Abstract
A patient receiving daptomycin developed asymptomatic transaminitis and hyperbilirubinemia without concurrent multiorgan dysfunction or elevation of his creatinine kinase level. After ruling out other etiologies, the liver injury was attributed to daptomycin and was subsequently resolved. A single-center retrospective cohort analysis of baseline and follow-up liver function panels (n = 614) from all admissions from 2008 to 2013 during which daptomycin was administered did not reveal any other cases of probable or definite drug-induced liver injury associated with daptomycin.
TEXT
Drug-induced liver injury (DILI) has been ascribed to >1,000 medications (1). While various scales have been developed to establish causality (e.g., the Naranjo probability scale, the Roussel Uclaf causality assessment method [RUCAM], the Maria and Victorino [M&V] scale, and the Council for International Organizations of Medical Sciences [CIOMS] scale), none has been adopted as a gold standard (2–5). The U.S. National Library of Medicine suggests that expert opinion is also suitable for attributing causality (see http://livertox.nih.gov/).
Daptomycin has been associated with transaminase elevations in 3% of subjects (6) but not with other findings indicating a hepatic origin (e.g., gamma-glutamyl transferase [GGT]) (Cubist Pharmaceuticals Medical Affairs, written communication). Therefore, the elevations likely reflect myocyte injury, a known effect that necessitates creatinine kinase (CK) monitoring. One previous case of daptomycin-associated DILI mirrored this pattern, while another report described a patient with multiorgan failure (7, 8). The case discussed below is unique because the patient developed isolated asymptomatic liver injury.
Case report.
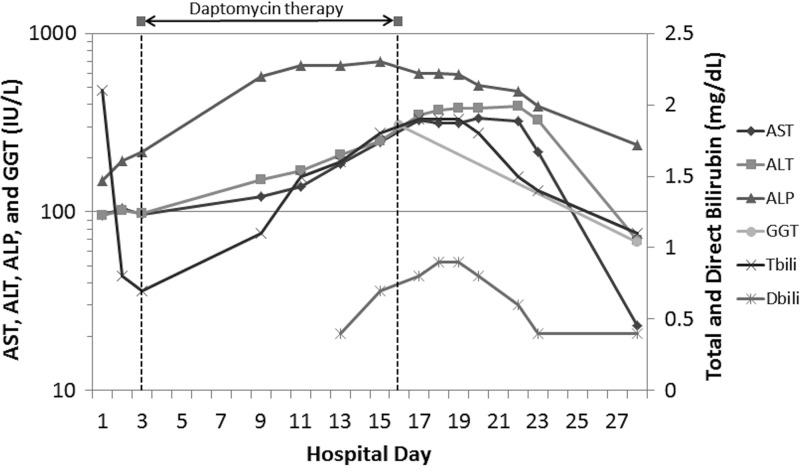
A 31-year-old man with a history of psychiatric disorders, degenerative disc disease with chronic back pain, and intravenous drug abuse presented with suicidal ideation. He also reported a productive cough, left-sided inspiratory chest pain, abdominal pain, and back pain. He denied using ethanol or drugs except previously prescribed oxycodone (30 mg every 4 h as needed) and alprazolam (2 mg daily). The physical exam revealed an abscess involving the left antecubital fossa, into which the patient eventually admitted to injecting crushed morphine tablets. No jaundice or hepatosplenomegaly was identified. He was febrile to 39.3°C. His urine drug screen was positive for opiates and benzodiazepines. His liver function test (LFT) values were elevated, including a total bilirubin level of 2.1 mg/dl, an aspartate aminotransferase (AST) level of 95 IU/liter, an alanine aminotransferase (ALT) level of 95 IU/liter, and an alkaline phosphatase (ALP) level of 150 IU/liter (Fig. 1). After ruling out vertebral osteomyelitis with a gallium-67-tagged white cell scan, the abdominal pain was attributed to opioid withdrawal, and the patient was treated with dicyclomine and an opioid taper.
FIG 1.
LFT results before, during, and after daptomycin administration.
On hospital day 3, the blood cultures started at admission grew Gram-positive cocci, and vancomycin was initiated. Treatment was escalated to 500 mg of daptomycin (6.9 mg per kg of body weight) daily on hospital day 4 for persistent Gram-positive bacteremia identified as methicillin-resistant Staphylococcus aureus (vancomycin MIC, 2 μg/ml). Echocardiography results were consistent with tricuspid valve endocarditis. On hospital day 10, the patient's LFT values were significantly elevated. His serum creatinine and blood urea nitrogen levels were stable at 0.8 and 6 mg/dl, respectively, his creatinine kinase level was 43 IU/liter, and his GGT level was 307 IU/liter (reference range, 5 to 65 IU/liter). Coagulation assay results remained normal, and his albumin level was stable at 2.8 g/dl. Left abdominal sonography and magnetic resonance tomography revealed no masses, edema, or any other focal lesions that explained the abnormal LFT results. The patient was seropositive for hepatitis A and B surface antibodies due to known immunization history, but he was seronegative for hepatitis A immunoglobulin M antibodies, hepatitis B core antibodies, and hepatitis C antibodies. There was also no evidence of antinuclear antibodies. A transthoracic echocardiogram revealed normal right- and left-sided filling pressures and normal biventricular geometry and septal wall motion, ruling out hepatic congestion from heart failure as a potential causative explanation for the abnormal LFT results.
At this point, DILI was considered. A medication review identified three possible causes, including daptomycin, senna, and dicyclomine. Since previous cases of DILI associated with daptomycin involved multiorgan dysfunction or CK elevation, the daptomycin was continued. Although this patient's presentation was not consistent with previous reports of senna- or dicyclomine-associated DILI, both medications were discontinued on hospital day 12. His LFT values continued to increase, so the gastrointestinal service was consulted. It was agreed that daptomycin-related DILI was the most likely etiology, so the daptomycin was discontinued on hospital day 17. Shortly thereafter, his transaminase and bilirubin values peaked and subsequently declined.
This patient's LFT values were consistent with a mixed hepatocellular and cholestatic liver injury that resolved after drug discontinuation. Given the temporal relationships without an alternative explanation, there is a probable association according to the Naranjo scale. Using the RUCAM, the M&V scale, and the CIOMS scale, causality varied from unlikely to probable depending upon points assigned for previous reports, the exclusion of other causes, and whether senna and dicyclomine are considered possible causes. Based on the opinion of all the health care providers involved, daptomycin-induced liver injury was diagnosed.
Retrospective cohort analysis.
Internal data were subsequently reviewed to identify any cases of DILI among patients receiving daptomycin between 1 May 2008 and 30 March 2013. The single-center retrospective cohort analysis was approved by the institutional review board of the Medical University of South Carolina. Admissions with LFTs performed prior to and during therapy were reviewed for new elevations in ALT or total bilirubin levels. ALT elevations were identified if the on-therapy value was at least two times the upper limit of the reference range and greater than the baseline value. For total bilirubin, an elevation was defined as an on-therapy value of at least 2.5 mg/dl and a baseline value within the reference range (<1.3 mg/dl). Eligible cases were assessed using the Naranjo scale. Among 759 admissions during which daptomycin was administered, 9 were associated with an ALT elevation, 10 with a total bilirubin elevation, and 5 with an elevation of ALT and total bilirubin (Table 1). A presumed etiology was noted in the discharge summary or progress notes for most patients, including graft-versus-host disease (GVHD), engraftment syndrome, hepatic fungal infection, primary biliary cirrhosis, and shock liver related to hypotension. The retrospective chart review was consistent with the noted etiology in all cases. Among the cases without an attribution, the two patients with ALT elevations improved while continuing daptomycin and had a Naranjo score of 0. Two of the five total bilirubin elevations resolved while on therapy, but the other 3 did not have another value measured while on therapy. The Naranjo scores for these 3 events were 2, 3, and 2; the first two cases involved a recent hematopoietic stem cell transplant and septic shock, and the third patient had recently undergone several procedures, red cell transfusion, and high-dose methotrexate therapy. The 5 cases with elevations of both ALT and total bilirubin levels were attributed to a combination of triazole or rifampin therapy for GVHD or sinusoidal obstructive syndrome.
TABLE 1.
Characterization of LFT elevations among patients receiving daptomycin
| Elevated LFT value(s) that met criteria | Days of daptomycin therapya | Day of first LFT value increasea | Day of LFT value peaka | Resolved on therapy (no. of patients) | Naranjo scorea | Attributed to other cause (no. of patients) |
|---|---|---|---|---|---|---|
| ALT (n = 9) | 10 (3–25) | 2 (1–10) | 2 (2–7)b | 6 | 1 (0–2) | 7 |
| Total bilirubin (n = 10) | 5 (3–11) | 2 (1–8) | 4 (2–9)b | 3 | 1 (0–3) | 5 |
| ALT and total bilirubin (n = 5) | 6 (2–13) | 2 (2–6)c | 6 (5–6)b,c | 1 | 2 (1–2) | 2 |
Values reported as median (range).
Peak values occurred after discontinuation of therapy in 2 patients in each group who were excluded from this column.
Day of first increase and day of peak were based on ALT levels.
The major limitation of this analysis is the lack of routine LFTs, so the timing of onset and peak was based on available data. While some cases included further diagnostic workups, i.e., imaging and/or biopsy, most patients had limited evaluations, especially if a cause was apparent. None of the cases appeared consistent with daptomycin-induced liver injury.
In conclusion, daptomycin appears to be a rare cause of DILI, but clinicians should be aware of the potential for isolated daptomycin-associated hepatotoxicity.
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Stirnimann G, Kessebohm K, Lauterburg B. 2010. Liver injury caused by drugs: an update. Swiss Med. Wkly. 140:w13080. 10.4414/smw.2010.13080 [DOI] [PubMed] [Google Scholar]
- 2.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. 1981. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30:239–245. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 3.Danan G, Benichou C. 1993. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol. 46:1323–1330. 10.1016/0895-4356(93)90101-6 [DOI] [PubMed] [Google Scholar]
- 4.Maria VA, Victorino RM. 1997. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 26:664–669. 10.1002/hep.510260319 [DOI] [PubMed] [Google Scholar]
- 5.Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. 2014. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J. Hepatol. 6:17–32. 10.4254/wjh.v6.i1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubist Pharmaceuticals. 2013. Cubicin package insert. Cubist Pharmaceuticals, Lexington, MA [Google Scholar]
- 7.Abraham G, Finkelberg D, Spooner LM. 2008. Daptomycin-induced acute renal and hepatic toxicity without rhabdomyolysis. Ann. Pharmacother. 42:719–721. 10.1345/aph.1K579 [DOI] [PubMed] [Google Scholar]
- 8.Echevarria K, Datta P, Cadena J, Lewis JS., II 2005. Severe myopathy and possible hepatotoxicity related to daptomycin. J. Antimicrob. Chemother. 55:599–600. 10.1093/jac/dki058 [DOI] [PubMed] [Google Scholar]