maturation, and production of antibodies. These findings suggest that an early and aggressive immune response to hantaviruses may lead to clearance in a reservoir host and suggest that a modest immune response may be a component of hantavirus ecology.
INTRODUCTION
Hantaviruses (family Bunyaviridae) are trisegmented, negative-stranded viruses that are hosted by rodents, moles, shrews, and, perhaps, bats (1). Several rodent-borne hantaviruses are human pathogens, but in reservoir hosts that have been experimentally examined, infection causes little or no pathology (2–4), although it may reduce survival in natural populations (5, 6). How hantaviruses evade sterilizing immune responses in their reservoirs is unknown, which is an obstacle for understanding the ecology and zoonotic potential of these viruses.
Hantaviruses cause two diseases in humans that share many pathological similarities: hemorrhagic fever with renal syndrome (HFRS) in Eurasia and hantavirus cardiopulmonary syndrome (HCPS) in the Americas (7, 8). About 200,000 cases of hantaviral disease occur each year, with fatality rates ranging from about 1% to more than 40%, depending on the virus (8, 9). Each pathogenic hantavirus is hosted by a single principal rodent reservoir, with occasional spillover to other rodent species (9–12), and infection of reservoirs results in persistence without signs of disease (2, 13). In contrast, human disease is characterized by a vascular leak syndrome and thrombocytopenia without signs of virus-induced damage to the endothelium (11, 14–18), the principal target of infection in both humans and rodents (2, 18). Inflammatory virus-specific T cells have been isolated from, and inflammatory cytokines detected in, hantavirus patients and autopsy specimens (19–21); thus, it has been speculated that the immune response contributes to pathogenesis.
While the role of the immune response in human hantaviral disease has been modestly addressed, little is known about how hantaviruses persist within their reservoirs without immune pathology, or how the viruses evade sterilizing immune responses to establish persistence (22). Naturally infected rodent reservoirs of hantaviruses produce virus-specific IgG, indicating that immune responses occur (10, 23–29). Experimental Seoul virus infection of its reservoir host, the Norway rat (Rattus norvegicus), also results in seroconversion, and signatures of regulatory T cell responses, including transforming growth factor β (TGF-β) and Fox-p3, are prominent (30). Infection of deer mice (Peromyscus maniculatus), the principal reservoir host of Sin Nombre virus (SNV), results in virus in many organs, including the lungs, heart, spleen, and kidneys, without conspicuous effects to the endothelium (2, 13). CD4+ T cells from acutely infected deer mice express many Th1 and Th2 cytokines, but TGF-β and Fox-p3 are expressed prominently from persistently infected deer mice, suggesting transition to a regulatory T cell response (31). Neutralizing antibodies are produced after several weeks; however, deer mice remain persistently infected (2). Thus, infection appears to lead to a modest immune response during acute infection, followed by a regulatory immune response that may mitigate disease but which also impairs virus clearance.
Recent work by us demonstrated that deer mice are experimentally susceptible to Andes hantavirus (ANDV) (32), which is naturally hosted by the long-tailed pygmy rice rat (Oligoryzomys longicaudatus) and which causes the great majority of HCPS cases in South America (8, 9). While deer mice remain persistently infected with SNV, they clear ANDV between 21 and 56 days postinfection without signs of disease. Antigen stimulation of lymph node cells (LNC) from ANDV-infected deer mice induces elevated expression of many cytokines and antiviral genes, including a preponderance of Th2 genes (33). Thus, deer mice provide an opportunity to experimentally examine how a reservoir host species is persistently infected with its homologous hantavirus but can clear a heterologous hantavirus.
Deer mice are about 25 million years divergent from laboratory mice (Mus musculus) and rats (Rattus norvegicus) (34); thus, few cross-reactive antibodies are available for assessing immune mobilization in deer mice. However, the deer mouse genome has been sequenced to 27× depth, but it has not yet been annotated. We developed a bioinformatics approach to identify cDNAs of interest from the deer mouse genomic trace archive for examination of deer mouse immune responses by real-time PCR gene expression analysis (33). In this study, we used this approach to identify differences in antigen-stimulated lymph node cell responses to SNV and ANDV in experimentally infected deer mice. While immune gene expression levels were elevated in response to SNV antigen, a substantially more robust response to ANDV occurred, including an antibody response and activation of helper T cells and B cells. Phosphorylation of STAT1Y701 occurred in all cultures of LNC from ANDV-infected mice examined but in only 3 of 5 cultures of LNC from SNV-infected mice. Together, these differences in lymphocyte activation may account for clearance of a heterologous hantavirus and may explain why homologous hantaviruses establish persistence.
MATERIALS AND METHODS
Ethics statement.
All procedures using deer mice were in compliance with the U.S. Animal Welfare Act and approved by the Rocky Mountain Laboratories (RML) institutional animal care and use committee (protocol 2013-042) and performed by following the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC), by certified staff in an AAALAC-approved facility.
Deer mouse infections.
All work involving infectious material was performed in the biosafety level 4 (BSL4) laboratory at RML. Male deer mice between 10 and 16 weeks of age were randomly divided into groups and were intramuscularly infected with 200 focus-forming units (FFU) of ANDV-9717869 (n = 8) that was propagated on Vero E6 cells (35) or 50 μl of clarified SNV 77734 (n = 5) deer mouse lung homogenate (2) in the hind limbs. Fourteen days later, the mice were anesthetized by inhalation of isoflurane for cardiac blood collection and subsequent euthanasia.
Hematology.
Hematological values and parameters were determined from EDTA blood with the HemaVet 950FS+ laser-based hematology analyzer (Drew Scientific, Dallas, TX). Complete blood counts (CBCs) with differentials were performed, and results for each group were compared by unpaired multiple t test (GraphPad Prism, San Diego, CA).
Viral RNA (vRNA) detection.
Tissue samples were excised and placed in 2-ml tubes containing 600 μl of RLT and a single stainless steel bead. Approximately 30 mg of tissue was homogenized using a TissueLyser (Qiagen, Redwood City, CA) prior to total RNA extraction with an RNeasy kit (Qiagen), according to the manufacturer's instructions. For quantitation of viral S-segment RNA, 40 ng of total RNA was added to the components of a 1-step Rotor-Gene reverse transcription-PCR (RT-PCR) kit (Qiagen) according to the manufacturer's instructions, along with forward and reverse primers and a gene-specific probe in a final reaction volume of 25 μl, as previously reported (13, 36). Sample cycle thresholds (CT) were compared to control cycle thresholds of dilutions of in vitro-transcribed S-segment RNA of known copy numbers used to generate a standard curve, and the number of absolute copies of S-segment RNA per mass of total RNA was extrapolated from the standard curve. Copy numbers were log10 transformed for statistical analysis (two-tailed t test with Welch's correction; GraphPad Prism).
Serology.
Recombinant SNV or ANDV nucleocapsid antigen was diluted to 1 μg/ml in phosphate-buffered saline (PBS), and 100 μl was dispensed into wells of a 96-well polyvinylchloride plate as previously described (37). Plates were incubated overnight at 4°C, washed with PBS-Tween 20, and blocked with 5% milk in PBS. Log2 serial dilutions of sera were made starting from 1:100 and added to the plates for 1 h at room temperature. After washing, goat anti-Peromyscus leucopus IgG(H+L)-horseradish peroxidase (HRP) conjugate (KPL, Gaithersburg, MD) was added for another hour prior to the addition of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate for 15 min. Absorbances were recorded at 405 nm, and the endpoint titers were determined as the reciprocal of the greatest dilutions that were 0.200 optical density (OD) units above the negative-control (uninfected deer mouse) serum sample (diluted 1:100).
Lymph node cell cultures.
Deer mice were euthanized 14 days postinfection, and cervical lymph nodes were collected in serum-free Hanks' balanced salt solution (HBSS) and made into single-cell suspensions. Cells were washed twice in HBSS and then once in complete medium (CM; 5% fetal bovine serum [FBS] in RPMI 1640) and adjusted to 6 × 106/ml in CM. For each deer mouse, 250 μl of cells (1.5 × 106) was pipetted into wells of a 48-well plate in quadruplicate; 2 wells received 250 μl of CM (basal expression), and the other 2 wells received 250 μl of recombinant homologous nucleocapsid (N) antigen (i.e., ANDV or SNV N antigen) in CM at a final concentration of 10 μg/ml. Cells were incubated for 72 h at 37°C and 5% CO2. One well without antigen and one well with antigen were collected for RNA extraction (RNeasy kit; Qiagen) for each deer mouse for gene expression profiling. The cells in the other two wells with or without antigen were collected in modified RIPA buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 1% NP-40, 1% SDS, 0.5% sodium deoxycholate, 0.2 mM sodium orthovanadate, 2 μg/ml each of leupeptin and aprotinin, 1 μg/ml of pepstatin, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 2 mM sodium pyrophosphate, and 1 mM glycerophosphate) for Western blot analysis. Samples were transferred from the BSL4 setting to a BSL2 setting according to standard operating procedures approved by the RML institutional biosafety committee and frozen at −80°C.
Gene expression profiling.
Real-time PCR arrays for 94 immune-related genes representing Th1, Th2, T regulatory (Treg), and Th17 cells and antiviral genes were performed as previously described (33). Briefly, 2 μg of total RNA from lymph node cell cultures was reverse transcribed using the RT2 cDNA synthesis kit (SABiosciences, Valencia, CA), which includes a genomic DNA elimination step. The cDNA was added to RT2 SYBR green I master mix and mixed thoroughly. Twenty microliters was dispensed into a 96-well real-time PCR plate using an 8-channel pipettor, and then 5 μl of primers (2 μM final concentration) was added. Cycling was performed at 95°C for 30 s and 60°C for 30 s for 40 cycles, followed by an 80-step melt curve analysis (iQ5 thermal cycler; Bio-Rad, Hercules, CA). The ΔΔCT method (38) was employed using the mean of Gapdh as the reference within samples for normalization, and comparison of same-gene normalized samples between antigen-stimulated and unstimulated cells was used to calculate fold change (e.g., normalized Ifng from antigen-stimulated LNC cDNA to normalized Ifng from medium-only LNC cDNA). Heat maps and hierarchical clusters were generated using the heatmap.2 (“gplots” package) from R statistical software (http://www.r-project.org/) (33). The distance matrix was calculated using the Euclidean method, and a complete linkage clustering method was used for generating the hierarchical clusters. Differential expression was examined between SNV and ANDV groups by two-tailed unpaired t test and between cluster groups by one-way analysis of variance (ANOVA) with Brown-Forsythe analysis of differences in standard deviations (GraphPad Prism 6). Functional gene classifications were based upon house mouse (Mus musculus) entries in the UniProt database (http://www.uniprot.org).
PCA.
Principal component analysis (PCA), a multivariate statistical technique, was used for summarizing relationships among the 13 deer mice in which relative expression of 42 genes was measured. All 546 relative expression values were entered into a 13 (deer mice) × 42 (genes) matrix. The Pearson's correlation coefficient (ρ) among expression values for each gene was calculated between each pair of deer mice to generate a symmetrical 13 × 13 correlation matrix. Biplot analysis is a facet of principal components (39) that requires little additional computation and identifies the magnitude of variances and covariances among individual genes. Principal component and biplot analyses were performed on R version 2.11.1 using the prcomp and biplot functions.
Detection of pSTAT1 and IRF8.
Cell lysates from LNC of all 5 SNV- and 4 ANDV-infected mice (insufficient cells were available for mice A2, A3, A4, and A8) were thawed on ice and centrifuged at 21,000 × g for 15 min, and protein concentrations of supernatants were determined with a Micro BCA kit (Pierce, Rockford, IL). Fifteen micrograms of cell lysate was separated under denaturing conditions on 4 to 12% NuPage (Life Technologies, Foster City, CA) polyacrylamide gels, transferred to Immobilon-P membranes (Millipore, Billerica, MA), and incubated with pSTAT1 (Tyr701; sc-135648; Santa Cruz Biotechnology, CA), STAT1 (p84/p91; E-23; sc-346; Santa Cruz), IRF8 (D20D8; 5628; Cell Signaling, Boston, MA), or β-actin (4967; Cell Signaling) antibodies overnight at 4°C. Blots were washed, incubated with anti-rabbit IgG antibody conjugated to horseradish peroxidase, and developed with 3,3′,5,5′-tetramethylbenzidine colorimetric substrate (KPL). Western blots were captured on a Visioneer 9420 reflective scanner with Photoshop CS 8.0. Composite images were assembled and levels of all parts of each panel adjusted at once with Photoshop CS5 Extended Version 12.0 ×32.
RESULTS
Hematology.
Complete blood counts with differentials were performed for each deer mouse. No differences were found in total white blood cell, red blood cell, platelet, neutrophil, monocyte, eosinophil, or basophil counts, hemoglobin, hematocrit or mean corpuscle concentrations, mean corpuscle volume, or red blood cell distribution width (Fig. 1).
FIG 1.
No hematological changes were found in deer mice infected with SNV or ANDV. CBCs were performed on each deer mouse, and the means and standard deviations were compared for ANDV-infected (A; n = 8), SNV-infected (S; n = 5), and uninfected (U; n = 3) animals. WBC, white blood count; Lymph, lymphocytes; Neut, neutrophils; Mono, monocytes, Eosin, eosinophils; Baso, basophils; Plt, platelets; MPV, mean platelet volume; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscle volume; MCH, mean corpuscle hemoglobin; MCHC, mean corpuscle hemoglobin concentration; RBC DW, red blood cell distribution width.
Viral load.
Viral RNA was detected in lungs and hearts of all SNV-infected deer mice but in only 3 of 5 spleens and 2 of 5 kidneys (Fig. 2). Variation of SNV load in the lungs was high (range, 14,096 to 1,609,662 copies of S), similar to our previous observation with deer mice (13). ANDV RNA was found in spleens of all infected deer mice, 7 of 8 lungs and hearts, and 4 of 8 kidneys. The variation was substantially lower in ANDV-infected deer mouse lungs (range, 0 to 20,518), which was the only tissue that was significantly different (P = 0.009) between ANDV- and SNV-infected deer mice.
FIG 2.
SNV loads are greater in the lungs but similar in other organs. Total RNA was extracted from each organ 14 days postinfection and copy number determined by TaqMan real-time RT-PCR against standards. Two-tailed t tests with Welch's correction were performed to determine statistical differences between deer mice. SNV RNA was greater in the lungs (P = 0.0009) but not different in heart, spleen, or kidneys.
Gene expression profiles in LNC cultures.
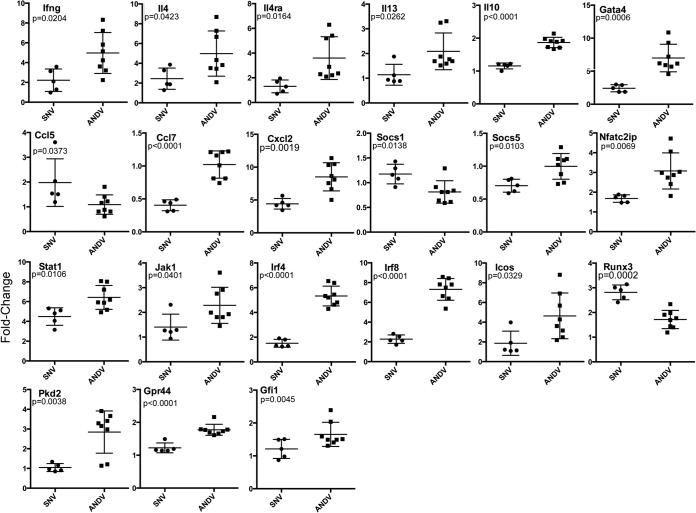
Lymph node cells from infected deer mice were incubated with or without homologous nucleocapsid antigen for 3 days, after which total RNA was extracted. Real-time RT-PCR was used to examine the differences in expression of 94 immune-related genes. Expression of most genes was not modulated, but 42 genes were substantially elevated or repressed in LNC cultures from at least one deer mouse. Twenty-one genes were differentially expressed in the two groups (two-tailed t test) (Fig. 3), and all but three (Ccl5, Runx3, and Socs1) were higher in ANDV LNC. Most that were modulated were elevated in both groups, but the increases were more apparent in LNC from ANDV-infected deer mice than from SNV-infected deer mice. Many lymphocyte activation genes, including those associated with antibody production, were elevated. Two differentially expressed antiviral genes (Pkd2 and Cxcl2), both of which are expressed by activated T cells, were more abundant in LNC from ANDV-infected deer mice. Ccl7 was repressed in SNV LNC but unchanged in ANDV LNC. Casp3 expression was repressed in LNC from all 13 infected mice (see the supplemental material).
FIG 3.
Differential gene expression in LNC from infected deer mice shows a robust response to ANDV. Total RNA was extracted from lymph node cell cultures, and gene expression levels were determined by real-time RT-PCR. The ΔΔCT method was used to assess expression levels by normalizing against Gapdh within unstimulated or antigen-stimulated samples (ΔCT), followed by division of the antigen-stimulated normalized value into the unstimulated normalized value for each gene (ΔΔCT) to determine fold changes. Expression levels were not significantly different for most genes; however, 21 genes were differentially expressed (unpaired t test), with most higher in LNC from ANDV-infected deer mice.
The expression data set of the 13 deer mice and the 42 genes was subjected to cluster analysis, which identified three broad groups: one with low Th1/Th2 gene expression, one with high Th1/Th2 expression, and an outgroup with high Th2 expression (Fig. 4). All five SNV LNC cultures were from the low-Th1/Th2 group (SNV group), six of the ANDV LNC cultures were from the high-Th1/Th2 group (ANDV1 group), and both LNC cultures in the high-Th2 group were from deer mice infected with ANDV (ANDV2 group). The SNV and ANDV1 groups clustered to the exclusion of the ANDV2 group. None of the SNV-infected deer mice had detectable IgG antibody titers (<100), while titers in the ANDV1 group ranged from undetectable to 200 (Fig. 4). The two deer mice in the ANDV2 group (A1 and A7) also had the highest IgG antibody titers (800 and 1,600, respectively).
FIG 4.
Cluster analysis of gene expression profiles in LNC identifies three immune profiles. Cluster analysis was performed on the 13 deer mice based upon the expression of 42 immune-related genes using gplots (R statistical software). The resulting dendrogram identified three broad groups: a low-Th1/Th2-expressing group of all 5 SNV-infected mice (SNV), a high-Th1/Th2-expressing group that included six of the eight ANDV-infected mice (ANDV1), and a Th2 group of the other two ANDV-infected mice (ANDV2). None of the SNV-infected deer mice seroconverted (titer < 100), while six of the eight ANDV-infected deer mice had seroconverted, with the two highest titers in the ANDV2 group.
Based upon the segregation of the deer mice by the cluster analysis of expression profiles, we examined gene expression levels in the three groups to identify additional expression patterns (ANOVA) (Fig. 5). In this analysis, 24 genes were differentially expressed, including 20 of the 21 genes that were differentially expressed in the analysis of SNV versus ANDV (compare to Fig. 3); only Ccl5 expression was not statistically different by this analysis. Four additional genes (Stat4, Il6, Cebpb, and Myb) were differentially expressed in this analysis compared to the SNV and ANDV group (t test) analysis. Many genes of the Th2, T follicular helper (Tfh), and B cell activation (Il4, Il13, Il4ra, Il6, Gata4, Jak1, Icos, Nfatc2ip, and Cebpb) pathways were higher in the ANDV2 group than in the ANDV1 group, whereas Ifng expression was higher in the ANDV1 group than in the ANDV2 group. Tgfb expression was elevated about 2-fold in both ANDV and SNV cultures, similar to our previous observations (13, 33), but the levels were not statistically different (see the supplemental material).
FIG 5.
Differential gene expression between cluster groups indicates a Th2 bias in ANDV2 group. Based upon the cluster analysis (Fig. 4), one-way ANOVA was performed on the three groups (SNV, n = 5; ANDV1, n = 6; ANDV, n = 2). One gene that was differentially expressed according to SNV/ANDV t test was no longer different (Ccl5), whereas four other genes was (Stat4, Il6, Cebpb, and Myb). Several genes denoting T cell/B cell activation events were higher in the ANDV2 than in the ANDV1 group (Gata4, Jak1, Il4, Il13, Il4ra, Il6, Icos, Nfatc2ip, and Gfi1).
Principal component analysis was used to identify genes with differences in expression profiles in individual deer mice (Fig. 6). Most genes had a minor impact on the distribution of LNC profiles. In contrast, expression of genes associated with lymphocyte activation accounted for a large proportion of the differences. All five LNC cultures from the SNV-infected deer mice clustered near Stat4, Runx3, Ccl5, and Irf1, although the expression levels of these genes were modest (i.e., short distance from biplot origin). Many more genes, most of which were expressed in greater abundance, were associated with LNC cultures from the ANDV-infected deer mice, and genes associated with germinal center formation, class switching, and affinity maturation clustered nearest to the two LNC cultures that exhibited a Th2 profile (deer mice A1 and A7, in the ANDV2 group). Although the SNV and ANDV1 group clustered together to the exclusion of the ANDV2 group (i.e., similar expression profiles), the positioning of those genes on the biplot was more heavily influenced by the six ANDV1 LNC cultures.
FIG 6.
Principal component analysis identifies a strong influence of genes of the T helper/B cell activation pathways in antigen-stimulated lymph node cells. PCA was used to generate a biplot of gene expression of the antigen-simulated lymph node cells cultured for 3 days. The LNC from SNV-infected mice (blue) clustered together with low expression levels; however, LNC from ANDV-infected mice were more diverse (red). Several genes of T cell/B cell effector functions (green oval) accounted for much of this distribution. Most genes had little influence on the distribution pattern (gray circle around origin).
Detection of pSTAT1 and IRF8.
The JAK-STAT signaling pathway is pivotal in directing the immune response to virus infection, and activation of the pathway is often antagonized by viral proteins. Increased expression of Stat1 mRNA was detected when lymph node cells were exposed to viral nucleocapsid ex vivo (Fig. 3). To evaluate whether increased gene expression correlated with activation of STAT1, Western blot analysis for total and phosphorylated STAT1Y701 was performed. Phosphorylation of STAT1Y701, a plasma membrane-proximal event, was detected in the LNC from all four ANDV-infected animals tested (insufficient numbers of cells were available for A2, A3, A4, and A8) but in LNC from only three of the five SNV-infected animals (S1, S5, and S6) (Fig. 7). LNC from the two SNV-infected deer mice that did not have pSTAT1 (S4 and S7) also had near-baseline Ifng expression, whereas the other three had elevated expression (2.1- to 3.6-fold) and all ANDV cultures had elevated expression (2.2-fold or greater). Expression of IRF8, another gene that was highly expressed in ANDV LNC cultures, was also detected. Increased expression of IRF8 was observed in all LNC cultures exposed to viral antigen, though more robustly in ANDV LNC.
FIG 7.

Detection of phosphorylated STAT1 and IRF8 in LNC from SNV-infected and ANDV-infected deer mice. All cultures were responsive to stimulation, as indicated by increased expression of IRF8 and STAT1 and by STAT1 activation (phospho-Y701). Cells were harvested at 14 days postinfection and cultured without or with recombinant nucleocapsid antigen stimulation for 72 h prior to lysis and Western analysis with the indicated antibodies. S, SNV infected; A, ANDV infected.
DISCUSSION
A prominent feature of hantavirus disease in humans is thrombocytopenia, which is used, in part, as a diagnostic indicator. Platelet counts from deer mice infected for 14 days with SNV or ANDV were not different than counts from uninfected deer mice (Fig. 1) and were similar to those obtained with ANDV infection of Syrian hamsters (Mesocricetus auratus) (36). Infection of human umbilical vein endothelial cells (HUVEC) with ANDV causes the adherence of quiescent human platelets to those cells, which is proposed to account for the observed thrombocytopenia in human hantavirus cases (40). Neutralizing antibodies to virus or blocking of β3-integrin (CD61), the receptor for pathogenic hantaviruses, on platelets or HUVEC prevents platelet adherence, suggesting that hantavirus Gn or Gc glycoproteins on the surface of infected cells are responsible for this event. Deer mouse endothelial cells are also the principal target of hantavirus infection (2); thus, the absence of thrombocytopenia suggests that quiescent platelets do not adhere to the endothelium of reservoir hosts. This is seemingly paradoxical since, presumably, SNV and ANDV also bind to β3-integrin on platelets or endothelial cells of infected deer mice. It may be that sequence differences between deer mouse and human β3-integrins account for this difference. All other hematological parameters were also nominal relative to uninfected deer mice, suggesting that no perturbation occurs during infection with either virus.
Viral S segment load was not different in the spleens, hearts, or livers of deer mice infected with ANDV or SNV (Fig. 2). However, SNV load in the lungs was significantly higher than ANDV load. One ANDV-infected deer mouse (A3) had substantially greater viral RNA in the lung (20,518 copies) than the other seven ANDV-infected deer mice; the next greatest was 2,138 copies. In contrast, only one SNV-infected deer mouse (S7) had fewer copies than did A3 deer mouse (14,096 copies), whereas one (S6) had more than a million copies of SNV S segment. We previously observed a similarly high variation of vRNA copies in the lungs of SNV-infected deer mice; this variation may contribute to increased shedding by some deer mice (“supershedders”) (13). In each organ, variation was substantially greater in SNV-infected deer mice than in ANDV-infected deer mice. It may be that abundant viral load in the lungs is a contributory factor for persistence. One SNV deer mouse (S1) had substantially higher RNA levels in the spleen than the other SNV-infected deer mice; exclusion of this animal from the viral load analysis resulted in statistically greater RNA abundance in the spleens of ANDV-infected deer mice (P = 0.008). It is possible that ANDV replicates more efficiently in secondary lymphoid tissues of deer mice, which could provide a substantially greater antigenic stimulus to the responding T and B cells that may contribute to clearance of ANDV.
Lymph node cells were examined for in vitro antigen recall responses. This assay historically has been used to assess CD4+ T cell proliferation and activation events, but it can also provide substantial information about other cells found in lymph nodes, including B cells, dendritic cells, follicular dendritic cells, and macrophages. The LNC cultured with antigen for 3 days had conspicuous proliferation in wells that received antigen but not in wells that were cultured without antigen (data not shown). Lymph nodes are a principal site of T cell/B cell collaboration for many essential aspects of the adaptive immune response. Many T cell-directed B cell activities, such as clonal expansion, class switching, and affinity maturation, occur in lymph nodes. These activities are facilitated by many cognate and noncognate signaling events between T cells and antigen-presenting B cells that capture antigen with surface B cell receptor (BCR) and process those antigens to peptide/major histocompatibility complex (MHC) class II complexes that are presented to helper T cells. The lymph nodes are also a critical site of CD4+ T cell maturation in response to antigenic stimulation, where naive T cells (Th0) differentiate into effector Th1 or Th2 cells, which can further differentiate into T follicular helper cells in germinal centers. Tfh cells provide the stimuli, in the form of cytokine signaling and receptor/counterreceptor ligation, that induce class switching and affinity maturation in antibody genes of B cells. Lymph nodes are also vascularized, and it is possible that lymph node endothelial cells may harbor virus; hantavirus RNA is detected in lymphocyte cultures from infected deer mice (31, 33).
We identified 25 genes that were differentially expressed (Table 1). Our initial analysis compared gene expression profiles between the two infection groups, SNV-infected and ANDV-infected deer mice (Fig. 3). Expression of genes in the LNC cultures from SNV-infected deer mice was low to modest, with less than 5-fold increases that were generally greater than our previous findings from whole spleens of SNV-infected deer mice (13). This greater abundance is likely because the lymph node cells were stimulated with antigen ex vivo, which led to clonal expansion of virus-specific lymphocytes during the 3-day incubation and a greater signal-to-noise ratio. However, expression levels in response to ANDV antigen were mostly greater than observed in SNV LNC, with some genes expressed 10-fold or more, similar to our previous findings (33). By this analysis, the difference in T cell responses appeared to be principally one of magnitude: ANDV elicits a more robust immune response in deer mice than does SNV. Ccl5 was slightly higher (P = 0.0373) in LNC from SNV-infected deer mice but near background for the cells from ANDV-infected deer mice. The Ccl5 product is a T cell-synthesized chemotactic factor for recruitment of basophils and can participate in the activation of NK cells. It is expressed in spleens of deer mice infected with SNV and of rats infected with Seoul virus (13, 41). Ccl2 was not expressed in LNC from either SNV- or ANDV-infected deer mice; however, it is detected in spleen RNA from deer mice at 15 days postinfection (13). It is unclear why they are different, but the difference suggests organ-specific patterns of expression, antigen-stimulated suppression, or that its levels are similarly elevated in antigen-stimulated and unstimulated lymph node cells from infected deer mice, which would be calculated near 1 using the ΔΔCT method (i.e., no change in expression).
TABLE 1.
Differentially expressed genes in antigen-stimulated lymph node cells from deer mice infected with SNV or ANDV
| Gene | Bias | Immune function(s) | P value | UniProt accession no. |
|---|---|---|---|---|
| Ccl5 | SNV | Chemoattractant for blood monocytes, memory T-helper cells, and eosinophils | 0.0373 | P30882 |
| Runx3 | SNV | T cell activation; cellular proliferation and differentiation | 0.0002 | Q64131 |
| Stat4 | SNV | IL-12 activation; Th1 development | 0.0363 | P42228 |
| Ccl7 | SNVa | Chemotactic factor that attracts monocytes and eosinophils but not neutrophils | <0.0001 | Q03366 |
| Cxcl2 | ANDV | Chemotaxis of polymorphonuclear leukocytes | 0.0019 | P10889 |
| Pkd2 | ANDV | T cell activation by NFAT pathway | 0.0038 | Q13563 |
| Gata4 | ANDV | Interacts with NFAT family members; IL-12 response; cytokine induction | 0.0006 | Q08369 |
| Stat1 | ANDV | IFN-αβγ pathway; Tfh activation and host cell response to infection | 0.0106 | P42225 |
| Il10 | ANDV | Inhibition of IFN-γ, IL-2, and tumor necrosis factor; produced by activated macrophages and T cells | <0.0001 | P18893 |
| Irf4 | ANDV | B cell activation, class switching, plasma cell formation, Th2 differentiation | <0.0001 | Q64287 |
| Irf8 | ANDV | Germinal center B cells; induces expression of more than 50 B cell genes | <0.0001 | P23611 |
| Gpr44 | ANDV | Prostaglandin D2 receptor; promotes inflammation | <0.0001 | Q9Z2J6 |
| Myb | ANDV | Activated T and B cells; induction of MIR-155 | 0.0478 | P06876 |
| Socs5 | ANDV | Inhibits IL-4 and IL-6 signaling pathways | 0.0103 | O54928 |
| Ifng | ANDV1 | Antiviral response; macrophage activation; promotes inflammation, produced by Th1 | 0.0070 | P01580 |
| Jak1 | ANDV2 | IFNαβγ/STAT1 signaling pathway; IL-4 signaling in B cells | 0.0033 | P52332 |
| Il4 | ANDV2 | B cell activation; upregulates MHC II expression; Ig synthesis | 0.0017 | P07750 |
| Il13 | ANDV2 | B cell activation; upregulates MHC II expression; suppresses inflammation | <0.0001 | P20109 |
| Il4ra | ANDV2 | B cell receptor for IL-4 and IL-13; Th2 maturation; B cell activation | 0.0005 | P16382 |
| Il6 | ANDV2 | Induction of maturation of B cells into antibody-secreting cells | 0.0013 | P08505 |
| Cebpb | ANDV2 | Induction of acute-phase genes and promotion of inflammation | 0.0336 | P28033 |
| Gfi1 | ANDV2 | Lymphocyte proliferation, TCR signaling | 0.0007 | P70338 |
| Icos | ANDV2 | Tfh/B cell interaction; germinal center formation and class switching; prevents T cell apoptosis; induces Il10 expression | 0.0008 | Q9WVS0 |
| Nfatc2ip | ANDV2 | Dimerizes with IRF4 in B cells; affinity maturation and class switching | <0.0001 | O09130 |
| Socs1 | ANDV2a | Inhibition of Il2, Il3, Csf2, and Ifng expression | 0.0425 | O35716 |
Repressed expression.
The deer mice in this study were outbred, and because of polymorphisms and epigenetic factors, assessment of host responses can complicate groupings that are routinely used with highly inbred animals (e.g., laboratory mice). For this reason, we performed cluster analysis of the expression array data on all 13 deer mice and identified three broad groups: one group that included all five SNV-infected deer mice (SNV group), a second group of six of the ANDV-infected deer mice (ANDV1 group), and an outgroup of the other two ANDV-infected deer mice (ANDV2 group) (Fig. 4). Although the expression levels were mostly lower for the SNV group, this group shared expression of many of the same Th1 genes as found in the ANDV1 group. The ANDV2 group expressed fewer Th1 genes, and 10 genes were substantially higher than in the ANDV1 group, including several genes involved in Tfh cell-mediated B cell activation events (Jak1, Il4, Il13, Il4ra, Il6, Icos, Nfatc2ip, and Gfi1) (Fig. 5) that are associated with affinity maturation and class switching (42, 43). We previously reported elevation of genes in the interleukin 4 (IL-4) signaling pathway in three of four ANDV-infected deer mice, similar to the findings presented here. Irf4, which is essential for Tfh cell function (44), was significantly higher in LNC from ANDV-infected deer mice. Irf8 expression was also elevated in LNC from ANDV-infected deer mice. It is expressed by a variety of immune cells and is associated with cytotoxic T lymphocyte (CTL) and B cell activation and Th17 repression (45–47). Irf8 is also implicated in dendritic cell development (48, 49). Without single-cell analysis (i.e., antibodies to lymphocyte markers), it will be challenging to identify its role in hantavirus infections of deer mice. We did not detect elevated expression of the Th1 maturation transcription factor encoded by Tbx21. We previously detected its expression in polyclonal T cell lines from spleens of SNV-infected deer mice 10 days postinfection but not 42 days postinfection (31); thus, its expression may have been tempered by day 14 postinfection, or its expression differs in spleens and lymph nodes.
Four additional genes were differentially expressed in the SNV-ANDV1-ANDV2 analysis (ANOVA) that were not differentially expressed in the SNV-ANDV analysis (t test): Stat4, Il6, Cebpb, and Myb (Fig. 5). Myb was elevated in ANDV-infected deer mice (P = 0.0478). Myb has been identified as an inducer of the microRNA MIR-155, which is expressed by activated T and B cells (50). In Treg cells, MIR-155 represses Socs1 expression (51), which was lower in LNC from ANDV-infected deer mice (Fig. 5). While deer mice produce a Treg-like response at persistence with SNV (31), other Treg signatures (e.g., Foxp3 and Tgfb) were not differentially expressed. Stat4, which encodes a Th1 differentiation factor (52), was slightly higher in the SNV group (P = 0.0363). Cebpb (NF-IL-6) was slightly higher in the ANDV2 group (P = 0.0336), and its product is a transcriptional activator of Il6 (53). Il6 expression was substantially higher in the ANDV2 group (P = 0.0017); IL-6 is an activator of STAT1, induces differentiation of Tfh cells, and facilitates the final activation stages of B cells for antibody synthesis. Stat1 expression was also slightly higher in LNC from ANDV-infected deer mice. We detected activated pSTAT1Y701 in all four cultures of LNC from ANDV-infected deer mice but only three of five cultures of LNC from SNV-infected deer mice (Fig. 7). The two without pSTAT1 lacked expression of Ifng (see the supplemental material); gamma interferon (IFN-γ) induces phosphorylation of STAT1, and its absence in these deer mouse LNC may account for the lack of pSTAT1. All eight cultures of cells from ANDV-infected deer mice had elevated Ifng expression. The work presented here is the first to examine phosphorylation of STAT1 in deer mouse cells. Studies conducted in various cell lines from other species showed that either ANDV glycoprotein or ANDV nucleocapsid protein could inhibit phosphorylation and nuclear translocation of STAT1, resulting in suppression of the type I interferon response (54). The differences between these studies likely contribute to the ability of ANDV-infected animals to clear infection or to promote pathogenesis.
During an immune response, antigens are transported to regional lymph nodes by draining lymph and professional antigen-presenting cells, such as dendritic cells. The lymph nodes thus act as depots for the accumulation of antigen that is then used to stimulate T cells and B cells, resulting in the formation of germinal centers with Tfh and B cell activation and in two critical B cell events: class switching and affinity maturation. Icos (which encodes a cell surface protein) and Nfatc2ip (which encodes a transcription factor) are involved in promoting class switching and affinity maturation (55, 56), and expression of both was greater in the ANDV2 group than in the ANDV1 or SNV group. In this study, IgG was detected in most ANDV-infected deer mice but not in the SNV-infected deer mice. It is likely those B cells that had undergone these two events, were more efficient at capturing antigen, by virtue of higher-affinity BCRs, and presented high-density peptide antigens to the Th cells (57). These cascading events, in turn, may account for the more robust responses observed in the cultures of LNC from ANDV-infected deer mice.
The partitioning of the ANDV-infected cultures into Th1/Th2 (ANDV1) and Th2 (ANDV2) groups likely reflects T cell maturation. Our previous work with polyclonal T cell lines from SNV-infected deer mice showed that during acute infection (10 days postinfection) a mixed Th1/Th2 response occurs, but at persistence (42 days postinfection) T cell lines appear to be Treg cells (31). It may be that the six ANDV1 group deer mice were in the process of producing Th2 cells, whereas the two deer mice in ADNV2 group had already produced mature Th2 cells. Additional studies of the temporal responses of ANDV-infected deer mice should shed light on this hypothesis.
Considering the long coevolutionary history of hantaviruses and their reservoir hosts (58), it is likely that selective pressures have shaped both the virus and the host response. Cultivating hantaviruses in cell culture can compromise infectivity of the natural host (59); thus, it is likely that subtle changes in viral genomes can alter the immunological outcomes. The mechanisms that govern hantavirus-rodent reservoir interactions are unknown. At the amino acid level, SNV and ANDV nucleocapsids are about 86% identical, and some of these differences could account for different host T cell responses through MHC class II binding or T cell receptor (TCR) engagement. However, it is also likely that the immune response was shaped during the 14 days of infection. Viral regulatory elements may also influence abundance of viral gene expression, which may limit the danger signal to SNV in deer mice but not to ANDV. Viral proteins may also modulate the host response; some hantavirus nucleocapsids, including that of ANDV, interfere with caspase 3 and granzyme B (60), which are important effector molecules of CD8+ CTLs, in primate cells, and the Gc polypeptides of pathogenic New World hantaviruses possess a conserved immunoreceptor tyrosine activation motif (61, 62) that has not been well characterized. Expression of Casp3 was repressed in all 13 LNC cultures, but not significantly differently, suggesting that deer mice may also have impaired CTL responses to these viruses. Some hantaviruses also encode a nonstructural NSs protein in frame 2 of the S segment that antagonizes the type I IFN response (63–65). NSs of ANDV and the putative NSs of SNV share 70% identity and 84% similarity; thus, these differences could influence the antiviral responses in deer mouse cells that lead to downstream adaptive responses. Importantly, none of these activities have been examined in cells from their reservoir hosts, where they have likely been under selective pressure and may modulate the host response in the virus's favor. Presumably, SNV's NSs is optimized for deer mice, whereas ANDV's is not. This difference could set the stage for clearance of ANDV from deer mice if its NSs fails to manipulate the type I IFN response in ANDV's favor.
In comparisons to Syrian hamster infections with ANDV, a pathogenesis model for human HCPS, we found similarities and differences (36, 66). Thrombocytopenia is a hallmark characteristic of HCPS; however, it is not a feature of either deer mice or hamsters infected with ANDV (36). By day 12, few signatures of B cell activation are present in hamster lymph nodes or spleens, with Il6, Ifng, Il10, and Tgfb expression near background or repressed, although Il4 expression is substantially elevated in the lymph nodes (36). The lack of Il6 and Il10 expression in hamsters differs from the LNC studies presented here, and these genes play instrumental roles in driving antibody secretion from B cells. It is also noteworthy that the magnitude of gene expression in tissues of infected hamsters is substantially greater than what we observe in deer mice infected with either virus, with some genes elevated more than 20-fold. Thus, in terms of overall expression levels of examined genes, that with SNV in deer mice is low, that with ANDV in deer mice is moderate, and that with ANDV in hamsters is high, which may account for some of the pathology observed in this model.
Important caveats of the system employed in this work are that the SNV used in these studies has only been propagated in deer mice and harvested from lungs, whereas the ANDV has only been propagated in Vero E6 cells since its isolation. It is possible that during the harvest of deer mouse lungs for stock virus production, some cytokines may be present that influence the host response. It is also possible that passaging of ANDV in Vero E6 cells has led to alterations in its behavior in animals; although we found no pathology in deer mice, Syrian hamsters develop fatal infections with this isolate.
The findings presented here demonstrate that two hantaviruses that are pathogenic in humans cause no hematological changes in deer mice, despite evidence of systemic infection. While SNV elicits only modest immune mobilization, ANDV induces a robust lymphocyte response, with the activation of the IL-4/IL-13 pathway, which likely leads to Tfh cell-mediated maturation of germinal center B cells, including class switching and affinity maturation, and clearance of ANDV. These events suggest that an early and aggressive antibody response is instrumental for clearance of ANDV from a heterologous reservoir host, whereas a modest immune response to SNV occurs that leads to persistent infection, a critical aspect of the ecology of hantaviruses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brian Hjelle for providing recombinant SNV and ANDV antigen for recall stimulation and enzyme-linked immunosorbent assay, Corey Rosenberg for assistance with statistical analysis, and Tim Shaw for cluster analysis R script.
This work was supported by NIH grant AI054461 and startup funds from the Department of Microbiology, Immunology and Pathology, CSU (to T.S.), and the Intramural Program of NIAID, NIH (H.F. and J.P.).
Footnotes
Published ahead of print 14 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00004-14.
REFERENCES
- 1.Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, Mustonen J. 2013. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 11:539–550. 10.1038/nrmicro3066 [DOI] [PubMed] [Google Scholar]
- 2.Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. 2000. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. U. S. A. 97:10578–10583. 10.1073/pnas.180197197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botten J, Mirowsky K, Kusewitt D, Ye C, Gottlieb K, Prescott J, Hjelle B. 2003. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J. Virol. 77:1540–1550. 10.1128/JVI.77.2.1540-1550.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PW, Yanagihara R, Gibbs CJ, Jr, Gajdusek DC. 1986. Pathogenesis of experimental Hantaan virus infection in laboratory rats. Arch. Virol. 88:57–66. 10.1007/BF01310890 [DOI] [PubMed] [Google Scholar]
- 5.Kallio ER, Voutilainen L, Vapalahti O, Vaheri A, Henttonen H, Koskela E, Mappes T. 2007. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 88:1911–1916. 10.1890/06-1620.1 [DOI] [PubMed] [Google Scholar]
- 6.Luis AD, Douglass RJ, Hudson PJ, Mills JN, Bjornstad ON. 2012. Sin Nombre hantavirus decreases survival of male deer mice. Oecologia 169:431–439. 10.1007/s00442-011-2219-2 [DOI] [PubMed] [Google Scholar]
- 7.Clement J, Maes P, Lagrou K, Van Ranst M, Lameire N. 2012. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur. J. Clin. Microbiol. Infect. Dis. 31:1–5. 10.1007/s10096-011-1456-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmaljohn C, Hjelle B. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95–104. 10.3201/eid0302.970202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson CB, Figueiredo LT, Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23:412–441. 10.1128/CMR.00062-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calisher CH, Root JJ, Mills JN, Rowe JE, Reeder SA, Jentes ES, Wagoner K, Beaty BJ. 2005. Epizootiology of Sin Nombre and El Moro Canyon hantaviruses, southeastern Colorado, 1995–2000. J. Wildl. Dis. 41:1–11. 10.7589/0090-3558-41.1.1 [DOI] [PubMed] [Google Scholar]
- 11.Johnson KM. 2001. Hantaviruses: history and overview. Curr. Top. Microbiol. Immunol. 256:1–14. 10.1007/978-3-642-56753-7_1 [DOI] [PubMed] [Google Scholar]
- 12.Rawlings JA, Torrez-Martinez N, Neill SU, Moore GM, Hicks BN, Pichuantes S, Nguyen A, Bharadwaj M, Hjelle B. 1996. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmodon hispidus). Am. J. Trop. Med. Hyg. 55:672–679 [DOI] [PubMed] [Google Scholar]
- 13.Schountz T, Acuna-Retamar M, Feinstein S, Prescott J, Torres-Perez F, Podell B, Peters S, Ye C, Black WC, IV, Hjelle B. 2012. Kinetics of immune responses in deer mice experimentally infected with Sin Nombre virus. J. Virol. 86:10015–10027. 10.1128/JVI.06875-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes JM, Peters CJ, Cohen ML, Mahy BW. 1993. Hantavirus pulmonary syndrome: an emerging infectious disease. Science 262:850–851. 10.1126/science.8235607 [DOI] [PubMed] [Google Scholar]
- 15.Lee HW. 1982. Korean hemorrhagic fever. Prog. Med. Virol. 28:96–113 [PubMed] [Google Scholar]
- 16.Morzunov SP, Feldmann H, Spiropoulou CF, Semenova VA, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. 1995. A newly recognized virus associated with a fatal case of hantavirus pulmonary syndrome in Louisiana. J. Virol. 69:1980–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters CJ, Simpson GL, Levy H. 1999. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu. Rev. Med. 50:531–545. 10.1146/annurev.med.50.1.531 [DOI] [PubMed] [Google Scholar]
- 18.Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, Rollin P, Ksiazek T, Nichol S, Mahy BW, Peters CJ. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552–579 [PMC free article] [PubMed] [Google Scholar]
- 19.Borges AA, Campos GM, Moreli ML, Moro Souza RL, Saggioro FP, Figueiredo GG, Livonesi MC, Moraes Figueiredo LT. 2008. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 10:1150–1157. 10.1016/j.micinf.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Ennis FA, Cruz J, Spiropoulou CF, Waite D, Peters CJ, Nichol ST, Kariwa H, Koster FT. 1997. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 238:380–390. 10.1006/viro.1997.8827 [DOI] [PubMed] [Google Scholar]
- 21.Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, Waite DC, Koster FT, Ennis FA. 1999. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 179:295–302. 10.1086/314597 [DOI] [PubMed] [Google Scholar]
- 22.Easterbrook JD, Klein SL. 2008. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 4:e1000172. 10.1371/journal.ppat.1000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott KD, Ksiazek TG, Mills JN. 1999. Long-term hantavirus persistence in rodent populations in central Arizona. Emerg. Infect. Dis. 5:102–112. 10.3201/eid0501.990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calisher CH, Sweeney W, Mills JN, Beaty BJ. 1999. Natural history of Sin Nombre virus in western Colorado. Emerg. Infect. Dis. 5:126–134. 10.3201/eid0501.990115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuenzi AJ, Morrison ML, Swann DE, Hardy PC, Downard GT. 1999. A longitudinal study of Sin Nombre virus prevalence in rodents, southeastern Arizona. Emerg. Infect. Dis. 5:113–117. 10.3201/eid0501.990113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills JN, Yates TL, Ksiazek TG, Peters CJ, Childs JE. 1999. Long-term studies of hantavirus reservoir populations in the southwestern United States: rationale, potential, and methods. Emerg. Infect. Dis. 5:95–101. 10.3201/eid0501.990111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monroe MC, Morzunov SP, Johnson AM, Bowen MD, Artsob H, Yates T, Peters CJ, Rollin PE, Ksiazek TG, Nichol ST. 1999. Genetic diversity and distribution of Peromyscus-borne hantaviruses in North America. Emerg. Infect. Dis. 5:75–86. 10.3201/eid0501.990109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Root JJ, Calisher CH, Beaty BJ. 1999. Relationships of deer mouse movement, vegetative structure, and prevalence of infection with Sin Nombre virus. J. Wildl. Dis. 35:311–318. 10.7589/0090-3558-35.2.311 [DOI] [PubMed] [Google Scholar]
- 29.Rowe JE, St Jeor SC, Riolo J, Otteson EW, Monroe MC, Henderson WW, Ksiazek TG, Rollin PE, Nichol ST. 1995. Coexistence of several novel hantaviruses in rodents indigenous to North America. Virology 213:122–130. 10.1006/viro.1995.1552 [DOI] [PubMed] [Google Scholar]
- 30.Easterbrook JD, Zink MC, Klein SL. 2007. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc. Natl. Acad. Sci. U. S. A. 104:15502–15507. 10.1073/pnas.0707453104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schountz T, Prescott J, Cogswell AC, Oko L, Mirowsky-Garcia K, Galvez AP, Hjelle B. 2007. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc. Natl. Acad. Sci. U. S. A. 104:15496–15501. 10.1073/pnas.0707454104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spengler JR, Haddock E, Gardner D, Hjelle B, Feldmann H, Prescott J. 2013. Experimental Andes virus infection in deer mice: characteristics of infection and clearance in a heterologous rodent host. PLoS One 8:e55310. 10.1371/journal.pone.0055310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schountz T, Shaw TI, Glenn TC, Feldmann H, Prescott J. 2013. Expression profiling of lymph node cells from deer mice infected with Andes virus. BMC Immunol. 14:18. 10.1186/1471-2172-14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsdell CM, Lewandowski AA, Glenn JL, Vrana PB, O'Neill RJ, Dewey MJ. 2008. Comparative genome mapping of the deer mouse (Peromyscus maniculatus) reveals greater similarity to rat (Rattus norvegicus) than to the lab mouse (Mus musculus). BMC Evol. Biol. 8:65. 10.1186/1471-2148-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meissner JD, Rowe JE, Borucki MK, St Jeor SC. 2002. Complete nucleotide sequence of a Chilean hantavirus. Virus Res. 89:131–143. 10.1016/S0168-1702(02)00129-6 [DOI] [PubMed] [Google Scholar]
- 36.Safronetz D, Zivcec M, Lacasse R, Feldmann F, Rosenke R, Long D, Haddock E, Brining D, Gardner D, Feldmann H, Ebihara H. 2011. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog. 7:e1002426. 10.1371/journal.ppat.1002426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schountz T, Calisher CH, Richens TR, Rich AA, Doty JB, Hughes MT, Beaty BJ. 2007. Rapid field immunoassay for detecting antibody to Sin Nombre virus in deer mice. Emerg. Infect. Dis. 13:1604–1607. 10.3201/eid1310.070356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 39.Gabriel K. 1971. The biplot graphic display of matrices with application to principal component analysis. Biometrika 58:453–467 [Google Scholar]
- 40.Gavrilovskaya IN, Gorbunova EE, Mackow ER. 2010. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 84:4832–4839. 10.1128/JVI.02405-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Klein SL. 2012. Seoul virus-infected rat lung endothelial cells and alveolar macrophages differ in their ability to support virus replication and induce regulatory T cell phenotypes. J. Virol. 86:11845–11855. 10.1128/JVI.01233-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nutt SL, Tarlinton DM. 2011. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 12:472–477. 10.1038/ni.2019 [DOI] [PubMed] [Google Scholar]
- 43.Nutt SL, Taubenheim N, Hasbold J, Corcoran LM, Hodgkin PD. 2011. The genetic network controlling plasma cell differentiation. Semin. Immunol. 23:341–349. 10.1016/j.smim.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 44.Bollig N, Brustle A, Kellner K, Ackermann W, Abass E, Raifer H, Camara B, Brendel C, Giel G, Bothur E, Huber M, Paul C, Elli A, Kroczek RA, Nurieva R, Dong C, Jacob R, Mak TW, Lohoff M. 2012. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 109:8664–8669. 10.1073/pnas.1205834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murty VV, Alobeid B. 2006. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J. Immunol. 177:6930–6939. 10.4049/jimmunol.177.10.6930 [DOI] [PubMed] [Google Scholar]
- 46.Miyagawa F, Zhang H, Terunuma A, Ozato K, Tagaya Y, Katz SI. 2012. Interferon regulatory factor 8 integrates T-cell receptor and cytokine-signaling pathways and drives effector differentiation of CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 109:12123–12128. 10.1073/pnas.1201453109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang X, Zhang R, Yang J, Li Q, Qin L, Zhu C, Liu J, Ning H, Shin MS, Gupta M, Qi CF, He JC, Lira SA, Morse HC, III, Ozato K, Mayer L, Xiong H. 2011. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat. Commun. 2:314. 10.1038/ncomms1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, III, Belardelli F, Gabriele L. 2002. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J. Exp. Med. 196:1415–1425. 10.1084/jem.20021263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. 2005. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 174:2573–2581. 10.4049/jimmunol.174.5.2573 [DOI] [PubMed] [Google Scholar]
- 50.Elton TS, Selemon H, Elton SM, Parinandi NL. 2013. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 532:1–12. 10.1016/j.gene.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 51.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. 2009. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30:80–91. 10.1016/j.immuni.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oestreich KJ, Weinmann AS. 2012. Transcriptional mechanisms that regulate T helper 1 cell differentiation. Curr. Opin. Immunol. 24:191–195. 10.1016/j.coi.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kishimoto T. 2005. Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 23:1–21. 10.1146/annurev.immunol.23.021704.115806 [DOI] [PubMed] [Google Scholar]
- 54.Levine JR, Prescott J, Brown KS, Best SM, Ebihara H, Feldmann H. 2010. Antagonism of type I interferon responses by New World hantaviruses. J. Virol. 84:11790–11801. 10.1128/JVI.00916-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621–663. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 56.Fathman JW, Gurish MF, Hemmers S, Bonham K, Friend DS, Grusby MJ, Glimcher LH, Mowen KA. 2010. NIP45 controls the magnitude of the type 2 T helper cell response. Proc. Natl. Acad. Sci. U. S. A. 107:3663–3668. 10.1073/pnas.0914700107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schountz T, Kasselman JP, Martinson FA, Brown L, Murray JS. 1996. MHC genotype controls the capacity of ligand density to switch T helper (Th)-1/Th-2 priming in vivo. J. Immunol. 157:3893–3901 [PubMed] [Google Scholar]
- 58.Plyusnin A, Morzunov SP. 2001. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunol. 256:47–75 [DOI] [PubMed] [Google Scholar]
- 59.Lundkvist A, Cheng Y, Sjolander KB, Niklasson B, Vaheri A, Plyusnin A. 1997. Cell culture adaptation of Puumala hantavirus changes the infectivity for its natural reservoir, Clethrionomys glareolus, and leads to accumulation of mutants with altered genomic RNA S segment. J. Virol. 71:9515–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S, Braun M, Tischler ND, Stoltz M, Sundstrom KB, Bjorkstrom NK, Ljunggren HG, Klingstrom J. 2013. Hantavirus-infection confers resistance to cytotoxic lymphocyte-mediated apoptosis. PLoS Pathog. 9:e1003272. 10.1371/journal.ppat.1003272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geimonen E, Fernandez I, Gavrilovskaya IN, Mackow ER. 2003. Tyrosine residues direct the ubiquitination and degradation of the NY-1 hantavirus G1 cytoplasmic tail. J. Virol. 77:10760–10868. 10.1128/JVI.77.20.10760-10768.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geimonen E, LaMonica R, Springer K, Farooqui Y, Gavrilovskaya IN, Mackow ER. 2003. Hantavirus pulmonary syndrome-associated hantaviruses contain conserved and functional ITAM signaling elements. J. Virol. 77:1638–1643. 10.1128/JVI.77.2.1638-1643.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinemann P, Schmidt-Chanasit J, Gunther S. 2013. The N terminus of Andes virus L protein suppresses mRNA and protein expression in mammalian cells. J. Virol. 87:6975–6985. 10.1128/JVI.00043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jääskeläinen KM, Kaukinen P, Minskaya ES, Plyusnina A, Vapalahti O, Elliott RM, Weber F, Vaheri A, Plyusnin A. 2007. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-beta promoter. J. Med. Virol. 79:1527–1536. 10.1002/jmv.20948 [DOI] [PubMed] [Google Scholar]
- 65.Vera-Otarola J, Solis L, Soto-Rifo R, Ricci EP, Pino K, Tischler ND, Ohlmann T, Darlix JL, Lopez-Lastra M. 2012. The Andes hantavirus NSs protein is expressed from the viral small mRNA by a leaky scanning mechanism. J. Virol. 86:2176–2187. 10.1128/JVI.06223-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper JW, Larsen T, Custer DM, Schmaljohn CS. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6–14. 10.1006/viro.2001.1133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.