Abstract
The IncA/C plasmids have been implicated for their role in the dissemination of β-lactamases, including gene variants that confer resistance to expanded-spectrum cephalosporins, which are often the treatment of last resort against multidrug-resistant, hospital-associated pathogens. A blaFOX-5 gene was detected in 14 Escherichia coli and 16 Klebsiella isolates that were cultured from perianal swabs of patients admitted to an intensive care unit (ICU) of the University of Maryland Medical Center (UMMC) in Baltimore, MD, over a span of 3 years. Four of the FOX-encoding isolates were obtained from subsequent samples of patients that were initially negative for an AmpC β-lactamase upon admission to the ICU, suggesting that the AmpC β-lactamase-encoding plasmid was acquired while the patient was in the ICU. The genomes of five E. coli isolates and six Klebsiella isolates containing blaFOX-5 were selected for sequencing based on their plasmid profiles. An ∼167-kb IncA/C plasmid encoding the FOX-5 β-lactamase, a CARB-2 β-lactamase, additional antimicrobial resistance genes, and heavy metal resistance genes was identified. Another FOX-5-encoding IncA/C plasmid that was nearly identical except for a variable region associated with the resistance genes was also identified. To our knowledge, these plasmids represent the first FOX-5-encoding plasmids sequenced. We used comparative genomics to describe the genetic diversity of a plasmid encoding a FOX-5 β-lactamase relative to the whole-genome diversity of 11 E. coli and Klebsiella isolates that carry this plasmid. Our findings demonstrate the utility of whole-genome sequencing for tracking of plasmid and antibiotic resistance gene distribution in health care settings.
INTRODUCTION
Multidrug resistance (MDR) plasmids have become an increasing concern due to their role in the spread of extended-spectrum β-lactamases (ESBLs), AmpC β-lactamases, and carbapenemases among pathogens causing health care-associated infections (1–5). In particular, the AmpC β-lactamases are frequently plasmid encoded and confer resistance to many β-lactams, including cephalosporins, penicillins, cephamycins, and monobactams (6). The FOX-encoding β-lactamases were identified as the most prevalent of the transferrable AmpC β-lactamases among a geographically distributed collection of nosocomial isolates from the United States (4). The FOX-1 AmpC β-lactamase was first detected in a Klebsiella pneumoniae strain isolated from a hospital in Buenos Aires, Argentina, in 1989 (7). Since the initial description of FOX-1, there have been 10 blaFOX alleles characterized to date that have been designated allele numbers by the Lahey Clinic (http://www.lahey.org/Studies/) (7–14). A recent study demonstrated that the blaFOX-1 gene was associated with an approximately 45-kb plasmid that could be conjugally transferred to an Escherichia coli laboratory isolate (7). In 2001, the blaFOX-5 allele was first described in a K. pneumoniae isolate from a urine culture from a New York City hospital (11). The blaFOX-5 allele has been associated with plasmids that are approximately 125 kb (11) to 200 kb (15). However, to our knowledge, there have been no complete sequences of FOX-encoding plasmids described to date.
Many of the MDR plasmids that have been characterized and carry β-lactamase genes belong to the IncA/C incompatibility group (16–18). Plasmids of the IncA/C incompatibility group are often large and self-mobilizable and have a broad host range (2, 3). IncA/C plasmids have frequently been detected in Enterobacteriaceae isolates that exhibit multidrug resistance (1, 19, 20). The IncA/C plasmids sequenced to date range in size from 131 to 195 kb and have been detected from nine different species of bacteria (E. coli, K. pneumoniae, Aeromonas hydrophila, Salmonella enterica, Yersinia pestis, Yersinia ruckeri, Providencia stuartii, Photobacterium damselae, and Enterobacter aerogenes), carrying at least seven different β-lactamase genes (2, 3, 16–18, 21–27). Comparative analysis of the sequenced IncA/C plasmids demonstrated that they share large conserved regions that bear genes involved in replication, stability, and conjugal transfer (2, 3, 21).
Typically, plasmid characterization is performed by isolation and sequencing of one or a few similar plasmids, combined with biochemical and molecular identification of the host bacteria (2, 3, 16–18, 21–27). This approach, while cost-effective, limits our ability to assess the genetic diversity of the host strain genomes that have acquired a particular plasmid of interest. However, as the cost of sequencing goes down, it becomes more economically feasible to sequence not only the plasmid but also the entire genome of bacterial strains that carry the multidrug resistance plasmids. Thus, comparative genomics is a powerful tool for examining the genetic diversity of multidrug resistance plasmids and their host bacteria as they are disseminated among individuals in a community or patients in a hospital. Several recent studies have used whole-genome sequencing and comparative genomics to investigate the dissemination of nosocomial pathogens and antimicrobial resistance genes (28–33).
In the following study, we used whole-genome sequencing of five E. coli and six Klebsiella isolates from a single health care facility to investigate both the diversity of the FOX IncA/C multidrug resistance plasmid carried by each of the isolates and the chromosomal diversity of these 11 isolates using phylogenomic analysis. These isolates were selected based on preliminary biochemical and molecular data, including their plasmid profiles, to represent phenotypically unique isolates. The contigs of the IncA/C plasmids from one of the E. coli genomes and two K. pneumoniae genomes sequenced were manually closed using PCR, and the genetic content of each of these plasmids was compared to that of each of the genomes sequenced. Multilocus sequence typing (MLST) demonstrated the genetic diversity among the remaining FOX-encoding isolates that were not selected for whole-genome sequencing. PCR was also used to investigate the distribution of select genes carried by these plasmids among all 30 of the FOX-encoding E. coli and Klebsiella isolates in this study. Overall, molecular analysis and whole-genome sequencing demonstrated that the blaFOX-5 gene is borne by an IncA/C plasmid that has been acquired by diverse E. coli and Klebsiella spp.
MATERIALS AND METHODS
Bacterial isolation, biochemical identification, and antimicrobial susceptibility testing.
The bacteria examined in this study were isolated from perianal swab cultures of patients in the surgical or medical intensive care unit (ICU) of the University of Maryland Medical Center (UMMC), which was part of a cohort study from 1 September 2001 through 1 June 2005 (34, 35) (Table 1). The E. coli and Klebsiella species isolates were cultured from the swabs as previously described (34). Biochemical identification of each isolate was performed using the VITEK2 and API20E biochemical assays (bioMérieux, Durham, NC). Antimicrobial susceptibilities were determined by identifying the MICs using VITEK2 in accordance with CLSI guidelines (36). This study was approved by the University of Maryland, Baltimore Institutional Review Board.
TABLE 1.
E. coli and Klebsiella spp. isolated from patients receiving treatments in a UMMC ICU, that carry the FOX beta-lactamase gene
| Species | Isolatea | Sample date (mo/day/yr)b | Presence of FOXc | Patient age (yr) | Patient genderd | ICUe | Length of hospital stay (days) | β-lactam(s) administered during stayf | Allele no. for amplicon PCR positive forg: |
No. of plasmids | Plasmid Inc type(s)h | Multilocus STi | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEM | SHV | KPC | ||||||||||||
| E. coli | 4541-1 | 02-16-2003 | Admission | 77 | F | MICU | 4 | PIP | 12 | 3 | A/C | 216 | ||
| 4552-1 | 02-18-2003 | Admission | 77 | F | MICU | 4 | PIP | 12 | 3 | A/C | 216 | |||
| 10635 | 12-24-2004 | Admission | 78 | F | MICU | 29 | IPM | 3 | A/C, Frep, B/O, L/M | 372 | ||||
| 9782 | 09-20-2004 | Admission | 82 | M | MICU | 9 | IPM, PIP | 7 | 3 | A/C, Frep, L/M | 372 | |||
| 2215 | 05-14-2002 | Admission | 74 | F | MICU | 9 | PIP | 1 | A/C | 599 | ||||
| 3866 | 12-03-2002 | Acquisition | 78 | M | SICU | 35 | CFZ, IPM, PIP, TIM | 1 | 9 | A/C, FIB, Frep | 69 | |||
| 9499-3 | 08-18-2004 | Admission | 49 | F | MICU | 14 | None | 1 | 7 | 3 | A/C, Frep, L/M | 372 | ||
| 7996-1 | 03-13-2004 | Admission | 54 | F | SICU | 14 | IPM | 2 | A/C | 127 | ||||
| 11366-2 | 03-22-2005 | Admission | 72 | M | MICU | 19 | PIP | 1 | A/C | 156 | ||||
| 11117 | 02-20-2005 | Admission | 77 | M | MICU | 10 | PIP | 1 | 5 | A/C, FIA, Frep | 131 | |||
| 10505 | 12-11-2004 | Admission | 76 | F | SICU | 8 | PIP | 5 | A/C, Frep | 167 | ||||
| 7856 | 02-28-2004 | Admission | 71 | M | MICU | 5 | IPM | 2 | A/C, FIA, Frep | 131 | ||||
| 10376 | 11-25-2004 | Admission | 69 | F | MICU | 1 | None | 4 | A/C, FIA, FIB, Frep | ND | ||||
| 10810 | 01-14-2005 | Admission | 77 | F | MICU | 15 | CRO | 1 | 10 | A/C, FIA, FIB, Frep | 131 | |||
| K. pneumoniae | 4006 | 12-18-2002 | Acquisition | 48 | M | SICU | 11 | None | 11 | 3 | A/C | 54 | ||
| 10830 | 01-19-2005 | Acquisition | 60 | M | SICU | 29 | FEP, IPM, PIP | 26 | 3 | A/C | 34 | |||
| 8746-1 | 06-04-2004 | Admission | 68 | F | MICU | 6 | PIP | 27 | 3 | A/C | 295 | |||
| 1830-2 | 03-28-2002 | Admission | 77 | M | MICU | 3 | TIM | 1 | 4 | A/C | 327 | |||
| 4541-2 | 02-16-2003 | Admission | 77 | F | MICU | 4 | PIP | 12 | 6 | A/C | 1125 | |||
| 11227-1 | 03-04-2005 | Admission | 53 | F | MICU | 10 | AMP | 26 | 2 | 9 | A/C, N | 228 | ||
| 11646 | 04-21-2005 | Admission | 44 | M | MICU | 6 | PIP | 26 | 2 | 9 | A/C, N | 228 | ||
| 9302 | 07-30-2004 | Admission | 60 | M | MICU | 4 | PIP | 11 | 2 | A/C | 429 | |||
| 8657-2 | 05-25-2004 | Admission | 75 | F | MICU | 23 | PIP | 7 | A/C, N | 1123 | ||||
| 7699 | 02-11-2004 | Admission | 63 | F | MICU | 9 | IPM | 11 | 7 | A/C, N | 1123 | |||
| 8068 | 03-23-2004 | Admission | 56 | F | MICU | 10 | IPM | 11 | 7 | A/C, N | 1123 | |||
| 11225-1 | 03-05-2005 | Admission | 81 | F | MICU | 2 | None | 11 | 2 | A/C | 664 | |||
| 11366-3 | 03-22-2005 | Admission | 72 | M | MICU | 19 | PIP | 1 | A/C | 628 | ||||
| 10332 | 11-20-2004 | Admission | 63 | M | MICU | 18 | IPM, PIP | 11 | 3 | A/C | 664 | |||
| Klebsiella sp. | 10982 | 02-02-2005 | Admission | 57 | M | SICU | 13 | CFZ | —j | 7 | A/C | 1155 | ||
| K. oxytoca | 11492-1 | 04-06-2005 | Acquisition | 64 | M | MICU | 57 | PIP, FEP | 5 | A/C | ND | |||
Isolates that were analyzed by genome sequencing are indicated in bold. Italics denote isolates obtained from the same patient, and underlining denotes isolates obtained from another patient.
The sample date denotes the date that swab sampling was performed on the patient.
“Admission” indicates that FOX was detected from a swab sampled shortly after admission to the ICU, while “acquisition” indicates that the FOX gene was not detected initially but was present in subsequent swabs.
F, female; M, male.
MICU, medical intensive care unit; SICU, surgical intensive care unit.
PIP, piperacillin; IPM, imipenem; CFZ, cefazolin; TIM, ticarcillin-clavulanic acid; CRO, ceftriaxone; FEP, cefepime; AMP, ampicillin.
The TEM, CTX-M, SHV, KPC, and CARB β-lactamases were detected by PCR as present or absent (cells with no data). Allele numbers are designated for positive PCR amplicons that were sequenced or extracted from the draft genome.
The plasmid incompatibility type (Inc type) was determined by PCR detection using genomic DNA of the wild-type isolates.
ST is the sequence type (MLST). ND, could not be determined.
—, the LEN-26 β-lactamase of 10982 was PCR amplified using the SHV primers.
Plasmid profiles.
The plasmid profile of each wild-type isolate was determined using an acid phenol extraction method (37, 38). The plasmid bands were visualized by running samples on a 0.7% agarose gel for 4 h at 100 V/cm, followed by visualization using ethidium bromide. The FOX plasmids were conjugally transferred to a rifampin-resistant E. coli laboratory strain, J53, as previously described (14). The J53 transconjugant colonies that were resistant to both rifampin and ceftazidime and had acquired a single plasmid were identified using a subsequent acid phenol extraction protocol followed by gel electrophoresis as described above.
MLST.
Multilocus sequence typing (MLST) was performed on the FOX-encoding E. coli and K. pneumoniae isolates using typing schemes that were previously developed for each species. The E. coli MLST was performed by following the guidelines at http://mlst.warwick.ac.uk/mlst/ as previously described (39). The K. pneumoniae MLST was performed by following the guidelines at http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html as previously described (40). The MLST loci were PCR amplified for sequencing using GoTaq polymerase (Promega) in a 50-μl reaction mixture with the following concentrations of reagents: 1× buffer, 2 to 2.5 mM MgCl2, 0.2 to 0.4 μM each primer, and 1 U of Taq polymerase. The PCR protocol included an initial denaturation at 95°C, followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and an elongation of 72°C for 1 min, with a final extension at 72°C for 7 min. The PCR amplicons were visualized by running samples on a 1% agarose gel containing ethidium bromide at 100 V for 60 to 75 min. Sequencing of PCR amplicons was performed using BigDye Terminator chemistry (Applied Biosystems) on a model 3130 genetic analyzer (Applied Biosystems).
PCR amplification and sequencing of β-lactamase genes.
The β-lactamase genes, other antimicrobial resistance genes, and the E. coli virulence genes were PCR amplified using primers listed in Table S1 in the supplemental material. The E. coli and Klebsiella isolates were PCR screened for the presence of SHV, TEM, KPC, FOX, CTX-M, and CARB β-lactamase genes (see Table S1 in the supplemental material). The PCR was performed using AmpliTaq Gold 360 polymerase (Life Technologies). The PCR was performed using the following final concentrations of reagents in a 15-μl final reaction volume: 1× buffer, 2 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.2 μM each primer, and 1.25 U of Taq polymerase. The PCR protocol included an initial denaturation at 95°C, followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and an elongation of 72°C for 1 min, with a final extension at 72°C for 7 min. The PCR amplicons were visualized by running samples on a 1% agarose gel containing ethidium bromide at 100 V for 60 to 75 min. The annealing temperatures used for each primer pair are listed in Table S1 in the supplemental material. The PCR amplicons were purified using the QIAquick PCR purification kit (Qiagen). Sequencing of PCR amplicons was performed using BigDye Terminator chemistry (Applied Biosystems) on a 3130 genetic analyzer (Applied Biosystems).
Plasmid incompatibility typing.
The plasmid incompatibility typing was performed on the wild type and the corresponding E. coli J53 transconjugants as previously described (41, 42). The PCR was performed using AmpliTaq Gold 360 polymerase (Life Technologies). The PCR was performed using the following final concentrations of reagents in a 15-μl final reaction volume: 1× buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.2 μM each primer, and 1.25 U of Taq polymerase. The PCR protocol included an initial denaturation at 95°C, followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and an elongation of 72°C for 1 min, with a final extension at 72°C for 7 min. The PCR amplicons were visualized by running samples on a 1% agarose gel containing ethidium bromide at 100 V for 60 to 75 min. The annealing temperatures used for each primer pair are listed in Table S1 in the supplemental material.
Genome sequencing and assembly.
The E. coli and Klebsiella isolates were grown overnight in Luria-Bertani (LB) broth (Difco), and their genomic DNA was isolated using the Sigma GenElute genomic kit (Sigma-Aldrich). The genome sequence of each isolate was generated using paired-end libraries with 300-bp inserts on the Illumina HiSeq2000 at the Institute for Genome Sciences, Genome Resource Center. The draft genomes were assembled using Minimus (43) to merge the contigs generated with the Velvet assembly program (44) (kmer values were determined using VelvetOptimiser v2.1.4 [http://bioinformatics.net.au/software.velvetoptimiser.shtml]) and the contigs generated with the Edena v3 assembler (45), and the assemblies were filtered to contain contigs of ≥500 bp. The average sequence coverage of the genomes analyzed in this study was 108 times (range, 64 to 156 times). Information regarding the genome assembly size, number of contigs, and accession numbers for each of the genomes sequenced in this study are listed in Table S2 in the supplemental material.
The genetic content of the FOX plasmids was determined by aligning all draft genome sequences using Mugsy (46). Homologous blocks that aligned from all the genomes were extracted from each genome and compared. The protein coding regions and putative functions encoded by the genome sequences and closed plasmids were predicted using Glimmer3 (47) and RAST (48). Contigs of the FOX plasmid were identified in the genomes of K. pneumoniae 4006, K. pneumoniae 4541-2, and E. coli 4552-1 by manual inspection of the annotation and comparison to previously sequenced IncA/C plasmids using BLASTN (49). The gaps of the FOX plasmids from the genomes of E. coli 4552-1 and K. pneumoniae 4541-2 and 4006 were manually closed by PCR amplification and sequencing. The p4006_FOX plasmid was completely closed by manual PCR. The p4552_1_FOX and p4541_2_FOX plasmids were assembled into a large linear sequence (∼171 kb) that contains the FOX gene and the IncA/C plasmid genes identified in p4006_FOX. However, a region encoding transposases that is adjacent to the second resistance region could not be uniquely PCR amplified. The p4006_FOX plasmid and the plasmid contigs of 4552-1 and 4541-2 were annotated using the RAST annotation server (48).
Alignments and phylogenetic analyses.
Phylogenomic analysis of the FOX-encoding E. coli genomes was performed as previously described (50) by aligning the current genomes with select reference E. coli genomes that are available in the public domain (see Table S2 in the supplemental material). Phylogenomic analysis of the FOX-encoding Klebsiella genomes was performed by aligning these genomes with select Klebsiella and Enterobacter genomes listed in Table S2. Phylogenomic analysis of the IncA/C plasmids was performed by aligning the FOX-encoding E. coli and Klebsiella genomes with previously sequenced IncA/C plasmids listed in Table S2. The genome alignments and plasmid alignments were performed using Mugsy (46). Homologous blocks that aligned from each genome were concatenated with the bx-python toolkit (https://bitbucket.org/james_taylor/bx-python). A maximum-likelihood phylogeny with 100 bootstrap replicates was generated using RAxML v7.2.8 (51) and visualized using FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
The β-lactamase gene phylogeny was constructed using the nucleotide sequences of all β-lactamase genes identified in the draft genome sequences of the FOX-encoding E. coli and Klebsiella isolates sequenced in this study (see Table S2 in the supplemental material). Additional β-lactamase genes (blaSHV, blaTEM, blaCTX-M, blaKPC) that could be PCR amplified and sequenced from the isolates in this study were also included in the phylogeny and are indicated by accession numbers. Briefly, the sequences were aligned in MEGA5 (52), and a maximum-likelihood phylogeny was constructed using the Kimura 2-parameter model of distance estimation with 1,000 bootstrap replications. The gene accession numbers are indicated below for any sequences that were PCR amplified and sequenced, while the genome accession numbers are designated for the sequences obtained from the draft genomes.
BSR analysis.
BLAST score ratio (BSR) analysis was performed as previously described (53). The predicted amino acid sequences of genes borne by the pFOX plasmid of K. pneumoniae 4006 were compared to all the FOX-encoding E. coli and Klebsiella genomes sequenced in this study by using TBLASTN (54). The ratio of TBLASTN scores was calculated by dividing the score for each protein for a single genome by the score obtained by TBLASTN for the nucleotide sequence for the protein. The proteins detected with high similarity had BSR values of ≥0.8. BSR analysis results were visualized in MeV (55, 56), and a cluster analysis comparison of the genomes and plasmid sequences was performed using the Pearson correlation coefficient. The circular plot of the FOX plasmid from K. pneumoniae 4006 compared to all the other FOX-encoding genomes sequenced in this study was generated using Circleator (J. Crabtree, S. Agrawal, A. Mahurkar, G. S. Myers, D. A. Rasko, and O. White, submitted for publication).
Nucleotide sequence accession numbers.
The draft genome sequences are deposited in GenBank under the accession numbers listed in Table 2. The blaTEM, blaSHV, and blaKPC sequences that were PCR amplified and sequenced from isolates that were not genome sequenced are deposited in GenBank under the accession numbers KF585134 to KF585147. The GenBank accession number of the Klebsiella sp. strain 10982 FOX-10 gene is JX049131, and that of the LEN-26 gene is JX124390.
TABLE 2.
Characteristics of the FOX-harboring E. coli and Klebsiella isolate draft genome sequences analyzed in this study
| Organism | Strain | No. of contigs | Genome size (Mb) | % GC | No. of genes (RAST)a | Beta-lactamases | Genome accession no. |
|---|---|---|---|---|---|---|---|
| E. coli | 4541-1 | 163 | 4.97 | 50.78 | 4,839 | FOX-5, CARB-2, SHV-12 | JDWU00000000 |
| 4552-1 | 129 | 5.08 | 50.87 | 4,979 | FOX-5, CARB-2, SHV-12 | JDWT00000000 | |
| 7996-1 | 83 | 5.31 | 50.55 | 5,280 | FOX-5, CARB-2 | JDWS00000000 | |
| 11117 | 182 | 5.26 | 50.96 | 5,239 | FOX-5, CARB-2, TEM-1 | JDWR00000000 | |
| 10810 | 644 | 5.03 | 51.50 | 4,913 | FOX-5, CARB-2, TEM-1 | JDWQ00000000 | |
| K. pneumoniae | 4006 | 65 | 5.56 | 57.28 | 5,533 | FOX-5, CARB-2, SHV-11 | JDWP00000000 |
| 4541-2 | 145 | 5.71 | 56.99 | 5,699 | FOX-5, CARB-2, SHV-12 | JDWO00000000 | |
| 11227-1 | 90 | 5.58 | 57.30 | 5,517 | FOX-5, CARB-2, SHV-26, KPC-2 | JDWN00000000 | |
| 7699 | 148 | 5.68 | 57.31 | 5,538 | FOX-5, CARB-2, SHV-11 | JDWM00000000 | |
| Klebsiella sp. | 10982 | 218 | 6.08 | 56.61 | 6,180 | FOX-10, CARB-2, LEN-26 | AKYX01000000 |
| K. oxytoca | 11492-1 | 212 | 6.17 | 54.92 | 6,147 | FOX-5, CARB-2 | AIEM00000000 |
This is the approximate number of protein-encoding genes predicted using the RAST annotation server.
RESULTS
E. coli and Klebsiella isolates that carry the FOX AmpC β-lactamase.
There were 4,866 patients admitted to the UMMC medical and surgical ICUs from 1 September 2001 to 1 September 2006 that were enrolled in a cohort study which investigated risk factors associated with colonization by multidrug-resistant bacteria while receiving treatment in the ICU (35). Perianal swabs were collected following admission to the ICU, weekly, and on discharge from the ICU, and all isolates resistant to expanded-spectrum cephalosporins were cultured (35). Of the isolates that were resistant to expanded-spectrum cephalosporins, 85 were positive by PCR for an AmpC β-lactamase gene, including 14 E. coli and 16 Klebsiella species isolates identified with the blaFOX gene (Table 1).
These blaFOX-bearing isolates were cultured from samples obtained between March 2002 and April 2005 from 27 different patients (Table 1). These patients included 14 females and 13 males whose ages ranged from 44 to 82 years, with a median age of 69 years (Table 1). Of these 27 patients, 23 were administered one or more β-lactams during their stay in the ICU (Table 1). There were two seemingly similar FOX-5-encoding E. coli isolates (4541-1 and 4552-1) from a single patient on different days and one FOX-5-encoding K. pneumoniae isolate (4541-2) from the same patient. Similarly, there was one FOX-5-encoding E. coli isolate (11366-2) and one FOX-5-encoding K. pneumoniae isolate (11366-3) that were obtained from a single patient (Table 1). Of the 30 isolates examined, six (three E. coli and three Klebsiella isolates) were cultured from perianal swabs collected from patients while they were in the surgical ICU, while the remaining 24 isolates were from patients in the medical ICU (Table 1). There was one E. coli isolate (3866) and three Klebsiella isolates (4006, 10830, 11492-1) that were identified as likely having been acquired or selected for in the ICU since these patients were negative for an AmpC β-lactamase-producing bacterium upon admission to the ICU (Table 1). The AmpC β-lactamase-producing isolates themselves may have been acquired by the patient following admission, or these isolates may have been present in the patient prior to admission and subsequently acquired the FOX-5 AmpC β-lactamase-encoding plasmid from other bacteria following admission to the ICU.
The 14 FOX-encoding E. coli isolates examined had nine different multilocus sequence types (STs) (ST69, ST127, ST131, ST156, ST167, ST216, ST372, ST599, and one ST that could not be identified due to a lack of PCR amplification of one of the housekeeping genes), demonstrating that the FOX-5 AmpC β-lactamase has been acquired by E. coli isolates with considerable chromosomal genetic diversity (Table 1). E. coli isolate 4552-1 was obtained from the same patient 2 days after E. coli 4541-1 and K. pneumoniae 4541-2 were obtained from this patient. Both E. coli 4541-1 and 4552-1 were determined to have the same multilocus sequence type, ST216 (Table 1). Other sequence types had multiple isolates, including three isolates that were ST372 and two isolates that were ST131 (Table 1). ST131 has been identified as the most prevalent multidrug-resistant clone of E. coli associated with clinical infections in the United States in recent years (57).
Similarly, the 15 FOX-encoding K. pneumoniae isolates exhibited significant genetic diversity based on an MLST analysis (Table 1). The 15 K. pneumoniae isolates had 11 different STs (ST34, ST54, ST228, ST295, ST327, ST429, ST628, ST664, ST1123, ST1125, and a novel ST designated ST1155 [14]). A few STs were represented by more than one isolate; of these, two isolates were ST228, two isolates were ST664, and three isolates were ST1123 (Table 1). This further confirms that the FOX gene is mobile and that the genomic backbone of E. coli or Klebsiella is not a determining factor in the acquisition of this feature.
In addition to determining the presence of the blaFOX gene, we examined the presence of five other prevalent β-lactamase genes (blaSHV, blaTEM, blaCARB, blaCTX-M, and blaKPC) in the FOX-encoding E. coli and Klebsiella isolates by PCR assay (Table 1). In addition to all of the isolates in this study being PCR positive for a blaFOX gene, all but one of the isolates was PCR positive for a blaCARB β-lactamase (see Table S6 in the supplemental material). Meanwhile, all of the E. coli and Klebsiella isolates tested PCR negative for blaCTX-M (data not shown). There were four E. coli isolates (3866, 9499-3, 11117, and 10810) that were PCR positive for a blaTEM β-lactamase. A blaSHV gene was detected by PCR in four (29%) of the E. coli isolates and 13 (81%) of the Klebsiella isolates. A blaKPC gene was detected by PCR in K. pneumoniae 11227-1 and 11646, and following sequencing, this amplicon was identified as blaKPC-2. The PCR amplicons were sequenced for confirmation and in some cases provided further information. Klebsiella sp. 10982 was PCR positive for a blaSHV gene; however, upon sequencing, it was determined that this amplicon is actually a LEN-26 β-lactamase (14).
The number of presumptive plasmids varied significantly from only 1 to approximately 10, depending on the isolate (Fig. 1). The FOX-encoding E. coli and Klebsiella isolates all contained at least one large plasmid of approximately the same size (>165 kb) (Fig. 1). PCR detection of the major plasmid incompatibility types (Inc types) identified eight different plasmid Inc types among the 30 FOX-encoding isolates examined (Table 1). All of the FOX-encoding isolates were positive for the A/C incompatibility type. The next most frequently detected Inc types were Frep (41), which was identified in nine E. coli isolates, and N, which was identified in five K. pneumoniae isolates (Table 1).
FIG 1.
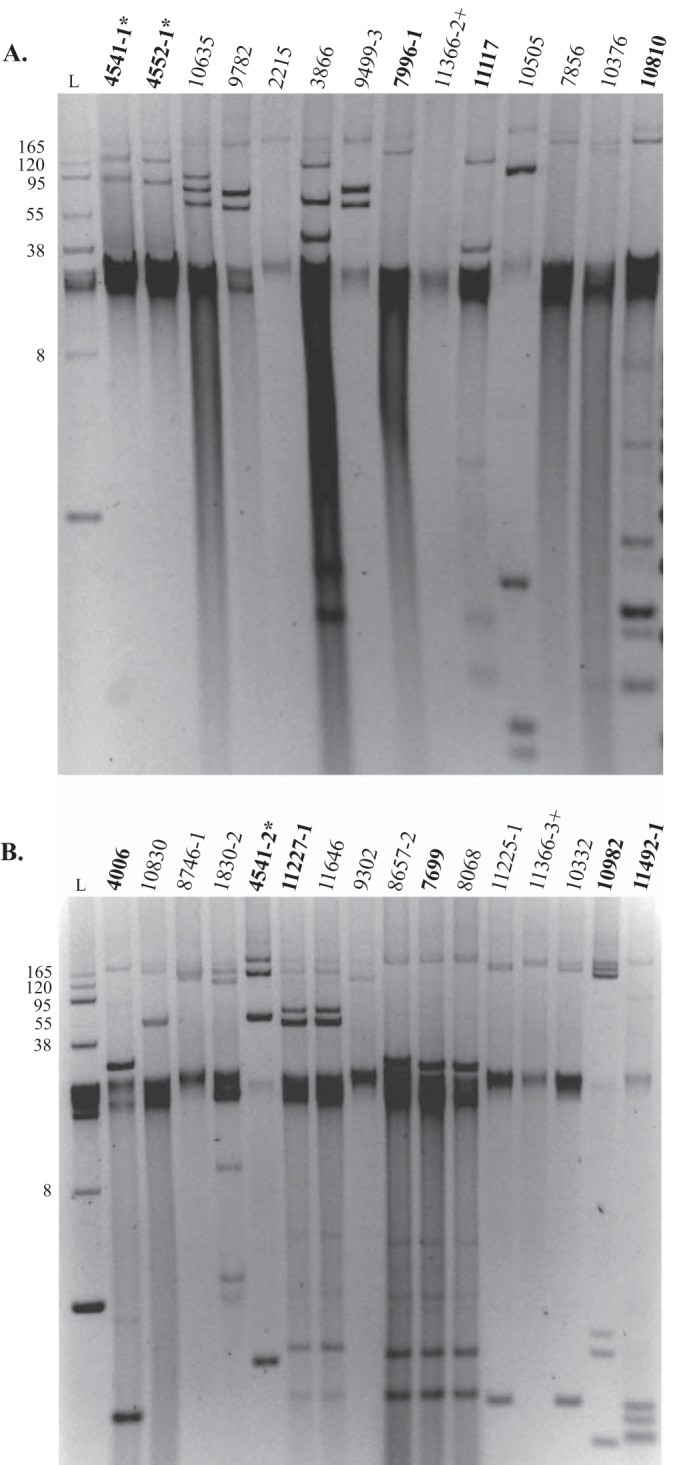
Plasmid profiles of E. coli (A) and Klebsiella (B) isolates that carry the FOX β-lactamase gene. The E. coli and Klebsiella isolates that were selected for genome sequencing are indicated in bold. The E. coli and Klebsiella strains that were isolated from the same patient are indicated with an asterisk or a plus sign. The first lane of each gel (L) contains the BAC-Tracker (Epicentre, Madison, WI) supercoiled DNA ladder, and the size (bp, in thousands) of each band in the ladder is indicated.
The MIC for and sensitivities of each wild-type isolate and its respective J53 transconjugant were determined for 10 β-lactam antibiotics (see Table S3 in the supplemental material). All of the wild-type and their pFOX transconjugant isolates were resistant to ampicillin and ampicillin-sulbactam, which is similar to the resistance reported for FOX-1 (7) (Table S3). Nearly all of the isolates were sensitive to carbapenems (ertapenem and meropenem), with the exception of wild-type K. pneumoniae 11227-1 and 11646, which were resistant to both antibiotics (Table S3). The blaKPC-2 gene was identified in both of these isolates by PCR and sequencing. However, the FOX IncA/C plasmid transconjugant strains of 11227-1 and 11646 were sensitive to both ertapenem and meropenem, suggesting that KPC-2 is likely encoded on a plasmid other than the FOX IncA/C plasmid. All of the FOX-encoding E. coli and Klebsiella isolates and their plasmid transconjugant isolates were resistant to the narrow-spectrum cephalosporin cephalothin and also the expanded-spectrum cephalosporin ceftazidime (Table S3), which is consistent with previous reports of the antimicrobial susceptibilities conferred by blaFOX genes (7–11). In addition, they were sensitive to the cephalosporin cefepime (Table S3).
Comparative genomics of select E. coli and Klebsiella isolates that carry the FOX plasmid.
Phylogenomic analysis of the E. coli and Klebsiella FOX-containing isolates reconfirms the genomic diversity inferred by the MLST previously completed (described above). Of the five FOX-encoding E. coli isolates, 4552-1 and 4541-1, which were isolated from the same patient sampled 2 days apart, were closely related in the whole-genome phylogeny. These isolates occur in E. coli phylogroup A, which includes HS, ATCC 8739, and 53638; these are commensal, lab-adapted, and enteroinvasive isolates (Fig. 2A). E. coli 10810 and 11117, which have different numbers of plasmids but are ST131 by MLST, are closely related based on the phylogenomic analysis, and both are within the B2 E. coli phylogroup (58, 59) with E. coli 7996-1 (Fig. 2A). This group includes the enteropathogenic E. coli (EPEC) strain E2348/69 and several uropathogenic/extraintestinal pathogenic E. coli (UPEC/ExPEC) isolates, UTI89, S88, 536, and CFT073 (Fig. 2A).
FIG 2.
Phylogenomic analysis of E. coli (A) and Klebsiella (B) isolates that carry the FOX-encoding IncA/C plasmids. The draft genomes were aligned with reference genomes using Mugsy, and a maximum-likelihood phylogeny was constructed in RAxML v7.2.8 (51) with 100 bootstrap replicates and visualized with FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/). The E. coli phylogeny was constructed using 2.78 Mb of aligned sequences, and the Klebsiella phylogeny was constructed with 2.1 Mb of aligned sequences. The scale bar indicates the approximate distance of 0.006 (A) or 0.04 (B) nucleotide substitution per site.
Phylogenomic analysis of the Klebsiella isolates sequenced in this study, compared with sequenced representative Klebsiella spp. and Enterobacter spp., demonstrated that the FOX-encoding Klebsiella isolates belong to three species (Fig. 2B; Table 2). Klebsiella 11492-1 was previously determined to be a Klebsiella oxytoca isolate based on housekeeping gene analyses and biochemical identification (60), and this isolate is in a clade separate from those of the other Klebsiella genomes analyzed. The isolates 4006, 4541-2, 11227-1, and 7699 formed a group that included the previously characterized K. pneumoniae genomes. Interestingly, Klebsiella isolate 10982 occupies its own branch and appears to be an intermediate between the human pathogen K. pneumoniae and endophytic Klebsiella variicola (Fig. 2B) (14). Klebsiella sp. 10982 also contains a novel FOX allele that has a single nonsynonymous nucleotide change compared to FOX-5 and has been designated the FOX-10 allele in the Lahey Clinic β-lactamase database (http://www.lahey.org/Studies/).
In summary, phylogenomic analysis of select FOX-encoding E. coli and Klebsiella isolates from a cohort study investigating the colonization of ICU patients by AmpC β-lactamase-producing bacteria (35) demonstrated that there is considerable genomic diversity among these isolates.
Characterization of the IncA/C multidrug resistance plasmid sequence encoding the FOX-5 β-lactamase.
The FOX IncA/C plasmid from K. pneumoniae 4006 is approximately 166.7 kb and contains 164 predicted protein coding DNA sequences (CDSs) (Fig. 3; see Table S4 in the supplemental material). The FOX IncA/C plasmid contig from the draft genomes of E. coli 4552-1 and K. pneumoniae 4541-2 were nearly identical (99% nucleotide identity) to each other, and both had a size of approximately 171 kb (Table S5). Comparison of the FOX IncA/C plasmid from K. pneumoniae 4006 (p4006_FOX) to the other 10 FOX-encoding isolates in this study that were analyzed by genome sequencing demonstrates that nearly all of the genes carried by this plasmid are present in the draft genomes analyzed (Fig. 3).
FIG 3.
Circular diagram of the 166.7-kb FOX-encoding IncA/C plasmid from K. pneumoniae 4006 and the presence of the plasmid-borne genes in the genomes of 10 E. coli and Klebsiella genomes determined using BLAST score ratio (BSR) analysis (53). Each plasmid gene is designated by a black rectangle in one of the two outermost rings, indicating the protein-coding strand. The GC content of the plasmid is designated in the green ring. Each of the 10 innermost rings indicates whether each of the p4006 genes is present with high similarity (yellow, BSR ≥ 0.8), present with lower similarity (black, BSR < 0.8 or > 0.4), or absent (blue, BSR ≤ 0.4). The diagram was constructed using Circleator (Crabtree et al., submitted).
The blaFOX-5 gene is immediately flanked on each side by genes that have ≥90% nucleotide identity to genes of Aeromonas salmonicida (61) that encode hypothetical proteins and mobile-element proteins, such as transposases, which is consistent with previous findings (11) (Fig. 4A). The blaCARB β-lactamase genes from all three of the plasmids were identical and had 100% nucleotide identity to the blaCARB-2 allele, which was characterized previously (M69058) (62). Meanwhile, the blaCARB-2 β-lactamase is carried within a different region of the plasmid, a region that carries additional predicted antimicrobial and heavy metal resistance genes (Fig. 4B). This region also carries a putative chloramphenicol resistance gene (cmlA) with 100% nucleotide identity to the cmlA gene (HQ380037) of an E. coli isolate from livestock (63) and a chloramphenicol acetyltransferase-like (cat-like) gene with 99% nucleotide identity to a cat-like gene carried by the K. pneumoniae plasmid pN5 (DQ831141) (64) (Fig. 4B). This region also carries a putative dihydropteroate synthase gene (sulI) with 100% nucleotide identity to the sulI gene from a K. pneumoniae isolate (EU780013) and a putative aminoglycoside resistance gene with 100% nucleotide identity to aacA4 (AB616660). Adjacent to these resistance genes in the plasmid from K. pneumoniae isolate 4006 are five mobile-element-associated genes encoding proteins of unknown function (Fig. 4B). These facts suggest that this region is a complex antimicrobial resistance cassette. Additionally, the plasmids from E. coli 4552-1 and K. pneumoniae 4541-2 carry putative heavy metal resistance genes, including a chromate transport protein adjacent to the antimicrobial resistance genes in this region (Fig. 4B).
FIG 4.
Diagram illustrating the genetic content and organization of the FOX-5-encoding region (A) and the second region of the FOX-5-encoding plasmids that carry the CARB-2 β-lactamase and additional antimicrobial and heavy metal resistance genes (B). The plasmids analyzed are the FOX-5-encoding plasmid p4006_FOX from K. pneumoniae 4006 and p4541_FOX from K. pneumoniae 4541-2. The size and direction of each arrow in the diagram denote the approximate amino acid sequence length and orientation of the sequences. Genes encoding predicted proteins involved in antibiotic resistance are red, heavy metal resistance genes are blue, transposable elements and other mobile-element-associated genes are green, and proteins with unknown function are white. A predicted Rhs family protein that is divided by the insertion of the transposable element genes and antimicrobial resistance and heavy metal resistance genes is indicated in orange. The gray rectangles connect genes with similarity ranging from approximately 80% (light gray) to 100% (darker gray) nucleotide identity. Genes that are divided by insertion of the resistance and mobile-element genes are indicated by stripes. The genes that are most similar to genes of Aeromonas salmonicida (61) are indicated with a pattern of rods.
Comparison of the previously sequenced IncA/C plasmids to the genomes of the FOX-encoding isolates by BSR analysis demonstrated that the FOX plasmids share the conserved IncA/C backbone with significant homology (Fig. 3; see Fig. S1 in the supplemental material). Cluster analysis of these genes confirmed that the FOX IncA/C plasmid genes are more similar to each other than to the few IncA/C plasmids that have been previously sequenced (Fig. S1). The FOX-encoding IncA/C plasmids are highly conserved, with two regions of variability compared to the previously sequenced IncA/C plasmids (Fig. S1). The regions of variability in the IncA/C plasmids are the locations of the antimicrobial resistance genes identified in the FOX-encoding plasmids in the current study (Fig. S1). Furthermore, alignment of the previously sequenced IncA/C plasmids to the FOX-encoding genomes identified a 37-kb region of significant homology representing the most highly conserved regions of the IncA/C plasmids (3). Phylogenetic analysis of this aligned region further demonstrated the significant similarity among the FOX-encoding plasmid genes (Fig. S2). The aligned FOX IncA/C regions were more closely related to IncA/C plasmids encoding diverse β-lactamases (CMY, TEM, OXA, SHV, VIM, CTX-M) than to the recently characterized NDM-encoding IncA/C plasmids, which formed a closely related phylogenetic group (Fig. S2).
PCR detection of these resistance genes in the FOX-encoding E. coli and Klebsiella isolates demonstrated that these genes were present in nearly all of the isolates (see Table S6 in the supplemental material). However, several of the E. coli isolates were PCR negative for one or more of the resistance genes (Table S6). E. coli 7856 and its FOX-encoding IncA/C plasmid transconjugant were PCR negative for cmlA, the aminoglycoside resistance gene, and the CARB-2 β-lactamase (Table S6). In addition, E. coli 3866 and its FOX IncA/C plasmid transconjugant were PCR negative for cmlA and the aminoglycoside resistance gene (Table S6), suggesting that these genes are not associated with the same plasmid as the FOX gene in this isolate. E. coli 7996-1 was PCR positive for all of the plasmid-borne FOX resistance genes except for the cmlA gene (Table S6). However, the FOX IncA/C plasmid transconjugant of 7996-1 was PCR negative for the aminoglycoside resistance gene and the blaCARB-2 β-lactamase gene, indicating that although the 7996-1 isolate carries both of these resistance genes, these genes may not be carried on the FOX IncA/C plasmid. These differences in the resistance gene content of this plasmid demonstrate variation of the FOX-encoding IncA/C plasmids.
Diversity of β-lactamase genes present in the E. coli and Klebsiella isolates that carry the FOX multidrug resistance plasmid.
To investigate the diversity of additional β-lactamase genes that the FOX-encoding E. coli and Klebsiella isolates have acquired, we phylogenetically compared all the β-lactamases detected in the sequenced genomes with those that could be PCR amplified from the isolates that were not sequenced. All of the β-lactamase genes identified in the E. coli and Klebsiella genomes sequenced in this study were compared to the SHV, TEM, and KPC genes that could be PCR amplified and sequenced from the additional FOX-encoding E. coli and Klebsiella isolates (Fig. 5). The E. coli 10810 and 11117 genomes contained a blaTEM β-lactamase with 100% amino acid identity to the blaTEM-1 allele β-lactamase (J01749) designated by the Lahey Clinic (http://www.lahey.org/Studies/). The sequenced E. coli genomes also encoded β-lactamases with similarity to the chromosomal ampC β-lactamases that have previously been described from E. coli (65) (Fig. 5). The chromosomal ampC genes from E. coli confer resistance to cephalosporins to various degrees, depending on their expression level (6, 65).
FIG 5.

Phylogenetic analysis of β-lactamase genes identified in the FOX plasmid-bearing E. coli and Klebsiella isolates examined in this study. All β-lactamase genes identified in the 11 sequenced genomes were included in the phylogeny. The accession numbers are indicated for the SHV, TEM, and KPC genes that could be PCR amplified and sequenced from the other 19 FOX-encoding isolates also included in the phylogeny. The reference sequence of each β-lactamase gene is indicated in bold. The nucleotide sequences were aligned using ClustalW in MEGA5 (52). A maximum-likelihood phylogeny was constructed using the Kimura 2-parameter model and 1,000 bootstrap replications using MEGA5 (52). Bootstrap values of ≥50 are indicated by a circle. The scale bar indicates 0.2 nucleotide change per site.
The presence of additional β-lactamase genes was not limited to the E. coli isolates. The blaKPC genes of K. pneumoniae isolates 11227-1 and 11646 have 100% nucleotide and amino acid identity to each other and to the characterized blaKPC-2 allele (AY034847) designated by the Lahey Clinic (http://www.lahey.org/Studies/). In silico analysis of the genome of K. oxytoca 11492-1 identified a gene with 100% nucleotide identity to the chromosomal β-lactamase of K. oxytoca, blaOXY-2, which has been characterized (AY077489) (66, 67). The blaOXY gene confers resistance to penicillins, monobactams, and cephalosporins (67, 68).
DISCUSSION
In the current study, we used comparative genomics to investigate the diversity of E. coli and Klebsiella isolates from a tertiary care academic medical center that carry an IncA/C multidrug resistance plasmid bearing a FOX-5 β-lactamase gene. The FOX β-lactamase gene was first characterized in K. pneumoniae in 1989 (7) and was identified as the most prevalent of the transferrable AmpC β-lactamase genes detected in a collection of nosocomial isolates from 2001 to 2002 (4). The 14 E. coli and 16 Klebsiella isolates characterized in this study that carry the FOX β-lactamase gene exhibit considerable diversity based on resistance gene content, plasmid content (Fig. 1), MLST (Table 1), and genomic comparisons (Fig. 2). The majority of the patients (85%, 23/27) from whom the FOX-encoding isolates were obtained had received a β-lactam antibiotic during their stay in the ICU (Table 1). This treatment may have selected for the maintenance of the FOX IncA/C plasmid in these isolates. Only four of the isolates likely acquired the FOX β-lactamase while the patient was in the ICU (3866, 4006, 10830, 11492-1), while the others were from patients that had an AmpC β-lactamase upon admission to the ICU. It is also possible that a FOX-encoding isolate was carried by the patient but undetected by sampling until the patient had been administered an antibiotic that selected for and resulted in increased abundance of the isolate to above a detectable threshold. Meanwhile, two patients were identified with both an E. coli and a K. pneumoniae isolate that carried the FOX IncA/C plasmid (Table 1). In particular, the FOX IncA/C plasmids of E. coli 4552-1 and K. pneumoniae 4541-2 were identical to each other but were different from the other FOX IncA/C plasmids characterized (Fig. 3 and 4), suggesting that this FOX IncA/C plasmid may have been transferred between these two isolates or that a third host may have been involved. We cannot determine the exact mechanism and direction of plasmid transfer among these isolates; however, many possibilities exist.
In addition to analyses of the FOX and CARB β-lactamases encoded by the IncA/C multidrug resistance plasmid, comparative genomics of select isolates and PCR detection of several prevalent β-lactamase genes (blaKPC, blaSHV, blaTEM) demonstrated that many of the isolates also carry additional plasmids with other β-lactamase genes (Fig. 1 and Table 1). These isolates were positive for multiple common plasmid incompatibility types that are identified in members of the Enterobacteriaceae (Table 1) (1, 41, 42). Among the isolates that carry plasmids of other Inc types were five Klebsiella isolates that were positive for the IncN incompatibility type, and four of these isolates also contained a blaSHV allele, although it is not known whether the blaSHV allele was carried by the IncN plasmid (Table 1). The IncN incompatibility group is another family of plasmids that has been linked to the spread of antimicrobial resistance genes among enteric bacteria (1). Klebsiella isolates 11227-1 and 11646 were ST-228 by MLST, were positive for the IncN incompatibility type, had identical plasmid profiles, carried a blaSHV-26 gene, and possessed a blaKPC-2 allele (Table 1). These isolates were obtained from patients upon admission to the ICU in March and April of 2005. These isolates demonstrate the potential for a Klebsiella isolate to disseminate four different β-lactamase genes (Fig. 4), some of which are known to be associated with a mobilizable IncA/C multidrug resistance plasmid, and potentially by a plasmid of the IncN incompatibility family. These findings highlight the ability of these plasmids to spread quickly in the bacterial population and disseminate the antimicrobial resistance phenotype.
Phylogenomic analysis demonstrated that the FOX-encoding E. coli isolates occur primarily in phylogroup A with E. coli commensal isolates or phylogroup B2 with the human-disease-associated UPEC/ExPEC and EPEC genomes (59, 69) (Fig. 2A). Further investigation is necessary to determine whether this E. coli isolate is capable of causing human disease or whether this isolate and the other FOX-encoding isolates investigated in this study are asymptomatic human colonizers. The Klebsiella isolates in this study represent three potential species, including K. pneumoniae and K. oxytoca, which have members capable of causing human disease (70). Klebsiella sp. isolate 10982 represents a potentially unique species within the Klebsiella isolates, as genome sequencing has identified that it shares similarities with previously sequenced genomes of the plant-colonizing K. variicola isolates, as well as the genomes of human disease-associated K. pneumoniae isolates (14).
Comparative genomics of the FOX IncA/C plasmid in each of the E. coli and Klebsiella genomes analyzed demonstrated that these plasmids are highly conserved, particularly in regions encoding proteins involved in stability and conjugal transfer (Fig. 3; see Fig. S1 in the supplemental material). This is consistent with the findings of previous studies that have analyzed the genetic content of IncA/C plasmids bearing diverse antimicrobial resistance genes and isolated from multiple species of bacteria and multiple locations (2, 3). However, we demonstrated that there is genetic variation within one of the regions of the FOX-5-encoding plasmids which contained antimicrobial and heavy metal resistance genes, indicating that this plasmid underwent additional gene exchange as it was transferred among different bacterial isolates (Fig. 4).
In conclusion, we have demonstrated the genetic diversity of E. coli and Klebsiella isolates that have an IncA/C multidrug resistance plasmid carrying two β-lactamase genes (blaFOX and blaCARB). Whole-genome sequencing is useful not only for investigating the transmission of an organism but also for examining the dissemination of multidrug resistance plasmids involved in both intra- and interspecies transmission of antimicrobial resistance genes. The comparative genomics of these isolates highlights the significant diversity of colonizing E. coli and Klebsiella isolates that serve as a reservoir of antibiotic resistance genes and IncA/C multidrug resistance plasmids.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jason Sahl and Jonathan Crabtree for technical assistance. We thank the team of curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles, and/or isolates at http://www.pasteur.fr/mlst.
This project is funded by NIH grants K12RR023250 (J.K.J.), 1K24AI079040-01A1 (A.D.H.), and 2R01AI060859-05 (A.D.H.) and Startup Funds from the State of Maryland (D.A.R.).
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02573-14.
REFERENCES
- 1.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238. 10.1128/AAC.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, White DG, Cebula TA, Ravel J. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J. Bacteriol. 191:4750–4757. 10.1128/JB.00189-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Alarcon C, Singer RS, Johnson TJ. 2011. Comparative genomics of multidrug resistance-encoding IncA/C plasmids from commensal and pathogenic Escherichia coli from multiple animal sources. PLoS One 6:e23415. 10.1371/journal.pone.0023415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moland ES, Hanson ND, Black JA, Hossain A, Song W, Thomson KS. 2006. Prevalence of newer beta-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J. Clin. Microbiol. 44:3318–3324. 10.1128/JCM.00756-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carattoli A. 2013. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 303:298–304. 10.1016/j.ijmm.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Jacoby GA. 2009. AmpC beta-lactamases. Clin. Microbiol. Rev. 22:161–182. 10.1128/CMR.00036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez Leiza M, Perez-Diaz JC, Ayala J, Casellas JM, Martinez-Beltran J, Bush K, Baquero F. 1994. Gene sequence and biochemical characterization of FOX-1 from Klebsiella pneumoniae, a new AmpC-type plasmid-mediated beta-lactamase with two molecular variants. Antimicrob. Agents Chemother. 38:2150–2157. 10.1128/AAC.38.9.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauernfeind A, Wagner S, Jungwirth R, Schneider I, Meyer D. 1997. A novel class C beta-lactamase (FOX-2) in Escherichia coli conferring resistance to cephamycins. Antimicrob. Agents Chemother. 41:2041–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bou G, Oliver A, Ojeda M, Monzon C, Martinez-Beltran J. 2000. Molecular characterization of FOX-4, a new AmpC-type plasmid-mediated beta-lactamase from an Escherichia coli strain isolated in Spain. Antimicrob. Agents Chemother. 44:2549–2553. 10.1128/AAC.44.9.2549-2553.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchese A, Arlet G, Schito GC, Lagrange PH, Philippon A. 1998. Characterization of FOX-3, an AmpC-type plasmid-mediated beta-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob. Agents Chemother. 42:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Queenan AM, Jenkins S, Bush K. 2001. Cloning and biochemical characterization of FOX-5, an AmpC-type plasmid-encoded beta-lactamase from a New York City Klebsiella pneumoniae clinical isolate. Antimicrob. Agents Chemother. 45:3189–3194. 10.1128/AAC.45.11.3189-3194.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miro E, Aguero J, Larrosa MN, Fernandez A, Conejo MC, Bou G, Gonzalez-Lopez JJ, Lara N, Martinez-Martinez L, Oliver A, Aracil B, Oteo J, Pascual A, Rodriguez-Bano J, Zamorano L, Navarro F. 2013. Prevalence and molecular epidemiology of acquired AmpC beta-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 32:253–259. 10.1007/s10096-012-1737-0 [DOI] [PubMed] [Google Scholar]
- 13.Perez-Llarena FJ, Kerff F, Zamorano L, Fernandez MC, Nunez ML, Miro E, Oliver A, Navarro F, Bou G. 2013. Characterization of the new AmpC beta-lactamase FOX-8 reveals a single mutation, Phe313Leu, located in the R2 loop that affects ceftazidime hydrolysis. Antimicrob. Agents Chemother. 57:5158–5161. 10.1128/AAC.00818-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazen TH, Zhao L, Sahl JW, Robinson G, Harris AD, Rasko DA, Johnson JK. 2014. Characterization of Klebsiella sp. 10982, a colonizer of humans that contains novel antibiotic resistance alleles and exhibits genetic similarities to plant and clinical Klebsiella isolates. Antimicrob. Agents Chemother. 58:1879–1888. 10.1128/AAC.01605-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez M, Tran JH, Chow N, Jacoby GA. 2004. Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob. Agents Chemother. 48:533–537. 10.1128/AAC.48.2.533-537.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. 2012. Evolution of IncA/C blaCMY-2-carrying plasmids by acquisition of the blaNDM-1 carbapenemase gene. Antimicrob. Agents Chemother. 56:783–786. 10.1128/AAC.05116-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGann P, Hang J, Clifford RJ, Yang Y, Kwak YI, Kuschner RA, Lesho EP, Waterman PE. 2012. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob. Agents Chemother. 56:1673–1679. 10.1128/AAC.05604-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekizuka T, Matsui M, Yamane K, Takeuchi F, Ohnishi M, Hishinuma A, Arakawa Y, Kuroda M. 2011. Complete sequencing of the bla(NDM-1)-positive IncA/C plasmid from Escherichia coli ST38 isolate suggests a possible origin from plant pathogens. PLoS One 6:e25334. 10.1371/journal.pone.0025334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsey RL, Fedorka-Cray PJ, Frye JG, Meinersmann RJ. 2009. Inc A/C plasmids are prevalent in multidrug-resistant Salmonella enterica isolates. Appl. Environ. Microbiol. 75:1908–1915. 10.1128/AEM.02228-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carattoli A, Miriagou V, Bertini A, Loli A, Colinon C, Villa L, Whichard JM, Rossolini GM. 2006. Replicon typing of plasmids encoding resistance to newer beta-lactams. Emerg. Infect. Dis. 12:1145–1148. 10.3201/eid1207.051555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Call DR, Singer RS, Meng D, Broschat SL, Orfe LH, Anderson JM, Herndon DR, Kappmeyer LS, Daniels JB, Besser TE. 2010. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob. Agents Chemother. 54:590–596. 10.1128/AAC.00055-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, Rasko DA, Mammel MK, Eppinger M, Rosovitz MJ, Wagner D, Rahalison L, Leclerc JE, Hinshaw JM, Lindler LE, Cebula TA, Carniel E, Ravel J. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. 10.1371/journal.pone.0000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Castillo CS, Hikima J, Jang HB, Nho SW, Jung TS, Wongtavatchai J, Kondo H, Hirono I, Takeyama H, Aoki T. 2013. Comparative sequence analysis of a multidrug-resistant plasmid from Aeromonas hydrophila. Antimicrob. Agents Chemother. 57:120–129. 10.1128/AAC.01239-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Hirono I, Kurokawa K, Maki T, Hawke J, Kondo H, Santos MD, Aoki T. 2008. Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R plasmids) from Photobacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob. Agents Chemother. 52:606–611. 10.1128/AAC.01216-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drieux L, Decre D, Frangeul L, Arlet G, Jarlier V, Sougakoff W. 2013. Complete nucleotide sequence of the large conjugative pTC2 multireplicon plasmid encoding the VIM-1 metallo-beta-lactamase. J. Antimicrob. Chemother. 68:97–100. 10.1093/jac/dks367 [DOI] [PubMed] [Google Scholar]
- 26.Doublet B, Boyd D, Douard G, Praud K, Cloeckaert A, Mulvey MR. 2012. Complete nucleotide sequence of the multidrug resistance IncA/C plasmid pR55 from Klebsiella pneumoniae isolated in 1969. J. Antimicrob. Chemother. 67:2354–2360. 10.1093/jac/dks251 [DOI] [PubMed] [Google Scholar]
- 27.Diene SM, Merhej V, Henry M, El Filali A, Roux V, Robert C, Azza S, Gavory F, Barbe V, La Scola B, Raoult D, Rolain JM. 2013. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new “killer bugs” are created because of a sympatric lifestyle. Mol. Biol. Evol. 30:369–383. 10.1093/molbev/mss236 [DOI] [PubMed] [Google Scholar]
- 28.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra116. 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, Connor TR, Harris SR, Fairley D, Bamford KB, D'Arc S, Brazier J, Brown D, Coia JE, Douce G, Gerding D, Kim HJ, Koh TH, Kato H, Senoh M, Louie T, Michell S, Butt E, Peacock SJ, Brown NM, Riley T, Songer G, Wilcox M, Pirmohamed M, Kuijper E, Hawkey P, Wren BW, Dougan G, Parkhill J, Lawley TD. 2013. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 45:109–113. 10.1038/ng.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Sun P, Vamathevan J, Li Y, Ingraham K, Palmer L, Huang J, Brown JR. 2011. Comparative genomics of Klebsiella pneumoniae strains with different antibiotic resistance profiles. Antimicrob. Agents Chemother. 55:4267–4276. 10.1128/AAC.00052-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa L, Feudi C, Fortini D, Garcia-Fernandez A, Carattoli A. 2014. Genomics of KPC-producing Klebsiella pneumoniae sequence type 512 clone highlights the role of RamR and ribosomal S10 protein mutations in conferring tigecycline resistance. Antimicrob. Agents Chemother. 58:1707–1712. 10.1128/AAC.01803-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAdam PR, Templeton KE, Edwards GF, Holden MT, Feil EJ, Aanensen DM, Bargawi HJ, Spratt BG, Bentley SD, Parkhill J, Enright MC, Holmes A, Girvan EK, Godfrey PA, Feldgarden M, Kearns AM, Rambaut A, Robinson DA, Fitzgerald JR. 2012. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 109:9107–9112. 10.1073/pnas.1202869109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris SR, Cartwright EJ, Torok ME, Holden MT, Brown NM, Ogilvy-Stuart AL, Ellington MJ, Quail MA, Bentley SD, Parkhill J, Peacock SJ. 2013. Whole-genome sequencing for analysis of an outbreak of methicillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect. Dis. 13:130–136. 10.1016/S1473-3099(12)70268-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson JK, Smith G, Lee MS, Venezia RA, Stine OC, Nataro JP, Hsiao W, Harris AD. 2009. The role of patient-to-patient transmission in the acquisition of imipenem-resistant Pseudomonas aeruginosa colonization in the intensive care unit. J. Infect. Dis. 200:900–905. 10.1086/605408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, Hebden JN, Morris JG., Jr 2007. Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg. Infect. Dis. 13:1144–1149. 10.3201/eid1308.070071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 37.Sobecky PA, Mincer TJ, Chang MC, Helinski DR. 1997. Plasmids isolated from marine sediment microbial communities contain replication and incompatibility regions unrelated to those of known plasmid groups. Appl. Environ. Microbiol. 63:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. 10.1016/0147-619X(84)90063-5 [DOI] [PubMed] [Google Scholar]
- 39.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 42.Johnson TJ, Wannemuehler YM, Johnson SJ, Logue CM, White DG, Doetkott C, Nolan LK. 2007. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 73:1976–1983. 10.1128/AEM.02171-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommer DD, Delcher AL, Salzberg SL, Pop M. 2007. Minimus: a fast, lightweight genome assembler. BMC Bioinformatics 8:64. 10.1186/1471-2105-8-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez D, Francois P, Farinelli L, Osteras M, Schrenzel J. 2008. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 18:802–809. 10.1101/gr.072033.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angiuoli SV, Salzberg SL. 2011. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics 27:334–342. 10.1093/bioinformatics/btq665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. 10.1093/bioinformatics/btm009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 50.Sahl JW, Steinsland H, Redman JC, Angiuoli SV, Nataro JP, Sommerfelt H, Rasko DA. 2011. A comparative genomic analysis of diverse clonal types of enterotoxigenic Escherichia coli reveals pathovar-specific conservation. Infect. Immun. 79:950–960. 10.1128/IAI.00932-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 52.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasko DA, Myers GS, Ravel J. 2005. Visualization of comparative genomic analyses by BLAST score ratio. BMC Bioinformatics 6:2. 10.1186/1471-2105-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gertz EM, Yu YK, Agarwala R, Schaffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol. 4:41. 10.1186/1741-7007-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. 2003. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378 [DOI] [PubMed] [Google Scholar]
- 56.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. 2006. TM4 microarray software suite. Methods Enzymol. 411:134–193. 10.1016/S0076-6879(06)11009-5 [DOI] [PubMed] [Google Scholar]
- 57.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin. Infect. Dis. 51:286–294. 10.1086/653932 [DOI] [PubMed] [Google Scholar]
- 58.Herzer PJ, Inouye S, Inouye M, Whittam TS. 1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J. Bacteriol. 172:6175–6181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8:207–217. 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- 60.Hazen TH, Robinson GL, Harris AD, Rasko DA, Johnson JK. 2012. Genome sequence of Klebsiella oxytoca 11492-1, a nosocomial isolate possessing a FOX-5 AmpC beta-lactamase. J. Bacteriol. 194:3028–3029. 10.1128/JB.00391-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reith ME, Singh RK, Curtis B, Boyd JM, Bouevitch A, Kimball J, Munholland J, Murphy C, Sarty D, Williams J, Nash JH, Johnson SC, Brown LL. 2008. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics 9:427. 10.1186/1471-2164-9-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huovinen P, Jacoby GA. 1991. Sequence of the PSE-1 beta-lactamase gene. Antimicrob. Agents Chemother. 35:2428–2430. 10.1128/AAC.35.11.2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis MA, Besser TE, Orfe LH, Baker KN, Lanier AS, Broschat SL, New D, Call DR. 2011. Genotypic-phenotypic discrepancies between antibiotic resistance characteristics of Escherichia coli isolates from calves in management settings with high and low antibiotic use. Appl. Environ. Microbiol. 77:3293–3299. 10.1128/AEM.02588-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez-Martinez JM, Velasco C, Garcia I, Cano ME, Martinez-Martinez L, Pascual A. 2007. Characterisation of integrons containing the plasmid-mediated quinolone resistance gene qnrA1 in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 29:705–709. 10.1016/j.ijantimicag.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 65.Mammeri H, Poirel L, Fortineau N, Nordmann P. 2006. Naturally occurring extended-spectrum cephalosporinases in Escherichia coli. Antimicrob. Agents Chemother. 50:2573–2576. 10.1128/AAC.01633-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Granier SA, Plaisance L, Leflon-Guibout V, Lagier E, Morand S, Goldstein FW, Nicolas-Chanoine MH. 2003. Recognition of two genetic groups in the Klebsiella oxytoca taxon on the basis of chromosomal beta-lactamase and housekeeping gene sequences as well as ERIC-1 R PCR typing. Int. J. Syst. Evol. Microbiol. 53:661–668. 10.1099/ijs.0.02408-0 [DOI] [PubMed] [Google Scholar]
- 67.Fournier B, Roy PH, Lagrange PH, Philippon A. 1996. Chromosomal beta-lactamase genes of Klebsiella oxytoca are divided into two main groups, blaOXY-1 and blaOXY-2. Antimicrob. Agents Chemother. 40:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fournier B, Lu CY, Lagrange PH, Krishnamoorthy R, Philippon A. 1995. Point mutation in the Pribnow box, the molecular basis of beta-lactamase overproduction in Klebsiella oxytoca. Antimicrob. Agents Chemother. 39:1365–1368. 10.1128/AAC.39.6.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaureguy F, Landraud L, Passet V, Diancourt L, Frapy E, Guigon G, Carbonnelle E, Lortholary O, Clermont O, Denamur E, Picard B, Nassif X, Brisse S. 2008. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genomics 9:560. 10.1186/1471-2164-9-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





