ABSTRACT
Interleukin-6 (IL-6) plays an important role in the development and progression of inflammatory responses, autoimmune diseases, and cancers. Many viral infections, including Theiler's murine encephalomyelitis virus (TMEV), result in the vigorous production of IL-6. However, the role of IL-6 in the development of virus-induced inflammatory responses is unclear. The infection of susceptible mice with TMEV induces the development of chronic demyelinating disease, which is considered a relevant infectious model for multiple sclerosis. In this study, we demonstrate that resistant C57BL/6 mice carrying an IL-6 transgene (IL-6 Tg) develop a TMEV-induced demyelinating disease accompanied by an increase in viral persistence and an elevated Th17 cell response in the central nervous system. Either IL-6 or IL-17 induced the expression of Bcl-2 and Bcl-xL at a high concentration. The upregulated expression of prosurvival molecules in turn inhibited target cell destruction by virus-specific CD8+ T cells. More interestingly, IL-6 and IL-17 synergistically promoted the expression of these prosurvival molecules, preventing cellular apoptosis at a much lower (<5-fold) concentration. The signals involved in the synergy appear to include the activation of both STAT3 and NF-κB via distinct cytokine-dependent pathways. Thus, the excessive IL-6 promotes the generation of Th17 cells, and the resulting IL-6 and IL-17 synergistically promote viral persistence by protecting virus-infected cells from apoptosis and CD8+ T cell-mediated target destruction. These results suggest that blocking both IL-6 and IL-17 functions are important considerations for therapies of chronic viral diseases, autoimmune diseases, and cancers.
IMPORTANCE This study indicates that an excessive level of IL-6 cytokine produced following viral infection promotes the development of IL-17-producing pathogenic helper T cells. We demonstrate here for the first time that IL-6 together with IL-17 synergistically enhances the expression of survival molecules to hinder critical host defense mechanisms removing virus-infected cells. This finding has an important implication in controlling not only chronic viral infections but also autoimmune diseases and cancers, which are associated with prolonged cell survival.
INTRODUCTION
Various viruses are known to utilize many different strategies to abrogate the function and/or induction of host antiviral responses. Theiler's murine encephalomyelitis virus (TMEV) of the Picornaviridae family (1) establishes a persistent infection in the central nervous system (CNS) of susceptible mice. The persistence of this virus in the CNS leads to the development of chronic demyelinating disease, which has been studied as a relevant viral model for human multiple sclerosis (2–4). Strategies utilized by TMEV to establish chronic viral persistence include the preferential induction of Th17 responses producing IL-17, which blocks the removal of virus-infected cells by inhibiting cytotoxic T cell function and apoptotic cell death (5). In addition, proinflammatory cytokines produced after TMEV infection via the host innate immune response involving TLR3 and TLR2, as well as the downstream responses such as IL-1, contribute to viral persistence and pathogenesis (6–9). In particular, IL-6 constitutes a major cytokine which promotes the induction of pathogenic Th17 responses (5). In addition, the level of IL-6 production after viral infection is well correlated with the susceptibility of mice to TMEV-induced demyelinating disease (5, 8, 10). Interestingly, such a chronic TMEV infection leads to the induction of autoimmune responses to the CNS target organs, which may also promote disease progression (11). Because both Th17 responses and IL-6 production are associated with the development of various autoimmune diseases and the progression of cancers (12–14), the effects of IL-17 and IL-6 are far reaching for many chronic diseases, such as chronic viral infection, autoimmunity and cancer development.
It has been repeatedly shown that IL-6 directly plays an important role in the inhibition of cellular apoptosis induced by various stimulations, such as transforming growth factor β (TGF-β) and IL-1β (15). In addition, IL-6 promotes growth and survival of various cells, including cancer cells (16). Furthermore, excessive levels of IL-6 promote the development of various autoimmune diseases such as EAE and diabetes (17, 18), in part by facilitating the generation of IL-17-producing Th17 cells (19, 20). Moreover, the production of IL-17 is elevated in patients with many autoimmune diseases, cancers, and viral infections, suggesting a tight positive-feedback amplification loop for the pathogenic functions of both IL-17 and IL-6 (20–22). Interestingly, both IL-17 and IL-6 promote the expression of prosurvival molecules, such as Bcl-2 and Bcl-xL, by utilizing STAT3 and NF-κB signaling (23–26). The elevation of these survival molecules plays a critical role in establishing viral persistence and the development of autoimmune diseases and cancers by permitting survival of virus-infected or pathogenic cells. However, it has not yet been established whether IL-17 and IL-6 function additively or synergistically in inhibiting cellular apoptosis, which promotes the development of chronic immune-mediated inflammation, autoimmune diseases, tumor growth, and viral persistence.
In this study, we have addressed the roles of IL-6 and IL-17 in the inhibition of cellular apoptosis using the TMEV infection-induced demyelinating disease system in conjunction with IL-6 knockout (KO) and IL-6 transgenic (Tg) mice. Our results indicate that resistant B6 mice become susceptible to TMEV-induced demyelinating disease when they carry an IL-6 transgene, resulting in the production of excessive IL-6. A high concentration of either IL-6 or IL-17 alone in the absence of viral infection was able to upregulate the expression of both Bcl-2 and Bcl-xL, indicating that IL-6 and IL-17 are capable of independently inducing the expression of these genes. Most interestingly, however, IL-6 and IL-17 synergistically promoted the upregulation of Bcl-2 and Bcl-xL expression at low concentrations, which failed to upregulate the prosurvival molecules separately. The upregulation of prosurvival molecules in turn inhibited the apoptotic cell death of virus-infected cells and the destruction of virus epitope-bearing target cells by virus-specific CD8+ T cells. The common signals involved in the upregulation caused by IL-6 and IL-17 were the activation of STAT3 and NF-κB, although the signaling pathways for the activation are different between IL-6 and IL-17 (20, 24, 25, 27–29). These synergistic effects appear to be operational in TMEV-infected mice, as viral persistence in the CNS is partially enhanced after IL-17 administration and reduced after anti-IL-17 antibody treatment. Based on these results, we hypothesize that the excessive presence of IL-6 in virus-infected mice promotes the generation of Th17 cells, and IL-17 together with IL-6 further synergistically promotes viral persistence by protecting virus-infected cells from apoptosis and T cell-mediated target destruction. We believe that this synergistic effect is particularly important as the individual levels of either IL-6 or IL-17 may not reach a significant phenotype. However, the combination of these cytokines may exert critical effects on viral pathogenesis, tumor growth, and various autoimmune diseases.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (B6) mice were purchased from Harlan Sprague Dawley, Madison, WI. IL-6 KO mice on a B6 background were obtained from the Jackson Laboratory. Human IL-6 Tg mice, on a B6 background from Tadamitsu Kishimoto (30), were kindly provided by Geoffrey Kansas at Northwestern University. Six- to 8-week-old mice were used in this study according to the protocols approved by the Northwestern University Animal Care and Use Committee.
Virus infection and treatment of mice.
Mice were intracerebrally inoculated with 30 μl containing 1 × 106 PFU of TMEV BeAn strain, and disease development was monitored weekly, as previously described (5). For treatment with lipopolysaccharide (LPS), wild-type (WT) or IL-6 KO mice were intraperitoneally injected with LPS (20 μg/100 μl/mouse; Escherichia coli 0111:B4; Sigma-Aldrich, St. Louis, MO) at 0 and 5 days relative to TMEV infection. For treatment with anti-IL-17 antibodies, IL-6 Tg mice were intraperitoneally injected (100 μg/200 μl/mouse) with a monoclonal anti-IL-17A antibody (clone eBioMM17F3; eBioscience, San Diego, CA) at 0 and 7 days after TMEV infection. For treatment with IL-17, WT or IL-6 KO mice were injected by intracerebral inoculation of 30 μl solution containing 100 ng of IL-17 (PeproTech, Rocky Hill, NJ) in combination with 1 × 106 PFU TMEV. Clinical symptoms of disease were assessed weekly on the following grading scale: grade 0, no clinical signs; grade 1, mild waddling gait; grade 2, severe waddling gait; grade 3, moderate hind-limb paralysis; grade 4, severe hind-limb paralysis.
Isolation of CNS-infiltrating cells.
Anesthetized mice were transcardially perfused with 30 ml of sterile Hanks' balanced salt solution (Sigma-Aldrich). To isolate CNS-infiltrating mononuclear cells, cell suspensions of brains and spinal cords were incubated at 37°C for 45 min in 250 μg/ml collagenase type 4 (Worthington Biochemical Corp.). An equal volume of 100% Percoll (Pharmacia) was added to the cell suspension to enrich infiltrating mononuclear cells in the bottom third of the gradient after centrifugation, without braking, at 15,000 rpm for 30 min. CNS-infiltrating mononuclear cells were stained and analyzed by flow cytometry. The expression of CD45 and CD11b was measured using anti-CD45-allophycocyanin (APC) and anti-CD11b-phycoerythrin (PE) antibodies (both from BD) to distinguish CNS-resident CD45int microglia from infiltrating CD45hi leukocytes, in conjunction with their expression of CD11b (macrophages) or lack of it (lymphocytes). To measure viral loads in the CNS, the CNS tissues were homogenized, freeze-thawed, and centrifuged at 8,000 rpm for 10 min, and the supernatant then was used to perform viral titer assays as previously described (5).
Assessment of T cell cytokine production.
The TMEV antigen-specific cytokine production by T cells was determined with in vitro recall responses as previously described (5). To measure the levels of IL-17 and IFN-γ, CNS-infiltrating mononuclear cells were restimulated with different doses of UV-TMEV or mixed major histocompatibility complex (MHC) class II-restricted epitopes (VP425-38 and VP2206-220) for 48 h. The culture supernatants were then assessed for the cytokines using cytokine-specific enzyme-linked immunosorbent assays (ELISAs) for IL-17A (R&D Systems kit) and IFN-γ (OptEIA kit; BD). To determine intracellular levels of the cytokines, CNS-infiltrating mononuclear cells or splenocytes from virus-infected mice were restimulated with 50 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (both from Sigma-Aldrich), or 2 μM mixed epitope peptides, for 6 h and then were stained with anti-IL-17-PE, anti-IFN-γ-APC, and anti-CD4-fluorescein isothiocyanate (FITC) (all from BD).
Intracellular staining of Bcl-2, Bcl-xL, and STAT3.
Bone marrow (BM) cells, cultured for 3 days in the presence of 20 ng/ml granulocyte-macrophage colony-stimulating factor (PeproTech), were used as previously described (5). The BM cells were treated with IL-6 (PeproTech), IL-17, or a combination of IL-6 and IL-17 for 24 h in the presence or absence of various inhibitors and then stained with anti-Bcl-2-FITC (eBioscience) or anti-Bcl-xL (Santa Cruz Biotechnology). The inhibitors that block specific signaling pathways included S31-201 (Sigma-Aldrich) for STAT3, TRAF6 inhibitory peptide (Imgenex) for TRAF6, SB202190 (EMD Chemicals Inc.) for p38, U0126 (EMD Chemicals Inc.) for ERK, pyrrolidone dithiocarbonate (PDTC; Sigma-Aldrich), and MG-132 (EMD Chemicals Inc.) for NF-κB. To assess the Bcl protein levels, isolated CNS-infiltrating cells were cultured for 6 h and then were stained with anti-CD45-APC, anti-CD11b-PE, and anti-Bcl-2-FITC or anti-Bcl-xL-FITC. To measure the intracellular levels of phosphorylated STAT3, BM cells were incubated with IL-6, IL-17, or their combination for 30 min. The treated cells were fixed with 2% (wt/vol) paraformaldehyde at 37°C for 10 min and then were permeabilized on ice for 30 min with 90% (vol/vol) methanol prior to staining for phosphorylated STAT3 using anti-phosphorylated STAT3 antibody (BD).
Assessment of cell apoptosis.
BM cells cultured for 3 days with GM-CSF were infected with TMEV at a multiplicity of infection (MOI) of 10 for 24 h in the presence of IL-6, IL-17, or the combination. The treated cells were washed, stained with propidium iodide (PI; Sigma-Aldrich) and annexin V-APC (Invitrogen), and then analyzed using flow cytometry.
T cell cytotoxicity assay.
The same number of red cell-removed spleen cells from naive mice was loaded with MHC class I-restricted epitope VP2121-130 or irrelevant ovalbumin (OVA) peptide OVA323-339 for 2 h and then were labeled for 10 min with low (0.5 μM) or high (10 μM) concentrations, respectively, of carboxyfluorescein succinimidyl ester (CFSE). The peptide-loaded CFSE-labeled cells were mixed in equal numbers, and the mixed labeled target cells were cocultured for 60 h with effector spleen cells from TMEV-infected mice at 8 days postinfection at different effector/target (E/T) ratios in the presence of IL-6, IL-17, or their combination.
Statistical analysis.
The data were expressed as the means ± standard deviations, where applicable. The differences in the incidence and severity of virally induced demyelinating diseases between the two experimental groups were determined between 21 and 84 or 98 days postinfection using a paired Student t test.
RESULTS
Reduction in the development of Th17 response and demyelinating disease in virus-infected IL-6 KO mice treated with LPS.
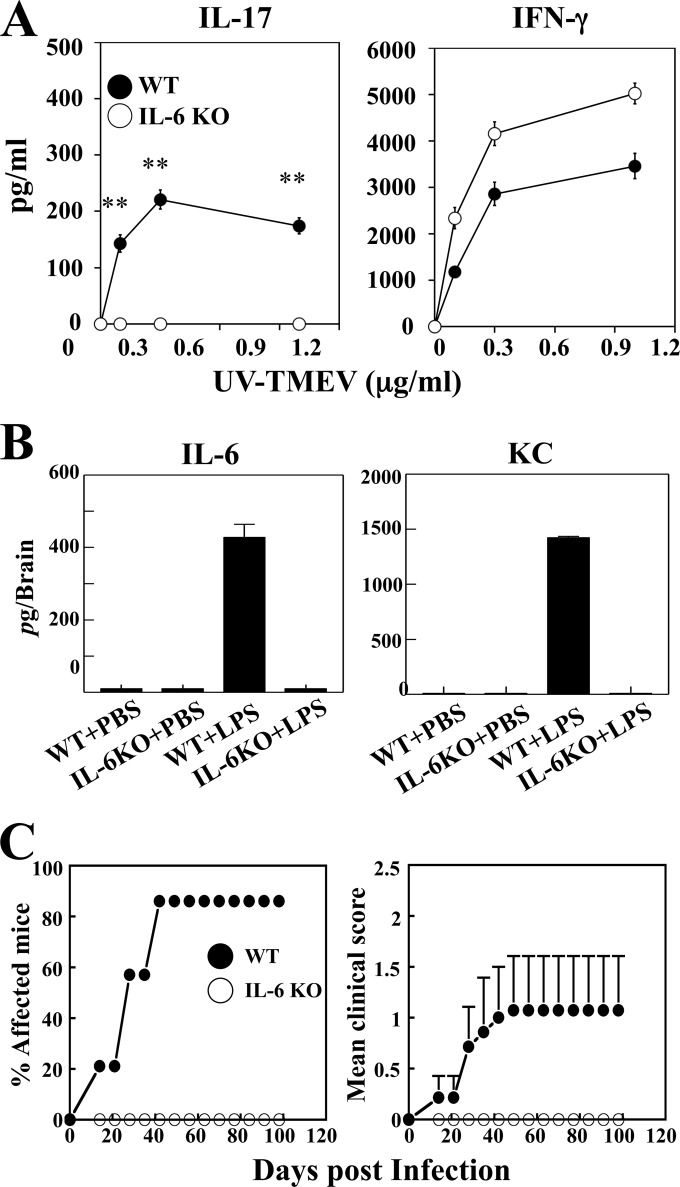
We have previously shown that Th17 cells promote viral persistence and induce the pathogenesis of chronic demyelinating disease via the IL-17-mediated inhibition of apoptosis, which enhances the survival of virus-infected cells and blocks target cell destruction by cytotoxic T cells. B6 mice, which are resistant to the development of demyelinating disease following TMEV infection, become susceptible to the disease after treatment with lipopolysaccharide (LPS) and produce markedly elevated levels of IL-17 compared to PBS-treated infected B6 mice (5, 10). In contrast, IL-6 KO mice on the B6 background developed similarly high numbers of Th1 cells but failed to develop Th17 cells in the CNS even after treatment with LPS. To further determine the role of IL-6 in the development of chronic viral diseases, we utilized these mice in conjunction with LPS treatment. Consistent with the previous results, the production of IFN-γ by the CNS cells in response to the same concentration of viral antigens was higher in IL-6 KO mice than in the WT mice (Fig. 1A). However, the production of IL-17 was not detectable in the IL-6 KO mice, while high levels were detected in the WT mice. The reduced production of IFN-γ in the WT mice may reflect the presence of counteracting IL-17 against the development of IFN-γ-producing Th1 cells, which is absent in IL-6 KO mice. Interestingly, LPS-treated WT B6 mice produced very high levels of IL-6 and IL-6-inducible CXCL1 (KC) in the brain compared to virus-infected WT mice treated with PBS or IL-6 KO mice treated with LPS (Fig. 1B), suggesting a critical role of IL-6 in the development of pathogenic Th17 cells in the CNS of virus-infected mice (5). We next examined the course of the development of demyelinating disease in these mice (Fig. 1C). As previously shown (5, 10), 90% of LPS-treated WT B6 mice developed clinical signs at 40 days postinfection with TMEV. However, none of the similarly LPS-treated IL-6 KO mice developed clinical signs, and they remained unaffected at 98 days after TMEV infection. The differential development of clinical disease was consistent with lower levels of viral load in the brain and spinal cord of IL-6 KO mice than those of WT mice at 8 and 21 days postinfection (31). Taken together, these results strongly suggest that IL-6 plays an important role in the pathogenesis of TMEV-induced demyelinating disease by promoting Th17 development and increasing viral loads in the CNS of virus-infected mice.
FIG 1.
IL-6 KO mice infected with TMEV exhibit reduced Th17 response and disease development. IL-6 KO and control B6 mice (n = 3) were treated with PBS or LPS at days 0 and 5 of TMEV infection. (A) Levels of IL-17 and IFN-γ produced by CD4+ T cells from the CNS of mice at 8 days after viral infection were assessed using ELISA after restimulation with UV-TMEV for 3 days. **, P < 0.01 for WT versus IL-6 KO mice. Data are representative of three independent experiments. (B) Levels of IL-6 and KC produced in the CNS of virus-infected WT and IL-6 KO mice treated with either PBS or LPS were determined at 8 days postinfection using specific ELISA. (C) The incidence and severity of demyelinating disease in LPS-treated WT B6 (n = 7) and IL-6 KO (n = 7) mice infected with TMEV was monitored weekly.
Development of TMEV-induced demyelinating disease in resistant B6 mice carrying an IL-6 transgene.
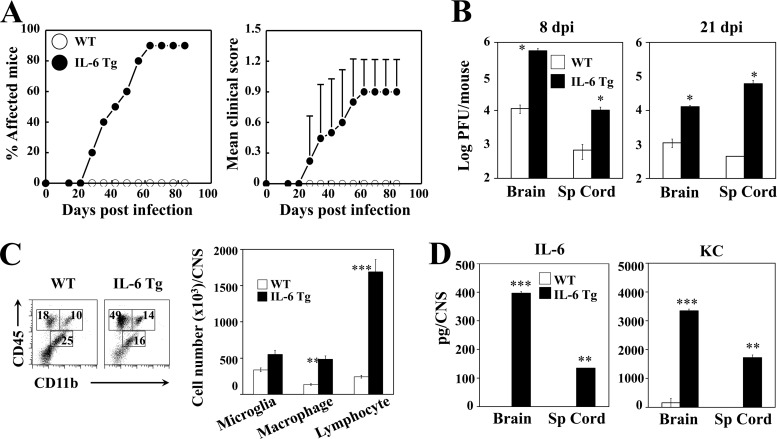
To verify the pathogenic role of IL-6 in the development of TMEV-induced demyelinating disease, we employed B6 transgenic mice carrying the human IL-6 gene. We compared the levels of Th17 induction, CNS inflammation, and viral disease development in WT B6 mice and IL-6 Tg mice after TMEV infection without LPS treatment (Fig. 2). Nine of 10 IL-6 Tg mice developed the virally induced demyelinating disease on day 63 postinfection and remained symptomatic until the experiment ended on day 84 (Fig. 2A). In contrast, TMEV-infected WT B6 mice did not develop the disease as expected during the time course. Consistent with the disease development pattern, the viral loads in the brains and spinal cords at 8 and 21 days postinfection were significantly higher in IL-6 Tg mice than in B6 mice (Fig. 2B). In addition, the infiltration of macrophages (CD45+ CD11bhi) and lymphocytes (CD45+ CD11b−) into the CNS of infected mice at 8 days postinfection was significantly higher in IL-6 Tg mice than in the WT mice (Fig. 2C). To verify whether the disease susceptibility in IL-6 Tg mice is attributable to the increased IL-6 level in the CNS of virus-infected IL-6 Tg mice, we compared the levels of IL-6 and KC in virus-infected WT control and IL-6 Tg mice (Fig. 2D). As shown previously (5), virus-infected WT B6 mice produced very low levels of cytokines, whereas infected IL-6 Tg mice produced high levels of both cytokines, similar to LPS-treated B6 mice. These results clearly demonstrate that mice resistant to TMEV-induced demyelinating disease become susceptible, accompanied by increased cellular infiltration to the CNS and elevated viral loads, when an excess level of IL-6 is present.
FIG 2.
B6 IL-6 Tg mice infected with TMEV develop demyelinating disease and exhibit elevated viral load. (A) Development of demyelinating disease in TMEV-infected WT (n = 10) and IL-6 Tg (n = 10) mice was monitored. (B) Viral loads in the CNS (n = 3) of infected mice were determined using plaque assays at 8 and 21 days postinfection. (C) Flow cytometry of CNS-infiltrating cells bearing CD45 and CD11b in WT (n = 3) and IL-6 Tg mice (n = 3) at 8 days after virus infection. The bar graph shows the number of CD45int CD11b+ microglia, CD45hi CD11b+ macrophages, and CD45hi CD11− lymphocytes in the CNS of infected mice. (D) Levels of IL-6 and KC in the CNS of virus-infected WT B6 and IL-6 Tg mice were assessed using specific ELISA at 8 days postinfection. Sp Cord, spinal cord.
Presence of elevated Th17 responses in the CNS of virus-infected IL-6 Tg mice.
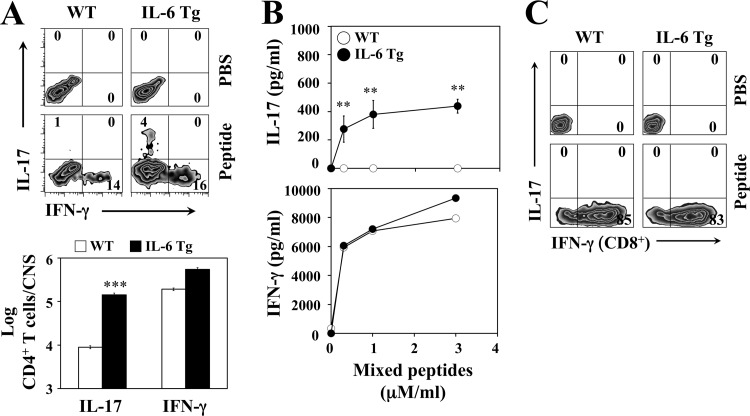
Infection of susceptible SJL mice with TMEV induces markedly elevated Th17 responses by utilizing IL-6 produced in response to the viral infection in antigen-presenting cells (31). To further investigate the role of IL-6 in the pathogenesis of TMEV-induced demyelinating disease, we determined the levels of Th17 development in the TMEV-infected WT and IL-6 Tg mice (Fig. 3). A higher proportion and number of CNS-infiltrating CD4+ T cells produced IL-17 in IL-6 Tg mice compared to the CD4+ T cells from WT mice on day 8 postinfection (Fig. 3A). However, the levels of IFN-γ-producing CD4+ T cells in the CNS were similar in these mice. Consistent with these results, the further restimulation of the CNS-infiltrating cells with viral epitope peptides for CD4+ T cells induced a markedly elevated production of IL-17 (P < 0.01) in IL-6 Tg mice compared to WT mice, whereas no difference was observed in IFN-γ production (Fig. 3B). Interestingly, the differential production of IL-17 in IL-6 Tg mice appeared to be limited to CD4+ T cells; hence, IL-17-producing CD8+ T cells were undetectable in the CNS of these mice, while similarly high levels of IFN-γ-producing CD8+ T cells (85 versus 83%) were observed (Fig. 3C). These results strongly suggest that mice with an intrinsically high level of IL-6 generate high numbers of IL-17-producing pathogenic Th17 cells following TMEV infection, which may contribute to the elevated pathogenesis of demyelinating disease.
FIG 3.
B6 IL-6 Tg mice infected with TMEV exhibit elevated Th17 response. (A) Flow-cytometric analysis of intracellular IL-17 and IFN-γ production by CNS-infiltrating CD4+ cells from WT and IL-6 Tg mice (n = 3 each) after restimulation with viral epitope peptides at 8 days postinfection. The bar graphs depict the quantity of cytokine-secreting cells among the CNS-infiltrating CD4+ cells. (B) ELISAs for IL-17 and IFN-γ levels produced by CNS-infiltrating cells at 8 days postinfection after restimulation with mixed CD4 epitope peptides. (C) Flow-cytometric analysis of intracellular production of IL-17 and IFN-γ by CNS-infiltrating CD8+ cells in WT and IL-6 Tg mice (n = 3) after restimulation with viral epitope peptides at 8 days postinfection. The presented data are representative of three independent experiments.
Upregulated expression of prosurvival molecules in the presence of IL-6 or IL-17A.
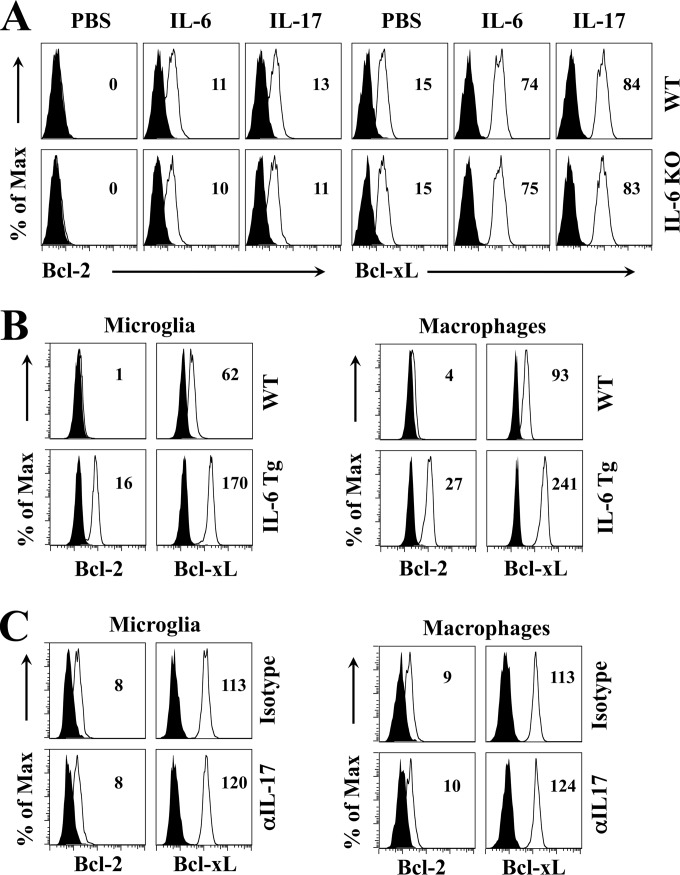
We have previously demonstrated that IL-17A produced by Th17 cells in TMEV-infected mice upregulates the expression of antiapoptotic molecules, resulting in viral persistence by enhancing the survival of virus-infected cells and blocking target cell destruction by cytotoxic T cells (5). Because the elevated Th17 response in virus-infected mice was associated with the presence of IL-6, we sought to examine whether the presence of excessive IL-6 independently promotes the viral persistence and pathogenesis of demyelinating disease or whether this occurs only via the generation of IL-17-producing Th17 cells (Fig. 4). To address this question, we first assessed the effects of IL-6 and IL-17 on the expression of antiapoptotic Bcl-2 and Bcl-xL molecules in bone marrow (BM) cells derived from naive WT B6 and IL-6 KO mice (Fig. 4A). BM cells from either WT mice or IL-6 KO mice expressed higher levels of both Bcl-2 and Bcl-xL, after treatment with IL-6 or IL-17, than BM cells similarly treated with PBS. The upregulation of Bcl expression by IL-6 or IL-17 treatment may not require the presence of endogenous IL-6; hence, similar expression levels of these molecules were observed in BM cells from WT and IL-6 KO mice. Consistent with the elevated viral loads in the CNS and the pathogenesis of demyelination in IL-6 Tg mice (Fig. 2), the major viral reservoirs in the CNS (CNS-infiltrating macrophages and CNS-resident microglia) of IL-6 Tg mice expressed higher levels of Bcl-2 and Bcl-xL at 8 days postinfection than those from WT mice (Fig. 4B). Because IL-6 Tg mice produced a higher level of IL-17 (Fig. 2D and E), IL-6 Tg mice were treated with either anti-IL-17 antibodies or isotype-matched control antibodies during the early stage of viral infection (Fig. 4C). However, treatment of virus-infected IL-6 Tg mice with anti-IL-17 antibodies did not affect Bcl-2 and Bcl-xL expression, suggesting that IL-6 also induces the upregulated expression of prosurvival molecules in virus-infected mice in an IL-17-independent manner.
FIG 4.
IL-6 promotes the expression of prosurvival proteins independently from IL-17. (A) Intracellular Bcl-2 and Bcl-xL expression in BM cells from naive WT and IL-6 KO mice was assessed after 24 h of stimulation with PBS, 100 ng/ml IL-6, or 100 ng/ml IL-17. (B) Comparison of Bcl-2 and Bcl-xL levels expressed by CNS-resident microglia and CNS-infiltrating macrophages in WT (n = 3) and IL-6 Tg mice (n = 3) at 8 days after TMEV infection. (C) Bcl-2 and Bcl-xL expression levels in microglia and macrophages from IL-6 Tg mice treated with either control antibody (n = 3) or anti-IL-17 antibody (n = 3) at 8 days postinfection. In each panel, the numbers in histograms represent relative median fluorescence intensity differences between the cells stained with isotype antibody (filled histogram) and anti-Bcl antibodies (open histogram). The data are representative of three independent experiments.
Synergistic upregulation of prosurvival protein expression by IL-6 and IL-17.
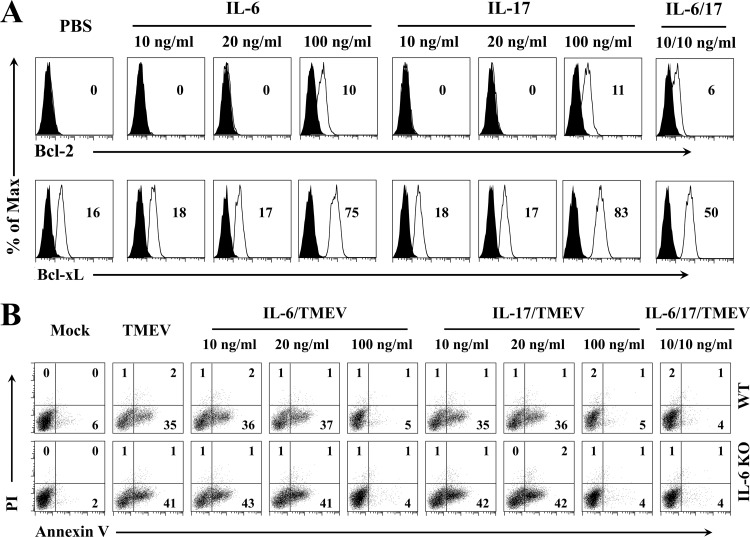
We further examined whether the presence of excessive IL-6 independently promotes the viral persistence leading to the pathogenesis of demyelinating disease or IL-6 functions only via the generation of IL-17-producing Th17 cells (Fig. 5). We first assessed the effects of IL-6 and IL-17 on the expression of Bcl-2 and Bcl-xL molecules in BM cells derived from naive IL-6 KO mice (Fig. 5A). Various concentrations of recombinant IL-6 or IL-17 were added to the BM cell cultures of IL-6 KO mice. A relatively high concentration (100 ng/ml) of IL-6 or IL-17 was able to upregulate the expression of Bcl-2 and Bcl-xL in the absence of viral infection, while low concentrations (10 and 20 ng/ml) failed. Interestingly, however, the mixture of low concentrations (10 ng/ml each) of IL-6 and IL-17, which does not upregulate Bcl expression independently, was capable of upregulating the expression of these molecules (Fig. 5A). The upregulation of Bcl-2 and Bcl-xL expression by the presence of both IL-6 and IL-17 was far greater than the additive effect, because 20 ng/ml of IL-6 or IL-17 independently failed to yield measurable upregulation. These results indicate that the presence of both IL-6 and IL-17 provides a synergistic effect on the upregulation of these prosurvival molecules.
FIG 5.
IL-6 and IL-17 synergistically promote the expression of prosurvival proteins to prevent virus infection-induced cellular apoptosis. (A) Flow-cytometric analysis of Bcl-2 and Bcl-xL expression in BM cells from IL-6 KO mice after 24 h of stimulation with IL-6, IL-17, or IL-6/IL-17 combination. (B) Flow-cytometric assessment for the effects of IL-6, IL-17, and IL-6/IL-17 combination on viral infection-induced cell apoptosis by staining with propidium iodide and allophycocyanin-conjugated annexin V. BM cells from WT or IL-6 KO mice were infected with TMEV in vitro at an MOI of 10 for 24 h in the presence or absence of the cytokines before flow cytometry analysis. The data are representative of two to three independent experiments.
Synergistic inhibition of virus infection-induced cellular apoptosis in the presence of both IL-6 and IL-17.
To further examine the functional consequence of the upregulated expression of prosurvival proteins, the effect of IL-6 and/or IL-17 on the survival of virus-infected cells, leading to viral persistence in infected mice, was assessed by measuring the levels of annexin V expression using flow cytometry (Fig. 5B). A high dose of IL-17 treatment inhibited TMEV infection-induced apoptosis of BM cells from both WT and IL-6 KO mice completely to the level of uninfected cells (from 35 to 41% to 4 to 5%). Similarly, the same high dose of IL-6 blocked BM cell apoptosis completely. Consistent with the synergistically upregulated expression of prosurvival molecules in the presence of a combination of low concentrations of IL-6 and IL-17 (Fig. 5A), low concentrations of IL-6 and IL-17 together resulted in the complete inhibition of apoptosis, although the low concentrations of IL-6 or IL-17 alone failed to block infection-induced apoptosis (Fig. 5B). Only a slight reduction in the apoptosis of BM cells from WT B6 mice was observed after TMEV infection compared to that of cells from IL-6 KO B6 mice. Therefore, BM cells from resistant WT B6 mice may not produce high enough levels of IL-6 after TMEV infection to significantly hinder the level of infection-induced apoptosis. These results are consistent with those of previous studies demonstrating low TMEV infectivity of cells from B6 mice, which are resistant to the development of demyelinating disease (31). However, the resistant B6 mice become susceptible after LPS treatment, which induces a high level of IL-6 (5). These results, together with results from IL-6 Tg mice shown in Fig. 2, strongly suggest that the presence of an excessive level of IL-6 promotes the pathogenesis of TMEV-induced demyelinating disease, in part by elevating viral persistence via blocking cellar apoptosis.
Synergistic inhibition of CD8+ T cell-mediated cytolysis by IL-6 and IL-17.
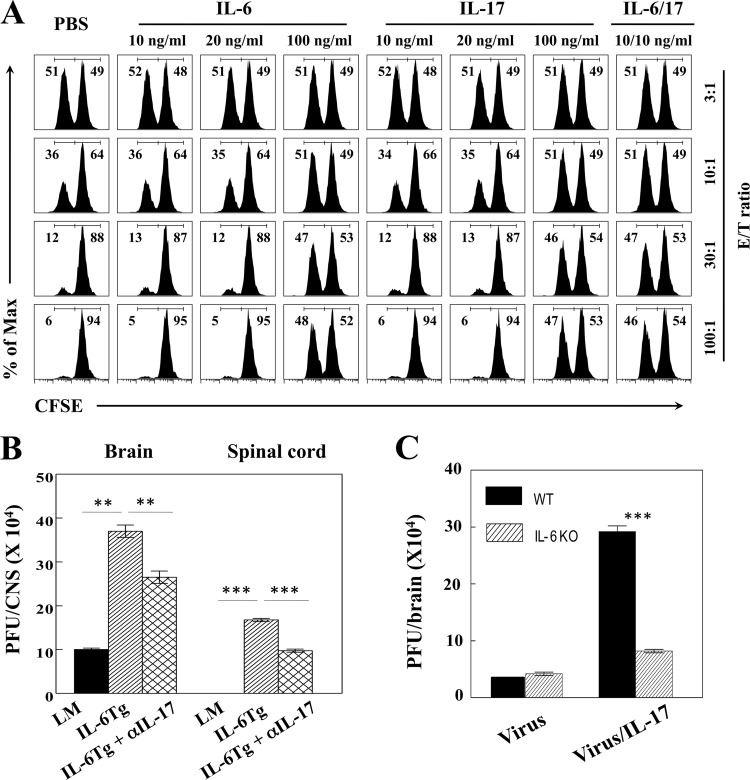
To extend the above-described synergistic effects of IL-6 and IL-17 to antiviral T cell function, we determined the potential inhibition of cytotoxic CD8+ T cell function in the presence of IL-6 and/or IL-17 (Fig. 6A). To exclude the effects of endogenous IL-6 on T cell function, we used splenic T cells from IL-6 KO mice infected with TMEV at 8 days postinfection as effector cells and CFSE-labeled peptide-loaded splenic cells from naive IL-6 KO mice as target cells in our in vitro killing assay (5). In line with the previous work, the high concentration (100 ng/ml) of IL-17, but not low concentrations (10 and 20 ng/ml), abrogated the cytolytic activity of CD8+ T cells against the predominant virus-specific target cells at levels above the 10:1 effector/target cell ratios (Fig. 6A). The same high concentration of IL-6 alone similarly inhibited cytolysis. However, the combination of low concentrations of IL-6 and IL-17 (10 ng/ml each) completely blocked cytolysis, whereas 10 and 20 ng/ml of IL-6 or IL-17 failed to inhibit cytolysis. These results clearly indicate that IL-6 and IL-17 synergistically inhibit the cytolysis of target cells mediated by virus-specific CD8+ T cells.
FIG 6.
IL-6 and IL-17 are synergistic with respect to the inhibition of antiviral cytotoxic T cell activity against target cells. (A) Spleen cells from naive IL-6 KO mice were pulsed with VP2121-130 or OVA323-339 peptides and labeled with a lower or higher concentration of CFSE as target cells, respectively. The labeled target cells and effector spleen cells isolated at 8 days from TMEV-infected IL-6 KO mice were cocultured for 60 h in the presence of different doses of cytokines and their combination. The numbers in each histogram show the percentages of the lower (VP2121-130) and higher (OVA323-339) concentrations of the overall CFSE-labeled cell population. (B) Levels of infectious virus in the CNS of TMEV-infected WT (littermate [LM]) mice and IL-6 Tg mice left untreated or treated with anti-IL-17 antibody (n = 3/group) were quantified using plaque assays at 8 days postinfection. (C) Levels of infectious virus in the CNS of WT and IL-6 KO mice infected with TMEV in PBS or 100 ng IL-17 (n = 3/group) were quantified using plaque assays at 7 days postinfection. **, P < 0.01; ***, P < 0.001.
We next employed IL-6 Tg mice to determine whether viral persistence in these animals is due mainly to the low level of antiviral CD8+ T cell function, which is attributable to the presence of high IL-17 levels. We administered anti-IL-17 antibodies into TMEV-infected IL-6 Tg mice and compared the viral load levels in the CNS of these antibody-treated IL-6 Tg mice to those of the WT control mice and untreated IL-6 Tg mice at 8 days postinfection (Fig. 6B). As shown earlier (Fig. 2), the viral load was significantly greater in virus-infected IL-6 Tg mice than in the WT control mice. Interestingly, the viral load in anti-IL-17 antibody-treated IL-6 Tg mice remained significantly higher than that of the WT control mice, although it was significantly lower than that of untreated IL-6 Tg mice. These results are consistent with the notion that excessive IL-6 alone is able to inhibit the function of antiviral CD8+ T cells in vivo. However, IL-6 appears to play a potent synergistic role in the inhibition of T cell function in combination with IL-17. In contrast, the level of viral load in IL-6 KO mice was similar to or lower than that of the WT control mice (Fig. 6C). Interestingly, the viral load in IL-6 KO mice infected with TMEV together with IL-17 was significantly elevated, although the increase in the viral load was lower than that in the WT control mice, which are capable of producing IL-6. These results strongly suggest that viral load is heightened in the presence of IL-17 alone in virus-infected mice and that it is further markedly elevated in the presence of both IL-6 and IL-17, consistent with the synergistically elevated survival of virus-infected cells in vitro (Fig. 5).
Signaling pathways of IL-6 and IL-17 for synergistic expression of Bcl-2 and Bcl-xL.
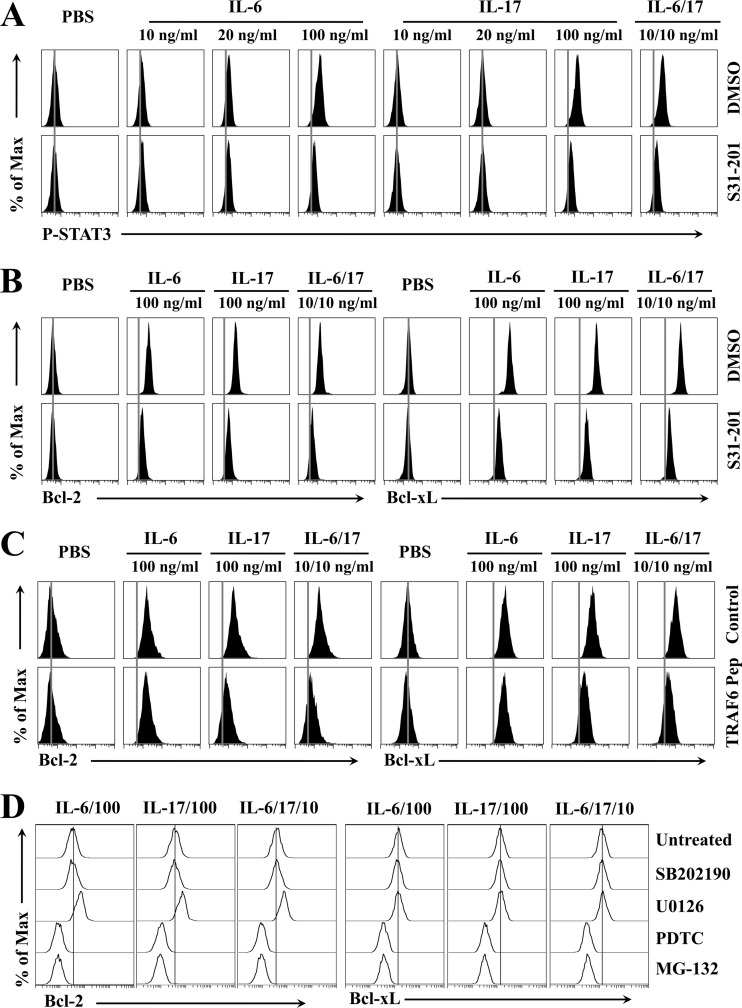
To understand the mechanisms underlying the synergistic function of IL-6 and IL-17 in combination, we first explored the possible involvement of the signal transducer and activator of transcription 3 (STAT3), which is phosphorylated when cells are treated with IL-6 (24) or IL-17 (20, 26, 27, 29, 32). Flow-cytometric assessment of phosphorylated intracellular STAT3 indicated that a high concentration (100 ng/ml) of IL-6 or IL-17, but not low concentrations (10 and 20 ng/ml), induced STAT3 activation in IL-6-deficient BM cells (Fig. 7A). The phosphorylation of STAT3 induced by IL-6 and IL-17 was completely abrogated in the presence of S31-201, a specific inhibitor against STAT3 activation (33), compared to that in the presence of diluent dimethylsulfoxide (DMSO) alone. In addition, we detected the synergistic activation of STAT3 in the presence of low concentrations of both IL-6 and IL-17 (Fig. 7A). To verify whether STAT3 activation is associated with the upregulation of intracellular Bcl-2 and Bcl-xL expression, the expression levels of Bcl-2 and Bcl-xL in IL-6-deficient BM cells were determined following treatment with cytokines in the presence or absence of S31-201 (Fig. 7B). The presence of S31-201 inhibited the IL-6- and/or IL-17-induced upregulation of Bcl-2 and Bcl-xL expression in the BM cells. These results indicate that the STAT3 pathway is involved in the upregulation of prosurvival molecules induced by IL-6 and IL-17 independently or cooperatively. We further assessed potential associations with other signaling pathways, such as tumor necrosis factor receptor-associated factor (TRAF) 6 (Fig. 7C), which is involved in the signaling pathway of IL-17 (34). It was previously shown that IL-17 induces the activation of nuclear factor (NF)-κB via the TRAF6 pathway. The specific inhibition of TRAF6 by the presence of an antagonistic peptide drastically abrogated the Bcl-2 and Bcl-xL expression induced by IL-17 alone or in combination with IL-6. In contrast, blocking TRAF6 activation did not affect the upregulation of survival molecules induced by IL-6. Thus, the NF-κB activation induced by IL-6 appears to utilize a different pathway. These results strongly suggest that the TRAF6 pathway for NF-κB activation is required for the IL-17-mediated upregulation of Bcl-2 and Bcl-xL expression and that IL-17 provides synergistic signaling for their upregulation via this pathway. Further experiments were conducted to determine the potential involvement of p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and NF-κB (Fig. 7D), many of which may be activated in different settings by IL-6 or IL-17 (24, 25). The increased expression of Bcl-2 and Bcl-xL induced by IL-6 and IL-17 alone or in combination was abrogated only by the NF-κB inhibitor pyrrolidone dithiocarbonate (PDTC) or MG-132 and not by p38 inhibitor SB202190 or ERK inhibitor U0126. These results are consistent with the notion that NF-κB activation is required for the upregulation of survival molecules by both IL-6 and IL-17, independently or cooperatively. Taken together, both STAT3 and NF-κB pathways are involved in the synergistic effects of IL-6 and IL-17 on the upregulation of prosurvival protein expression, and the NF-κB activation signaling provided by IL-17 is necessary for the synergistic upregulation.
FIG 7.
Common (STAT3 and NF-κB) and distinct (TRAF6) signaling pathways by IL-6 and IL-17 are involved in the upregulation of Bcl molecules. (A) Flow cytometry of intracellular phosphorylated STAT3 in IL-6 KO BM cells pretreated with the control (DMSO) or an inhibitor of STAT3, S31-201 (100 μM), for 10 min prior to stimulation with IL-6, IL-17, or IL-6/IL-17 for 15 min. (B) Flow cytometry of intracellular Bcl-2 and Bcl-xL in IL-6 KO BM cells pretreated with DMSO or S31-201 for 10 min prior to stimulation with the cytokines listed for panel A for 24 h. (C) Expression of Bcl-2 and Bcl-xL in IL-6 KO BM cells was determined after pretreatment with 100 μM control peptide or 100 μM TRAF6 inhibitory peptide prior to stimulation with the cytokines for 24 h. (D) Expression of Bcl-2 and Bcl-xL was determined as described for panel C after pretreatment with 10 μM SB202190, 10 μM U0126, 100 μM PDTC, or 10 μM MG-132 for 2 h and then stimulated with the cytokines for 24 h. The presented data are representatives of at least two separate experiments.
DISCUSSION
It has been shown previously that infections with many different microbes, including viruses, induce various innate immune cytokines via signaling mediated by pattern recognition molecules (35). Among the innate immune cytokines, IL-6 is one of the most pronounced cytokines produced following such infections. IL-6 is known to play an important role in initiating and maintaining inflammatory responses during infections as well as in autoimmune diseases (17, 18). However, the precise roles of IL-6 in the protection from and/or the development of virus-induced inflammatory disease remain unclear. Some studies suggest that IL-6 plays a protective role in viral infections, including TMEV-induced demyelinating disease (36, 37). In contrast, our previous studies suggested that the infection of susceptible mice with TMEV induces vigorous IL-6 production in macrophages, microglia, and dendritic cells, which are potential antigen-presenting cells in the CNS, and that these virus-infected cells preferentially promote the induction of pathogenic Th17 cells in the development of TMEV-induced demyelinating disease (5). In addition, mice that produced high levels of IL-6 following administration of certain TLR ligands [i.e., LPS for TLR4 and poly(I·C) for TLR3] display exacerbated development of the disease (8, 10). Therefore, IL-6 may play a protective or pathogenic role in viral clearance and the development of demyelinating disease, depending on the level, distribution, and/or timing of this cytokine in infected mice.
In this study, we utilized IL-6 Tg mice and IL-6 KO mice to demonstrate that IL-6 plays a pathogenic role in the development of TMEV-induced demyelinating disease. Our results indicate that IL-6 Tg mice with the resistant B6 background are susceptible to TMEV-induced demyelinating disease accompanied by increased viral persistence in the CNS. These results support the pathogenic role of IL-6. IL-6 appears to exert pathogenic roles using 2 different mechanisms. One is by enhancing the production of IL-17 via the generation of Th17 cells, which results in elevated viral persistence in the CNS (5) (Fig. 1 to 3). In addition, the produced IL-17 promotes the production of IL-6 via a positive amplification loop (20–22). The other mechanism involves a direct (Fig. 4) and, more importantly, synergistic upregulation of the expression of Bcl-2 and Bcl-xL with IL-17 at low concentration (Fig. 5), which inhibits the apoptosis of virus infected cells (Fig. 5) and antiviral cytotoxic T cell function (Fig. 6). We have previously demonstrated that IL-17 alone at a high concentration induces similar upregulation of the expression of these molecules (5). However, these results somewhat contradict the previous observation that the subcutaneous administration of recombinant IL-6 into susceptible SJL mice suppresses the development of demyelination induced by TMEV infection (36). Therefore, the sites where IL-6 is available, i.e., CNS versus subcutaneous lesions, may be a critical factor for the pathogenic function of IL-6 in the development of demyelinating disease.
The synergistic effect of IL-6 and IL-17 on viral persistence apparently is operational in TMEV-infected mice, as viral persistence is partially enhanced in IL-6 Tg mice after IL-17 administration and reduced after anti-IL-17 antibody treatment (Fig. 4C). These results suggest that an excessive level of IL-6 during early viral infection promotes the generation of IL-17-producing Th17 cells. The production of these cytokines may be further amplified by each other, as previously suggested (20–22). Moreover, IL-6 and IL-17 either independently or synergistically promote viral persistence by protecting virus-infected cells from apoptosis (Fig. 5) and/or by inhibiting T cell-mediated target cell destruction (Fig. 6). These results are consistent with previous reports indicating that both IL-6 and IL-17 independently inhibit cellular apoptosis by upregulating the expression of Bcl-2 and Bcl-xL survival molecules (5, 38). The initial level of IL-6 production appears to be determined by the susceptibility of the cells to viral infection, which is associated with genetic background. Cells from susceptible mice are dramatically more permissive to TMEV infection (31, 39) and, consequently, the infected cells from susceptible mice produce higher levels of IL-6, mainly via TLR and MDA-5 signaling (6–8, 40). Furthermore, higher levels of IL-1, a potent IL-6 and IL-17 inducer, are produced in virus-infected cells from susceptible mice than from resistant mice (9). Therefore, the initial levels of these cytokines, which reflect viral permissiveness levels, are critically important in determining the susceptibility of mice to the development of demyelinating disease associated with viral persistence.
The signaling of IL-6 and IL-17 involved in the upregulation of the Bcl molecules apparently depends on the activation of STAT3 and NF-κB, because the inhibition of STAT3 phosphorylation or the blocking of NF-κB activation abrogates the IL-6- and IL-17-dependent upregulation of Bcl-2 and Bcl-xL expression (Fig. 7). These results are consistent with previous reports indicating that both IL-6 and IL-17 activate STAT3 and NF-κB (27, 29, 41). In addition, it was previously shown that the activation of both STAT3 and NF-κB leads to the upregulation of Bcl-2 and Bcl-xL expression (26, 42). However, the individual components associated with the activation of STAT3 and NF-κB may differ between IL-6- and IL-17-mediated signaling. IL-17 signaling utilizes JAK2 for STAT3 phosphorylation and Act1/TRAF6 for NF-κB activation (34, 43). Conversely, IL-6 signaling involves JAK3 for STAT3 phosphorylation and phosphatidylinositol 3-kinase (PI3K)/Akt for NF-κB activation (16, 44). PI3K/Akt inhibition leads to an increased activation of p21waf1 (45), which interferes with the expression of Bcl proteins (46–48). However, the upregulation of P21 may not necessarily alter the expression of Bcl proteins (49). In addition, Bcl-2 expression induced by IL-6 is regulated mainly by the JAK/STAT3 pathway associated with Pim-1/2 and c-Myc rather than by NF-κB activation (50, 51). Therefore, the PI3K/Akt pathway for the activation of NF-κB by IL-6 may poorly contribute to the expression of Bcl proteins, although a high concentration of IL-6 may upregulate the expression via this pathway (Fig. 7). In contrast, the inhibition of TRAF-6 function, which is required for IL-17-mediated NF-κB activation, abrogated the synergistic upregulation of Bcl-2 and Bcl-xL by IL-6 and IL-17 (Fig. 7). Therefore, the TRAF6-mediated signaling of IL-17-induced NF-κB activation is associated with this synergism, in addition to the activation of STAT3, which is provided mainly by IL-6 or both cytokines. The activation of NF-κB subunits may differ between IL-6- and IL-17-induced NF-κB activation, and the difference may affect the transcription of Bcl-2 and Bcl-xL via recruitment of C/EBPA, which cooperates with NF-κB p50 (52).
Nevertheless, the synergistic function between IL-6 and IL-17 for the upregulation of Bcl-2 and Bcl-xL expression implies a potentially important role for this combination in the development of chronic viral inflammation, autoimmune diseases, and cancers. Furthermore, chronic viral infections may also negatively affect the development or the progression of autoimmune diseases and cancers by altering the survival of cells (12–14). These cytokines have been targets for the treatment of many autoimmune diseases and cancers, although the approaches involve blocking the function of a single cytokine, IL-6 or IL-17 (53, 54). However, these approaches may not yield satisfactory results, because the residual low levels of IL-6 or IL-17 may provide the combination of these cytokines sufficiently contributing to the pathogenesis of diseases. Therefore, it may be beneficial to block both IL-6 and IL-17 signals in the treatment and/or prevention of disease development.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (RO1 NS28752 and RO1 NS33008) and the National Multiple Sclerosis Society (RG 4001-A6).
We thank Liang Zhou at the Department of Microbiology-Immunology and Pathology, Northwestern University Medical School, for his helpful comments.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Pevear DC, Calenoff M, Rozhon E, Lipton HL. 1987. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J. Virol. 61:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipton HL, Dal Canto MC. 1976. Chronic neurologic disease in Theiler's virus infection of SJL/J. mice. J. Neurol. Sci. 30:201–207. 10.1016/0022-510X(76)90267-7 [DOI] [PubMed] [Google Scholar]
- 3.Dal Canto MC, Lipton HL. 1977. Multiple sclerosis. Animal model: Theiler's virus infection in mice. Am. J. Pathol. 88:497–500 [PMC free article] [PubMed] [Google Scholar]
- 4.Dal Canto MC, Kim BS, Miller SD, Melvold RW. 1996. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelination: a model for human multiple sclerosis. Methods 10:453–461. 10.1006/meth.1996.0123 [DOI] [PubMed] [Google Scholar]
- 5.Hou W, Kang HS, Kim BS. 2009. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 206:313–328. 10.1084/jem.20082030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.So EY, Kang MH, Kim BS. 2006. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858–867. 10.1002/glia.20346 [DOI] [PubMed] [Google Scholar]
- 7.So EY, Kim BS. 2009. Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia 57:1216–1226. 10.1002/glia.20843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin YH, Kaneyama T, Kang MH, Kang HS, Koh CS, Kim BS. 2011. TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection. J. Neuroinflammation 8:178. 10.1186/1742-2094-8-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BS, Jin YH, Meng L, Hou W, Kang HS, Park HS, Koh CS. 2012. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J. Neuroinflammation 9:217. 10.1186/1742-2094-9-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pullen LC, Park SH, Miller SD, Dal Canto MC, Kim BS. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497–4503 [PubMed] [Google Scholar]
- 11.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3:1133–1136. 10.1038/nm1097-1133 [DOI] [PubMed] [Google Scholar]
- 12.Schattner A, Rager-Zisman B. 1990. Virus-induced autoimmunity. Rev. Infect. Dis. 12:204–222. 10.1093/clinids/12.2.204 [DOI] [PubMed] [Google Scholar]
- 13.Horwitz MS, Sarvetnick N. 1999. Viruses, host responses, and autoimmunity. Immunol. Rev. 169:241–253. 10.1111/j.1600-065X.1999.tb01319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arzumanyan A, Reis HM, Feitelson MA. 2013. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat. Rev. Cancer 13:123–135. 10.1038/nrc3449 [DOI] [PubMed] [Google Scholar]
- 15.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. 1999. Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J. Biol. Chem. 274:23013–23019. 10.1074/jbc.274.33.23013 [DOI] [PubMed] [Google Scholar]
- 16.Hideshima T, Nakamura N, Chauhan D, Anderson KC. 2001. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 20:5991–6000. 10.1038/sj.onc.1204833 [DOI] [PubMed] [Google Scholar]
- 17.Graeve L, Baumann M, Heinrich PC. 1993. Interleukin-6 in autoimmune disease. Role of IL-6 in physiology and pathology of the immune defense. Clin. Investig. 71:664–671 [DOI] [PubMed] [Google Scholar]
- 18.Ishihara K, Hirano T. 2002. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 13:357–368. 10.1016/S1359-6101(02)00027-8 [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 20.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 21.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390–1397. 10.1038/ni1539 [DOI] [PubMed] [Google Scholar]
- 22.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593–2603. 10.1084/jem.183.6.2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. 1993. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597–608. 10.1016/0092-8674(93)90508-N [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto T. 2005. Interleukin-6: from basic science to medicine–40 years in immunology. Annu. Rev. Immunol. 23:1–21. 10.1146/annurev.immunol.23.021704.115806 [DOI] [PubMed] [Google Scholar]
- 25.Gaffen SL. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556–567. 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SY, Kwok SK, Son HJ, Ryu JG, Kim EK, Oh HJ, Cho ML, Ju JH, Park SH, Kim HY. 2013. IL-17-mediated Bcl-2 expression regulates survival of fibroblast like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthr. Res. Ther. 15:R31. 10.1186/ar4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. 2004. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthr. Res. Ther. 6:R120–R128. 10.1186/ar1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanai Y, Tokuda H, Ohta T, Matsushima-Nishiwaki R, Takai S, Kozawa O. 2006. Phosphatidylinositol 3-kinase/Akt auto-regulates PDGF-BB-stimulated interleukin-6 synthesis in osteoblasts. J. Cell. Biochem. 99:1564–1571. 10.1002/jcb.21007 [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. 2009. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 206:1457–1464. 10.1084/jem.20090207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. 1992. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 89:232–235. 10.1073/pnas.89.1.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou W, So EY, Kim BS. 2007. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 3:e124. 10.1371/journal.ppat.0030124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramaniam SV, Cooper RS, Adunyah SE. 1999. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem. Biophys. Res. Commun. 262:14–19. 10.1006/bbrc.1999.1156 [DOI] [PubMed] [Google Scholar]
- 33.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. 2007. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. U. S. A. 104:7391–7396. 10.1073/pnas.0609757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwandner R, Yamaguchi K, Cao Z. 2000. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 191:1233–1240. 10.1084/jem.191.7.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez M, Pavelko KD, McKinney CW, Leibowitz JL. 1994. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J. Immunol. 153:3811–3821 [PubMed] [Google Scholar]
- 37.Ramshaw IA, Ramsay AJ, Karupiah G, Rolph MS, Mahalingam S, Ruby JC. 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159:119–135. 10.1111/j.1600-065X.1997.tb01011.x [DOI] [PubMed] [Google Scholar]
- 38.Leu CM, Wong FH, Chang C, Huang SF, Hu CP. 2003. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene 22:7809–7818. 10.1038/sj.onc.1207084 [DOI] [PubMed] [Google Scholar]
- 39.Kang MH, So EY, Park H, Kim BS. 2008. Replication of Theiler's virus requires NF-kappaB-activation: higher viral replication and spreading in astrocytes from susceptible mice. Glia 56:942–953. 10.1002/glia.20668 [DOI] [PubMed] [Google Scholar]
- 40.Jin YH, Kim SJ, So EY, Meng L, Colonna M, Kim BS. 2012. Melanoma differentiation-associated gene 5 is critical for protection against Theiler's virus-induced demyelinating disease. J. Virol. 86:1531–1543. 10.1128/JVI.06457-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong Z, Wen Z, Darnell JE., Jr 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95–98. 10.1126/science.8140422 [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharya S, Ray RM, Johnson LR. 2005. STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem. J. 392:335–344. 10.1042/BJ20050465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. 2007. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8:247–256. 10.1038/ni1439 [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Walia B, Evans J, Gewirtz AT, Merlin D, Sitaraman SV. 2003. IL-6 induces NF-kappa B activation in the intestinal epithelia. J. Immunol. 171:3194–3201. 10.4049/jimmunol.171.6.3194 [DOI] [PubMed] [Google Scholar]
- 45.Barre B, Avril S, Coqueret O. 2003. Opposite regulation of myc and p21waf1 transcription by STAT3 proteins. J. Biol. Chem. 278:2990–2996. 10.1074/jbc.M210422200 [DOI] [PubMed] [Google Scholar]
- 46.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241. 10.1016/S0092-8674(00)80405-5 [DOI] [PubMed] [Google Scholar]
- 47.Strasberg Rieber M, Welch DR, Rieber M. 2001. Suppression of C8161 melanoma metastatic ability by chromosome 6 induces differentiation-associated tyrosinase and decreases proliferation on adhesion-restrictive substrates mediated by overexpression of p21WAF1 and down-regulation of bcl-2 and cyclin D3. Biochem. Biophys. Res. Commun. 281:159–165. 10.1006/bbrc.2001.4330 [DOI] [PubMed] [Google Scholar]
- 48.Wu Q, Kirschmeier P, Hockenberry T, Yang TY, Brassard DL, Wang L, McClanahan T, Black S, Rizzi G, Musco ML, Mirza A, Liu S. 2002. Transcriptional regulation during p21WAF1/CIP1-induced apoptosis in human ovarian cancer cells. J. Biol. Chem. 277:36329–36337. 10.1074/jbc.M204962200 [DOI] [PubMed] [Google Scholar]
- 49.Sheikh MS, Garcia M, Zhan Q, Liu Y, Fornace AJ., Jr 1996. Cell cycle-independent regulation of p21Waf1/Cip1 and retinoblastoma protein during okadaic acid-induced apoptosis is coupled with induction of Bax protein in human breast carcinoma cells. Cell Growth Differ. 7:1599–1607 [PubMed] [Google Scholar]
- 50.Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T. 1999. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity 11:709–719. 10.1016/S1074-7613(00)80145-4 [DOI] [PubMed] [Google Scholar]
- 51.Sepulveda P, Encabo A, Carbonell-Uberos F, Minana MD. 2007. BCL-2 expression is mainly regulated by JAK/STAT3 pathway in human CD34+ hematopoietic cells. Cell Death Differ. 14:378–380. 10.1038/sj.cdd.4402007 [DOI] [PubMed] [Google Scholar]
- 52.Dooher JE, Paz-Priel I, Houng S, Baldwin AS, Jr, Friedman AD. 2011. C/EBPalpha, C/EBPalpha oncoproteins, or C/EBPbeta preferentially bind NF-kappaB p50 compared with p65, focusing therapeutic targeting on the C/EBP:p50 interaction. Mol. Cancer Res. 9:1395–1405. 10.1158/1541-7786.MCR-11-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou W, Restifo NP. 2010. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10:248–256. 10.1038/nri2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. 2012. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 38:904–910. 10.1016/j.ctrv.2012.04.007 [DOI] [PubMed] [Google Scholar]