ABSTRACT
Infectious laryngotracheitis (ILT) is a highly contagious acute respiratory disease of chickens caused by infectious laryngotracheitis virus (ILTV). The disease is controlled mainly through biosecurity and vaccination with live attenuated strains of ILTV and vectored vaccines based on turkey herpesvirus (HVT) and fowlpox virus (FPV). The current live attenuated vaccines (chicken embryo origin [CEO] and tissue culture origin [TCO]), although effective, can regain virulence, whereas HVT- and FPV-vectored ILTV vaccines are less efficacious than live attenuated vaccines. Therefore, there is a pressing need to develop safer and more efficacious ILTV vaccines. In the present study, we generated Newcastle disease virus (NDV) recombinants, based on the LaSota vaccine strain, expressing glycoproteins B (gB) and D (gD) of ILTV using reverse genetics technology. These recombinant viruses, rLS/ILTV-gB and rLS/ILTV-gD, were slightly attenuated in vivo yet retained growth dynamics, stability, and virus titers in vitro that were similar to those of the parental LaSota virus. Expression of ILTV gB and gD proteins in the recombinant virus-infected cells was detected by immunofluorescence assay. Vaccination of specific-pathogen-free chickens with these recombinant viruses conferred significant protection against virulent ILTV and velogenic NDV challenges. Immunization of commercial broilers with rLS/ILTV-gB provided a level of protection against clinical disease similar to that provided by the live attenuated commercial vaccines, with no decrease in body weight gains. The results of the study suggested that the rLS/ILTV-gB and -gD viruses are safe, stable, and effective bivalent vaccines that can be mass administered via aerosol or drinking water to large chicken populations.
IMPORTANCE This paper describes the development and evaluation of novel bivalent vaccines against chicken infectious laryngotracheitis (ILT) and Newcastle disease (ND), two of the most economically important infectious diseases of poultry. The current commercial ILT vaccines are either not safe or less effective. Therefore, there is a pressing need to develop safer and more efficacious ILT vaccines. In the present study, we generated Newcastle disease virus (NDV) recombinants expressing glycoproteins B (gB) and D (gD) of infectious laryngotracheitis virus (ILTV) using reverse genetics technology. These recombinant viruses were safe, stable, and immunogenic and replicated efficiently in birds. Vaccination of chickens with these recombinant viruses conferred complete protection against ILTV and NDV challenge. These novel bivalent vaccines can be mass administered via aerosol or drinking water to large chicken populations at low cost, which will have a direct impact on poultry health, fitness, and performance.
INTRODUCTION
Infectious laryngotracheitis (ILT) is a highly contagious acute respiratory disease that has become a major problem in the U.S. poultry industry in recent years (1). Chickens are vaccinated multiple times with live infectious laryngotracheitis virus (ILTV) strains that were attenuated by multiple passages either in embryonated eggs (chicken embryo origin [CEO]) or in tissue culture (tissue culture origin [TCO]) (2, 3). Although these vaccines protect against clinical disease, they have residual virulence which is exacerbated by continued infections of naive birds from productively infected animals and latent carriers (4–6). Moreover, the CEO vaccine strain has been demonstrated to mutate and become more virulent simply by bird-to-bird passage (7). In high-density poultry rearing facilities there is a continuous reservoir of viruses, both virulent and vaccinal, evolving to higher levels of virulence. These “revertants” have become the dominant field strains in poultry populations and are the cause of field outbreaks (8, 9).
To overcome these problems associated with live attenuated ILTV vaccine strains, inactivated whole-virus vaccines and turkey herpesvirus (HVT) and fowlpox virus (FPV) vectors encoding ILTV antigens have been developed (10–15). Although in protection studies these vaccines are completely safe when administered at different ages, they induce only partial protection compared with that induced by live attenuated vaccines (16). Thus, there is a significant need to revise the ILT control strategies, particularly regarding the development of next-generation vaccines that are safe and protective.
Newcastle disease (ND), caused by infection with virulent Newcastle disease virus (NDV), is one of the most serious infectious diseases in poultry (17). Vaccination combined with strict biosecurity practices has been the recommended strategy for controlling NDV outbreaks for over 60 years (18). The NDV LaSota strain, a naturally occurring low-virulence NDV strain, has been routinely used as a live vaccine throughout the world (19). This vaccine strain induces strong immunity both locally and systemically and can be readily administered through drinking water supplies or by direct spray (20). The LaSota vaccine has been proven to be safe and stable, with no reports of virulence reversion or recombination with field strains. During the past decade, the LaSota vaccine and other NDV strains have been developed as vectors using reverse genetics technology in order to express foreign antigens for vaccine or gene therapy purposes (21–25). Previously our group has generated LaSota strain-based recombinant viruses expressing avian metapneumovirus (aMPV) G protein (26, 27) and infectious bronchitis virus (IBV) S2 protein (28) as vaccines for protection of birds against aMPV and IBV challenge. In the present study, we further engineered the NDV LaSota vector to construct bivalent vaccines expressing the immunogenic proteins of ILTV.
ILTV, an alphaherpesvirus, possesses at least 10 envelope glycoprotein genes, including the UL27 and US6 genes, encoding glycoprotein B (gB) and glycoprotein D (gD), respectively, which are highly conserved herpesvirus structural glycoproteins (29). Glycoprotein B is essential for infectivity and is involved in membrane fusion and virus penetration (30, 31). Glycoprotein D is essential for most herpesviruses and functions as a receptor for virus binding to susceptible cells (32, 33). In addition, gB in gD elicits neutralizing antibodies and cell-mediated immune responses and has been shown to be a candidate antigen for recombinant vaccines (34–36). Therefore, both ILTV gB and gD were selected as putative protective antigens in this study. Using reverse genetics techniques, we engineered the NDV genome to contain either the gB or gD open reading frames (ORF) of ILTV. These recombinant viruses (rLS/ILTV-gB and rLS/ILTV-gD) were evaluated in vitro and in vivo for levels of protein expression, stability, safety, and protection against ILTV and NDV challenge in chickens.
MATERIALS AND METHODS
Cells, viruses, and nucleic acid isolation.
The HEp-2 (CCL-81; ATCC) and DF-1 (CRL-12203; ATCC) cell lines were grown in Dulbecco's modified Eagle medium (DMEM) (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA) and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B; Thermo Scientific, Suwanee, GA) at 37°C in a 5% CO2 atmosphere. DF-1 cells were maintained in DMEM supplemented with 10% allantoic fluid (AF) from 10-day-old specific-pathogen-free (SPF) chicken embryos for all subsequent infections unless otherwise indicated. The NDV LaSota strain was obtained from ATCC (Manassas, VA) and propagated in 9-day-old SPF chicken embryos. The velogenic strain of NDV, California 2002 [NDV/CA02; game chicken/US(CA)/S0212676/02], was obtained from the pathogen repository bank at the Southeast Poultry Research Laboratory (SEPRL), USDA-ARS, Athens, GA, USA. The ILTV strain (63140/C/08/BR) was obtained from the pathogen repository bank at the Poultry Diagnostic and Research Center (PDRC), University of Georgia, Athens, GA (37). The modified vaccinia Ankara/T7 recombinant virus (MVA/T7) used during virus rescue to provide the bacteriophage T7 RNA polymerase was a kind gift from B. Moss, National Institutes of Health (38). The commercial live attenuated ILTV vaccines, Trachivax (CEO) and LT-Ivax (TCO), were purchased from Merck Animal Health (Summit, NJ).
Viral RNA was isolated from the allantoic fluid of NDV-infected chicken embryos and infected DF-1 cells using the TRIzol-LS reagent according to the manufacturer's instructions (Life Technologies, Carlsbad, CA). Viral DNA extraction from tracheal and ocular swab samples was performed using the MagaZorbH DNA Miniprep 96-well kit (Promega, Madison, WI) as described previously (12).
Construction of a recombinant LaSota cDNA clone containing the GFP gene.
The LaSota infectious clone, pFLC-LaSota (26), was used to generate a recombinant LaSota cDNA clone with the green fluorescent protein (GFP) gene inserted between the phosphoprotein (P) and matrix (M) genes as an additional transcription unit (Fig. 1). To facilitate this, a two-step approach was initiated using infusion cloning of PCR products that were generated using Pfu Ultra II Fusion HS DNA polymerase (Agilent Technologies, La Jolla, CA). First, a cDNA fragment spanning the LaSota P and M gene junction region was amplified by PCR from the pFLC-LaSota clone with a pair of primers (C5-M F and C5-M R [Table 1]) and cloned into the TOPO TA Cloning vector (Life Technologies, Carlsbad, CA) to generate pT-LS-M. The LaSota M coding sequences were then replaced with the ORF of the GFP gene using the In-Fusion PCR cloning kit (Clontech, Mountain View, CA) with PCR products generated in amplification reactions using paired vector-specific primers (Insert vec Up and Insert vec Down [Table 1]) and PCR products generated in amplification reactions using the GFP F and GFP R primers (Table 1) with pAAV-hrGFP plasmid (Agilent Technologies, La Jolla, CA) as a template. To enhance GFP expression, a Kozak consensus sequence was added to the forward primer (GFP F) at a position upstream of the GFP gene translation start codon. Second, the GFP ORF along with the NDV trans-acting signal sequences, including the gene end (GE), intergenic (IG), and gene start (GS) sequences, was amplified using the resulting recombinant subclone, pT-LS-GFP, as a template with the C5-M F and C5-M R primers (Table 1), and the vector sequences of pFLC-LaSota were amplified using C5 M vec Up and LS M vec Down primers (Table 1). Subsequently, the PCR product containing the GFP ORF along with the NDV trans-acting signal sequences was cloned into the noncoding region downstream of the P gene of the pFLC-LaSota vector using the In-Fusion PCR cloning kit (Clontech, Mountain View, CA). The resulting recombinant, designated pLS-GFP, was propagated in Stbl2 cells at 30°C for 24 h and purified using a QIAprep Spin Miniprep kit (Qiagen, Valencia, CA).
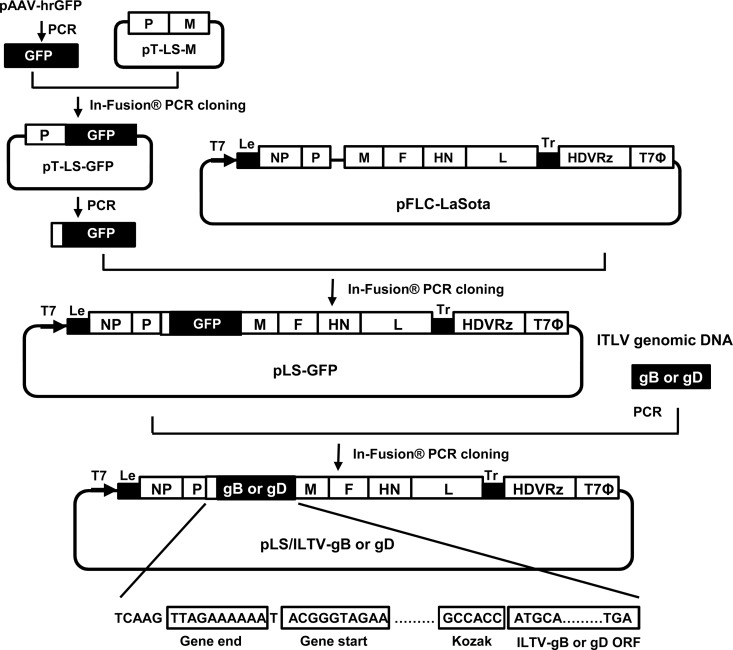
FIG 1.
Scheme of pLS-GFP, pLS/ILTV-gB, and pLS/ILTV-gD clone construction. The open reading frame (ORF) of the GFP gene was amplified from plasmid pAAV-hrGFP (Agilent Technologies, La Jolla, CA) and cloned into the pT-LS-M vector to replace the NDV M gene coding sequences. Subsequently, the GFP ORF along with the NDV trans-acting signal sequences was amplified from pT-LS-GFP and inserted into the pFLC-LaSota vector in the noncoding region downstream of the P gene using the In-Fusion PCR cloning kit (Clontech, Mountain View, CA), resulting in the pLS-GFP clone. The ORFs of the ILTV gB and gD genes were amplified from ILTV genomic DNA and cloned into the pLS-GFP vector to replace the GFP ORF, resulting in the pLS/ILTV-gB and -gD clones. The direction of the T7 promoter is indicated by a bold black arrow. HDVRz and T7Φ represent the sites of the hepatitis delta virus ribozyme and the T7 terminator sequences, respectively. The NDV gene start and gene end signal sequences, Kozak sequences, and ILTV-gB and -gD ORFs are boxed.
TABLE 1.
Primer sequences used
| Primera | Sequenceb | Name |
|---|---|---|
| 1 | 5′CAACTCTCCAAGCGGCAATC | C5-M F |
| 2 | 5′AGCGAGAGAGGTAACGATTAGTTTTTTGTGTC | C5-M R |
| 3 | 5′atagttgtagccaccATGGTGAGCAAGCAGA | GFP F |
| 4 | 5′acggtagttacacacTCACACCCACTCGTG | GFP R |
| 5 | 5′CCGCTTGGAGAGTTGGACCTTG | C5 M vec Up |
| 6 | 5′GTTACCTCTCTCGCTTCCTCAG | LS M vec Down |
| 7 | 5′GGTGGCTACAACTATCAACTAAACT | Insert vec Up |
| 8 | 5′GTGTGTAACTACCGTGTACTAAGC | Insert vec Down |
| 9 | 5′atagttgtagccaccATGCAATCCTACATCG | Plant-gB/GFP F |
| 10 | 5′gtagttacacacagcTTATTCGTCTTCGCTTTC | Plant-gB/GFP R |
| 11 | 5′atagttgtagccaccATGCACCGTCCTCATC | Plant-gD/GFP F |
| 12 | 5′gtagttacacacagcTTAGCTACGCGCGCAT | Plant-gD/GFP R |
Primers 1 and 2 were used to PCR amplify a cDNA fragment spanning the LaSota P and M gene junction region, primers 3 and 4 were used to PCR amplify a cDNA fragment containing the GFP gene ORF, primers 5 and 6 were used to PCR amplify or linearize the pFLC-LaSota vector in the noncoding region of the M gene end region, primers 7 and 8 were used to amplify or linearize the pT-LS-M, pT-LS-GFP, and pLS-GFP vectors, primers 9 and 10 were used to amplify a cDNA fragment containing the ILTV gB gene ORF, and primers 11 and 12 were used to amplify a cDNA fragment containing the ILTV gD gene ORF.
Nucleotides shown in lowercase letters represent homology sequences with a vector backbone, which were used to facilitate the restriction endonuclease site (RE)-independent cloning using the In-Fusion PCR cloning kit (Clontech).
Construction of recombinant LaSota cDNA clones containing the gB and gD genes of ILTV.
The recombinant LaSota cDNA clones containing the gB and gD genes of ILTV were constructed by swapping the GFP ORF in the pLS-GFP vector with that of either the ILTV gB or gD ORF (Fig. 1). In brief, vector-specific primers (Insert vec Up and Insert vec Down [Table 1]) which flank the GFP ORF were used in an amplification reaction with Pfu Ultra II Fusion HS DNA polymerase (Agilent Technologies, La Jolla, CA) and the pLS-GFP plasmid. The open reading frames of the ILTV gB and gD genes were amplified using the same polymerase with genomic DNA of ILTV strain 63140/C/08/BR (37) and gene-specific primers (Plant-gB/GFP F and Plant-gB/GFP R for gB and Plant-gD/GFP F and Plant-gD/GFP R for gD) and subsequently cloned together with amplified pLS-vector sequences using the In-Fusion PCR cloning kit (Clontech, Mountain View, CA). The resulting recombinant cDNA clones, designated pLS/ILTV-gB and pLS/ILTV-gD, were propagated and purified as described above.
Virus rescue and propagation.
Rescue of the recombinant viruses was performed by transfection of the full-length cDNA clone, pLS/ILTV-gB, pLS/ILTV-gD, or pLS-GFP, and supporting plasmids that express the NDV NP, P, and L proteins into HEp-2 cells as described previously (39). The rescued viruses rLS/ILTV-gB, rLS/ILTV-gD, and rLS-GFP were screened using hemagglutination assay (HA) (40). HA-positive viruses were diluted in phosphate-buffered saline (PBS) and inoculated into chicken embryos. Stocks were then prepared from harvested allantoic fluids, aliquoted, and stored at −80°C. The complete genomic sequences of the rescued viruses were determined by direct sequencing of the reverse transcription-PCR (RT-PCR) products amplified from the viral genomic RNA as described previously (26).
Virus titration and pathogenicity assessment.
Recombinant viruses were characterized using the standard HA and the 50% tissue infectious dose (TCID50) assay on DF-1 cells in 96-well formats and the 50% egg infective dose (EID50) assay in 9-day-old SPF chicken embryos (40). Pathogenicity of the recombinant viruses was assessed by performing the standard mean death time (MDT) and intracerebral pathogenicity index (ICPI) tests (40).
IFA.
Expression of the gB and gD glycoproteins from DF-1 cells infected with the rLS/ILTV-gB and rLS/ILTV-gD viruses, respectively, was examined by immunofluorescence assay (IFA) with anti-ILTV chicken serum (M. Garcia, PDRC, University of Georgia, Athens, GA) and an NDV-specific monoclonal antibody (MAb) against the HN protein (a gift from Ron Iorio, University of Massachusetts Medical School). Briefly, confluent monolayers of DF-1 cells were infected with recombinant viruses at a multiplicity of infection (MOI) of 0.01. After 24 h, the infected cells and control cells were washed with phosphate-buffered saline (PBS) and fixed with 10% zinc formalin (Fisher Scientific, Pittsburgh, PA) for 15 min at room temperature, followed by addition of 0.5% Triton X-100 (Sigma, St. Louis, MO) to permeabilize the cells at room temperature for 10 min. The permeabilized cells were blocked with 5% goat serum (Southern Biotech, Birmingham, AL) for 30 min at 37°C. After blocking, the cells were incubated for 1 h with a mixture of anti-ILTV serum (1:100 dilution) and mouse anti-NDV HN MAb (1:100 dilution). Cells were washed with PBS and incubated with a mixture of fluorescein isothiocyanate (FITC)-labeled goat anti-chicken IgG(H+L) (Southern Biotech, 1:1,000 dilution) and Alexa Fluor 568-conjugated goat anti-mouse IgG (Life Technologies, 1:1,000 dilution) for 1 h at 37°C. Fluorescence images (Fig. 2) were monitored and photographed using an inverted fluorescence microscope at a magnification of ×100 with matching excitation/emission filters for FITC or Alexa Fluor 568 (Eclipse Ti; Nikon, Melville, NY).
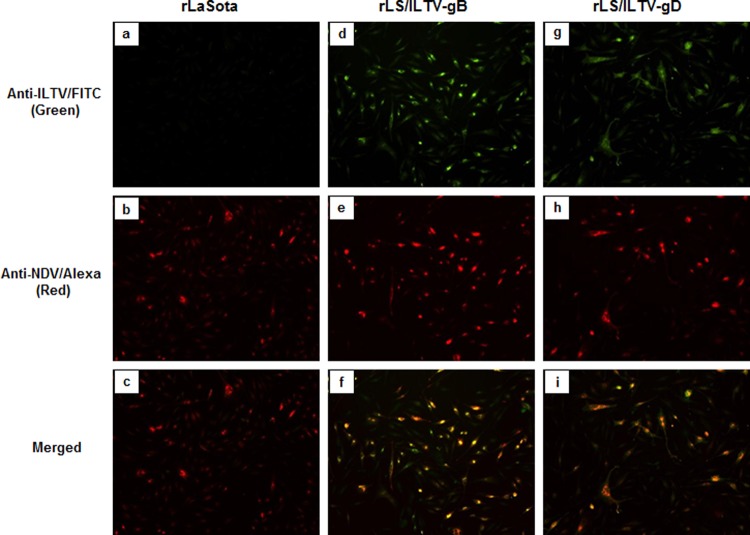
FIG 2.
Detection of ILTV gB and gD protein expression by IFA. DF-1 cells were infected with the recombinant LaSota, LS/ILTV-gB, and LS/ILTV-gD viruses at a multiplicity of infection (MOI) of 0.01. At 24 h postinfection, the cells were fixed and stained with a mixture of chicken anti-ILTV serum and mouse anti-NDV HN MAb, followed by a mixture of the FITC-labeled goat anti-chicken IgG and Alexa Fluor 568-labeled goat anti-mouse IgG. Fluorescence was monitored and photographed using an inverted fluorescence microscope at a magnification of ×100 under UV light with matching excitation/emission filters for FITC or Alexa Fluor 568 (Eclipse Ti; Nikon, Melville, NY). Green and Reagan red fluorescent images that were photographed from the same field of the rLaSota (a and b), rLS-ILTV-gB (d and e), and rLS-ILTV-gD (g and h) virus-infected cells were merged into single images (c, f, and i).
Immunization and challenge experiments.
To evaluate the protective efficacy conferred by the NDV/ILTV recombinant viruses against virulent ILTV and NDV challenges, three animal experiments were initiated. Experiments 1 and 2 were conducted using specific-pathogen-free (SPF) Leghorn chickens and commercial broiler chickens in the biosafety level 2E (BSL2E) animal facilities at the Southeast Poultry Research Laboratory (SEPRL) for the evaluation of protection against virulent ILTV challenge. Experiment 3 was conducted with SPF chickens in the BSL3E animal facilities at SEPRL for the evaluation of protection against lethal NDV challenge. Birds were housed in Horsfal isolators (Federal Designs, Inc., Comer, GA) with ad libitum access to feed and water. At the termination of the experiments, all birds were humanely euthanized in accordance with an SEPRL's Institutional Animal Care and Use Committee-approved animal use protocol.
(i) Experiment 1.
One hundred five 1-day-old SPF Leghorn chickens were randomly divided into seven groups of 15 birds. Each bird in group 1 was inoculated with 100 μl of phosphate-buffered saline (PBS) via the intranasal (i.n.) and intraocular (i.o.) routes, and these served as unvaccinated controls. Birds in groups 2 and 3 were vaccinated with 100 μl of rLS-GFP (1.0 × 107 TCID50/ml) and served as LaSota vector controls, birds in groups 4 and 5 were vaccinated with 100 μl of rLS/ILTV-gB (1.0 × 107 TCID50/ml), and birds in groups 6 and 7 were vaccinated with 100 μl of rLS/ILTV-gD (1.0 × 107 TCID50/ml) per bird via i.n./i.o. routes. At 21 days postvaccination (dpv), the birds in groups 1, 2, 4, and 6 were challenged with the virulent ILTV (strain 63140/C/08/BR) at a dose of 104 TCID50/per bird. At 28 dpv, birds in groups 3, 5, and 7 were challenged with the virulent ILTV at the same dose. After challenge, clinical signs of ILT were recorded daily for 9 days as previously described (16). Briefly, breathing patterns, conjunctivitis, and level of depression were scored on a scale of 0 to 3: normal (0), mild (1), moderate (2), and severe (3). Mortality was given a total score of nine. The median clinical sign score per group per day was calculated, and differences among groups were analyzed statistically. Blood samples were collected from each bird prior to challenge for detecting NDV-specific antibody by the hemagglutination inhibition (HI) test (40) and ILTV-specific antibody by the enzyme-linked immunosorbent assay (ELISA) using a fowl laryngotracheitis virus antibody test kit (BioChek, London, United Kingdom). Tracheal and ocular swabs were collected from each bird at 4 days postchallenge (dpc) and stored in brain heart infusion (BHI) medium (Becton, Dickinson and Company, Sparks, MD) at −80°C until needed to measure the amount of virus shed using a quantitative real-time PCR (qPCR) assay.
(ii) Experiment 2.
One hundred twenty 3-week-old commercial broilers, obtained from Moyer's Chicks, Inc. (Quakertown, PA), were randomly divided into six groups of 20 birds. Each bird was inoculated via the i.n./i.o. routes with 100 μl of PBS (group 1), rLS-GFP (1.0 × 107 TCID50/ml) (group 2), or rLS/ILTV-gB (1.0 × 107 TCID50/ml) (group 3). Birds in groups 4 and 5 were vaccinated with one dose of commercial ILTV vaccine, either CEO (2.8 log10 TCID50/dose) or TCO (3.0 log10 TCID50/dose), per bird via the eye drop route. Birds in group 6 were untreated and served as body weight gain controls. At 21 dpv, the birds in groups 1 to 5 were challenged with the virulent ILTV (strain 63140/C/08/BR) at a dose of 104 TCID50 per bird. Clinical signs were observed daily for 9 days. Body weights were measured prior to challenge and at 9 dpc. Serum samples were collected prior to vaccination and prior to challenge for use in HI and ELISA testing. Tracheal and ocular swab samples were collected at 4 dpc, stored and tested as described for experiment 1.
(iii) Experiment 3.
Forty 1-day-old SPF chickens were randomly divided into four groups of 10 birds. Birds were inoculated with 100 μl of PBS (group 1), the LaSota vaccine (1.0 × 107 TCID50/ml) (group 2), rLS/ILTV-gB (1.0 × 107 TCID50/ml) (group 3), and rLS/ILTV-gD (1.0 × 107 TCID50/ml) (group 4), via the i.n./i.o. routes. At 14 days dpv, the birds were challenged with a lethal dose of the NDV/CA02 virus as described previously (41). The serum samples were collected immediately before challenge for NDV antibody detection using the HI test. After challenge, the birds were monitored daily for clinical signs and mortality for 2 weeks.
Quantitation of ILTV load in tracheal and ocular swabs.
The ILTV viral load in each tracheal and ocular swab sample was quantified using a relative quantitative real-time PCR (qPCR) assay with the host α2-collagen gene as a reference (42, 43). The real-time PCR assays were carried out on an Applied Biosystems 7500 Fast real-time PCR system (Life Technologies, Carlsbad, CA). The multiplex reaction mixture contained specific primers and probes for the detection of the UL44 gene of ILTV (encoding glycoprotein C) and the endogenous control gene (avian α2-collagen gene) (43). The UL44 forward primer and UL44 reverse primer generated a 103-bp amplicon. The α2-collagen forward primer and α2-collagen reverse primer generated a 96-bp amplicon. Duplex PCRs were set up in a final volume of 25 μl as follows: 1× Universal TaqMan master mix with uracil-DNA glycosylase (UDG) (Life Technologies), 500 nM UL44 forward primer, 500 nM UL44 reverse primer, 500 nM α2-collagen forward primer, 500 nM α2-collagen reverse primer, 100 nM UL44–6-carboxyfluorescein (FAM)–6-carboxytetramethylrhodamine (TAMRA) probe, 100 nM α2-collagen-VIC-TAMRA probe, and 5.0 μl of DNA template (approximately 10 to 300 ng). The reactions were carried out in a thermal cyclical program with the following parameters: 50°C for 2 min followed by 95°C for 10 min followed by a two-step PCR with 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. A threshold value of 0.05 was used for consistent evaluation of different 96-well plates. Samples with any recorded threshold cycle number (CT) value were considered positive, and samples with no recorded CT value were considered negative.
Statistical analysis.
The percentage of body weight gain in each of the vaccinated/challenged bird groups was compared with that in the control (unvaccinated/nonchallenged) group using the Student t test with a 5% level of significance (Microsoft Excel). For clinical score analysis, the Kruskal-Wallis test was independently used to compare the median clinical scores for each day postchallenge, and then multiple pairwise comparisons were performed for post hoc comparisons. The relative reduction in virus load in DNA isolated from swabs of the rLS-GFP versus vaccinated/challenged groups was measured using one-way analysis of variance (ANOVA) at a 5% level of significance.
RESULTS
Generation of the rLS-GFP and rLS/ILTV-gB and -gD viruses.
Three full-length cDNA clones carrying the complete antisense genome of the NDV LaSota vaccine strain with the ILTV gB, gD, and GFP ORFs were constructed using PCR and In-Fusion PCR cloning (Fig. 1). The insertion of the transcription “cassettes” containing NDV trans-acting elements and the ORF of the GFP, ILTV-gD, and ILTV-gB increased the length of the recombinant clones by 948, 1,536, and 2,880 nucleotides (nt), respectively. Thus, the total lengths of cDNA clones in the pLS-GFP, pLS/ILTV-gD, and pLS/ILTV-gB plasmids are 16,104, 16,692, and 18,036 nt, respectively, and are divisible by 6, abiding by the “rule of six” (44). After cotransfection of these full-length cDNA clones and supporting plasmids into HEp-2 cells and subsequent propagation in SPF chicken embryonated eggs, the LaSota strain-based recombinant viruses vectoring the GFP, ILTV gB, or ILTV gD gene were successfully generated. The nucleotide sequence analysis of the RT-PCR products of the viral genome confirmed the fidelity of the rescued viruses.
Biological characterization of the rLS/ILTV-gB and -gD viruses.
To determine whether the insertion of the GFP, gB, and gD ORFs into the NDV LaSota genome affects the biological properties of the recombinant viruses, the pathogenicities and growth abilities of the rLS-GFP and rLS/ILTV-gB and -gD viruses were examined in vitro and in vivo by conducting titration assays and MDT and ICPI tests. As shown in Table 2, the recombinant viruses were slightly more attenuated, with a lower ICPI (0.0) than the parental LaSota virus. The titers of the recombinant viruses grown either in embryonated eggs or in DF-1 cells, as measured by EID50, TCID50, and HA, were comparable to the titers of the parental LaSota strain (Table 2). The recombinant viruses appeared to be stable, with no apparent changes in MDT and virus titers after 10 passages in SPF chicken embryos.
TABLE 2.
Biological assessments of the recombinant virusesa
| Virus | MDT (h) | ICPI | HA | EID50 | TCID50 |
|---|---|---|---|---|---|
| rLaSota | 110 | 0.15 | 1,024 | 6.8 × 108 | 3.5 × 107 |
| rLS-GFP | 120 | 0.00 | 2,048 | 3.1 × 108 | 9.8 × 107 |
| rLS/ILTV-gB | 120 | 0.00 | 2,048 | 3.8 × 108 | 1.6 × 107 |
| rLS/ILTV-gD | 112 | 0.01 | 2,048 | 6.8 × 108 | 3.8 × 107 |
Abbreviations: MDT, mean death time in embryonated eggs; ICPI, intracerebral pathogenicity index in 1-day-old chickens; HA, hemagglutination titer; EID50, 50% egg infectious dose in embryonated eggs; TCID50, 50% tissue infectious dose on DF-1 cells.
Expression of the ILTV gB and gD proteins in cells infected with the rLS/ILTV-gB and rLS/ILTV-gD viruses.
Expression of the ILTV gB and gD proteins from the recombinant-virus-infected DF-1 cells was examined by IFA using chicken anti-ILTV serum and FITC-labeled goat anti-chicken IgG. Reactivities to mouse anti-NDV HN monoclonal antibody (MAb) and Alexa Fluor 568-conjugated goat anti-mouse IgG were also tested. As shown in Fig. 2, NDV LaSota-infected cells were stained with mouse anti-NDV HN MAb and Alexa conjugates (Fig. 2b) but not with chicken anti-ILTV serum and FITC conjugate (Fig. 2a), demonstrating the specificity of the antibodies and conjugates. When examining rLS/ILTV-gB- and rLS/ILTV-gD-infected DF-1 cells stained with a mixture of anti-ILTV/FITC and anti-NDV HN/Alexa 568 antibodies, both green (Fig. 2d and g) and red (Fig. 2e and h) fluorescences were observed by fluorescence microscopy, respectively. After merging both fluorescent images, green and red fluorescence colocalized to the same cells (Fig. 2f and i). These results confirm that the ILTV gB and gD proteins were coexpressed with the NDV HN protein from the rLS/ILTV-gB and rLS/ILTV-gD recombinant-infected cells.
Protection against virulent ILTV challenge.
To examine whether the rLS/ILTV-gB and rLS/ILTV-gD viruses induce protective immunity against virulent ILTV challenge, two animal experiments were carried out with SPF 1-day-old chickens and 3-week-old commercial broiler chickens. After vaccination with the recombinant viruses, the chickens appeared healthy, with no signs of vaccine-induced side effects. Chicken sera collected immediately before challenge were analyzed for NDV antibodies by the HI test (Table 3). NDV HI titers ranging around 3.8 log2, 3.3 log2, and 3.47 log2 were determined in sera from chickens inoculated with rLS/ILTV-GFP, rLS/ILTV-gB, and rLS/ILTV-gD, respectively. Thus, all vaccinated birds seroconverted and developed an antibody response to NDV with titers similar to those detected in sera of rLS-GFP-vaccinated birds. However, no ILTV antibody was detected in the recombinant-virus-vaccinated birds by ELISA (data not shown).
TABLE 3.
Serum NDV-specific antibody responses of chickens following vaccination
| Expt | Treatment | No. of seropositive birds/total | NDV HI titera |
|---|---|---|---|
| 1 | PBS | 0/15 | 0 |
| rLS-GFP | 30/30 | 3.83 ± 0.95 | |
| rLS/ILTV-gB | 30/30 | 3.33 ± 0.66 | |
| rLS/ILTV-gD | 30/30 | 3.47 ± 1.25 | |
| 2 | PBS | 0/20 | 0 |
| rLS-GFP | 20/20 | 3.85 ± 0.93 | |
| rLS/ILTV-gB | 20/20 | 3.05 ± 0.83 | |
| CEO | 0/20 | 0 | |
| TCO | 0/20 | 0 |
The hemagglutination inhibition (HI) titer is expressed as log2 mean ± standard deviation.
After challenge with the virulent ILTV, the chickens were examined daily in two experiments for clinical signs. In experiment 1, SPF chickens in the nonvaccinated (PBS) and the LaSota vaccine vector control (rLS-GFP) groups exhibited typical clinical signs of the disease from 3 dpc onward (Fig. 3a and b), showing depression, conjunctivitis, or respiratory signs, which were scored 1 to 3 depending on the severity of disease signs. The infected birds showed peak clinical signs between 4 and 6 dpc, with 10 to 20% mortality, and the signs gradually decreased in severity thereafter. In contrast, more than 90% of the rLS/ILTV-gB or -gD-vaccinated chickens showed no disease signs, and only 2 to 5 birds displayed very mild clinical signs at 4 to 7 dpc in the recombinant vaccine groups.
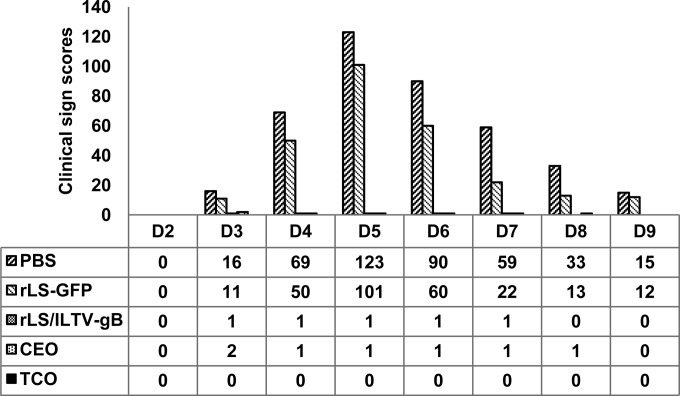
FIG 3.
Daily total clinical sign scores of chickens postchallenge. One-day-old SPF chickens were vaccinated with rLS/ILTV-gB, rLS/ILTV-gD, rLS-GFP, or PBS and challenged with the virulent ILTV strain 63140/C/08/BR at 21 (a) or 28 (b) days postvaccination. The birds were examined daily for clinical signs, and the total scores from each group of birds were plotted.
In experiment 2, all birds vaccinated with the recombinant NDV expressing gB and the “gold standard” ILTV vaccines, CEO and TCO, appeared healthy, without any signs of vaccine side effects. Detection of antibody responses from the sera collected immediately before challenge showed that all of the LaSota vector- and rLS/ILTV-gB-immunized birds became NDV seropositive (Table 3). As expected, the unvaccinated (PBS control) and the ILTV CEO- and TCO-vaccinated birds did not have NDV-specific antibodies. Unexpectedly, low percentages of TCO-vaccinated (15%) and CEO-vaccinated (55%) birds became ILTV seropositive, and none of the rLS/ILTV-gB-vaccinated birds had detectable ILTV antibody by ELISA (data not shown). As shown in Fig. 4 the commercial broiler chickens in the nonvaccinated (PBS) and the vaccine vector control (rLS-GFP) groups exhibited typical clinical signs of the disease from 3 dpc. The total scores of clinical signs peaked at 5 dpc and gradually decreased thereafter. There were 50% mortality in birds within the PBS group and 5% mortality in the LaSota vector group. Very mild clinical signs were observed only in two birds from the CEO vaccinated group and only one bird from the rLS/ILTV-gB vaccinated group, whereas the TCO vaccinated broiler chickens did not show any clinical signs of the disease. In addition to typical clinical signs, ILTV infection also causes a decrease in body-weight gains (1). This was determined by measuring the weight of the broiler chickens prior to challenge (42 days of age) and at the termination of the experiment at 51 days of age. As shown in Table 4, the rate of body weight gain of the birds within the rLS/ILTV-gB-vaccinated group (22%) was similar to those of unvaccinated/unchallenged birds (21.8%) and the birds vaccinated with either TCO or CEO (average, 21.85%) (P > 0.05) but significantly higher than those within the PBS and LaSota vector groups (P < 0.05).
FIG 4.
Total clinical sign scores of broiler chickens recorded daily postchallenge. Three-week-old commercial broiler chickens were vaccinated with rLS/ILTV-gB, commercial ILTV vaccines CEO and TCO, rLS-GFP, and PBS and challenged with the virulent ILTV strain 63140/C/08/BR at 21 dpv. The birds were examined daily for clinical signs, and the total scores from each group of birds were plotted.
TABLE 4.
Percentage of body weight gained from 42 to 51 days of age
| Treatment group | Mean % body wt gaina |
|---|---|
| PBS | 5.1 (2.2E−08) A |
| rLS-GFP | 12.8 (8.7E−05) B |
| rLS/ILTV-gB | 22.0 (0.91) C |
| CEO | 24.6 (0.14) C |
| TCO | 19.1 (0.18) C |
| Control (nonvaccinated, nonchallenged) | 21.8 (1.00) C |
(Mean body weight postchallenge [51 days of age] − mean body weight prechallenge [42 days of age]/mean body weight prechallenge) × 100. The numbers in parentheses indicate the P value. The values with same letters were not statistically significantly different (P > 0.05).
Reduction in shedding of the challenge ILTV in vaccinated birds.
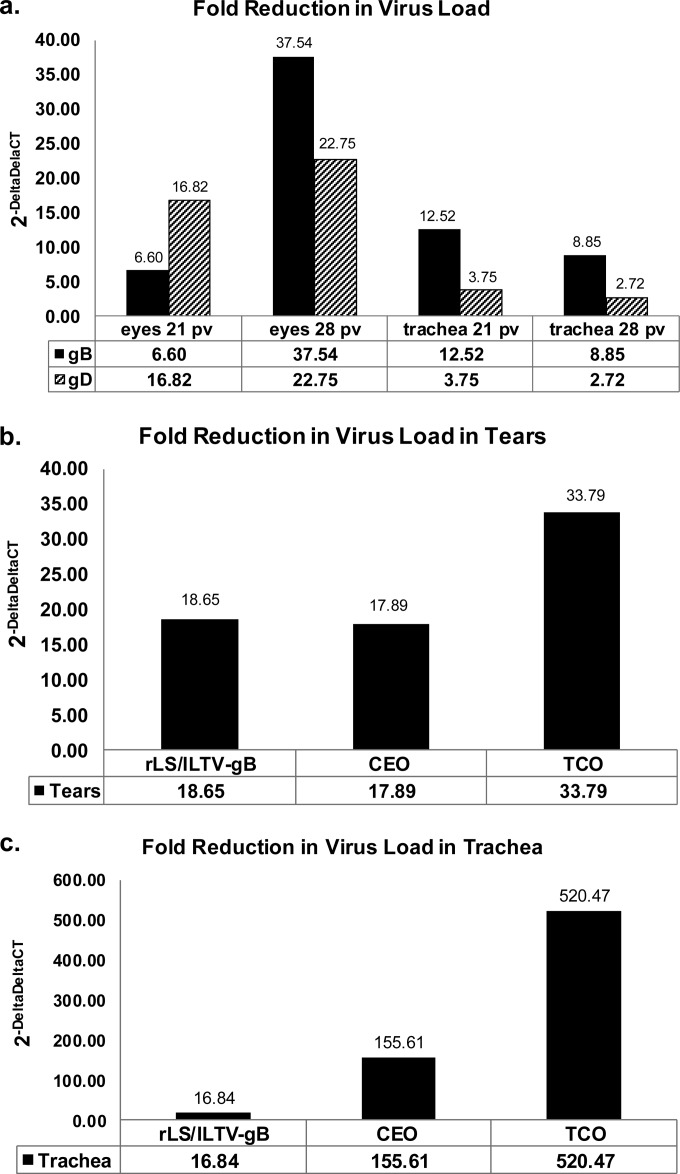
Reduction of challenge virus loads or shedding in vaccinated birds after challenge was another important criterion to evaluate the efficacy of the vaccine. Detection of the presence of the ILTV DNA in the tracheal lumen and tears of chickens was measured by relative qPCR using the 2−ΔΔCT method. The fold reduction in virus load was measured relative to CT values of samples from chickens vaccinated with rLS-GFP. The fold reduction in virus load in tear samples from rLS/ILTV-gB-vaccinated SPF birds (experiment 1) was greater at 28 dpv (37-fold reduction; P < 0.01) than the reduction measured at 21 dpv (6.6-fold reduction) (Fig. 5a). Similarly a greater fold reduction in virus load in ocular samples from rLS/ILTV-gD-vaccinated birds was also noted at the later time point. This was in contrast to the shedding pattern from tracheal swabs. A greater fold reduction in virus load occurred at the earlier time point (21 dpv), as measured from tracheal swabs of rLS/ILTV-gB- and rLS/ILTV-gD-vaccinated birds.
FIG 5.
Fold reduction in the amount of challenge infectious laryngotracheitis virus shed in tracheal and ocular samples. The relative 2−ΔΔCT method was used to calculate the fold reduction in virus shedding between ITLV vaccines (rLS/ILTV-gB, rLS/ILTV-gD, CEO, and TCO) relative to rLS-GFP. ΔCT values were calculated from CT values measured for the gC amplicon minus those of the endogenous control (collagen amplicon). (a) Results from tracheal and ocular samples from SPF birds vaccinated with rLS/ILTV-gB and rLS/ILTV-gD relative to rLS-GFP. Samples were collected at 21 dpv and 28 dpv. (b) Fold reduction in the amount of challenge virus shed in ocular samples from broiler chickens vaccinated with rLS/ILTV-gB and the commercial ILTV vaccines CEO and TCO. Ocular samples were collected at 21 dpv. (c) Fold reduction in the amount of challenge virus shed in tracheal samples from broiler chickens vaccinated with rLS/ILTV-gB and the commercial ILTV vaccines CEO and TCO. Tracheal samples were collected at 21 dpv.
In experiment 2, commercial broiler chickens vaccinated with the rLS/ILTV-gB virus shed a level of the challenge virus comparable to that for the birds in the CEO-vaccinated groups when ocular samples were assayed (fold reduction, 18.65 versus 17.89) (Fig. 5b). However, in tracheal samples (Fig. 5c), there was a log fold reduction between rLS/ILTV-gB and CEO (16.84 versus 155.64). Although the fold reduction in birds vaccinated with the attenuated live ILTV vaccines (CEO and TCO) was significantly greater than the average 16.84-fold reduction with rLS/ILTV-gB tracheal samples, all vaccinated and challenged birds showed few clinical signs, without any loss in weight gains (Table 4).
Immune response and protection against velogenic NDV challenge.
To evaluate the protective efficacy conferred by the NDV/ILTV recombinant viruses against Newcastle disease, groups of SPF chickens in experiment 3 were inoculated by i.n./i.o. routes with rLS/ILTV-gB, rLS/ILTV-gD, LaSota vaccine, or PBS and challenged with a lethal dose of the NDV/CA02 virus at 14 dpv. As shown in Table 5, all of the chickens that had been immunized with the LaSota vector or the recombinant viruses were completely protected against NDV challenge, showing no signs of disease. In contrast, all of the birds in the unvaccinated control group (inoculated with PBS) displayed disease signs, with conjunctivitis and severe depression from 2 to 4 dpc and 100% mortality at 5 dpc. The HI assay using sera collected immediately before challenge indicated that chickens immunized with recombinant viruses and the parental LaSota vaccine induced NDV-specific humoral immunity.
TABLE 5.
Serum antibody responses of chickens following vaccination and number of survivors after NDV challenge
| Treatment group | No. of seropositive birds/total | NDV HI titera | No. of survivors/total |
|---|---|---|---|
| PBS | 0/10 | 0 | 0/10 |
| LaSota | 10/10 | 4.8 ± 1.4 | 10/10 |
| rLS/ILTV-gB | 10/10 | 3.4 ± 1.3 | 10/10 |
| rLS/ILTV-gD | 10/10 | 3.8 ± 1.2 | 10/10 |
The hemagglutination inhibition (HI) titer is expressed as log2 mean ± standard deviation.
DISCUSSION
This study describes the development of live, mucosally delivered NDV-vectored vaccines containing glycoproteins gB and gD of ILTV and their evaluation for pathogenicity, immunogenicity, and protective efficacy against virulent ILTV and lethal NDV challenges in SPF and commercial broiler chickens. To control infectious laryngotracheitis in poultry, there is a need to improve the current attenuated live and vectored vaccines and to develop highly efficacious vaccines that are incapable of virulence reversion in order to provide broad and effective protection against ILT.
One vector that has shown promising results with its ability to infect birds efficiently via the intranasal route and induce innate immunity and mucosal immunity with local IgA and systemic IgG antibodies and to elicit cell-mediated immune responses, characteristics of importance for protection of animals against respiratory viral diseases, is Newcastle disease virus (17). In contrast to virus vectors that encode a large number of proteins, such as Turkey herpesvirus and fowlpox virus, NDV encodes only 8 proteins; therefore, there is less competition for immune responses between vector proteins and the expressed foreign antigen (45). Moreover, development of an efficacious live bivalent vaccine against ILTV and NDV has several advantages, including price per dose, growth to high titers in chicken embryos, stability, ability to be lyophilized, ease of administration, and ability to differentiate infected from vaccinated animals (DIVA).
Previously we have used NDV as a vector to express the G gene of avian metapneumovirus (aMPV). In this first-generation NDV vector, the G gene was inserted between the F and HN genes, and in clinical trials only partial protection against aMPV challenge was achieved (26, 27). To improve upon this, we developed a second-generation NDV vector by inserting the foreign gene, the gB or gD gene of ILTV as reported in this study, into the noncoding region downstream of the P gene, and we added the Kozak consensus sequence immediately upstream of the foreign gene's translation start codon. These modifications significantly improved the foreign gene's expression as evidenced by IFA. Vaccination of SPF chickens and commercial broiler chickens with rLS/ILTV-gB and rLS/ILTV-gD conferred complete protection against challenge with virulent ILTV, the causative agent of a severe respiratory disease in chickens that has a mortality rate of 70% (46). Other vectored vaccines for ILTV, including fowlpox viruses expressing glycoprotein B and HVT expressing glycoprotein I and D, are commercially available. These viral vector vaccines expressing ILTV glycoproteins have proven to be safe, and in particular the HVT vectored construct can significantly reduce the clinical disease; however, they are not as effective as live attenuated vaccines in reducing the shedding of challenge virus (12–16, 47).
Both recombinant viruses, rLS/ILTV-gB and rLS/ILTV-gD, described in this paper grew at titers similar to those of LaSota vaccine and induced a comparable antibody response, as strong as that of the LaSota vaccine, as evidenced by high NDV HI titers and a level of ILTV antibody response that was undetectable by ELISA. It is well documented that the ILTV antibody response does not necessarily correlate with the disease protection (1). Therefore, in this study the ILTV antibody response was not used as a major criterion to evaluate the vaccine effectiveness. Importantly, in our experiments using either 1-day-old SPF or 3-week-old commercial broiler chickens, all birds appeared healthy and without any signs of vaccine-induced side effects following vaccine inoculation, indicating that these vaccines are completely safe to administer even early in life.
In the first part of this study to determine the protective nature of vectored NDV/ILTV recombinants in 1-day-old SPF Leghorn chickens, clinical signs of ILT were noticed on day 3 postchallenge in all the control birds in the sham-vaccinated (inoculated with PBS) and the LaSota vector (rLS-GFP)-vaccinated groups. In contrast, birds vaccinated with either the rLS/ILTV-gB or rLS/ILTV-gD virus and challenged at either 21 or 28 dpv displayed few or very mild clinical signs. Virus shedding was significantly reduced but not totally eliminated in ocular and tracheal samples from rLS/ILTV-gB- and rLS/ILTV-gD-vaccinated birds, relative to that in the rLS-GFP- and sham (PBS)-vaccinated birds. Overall, the rLS/ILTV-gB construct outperformed rLS/ILTV-gD in decreasing the amount of virus shed in both ocular and tracheal samples from SPF birds. Due to the viral shedding reduction patterns and the encouraging protection data, only the rLS/ILTV-gB vaccine candidate was examined in subsequent protection studies using maternal antibody-negative 3-week-old commercial broiler chickens.
While the commercial broiler chickens in the nonvaccinated (PBS) and the vaccine vector control (rLS-GFP) groups exhibited typical clinical signs of the disease, only one bird from the rLS/ILTV-gB-vaccinated broiler group and two from the CEO-vaccinated broiler chickens showed very mild clinical signs at 4 to 5 dpc. Interestingly, there was a higher percent mortality (50%) for birds in the challenged PBS group than in the challenged LaSota vector group (5%), suggesting a vector-induced immunomodulating effect. This is not surprising since it is well known that NDV elicits an innate immune response (48).
Although rLS/ILTV-gB-, CEO-, and TCO-vaccinated birds were protected against clinical signs and maintained similar weight gains relative to unvaccinated/unchallenged controls, there were notable differences in virus shedding between the groups. Vaccination with rLS/ILTV-gB was sufficient to cause a 17-fold reduction in virus shedding in the trachea, and 156-fold and 520-fold reductions in virus shedding were measured in tracheal samples from CEO- and TCO-vaccinated birds, respectively. It has been reported that challenged CEO- and TCO-vaccinated birds are unable to infect sentinel birds with the challenge virus; however, it is unknown whether vaccination with rLS/ILTV-gB, even at a higher dose than that used in this study, will reduce virus shedding to a level at which sentinel birds will not become infected (49).
Because expression of glycoproteins gB and gD from a virulent strain of ILTV could alter the virulence of the NDV vector, the pathogenicities of the recombinant viruses were examined using the MDT and ICPI methods. Our results did not show any increase in the pathogenicities of the recombinants compared to that of the parental strain LaSota. Chickens that were immunized with the LaSota vector or the recombinant viruses were completely protected against NDV challenge, without showing any signs of disease. Birds vaccinated with rLS/ILTV-gB or rLS/ILTV-gD developed an NDV-specific serum antibody response, with slightly lower HI titers than those in serum samples collected from LaSota-vaccinated birds. This was interesting, since all 3 viruses had similar replication rates in tissue culture as measured by TCID50, but might be the result of variable replication rates of NDV recombinants in vivo.
In summary, for the first time we have constructed and evaluated the potential of the NDV LaSota strain as a vaccine vector for the expression of glycoproteins of ILTV. Our results demonstrated that the recombinant NDV-vectored gB and gD vaccines generated in this study could be used as a bivalent vaccines against NDV and ILTV in chickens. Immunization was performed by the ocular/nasal route, which mimics the natural route of infection. Thus, the NDV/ILTV recombinants should be quite effective when applied en masse via spray or drinking water.
ACKNOWLEDGMENTS
We thank Xiuqin Xia, Teresa Ross, Fenglan Li, and Sylva Riblet for excellent technical assistance, Bernard Moss for the gift of MVA/T7 recombinant virus, and Ron Iorio for a gift of mouse anti-NDV HN monoclonal antibody.
This research was supported by USDA, ARS CRIS project 6612-32000-065-00D. W. Zhao and Z. Zhang were sponsored by a scholarship from the China Scholarship Council, and G. Wen was sponsored by a scholarship from the Hubei Academy of Agricultural Sciences, China.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Garcia M, Spatz S, Guy J. 2013. Infectious laryngotracheitis, p 161–179 In Swayne D. (ed), Diseases of poultry, vol 13 John Wiley & Sons, Inc., Ames, IA [Google Scholar]
- 2.Gelenczei E, Marty E. 1962. Strain stability and immunologic characterizatics of tissue-culture modified infectious laryngotracheitis virus. Avian Dis. 9:44–56 [PubMed] [Google Scholar]
- 3.Samberg Y, Aronovici I. 1969. The devlopment of a vaccine against avian infectious laryngotracheitis. I. Modification of a laryngotracheitis virus. Refu. Vet. 26:54–59 [PubMed] [Google Scholar]
- 4.Menendez KR, Garcia M, Spatz S, Tablante NL. 2014. Molecular epidemiology of infectious laryngotracheitis: a review. Avian Pathol. 43:108–117. 10.1080/03079457.2014.886004 [DOI] [PubMed] [Google Scholar]
- 5.Hughes CS, Gaskell RM, Jones RC, Bradbury JM, Jordan FT. 1989. Effects of certain stress factors on the re-excretion of infectious laryngotracheitis virus from latently infected carrier birds. Res. Vet. Sci. 46:274–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes CS, Williams RA, Gaskell RM, Jordan FT, Bradbury JM, Bennett M, Jones RC. 1991. Latency and reactivation of infectious laryngotracheitis vaccine virus. Arch. Virol. 121:213–218. 10.1007/BF01316755 [DOI] [PubMed] [Google Scholar]
- 7.Guy JS, Barnes HJ, Smith L. 1991. Increased virulence of modified-live infectious laryngotracheitis vaccine virus following bird-to-bird passage. Avian Dis. 35:348–355. 10.2307/1591188 [DOI] [PubMed] [Google Scholar]
- 8.Guy JS, Barnes HJ, Morgan LM. 1990. Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates. Avian Dis. 34:106–113. 10.2307/1591340 [DOI] [PubMed] [Google Scholar]
- 9.Kotiw M, Wilks CR, May JT. 1995. The effect of serial in vivo passage on the expression of virulence and DNA stability of an infectious laryngotracheitis virus strain of low virulence. Vet. Microbiol. 45:71–80. 10.1016/0378-1135(94)00115-D [DOI] [PubMed] [Google Scholar]
- 10.Barhoom S, Forgacs A, Solyom F. 1986. Development of an inactivated vaccine against laryngotracheitis (ILT)—serological and protection studies. Avian Pathol. 15:213–221. 10.1080/03079458608436282 [DOI] [PubMed] [Google Scholar]
- 11.York JJ, Fahey KJ. 1991. Vaccination with affinity-purified glycoproteins protects chickens against infectious laryngotracheitis herpesvirus. Avian Pathol. 20:693–704. 10.1080/03079459108418808 [DOI] [PubMed] [Google Scholar]
- 12.Johnson DI, Vagnozzi A, Dorea F, Riblet SM, Mundt A, Zavala G, Garcia M. 2010. Protection against infectious laryngotracheitis by in ovo vaccination with commercially available viral vector recombinant vaccines. Avian Dis. 54:1251–1259. 10.1637/9401-052310-Reg.1 [DOI] [PubMed] [Google Scholar]
- 13.Davison S, Gingerich EN, Casavant S, Eckroade RJ. 2006. Evaluation of the efficacy of a live fowlpox-vectored infectious laryngotracheitis/avian encephalomyelitis vaccine against ILT viral challenge. Avian Dis. 50:50–54. 10.1637/7398-062105R.1 [DOI] [PubMed] [Google Scholar]
- 14.Saif YM, Rosenberger J, Cloud S. 1994. Efficacy and safety of a recombinant herpesvirus of turkeys containing genes from infectious laryngotracheitis virus, p 154 In Proceeding of the 130th Annual Meeting of the American Veterinary Medical Association, Minneapolis, MN. American Veterinary Medical Association, Schaumburg, IL [Google Scholar]
- 15.Tong GZ, Zhang SJ, Wang L, Qiu HJ, Wang YF, Wang M. 2001. Protection of chickens from infectious laryngotracheitis with a recombinant fowlpox virus expressing glycoprotein B of infectious laryngotracheitis virus. Avian Pathol. 30:143–148. 10.1080/03079450120044542 [DOI] [PubMed] [Google Scholar]
- 16.Vagnozzi A, Zavala G, Riblet SM, Mundt A, Garcia M. 2012. Protection induced by commercially available live-attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathol. 41:21–31. 10.1080/03079457.2011.631983 [DOI] [PubMed] [Google Scholar]
- 17.Alexander DJ, Senne DA. 2008. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections, p 75–100 In Saif YM, Fadly AM, Glisson JR, McDougald LR, Nolan LK, Swayne DE. (ed), Diseases of poultry, 12th ed. Blackwell Publishing, Ames, IA [Google Scholar]
- 18.Hitchner SB. 2004. History of biological control of poultry diseases in the USA. Avian Dis. 48:1–8. 10.1637/6100 [DOI] [PubMed] [Google Scholar]
- 19.Goldhaft TM. 1980. Historical note on the origin of the LaSota strain of Newcastle disease virus. Avian Dis. 24:297–301. 10.2307/1589696 [DOI] [PubMed] [Google Scholar]
- 20.Meulemans G. 1988. Control by vaccination, p 318–332 In Alexander DJ. (ed), Newcastle disease. Kluwer Academic Publishers, Boston, MA [Google Scholar]
- 21.Bukreyev A, Collins PL. 2008. Newcastle disease virus as a vaccine vector for humans. Curr. Opin. Mol. Ther. 10:46–55 [PubMed] [Google Scholar]
- 22.Huang Z, Elankumaran S, Panda A, Samal SK. 2003. Recombinant Newcastle disease virus as a vaccine vector. Poultry Sci. 82:899–906. 10.1093/ps/82.6.899 [DOI] [PubMed] [Google Scholar]
- 23.Schirrmacher V, Fournier P. 2009. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol. Biol. 542:565–605. 10.1007/978-1-59745-561-9_30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigil A, Martinez O, Chua MA, Garcia-Sastre A. 2008. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol. Ther. 16:1883–1890. 10.1038/mt.2008.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Peeters BP. 2003. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J. Gen. Virol. 84:781–788. 10.1099/vir.0.18884-0 [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Roth JP, Estevez CN, Zsak L, Liu B, Yu Q. 2011. Generation and evaluation of a recombinant Newcastle disease virus expressing the glycoprotein (G) of avian metapneumovirus subgroup C as a bivalent vaccine in turkeys. Vaccine 29:8624–8633. 10.1016/j.vaccine.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 27.Yu Q, Roth JP, Hu H, Estevez CN, Zhao W, Zsak L. 2013. Protection by recombinant Newcastle disease viruses (NDV) expressing the glycoprotein (G) of avian metapneumovirus (aMPV) subtype A or B against challenge with virulent NDV and aMPV. World J. Vaccines 3:130–139. 10.4236/wjv.2013.34018 [DOI] [Google Scholar]
- 28.Toro H, Zhao W, Breedlove C, Zhang Z, van Santen V, Yu Q. 2014. Infectious bronchitis virus S2 expressed from recombinant virus confers broad protection against challenge. Avian Dis. 58:83–89 [DOI] [PubMed] [Google Scholar]
- 29.Fuchs W, Veits J, Helferich D, Granzow H, Teifke JP, Mettenleiter TC. 2007. Molecular biology of avian infectious laryngotracheitis virus. Vet. Res. 38:261–279. 10.1051/vetres:200657 [DOI] [PubMed] [Google Scholar]
- 30.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9:369–381. 10.1038/nrmicro2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear PG, Longnecker R. 2003. Herpesvirus entry: an update. J. Virol. 77:10179–10185. 10.1128/JVI.77.19.10179-10185.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog. 7:e1002277. 10.1371/journal.ppat.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. 10.3390/v4050800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J. Virol. 86:1563–1576. 10.1128/JVI.06480-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanberry L. 2013. Pathogens and vaccines: genital and perinatal herpes simplex virus infections, p 273–314 In Stanberry L, Rosenthal S. (ed), Sexually-transmitted diseases: vaccines, prevention, and control. Academic Press, London, United Kingdom [Google Scholar]
- 36.Rouse BT, Kaistha SD. 2006. A tale of 2 alpha-herpesviruses: lessons for vaccinologists. Clin. Infect. Dis. 42:810–817. 10.1086/500141 [DOI] [PubMed] [Google Scholar]
- 37.Spatz SJ, Volkening JD, Keeler CL, Kutish GF, Riblet SM, Boettger CM, Clark KF, Zsak L, Afonso CL, Mundt ES, Rock DL, Garcia M. 2012. Comparative full genome analysis of four infectious laryngotracheitis virus (Gallid herpesvirus-1) virulent isolates from the United States. Virus Genes 44:273–285. 10.1007/s11262-011-0696-3 [DOI] [PubMed] [Google Scholar]
- 38.Wyatt LS, Moss B, Rozenblatt S. 1995. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202–205. 10.1006/viro.1995.1332 [DOI] [PubMed] [Google Scholar]
- 39.Estevez CN, King DJ, Seal B, Yu Q. 2007. Evaluation of Newcastle disease virus chimeras expressing the hemagglutinin-neuraminidase protein of velogenic strains in the context of a mesogenic recombinant virus backbone. Virus Res. 129:182–190. 10.1016/j.virusres.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 40.Alexander DJ. 1998. Newcastle disease virus and other avian paramyxoviruses, p 156–163 In Swayne D, Glisson JR, Jackwood MW, Pearson JE, Reed WM. (ed), A laboratory manual for the isolation and identification of avian pathogens, 4th ed. American Association of Avian Pathologists, Kennett Square, PA [Google Scholar]
- 41.Kapczynski DR, King DJ. 2005. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine 23:3424–3433. 10.1016/j.vaccine.2005.01.140 [DOI] [PubMed] [Google Scholar]
- 42.Callison SA, Riblet SM, Oldoni I, Sun S, Zavala G, Williams S, Resurreccion RS, Spackman E, Garcia M. 2007. Development and validation of a real-time Taqman PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J. Virol. Methods 139:31–38. 10.1016/j.jviromet.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 43.Vagnozzi A, Riblet SM, Zavala G, Garcia M. 2012. Optimization of a duplex real-time PCR method for relative quantitation of infectious laryngotracheitis virus. Avian Dis. 56:406–410. 10.1637/9883-081111-ResNote.1 [DOI] [PubMed] [Google Scholar]
- 44.Kolakofsky D, Roux L, Garcin D, Ruigrok RW. 2005. Paramyxovirus mRNA editing, the “rule of six” and error catastrophe: a hypothesis. J. Gen. Virol. 86:1869–1877. 10.1099/vir.0.80986-0 [DOI] [PubMed] [Google Scholar]
- 45.Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A, Villar E, Garcia-Sastre A, Palese P. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868–11873. 10.1128/JVI.75.23.11868-11873.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guy JS, Bagust TJ. 2003. Laryngotracheitis, p 121–134 In Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE. (ed), Diseases of poultry, 11th ed. Iowa State Press, Ames, IA [Google Scholar]
- 47.Mebatsion T, Nelson L, Hein R. 2008. XXIII World Poultry Conference, p 42–44 World Poultry Science Association, Brisbane, Australia [Google Scholar]
- 48.Kapczynski DR, Afonso CL, Miller PJ. 2013. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 41:447–453. 10.1016/j.dci.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Avila A, Oldoni I, Riblet S, Garcia M. 2007. Replication and transmission of live attenuated infectious laryngotracheitis virus (ILTV) vaccines. Avian Dis. 51:905–911. 10.1637/8011-041907-REGR.1 [DOI] [PubMed] [Google Scholar]