Abstract
The emergence and spread of New Delhi metallo-β-lactamase 1 (NDM-1)-producing carbapenem-resistant Enterobacteriaceae (CRE) present an urgent threat to human health. In China, the blaNDM-1 gene has been reported mostly in Acinetobacter spp. but is rarely found in Enterobacteriaceae. Here, we report a high incidence and endemic spread of NDM-1-producing CRE in Henan Province in China. Sixteen (33.3%) of the 48 CRE isolates obtained from patients during June 2011 to July 2012 were positive for blaNDM-1, and the gene was found to be carried on plasmids of various sizes (∼55 to ∼360 kb). These plasmids were readily transferrable to recipient Escherichia coli by conjugation, conferred resistance to multiple antibiotics, and belonged to multiple replicon types. The blaNDM-1-positive CRE isolates were genetically diverse, and six new multilocus sequence typing (MLST) sequence types were linked to the carriage of NDM-1. Five of the isolates were classified as extensively drug-resistant (XDR) isolates, four of which also carried the fosA3 gene conferring resistance to fosfomycin, an alternative drug for treating infections by CRE. In each blaNDM-1-positive CRE isolate, the blaNDM-1 gene was downstream of an intact ISAba125 element and upstream of the bleMBL gene. Furthermore, gene environment analysis suggested the possible transmission of blaNDM-1-containing sequences from Acinetobacter spp. to Klebsiella pneumoniae and Klebsiella oxytoca. These findings reveal the emergence and active transmission of NDM-1-positive CRE in China and underscore the need for heightened measures to control their further spread.
INTRODUCTION
Carbapenems are the last-resort antibiotics against drug-resistant Gram-negative bacterial pathogens (1). However, carbapenem-resistant Enterobacteriaceae (CRE) are increasingly reported in health care-associated infections (HAIs) and are considered an urgent threat to human health by the Centers for Disease Control in the United States (2). Multiple genes conferring carbapenem resistance have been reported, which encode various types of carbapenemases (1). New Delhi metallo-β-lactamase 1 (NDM-1), an Ambler class B metallo-β-lactamase (MBL), hydrolyzes all β-lactams (including carbapenems) but monobactams and was first identified in Klebsiella pneumoniae and Escherichia coli isolated from a Swedish patient who was hospitalized in India in 2008 (3). Subsequently, the wide distribution of NDM-1-producing Enterobacteriaceae in India, Pakistan, and the United Kingdom was reported (4), and the Indian subcontinent was considered the main reservoir of NDM-1 producers (5). To date, the NDM-1-producing pathogens have been reported in more than 40 countries and have become a significant threat for public health worldwide (6).
In China, the blaNDM-1 gene was first identified in four nonclonal Acinetobacter baumannii isolates (7). Subsequent studies found that Acinetobacter spp. were the main species carrying this gene, but the carriage of blaNDM-1 was at a low frequency (<1.5%) (7–9). Carbapenem resistance in Enterobacteriaceae in China has been mainly associated with Klebsiella pneumoniae carbapenemases (KPCs), such as KPC-2, and MBLs (such as IMP-4) other than NDM-1 (10, 11). NDM-1-mediated carbapenem resistance in Enterobacteriaceae in China has been rarely reported; so far there have been only two confirmed cases of NDM-1-producing K. pneumoniae and E. coli infections in China (12, 13). A recent study detected a high infection rate (14.8%) of NDM-1-producing bacteria in clinical fecal samples from multiple hospitals in China, but none of the NDM-1-carrying bacteria belonged to Enterobacteriaceae (14). These observations suggested that NDM-1 was not prevalent in CRE isolates from China. However, in this study, we demonstrated the high incidence and endemic spread of NDM-1-producing Enterobacteriaceae in Henan Province in China. Additionally, we conducted molecular characterization of the blaNDM-1-positive CRE isolates, revealing new molecular epidemiological features of CRE in China.
MATERIALS AND METHODS
Bacterial isolates, identification, and antimicrobial susceptibility testing.
A total of 48 CRE (imipenem [MIC, ≥4 μg/ml] or meropenem [MIC, ≥2 μg/ml]) isolates, including 6 E. coli, 33 K. pneumoniae, 1 Klebsiella oxytoca, 5 Enterobacter cloacae, and 3 Citrobacter freundii isolates, were collected from diagnostic laboratories in three hospitals located in the middle (Zhengzhou, n = 40), western (Sanmenxia, n = 4), and southern (Zhumadian, n = 4) regions of Henan Province (north-central China). These isolates were obtained from June 2011 to July 2012. All isolates were identified using the Vitek 2 system (bioMérieux, France) and 16S rRNA gene sequencing. Antimicrobial susceptibilities for the blaNDM-1-positive isolates and transconjugants were initially tested using the Vitek 2 system (bioMérieux, France) and then were followed by measuring the MIC using the broth microdilution method (for ampicillin-sulbactam, piperacillin-tazobactam, cefazolin, cefotetan, ceftazidime, cefepime, imipenem, ertapenem, ciprofloxacin, levofloxacin, gentamicin, amikacin, and aztreonam), the Etest (AB bioMérieux, Sweden) (for chloramphenicol, tetracycline, tigecycline, and colistin), and the agar dilution method (for fosfomycin), respectively. The standard microbroth and agar dilution methods were performed according to the guideline M07-A9 of the Clinical and Laboratory Standards Institute (CLSI) (15). Etests were conducted according to packet insert instructions using Mueller-Hinton agar (MHA). Fresh bacterial colonies taken directly from MHA plates that were incubated at 37°C for 16 to 20 h were resuspended in sterile Mueller-Hinton broth to obtain a suspension with McFarland standard 0.5 turbidity. E. coli ATCC 25922 was used as the quality control. The MIC results were interpreted according to the CLSI guideline M100-S22 (15). The 2013 European Committee on Antimicrobial Susceptibility Testing breakpoints were used (available at http://www.eucast.org/clinical_breakpoints/) for colistin and tigecycline.
Detection of resistance determinants.
The modified Hodge test and the imipenem-EDTA double-disk synergy test were performed according to the CLSI guidelines to detect carbapenemase activity (15). PCR and nucleotide sequencing were employed to screen for the presence of carbapenemase-encoding genes, including blaOXA-48-like, blaIMP, blaVIM, blaKPC, and blaNDM. In addition, extended-spectrum β-lactamase (ESBL) genes, plasmid-mediated AmpC genes, 16S rRNA methyltransferase genes, and fosfomycin resistance determinants were also detected by use of the methods described previously (16–20).
Bacterial genotyping.
Pulsed-field gel electrophoresis (PFGE) of XbaI-digested genomic DNA of blaNDM-1-positive CRE and reference marker Salmonella H9812 was performed using a contour-clamped homogeneous electric field (CHEF)-Mapper XA PFGE system (Bio-Rad, USA) with a 5- to 35-s linear ramp for 22 h at 6 V/cm at 14°C. The running buffer was 0.5× Tris-boric acid-EDTA (TBE) without thiourea. The gel image was captured using a Bio-Rad Universal Hood II gel imaging system (Bio-Rad, USA). The dendrograms were constructed from the PFGE data by the unweighted-pair group method with arithmetic mean (UPGMA) with the Dice coefficient using InfoQuest FP software version 4.5 (Bio-Rad Laboratories, USA). Multilocus sequence typing (MLST) for clinical K. pneumoniae and E. coli isolates was performed following the methods described previously (21, 22). The allelic profiles and sequence types (STs) were assigned using online databases (see http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html for K. pneumoniae and http://mlst.warwick.ac.uk/mlst/dbs/Ecoli for E. coli).
Conjugation and plasmid analysis.
Conjugative assays were performed according to the method described previously (23). The blaNDM-1-positive CRE served as the donors, while E. coli J53 (sodium azide resistant) was used as the recipient strain. Transconjugants were selected on Mueller-Hinton (MH) agar supplemented with sodium azide (100 μg/ml) and imipenem (1 μg/ml). The presence of the blaNDM-1 gene and other resistance genes in transconjugants were identified using PCR and DNA sequencing, and antimicrobial susceptibility was also determined.
S1-PFGE and Southern blotting were conducted to estimate sizes of blaNDM-1 plasmids (24). Briefly, whole-cell DNA of clinical and transconjugant strains in agarose gel plugs was treated with S1 nuclease (TaKaRa, Dalian, China), then separated by PFGE under the following conditions: 0.5× Tris-borate-EDTA, 1% agarose solution for 18 h at 6 V/cm and 14°C, with a pulse angle of 120° and the pulse time linearly ramped from 2.16 s to 63.8 s. Linear plasmids generated by S1-PFGE were transferred to nylon membranes (Millipore, USA), hybridized with digoxigenin-labeled blaNDM-1-specific probes, and detected using a nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) color detection kit (Roche Applied Sciences, Germany) according to the recommendations of the supplier. Plasmid replicons were determined using the PCR-based replicon typing method (25).
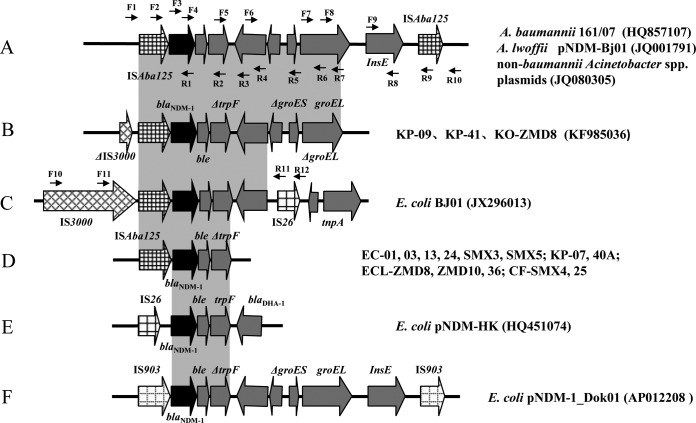
The genetic environment surrounding blaNDM-1 was investigated by PCR mapping and sequencing, and the Acinetobacter lwoffii plasmid of pNDM-BJ01 (accession no. JQ001791) and E. coli plasmid of pBJ01 (GenBank accession no. JX296013) were used as the references. PCR primers were designed from the reference sequences and are listed in Table S1 in the supplemental material. The locations of the primers are also shown (see Fig. 3).
FIG 3.
Comparison of the blaNDM-1 gene environments identified in this study with others published previously. (A) blaNDM-1-surrounding sequences in A. baumannii 161/07 (GenBank accession no. HQ857107), A. lwoffii pNDM-Bj01 (JQ001791), and non -baumannii Acinetobacter spp. plasmids (JQ080305). (B and D) the blaNDM-1 gene environments identified in this study; the names of the isolates are listed on the right of each panel.(C, E and F) blaNDM-1-surrounding sequences in E. coli BJ01 (JX296013), E. coli pNDM-HK (HQ451074), and E. coli pNDM-1_Dok01 (AP012208), respectively. The boxed arrows indicate the positions and directions of transcription of the genes. The gray-shaded areas represent regions sharing >99% DNA identity. Positions of the primers used for PCR mapping are indicated by arrows.
Nucleotide sequence accession number.
The sequence described in this paper has been submitted to GenBank under the accession no. KF985036 (K. oxytoca isolate ZMD8).
RESULTS AND DISCUSSION
Identification of blaNDM-1-positive isolates from hospital patients.
Among the 48 CRE, 16 (16/48 [33.3%]) were identified as blaNDM-1 positive, including 6 E. coli, 4 K. pneumoniae, 1 K. oxytoca, 3 E. cloacae, and 2 C. freundii isolates, which were obtained from specimens of blood (n = 7), urine (n = 6), and sputum (n = 2) and a burn site (n = 1) (Table 1). Additionally, 26 K. pneumoniae isolates harbored blaKPC-2, 2 isolates (1 K. pneumoniae and 1 E. cloacae) carried blaIMP-4, and the remaining isolates did not contain the carbapenemase genes (blaNDM, blaKPC, blaVIM, blaIMP, and blaOXA-48-like) screened in this study. The 16 blaNDM-1-positive CRE were from three hospitals in three different cities in Henan Province. The clinical data associated with the 16 isolates were summarized in Table 1. These patients were diagnosed with different clinical diseases, but none of the patients had a history of foreign travel. Notably, 5 patients, including 3 young children, died of infections. All of the patients who died, except an infant, had a positive blood culture for CRE and two of the patients had septicemia (Table 1). Thus, the death rate among the patients infected with a blaNDM-1-positive isolate was 31%, which is higher than previously reported rates in confirmed cases associated with NDM-1-producing bacteria worldwide (26). These results revealed the high prevalence of blaNDM-1-positive CRE in the hospitals of Henan Province and their association with a high mortality rate.
TABLE 1.
Characteristics of blaNDM-1-positive CRE isolates
| Isolatea | Clinical features |
Additional resistance determinantsb |
MLSTc | Plasmid type carrying blaNDM-1/plasmid size (kb) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patient age/sex | Specimen | Diagnosis/wardd | Outcome | β-Lactamases | 16S rRNA methylase | Others | |||
| EC-01 | 76 yr/female | Urine | Pyelonephritis complicated with diabetes/endocrinology | Discharge | − | − | − | ST1237 | Untypeable/230 |
| EC-03 | 58 yr/male | Blood | Severe acute pancreatitis/ICU | Death | TEM-1, CTX-M-55, CMY-30 | RmtB | FosA3 | ST361 | A/C/180 |
| EC-13 | 6 days/male | Blood | Neonatal sepsis/NICU | Discharge | TEM-1, CMY-30 | − | − | ST40 | FIB/310 |
| EC-24 | 56 yr/female | Urine | Diabetes and urinary tract infections/endocrinology | Discharge | CTX-M-15 | − | FosA3 | ST205 | A/C/230 |
| EC-SMX3 | 42 yr/male | Blood | Multiple injuries/EICU | Discharge | TEM-1, CTX-M-15, CMY-30 | − | − | ST410 | I1/60 |
| EC-SMX5 | 63 yr/female | Sputum | Lung cancer/oncology | Discharge | CTX-M-15, CMY-30 | − | − | ST361 | A/C/260 |
| KP-07 | 72 yr/female | Blood | Intracranial hemorrhage associated with cerebral infections/neurosurgery | Discharge | − | − | − | ST11 | Untypeable/55 |
| KP-09 | 1 yr/male | Urine | Multiple contusions as a result of a car accident/PICU | Discharge | TEM-1 | RmtB | − | ST889 | A/C/245 |
| KP-40A | 10 days/male | Blood | Neonatal sepsis/NICU | Death | TEM-1, CTX-M-15 | − | − | ST966 | A/C/−e |
| KP-41 | 9 mo/female | Blood | Septicemia/PICU | Death | − | − | − | ST113 | N/55 |
| KO-ZMD8 | 75 yr/male | Urine | Nephrotic syndrome/nephrology | Discharge | − | − | − | ND | Untypeable/55 |
| ECL- ZMD10 | 49 yr/male | Wound | Extensive burns/burn unit | Discharge | − | ArmA | FosA3 | ND | Untypeable/360 |
| ECL- ZMD12 | 21 yr/female | Blood | Severe aplastic anemia/hematology | Death | − | ArmA | − | ND | A/C/55 |
| ECL-36 | 15 days/male | Sputum | Lung infections and asphyxia/NICU | Death | MIR-2 | − | − | ND | A/C/160 |
| CF-SMX4 | 67 yr/male | Urine | Nephropathy/nephrology | Discharge | CMY-73 | − | − | ND | Untypeable/55 |
| CF-25 | 62 yr/male | Urine | Cerebral hemorrhage and lung infection/neurosurgery | Discharge | CMY-73 | ArmA | FosA3 | ND | A/C/170 |
EC, E. coli strains; KP, K. pneumoniae strains; KO, K. oxytoca strains; ECL, E. cloacae strains; CF, C. freundii strains.
Resistance markers that are cotransferred with blaNDM-1 by conjugation are underlined. Minus signs indicate negative results. PCR screening included blaTEM; blaSHV; blaOXA-1-like; blaCTX-M groups 1, 2, 8, 9, and 26; blaOXA-48-like; blaIMP; blaVIM; blaKPC; blaNDM; 6 groups of blaAmpC β-lactamase genes; and fosfomycin resistance genes fosA, fosB, fosC, and fosX as well as the armA, rmtA-rmtE, and npmA 16S methylase genes.
STs that are newly identified harboring blaNDM-1 in this study are underlined. MLST, multilocus sequence typing; ND, not determined.
ICU, intensive care unit; NICU, newborn ICU; EICU, emergency ICU; PICU, pediatric ICU.
S1-PFGE and Southern blotting failed to detect the blaNDM-1 plasmid in KP-40A and its transconjugant, although the blaNDM-1 gene was detected in them by PCR and sequencing.
Antimicrobial susceptibility patterns and resistance determinants.
All of the NDM-1-positive CRE isolates were resistant to carbapenems and cephalosporins but susceptible to colistin (MICs of ≤2 mg/liter) (Table 2). Molecular characterization showed that most of the E. coli (5/6) and half of the K. pneumoniae isolates (2/4) harbored ESBL genes, AmpC genes, or both (Table 1). Other carbapenemase-encoding genes, including blaKPC, blaVIM, blaIMP, and blaOXA-48-like, were not detected in any of the NDM-1-positive CRE isolates. Six isolates (EC-24, KP-07, KP-09, KP-40A, KP-41, and ZMD12) demonstrated tigecycline resistance according to the EUCAST clinical breakpoint, with MICs of ≥2 μg/ml (Table 2). More significantly, five isolates, including EC-03, CF-25, ECL-ZMD10 (susceptible only to tigecycline and colistin), EC-24 (susceptible only to amikacin and colistin), and ECL-ZMD12 (susceptible only to fosfomycin and colistin), were identified as extensively drug-resistant (XDR) bacteria (nonsusceptible to ≥1 agent in all of the 17 but ≤2 antimicrobial categories, including glycylcyclines and polymyxins used for treatment of infections caused by Enterobacteriaceae) according to the definitions described by Magiorakos et al. (27). In addition, four isolates (EC-03, EC-24, ECL-ZMD10, and CF-25) harbored a plasmid-mediated fosfomycin resistance gene, fosA3. This gene was found only in CTX-M-producing E. coli isolated from Asian countries (28), and our finding represents the first report of NDM-1-producing CRE harboring the fosA3 gene encoding fosfomycin resistance. Generally, the resistance rate of fosfomycin in E. coli was low (1% to 3%) worldwide, and fosfomycin is also used as an alternative choice for treating infections caused by ESBL-producing and even carbapenemase-producing Enterobacteriaceae (29). However, the occurrence of fosfomycin resistance in NDM-1-producing CRE observed in this study will further limit clinical therapeutic options.
TABLE 2.
Antibiotic susceptibilities of blaNDM-1-positive CRE and their transconjugants
| Isolatea | Antibioticb susceptibility (μg/ml) to: |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAM | TZP | CFZ | CTT | CAZ | FEP | IPM | ETP | CIP | LVX | GEN | AMK | SXT | ATM | CHL | TET | FOF | TGC | CST | |
| EC-01 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | 16 | 2 | 2 | 64 | 16 | 40 | 8 | 256 | 256 | 8 | 0.75 | 0.5 |
| EC-03 | >256 | >256 | >256 | >256 | >256 | 256 | >32 | 32 | >32 | >32 | >256 | >256 | >320 | 256 | 256 | 256 | 512 | 0.5 | 0.5 |
| EC-13 | >256 | >256 | >256 | >256 | >256 | >256 | 4 | 16 | 8 | 2 | 32 | 16 | >320 | 64 | 256 | 256 | 8 | 0.38 | 1 |
| EC-24 | >256 | >256 | >256 | >256 | >256 | >256 | >32 | 32 | >32 | >32 | 32 | 8 | >320 | 128 | 256 | 256 | 512 | 3 | 1 |
| EC-SMX3 | >256 | >256 | >256 | >256 | >256 | >256 | 4 | 8 | >32 | >32 | 64 | 128 | >320 | 256 | 4 | 2 | 32 | 0.5 | 2 |
| EC-SMX5 | >256 | >256 | >256 | >256 | >256 | >256 | 32 | 16 | >32 | >32 | 32 | <2 | >320 | 128 | 256 | 256 | 8 | 0.5 | 2 |
| KP-07 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | >32 | >32 | >32 | <1 | <2 | >320 | 256 | 128 | 2 | 8 | 3 | 1 |
| KP-09 | >256 | >256 | >256 | >256 | >256 | >256 | >32 | 4 | 1 | 1 | >256 | >256 | >320 | 32 | 256 | 256 | 32 | 3 | 1 |
| KP-40A | >256 | >256 | >256 | >256 | >256 | >256 | >32 | 16 | <0.25 | <0.25 | 64 | <2 | >320 | >256 | 256 | 256 | 32 | 6 | 1 |
| KP-41 | >256 | >256 | >256 | >256 | >256 | >256 | >32 | >32 | <0.25 | <0.25 | <1 | <2 | >320 | <1 | 6 | 2 | 8 | 3 | 0.5 |
| KO-ZMD8 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | 16 | <0.25 | 0.5 | 32 | <2 | >320 | 128 | 256 | 256 | 32 | 0.5 | 0.5 |
| ECL-ZMD10 | ND | 64 | ND | ND | >256 | >256 | 8 | 32 | >32 | >32 | >256 | >256 | >320 | 256 | 256 | 256 | 128 | 1 | 1 |
| ECL-ZMD12 | ND | >256 | ND | ND | >256 | >256 | 8 | 32 | >32 | >32 | >256 | >256 | >320 | >256 | 256 | 256 | 32 | 3 | 2 |
| ECL-36 | ND | >256 | ND | ND | >256 | >256 | 32 | >32 | <0.25 | <0.25 | 32 | <2 | >320 | 256 | 8 | 4 | 8 | 2 | 1 |
| CF-SMX4 | ND | >256 | ND | ND | >256 | >256 | >32 | >32 | 8 | 4 | 16 | <2 | <20 | 256 | 6 | 128 | 8 | 1 | 1 |
| CF-25 | ND | >256 | ND | ND | >256 | >256 | >32 | >32 | >32 | >32 | >256 | >256 | >320 | 64 | 48 | 256 | 512 | 0.75 | 0.5 |
| E. coli transconjugant strains | |||||||||||||||||||
| EC-01-J53 | >256 | 64 | >256 | >256 | >256 | 32 | 8 | 16 | <0.25 | <0.25 | 32 | <2 | <20 | 4 | 256 | 256 | 8 | 1 | 0.5 |
| EC-03-J53 | >256 | 32 | >256 | >256 | >256 | 8 | >32 | 8 | <0.25 | <0.25 | >256 | >256 | >320 | 128 | 256 | 2 | 512 | 0.19 | 1 |
| EC-13-J53 | >256 | 64 | >256 | 32 | >256 | 4 | 4 | 4 | <0.25 | <0.25 | 16 | <2 | >320 | 32 | 128 | 128 | 8 | 0.25 | 1 |
| EC-24-J53 | >256 | >256 | >256 | >256 | >256 | >256 | 32 | 16 | <0.25 | <0.25 | 8 | <2 | <20 | 2 | 256 | 256 | 8 | 0.19 | 1 |
| EC-SMX3-J53 | >256 | >256 | >256 | >256 | >256 | >256 | 4 | 8 | 8 | 16 | 32 | 32 | >320 | 64 | 4 | 0.75 | 8 | 0.19 | 1 |
| EC-SMX5-J53 | >256 | 64 | >256 | >256 | >256 | 16 | 32 | 16 | <0.25 | <0.25 | 32 | <2 | <20 | <1 | 256 | 8 | 16 | 1 | 0.5 |
| KP-07-J53 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | 16 | 8 | 16 | <1 | <2 | >320 | 128 | 24 | 2 | 8 | 0.5 | 1 |
| KP-09-J53 | >256 | 64 | >256 | 4 | >256 | 2 | 4 | 4 | <0.25 | <0.25 | <1 | <2 | <20 | <1 | 8 | 256 | 32 | 2 | 1 |
| KP-40A-J53 | >256 | >256 | >256 | 32 | >256 | 8 | 8 | 4 | <0.25 | <0.25 | 32 | <2 | >320 | 128 | 256 | 256 | 8 | 1 | 1 |
| KP-41-J53 | >256 | >256 | >256 | >256 | >256 | 8 | 8 | 16 | <0.25 | <0.25 | <1 | <2 | 40 | <1 | 4 | 2 | 8 | 0.75 | 1 |
| KO-ZMD8-J53 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | 16 | <0.25 | <0.25 | 8 | <2 | >320 | 128 | 256 | 256 | 16 | 0.75 | 1 |
| ECL-ZMD10-J53 | >256 | 64 | >256 | >256 | >256 | >256 | 4 | 16 | 32 | 16 | >256 | >256 | >320 | 128 | 256 | 256 | 128 | 1.5 | 0.5 |
| ECL-ZMD12-J53 | >256 | >256 | >256 | >256 | >256 | >256 | 8 | 32 | 32 | 32 | >256 | 16 | <20 | >256 | 256 | 256 | 32 | 3 | 1 |
| ECL-36-J53 | >256 | 64 | >256 | >256 | >256 | >256 | 32 | >32 | <0.25 | <0.25 | 32 | <2 | >320 | <1 | 8 | 8 | 8 | 1.5 | 1 |
| CF-SMX4-J53 | >256 | 64 | >256 | 32 | >256 | >256 | 8 | 16 | <0.25 | <0.25 | 2 | <2 | <20 | 2 | 4 | 128 | 8 | 1 | 1 |
| CF-25-J53 | >256 | 64 | >256 | 16 | >256 | 2 | 8 | 4 | <0.25 | <0.25 | 32 | <2 | <20 | 4 | 48 | 128 | 128 | 0.25 | 1 |
| EC J53 | <2 | <4 | <4 | <4 | <1 | <1 | <1 | <0.5 | <0.25 | <0.25 | <1 | <2 | <20 | <1 | 8 | 2 | 2 | 0.25 | 0.5 |
All of the blaNDM-1-positive isolates were multidrug-resistant (MDR) strains, and the XDR isolates are highlighted in bold type. EC, E. coli strains; KP, K. pneumoniae strains; KO, K. oxytoca strains; ECL, E. cloacae strains; CF, C. freundii strains. For the transconjugants, all were E. coli J53 harboring plasmids from the respective clinical isolates.
SAM, ampicillin-sulbactam (1/0.5–256/128) (the numbers in parentheses indicate the test range [μg/ml] for each agent); TZP, piperacillin-tazobactam (0.5/4–256/4); CFZ, cefazolin (0.5–256); CTT, cefotetan (0.03–256); CAZ, ceftazidime (0.03–256); FEP, cefepime (0.015–256); IPM, imipenem (0.06–32); ETP, ertapenem (0.004–32); CIP, ciprofloxacin (0.004–32); LVX, levofloxacin (0.008–32); GEN, gentamicin (0.25–256); AMK, amikacin (0.5–256); ATM, aztreonam (0.06–256); CHL, chloramphenicol (0.016–256); TET, tetracycline (0.016–256); FOF, fosfomycin (0.25–512); TGC, tigecycline (0.016–256); CST, colistin (0.016–256). The MICs of trimethoprim-sulfamethoxazole (SXT) were obtained by the Vitek 2 system. ND, not determined (E. cloacae and C. freundii are intrinsically resistant to SAM, CFZ, and CTT).
Genotyping.
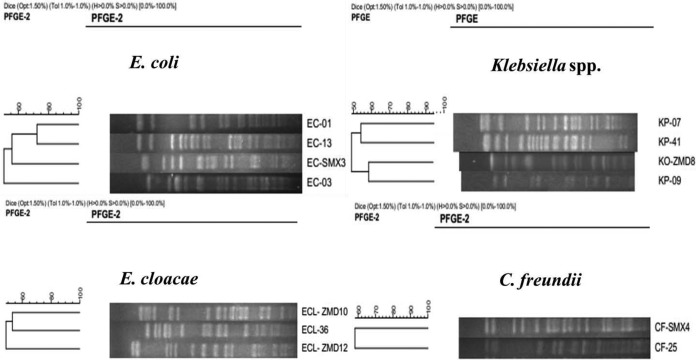
PFGE was successfully performed with 4 E. coli, 3 K. pneumoniae, 1 K. oxytoca, 3 E. cloacae, and 2 C. freundii isolates, and their patterns were completely different (Fig. 1). Three isolates, including EC-24, EC-SMX5, and KP-40A, were nontypeable by PFGE, as their XbaI-digested genomic DNA failed to yield distinct bands despite multiple efforts to repeat the experiment. MLST was performed for all of the blaNDM-1-positive E. coli and K. pneumoniae isolates, since the two species of Enterobacteriaceae were the major ones carrying the blaNDM-1 gene. Five MLST types were identified among the six E. coli isolates. Similarly, four MLST types were identified among the four K. pneumoniae isolates (Table 1). Two E. coli isolates (EC-03 and EC-SMX5) obtained from two different hospitals located in geographically separated areas (Zhengzhou and Sanmenxia) shared the same ST type (ST361), suggesting they were clonally related. Overall, our data showed that clonally diverse NDM-1-producing Enterobacteriaceae contributed to the dissemination of blaNDM-1in Henan Province.
FIG 1.
PFGE-based dendrograms showing the genetic relationships of 13 blaNDM-1-positive CRE isolates. EC-24, EC-SMX5, and KP-40A failed to yield bands by XbaI-PFGE and were not included.
This study linked for the first time six new STs (ST40, ST205, and ST1237 [E. coli] and ST113, ST889, and ST966 [K. pneumoniae]) to the production of NDM-1. E. coli ST410 and K. pneumoniae ST11 were frequently detected in clinical isolates of NDM-1-producing E. coli and K. pneumoniae from different countries, suggesting that they play an important role in the dissemination of blaNDM-1 (30). In addition, the NDM-1-producing E. coli ST361 isolate was also found in New Zealand (31).
Plasmid analysis.
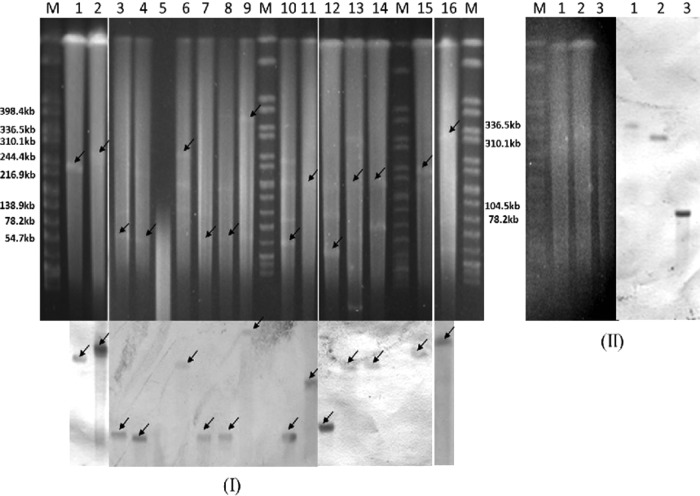
S1-PFGE and Southern blot analysis showed that the presence of the blaNDM-1 gene in the 16 CRE isolates was located on diverse plasmids, with sizes ranging from ∼55 to ∼360 kb. Furthermore, half of the blaNDM-1 plasmids belonged to plasmid replicon type IncA/C (Table 1 and Fig. 2). This broad-host-range plasmid was most frequently reported for carrying blaNDM-1 and other resistance genes, and it is widely disseminated among Gram-negative bacteria worldwide (6, 32).
FIG 2.
Detection of blaNDM-1- (I) and fosA3- (II) carrying plasmids by PFGE and Southern hybridization. (I) S1-PFGE (top) and Southern blotting (bottom) with blaNDM-1 specific probe. Lanes M, marker (Salmonella H9812); lane 1, EC-01-J53; lane 2, EC-SMX5-J53; lane 3, EC-SMX3; lane 4, KP-41; lane 5, KP-40A (untypeable); lane 6, KP-09; lane 7, KP-07; lane 8, KO-ZMD8; lane 9, ECL-ZMD10; lane 10, ECL-ZMD12; lane 11, ECL-36; lane 12, CF-SMX4; lane 13,CF-25; lane 14, EC-03-J53; lane 15, EC-24-J53; lane 16, EC-13. The arrows indicate the locations of the plasmids hybridized to the NDM-1 probe. (II) S1-PFGE (left) and Southern blotting (right) with fosA3-specific probe. Lane M, marker (Salmonella H9812); lanes 1, ECL-ZMD10-J53; lanes 2, CF-25-J53; lanes 3, EC-03-J53.
Conjugative assays revealed that all of the blaNDM-1 plasmids were successfully transferred to E. coli J53 from the 16 donors by conjugation. The 16 transconjugants all showed resistance to carbapenems and cephalosporins (Table 2). Moreover, the blaNDM-1 plasmids in all the transconjugants remained stable after 10 passages in the absence of imipenem selection. In addition, resistance genes such as blaTEM-1, blaCTX-M-15/55, blaCMY-30, rmtB, armA, and fosA3 were cotransferred to E. coli J53 with the blaNDM-1 gene in several isolates (Table 2). Interestingly, we found that the blaNDM-1 and fosA3 genes were carried by distinct IncA/C plasmids in EC-03 (∼180 kb for blaNDM-1 and ∼90 kb for fosA3) and CF-25(∼170 kb for blaNDM-1 and ∼310 kb for fosA3) (Fig. 2), but the two plasmids in each isolate were cotransferred to the E. coli J53 recipient, suggesting that both were mobile or one of them acted as a helper plasmid for the other to transfer. In contrast to the above two isolates, ECL-ZMD10 harbored a large plasmid (∼360 kb) of an untypeable replicon type, which carried both blaNDM-1 and fosA3 and was transferred to E. coli J53 by conjugation (Fig. 2 and Table 2). Several recent studies demonstrated that the fosA3 gene was associated with blaCTX-M genes and carried by plasmids belonging to different replicon types, including F, N, B/O, and I1 in E. coli and K. pneumonia (33–35). In our study, this gene was found to be carried by a conjugative blaNDM-1 plasmid in a species (E. cloacae) other than E. coli and K. pneumonia, implying the further spread of fosA3 among Enterobacteriaceae. Moreover, the E. coli J53 recipient became an XDR strain after accepting the ECL-ZMD10 plasmid by conjugation, indicating multiple resistance determinants might be carried by this blaNDM-1 plasmid. Additional studies are ongoing to characterize this plasmid. In vivo interspecies dissemination of IncA/C plasmids carrying blaNDM-1was described in previous studies (23, 36). However, all of the NDM-1 plasmids identified in the 16 blaNDM-1-positive CRE isolates were different either in size or replicon type, suggesting that insertion elements or transposons may play important roles in the mobilization of blaNDM-1.
Genetic environments of blaNDM-1.
The genetic environments surrounding the blaNDM-1 gene were detected by PCR mapping and DNA sequencing. The bleomycin resistance gene bleMBL, followed by a truncated trpF gene, was located immediately downstream of the blaNDM-1 gene in all of the 16 CRE isolates (Fig. 3). The three-gene cluster (blaNDM-1-bleMBL-ΔtrpF) was highly conserved and was also previously reported in E. coli plasmids (pNDM-HK, pBJ01, and pNDM_Dok01) and A. lwoffii plasmids (pNDM-BJ01 and pNDM-BJ02) (37) (Fig. 3). It was reported that blaNDM-1 and bleMBL are under the control of the same promoter in front of blaNDM-1, and this structure may facilitate the spread of NDM-1 when under the selective pressure of bleomycin-like molecules in the environment (38). The entire ISAba125 element was detected immediately upstream of the blaNDM-1 gene in each of the 16 isolates. It has been documented that ISAba125 provides the −35 region of the promoter sequence for blaNDM-1 in all reported cases (37). For two K. pneumonia isolates (KP-09 and KP-41) and one K. oxytoca isolate (KO-ZMD8), a 5,859-bp DNA segment was sequenced and it contained eight genes, including ISAba125, blaNDM-1, bleMBL, ΔtrpF, dsbC, cutA1, groES, and groEL (Fig. 3). This DNA segment is highly homologous (>99%) to previously identified sequences in Acinetobacter spp. from China (9, 39) (Fig. 3), suggesting the possible transmission of blaNDM-1-containing sequences between Acinetobacter spp. and Enterobacteriaceae. Considering that blaNDM-1 was mostly reported in Acinetobacter spp. in China, our finding suggests that Acinetobacter may have served as a reservoir for the dissemination of NDM-1 toward Enterobacteriaceae.
The sporadic emergence of NDM-1-producing E. coli and K. pneumoniae in mainland China has been reported in two recent studies (12, 13). Additionally, several cases of NDM-1-producing CRE reported from Hong Kong and Taiwan were also epidemiologically linked to mainland China (40–42). Here, we report our study of a cluster of blaNDM-1-positive CRE from patients in Henan Province. Together, these findings strongly suggest that NDM-1-producing CRE is spreading in mainland China. Compared to previously reported studies, our work revealed several important findings on CRE in China. First, a high incidence (33.3%) of NDM-1-producing Enterobacteriaceae was observed among the examined CRE, which is much higher than previously realized and suggests that blaNDM-1-positive CRE is endemic in Henan Province. Second, none of the patients carrying NDM-1-producing CRE had a history of foreign travel, suggesting that the NDM-1-carrying CRE were endogenous to the region and likely originated locally due to antibiotic selection and spread of the resistance gene. Third, most of the NDM-1-producing E. coli and K. pneumoniae isolates identified in this study belong to new MLST sequence types that have not been reported previously, suggesting the active spread of blaNDM-1 among Enterobacteriaceae in China. These findings provide new insights into the epidemiology of CRE in China.
In summary, our study demonstrated a high incidence of NDM-1-producing CRE from patients with different clinical diseases in Henan Province. These NDM-1-positive isolates were genetically diverse and carried plasmids of various sizes that were readily transferred by conjugation. Our results suggest that mainland China is becoming an endemic reservoir for NDM-1-producing Enterobacteriaceae. In addition, the emergence of XDR Enterobacteriaceae carrying conjugative blaNDM-1 plasmids coharboring other resistance determinants such as fosA3 is alarming, as spread of these isolates will seriously limit options for clinical treatment in future. Thus, enhanced efforts are urgently needed to control the further spread of NDM-1-producing Enterobacteriaceae.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 81172937), the Scientific and Technologic Development Program of Henan Province (grant no. 112102310165), and the Medical Science and Technique Foundation of Henan Province (grant no. 201303046).
We thank the two hospitals in Zhumadian and Sanmenxia for providing the CRE isolates examined in this study.
Footnotes
Published ahead of print 28 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02813-13.
REFERENCES
- 1.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/ [Google Scholar]
- 3.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moellering RC., Jr 2010. NDM-1—a cause for worldwide concern. N. Engl. J. Med. 363:2377–2379. 10.1056/NEJMp1011715 [DOI] [PubMed] [Google Scholar]
- 6.Johnson AP, Woodford N. 2013. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol. 62:499–513. 10.1099/jmm.0.052555-0 [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Zhou Z, Jiang Y, Yu Y. 2011. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Antimicrob. Chemother. 66:1255–1259. 10.1093/jac/dkr082 [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Chen Y, Jia X, Luo Y, Song Q, Zhao W, Wang Y, Liu H, Zheng D, Xia Y, Yu R, Han X, Jiang G, Zhou Y, Zhou W, Hu X, Liang L, Han L. 2012. Dissemination and characterization of NDM-1-producing Acinetobacter pittii in an intensive care unit in China. Clin. Microbiol. Infect. 18:E506–E513. 10.1111/1469-0691.12035 [DOI] [PubMed] [Google Scholar]
- 9.Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. 2012. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J. Antimicrob. Chemother. 67:2114–2122. 10.1093/jac/dks192 [DOI] [PubMed] [Google Scholar]
- 10.Yu F, Ying Q, Chen C, Li T, Ding B, Liu Y, Lu Y, Qin Z, Parsons C, Salgado C, Qu D, Pan J, Wang L. 2012. Outbreak of pulmonary infection caused by Klebsiella pneumoniae isolates harbouring blaIMP-4 and blaDHA-1 in a neonatal intensive care unit in China. J. Med. Microbiol. 61:984–989. 10.1099/jmm.0.043000-0 [DOI] [PubMed] [Google Scholar]
- 11.Wei Z, Yu T, Qi Y, Ji S, Shen P, Yu Y, Chen Y. 2011. Coexistence of plasmid-mediated KPC-2 and IMP-4 carbapenemases in isolates of Klebsiella pneumoniae from China. J. Antimicrob. Chemother. 66:2670–2671. 10.1093/jac/dkr330 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Li W, Wang J, Pan J, Sun S, Yu Y, Zhao B, Ma Y, Zhang T, Qi J, Liu G, Lu F. 2013. Identification and characterization of the first Escherichia coli strain carrying NDM-1 gene in China. PLoS One 8:e66666. 10.1371/journal.pone.0066666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Zhong Q, Tu J, Xu Y, Qin Z, Parsons C, Zhang B, Hu X, Wang L, Yu F, Pan J. 2013. Emergence of blaNDM-1 among Klebsiella pneumoniae ST15 and novel ST1031 clinical isolates in China. Diagn. Microbiol. Infect. Dis. 75:373–376. 10.1016/j.diagmicrobio.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Liu W, Zou D, Li X, Wei X, Shang W, Wang Y, Li H, Huan Li YW, He X, Huang L, Yuan J. 2013. High rate of New Delhi metallo-β-lactamase 1-producing bacterial infection in China. Clin. Infect. Dis. 56:161–162. 10.1093/cid/cis782 [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 16.Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88-94. 10.1086/518605 [DOI] [PubMed] [Google Scholar]
- 17.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J. Clin. Microbiol. 50:3877–3880. 10.1128/JCM.02117-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leflon-Guibout V, Jurand C, Bonacorsi S, Espinasse F, Guelfi MC, Duportail F, Heym B, Bingen E, Nicolas-Chanoine MH. 2004. Emergence and spread of three clonally related virulent isolates of CTX-M-15-producing Escherichia coli with variable resistance to aminoglycosides and tetracycline in a French geriatric hospital. Antimicrob. Agents Chemother. 48:3736–3742. 10.1128/AAC.48.10.3736-3742.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162. 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 57:154–155. 10.1093/jac/dki412 [DOI] [PubMed] [Google Scholar]
- 21.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgia S, Lastovetska O, Richardson D, Eshaghi A, Xiong J, Chung C, Baqi M, McGeer A, Ricci G, Sawicki R, Pantelidis R, Low DE, Patel SN, Melano RG. 2012. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin. Infect. Dis. 55:e109–e117. 10.1093/cid/cis737 [DOI] [PubMed] [Google Scholar]
- 24.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235–240. 10.1006/abio.1995.1220 [DOI] [PubMed] [Google Scholar]
- 25.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 26.Bushnell G, Mitrani-Gold F, Mundy LM. 2013. Emergence of New Delhi metallo-β-lactamase type 1-producing Enterobacteriaceae and non-Enterobacteriaceae: global case detection and bacterial surveillance. Int. J. Infect. Dis. 17:e325–e333. 10.1016/j.ijid.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 27.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18:268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 28.Hou J, Yang X, Zeng Z, Lv L, Yang T, Lin D, Liu JH. 2013. Detection of the plasmid-encoded fosfomycin resistance gene fosA3 in Escherichia coli of food-animal origin. J. Antimicrob. Chemother. 68:766–770. 10.1093/jac/dks465 [DOI] [PubMed] [Google Scholar]
- 29.Raz R. 2012. Fosfomycin: an old-new antibiotic. Clin. Microbiol. Infect. 18:4–7. 10.1111/j.1469-0691.2011.03636.x [DOI] [PubMed] [Google Scholar]
- 30.Poirel L, Dortet L, Bernabeu S, Nordmann P. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 55:5403–5407. 10.1128/AAC.00585-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson DA, Sidjabat HE, Freeman JT, Roberts SA, Silvey A, Woodhouse R, Mowat E, Dyet K, Paterson DL, Blackmore T, Burns A, Heffernan H. 2012. Identification and molecular characterisation of New Delhi metallo-β-lactamase-1 (NDM-1)- and NDM-6-producing Enterobacteriaceae from New Zealand hospitals. Int. J. Antimicrob. Agents 39:529–533. 10.1016/j.ijantimicag.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 32.Johnson TJ, Lang KS. 2012. IncA/C plasmids: an emerging threat to human and animal health? Mob. Genet. Elements 2:55–58. 10.4161/mge.19626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho PL, Chan J, Lo WU, Lai EL, Cheung YY, Lau TC, Chow KH. 2013. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J. Med. Microbiol. 62(Pt 11):1707–1713. 10.1099/jmm.0.062653-0 [DOI] [PubMed] [Google Scholar]
- 34.Ho PL, Chan J, Lo WU, Law PY, Li Z, Lai EL, Chow KH. 2013. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant Escherichia coli from livestock and other animals. J. Appl. Microbiol. 114:695–702. 10.1111/jam.12099 [DOI] [PubMed] [Google Scholar]
- 35.Lee SY, Park YJ, Yu JK, Jung S, Kim Y, Jeong SH, Arakawa Y. 2012. Prevalence of acquired fosfomycin resistance among extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae clinical isolates in Korea and IS26-composite transposon surrounding fosA3. J. Antimicrob. Chemother. 67:2843–2847. 10.1093/jac/dks319 [DOI] [PubMed] [Google Scholar]
- 36.Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. 2011. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 17:103–106. 10.3201/eid1701.101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge SR, Iredell JR. 2012. Genetic contexts of blaNDM-1. Antimicrob. Agents Chemother. 56:6065–6067. 10.1128/AAC.00117-12 (Reply, 56: 6071. ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dortet L, Nordmann P, Poirel L. 2012. Association of the emerging carbapenemase NDM-1 with a bleomycin resistance protein in Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 56:1693–1697. 10.1128/AAC.05583-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu H, Hu Y, Pan Y, Liang H, Wang H, Wang X, Hao Q, Yang X, Xiao X, Luan C, Yang Y, Cui Y, Yang R, Gao GF, Song Y, Zhu B. 2012. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob. Agents Chemother. 56:1698–1702. 10.1128/AAC.06199-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho P-L, Li Z, Lo W-U, Cheung Y-Y, Lin C-H, Sham P-C, Chi-Chung Cheng V, Ng T-K, Que T-L, Chow K-H. 2012. Identification and characterization of a novel incompatibility group X3 plasmid-carrying blaNDM-1 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. EMI 1:e39. 10.1038/emi.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho PL, Li Z, Lai EL, Chiu SS, Cheng VC. 2012. Emergence of NDM-1-producing Enterobacteriaceae in China. J. Antimicrob. Chemother. 67:1553–1555. 10.1093/jac/dks095 [DOI] [PubMed] [Google Scholar]
- 42.Lai CC, Lin TL, Tseng SP, Huang YT, Wang JT, Chang SC, Teng LJ, Hsueh PR. 2011. Pelvic abscess caused by New Delhi metallo-β-lactamase-1-producing Klebsiella oxytoca in Taiwan in a patient who underwent renal transplantation in China. Diagn. Microbiol. Infect. Dis. 71:474–475. 10.1016/j.diagmicrobio.2011.09.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.