Abstract
Amphotericin B (AMB) has been a mainstay therapy for fungal infections of the central nervous system, but its use has been limited by its poor penetration into the brain, the mechanism of which remains unclear. In this study, we aimed to investigate the role of P-glycoprotein (P-gp) in AMB crossing the blood-brain barrier (BBB). The uptake of AMB by primary brain capillary endothelial cells in vitro was significantly enhanced after inhibition of P-gp by verapamil. The impact of two model P-gp inhibitors, verapamil and itraconazole, on brain/plasma ratios of AMB was examined in both uninfected CD-1 mice and those intracerebrally infected with Cryptococcus neoformans. In uninfected mice, the brain/plasma ratios of AMB were increased 15 min (3.5 versus 2.0; P < 0.05) and 30 min (5.2 versus 2.8; P < 0.05) after administration of verapamil or 45 min (6.0 versus 3.9; P < 0.05) and 60 min (5.4 versus 3.8; P < 0.05) after itraconazole administration. The increases in brain/plasma ratios were also observed in infected mice treated with AMB and P-gp inhibitors. The brain tissue fungal CFU in infected mice were significantly lower in AMB-plus-itraconazole or verapamil groups than in the untreated group (P < 0.005), but none of the treatments protected the mice from succumbing to the infection. In conclusion, we demonstrated that P-gp inhibitors can enhance the uptake of AMB through the BBB, suggesting that AMB is a P-gp substrate.
INTRODUCTION
Fungal infection of the central nervous system (CNS) is one of the most deadly forms of invasive fungal diseases. The most common pathogens that involve the CNS included Cryptococcus neoformans, Candida spp., and Aspergillus spp., while numerous other fungi had been reported as less common causes. New-generation triazoles with both broad antifungal spectrum and good CNS penetration, such as voriconazole and posaconazole, have expanded the choice for treatment of CNS fungal infections (1). Nonetheless, amphotericin B (AMB) remains the drug of choice for many forms of CNS fungal infection. In fact, AMB is strongly recommended for treatment of CNS cryptococcosis, histoplasmosis, blastomycosis, and mucormycosis (2). AMB plus flucytosine has demonstrated favorable efficacy in AIDS-associated cryptococcal meningitis and has been recommended as the first-line induction therapy (3). Ironically, AMB is well known for its poor ability to penetrate the blood-brain barrier (BBB), as the concentrations in the cerebrospinal fluid and brain tissue are almost undetectable (4). This has posed a dilemma to physicians dealing with CNS fungal infections using AMB, because increasing the dose of AMB in an effort to raise the CNS concentration would aggravate the severity of its multisystemic peripheral toxicities, especially nephrotoxicity.
The manipulation of AMB penetration at the BBB is one strategy to improve outcome in CNS fungal infections, since the ability of antifungal drugs to achieve adequate concentrations in the CNS is one of the key factors influencing efficacy. However, despite advances in the understanding of action mechanisms, a great gap still exists in knowledge on the pharmacokinetics of AMB (5–7).
The BBB consists of a physical barrier, the metabolic barrier, and the transport barrier, including ATP-binding cassette (ABC) efflux transporters (8). P-glycoprotein (P-gp), a member of the ABC transporter protein family, is abundantly distributed on the BBB and serves as a key transporter which actively effluxes a huge variety of substances across the BBB (9, 10). P-gp at the BBB have become an area of great interest for the purpose of developing new drugs targeting the CNS, as well as improving BBB penetration of older ones through interaction with P-gp (11). Drug-drug interactions at the BBB that are mediated by P-gp for various combinations have been demonstrated by both in vitro and animal studies (12). The inhibition of P-gp has been demonstrated to increase the CNS concentrations of numerous agents, highlighting the therapeutic potential for P-gp inhibitors as synergists to improve BBB penetration of substance drugs (11). It is currently unclear whether P-gp is also involved in the BBB transportation of AMB, except that two earlier studies suggested relationships between the rat intestinal P-gp transporter and AMB (13, 14). This study aims to further investigate the effect of P-gp inhibitors in the brain uptake of AMB in vitro and in vivo and to explore the potential therapeutic effect of AMB with P-gp inhibition in a cryptococcal meningitis murine model.
MATERIALS AND METHODS
Materials.
AMB for injection (50 mg) was purchased from Asia Pioneer Pharmaceuticals, Shanghai, China. Verapamil for intravenous use (2.5 mg/ml) was purchased from Fenghe Pharmaceuticals, Shanghai, China. Itraconazole injection (100 mg/ml) was provided by Janssen-Cilag (Xi'an, China). Chemical agents were purchased from Sinopharm (Shanghai, China) as analytical-grade preparations. High-performance liquid chromatography (HPLC)-grade acetonitrile was purchased as from Dikma (Lake Forest, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and 100 U/ml penicillin-100 μg/ml streptomycin were purchased from Gibco Laboratories (Grand Island, NY, USA). Fetal bovine serum (FBS) was from Sijiqing (Hangzhou, China). Gelatin and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (USA).
Cells.
Primary brain capillary endothelial cells (BCECs) isolated from cerebral gray matter of CBA/J mouse brain were kindly provided by J. N. Lou from the Clinical Medicine Research Institute of the Chinese-Japanese Friendship Hospital and cultured as described previously (15–17).
Animals.
Male CD-1 mice (the Sino-British SIPPR/BK Laboratory), 6 to 8 weeks of age, were housed in an animal biosafety level 2 laboratory (Institute Pasteur of Shanghai Chinese Academy of Sciences), with five mice per cage, and were provided irradiated food and acidified water. Manipulations of animals were performed in accordance with guidelines from the Pasteur Animal Ethics Committee.
Uptake of amphotericin B by BCECs.
An MTT test was used to measure the effects of AMB and verapamil on BCEC viability. BCECs then were incubated with 2 μg/ml AMB in the presence or absence of 100 μmol/liter verapamil at 37°C for 10 to 150 min. Cells were washed three times with cold Hanks balanced salt solution and further incubated with Triton X-100 (1%, vol/vol) overnight at 4°C for cellular lysis. The cell lysates were centrifuged at 12,000 × g for 2 min at 4°C. The clear supernatant was collected for quantification of AMB and cell protein. BCECs also were coincubated with 2 μg/ml AMB and serial concentrations of verapamil (2 to 100 μmol/liter) for 90 min to test the dose effect of verapamil on the BCEC uptake of AMB. All experiments were performed in quadruplicates.
Murine model with cryptococcal infection.
A clinical strain of Cryptococcus neoformans, isolated from a patient with cryptococcal meningitis and stored at the clinical mycology laboratory at Huashan Hospital, was used for all experiments. This strain was susceptible to both AMB and itraconazole in vitro, as determined with the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) standard M27-A3 (18). Before use, the number of CFU in the stock was measured by cultures of serial dilutions on Sabouraud's dextrose agar (SDA) plates at 35°C for a minimum of 3 days. On the day of the experiment, the inoculum was adjusted to the desired concentrations with sterile saline, and the CFU were confirmed at the end of the experiment.
A murine model of intracerebral cryptococcal infection was established as described previously (19–21). In brief, mice were given cyclophosphamide intraperitoneally at a dose of 200 mg/kg of body weight beginning 3 days prior to inoculation and then every 6 days thereafter. Pancytopenia was documented by peripheral white blood cell counts beginning on the day of inoculation and throughout the experimental period (22). On the day of inoculation (day 0), mice were anesthetized by diethyl ether inhalation. A total of 105 yeast cells in a volume of 20 μl then were inoculated intracerebrally.
Treatment regimens in uninfected mice.
Uninfected mice were given intravenous AMB at 3 mg/kg/day for 4 days. At day 5 (24 h after the last dose of AMB), the animals were randomized to 3 groups (n = 15 for each group) that received a single dose of intravenous normal saline, verapamil at 10 mg/kg, or itraconazole at 10 mg/kg. Mice were anesthetized at 5 min, 15 min, 30 min, 45 min, and 60 min after administration. After 500-μl blood samples were collected by retro-orbital puncture, mice were perfused heart to brain with 20 ml normal saline. Brain tissues then were removed and stored at −80°C for further detection of AMB concentration by HPLC.
Treatment regimens in Cryptococcus-infected mice.
One day after inoculation, the Cryptococcus-infected mice were treated with intravenous AMB at 3 mg/kg/day consecutively for 4 days. At day 5 (24 h after the last dose of AMB), the animals were given a single dose of normal saline, verapamil at 10 mg/kg, or itraconazole at 10 mg/kg. Ten mice from each group were observed for survival conditions twice daily for a total period of 35 days postinfection, and weight change was recorded every day. Another 10 mice from each group were subjected to further measurement of brain fungal burden and AMB concentration. Blood samples and brain tissues were collected aseptically 45 min after administration of inhibitors at day 5. Brains were placed in sterile preweighed Eppendorf tubes for further measurement of CFU and AMB concentrations.
Fungal burden measurement.
After brain weights were determined, 1 ml saline for 1 g of brain tissue was added and homogenized. Serial dilutions of the whole-brain homogenate were prepared for quantitative CFU counts. The dilutions were plated as triplicates on yeast extract peptone dextrose (YEPD; 1% yeast extract, 2% dextrose, and 2% peptone) agar plates. The plates were incubated at 30°C for 48 h, and the numbers of CFU were recorded as the mean numbers of the triplicates.
Brain and plasma sample processing for HPLC.
After the weight of brain tissue was determined, saline was added at 1 ml per gram of brain tissue and then homogenized by ultrasonication. Methanol was added to the homogenate at 400 μl per gram of tissue and vortexed for 2 min. The mixture was centrifuged at 16,000 × g for 10 min at 4°C. The supernatant was collected and stored at −20°C. The plasma sample was added to methanol at 400 μl per 100 μl of plasma and then vortexed for 2 min. The mixture was centrifuged at 9,000 × g for 10 min at 4°C. The supernatant was collected and stored at −20°C. Cell lysate of BCECs was centrifuged at 12,000 × g for 2 min at 4°C, and then the supernatant was collected.
HPLC method for determination of AMB concentration.
HPLC analysis was performed with a modular liquid chromatograph system (Agilent Technologies, Wilmington, DE). Twenty microliters of supernatant was loaded onto an Agilent TC-C18 column (4.6 by 250 mm; 5-μm volume). The mobile phase consisted of acetonitrile and 10 mM sodium acetate buffer, pH 4.0 (40:60, vol/vol), and the flow rate was kept at 1 ml/min. The effluent was monitored at 408 nm.
The absorbance peak appeared at 5 min after loading. The lower limit of detection for the assay was 10 ng/ml.
Statistical analysis.
A statistical analysis of CFU results was done by a Mann-Whitney U test. The comparative survival rates were analyzed by a log-rank test. Statistical comparisons of AMB levels in brain tissues were done by analysis of variance (ANOVA) by Stata 9.0 (StataCorp).
RESULTS
Verapamil increased amphotericin B uptake by brain capillary endothelial cells.
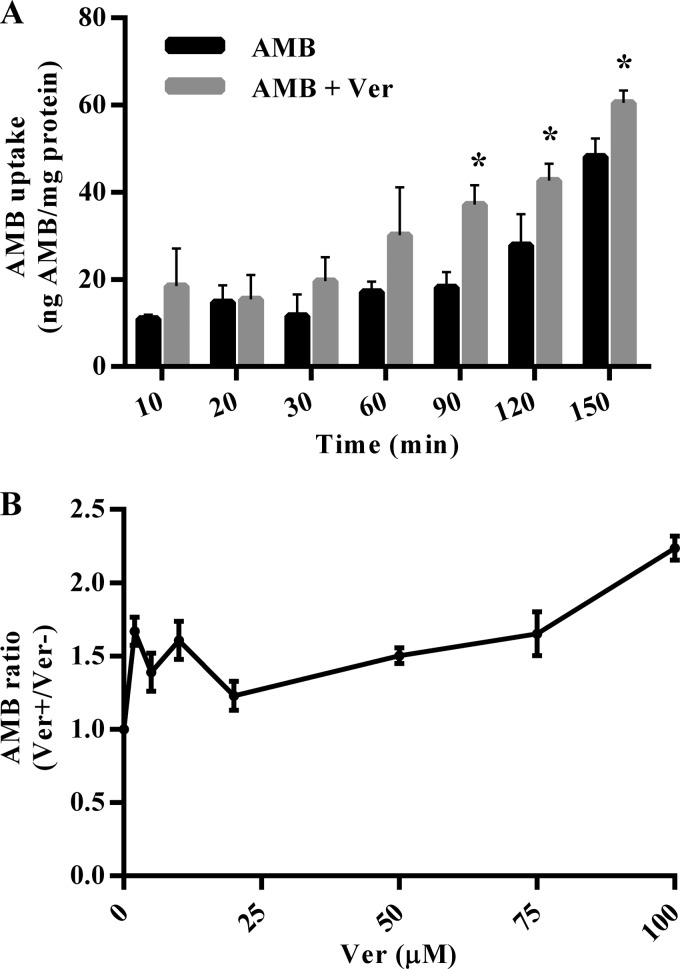
The effects of AMB and verapamil on BCEC viability were evaluated by MTT tests, which showed that BCECs retained a viability rate of >80% over a 3-h period when incubated with a wide concentration range of verapamil (0.2 to 400 μmol/liter). BCECs coincubated with AMB at a concentration of ≤10 μg/ml had a viability rate of >80%. When AMB concentrations exceeded 10 μg/ml, the viability rates decreased to less than 80% in a dose-dependent manner (data not shown). Therefore, an AMB concentration of 2 μg/ml was used for further in vitro experiments. After incubation with AMB, the BCEC uptake of AMB was increased as time passed (Fig. 1A). Compared to that of the AMB-only group, the verapamil-coincubated group had a significant higher cellular uptake of AMB at 90, 120, and 150 min. The BCEC uptake of AMB also was influenced by the concentration of coincubated verapamil. The AMB uptake level increased gradually as the concentration of verapamil increased from 20 to 100 μmol/liter. However, at lower concentrations of verapamil (0 to 20 μmol/liter), the dose-dependent manner was not evident (Fig. 1B).
FIG 1.
Amphotericin B uptake by mouse brain capillary endothelial cells was increased after coincubation with verapamil. (A) Mouse brain capillary endothelial cells were incubated with 2 μg/ml amphotericin B (AMB) with or without 100 μmol/liter verapamil (Ver). (B) Cells were coincubated with 2 μg/ml AMB and serial concentrations of Ver for 90 min. Data are means ± standard errors of the means (SEM). *, P < 0.05 compared to results at the same time point for the control (Student's t tests).
P-gp inhibitors increased the brain/plasma ratio of AMB in uninfected mice.
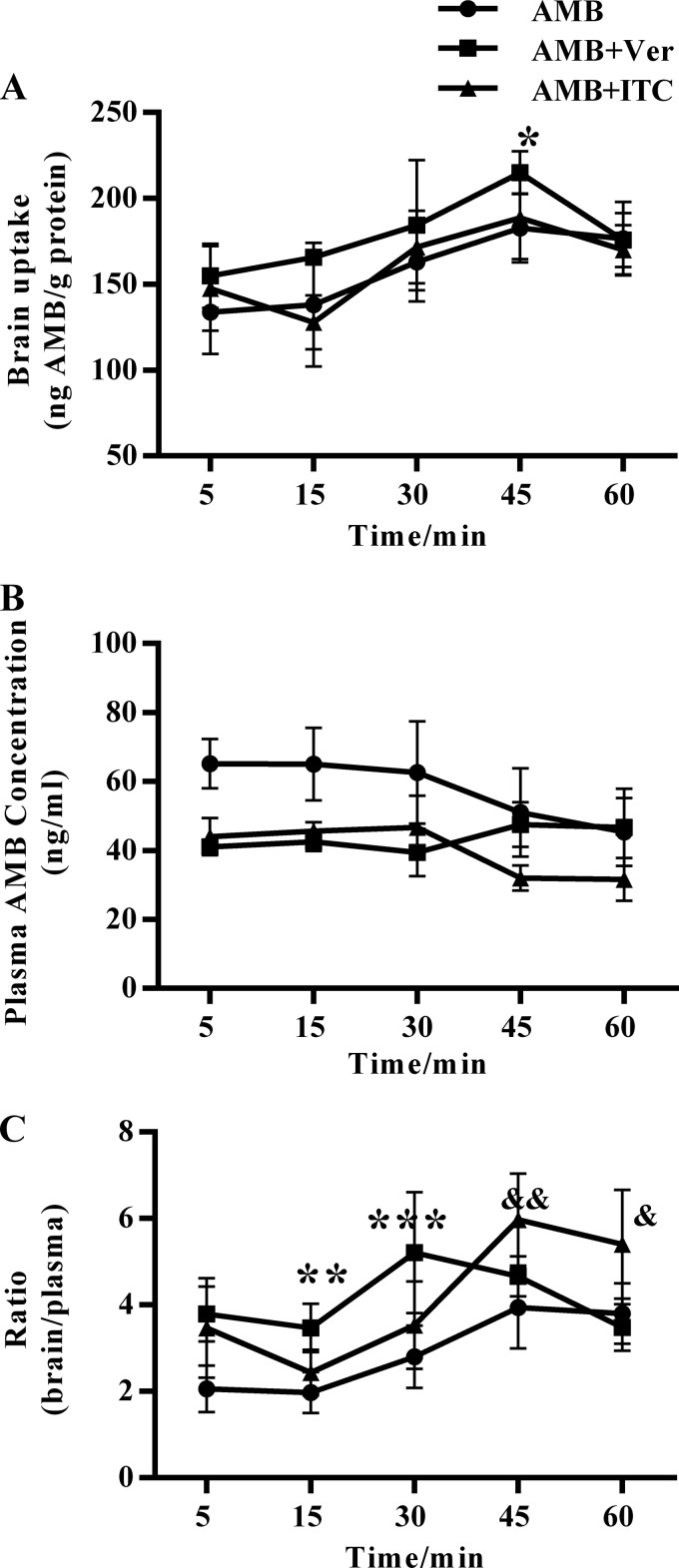
To investigate the effect of P-gp inhibitors on the brain/plasma ratio of AMB in vivo, we measured the brain and plasma AMB concentrations in uninfected CD-1 mice treated with 3 mg/kg/day AMB consecutively for 4 days, with or without additional administration of a single dose of verapamil or itraconazole at day 5. For the verapamil-cotreated group, there was a tendency toward higher brain AMB concentrations than those with the AMB-only group, and the differences was statistically significant at 45 min. For the itraconazole-cotreated group, the brain AMB concentration was similar to that of the AMB-only group (Fig. 2A). As for the AMB plasma concentrations, the verapamil-cotreated group had lower plasma levels at 5 min, 15 min, and 30 min and similar plasma levels at 45 min and 50 min compared to those of the AMB group (Fig. 2B), while the itraconazole-cotreated group had lower plasma levels at all time points from 5 min to 50 min. When brain AMB levels were explained by the plasma concentration (Fig. 2C), the brain/plasma ratios were significantly higher in the verapamil-cotreated group at 15 min (3.5 versus 2.0; P < 0.05) and 30 min (5.2 versus 2.8; P < 0.05) than in the AMB-only group. Higher brain/plasma ratios also were observed in the itraconazole-cotreated group than in the AMB-only group at 45 min (6.0 versus 3.9; P < 0.05) and 60 min (5.4 versus 3.8; P < 0.05).
FIG 2.
Verapamil and itraconazole increased the brain/plasma ratio of amphotericin B in uninfected mice. CD-1 mice were given 3 mg/kg/day amphotericin B (AMB) for 4 days. At day 5 (24 h after the last dose of AMB), the animals received a single intravenous dose of 10 mg/kg verapamil (Ver) and 10 mg/kg itraconazole (ITC). Mice were anesthetized at 5, 15, 30, 45, and 60 min after administration, and plasma and brain tissue were collected. (A) Concentrations of AMB in brain tissue were determined by HPLC. (B) Concentrations of AMB in plasma were determined by HPLC. (C) Brain/plasma ratios were calculated as AMB concentrations in brain tissue divided by those in plasma. Data are means ± standard deviations (SD). An asterisk indicates significant differences between the AMB plus Ver and AMB-only groups; an ampersand indicates significant differences between the AMB plus ITC and AMB-only groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; &, P < 0.05; &&, P < 0.01. Significance was determined by two-way ANOVA.
Verapamil and itraconazole increased AMB brain/plasma ratios and reduced brain fungal loads in Cryptococcus-infected murine model.
To explore the effect of P-gp inhibitors on AMB brain uptake in the setting of CNS infection, we used a murine model of Cryptococcus intracerebral infection treated with AMB monotherapy, AMB plus verapamil, or AMB plus itraconazole and examined the changes in brain and plasma AMB concentrations, weight, length of survival in days, and brain tissue fungal burden.
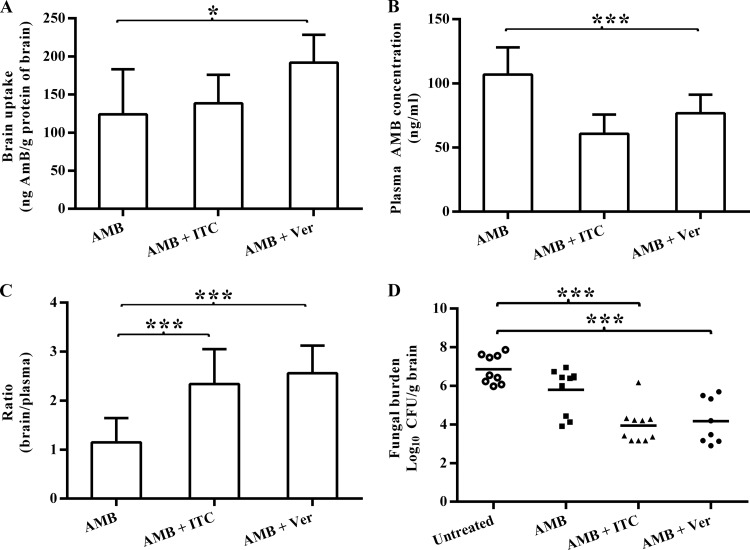
Among the three treated groups, the brain AMB concentrations were highest in the verapamil-cotreated group, followed by the itraconazole-cotreated group, and were lowest in the AMB monotherapy group (Fig. 3A). The difference in brain uptake level was statistically significant between the verapamil-cotreated group and the AMB monotherapy group (P < 0.05). Consistent with those observed in uninfected mice, the plasma concentrations of AMB were lowered after administration of either verapamil or itraconazole (Fig. 3B). The brain/plasma ratio was significantly higher in the verapamil-cotreated group (2.6 ± 0.6) and itraconazole-cotreated group (2.3 ± 0.7) than in the AMB monotherapy group (1.1 ± 0.5) (Fig. 3C).
FIG 3.
Brain/plasma ratio of amphotericin B and brain fungal burden in cryptococcal meningitis mice cotreated with P-glycoprotein inhibitors. After intracranial infection, the mice were treated with intravenous amphotericin B (AMB) at 3 mg/kg/day consecutively for 4 days and then given a single dose of saline, verapamil (Ver) at 10 mg/kg, or itraconazole (ITC) at 10 mg/kg on day 5. After 45 min, mice were anesthetized and samples were collected. (A to C) Data are expressed as the means ± SEM. (D) Each data point corresponds to the log10 CFU/g brain for an individual mouse. The bars represent median group values. P < 0.05 (*) and P < 0.001 (***) by one-way ANOVA for panels A to C and by Mann-Whitney U test for panel D.
The fungal burden of the cryptococcal meningitis murine model was presented as CFU recovered from brain tissues (Fig. 3D). Among the two groups cotreated with verapamil or itraconazole, the levels of Cryptococcus CFU in the brain tissue were significantly lower than those in the untreated group. There was no significant difference in brain CFU between the verapamil-cotreated and the itraconazole-cotreated groups.
In the survival study (Fig. 4A), fatal outcomes for 100% of the untreated mice were observed between 6 and 14 days postinfection (median survival time, 8 days), consistent with our previous study (19). For the treated group, the median survival time was 17 days for the AMB monotherapy group, 19 days for the verapamil-cotreated group, and 22 days for the itraconazole-cotreated group. Treated groups exhibited significantly better survival than the untreated group (P < 0.01 for the AMB-treated group versus the untreated group by log-rank tests). The median survival time of the verapamil-cotreated group and itraconazole-cotreated group was 2 and 5 days longer than that of the AMB group, and the differences were not statistically significant. At day 35 postinfection, the termination of observation, all animals were deceased except for two animals from the itraconazole-cotreated group, indicating that none of the treatments produced adequate long-term protection against this infection. The weight changes in these groups showed a pattern consistent with the survival curve (Fig. 4B).
FIG 4.
Survival and weight changes in the cryptococcal meningitis murine model treated with amphotericin B and P-glycoprotein inhibitors. The mice were treated with amphotericin B (AMB) at 3 mg/kg/day consecutively for 4 days and then given a single dose of normal saline, verapamil (Ver) at 10 mg/kg, or itraconazole (ITC) at 10 mg/kg on day 5. Data are means ± SEM in panel B. **, P < 0.01 by log-rank test for comparison between the untreated group and the AMB monotherapy group.
DISCUSSION
AMB has been tested in previous studies for its ability to reverse P-gp-mediated drug resistance in P-gp-overexpressing cells. One study evaluated the effect of AMB on vinblastine and paclitaxel resistance, where no reversal of resistance was observed (23). Another study tested the effect of several antifungals on P-gp transport activity using [3H]digoxin and found that the P-gp mediated transport of [3H]digoxin was not inhibited by AMB (24). Nevertheless, the lack of inhibitory effect on P-gp does not exclude AMB as a P-gp substrate, as the P-gp substrate is not necessarily correlated with P-gp inhibitors. In the present study, we hypothesized that the low concentration of AMB in the brain was a result of P-gp-mediated active transportation at the BBB. Verapamil and itraconazole were used as P-gp inhibitors in this study to test their drug-drug interaction with AMB. Our data showed that the uptake of AMB by BCECs was increased with the presence of verapamil, providing the first evidence that AMB is transported through the BBB by P-gp (Fig. 1).
To test our hypothesis in vivo, we further examined the effect of P-gp inhibitors on the brain/plasma ratio of AMB in CD-1 mice. As shown in our data, the AMB brain/plasma ratio was significantly increased after administration of verapamil and itraconazole, indicating that P-gp-mediated efflux plays an important role in the transportation of AMB at the BBB (Fig. 2). Consistent with our results, interaction between AMB and P-gp has been suggested by other pieces of evidence from studies on animal intestines (13, 14, 25). In a study by Ishizaki and colleagues, the oral bioavailability of cyclosporine was decreased after coadministration of AMB in Wistar rats, presumably due to the increased P-gp expression induced by AMB at the duodenum (14). Second, another research group demonstrated enhanced gastrointestinal absorption of AMB through formulating it in the oral lipid-based delivery system Peceol, which was able to decrease P-gp-mediated drug efflux by downregulating mdr1 mRNA in Caco-2 cells, also suggesting an interaction between this agent and P-gp (13, 26).
It was also observed in our results that the plasma concentrations of AMB were substantially decreased after administration of verapamil or itraconazole, which was unexpected, since elevated blood concentrations have been reported for other P-gp substrate agents in the presence of P-gp inhibitors due to the suppressed P-gp mediated efflux at the intestine and the kidney (11). Possible explanations for the decreased AMB plasma concentrations include that verapamil and itraconazole lead to redistribution of AMB in other organs through inhibition of the P-gp transporter elsewhere. Further investigations will be required to explain this phenomenon of decrease in plasma concentration of AMB.
Since AMB is used in the setting of fungal infections, it is important to know whether the improvement in BBB penetration observed in uninfected mice could be duplicated in those with CNS fungal infection. Therefore, we used a murine model of cryptococcal meningitis to examine the effect of verapamil and itraconazole on brain accumulation of AMB. Consistent with results observed in uninfected mice, the brain uptake of AMB in infected mice was increased after administration of either verapamil or itraconazole (Fig. 3). Although the fungal burden was decreased by the AMB and P-gp treatments compared to the burden in untreated mice, the burden was still high; thus, the animals still died. To actually effect a cure, higher doses, a longer treatment course of AMB, or multiple P-gp inhibitor administrations at higher doses would be required to maintain higher levels in the brain long enough to really be effective in the long run.
Verapamil, as the first discovered P-gp inhibitor, has been examined for clinical efficacy as a synergist of chemotherapy in several clinical trials but fails to demonstrate efficacy (11). Itraconazole not only is a P-gp inhibitor but also is an antifungal agent with activity against Cryptococcus spp. A previous study has found that the combination of itraconazole and AMB showed synergistic or additive in vitro interaction for 80% of clinical isolates of Cryptococcus neoformans, and no antagonism was observed (27). Itraconazole had been proved to be efficacious in the treatment of cryptococcal meningitis, although data concerning the combination of AMB and itraconazole as an induction therapy for cryptococcal meningitis is sparse (3, 28, 29). Our results suggest that AMB and itraconazole combination is likely to a strategy in the initial treatment of cryptococcal meningitis with improved efficacy over AMB monotherapy through dual mechanisms of additional antifungal activity and enhanced AMB accumulation due to inhibition of P-gp at the BBB by itraconazole. Further study is warranted to examine the efficacy and safety of this combination in cryptococcal meningitis patients against AMB monotherapy.
In summary, the present study has demonstrated for the first time that AMB is likely to be a substrate of P-gp, and that P-gp inhibitors can enhance the uptake of AMB through the BBB, leading to reduced fungal burden in the brain. The combination of AMB and P-gp inhibitors might provide a novel strategy in improving the efficacy of AMB in treatment of CNS fungal infections.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key Basic Research Program of China (grant number 2013CB531600), the Shanghai Natural Science Foundation (grant number 10ZR1405700), and the National Natural Science Foundation of China (grant number 81271803).
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.Black KE, Baden LR. 2007. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs 21:293–318. 10.2165/00023210-200721040-00004 [DOI] [PubMed] [Google Scholar]
- 2.Kuyucu N. 2011. Amphotericin B use in children: conventional and lipid-based formulations. Expert Rev. Anti Infect. Ther. 9:357–367. 10.1586/eri.11.5 [DOI] [PubMed] [Google Scholar]
- 3.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 50:291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kethireddy S, Andes D. 2007. CNS pharmacokinetics of antifungal agents. Expert Opin. Drug Metab. Toxicol. 3:573–581. 10.1517/17425255.3.4.573 [DOI] [PubMed] [Google Scholar]
- 5.Palacios DS, Anderson TM, Burke MD. 2007. A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J. Am. Chem. Soc. 129:13804–13805. 10.1021/ja075739o [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Ami R, Lewis RE, Kontoyiannis DP. 2008. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin. Infect. Dis. 47:226–235. 10.1086/589290 [DOI] [PubMed] [Google Scholar]
- 7.Mesa-Arango AC, Scorzoni L, Zaragoza O. 2012. It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 3:286. 10.3389/fmicb.2012.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. 2010. Structure and function of the blood-brain barrier. Neurobiol. Dis. 37:13–25. 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 9.Schinkel AH, Wagenaar E, Mol van Deemter CAL. 1996. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Investig. 97:2517–2524. 10.1172/JCI118699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. 1989. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. U. S. A. 86:695–698. 10.1073/pnas.86.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. 2012. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr. Med. Chem. 19:1946–2025. 10.2174/092986712800167392 [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Chen C, Smith BJ. 2008. Progress in brain penetration evaluation in drug discovery and development. J. Pharmacol. Exp. Ther. 325:349–356. 10.1124/jpet.107.130294 [DOI] [PubMed] [Google Scholar]
- 13.Risovic V, Sachs-Barrable K, Boyd M, Wasan KM. 2004. Potential mechanisms by which Peceol increases the gastrointestinal absorption of amphotericin B. Drug Dev. Ind. Pharm. 30:767–774. 10.1081/DDC-120039793 [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki J, Ito S, Jin M, Shimada T, Ishigaki T, Harasawa Y, Yokogawa K, Takami A, Nakao S, Miyamoto K. 2008. Mechanism of decrease of oral bioavailability of cyclosporin A during immunotherapy upon coadministration of amphotericin B. Biopharm. Drug Dispos. 29:195–203. 10.1002/bdd.604 [DOI] [PubMed] [Google Scholar]
- 15.Lou J, Gasche Y, Zheng L, Critico B, Monso-Hinard C, Juillard P, Morel P, Buurman WA, Grau GE. 1998. Differential reactivity of brain microvascular endothelial cells to TNF reflects the genetic susceptibility to cerebral malaria. Eur. J. Immunol. 28:3989–4000 [DOI] [PubMed] [Google Scholar]
- 16.Xie Y, Ye LY, Zhang XB, Hou XP, Lou JN. 2004. Establishment of an in vitro model of brain-blood barrier. Beijing Da Xue Xue Bao 36:435–438 [PubMed] [Google Scholar]
- 17.Shao K, Huang R, Li J, Han L, Ye L, Lou J, Jiang C. 2010. Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J. Control Release 147:118–126. 10.1016/j.jconrel.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19.Shao K, Wu J, Chen Z, Huang S, Li J, Ye L, Lou J, Zhu L, Jiang C. 2012. A brain-vectored angiopep-2 based polymeric micelles for the treatment of intracranial fungal infection. Biomaterials 33:6898–6907. 10.1016/j.biomaterials.2012.06.050 [DOI] [PubMed] [Google Scholar]
- 20.Capilla J, Clemons KV, Stevens DA. 2007. Animal models: an important tool in mycology. Med. Mycol. 45:657–684. 10.1080/13693780701644140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemons KV, Schwartz JA, Stevens DA. 2012. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob. Agents Chemother. 56:4439–4449. 10.1128/AAC.06015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiller TM, Luque JC, Sobel RA, Farrokhshad K, Clemons KV, Stevens DA. 2002. Development of a murine model of cerebral aspergillosis. J. Infect. Dis. 186:574–577. 10.1086/341567 [DOI] [PubMed] [Google Scholar]
- 23.Iida N, Takara K, Ohmoto N, Nakamura T, Kimura T, Wada A, Hirai M, Sakaeda T, Okumura K. 2001. Reversal effects of antifungal drugs on multidrug resistance in MDR1-overexpressing HeLa cells. Biol. Pharm. Bull. 24:1032–1036. 10.1248/bpb.24.1032 [DOI] [PubMed] [Google Scholar]
- 24.Sakaeda T, Iwaki K, Kakumoto M, Nishikawa M, Niwa T, Jin JS, Nakamura T, Nishiguchi K, Okamura N, Okumura K. 2005. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J. Pharm. Pharmacol. 57:759–764. 10.1211/0022357056118 [DOI] [PubMed] [Google Scholar]
- 25.Ashbee HR, Gilleece MH. 2012. Has the era of individualised medicine arrived for antifungals? A review of antifungal pharmacogenomics. Bone Marrow Transplant. 47:881–894. 10.1038/bmt.2011.146 [DOI] [PubMed] [Google Scholar]
- 26.Sachs-Barrable K, Lee SD, Wasan EK, Thornton SJ, Wasan KM. 2008. Enhancing drug absorption using lipids: a case study presenting the development and pharmacological evaluation of a novel lipid-based oral amphotericin B formulation for the treatment of systemic fungal infections. Adv. Drug Deliv. Rev. 60:692–701. 10.1016/j.addr.2007.08.042 [DOI] [PubMed] [Google Scholar]
- 27.Barchiesi F, Schimizzi AM, Caselli F, Novelli A, Fallani S, Giannini D, Arzeni D, Di Cesare S, Di Francesco LF, Fortuna M, Giacometti A, Carle F, Mazzei T, Scalise G. 2000. Interactions between triazoles and amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2435–2441. 10.1128/AAC.44.9.2435-2441.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denning DW, Tucker RM, Hanson LH, Hamilton JR, Stevens DA. 1989. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch. Intern. Med. 149:2301–2308 [PubMed] [Google Scholar]
- 29.Zhu LP, Wu JQ, Xu B, Ou XT, Zhang QQ, Weng XH. 2010. Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997–2007. Med. Mycol. 48:570–579. 10.3109/13693780903437876 [DOI] [PubMed] [Google Scholar]