Abstract
Cefoxitin could be an alternative to carbapenems in extended-spectrum-beta-lactamase-producing Escherichia coli (ESBL-EC) infections. However, pharmacological and clinical data regarding cefoxitin are limited. Using a recent pharmacological model and the MICs of ESBL-EC collected from pyelonephritis, we determined the probabilities to reach four pharmacological targets: free cefoxitin concentrations above the MIC during 50% and 100% of the administration interval (T>MIC = 50% and T>MIC = 100%, respectively) and free cefoxitin concentrations above 4× MIC during 50% and 100% of the administration interval (T>4MIC = 50% and T>4MIC = 100%, respectively). Cefoxitin could be used to treat ESBL-EC pyelonephritis, but administration modalities should be optimized according to MICs in order to reach pharmacological targets.
TEXT
Extended-spectrum-beta-lactamase-producing Enterobacteriaceae (ESBL-PE), especially Escherichia coli, have become a major public health issue worldwide (1, 2). Since they are often resistant to other antibiotics, ESBL-PE require carbapenems prescription (3). The increasing rate of ESBL-PE infections runs the risk of carbapenems overconsumption (4) and thus to the spread of last-resort antibiotic-resistant Enterobacteriaceae strains (5). Alternatives to carbapenems are urgently needed. As cephamycins demonstrate in vitro activity against ESBL-PE, some authors suggest that this class of antibiotics could be used in ESBL-producing E. coli (ESBL-EC) infections (6, 7). However, data concerning the use of cefoxitin for the treatment of ESBL-PE infections are scarce, and pharmacodynamic target attainment probabilities in pyelonephritis have not been studied so far.
Our aim was to assess the probability of target attainment for cefoxitin in adult patients with ESBL-EC pyelonephritis. Since the renal parenchyma itself is infected in pyelonephritis, we assumed that therapeutic success was dependent on cefoxitin plasma concentration. Between 2008 and 2012, we collected 145 nonduplicate ESBL-EC strains from the urine of patients with proven pyelonephritis in 2 French university hospitals. The antimicrobial susceptibility testing was performed by the conventional disk diffusion methodology as described by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (8). Strains were considered being susceptible to cefoxitin when the inhibition diameter zone measurement was ≥19 mm (30-μg disk; Bio-Rad, Marne-la-Coquette, France). ESBL production was authenticated by a double-disk synergy test using amoxicillin-clavulanic acid application next to ceftriaxone and cefepime disks (Bio-Rad, Marne-la-Coquette, France) (9). Cefoxitin MICs were determined by the Etest method according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France). No EUCAST cefoxitin MIC susceptibility breakpoint is available. Thus, as suggested by the Antibiogram Committee of the French Microbiology Society (CA-SFM), we chose 8 mg/liter to this end (10).
The probability of maintaining free cefoxitin plasma concentrations above the MIC was determined by simulating the pharmacokinetic profile of 10,000 subjects with creatinine clearance of 120 ml/min (NONMEM Software), using the model described by Isla et al. (11). A protein binding of 70% was used. The dosage regimen was 2 g 4 times/day, and either 1-h, 4-h, or continuous infusion durations were tested. Four pharmacological targets were chosen: free cefoxitin concentrations above the MIC during 50% and 100% of the administration interval (T>MIC = 50% and T>MIC = 100%, respectively) and free cefoxitin concentrations above 4× MIC during 50% and 100% of the administration interval (T>4MIC = 50% and T>4MIC = 100%, respectively). The MIC distribution used was the MICs of the cefoxitin-susceptible strains (≥19-mm inhibition diameter).
Table 1 displays ESBL-EC strains' susceptibilities to different antibiotic classes. According to the inhibition diameter measurement zones and to the MICs, 142 (97.9%) and 121 (83.4%) strains were considered to be susceptible to cefoxitin (Fig. 1).
TABLE 1.
Antibiotic susceptibility profiles of ESBL-EC strains, according to EUCAST breakpoints (n = 145)
| Antibiotic class | No. (%) of susceptible strains |
|---|---|
| Third-generation cephalosporins | 15 (10.3) |
| Fourth-generation cephalosporins | 44 (30.3) |
| Imipenem | 145 (100) |
| Amoxicillin-clavulanate | 129 (88.9) |
| Piperacillin-tazobactam | 140 (96.5) |
| Cefoxitin | 142 (97.9) |
| Gentamicin | 95 (65.5) |
| Amikacin | 141 (97.2) |
| Any aminoside | 142 (97.9) |
| Ciprofloxacin | 62 (42.7) |
| Trimethoprim-sulfamethoxazole | 26 (17.9) |
| Any “classic” alternative | 78 (53.8) |
FIG 1.
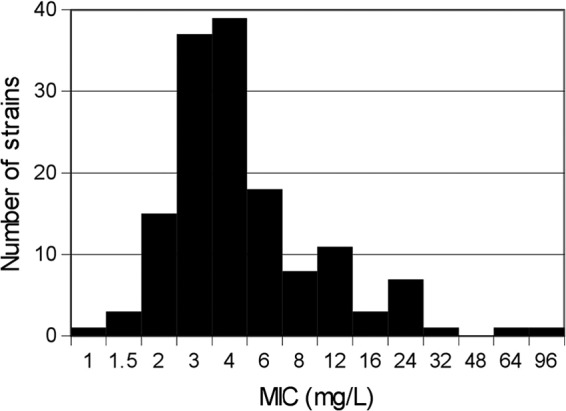
Distribution of cefoxitin MICs (n = 145).
The probabilities of target attainment (PTA) for a dose of 2 g 4 times/day, with respect to MIC value and infusion duration, are presented in Fig. 2.
FIG 2.
Probability of target attainment (PTA), depending on the MIC, for a dosage of 2 g 4 times/day and the following targets: T>MIC = 50% (A), T>MIC = 100% (B), T>4MIC = 50% (C), and T>4MIC = 100% (D).
For a target of T>MIC = 50% (Fig. 2A), 1 h of infusion enabled target attainment with a probability of ≥80% when MICs were ≤8 mg/liter. Four hours of infusion enabled target attainment with a 100% probability when MICs were ≤32 mg/liter.
The probability of reaching T>MIC = 100% (Fig. 2B) with a 1-h infusion was always lower than 50%, regardless of the MIC value. With a 4-h infusion, the PTA was ≥80% when MICs were ≤2 mg/liter. Continuous infusion allowed target attainment with a 100% probability for MICs of ≤32 mg/liter.
Next, for a target of T>4MIC = 50% (Fig. 2C), a 1-h infusion enabled target attainment with a probability of ≥80% for MICs of ≤2 mg/liter. A 4-h infusion allowed target attainment with a 100% probability when MICs were ≤32 mg/liter.
Last, the probabilities of reaching T>4MIC = 100% (Fig. 2D) did not exceed 80% with 1-h and 4-h infusion, regardless of the MIC value. Continuous infusion enabled target attainment with a 100% probability when MICs were ≤8 mg/liter.
The probabilities of achieving T>MIC = 50%, T>MIC = 100%, T>4MIC = 50%, and T>4MIC = 100% considering the distribution of cefoxitin MICs in our E. coli population are reported in Table 2.
TABLE 2.
Probability of pharmacological success
| Dosage | Duration of infusion | % of strains with pharmacological success by targeta |
|||
|---|---|---|---|---|---|
| T>MIC = 50% | T>MIC = 100% | T>4MIC = 50% | T>4MIC = 100% | ||
| 2 g 4 times/day | 1 h | 92 | 22 | 70 | 5.4 |
| 2 g 4 times/day | 4 h | 100 | 76 | 99 | 38 |
| 8 g/day | Continuous | 100 | 100 | 100 | 98.5 |
The probability of target attainment was calculated with all strains susceptible to cefoxitin according to antimicrobial susceptibility testing (n = 142).
Given its pharmacological properties, cefoxitin appears to be a good theoretical alternative to carbapenems in the treatment of ESBL-EC urinary tract infections (UTI). Indeed, unlike oxyimino cephalosporins, cefoxitin has shown to be stable against ESBL (3). Furthermore, cefoxitin is more rapidly bactericidal and less susceptible to inoculum effect than other beta-lactam antibiotics (12). Besides, from an epidemiological point of view, this drug also seems to be a reliable carbapenem-sparing antibiotic, since 83.4% of our strains were sensitive to cefoxitin. This finding is in agreement with recent data suggesting that 90% of ESBL-EC strains remained sensitive to cefoxitin (13).
To our knowledge, there are no randomized controlled trials assessing the clinical efficacy of cefoxitin in ESBL-PE pyelonephritis. However, two publications recently suggested that this antibiotic could indeed be an alternative to carbapenems in the treatment of ESBL-PE UTI. The first paper is a case report of a patient with bacteremia due to ESBL-producing Klebsiella pneumoniae (ESBL-KP) prostatitis who was successfully treated with cefoxitin (2 g 2 times/day for 3 weeks) and amikacin (6). In the second paper, similar in vitro bactericidal activities for cefoxitin and carbapenems were demonstrated in an animal model of ESBL-EC UTI (7).
Our work emphasizes the clinical benefits of continuous beta-lactam infusions, as this route of administration is the sole route in our model to reach T>MIC = 100% and T>4MIC = 100% with a close to 100% probability. Based on our results, we thus also recommend to avoid using 1-h infusion regimens and believe that 4-h infusions should be considered only in mild infections.
Our work has some limitations. First, it was performed in silico. Confirmatory clinical studies are thus mandatory. Second, its results cannot be generalized per se, since the distributions of MICs are not homogeneous among and even throughout countries (14). Last, a limitation to cefoxitin use is the threat of drug resistance emergence under treatment. Indeed, cefoxitin resistance secondary to loss of porin has already been described in vivo in ESBL-producing Klebsiella pneumoniae (ESBL-KP) (15, 16) and in vitro in ESBL-EC (17, 18).
To conclude, our study suggests that cephamycins could be used as an alternative to carbapenems in the treatment of ESBL-EC pyelonephritis. Constant infusion with a daily dose of 8 g is necessary within our range of MICs. For intermittent infusions, prior determination of the MIC is mandatory.
Footnotes
Published ahead of print 28 April 2014
REFERENCES
- 1.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin. Infect. Dis. 56:641-648. 10.1093/cid/cis942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686. 10.1128/CMR.18.4.657-686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prinapori R, Guinaud J, Khalil A, Lecuyer H, Gendrel D, Lortholary O, Nassif X, Viscoli C, Zahar JR. 2013. Risk associated with a systematic search of extended-spectrum beta-lactamase-producing Enterobacteriaceae. Am. J. Infect. Control 41:259–260. 10.1016/j.ajic.2012.03.035 [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyer M, Bignon A, Dessein R, Faure K, Guery B, Kipnis E. 2012. Cefoxitin and ESBL. Med. Mal. Infect. 42:126–128. 10.1016/j.medmal.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 7.Lepeule R, Ruppe E, Le P, Massias L, Chau F, Nucci A, Lefort A, Fantin B. 2012. Cefoxitin as an alternative to carbapenems in a murine model of urinary tract infection due to Escherichia coli harboring CTX-M-15-type extended-spectrum beta-lactamase. Antimicrob. Agents Chemother. 56:1376–1381. 10.1128/AAC.06233-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing. 2012. Breakpoint tables for interpretation of MICs and zone diameters. EUCAST, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing. 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. EUCAST, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf [Google Scholar]
- 10.Comité de l'Antibiogramme de la Société Française de Microbiologie. 2012. Recommandations 2012. Société Française de Microbiologie, Paris, France: http://www.sfm-microbiologie.org/ [Google Scholar]
- 11.Isla A, Troconiz IF, de Tejada IL, Vazquez S, Canut A, Lopez JM, Solinis MA, Rodriguez Gascon A. 2012. Population pharmacokinetics of prophylactic cefoxitin in patients undergoing colorectal surgery. Eur. J. Clin. Pharmacol. 68:735–745. 10.1007/s00228-011-1206-1 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein EJ, Citron DM, Cherubin CE. 1991. Comparison of the inoculum effects of members of the family Enterobacteriaceae on cefoxitin and other cephalosporins, beta-lactamase inhibitor combinations, and the penicillin-derived components of these combinations. Antimicrob. Agents Chemother. 35:560–566. 10.1128/AAC.35.3.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier D, Chirouze C, Leroy J, Cholley P, Talon D, Plesiat P, Bertrand X. 2013. Alternatives to carbapenems in ESBL-producing Escherichia coli infections. Med. Mal. Infect. 43:62–66. 10.1016/j.medmal.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933–951. 10.1128/CMR.14.4.933-951.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pangon B, Bizet C, Bure A, Pichon F, Philippon A, Regnier B, Gutmann L. 1989. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 beta-lactamase. J. Infect. Dis. 159:1005–1006. 10.1093/infdis/159.5.1005 [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Martinez L, Hernandez-Alles S, Alberti S, Tomas JM, Benedi VJ, Jacoby GA. 1996. In vivo selection of porin-deficient mutants of Klebsiella pneumoniae with increased resistance to cefoxitin and expanded-spectrum-cephalosporins. Antimicrob. Agents Chemother. 40:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananthan S, Subha A. 2005. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J. Med. Microbiol. 23:20–23. 10.4103/0255-0857.13867 [DOI] [PubMed] [Google Scholar]
- 18.Palasubramaniam S, Muniandy S, Navaratnam P. 2009. Resistance to extended-spectrum beta-lactams by the emergence of SHV-12 and the loss of OmpK35 in Klebsiella pneumoniae and Escherichia coli in Malaysia. J. Microbiol. Immunol. Infect. 42:129–133 [PubMed] [Google Scholar]



