Abstract
The development of topical anti-human immunodeficiency virus (HIV) microbicides may provide women with strategies to protect themselves against sexual HIV transmission. Pericoital drug delivery systems intended for use immediately before sex, such as microbicide gels, must deliver high drug doses for maximal effectiveness. The goal of achieving a high antiretroviral dose is complicated by the need to simultaneously retain the dose and quickly release drug compounds into the tissue. For drugs with limited solubility in vaginal gels, increasing the gel volume to increase the dose can result in leakage. While solid dosage forms like films and tablets increase retention, they often require more than 15 min to fully dissolve, potentially increasing the risk of inducing epithelial abrasions during sex. Here, we demonstrate that water-soluble electrospun fibers, with their high surface area-to-volume ratio and ability to disperse antiretrovirals, can serve as an alternative solid dosage form for microbicides requiring both high drug loading and rapid hydration. We formulated maraviroc at up to 28 wt% into electrospun solid dispersions made from either polyvinylpyrrolidone or poly(ethylene oxide) nanofibers or microfibers and investigated the role of drug loading, distribution, and crystallinity in determining drug release rates into aqueous media. We show here that water-soluble electrospun materials can rapidly release maraviroc upon contact with moisture and that drug delivery is faster (less than 6 min under sink conditions) when maraviroc is electrospun in polyvinylpyrrolidone fibers containing an excipient wetting agent. These materials offer an alternative dosage form to current pericoital microbicides.
INTRODUCTION
The development of more effective topical anti-human immunodeficiency virus (HIV) microbicides might provide women with products that reduce the risk of HIV-1 transmission during heterosexual intercourse. Recent clinical trials of anti-HIV microbicides and oral preexposure prophylaxis (PREP) have demonstrated that prophylactic delivery of small-molecule anti-HIV compounds can significantly reduce HIV acquisition rates and that adherence to microbicide use is correlated with protective outcomes (1–5). Current lead delivery systems for microbicides include intravaginal rings, gels, tablets, and films. While rings provide the advantage of long-term delivery, they may not be most appropriate for use during infrequent sex acts. Tablets and films, while they can be used immediately before sex, often require 30 min to dissolve fully before the start of intercourse. Moreover, microbicide films reported in literature have low drug loadings (less than 1.5 wt%) (6, 7), which when coupled with their small dosage size limits their application to only highly potent antiretroviral (ARV) drugs. Recently, Ball et al. and Huang et al. demonstrated that electrospun fibers could release a range of active ARV drugs and spermicides (8) and that such release could occur in response to stimuli, such as a semen-induced rise in pH (9). Furthermore, Huang et al. demonstrated high loading (15 wt%) of tenofovir disoproxil into electrospun fibers (9), which would reduce the required dosage size.
Maraviroc, another antiretroviral drug, is of interest for use in a microbicide due to its effectiveness at treating HIV (10), high potency in animal models (11), and favorable toxicity profile. Maraviroc works by binding to CCR5 coreceptors on HIV target cells, thereby inhibiting viral entry (12). Since the majority of new HIV infections are CCR5 tropic, maraviroc may be particularly suited for use as a microbicide. Maraviroc is also not a first-line HIV medication, so the development of HIV resistance to maraviroc (13) would not necessarily preclude the use of frontline highly active antiretroviral therapy (HAART) treatments. While maraviroc has been loaded into a hydroxyethyl cellulose (HEC) gel (11), two vaginal rings (one also containing dapivirine) (14, 15), and a silicone-based elastomer gel (16) for testing as a microbicide, it has yet to be formulated into fully soluble disintegrable fibers for the purpose of pericoital protection. Previous pharmacokinetic and efficacy studies have shown that maraviroc can be delivered and absorbed intravaginally but that protection against high-dose simian-human immunodeficiency virus (SHIV)-162P3 challenge in macaques requires a large dose (3.3 wt% in HEC gel) just 30 min prior to viral exposure (11, 17). Thus, solid pericoital delivery systems should be designed in part to maximize drug release speed. Within pharmaceutics, it is often the case that drugs with melting temperatures near or above 200°C will display improved solubility and release rates when formulated into amorphous solid dispersions, since crystalline active pharmaceutical ingredients must overcome the enthalpic energy barrier of “melting” their crystalline lattice structure before solvating (18, 19). The melting point of crystalline maraviroc is near 195°C (15), suggesting that dispersion of maraviroc within appropriately compatible polymer fibers might aid in achieving high drug dosing in less than 30 min prior to viral challenge. Solid dispersions most often utilize polymers like poly(ethylene oxide) (PEO; also known as PEG) and polyvinylpyrrolidone (PVP) to serve as carriers that can formulate active compounds as a “dissolved solid.” Our previous, unpublished work demonstrated that maraviroc existed in a semicrystalline state within PEO fibers even at loadings as low as 1 wt%. PVP, which has often been used to accelerate drug release or increase apparent drug solubility in both electrospun fibers and oral tablets (20–22), may improve the extent of amorphous dispersion of maraviroc within electrospun materials, even at 15 wt% or higher drug content. Additionally, incorporating hydrophilic surfactants like Tween 20 into electrospun microbicides might lower the entropic cost of solvating maraviroc's hydrophobic moieties through complexation with drug molecules in solution, thereby further accelerating drug release. However, the incorporation of a surfactant into a potential microbicide raises the potential for increased risk of HIV transmission, so it is important to investigate how their use to alter release behaviors may be balanced with toxicity concerns.
Here, we design and evaluate electrospun fibers containing the antiretroviral drug maraviroc for rapid, pericoital prevention of HIV-1. We demonstrate that maraviroc may be loaded to at least 28 wt% into PVP and PEO nanofibers and microfibers. These materials hydrate rapidly (in less than 15 min) and release high levels (106 × 50% inhibitory concentration [IC50], in vitro, for maraviroc [11]) of active agents both in sink conditions (defined as the volume of medium at least greater than three times that required to form a saturated solution of a drug substance) and on agar gels. Since maraviroc constituted such a large portion of the fiber mass, we reasoned that maraviroc's ability to hydrate and enter solution would have a large influence on the time required to complete release. We also examine how drug loading, polymer choice, and inclusion of Tween 20 (at levels deemed to be an upper limit for safe use) would affect the crystallinity, distribution, and release rates of maraviroc from the fibers. Our studies show that the inclusion of the wetting agent Tween 20, drug loading, and polymer choice impacted the distribution, crystallinity, drug solubility, and release rates of maraviroc from electrospun fibers. While all fiber formulations achieved rapid drug delivery through dispersion of maraviroc (increased surface area for drug dissolution), the most rapid delivery of maraviroc was achieved through a combination of PVP and 2.5 wt% Tween 20, which allowed for the complete release of 28 wt% maraviroc in just 6 min under sink conditions. Crucially, the maraviroc released from electrospun fibers was shown to retain its original potency against HIV in vitro. These rapidly dissolving fibers could be ideally suited for use as a pericoital delivery system, since they have a very high surface area-to-volume ratio, can be made of the same polymers as films and tablets, and, unlike other nanosystems, like nanoparticles, are easily handled. Electrospun fibers are smooth (materials appear textured on a micrometer scale), fabric-like materials (capable of repeated folding without breakage and postproduction shaping) that could be inserted vaginally either with a finger or with an applicator (8).
MATERIALS AND METHODS
Preparation and composition of electrospinning solutions.
Maraviroc was purchased through the University of Washington's Investigative Drug Services facility and was purified and recrystallized from Selzentry (ViiV Healthcare) through extraction into dichloromethane and recrystallization in ethyl acetate (see the supplemental material). Drug identity and purity (>99 mol%) were verified by nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), differential scanning calorimetry (DSC), and X-ray photoelectron spectroscopy (XPS). Polyvinylpyrrolidone (PVP) with an MW of ∼1,300,000 Da was purchased from Sigma-Aldrich (St. Louis, MO). Poly(ethylene oxide) (PEO) with an Mw of ∼400,000 Da was purchased from Scientific Polymer Products, Inc. (Ontario, NY). One-hundred-percent ethanol (USP grade) was purchased from the University of Washington's biochemistry supply store. Distilled, deionized water was obtained using a Milli-Q water purifier (Millipore). Tween 20 (polysorbate 20) was purchased from Fisher Scientific. PVP, PEO, Tween 20, maraviroc, and solvents were added to 20-ml glass scintillation vials with gas-tight lids. Solutions were mixed by gentle tumbling overnight on a rotisserie shaker (Labquake; Thermo Scientific). Solution density was measured by massing triplicate 500-μl samples, taking the mean of the measurements, and dividing by the sample volume. Solution conductivity was measured using a calibrated conductivity probe (Thermo Scientific). Solution surface tensions were measured using an AquaPi surface tensiometer (Kibron). Rheological data were measured using an AR G2 series rheometer (TA Instruments) with a 40-mm 2° cone geometry in frequency sweep oscillation mode with a constant small strain of 4%. Measurements of G″ (the shear loss modulus) were converted to viscosity measurements by dividing G″ by the angular frequency. Comparisons of solution parameters between solutions with and without Tween 20 were made using paired, two-sided t tests with α = 0.05, matching for drug loading. Correlations between solution parameters and drug loading were tested using a Pearson's correlation test with α = 0.05.
Electrospinning and scanning electron microscopy (SEM) characterization.
PVP solutions were electrospun at 15 kV over a 20-cm gap using flow rates of 25, 50, and 100 μl/min. PEO solutions were electrospun at 15 kV over a 25-cm gap using flow rates of 10, 25, and 50 μl/min. Solutions were spun with a charged 15-cm by 15-cm metal screen at the base of the needle to promote a spatially homogeneous electric field and increase polymer recovery. For each run, 1 ml of solution was spun from a glass syringe fitted with a 2.54-cm-long 25-gauge stainless steel blunt dispensing needle. Fibers were collected onto a flat, grounded, aluminum surface covered with a single layer of wax paper. The wax paper substrate facilitated easy removal of the fiber samples from the collector with tweezers. After being electrospun, fiber samples were lyophilized for at least 24 h, and then their final mass was recorded to determine yield.
Electrospun fibers were examined by SEM using a Sirion SEM (NanoTech User Facility, University of Washington). Fibers were examined after sputtering with gold and palladium for 90 s to minimize charge buildup on fibers. Imaging settings of 5 kV, spot size of 3, and working distance of 6.5 mm were used to obtain images. Fields of view were randomly selected in order to eliminate bias in selecting which fibers to image. Fiber diameters were measured in ImageJ (NIH) by bisecting the SEM image diagonally with a line and manually measuring the diameters of fibers that intersected that bisecting line. At least 25 fibers were measured per sample, and fiber diameters were reported using the median and coefficient of variation as measures of the population's center and distribution, respectively. Correlations between median fiber diameters and drug loading were tested using a Pearson's correlation test with α = 0.05.
Measuring drug loading by HPLC.
A Shimadzu prominence LC20AD UV-HPLC system equipped with a Phenomenex Luna C18 column (5 μm, 250 by 4.6 mm) and LCSolutions software were used to quantify drug levels in samples. The actual loading of maraviroc in electrospun fibers was measured with UV-HPLC (Shimadzu) by dissolving 2.5-mg pieces of mesh (n = 1, containing 0.25 mg to 1 mg of maraviroc) in 50 ml of the HPLC mobile phase, which consisted of a 60% 10 mM KH2PO4 buffer and 40% acetonitrile, filtered through 0.45-μm, 0.22-μm, and glass frit filters to remove particulates. Polymers and maraviroc were freely soluble in the mobile phase. A fresh maraviroc standard curve was prepared by dissolving 20 mg of maraviroc in mobile phase to a concentration of 1 mg/ml and diluting serially at 1:2 until concentrations of approximately 10 ng/ml were achieved. Spiked samples were prepared by adding maraviroc from 1 mg/ml stock solution to dissolved blank fibers with and without Tween 20. The maraviroc standard, spiked samples, and unknown samples were detected by UV-HPLC as described previously (8, 23). The calibration curve was prepared in Prism (GraphPad) using 1/C2 weighting to minimize residuals. The linear range was found to be from 64,000 ng/ml to 400 ng/ml.
Thermal analysis by DSC.
Samples with a mass of 5 to 10 mg (n = 1 sample tested by DSC per fiber type) were placed into aluminum pans (T-Zero; TA Instruments) and analyzed with a TA Auto Q20 DSC instrument (TA Instruments). Samples were heated from 30°C to 250°C at a rate of 10°C/min with a nitrogen flow of 50 ml/min. Peak integration was performed using TA thermal analysis software and sigmoidal tangential integration. Percent crystallinity of the polymer or the drug was calculated by dividing the measured specific heat of fusion for the sample by the mass fraction of drug or polymer and the reference heat of fusion of the pure crystalline substance (reference value for maraviroc was measured directly; reference value for purely crystalline PEO was estimated as 197 J/g, according to TA Instruments' thermal application note TN048 [24]). The lower limit of detection of crystalline maraviroc was approximately 0.5 wt%. Correlations between melting temperature (Tm) values or PEO crystallinity and drug loading were tested using a Pearson's correlation test with α = 0.05. The impact of Tween 20 on the Tm or percent crystallinity of PEO or maraviroc was tested using paired, two-sided t tests with α = 0.05, matching for drug loading.
Fiber surface analysis by XPS.
XPS was performed using a Surface Science Instruments S-Probe at the University of Washington's NESAC/BIO surface analysis recharge center. Due to the surface sensitivity of XPS measurements, care was taken to prepare samples with no surface contamination. Freshly electrospun materials were collected onto aluminum foil and immediately lyophilized. Samples were analyzed in triplicate and illuminated with low-intensity electrons to reduce charging of the insulated materials. In addition to survey scans, high-resolution carbon scans and detailed nitrogen and fluorine scans were taken for all materials. Peak assignment and integration were performed using XPS analysis software (CasaXPS). Theoretical atomic percentages for C, N, O, and F were calculated assuming a uniform distribution of materials within electrospun fibers. The percentage of the fiber surface covered by maraviroc molecules was calculated by normalizing the total fluorine content in each fiber sample to the fluorine content in pure maraviroc crystal. Enrichment of Tween 20 in PVP materials was assessed using atomic percentage of oxygen and was calculated only for blank PVP fiber materials. Pure PVP fibers and PVP fibers with Tween 20 were compared by subtracting atomic percentage of O and based on the 95% confidence intervals after error propagation.
Determination of in vitro maraviroc solubility following release from fibers.
Drug release from fibers into saturated drug conditions was carried out to assess the solubility limit of maraviroc following release from electrospun fibers loaded with approximately 28 wt% maraviroc. A total of 21 ± 3.5 mg or 42 ± 5.3 mg (mean ± standard deviation [SD]) of crystalline maraviroc or maraviroc-loaded fibers, respectively, was added to pH 4.0 10 mM citrate (sodium) buffer with 154 mM ionic strength at 50 mg of drug per 1 ml of buffer (n = 3 samples per group). Fibers were vortexed for 1 min, heated to 37°C for 24 h, vortexed again for 1 min, and centrifuged to pellet insoluble drug and polymer. A clear, viscous supernatant was present in all samples. This supernatant was diluted 1:1,000 into citrate buffer for quantification of drug concentration by UV-HPLC. Pure PVP and PEO were also added to identical buffer to verify that they did not alter solution pH. A one-way analysis of variance (ANOVA) was used to test the null hypothesis that drug solubility was equivalent across all formulations of maraviroc (α = 0.05), and Dunnett's posttest comparisons were performed to test for solubility differences between fiber formulation groups with the pure drug control group (α = 0.05).
In vitro drug release into sink conditions.
Sink release was studied in ambient conditions (20°C, 50% relative humidity) in a pH 4.0 10 mM citrate (sodium) buffer with 154 mM ionic strength. Phosphate-buffered saline (PBS; pH 7.0) was also used to assess the effect of drug ionization on release rate from highly loaded PVP fibers without Tween 20. Medium was filter sterilized before use. Studies were carried out by adding 5 mg of fiber to a 50-ml conical tube secured to a rotisserie shaker (Labquake; Thermo Scientific). A total of 25 ml of medium was added (maximum [maraviroc] = 0.06 mg/ml, >50 times lower than the drug's solubility limit), and a timer was started when fibers first touched fluid. Materials were tumbled gently at 7 rpm, and 50-μl samples were removed at 2-min intervals for 20 min (total of 500 μl). A 24-h time point was taken the next day as an approximation of infinite time. Spiked samples as well as pure drug crystal controls were also analyzed to validate quantitative methods. Spiked drug controls yielded full drug recovery at the 2-min time point. Purified maraviroc was micronized using a mortar and pestle, and particle size was assessed by SEM and imaging software (ImageJ; NIH). Release samples were quantified by UV-HPLC as described above.
In vitro dissolution of electrospun fibers on a moist, porous surface.
Electrospun fibers were cut into 1.27-cm-diameter circles using a metal die (Grainger), and their mass and thickness were measured with an analytical balance and calipers. PVP samples had a mass of 8.2 ± 4.2 mg (thickness of 0.49 ± 0.25 mm, basis weight of 65 ± 35 g/m2, density of 0.13 ± 0.02 g/cm3), and PEO samples had a mass of 2.0 ± 0.89 mg (thickness of 0.11 ± 0.04 mm, basis weight of 16 ± 7 g/m2, density of 0.15 ± 0.07 g/cm3). Fiber discs were gently dropped onto black agar plates (1.5% agar with 1% [vol/vol] of India ink) incubated at 37°C. No pressure was applied to fibers to force contact with the gels. When the fibers absorbed water from the gels, the black plate clearly showed through the hydrated fibers (opaque and white prior to wetting). The degree of fiber hydration was assessed visually every 30 s from time lapse photos taken on a smartphone (iPhone 4; Apple).
In vitro Tween 20 cytotoxicity anti-HIV activity of dissolved electrospun fibers.
The in vitro cytotoxicity of the surfactants Tween 20 (Fisher), Tween 80 (Fisher), glycerol monolaurate (Sigma-Aldrich), and nonoxynol-9 (Options Conceptrol) were evaluated using a cell titer blue assay (Promega) according to the manufacturer's instructions. The in vitro activity of maraviroc in fiber eluates (from sink release assays) was evaluated using a cell-based reporter assay described previously (8, 25). Briefly, TZM-bl cells and an HIV-1 BaL isolate were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (http://www.aidsreagent.org/). TZM-bL cells, a derived HeLa cell line that expresses CD4, CCR5, and CXCR4 (26–29), were added to black 96-well plates (Corning Inc., Corning, NY) with Dulbecco's modified Eagle medium (DMEM) (Gibco Life Technologies) with 10% fetal bovine serum (HyClone), 1% 100× penicillin-streptomycin (Invitrogen), and 1% 200 mM l-glutamine (Invitrogen) with 50 μl/well at a density of 5,000 cells/well. Cells were incubated in 5% CO2 and 37°C for 24 h prior to exposure to surfactants or fiber eluates. For the surfactant cytotoxicity assay, compounds were dissolved or suspended with slight heating in cDMEM at 5% (wt/vol) and then diluted serially. Fifty-microliter volumes of the dilutions were added into duplicate wells, and cells were incubated an additional 24 h. Finally, per manufacturer specifications, 20 μl of cell titer blue reagent was added to each well and incubated for 4 h before fluorescence was read on a plate reader (Tecan). Fifty-percent lethal dose (LD50) values of the surfactants were estimated using sigmoidal regression in Prism (GraphPad Software, Inc.) and compared using an extra sum-of-squares F-test (α = 0.05). For the antiviral activity assay, treatments were diluted serially. A second operator, blinded to the identity of the treatments, added each in 50-μl volumes into duplicate wells (PVP and PEO Tween 20 formulation eluates were added into single wells due to space limitations on the 96-well plate). Polymer, surfactant, and drug concentrations were at least 1,000 times below toxic concentrations. A total of 100 μl of HIV-BaL (240 tissue culture infective doses [TCID]/well) was added to wells 1 h after drug treatment. Medium was removed from cells after 48 h posttreatment, and 100 μl of phosphate-buffered saline (Gibco Life Technologies) and 100 μl of Bright-Glo Luciferase reagent (Promega) were added to wells. Infectious activity was quantified by measuring luminescence on a plate reader (Tecan). IC50s of the 5 drug formulations were estimated using sigmoidal regression in Prism (GraphPad Software, Inc.) and compared using an extra sum-of-squares F-test (α = 0.05).
RESULTS
Electrospinning solutions, fiber characterization, and drug loading determination.
Our results show that it is possible to incorporate high levels of maraviroc into PVP and PEO solutions without compromising fiber production by electrospinning. Electrospinning solution properties (see Table S1 in the supplemental material) correlated with finished fiber characteristics (see Table S2) and drug loading (see Table S3). HPLC analysis of PVP and PEO fibers incorporating maraviroc had measured drug loadings of 94.2% ± 1.2% (n = 8) and 98.0% ± 1.8% (n = 8), respectively. Maraviroc loading efficiency was not reduced even when drug precipitate was visually detected in the formulation solutions, such as in the case with solutions containing both Tween 20 and 40% (wt/wt) drug-polymer maraviroc. Material efficiency was high for all PVP and PEO formulations, with measured polymer recoveries of up to 95%. As expected, the lower PEO concentrations used in the solution formulations resulted in a reduced basis weight for PEO fiber materials (16 ± 7 g/m2, thickness of 0.11 ± 0.04 mm) compared to that of PVP materials (65 ± 35 g/m2, thickness of 0.49 ± 0.25 mm). PVP fibers loaded with maraviroc appeared large, cylindrical, and slightly wrinkled (Fig. 1). We observed that increasing maraviroc content correlated highly with increased conductivity of PVP solutions with and without Tween 20 (Pearson's R = 0.95 and 0.91, respectively, P < 0.05) and predicted that drug incorporation would lead to the formation of smaller-diameter fibers. While we did observe that PVP fiber diameter decreased with increasing maraviroc concentration for formulations without Tween 20, the correlation was not statistically significant (Pearson's R = −0.80, P = 0.11). In contrast, when maraviroc was added to formulations containing Tween 20, the median fiber diameter decreased by up to 3-fold, and SEM images revealed a distinct population of both large (diameter of ∼2,000 nm) and small (diameter of ∼400 nm) fibers (Fig. 1).
FIG 1.
Maraviroc may be loaded into PVP and PEO fibers with and without the surfactant Tween 20 up to at least 28 wt%. The size and surface structure of the fibers depend upon the specific combination of polymer choice, drug loading, and surfactant incorporation. SEM images of fibers are organized into a matrix with the row representing either 9 wt% or 28 wt% drug loading and the column representing the polymer (PVP or PEO) and inclusion of Tween 20. Scale bar = 5 μm.
All PEO solutions containing maraviroc produced electrospun fibers that showed narrow size distributions. Increasing maraviroc content by 4-fold in these PEO formulations also led to higher solution conductivity, but median fiber diameter did not decrease as might be expected. Rather, fiber diameter was positively correlated with maraviroc content in fibers without Tween 20 (Pearson's R = 0.97, P = 0.0042), where we observed up to a 30% change in solution conductivity with increasing maraviroc in solution. In samples containing Tween 20, fiber diameter increased until 16 wt% maraviroc loading and then remained fairly constant at ∼600 nm. SEM micrographs of PEO fibers without Tween 20 revealed a rough surface morphology, whereas fibers containing Tween 20 displayed a smooth, highly fused surface morphology (Fig. 1). The observed increase in diameter size by hundreds of nanometers with increasing maraviroc content suggests that a complex interplay of solution characteristics and electrospinning processing parameters may make it challenging to predict physical properties of the finished fibers from measuring viscosity, conductivity, and surface tension alone. In summary, our results demonstrate that electrospinning efficiently and reproducibly formulates maraviroc into fibers. We performed all subsequent studies with 9 wt% and 28 wt% formulations, and final Tween 20 content in fibers ranged from 0 wt% to 3.7 wt% or 2.5 wt% (decreasing at the high end with increasing maraviroc content) (see Table S3). These formulations were chosen as comparator groups to explore the impact that a large range in drug loading and Tween content might have on drug release.
Thermal analysis by DSC.
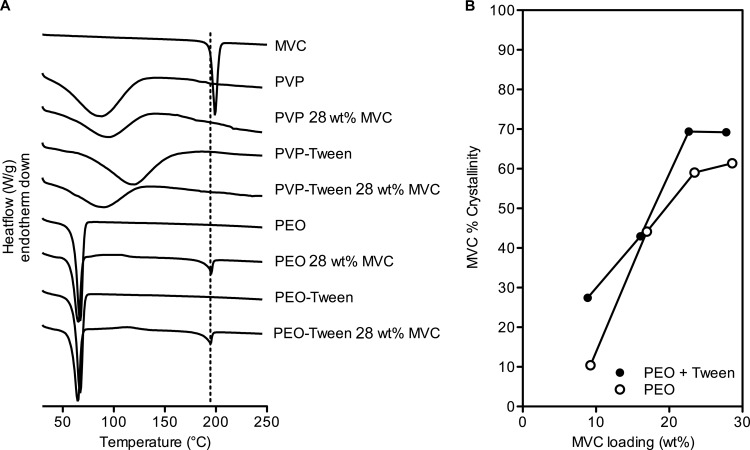
We hypothesized that the extent of drug crystallinity might ultimately affect release rates in vitro. Differential scanning calorimetry (DSC) analysis of PVP fibers containing maraviroc indicated that PVP and maraviroc were amorphously distributed throughout the electrospun fibers irrespective of Tween 20 content (Fig. 2). In contrast, drug-loaded PEO fibers displayed melting endotherms near the Tm of pure maraviroc (195°C) (Fig. 2). There was a significant, positive correlation between drug loading and percent maraviroc crystallinity in PEO fibers with and without Tween 20 (two-sided Pearson's correlation test, R > 0.95, P < 0.05, n = 4 ordered pairs for each formulation type). For example, drug crystallinity was minimal in PEO fibers loaded at 9 wt% maraviroc but peaked at ∼70% in PEO fibers above a loading of 23 wt% maraviroc. Interestingly, PEO formulations containing Tween 20 led to 9.9% greater maraviroc crystallinity in these finished fibers (Fig. 2; see also Table S4 in the supplemental material) (P = 0.035; n = 4 paired observations). We also observed that the Tm of maraviroc was positively correlated to maraviroc loading in fibers (Pearson's R = 0.95 and 0.95, P = 0.053 and 0.046, n = 5 ordered pairs for both fibers with and without Tween 20) and increased by 12°C as maraviroc content went from 9 wt% to 28 wt%. Small exotherms suggestive of maraviroc recrystallization during heating (near 120°C) were present in many PEO fibers containing drug, but they were too minute to accurately integrate and quantify. Therefore, estimates of maraviroc crystallinity may be negligibly overestimated. All PEO fibers had endotherms near 66°C corresponding to the Tm of PEO (Fig. 2). The crystallinity of PEO in fibers was estimated to be ∼55% and was unaffected by drug loading or inclusion of Tween (P > 0.05). However, the Tm of PEO was negatively correlated with maraviroc content in fibers (Pearson's R = −0.94 and −0.97, P = 0.0183 and 0.0048, n = 5 ordered pairs for both fibers with and without Tween 20) (Fig. 2; see also Table S4). In summary, thermal analysis of electrospun materials by DSC revealed that maraviroc was amorphous in PVP fibers and semicrystalline in PEO materials and that the degree of drug crystallinity in PEO increased with drug loading and Tween 20 incorporation.
FIG 2.
Maraviroc exists in different physical states depending upon the polymer choice and drug loading. DSC thermograms are shown in panel A. Pure crystalline maraviroc has a melting endotherm around 195°C (dashed line, panel A), which is observed in formulations of maraviroc into PEO-based fibers. No maraviroc endotherm was observed in PVP formulations. In PEO fibers, the crystallinity of the drug content was a function of drug loading and was similar between fibers with and without Tween 20 (B). Data represent values from single experiments.
Fiber surface analysis by XPS.
X-ray photoelectron spectroscopy (XPS) of electrospun fibers was used to estimate the degree of enrichment of maraviroc and Tween 20 at the surface (top 10 Å) of electrospun fibers. Atomic percent data for carbon, nitrogen, oxygen, and fluorine showed that the content of maraviroc varied with the polymer type, drug loading, and presence or absence of Tween 20 (Fig. 3). All PVP and PEO formulations showed enrichment of maraviroc on fiber surfaces compared to expected values for theoretical fibers with radially homogeneous distributions of maraviroc. The degree of surface enrichment was dependent upon drug loading and formulation type. PEO fibers without Tween showed the highest surface concentrations of maraviroc, approaching complete surface coverage by 17 wt% maraviroc loading. The surface of PVP fibers with Tween 20 became saturated with maraviroc at the higher loading of 28 wt% maraviroc. However, neither PEO with Tween 20 nor PVP without Tween 20 had maraviroc-saturated surfaces when loaded at 28 wt%. Tween 20 was enriched 4.8- to 7.8-fold on the surface of PVP fibers without maraviroc. We were not able to determine the surface concentration of Tween 20 in either drug-loaded PVP fibers or PEO fibers due to limitations in instrument sensitivity. The surface enrichment of maraviroc in fibers suggests that maraviroc's physicochemical properties may play a large role in determining the rate of fiber hydration and subsequent drug release.
FIG 3.
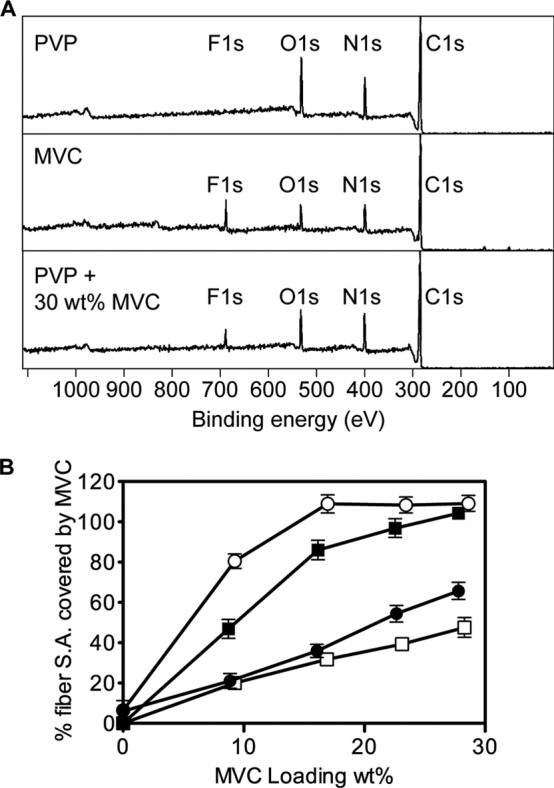
Maraviroc content was enriched at the surface of PVP and PEO fibers, as detected by XPS. Representative spectra are shown (A) for pure PVP polymer fibers (top), pure maraviroc (middle), and PVP fibers loaded to 28 wt% with maraviroc (bottom), showing the shifting of C, N, and O atomic ratios due to the presence of maraviroc in the top 1 nm of the fiber surface. The amount of fluorine detected in samples was normalized to that found in pure maraviroc and plotted against drug loading as the percentage of surface area covered by maraviroc (B). “100%” means that the fluorine peak in the sample was as large as that in pure maraviroc samples. Data in panel B represent the means from n = 3 independent experiments. Error bars represent SD. ○, PEO; ●, PEO and Tween; □, PVP; ◼, PVP and Tween.
In vitro drug release under sink conditions.
We tested the solubility limits of maraviroc following incubation of an excess of fibers or pure drug in sodium citrate buffer (10 mM, pH 4.0) for 24 h (Fig. 4). There was no change in solution pH after dissolving the fibers in the citrate buffer. Nevertheless, we found that drug solubility differed between all formulations tested (P = 0.0015). The solubility limit of pure maraviroc in the absence of polymer or surfactant was 21.3 ± 6.4 mg/ml (n = 3). While PVP and Tween 20 did not affect drug solubility, there was a large decrease in drug solubility after release from PEO formulations with or without Tween 20 (P < 0.01) (Fig. 4). Therefore, in low fluid volumes, PVP fiber formulations maintained drug solubility in pH 4.0 buffer, whereas PEO formulations reduced drug solubility, and Tween 20 had no effect on drug solubility with either polymer.
FIG 4.

The solubility limit of maraviroc after release from fiber formulations into very low fluid volumes is maintained in PVP fiber formulations but greatly reduced in PEO fiber formulations. Solubility limits were assessed in pH 4.0 10 mM citrate (sodium) buffer at 154 mM ionic strength, 37°C, and 24 h. Data are shown as the means from n = 3 independent experiments. Error bars represent SD.
Drug release into sink conditions at pH 4.0 was evaluated for PVP and PEO fibers loaded with 9 wt% or 28 wt% maraviroc (Fig. 5). We observed that the time to >95% release of maraviroc from 28 wt% drug materials was 14 min, 6 min, 18 min, and 6 min for PVP, PVP with Tween 20, PEO, and PEO with Tween 20 fibers, respectively (Fig. 5). All 9 wt% formulations released >95% of the maraviroc within 2 to 4 min, except for 9 wt% maraviroc in PEO with Tween 20, which required 10 min to fully release maraviroc. The time required to release >95% of loaded maraviroc generally increased with higher drug loading. Fibers loaded at 28 wt% maraviroc and 2.5 wt% Tween 20 released maraviroc more rapidly than those fibers without Tween 20. We also examined the effect on release from PVP fibers without Tween by changing the release medium from a pH 4.0 citrate buffer, meant to mimic the mildly acidic pH of the vagina, to a pH 7.0 PBS. The result was that PVP fibers (no Tween 20) required a full 20 min to completely release a 28 wt% maraviroc payload and 12 min to completely release a 9 wt% maraviroc payload. The time to >95% drug release from fibers was also compared to that from pure maraviroc particles under pH 4.0 and pH 7.0 sink conditions in vitro. At pH 4.0, micronized (50-nm to 5-μm) maraviroc particles dissolved completely within 2 min despite being >99% crystalline, while it took 20 min to dissolve 90% of a 2-mm-diameter piece of crystalline maraviroc. At pH 7.0, however, large pieces of crystalline maraviroc required overnight mixing to fully dissolve (data not shown). These results demonstrate that solid dispersion of maraviroc into fibers dramatically accelerated drug dissolution, especially relative to dissolution rates at higher pH. Sink release data demonstrated that all fiber formulations of maraviroc were capable of rapid drug release but that fibers incorporating Tween 20 as a wetting agent released maraviroc far more rapidly.
FIG 5.

The release rate of maraviroc from electrospun fibers in vitro was dependent upon the drug loading, the inclusion of Tween 20, the pH of the release medium, and the polymer. (A) Release from PVP fibers with 28 wt% maraviroc (□) into pH 4.0 citrate buffer (solid line) or pH 7.0 PBS (dashed line) and 9 wt% maraviroc (□ with dot) into pH 4.0 citrate buffer (solid line) or pH 7.0 PBS (dashed line); (B) release from PVP fibers with Tween 20 with 28 wt% maraviroc (◼) and 9 wt% maraviroc (⊠); (C) release from PEO fibers with 28 wt% maraviroc (○) and 9 wt% maraviroc (⊙); (D) release from PEO fibers with Tween 20 with 28 wt% maraviroc (filled ○) and 9 wt% maraviroc (○ with x). Data are shown as the means from n = 3 independent experiments. Error bars represent SD.
In vitro dissolution of electrospun fibers on a moist, porous surface.
PVP and PEO fibers containing 9 wt% or 28 wt% maraviroc and 0 wt% or 3 wt% Tween 20 were easily visualized as white materials on a black background using black agar plates (Fig. 6). Fiber hydration was rapid, despite not being immersed in a bath of fluid and not applying pressure to fiber samples on the agar plates. Fibers containing 9 wt% maraviroc significantly hydrated, contracted, or dissolved in fewer than 10 s (see Fig. S2 in the supplemental material). Fibers containing 28 wt% maraviroc had slower hydration rates and displayed two distinct behaviors depending upon Tween 20 incorporation. Fibers without Tween 20 absorbed water and rapidly contracted within 30 s and then continued to hydrate over 20 min, at which point the resulting hydrogel could flow if the agar plate were tipped at an angle. A visible precipitate, thought to be undissolved maraviroc, was present in this gel. In contrast, fibers with Tween 20 hydrated more rapidly than fibers without Tween 20. Between 5 s and 30 s, the fibers wet out in place without significant matrix contraction or shrinkage. By the end of the 20-min monitoring period, no precipitated maraviroc or undissolved polymer was visible. Overall, fibers hydrated more rapidly when they contained less maraviroc or contained Tween 20.
FIG 6.

Incorporating Tween 20 into PVP or PEO fibers containing 28 wt% maraviroc led to more rapid and total dissolution of fibers and encapsulated maraviroc on a moist porous surface of 1.5% agar at 37°C. The original perimeters of the 28 wt% maraviroc fiber discs are shown as dashed circles. Images are arranged by fiber type (row) as seen over time (column). A visible precipitate remained in hydrated fiber gels after 20 min for both PVP and PEO fibers without Tween 20 (white arrows). Images are representative samples from n = 3 independent experiments.
Tween 20 cytotoxicity and antiviral activity of maraviroc following sink release.
The in vitro LD50 values of the tested surfactants were Tween 20 = 0.24% (wt/vol), Tween 80 = 0.32% (wt/vol), glycerol monolaurate = 0.022% (wt/vol), and nonoxynol-9 = 0.0053% (wt/vol) when exposed to TZM-bL cells in cDMEM for 24 h. The antiviral activities of pure maraviroc and maraviroc released from electrospun formulations were identical in vitro (Fig. 7) and consistent with values reported in literature. No toxic effects due to drug eluates were observed. The estimated IC50s for each individual dose-response curve were not significantly different from one another (P = 0.924). The globally shared best-fit IC50 for maraviroc was found to be 6.96 ng/ml (13.5 nM), which is consistent with previous findings for maraviroc using this in vitro assay (8). These results show that maraviroc was fully potent following release from electrospun fibers into release medium.
FIG 7.

Incorporation of maraviroc into electrospun fibers does not decrease drug potency against HIV in vitro. Eluates from 28 wt% maraviroc fibers in pH 4.0 citrate buffer were tested alongside neat maraviroc for antiviral activity against HIV-BaL using a TZM-bL cell reporter assay. There was no difference between the IC50s of neat and formulated maraviroc. All treatments were diluted serially 10 times. Data represent mean values from duplicate wells (n = 2), except for eluates from PVP and PEO-Tween fibers (n = 1). Error bars represent SD.
DISCUSSION
Maraviroc is promising as a microbicide because of its potency, good toxicity profile, and CCR5 tropism (15–17). In recent years, the pharmacokinetics of maraviroc in nonhuman primates following the administration of maraviroc-containing vaginal gels (11, 16, 17) and vaginal rings (14, 15) have been reported. These pharmacokinetic and efficacy studies have demonstrated that intravaginal maraviroc is rapidly absorbed in macaques and can provide high levels of protection against high-dose SHIV-162P3 challenge. However, the efficacy of maraviroc in these trials has been strongly dependent on both the dose and the time of administration. For example, formulations of maraviroc into hydroxyethylcellulose gels poses challenges since a minimum drug loading of 3.3 wt% is required to approach complete efficacy, but the drug is not soluble in the gel at this level. Achieving higher doses with a vaginal gel would require greater gel volumes, which would likely result in dose leakage (30). Solid dosage forms, like films, could provide higher doses without causing leakage problems, but films have not yet demonstrated high antiretroviral drug loading capacity (6, 7). Furthermore, the efficacy of maraviroc gels decreased when they were applied 1 h instead of 30 min before challenge (11), suggesting that maraviroc microbicides should be designed for use as close to the time of viral exposure as possible. In other words, a dry dose maraviroc microbicide should dissolve quickly. Since electrospun fibers possess a large surface area for hydration and drug dissolution, they may be ideally suited for pericoital administration of maraviroc.
We showed that electrospun PVP fibers released maraviroc rapidly enough to warrant further investigation as a female self-administered pericoital anti-HIV microbicide product. The time to >95% drug release could be reduced by either lowering drug loading or adding Tween 20 as a wetting agent. Furthermore, the fibers rapidly hydrated in low fluid volumes, transforming the solid fiber dosage into an apparently spreadable gel. Such rapid hydration and drug release might simultaneously accomplish three important tasks. First, rapidly dissolving fibers might be ideally suited for pericoital release of protective levels of maraviroc (132 mg) to the vagina less than 30 min before sex, which has been shown in gel efficacy studies to maximize protective efficacy of maraviroc-loaded HEC gels (11, 17). Due to the high drug loading in electrospun fibers, 132 mg of maraviroc could be delivered with less than 500 mg of electrospun fibers. Second, rapid hydration rates of fibers may minimize the risk of epithelial abrasions caused by the intravaginal dosage form if applied immediately before sex. The observation that fibers transform into a semisolid with gel-like properties could prevent the sensation of “dry” sex often attributed to dry-dose microbicides (31). Also, since high-Mw polymers (Mw > 100 kDa) are utilized for electrospinning, the osmolality of dissolved fibers may be significantly lower than that of dissolved films incorporating lower-Mw polymers (polyvinyl alcohol with Mw of ∼10 kDa) and excipients like PEG 400 and glycerol (∼10 to 100 times lower moles of solute per volume in fibers than in films). However, this observation depends on several complex factors and should be further tested. Third, since the microbicide products would be applied as a dry-dosage form, retention of the drug dose may be higher than if it were applied in a 3- to 4-ml volume of gel. While the form of a fiber-based microbicide is still undefined, numerous product forms may be envisaged. Fibers could be shaped for insertion with or without an applicator. Disadvantages of an applicator are the added cost, complexity, and potential for abrasions. Likewise, finger insertion may be complicated by moist fingers preventing full insertion of the dose should it hydrate too rapidly. Despite these complexities, the soft, flexible nature of electrospun materials, which resemble fabrics with a microscopically fine texture, may help make them acceptable to users in a number of different delivery formats. For these reasons, local maraviroc delivery from microbicide fibers may fill a gap in the current microbicide product portfolio.
In order to understand the drug release properties from different formulations used in our study, we examined fibers using electron microscopy, chemical analysis, and calorimetric techniques. We observed clear trends in drug distribution, crystallinity, and fiber properties on drug release kinetics, which will be useful for informing the design of future electrospun microbicides. While some of these properties, such as drug crystallinity, had little impact for the delivery of maraviroc from fibers, they have been shown to be of considerable significance for other drugs used in microbicides. For example, Johnson et al. showed that greater crystallinity of tenofovir in the core of an intravaginal ring led to lower daily release rates of the drug (32). In this study, our characterization suggested that water penetration into and subsequent drug release from materials slowed in response to higher drug loading due to a combination of reduced hydrophilicity of maraviroc versus the base polymers, increased drug crystallinity, and drug surface enrichment.
Visual observations of the fibers during release experiments and images of fibers hydrating and disintegrating on agar plates (Fig. 6) revealed correlations between the apparent extent of fiber hydration and the percentage of maraviroc released from fibers. While we did not directly measure polymer hydration, swelling, or dissolution, it is worth discussing the role that the composition of the fibers had on the hydration and subsequent release of maraviroc. Our electrospun materials contained high levels of drug, which significantly altered the hydration behavior of the polymer matrix. Maraviroc was formulated into fibers as a neutral compound, and no salts were present in the electrospinning solutions. Once hydrated, however, protonation would be rapid, and maraviroc would be >99% ionized at pH 4.0. Despite this predicted ionization, increasing the proportion of the drug in PVP and PEO fibers led to longer times to >95% drug release (Fig. 5), which is likely due to maraviroc being less hydrophilic than either the PVP or PEO base polymers alone.
The impact of media pH on drug release from anti-HIV microbicides is important for evaluating the potential impact of elevated vaginal pH (due to natural variation, infection, the presence of semen, tampon use, heavy menstruation, douching, etc.) on device performance. We found that increasing the pH of release medium from 4.0 to 7.0 significantly increased the required time to dissolve micronized maraviroc crystals under sink conditions from less than 2 min to overnight. This might correspond to a decrease in the percentage of maraviroc molecules that are ionized at the tropane ring (pKa = 7.84), and we estimated that only 86% of maraviroc is ionized at pH 7.0, compared to >99% of maraviroc at pH 4.0. In contrast to micronized drug, maraviroc release from PVP fibers with 28 wt% loading required 4.6 min to release 50% drug content at pH 7.0 compared to 1.9 min at pH 4.0 (20 min versus 14 min for >95% release). Thus, while reduced ionization could have slowed hydration and retarded release rates from fibers, especially at loadings as high as 9 to 28 wt%, solid dispersion within electrospun fibers might offer clear dissolution advantages over the delivery of micronized drug particles. Such pH dependencies imply that release will likely be slower in animal models with higher vaginal pH (e.g., macaques) or if used for rectal application to prevent transmission during receptive anal intercourse among either heterosexual populations or men who have sex with men. Other ionizable antiretrovirals would likely show analogous pH dependencies.
Incorporation of Tween 20 into fibers accelerated drug release (Fig. 5) and reduced the apparent dissolution time on agar plates (Fig. 6). Since Tween 20 did not appear to have a plasticizing effect (defined as significantly lowering polymer melting or glass transition temperatures or reducing polymer crystallinity) on PVP or PEO, we expect that this was not the major mechanism behind the accelerated drug release from surfactant-loaded fibers (33). It is also possible that Tween 20 on or within fibers allowed water to penetrate the matrix more quickly given that Tween 20 is more hydrophilic than maraviroc. While Tween 20 accelerates drug release, it does not seem to enhance maraviroc's solubility in the release medium (Fig. 4). The use of surfactants in a microbicide formulation is a rightly controversial issue, as surfactants may be toxic to epithelial tissue. Increased risk for HIV infection can result from repeated epithelial disruption by detergents such as noonoxynol-9 (34). Therefore, when designing a vaginal drug delivery system, it is of paramount importance to select a surfactant that poses little to no risk to vaginal and cervical tissue when applied topically. Tween 20 is a common wetting agent that may be useful for increasing the rate of drug release and could have an acceptable threshold for toxicity in vivo but was not tested here. There is limited but insufficient precedent for the safe repeated use of Tween 20 in vaginal formulations. The gel Conceival (a potential microbicide for lipophilic drug delivery) contains 2 to 4 wt% Tween 20 and is nontoxic in rabbits and to rabbit and human sperm (35). Our rationale for formulating Tween 20 into fibers at 4% (wt/wt) Tween-polymer was to examine the effects of an upper limit of surfactant levels on drug release from fibers. It is possible that much lower concentrations could be used to preserve the beneficial effects of Tween on drug release rates while minimizing concerns about toxicity. Our in vitro testing of surfactant cytotoxicity shows that Tween 20 and Tween 80 are tolerated 10 times better than glycerol monolaurate (shown to be safe in macaques [36] but potentially harmful in mice [37]) and 50 times better than nonoxynol-9 by TZM-bL cells, with an LD50 for Tween 20 of 0.24% (wt/vol) in cell culture medium. The in vivo toxicity of electrospun fibers containing small amounts of Tween 20 remains to be evaluated. Depending upon the required drug dosage and drug loading, it may not be necessary to include wetting agents to achieve rapid delivery with electrospun fibers.
Maraviroc was amorphous in PVP and semicrystalline in PEO; yet despite this difference in physical state, releases of maraviroc were similar from the two polymer types (Fig. 5). In addition, the crystallinity of maraviroc in electrospun PEO materials had an insignificant impact on the dissolution of maraviroc into pH 4 medium on the time scale of 15 min, which we attribute to the small physical size of crystalline drug present in the fibers. Consequently, it can be argued that drug crystallinity was not the principal determinant of either the rate of hydration or the time to >95% release. Previous work by Korsmeyer et al. has shown that the crystallinity of hydrophilic polymers can have a large effect on drug release rates (38). Since the crystallinity of the PEO in our fibers was effectively constant over the range of drug loadings tested, neither drug nor polymer crystallinity likely explains the significantly retarded water uptake at higher drug loadings. In fact, our observations imply that as long as the size of the crystalline domains is small and the drug is readily ionizable in the medium, the dissolution of maraviroc will not likely be slow due to the crystalline state of the drug.
The highly loaded materials used in this study were electrospun using a mild process with high productivity and material efficiency. In addition to solvent safety, no high temperatures were needed to create these materials, and electrospinning proceeded at ambient conditions (19 to 23°C, 50 to 70% RH). Although electrospinning relies upon high voltage, spinning requires a very low current and therefore little energy. The materials in this study were successfully loaded with up to 28 wt% maraviroc. Higher drug loading is possible and would result in smaller applied fiber doses but may be an important cost constraint. Increased drug loading did not reduce productivity, and we observed high material efficiency (88 to 94% for PVP materials) resulting in a fiber production rate of ∼7 mg/min using a benchtop instrument with a single nozzle. We recently reviewed the scale-up potential and expected cost of electrospun microbicides, finding that current electrospinning technologies are capable of producing up to 6.5 kg of fiber every hour (10 to 20 million doses annually), at a projected cost of $0.50 to $3.00 per dose depending upon a large number of factors such as initial investments in capital, material costs, and production volume (39).
Although this study focused on the intravaginal delivery of maraviroc fibers, similar materials may be developed for rectal delivery or for indications other than HIV prevention. Recent work by Massud et al. suggested that rectal delivery of maraviroc gels lacks efficacy in a macaque SHIV challenge model and may be complicated by effects of rectally applied maraviroc on populations of CD3+/CCR5+ cells (40). It remains to be seen if maraviroc can serve as an effective microbicide in humans, either intravaginally or rectally. Previous work on electrospun microbicides has demonstrated the ability to load and release other APIs, including acyclovir (anti-HSV-2), tenofovir (anti-HIV/HSV-2), glycerol monolaurate (antisperm, antimicrobial, anti-inflammatory), and even whole live lactobacilli (antibacterial vaginosis) (8, 9, 41). We expect that fibers will be capable of delivering a full range of APIs for not only anti-HIV microbicides but also for treatment and prevention of bacterial, fungal, and viral infections.
Conclusions.
Electrospun PVP fibers can deliver a high dose of maraviroc under mildly agitated sink conditions within minutes following initial contact with release media. Maraviroc is currently a lead antiretroviral compound for formulation into anti-HIV topical microbicides, including rings and gels. It is highly potent against CCR5-tropic viruses and has no major side effects. Based on clinical and preclinical studies of the drug's pharmacokinetics and ability to prevent viral transmission, it seems likely that maraviroc will be most effective when incorporated into a microbicide platform that allows for a rapid release of high levels of maraviroc within 30 min before viral challenge. In particular, the microbicide product should act locally, achieve high vaginal retention, minimize leakage, and enable coformulation with other APIs. Our current work demonstrates the need for rigorous physical and chemical characterization of new materials in order to better understand and engineer such novel anti-HIV vaginal microbicides.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge G. Hammer and L. Gamble from NESAC/BIO (NIH P41 EB-002027) for conducting XPS experiments, the UW NNIN for assistance with SEM imaging, and Y. Jiang for performing the in vitro anti-HIV activity assay.
C.B. was supported by a National Science Foundation Graduate Research Fellowship, and the research was funded by NIH grant AI098648 awarded to K.A.W.
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02564-14.
REFERENCES
- 1.Abdool Karim Q, Abdool Karim S, Frohlich J. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. 10.1126/science.1193748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT, TDF2 Study Group 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367:423–434. 10.1056/NEJMoa1110711 [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D, FEM-PrEP Study Group 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422. 10.1056/NEJMoa1202614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Microbicide Trials Network. 2013. VOICE fact sheet—understanding the results of VOICE. MTN, Pittsburgh, PA [Google Scholar]
- 6.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, Rohan LC. 2011. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 1:209–222. 10.1007/s13346-011-0022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ham AS, Rohan LC, Boczar A, Yang L, Buckheit W K, Buckheit RW. 2012. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm. Res. 29:1897–1907. 10.1007/s11095-012-0715-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. 2012. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS One 7:e49792. 10.1371/journal.pone.0049792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, Vervaet C, Coenye T, Verstraelen H, Temmerman M, Demeester J, De Smedt SC. 2012. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 33:962–969. 10.1016/j.biomaterials.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 10.Emmelkamp JM, Rockstroh JK. 2008. Maraviroc, risks and benefits: a review of the clinical literature. Expert Opin. Drug Saf. 7:559–569. 10.1517/14740338.7.5.559 [DOI] [PubMed] [Google Scholar]
- 11.Malcolm RK, Forbes CJ, Geer L, Veazey RS, Goldman L, Johan Klasse P, Moore JP. 2013. Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J. Antimicrob. Chemother. 68:678–683. 10.1093/jac/dks422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721–4732. 10.1128/AAC.49.11.4721-4732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roche M, Jakobsen MR, Ellett A, Salimiseyedabad H, Jubb B, Westby M, Lee B, Lewin SR, Churchill MJ, Gorry PR. 2011. HIV-1 predisposed to acquiring resistance to maraviroc (MVC) and other CCR5 antagonists in vitro has an inherent, low-level ability to utilize MVC-bound CCR5 for entry. Retrovirology 8:89. 10.1186/1742-4690-8-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malcolm RK, Veazey RS, Geer L, Lowry D, Fetherston SM, Murphy DJ, Boyd P, Major I, Shattock RJ, Klasse PJ, Doyle LA, Rasmussen KK, Goldman L, Ketas TJ, Moore JP. 2012. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob. Agents Chemother. 56:2251–2258. 10.1128/AAC.05810-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetherston SM, Boyd P, McCoy CF, McBride MC, Edwards K-L, Ampofo S, Malcolm RK. 2012. A silicone elastomer vaginal ring for HIV prevention containing two microbicides with different mechanisms of action. Eur. J. Pharm. Sci. 48:406–415. 10.1016/j.ejps.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Forbes CJ, Lowry D, Geer L, Veazey RS, Shattock RJ, Klasse PJ, Mitchnick M, Goldman L, Doyle LA, Muldoon BCO, Woolfson AD, Moore JP, Malcolm RK. 2011. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J. Control. Release 156:161–169. 10.1016/j.jconrel.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veazey RS, Ketas TJ, Dufour J, Moroney Rasmussen T, Green LC, Klasse PJ, Moore JP. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J. Infect. Dis. 202:739–744. 10.1086/655661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yalkowsky SH. 1981. Techniques of solubilization of drugs. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 19.Leuner C. 2000. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 50:47–60. 10.1016/S0939-6411(00)00076-X [DOI] [PubMed] [Google Scholar]
- 20.Yu D-G, Zhu L-M, Branford-White CJ, Yang J-H, Wang X, Li Y, Qian W. 2011. Solid dispersions in the form of electrospun core-sheath nanofibers. Int. J. Nanomedicine 6:3271–3280. 10.2147/IJN.S27468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank KJ, Westedt U, Rosenblatt KM, Hölig P, Rosenberg J, Mägerlein M, Fricker G, Brandl M. 2012. The amorphous solid dispersion of the poorly soluble ABT-102 forms nano/microparticulate structures in aqueous medium: impact on solubility. Int. J. Nanomedicine 7:5757–5768. 10.2147/IJN.S36571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlberg C, Dvinskikh SV, Schuleit M, Furó I. 2011. Polymer swelling, drug mobilization and drug recrystallization in hydrating solid dispersion tablets studied by multinuclear NMR microimaging and spectroscopy. Mol. Pharmaceutics 8:1247–1256. 10.1021/mp200051e [DOI] [PubMed] [Google Scholar]
- 23.Notari S, Tommasi C, Nicastri E, Bellagamba R, Tempestilli M, Pucillo LP, Narciso P, Ascenzi P. 2009. Simultaneous determination of maraviroc and raltegravir in human plasma by HPLC-UV. IUBMB Life 61:470–475. 10.1002/iub.181 [DOI] [PubMed] [Google Scholar]
- 24.Blaine RL. 2002. Thermal applications note TN048, TA Instruments, New Castle, DE [Google Scholar]
- 25.Chaowanachan T, Krogstad E, Ball C, Woodrow KA. 2013. Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis. PLoS One 8:e61416. 10.1371/journal.pone.0061416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905. 10.1128/AAC.46.6.1896-1905.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358–8367. 10.1128/JVI.74.18.8358-8367.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J. Virol. 82:12585–12588. 10.1128/JVI.01726-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnhart K, Pretorius E, Timbers K, Shera D. 2004. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception 70:498–505. 10.1016/j.contraception.2004.06.013 [DOI] [PubMed] [Google Scholar]
- 31.Nel AM, Mitchnick LB, Risha P, Muungo LTM, Norick PM. 2011. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J. Womens Health 20:1207–1214. 10.1089/jwh.2010.2476 [DOI] [PubMed] [Google Scholar]
- 32.Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. 2010. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 39:203–212. 10.1016/j.ejps.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Wypych G. 2013. Handbook of plasticizers. William Andrew, Norwich, NY [Google Scholar]
- 34.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiègne-Traoré V, Uaheowitchai C, Karim SSA, Mâsse B, Perriëns J, Laga M, COL-1492 Study Group 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971–977. 10.1016/S0140-6736(02)11079-8 [DOI] [PubMed] [Google Scholar]
- 35.D'Cruz OJ, Samuel P, Uckun FM. 2005. Conceival, a novel noncontraceptive vaginal vehicle for lipophilic microbicides. AAPS PharmSciTech 6:E56–E64. 10.1208/pt060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlievert PM, Strandberg KL, Brosnahan AJ, Peterson ML, Pambuccian SE, Nephew KR, Brunner KG, Schultz-Darken NJ, Haase AT. 2008. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob. Agents Chemother. 52:4448–4454. 10.1128/AAC.00989-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moench T, Mumper R, Hoen T, Sun M, Cone R. 2010. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect. Dis. 10:331. 10.1186/1471-2334-10-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. 1983. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharmaceutics 15:25–35. 10.1016/0378-5173(83)90064-9 [DOI] [Google Scholar]
- 39.Blakney AK, Ball C, Krogstad EA, Woodrow KA. 2013. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral Res. 100:S9–S16. 10.1016/j.antiviral.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 40.Massud I, Aung W, Martin A, Bachman S, Mitchell J, Aubert R, Tsegaye TS, Kersh E, Pau CP, Heneine W, Garcia-Lerma JG. 2013. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J. Virol. 87:8952–8961. 10.1128/JVI.01204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy ZK, Wagner I, Suhajda Á, Tobak T, Harasztos AH, Vigh T, Sóti PL, Pataki H, Molnár K, Marosi G. 2014. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. Express Polym. Lett. 8:352–361. 10.3144/expresspolymlett.2014.39 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.