Abstract
Fluoroquinolones are avoided during pregnancy due to developmental toxicity in animals. The aim of this study was to assess the fetal risk after intrauterine fluoroquinolone exposure. We performed an observational study of a prospectively ascertained cohort of pregnant women exposed to a fluoroquinolone during the first trimester. Pregnancy outcomes were compared to those of a cohort exposed to neither fluoroquinolones nor teratogenic or fetotoxic drugs. The outcomes evaluated were major birth defects (structural abnormalities of medical, surgical, or cosmetic relevance), spontaneous abortion, and elective termination of pregnancy. Pregnancy outcomes of 949 women with fluoroquinolone treatment were compared with those of 3,796 nonexposed controls. Neither the rate of major birth defects (2.4%; adjusted odds ratio [ORadj], 0.91; 95% confidence interval [CI], 0.6 to 1.5) nor the risk of spontaneous abortion (adjusted hazard ratio [HRadj], 1.01; 95% CI, 0.8 to 1.3) was increased. However, there was a nonsignificant increase in major birth defects after exposure to moxifloxacin (6/93, 6.5%; crude odds ratio [ORcrude], 2.40; 95% CI, 0.8 to 5.6). Neither a critical exposure time window within the first trimester nor a specific pattern of birth defects was demonstrated for any of the fluoroquinolones. The rate of electively terminated pregnancies was increased among the fluoroquinolone-exposed women (HRadj, 1.32; 95% CI, 1.03 to 1.7). The gestational ages at delivery and birth weights did not differ between groups. Our study did not detect an increased risk of spontaneous abortion or major birth defects. These reassuring findings support the recommendation to allow fluoroquinolone use in early pregnancy in selected cases. After the use of moxifloxacin, a detailed fetal ultrasound examination should be considered.
INTRODUCTION
Bacterial infections need effective treatment during pregnancy because they may be hazardous to the health of the mother and the unborn child. Often the bacterial spectrum requires the use of second-line agents like fluoroquinolones, but there is uncertainty concerning the risk for the unborn child. Furthermore, increasing numbers of fluoroquinolone prescriptions have led to increasing exposure during unplanned pregnancies (1, 2).
Fluoroquinolones are a class of broad-spectrum anti-infective agents, which act by inhibiting bacterial DNA gyrase. They have not been found to be teratogenic in animals (3, 4). Fluoroquinolones have a high affinity to bone tissue and cartilage. In 1977, Ingham et al. reported on arthropathy of weight-bearing joints in juvenile animals after postnatal exposure to narrow-spectrum quinolones (5). This effect has also been observed in animal experiments with newer fluoroquinolones (6, 7). Therefore, they are generally avoided during pregnancy although studies have not shown any significant risk of cartilage damage in children (8).
Fluoroquinolones cross the human placenta and are found in the amniotic fluid at low concentrations (9, 10). A few publications have raised concerns about intrauterine fluoroquinolone exposure. Bach et al. published two cases with abdominal wall defects after fluoroquinolone treatment in early pregnancy (11). A Danish cohort study based on prescription data raised the concern that prenatal fluoroquinolone exposure might be associated with an increased risk of bone malformation (12). The manufacturer published three cases with limb defects after ciprofloxacin exposure among the 103 pregnancies from their database (13). In contrast to these reports, other studies with a total of approximately 1,000 first-trimester exposures did not show an increased risk of adverse pregnancy outcome after the use of fluoroquinolones (14–18).
The objectives of this study were to evaluate the risks of spontaneous abortion (SAB), elective termination of pregnancy (ETOP), and major birth defects after first-trimester exposure to fluoroquinolones.
MATERIALS AND METHODS
Study design.
The study design corresponded to a prospective observational cohort study based on archived pregnancy courses. The exposed and nonexposed women enrolled between January 1995 and December 2012 were followed prospectively; i.e., neither the outcome of the pregnancy nor the results of prenatal diagnostics were known at the time of enrollment. The study was performed in accordance with the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement adapted to the needs of pregnancy outcome studies (19, 20). Ethics approval was obtained from the ethics committee of the Charité Universitätsmedizin Berlin (no. EA4/011/13). The study was registered at the German Clinical Trials Register (no. DRKS00004864).
Cohorts.
The Berlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy offers risk assessment to pregnant women, physicians, and other health care professionals who spontaneously contact the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy for consultation at any time during pregnancy. The patients are asked for informed consent to monitor the course of their pregnancies. Data collection is performed using two structured questionnaires at the first contact and 8 weeks after the estimated date of birth and includes a detailed history of drug use, demographic characteristics, information on previous and current obstetrical history, family history, maternal chronic diseases, and other risks or exposures. The follow-up is focused on pregnancy complications, congenital anomalies, and postnatal disorders. Details of delivery, gestational age at birth, sex, birth weight, length, head circumference, pH, Apgar score, and, if applicable, pregnancy loss, are also collected.
In our study, the exposed group consisted of women who had been treated orally or intravenously with a fluoroquinolone between gestational week 2 + 0 days and week 12 + 6 days after their last menstrual period. The cases with concomitant exposure to established teratogens or fetotoxicants were discussed separately.
The exclusion criteria for the comparison group were exposure to a fluoroquinolone or to established teratogens or fetotoxicants (acitretin, carbamazepine, isotretinoin, lenalidomide, methotrexate, mycophenolate, phenobarbital, phenprocoumon, phenytoin, thalidomide, valproate, warfarin, angiotensin type 1 [AT1] receptor antagonists, and angiotensin-converting enzyme [ACE] inhibitors), the presence of a malignancy, or treatment with an antiepileptic drug. Subjects in the comparison group were randomly chosen from all eligible pregnant women who met the study criteria. They were frequency matched to the fluoroquinolone-exposed cohort for the year of counseling with an approximate ratio of 4:1.
Outcomes.
The primary endpoints of this study were the risks of major birth defects and spontaneous abortions. In addition, we looked for substance-specific risks, specific patterns of birth defects, and a critical exposure time window within the first trimester. The rate of elective termination of pregnancy was evaluated as a secondary endpoint.
Congenital malformations were classified as major or minor by two experts according to established standards (21–23). Major birth defects were defined as structural abnormalities of medical, surgical, or cosmetic relevance. Classifications were performed independently and blind to exposure data.
Weeks of gestation were calculated from an ultrasound examination during the first trimester or, if not available, from the last menstrual period. If possible, gestational age was given in completed weeks plus days. Spontaneous abortion (SAB) was defined as spontaneous pregnancy loss of a fetus <500 g or, if the weight was not known, at <23 completed gestational weeks. The birth weight was adjusted to gestational age at birth and sex and classified according to the percentile table derived from the German Perinatal Study (24).
Statistical analysis.
To assess the effects of fluoroquinolone exposure on the risk of SAB and elective termination of pregnancy (ETOP), Cox proportional hazards modeling was applied, considering SAB, ETOP, and live birth as competing events. As the pregnant women were enrolled at various stages of pregnancy, delayed study entry was taken into account (25). The analyses were adjusted using maternal age, alcohol consumption, smoking habits, number of previous SABs, number of previous deliveries, and number of previous children with congenital abnormalities as covariates. As the time of exposure varied within the exposed group, no cumulative incidences of spontaneous abortion were provided.
The effect of fluoroquinolone exposure on the risk of birth defects was assessed through logistic regression. Crude birth defect rates were calculated by dividing the total number of infants and fetuses with birth defects by the number of all live-born infants plus the number of pregnancy losses with birth defects. Chromosomal or genetic anomalies were considered separately. The final analyses used propensity score methods for bias reduction (26). The propensity score was estimated using boosted regression trees based on maternal age, alcohol consumption, smoking habits, number of previous spontaneous abortions, number of previous deliveries, and number of previous children with congenital abnormalities as covariates (27). The logit of the propensity score was included as a covariate in the logistic regression. Relative risks are stated as adjusted odds ratios (ORadj) or crude odds ratios (ORcrude). To investigate whether the risk of birth defects changed with the gestation period at the time of treatment (critical exposure time window), exposure week was added as a category to the model described above.
For all analyses including covariates, the missing values were addressed using multiple imputations by a chained equation, assuming that the data were missing at random (28). Ten imputed data sets were generated per outcome, including the respective outcomes and the covariates used for model adjustment. For each imputed data set, analyses were performed as described above. The results were then combined using Rubin's rule (29). All analyses were done with R version 2.13 (R Development Core Team).
RESULTS
Cohort size, exposures, and maternal characteristics.
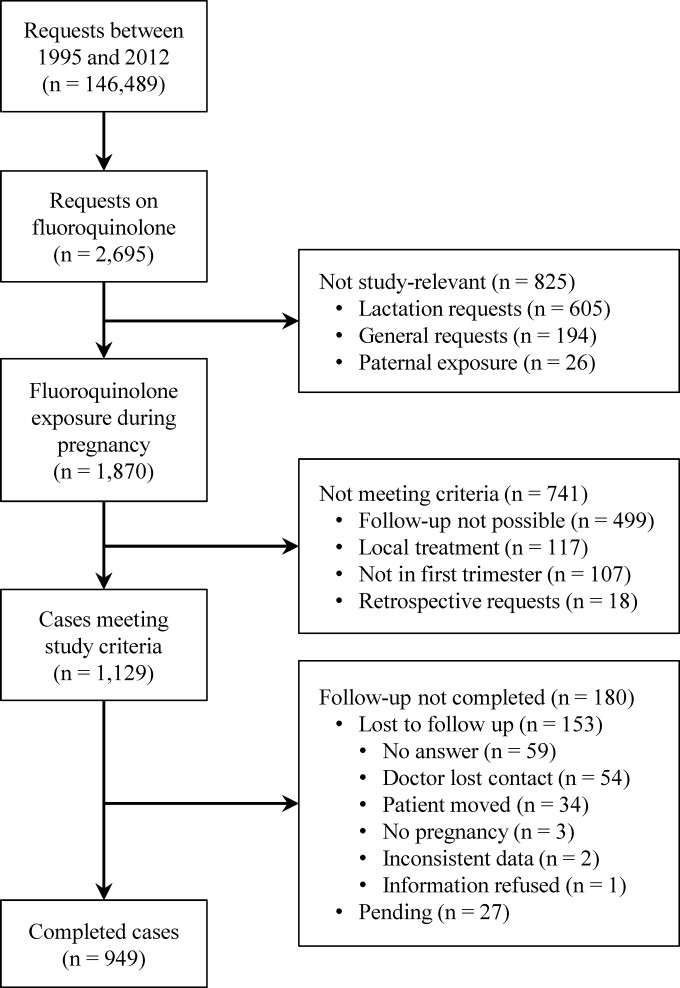
Between 1995 and 2012, the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy performed 146,489 consultations on the reproductive safety of medicinal products and other agents. Inclusion criteria for the fluoroquinolone group were met in 949 completed cases (Fig. 1). A total of 25,373 cases fulfilled the requirements for the comparison group. Of these, a total of 3,796 pregnant women were randomly chosen as described above.
FIG 1.
Number of fluoroquinolone-exposed cases.
The most frequent treatment indications for fluoroquinolones were urinary tract infections (50.4%), followed by respiratory tract infections (18.4%) (see Table S1 in the supplemental material). Nineteen patients were exposed to two or more fluoroquinolones during the first trimester. Ciprofloxacin (n = 407) was the most prevalent fluoroquinolone, followed by ofloxacin (n = 170), levofloxacin (n = 136), moxifloxacin (n = 108), norfloxacin (n = 97), enoxacin (n = 20), gatifloxacin (n = 12), fleroxacin (n = 5), grepafloxacin (n = 5), trovafloxacin (n = 5), pefloxacin (n = 2), and lomefloxacin (n = 1). The vast majority of women received their antibiotics orally; only eight of them had an intravenous treatment. Duration of therapy was between 1 day and long-term therapy throughout pregnancy in a case of cystic fibrosis. Only 17% of the women were treated longer than 7 days. As shown in Table 1, the maternal characteristics were comparable between the cohorts.
TABLE 1.
Maternal characteristics
| Characteristic | Result for: |
|
|---|---|---|
| Study cohort | Comparison cohort | |
| No. of pregnancies | 949 | 3,796 |
| Maternal age (total no.) | 937 | 3,732 |
| Maternal age (median [interquartile range]) (yr) | 31 (26–34) | 31 (27–35) |
| BMIa (total no.) | 434 | 1,721 |
| BMI (median [interquartile range]) (kg/m2) | 22 (20.3–25) | 22.5 (20.5–25.1) |
| Mother's education (total no.) | 369 | 1,552 |
| No graduation (no. [%]) | 4 (1.1) | 13 (0.8) |
| 9-yr exam (no. [%]) | 15 (4.1) | 105 (6.8) |
| 10-yr exam (no. [%]) | 131 (35.5) | 452 (29.1) |
| 13-yr exam (Abitur) (no. [%]) | 84 (22.8) | 371 (23.9) |
| Academic study (no. [%]) | 135 (36.6) | 611 (39.4) |
| Smoking (total no.) | 922 | 3,668 |
| No (no. [%]) | 766 (83.1) | 2,982 (81.3) |
| ≤5 cigarettes/day (no. [%]) | 57 (6.2) | 181 (4.9) |
| >5 cigarettes/day (no. [%]) | 99 (10.7) | 505 (13.8) |
| Alcohol (total no.) | 921 | 3,657 |
| No (no. [%]) | 864 (93.8) | 3,452 (94.4) |
| ≤1 drink/day (no. [%]) | 46 (5) | 128 (3.5) |
| >1 drink/day (no. [%]) | 11 (1.2) | 77 (2.1) |
| Illicit drugs (total no.) | 912 | 3,636 |
| No (no. [%]) | 909 (99.7) | 3,552 (97.7) |
| Yes (no. [%]) | 3 (0.3) | 84 (2.3) |
| Folic acid (total no.) | 447 | 1,822 |
| Yes (no. [%]) | 402 (89.9) | 1,673 (91.8) |
| No (no. [%]) | 45 (10.1) | 149 (8.2) |
| Positive attitude toward pregnancy (total no.) | 834 | 3,195 |
| Yes (no. [%]) | 715 (85.7) | 2,845 (89) |
| No (no. [%]) | 24 (2.9) | 62 (1.9) |
| Indifferent (no. [%]) | 95 (11.4) | 288 (9) |
| Previous pregnancies (total no.) | 926 | 3,704 |
| 0 (no. [%]) | 447 (48.3) | 1,673 (45.2) |
| 1 (no. [%]) | 256 (27.6) | 1,117 (30.2) |
| 2 (no. [%]) | 146 (15.8) | 537 (14.5) |
| ≥3 (no. [%]) | 77 (8.3) | 377 (10.2) |
| Previous deliveries (total no.) | 925 | 3,692 |
| 0 (no. [%]) | 514 (55.6) | 1,986 (53.8) |
| 1 (no. [%]) | 282 (30.5) | 1,151 (31.2) |
| 2 (no. [%]) | 105 (11.4) | 409 (11.1) |
| ≥3 (no. [%]) | 24 (2.6) | 146 (4) |
| Previous SABsb (total no.) | 924 | 3,629 |
| 0 (no. [%]) | 815 (88.2) | 3,108 (85.6) |
| 1 (no. [%]) | 83 (9) | 389 (10.7) |
| ≥2 (no. [%]) | 26 (2.8) | 132 (3.6) |
| Previous ETOPsc (total no.) | 924 | 3,622 |
| 0 (no. [%]) | 850 (92) | 3,335 (92.1) |
| 1 (no. [%]) | 60 (6.5) | 233 (6.4) |
| ≥2 (no. [%]) | 14 (1.5) | 54 (1.5) |
| Previous children with birth defects (total no.) | 924 | 3,615 |
| 0 (no. [%]) | 908 (98.3) | 3,546 (98.1) |
| 1 (no. [%]) | 15 (1.6) | 65 (1.8) |
| ≥2 (no. [%]) | 1 (0.1) | 4 (0.1) |
| Gestational wk at first contact (total no.) | 949 | 3,796 |
| Gestational wk at first contact (median [interquartile range]) | 7.1a (6–9) | 8.1 (6–13) |
BMI, body mass index.
SAB, spontaneous abortion.
ETOP, elective termination of pregnancy.
Pregnancy outcome and neonatal characteristics.
The pregnancy outcomes and neonatal characteristics are shown in Table 2. The rate of live births was lower in the study cohort than in the comparison cohort due to more frequent SABs and ETOPs. SABs occurred in 87/949 (9.2%) cases of the fluoroquinolone cohort and in 292/3,796 (7.7%) of the comparison cohort. After adjustment, the risk of spontaneous abortion was not significantly increased in the fluoroquinolone cohort (adjusted hazard ratio [HRadj], 1.01; 95% confidence interval [CI], 0.8 to 1.3), whereas ETOPs occurred more frequently (HRadj, 1.32; 95% CI, 1.03 to 1.7). The gestational week at ETOP and the reason for the ETOP did not differ considerably between groups (see Table S2 in the supplemental material). Gestational age at birth, sex, and birth weight did not differ between groups (see also Fig. S1 in the supplemental material).
TABLE 2.
Pregnancy outcomes and neonatal characteristics
| Outcome or characteristic | Result for: |
|
|---|---|---|
| Study cohort | Comparison cohort | |
| No. of pregnancies | 949 | 3,796 |
| SABsa (no. [%]) | 87b (9.2) | 292 (7.7) |
| ETOPsc (no. [%]) | 94 (9.9) | 233 (6.1) |
| Stillbirths (no. [%]) | 1 (0.1) | 10d (0.3) |
| Live births (no. [%]) | 768b (80.9) | 3,262d (85.9) |
| No. of live-born infants | 775e | 3,318f |
| Gestational wk at birth (total no.) | 771 | 3,302 |
| Gestational wk at birth (median [interquartile range]) | 40 (38–40) | 39 (38–40) |
| Preterm (<37 wk) (no. [%]) | 69 (8.9) | 316 (9.6) |
| Term (≥37 wk) (no. [%]) | 702 (91.1) | 2,986 (90.4) |
| Sex (total no.) | 765 | 3,279 |
| Female (no. [%]) | 370 (48.4) | 1,582 (48.2) |
| Male (no. [%]) | 395 (51.6) | 1,697 (51.8) |
| Weight (total no.) | 769 | 3,277 |
| Weight (median [interquartile range]) (g) | 3,375 (3,060–3,700) | 3,340 (2,995–3,680) |
| Length (total no) | 753 | 3,230 |
| Length (median [interquartile range]) (cm) | 51 (50–53) | 51 (49–53) |
| Head circumference (total no.) | 666 | 2,889 |
| Head circumference (median [interquartile range]) (cm) | 35 (34–36) | 35 (34–36) |
SAB, spontaneous abortion.
Including a multiple pregnancy ending in an SAB and in 1 live-born child.
ETOP, elective termination of pregnancy.
Including a set of twins ending in a live-born and stillborn infant at gestational week 25.
Including 7 sets of twins.
Including 54 sets of live-born twins and 1 live-born triplet.
Congenital malformations.
The proportion of infants with birth defects in each group is shown in Table 3. Major birth defects were present in 19/779 (2.4%) children in the fluoroquinolone group and in 93/3,329 (2.8%) in the comparison group (ORadj, 0.91; 95% CI, 0.6 to 1.5). Table 4 presents details on all infants with major birth defects in the exposed group (for minor birth defects, see Table S3 in the supplemental material, and for congenital anomalies with a chromosomal or genetic background, see Table S4 in the supplemental material). Fig. S2 in the supplemental material illustrates the courses of pregnancies with birth defects in the fluoroquinolone cohort. The distribution of major birth defects by organ system was not different between the two cohorts (see Table S5 in the supplemental material). Table 5 presents the major birth defect rate by substance. A higher but still not significant risk of major birth defects was found in association with moxifloxacin (6.5%; ORcrude, 2.4; 95% CI, 0.8 to 5.6).
TABLE 3.
Birth defect rates and adjusted odds ratios
| Birth defect | Birth defect rates (no./total no. [%]) for: |
ORadj (95% CI)a | |
|---|---|---|---|
| Study cohort | Comparison cohort | ||
| All birth defects | 45/783b (5.7) | 227/3,343b (6.8) | 0.89 (0.6–1.2) |
| Major birth defects | 19/779b (2.4) | 93/3,329b (2.8) | 0.91 (0.6–1.5) |
| Minor birth defects | 17/775b (2.2) | 106/3,318b (3.2) | 0.70 (0.4–1.2) |
| Congenital anomalies with chromosomal or genetic background | 9/779b (1.2) | 27/3,332b (0.8) | 1.73 (0.8–3.8) |
ORadj, adjusted odds ratio; CI, confidence interval.
Various denominators are due to various numbers of elective terminations of pregnancy/spontaneous abortions with major, minor, or genetic birth defects considered in numerators and denominators.
TABLE 4.
Details of major birth defects in the fluoroquinolone cohort
| Infant no. | GW at birth/ETOPa | Birth defect(s)b | Substance(s) | Dosage (mg/day) | GW at exposure | Coexposure (trimester) |
|---|---|---|---|---|---|---|
| 1 | 38 | VSD | Ciprofloxacin | 2 × 250 | 3–4 | Salbutamol (1) |
| 2 | 39 | Polydactyly, microcephaly | Ciprofloxacin | Unknown | 3–4 | Ibuprofen (1), clindamycin (1), head X-ray (1), nicotine (1–3) |
| 3 | 40 | VSD | Ciprofloxacin | 2 × 250 | 4–5 | Ibuprofen (1) |
| 4 | 34 | Gastroschisis | Ciprofloxacin | 2 × 500 | 1–2 | Doxycycline (1), ambroxol (1) |
| 5 | 38 | Hypospadias (degree unknown, operation recommended) | Ciprofloxacin | 500 | 3 | Acyclovir (1), erythromycin (1), budesonide (1), oxytetracycline (1), nicotine (1–3) |
| 6 | 36 | ASD | Ciprofloxacin; levofloxacin | 2 × 500; unknown | 0–36; 3rd trimester | Azithromycin (1–3), aztreonam (1–3), colistin (1–3), fenoterol (1–3), ipratropium (1–3), pancreatin (1–3), salbutamol (1–3), vitamin K (1–3), insulin (2–3), ceftazidime (3), ivacaftor (3), meropenem (3) |
| 7 | 19 (ETOP) | Spina bifida occulta with hydrocephaly | Ciprofloxacin | 500 | 3–5 | Metamizole (1) |
| 8 | 15 (ETOP) | Univentricular heart | Ciprofloxacin | Unknown | 7–8 | Penicillin (1) |
| 9 | 12 (ETOP) | Atrioventricular canal | Levofloxacin; moxifloxacin | 500; 400 | 5–6; 5 | Glatiramer (1–2), corticosteroid (1) |
| 10 | 32 | Cleft lip and palate | Moxifloxacin | Unknown | 7–8 | Ibuprofen (1), nicotine (1–3) |
| 11 | 40 | VSD, tricuspidal valve insufficiency | Moxifloxacin | Unknown | 2–3 | |
| 12 | 41 | VSD, PFO | Moxifloxacin | 400 | 5 | Topical nifuratel (1), topical hexetidine (2) |
| 13 | 27 (ETOP) | Arnold-Chiari malformation, myelomeningocele | Moxifloxacin | 400 | 6–7 | Chest X-ray (1), myrtol (1) |
| 14 | 40 | Tetralogy of Fallot | Moxifloxacin | Unknown | 6–7 | Ramipril (1), xipamide (1–2) |
| 15 | 38 | Hemangioma on forehead | Norfloxacin | Unknown | 3–4 | Topical clotrimazole (1–2) |
| 16 | 37 | Finger II–V left hand without bones, hypoplastic thumb | Ofloxacin | Unknown | 4 | Topical dequalinium (1), ampicillin (3), fenoterol (3), corticosteroid (3), metoprolol (3) |
| 17 | 38 | Hydronephrosis, skin tag on digit V both hands (ligated) | Ofloxacin | 400 | 3–4 | Intravenous pyelogram with contrast agent (1) |
| 18 | 41 | Poland syndrome | Ofloxacin | 100 | 4 | Clemastine (1), salbutamol (1–3), beclomethasone (1–3) |
| 19 | 40 | Aortic coarctation, persistent left superior vena cava, ASD, PDA | Ofloxacin | Unknown | 5–6 | Citalopram (1), ibuprofen (1), arbutin (1), amoxicillin (1), topical acyclovir (1) |
GW, gestational week; ETOP, elective termination of pregnancy.
ASD, atrial septal defect; PDA, patent ductus arteriosus; PFO, patent foramen ovale; VSD, ventricular septal defect.
TABLE 5.
Major birth defect rates by specific fluoroquinolones
| Fluoroquinolone | No. (%; 95% CI) of major birth defects |
|---|---|
| Ciprofloxacin | 8/336 (2.4; 1.0–4.6) |
| Ofloxacin | 4/137 (2.9; 0.8–7.3) |
| Levofloxacin | 1/112a (0.9; 0.02–4.9) |
| Moxifloxacin | 6/93a (6.5; 2.4–13.5) |
| Norfloxacin | 1/77 (1.3; 0.03–7.0) |
One case that was exposed to moxifloxacin and levofloxacin is included under both agents.
Critical exposure time window within the first trimester.
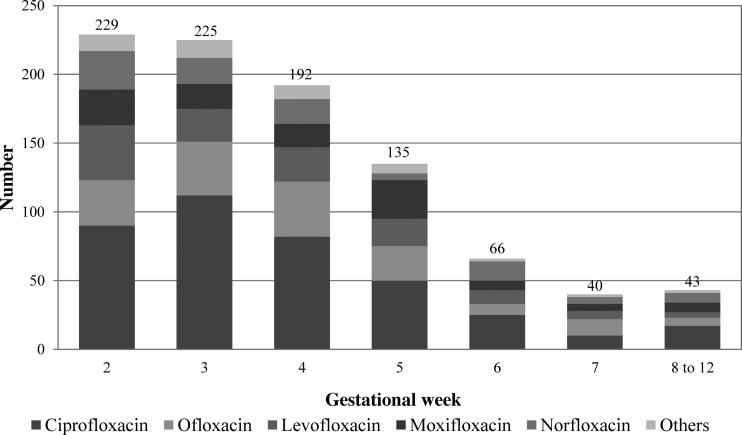
To screen for a teratogenic time window within the first trimester, the data were analyzed according to the onset of fluoroquinolone exposure. Nineteen cases were excluded from this analysis because the exact week of treatment was unknown (n = 1) or the exposure lasted longer than 14 days (n = 18). Most women (84%) began therapy within the first 5 weeks of pregnancy (Fig. 2). The major birth defect rate was slightly increased after onset of therapy within gestational week 7. However, the absolute numbers are low, and in relation to the comparison group, the differences did not reach significance (Table 6).
FIG 2.
Gestational week at onset of fluoroquinolone exposure.
TABLE 6.
Birth defect rates and adjusted odds ratios by onset of fluoroquinolone exposure
| Gestational wk at onset of therapy | No. of pregnancies | No. of live-born infants | No. of birth defects/total no. (%) | ORadj (95% CI)a | No. of major birth defects/total no. (%) | ORadj (95% CI) |
|---|---|---|---|---|---|---|
| 2 | 229 | 182 | 11/184b (6.0) | 0.92 (0.5–1.7) | 2/182b (1.1) | 0.40 (0.1–1.6) |
| 3 | 225 | 187 | 13/188b (6.9) | 1.09 (0.6–2.0) | 6/188b (3.2) | 1.20 (0.5–2.8) |
| 4 | 192 | 149 | 7/151b (4.6) | 0.70 (0.3–1.5) | 3/149b (2.0) | 0.74 (0.2–2.4) |
| 5 | 135 | 113 | 4/114b (3.5) | 0.54 (0.2–1.5) | 3/114b (2.6) | 0.98 (0.3–3.2) |
| 6 | 66 | 59 | 3/60b (5.0) | 0.78 (0.2–2.5) | 2/60b (3.3) | 1.26 (0.3–5.3) |
| 7 | 40 | 36 | 4/37b (10.8) | 1.81 (0.6–5.2) | 2/37b (5.4) | 2.10 (0.5–8.9) |
| 8–12 | 43 | 36 | 1/36b (2.8) | 0.44 (0.1–3.3) |
ORadj, adjusted odds ratio; CI, confidence interval.
Various denominators are due to various numbers of elective terminations of pregnancy/spontaneous abortions with major, minor, or genetic birth defects considered in numerators and denominators.
DISCUSSION
This study prospectively evaluated a cohort of 949 pregnant women after systemic first-trimester exposure to fluoroquinolones. To the best of our knowledge, it is the largest study on fluoroquinolones and pregnancy outcome published so far. Except for the rate of ETOP, we did not find an increased risk for any of the endpoints studied. The higher rate of ETOP might be due to more unplanned pregnancies and fear of malformations in the exposed cohort. However, these variables are not ascertained regularly, so it was not possible to include them as confounders to the analyses.
The rate of major birth defects after first-trimester exposure to fluoroquinolones was not increased compared to that for the nonexposed group, which is consistent with the results of most publications (14–18). Bach et al. described abdominal wall defects after fluoroquinolone exposure (11). In the present study, only one exposed child with gastroschisis was observed (ciprofloxacin from week 1 + 4 days until week 2 + 1 day) (Table 4, infant no. 4). A causal relationship seems unlikely because of the early exposure and the short half-life of ciprofloxacin of 3 to 5 h. Two publications discussed an increased risk of skeletal malformations after intrauterine fluoroquinolone exposure. In a prescription study by Wogelius et al., based on 130 exposed cases, the total malformation rate was not increased. Although not resembling each other, in three out of four birth defects the skeleton was affected: asymmetrical closure of the cranial sutures, polydactyly, and clubfoot (12). Among 103 pregnancies which were reported to the manufacturer Bayer, 3 children were born with different limb reduction defects (aplasia of the right femur, amelia of the forearm, and a femur-fibular-ulna complex) (13). The two children with skeletal malformations in the present study, namely, polydactyly and a limb reduction defect also did not show any specific pattern (Table 4, infant no. 2 and 16). Furthermore, there was one child with Poland syndrome including involvement of the limbs (Table 4, no. 18). In conclusion, neither the risk of abdominal wall nor the risk of skeletal defects differed between cohorts (see Table S5 in the supplemental material).
It is important to note that the sample size of this study was sufficient to detect a 1.5- to 2-fold increase in the risk of major birth defects, given a baseline risk of 3% with a power of approximately 80%. The pregnancies exposed to established teratogens or fetotoxicants were excluded from the comparison group but not from the exposed group in order to observe any effects possibly caused by fluoroquinolones. This different approach did not seem to have altered the results, as the birth defect rate was not increased after fluoroquinolone exposure. Only one child with a major birth defect (tetralogy of Fallot) in the exposed group was concomitantly exposed to a developmental toxicant (ramipril until gestational week 11) (Table 4, no. 14). Although first-trimester effects of ACE inhibitors were suggested by one study, the majority of data supports a fetotoxic hazard only beyond the first trimester (30–32).
Although not significant, the major birth defect rate of 6.5% after moxifloxacin exposure is noteworthy. To our knowledge, the pregnancy outcome after first-trimester exposure to moxifloxacin has not yet been reported. As the number of moxifloxacin-exposed women in our study (n = 108) is still limited, further studies are needed to evaluate the potential risks of this drug.
Most women received short-time therapy. Therefore, each exposed pregnancy is informative only for a limited period of organogenesis within the first trimester and not for the entire first trimester. To address this problem, in this study, we used advanced methods to assess the influence of particular exposure periods within the first trimester on the risk of malformations. No specific risk of fluoroquinolones for any particular exposure time was identified. However, the number of exposed women decreased with advancing gestation, especially after week 6.
Assessing the risk of chondrotoxicity after intrauterine fluoroquinolone exposure was not an objective of this study. If there is a risk at all, it is assumed that this effect occurs after exposure in later pregnancy. Furthermore, arthropathy, as observed in postnatally exposed animals, is usually diagnosed later in life and not during the first 8 weeks after delivery.
The strengths and limitations of prospective observational pregnancy outcome studies have been described in detail elsewhere (20). The prospective approach of this study with similar procedures of ascertainment across cohorts ensures that a substantial bias of exposure and outcome data is unlikely. While this is the largest prospective cohort study on fluoroquinolones during pregnancy, the sample size is still too small to reach a final assessment of the safety and risks of this group of antibiotics in pregnancy.
In conclusion, in this study, we did not observe an increased risk for the unborn after fluoroquinolone therapy during the first trimester. These reassuring findings support the recommendation to allow fluoroquinolone use in early pregnancy in selected cases of bacterial resistance or intolerance to first-line antibiotics. A detailed fetal ultrasound examination should be considered in cases of first-trimester exposure to moxifloxacin, due to the observed trend toward an increased risk of birth defects with this drug.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the German Federal Institute for Drugs and Medical Devices (BfArM).
We thank the staff of the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy for counseling, documentation, and data handling. We thank Eugen Salzmann for his assistance with the data analysis, and we especially thank Jessica Rotty and Ian Mitchell, Melbourne, Australia, for critical reading of the manuscript.
Footnotes
Published ahead of print 19 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02413-14.
REFERENCES
- 1.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. 2005. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am. J. Med. 118:259–268. 10.1016/j.amjmed.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 2.Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, Goossens H. 2006. European Surveillance of Antimicrobial Consumption (ESAC): outpatient quinolone use in Europe. J. Antimicrob. Chemother. 58:423–427. 10.1093/jac/dkl183 [DOI] [PubMed] [Google Scholar]
- 3.Schluter G. 1989. Ciprofloxacin: toxicologic evaluation of additional safety data. Am. J. Med. 87:37S–39S. 10.1016/0002-9343(89)90018-1 [DOI] [PubMed] [Google Scholar]
- 4.Corrado ML, Struble WE, Peter C, Hoagland V, Sabbaj J. 1987. Norfloxacin: review of safety studies. Am. J. Med. 82:22–26. 10.1016/0002-9343(87)90614-0 [DOI] [PubMed] [Google Scholar]
- 5.Ingham B, Brentnall DW, Dale EA, McFadzean JA. 1977. Arthropathy induced by antibacterial fused N-alkyl-4-pyridone-3-carboxylic acids. Toxicol. Lett. 1:21–26. 10.1016/0378-4274(77)90016-9 [DOI] [Google Scholar]
- 6.Gough AW, Kasali OB, Sigler RE, Baragi V. 1992. Quinolone arthropathy—acute toxicity to immature articular cartilage. Toxicol. Pathol. 20:436–449 [DOI] [PubMed] [Google Scholar]
- 7.von Keutz E, Ruhl-Fehlert C, Drommer W, Rosenbruch M. 2004. Effects of ciprofloxacin on joint cartilage in immature dogs immediately after dosing and after a 5-month treatment-free period. Arch. Toxicol. 78:418–424. 10.1007/s00204-004-0551-6 [DOI] [PubMed] [Google Scholar]
- 8.Bradley JS, Jackson MA. 2011. The use of systemic and topical fluoroquinolones. Pediatrics 128:e1034–e1045. 10.1542/peds.2011-1496 [DOI] [PubMed] [Google Scholar]
- 9.Giamarellou H, Kolokythas E, Petrikkos G, Gazis J, Aravantinos D, Sfikakis P. 1989. Pharmacokinetics of three newer quinolones in pregnant and lactating women. Am. J. Med. 87:49S–51S. 10.1016/0002-9343(89)90021-1 [DOI] [PubMed] [Google Scholar]
- 10.Ozyüncü O, Beksac MS, Nemutlu E, Katlan D, Kir S. 2010. Maternal blood and amniotic fluid levels of moxifloxacin, levofloxacin and cefixime. J. Obstet. Gynaecol. Res. 36:484–487. 10.1111/j.1447-0756.2010.01246.x [DOI] [PubMed] [Google Scholar]
- 11.Bach C, López E, Sabriá J. 1993. Defectos congénitos de la pared abdominal poco frecuentes. A propósito de dos casos en los que se constata la exposición embrionaria precoz a las quinolonas. Progr. Diagn. Prenatal 5:13–17 [Google Scholar]
- 12.Wogelius P, Norgaard M, Gislum M, Pedersen L, Schonheyder HC, Sorensen HT. 2005. Further analysis of the risk of adverse birth outcome after maternal use of fluoroquinolones. Int. J. Antimicrob. Agents 26:323–326. 10.1016/j.ijantimicag.2005.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Bomford JAL, Ledger JC, O'Keeffe BJ, Reiter C. 1993. Ciprofloxacin use during pregnancy. Drugs 45:461–462. 10.2165/00003495-199300453-00206 [DOI] [Google Scholar]
- 14.Berkovitch M, Pastuszak A, Gazarian M, Lewis M, Koren G. 1994. Safety of the new quinolones in pregnancy. Obstet. Gynecol. 84:535–538 [PubMed] [Google Scholar]
- 15.Schaefer C, Amoura-Elefant E, Vial T, Ornoy A, Garbis H, Robert E, Rodriguez-Pinilla E, Pexieder T, Prapas N, Merlob P. 1996. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS). Eur. J. Obstet. Gynecol. Reprod. Biol. 69:83–89 [DOI] [PubMed] [Google Scholar]
- 16.Wilton LV, Pearce GL, Mann RD. 1996. A comparison of ciprofloxacin, norfloxacin, ofloxacin, azithromycin and cefixime examined by observational cohort studies. Br. J. Clin. Pharmacol. 41:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loebstein R, Addis A, Ho E, Andreou R, Sage S, Donnenfeld AE, Schick B, Bonati M, Moretti M, Lalkin A, Pastuszak A, Koren G. 1998. Pregnancy outcome following gestational exposure to fluoroquinolones: a multicenter prospective controlled study. Antimicrob. Agents Chemother. 42:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-Oz B, Moretti ME, Boskovic R, O'Brien L, Koren G. 2009. The safety of quinolones—a meta-analysis of pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 143:75–78. 10.1016/j.ejogrb.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 20.Schaefer C, Ornoy A, Clementi M, Meister R, Weber-Schoendorfer C. 2008. Using observational cohort data for studying drug effects on pregnancy outcome—methodological considerations. Reprod. Toxicol. 26:36–41. 10.1016/j.reprotox.2008.05.064 [DOI] [PubMed] [Google Scholar]
- 21.EUROCAT. 2005. EUROCAT guide 1.3 and reference documents: instructions for the registration and surveillance of congenital anomalies. EUROCAT Central Registry, Antrim, Northern Ireland: http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf [Google Scholar]
- 22.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA. 2003. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res. A Clin. Mol. Teratol. 67:193–201. 10.1002/bdra.10012 [DOI] [PubMed] [Google Scholar]
- 23.Merks JH, van Karnebeek CD, Caron HN, Hennekam RC. 2003. Phenotypic abnormalities: terminology and classification. Am. J. Med. Genet. A 123A:211–230. 10.1002/ajmg.a.20249 [DOI] [PubMed] [Google Scholar]
- 24.Voigt M, Rochow N, Hesse V, Olbertz D, Schneider KT, Jorch G. 2010. Kurzmitteilung zu den Perzentilwerten für die Körpermaße der Neugeborenen. Z. Geburtshilfe Neonatol. 214:24–29. 10.1055/s-0029-1241833 [DOI] [PubMed] [Google Scholar]
- 25.Meister R, Schaefer C. 2008. Statistical methods for estimating the probability of spontaneous abortion in observational studies—analyzing pregnancies exposed to coumarin derivatives. Reprod. Toxicol. 26:31–35. 10.1016/j.reprotox.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 26.D'Agostino RB., Jr 1998. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey DF, Ridgeway G, Morral AR. 2004. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol. Methods 9:403–425. 10.1037/1082-989X.9.4.403 [DOI] [PubMed] [Google Scholar]
- 28.Little RJA, Rubin RB. 1987. Statistical analysis with missing data. Wiley, New York, NY [Google Scholar]
- 29.Rubin DB. 1987. Multiple imputation for nonresponse in surveys. Wiley, New York, NY [Google Scholar]
- 30.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. 2006. Major congenital malformations after first-trimester exposure to ACE inhibitors. N. Engl. J. Med. 354:2443–2451. 10.1056/NEJMoa055202 [DOI] [PubMed] [Google Scholar]
- 31.Scialli AR, Lione A. 2006. ACE inhibitors and major congenital malformations. N. Engl. J. Med. 355:1280. 10.1056/NEJMc061798 [DOI] [PubMed] [Google Scholar]
- 32.Diav-Citrin O, Shechtman S, Halberstadt Y, Finkel-Pekarsky V, Wajnberg R, Arnon J, Di GE, Clementi M, Ornoy A. 2011. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod. Toxicol. 31:540–545. 10.1016/j.reprotox.2011.02.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.