ABSTRACT
VSV-FH is a hybrid vesicular stomatitis virus (VSV) with a deletion of its G glycoprotein and encoding the measles virus (MV) fusion (F) and hemagglutinin (H) envelope glycoproteins. VSV-FH infects cells expressing MV receptors and is fusogenic and effective against myeloma xenografts in mice. We evaluated the fusogenic activities of MV and VSV-FH in relationship to the density of receptor on the target cell surface and the kinetics of F and H expression in infected cells. Using a panel of cells expressing increasing numbers of the MV receptor CD46, we evaluated syncytium size in MV- or VSV-FH-infected cells. VSV-FH is not fusogenic at low CD46 density but requires less CD46 for syncytium formation than MV. The size of each syncytium is larger in VSV-FH-infected cells at a specific CD46 density. While syncytium size reached a plateau and did not increase further in MV-infected CHO cells expressing ≥4,620 CD46 copies/cell, there was a corresponding increase in syncytium size with increases in CD46 levels in VSV-FH-infected CD46-expressing CHO (CHO-CD46) cells. Further analysis in VSV-FH-infected cell lines shows earlier and higher expression of F and H mRNAs and protein. However, VSV-FH cytotoxic activity was reduced by pretreatment of the cells with type I interferon. In contrast, the cytopathic effects are not affected in MV-infected cells. In summary, VSV-FH has significant advantages over MV as an oncolytic virus due to its higher viral yield, faster replication kinetics, and larger fusogenic capabilities but should be used in cancer types with defective interferon signaling pathways.
IMPORTANCE We studied the cytotoxic activity of a vesicular stomatitis/measles hybrid virus (VSV-FH), which is superior to that of measles virus (MV), in different cancer cell lines. We determined that viral RNA and protein were produced faster and in higher quantities in VSV-FH-infected cells. This resulted in the formation of larger syncytia, higher production of infectious particles, and a more potent cytopathic effect in permissive cells. Importantly, VSV-FH, similar to MV, can discriminate between low- and high-expressing CD46 cells, a phenotype important for cancer therapy as the virus will be able to preferentially infect cancer cells that overexpress CD46 over low-CD46-expressing normal cells.
INTRODUCTION
The Edmonston strain of measles virus (MV) has been used as a vaccine since the 1960s and has proven to be safe, with millions of doses given worldwide to protect children from infection by wild-type measles (1). Engineered versions of Edmonston lineage MV are being used as an oncolytic agent in human clinical trials against ovarian cancer, glioblastoma, multiple myeloma, mesothelioma, and head and neck cancer (2, 3). The data from preclinical rodent studies and human clinical trials show that oncolytic MV is a safe virus, with low toxicity and restricted tropism to cancer cells. To produce a more potent virus with a similar tropism, we generated a new hybrid virus by deleting the G glycoprotein from the full-length infectious cDNA clone of a potent oncolytic virus, vesicular stomatitis virus (VSV), and replacing it with the fusion (F) and hemagglutinin (H) envelope glycoproteins of MV. While parental VSV is neurovirulent when inoculated experimentally into research rodents, this new oncolytic VSV-FH virus shows reduced neurotoxicity and increased efficacy against plasmacytomas compared to parental MV and VSV-M51, which has a deletion in the matrix protein (4).
The MV-F and MV-H proteins are responsible for the attachment and entry events of the virus through either one of the three measles cellular receptors, CD46, signaling lymphocyte activation molecule (SLAM), or nectin-4 (5–9). Hence, both MV and VSV-FH use the same cellular receptors to infect target cells. SLAM is expressed on activated T cells, B cells, or dendritic cells (10). Nectin-4 (PVRL-4), recently identified as a receptor important for shedding and transmission of wild-type MV, is found on epithelial cells but is overexpressed in breast, lung, and ovarian cancer (11–13). CD46, a negative regulator of complement activation, is expressed at low levels on all nucleated cells but is overexpressed on numerous malignancies, including B cell malignancies, hepatocellular carcinoma, and head and neck cancer (14). In contrast to wild-type VSV that is not fusogenic, VSV-FH is also able to induce fusion between infected and uninfected neighboring cells to form multinucleated structures (syncytia) due to expression of F and H proteins on the infected cells. The fusogenic activity increases the bystander killing ability of this hybrid virus and may help to enhance its intratumoral spread and oncolytic activity. We previously showed that the extent of MV-induced intercellular fusion is in part controlled by numbers of CD46 receptors found on the target cells (15). Thus, at low CD46 receptor density, minimal infection and syncytia were observed but both were increased significantly above a threshold level of CD46 on the target cells. Since expression of F and H proteins in VSV-FH is driven by VSV elements, it is possible that syncytium formation will occur at a different threshold level of CD46 receptor density than in MV. To further characterize this newly described virus as a potential oncolytic agent for clinical testing, we compared the phenotypes, fusogenic activities, and expression kinetics of VSV-FH and MV in a panel of cancer cells and CHO cells expressing increasing levels of CD46 in this study.
MATERIALS AND METHODS
Cells and viruses.
Recombinant CHO cells stably expressing the human CD46 receptor (C1 isoform) have been previously described (15). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) plus 1 mg/ml G418. Vero (ATCC CCL-81), LoVo (ATCC CCL-229), SW480 (ATCC CCL-228), SW579 (ATCC HTB-107), and SW620 (ATCC CCL-227) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The MV Edmonston strain was used for all of the experiments, while the parental virus for VSV-FH is the VSV Indiana serotype.
To rescue VSV-FH tagged with enhanced green fluorescent protein (eGFP), MluI and AvrII restriction sites were introduced by PCR into MV-H (from pCGH plasmid). The VSV-G gene was deleted from pVSV-MC11-eGFP (16) by MluI-AvrII digestion, and then MV-H was ligated into this plasmid. SphI restriction sites were introduced into MV-F (from pCGF plasmid) and ligated into the SphI site of pMC11-VSV-MV-H-eGFP. The resulting plasmid, pMC11-VSVFH-eGFP, was used to rescue a replication-competent virus, as previously described (4).
Syncytium analysis.
For CHO cells, 1.5 × 106 or 4 × 106 cells (for MV or VSV-FH infections, respectively) were plated in a 10-cm dish. Cells were infected for 2 h with MV or VSV-FH at a multiplicity of infection (MOI) of 0.01 in OptiMEM. Medium was then replaced with 8 ml of complete medium with 1% methyl cellulose. At 1 or 2 days postinfection (for VSV-FH or MV, respectively), cells were fixed with 4% paraformaldehyde (PFA) for 30 min. Fixed cells were observed under the microscope to count the total number of syncytia per plate. Pictures of the infected cells were used to calculate the size of the syncytia using the NIH ImageJ software (17).
For human cell lines, the following numbers of cells were plated (half of the amount was plated for MV infections): 1 × 106 for LoVo, SW480, and SW620 cells; 2 × 106 for SW579 cells. Cells were infected with VSV-FH (MOI of 0.0001) or MV (MOI of 0.01) for 3 h. Medium was replaced with 8 ml of complete medium with 1% methylcellulose. At 24 or 48 h postinfection (hpi), pictures were taken, and the sizes of the syncytia were calculated.
Cell susceptibility.
MV and VSV-FH stocks were diluted in OptiMEM to get a titer of around 1 × 106 50% tissue culture infective doses (TCID50)/ml. Virus titers were determined in 96-well plates seeded with the following amounts of cells: for Vero cells, 7,500 cells/well; for LoVo, SW480, and SW620 cells, 15,000 cells/well; for SW579 cells, 6,000 cells/well. The viral titer of each cell line was calculated as previously described (18).
Cell viability and IFN sensitivity assays.
Ninety-six-well plates were seeded with the same number of cells as for the cell susceptibility assays. Cells were infected with MV or VSV-FH at MOIs of 1, 0.1, 0.01, and 0.001. For interferon (IFN) sensitivity assays, cells were treated with universal type I interferon (PBL Interferon Source, Piscataway, NJ) 16 h prior to the infection. At 72 hpi, cells were washed once with phosphate-buffered saline (PBS), trypsinized, and resuspended in 40 ml of complete medium. Cell viability was measured using a CellTiter 96 AQueous Assay (Promega, Fitchburg, WI), according to the manufacturer instructions.
Kinetics of viral production.
A total of 2.5 × 105 (Vero cells), 2 × 106 (LoVo, SW480, and SW620 cells), or 5 × 105 (SW579 cells) cells in six-well plates were infected with MV or VSV-FH at an MOI of 0.03 for 3 h in OptiMEM. Medium was replaced with 2 ml of complete medium, and samples were incubated at 37°C. At 24, 48, and 72 hpi, cells and supernatant were collected and subjected to two freeze-thaw cycles. Numbers of infectious particles were calculated as described above.
Analysis of viral RNA and protein accumulation.
A total of 5 × 105 (LoVo and SW620 cells) or 2.5 × 105 (SW480 and SW579 cells) cells per well of a 24-well plate were infected with MV or VSV-FH at an MOI of 0.5 for 3 h at 37°C. Virus inoculum was then removed, complete medium was added, and the cells were incubated at 37°C. At 6, 12, 24, and 48 hpi, medium was removed, and cells were harvested using buffer RLT (Qiagen, Venlo, Netherlands) or Laemmli buffer (Bio-Rad, Hercules, CA). For RNA analysis, RNA was purified using an RNeasy kit (Qiagen, Venlo, Netherlands), and 250 ng of total RNA was reverse transcribed using a SuperScript III kit and oligo(dT) primer (Life Technologies, Carlsbad, CA). A semiquantitative PCR (26 cycles, optimized to stop the PCR within the logarithmic phase) was done using Taq DNA polymerase (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. The primers used have been previously described (19, 20).
PCR products were analyzed in 1% agarose gels. For protein analysis, samples in Laemmli buffer were fractionated by PAGE in Criterion 10% Tris-HCl gels (Bio-Rad, Hercules, CA). Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA). Membranes were blocked with 5% powdered milk in Tris-buffered saline buffer with Tween 20 (TBST) for 1 h. MV-H or MV-F proteins were detected using specific antibodies as previously described (18); a goat polyclonal actin-specific horseradish peroxidase-conjugated antibody (I-19) was used to detect actin (Santa Cruz Biotechnology, Inc., Dallas, TX). Signal was developed using a Pierce ECL Western blotting substrate kit (Thermo Scientific, Waltham, MA).
RESULTS
VSV-FH induces larger syncytia than MV at a specific CD46 receptor density.
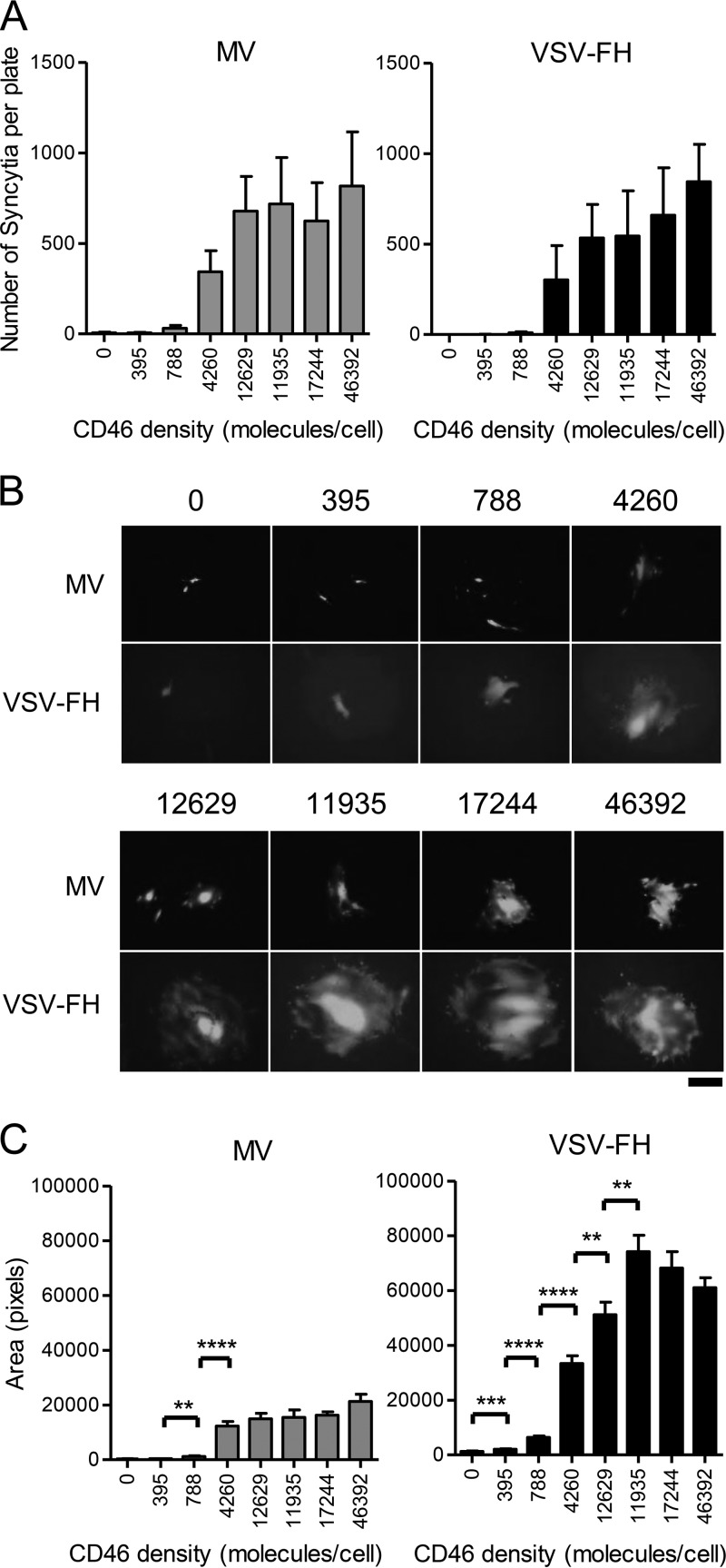
We previously generated a panel of CHO clones stably expressing 395 to 46,392 copies of human CD46 on the cell surface (Table 1) and showed that a threshold number of CD46 copies is required for syncytia to form (15). The panel of CHO-CD46 clones was infected with MV and VSV-FH at a low multiplicity of infection (MOI). An MOI of 0.01 was chosen to prevent syncytia from merging into each other. Methylcellulose was added to the complete medium to restrict viral release and formation of new syncytia at later time points postinfection. The number and size of syncytia present at 24 h (VSV-FH) or 48 h (MV) per plate postinfection were determined and plotted. An earlier time point was chosen for VSV-FH as the virus was much faster in its replication, and all cells in the monolayer were dead by 48 h (data not shown). Minimal syncytia or infection was seen at low CD46 density. The number of syncytia in low-density CD46 clones (395 or 788 CD46 copies/cell) was not different from that in parental CHO cells in both MV and VSV-FH infections (Fig. 1A). From CHO cells expressing 4,260 CD46 copies/cell, there was a significant increase in the numbers of syncytia induced by both viruses. From a density of 12,629 CD46 copies/cell onwards, syncytium numbers reached a plateau and did not significantly increase even in the clone expressing 46,392 CD46 copies/cell. Importantly, these results indicate that the two viruses, with very different morphologies (pleiomorphic measles and bullet-shaped VSV) but bearing the same F and H envelope glycoproteins, require a similar minimum receptor density to infect receptor-positive target cells.
TABLE 1.
Number of CD46 molecules per cell of the recombinant CHO cells
| CHO cell line | No. of CD46 molecules/cell |
|---|---|
| CHO | 0 |
| Clone 64 | 395 |
| Clone 41 | 788 |
| Clone 78 | 4,260 |
| Clone 25 | 12,629 |
| Clone 42 | 11,935 |
| Clone 62 | 17,244 |
| Clone 63 | 46,392 |
FIG 1.
Characterization of MV- or VSV-FH-induced syncytia in a panel of CHO-CD46 cells. A total of 1.5 × 106 (for MV infection) or 4 × 106 (for VSV-FH infection) CHO cells expressing increasing levels of the MV receptor CD46 were infected with MV or VSV-FH at an MOI of 0.01. (A) The numbers of syncytia per plate were counted at 24 hpi (VSV-FH) or 48 (MV) hpi. (B) Representative photographs of syncytia present in CHO-CD46 clones at 24 or 48 hpi. (C) Average size of MV- or VSV-FH-induced syncytia was measured using NIH ImageJ software. In all panels, CHO clones are arranged according to CD46 expression levels, starting from the left with the negative control (CHO) and finishing with the highest CD46-expressing clone; numbers below each bar or above each photograph indicate the number of CD46 molecules per cell. Scale bar, 100 μm. Bars represent averages ± standard deviations of three independent experiments. **, P ≤ 0.005; ***, P ≤ 0.0005; ****, P ≤ 0.00005.
It was observed that syncytia induced by VSV-FH appeared sooner (24 hpi versus 48 hpi for MV) and covered a larger area than MV-induced syncytia (representative images are shown in Fig. 1B). Measurements of syncytium size (area) in VSV-FH-infected cells show that as the CD46 levels increased from 395 copies per cell, the area covered by the syncytia increased significantly until a plateau was reached at 11,935 CD46 copies/cell. Syncytium size in the clone that expresses 46,392 CD46 copies/cell was not significantly larger than at 11,935 CD46 copies/cell. In contrast, no syncytia were seen at a low CD46 density in MV-infected cells, but they became apparent when CD46 levels reached 4,260 copies/cell. There was no significant increase in syncytium size thereafter with the increase in CD46 levels (Fig. 1C).
Extensive intercellular fusion is induced by VSV-FH in human cancer cell lines.
The studies on the panel of CHO cells indicate that syncytium formation of both viruses is differentially affected by receptor density even though both viruses express the same surface glycoproteins. We expanded the evaluation in a panel of human cancer cell lines, which not only express different numbers of CD46 and nectin-4 receptors (Table 2) but also are likely to be differentially permissive to the viruses as a result of their transformed state. Four human cancer cell lines (LoVo, SW480, SW579, and SW620) were chosen to study the different phenotypes of MV and VSV-FH, with the aim of evaluating relative syncytium sizes and viral fusogenic abilities.
TABLE 2.
Amount of CD46 and nectin-4 on the cell surface of the tested cancer cells
| Cell line | No. of receptormolecules/cell for:a |
|
|---|---|---|
| CD46 | Nectin-4 | |
| HCT-116 | 40,791 | 383 |
| LoVo | 53,923 | 196 |
| SW480 | 62,037 | ND |
| SW579 | 1,063 | 1,886 |
| SW620 | 51,364 | ND |
ND, nondetectable level.
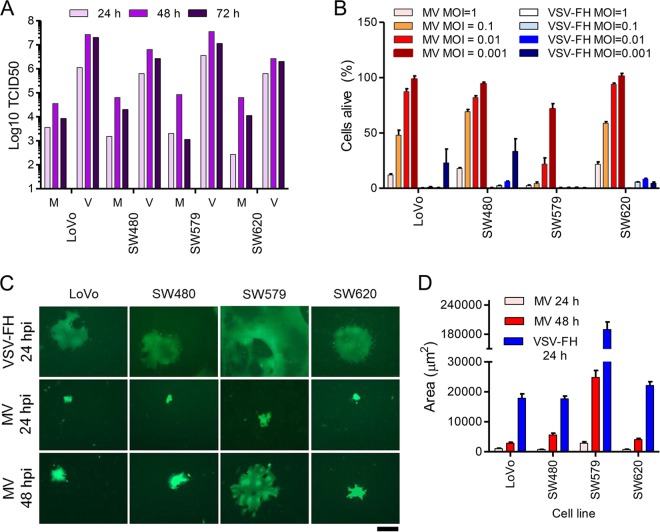
First, the permissivity of these cell lines to VSV-FH and MV was analyzed. Cells were infected with the viruses at an MOI of 0.03, and the total number of intra- and extracellular infectious particles produced over 24, 48, and 72 h was determined (Fig. 2A). The progeny titers obtained for VSV-FH were approximately 2 logs higher than for MV in all the cell lines tested (Fig. 2A). The cell-killing abilities of both viruses were tested at various MOIs. Cell viability assays show that even though both MV and VSV-FH were effective at killing cancer cells, VSV-FH induced more cell death at lower MOIs than MV for most of the cell lines tested (Fig. 2B).
FIG 2.
Phenotypic characterization of MV and VSV-FH in a panel of human cancer cell lines. (A) Viral particle production. Representative experiment measuring viral progeny in cells infected with MV (M) or VSV-FH (V) at 24, 48, and 72 hpi. (B) Cytotoxicity of MV and VSV-FH. Cells were infected with MV or VSV-FH at different MOIs. At 3 days postinfection, cell viability was determined by MTS assay. The amount of cells alive after each treatment is expressed as a percentage of the noninfected wells (100% cells alive). (C) Representative images of syncytia in cells infected with VSV-FH or MV at 24 and/or 48 hpi. (D) Syncytium area was determined by NIH ImageJ software and plotted. Scale bar, 100 μm. Bars represent averages ± standard deviations of three independent experiments.
Syncytium size was determined in VSV-FH or MV-infected cell lines at 24 hpi (for VSV-FH and MV) or 48 hpi (for MV only as VSV-FH-induced syncytia were no longer attached to the plates at this time) in the human cancer cell lines. Representative images used to calculate the syncytium size are shown in Fig. 2C, along with the results of the measurements (Fig. 2D). VSV-FH is clearly more fusogenic than MV; VSV-FH is capable of inducing large syncytia within 24 h and inducing them to a size that is not reached or feasible with MV even at 48 hpi (Fig. 2C, bottom panel, and D). The sizes of VSV-FH-induced syncytia were 1.5 to 6 times larger than those of MV.
VSV-FH expresses high levels of protein and RNA for the foreign MV glycoprotein genes.
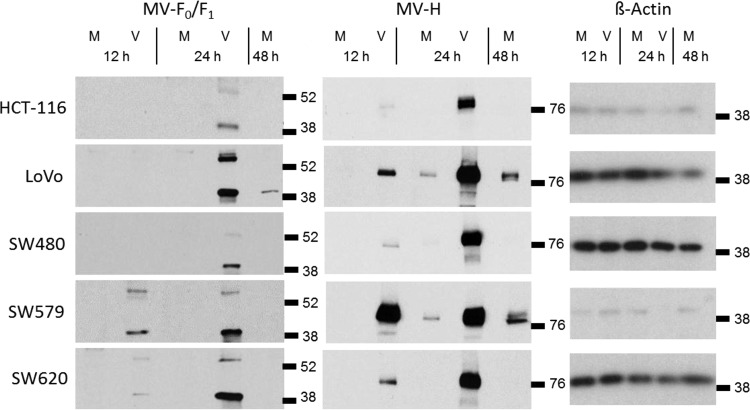
The formation of larger syncytia by VSV-FH could be the result of a higher production of MV-F and MV-H proteins than produced by MV. To gain more understanding of the kinetics of viral transcription and translation of VSV-FH and MV, the levels of MV-F and MV-H proteins in infected cells were determined. The panel of human cancer cells was infected with MV or VSV-FH at an MOI of 0.5, and the cells were harvested at early (6 and 12 h) and late times (24 and 48 h) postinfection. The accumulation of glycoproteins was determined by Western blot analysis using specific antibodies. Cells infected by VSV-FH expressed higher levels of F and H proteins than MV and expressed them at earlier times than MV (Fig. 3). MV-H protein expression in VSV-FH-infected cells is easily detectable at 12 hpi, with a slight increase at 24 hpi. MV-F protein is detectable in VSV-FH-infected cells at 24 hpi for most of the cell lines except for SW579 and SW620, where it is visible at 12 hpi. In MV-infected cells, MV-H is detected at 24 hpi while the maximum accumulation of this protein is observed at 48 hpi. MV-F accumulation is barely detectable under these conditions at 48 hpi. Overall, F and H proteins were translated earlier and accumulated to higher levels in VSV-FH-infected cells than in MV-infected cells.
FIG 3.
Kinetics of viral translation of F and H proteins in infected cells. Cells were infected with MV (M) or VSV-FH (V) at an MOI of 0.5. At the indicated hours cells were harvested. Proteins were fractionated by PAGE and detected by immunoblotting. Locations and molecular weights (in thousands) of the molecular weight markers are indicated at the right of each panel.
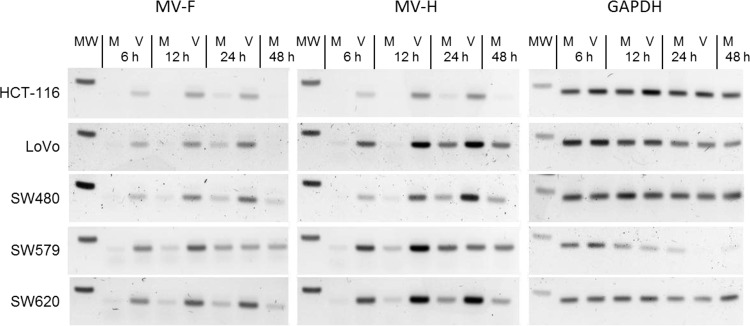
We hypothesized that the high levels of viral protein translation in VSV-FH are related to a higher production of mRNA due to faster and higher VSV viral replication and/or transcription. In parallel to the previous experiment, another set of cells was harvested at the same time to quantify the levels of mRNA for MV-F and MV-H genes. Results in Fig. 4 show that the mRNAs of MV-F and MV-H encoded by VSV-FH are detectable by 6 hpi, with a peak at 12 hpi. In contrast, F and H mRNAs in MV-infected cells did not appear until 12 hpi, with a peak at 24 hpi. In addition, MV-F and MV-H mRNAs accumulated about 2-fold more in VSV-FH-infected cells than in MV-infected cells, which led to a large difference in protein expression levels by the two viruses (Fig. 3).
FIG 4.
Kinetics of F and H mRNA production in infected cells. MV (M)- or VSV-FH (V)-infected cells were harvested at 6, 12, 24, or 48 hpi. Semiquantitative PCR was done to detect the levels of F, H, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA present at these times. Molecular weight band (MW) corresponds to the 250-nucleotide band.
MV cytopathic effect is not severely affected by IFN.
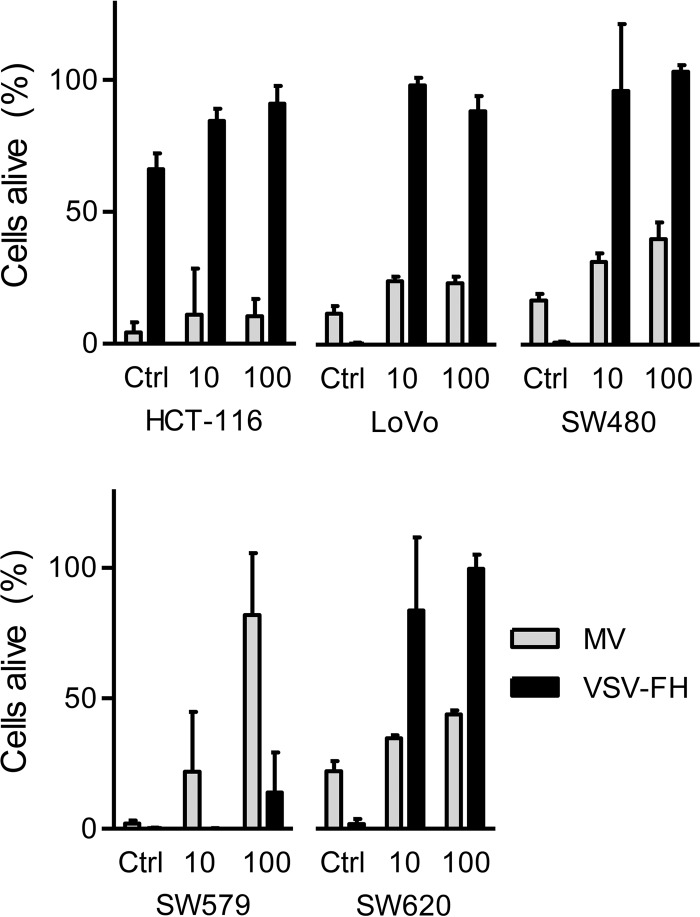
Data obtained so far indicate that VSV-FH is a potent cytotoxic virus, is able to kill permissive cells at a low MOI, and is capable of forming large syncytia that in turn might help viral spread within tumors. However, previous reports suggest that some tumors exist in an interferon (IFN)-activated antiviral state that limits the cell-killing activity of IFN-sensitive viruses such as VSV (21). We thus proceeded to evaluate the cytotoxic activity of MV and VSV-FH in interferon-treated cells. The panel of human cancer cell lines was incubated with a low or high dose of IFN (10 or 100 units, respectively), and the cells were infected with VSV-FH or MV at an MOI of 1.0. The percentage of live cells was measured at 3 days postinfection by an MTS [3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt] cell proliferation assay (Fig. 5). In general, MV was able to kill at least 70% of cells even at higher doses of IFN, except in SW579 cells, where MV cytotoxicity was impaired. In contrast, the cell-killing activity of VSV-FH was significantly compromised if the cell lines were pretreated with 10 or 100 units of IFN. Interestingly, the cell line HCT-116 was resistant to VSV-FH infection even without IFN treatment, and IFN-treated SW579 cells were resistant to MV but not to VSV-FH infection.
FIG 5.
Impact of IFN pretreatment on MV and VSV-FH cytotoxicity. IFN-pretreated cells (10 or 100 units) or nontreated control (Ctrl) cells were infected with MV or VSV-FH at an MOI of 1, and cell viability was calculated at 72 hpi by MTS assay. The amount of cells alive is expressed as a percentage of live cells in the noninfected wells (100% cells alive). Bars represent averages ± standard deviations of three independent experiments.
DISCUSSION
Use of fusogenic membrane glycoproteins as therapeutic genes to increase virus potency has been performed in different viruses. For instance, oncolytic herpes simplex virus has been engineered to express the constitutively fusogenic protein of the gibbon ape leukemia virus (GALV). The killing activity of this fusogenic virus was improved, as shown in a panel of tumor cell lines in vitro and against solid tumors (22, 23). VSV is another virus whose oncolytic activity was improved by inserting a fusogenic gene; for this, its genome was engineered to express the gene for the fusion protein of Newcastle disease virus (NDV-F; with the mutation L289A to render it fusogenic without the expression of the hemagglutinin-neuraminidase protein). Compared to parental virus, the mutant showed increased oncolytic activity and increased overall mouse survival against hepatocellular carcinoma, squamous cell carcinoma of the head and neck, and murine orthotropic floor-of-mouth squamous cell carcinoma (24, 25).
Recombinant VSV used in cancer therapy is often designed to express or induce the expression of IFN in infected cells as a mechanism to allow preferential virus replication in cancer cells but not in normal cells. However, a number of tumor types are not totally defective in their IFN responses and can still respond to the protective effects of IFN. As a result, the oncolytic activity of IFN-inducing VSV can often be blunted in IFN-responsive cancers. We recently generated VSV-FH as an alternative strategy to redirect virus tropism to cancer cells. Oncolytic measles virus has shown excellent safety in numerous phase I clinical trials in patients with recurrent ovarian cancer, glioma, and relapsed myeloma. Of the three receptors that can be used by MV to infect cells, CD46 has the broadest expression profile since it is ubiquitously expressed on all nucleated cells to protect them from self-lysis by the complement system. Interestingly, CD46 is overexpressed on numerous cancer types, and high expression of CD46 on cancer cells contributes in part to the extensive fusion seen in oncolytic MV-infected cancer cells compared to nontransformed cells.
The in vitro fusogenic properties of MV are correlated to the density of receptor expressed on the target cells. Using parental MV or HER-2 receptor-specific MV, we showed that syncytium size and the extent of cytopathic effects increase as a function of receptor (CD46 or HER-2) density. Importantly, there is a threshold receptor density below which cell fusion does not occur and above which syncytium size does not increase further (15, 26). With regard to VSV-FH, we were intrigued by the possibility that the hybrid virus might not be subject to the same rules of engagement in relation to receptor density as VSV has much faster replication kinetics and drives a higher level of gene expression. MV and VSV-FH cannot infect CHO cells as the cells do not express any of the measles receptors. Using a panel of CHO cells genetically engineered to express CD46, we compared the fusogenic properties of MV and VSV-FH, and it was clear that VSV-FH-infected cells can fuse at a lower CD46 level (at 788 CD46 copies) than MV-infected cells (at 4,620 CD46 copies). The size per syncytium was also different; syncytia in VSV-FH-infected cells were generally larger those of MV-infected cells in the same CHO-CD46 clone. The same result was seen on human cancer cells; VSV-FH-induced syncytia were between 4 and 6 times larger than those of MV in the corresponding cell line and occurred about 24 h earlier.
Since the panel of CHO recombinant cell lines is based on the same CHO cell type, we can reasonably attribute the differences in syncytium size to the differences in receptor density. These results, however, cannot be easily extrapolated to human cancer cells, where the intrinsic transformed characteristics of each cell line would play a role in modulating the fusogenic properties and cytopathic effects of the viruses. We selected cancer cell lines from the same malignancy (LoVo, SW480, and SW620 are all from colorectal carcinoma) to observe the differences in cytotoxic activity of VSV-FH or MV in a specific cancer type. Results showed that LoVo, SW480, and SW620 cells were all similarly susceptible to VSV-FH and MV infections. Cell-killing abilities and production of viral particles were different between VSV-FH and MV. Peak production of infectious MV viral particles was 1 to 2 logs less than that of VSV-FH. MV requires a significantly higher MOI (2 to 3 logs higher) to achieve cell killing similar to that of VSV-FH.
One of the most striking differences between VSV-FH and MV is rate of syncytium formation and the speed at which syncytia grow and coalesce. Close monitoring of the kinetics of transcription and translation of F/H mRNA and proteins revealed that these processes were much more rapid in VSV-FH-infected cells. Both F and H proteins were detected as early as 12 hpi in VSV-FH-infected cells, whereas F and H expression by MV were detected only at 24 hpi, even in the highly susceptible cell line SW579. In this cell line, VSV-FH grows at high titers and induces the formation of large syncytia (Fig. 2). This phenotype might be due to higher viral translation, particularly F translation, as it has been demonstrated that an increment in the translation of MV-F does not affect viral replication but enhances MV cytopathogenicity (27).
In addition to the different viral polymerases, an important factor in play here is that the Edmonston strain of MV does not induce a shutoff of host protein translation (28, 29). In contrast, VSV induces a rapid shutoff of cellular translation, usurping the cellular machinery for synthesis of VSV proteins and virions (30, 31). Overall, high levels of viral transcription and translation combined with a rapid shutoff of viral proteins contribute to the enhanced formation of syncytia in VSV-FH-infected cells.
VSV is highly sensitive to the antiviral effects of IFN, and the blocking of nuclear transport of cellular mRNA by the matrix protein of VSV prevents the induction of IFN-responsive genes in the infected cell. As such, VSV sensitivity to IFN is a trait that was exploited to target the parental virus to cancer cells presenting defects in the IFN pathway (32). Unfortunately, it is becoming more evident that some cancer cell lines retain some of this IFN responsiveness and in some cases play an important role in obstructing oncolytic activity of VSV (21). When the cytopathic effects of VSV-FH and MV were analyzed in IFN-treated cells, we found that MV, but not VSV-FH, was able to kill the cells even at the highest dose (100 units) of IFN tested. In HCT-116 cells, VSV-FH cell killing was low even in nontreated cells, perhaps due to a preexisting antiviral state making it constitutively resistant to VSV infection, as observed for other cell lines (33). In contrast, MV cytotoxicity was not severely affected in most of the IFN-treated cells, probably due to the expression of the viral proteins P, V, and C. These proteins have a role in preventing the activation of the IFN pathway by a mechanism such as preventing the activation of NF-κB, blocking the phosphorylation of Jak1, or downregulating IFN-β transcription (34). Unexpectedly, IFN-treated SW579 cells are MV resistant but VSV-FH sensitive, suggesting that activation of the IFN response and of the response to viral infection is different among the tested cell lines.
These experiments utilizing pretreatment of cancer cells with IFN show that some cancer cells exist in a constitutively activated antiviral state or have intact IFN antiviral response pathways, highlighting one of the major limiting factors in VSV virotherapy. There is an ongoing search for clinically viable drugs, such as Jak1/2 inhibitors (e.g., ruxolitinib), that could be combined with VSV virotherapy such that cellular antiviral resistance to VSV could be reversed (20).
In summary, the fast replication machinery of VSV combined with fusogenic MV-derived F/H proteins results in a virus with increased cytotoxicity and production of viral particles and with better intracellular spreading. Although the heterogenic nature of cancer makes it difficult to predict the behavior of the oncolytic viruses in the different types of tumors, the results in this report show the potential of VSV-FH as a promising cancer therapeutic. Future experiments are needed to determine the cancer type in which VSV-FH could be applied for clinical testing.
ACKNOWLEDGMENTS
This work was funded by grants from the NIH/NCI (CA129193 and CA129966).
S.J.R., K.-W.P., and the Mayo Clinic have a financial interest in this research.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Hilleman MR. 2001. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651–665. 10.1016/S0264-410X(01)00384-X [DOI] [PubMed] [Google Scholar]
- 2.Msaouel P, Dispenzieri A, Galanis E. 2009. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr. Opin. Mol. Ther. 11:43–53 [PMC free article] [PubMed] [Google Scholar]
- 3.Galanis E. 2010. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin. Pharmacol. Ther. 88:620–625. 10.1038/clpt.2010.211 [DOI] [PubMed] [Google Scholar]
- 4.Ayala-Breton C, Suksanpaisan L, Mader EK, Russell SJ, Peng KW. 2013. Amalgamating oncolytic viruses to enhance their safety, consolidate their killing mechanisms, and accelerate their spread. Mol. Ther. 21:1930–1937. 10.1038/mt.2013.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatsuo H, Ono N, Tanaka K, Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893–897. 10.1038/35022579 [DOI] [PubMed] [Google Scholar]
- 6.Dorig RE, Marcil A, Chopra A, Richardson CD. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295–305. 10.1016/0092-8674(93)80071-L [DOI] [PubMed] [Google Scholar]
- 7.Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, Richardson CD. 2011. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7:e1002240. 10.1371/journal.ppat.1002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlebach MD, Mateo M, Sinn PL, Prufer S, Uhlig KM, Leonard VH, Navaratnarajah CK, Frenzke M, Wong XX, Sawatsky B, Ramachandran S, McCray PB, Jr, Cichutek K, von Messling V, Lopez M, Cattaneo R. 2011. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480:530–533. 10.1038/nature10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avota E, Gulbins E, Schneider-Schaulies S. 2011. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 7:e1001290. 10.1371/journal.ppat.1001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, Bliss RL, Geller MA, Argenta PA, Harrington KM, Skubitz AP. 2010. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am. J. Clin. Pathol. 134:835–845. 10.1309/AJCPGXK0FR4MHIHB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, Finetti P, Sauvan R, Adelaide J, Geneix J, Lecocq E, Popovici C, Dubreuil P, Viens P, Goncalves A, Charafe-Jauffret E, Jacquemier J, Birnbaum D, Lopez M. 2007. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 7:73. 10.1186/1471-2407-7-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, Nishimura H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y. 2009. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 69:6694–6703. 10.1158/0008-5472.CAN-09-0016 [DOI] [PubMed] [Google Scholar]
- 14.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. 2003. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol. Immunol. 40:109–123. 10.1016/S0161-5890(03)00112-3 [DOI] [PubMed] [Google Scholar]
- 15.Anderson BD, Nakamura T, Russell SJ, Peng KW. 2004. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 64:4919–4926. 10.1158/0008-5472.CAN-04-0884 [DOI] [PubMed] [Google Scholar]
- 16.Ammayappan A, Nace R, Peng KW, Russell SJ. 2013. Neuroattenuation of vesicular stomatitis virus through picornaviral internal ribosome entry sites. J. Virol. 87:3217–3228. 10.1128/JVI.02984-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadac EM, Peng KW, Nakamura T, Russell SJ. 2004. Reengineering paramyxovirus tropism. Virology 329:217–225. 10.1016/j.virol.2004.08.036 [DOI] [PubMed] [Google Scholar]
- 19.Hummel KB, Lowe L, Bellini WJ, Rota PA. 2006. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J. Virol. Methods 132:166–173. 10.1016/j.jviromet.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Escobar-Zarate D, Liu YP, Suksanpaisan L, Russell SJ, Peng KW. 2013. Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors. Cancer Gene Ther. 20:582–589. 10.1038/cgt.2013.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YP, Suksanpaisan L, Steele MB, Russell SJ, Peng KW. 2013. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci. Rep. 3:2375. 10.1038/srep02375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu X, Tao L, Jin A, Vile R, Brenner MK, Zhang X. 2003. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol. Ther. 7:748–754. 10.1016/S1525-0016(03)00092-3 [DOI] [PubMed] [Google Scholar]
- 23.Simpson GR, Han Z, Liu B, Wang Y, Campbell G, Coffin RS. 2006. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 66:4835–4842. 10.1158/0008-5472.CAN-05-4352 [DOI] [PubMed] [Google Scholar]
- 24.Ebert O, Shinozaki K, Kournioti C, Park MS, Garcia-Sastre A, Woo SL. 2004. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 64:3265–3270. 10.1158/0008-5472.CAN-03-3753 [DOI] [PubMed] [Google Scholar]
- 25.Shin EJ, Chang JI, Choi B, Wanna G, Ebert O, Genden EM, Woo SL. 2007. Fusogenic vesicular stomatitis virus for the treatment of head and neck squamous carcinomas. Otolaryngol. Head Neck Surg. 136:811–817. 10.1016/j.otohns.2006.11.046 [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ, Peng KW. 2007. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J. Virol. 81:13149–13157. 10.1128/JVI.01415-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda M, Ohno S, Seki F, Nakatsu Y, Tahara M, Yanagi Y. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 79:14346–14354. 10.1128/JVI.79.22.14346-14354.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler SL, Fields BN. 1978. Intracellular synthesis of measles virus-specified polypeptides. J. Virol. 25:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves MC. 1981. Measles virus polypeptides in infected cells studied by immune precipitation and one-dimensional peptide mapping. J. Virol. 38:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor JH, Lyles DS. 2005. Inhibition of host and viral translation during vesicular stomatitis virus infection. eIF2 is responsible for the inhibition of viral but not host translation. J. Biol. Chem. 280:13512–13519. 10.1074/jbc.M501156200 [DOI] [PubMed] [Google Scholar]
- 31.Wertz GW, Youngner JS. 1970. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J. Virol. 6:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber GN. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710–7719. 10.1038/sj.onc.1209042 [DOI] [PubMed] [Google Scholar]
- 33.Paglino JC, van den Pol AN. 2011. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J. Virol. 85:9346–9358. 10.1128/JVI.00723-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delpeut S, Noyce RS, Siu RW, Richardson CD. 2012. Host factors and measles virus replication. Curr. Opin. Virol. 2:773–783. 10.1016/j.coviro.2012.10.008 [DOI] [PubMed] [Google Scholar]