Abstract
The N terminus of arenavirus L protein contains an endonuclease presumably involved in “cap snatching.” Here, we employed the Lassa virus replicon system to map other L protein sites that might be involved in this mechanism. Residues Phe-1979, Arg-2018, Phe-2071, Asp-2106, Trp-2173, Tyr-2179, Arg-2200, and Arg-2204 were important for viral mRNA synthesis but dispensable for genome replication. Thus, the C terminus of L protein is involved in the mRNA synthesis process, potentially by mediating cap binding.
TEXT
Lassa virus is a segmented negative-strand RNA virus of the family Arenaviridae causing hemorrhagic fever in humans. The genome consists of two single-stranded RNA segments, each containing two genes in opposite directions (1). The S RNA segment encodes the nucleoprotein (NP) and the glycoprotein precursor. The L RNA encodes the small matrix protein Z (2, 3) and the 200-kDa L protein (4). NP and L protein are the minimal viral trans-acting factors required for replication and transcription of the genome (5–7). L protein mediates the synthesis of two RNA species, mRNA terminating in the intergenic region and noncapped genomic or antigenomic RNA representing a full-length copy of the genome (8, 9). The central domain of L protein harbors the RNA-dependent RNA polymerase (RdRp) (10–14), and the N terminus contains an endonuclease essential for transcription (15, 16). The latter presumably cleaves off from cellular mRNAs the cap structure with 4 to 5 nucleotides, which serves as a primer for viral mRNA synthesis (9, 17, 18). Influenza virus, the prototype of “cap-snatching” viruses, expresses a cap-binding protein (PB2) (19) in addition to an endonuclease (PA) (20, 21). Assuming the cap-snatching mechanism in arenaviruses corresponds to that in influenza virus, it is tempting to speculate that the L protein also contains a cap-binding domain. We have conducted a mutagenesis study to identify residues that might be involved in cap binding.
MATERIALS AND METHODS
The experiments were performed in the context of the T7 RNA polymerase-based Lassa virus replicon system essentially as described previously (5, 14, 16). In brief, L gene mutants were generated by mutagenic PCR using pCITE-L as a template. The PCR products containing the functional cassette for expression of mutant L protein were purified, quantified spectrophotometrically, and used for transfection without prior cloning. As the Phusion polymerase used for PCR has an extremely low error rate (22), more than 95% of the amplified L genes are predictably free of random polymerization errors (23). The presence of the desired artificial mutation was ascertained by sequencing. BSR-T7/5 cells stably expressing T7 RNA polymerase (24) were transfected per well of a 24-well plate with 250 ng of minigenome expressing Renilla luciferase (Ren-Luc), 250 ng of L gene PCR product, 250 ng of pCITE-NP expressing NP, and 10 ng of pCITE-FF-luc expressing firefly luciferase as an internal transfection control. One day after transfection, total RNA was purified for Northern blotting using an RNeasy minikit (Qiagen) or cells were lysed in 100 μl of passive lysis buffer (Promega) per well and assayed for firefly luciferase and Ren-Luc activity using the dual-luciferase reporter assay system (Promega). Ren-Luc levels were corrected with the firefly luciferase levels (resulting in standardized relative light units [sRLU]) to compensate for differences in transfection efficiency or cell density.
For Northern blot analysis, 500 ng of RNA was separated in a 1.5% agarose–formaldehyde gel and transferred onto a Hybond N+ membrane (Amersham Pharmacia Biotech). Blots were hybridized with a 32P-labeled riboprobe targeting the Ren-Luc gene, and RNA bands were visualized by autoradiography using an FLA-7000 phosphorimager (Fujifilm).
To test the stability of L protein mutants, BSR-T7/5 cells in a well of a 24-well plates were transfected with 500 ng of PCR product expressing L protein mutants tagged at the C terminus with a 3× FLAG sequence. The cells were additionally inoculated with modified vaccinia virus Ankara expressing T7 RNA polymerase (MVA-T7) (25) to enhance expression of L protein and, thus, facilitate its detection by immunoblotting. Cytoplasmic lysate was separated in a 3 to 8% Tris-acetate polyacrylamide gel, transferred to a nitrocellulose membrane (Schleicher & Schuell), and detected by immunoblotting using peroxidase-conjugated anti-FLAG M2 antibody (1:10,000) (A8592; Sigma-Aldrich). L protein bands were visualized by chemiluminescence using SuperSignal West Femto substrate (Pierce) and a FUSION SL image acquisition system (Vilber Lourmat).
RESULTS
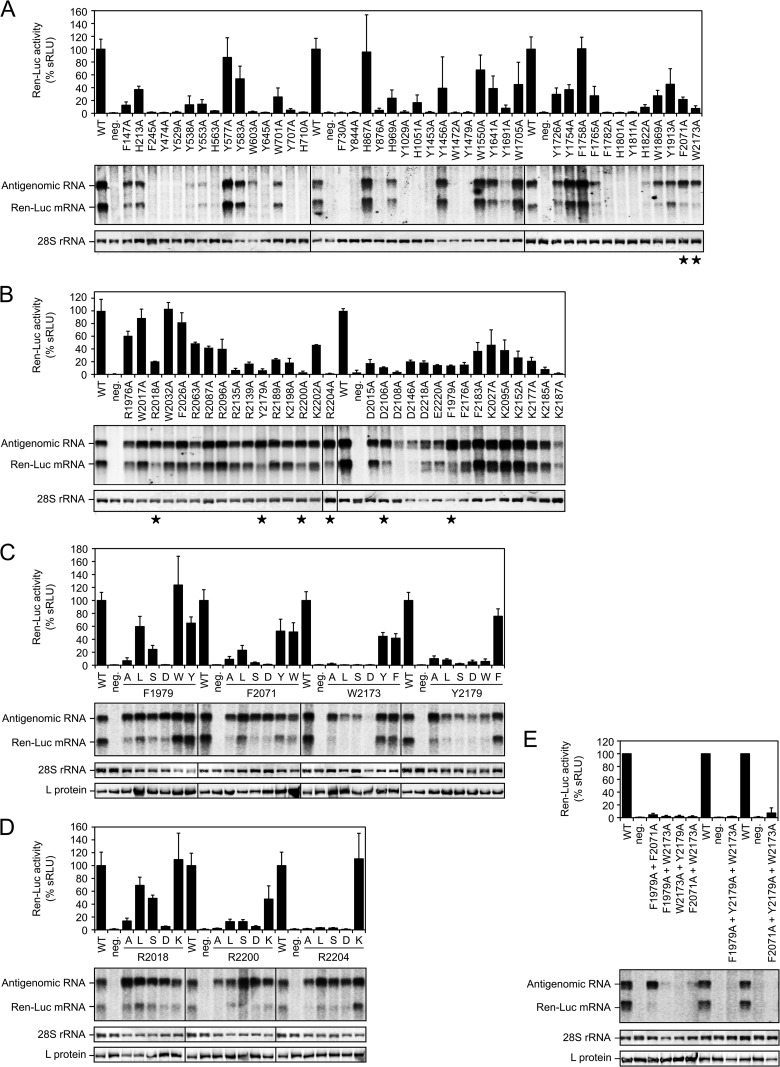
A structural hallmark of the cap-binding pocket in influenza virus PB2 and other cap-binding proteins is a sandwich of two aromatic residues (Trp, Phe, Tyr, His) which binds in between the m7G of the cap structure (19, 26). Therefore, the first mutagenesis screening was focused on aromatic residues that (i) are conserved—within the chemical class—among arenaviruses, (ii) are located outside the RdRp and endonuclease domains, and (iii) had not yet been tested in our previous study (16). Forty-two Trp, Phe, Tyr, and His residues fulfilling these criteria were changed to Ala, and the functionality of the mutants was tested in a replicon system. Transcriptional activity was measured via expression of the minigenome-encoded Ren-Luc reporter gene (Ren-Luc activity). Synthesis of Ren-Luc mRNA and antigenomic RNA was analyzed by Northern blotting. A defect in cap binding or any other step in cap snatching is expected to manifest in a reduction of Ren-Luc activity in spite of wild-type-like antigenome synthesis as well as a reduction in the Ren-Luc mRNA level relative to the antigenomic RNA level (collectively called the mRNA-defective phenotype). This phenotype corresponds to that of influenza virus PB2 mutants with exchanges in the cap-binding site (19, 27). Ren-Luc activity, antigenome level, and the Ren-Luc mRNA/antigenome ratio are listed for all mutants tested in this study in Table S1 in the supplemental material.
Most of the Trp/Phe/Tyr/His-to-Ala mutants showed a wild-type-like RNA expression pattern or a partial or complete defect in global RNA synthesis (Fig. 1A). An mRNA-defective phenotype was observed with two mutants: F2071A and W2173A (Fig. 1A, marked with an asterisk). As both residues Phe-2071 and Trp-2173 were located in the C terminus of L protein, we focused further experiments on this part of the protein.
FIG 1.
Influence of L protein mutations on viral RNA synthesis as tested in the Lassa virus replicon system. Transcriptional activity of L protein mutants was measured via Ren-Luc reporter gene expression. The Ren-Luc activity is shown in the bar graphs (mean and range [n = 2 independent transfection experiments, panel B] or standard deviation [n ≥ 3 independent transfection experiments, panels A, C, D, and E] of standardized relative light units [sRLU] as a percentage of the wild type). Synthesis of antigenome and Ren-Luc mRNA was evaluated by Northern blotting using a radiolabeled riboprobe hybridizing to the Ren-Luc gene. A defective L protein with a mutation in the catalytic site of the RdRp served as a negative control (neg.). The methylene blue-stained 28S rRNA is shown as a marker for gel loading and RNA transfer. Immunoblot analysis of FLAG-tagged L protein mutants is shown for selected experiments. (A) Conserved aromatic residues (Phe, Tyr, Trp, His) along the L protein sequence, with the exception of the N-terminal endonuclease and the central RdRp domain, were exchanged with alanine. Mutants with an mRNA-defective phenotype are marked with an asterisk. (B) Aromatic residues (Phe, Tyr, Trp) as well as positively (Lys, Arg) and negatively (Asp, Glu) charged residues in the C terminus of L protein were exchanged with alanine. An mRNA-defective phenotype is marked with an asterisk. (C) Aromatic residues Phe-1979, Phe-2071, Trp-2173, and Tyr-2179 were exchanged with Ala, Leu, Ser, Asp, Trp, Phe, and/or Tyr. (D) Positively charged residues Arg-2018, Arg-2200, and Arg-2204 were exchanged with Ala, Leu, Ser, Asp, and Lys. (E) Double and triple mutation of Phe-1979, Phe-2071, Trp-2173, and Tyr-2179.
In addition to aromatic residues, positively charged residues (Arg, Lys) play an important role in stabilizing cap binding via interaction with the triphosphate moiety of m7Gppp (19, 26). However, as the C terminus of L protein from position 1900 is poorly conserved (Fig. S1)—actually it is the least conserved part of the protein (13)—it was difficult to establish criteria for selection of residues to be mutated. Eventually, we selected 18 Arg and Lys residues, which were conserved among Lassa virus strains but showed a variable degree of conservation among other arenavirus species (see Fig. S1 in the supplemental material). In addition, a few negatively charged residues (Asp and Glu) were selected, as such residues are known to interact with nitrogen atoms of the guanine moiety in cap-binding proteins (19, 26). Finally, some partially conserved Trp, Phe, and Tyr residues were selected to test if more aromatic residues, in addition to Phe-2071 and Trp-2173, might be involved in a putative hydrophobic cap-binding pocket. This second alanine screening revealed six more mutants with an mRNA-defective phenotype: F1979A, R2018A, D2106A, Y2179A, R2200A, and R2204A (Fig. 1B, marked with an asterisk).
In a third set of experiments, the structural requirements at the positions for aromatic and charged residues, which were found to be important for mRNA synthesis, were studied in more detail. Phe-1979, Phe-2071, Trp-2173, and Tyr-2179 were exchanged with Leu, Ser, Asp, Trp, Phe, and/or Tyr; Arg-2018, Arg-2200, and Arg-2204 were exchanged with Leu, Ser, Asp, and Lys; and Asp-2106 was exchanged with Val, Ser, Asn, Glu, and Lys. Alanine mutants were included for confirmatory purposes.
An exchange of Phe-1979 and Phe-2071 hardly influenced the antigenome level but had variable effects on the mRNA level (Fig. 1C). Mutation to Trp and Tyr led to a wild-type-like phenotype. Leu still facilitated residual transcriptional activity, while Ala, Ser, and Asp strongly reduced transcriptional activity. The mRNA-defective phenotype was most pronounced with mutants F1979D and F2071D, which showed wild-type-like antigenome synthesis but were completely defective in Ren-Luc expression.
Trp-2173 and Tyr-2179 were more sensitive to mutation than Phe-1979 and Phe-2071 (Fig. 1C). While Tyr and Phe residues were functional at positions 2173 and 2179, a bulky Trp at position 2179 resulted in an mRNA-defective phenotype. Similarly, Leu, Ser, and Asp specifically reduced transcriptional activity, though the antigenome level was also somewhat reduced. Mutants W2173A and Y2179A showed a clear mRNA-defective phenotype with strongly reduced Ren-Luc levels and wild-type-like antigenome levels.
Replacement of the positively charged residues Arg-2018, Arg-2200, and Arg-2204 by the related Lys hardly influenced L protein activity (Fig. 1D). However, an mRNA-defective phenotype was clearly seen when the Arg residues were replaced by Asp or Ala. Exchange of Arg-2200 by Ser and Arg-2204 by Leu and Ser also led to an mRNA-defective phenotype, while replacement of Arg-2200 by Leu and Arg-2018 by Leu and Ser resulted in an intermediate phenotype.
Replacement of the negatively charged residue Asp-2106 by Val and Lys resulted in an mRNA-defective phenotype, the repeat experiment with Ala resulted only in an intermediate phenotype, and Ser, Asn, and Glu exchanges had no major effect (data not shown; see Table S1 in the supplemental material). Overall, the phenotype seen with Asp-2106 mutants was less pronounced, with none of the mutants showing <10% of wild-type Ren-Luc activity.
Double and triple alanine mutants were generated for residues Phe-1979, Phe-2071, Trp-2173, and Tyr-2179 (Fig. 1E). However, with the exception of the F1979A F2071A double mutant, all other mutants were completely defective. The F1979A F2071A mutant showed a clear mRNA-defective phenotype.
DISCUSSION
Our data indicate that residues Phe-1979, Arg-2018, Phe-2071, Asp-2106, Trp-2173, Tyr-2179, Arg-2200, and Arg-2204 play an important role in mRNA synthesis but are dispensable for genome replication. There were minor differences in the phenotypes of the respective mutants. Mutation of Phe-1979 and Phe-2071 impaired mRNA synthesis with negligible effect on antigenome synthesis, and even simultaneous removal of both aromatic side chains was still compatible with de novo primed RNA synthesis. This suggests that these residues have hardly any function in maintaining the overall structure of L protein. In contrast, residues Trp-2173 and Tyr-2179 seem to be important for the structural integrity of the protein as well, as their mutation also impaired de novo primed RNA synthesis, and simultaneous removal of both aromatic side chains was incompatible with L protein function.
This study was designed to specifically search for residues that are potentially involved in cap binding. However, definitive evidence for the involvement of a residue in this activity cannot be provided by the replicon data, and, unfortunately, preliminary efforts to demonstrate cap binding of the C-terminal domain or the full-length L protein in biochemical assays have not met with success so far (unpublished data). While this does not exclude a function of the C terminus in cap binding, it underlines that various hypotheses have to be taken into account to explain the observed phenotypes. The cap-binding hypothesis would imply that the aromatic residues Phe-1979, Phe-2071, Trp-2173, and/or Tyr-2179 could be involved in sandwiching the m7G moiety, while Arg-2018, Arg-2200, and/or Arg-2204 may bind the triphosphate moiety of the cap or adjacent bases of the RNA. The fact that four rather than two aromatic residues were found to be essential for mRNA synthesis may indicate that the cap-snatching mechanism or the architecture of the binding pocket in arenaviruses differs from that of influenza virus. Alternatively, the function of the C terminus in mRNA synthesis is not related to cap binding at all. A cap-binding site has recently been proposed in Lassa virus NP based on structural studies (28), and also hantavirus nucleocapsid protein has been implicated in cap snatching (29, 30). The hypothesis that the primary binding site for cap structures resides in NP and not in L protein would imply that the identified residues contribute to a step in mRNA synthesis that lacks a known counterpart in influenza virus. If the pathway is thus complex, it will be quite demanding to decipher the individual steps in their molecular detail by biochemical and structural experimentation.
The C-terminal domain, including the identified key residues, is poorly conserved among arenavirus species (Fig. 2; see also Fig. S1 in the supplemental material). Phe-2071, Trp-2173, Tyr-2179, and Arg-2200 are largely conserved (within the chemical class) among Old World and New World arenaviruses; notable exceptions are Ile-2071 in Ippy virus and Thr/Cys-2173 in Lunk/lymphocytic choriomeningitis virus. Conserved homologous residues to Phe-1979, Arg-2018, Asp-2106, and Arg-2204 are not at all or rarely found in New World viruses. Arg-2018 and Asp-2106 are even not conserved among Old World viruses. This would imply that the specific arrangement of the functional site, to which the residues contribute, differs among arenavirus species.
FIG 2.
Amino acid sequence alignment of the C terminus of L protein of Old and New World arenaviruses and summary of the mutagenesis experiments. Residues subjected to mutagenesis are marked above the Lassa virus sequence. The data from Northern blotting are coded as follows: inverted black triangle, mutant with mRNA-defective phenotype; circle, mutant with wild-type mRNA/antigenome ratio or no RNA signals at all. The Ren-Luc data are coded as follows: black circle, inactive mutant (<5% activity); gray circle, mutant with reduced activity (5 to 30%); white circle, mutant with wild-type-like activity (>30%) (see Table S1 in the supplemental material for the data). The sequences are part of a larger alignment in Fig. S1in the supplemental material that was generated with PRALINE (http://www.ibi.vu.nl/programs/pralinewww/) (31). Residues are colored according to chemical type: aromatic (Phe, Tyr, Trp, His) in green, positively charged (Lys, Arg) in blue, and negatively charged (Asp, Glu) in orange. The secondary structure (arrow, sheet; filled box, helix) of the Lassa virus L protein as shown below the alignment was predicted by Jpred3 (http://www.compbio.dundee.ac.uk/www-jpred/) (32).
In conclusion, this study indicates that the C terminus of Lassa virus L protein plays an important role in mRNA synthesis, potentially by mediating cap binding or being otherwise involved in cap snatching. The data will facilitate structural and biochemical investigations addressing this role in detail.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nina Schmalstieg for technical assistance.
This study was supported by grant GU 883/1-1 from the German Research Foundation (DFG). The Department of Virology of the Bernhard-Nocht-Institute is a WHO Collaborating Centre for Arbovirus and Haemorrhagic Fever Reference and Research (DEU-115).
Footnotes
Published ahead of print 14 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00652-14.
REFERENCES
- 1.Auperin DD, Romanowski V, Galinski M, Bishop DH. 1984. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J. Virol. 52:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strecker T, Eichler R, Meulen J, Weissenhorn W, Klenk HD, Garten W, Lenz O. 2003. Lassa virus Z protein is a matrix protein sufficient for the release of virus-like particles. J. Virol. 77:10700–10705. 10.1128/JVI.77.19.10700-10705.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez M, Craven RC, de la Torre JC. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc. Natl. Acad. Sci. U. S. A. 100:12978–12983. 10.1073/pnas.2133782100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh MK, Fuller-Pace FV, Buchmeier MJ, Southern PJ. 1987. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology 161:448–456. 10.1016/0042-6822(87)90138-3 [DOI] [PubMed] [Google Scholar]
- 5.Hass M, Gölnitz U, Müller S, Becker-Ziaja B, Günther S. 2004. Replicon system for Lassa virus. J. Virol. 78:13793–13803. 10.1128/JVI.78.24.13793-13803.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KJ, Novella IS, Teng MN, Oldstone MB, de La Torre JC. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470–3477. 10.1128/JVI.74.8.3470-3477.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez N, Jacamo R, Franze-Fernandez MT. 2001. Transcription and RNA replication of Tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol. 75:12241–12251. 10.1128/JVI.75.24.12241-12251.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcin D, Kolakofsky D. 1990. A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J. Virol. 64:6196–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer BJ, Southern PJ. 1993. Concurrent sequence analysis of 5′ and 3′ RNA termini by intramolecular circularization reveals 5′ nontemplated bases and 3′ terminal heterogeneity for lymphocytic choriomeningitis virus mRNAs. J. Virol. 67:2621–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller-Pace FV, Southern PJ. 1989. Detection of virus-specific RNA-dependent RNA polymerase activity in extracts from cells infected with lymphocytic choriomeningitis virus: in vitro synthesis of full-length viral RNA species. J. Virol. 63:1938–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcin D, Kolakofsky D. 1992. Tacaribe arenavirus RNA synthesis in vitro is primer dependent and suggests an unusual model for the initiation of genome replication. J. Virol. 66:1370–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukashevich IS, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol ST, Salvato MS. 1997. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J. Gen. Virol. 78(Part 3):547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieth S, Torda AE, Asper M, Schmitz H, Günther S. 2004. Sequence analysis of L RNA of Lassa virus. Virology 318:153–168. 10.1016/j.virol.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 14.Hass M, Lelke M, Busch C, Becker-Ziaja B, Günther S. 2008. Mutational evidence for a structural model of the Lassa virus RNA polymerase domain and identification of two residues, Gly1394 and Asp1395, that are critical for transcription but not replication of the genome. J. Virol. 82:10207–10217. 10.1128/JVI.00220-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin B, Coutard B, Lelke M, Ferron F, Kerber R, Jamal S, Frangeul A, Baronti C, Charrel R, de Lamballerie X, Vonrhein C, Lescar J, Bricogne G, Günther S, Canard B. 2010. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog. 6:e1001038. 10.1371/journal.ppat.1001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lelke M, Brunotte L, Busch C, Günther S. 2010. An N-terminal region of Lassa virus L protein plays a critical role in transcription but not replication of the virus genome. J. Virol. 84:1934–1944. 10.1128/JVI.01657-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polyak SJ, Zheng S, Harnish DG. 1995. 5′ Termini of Pichinde arenavirus S RNAs and mRNAs contain nontemplated nucleotides. J. Virol. 69:3211–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raju R, Raju L, Hacker D, Garcin D, Compans R, Kolakofsky D. 1990. Nontemplated bases at the 5′ ends of Tacaribe virus mRNAs. Virology 174:53–59. 10.1016/0042-6822(90)90053-T [DOI] [PubMed] [Google Scholar]
- 19.Guilligay D, Tarendeau F, Resa-Infante P, Coloma R, Crepin T, Sehr P, Lewis J, Ruigrok RW, Ortin J, Hart DJ, Cusack S. 2008. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 15:500–506. 10.1038/nsmb.1421 [DOI] [PubMed] [Google Scholar]
- 20.Dias A, Bouvier D, Crepin T, McCarthy AA, Hart DJ, Baudin F, Cusack S, Ruigrok RW. 2009. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458:914–918. 10.1038/nature07745 [DOI] [PubMed] [Google Scholar]
- 21.Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, Lv Z, Ge R, Li X, Deng T, Fodor E, Rao Z, Liu Y. 2009. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458:909–913. 10.1038/nature07720 [DOI] [PubMed] [Google Scholar]
- 22.Li M, Diehl F, Dressman D, Vogelstein B, Kinzler KW. 2006. BEAMing up for detection and quantification of rare sequence variants. Nat. Methods 3:95–97. 10.1038/nmeth850 [DOI] [PubMed] [Google Scholar]
- 23.Gunther S, Sommer G, Von Breunig F, Iwanska A, Kalinina T, Sterneck M, Will H. 1998. Amplification of full-length hepatitis B virus genomes from samples from patients with low levels of viremia: frequency and functional consequences of PCR-introduced mutations. J. Clin. Microbiol. 36:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutter G, Ohlmann M, Erfle V. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 371:9–12. 10.1016/0014-5793(95)00843-X [DOI] [PubMed] [Google Scholar]
- 26.Fechter P, Brownlee GG. 2005. Recognition of mRNA cap structures by viral and cellular proteins. J. Gen. Virol. 86:1239–1249. 10.1099/vir.0.80755-0 [DOI] [PubMed] [Google Scholar]
- 27.Fechter P, Mingay L, Sharps J, Chambers A, Fodor E, Brownlee GG. 2003. Two aromatic residues in the PB2 subunit of influenza A RNA polymerase are crucial for cap binding. J. Biol. Chem. 278:20381–20388. 10.1074/jbc.M300130200 [DOI] [PubMed] [Google Scholar]
- 28.Qi X, Lan S, Wang W, Schelde LM, Dong H, Wallat GD, Ly H, Liang Y, Dong C. 2010. Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature 468:779–783. 10.1038/nature09605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mir MA, Duran WA, Hjelle BL, Ye C, Panganiban AT. 2008. Storage of cellular 5′ mRNA caps in P bodies for viral cap-snatching. Proc. Natl. Acad. Sci. U. S. A. 105:19294–19299. 10.1073/pnas.0807211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng E, Mir MA. 2012. Signatures of host mRNA 5′ terminus for efficient hantavirus cap snatching. J. Virol. 86:10173–10185. 10.1128/JVI.05560-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simossis VA, Heringa J. 2005. PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 33:W289–W294. 10.1093/nar/gki390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuff JA, Barton GJ. 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40:502–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.