ABSTRACT
Some animal influenza A viruses (IAVs) bind not only to N-acetylneuraminic acid (Neu5Ac) but also to N-glycolylneuraminic acid (Neu5Gc), which has been discussed as a virus receptor. Human cells cannot synthesize Neu5Gc due to dysfunction of the CMP-Neu5Ac hydroxylase (CMAH) gene, which converts CMP-Neu5Ac to CMP-Neu5Gc. However, exogenous Neu5Gc from Neu5Gc-rich dietary sources is able to be metabolically incorporated into surfaces of tissue cells and may be related to enhancement of the infectivity and severity of IAV. Here, we investigated the receptor function of Neu5Gc on IAV infection in Neu5Gc-expressing cells by transfection of the monkey CMAH gene into human cells or by incubation with human cells in the presence of N-glycolylmannosamine. Expression of Neu5Gc on human cells clearly suppressed infectivity of IAVs that possess Neu5Gc binding ability. Furthermore, there was no difference in infectivity of a transfectant virus that included the wild-type HA gene from A/Memphis/1/1971 (H3N2), which shows no Neu5Gc binding, between parent MCF7 cells and cells stably expressing the monkey CMAH gene (CMAH-MCF7 cells). On the other hand, cell entry of the transfectant virus that included the Neu5Gc-binding HA gene with a single mutation to Tyr at position Thr155 was arrested at the stage of internalization from the plasma membrane of the CMAH-MCF7 cells. These results indicate that expression of Neu5Gc on the surface of human epithelial cells suppresses infection of IAVs that possess Neu5Gc binding ability. Neu5Gc is suggested to work as a decoy receptor of Neu5Gc-binding IAVs but not a functional receptor for IAV infection.
IMPORTANCE Influenza A viruses (IAVs) bind to the host cell surfaces through sialic acids at the terminal of glycoconjugates. For IAV binding to sialic acids, some IAVs bind not only to N-acetylneuraminic acid (Neu5Ac) as a receptor but also to N-glycolylneuraminic acid (Neu5Gc). Neu5Gc has been discussed as a receptor of human and animal IAVs. Our results showed that Neu5Gc expression on human epithelial cells suppresses infection of IAVs that possess Neu5Gc binding ability. Neu5Gc is suggested to be a “decoy receptor” of Neu5Gc-binding IAVs but not a functional receptor for IAV infection. Human cells cannot synthesize Neu5Gc because of dysfunction of the CMP-N-acetylneuraminic acid hydroxylase gene but can exogenously and metabolically incorporate Neu5Gc from dietary sources. The expression of Neu5Gc on human epithelial cells by taking in exogenous Neu5Gc from Neu5Gc-rich dietary sources may be related to restriction of the infection of IAVs that have acquired Neu5Gc binding ability.
INTRODUCTION
Influenza A virus (IAV) utilizes various hosts, including humans, aquatic birds, ground birds, pigs, and horses. IAV recognizes terminal sialic acid moieties of sialosaccharides on host cells through the viral envelope glycoprotein hemagglutinin (HA), followed by initiation of entry into the cells through an endocytic pathway. Generally, human IAV preferentially recognizes sialic acid of galactose by the α2,6 linkage (SAα2,6Gal), while avian and equine IAVs recognize sialic acid of galactose by the α2,3 linkage (SAα2,3Gal). Swine IAV recognizes both SAα2,6Gal and SAα2,3Gal (1). Molecular species of sialic acid are largely divided into two moieties: N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Almost all equine IAVs (2) and some human (3, 4), swine (5–8), and duck IAVs (9) bind not only to Neu5Ac but also to Neu5Gc. Neu5Gc is synthesized from the addition of a hydroxyl group to the N-acetyl group of Neu5Ac by CMP-Neu5Ac hydroxylase (CMAH; EC 1.14.18.2) with the cofactors cytochrome b5 reductase and NADH (10, 11) (Fig. 1). Human CMAH is a nonfunctional protein because of a frameshift from a 92-bp deletion in the coding region nucleotide sequence (12, 13) (Fig. 2A), and Neu5Gc has not been detected as a main component in human tracheal epithelia (5). However, exogenous Neu5Gc from Neu5Gc-rich dietary sources (particularly red meat and milk) is able to be metabolically incorporated into human tissue cells (14, 15). In nonhuman animals, Neu5Gc has been detected in swine tracheal epithelia (5), on crypt epithelial cells of the duck colon (9) and equine tracheal epithelia (2), and in the trachea and intestine of pigeons (16). In chickens, Neu5Gc has been detected in the trachea and intestine by high-performance liquid chromatography (HPLC) analysis (16) but not in the tracheal epithelia by histological immunostaining (2). The ratios of Neu5Ac and Neu5Gc have been reported to be 47:53 (mol/mol) in swine tracheal epithelia (5), with more than 90% of Neu5Gc in equine tracheal epithelia (2), 1.8:98.2 (mol/mol) in chicken trachea, and 1.8 to 13.8 Neu5Ac to 86.2 to 98.2 Neu5Gc (mol/mol) in chicken intestines (16). Since ground bird, swine, and horse tracheas and the duck colon are the main replication sites of mammalian and avian IAVs, Neu5Gc in these sites has been discussed to be a functional receptor for IAV infection. If Neu5Gc is a functional receptor, it may be related to enhancement of the infectivity and severity of IAVs that can bind to Neu5Gc. Moreover, some human IAVs that can bind to Neu5Gc may be easily transmitted to intermediate hosts, such as pigs, and then may lead to the occurrence of a new type of IAV reassorted between the human IAV genome and nonhuman animal IAV genome that can become a pandemic IAV in humans (17). However, the receptor function of Neu5Gc has not been evaluated in detail.
FIG 1.
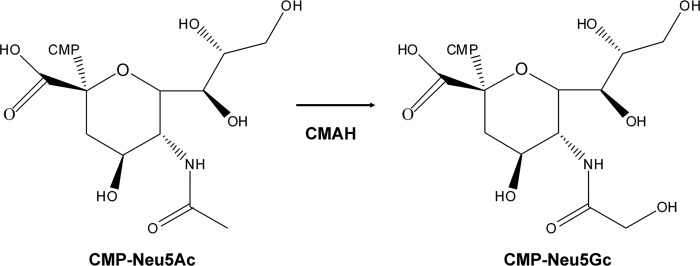
Conversion of CMP-Neu5Ac to CMP-Neu5Gc by CMAH.
FIG 2.
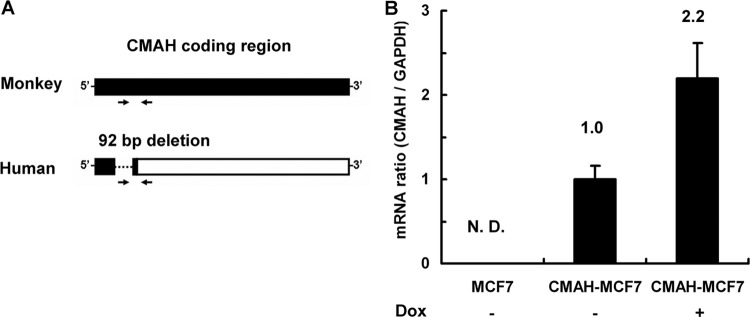
mRNA expression levels of the transfected CMAH gene in MCF7 cells and CMAH-MCF7 cells. (A) Primer positions for detection of monkey CMAH mRNA. The coding region is shown as a filled column. Human CMAH mRNA includes a noncoding region (empty column) because of a 92-bp nucleotide deletion (dotted line). Arrows indicate a pair of primers for quantitative PCR. The forward primer cannot detect human CMAH mRNA because it recognizes base pairs corresponding to the deletion region of human CMAH. (B) Quantitative PCR of CMAH mRNA. CMAH mRNA ratios were calculated as a proportion of the levels in CMAH-MCF7 cells in the absence of doxycycline (Dox). The amount of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA expression was used for normalization. Standard deviations were calculated from data from three independent experiments. N. D., not detected.
Here, we evaluated the receptor function of Neu5Gc in IAV infection by comparison of IAV infectivity in Neu5Gc-expressing cells to that in parent cells. We generated cells that stably express the monkey CMAH gene (CMAH-MCF7 cells) by transfection of the CMAH gene to human breast adenocarcinoma MCF7 cells. We also generated Neu5Gc-expressing A549 cells, which are human lung adenocarcinoma epithelial cells that are often used in experiments on influenza A virus infection, by incubation with the Neu5Gc precursor N-glycolylmannosamine (ManNGc). Experiments using CMAH-MCF7 cells and Neu5Gc-expressing A549 cells showed that expression of Neu5Gc had a negative effect on infection of human and equine IAVs that exhibit Neu5Gc binding ability. Furthermore, for comparison of virus infectivity, we used HA mutant IAVs, i.e., strain A/WSN/1933 with a wild-type or single-amino-acid-mutated HA gene of human influenza virus A/Memphis/1/1971 (H3N2). In our previous study, HA of A/Memphis/1/1971, which showed no binding to Neu5Gc, acquired Neu5Gc binding ability by a single-amino-acid mutation, threonine to tyrosine at position 155 in HA (18, 19). All of the Neu5Gc-binding IAVs tested here and Neu5Gc-binding mutant IAVs showed significantly less infectivity in Neu5Gc-expressing cells than in parent MCF7 cells. These results indicate that Neu5Gc expression in human cells suppresses infection of IAVs possessing Neu5Gc binding ability.
MATERIALS AND METHODS
Cells and viruses.
Human breast adenocarcinoma MCF7 cells (MCF7 Tet-On advanced cell line) were purchased from Clontech Laboratories Inc., and human lung adenocarcinoma epithelial A549 cells were purchased from Riken Cell Bank, Saitama, Japan. The cells were maintained in high-glucose Dulbecco's modified Eagle's minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 μg/ml of G418 sulfate (Promega, Madison, WI) according to the instruction manual. African green monkey kidney COS7 cells were maintained in DMEM supplemented with 10% FBS. IAVs [A/Aichi/2/1968 (H3N2), A/Memphis/1/1971 (H3N2), A/Memphis/102/1972 (H3N2), A/equine/Miami/1963 (H3N8), A/equine/Fontainebleau/1/1979 (H3N8), A/equine/Tennessee/5/1986 (H3N8), A/equine/Ibaraki/1/2007 (H3N8), and two Mem71HA-possessing WSN strains, WT and T155Y] were propagated in 10-day-old embryonated hen's eggs for 2 days at 34°C. Mem71HA-WSN is an IAV with the HA gene of A/Memphis/1/1971 (H3N2) together with seven genes of A/WSN/1933 (H1N1) other than the HA gene, as we reported previously (19). The two Mem71HA-WSN strains, WT and T155Y, have the wild-type HA of A/Memphis/1/1971 and the HA with a single-amino-acid change of threonine to tyrosine at position 155, respectively (18, 19).
Cloning of the CMAH gene and selection of cells transfected with the CMAH gene.
mRNA of COS7 cells was extracted with TRIzol reagent (Invitrogen Corp., Carlsbad, CA) and was converted to cDNA by using a TaKaRa RNA PCR kit (AMV), version 3.0 (TaKaRa Bio Inc., Shiga, Japan). The monkey CMAH gene was amplified by the nested PCR method with primers 5′-GGCTTATGGGAGCAAATGAGCCACAG-3′ and 5′-AATTCTTCTTGAAATTTTCATGTGGTTTTGCATTCC-3′ (first PCR) as well as 5′-GGAATTCCCATGGGCAGCACTGAACAAACAACTG-3′ and 5′-CGGAATTCCGTCATGTGGTTTTGCATTCCTGCG-3′ (second PCR), each containing an EcoRI site, using PFU Ultra high-fidelity DNA polymerase (Agilent Technologies Inc., Palo Alto, CA). The PCR product of the CMAH gene was digested with EcoRI enzyme and then inserted into a multicloning site between the two EcoRI sites of the pGEM-T easy vector (Promega, Madison, WI). The CMAH gene between the two NotI sites within the multicloning site of the pGEM-T easy vector was transferred into the NotI site of the pTRE2hyg vector (Clontech Laboratories Inc., Mountain View, CA) by digestion with NotI enzyme. The nucleotide sequence of the CMAH gene in all plasmids was confirmed by using an ABI Prism 310NT Genetic Analyzer (Applied Biosystems, Foster City, CA). MCF7 cells were transfected with the pTRE2hyg vector containing the CMAH gene using transfection reagent TransIT-293 (Mirus, Madison, WI) according to the instruction manual. Since MCF7 cells lacked expression of caspase-3 (20), which plays an essential role in IAV production and replication (21), they were expected to be suitable for evaluation of virus infectivity at the initial stage of the IAV life cycle. The transfected cells were selected and cloned in the presence of 200 μg/ml hygromycin B (Invitrogen Corp., Carlsbad, CA) for more than 2 months. The selected cells were maintained in DMEM supplemented with 10% FBS, 100 μg/ml hygromycin B, and 100 μg/ml G418 sulfate. After induction of CMAH gene expression using 1 μg/ml doxycycline (Clontech Laboratories Inc., Mountain View, CA) for 4 or 5 days, mRNA of the cells was extracted and converted to cDNA. After screening of CMAH mRNA expression using the PCR method, a single clone stably expressing the monkey CMAH gene (CMAH-MCF7 cells) was obtained.
Quantitative real-time PCR.
MCF7 cells and CMAH-MCF7 cells (2 × 105 cells/well) in the absence or presence of 1 μg/ml doxycycline were cultured in a 6-well plate at 37°C for 4 days. The cells were placed on ice for 5 min and washed with 1 ml/well of cold phosphate-buffered saline (PBS). The washed cells were harvested with 800 μl/well of cold PBS using a cell scraper. The cells were centrifuged at 2,000 × g for 6 min at 4°C and suspended in 300 μl of cold PBS. mRNA was extracted from 100 μl of the cell suspension using an RNA easy kit (Invitrogen Corp., Carlsbad, CA) and converted to cDNA (50 μl) using a PrimeScript reverse transcription-PCR (RT-PCR) kit (TaKaRa Bio Inc., Shiga, Japan). Endogenous mRNA of the monkey CMAH gene was measured by quantitative real-time RT-PCR (LightCycler 2.0; Roche Applied Science, Tokyo, Japan) using the primer pair 5′-GTCCGTTAAATGCACAAAGC-3′ (specific only for monkey CMAH mRNA due to a 92-bp nucleotide deletion in human CMAH mRNA) and 5′-AAAGTCCGTTGTTTTCATCCAT-3′ (specific for both human and monkey CMAH mRNA) (Fig. 2A). Endogenous mRNA of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene, as a housekeeping gene, was measured using the primer pair 5′-TCAACGGATTTGGCCGTATTGG-3′ and 5′-TGAAGGGGTCATTGATGGCG-3′ and was used with the ΔΔCT method (where CT is threshold cycle). The relative amount of CMAH mRNA was calculated as a proportion of that in CMAH-MCF7 cells in the absence of doxycycline.
Fluorescence microscopy.
During and after this experiment, CMAH-MCF7 cells were maintained in the presence of 1 μg/ml doxycycline for more than 2 weeks. To visualize Neu5Gc expression in cells, the cells (5 × 103 cells/well) were grown on 8-well glass coverslips (Teflon-printed glass slides; Erie Scientific Company, Portsmouth, NH) overnight and washed with PBS (pH 7.2; 131 mM NaCl, 14 mM Na2HPO4, 1.5 mM KH2PO4, and 2.7 mM KCl). The cells were fixed with 4% paraformaldehyde (PFA)-PBS at room temperature for 10 min, washed with PBS, and then incubated with chicken anti-Neu5Gc antibody, which recognizes Neu5Gcα2,3Gal but not Neu5Acα2,3Gal (2, 9, 22, 23), on ice for 1 h and with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-chicken IgY secondary antibody on ice for 1 h. Nuclei were visualized with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Dojindo Laboratories, Kumamoto, Japan). For sialidase treatment, cells were incubated with 30 μl/well of 2,500 mU/ml Salmonella enterica serovar Typhimurium sialidase (New England BioLabs Inc., Ipswich, MA) at 37°C for 1 h before fixation. All pictures were taken by an Olympus IX71 fluorescence microscope equipped with an Olympus DP70 camera (Olympus Corp., Shinjuku, Japan).
Analysis of sialic acid species using HPLC.
Confluent MCF7 cells and CMAH-MCF7 cells in a 100-mm dish were harvested using a cell scraper. The amount of protein in cells was measured using bicinchoninic acid (BCA) protein assay reagent (Thermo Fisher Scientific Inc., Rockford, IL). To investigate the total amounts of Neu5Ac and Neu5Gc in MCF7 cells and CMAH-MCF7 cells, fluorometric determination of Neu5Ac and Neu5Gc was conducted by the modified HPLC method using 1,2-diamino-4,5-methylenedioxybenzene (DMB) (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as previously described (5, 24). Briefly, the cells (50 μl) were mixed with 50 mM H2SO4 (75 μl) and 2 μM N-propylneuraminic acid (25 μl) as an internal control. After incubation at 80°C for 12 h (hydrolysis of the glycochain), the mixture was filtrated by centrifugation using Amicon Ultra centrifugal filters (Ultracel 3K membrane) (Millipore, Billerica, MA) at 15,100 × g for 90 min. The flowthrough (60 μl) was mixed with DMB reagent (60 μl) consisting of 7 mM DMB, 1 M β-mercaptoethanol, and 18 mM sodium hydrosulfite (Sigma Aldrich Corp., St. Louis, MO) in water. After incubation at 60°C for 2.5 h under protection from light (fluorescent derivatization of sialic acid), the mixture was cooled on ice (derivatization reaction stop). A 100-μl aliquot of the supernatant was injected into the HPLC system with a TSKgel ODS-100V 5-μm (4.6- by 150-mm) column (Tosoh Inc., Tokyo, Japan) at 40°C at a flow rate of 1.2 ml/min of methanol-water at 25:75 (vol/vol). The fluorescent intensity of sialic acid derivatives (excitation at 373 nm and emission at 448 nm) was measured by an FP-2020 Plus fluorescent detector (Jasco Corp., Tokyo, Japan). For the establishment of calibration curves, standard mixtures of Neu5Ac and Neu5Gc (0.1, 1, and 10 μM) (Sigma Aldrich Corp., St. Louis, MO) or water only were used. Each sample was compensated by the internal control.
Flow-cytometric analysis of anti-Neu5Gc staining on MCF7 cells and CMAH-MCF7 cells.
MCF7 cells and CMAH-MCF7 cells (2 × 105 cells/well) were cultured in a 12-well plate for 2 days at 37°C. After washing with 500 μl/well of PBS, the cells were harvested by treatment with 0.125% trypsin. The cells were centrifuged and suspended in 200 μl/well of PBS and then fixed by adding 200 μl/well of 4% PFA-PBS at room temperature for 10 min. The fixed cells were incubated with chicken anti-Neu5Gc antibody at room temperature for 30 min and FITC-conjugated rabbit anti-chicken IgY secondary antibody at room temperature for 30 min. Fluorescence for cells was excited with the 488-nm line of an argon laser on a FACSCanto II flow cytometer (BD, Franklin Lakes, NJ). At least 1 × 104 cells were analyzed for each sample. As a control, fixed cells were incubated with the secondary antibody only.
Flow-cytometric analysis of lectin staining on MCF7 cells and CMAH-MCF7 cells.
MCF7 cells and CMAH-MCF7 cells (4 × 105 cells/well) were cultured in a 12-well plate at 37°C for 24 h. After washing with 500 μl/well of PBS, the cells were harvested by treatment with 0.125% trypsin. The cells were centrifuged and suspended in 100 μl/well of PBS and then fixed by adding 300 μl/well of 4% PFA-PBS at room temperature for 15 min. The fixed cells were incubated with FITC-conjugated Sambucus nigra (elderberry) lectin SNA (Anaspec, Fremont, CA) in order to stain sialic acid of SAα2,6Gal or with FITC-conjugated Maackia amurensis lectin MAL-I (Anaspec, Fremont, CA) in order to stain sialic acid of SAα2,3Gal at room temperature for 30 min. Measurement in the absence of lectins was performed as a negative control. Fluorescence for cells was excited with the 488-nm line of an argon laser on a FACSCanto II flow cytometer. At least 1 × 104 cells were analyzed for each sample.
Measurement of viral infectivity by TCID50.
MCF7 cells and CMAH-MCF7 cells (2.5 × 104 cells/well) were cultured in a 96-well plate at 37°C for 24 h. The cells were washed with 100 μl of PBS and infected with a 10-times serial dilution of virus suspension in 100 μl/well of the serum-free medium hybridoma-SFM (SFM; Invitrogen Corp., Carlsbad, CA) from an undiluted-virus concentrated suspension at 37°C for 14 to 20 h. The cells were fixed with 50 μl/well of methanol for 30 s and washed with 100 μl/well of PBS. The infected cells were incubated with mouse anti-IAV nucleoprotein (NP) monoclonal antibody (4E6) (25, 26) at room temperature for 30 min and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and IgM secondary antibody (Jackson Immuno Research, West Grove, PA) at room temperature for 30 min, and then they were developed by adding H2O2, N,N-diethyl-p-phenylenediamine sulfate (Sigma Aldrich Corp., St. Louis, MO), and 4-chloro-1-naphtol (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as described previously (26). The 50% tissue culture infectious dose (TCID50) was calculated by counting virus infection-positive or -negative wells under an Olympus IX71 microscope.
Virus infection of MCF7 cells and CMAH-MCF7 cells.
MCF7 cells and CMAH-MCF7 cells (1 × 105 cells/well) were cultured in a 24-well plate at 37°C for 2 days. The cells were washed with 250 μl/well of PBS and infected with a virus suspension (9.39 × 103 to 1.27 × 105 TCID50 for MCF7 cells) in 250 μl/well of SFM at 37°C for 30 min. After washing with 250 μl/well of PBS, the infected cells were cultured in 500 μl/well of SFM at 37°C for 20 to 22 h. The cells were fixed with 400 μl/well of methanol for 30 s and washed with 400 μl/well of PBS. The infected cells were incubated with mouse anti-NP (4E6) at room temperature for 30 min and HRP-conjugated goat anti-mouse IgG and IgM secondary antibody at room temperature for 30 min and then developed as described for TCID50 measurement. All pictures were taken by using an Olympus IX71 microscope equipped with an Olympus DP70 camera at ×40 magnification.
Treatment of A549 cells with ManNGc.
To increase expression of Neu5Gc in a cell line other than MCF7 cells, human lung adenocarcinoma epithelial A549 cells were grown in the presence of Neu5Ac and Neu5Gc precursors (N-acetylmannosamine [ManNAc] and ManNGc, respectively). The cells (0.7 × 105 cells/well in a 24-well plate) were cultured in DMEM supplemented with 10% FBS at 37°C overnight. The adherent cells on a plate were washed with PBS and treated with 250 μl/well of SFM containing 10 mU/ml Arthrobacter ureafaciens sialidase (Marukin Bio, Kagawa, Japan) at 37°C for 1 h. After washing the cells with PBS, they were cultured in 500 μl/well of SFM in the presence of ManNAc (5 mM) or ManNGc (0.5 or 2 mM) at 37°C for 3 days. Control cells were cultured in SFM mixed with dimethyl sulfoxide (500 dilutions) in the same manner, because stock solutions of ManNAc and ManNGc were prepared in dimethyl sulfoxide and the dilution from stock solutions was 500 times at maximum.
For the infection experiment, after washing the cells with PBS, ManNAc- and ManNGc-treated cells were infected with 5 × 103 to 6 × 103 TCID50 of A/Memphis/102/72 (H3N2) and A/equine/Fontainebleau/1979 (H3N8) in 250 μl/well of SFM at 37°C for 30 min. After washing the cells with PBS, the cells were incubated in SFM at 37°C for 10 h. The cells were fixed with 400 μl/well of methanol for 30 s and washed with 400 μl/well of PBS. The infected cells were incubated with mouse anti-NP (4E6) at room temperature for 30 min and HRP-conjugated goat anti-mouse IgG and IgM secondary antibody at room temperature for 30 min, and then they were developed as described for TCID50 measurement. The number of infected cells was counted in eight arbitrary fields under an Olympus IX71 microscope at a magnification of ×100. Levels of infected cells in the ManNAc- and ManNGc-treated cell groups are expressed as percentages relative to the number of infected cells in the control group.
For flow-cytometric analysis, ManNAc- and ManNGc-treated cells were harvested by treatment with 0.125% trypsin after washing with PBS. The cells were centrifuged and suspended in 200 μl/well of PBS and then fixed by adding 200 μl/well of 4% PFA-PBS at room temperature for 10 min. The fixed cells were incubated with chicken anti-Neu5Gc antibody at room temperature for 30 min and FITC-conjugated rabbit anti-chicken IgY secondary antibody at room temperature for 30 min. Fluorescence for cells was excited with the 488-nm line of an argon laser on a FACSCanto II flow cytometer. At least 1 × 104 cells were analyzed for each sample. Mean fluorescence intensity (MFI) ratios of ManNAc- and ManNGc-treated cells were calculated relative to those of control cells.
Confocal microscopy.
MCF7 cells and CMAH-MCF7 cells (5.0 × 104 cells/well) were cultured in 8-well glass coverslips at 37°C for 24 h. After washing the cells with cold PBS, the cells were adsorbed with 30 μl/well of HA mutant virus WT or T155Y (5.0 × 105 TCID50) in cold DMEM on ice for 1.5 h. After washing the cells with cold PBS, the cells were incubated at 37°C for 10 min. After cooling the cells on ice for 5 min, the cells were fixed with 4% PFA-PBS (30 μl/well) at room temperature for 15 min and permeabilized with 0.05% Triton X-100-PBS (30 μl/well) at room temperature for 15 min. To visualize viral NP and cell outlines, the cells were reacted with mouse anti-NP (4E6) and biotinylated wheat germ agglutinin (WGA) (J-Oil Mills Inc., Tokyo, Japan) at 4°C for 1 h, followed by incubation with goat tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)-conjugated anti-mouse IgG (Sigma Aldrich Corp., St. Louis, MO), Cy5-conjugated streptavidin (GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, England), and DAPI for visualization of nuclei at 4°C for 1 h. After dropping the antifade reagent SlowFade onto the cells, the cells were observed by using an LSM510 meta confocal microscope (Carl Zeiss Inc., Thornwood, NY).
Flow-cytometric analysis of MAL-II staining on MCF7 cells and CMAH-MCF7 cells.
MCF7 cells and CMAH-MCF7 cells (4 × 105 cells/well) were cultured in a 12-well plate at 37°C for 24 h. After washing with 500 μl/well of PBS, the cells were harvested by treatment with 0.125% trypsin. The cells were centrifuged and suspended in 100 μl/well of PBS and then fixed by adding 300 μl/well of 4% PFA-PBS at room temperature for 15 min. The fixed cells were incubated with biotinylated Maackia amrensis lectin MAL-II (Vector Laboratories, Burlingame, CA) in order to stain sialic acid of SAα2,3Gal of O-glycans at room temperature for 30 min, followed by incubation with FITC-labeled streptavidin (Thermo Fisher Scientific Inc., Rockford, IL). The negative control was measured by incubation with FITC-labeled streptavidin only in the absence of MAL-II. Fluorescence for cells was excited with the 488-nm line of an argon laser on a FACSCanto II flow cytometer. At least 1 × 104 cells were analyzed for each sample.
RESULTS
Generation of human cells stably expressing the monkey CMAH gene.
To express Neu5Gc in human cells through introduction of functional CMAH (Fig. 1), we transfected the monkey CMAH gene from COS7 cells to human MCF7 cells. We obtained a single cell clone that stably expressed the monkey CMAH gene (CMAH-MCF7 cells) by selection and cloning in the presence of the antibiotic reagent hygromycin B. These MCF7 cells have the Tet-On system, which is able to induce expression of the gene of interest by the antibiotic reagent doxycycline. In MCF7 cells and CMAH-MCF7 cells in the absence or presence of doxycycline, endogenous expression levels of monkey CMAH mRNA were measured by quantitative real-time PCR using a primer specific for monkey CMAH mRNA, corresponding to a 92-bp nucleotide deletion in human CMAH mRNA (Fig. 2A). The monkey CMAH mRNA was detected in CMAH-MCF7 cells but not in MCF7 cells (Fig. 2B). Since monkey CMAH mRNA was detected without doxycycline and its expression level was 2.2 times higher in the presence of doxycycline than in the absence of doxycycline, CMAH-MCF7 cells were used after culture in the presence of doxycycline.
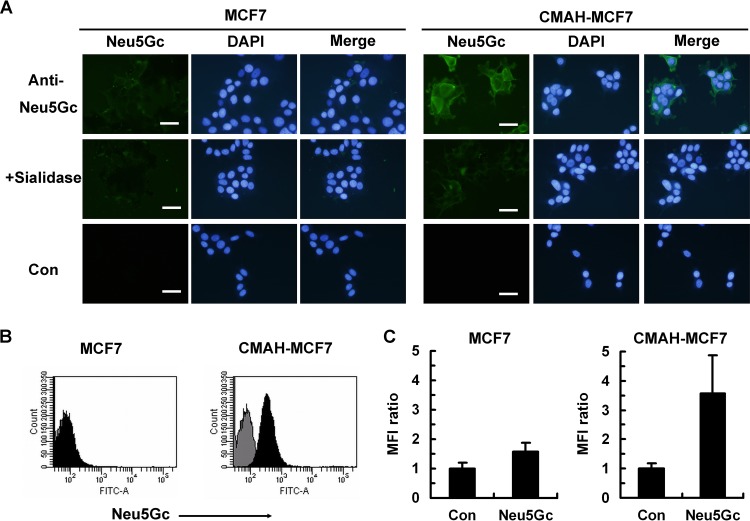
Detection of Neu5Gc expression in CMAH-MCF7 cells.
To confirm Neu5Gc expression in CMAH-MCF7 cells, we performed immunostaining of Neu5Gc and chemical analysis of sialic acid molecular species using HPLC. The presence of Neu5Gc was observed by a chicken anti-Neu5Gc antibody, which recognized Neu5Gc of galactose by the α2,3 linkage (Neu5Gcα2,3Gal) but not Neu5Ac of galactose by the α2,3 linkage (Neu5Acα2,3Gal) (2, 9, 22, 23) in CMAH-MCF7 cells but not in MCF7 cells (Fig. 3A). Sialidase treatment of CMAH-MCF7 cells diminished fluorescent staining of Neu5Gc, indicating that the antibody binding depended on sialic acid. We also measured the fluorescent signal of Neu5Gcα2,3Gal detected by the antibody using a flow cytometer. The flow-cytometric data may result in loss of sialic acid-containing glycopeptides because of the lack of recovery time for the cells after trypsin treatment. However, the Neu5Gcα2,3Gal signal on CMAH-MCF7 cells was clearly stronger than that on the MCF7 cells (Fig. 3B). MFI ratios of the Neu5Gcα2,3Gal signal were 3.57 and 1.57 on CMAH-MCF7 cells and MCF7 cells, respectively (Fig. 3C). Fluorescent derivatives of standard Neu5Ac and Neu5Gc were detected as separable peaks by HPLC (Fig. 4A). The level of Neu5Gc was hardly detectable in MCF7 cells (Fig. 4B), whereas abundant Neu5Gc was observed in CMAH-MCF7 cells (Fig. 4C). The Neu5Gc content in CMAH-MCF7 cells was slightly higher than that of Neu5Ac (Table 1). On the other hand, the Neu5Ac content was slightly lower than that in MCF7 cells, possibly due to consumption of Neu5Ac as a substrate of CMAH. Although a small amount of Neu5Gc was detected in MCF7 cells, it is likely that it was derived from incorporation of sialic acids into cells from 10% FBS in the culture medium (14), in which the ratio of Neu5Ac to Neu5Gc was shown by HPLC analysis to be 96.9:3.09.
FIG 3.
Anti-Neu5Gc antibody staining on MCF7 cells and CMAH-MCF7 cells. (A) Fluorescent immunostaining of Neu5Gc expression on MCF7 cells and CMAH-MCF7 cells. Cells were fixed with 4% paraformaldehyde (PFA). Neu5Gc was stained with chicken anti-Neu5Gc antibody and fluorescein isothiocyanate (FITC)-labeled anti-chicken IgY. Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). Sialidase treatment was performed at 37°C for 1 h before fixation of cells. Bars, 50 μm. (B) Flow-cytometric histogram of Neu5Gc expression on MCF7 cells and CMAH-MCF7 cells. The cells were stained with anti-Neu5Gc antibody (filled histogram) or a secondary antibody only (gray histogram) after fixation with 2% PFA. (C) Mean fluorescence intensity (MFI) ratio of Neu5Gc expression by flow cytometry. MFI ratios were calculated as proportions of levels for the control (secondary antibody only). Standard deviations were calculated from data from three independent experiments.
FIG 4.
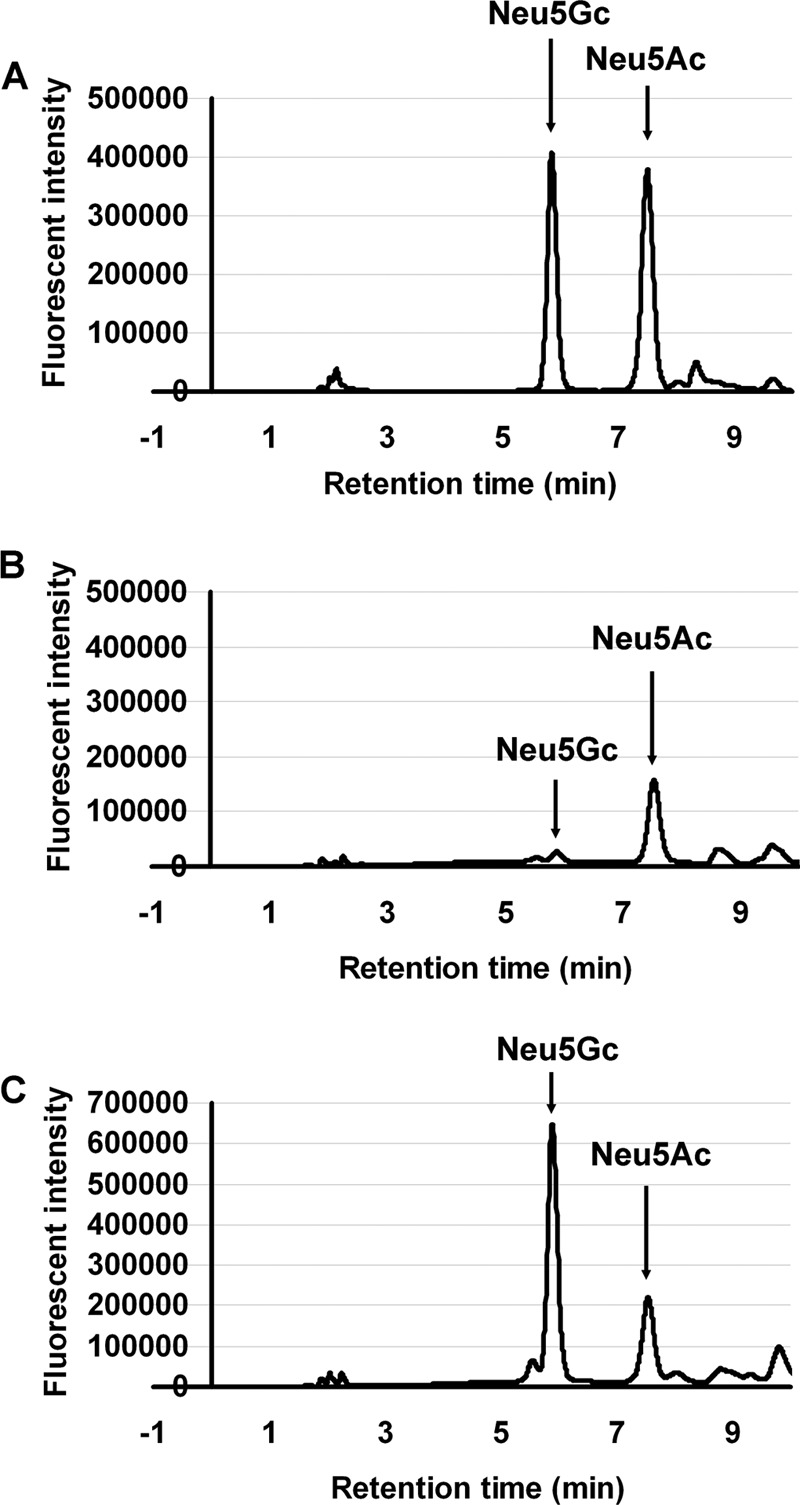
HPLC analysis of Neu5Gc and Neu5Ac contents in MCF7 cells and CMAH-MCF7 cells. (A) Standard peaks of Neu5Gc and Neu5Ac on HPLC. Peaks for Neu5Gc and Neu5Ac are indicated by arrows at retention times of 5.9 min and 7.5 min, respectively. (B) HPLC analysis of MCF7 cells. (C) HPLC analysis of CMAH-MCF7 cells.
TABLE 1.
Contents of Neu5Gc and Neu5Ac in MCF7 cells and CMAH-MCF7 cellsa
| Cell line | Sialic acid content (±SD; nmol/mg of protein) |
Neu5Gc/Neu5Ac ratio | |
|---|---|---|---|
| Neu5Gc | Neu5Ac | ||
| MCF7 | 0.15 ± 0.03 | 1.90 ± 0.27 | 0.08 |
| CMAH-MCF7 | 1.71* ± 0.28 | 1.54 ± 0.70 | 1.11 |
Standard deviations (SD) were calculated from data from three independent experiments. *, P < 0.05 by independent t test for MCF7 cells versus CMAH-MCF7 cells.
Flow-cytometric analysis of SAα2,6Gal linkage and SAα2,3Gal linkage showed that the MFI of SAα2,6Gal linkage on CMAH-MCF7 cells was increased compared to that on MCF7 cells, but MFIs of SAα2,3Gal linkage on MCF7 cells and CMAH-MCF7 cells were similar (Fig. 5). These fluorescent intensities contain both Neu5Ac and Neu5Gc as terminal sialic acids because of recognition of both Neu5Ac and Neu5Gc by the lectins SNA and MAL-I (27). However, MAL-I cannot detect SAα2,3Gal linkage of O-glycan, which is recognized by MAL-II (28). MFI ratios with MAL-II were 9.19 for MCF7 cells and 12.84 for CMAH-MCF7 cells (data not shown). MAL-II shows 1.49 times stronger binding to Neu5Gcα2,3Galβ1,3GalNAc than to Neu5Acα2,3Galβ1,3GalNAc (28), which are known to be in the core 1 structure of O-glycan. MAL-II binding signal on CMAH-MCF7 cells is 1.40 times higher than that on MCF7 cells. Therefore, terminal sialic acids of most O-glycans may convert from Neu5Ac to Neu5Gc.
FIG 5.
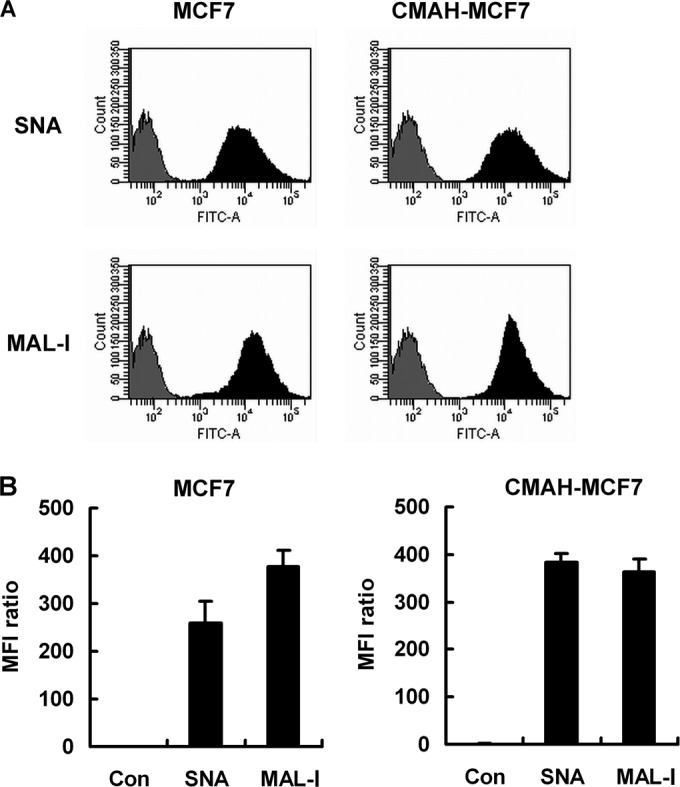
Flow-cytometric analysis of SNA or MAL-I lectin staining on MCF7 cells and CMAH-MCF7 cells. (A) Flow-cytometric histogram of SNA or MAL-I lectin staining on MCF7 cells and CMAH-MCF7 cells. The cells were stained with each lectin (filled histogram) or no lectin (gray histogram) as a control after fixation with 3% PFA. (B) MFI ratios of SNA or MAL-I staining by flow cytometry. MFI ratios were calculated relative to the levels for control cells. Standard deviations were calculated from data from four independent experiments.
Comparison of the infectivity of IAVs in MCF7 cells to that in CMAH-MCF7 cells.
We compared the numbers of infected MCF7 cells and CMAH-MCF7 cells inoculated with two human IAVs, A/Memphis/1/1971 (H3N2) and A/Memphis/102/1972 (H3N2), and two equine IAVs, A/equine/Fontainebleau/1/1979 (H3N8) and A/equine/Tennessee/5/1986 (H3N8). A/Memphis/102/1972 (3, 4) and the two equine IAVs (2), but not A/Memphis/1/1971 (4, 5, 19), have been shown to bind to Neu5Gcα2,6Gal and Neu5Gcα2,3Gal, respectively. These three Neu5Gc-binding IAVs, A/Memphis/102/1972, A/equine/Fontainebleau/1/1979, and A/equine/Tennessee/5/1986, showed 36.3%, 16.8%, and 14.0% relative infectivity, respectively, in CMAH-MCF7 cells and 100% infectivity in MCF7 cells. Infectivity of A/Memphis/1/1971 in CMAH-MCF7 cells was similar to that in the MCF7 cells (Fig. 6). Furthermore, we compared the TCID50 values of each of three human IAVs and four equine IAVs in MCF7 cells and CMAH-MCF7 cells. Human IAV A/Aichi/2/1968 (H3N2) binds mainly to Neu5Gcα2,6Gal and sometimes to Neu5Gcα2,3Gal (4). Equine IAVs A/equine/Miami/1963 (H3N8) (2) and A/equine/Ibaraki/1/2007 (H3N8) (29) also bind to Neu5Gcα2,3Gal. The TCID50 of A/equine/Ibaraki/1/2007 was 5.41 in MCF7 cells, but it could not be measured in CMAH-MCF7 cells because of low infectivity. All Neu5Gc-binding IAVs showed a significant reduction of TCID50 in CMAH-MCF7 cells compared to that in MCF7 cells, but the TCID50 of A/Memphis/1/1971 was not reduced (Table 2). For Neu5Gc-bnding IAVs, Neu5Gc expression had a negative effect on infectivity in host cells.
FIG 6.
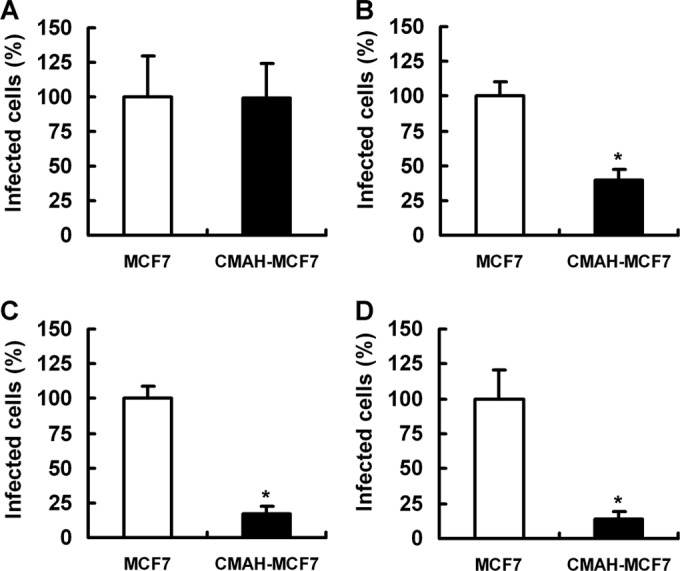
Infectivity of IAVs in MCF7 cells and CMAH-MCF7 cells. Cells were infected with A/Memphis/1/1971 (H3N2) (A), A/Memphis/102/1972 (H3N2) (B), A/equine/Fontainebleau/1/1979 (H3N8) (C), or A/equine/Tennessee/5/1986 (H3N8) (D). Infected cells were fixed with methanol and stained with anti-NP monoclonal antibody. Numbers of infected cells were counted in six arbitrary fields per well. The number of infected CMAH-MCF7 cells is expressed as a percentage relative to the level of infection in MCF7 cells. *, P < 0.01 by independent t test for percentages of infected MCF7 cells compared to the level of CMAH-MCF7 cell infection.
TABLE 2.
TCID50 values of IAVs to MCF7 and CMAH-MCF7 cellsa
| Virus strain | TCID50/ml (±SD; log10) for: |
TCID50 ratio (CMAH-MCF7/MCF7) | |
|---|---|---|---|
| MCF7 | CMAH-MCF7 | ||
| Human | |||
| A/Aichi/2/1968 (H3N2) | 9.56 ± 0.51 | 9.22 ± 0.48 | 0.46 |
| A/Memphis/1/1971 (H3N2) | 5.67 ± 0.00 | 5.67 ± 0.29 | 1.00 |
| A/Memphis/102/1972 (H3N2) | 8.83 ± 0.44 | 8.08 ± 0.36 | 0.18 |
| Equine (Eq) | |||
| A/Eq/Miami/1963 (H3N8) | 9.83 ± 0.29 | 8.89 ± 0.19 | 0.11 |
| A/Eq/Fontainebleau/1/1979 (H3N8) | 7.39 ± 0.35 | 6.06 ± 0.42 | 0.05 |
| A/Eq/Tennessee/5/1986 (H3N8) | 8.61 ± 0.10 | 7.78 ± 0.19 | 0.15 |
SD were calculated from data from three independent experiments. TCID50 ratios are presented as values for CMAH-MCF7 cells relative to those for MCF7 cells as control cells.
To confirm the effect of Neu5Gc expression on virus infectivity, A549 cells, which are human lung adenocarcinoma epithelial cells and are often used in experiments on IAV infection, were cultured in the presence of Neu5Ac and Neu5Gc precursors, ManNAc and ManNGc, respectively. The infectivity of A/Memphis/102/1972 (H3N2) and A/equine/Fontainebleau/1/1979 (H3N8) was decreased in ManNGc-treated cells, but not in ManNAc-treated cells, compared to that in nontreated cells (Fig. 7A). Neu5Gc expression on the cell surface with ManNGc was confirmed by flow-cytometric analysis (Fig. 7B and C). The effect of Neu5Gc expression in A549 cells infected with IAVs was similar to that of MCF7 cells. The results indicate that expression of Neu5Gc on the surface of human epithelial cells suppresses infection of IAVs with Neu5Gc binding ability.
FIG 7.
Virus infectivity and Neu5Gc expression in A549 cells treated with ManNGc. A549 cells were cultured in SFM containing ManNGc or ManNAc at 37°C for 3 days. Control cells (Con) were cultured in SFM in the absence of mannosamine at 37°C for 3 days. (A) Virus infectivity in ManNAc- and ManNGc-treated A549 cells. The cells were infected with each virus and cultured at 37°C for 10 h. After fixation of the cells with methanol, infected cells were stained with anti-NP monoclonal antibody and counted. Percentages of infected cells in ManNAc- and ManNGc-treated cells are expressed as a percentage relative to the number of infected cells in the control cell group. (B) Flow-cytometric histogram of anti-Neu5Gc antibody staining on the surface of ManNAc- and ManNGc-treated A549 cells. Neu5Gc on the surface of cells was stained with anti-Neu5Gc antibody after fixation with 2% PFA. 2nd antibody indicates results for control cells treated with a secondary antibody only without reaction of anti-Neu5Gc antibody. (C) MFI ratios of anti-Neu5Gc antibody staining on the surface of ManNAc- and ManNGc-treated A549 cells by flow cytometry. MFI ratios were calculated relative to levels for control cells. Standard deviations were calculated from data from four independent experiments. *, P < 0.01 by independent t test compared to results for control cells.
Comparison of infectivities of HA mutant IAVs that have acquired Neu5Gc binding ability in MCF7 cells and CMAH-MCF7 cells.
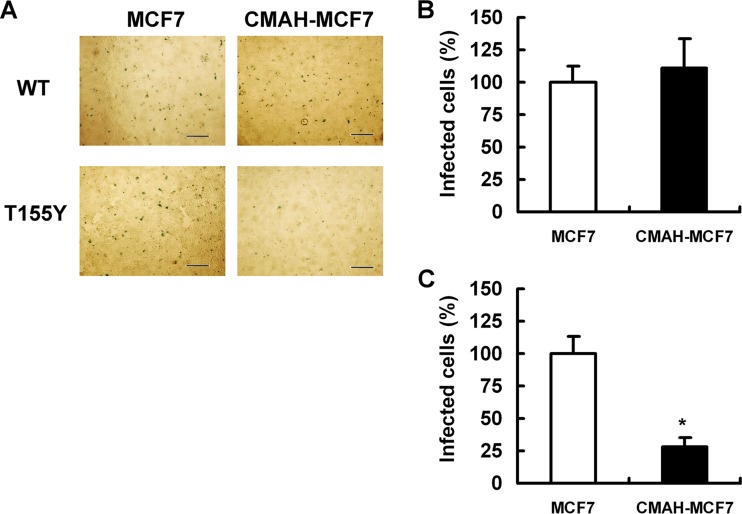
We previously showed that amino acid alteration of threonine to tyrosine at position 155 in H3 HA facilitated virus binding to Neu5Gc. We generated two HA mutant IAVs, wild-type HA of A/Memphis/1/1971 (named WT) and HA with a single-amino-acid change of threonine to tyrosine at position 155 (named T155Y), together with the backbone of the A/WSN/1933 strain (19). T155Y strongly bound to Neu5Gcα2,6Gal linkage in addition to Neu5Acα2,6Gal linkage (18). In contrast, WT bound to Neu5Acα2,6Gal linkage but not to Neu5Gcα2,6Gal, Neu5Gcα2,3Gal, and Neu5Acα2,3Gal linkages, as in the case of A/Memphis/1/1971 (19). We compared the numbers of MCF7 cells and CMAH-MCF7 cells infected by these HA mutant IAVs. T155Y showed 27.9% relative infectivity in CMAH-MCF7 cells and 100% infectivity in MCF7 cells. The infectivity of WT in CMAH-MCF7 cells was similar to that in MCF7 cells (Fig. 8). We also compared TCID50 values of these HA mutant IAVs in MCF7 cells to those in CMAH-MCF7 cells. T155Y showed a significant reduction of TCID50 in CMAH-MCF7 cells compared to the level in MCF7 cells, but WT did not (Table 3).
FIG 8.
Infectivity of HA mutant IAVs WT and T155Y in MCF7 cells and CMAH-MCF7 cells. (A) Images of infected cells inoculated with the indicated viruses. Cells were infected with HA mutant IAVs WT (B) and T155Y (C). Infected cells were fixed with methanol and stained with anti-NP monoclonal antibody. Bars are 500 μm. (B and C) Numbers of infected cells were counted in six arbitrary fields per well. The number of infected cells in the CMAH-MCF7 cell group is expressed as a percentage relative to the level of infection in MCF7 cells. *, P < 0.01 by independent t test for percentages of infected MCF7 cells versus infected CMAH-MCF7 cells.
TABLE 3.
TCID50 values of HA mutant IAVs WT and T155Y for MCF7 and CMAH-MCF7 cellsa
| Mutant virus | Neu5Gc binding | TCID50/ml (±SD; log10) for: |
TCID50 ratio (CMAH-MCF7/MCF7) | |
|---|---|---|---|---|
| MCF7 | CMAH-MCF7 | |||
| WT | − | 8.08 ± 0.38 | 8.22 ± 0.19 | 1.37 |
| T155Y | + | 8.61 ± 0.35 | 7.89 ± 0.19 | 0.19 |
SD were calculated from data from three independent experiments. TCID50 ratios are presented as values for CMAH-MCF7 cells relative to those for MCF7 cells as control cells.
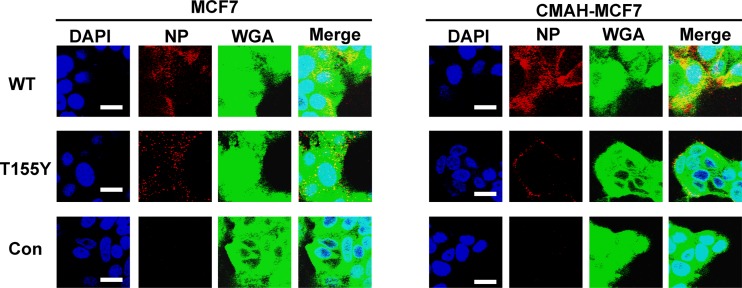
IAV enters cells through endocytosis after binding to receptors on the cell surface membranes. We observed entry of WT and T155Y into the cells. WT and T155Y were observed in MCF7 cells by incubation at 37°C for 10 min. WT entered CMAH-MCF7 cells during that incubation, but T155Y was observed mainly on the cell surface (Fig. 9). These results indicate that Neu5Gc, which was expressed on the surface of human epithelial cancer cells, inhibits infection of IAV that has acquired Neu5Gc binding ability through suppression of entry into the cells.
FIG 9.
Entry of WT and T155Y into MCF7 cells and CMAH-MCF7 cells. After adsorption of the virus to the cells on ice, the cells were incubated at 37°C for 10 min. The cells were fixed with 4% PFA and permeabilized with 0.05% Triton X-100. Bars are 10 μm. DAPI, blue; viral NP, red; WGA, green.
DISCUSSION
Neu5Gc has been thought to play an important role in IAV infection in horses and ducks (2, 9). Humans cannot synthesize Neu5Gc due to inactivation of CMAH. However, exogenous Neu5Gc from Neu5Gc-rich dietary sources (particularly red meat and milk) is metabolically incorporated into human tissue cells (14, 15). Therefore, we evaluated the receptor function of Neu5Gc on IAV infection in human epithelial cells by employing glycobiological and virological approaches: use of Neu5Gc-expressing cells and HA mutant IAVs that have acquired Neu5Gc binding ability. Our results indicated that Neu5Gc expressed on the surface of human epithelial cancer cells is not a functional receptor and that it has a negative effect on infectivity of IAV possessing Neu5Gc binding ability. Bateman et al. also reported that addition of ManNGc, a material of the biosynthesis pathway to Neu5Gc, to primary swine respiratory epithelial cells inhibited infection of six swine IAVs, emphasizing the importance of Neu5Ac as a functional receptor of swine IAV (30). Two swine IAVs [A/swine/Minnesota/593/1999 (H3N2) and A/swine/Ontario/00130/1997 (H3N2)] in that study showed no binding to Neu5Gcα2,3Gal linkage of 5 glycoconjugates and Neu5Gcα2,6Gal linkage of 3 glycoconjugates on a glycoarray. Avian IAV specifically binds to Neu5Acα2,3Gal linkage regardless of the structure of the asialo portion. In contrast, human IAV preferentially binds to long Neu5Acα2,6Gal glycan with penta- or heptasaccharides in a glycan length-dependent manner (31). In our previous study, Neu5Ac and Neu5Gc binding abilities of human IAVs and HA mutant IAVs, including IAVs used in the present study, were determined by pentasaccharides containing terminal Neu5Ac or Neu5Gc (4, 19). A glycoarray reported by Bateman et al. uses mono- to trisaccharides (except an internal linkage of fucose) containing terminal Neu5Gc. Since the property of swine IAV (especially the human-lineage IAV A/swine/Ontario/00130/1997) is thought to be close to that of human IAV rather than avian IAV, these two swine IAVs may have binding activity to penta- to heptasaccharides containing terminal Neu5Gc.
Generally, human IAV also shows preferential binding to SAα2,6Gal linkage, whereas avian and equine IAVs show preferential binding to SAα2,3Gal. Swine IAV shows binding to both SAα2,6Gal and SAα2,3Gal linkages (1). Flow-cytometric analysis of SAα2,6Gal linkage and SAα2,3Gal linkage showed that MFI of SAα2,6Gal linkage on CMAH-MCF7 cells was increased compared to that on MCF7 cells, but MFIs of SAα2,3Gal linkage on MCF7 cells and CMAH-MCF7 cells were similar (Fig. 5). These fluorescent intensities contain both Neu5Ac and Neu5Gc as terminal sialic acids. Possible reasons for the increased MFI of SAα2,6Gal linkage on CMAH-MCF7 cells but similar MFIs of SAα2,3Gal linkage on both MCF7 cells and CMAH-MCF7 cells are the following. (i) α2,6-Sialyltransferases are more sensitive to CMP-Neu5Gc than to CMP-Neu5Ac. α2,3-Sialyltransferases have similar reactivities for CMP-Neu5Gc and CMP-Neu5Ac. (ii) Increased expression of CMAH and/or CMP-Neu5Gc increases the expression of α2,6-sialyltransferases and has no effect on the expression of α2,3-sialyltransferases. (iii) The degradation rate of SAα2,6Gal linkage but not that of SAα2,3Gal linkage was decreased by Neu5Gc expression. (iv) The difference is derived from reactivities of SNA and MAL-I. According to supplemental material of Song et al. (27), SNA bound to Neu5Gc- and Neu5Ac-α2,6Galβ1,4GlcNAcβ1,2Manα1,3(Neu5Gc- and Neu5Ac-α2,6Galβ1,4GlcNAcβ1,2Manα1,6)Manβ1,4GlcNAcβ1,4GlcNAcitol (with binding signals being 26,327 and 22,218, respectively, for 10 μg/ml SNA) and to Neu5Gc- and Neu5Ac-α2,6Galβ1,4GlcNAcβ1,3Galβ1,4Glcitol (with binding signals being 8,544 and 9,187, respectively, for 10 μg/ml SNA) but not to Neu5Gc- and Neu5Ac-α2,6Galβ1,3GlcNAcβ1,3Galβ1,4Glcitol (with binding signals being 56 and 96, respectively, for 10 μg/ml SNA). Similar structures containing Neu5Gc or Neu5Ac are suggested to be recognized equivalently by SNA. However, if CMAH expression and/or Neu5Gc expression results in an increase of SAα2,6Galβ1,4GlcNAc and/or decrease of SAα2,6Galβ1,3GlcNAc, use of SNA would increase the MFI of SAα2,6Gal linkage on CMAH-MCF7 cells. MAL-I bound to Neu5Gc- and Neu5Ac-α2,3Galβ1,4GlcNAcβ1,2Manα1,3(Neu5Gc- and Neu5Ac-α2,3Galβ1,4GlcNAcβ1,2Manα1,6)Manβ1,4GlcNAcβ1,4GlcNAcitol (with binding signals being 3,383 and 15,783, respectively, for 10 μg/ml MAL-I) and to Neu5Gc- and Neu5Ac-α2,3Galβ1,4GlcNAcβ1,3Galβ1,4Glcitol (with binding signals being 4,635 and 3,200, respectively, for 10 μg/ml MAL-I) but not to Neu5Gc- and Neu5Ac-α2,3Galβ1,3GlcNAcβ1,3Galβ1,4Glcitol (with binding signals being 27 and 6, respectively, for 10 μg/ml MAL-I). Neu5Gc binding of MAL-I is estimated to be about 4 to 5 times weaker than Neu5Ac binding. As one example, even though MFIs of MAL-I are similar on both MCF7 cells and CMAH-MCF7 cells, the Neu5Gcα2,3Gal expression level on CMAH-MCF7 cells may be much higher than the Neu5Acα2,3Gal expression level. As another example, CMAH expression may result in no change in the total amount of SAα2,3Galβ1,4GlcNAc but an increase in Neu5Gcα2,3Galβ1,3GlcNAc. Although relative expression of SAα2,6Gal linkage appeared to be increased in CMAH-MCF7 cells, human IAV A/Memphis/1/1971, which was reported to preferentially bind to Neu5αAc2,6Gal linkage (4, 19), showed no difference in infectivity between MCF7 cells and CMAH-MCF7 cells. Therefore, an increased level of SAα2,6Gal linkage does not affect the infectivity of IAV showing only Neu5Ac binding. The HA mutant IAV T155Y, which has binding ability for Neu5Gcα2,6Gal linkage in addition to Neu5Acα2,6Gal linkage, showed decreased infectivity in CMAH-MCF7 cells (Fig. 8 and Table 3). The results indicate that the decrease of viral infectivity in CMAH-MCF7 cells probably is due to Neu5Gc expression rather than differences in sialic acid linkage to galactose. Additionally, in CMAH-MCF7 cells and ManNGc-treated A549 cells, an obvious reduction in infectivity of equine IAVs, which show preferential binding to Neu5Gc rather than Neu5Ac, is thought to be dependent on Neu5Gc expression (Fig. 6C, 6D, and 7A and Table 2).
IAV neuraminidase (NA) hydrolyzes α-glycosidic linkages of terminal sialic acid residues. Sialidase activity of NA plays a critical role not only in release of progeny virus from IAV-infected cells (32, 33) but also in the initial stage of IAV infection (34–37). Human airway epithelial cells secrete mucins that protect them from pathogenic insult (38). Matrosovich et al. demonstrated that NA was important for the initiation of IAV infection in cultures of primary human tracheobronchial and nasal epithelial cells from inhibition of IAV infection by pretreatment of IAV with the NA inhibitor oseltamivir carboxylate (34). Ohuchi et al. reported that NA inhibitors prevented IAV entry at the initial stage into A549 cells and Madin-Darby canine kidney cells, which exhibit no mucin secretion (37). Vries et al. showed that NA inhibitors strongly reduced IAV entry into N-acetylglucosamine transferase 1-deficient cells lacking sialylated N-glycans (expressing sialylated O-glycans and gangliosides) in the presence of serum decoy receptors, including sialoglycoconjugates (36). These reports indicate that NA contributes to IAV access to functional receptors in the entry step to target cells without trapping by sialo-decoy receptors on mucins, serum, and cellular glycocalix. In a previous study, we investigated the substrate specificities of NA for sialyl linkages and different molecular species of sialic acids. Avian viruses and most of the human IAVs tested exhibited low specificity for Neu5Acα2,6Gal linkage (5 to 35% of Neu5Acα2,3Gal in sialidase activity) versus Neu5Acα2,3Gal linkage and low specificity for Neu5Gcα2,3Gal linkage (5 to 35% of Neu5Acα2,3Gal in sialidase activity of avian and some human IAVs, including A/Aichi/2/1968) versus Neu5Acα2,3Gal linkage (39). Therefore, our results suggest that IAVs possessing Neu5Gc binding ability were trapped by Neu5Gc-containing decoy receptors, which are not susceptible to sialidase activity of IAV and are not functional receptors for the entry step of IAV infection in CMAH-MCF7 cells and Neu5Gc-expressing A549 cells.
How does Neu5Gc function as a decoy receptor of IAV possessing Neu5Gc binding ability? Infection of IAV requires endocytosis pathways. Neu5Gc expression arrested IAV on the cell surface. The data suggest that endocytosis pathways are not induced by IAV binding to Neu5Gc. After IAV binding to cellular receptors, IAV endocytosis may be associated with some glycoconjugates linked with Neu5Ac but not Neu5Gc. The role of sialic acids in endocytosis remains unknown. Alternatively, there may be an endocytosis initiation switch on HA, which functions for binding to Neu5Ac but not for binding to Neu5Gc. If 10% of the IAV surface makes contact with the cell surface, it is estimated that 600 to 4,500 sialic acids would be covered by 50 HAs on an IAV particle (40). An IAV particle possessing Neu5Gc binding ability should bind to the sialic acids, including both Neu5Ac and Neu5Gc, on the cell surface of Neu5Gc-expressing cells. When an IAV particle increases the ratio of Neu5Gc binding as sialic acid on Neu5Gc-expressing cells, the virus may less efficiently proceed to endocytosis. In primary swine respiratory epithelial cells that naturally express Neu5Gc, Neu5Gc expression with ManNGc treatment also suppresses swine IAV infection (30). Neu5Gc is thought to function as a decoy receptor of IAV not only in human cells but also at least in swine cells. Neu5Gc expression in swine respiratory epithelial cells may be a factor that protects against transmission of human IAV possessing Neu5Gc binding activity, resulting in avoiding the occurrence of a new subtype human IAV by genetic reassortment between human IAV and animal/avian IAV, which has a pandemic potential in humans. Further studies are needed to delineate the mechanism of the decoy function of Neu5Gc.
In the present study, Neu5Gc is suggested to work as a decoy receptor of Neu5Gc-binding IAVs but not a functional receptor for IAV infection. Human and avian IAVs are subjected to restriction for targeting to a certain type of primary culture human airway epithelial cell, depending on viral preferential binding ability and cell expression of SAα2,3Gal and SAα2,6Gal linkages (41). The expression of Neu5Gc on human epithelial cells by taking in exogenous Neu5Gc from dietary sources may be related to the restriction of infection of IAVs that have acquired Neu5Gc binding ability.
ACKNOWLEDGMENTS
This work was supported by a Sasakawa Scientific Research Grant from the Japan Science Society (23-439), an SRI (Shizuoka Research Institute) academic research grant, Hokuto Foundation for Bioscience, a research grant from Institution for Fermentation, Osaka, MEXT/JSPS KAKENHI (Scientific Research C, 23590549; challenging Exploratory Research, 26670064), and the Global COE Program from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Suzuki Y. 2005. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 28:399–408. 10.1248/bpb.28.399 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Ito T, Suzuki T, Holland RE, Jr, Chambers TM, Kiso M, Ishida H, Kawaoka Y. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74:11825–11831. 10.1128/JVI.74.24.11825-11831.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers GN, Herrler G, Paulson JC, Klenk HD. 1986. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J. Biol. Chem. 261:5947–5951 [PubMed] [Google Scholar]
- 4.Masuda H, Suzuki T, Sugiyama Y, Horiike G, Murakami K, Miyamoto D, Hidari KI, Ito T, Kida H, Kiso M, Fukunaga K, Ohuchi M, Toyoda T, Ishihama A, Kawaoka Y, Suzuki Y. 1999. Substitution of amino acid residue in influenza A virus hemagglutinin affects recognition of sialyl-oligosaccharides containing N-glycolylneuraminic acid. FEBS Lett. 464:71–74. 10.1016/S0014-5793(99)01575-6 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Horiike G, Yamazaki Y, Kawabe K, Masuda H, Miyamoto D, Matsuda M, Nishimura SI, Yamagata T, Ito T, Kida H, Kawaoka Y, Suzuki Y. 1997. Swine influenza virus strains recognize sialylsugar chains containing the molecular species of sialic acid predominantly present in the swine tracheal epithelium. FEBS Lett. 404:192–196. 10.1016/S0014-5793(97)00127-0 [DOI] [PubMed] [Google Scholar]
- 6.Gambaryan AS, Karasin AI, Tuzikov AB, Chinarev AA, Pazynina GV, Bovin NV, Matrosovich M. 2005. Receptor-binding properties of swine influenza viruses isolated and propagated in MDCK cells. Virus Res. 114:15–22. 10.1016/j.virusres.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 7.Takemae N, Ruttanapumma R, Parchariyanon S, Yoneyama S, Hayashi T, Hiramatsu H, Sriwilaijaroen N, Uchida Y, Kondo S, Yagi H, Kato K, Suzuki Y, Saito T. 2010. Alterations in receptor-binding properties of swine influenza viruses of the H1 subtype after isolation in embryonated chicken eggs. J. Gen. Virol. 91:938–948. 10.1099/vir.0.016691-0 [DOI] [PubMed] [Google Scholar]
- 8.Bradley KC, Jones CA, Tompkins SM, Tripp RA, Russell RJ, Gramer MR, Heimburg-Molinaro J, Smith DF, Cummings RD, Steinhauer DA. 2011. Comparison of the receptor binding properties of contemporary swine isolates and early human pandemic H1N1 isolates (novel 2009 H1N1). Virology 413:169–182. 10.1016/j.virol.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Suzuki Y, Suzuki T, Takada A, Horimoto T, Wells K, Kida H, Otsuki K, Kiso M, Ishida H, Kawaoka Y. 2000. Recognition of N-glycolylneuraminic acid linked to galactose by the α2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J. Virol. 74:9300–9305. 10.1128/JVI.74.19.9300-9305.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchmore EA, Milewski M, Varki A, Diaz S. 1989. Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool. J. Biol. Chem. 264:20216–20223 [PubMed] [Google Scholar]
- 11.Kawano T, Kozutsumi Y, Kawasaki T, Suzuki A. 1994. Biosynthesis of N-glycolylneuraminic acid-containing glycoconjugates. Purification and characterization of the key enzyme of the cytidine monophospho-N-acetylneuraminic acid hydroxylation system. J. Biol. Chem. 269:9024–9029 [PubMed] [Google Scholar]
- 12.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. U. S. A. 95:11751–11756. 10.1073/pnas.95.20.11751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varki A. 2001. N-glycolylneuraminic acid deficiency in humans. Biochimie 83:615–622. 10.1016/S0300-9084(01)01309-8 [DOI] [PubMed] [Google Scholar]
- 14.Bardor M, Nguyen DH, Diaz S, Varki A. 2005. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J. Biol. Chem. 280:4228–4237. 10.1074/jbc.M412040200 [DOI] [PubMed] [Google Scholar]
- 15.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. 2003. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. U. S. A. 100:12045–12050. 10.1073/pnas.2131556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Han C, Wang X, Lin J, Ma M, Shu Y, Zhou J, Yang H, Liang Q, Guo C, Zhu J, Wei H, Zhao J, Ma Z, Pan J. 2009. Influenza A virus receptors in the respiratory and intestinal tracts of pigeons. Avian Pathol. 38:263–266. 10.1080/03079450903055363 [DOI] [PubMed] [Google Scholar]
- 17.Yasuda J, Shortridge KF, Shimizu Y, Kida H. 1991. Molecular evidence for a role of domestic ducks in the introduction of avian H3 influenza viruses to pigs in southern China, where the A/Hong Kong/68 (H3N2) strain emerged. J. Gen. Virol. 72:2007–2010. 10.1099/0022-1317-72-8-2007 [DOI] [PubMed] [Google Scholar]
- 18.Anders EM, Scalzo AA, Rogers GN, White DO. 1986. Relationship between mitogenic activity of influenza viruses and the receptor-binding specificity of their hemagglutinin molecules. J. Virol. 60:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Hashimoto A, Maruyama M, Ishida H, Kiso M, Kawaoka Y, Suzuki Y, Suzuki T. 2009. Identification of amino acid residues of influenza A virus H3 HA contributing to the recognition of molecular species of sialic acid. FEBS Lett. 583:3171–3174. 10.1016/j.febslet.2009.08.037 [DOI] [PubMed] [Google Scholar]
- 20.Kagawa S, Gu J, Honda T, McDonnell TJ, Swisher SG, Roth JA, Fang B. 2001. Deficiency of caspase-3 in MCF7 cells blocks Bax-mediated nuclear fragmentation but not cell death. Clin. Cancer Res. 7:1474–1480 http://clincancerres.aacrjournals.org/content/7/5/1474.long [PubMed] [Google Scholar]
- 21.Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzahn T, Pleschka S, Ludwig S. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 22:2717–2728. 10.1093/emboj/cdg279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirabayashi Y, Suzuki T, Suzuki Y, Taki T, Matsumoto M, Higashi H, Kato S. 1983. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J. Biochem. 94:327–330 [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi Y, Higashi H, Kato S, Taniguchi M, Matsumoto M. 1987. Occurrence of tumor-associated ganglioside antigens with Hanganutziu-Deicher antigenic activity on human melanomas. Jpn. J. Cancer Res. 78:614–620 [PubMed] [Google Scholar]
- 24.Hara S, Takemori Y, Yamaguchi M, Nakamura M, Ohkura Y. 1987. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal. Biochem. 164:138–145. 10.1016/0003-2697(87)90377-0 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Moriyama Y, Ikari A, Sugatani J, Suzuki T, Miwa M. 2008. Surface localization of the nuclear receptor CAR in influenza A virus-infected cells. Biochem. Biophys. Res. Commun. 368:550–555. 10.1016/j.bbrc.2008.01.145 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Murakami K, Nagakura M, Kishita H, Watanabe S, Honke K, Ogura K, Tai T, Kawasaki K, Miyamoto D, Hidari KI, Guo CT, Suzuki Y, Suzuki T. 2008. Sulfatide is required for efficient replication of influenza A virus. J. Virol. 82:5940–5950. 10.1128/JVI.02496-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, Tiwari VK, Cao H, Chokhawala HA, Zheng H, Cummings RD, Smith DF. 2011. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J. Biol. Chem. 286:31610–31622. 10.1074/jbc.M111.274217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padler-Karavani V, Song X, Yu H, Hurtado-Ziola N, Huang S, Muthana S, Chokhawala HA, Cheng J, Verhagen A, Langereis MA, Kleene R, Schachner M, de Groot RJ, Lasanajak Y, Matsuda H, Schwab R, Chen X, Smith DF, Cummings RD, Varki A. 2012. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J. Biol. Chem. 287:22593–22608. 10.1074/jbc.M112.359323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamanaka T, Tsujimura K, Kondo T, Matsumura T, Ishida H, Kiso M, Hidari KI, Suzuki T. 2010. Infectivity and pathogenicity of canine H3N8 influenza A virus in horses. Influenza Other Respir. Viruses 4:345–351. 10.1111/j.1750-2659.2010.00157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bateman AC, Karamanska R, Busch MG, Dell A, Olsen CW, Haslam SM. 2010. Glycan analysis and influenza A virus infection of primary swine respiratory epithelial cells: the importance of NeuAcα2-6 glycans. J. Biol. Chem. 285:34016–34026. 10.1074/jbc.M110.115998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogata M, Hidari KI, Kozaki W, Murata T, Hiratake J, Park EY, Suzuki T, Usui T. 2009. Molecular design of spacer-N-linked sialoglycopolypeptide as polymeric inhibitors against influenza virus infection. Biomacromolecules 10:1894–1903. 10.1021/bm900300j [DOI] [PubMed] [Google Scholar]
- 32.Palese P, Tobita K, Ueda M, Compans RW. 1974. Characterization of temperature sensitive influenza virus mutant defective in neuraminidase. Virology 61:397–410. 10.1016/0042-6822(74)90276-1 [DOI] [PubMed] [Google Scholar]
- 33.Palese P, Compans RW. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoro-acetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 33:159–163. 10.1099/0022-1317-33-1-159 [DOI] [PubMed] [Google Scholar]
- 34.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. 2004. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 78:12665–12667. 10.1128/JVI.78.22.12665-12667.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki T, Takahashi T, Guo CT, Hidari KI, Miyamoto D, Goto H, Kawaoka Y, Suzuki Y. 2005. Sialidase activity of influenza A virus in an endocytic pathway enhances viral replication. J. Virol. 79:11705–11715. 10.1128/JVI.79.18.11705-11715.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vries E, de Vries RP, Wienholts MJ, Floris CE, Jacobs MS, van den Heuvel A, Rottier PJ, de Haan CA. 2012. Influenza A virus entry into cells lacking sialylated N-glycans. Proc. Natl. Acad. Sci. U. S. A. 109:7457–7462. 10.1073/pnas.1200987109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohuchi M, Asaoka N, Sakai T, Ohuchi R. 2006. Roles of neuraminidase in the initial stage of influenza virus infection. Microbes Infect. 8:1287–1293. 10.1016/j.micinf.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 38.Strous GJ, Dekker J. 1992. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. Biol. 27:57–92. 10.3109/10409239209082559 [DOI] [PubMed] [Google Scholar]
- 39.Kobasa D, Kodihalli S, Luo M, Castrucci MR, Donatelli I, Suzuki Y, Suzuki T, Kawaoka Y. 1999. Amino acid residues contributing to the substrate specificity of the influenza A virus neuraminidase. J. Virol. 73:6743–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong X, Coombs PJ, Martin SR, Liu J, Xiao H, McCauley JW, Locher K, Walker PA, Collins PJ, Kawaoka Y, Skehel JJ, Gamblin SJ. 2013. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497:392–396. 10.1038/nature12144 [DOI] [PubMed] [Google Scholar]
- 41.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 101:4620–4624. 10.1073/pnas.0308001101 [DOI] [PMC free article] [PubMed] [Google Scholar]