ABSTRACT
Naturally occurring Newcastle disease virus (NDV) strains vary greatly in virulence. The presence of multibasic residues at the proteolytic cleavage site of the fusion (F) protein has been shown to be a primary determinant differentiating virulent versus avirulent strains. However, there is wide variation in virulence among virulent strains. There also are examples of incongruity between cleavage site sequence and virulence. These observations suggest that additional viral factors contribute to virulence. In this study, we evaluated the contribution of each viral gene to virulence individually and in different combinations by exchanging genes between velogenic (highly virulent) strain GB Texas (GBT) and mesogenic (moderately virulent) strain Beaudette C (BC). These two strains are phylogenetically closely related, and their F proteins contain identical cleavage site sequences, 112RRQKR↓F117. A total of 20 chimeric viruses were constructed and evaluated in vitro, in 1-day-old chicks, and in 2-week-old chickens. The results showed that both the envelope-associated and polymerase-associated proteins contribute to the difference in virulence between rBC and rGBT, with the envelope-associated proteins playing the greater role. The F protein was the major individual contributor and was sometimes augmented by the homologous M and HN proteins. The dramatic effect of F was independent of its cleavage site sequence since that was identical in the two strains. The polymerase L protein was the next major individual contributor and was sometimes augmented by the homologous N and P proteins. The leader and trailer regions did not appear to contribute to the difference in virulence between BC and GBT.
IMPORTANCE This study is the first comprehensive and systematic study of NDV virulence and pathogenesis. Genetic exchanges between a mesogenic and a velogenic strain revealed that the fusion glycoprotein is the major virulence determinant regardless of the identical virulence protease cleavage site sequence present in both strains. The contribution of the large polymerase protein to NDV virulence is second only to that of the fusion glycoprotein. The identification of virulence determinants is of considerable importance, because of the potential to generate better live attenuated NDV vaccines. It may also be possible to apply these findings to other paramyxoviruses.
INTRODUCTION
Newcastle disease (ND) is an important cause of poultry disease worldwide that frequently is fatal and accounts for severe economic losses (1, 2). The causative agent, Newcastle disease virus (NDV), can infect a majority of the avian species in the class Aves and has been isolated from domesticated birds, wild birds, and waterfowl worldwide. NDV strains show a wide spectrum of virulence that varyies from inapparent infection to severe systemic disease resulting in 100% mortality. Based on the severity of disease in chickens, NDV strains are grouped into lentogenic, mesogenic, and velogenic pathotypes, representing low, moderate, and high virulence, respectively (1–3).
NDV is a pleomorphic, enveloped, cytoplasmic virus containing a single-stranded negative-sense RNA genome. NDV belongs to the genus Avulavirus in the subfamily Paramyxovirinae in the family Paramyxoviridae (4, 5). Three genome-size categories have been identified, with lengths of 15,186, 15,192, and 15,198 nucleotides (nt) (6–10). The genome contains six genes that are flanked at the 3′ and 5′ termini by short extragenic leader and trailer regions, with the following gene order: 3′ leader-N-P-M-F-HN-L-5′ trailer. The genes code for, respectively, the nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion glycoprotein (F), hemagglutinin-neuraminidase glycoprotein (HN), and large polymerase protein (L). The P gene also codes for an additional protein called V from a second open reading frame that is accessed by a cotranscriptional frameshifting mechanism known as RNA editing (11). All NDV strains belong to a single serotype but are segregated into class I, containing a single genotype, and class II, containing at least 18 genotypes, I to XVIII. Class I strains are usually avirulent, whereas class II strains include representatives of all pathotypes (6–10).
Our understanding of the viral factors responsible for NDV virulence and pathogenicity is incomplete. The amino acid sequence at the F protein cleavage site has been identified as a primary determinant of NDV virulence that usually differentiates virulent strains from avirulent strains (12–15). Virulent NDV strains typically have multiple basic residues and include the cleavage motif (Arg-X-Arg/Lys-Arg↓), which is optimal for the intracellular protease furin that is present in most cell types. In contrast, the F protein cleavage site of avirulent NDV strains typically contains fewer basic residues, lacks the furin motif, and is cleaved at a single basic residue by extracellular protease present in secretions of the respiratory and enteric tracts. The presence of the furin motif at the F protein cleavage site of virulent strains confers the ability to replicate in a wide variety of tissues, whereas the dependence of avirulent strains on extracellular secretory protease restricts viral replication to the respiratory and enteric tracts. However, NDV strains that contain identical F protein cleavage sites sometimes can differ substantially in virulence. For example, strains GB Texas (GBT) and Beaudette C (BC) have identical F protein cleavage sites (112RRQKR↓F117), but GBT is a velogenic strain and BC is a mesogenic strain. Also, in some cases the structure of the cleavage site does not predict the pathotype. For example, there are strains of NDV that have lentogenic cleavage site motifs but that are highly virulent in chickens (16), as well as strains with velogenic motifs that do not appear to cause disease (17). These observations suggest that viral factors other than the F protein cleavage site contribute to the differences in virulence of NDV strains.
Several studies have been conducted to investigate the contributions of individual viral genes to NDV virulence by exchanging genes between strains (18–23). Although those studies have increased our understanding of NDV virulence, the results have not been consistent or conclusive. One limitation is that these studies involved strain comparisons that were made based on the available reverse genetic systems rather than ideal comparisons, and thus they were carried out between NDV strains that may have been too divergent genetically or biologically to be compatible for gene swaps. For example, genes were exchanged between lentogenic and mesogenic strains (23), or between strains of chicken origin versus pigeon origin (18, 19), or of turkey origin versus game fowl origin (21), or between NDV and the heterologous avian paramyxovirus serotype 2 (APMV-2) (20), or between two phylogenetically distantly related strains (18, 19, 21, 22). Therefore, it was unclear whether the observed effects of the gene swaps reflected genuine virulence determinant differences versus incompatibility due to excessive biological or phylogenetic divergence. Another limitation was that previous studies did not directly compare the contributions of the viral proteins that are associated with the viral envelope versus those that are associated with the nucleocapsid and polymerase complex.
In the present report, we conducted a systematic study of NDV virulence and pathogenesis by exchanging the full repertoire of viral genes, individually and in a number of combinations, between the two NDV strains noted above, mesogenic BC and velogenic GBT. These two strains are well-suited for this study. They are closely related phylogenetically, and both viruses belong to genotype II of class II. They have identical genome lengths of 15,186 nt. They share 99.1% genome-wide nucleotide sequence identity, and the amino acid sequence identity between the respective proteins of these two strains is 99.8% for N, 98.7% for P, 98.9% for M, 98.6% for F, 98.1% for HN, and 99.4% for L. The two strains are of chicken origin, and both viruses were isolated in the United States in the late 1940s. Importantly, as already noted, they contain identical multibasic F protein cleavage site sequences with the furin motif. However, the two strains differ greatly in virulence and pathogenesis: BC causes mild clinical illness in young chickens and rarely fatal disease in older chickens, whereas GBT is a highly virulent virus with a pronounced neurological tropism, is associated with 100% mortality in older chickens, and is used as a standard challenge virus in the United States.
For this study, we constructed de novo reverse genetic systems for the two strains in which each of the two antigenomic cDNAs had the same array of unique restriction sites located at the same nucleotide sequence positions flanking each gene, so that each gene could be exchanged using the same restriction sites and involving exactly the same genome coordinates. We examined the contributions to NDV virulence of the envelope-associated protein genes (M, F, and HN) individually and in combination, as well as the polymerase-associated protein genes (N, P, and L) individually and in combination, as well as the extragenic trailer region (the leader region was not swapped, because it is identical in the two strains). A total of 20 chimeric viruses, plus the two parent strains, was constructed and recovered. In vitro, we analyzed virus replication, plaque formation, and fusogenicity. In vivo, we performed the standard intracerebral pathogenicity index (ICPI) test in 1-day-old chicks, and we performed natural infection in 2-week-old chickens in which viral load, clinical disease signs, histopathology, and lethality were examined. Our results showed that both the envelope-associated proteins and polymerase-associated proteins contribute to the virulence of NDV, with the former playing the greater role. The F and L proteins were the major individual contributors to the difference in virulence between recombinant BC (rBC) and rGBT.
MATERIALS AND METHODS
Cells and viruses.
A chicken embryo fibroblast cell line (DF-1), an African green monkey kidney cell line (Vero), and a human epidermoid carcinoma cell line (HEp-2) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cell lines were grown in Dulbecco's minimal essential medium (DMEM) with 10% fetal bovine serum (FBS) and maintained in DMEM with 2% FBS. The velogenic virulent strain GBT and the mesogenic strain BC were obtained from the National Veterinary Services Laboratory (NVSL), Ames, IA. The modified vaccinia virus strain Ankara (MVA) expressing T7 RNA polymerase was kindly provided by Bernard Moss (NIH, Bethesda, MD) and propagated in primary chicken embryo fibroblast cells in DMEM with 2% FBS. The NDV strains BC and GBT and their recombinant derivatives were grown in 9-day-old embryonated specific-pathogen-free (SPF) chicken eggs. Viral titers in harvested allantoic fluid were determined by plaque assay. Briefly, samples were serially diluted, and 100 μl of each serial dilution was added per well to confluent DF-1 cells in 12-well plates. After 60 min of adsorption, cells were overlaid with DMEM (containing 2% FBS and 0.81% methylcellulose) and then incubated at 37°C for 4 days. The cells were then fixed with absolute methanol and stained with 1% crystal violet for observation of plaques.
All in vitro and in vivo studies were conducted in an enhanced biosafety level 3 (BSL-3+) containment facility certified by the USDA following the guidelines of the IACUC, University of Maryland.
Plasmid construction, transfection, virus rescue, and sequence analysis.
Full-length antigenomic cDNA clones of NDV strains BC and GBT were constructed from eight fragments that were made by reverse transcription-PCR (RT-PCR) of RNA that had been purified from allantoic fluid from infected eggs. The primer sequences used for the RT-PCR and for the cloning of antigenomic cDNA of NDV strains BC and GBT is available on request. The antigenome cDNAs were designed so that the leader region was preceded by a T7 RNA polymerase promoter that added three G residues to the 5′ end of the antigenome, and the trailer region was flanked by a hepatitis delta virus ribozyme that cleaved following the last NDV-specific residue. Each antigenome and its flanking sequences were designed to have the following nine restriction sites placed at identical positions (Fig. 1): AscI, located in the flanking plasmid sequence upstream of the T7 promoter; PacI, PmeI, AsiSI, AgeI, and SnaBI, located in the downstream noncoding region of the N, P, M, F, and HN gene, respectively; BstBI and MluI, located within the L open reading frame (ORF); RsrII, located in the flanking ribozyme sequence downstream of the trailer region. Of the seven sites in the NDV sequence, the AgeI site occurred naturally in each virus; the others were introduced during RT-PCR. In addition, a naturally occurring RsrII site in the P ORF was eliminated without changing amino acid coding in order for the RsrII site in the ribozyme to be unique. These changes were the same in each of the two NDV strains and are summarized in Table 1; they involved 16 nt changes and no amino acid changes. The BC and GBT antigenome cDNAs were completely sequenced and were confirmed to be identical to the biological viruses, except for these 16 nt changes.
FIG 1.
Viral gene maps. The two gene maps at the top are recombinant versions of NDV strains BC and GBT (rBC and rGBT) and show the nine unique restriction sites used in constructing the antigenomic cDNAs and for swapping genes. The next 20 gene maps illustrate chimeric viruses in which one or more genes have been swapped (in gray). The first five chimeric viruses consist of the rBC backbone with the envelope-associated protein genes M, F, HN, F+HN, and M+F+HN replaced by those of strain rGBT. The next five viruses are the converse, with the rGBT backbone and the corresponding M, F, HN, F+HN, and M+F+HN replacements from strain rBC. The next five viruses consist of the rBC backbone with the polymerase-associated protein genes N, P, L, N+P+L, and the trailer (Tr) replaced by those of strain rGBT. The bottom five viruses are the converse, with the rGBT backbone and replacements of N, P, L, N+P+L, and Tr from rBC. The leader region was not swapped because it is identical in the two strains. Abbreviations: 5′NTR, 5′-nontranslated gene region; IGS, intergenic sequence.
TABLE 1.
The position and nucleotide changes introduced into the antigenome cDNA clones of NDV strains BC and GBT
| Location | Nucleotide change(s)a | RE site | Change to ORFe |
|---|---|---|---|
| N gene 5′-NTRb | A(1768)T, C(1769)T, C(1773)T | PacIc | None |
| P gene 5′-NTRb | A(3221)G, A(3222)T, T(3227)A | PmeIc | None |
| M gene 5′-NTRb | T(4452)G, T(4453)C, T(4457)C, A(4458)G, T(4459)C | AsiSIc | None |
| HN-L IGS | A(8327)C, G(8330)A | SnaBIc | None |
| L ORF | G(10396)A, A(13537)G | BstBIc | GAG to GAA, silent |
| MluIc | GCA to GCG, silent | ||
| P ORF | A(13537)G | RsrIId | CGG to CGA, silent |
Nucleotide changes are relative to the full-length antigenome nucleotide sequence and were the same in both strains. The GenBank accession number for GBT is GU978777. The BC genome sequence was first determined in our laboratory, as described in reference 10.
The 5′-NTR is the nontranslated gene region downstream of the respective ORF.
The restriction site was added to the antigenome.
The restriction site was removed from the antigenome.
The mutated nucleotide is shown in bold.
The N, P, M, F, HN, and L genes were reciprocally exchanged between the BC and GBT antigenome cDNAs by using the nine restriction enzyme (RE) sites noted above (Fig. 1). The extragenic leader sequences were identical in BC and GBT and hence were not exchanged between the two strains. The extragenic trailer sequence was constructed using overlap PCR and cloned using the MluI and RsrII sites. NDV strain BC has 7 U residues in the gene end (GE) signals of the N and L genes, compared to 6 U residues for strain GBT. Since we did not know the significance of GE length differences in pathogenesis, we exchanged the N and L genes together with their matching GE sequences. In the case of the N gene, the PacI site used in the gene exchange is upstream of the N GE signal. Therefore, after exchange of the N gene, we also modified the fragment containing the P gene by subcloning and primer mutagenesis in order to incorporate the strain-specific N GE signal. In the case of the L gene, the MluI-RsrII fragment was subcloned and mutagenized to incorporate the correct L GE signal. The other gene start (GS) and GE signals were identical between the two strains.
The recombinant parental and chimeric viruses were recovered in HEp-2 cells by using MVA-T7 as a source of T7 polymerase (26). Viruses were subjected to five passages in 9-day-old SPF chicken embryos. From the fifth passage, total RNA was isolated using an RNeasy RNA purification kit (Invitrogen, Life Technologies, Grand Island, NY). The rBC and rGBT parents were completely sequenced, and chimeric derivatives were sequenced over the swapped genes and flanking sequences. No adventitious mutations were detected. These passage 5 virus stocks were used in the experiments.
Virus growth kinetics.
The growth kinetics of rBC and rGBT and their mutant viruses were determined under multiple-cycle growth conditions in DF-1 cells. The virus was inoculated at a multiplicity of infection (MOI) of 0.01 PFU into DF-1 cells grown in DMEM with 10% FBS at 37°C. The supernatant was collected at 8-h intervals and replaced by equal volumes of fresh medium until 64 h postinfection. The virus content in the samples was quantified via limiting dilution on DF-1 cells by using the method of Reed and Muench (24) and expressed as the 50% tissue culture infective dose (TCID50).
Fusion index assay.
The fusogenic abilities of the chimeras involving the envelope-associated protein genes, M, F, HN, F plus HN (F+HN), and M plus F plus HN (M+F+HN), were evaluated by the fusion index assay described by Kohn (25). Virus was inoculated onto confluent Vero cells in 6-well plates at an MOI of 0.1 PFU. Cells were maintained in 2% DMEM at 37°C under 5% CO2. At 36 h postinfection, the medium was removed and cells were fixed with methanol for 20 min at room temperature and stained with hematoxylin-eosin (Hema 3; Thermo Fisher Scientific Inc., Rockville, MD). Nuclei and cells were counted in eight microscope fields in each of three replicate wells per virus. The fusion index was calculated as the ratio of the number of nuclei to the number of cells and was normalized to the parent virus, rBC or rGBT, set as 100.
ICPI test in 1-day-old chicks.
Following standard procedures (3), 0.05 ml of a 10−1 dilution of fresh allantoic fluid (29 hemagglutination [HA] units) of each virus/chicken was inoculated intracerebrally into 10 1-day-old SPF chicks per virus. The birds were observed for clinical signs and mortality once every 12 h for 8 days. ICPI values were calculated as described by Alexander (3). Briefly, the birds were scored 0 if normal, 1 if sick, and 2 if dead. The ICPI value was the mean score per bird per observation. Highly virulent (velogenic) viruses give values approaching 2; avirulent (lentogenic) viruses give values close to 0.
Pathogenicity in 2-week-old chickens, virus titration, and histopathology.
Two-week-old SPF chickens in groups of 12 were inoculated with 0.2 ml containing 106 PFU of virus per bird by the oculonasal route. The birds were observed daily and scored for clinical signs for 14 days postinfection (dpi). Two birds from each group were euthanized at 3 dpi, and lung, trachea, spleen, brain, and intestine were collected in two parts. One part was used for virus titration and other part was fixed in 10% buffered formalin for histopathology. For virus titration, the tissue samples were homogenized and the supernatant was serially 10-fold diluted and used to infect DF-1 cells, with duplicate wells per dilution. Infected wells were identified by HA assay of the supernatant, and the TCID50/g of tissue was calculated using the method of Reed and Muench (24). For histopathology, tissue samples were fixed in 10% neutral buffered formalin. The fixed tissues were sectioned and stained with hematoxylin-eosin (Histoserv Inc., Germantown, MD). The tissue sections were scored for the histopathological changes. The remaining 10 chickens in each virus group were given daily clinical scores: 0 for normal, 1 for sick, 2 for paralysis/twitching/wing drop, 3 for prostration, and 4 for death, with scores taken daily until 14 dpi. A mean score per virus group per day was generated for comparisons. Survival was monitored until 14 dpi.
Statistical analysis.
The survival patterns and median survival times were compared using the log-rank test and chi-square statistics. In the log-rank test, survival curves compare the cumulative probability of survival at any specific time, and the assumption of proportional deaths per time is the same at all time points. Survival data and one-way analysis of variance were used within the Prism 5.0 program (GraphPad Software Inc., San Diego, CA) with a significance level of P < 0.05. Error bars in the data from virus growth kinetics, fusion index assay, and virus titration indicate standard errors of the means, and a two-tailed, unpaired t test was used to calculate the P value to test the level of significance between two data sets.
RESULTS
Recovery of recombinant NDV strains rBC and rGBT and their chimeric viruses.
We made reverse genetic systems for the mesogenic NDV strain BC and the velogenic NDV strain GBT in which the two antigenomic cDNAs were constructed using matched unique restriction sites placed in identical genome locations flanking the viral genes, within the L gene and flanking both ends of the antigenome (Fig. 1). The unique AgeI site occurred naturally in the NDV sequences; the unique PacI, PmeI, AsiSI, SnaBI, BstBI, and MluI sites were added to the antigenome during construction, and an existing RsrII site in the P ORF was removed (Materials and Methods) (Table 1). These changes involved a total of 16 nt substitutions in the antigenome with no amino acid changes, compared to the corresponding biological viruses (Table 1). We recovered the wild-type cDNA-derived rBC and rGBT viruses and completely sequenced each genome, which confirmed a lack of adventitious mutations. Next, we evaluated the virulence of rBC and rGBT by performing ICPI tests in 1-day-old chicks. The ICPI values of rBC and rGBT were 1.58 and 1.91, respectively, whereas those of biological BC and GBT were 1.6 and 1.9, respectively (data not shown). These results showed that the cDNA-derived strains rBC and rGBT were equivalent in virulence to their biological parents and, in particular, the creation of six new restriction sites and the elimination of another did not alter their pathogenicity.
We then used the matched restriction sites to make reciprocal gene swaps between the parental rBC and rGBT strains, resulting in 20 chimeric viruses, which are shown in Fig. 1. The top five chimeric viruses in Fig. 1 were derivatives of strain rBC in which the envelope-associated protein genes M, F, HN, F+HN, and M+F+HN were replaced with those of strain rGBT. The next five chimeras in Fig. 1 were the converse: specifically, they were derivatives of strain rGBT in which the M, F, HN, F+HN, and M+F+HN genes were replaced with those of strain rBC. The next five viruses were derivatives of strain rBC in which the polymerase-associated protein genes N, P, L, and N plus P plus L (N+P+L) and the trailer were replaced with those of strain rGBT (the leader region is identical between the two strains and thus could not be exchanged). Finally, the bottom five viruses in Fig. 1 were the converse: they were derivatives of strain rGBT in which the N, P, L, and N+P+L genes and the trailer were replaced with those of strain rBC. Each of the 20 chimeras was readily recovered and was passaged five times in embryonated eggs. To confirm the presence and integrity of the exchanged genes, they were amplified by RT-PCR and sequenced, which confirmed the correct structure of each swap and a lack of adventitious mutations.
In vitro biological activities of recombinant chimeric viruses.
We investigated whether the exchange of genes between rBC and rGBT affected growth, plaque formation, and fusion activity in cell culture. The growth kinetics of rBC and rGBT and their chimeric derivatives were determined in DF-1 cells infected at an MOI of 0.01 PFU (Fig. 2). Between the parental viruses, rGBT showed early initiation of cytopathic effects (data not shown) and faster growth kinetics (Fig. 2a and c) than rBC.
FIG 2.
Multicycle growth kinetics of parental rBC and rGBT and their chimeric derivatives in chicken embryo fibroblast (DF-1) cells. (a and b) rBC-based chimeras in which the envelope-associated protein genes M, F, and HN were replaced by their counterparts from rGBT individually (a) or in combination (b). (c and d) The converse: rGBT-based chimeras in which the M, F, and HN genes were replaced by their counterparts from rBC individually (c) or in combination (d). (e and f) rBC-based chimeras in which the polymerase-associated N, P, and L protein genes and the trailer (Tr) were replaced by their counterparts from rGBT individually (e) or in combination (f). (g and h) rGBT-based chimeras in which N, P, L, and Tr were replaced by their counterparts from rBC individually (g) or in combination. Briefly, DF-1 cells were infected at a multiplicity of infection of 0.01, supernatant samples were collected at 8-h intervals until 64 h postinfection, and virus titers were determined by TCID50 limiting dilution assay and calculated using the method of Reed and Muench (24). Each time the supernatant was collected, an equal volume of maintenance medium was replaced. Asterisks indicate test of significance of the virus titer of a chimeric virus compared to the parental virus of that group at 24 h; P values were calculated based on a two-tailed, unpaired t test (95% confidence levels). ***, P = 0.0001 to 0.001, extremely significant; **, P = 0.001 to 0.01, very significant; *, P = 0.01 to 0.05, significant.
For the rBC derivatives bearing rGBT-derived envelope-associated protein genes, those bearing the rGBT F gene alone or in the combination of F+HN had increased replication compared to parental rBC (Fig. 2a and b; see Table 2 for an overview of the biological properties of the chimeras). At 24 h postinfection (during the period of exponential replication), the two chimeras with the most increased replication were rBC-GBT(F+HN) (Fig. 2b) followed by rBC-GBT(F) (Fig. 2a), which had titers of 8.6 × 108 TCID50 and 7.8 × 107 TCID50, respectively, compared to 6.7 × 106 TCID50 for rBC. The rBC chimera with all three envelope proteins derived from rGBT, rBC-GBT(M+F+HN), had growth kinetics that were similar to those of rBC at 24 h but achieved final titers at 48 to 64 h that were greater than those of rBC (Fig. 2b). In contrast, replication of rBC-GBT(M) and rBC-GBT(HN) was indistinguishable from that of parental rBC (Fig. 2a).
TABLE 2.
Virulence scores from the six different assays with parental rBC and rGBT and their chimeric viruses
| Swap | Virulence scorea |
|||||
|---|---|---|---|---|---|---|
| In vitro replication | Plaque size | Fusion index | ICPI | In vivo replication | Clinical signs/mortality | |
| rBC derivatives, rGBT envelope-associated protein genes | ||||||
| M | Ø | Ø | Ø | Ø | Ø | - |
| F | +++ | +++ | +++ | +++ | +++ | +++ |
| HN | Ø | + | + | + | Ø | - |
| F+HN | +++ | ++ | +++ | +++ | +++ | +++ |
| M+F+HN | ++ | ++++ | ++++ | +++ | ++ | ++ |
| rGBT derivatives, rBC envelope-associated protein genes | ||||||
| M | Ø | --- | Ø | - | Ø | Ø |
| F | --- | - | --- | --- | --- | --- |
| HN | Ø | ++ | Ø | Ø | Ø | Ø |
| F+HN | --- | --- | --- | -- | -- | --- |
| M+F+HN | --- | --- | ---- | -- | --- | - |
| rBC derivatives, rGBT polymerase-associated protein genes | ||||||
| N | Ø | --- | ND | Ø | Ø | - |
| P | Ø | -- | ND | Ø | Ø | - |
| L | + | -- | ND | ++ | ++ | +++ |
| N+P+L | ++ | +++ | ND | ++ | +++ | ++ |
| Tr | Ø | Ø | ND | Ø | Ø | Ø |
| rGBT derivatives, rBC polymerase-associated protein genes | ||||||
| N | - | - | ND | Ø | Ø | Ø |
| P | - | Ø | ND | Ø | Ø | Ø |
| L | -- | --- | ND | -- | -- | -- |
| N+P+L | --- | ---- | ND | -- | --- | --- |
| Tr | -- | Ø | ND | Ø | Ø | Ø |
Increases and decreases in virulence relative to the respective parent are indicated as + and - symbols, respectively. The scoring is for a descriptive comparison and was not intended to be quantitative. ND, not determined; Ø, no observable effect.
For the rGBT derivatives bearing rBC-derived envelope-associated protein genes, those bearing the rBC F gene alone or in the combinations F+HN and M+F+HN had decreased replication compared to rGBT (Fig. 2c and d). In contrast, replication of rGBT-BC(M) and rGBT-BC(HN) was indistinguishable from that of parental rGBT (Fig. 2c).
Thus, transfer of the F gene from the velogenic rGBT strain into the mesogenic rBC strain increased the replication of rBC, and conversely, transfer of the F gene from the mesogenic rBC strain into the velogenic rGBT strain decreased the replication of rGBT. This indicated that, among the envelope-associated proteins, the F protein plays the major role in determining the level of growth of NDV in vitro.
For the rBC derivatives bearing the polymerase-associated protein genes or the trailer from rGBT, transfer of the L gene alone, as in rBC-GBT(L), or in combination with N and P, as in rBC-GBT(N+P+L), resulted in increased growth (Fig. 2e and f). The combination of the three genes, N+P+L, had a greater effect than L alone. Transfer of the rGBT trailer into rBC had no effect on replication (Fig. 2f). Conversely, transfer of any of the polymerase-associated protein genes or the trailer from rBC into rGBT resulted in a modest reduction in replication, with the effect among the individual components being greatest for the L gene (Fig. 2g and h). The effect was even greater when N, P, and L were transferred together (Fig. 2h). These results indicated that, among the polymerase-associated proteins, the L protein has the greatest effect on modulating the level of growth of NDV and that this effect is enhanced by the homologous N and P proteins.
We also evaluated growth by measuring the plaque sizes of the chimeric viruses on DF-1 cell monolayers in comparison with those of their parental viruses (Fig. 3). For the rBC derivatives bearing the envelope-associated protein genes of rGBT, the plaque sizes (diameters, in mm) were as follows: rBC, 1.55 ± 0.06; rBC-GBT(M), 1.54 ± 0.12; rBC-GBT(F), 2.08 ± 0.18; rBC-GBT(HN), 1.71 ± 0.13; rBC-GBT(F+HN), 1.85 ± 0.13; rBC-GBT(M+F+HN), 2.32 ± 0.32 (Fig. 3a). Thus, transfer of the rGBT F gene had the greatest effect among the individual M, F, and HN genes, and the effect was greater when F was transferred together with the homologous M and HN genes. Conversely, for the rGBT derivatives bearing the envelope-associated protein genes of rBC, the plaque sizes (diameters, in mm) were as follows: rGBT, 2.06 ± 0.14; rGBT-BC(M), 1.73 ± 0.07; rGBT-BC(F), 1.95 ± 0.08; rGBT-BC(HN), 2.28 ± 0.25; rGBT-BC(F+HN), 1.67 ± 0.05; rGBT-BC(M+F+HN), 1.77 ± 0.07 (Fig. 3a). In this case, transfer of the rBC M gene had the greatest effect among the individual genes, followed by the F gene, and the effect of F was increased when transferred in the combination F+HN or M+F+HN.
FIG 3.
Plaque morphologies of parental rBC and rGBT and their chimeric derivatives in DF-1 cells. Envelope-associated protein gene chimeras of rBC and rGBT (a) and the polymerase-associated protein gene and Tr chimeras of rBC and rGBT (b) are shown in separate panels.
For rBC derivatives bearing the polymerase-associated protein genes or the trailer of rGBT, the plaque sizes (diameters, in mm) were as follows: rBC, 1.73 ± 0.17; rBC-GBT(N), 1.44 ± 0.09; rBC-GBT(P), 1.4 ± 0.07; rBC-GBT(L), 1.43 ± 0.13; rBC-GBT(N+P+L), 2.01 ± 0.11; rBC-GBT(Tr), 1.4 ± 0.18 (Fig. 3b). This showed that transfer of the individual rGBT components into rBC did not increase plaque size, but transfer of N+P+L did. For rGBT derivatives bearing the polymerase-associated protein genes or trailer of rBC, the plaque sizes (diameters, in mm) were as follows: rGBT, 2.19 ± 0.1; rGBT-BC(N), 1.99 ± 0.23; rGBT-BC(P), 2.2 ± 0.12; rGBT-BC(L), 1.58 ± 0.16; rGBT-BC(N+P+L), 1.34 ± 0.08; rGBT-BC(Tr), 2.12 ± 0.09 (Fig. 3b). This showed that transfer of the rBC L gene had the greatest effect among the individual components, and this effect was greater when the L gene was transferred with its homologous N and P genes.
The cell fusion activities of the recombinant chimeric viruses were determined by infecting monolayer cultures of Vero cells with an MOI of 0.1 PFU and evaluating fusion formation 36 h later. The fusion index was calculated as the ratio of the total number of nuclei divided by the number of cells in which these nuclei were observed, expressed relative to rBC or rGBT as 100%. For rBC derivatives bearing the envelope-associated protein genes of rGBT, the indices (relative to rBC, as 100%) were as follows: rBC-GBT(M), 83%; rBC-GBT(F), 412%; rBC-GBT(HN), 156%; rBC-GBT(F+HN), 478%; rBC-GBT(M+F+HN), 614% (Fig. 4a). The fusion index of rBC-GBT(M+F+HN) was 11% higher than that of rGBT (data not shown). These results indicated that transfer of the GBT F gene into the rBC backbone increased the fusogenicity of the chimeric virus and that this effect was greater when F was transferred together with its homologous M and HN genes. For rGBT derivatives bearing the envelope-associated protein genes of rBC, the indices (relative to rGBT, as 100%) were as follows: rGBT-BC(M), 98%; rGBT-BC(F), 17%; rGBT-BC(HN), 95%; rGBT-BC(F+HN), 13%; rGBT-BC(M+F+HN), 10% (Fig. 4b). This showed that transfer of the BC F gene into the rGBT backbone resulted in reduced fusogenicity, and this effect was greater when the F gene was transferred with the homologous M and HN genes. These results indicated that the fusogenicity of NDV is determined primarily by the F gene but also is influenced by M and HN.
FIG 4.
Comparison of the fusion indices of parental rBC and rGBT and their chimeric derivatives involving the envelope-associated protein genes. Vero cells were infected with each virus at an MOI of 0.1, fixed at 36 h postinfection, and stained with hematoxylin-eosin. The fusion index was calculated as the ratio of the total number of nuclei to the total number of nuclei in the field, normalized to the respective parental virus, as 100. Data represent the means of the results from three independent experiments. Asterisks indicate test of significance of the fusion index of a chimeric virus compared to the parental virus of that group; P values were calculated based on a two-tailed, unpaired t test (95% confidence levels). ***, P = 0.0001 to 0.001, extremely significant; **, P = 0.001 to 0.01, very significant; *, P = 0.01 to 0.05, significant.
Evaluation of the pathogenicity of parental and chimeric viruses by ICPI assay.
The pathogenicity of the chimeric viruses was assessed by a standard ICPI test in 1-day-old chicks. The respective ICPI values of parental rBC and rGBT in two separate assays were as follows: rBC, 1.61 (Table 3) and 1.58 (Table 4); rGBT, 1.91 in both assays (Tables 3 and 4). These values are consistent with the designations of rBC as mesogenic and rGBT as velogenic. For the rBC derivatives bearing the envelope-associated protein genes of rGBT, the ICPI values were as follows: rBC, 1.61; rBC-GBT(M), 1.58; rBC-GBT(F), 1.88; rBC-GBT(HN), 1.71; rBC-GBT(F+HN), 1.8; rBC-GBT(M+F+HN), 1.82 (Table 3). This showed that transfer of the rGBT F protein into rBC had the greatest effect on increasing the ICPI value, with transfer of HN having a lesser effect. For the rGBT derivatives bearing the envelope-associated protein genes of rBC, the values were as follows: rGBT, 1.91; rGBT-BC(M), 1.85; rGBT-BC(F), 1.62; rGBT-BC(HN), 1.88; rGBT-BC(F+HN), 1.78; rGBT-BC(M+F+HN), 1.75 (Table 3). This showed that transfer of the rBC F protein into rGBT had the greatest effect on decreasing the ICPI value.
TABLE 3.
ICPI scores for parental rBC and rGBT and their envelope-associated protein gene chimeras
| Virus | ICPI scorea |
|---|---|
| rBC | 1.61 |
| rBC-GBT(M) | 1.58 |
| rBC-GBT(F) | 1.88*** |
| rBC-GBT(HN) | 1.71** |
| rBC-GBT(F+HN) | 1.80*** |
| rBC-GBT(M+F+HN) | 1.82*** |
| rGBT | 1.91 |
| rGBT-BC(M) | 1.85** |
| rGBT-BC(F) | 1.62*** |
| rGBT-BC(HN) | 1.88 |
| rGBT-BC(F+HN) | 1.78*** |
| rGBT-BC(M+F+HN) | 1.75*** |
Pathotype definitions by ICPI: velogenic strains approach the maximum score of 2.00, whereas lentogenic strains give values close to 0. Asterisks indicate the statistical significance of the ICPI score of a chimeric virus compared to the parental virus of that group; P values were calculated based on a two-tailed, unpaired t test (95% confidence levels). ***, P = 0.0001 to 0.001, extremely significant; **, P = 0.001 to 0.01, very significant.
TABLE 4.
ICPI scores for parental rBC and rGBT and their polymerase-associated protein gene chimeras
| Virus | ICPI scorea |
|---|---|
| rBC | 1.58 |
| rBC-GBT(N) | 1.62 |
| rBC-GBT(P) | 1.60 |
| rBC-GBT(L) | 1.71** |
| rBC-GBT(N+P+L) | 1.75*** |
| rBC-GBT(Tr) | 1.61 |
| rGBT | 1.91 |
| rGBT-BC(N) | 1.88 |
| rGBT-BC(P) | 1.90 |
| rGBT-BC(L) | 1.83** |
| rGBT-BC(N+P+L) | 1.78*** |
| rGBT-BC(Tr) | 1.90 |
Pathotype definitions by ICPI: velogenic strains approach the maximum score of 2.00, whereas lentogenic strains give values close to 0. Asterisks indicate the statistical significance of the ICPI score of a chimeric virus compared to the parental virus of that group; P values were calculated based on a two-tailed, unpaired t test (95% confidence levels). ***, P = 0.0001 to 0.001, extremely significant; **, P = 0.001 to 0.01, very significant.
For rBC derivatives bearing the polymerase-associated protein genes of rGBT, the ICPI values were as follows: rBC, 1.58; rBC-GBT(N), 1.62; rBC-GBT(P), 1.6; rBC-GBT(L), 1.71; rBC-GBT(N+P+L), 1.75; rBC-GBT(Tr), 1.61 (Table 4; Tr indicates the trailer sequence). For rGBT derivatives bearing the polymerase proteins of rBC, the ICPI values were as follows: rGBT, 1.91; rGBT-BC(N), 1.88; rGBT-BC(P), 1.9; rGBT-BC(L), 1.83; rGBT-BC(N+P+L), 1.78; rGBT-BC(Tr), 1.9 (Table 4). Thus, in both series, the ICPI value was affected by transfer of the L gene but not by the other individual components, and the effect of L was increased when transferred with N and P.
Replication and pathogenicity of parental and chimeric viruses in 2-week-old chickens.
To further evaluate the pathogenicity of rBC and rGBT and their chimeric viruses, 2-week-old chickens in groups of 12 were infected with 1 × 106 PFU of virus per bird via the oculonasal route, mimicking natural infection. At 3 dpi, two chickens from each group were sacrificed and tissue samples were collected from trachea, lung, spleen, intestine, and brain. Half of each tissue sample was homogenized for virus titration and the other half was processed for histopathology.
For the rBC derivatives bearing the envelope-associated protein genes of rGBT, the tissue titers were higher with rBC-GBT(F), rBC-GBT(F+HN), and rBC-GBT(M+F+HN) than with other chimeric viruses (Fig. 5a). For the rGBT derivatives bearing the envelope-associated protein genes, the virus titers were lower with rGBT-BC(F), rGBT-BC(F+HN), and rGBT-BC(M+F+HN) than with other chimeric viruses (Fig. 5b). Thus, in both the rBC and rGBT series, transfer of the F gene had a substantial effect on the magnitude of virus replication.
FIG 5.
Virus titers in trachea, lungs, spleen, intestine, and brain of 2-week-old chickens following oculonasal inoculation with parental rBC and rGBT and their chimeric derivatives. Virus titers of the envelope-associated protein gene chimeras of rBC (a) and rGBT (b) and the polymerase-associated protein gene chimeras of rBC (c) and rGBT (d) are shown in separate panels. Virus titers were determined from 2 chickens from each indicated virus group at 3 dpi. The average virus titers are shown. Asterisks indicate test of significance of the tissue-specific virus titer of a chimeric virus compared to the parental virus of that group; P values were calculated based on a two-tailed, unpaired t test (95% confidence levels). ***, P = 0.0001 to 0.001, extremely significant; **, P = 0.001 to 0.01, very significant; *, P = 0.01 to 0.05, significant.
For the rBC derivatives bearing the polymerase-associated protein genes of rGBT, the tissue titers were higher for rBC-GBT(L) and rBC-GBT(N+P+L) than for the other chimeric viruses (Fig. 5c). For the rGBT derivatives bearing the polymerase-associated protein genes of rBC, the virus titers were lower in rGBT-BC(L) and rGBT-BC(N+P+L) than for the other chimeric viruses (Fig. 5d). This showed that, for both series, transfer of the L protein had the greatest effect on virus replication among the individual components, and this effect was greater when the L gene was cotransferred with the N and P genes.
The remaining 10 chickens in each group were observed daily for clinical signs and mortality until day 14; the results for the envelope-associated protein genes are shown in Fig. 6, and the results for the polymerase-associated proteins are shown in Fig. 7. The pathogenicity of the two parental viruses differed greatly, consistent with their respective pathotypes. Specifically, the birds infected with rBC developed mild illness (Fig. 6a and 7a) and all survived (Fig. 6b and 7b), whereas the birds infected with rGBT developed severe illness by day 3 to 5 (Fig. 6c and 7c) and all died by day 5 (Fig. 6d and 7d).
FIG 6.
Clinical scores and survival curves of 2-week-old chickens infected with parental rBC and rGBT and their envelope-associated protein gene chimeras. The clinical scores of envelope-associated protein gene chimeras of rBC (a) and rGBT (c) and the survival curves for envelope-associated protein gene chimeras of rBC (b) and rGBT (d) are given as separate panels and are based on 10 birds per group. Clinical scoring: 0, normal; 1, sick; 2, paralysis/torticollis/wing drop/incoordination; 3, prostration; 4, death. The mean scores per group per day are shown.
FIG 7.
Clinical scores and survival curves of 2-week-old chickens infected with parental rBC and rGBT and their polymerase-associated protein gene chimeras. The clinical scores of polymerase-associated protein gene chimeras of rBC (a) and rGBT (c) and the survival curves for polymerase-associated protein gene chimeras of rBC (b) and rGBT (d) are shown in separate panels and are based on 10 birds per group. Clinical scoring: 0, normal; 1, sick; 2, paralysis/torticollis/wing drop/incoordination; 3, prostration; 4, death. The mean scores per group per day are shown.
For rBC derivatives bearing the envelope-associated protein genes of rGBT, chimeras bearing the rGBT F, F+HN, or M+F+HN genes caused greater clinical scores than the rBC parent, whereas chimeras bearing the rGBT M or HN genes caused lower clinical scores than the rBC parent (Fig. 6a). rBC-GBT(F+HN) was the most virulent chimera, reaching a maximum score of 4.0 by 6 dpi, followed by rBC-GBT(F), which reached a maximum clinical score of 4.0 by 9 dpi. Unexpectedly, the clinical scores for rBC-GBT(M+F+HN) were less than those of rBC-GBT(F) and rBC-GBT(F+HN), which may indicate an element of incompatibility for this particular combination. The mortality pattern (Fig. 6b) mirrored the clinical disease pattern (Fig. 6a). Thus, these findings indicate that transfer of the rGBT F protein into rBC resulted in a substantial increase in virulence, although this was influenced by the M and HN proteins.
For rGBT derivatives bearing the envelope-associated genes of rBC, transfer of the rBC F or F+HN genes resulted in substantial reductions in clinical score compared to parental rGBT (Fig. 6c). Unexpectedly, the further inclusion of the rBC M gene, creating rGBT-BC(M+F+HN), largely reversed the attenuating effect of the rBC F gene, such that maximal clinical scores were reached by day 6 (Fig. 6c) and all of the birds died by that day (Fig. 6d). Thsese findings indicated that certain combinations of genes can have unexpected synergies. Transfer of rBC M or HN individually to rGBT had little or no effect on its high level of virulence, indicating that these rBC genes were compatible with the rGBT backbone but apparently were not determinants of virulence. The mortality pattern (Fig. 6d) resembled the clinical score pattern (Fig. 6c). These results reinforced our observation that the F gene plays a major role in the pathogenicity of NDV.
For rBC derivatives bearing the polymerase-associated protein genes of rGBT, transfer of rGBT L alone or in the combination N+P+L resulted in increased clinical disease, whereas the other individual rGBT components (N, P, and Tr) resulted in marginally reduced scores. rBC-GBT(L) was the most virulent of these chimeras, reaching a maximum clinical score of 2.9 in 12 days (Fig. 7a). rBC-GBT(N+P+L) was the next most virulent, with a clinical score of 1.9 on day 12. These findings support the idea that L makes the biggest contribution to virulence among the polymerase-associated proteins, although it was paradoxical that the further addition of rGBT N and P resulted in a somewhat lower clinical score. These findings may reflect some incompatibility of the combination of rGBT N, P, and L with the rBC backbone. The survival curves (Fig. 7b) reflected the clinical scores (Fig. 7a).
For rGBT derivatives bearing the polymerase-associated protein genes of rBC, transfer of rBC L alone or in the combination N+P+L resulted in a reduction in clinical disease scores compared to the rGBT parent (Fig. 7c). The combination of rBC N+P+L had the greatest attenuating effect, which may might indicate an additive contribution to attenuation but also could be an indication of some incompatibility of this combination with the rGBT backbone. The transfer of rGBT P alone had a marginal attenuating effect, while transfer of N or Tr alone had no effect. The survival curves (Fig. 7d) reflected the clinical scoring (Fig. 7c).
Histopathological examination of tissue samples from 2-week-old chickens infected with parental and chimeric viruses.
Tissue samples from the trachea, lungs, spleen, intestine, and brains of two birds from each group infected with parental rBC and rGBT and their chimeric viruses were collected on day 3 postinfection for histopathological examination. Representative specimens for certain tissues are shown in Fig. 8 and 9, and summaries of histopathologic lesions and scores are provided in Tables 5 and 6. Overall, lesions in the tissue sections from the infected chickens were subacute in nature and exhibited considerable variability in severity and distribution within samples. For this reason, the histopathology provided a qualitative rather than quantitative picture of disease associated with the various viruses.
FIG 8.
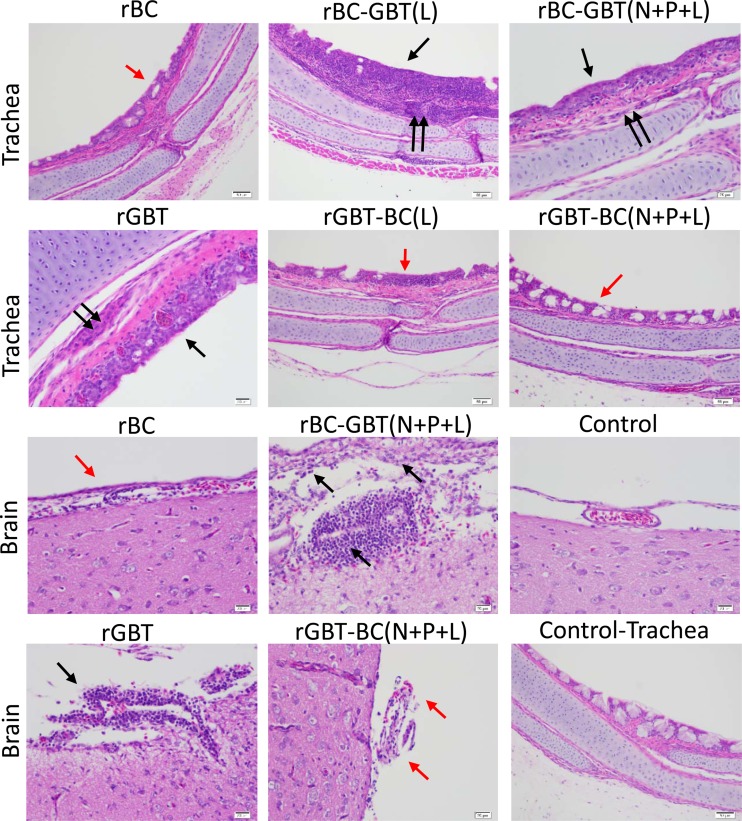
Histopathology of trachea, lungs, and brain tissues of 2-week-old chickens infected oculonasally with parental and chimeras of NDV strains BC and GBT after swapping of the envelope-associated protein genes. Birds were sacrificed at 3 dpi, and tissue was fixed with formalin, sectioned, and stained with hematoxylin and eosin. (a, trachea) The rGBT parent caused severe inflammation of the tracheal mucosa (black arrows), which also was observed with rBC-GBT(F), whereas the rBC parent and rGBT-BC(F) caused attenuation of the tracheal epithelium (red arrows) with minimal inflammation. (a, lungs) There was no significant difference observed among rBC and rGBT and their F gene swap constructs. (a, brain) Strain GBT caused substantial lymphoplasmacytic infiltration in the meninges (black arrows), which also was observed with rBC-GBT(F), whereas rBC and rGBT-BC(F) were associated with reduced lymphoplasmacytic infiltration (red arrows). (b, trachea) rBC-GBT(F+HN) caused severe inflammation of the tracheal mucosa (black arrow) and submucosa (double black arrows) compared to mucosal attenuation in the rBC-GBT(HN), rBC-GBT(M+F+HN), and rGBT-BC(M+F+HN) groups (red arrows) but the rGBT-BC(F+HN) group showed moderate mucosal inflammation. (b, lungs) rBC-GBT(F+HN) caused hyperplasia of the parabronchi (black arrows), and rBC-GBT(M+F+HN) caused severe parabronchial inflammation (red arrows), whereas this was minimal with rBC-GBT(HN), rGBT-BC(F+HN), and rGBT-BC(M+F+HN). In these images it is evident that when GBT F was exchanged with that of BC, this increased the virulence of BC alone or in combination with GBT HN. In contrast, when BC F was exchanged with that of GBT it reduced the virulence of GBT.
FIG 9.
Histopathology of sections of trachea and brain of 2-week-old chickens infected oculonasally with parental and chimeras of NDV strains BC and GBT, involving swaps of the polymerase-associated protein genes. (Trachea) Severe inflammation in the tracheal mucosa (black arrows) and submucosa (double black arrows) was observed with parental rGBT, rBC-GBT(L), and rBC-GBT(N+P+L), whereas inflammation was reduced (red arrows) with rBC, rGBT-BC(L), and rBC-GBT(N+P+L). (Brain) Severe inflammation and marked perivascular aggregates of lymphocytes and fewer plasma cells were observed in meninges in response to rGBT and rBC-GBT(N+P+L) (black arrows), whereas inflammation and lesions were reduced with rBC and rGBT-BC(N+P+L) (red arrows). The trachea and the brain lesions indicate that rGBT L alone or together with N and P increased the virulence of rBC, and the converse swap increased the virulence of rBC.
TABLE 5.
Summary of histopathological lesions in lung, trachea, spleen, intestine, and brain from chickens infected with M, F, and HN gene chimeras of NDV strains BC and GBTa
| Tissue and histopathological lesion | Control |
rBC |
rBC-GBT(M) |
rBC-GBT(F) |
rBC-GBT(HN) |
rBC-GBT(F+HN) |
rBC-GBT(M+F+HN) |
rGBT |
rGBT-BC(M) |
rGBT-BC(F) |
rGBT-BC(HN) |
rGBT-BC(F+HN) |
rGBT-BC(M+F+HN) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | |
| Lung | ||||||||||||||||||||||||||
| Bronchial lymphoid aggregates | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 |
| Parabronchitis | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 0 | 2 |
| Epithelial hyperplasia | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 1–2* | 2* | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 2* | 2* |
| Trachea | ||||||||||||||||||||||||||
| Tracheitis | 0 | 0 | 0 | 0 | 1 | 2 | 1–3 | 2 | 1 | 2 | 1–3 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 3* | 2* | 1 | 2 |
| Mucosal attenuation | 0 | 0 | 2 | 2 | 2 | 2 | 2* | 2* | 2–3 | 2 | 0 | 0 | 2 | 2 | 3* | 2* | 2* | 2* | 1 | 2 | 0 | 0 | 2–3 | 2 | 1–3 | 2 |
| Mucosal hyperplasia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 3* | 2* | 0 | 0 |
| Necrotic/apoptotic mucosal cells | − | − | − | − | − | − | − | + | + | − | + | + | + | |||||||||||||
| Spleen | ||||||||||||||||||||||||||
| Lymphoid hyperplasia | 0 | 0 | 3 | 5 | 3 | 5 | 2–3 | 5 | 0 | 5 | 3 | 5 | 0 | 5 | 4 | 5 | 0 | 5 | 0 | 5 | 3 | 5 | 0 | 5 | 0 | 5 |
| Intestine | ||||||||||||||||||||||||||
| Lymphohistiocytic enteritis | 0 | 0 | 2–4 | 1–3 | 3 | 2 | 1–4 | 2–4 | 2–3 | 2 | 3* | 2* | 0 | 0 | 0 | 0 | 2* | 1* | 2* | 2* | 3* | 1* | 1–2* | 2* | 1–2* | 1–2* |
| Mucosal hyperplasia | 0 | 0 | 2–3 | 2 | 0 | 2 | 2–3 | 2 | 2–3 | 2 | 2–3 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 2 |
| Brain | ||||||||||||||||||||||||||
| Meningitis | 0 | 0 | 1* | 1* | 1–2* | 2* | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1* | 2* | 1* | 2* | 0 | 0 | 0 | 2 | 3* | 2* |
| Encephalitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2* | 1* | 3* | 2* |
The severity (S) and distribution (D) of the histopathological lesions were scored using scales of 0 to 5. For severity, the scores indicate the following: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked; 5, severe. For distributions, the numbers indicate the following: 0, absent; 1, focal; 2, multifocal; 3, locally extensive; 4, multifocal and coalescing; 5, diffuse. +, present; −, absent. *, the lesion was not present in all specimens.
TABLE 6.
Summary of histopathological lesions in lung, trachea, spleen, intestine, and brain from chickens infected with N, P, L, and trailer sequence chimeras of NDV strains BC and GBTa
| Tissue and histopathological lesion | Control |
rBC |
rBC-GBT(N) |
rBC-GBT(P) |
rBC-GBT(L) |
rBC-GBT(N+P+L) |
rBC-GBT (Tr) |
rGBT |
rGBT-BC(N) |
rGBT-BC(P) |
rGBT-BC(L) |
rGBT-BC(N+P+L) |
rGBT-BC(Tr) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | S | D | |
| Lung | ||||||||||||||||||||||||||
| Bronchial lymphoid aggregates | 1 | 1 | 0 | 0 | 1* | 1* | 1–2 | 2 | 1* | 1* | 2* | 2* | 1–3 | 2 | 2* | 2* | 1* | 2* | 0 | 0 | 2–3 | 2 | 3* | 2* | 1* | 2* |
| Lymphocytic parabronchitis | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1–2 | 2 | 1 | 2 | 1–2 | 2 | 1–2 | 2 | 2 | 2 | 1–2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 |
| Alveolar duct epithelial hyperplasia | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 1* | 2* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trachea | ||||||||||||||||||||||||||
| Lymphocytic tracheitis | 0 | 0 | 0 | 0 | 0 | 0 | 2* | 1* | 1–3* | 1–2* | 1* | 1* | 1 | 2 | 1* | 2* | 2* | 2* | 1–2 | 2 | 0 | 0 | 1* | 1* | 1 | 2 |
| Mucosal attenuation | 0 | 0 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 3* | 2* | 0 | 0 | 1–3 | 2 | 0 | 0 | 1* | 2* | 0 | 0 | 0 | 0 | 0 | 0 |
| Necrotic/apoptotic mucosal cells | − | − | − | − | − | + | − | + | − | − | − | − | + | |||||||||||||
| Spleen | ||||||||||||||||||||||||||
| Lymphoid hyperplasia | 0 | 0 | 3 | 5 | 3–4 | 5 | 3 | 5 | 3 | 5 | 3–4 | 5 | 3 | 5 | 4 | 5 | 3 | 5 | 3 | 5 | 3–4 | 5 | 3–4 | 5 | 3 | 5 |
| Intestine | ||||||||||||||||||||||||||
| Lymphocytic enteritis | 0 | 0 | 2–4 | 1–3 | 2* | 1* | 1* | 1* | 2* | 2* | 2* | 2* | 1* | 2* | 1* | 2* | 0 | 0 | 1–2* | 2* | 2* | 2* | 0 | 0 | 1 | 2 |
| Mucosal hyperplasia | 0 | 0 | 2–3 | 2 | 2–3 | 2 | 2 | 2 | 2–3 | 2 | 3 | 2 | 2* | 2* | 2 | 2 | 1–2 | 2 | 1–2 | 2 | 2 | 2 | 1 | 2 | 1 | 2 |
| Brain | ||||||||||||||||||||||||||
| Lymphocytic perivascular meningitis | 0 | 0 | 1 | 1 | 1–2* | 2* | 0 | 0 | 0 | 0 | 3* | 2* | 0 | 0 | 2–3* | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1–2 | 1–2* | 2* |
| Lymphocytic perivascular encephalitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3* | 2* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The severity (S) and distribution (D) of the histopathological lesions were scored using scales of 0 to 5. For severity, the scores indicate the following: 0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked; 5, severe. For distributions, the numbers indicate the following: 0, absent; 1, focal; 2, multifocal; 3, locally extensive; 4, multifocal and coalescing; 5, diffuse. +, present; −, absent. *, the lesion was not present in all specimens.
In the trachea, parental rBC showed mild, multifocal, mucosal attenuation with reduction in density of mucous glands, whereas parental rGBT caused moderate, multifocal, lymphocytic tracheitis with inflammatory cells present in the lamina propria (Fig. 8a). The tracheal sections of the rBC-GBT(F) group exhibited general similarities to the rGBT group (Fig. 8a). Also, rBC-GBT(F+HN) and rBC-GBT(M+F+HN) caused multifocal lymphocytic tracheitis with inflammatory cells present in the lamina propria, indicative of increased virulence compared to the rBC parent (Fig. 8b). In contrast, the rGBT-BC(F) group showed minimal, multifocal, lymphocytic tracheitis and also exhibited minimal mucosal attenuation with decreased mucous glands (Fig. 8a and Table 5). rBC-GBT(M) and rBC-GBT(HN) similarly caused mild, multifocal tracheitis. Thus, the presence of the F protein of rBC or rGBT was associated with mild or moderate disease, respectively, consistent with the importance of F in virulence.
For the chimeras involving the polymerase-associated protein genes, tracheal lesions of the rBC-GBT(N) and rBC-GBT(P) groups were similar to those of the rBC group and did not exhibit increased virulence. In contrast, rBC-GBT(L) showed increased pathology with a focal, dense submucosal aggregate of lymphocytes and fewer macrophages that expanded the submucosa and extended beyond the tracheal cartilage into the tracheal interstitium (Fig. 9). rBC-GBT(N+P+L) showed multifocal, mucosal attenuation with reduction in the mucous glands. There were multiple individually apoptotic cells in the mucosa and perivascular and submucosal infiltrates of lymphocytes (Fig. 9). Thus, transfer of rGBT L or N+P+L (but not N or P individually) to the rBC group resulted in increased virulence. Conversely, rGBT-BC(N) and rGBT-BC(P) caused lesions of severe tracheal inflammation, similar to parental rGBT, whereas this was substantially reduced for rGBT-BC(L) and rGBT-BC(N+P+L) (Fig. 9 and Table 6).
In the lungs, rBC, rBC-GBT(M), rBC-GBT(F), and rBC-GBT(HN) caused mild, lymphocytic parabronchitis with inflammatory cells infiltrating the parabronchial interstitium, and mild multifocal epithelial hyperplasia primarily affecting the parabronchi atria (Fig. 8a and Table 5). In the rBC-GBT(F+HN) group, in addition to inflammatory lesions, there was a focally extensive area of parabronchial epithelial hyperplasia with distended atria that contained small amounts of proteinaceous material (Fig. 8b). In the rBC-GBT(M+F+HN) group, in addition to signs of inflammation, there was an extensive lymphocytic bronchitis with extension of inflammatory cells from the lamina propria into the submucosa and around the bronchus (Fig. 8b). Among the rGBT derivatives, rGBT, rGBT-BC(M), rGBT-BC(F), rGBT-BC(HN), rGBT-BC(F+HN), and rGBT-BC(M+F+HN) caused multifocal lymphocytic parabronchitis with inflammatory cells infiltrating the parabronchial and perivascular interstitium (Fig. 8a and b and Table 5). And, also, there was epithelial hyperplasia that affected atria of parabronchi and the bronchia-atria junction. There were no significant differences noticed among the lung tissue sections in the rGBT chimeric viruses groups, and hence the differences that were observed in the various other virulence assays were not always reflected in the histopathologic findings.
In the brain, rBC, rBC-GBT(M), and rBC-GBT(HN) caused minimal, focal lymphoplasmacytic perivascular meningitis (Fig. 8a and Table 5). rBC-GBT(F), rBC-GBT(F+HN), and rBC-GBT(M+F+HN) caused focal infiltrates of lymphoid cells in the interstitium of the choroid plexus in addition to increased lymphoplasmacytes in areas of perivascular meningitis (Fig. 8a and b). In general the rGBT chimeric virus group exhibited multifocal areas of perivascular lymphoplasmacytic meningitis. Meningitis and encephalitis lesions were greater among the rGBT derivatives. The rGBT-BC(F) group showed a relative reduction in lymphoplasmacytes in perivascular meningitis compared to the rGBT group (Fig. 8a).
For the brain specimens for the polymerase protein gene chimeras, rBC-GBT(N) and rBC-GBT(P) resembled the rBC parent in causing minimal, focal, lymphoplasmacytic perivascular meningitis (Tables 5 and 6). In contrast, the rBC-GBT(L) group showed aggregates of lymphoid cells in the choroid plexus that were interpreted to represent migration to the pineal gland. rBC-GBT(N+P+L) showed numerous vessels in the meninges and brainstem that exhibited moderate, dense, perivascular aggregates of lymphocyptes and fewer plasma cells, indicating an increase in severity of meningitis compared to rBC. There were no appreciable changes in the rGBT-BC(N), rGBT-BC(P), and rGBT-BC(L) groups compared to the rGBT group, but the rGBT-BC(N+P+L) group showed reduced meningitis and encephalitis.
DISCUSSION
In this study we chose two viruses, BC and GBT, which differ substantially in virulence but are a genetically closely related pair. Specifically, both viruses are of chicken origin from the United States from the late 1940s, belong to genotype II of class II, have the same genome length, have a high degree of nucleotide and amino acid sequence identity, and have an identical F protein cleavage site sequence (RRQKR↓F). Using reverse genetics, we swapped each NDV gene alone and in a number of combinations in order to investigate the NDV genes responsible for the difference in virulence between rBC and rGBT. The chimeric viruses were examined for their virulence in chickens in six different assays: (i) in vitro replication kinetics and magnitude; (ii) plaque size; (iii) fusion index (this was performed only for chimeras involving the membrane-associated protein genes); (iv) ICPI; (v) magnitude of replication in five different tissues in 2-week-old chickens; (vi) clinical scores and mortality in 2-week-old chickens. The velogenic rGBT parent had higher scores in all of these assays compared to the mesogenic rBC parent, indicating that all of these parameters are measures of virulence, at least for these viruses. In addition, we evaluated tissue histopathology, which provided direct visualization of viral pathogenesis but proved to be too variable within groups for clear quantification. We found that the three in vivo assays, namely, the ICPI assay, replication in 2-week-chickens, and clinical scores and mortality in 2-week-old chickens, were the most relevant with regard to predicted virulence in nature.
When the envelope-associated proteins of rGBT were transferred into the rBC backbone, the individual transfer of the F protein caused chimeric rBC to score substantially higher in each of the six virulence assays. Transfer of rGBT HN alone gave marginal increases in several assays, namely, plaque size, fusion, and ICPI, but transfer of rGBT M alone did not give increases in any assay. Thus, among the individual envelope-associated protein genes, F had the greatest impact on virulence. The combinations of F+HN and M+F+HN usually gave a similar, and sometimes greater, increase than did F alone. For example, the plaque size and fusion index were greatest for M+F+HN. This suggests that the combination of M+F+HN is optimal for fusion activity. On the other hand, the combination of M+F+HN scored somewhat lower than F or F+HN with regard to replication under liquid overlay, replication in birds, and clinical scores and mortality in birds. It may be that the increased fusion achieved by transfer of rGBT(M+F+HN) into rBC was somewhat detrimental for rBC replication, perhaps leading to enhanced cytopathogenicity that could reduce virus yield. The idea that a high level of fusion might be detrimental is suggested by the observation that a number of paramyxoviruses have evolved mechanisms for downregulating expression of the F gene (27). Alternatively, it may be that the combination of rGBT M+F+HN was somewhat incompatible with the rBC backbone and that this effect was more evident in assays requiring extensive replication. In summary, these results showed that the rGBT F protein plays the major role among the envelope-associated NDV proteins in the greater virulence of strain GBT compared to that of BC and that M and HN can sometimes modulate that role.
Conversely, when the envelope-associated protein genes of rBC were transferred into the rGBT backbone, the individual transfer of the F gene caused chimeric rGBT to score substantially lower in most of the virulence assays (the one exception was the plaque size assay; see below), whereas the individual transfer of rGBT M or HN had little effect in most cases. Thus, among the individual envelope-associated protein genes, transfer of the rBC F gene into rGBT had the greatest effect on virulence (in this case, decreasing the virulence of the velogenic backbone). This provided reciprocal confirmation of the findings with the rBC derivatives described above. Interestingly, the dramatic difference in virulence afforded by the rBC F protein versus the rGBT F protein is independent of the cleavage site sequence, since this is identical in the two strains. Introduction of the combination of rBC F+HN or M+F+HN into rGBT also yielded a substantial decrease in virulence in most of the assays, consistent with the importance of F. However, two findings were unexpected. First, in the plaque size assay, it was the rBC M gene rather than the F gene that gave the greatest reduction in size, and the rBC HN gene gave an increase in size. However, the combination of rBC F+HN or M+F+HN was each more consistent with expectations and yielded substantial decreases in plaque sizes. The second unexpected finding was that, whereas transfer of rBC F or F+HN into rGBT yielded substantial decreases in virulence, the further addition of rBC M largely restored virulence. This gain of function remains unexplained but presumably is due to interactions specific to this particular gene constellation. That unexpected synergies can occur perhaps is not unexpected, given how few amino acid differences there are between the proteins of the two strains.
As noted in the introduction, the cleavage site of the F protein was already known to be a major determinant of NDV virulence in general. However, in the present study, the cleavage site sequence was identical between rBC and rGBT and was left unchanged. Thus, the finding in the present study that the F protein was the major determinant of the difference in virulence between rBC and rGBT was somewhat surprising, because it indicated that other aspects of the F protein beyond the cleavage site contribute to virulence, and that contribution is substantial. The basis for the differences in phenotype between the F proteins of rBC and rGBT remains unknown, although it seems likely that it includes structural differences that may facilitate fusion in rGBT compared to rBC, such as by promoting the conformational rearrangements involved in fusion. The F proteins of these two strains are highly related, differing by only 8 amino acid assignments. The regions of the F protein in which these amino acid assignment differences occur between strains BC and GBT are the following: the F2 region (P10S and V11A), the F1 ectodomain region (G265S, G304E, and I457T), the transmembrane region (I510T and V520A), and the cytoplasmic tail (T550A) (28). The 3 amino acid assignment differences that occur in the F1 ectodomain region did not fall in the heptad repeat (HR) domains, HR1 and HR2. Thus, the importance of these differences between the two F proteins should be readily amenable to investigation by reverse genetics.
The finding in the present study that the F protein was the major determinant of NDV virulence is not in agreement with the results of a previous study in which the F gene did not appear to play a prominent role in NDV virulence (18). However, that study was performed by exchanging F genes between a pigeon paramyxovirus and a virulent NDV strain. Therefore, the results might have been confounded by incompatibility between NDV and the pigeon paramyxovirus. Another previous study indicated an important role for the HN protein in virulence (29, 30), whereas our present results showed that HN either does not play any role or has a minimal role in determining NDV virulence. Our results also contradict the results of a previous study in which F and HN together were found to determine the virulence of NDV (20). However, the previous study involved chimeras between NDV and APMV-2 and thus involved chimeras between two different serotypes of avian paramyxoviruses. In particular, chimeras involving swaps of HN could not be recovered, probably due to an absence of homotypic interactions due to divergence between the serotypes. Therefore, the individual contribution of HN to virulence could not be determined (20). To avoid these confounding factors, we performed the present study with a closely matched pair of viruses and employed reverse genetic systems that allowed for precise reciprocal swaps.
We also sought to determine the relative contributions of the polymerase-associated proteins to NDV virulence. There is precedence for polymerase-associated proteins of paramyxoviruses to be involved in pathogenicity (19, 23). We exchanged the N, P, and L genes individually and in combination between the rBC and rGBT viruses. When the polymerase-associated protein genes of rGBT were transferred into the rBC backbone, the individual transfer of the L gene usually resulted in a substantial increase in virulence, and the combined transfer of N+P+L gave a substantially increased response in all of the assays. Conversely, transfer of rBC L or N+P+L into rGBT resulted in decreased virulence in all of the assays, and this usually was greater for N+P+L than for L alone. In contrast, there was no significant change in pathogenicity of chimeric viruses compared to their parental viruses when the N or the P gene was exchanged between BC and GBT viruses. These results indicated that, among the polymerase-associated proteins, the L protein contributes the most to viral virulence and that this can be enhanced by the presence of homotypic N, P, and L proteins.
The finding that the L protein of NDV is an important determinant of virulence is in agreement with the results of a previous study in which the L protein was found to be the only determinant among polymerase-associated proteins that contributed to virulence (23). But our results differed from the finding of another study which showed that all three replication proteins (N, P, and L) were associated with virulence (19). An explanation for this difference might be that, in that study, one of the virus strains was from pigeons and the other strain was from chickens and those two strains belonged to different lineages and genotypes, and thus the apparent attenuation observed with gene swaps might have included effects from gene incompatibility. In contrast, the strains used in the present study were genetically closely related and amenable to gene swapping.
The molecular mechanism for increased virulence caused by the L protein of GBT in a BC backbone is not known. It may be that the L protein of GBT virus directs increased RNA synthesis, leading to higher levels of virus replication in vitro and in vivo. A higher level of virus replication in vivo can increase virulence by overwhelming the host immune responses. A previous study showed that avirulent NDV strains had reduced levels of RNA synthesis (31). Therefore, it will be interesting to compare the RNA synthesis activities of the L proteins of BC and GBT viruses. Between the L proteins of BC and GBT, 14 amino acid assignment differences occur, and they include A75T, V89I, D265N, R698C, N889K, Y1379F, N1531D, S1643N, K1668R, L1706F, P1734L, A1758S, I1785M, and V2067I (28). These differences are distributed throughout the multidomain L protein. The role of these amino acid residues in L protein function can be determined by site-directed mutagenesis using reverse genetics.
It was somewhat surprising that transfer of the P gene did not result in substantial changes in virulence. In addition to encoding the P protein, which is part of the polymerase complex, the NDV P gene also encodes a V protein and possibly a W protein (2). Studies with a number of members of this subfamily have shown that the V protein interferes with the induction of host cell type I interferon and with signaling from the type I interferon receptor. V also may have other functions, such as blocking apoptosis, whereas the significance of W remains unknown. Loss of expression of the V protein is highly attenuating in vitro and in vivo, indicating that it plays an important role in NDV virulence. However, the results of the present study indicate that the V protein does not contribute significantly to the difference in virulence between strains rBC and rGBT.
Previously, the relative contributions of the envelope-associated proteins versus the polymerase-associated proteins to NDV virulence had not been compared side by side. Several reverse genetic studies addressed the contributions of either the envelope-associated proteins (12–15, 18, 20–22) or the polymerase-associated proteins (19–23), but not both groups of proteins in the same study. The present study was designed to compare the relative contributions of these groups of proteins to NDV virulence. The results of the present study showed that both envelope-associated and polymerase-associated proteins contribute to the overall virulence of NDV, but the contributions of envelope-associated proteins are substantially more than those of the polymerase-associated proteins. For example, transferring the rGBT F or F and HN genes into rBC was sufficient to change the percent survival from 100% for rBC to 0% for rBC-GBT(F) and rBE-GBT(F+HN). Conversely, the transfer of the rBC F or F and HN genes to rGBT changed the percent survival from 0% to 100% and 75%, respectively. In contrast, the maximum effects observed with transfer of the polymerase-associated proteins were not as high. For example, the transfer of the rGBT L or N, P, and L genes into rBC changed the percent survival from 100% to 75%, and the converse transfer of rBC L or the N, P, and L genes into rGBT changed the percent survival from 0% to 0% (with death delayed by 3 days) and 40%, respectively.
The genomic RNA of NDV contains a 55-nt 3′ extragenic leader region and a 114-nt 5′ extragenic trailer region. The leader and trailer are control regions essential not only for transcription and replication but also encapsidation of newly synthesized RNAs into nucleocapsids. However, the roles of these regions in pathogenesis of NDV have never been investigated. The leader region is identical between BC and GBT, and therefore it was not exchanged in this study. But there are 5 nt differences between the trailer region of BC and GBT. Hence, the extragenic trailer region was exchanged between BC and GBT. However, for the most part, we did not detect any noticeable change in the pathogenicity of BC or GBT chimeric viruses compared to their parental viruses due to swapping the trailer region, suggesting that it does not play a significant role in virulence.
In summary, we have shown that the variation in virulence of NDV is contributed by multiple viral proteins. Among all NDV viral proteins, the F protein, independent of the cleavage site, is the major contributor to virulence. The L protein also contributes to the overall virulence of NDV. Although the other viral proteins usually did not make substantial contributions to virulence, they did affect the overall virulence of the virus. Further studies are needed to identify the specific amino acids or the domains in the F and L proteins that are responsible for the virulence. The identification of virulence determinants is of considerable importance because of the potential to generate better live attenuated NDV vaccines. It may also be possible to apply these findings to other paramyxoviruses.
ACKNOWLEDGMENTS
We thank Daniel Rockemann, Girmay Gebreluul, Yonas Araya, and our laboratory members for excellent technical assistance and Bernard Moss (NIAID, NIH) for providing the vaccinia T7 recombinant virus. We also thank Andrea Ferrero-Perez for BSL-3+ facility coordination.
This research was supported by NIAID contract N01A060009 (85% support) and the NIAID, NIH Intramural Research Program (15% support).
The views expressed herein do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Footnotes
Published ahead of print 21 May 2014
REFERENCES
- 1.Alexander DJ. 2003. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections, p 63–99 In Saif JM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE. (ed), Diseases of poultry, 11th ed. Iowa State University Press, Ames, IA [Google Scholar]
- 2.Samal SK. 2011. The biology of paramyxoviruses, 1st ed, p 69–114 Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 3.Alexander DJ. 1998. Newcastle disease virus and other avian paramyxoviruses, p 156–163 In Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM. (ed), A laboratory manual for the isolation and identification of avian pathogens, 4th ed. American Association of Avian Pathologists, Inc., Kennett Square, PA [Google Scholar]
- 4.Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1449–1496 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 5.Mayo MA. 2002. Virus taxonomy: Houston. Arch. Virol. 147:1071–76. 10.1007/s007050200036 [DOI] [PubMed] [Google Scholar]
- 6.Aldous EW, Mynn JK, Banks J, Alexander DJ. 2003. A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathol. 32:239–256. 10.1080/030794503100009783 [DOI] [PubMed] [Google Scholar]
- 7.Czeglédi A, Ujvári D, Somogyi E, Wehmann E, Werner O, Lomniczi B. 2006. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 120:36–48. 10.1016/j.virusres.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 8.de Leeuw O, Peeters B. 1999. Complete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily Paramyxovirinae. J. Gen. Virol. 80:131–136 9934695 [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Wan HQ, Liu HQ, Wu YT, Liu XF. 2004. Genomic sequence of an isolate of Newcastle disease virus isolated from an outbreak in geese: a novel six nucleotide insertion in the non-coding region of the nucleoprotein gene. Arch. Virol. 149:1445–1457. 10.1007/s00705-004-0297-8 [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy S, Samal SK. 1998. Nucleotide sequences of the trailer, nucleocapsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequence. J. Gen. Virol. 79:2419–2424 [DOI] [PubMed] [Google Scholar]
- 11.Miller PJ, Decanini EL, Afonso CL. 2010. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 10:26–35. 10.1016/j.meegid.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 12.de Leeuw OS, Koch G, Hartog L, Ravenshorst N, Peeters BPH. 2005. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J. Gen. Virol. 86:1759–1769. 10.1099/vir.0.80822-0 [DOI] [PubMed] [Google Scholar]
- 13.Panda A, Huang Z, Elankumaran S, Rockemann DD, Samal SK. 2004. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 36:1–10. 10.1016/j.micpath.2003.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Römer-Oberdörfer A, Veits J, Werner O, Mettenleiter TC. 2006. Enhancement of pathogenicity of Newcastle disease virus by alteration of specific amino acid residues in the surface glycoproteins F and HN. Avian Dis. 50:259–263. 10.1637/7471-111505R.1 [DOI] [PubMed] [Google Scholar]
- 15.Samal S, Kumar S, Khattar SK, Samal SK. 2011. A single amino acid change, Q114R, in the cleavage-site sequence of Newcastle disease virus fusion protein attenuates viral replication and pathogenicity. J. Gen. Virol. 92:2333–2338. 10.1099/vir.0.033399-0 [DOI] [PubMed] [Google Scholar]
- 16.Tan LT, Xu HY, Wang YL, Qin ZM, Sun L, Liu WJ, Cui ZZ. 2008. Molecular characterization of three new virulent Newcastle disease virus variants isolated in China. J. Clin. Microbiol. 46:750–753. 10.1128/JCM.01587-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Servan de Almeida R, Maminiaina OF, Gil P, Hammoumi S, Molia S, Chevalier V, Koko M, Andriamanivo HR, Traoré A, Samaké K, Diarra A, Grillet C, Martinez D, Albina E. 2009. Africa, a reservoir of new virulent strains of Newcastle disease virus? Vaccine 27:3127–3129. 10.1016/j.vaccine.2009.03.076 [DOI] [PubMed] [Google Scholar]
- 18.Dortmans JC, Koch G, Rottier PJ, Peeters BP. 2009. Virulence of pigeon paramyxovirus type 1 does not always correlate with the cleavability of its fusion protein. J. Gen. Virol. 90:2746–2750. 10.1099/vir.0.014118-0 [DOI] [PubMed] [Google Scholar]
- 19.Dortmans JC, Rottier PJ, Koch G, Peeters BP. 2010. The viral replication complex is associated with the virulence of Newcastle disease virus. J. Virol. 84:10113–10120. 10.1128/JVI.00097-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Subbiah M, Samuel AS, Collins PL, Samal SK. 2011. Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses. J. Virol. 85:8582–8596. 10.1128/JVI.00652-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Susta L, Miller PJ, Afonso CL, Estevez C, Yu Q, Zhang J, Brown CC. 2010. Pathogenicity evaluation of different Newcastle disease virus chimeras in 4-week-old chickens. Trop. Anim. Health Prod. 42:1785–1795. 10.1007/s11250-010-9638-7 [DOI] [PubMed] [Google Scholar]
- 22.Estevez C, King D, Seal B, Yu Q. 2007. Evaluation of Newcastle disease virus chimeras expressing the hemagglutinin-neuraminidase protein of velogenic strains in the context of a mesogenic recombinant virus backbone. Virus Res. 129:182–190. 10.1016/j.virusres.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 23.Rout SN, Samal SK. 2008. The large polymerase protein is associated with the virulence of Newcastle disease virus. J. Virol. 82:7828–7836. 10.1128/JVI.00578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed LJ, Muench H. 1938. A simple method of estimation of 50% end points. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 25.Kohn A. 1965. Polykaryocytosis induced by Newcastle disease virus in monolayers of animal cells. Virology 26:228–245. 10.1016/0042-6822(65)90050-4 [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy S, Huang Z, Samal SK. 2000. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology 278:168–182. 10.1006/viro.2000.0618 [DOI] [PubMed] [Google Scholar]
- 27.Karron RA, Collins PL. 2013. Parainfluenza viruses, p 996–1023 In Knipe DM, Howley PM. (ed), Fields virology, 6th ed, vol 1 Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 28.Paldurai A, Kumar S, Nayak B, Samal SK. 2010. Complete genome sequence of highly virulent neurotropic Newcastle disease virus strain Texas GB. Virus Genes 41:67–72. 10.1007/s11262-010-0486-3 [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Panda A, Elankumaran S, Govindarajan D, Rockemann DD, Samal SK. 2004. The hemagglutinin-neuraminidase protein of Newcastle disease virus determines tropism and virulence. J. Virol. 78:4176–4184. 10.1128/JVI.78.8.4176-4184.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panda A, Elankumaran S, Krishnamurthy S, Huang Z, Samal SK. 2004. Loss of N-linked glycosylation from the hemagglutinin-neuraminidase protein alters virulence of Newcastle disease virus. J. Virol. 78:4965–4975. 10.1128/JVI.78.10.4965-4975.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madansky CH, Bratt MA. 1981. Noncytopathic mutants of Newcastle disease virus are defective in virus-specific RNA synthesis. J. Virol. 37:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]