Abstract
Linezolid-dependent growth was recently reported in Staphylococcus epidermidis clinical strains carrying mutations associated with linezolid resistance. To investigate this unexpected behavior at the molecular level, we isolated active ribosomes from one of the linezolid-dependent strains and we compared them with ribosomes isolated from a wild-type strain. Both strains were grown in the absence and presence of linezolid. Detailed biochemical and structural analyses revealed essential differences in the function and structure of isolated ribosomes which were assembled in the presence of linezolid. The catalytic activity of peptidyltransferase was found to be significantly higher in the ribosomes derived from the linezolid-dependent strain. Interestingly, the same ribosomes exhibited an abnormal ribosomal subunit dissociation profile on a sucrose gradient in the absence of linezolid, but the profile was restored after treatment of the ribosomes with an excess of the antibiotic. Our study suggests that linezolid most likely modified the ribosomal assembly procedure, leading to a new functional ribosomal population active only in the presence of linezolid. Therefore, the higher growth rate of the partially linezolid-dependent strains could be attributed to the functional and structural adaptations of ribosomes to linezolid.
INTRODUCTION
Oxazolidinone antibiotics inhibit protein synthesis by binding to the peptidyltransferase center (PTC) of the ribosome and inhibiting the growth of bacteria (1). Although it has been suggested that they are involved in the initiation of translation, many reports have been contradictory, mainly because the ratio of drug to ribosome that was used was extremely high (2). Moreover, the inhibitory effect of oxazolidinones on peptide bond formation has not been demonstrated so far, despite structural data suggesting the binding of linezolid in the peptidyltransferase center (3–5). In contrast, linezolid perturbs translational accuracy in vivo, even at concentrations lower than the MIC (6).
Linezolid (LZD) was the first FDA-approved oxazolidinone used to treat serious infections due to Gram-positive bacteria. Although LZD was completely synthetic (7), resistance readily emerged and was attributed mainly to mutations in 23S rRNA and ribosomal proteins L3 and L4. Mutations in 23S rRNA implicated in linezolid resistance include not only bases near the binding site, like G2061, C2452, A2503, U2504, and G2505, but also bases that are located more distantly from the binding site, such as A2062, G2447, A2453, C2499, U2500, and G2576 (reviewed in reference 8). Additional mechanisms include acquisition of the cfr gene, which encodes a methyltransferase which modifies A2503 in 23S rRNA, and a mutation in the RlmN gene, which naturally modifies A2503 (8–14). Recently, four nosocomial Staphylococcus epidermidis isolates belonging to the same pulsed-field gel electrophoresis type were described to exhibit partial linezolid dependence, an adaptation reported for only a few antibiotics and bacterial species in the past (15). These strains carried the same mutations (U2504A and C2534U in 23S rRNA) and two potentially important amino acid substitutions (G152D and D159Y), along with the L101V substitution in the L3 protein. All the mutations described above had previously been associated with LZD resistance, mainly through a synergistic effect associated with 23S rRNA mutations at or near the PTC (8).
Given that these strains were not only highly resistant to linezolid but additionally were growing significantly faster in the presence of the antibiotic, we attempted to gain insights into the underlying mechanisms that could account for this unexpected finding. Toward this aim, we carried out biochemical and structural studies on ribosomes derived from both a linezolid-dependent (LZDD) strain and a wild-type (wt) strain in the presence and absence of linezolid. More specifically, we isolated ribosomes from a wild-type linezolid-susceptible S. epidermidis strain and partially linezolid-dependent strain A2864 (15). The latter strain was grown in the presence of linezolid (LZDD+L) and in the absence of linezolid, and the in vitro peptidyltransferase activity of isolated ribosomes was studied, again in the absence and presence of the antibiotic. In order to get an estimate of the ratio of 30S (small) to 50S (large) ribosomal subunits in the general population of isolates, we studied the sedimentation distribution of ribosomes isolated in sucrose gradients, which revealed an abnormal subunit dissociation profile for the LZDD+L ribosomes. Finally, we discuss the possible mechanisms of adaptation to linezolid dependence for faster growth, on the basis of the available crystal structure data describing the antibiotic bound on the ribosome.
MATERIALS AND METHODS
Materials.
GTP, ATP, poly(U), puromycin, linezolid, and total Escherichia coli tRNA were purchased from Sigma (St. Louis, MO). l-[2,3,4,5,6-3H]phenylalanine was obtained from Amersham Pharmacia Biotech (Piscataway, NJ).
Biochemical preparations.
S. epidermidis cells were grown in LB broth either in the presence or in the absence of linezolid (final concentration, 128 mg/liter, equal to 0.38 mM) and were collected after centrifugation (10,000 × g, 10 min, 4°C). Cells were washed in buffer A [10 mM Tris-HCI (pH 7.6), 10 mM Mg(CH3COO)2, 60 mM KCI, 6 mM β-mercaptoethanol] and resuspended again in the same buffer with the addition of lysostaphin (100 mg/liter), lysozyme (250 mg/liter), and DNase I (10 mg/liter). After cell disruption, the cell debris was removed through centrifugation (10,000 × g, 10 min, 4°C) and the lysate was placed on the top of a 15% sucrose cushion in buffer A. Centrifugation followed at 70,000 × g for 18 h at 4°C, and the pellet was again resuspended in buffer A, dialyzed over the same buffer, and then stored at −70°C in aliquots. The ribosomal subunit distribution was analyzed by sucrose gradient centrifugation. Crude ribosomes (8 units/A260 unit) were placed on the top of 10 to 30% sucrose gradients in buffer A or in buffer B [10 mM Tris-HCI (pH 7.5), 1 mM Mg(CH3COO)2, 200 mM NH4CI, 6 mM β-mercaptoethanol], which favors subunit dissociation, which was followed by centrifugation at 120,000 × g (SW41 Beckman rotor, 4°C, 4 h). Acetylated [3H]Phe-tRNA (Ac[3H]Phe-tRNA) was prepared using total E. coli tRNA and a mix of aminoacyl-tRNA synthetases from the S-100 fraction (the supernatant of E. coli ribosome precipitation after centrifugation at 100,000 × g) (16).
Poly(U)-dependent poly(Phe) synthesis.
The poly(U)-dependent poly(Phe) synthesis assay was carried out in the presence of buffer A in two separate steps. In the first step, 70S ribosomes (final concentration, 0.5 μM) were incubated for 10 min at 37°C in the presence or absence of linezolid (at concentrations ranging from 1 up to 300 mg/liter). In the second step, the reaction took place in a final volume of 15 μl, which included 25 μg of poly(U) mRNA, 5 nmol (50 dpm/pmol) [3H]phenylalanine, 1 unit A260 bulk tRNA (E. coli), 3 mM ATP, 1.5 mM GTP, 5 mM acetyl phosphate, and an optimized S-100 fraction to maximize the number of picomoles of phenylalanine polymerized per picomole of 70S ribosomes. After 60 min of incubation at 37°C, the mixture was precipitated with hot trichloroacetic acid (TCA; 5%, wt/vol) and the precipitate was filtered through glass fiber filters (17). The radioactivity retained on the filters, representing the amount (pmol) of phenylalanine incorporated into the polypeptide chains per pmol of mature (70S) ribosomes used, was measured in a liquid scintillation counter.
Ribosomal complex formation.
Postribosomal complexes were prepared as previously described, and their formation was calculated after measuring the radioactivity that remained on the cellulose nitrate filter, while the P site-bound Ac[3H]Phe-tRNA was titrated with a puromycin reaction (18).
Puromycin reaction.
A puromycin reaction was used either for the titration of P site- or A site-bound Ac[3H]Phe-tRNA or for the measurement of peptidyltransferase activity. In the first case, the isolated ribosomal complex was incubated in the presence of puromycin under saturation conditions (2 mM) for 2 min. In the second case, the complex was incubated with different puromycin concentrations (0.08, 0.12, 0.25, and 0.50 mM) and the progression of the reaction was monitored over time. The data for product (acetylated Phe [AcPhe]-puromycin) formation are presented in the form ln[Co/(Co − P)] = f(t), where Co represents the total amount of the postribosomal complex isolated, P represents the amount of product formed after each time (t) of incubation with puromycin (S), according to the first-order rate law ln[C0/(C0 − P)] = [kcatS/(Ks + S)]t (19).
Statistical analysis.
All measurements were replicated at least five times, and the data are indicated as means ± standard deviations (SDs). Significant differences between mean values were measured by the F-Scheffé test (SPSS Statistics program, version 19, for Windows).
RESULTS
Analysis of the data from the phenylalanine polymerization assay (Fig. 1A) showed that both linezolid-susceptible and linezolid-dependent ribosomes exhibited the same level of protein synthesis activity in the presence of linezolid. This is in agreement with previous data, according to which oxazolidinones did not inhibit peptide elongation using the same assay, namely, poly(U)-dependent phenylalanine incorporation (20). However, the ribosomes derived from the LZDD strain grown in the presence of linezolid (LZDD+L) exhibited increased poly(Phe) synthesis per ribosome, a clear indication of a higher protein synthesis rate.
FIG 1.
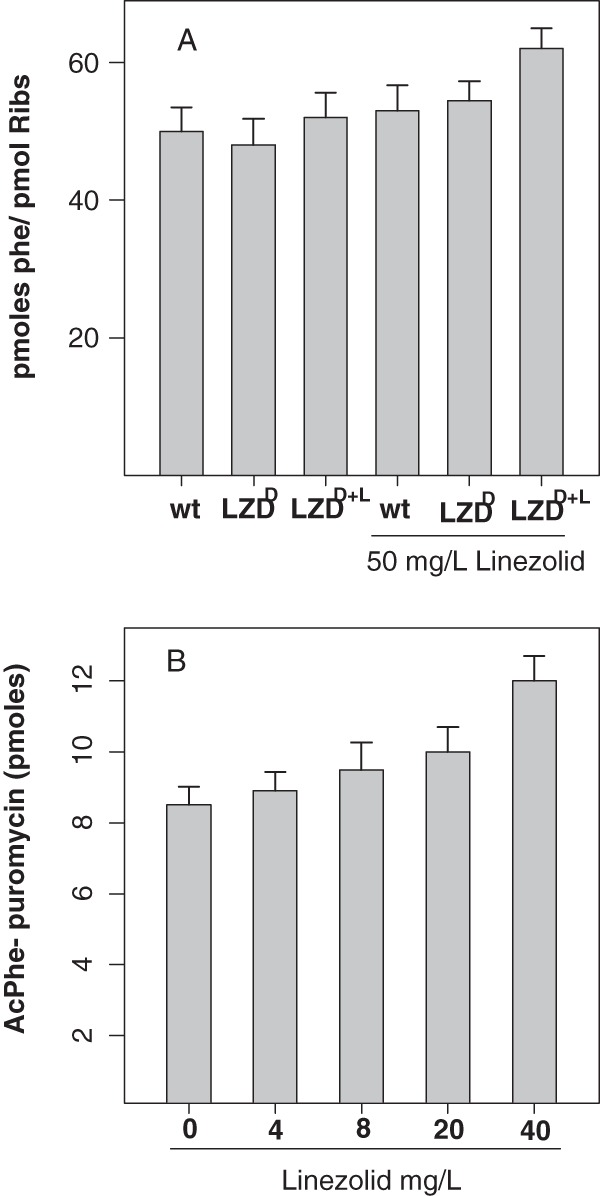
(A) Poly(U)-dependent phenylalanine polymerization using ribosomes from wild-type and linezolid-dependent strains (grown either in the absence of LZD [LZDD] or in the presence of LZD [LZDD+L]) in the absence and presence of 50 mg/liter linezolid. (B) Puromycin reaction with ribosomes from the LZD-dependent strain grown in the presence of linezolid (LZDD+L) in the presence of increasing LZD concentrations. Values represent the means from five independently performed experiments. Statistically significant different values (P < 0.05) with and without growth in the presence of linezolid were found only for the LZDD+L strain (A).
To identify further the specific step which is responsible for the stimulation of protein synthesis, we carried out the puromycin reaction in the absence and presence of linezolid (Fig. 1B). Ribosomes isolated from the linezolid-dependent strain again exhibited the same protein synthesis activity in the presence of linezolid, even at considerably higher concentrations (1 mM; data not shown). In contrast, the ribosomes isolated from the same strain grown in the presence of linezolid (LZDD+L) showed a 50% increase in AcPhe-puromycin production compared to the level for the same ribosomes in the absence of the antibiotic (Fig. 1B). It has been reported previously that, using the same methodology, peptidyltransferase activity is not inhibited by linezolid even at an extremely high concentration (1 mM) (3). Given that AcPhe-puromycin is formed through two separate reaction steps, we discriminated the first step (binding of the first substrate, AcPhe-tRNA) from the second step (puromycin binding and reaction). According to our data (Fig. 2), neither type of ribosome exhibited any statistically significant difference in the binding ratio, thus excluding any possible effect of linezolid on the AcPhe-tRNA binding also found for wt ribosomes (data not shown). The completion of the puromycin reaction allowed the calculation of the observed relative rate constant (kobs) of the reaction in the presence of linezolid using different puromycin concentrations (Fig. 3A). Interestingly, only ribosomes derived from the LZDD strain grown in the presence of linezolid exhibited a faster puromycin reaction. To discriminate whether the difference was due to the binding of puromycin (expressed with the Ks value) or the catalytic step (expressed with the kcat value), we tested many puromycin concentrations, and the data are presented in Table 1. The replot of the previous data (Table 1) in the form 1/kobs versus 1/S (Fig. 3B) allowed us to calculate the Ks and kcat values and, additionally, in each case, the catalytic power of the peptidyltransferase activity, which is expressed by the ratio kcat/Ks (Table 2). According to the values in Table 2, the antibiotic activates enzymatic activity, increasing the catalytic rate constant while keeping the Michaelis constant (Ks) almost unvaried. This activity is much higher only when the linezolid-dependent strain is grown in the presence of linezolid (LZDD+L). Actually, the peptidyltransferase catalytic efficiency was found to be increased almost 2-fold, showing that peptide bond formation is much faster in the presence than in the absence of linezolid.
FIG 2.
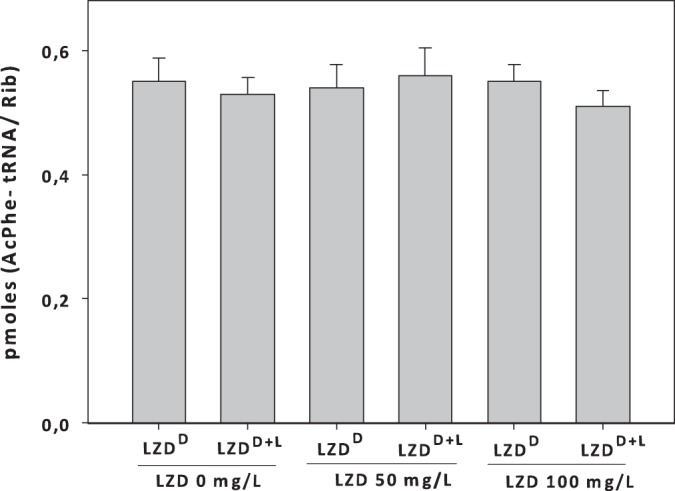
AcPhe-tRNA binding to poly(U) programmed ribosomes in the absence and presence of LZD. Ribosomes were isolated from a linezolid-dependent strain grown either in the absence (LZDD) or in the presence (LZDD+L) of linezolid. The final linezolid concentrations were 150 and 300 μM (or 50 and 100 mg/liter, respectively). Values represent the means ± SDs and were not found to be statistically significantly different with or without growth in the presence of linezolid (P < 0.05).
FIG 3.
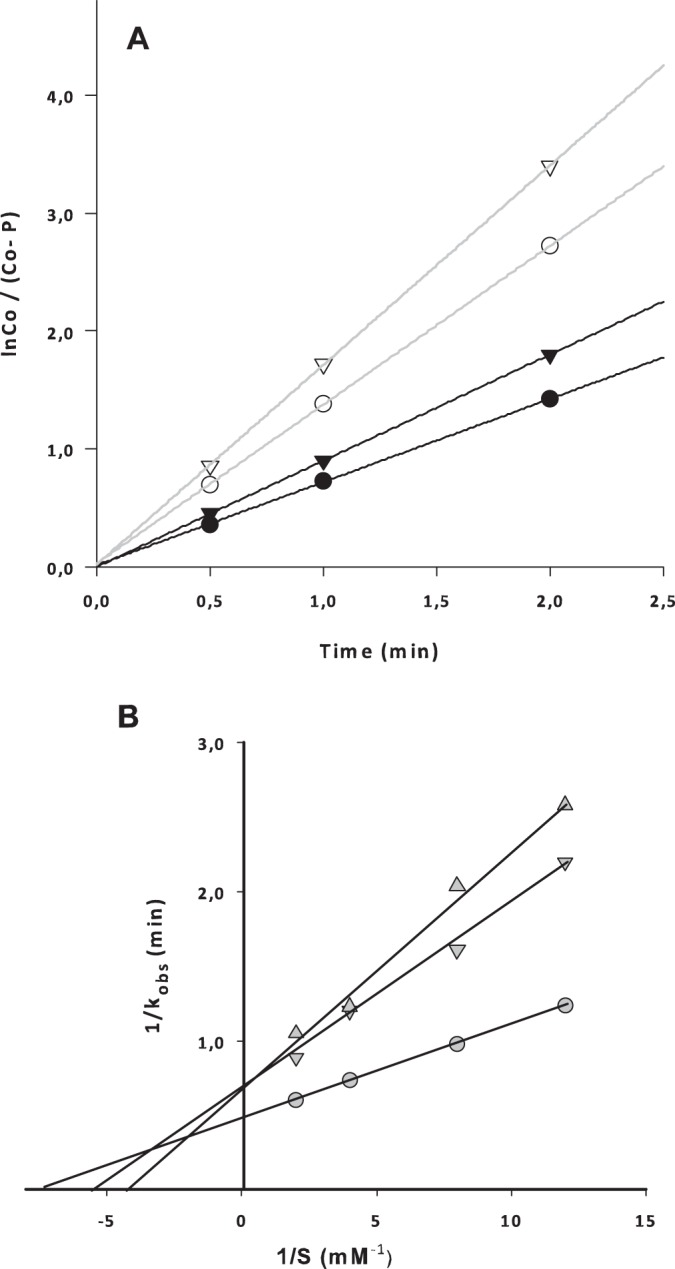
(A) Progression of the puromycin reaction with ribosomes from the linezolid-dependent strain (LZDD; filled symbols) and the linezolid-dependent strain grown in the presence of linezolid (LZDD+L; empty symbols) in the presence of 0.12 mM linezolid. Circles, puromycin concentration of 0.25 mM; triangles, puromycin concentration of 0.50 mM. (B) Double-reciprocal plot of the puromycin reaction with different ribosomal species, allowing the calculation of the catalytic rate constant (kcat) and the Michaelis constant (Ks). The y-axis intercept gives the reciprocal of kcat, and the x-axis intercept gives the reciprocal of Ks. The three lines represent ribosomal species from the wild-type strain (△), the LZDD strain (▽), and the LZDD+L strain (○). All constants calculated from the plot are presented in Table 2.
TABLE 1.
Values of kobsa
| Puromycin concn (mM) |
kobsa (min−1) for ribosomes from: |
||
|---|---|---|---|
| wt | LZDD | LZDD+L | |
| 0.08 | 0.41 | 0.38 | 0.80 |
| 0.12 | 0.62 | 0.51 | 1.02 |
| 0.25 | 0.83 | 0.73 | 1.38 |
| 0.50 | 1.10 | 0.90 | 1.72 |
The values of the apparent rate constant (kobs) were calculated from the slopes of the logarithmic plots (like those in Fig. 3A) for many puromycin concentrations and different ribosomal sources.
TABLE 2.
kcat and Ks values and catalytic activity (kcat/Ks) for ribosomes from different sourcesa
| Ribosome | kcat (min−1) | Ks (mM) | kcat/Ks (min−1 mM−1) |
|---|---|---|---|
| wt | 1.52 | 0.24 | 6.33 |
| LZDD | 1.45 | 0.20 | 7.25 |
| LZDD+L | 2.12 | 0.14 | 15.14 |
The kcat and Ks values were calculated from the double-reciprocal plots (Fig. 3B), and the catalytic activity of peptidyltransferase is expressed by the ratio kcat/Ks.
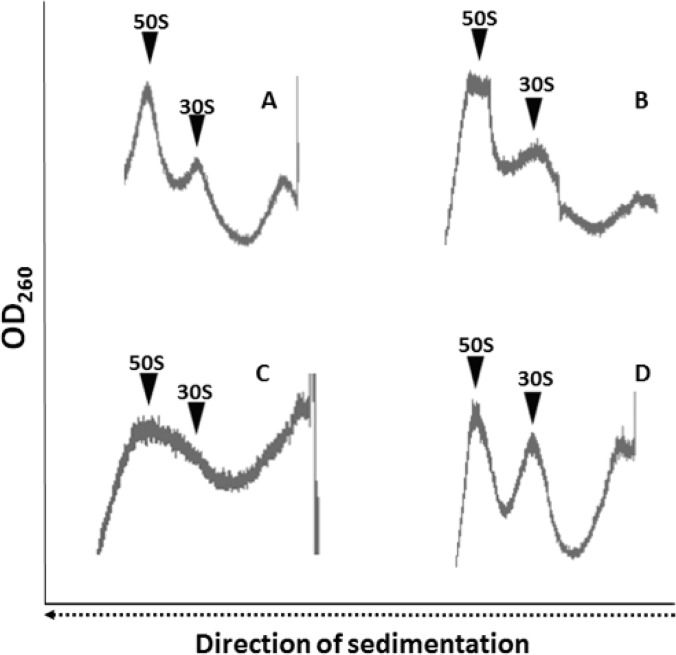
Previous data have shown that linezolid also inhibits 50S subunit assembly (21). Therefore, we analyzed the ribosomal subunit contribution ratio to the total amount of ribosomes after centrifugation onto a 10 to 30% sucrose gradient. The ribosomal subunit contribution ratio in the linear sucrose gradient of the linezolid-susceptible and the LZDD strains in the absence of LZD during growth was found to be within the expected normal range of ratios (Fig. 4A and B). In contrast, the ribosomal subunit dissociation pattern for the LZDD strain grown in the presence of linezolid was found to be significantly disturbed (Fig. 4C). More specifically, the subunit dissociation profile was not clear for the 50S and 30S subunits. The abnormal profile that we observed could be attributed to possible structural changes in the presence of LZD during growth, resulting in a mixture of populations of 50S and 30S subunit species as we move from the top to the bottom. The heterogeneity of the subunit population due to the absence of linezolid is probably the reason why that, although we observed a peak possibly corresponding to a mixture of both assembled and defective 50S subunits, we could not observe a clear peak for assembled 30S subunits. However, when the same ribosomal fraction was incubated in the presence of linezolid for 10 min at 37°C before loading onto the gradient, the subunit dissociation profile was restored, although the 30S subunits were more abundant than the 50S subunits (Fig. 4D). The observations from the sucrose gradient analysis indicated that LZDD ribosomes that assemble in the presence of LZD have undergone significant structural modifications compared to normal ribosomes (Fig. 4A); however, this finding favors higher protein synthesis rates.
FIG 4.
Sucrose gradient profile of ribosomal subunits. Ribosomes were loaded on a 15 to 30% sucrose gradient in buffer B, centrifuged for 4 h in a Ti SW41 rotor at 120,000 × g, and analyzed in a Cary spectrophotometer that measured the absorbance at 260 nm with continuous flow (from the bottom to the top). (A) Ribosomes were isolated from a wt strain susceptible to linezolid (control) (A), an LZDD strain (B), or the LZDD strain grown in the presence of LZD (LZDD+L) (C) or were isolated exactly as described above but, before the gradient was loaded, with incubation of the ribosomes with linezolid (128 mg/liter, or 0.38 mM) for 10 min in buffer B at 37°C. OD260, optical density at 260 nm.
Overall, it appears that the ribosomes from the LZDD strain exhibit a completely different profile in the presence and absence of linezolid. First, the catalytic activity of peptidyltransferase was increased in the presence of linezolid, while the same ribosomes were unable to efficiently dissociate into intact subunits in the absence of the antibiotic. Second, and interestingly, this inability was waived only when linezolid was exogenously added. Both findings could explain the peculiar ribosome behavior and are also compatible with the observed increased bacterial growth that has previously been reported (15) for the linezolid-dependent strain.
DISCUSSION
Antibiotic dependence leading to faster bacterial growth is a rare observation, and so far this phenomenon has been described in only a few reports (22–24), which have described antibiotic dependence leading to faster bacterial growth only for kasugamycin (22), streptomycin (23), and vancomycin (24). Although vancomycin dependence has not been associated with protein synthesis, streptomycin (23) and kasugamycin (22) dependence has been attributed to specific ribosomal protein and/or rRNA mutations. Additionally, some Bacillus subtilis strains that were dependent on kasugamycin for growth exhibited defects in ribosomal subunit assembly (22). Moreover, this antibiotic induces the assembly of a 61S ribosome particle (instead of the normal 70S particle) missing at least six ribosomal proteins that translates leaderless mRNA (25). Therefore, the elucidation of the underlying mechanisms is crucial for the appropriate use of antibiotics and the effective treatment of patients.
In the case of linezolid, although the detailed structural data for the ribosome-linezolid complex are known (Fig. 5), the exact mechanism of action of the antibiotic remains unclear. This is due to the lack of a reliable in vitro biochemical assay that accurately measures the inhibitory effect of linezolid at concentrations near the MIC value. Nevertheless, it has become clear that, until now, the only ribosomal functions inhibited at concentrations near the MIC are frameshifting and nonsense suppression (6). Finally, linezolid inhibition of the initiation of translation or peptide bond formation could be attributed to indirect effects due to the extremely high concentrations used (2, 26).
FIG 5.
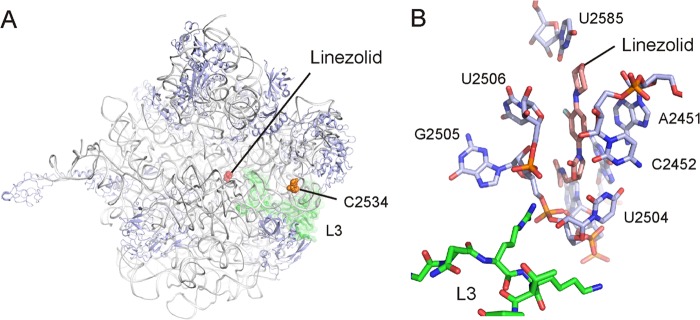
The binding site of oxazolidinones on the 50S ribosomal subunit. (A) Interface view of the Deinococcus radiodurans 50S subunit with the binding position of linezolid relative to the positions of mutated bases U2504 and C2534 and ribosomal protein L3. (B) View of linezolid (pink) within the binding pocket formed by eight universally conserved nucleotides (blue) of the 23S rRNA (PDB accession number 3DLL) (4). (Courtesy of Daniel N. Wilson, reproduced with permission.)
In the reported structures (4, 5), specific bases on 23S rRNA participate in the formation of the linezolid binding pocket (Fig. 5B). On the basis of its binding position, linezolid should occupy the A site of the ribosome and by doing so could inhibit the initial steps of peptide bond formation (4, 5, 27). However, the increased peptidyltransferase activity observed for the ribosomes from the linezolid-dependent strain that were assembled in the presence of the antibiotic depends entirely on the presence of linezolid. The new ribosomal population then exhibits a structural and functional adaptation which is linezolid dependent. This fitness cost adaptation seems to emerge from the successful combination of the mutations identified in the presence of linezolid. As exposure to linezolid is very common among hospitalized patients, particularly in intensive care units, the increased peptidyltransferase activity seen when these strains are exposed to linezolid could offer them a significant selective advantage and might contribute to their ongoing expansion (15).
It should be noted that the U2504C and C2534U mutations in the LZDD strain exist simultaneously with additional mutations in the L3 protein. Although U2504 is very close to the linezolid binding site, C2534 is distant from that site (Fig. 5). However, both bases in combination with the L3 protein possibly contribute to a local network of interactions, thus affecting ribosome assembly and function. Although the main part of ribosomal protein L3 is positioned on the surface of the 50S subunit, a loop ending in two tips extends into the PTC (Fig. 5). Such networks of interactions are known to exist, especially for the communication of the exit tunnel with the peptidyltransferase center, and are activated by small molecules, such as amino acids or macrolide antibiotics (28, 29). In addition, the inability of the linezolid-dependent ribosomes to successfully assemble their subunits in the absence of linezolid offers additional evidence that the ribosomal population is modified and largely relies on the presence of linezolid. Moreover, the observation that the presence of linezolid is required for a normal subunit profile provides further support for the linezolid dependence of the new ribosomal populations that have emerged. The latter observation, taken together with our findings, indicates that under stress conditions, dynamic and catalytically efficient unconventional ribosomal assemblies can be induced in vivo for specific reasons (30), a behavior which might reflect, from an evolutionary point of view, the formation of ancient bacterial protoribosomes (25). At this time and based on our observations, we can only speculate that if linezolid could interfere with peptide release, then most likely we would expect a decrease in the growth rate of the mutant strain rather than a significant increase. Furthermore, since linezolid has also been reported to affect frameshifting and stop codon read-through, the possibility of such an effect cannot be excluded.
In this study, we present evidence supporting the notion that the faster linezolid-dependent growth of linezolid-resistant S. epidermidis involves functional and possibly structural adaptations of the ribosomes, and these in turn affect the overall protein synthesis rates. Our data indicate that increased peptidyltransferase activity could account for this abnormal phenotype. During treatment and prior to the administration of LZD to patients, physicians should meticulously evaluate bacterial strains and seriously consider the possibility of a linezolid-dependent phenotype for bacterial growth. Moreover, the contribution of the functional adaptation occurring with linezolid exposure to the ongoing dissemination of linezolid-resistant S. epidermidis strains in Greece is under investigation at a national level. Finally, a more thorough survey of many strains with similar behavior and further experiments are required to identify any possible mechanisms of linezolid dependence, in addition to those proposed herein.
ACKNOWLEDGMENTS
We are grateful to Daniel N. Wilson for preparing Fig. 5 and Marios Krokidis for his help during the initial phases of the project. We also thank George Spyroulias (NMR Center, University of Patras) for helpful comments.
This work was supported in part by the University of Patras Research Committee (K. Karatheodoris grant D164 to C.S.).
Footnotes
Published ahead of print 2 June 2014
REFERENCES
- 1.Colca JR, McDonald WG, Waldon DJ, Thomasco LM, Gadwood RC, Lund ET, Cavey GS, Mathews WR, Adams LD, Cecil ET, Pearson JD, Bock JH, Mott JE, Shinabarger DL, Xiong L, Mankin AS. 2003. Crosslinking in the living cell locates the site of action of oxazolidinone antibiotics. J. Biol. Chem. 278:21972–21979. 10.1074/jbc.M302109200 [DOI] [PubMed] [Google Scholar]
- 2.Aoki H, Ke L, Poppe SM, Poel TJ, Weaver EA, Gadwood RC, Thomas RC, Shinabarger DL, Ganoza MC. 2002. Oxazolidinone antibiotics target the P site on Escherichia coli ribosomes. Antimicrob. Agents Chemother. 46:1080–1085. 10.1128/AAC.46.4.1080-1085.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin AH, Murray RW, Vidmar TJ, Marotti KR. 1997. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 41:2127–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DN, Schluenzen F, Harms JM, Starosta AL, Conell SR, Fucini P. 2008. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc. Natl. Acad. Sci. U. S. A. 105:13339–13344. 10.1073/pnas.0804276105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM. 2008. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 51:3353–3356. 10.1021/jm800379d [DOI] [PubMed] [Google Scholar]
- 6.Thompson J, O'Connor M, Mills JA, Dahlberg AE. 2002. The protein synthesis inhibitors, oxazolidinones and chloramphenicol, cause extensive translational inaccuracy in vivo. J. Mol. Biol. 322:273–279. 10.1016/S0022-2836(02)00784-2 [DOI] [PubMed] [Google Scholar]
- 7.Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093. 10.1126/science.1176667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long KS, Vester B. 2012. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob. Agents Chemother. 56:603–612. 10.1128/AAC.05702-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla H, Huband MD, Seidel J, Schmidt H, Lescoe M, McCurdy SP, Lemmon MM, Brennan LA, Tait-Kamradt A, Puzniak L, Quinn JP. 2010. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin. Infect. Dis. 51:796–800. 10.1086/656281 [DOI] [PubMed] [Google Scholar]
- 10.Mulanovich VE, Huband MD, McCurdy SP, Lemmon MM, Lescoe M, Jiang Y, Rolston KV, LaSala PR. 2010. Emergence of linezolid-resistant coagulase-negative Staphylococcus in a cancer centre linked to increased linezolid utilization. J. Antimicrob. Chemother. 65:2001–2004. 10.1093/jac/dkq238 [DOI] [PubMed] [Google Scholar]
- 11.Wong A, Reddy SP, Smyth DS, Aguero-Rosenfeld ME, Sakoulas G, Robinson DA. 2010. Polyphyletic emergence of linezolid-resistant staphylococci in the United States. Antimicrob. Agents Chemother. 54:742–748. 10.1128/AAC.00621-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Tenover FC, Limor J, Lonsway D, Prince D, Dunne WM, Jr, Patel JB. 2007. Use of pyrosequencing to identify point mutations in domain V of 23S rRNA genes of linezolid-resistant Staphylococcus aureus and Staphylococcus epidermidis. Eur. J. Clin. Microbiol. Infect. Dis. 26:161–165. 10.1007/s10096-007-0261-0 [DOI] [PubMed] [Google Scholar]
- 13.Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, Jones RN. 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother. 52:2244–2246. 10.1128/AAC.00231-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMarre JM, Howden BP, Mankin AS. 2011. Inactivation of the indigenous methyltransferase RlmN in Staphylococcus aureus increases linezolid resistance. Antimicrob. Agents Chemother. 55:2989–2991. 10.1128/AAC.00183-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pournaras S, Ntokou E, Zarkotou O, Ranellou K, Themeli-Digalaki K, Stathopoulos C, Tsakris A. 2013. Linezolid dependence in Staphylococcus epidermidis bloodstream isolates. Emerg. Infect. Dis. 19:129–132. 10.3201/eid1901.111527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinos G, Wilson D, Terαoka Y, Szafrarski W, Fucini P, Kalpaxis D, Nierhaus K. 2004. Dissecting the ribosomal inhibition mechanisms of edeine and pactamycin: the universally conserved residues G693 and C795 regulate P-site RNA binding. Mol. Cell 13:113–124. 10.1016/S1097-2765(04)00002-4 [DOI] [PubMed] [Google Scholar]
- 17.Bommer U, Burkhardt N, Junemann R, Spahn CM, Triana-Alonso FJ, Nierhaus KH. 1996. Ribosomes and polysomes, p 271–301 In Graham J, Rickwoods D. (ed), Subcellular fractionation. A practical approach. Oxford University Press, New York, NY [Google Scholar]
- 18.Dinos G, Kalpaxis DL. 2000. Kinetic studies on the interaction between a ribosomal complex active in peptide bond formation and the macrolide antibiotics tylosin and erythromycin. Biochemistry 39:11621–11628. 10.1021/bi000811f [DOI] [PubMed] [Google Scholar]
- 19.Karahalios P, Kalpaxis DL, Fu H, Katz L, Wilson DN, Dinos GP. 2006. On the mechanism of action of 9-O-arylalkyloxime derivatives of 6-O-mycaminosyltylonolide, a new class of 16-membered macrolide antibiotics. Mol. Pharmacol. 70:1271–1280. 10.1124/mol.106.026567 [DOI] [PubMed] [Google Scholar]
- 20.Eustice DC, Feldman PA, Slee AM. 1988. The mechanism of action of DuP 721, a new antibacterial agent: effects on macromolecular synthesis. Biochem. Biophys. Res. Commun. 150:965–971. 10.1016/0006-291X(88)90723-1 [DOI] [PubMed] [Google Scholar]
- 21.Champney WS, Miller M. 2002. Linezolid is a specific inhibitor of 50S ribosomal subunit formation in Staphylococcus aureus cells. Curr. Microbiol. 44:350–356. 10.1007/s00284-001-0023-7 [DOI] [PubMed] [Google Scholar]
- 22.Pai Y, Dabbs ER. 1981. Conditional lethal mutants of Bacillus subtilis dependent on kasugamycin for growth. Mol. Gen. Genet. 183:478–483. 10.1007/BF00268768 [DOI] [PubMed] [Google Scholar]
- 23.Birge EA, Kurland CG. 1969. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science 166:1282–1284. 10.1126/science.166.3910.1282 [DOI] [PubMed] [Google Scholar]
- 24.Tambyah PA, Marx JA, Maki DJ. 2004. Nosocomial infection with vancomycin-dependent enterococci. Emerg. Infect. Dis. 10:1277–1281. 10.3201/eid1007.030993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaberdina AC, Szaflarski W, Nierhaus KH, Moll I. 2009. An unexpected type of ribosomes induced by kasugamycin: a look into ancestral times of protein synthesis? Mol. Cell 33:227–236. 10.1016/j.molcel.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghardt H, Schimz K-L, Müller M. 1998. On the target of a novel class of antibiotics, oxazolidinones, active against multidrug-resistant Gram-positive bacteria. FEBS Lett. 425:40–44. 10.1016/S0014-5793(98)00194-X [DOI] [PubMed] [Google Scholar]
- 27.Leach KL, Swaney SM, Colca JR, McDonald WG, Blinn JR, Thomasco LM, Gadwood RC, Shinabarger D, Xiong L, Mankin AS. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26:393–402. 10.1016/j.molcel.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Vera LR, Sachs MS, Squires CL, Yanofsky C. 2011. Nascent polypeptide sequences that influence ribosome function. Curr. Opin. Microbiol. 14:160–166. 10.1016/j.mib.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 29.Vázquez-Laslop N, Klepacki D, Mulhearn DC, Ramu H, Krasnykh O, Franzblau S, Mankin AS. 2011. Role of antibiotic ligand in nascent peptide-dependent ribosome stalling. Proc. Natl. Acad. Sci. U. S. A. 108:10496–10501. 10.1073/pnas.1103474108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodmel JS, Gutell RR, Dahlberg AE. 1995. Genetic and comparative analyses reveal an alternative secondary structure in the region of nt912 of Escherichia coli 16S rRNA. Proc. Natl. Acad. Sci. U. S. A. 92:10555–10559. 10.1073/pnas.92.23.10555 [DOI] [PMC free article] [PubMed] [Google Scholar]