Abstract
Stable resistance to metronidazole in a nontoxigenic Clostridium difficile strain was investigated at both the genomic and proteomic levels. Alterations in the metabolic pathway involving the pyruvate-ferredoxin oxidoreductase were found, suggesting that reduction of metronidazole, required for its activity, may be less efficient in this strain. Proteomic studies also showed a cellular response to oxidative stress.
TEXT
Clostridium difficile infection (CDI), the main cause of nosocomial infectious diarrhea, has increased in recent years (1). Metronidazole, a prodrug activated after the reduction of its nitro group (2), is recommended for the treatment of initial and moderate CDI cases.
A direct association between in vitro susceptibility to metronidazole and treatment efficacy is not always observed in CDI (3).
C. difficile strains showing resistance to metronidazole are rare, and laboratory manipulation of these strains frequently results in a MIC decrease toward the susceptibility range (4, 5). Therefore, the investigation of resistance mechanisms in C. difficile has proved difficult.
In other anaerobes, metronidazole resistance has been associated with drug inactivation by nim genes, altered activity of nitroreductases such as pyruvate-ferredoxin oxidoreductase (Pfo), a proteomic response to oxidative stress (2), and mutations of the ferric uptake regulator (fur) and oxygen-independent coproporphyrinogen III oxidase (hemN), as recently reported in a North American pulsed-field gel electrophoresis type 1 (NAP1) C. difficile strain (5).
In a previous study, we investigated metronidazole susceptibility in different C. difficile PCR ribotypes (6). In that study, the average MIC of metronidazole for PCR ribotype 010 strains was higher than that for widespread epidemic PCR ribotype strains, i.e., 7-fold compared to PCR ribotype 027 and 9-fold compared to PCR ribotype 001 or 078.
In the present study, C. difficile 7032989, a nontoxigenic strain with stable resistance to metronidazole, was analyzed by genome sequencing and quantitative proteomic analysis in comparison with C. difficile 7032985, a strain showing reduced susceptibility to metronidazole. Both strains were isolated in Spain and were typed as PCR ribotype 010 at the Cardiff Reference Centre (6). C. difficile 7032994, a metronidazole-susceptible strain also belonging to PCR ribotype 010, and C. difficile 630, a toxigenic reference strain (7), were used as controls in this study.
All C. difficile strains were stored at −80°C. Susceptibility assays were performed immediately after thawing and also after serial passages in medium without antibiotics by the agar dilution method (8). Antibiotic resistance was defined as a MIC of ≥32 μg/ml, according to the Clinical and Laboratory Standards Institute (7). The MICs were 0.25, 2, 4, and 32 μg/ml for C. difficile 630, 7032994, 7032985, and 7032989, respectively (6). PCR ribotype 010 strains were sequenced by Next-Generation Sequencing, 7032985 and 7032994 were sequenced via Illumina HiSeq, and 7032989 was sequenced via Roche 454. Contigs were assembled by using Velvet software (kmer 55 and 99; options, -short -fastq -unused_reads -read_trkg) (9) and reorganized on the basis of C. difficile reference strain 630 (7). Contigs that did not match the reference were placed at the end to obtain each genome scaffold. The genomes of strains 7032989, 7032985, and 7032994 are available in the MicroScope database with the links https://www.genoscope.cns.fr/agc/microscope/mage/viewer.php?S_id=2122, https://www.genoscope.cns.fr/agc/microscope/mage/viewer.php?S_id=2341, and https://www.genoscope.cns.fr/agc/microscope/mage/viewer.php?S_id=3220, respectively. Mauve Software (10) was used to compare the whole-genome sequences of PCR ribotype 010 strains 7032994, 7032985, and 7032989 with the available genome sequences of several C. difficile PCR ribotype strains (GenBank database accession numbers CAMY00000000, CAMJ00000000, AM180355.1, CAMS00000000, CAMH00000000, FN665652.1, CAMP00000000, FN545816.1, CAMM00000000, CAMC00000000, CAMB00000000, CAMR00000000, CAMZ00000000, CAMD00000000, CANC00000000, and CANB00000000 for PCR ribotypes 001, 005, 012, 014, 015, 017, 020, 027, 075, 078, 079, 095, 106, 126, 156, and 165, respectively).
The phylogenetic analysis of C. difficile strains 7032994, 7032985, and 7032989 in comparison with strains belonging to other PCR ribotypes showed that the three PCR ribotype 010 strains were closely related, with distances between the strains ranging from 0.02 to 0.06 (Fig. 1).
FIG 1.
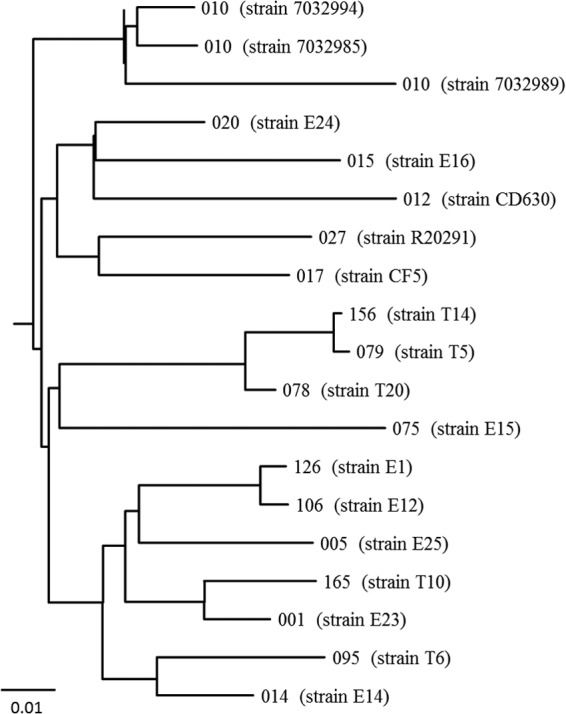
Phylogenetic tree showing relationships between C. difficile strains 7032994, 7032985, and 7032989, belonging to PCR ribotype 010, and other C. difficile PCR ribotypes. PCR ribotype 012 is represented by C. difficile reference strain 630. The GenBank database accession numbers of PCR ribotypes 001, 005, 012, 014, 015, 017, 020, 027, 075, 078, 079, 095, 106, 126, 156, and 165 are CAMY00000000, CAMJ00000000, AM180355.1, CAMS00000000, CAMH00000000, FN665652.1, CAMP00000000, FN545816.1, CAMM00000000, CAMC00000000, CAMB00000000, CAMR00000000, CAMZ00000000, CAMD00000000, CANC00000000, and CANB00000000, respectively.
DNA sequencing did not show the presence of the pathogenicity locus (PaLoc) in any of the PCR ribotype 010 strains investigated. Additionally, the genomic analysis did not show the presence of nim genes or deletions or mutations in the pfo, hemN, or fur gene in any of the strains (data not shown). Thus, quantitative proteomic experiments were undertaken.
Strains were grown in brucella agar (Oxoid) plates containing vitamin K1 (0.5 mg/liter), hemin (5 mg/liter), and 5% defibrinated sheep red blood cells. Proteins were extracted from cultures grown until mid-log phase (optical density at 600 nm of 0.5) in brain heart infusion broth (BHI; Oxoid) (11) without antibiotic or in BHI containing metronidazole at 0.1 μg/ml for C. difficile 630, 0.4 μg/ml for 7032994, 1 μg/ml for 7032985, and 10 μg/ml for 7032989. Extraction assays were performed in triplicate. Following centrifugation, pellets were washed with Tris-EDTA (pH 7.5) containing a protease inhibitor cocktail (Roche) and maintained at −80°C. Pellets were resuspended in Tris-EDTA, sonicated on ice, and cleared by centrifugation.
Following denaturation and reduction by 0.1% RapiGest (Waters) and 5 mM dithiothreitol, respectively, 50 μg of each extract was trypsin digested (Promega) overnight at 37°C. The digestion reaction was stopped with 0.1% formic acid, and peptide mixtures were desalted with Oasis cartridges (Waters). Label-free analysis was performed with a nanoACQUITY UPLC column coupled to a Synapt G2 mass spectrometer (Waters) by the Hi3 method (12). Data were processed with the ProteinLynx Global Server 2.5.2 software (Waters) for protein identification and quantification. Each extract was digested twice, and each digestion product was analyzed in triplicate by data-independent liquid chromatography-mass spectrometry. Significant protein level differences were established at >1.5-fold (13).
Although the amounts of 95% of the proteins were similar, some alterations were observed in both 7032989 and 7032985 before and after exposure to metronidazole (Table 1), suggesting a common cellular response. In particular, the DNA repair protein RecA increased following antibiotic pressure in both 7032989 and 7032985 but not in the control strains and the polyribonucleotide nucleotidyltransferase (PNP) protein was detected in both 7032989 and 7032985 only in the absence of antibiotic. PNP is associated with RNA processing and mRNA quality control (14). Alterations in transcription due to a decrease in this protein's activity could interfere with the response of strains 7032989 and 7032985 to metronidazole stress.
TABLE 1.
Protein quantification in C. difficile strains 630, 7032994, 7032985, and 7032989 before and after metronidazole exposure
| Proteina | Normalized valueb |
|||||||
|---|---|---|---|---|---|---|---|---|
| 630 |
7032994 |
7032985 |
7032989 |
|||||
| Beforec MZ | Afterd MZ | Before MZ | After MZ | Before MZ | After MZ | Before MZ | After MZ | |
| Pfo | 7.4 | 8.34 | 7.89 | 8.03 | 5.65 | 9.65 | 4.40 | 4.92 |
| Pnp | NDe | ND | 0.9 | 0.86 | 0.92 | ND | 0.89 | ND |
| Bcd | 0.27 | ND | 0.23 | 0.21 | 0.52 | 0.47 | 0.52 | 0.27 |
| CysS | ND | ND | 0.35 | 0.28 | 0.56 | ND | 0.56 | ND |
| SerS | ND | ND | ND | ND | 0.32 | ND | 0.43 | ND |
| FtnA | 0.13 | 0.13 | 0.15 | 0.16 | 0.19 | 0.22 | 0.22 | ND |
| RecA | ND | 0.14 | ND | 0.4 | 0.15 | 0.58 | 0.13 | 0.46 |
Pfo, pyruvate ferredoxin oxidoreductase; Pnp, polyribonucleotide nucleotidyltransferase; Bcd, butyryl coenzyme A dehydrogenase; CysS, cysteinyl-tRNA synthetase; SerS, seryl-tRNA synthetase 1; FtnA, ferritin; RecA, protein recombinase A.
Nanograms of protein/nanograms on column.
Before metronidazole (MZ) exposure.
After metronidazole (MZ) exposure.
ND, not detected.
In this study, the aminoacyl-tRNA proteins cysteinyl-tRNA synthetase (CysS) and seryl-tRNA synthetase (SerS) were not detected in strains 7032989 and 7032985 in the presence of antibiotic. On the contrary, in susceptible strain 7032994, CysS was observed under both conditions, together with tyrosyl-tRNA synthetase and threonyl-tRNA synthetase (data not shown). In other bacteria, a decrease in the concentration of aminoacyl-tRNAs has been associated with slower cell growth (15), a characteristic also reported for C. difficile colonies showing higher metronidazole MICs (4, 5). Impaired activity of aminoacyl-tRNAs in strains 7032989 and 7032985 could result in posttranslational variations of proteins relevant for metronidazole activation.
The protein ferritin was not detected in metronidazole-resistant strain 7032989 under antibiotic pressure, suggesting restriction of the storage of iron, an element relevant to the oxidation-reduction balance of bacterial cells (16). Under the same experimental conditions, a significant butyryl coenzyme A dehydrogenase (Bcd) concentration decrease was also observed. It has been proposed that a cytoplasmic complex formed by Bcd and electron transport flavoproteins could catalyze the reduction of ferredoxin in other Clostridium species. This process could result in a decreased amount of reduced ferredoxin in the metronidazole-resistant strain (17).
Interestingly, the Pfo concentration was stable in resistant strain 7032989 before and after metronidazole exposure (4.4 and 4.9 ng, respectively), whereas it increased significantly from 5.6 to 9.6 ng in strain 7032985 following antibiotic pressure. In C. difficile 630 and 7032994, the concentration of Pfo was significantly higher than in the metronidazole-resistant strain, both in the presence and in the absence of metronidazole. Gene expression of the locus including the pfo gene (18 genes in total) was investigated by real-time quantitative reverse transcription (qRT)-PCR in both 7032989 and 7032985. cDNA synthesis and qRT-PCR were performed as previously described (18). The qRT-PCR analysis did not show significant differences in the expression of the pfo gene or of surrounding genes in either strain before and after antibiotic exposure (data not shown), suggesting that the variations observed in C. difficile 7032985 could be posttranslational.
In other anaerobes, metronidazole activation has been related to higher enzymatic activity of electron carriers mediating the pyruvate-oxidizing pathway, such as Pfo (trichomonads), pyruvate dehydrogenase (C. perfringens), or flavodoxins and hydrogenases (C. acetobutylicum) (2). A recent proteomic study of a NAP1 C. difficile strain reported a reduction in iron uptake under antibiotic pressure, possibly because of mutations in the genes hemN and fur, which could result in a defect in the electron transport required to metronidazole activation (19). In this study, we did not observe variations in those genes and the production of relative products. Instead, we found that the Pfo concentration in resistant strain 7032989 remained stable at a low concentration, with or without antibiotic, so it could be hypothesized that the electron transfer required for metronidazole activation is less efficient in this strain than in the other strains investigated. These results also suggest that C. difficile strains resistant to metronidazole can show peculiar alterations in their enzymes or metabolic pathways.
The average MIC of metronidazole for the PCR ribotype 010 strains we have analyzed so far was higher than that for epidemic PCR ribotypes (6). According to the European Committee on Antimicrobial Susceptibility Testing epidemiological cutoff (MIC, >2 μg/ml) (20), the majority of those strains can be classified as having reduced susceptibility to metronidazole. PCR ribotype 010 strains are mostly nontoxigenic, but the possibility that they acquire the PaLoc in vivo cannot be excluded. Actually, it was recently shown that the PaLoc was successfully transferred to nontoxigenic isolates in vitro by genetic recombination (21).
In conclusion, our results indicate that a metabolic pathway associated with pfo expression is altered in stably metronidazole-resistant strain 7032989, opening new perspectives in the complete characterization of this intriguing and complex resistance mechanism in C. difficile.
Nucleotide sequence accession numbers.
The sequences for strains 7032989, 7032985, and 7032994 can be found under EMBL accession numbers PRJEB6600, PRJEB6601, and PRJEB6602, respectively.
ACKNOWLEDGMENTS
We thank Federico Giannoni and François Wasels for technical advice and support.
The research leading to these results received funding from the European Community's Seventh Framework Programme FP7/2007-2013 under grant agreement 2378942.
Footnotes
Published ahead of print 9 June 2014
REFERENCES
- 1.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. 2010. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 23:529–549. 10.1128/CMR.00082-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Land KM, Johnson PJ. 1999. Molecular basis of metronidazole resistance in pathogenic bacteria and protozoa. Drug Resist. Updat. 2:289–294. 10.1054/drup.1999.0104 [DOI] [PubMed] [Google Scholar]
- 3.Venugopal AA, Riederer K, Patel SM, Szpunar S, Jahamy H, Valenti S, Shemes SP, Khatib R, Johnson LB. 2012. Lack of association of outcomes with treatment duration and microbiologic susceptibility data in Clostridium difficile infections in a non-NAP1/BI/027 setting. Scand. J. Infect. Dis. 44:243–249. 10.3109/00365548.2011.631029 [DOI] [PubMed] [Google Scholar]
- 4.Peláez T, Cercenado E, Alcalá L, Marín M, Martín-López A, Martínez-Alarcón J, Catalán P, Sánchez-Somolinos M, Bouza E. 2008. Metronidazole resistance in Clostridium difficile is heterogeneous. J. Clin. Microbiol. 46:3028–3032. 10.1128/JCM.00524-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch T, Chong P, Zhang J, Hizon R, Du T, Graham MR, Beniac DR, Booth TF, Kibsey P, Miller M, Gravel D, Mulvey MR, Canadian Nosocomial Infection Surveillance Program (CNISP) 2013. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS One 8(1):e53757. 10.1371/journal.pone.0053757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moura I, Spigaglia P, Barbanti F, Mastrantonio P. 2013. Analysis of metronidazole susceptibility in different Clostridium difficile PCR ribotypes. J. Antimicrob. Chemother. 68:362–365. 10.1093/jac/dks420 [DOI] [PubMed] [Google Scholar]
- 7.Vallenet D, Engelen S, Mornico D, Cruveiller S, Fleury L, Lajus A, Rouy Z, Roche D, Salvignol G, Scarpelli C, Médigue C. 2009. MicroScope: a platform for microbial genome annotation and comparative genomics. Database (Oxford) 2009:bap021. 10.1093/database/bap021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th ed. Approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss, and rearrangement. PLoS One 5:e11147. 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S, Graham RL, McMullan G, Ternan NG. 2010. Proteomic analysis of the insoluble subproteome of Clostridium difficile strain 630. FEMS Microbiol. Lett. 312:151–159. 10.1111/j.1574-6968.2010.02111.x [DOI] [PubMed] [Google Scholar]
- 12.Silva JC, Denny R, Dorschel C, Gorenstein MV, Li GZ, Richardson K, Wall D, Geromanos SJ. 2006. Simultaneous qualitative and quantitative analysis of the Escherichia coli proteome: a sweet tale. Mol. Cell. Proteomics 5:589–607. 10.1074/mcp.M500321-MCP200 [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, VerBerkmoes NC, Langston MA, Uberbacher E, Hettich RL, Samatova NF. 2006. Detecting differential and correlated protein expression in label-free shotgun proteomics. J. Proteome Res. 5:2909–2918. 10.1021/pr0600273 [DOI] [PubMed] [Google Scholar]
- 14.Oussenko IA, Abe T, Ujiie H, Muto A, Bechhofer DH. 2005. Participation of 3′-to-5′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 187:2758–2767. 10.1128/JB.187.8.2758-2767.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurdle JG, O'Neill AJ, Chopra I. 2005. Prospects for aminoacyl-tRNA synthetase inhibitors as new antimicrobial agents. Antimicrob. Agents Chemother. 49:4821–4833. 10.1128/AAC.49.12.4821-4833.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandal A, Huggins CC, Woodhall MR, McHugh J, Rodríguez-Quiñones F, Quail MA, Guest JR, Andrews SC. 2010. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe(2+)-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol. Microbiol. 75:637–657. 10.1111/j.1365-2958.2009.06977.x [DOI] [PubMed] [Google Scholar]
- 17.Seedorf H, Fricke WF, Veith B, Brüggemann H, Liesegang H, Strittmatter A, Miethke M, Buckel W, Hinderberger J, Li F, Hagemeier C, Thauer RK, Gottschalk G. 2008. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. U. S. A. 105:2128–2133. 10.1073/pnas.0711093105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antunes A, Martin-Verstraete I, Dupuy B. 2011. CcpA-mediated repression of Clostridium difficile toxin gene expression. Mol. Microbiol. 79:882–899. 10.1111/j.1365-2958.2010.07495.x [DOI] [PubMed] [Google Scholar]
- 19.Chong PM, Lynch T, McCorrister S, Kibsey P, Miller M, Gravel D, Westmacott GR, Mulvey MR, the Canadian Nosocomial Infection Surveillance Program (CNISP) 2014. Proteomic analysis of a NAP1 Clostridium difficile clinical isolate resistant to metronidazole. PLoS One 9(1):e82622. 10.1371/journal.pone.0082622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing. 2013. Clinical breakpoint tables, version 1.3. European Committee on Antimicrobial Susceptibility Testing, London, United Kingdom: http://www.eucast.org/clinical_breakpoints/ Accessed 11 December 2013 [Google Scholar]
- 21.Brouwer MS, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. 2013. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat. Commun. 4:2601. 10.1038/ncomms3601 [DOI] [PMC free article] [PubMed] [Google Scholar]


