Abstract
Arylimidamides (AIAs) are inspired by diamidine antimicrobials but show superior activity against intracellular parasites. The AIA DB766 {2,5-bis[2-(2-i-propoxy)-4-(2-pyridylimino)aminophenyl]furan hydrochloride} displays outstanding potency against intracellular Leishmania parasites and is effective in murine and hamster models of visceral leishmaniasis when given orally, but its mechanism of action is unknown. In this study, through the use of continuous DB766 pressure, we raised Leishmania donovani axenic amastigotes that displayed 12-fold resistance to this compound. These DB766-resistant (DB766R) parasites were 2-fold more sensitive to miltefosine than wild-type organisms and were hypersensitive to the sterol 14α-demethylase (CYP51) inhibitors ketoconazole and posaconazole (2,000-fold more sensitive and over 12,000-fold more sensitive than the wild type, respectively). Western blot analysis of DB766R parasites indicated that while expression of CYP51 is slightly increased in these organisms, expression of CYP5122A1, a recently identified cytochrome P450 associated with ergosterol metabolism in Leishmania, is dramatically reduced in DB766R parasites. In vitro susceptibility assays demonstrated that CYP5122A1 half-knockout L. donovani promastigotes were significantly less susceptible to DB766 and more susceptible to ketoconazole than their wild-type counterparts, consistent with observations in DB766R parasites. Further, DB766-posaconazole combinations displayed synergistic activity in both axenic and intracellular L. donovani amastigotes. Taken together, these studies implicate CYP5122A1 in the antileishmanial action of the AIAs and suggest that DB766-azole combinations are potential candidates for the development of synergistic antileishmanial therapy.
INTRODUCTION
Designated by the World Health Organization (WHO) as a neglected tropical disease, leishmaniasis is a diverse and complex vector-borne infection caused by over 20 different species of protozoan parasites of the genus Leishmania. Depending upon the causative species, the disease has four major clinical manifestations: (i) a self-healing cutaneous form resulting in skin lesions, (ii) a disseminated cutaneous manifestation that is more chronic in nature, (iii) a mucocutaneous form affecting the mucosal lining, and (iv) a fatal visceral form with spleen and liver involvement caused by parasites of the Leishmania donovani-Leishmania infantum complex (http://www.who.int/tdr/diseases-topics/leishmaniasis/en/). Leishmaniasis is endemic in 98 countries across five continents, and the estimated numbers of new cases of visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) are in the range of 300,000 and 1,000,000 per year, respectively (1). Although pentavalent antimonials have long served as the first line of treatment for both VL and CL, they are no longer effective against Indian VL because of the emergence of drug-resistant strains. Liposomal amphotericin B formulations, while very effective, are limited by the route of drug administration and costs associated with treatment. Miltefosine, the first oral antileishmanial drug, and paromomycin are effective and have been approved for the treatment of VL in India. However, the use of the former is limited due to its gastrointestinal toxicity, teratogenicity, and relatively high cost (2, 3), and the latter must be given by injection over a period of 3 weeks (4). Thus, while there have been some recent advances in VL chemotherapy, the need for new, inexpensive oral agents with improved efficacy against existing drug-resistant strains and reduced toxicity is urgent.
Arylimidamides (AIAs) are potent antiprotozoal agents that are members of a library of cationic diamidines and their analogs. Although the design of AIAs was inspired by diamidine antimicrobials such as pentamidine, AIAs possess physicochemical properties that are distinct from those of diamidines (5). These differences are believed to translate into improved activity against intracellular pathogens, such as Mycobacterium tuberculosis (6), Trypanosoma cruzi (7), and Leishmania (8). The lead AIA in this series, DB766 {2,5-bis[2-(2-i-propoxy)-4-(2-pyridylimino)aminophenyl]furan hydrochloride}, displayed outstanding potency against Leishmania donovani intracellular amastigotes (50% inhibitory concentration [IC50] = 0.036 μM) in vitro as well as oral efficacy in murine and hamster models of visceral leishmaniasis (71% and 89% reductions in liver parasitemia when given orally at 100 mg/kg of body weight/day for 5 days, respectively) (5). Unfortunately, neither DB766 nor its corresponding mesylate salt, DB1960, possesses a sufficient therapeutic window to permit further development of this molecule as an antileishmanial drug (9), and none of the newer bis-AIAs that have been prepared are superior to DB766 as antileishmanial candidates (10, 11).
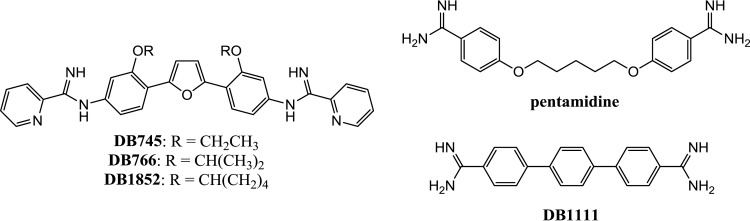
In an attempt to capitalize on the antileishmanial potency of AIAs for the development of drug candidates with improved activity against leishmaniasis, a series of experiments was performed with the initial goal of obtaining an understanding of the antileishmanial mechanism of action of AIAs. These findings shed light on the action of DB766 in Leishmania, may provide further insight into the effects of azoles on these parasites, and point toward a new strategy for antileishmanial drug development. The structures of the arylimidamides and diamidines used in this study are given in Fig. 1.
FIG 1.
Structures of arylimidamides and diamidines.
MATERIALS AND METHODS
Parasites and culture conditions.
Leishmania donovani MHOM/SD/62/1S-CL2D promastigotes were adapted to axenic amastigote forms by culturing the former at 37°C in a humidified 5% CO2 atmosphere in axenic amastigote medium as described previously (12). For intracellular assays, β-lactamase-expressing L. donovani promastigotes (provided by Frederick Buckner, University of Washington) were maintained as outlined earlier (13). Wild-type and CYP5122A1 half-knockout (HKO) promastigotes of L. donovani MHOM/IN/80/DD8 (14) were used in DB766 and ketoconazole susceptibility assays. Both wild-type and CYP5122A1 HKO L. donovani promastigotes were grown and cultured as described previously (15).
Drugs and reagents.
The CellTiter reagent was obtained from Promega (Madison, WI), miltefosine was purchased from Cayman Chemical Company (Ann Arbor, MI), and DB1111, DB766, DB745, and DB1852 were synthesized according to known methods (5, 8, 16). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated.
Selection of a DB766-resistant Leishmania donovani cell line.
Axenically grown Leishmania donovani amastigotes were exposed to increasing DB766 pressure starting at a concentration of 0.05 μM and rising to a final concentration of 8 μM. A stepwise increase in the DB766 concentration was applied only when pressured cultures showed a growth rate equivalent to that of untreated cultures.
In vitro differentiation and growth curve.
The transformation of L. donovani axenic amastigote forms to promastigotes was initiated by inoculating 5 × 106 parasites/ml in 4 ml of RPMI 1640 medium containing 20% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin at pH 6.88 and 23°C. The cell density and number of promastigote-like slender forms were determined by hemocytometer-based counting every 24 h for 72 h in three separate experiments.
In vitro susceptibility studies.
The in vitro susceptibility of the DB766-resistant L. donovani cell line was evaluated after the cells were allowed to grow in the absence of DB766 for at least 3 days. Briefly, 106 DB766-sensitive or DB766-resistant axenic amastigotes per ml in a total volume of 60 μl were treated with a 2-fold dilution series of each compound in a 96-well plate at 37°C for 72 h. At the end of the treatment, cell viability was determined using the tetrazolium dye-based CellTiter reagent (Promega, Madison, WI). IC50s were calculated using a four-parameter curve with SoftMax Pro software (Amersham Biosciences, Piscataway, NJ). Each compound was tested in at least three separate experiments.
The nature of the interaction between DB766 and posaconazole was determined according to the modified fixed-ratio isobologram method (17). In assays employing L. donovani MHOM/SD/62/1S-CL2D axenic amastigotes, a series of solutions was prepared by making 10 2-fold dilutions of fixed-ratio solutions of posaconazole and DB766 (5:0; 4:1; 3:2; 2:3; 1:4, and 0:5); the highest concentration of posaconazole and DB766 used in these assays was 25 μM each. This allowed determination of the IC50 of each drug alone against wild-type axenic amastigotes from fixed-ratio solutions of 5:0 and 0:5, as well as the IC50s of drug combinations from fixed-ratio solutions of 4:1, 3:2, 2:3, and 1:4. Each point was tested in triplicate. Endpoints were determined as described previously in drug susceptibility assays. The fractional inhibitory concentration (FIC) of DB766 was defined as the IC50 of DB766 in combination/IC50 of DB766 alone; the FIC of posaconazole was defined as the IC50 of posaconazole in combination/IC50 of posaconazole alone. FICs were used for constructing the isobolograms, with the sum of the FICs (ΣFICs) being equal to the FIC of DB766 plus the FIC of posaconazole, thus allowing the determination of the nature of the DB766-posaconazole interaction. The susceptibility of intracellular β-lactamase-expressing L. donovani to DB766, posaconazole, and fixed-ratio combinations of these two compounds was determined as outlined previously (13). FIC, ΣFIC, and mean ΣFIC values were calculated as described above for L. donovani axenic amastigotes. In both assays, the interaction between DB766 and posaconazole was classified as synergistic if the ΣFIC was ≤0.5, indifferent if the ΣFIC was >0.5 and <4, and antagonistic if the ΣFIC was >4 (17).
To evaluate the susceptibility of wild-type and CYP5122A1 HKO L. donovani MHOM/IN/80/DD8 promastigotes to DB766 or ketoconazole, these parasites were incubated with or without DB766 (50 to 750 nM) or ketoconazole (10 and 30 μM) at a seeding density of 106 cells/ml at 23°C for 24 h. For assessment of cell viability, parasites were harvested by centrifugation at 1,100 × g for 5 min, followed by resuspension in phosphate-buffered saline. Propidium iodide was added at a final concentration of 2 μg/ml, and the mixture was incubated for 5 min before analyzing the fluorescence on the FL2 channel of a BD FACSCalibur flow cytometer.
Western blotting.
Cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer (Pierce), and protein determinations were performed using a bicinchoninic acid protein assay kit (Pierce) according to the manufacturer's instructions. Proteins were electrophoresed on 10% polyacrylamide gels by standard denaturing SDS-PAGE using precast gels from Bio-Rad. For Western blotting, proteins were transferred to a polyvinylidene difluoride membrane (GE Life Sciences) at a constant voltage of 80 kV for 2 h. Tris-buffered saline containing 5% nonfat milk in 0.1% Tween was used for blocking and probing the membrane with a 1:20,000 dilution of anti-CYP5122A1 antibody, a 1:500 dilution of anti-CYP51 antibody (provided by Frederick Buckner, University of Washington, Seattle, WA), or a 1:1,000 dilution of anti-α-enolase antibody (provided by Paul Michels, Catholic University of Louvain, Brussels, Belgium). To visualize the bands, enhanced chemiluminescence was performed according to the manufacturer's instructions (Cell Signaling Technologies, Danvers, MA).
RESULTS
The trypanosomatid mitochondrion has been shown to be the main subcellular target of pentamidine and other diamidines (18). Since AIAs contain amidine functional groups, the ultrastructural effects caused by the lead AIA DB766 were compared with those caused by the diamidine DB1111 in Leishmania donovani axenic amastigotes. While DB1111 caused dilation of the L. donovani mitochondrion, as observed previously (16), no changes in mitochondrial morphology were observed upon DB766 treatment. Instead, other ultrastructural alterations were noted in other organelles, including an increased number of vesicles in the flagellar pocket, damage to the flagellar membrane, and increased cytoplasmic vacuolization (data not shown).
Generation of DB766-resistant L. donovani.
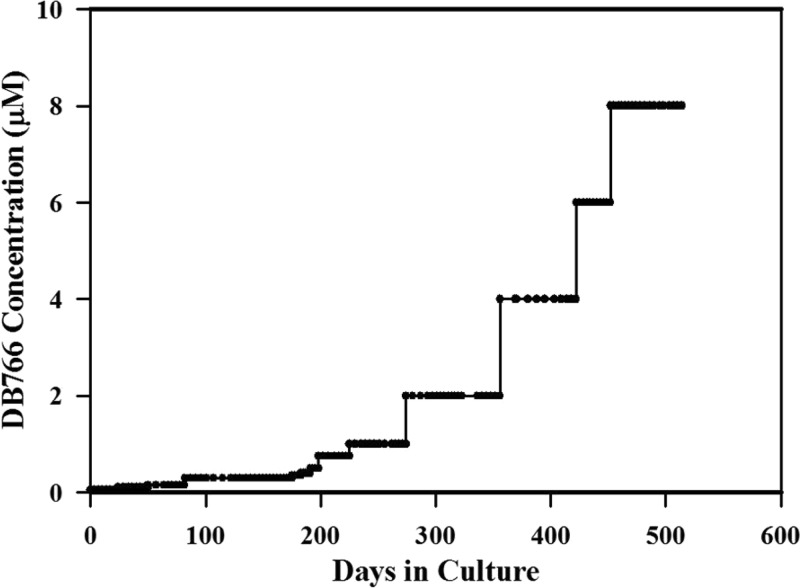
In an attempt to obtain mechanistic information concerning AIAs, we generated L. donovani axenic amastigotes that were approximately 12-fold resistant to DB766 by culturing parasites in the presence of increasing DB766 concentrations. As indicated in Fig. 2, the development of resistance to DB766 occurred with difficulty in culture. The time required to induce ∼12-fold resistance to DB766 through increasing pressure (assessed by comparing the IC50s of resistant parasites with those of wild-type parasites) was about 18 months. Further, this resistance was maintained for at least 5 months in the absence of DB766 pressure, indicating a stable chemoresistant phenotype.
FIG 2.
Generation of a DB766-resistant L. donovani cell line. Leishmania donovani axenic amastigotes were cultured under increasing DB766 pressure starting at a concentration of 0.05 μM and rising to a final concentration of 8 μM.
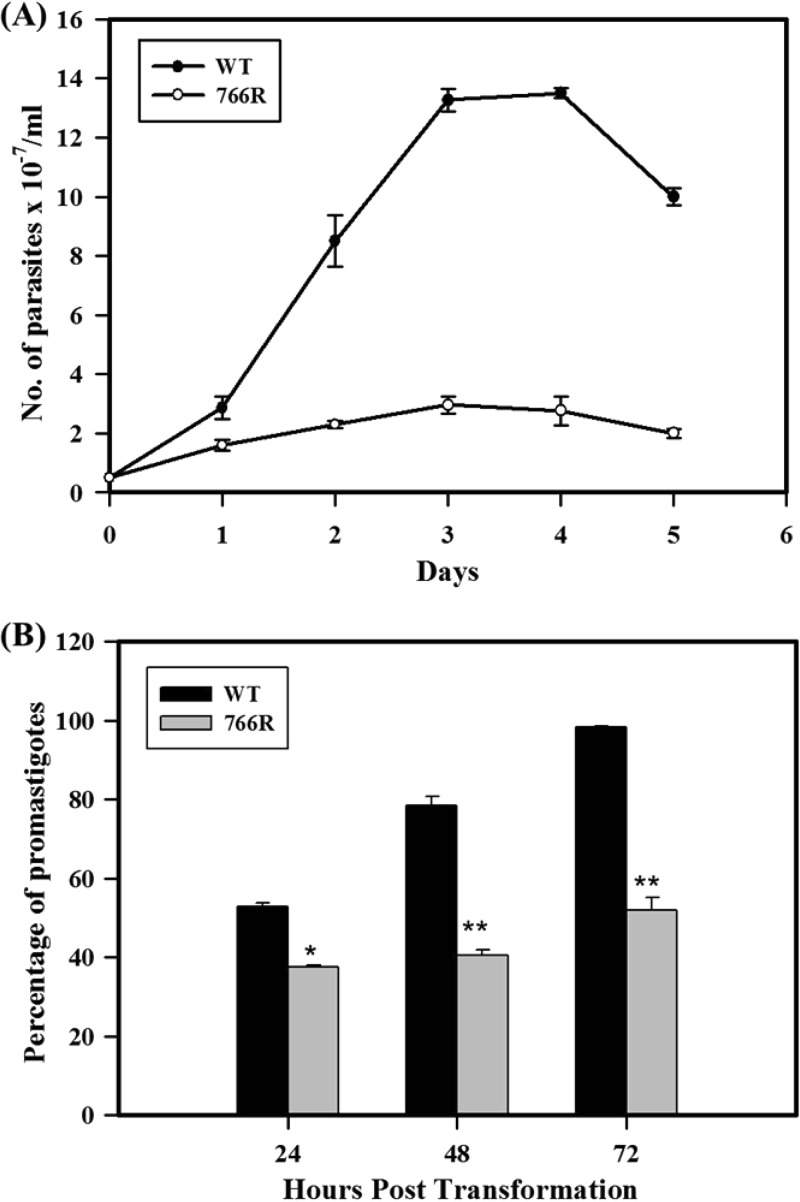
DB766-resistant parasites show defects in amastigote-to-promastigote differentiation.
Wild-type and DB766-resistant axenic amastigotes had comparable growth rates (doubling time, ∼12 h). To test their ability to differentiate into promastigotes, axenically grown amastigotes were cultured in promastigote medium in the absence of DB766 at 23°C. The growth rate (doubling time, ∼24 h) and the maximum cell density of promastigotes adapted from DB766-resistant axenic amastigotes were significantly lower than those of the wild-type cells (doubling time, ∼12 h; Fig. 3A), and these cells were much smaller and less motile than their wild-type counterparts. About 80% of the population of wild-type axenic amastigotes transformed into promastigotes within 48 h; the transformation was complete at 72 h. Under the same experimental conditions, the transformation of DB766-resistant amastigotes was incomplete, with these cultures containing only about 60% promastigotes at the end of 72 h (Fig. 3B).
FIG 3.
In vitro differentiation efficacy and growth curve. (A) Growth curve of slender forms adapted from wild-type (WT) and DB766-resistant (766R) axenic amastigotes over a period of 5 days in culture. A total of 5 × 106 axenic amastigote forms/ml were cultured in promastigote medium in the absence of DB766. The number of slender forms arising from transformation of DB766-resistant and wild-type axenic amastigotes was determined by hemocytometer-based counting every 24 h for 5 days. Results indicate the mean ± SE of three separate measurements. (B) Efficiency of transformation of axenic amastigotes to promastigotes adapted from wild-type and DB766-resistant L. donovani. A total of 5 × 106 axenic amastigotes/ml were cultured in promastigote medium in the absence of DB766. Total cell density and the number of slender forms arising from DB766-resistant and wild-type axenic amastigotes were determined by hemocytometer-based counting every 24 h for 72 h. Values are expressed as the percentage of slender forms relative to the total cell density. Results indicate the mean ± SE of three separate measurements. *, P < 0.01; **, P < 0.005.
Resistance to DB766 alters drug susceptibility in L. donovani axenic amastigotes.
The susceptibility profile of the DB766-resistant cell line to other structurally related and unrelated drugs is summarized in Table 1. There was no statistically significant difference between resistant and wild-type axenic amastigotes in susceptibility to pentamidine, amphotericin B, fluconazole, or terbinafine. Resistance to DB766 was not reversed by verapamil, a calcium channel blocker known to reverse multidrug resistance associated with overexpression of P glycoprotein (PgP)-type efflux pumps. Further, DB766-resistant parasites are cross resistant to the bis-AIAs DB745 (∼8-fold) and DB1852 (∼5-fold). DB766-resistant parasites were twice as sensitive to miltefosine and, remarkably, were more than 2,000-fold more sensitive to ketoconazole and over 12,000-fold more sensitive to posaconazole than wild-type organisms.
TABLE 1.
Susceptibility profiles of wild-type and DB766-resistant L. donovani axenic amastigotes at 72 h posttreatment
| Compound | IC50a (μM) |
Fold difference | |
|---|---|---|---|
| Wild type | DB766R | ||
| DB766 | 0.66 ± 0.15 | 7.7 ± 1.4 | +11.7b |
| DB745 | 0.67 ± 0.15 | 5.5 ± 1.3 | +8.2b |
| DB1852 | 1.3 ± 0.3 | 6.5 ± 0.0 | +5.0c |
| Pentamidine | 1.3 ± 0.2 | 1.2 ± 0.0 | 1.1 |
| Verapamil | >100 | >100 | |
| DB766 + 50 μM verapamil | 1.1 ± 0.7 | 11 ± 2 | +9.8b |
| Amphotericin B | 0.15 ± 0.04 | 0.14 ± 0.04 | 1.1 |
| Miltefosine | 2.7 ± 0.1 | 1.2 ± 0.3 | −2.3b |
| Ketoconazole | 45 ± 1 | 0.016 ± 0.005 | −2,800c |
| Fluconazole | 140 ± 50 | 120 ± 30 | 1.2 |
| Posaconazole | 12 ± 0 | 0.0010 ± 0.0005 | −12,000c |
| Terbinafine | 99 ± 25 | 77 ± 5 | 1.3 |
Data are means ± standard deviations from ≥3 separate determinations. DB766R, DB766-resistant strain.
P < 0.005.
P < 0.0005.
DB766-resistant and DB766-treated parasites have significantly reduced expression of CYP5122A1.
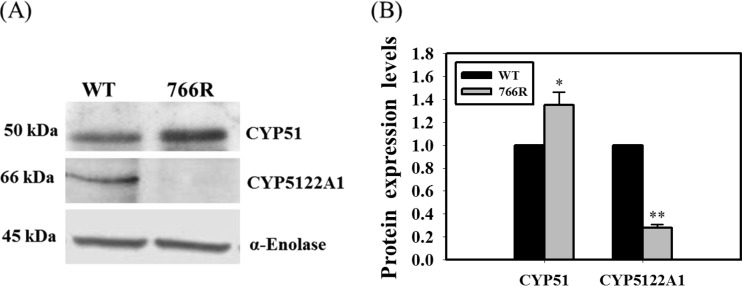
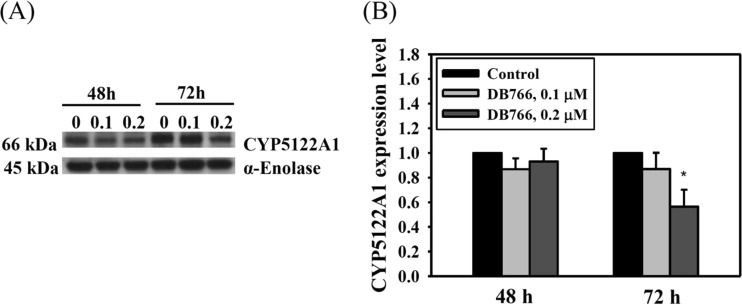
Miltefosine and antifungal azoles are known to alter lipid and sterol metabolism in Leishmania (19, 20). It is conceivable that reduced expression of key sterol biosynthetic enzymes, such as CYP51 (sterol 14α-demethylase, an antifungal azole target), would hypersensitize these organisms to the lethal effects of azoles, consistent with the earlier observations of enhanced sensitivity to antifungal azoles in CYP51 knockout fungi (21, 22). Recently, a novel cytochrome P450, CYP5122A1, essential for survival, virulence, drug response, and ergosterol metabolism in Leishmania, was identified (14). Although strains with double knockouts were not viable in culture, knockout of a single allele of CYP5122A1 in L. donovani (half knockout [HKO]) resulted in a 3.5-fold decrease in ergosterol levels and significant growth defects. The observed growth defects of CYP5122A1 HKOs were partially rectified upon supplementation with ergosterol in the growth medium and also upon complementation with episomally expressed CYP5122A1 in HKO parasites. These observations provide strong evidence for the role of CYP5122A1 in ergosterol metabolism in Leishmania. On the basis of the observations described above, modulation of ergosterol-metabolizing enzymes could occur as a consequence of acquired resistance to DB766. CYP51 and CYP5122A1 were chosen for investigation on the basis of the hypersensitivity of the DB766-resistant parasites to ketoconazole and posaconazole (Table 1). There were no significant differences in the transcript levels of CYP51 and CYP5122A1 in wild-type versus DB766-resistant Leishmania parasites, as assessed by real-time quantitative PCR (data not shown). While there was a slight increase in expression of the CYP51 protein in the resistant parasites (1.35-fold, P < 0.05), CYP5122A1 protein levels were dramatically reduced in the resistant parasites (3.80-fold, P < 0.001) compared to those in their wild-type counterparts (Fig. 4). To obtain further information about CYP5122A1 expression in DB766-treated parasites, L. donovani axenic amastigotes were exposed to different concentrations of DB766 for different time periods, and then CYP5122A1 expression levels were measured as described above. CYP5122A1 levels were significantly reduced (1.80-fold, P < 0.05) in parasites treated with 0.2 μM DB766 for 72 h (Fig. 5).
FIG 4.
Expression profile of CYP5122A1 and CYP51 in wild-type and DB766-resistant L. donovani axenic amastigotes. (A) A Western blot of 10 μg of total protein from wild-type and DB766-resistant L. donovani axenic amastigotes probed with anti-CYP51 (top) and anti-CYP5122A1 (middle) antibodies is shown. α-Enolase was used as a loading control (bottom). The figure is representative of three separate experiments. (B) Histograms representing normalized means from densitometric analysis of the immunoblots shown in panel A and in two other experiments, as quantified using ImageJ software (public domain; National Institutes of Health). *, P < 0.05; **, P < 0.001.
FIG 5.
Expression of CYP5122A1 in wild-type L. donovani axenic amastigotes treated with DB766. (A) A Western blot of 10 μg of total protein from L. donovani axenic amastigotes treated with 0.1 μM and 0.2 μM DB766 for 48 h and 72 h is shown. α-Enolase was used as a loading control. The figure is representative of three separate experiments. (B) Histograms representing normalized CYP5122A1 expression levels from densitometric analysis of immunoblots shown in panel A and from two additional experiments, as quantified using ImageJ software (public domain; National Institutes of Health). *, P < 0.05.
CYP5122A1 HKO L. donovani promastigotes display reduced susceptibility to DB766 and heightened sensitivity to ketoconazole compared to their wild-type counterparts.
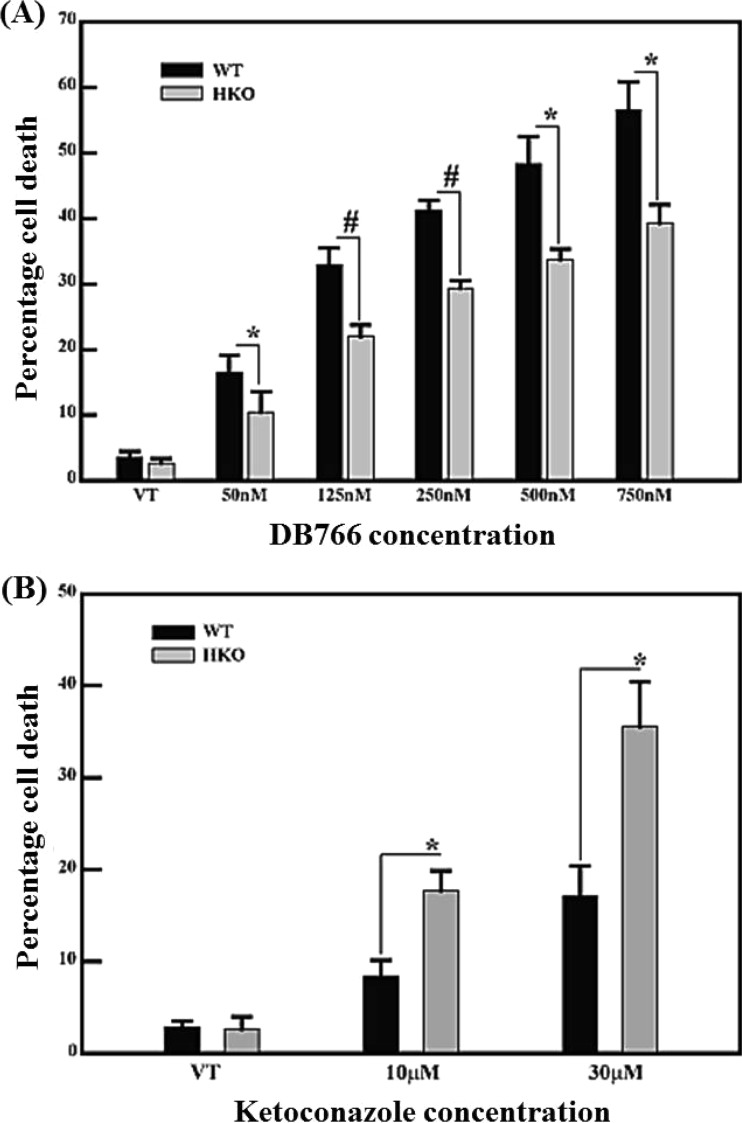
To test whether a reduction in CYP5122A1 expression alone caused reduced susceptibility to DB766 and increased sensitivity to ketoconazole, the susceptibility of CYP5122A1 HKO L. donovani promastigotes to both of these agents was measured. As shown in Fig. 6A, CYP5122A1 HKO parasites exhibited significantly less cell death (fewer propidium iodide [PI]-positive cells) than wild-type organisms at all the tested concentrations of DB766 (50 nM to 750 nM). Figure 6B shows that these half-knockout cells undergo significantly more cell death (more PI-positive cells) when incubated with 10 μM and 30 μM ketoconazole. The CYP51222A1 HKO cell line is thus less susceptible to DB766 and more susceptible to ketoconazole than wild-type L. donovani, similar to the DB766-resistant L. donovani axenic amastigotes.
FIG 6.
Profile of susceptibility of wild-type and CYP5122A1 HKO Leishmania donovani promastigotes to DB766 and ketoconazole. A total of 106 parasites/ml from wild-type and CYP5122A1 HKO Leishmania donovani promastigotes were exposed to various concentrations of DB766 (50 to 750 nM) (A) or ketoconazole (10 or 30 μM) (B) for 24 h. Cell viability was determined by propidium iodide staining by flow cytometry. The values represent percentage cell death relative to untreated controls. Results indicate the mean ± SE of three separate measurements. *, P < 0.05; #, P < 0.001. VT, vehicle treatment.
DB766 is synergistic with posaconazole against L. donovani in vitro.
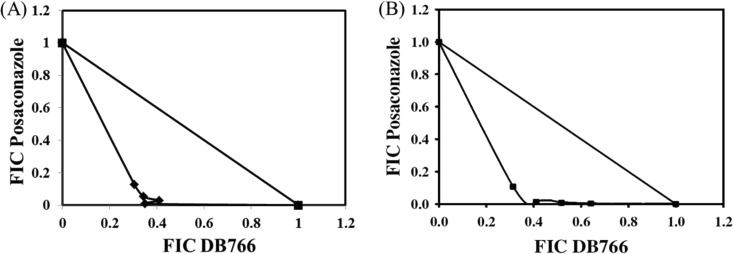
Since DB766-resistant parasites were hypersensitive to ketoconazole and posaconazole, the in vitro interaction between DB766 and posaconazole was assessed in L. donovani axenic amastigotes and intracellular amastigotes using the fixed-ratio isobologram method (17). On the basis of the mean ΣFIC values of 0.51 against axenic amastigotes and 0.41 against intracellular amastigotes (Table 2) and the concave isobolograms observed (Fig. 7), the DB766-posaconazole interaction was classified as borderline synergistic for axenic amastigotes and synergistic for intracellular amastigotes.
TABLE 2.
Mean ΣFICs for the interaction between DB766 and posaconazole in L. donovani at the IC50
| Expt no. |
L. donovani axenic amastigotes |
Intracellular L. donovani |
||||
|---|---|---|---|---|---|---|
| IC50a (μM) |
Mean ∑FIC ± SD at IC50 | IC50 (μM) |
Mean ∑FIC ± SD at IC50 | |||
| DB766 | Posaconazole | DB766 | Posaconazole | |||
| 1 | 0.46 | 9.8 | 0.61 ± 0.22 | 0.046 | 6.1 | 0.34 ± 0.08 |
| 2 | 0.49 | 13 | 0.41 ± 0.04 | 0.036 | 6.0 | 0.47 ± 0.14 |
The IC50 of each compound alone was used to calculate the FICs in the individual experiments.
FIG 7.
Isobolograms showing in vitro interactions between DB766 and posaconazole at the IC50 in L. donovani. (A) Analysis of L. donovani axenic amastigotes. (B) Analysis of intracellular L. donovani amastigotes using mouse peritoneal macrophages as host cells. The data shown in these panels are from representative experiments performed on two separate occasions (Table 2).
DISCUSSION
While kinetoplast DNA (kDNA) binding and disruption of mitochondrial function are among the likely mechanisms of action of diamidines in kinetoplastids (23), targets for the antiparasitic action of AIAs have not been clearly defined. The activity of AIAs against T. cruzi does not correlate with the ability of these compounds to bind kDNA (24), and treatment of T. cruzi intracellular amastigotes with AIAs resulted in not only swelling of the mitochondrion and disorganization of kDNA but also vacuolization and the appearance of electron-dense bodies in the cytoplasm, the development of vesicles in the flagellar pocket, and disorganization of subpellicular microtubules (7). In terms of apicomplexan parasites, exposure to AIAs compromised the viability of intracellular forms of both Neospora caninum and Toxoplasma gondii specifically through the modulation of host cell processes (25). Considering that few mechanistic studies have been conducted with AIAs and no target proteins or pathways have been identified, the antiprotozoal mechanism of action of these compounds is poorly understood. In the present investigation, we show that (i) the mechanism of action of DB766 is distinct from that of diamidines in Leishmania, (ii) CYP5122A1, a novel P450 enzyme involved in Leishmania ergosterol metabolism, plays an important role in susceptibility and resistance to DB766 and azoles in L. donovani, and (iii) DB766 synergizes the antileishmanial potency of azoles, CYP51 inhibitors that disrupt sterol biosynthesis in fungi and trypanosomatids.
In vitro susceptibility assays with the DB766-resistant cell line (Table 1) showed that (i) there is no significant difference in susceptibility to pentamidine between wild-type and DB766-resistant Leishmania parasites, (ii) resistance to DB766 is not reversed by verapamil, indicating that the overexpression of PgP-type efflux pumps is unlikely to be responsible for resistance, (iii) DB766-resistant parasites are cross resistant to other AIAs, indicating that AIAs share a target or targets, and (iv) DB766-resistant parasites are twice as sensitive as wild-type axenic amastigotes to miltefosine and over 3 orders of magnitude more sensitive to ketoconazole and posaconazole than wild-type axenic amastigotes. These susceptibility data are consistent with the findings of ultrastructural studies, suggesting that the target of AIAs is different from that of diamidines in L. donovani. In addition, the hypersensitivity of the DB766-resistant cell line to ketoconazole and posaconazole led to an investigation of the role of sterol biosynthesis enzymes, particularly sterol 14α-demethylase, in the mechanism of action of and resistance to DB766 in L. donovani.
While reduced expression of CYP51 has been shown to enhance the susceptibility to antifungal azoles in fungi (21, 22), reduced expression of CYP5122A1 increased the sensitivity of L. donovani to miltefosine (14). Consistent with the hypothesis that resistance to DB766 was caused by altered expression of CYP5122A1 in L. donovani, Western blot analysis of these proteins indicated that expression of CYP5122A1 was dramatically reduced in the resistant organisms compared to that in their wild-type counterparts (Fig. 4). Further, CYP5122A1 HKO L. donovani promastigotes are less susceptible to DB766 (Fig. 6A) and more susceptible to ketoconazole (Fig. 6B) than the corresponding wild-type promastigotes, consistent with observations made with CYP5122A1-deficient DB766-resistant parasites (Table 1). Besides the similarities noted between CYP5122A1-deficient, DB766-resistant L. donovani parasites and CYP5122A1 HKO L. donovani parasites in their susceptibilities to DB766 and azoles, these two parasite lines also display significantly reduced growth rates compared with those of their wild-type counterparts and fail to differentiate completely to promastigotes (14) (Fig. 3). Taken together, these data indicate that CYP5122A1 plays a critical role in determining the susceptibility of L. donovani to both DB766 and antifungal azoles. On the basis of the available data, two mechanistic possibilities for the antileishmanial effects of DB766 involving CYP5122A1 appear to be plausible: (i) DB766 disrupts sterol metabolism in L. donovani by interfering with the action of CYP5122A1, or (ii) CYP5122A1 metabolizes DB766 to a more active form, resulting in toxicity to the parasite through an unknown mechanism. Preliminary experiments revealed minor differences in sterol composition between DB766-treated and untreated parasites (data not shown). These differences were not as dramatic as those observed when Leishmania parasites were exposed to azoles, where synthesis of 14-demethylated sterols could be almost completely blocked (20). Given its sequence similarity to mammalian CYP4A10 (25%), a CYP450 involved in drug metabolism, it is possible that CYP5122A1 may also play a role in Leishmania xenobiotic metabolism. Decreased expression of CYP5122A1 may also force the parasite to depend more heavily on CYP51 for sterol biosynthesis, providing a possible explanation for the synergism observed between DB766 and posaconazole (Fig. 7). While the data shown here provide support for the proposed mechanism responsible for the azole hypersensitivity seen in DB766-resistant Leishmania parasites and the posaconazole-DB766 synergy seen in wild-type parasites, extensive sterol analysis in DB766- and DB766-azole-treated L. donovani parasites, as well as expression and biochemical characterization of CYP5122A1, will be required to distinguish different mechanistic hypotheses for the antileishmanial action of DB766.
Aside from CYP51 and CYP5122A1, the expression of other enzymes as well as the modulation of genes other than those of the sterol biosynthetic pathway could also contribute to azole hypersensitivity and resistance to DB766 in Leishmania and warrant further investigation. However, given the important role of the CYP5122A1 protein in survival, virulence, drug response, and ergosterol metabolism in Leishmania, the dramatic downregulation of CYP5122A1 in DB766-resistant parasites at least partially explains their resistance to DB766 and hypersensitivity to antifungal azoles. The present studies suggest that CYP5122A1 is a critical modulator of DB766 activity, downregulation of which offers a survival advantage during the acquisition of resistance to AIAs in L. donovani, and provide additional evidence of a role for CYP5122A1 in ergosterol biosynthesis and drug metabolism in these parasites. Reduced expression of CYP5122A1 may also be responsible for the synergism observed between DB766 and posaconazole in this organism. In addition, this work further highlights the importance of sterol metabolism in the action of antileishmanial drugs and drug candidates and suggests that AIA-azole combinations could have therapeutic potential against Leishmania.
ACKNOWLEDGMENTS
This work was supported in part by the Bill and Melinda Gates Foundation through the Consortium for Parasitic Drug Development.
We thank Richard Montione at the OSU Campus Microscopy and Imaging Facility for technical assistance with electron microscopy studies, Paul Michels (Catholic University of Louvain, Brussels, Belgium) for providing α-enolase antiserum, Jean-Christophe Cocuron at the OSU Targeted Metabolomics Laboratory for technical assistance with gas chromatography-mass spectrometry analysis of Leishmania sterol samples, and Frederick Buckner for the CYP51 antibody and for a critical reading of the manuscript.
Footnotes
Published ahead of print 2 June 2014
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman JD. 2006. Development of miltefosine for the leishmaniases. Mini. Rev. Med. Chem. 6:145–151. 10.2174/138955706775475993 [DOI] [PubMed] [Google Scholar]
- 3.Olliaro P, Sundar S. 2009. Anthropometrically derived dosing and drug costing calculations for treating visceral leishmaniasis in Bihar, India. Trop. Med. Int. Health 14:88–92. 10.1111/j.1365-3156.2008.02195.x [DOI] [PubMed] [Google Scholar]
- 4.Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. 2007. Injectable paromomycin for visceral leishmaniasis in India. N. Engl. J. Med. 356:2571–2581. 10.1056/NEJMoa066536 [DOI] [PubMed] [Google Scholar]
- 5.Wang MZ, Zhu X, Srivastava A, Liu Q, Sweat JM, Pandharkar T, Stephens CE, Riccio E, Parman T, Munde M, Mandal S, Madhubala R, Tidwell RR, Wilson WD, Boykin DW, Hall JE, Kyle DE, Werbovetz KA. 2010. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 54:2507–2516. 10.1128/AAC.00250-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens CE, Tanious F, Kim S, Wilson WD, Schell WA, Perfect JR, Franzblau SG, Boykin DW. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741–1748. 10.1021/jm000413a [DOI] [PubMed] [Google Scholar]
- 7.Silva CF, Meuser MB, De Souza EM, Meirelles MN, Stephens CE, Som P, Boykin DW, Soeiro MN. 2007. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob. Agents Chemother. 51:3803–3809. 10.1128/AAC.00047-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens CE, Brun R, Salem MM, Werbovetz KA, Tanious F, Wilson WD, Boykin DW. 2003. The activity of diguanidino and ‘reversed' diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg. Med. Chem. Lett. 13:2065–2069. 10.1016/S0960-894X(03)00319-6 [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Liu Q, Yang S, Parman T, Green CE, Mirsalis JC, de Nazare Correia Soeiro M, Mello de Souza E, da Silva CF, da Gama Jaen Batista D, Stephens CE, Banerjee M, Farahat AA, Munde M, Wilson WD, Boykin DW, Wang MZ, Werbovetz KA. 2012. Evaluation of arylimidamides DB1955 and DB1960 as candidates against visceral leishmaniasis and Chagas' disease: in vivo efficacy, acute toxicity, pharmacokinetics, and toxicology studies. Antimicrob. Agents Chemother. 56:3690–3699. 10.1128/AAC.06404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid CS, Farahat AA, Zhu X, Pandharkar T, Boykin DW, Werbovetz KA. 2012. Antileishmanial bis-arylimidamides: DB766 analogs modified in the linker region and bis-arylimidamide structure-activity relationships. Bioorg. Med. Chem. Lett. 22:6806–6810. 10.1016/j.bmcl.2012.06.037 [DOI] [PubMed] [Google Scholar]
- 11.Banerjee M, Farahat AA, Kumar A, Wenzler T, Brun R, Munde MM, Wilson WD, Zhu X, Werbovetz KA, Boykin DW. 2012. Synthesis, DNA binding and antileishmanial activity of low molecular weight bis-arylimidamides. Eur. J. Med. Chem. 55:449–454. 10.1016/j.ejmech.2012.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werbovetz KA, Sackett DL, Delfin D, Bhattacharya G, Salem M, Obrzut T, Rattendi D, Bacchi C. 2003. Selective antimicrotubule activity of N1-phenyl-3,5-dinitro-N4,N4-di-n-propylsulfanilamide (GB-II-5) against kinetoplastid parasites. Mol. Pharmacol. 64:1325–1333. 10.1124/mol.64.6.1325 [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Pandharkar T, Werbovetz K. 2012. Identification of new antileishmanial leads from hits obtained by high-throughput screening. Antimicrob. Agents Chemother. 56:1182–1189. 10.1128/AAC.05412-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma S, Mehta A, Shaha C. 2011. CYP5122A1, a novel cytochrome P450 is essential for survival of Leishmania donovani. PLoS One 6:e25273. 10.1371/journal.pone.0025273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee SB, Das M, Sudhandiran G, Shaha C. 2002. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J. Biol. Chem. 277:24717–24727. 10.1074/jbc.M201961200 [DOI] [PubMed] [Google Scholar]
- 16.Hu L, Arafa RK, Ismail MA, Wenzler T, Brun R, Munde M, Wilson WD, Nzimiro S, Samyesudhas S, Werbovetz KA, Boykin DW. 2008. Azaterphenyl diamidines as antileishmanial agents. Bioorg. Med. Chem. Lett. 18:247–251. 10.1016/j.bmcl.2007.10.091 [DOI] [PubMed] [Google Scholar]
- 17.Seifert K, Munday J, Syeda T, Croft SL. 2011. In vitro interactions between sitamaquine and amphotericin B, sodium stibogluconate, miltefosine, paromomycin and pentamidine against Leishmania donovani. J. Antimicrob. Chemother. 66:850–854. 10.1093/jac/dkq542 [DOI] [PubMed] [Google Scholar]
- 18.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. 2008. Antiparasitic compounds that target DNA. Biochimie 90:999–1014. 10.1016/j.biochi.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. 2007. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 51:1425–1430. 10.1128/AAC.01123-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beach DH, Goad LJ, Holz GG., Jr 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31:149–162. 10.1016/0166-6851(88)90166-1 [DOI] [PubMed] [Google Scholar]
- 21.Mellado E, Garcia-Effron G, Buitrago MJ, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2005. Targeted gene disruption of the 14α-sterol demethylase (cyp51A) in Aspergillus fumigatus and its role in azole drug susceptibility. Antimicrob. Agents Chemother. 49:2536–2538. 10.1128/AAC.49.6.2536-2538.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan X, Ma WB, Li Y, Wang H, Que YW, Ma ZH, Talbot NJ, Wang ZY. 2011. A sterol 14α-demethylase is required for conidiation, virulence and for mediating sensitivity to sterol demethylation inhibitors by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 48:144–153. 10.1016/j.fgb.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Werbovetz K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147–157 [PubMed] [Google Scholar]
- 24.Daliry A, Pires MQ, Silva CF, Pacheco RS, Munde M, Stephens CE, Kumar A, Ismail MA, Liu Z, Farahat AA, Akay S, Som P, Hu Q, Boykin DW, Wilson WD, De Castro SL, Soeiro MN. 2011. The trypanocidal activity of amidine compounds does not correlate with their binding affinity to Trypanosoma cruzi kinetoplast DNA. Antimicrob. Agents Chemother. 55:4765–4773. 10.1128/AAC.00229-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leepin A, Stüdli A, Brun R, Stephens CE, Boykin DW, Hemphill A. 2008. Host cells participate in the in vitro effects of novel diamidine analogues against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob. Agents Chemother. 52:1999–2008. 10.1128/AAC.01236-07 [DOI] [PMC free article] [PubMed] [Google Scholar]