Abstract
Infective endocarditis due to methicillin-resistant Staphylococcus aureus (MRSA IE) is associated with high morbidity and mortality. Vancomycin continues to be the primary treatment for this disease. The emergence of heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA), defined as a modified population analysis profile (PAP) of ≥0.9, may affect patient outcomes. The objective of this study was to evaluate the relationship of vancomycin subpopulation susceptibility and the clinical outcomes of MRSA IE. We conducted a retrospective cohort study of patients treated with vancomycin for MRSA IE from 2002 to 2013 at the Detroit Medical Center. A modified PAP was used to measure the vancomycin PAP MIC and the PAP-to-area under the curve (AUC) ratio. Treatment failure was defined as bacteremia for ≥7 days or death attributed to MRSA. Classification and regression tree (CART) analysis was used to select a failure breakpoint between the PAP-AUC ratios and the PAP MIC. A total of 202 patients were included in the study. Twenty-seven percent of the patients had left-sided IE, 19% of the strains were hVISA, and 70% of the strains were staphylococcal cassette chromosome mec element (SCCmec) type IV. Overall treatment failure was observed in 64%; 59% had persistent bacteremia, and the 30-day attributable mortality rate was 21%. The CART breakpoint between failure and success in terms of the PAP-AUC ratio was 0.9035. On logistic regression analysis, intensive care unit (ICU) admission (adjusted odds ratio [aOR], 2.8; 95% confidence interval [CI], 1.5 to 5.2) and a PAP MIC of ≥4 mg/liter (aOR, 3.2; 95% CI, 1.3 to 8.4) were associated with failure (P = 0.001 and 0.015, respectively). A PAP MIC of ≥4 mg/liter and ICU admission were significant for treatment failure for patients with MRSA IE. The PAP-AUC ratio of ≥0.9035 predicted failure consistent with the hVISA definition. The role of population MIC analysis in predicting outcome with MRSA infections warrants further investigation.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a pathogen that causes serious infections in both the community and hospital settings (1–3). Infective endocarditis (IE) is one of the most complicated infections caused by MRSA and is associated with high morbidity and mortality (4–7). National discharge data reported an annual incidence of IE-related hospitalization in the United States of as high as 12.7 per 100,000 patients, which is considerably more common now than was previously identified (8). A study by the International Collaboration on Endocarditis (ICE) investigated a cohort of 65 MRSA IE patients and reported hospital mortality rates of as high as 37% and a persistent bacteremia rate of as high as 46% (9).
Vancomycin is a glycopeptide that has been considered the primary therapy for Gram-positive infections, including severe infections caused by MRSA bacteremia and IE (10). A recent meta-analysis and systematic review of complicated MRSA bloodstream infections (BSI) has described MRSA strains with vancomycin MICs at the high end of the susceptibility range (>1 mg/liter) as being associated with a higher mortality rate (11). Previous literature has reported vancomycin failure rates ranging from 31 to 53% and a general reduction in vancomycin susceptibility (12–15). Prolonged use and suboptimal dosing of vancomycin may possibly have led to the emergence of MRSA strains with reduced susceptibility, including heterogeneous vancomycin-intermediate S. aureus (hVISA) and vancomycin-intermediate S. aureus (VISA) strains (16). It has been reported that patients with high-inoculum infections, such as IE, appear to have a higher proportion of hVISA (9).
Heterogeneous susceptibility to vancomycin (e.g., hVISA) has been defined in the literature as a modified population analysis profile (PAP)-to-area under the concentration curve (AUC) ratio for the hVISA S. aureus ATCC strain 700698 (Mu3) of ≥0.9 (17). Several studies have evaluated the presence of hVISA in various types of MRSA infections and reported high frequencies of treatment failure, prolonged length of hospital stay, and persistent bacteremia (12, 18). However, this heterogeneity in vancomycin susceptibility has never been directly correlated with patient outcomes within a specific cohort of patients. Although S. aureus strains with higher vancomycin MIC values by definition exist within the subpopulations of hVISA organisms, it is unknown whether the use of these MIC values is a better predictor of patient outcome in high-inoculum infections, such as infective endocarditis. The characterization of treatment outcomes with respect to patient underlying conditions, pathogen susceptibility, and overall antimicrobial exposure is important in order to understand the reason for poor drug performance and discover a potential for alternative management strategies; therefore, our objective was to evaluate the relationship of a vancomycin subpopulation susceptibility profile and the clinical outcomes of patients treated with vancomycin for MRSA IE.
MATERIALS AND METHODS
This was an investigational review board-approved retrospective cohort study conducted from January 2002 to July 2013 at the Detroit Medical Center. Adult patients ≥18 years old who received ≥72 h of vancomycin therapy for MRSA IE were included for analysis. A diagnosis of IE included possible, probable, or definite IE as documented by the treating physician, according to modified Duke criteria with MRSA bloodstream infection (BSI) (19). MRSA BSI was defined as MRSA in blood cultures that met the Centers for Disease Control and Prevention (CDC) criteria for primary bloodstream infections (20).
MRSA isolates were retrieved from initial patient positive blood cultures, and vancomycin MICs were determined by the broth microdilution (BMD) and Etest (bioMérieux, Durham, NC) methods, performed in agreement with the Clinical and Laboratory Standards Institute (CLSI) guidelines and per the manufacturer's instructions, respectively (21). The isolates were screened and confirmed by a modified population analysis profile (mPAP) to be hVISA. Vancomycin mPAPs were determined at an inoculum of approximately 1 × 109 CFU/ml, adjusted to a 1 × 108 CFU/ml density, and spiral plated (Don Whitley Scientific Limited, West Yorkshire, England) onto brain heart infusion (BHI) agar (Difco, Detroit, MI) plates containing 0, 0.5, 1, 1.5, 2, 3, 4, 8, or 16 mg/liter of vancomycin. The mPAPs of all MRSA isolates were compared to that of the reference strain Mu3 (ATCC 700689), and a MRSA isolate was considered hVISA if the population analysis profile-to-area under the curve (PAP-AUC) ratio to Mu3 was ≥0.9, as previously described (17). A vancomycin population analysis profile MIC (PAP MIC) was determined from the vancomycin mPAP and was defined as the lowest vancomycin concentration that inhibited growth to below the level of detection. The staphylococcal cassette chromosome mec (SCCmec) type, accessory gene regulator (agr) genotype group, and USA300 or USA400 grouping for the isolates were characterized using multiplex PCR, as described previously (22–24). The expression of the agr gene cluster was determined by quantitating delta-hemolysin production, utilizing a previously described method by cross-streaking test strains perpendicular to S. aureus strain RN4220 (25).
Data collection included patient characteristics, the presence of comorbid conditions (e.g., diabetes and renal disease), Acute Physiology and Chronic Health Evaluation (APACHE) II score (26), and Charlson comorbidity index (27) at the time the first positive blood cultures were drawn. Additional data collected included the duration of bacteremia, antimicrobial therapy, initial vancomycin steady-state trough serum concentration, length of hospital stay, and 30-day attributable mortality rate.
The primary outcome was based on vancomycin treatment failure, defined as a composite by involving at least one of the following criteria: persistent bacteremia for ≥7 days from the first initial positive MRSA blood culture or death attributable to MRSA within 30 days after discharge. Death was considered to be attributed to MRSA infection if one of the following criteria were present: (i) blood cultures were positive for MRSA at the time of death, (ii) death occurred before the resolution of signs or symptoms of MRSA infection, (iii) death occurred 14 days after the onset of MRSA without another explanation, (iv) autopsy findings indicated MRSA as a cause of death, or (v) MRSA was indicated as a cause of death on the death certificate (12). The study data were collected and managed using Research Electronic Data Capture (REDCap), Vanderbilt University, with the electronic data capture tools hosted at Wayne State University (28).
Categorical variables were compared by chi-square or Fisher's exact test, if appropriate. Continuous variables were compared by Student's t test or the Mann-Whitney U test for parametric or nonparametric variables, respectively. Multivariable analyses were performed to determine the independent association of vancomycin treatment failure while adjusting for confounding variables. All variables significantly associated with the outcome on bivariate analysis (P ≤ 0.2) and with clinical rationale were considered for inclusion in the explanatory multivariable model using a stepwise logistic regression backward approach. Classification and regression tree (CART) analysis was used to select the vancomycin PAP-AUC ratio to Mu3 and the PAP MIC in order to determine the influence of the vancomycin treatment failure rate. The nodes in the CART were constrained to have a minimum size of 100 cases in the parent nodes and 50 cases in the terminal nodes. A split-sample validation was performed using a random assignment of 50% training samples and 50% test samples to assess the predictive ability of the regression tree model. The maximum number of tree depth levels was set at five. A Gini impurity measure was set at a minimum change in improvement of 0.0001. All tests were two-tailed, and a P value of <0.05 was considered to be statistically significant. SPSS Statistics, version 21.0 (SPSS, Inc., Chicago, IL) was used for all calculations.
RESULTS
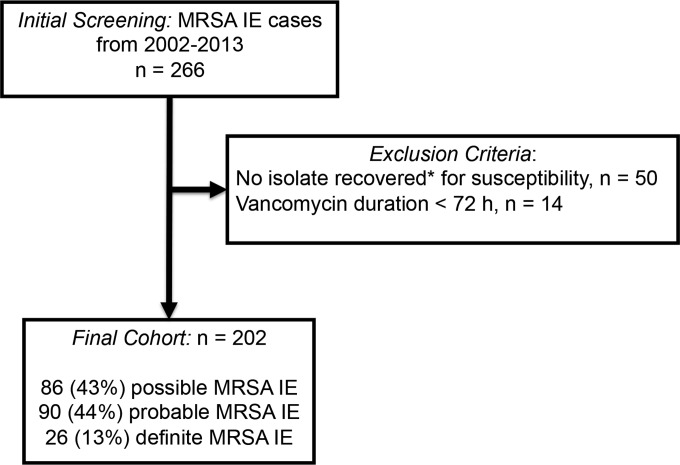
A total of 266 patients had MRSA IE from 2002 to 2013. Of these, 202 (75.9%) patients were included for analysis (Fig. 1). The median age was 53 years, with an interquartile range (IQR) of 45 to 59 years, and the median APACHE II score was 12 (IQR, 8 to 18). The manual broth microdilution (BMD) vancomycin MIC50 and MIC90 values were 1 mg/liter and 2 mg/liter, respectively. The vancomycin Etest MIC50 and MIC90 values were 1.5 mg/liter and 2 mg/liter, respectively. By the BMD definition (>2 mg/liter and <16 mg/liter), three (1.5%) isolates were identified as VISA. As confirmed by population analysis, 38 (18.8%) were hVISA, with a modified PAP-AUC ratio to Mu3 of ≥0.9. The vancomycin PAP MIC50 and PAP MIC90 values were 3 mg/liter and 4 mg/liter, respectively. The majority of isolates were either SCCmec type II or IV, with frequencies of 57 (28.2%) and 141 (69.8%), respectively. One hundred twelve (55.4%) MRSA isolates were identified as USA300 strains. The most common agr genotypes were 1 and 2, with proportions of 124 (61.4%) and 71 (35.1%), respectively. Of all the MRSA strains, 36 (17.8%) were identified as having agr dysfunction.
FIG 1.
Study population flow chart. *, isolates that were not recovered from the clinical laboratory to do central vancomycin susceptibility (e.g., MIC and PAP) were excluded.
During hospitalization, the total median duration of antimicrobial therapy for MRSA IE was 17 days (IQR, 12 to 27). Of interest, all patients were initially treated with vancomycin, and the median duration of inpatient vancomycin treatment was 9 days (IQR, 4 to 16 days). One hundred eighty-eight (93.1%) patients had available initial vancomycin steady-state trough concentration information; the median initial trough concentration was 14.9 mg/liter (IQR, 10.2 to 20.15 mg/liter). Ninety-three (49.5%) patients had an initial trough concentration value of ≥15 mg/liter, and 46 (24.5%) had initial vancomycin trough concentrations between 15 and 20 mg/liter. A total of 103 (51.0%) patients switched from vancomycin to another anti-MRSA agent. Of these, 89 (86.4%) were switched to daptomycin and 9 (8.7%) were switched to linezolid. A total of 129 (63.9%) continued antimicrobials as outpatient therapy, with the two most common antimicrobials prescribed being vancomycin and daptomycin, in 57 (44.2%) and 47 (36.4%) patients, respectively.
Overall, the median duration of bacteremia was 7 days (IQR, 5 to 11 days), and the total length of hospital stay was 17 days (IQR, 12 to 30 days). One hundred thirty (64.4%) MRSA IE patients failed vancomycin therapy; 42 (20.8%) were found to have mortality attributed to MRSA, and 119 (58.9%) had persistent bacteremia. See Table 1 for the bivariate comparison of the clinical and microbiological characteristics between vancomycin treatment failure and success.
TABLE 1.
Clinical characteristics and microbiological data with vancomycin treatment
| Characteristic | VAN effectivenessa |
P value | |
|---|---|---|---|
| Success (n = 72) | Failure (n = 130) | ||
| Patient characteristics | |||
| Age (yr) | 51 (38–60.5) | 54 (48–59) | 0.234 |
| APACHE II score | 11.5 (8–17.5) | 12 (8–18) | 0.766 |
| Charlson comorbidity score | 2 (0.5–4) | 2 (1–4) | 0.304 |
| Actual body wt (kg) | 69.8 (61.9–80.5) | 71.9 (60.3–85.8) | 0.503 |
| Creatinine clearance (ml/min) | 49.2 (22.9–81.3) | 45.4 (16.2–80.2) | 0.566 |
| Prior hospitalization (1 yr) | 46 (63.9) | 70 (53.8) | 0.167 |
| Prior VAN (30 days) | 33 (45.8) | 62 (47.7) | 0.800 |
| Female | 28 (38.9) | 45 (34.6) | 0.545 |
| ICU admission | 22 (30.6) | 72 (55.4) | 0.001 |
| IDUb | 41 (56.9) | 68 (52.3) | 0.527 |
| Diabetes | 14 (19.4) | 39 (30.0) | 0.102 |
| Heart disease | 16 (22.2) | 30 (23.1) | 0.890 |
| Chronic kidney disease | 25 (34.7) | 39 (30.0) | 0.490 |
| Hemodialysis | 22 (30.6) | 41 (31.5) | 0.885 |
| Cerebral vascular accident | 6 (8.3) | 20 (15.4) | 0.152 |
| Liver disease | 15 (20.8) | 38 (29.2) | 0.194 |
| Left-sided IE | 18 (25.0) | 36 (27.7) | 0.679 |
| MRSA isolate characteristics | |||
| hVISA | 6 (8.3) | 32 (24.6) | 0.005 |
| VAN PAP MIC ≥ 4 mg/liter | 6 (8.3) | 30 (23.1) | 0.009 |
| VAN BMD MIC > 1 mg/liter | 9 (15.5) | 25 (20.5) | 0.047 |
| VAN Etest MIC > 1 mg/liter | 44 (61.1) | 88 (67.7) | 0.346 |
| SCCmec type | 0.540 | ||
| II | 19 (26.4) | 38 (29.2) | 0.667 |
| III | 0 (0.0) | 2 (1.5) | 0.539 |
| IV | 51 (70.8) | 90 (69.2) | 0.812 |
| agr genotype group | 0.314 | ||
| I | 45 (62.5) | 79 (60.8) | 0.809 |
| II | 24 (33.3) | 47 (36.2) | 0.688 |
| III | 1 (1.4) | 0 (0.0) | 0.350 |
| IV | 0 (0.0) | 4 (3.1) | 0.142 |
| agr defective function | 10 (13.9) | 26 (20.0) | 0.316 |
| USA300 | 39 (54.2) | 73 (56.2) | 0.786 |
| Clinical characteristics | |||
| Initial VAN trough (mg/liter) | 18.1 (13.1–22.9) | 13.9 (9.8–18) | 0.001 |
| Initial VAN trough < 15 mg/liter | 20 (27.8) | 75 (57.7) | <0.001 |
| VAN duration (days) | 8.5 (4–14) | 9 (4–17) | 0.656 |
| Infectious disease consult | 63 (87.5) | 105 (80.8) | 0.221 |
| Surgical intervention | 4 (5.6) | 18 (13.8) | 0.070 |
| Outcome | |||
| Days of bacteremia | 4 (3–5) | 10 (8–14) | <0.001 |
| Total length of stay (days) | 14 (10–21.5) | 21 (13–39) | <0.001 |
All data are presented as n (%) or median (IQR). VAN, vancomycin.
IDU, injection drug use.
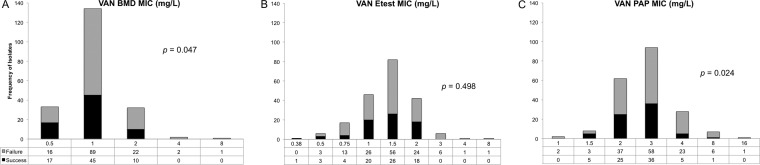
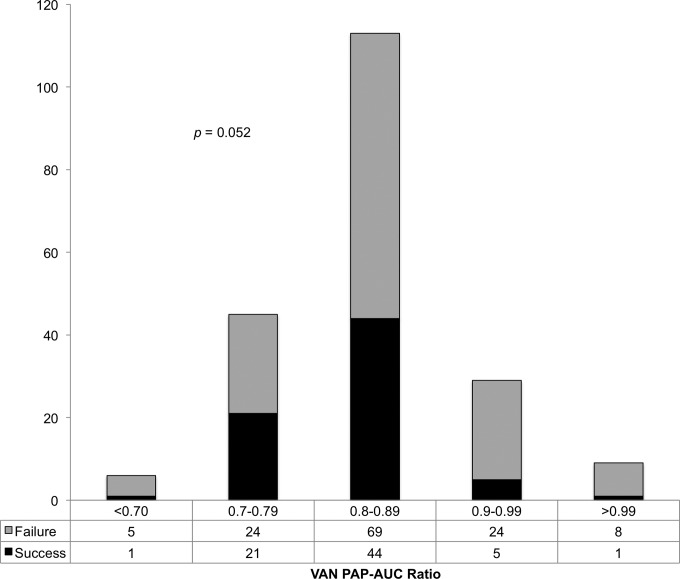
Treatment responses according to vancomycin MIC distributions by the BMD, Etest, and PAP methods are shown in Fig. 2. There was a trend in treatment failure as a function of both BMD and PAP methods for vancomycin MIC. The PAP MIC breakpoint derived by CART analysis for treatment failure was ≥4 mg/liter. Thirty-six (17.8%) patients had a PAP MIC of ≥4 mg/liter and 166 (82.1%) patients had a PAP MIC of <4 mg/liter. Bivariate comparison of the treatment failure between the groups demonstrated an odds ratio of 3.3 (relative risk, 1.4) of treatment failure in the group with a PAP MIC of ≥4 mg/liter compared to the group with a PAP MIC of <4 mg/liter (83.3% versus 60.2%, respectively; P = 0.009). The distribution of the PAP-AUC ratio to Mu3 is provided in Fig. 3, which reports the association of the ratio with treatment success and failure. The PAP-AUC ratio breakpoint derived by CART analysis was set for treatment failure at 0.9035. Multiple variables, including previous hospitalization, admission to the ICU, comorbid conditions (diabetes, cerebral vascular accident, and liver disease), surgical intervention, and PAP MIC of ≥4 mg/liter were all predictors of treatment failure on bivariate analysis and were put into the multivariable logistic regression analysis. In the logistic regression analysis of treatment failure, a PAP MIC of ≥4 mg/liter (adjusted odds ratio [aOR], 3.24; 95% confidence interval [CI], 1.25 to 8.35; P = 0.015) and admission to the ICU (aOR 2.79; 95% CI, 1.50 to 5.18; P = 0.001) remained in the model and were found to be independent predictors of treatment failure. The final multivariable model was able to distinguish between treatment failure and success, with no evidence of a lack-of-fit (P = 0.997) determined by the Hosmer-Lemeshow goodness-of-fit test.
FIG 2.
MIC distributions and treatment outcome. (A) Vancomycin broth microdilution MIC distribution and treatment outcome. The statistical test performed was a Mann-Whitney U test. The failure rate distributions were 48% for 0.5 mg/liter, 66% for 1 mg/liter, 69% for 2 mg/liter, 100% for 4 mg/liter, and 100% for 8 mg/liter. (B) Vancomycin Etest MIC distribution and treatment outcome. The statistical test performed was a Mann-Whitney U test. The failure rate distributions were 0% for 0.38 mg/liter, 50% for 0.5 mg/liter, 76% for 0.75 mg/liter, 57% for 1 mg/liter, 68% for 1.5 mg/liter, 57% for 2 mg/liter, 100% for 3 mg/liter, 100% for 4 mg/liter, and 100% for 8 mg/liter. (C) Vancomycin PAP MIC distribution and treatment outcome. The statistical test performed was a Mann-Whitney U test. The failure rate distributions were 100% for 1 mg/liter, 38% for 1.5 mg/liter, 60% for 2 mg/liter, 62% for 3 mg/liter, 82% for 4 mg/liter, 86% for 8 mg/liter, and 100% for 16 mg/liter.
FIG 3.
Vancomycin population analysis profile AUC ratio and treatment outcome.
DISCUSSION
Our study found that patients with MRSA IE were 1.4 times more likely to fail vancomycin therapy if the subpopulation PAP MIC of the isolate was ≥4 mg/liter. Heterogeneous vancomycin susceptibility has previously been associated with treatment failure (18). Specifically, there appears to be a higher occurrence of hVISA in IE (9). In addition, high MICs of vancomycin within the susceptibility range may be a marker for hVISA (as we documented in this database) (29–31). Of interest, our data with the CART analysis breakpoint for failure for the PAP-AUC ratio was set at 0.9, which is currently the accepted ratio for defining hVISA by using the population AUC-to-Mu3 AUC ratio (17, 32). Within our data, we found hVISA to be associated with treatment failure; however, the vancomycin susceptibility method by modified population analysis displayed a stronger association than did BMD MIC, and there was not a significant association with the Etest MIC. This is of interest since the Etest methodology for vancomycin susceptibility has routinely been found to have a statistical association with treatment failure in other MRSA BSI cohort studies (33, 34). Our data were similar to those of the International Collaboration on Endocarditis-Prospective Cohort Study (9), as we found no independent association between treatment failure and a vancomycin MIC of ≥1.5 mg/liter by the Etest method.
In our study population, the hVISA phenotype as determined by the PAP-AUC ratio and PAP MIC was associated with clinical outcomes. Only PAP MIC was evaluated in multivariable analysis because of the collinear properties of the PAP MIC and the PAP-AUC ratio with hVISA. The majority of the isolates with a PAP MIC of ≥4 mg/liter were hVISA, and this may partially explain why patients with hVISA treated with vancomycin have poor outcomes. Although reported susceptible by the laboratory, it is thought that these organisms within the population that have higher MIC values are selected out upon treatment and hence respond poorly to vancomycin. Most of the types of infections related to higher MIC values tend to be higher-inoculum infections. An evaluation of S. aureus vancomycin heteroresistance with other sources of BSI or types of infections may be of interest to determine the relationship between vancomycin treatment and patient outcome. The relationship between vancomycin MIC and outcome may be confounded by the presence of heteroresistance and therefore lead to erroneous conclusions. The contribution of heteroresistance to other sources of infection is of interest since the risk of mortality or treatment failure is lower in other sources of BSI, such as intravenous catheter-related and skin tissue sources, compared to infective endocarditis (35).
Molecular characteristics have been shown to be associated with heteroresistance to vancomycin (36). The majority of our MRSA isolates had SCCmec type IV, indicating these isolates may have originated from the community rather than from hospital settings (37). These data have been in contrast to what has been published previously with MRSA IE and heteroresistance to vancomycin being associated with a higher frequency of SCCmec type II (9, 36). Of interest, 112 patient isolates were USA300, leaving approximately 20% of isolates coming from another USA genotype associated with SCCmec type IV (38). The only molecular characteristic that demonstrated an association with a PAP MIC of ≥4 mg/liter was the presence of another genotype of USA versus the presence of USA300 (24% [22/90] versus 13% [14/112]; P = 0.027). Although we did not further differentiate the 22 non-USA300 genotypes that had a PAP MIC of ≥4 mg/liter, the majority of these were either USA100 (SCCmec type II, agr genotype II) or USA500/600 (SCCmec type IV, agr genotype I) (39).
This study has a large diverse population of patients with MRSA IE; however, by its retrospective observational nature, it has some limitations. First, this is a single-center study that may lack the external validity required to support widespread changes in practice. Second, the misclassification of a PAP MIC of 4 mg/liter may be a factor even if it was done in duplicate, since the initial MRSA isolates were collected from blood cultures and therefore may not represent the population at the site of infection. Subsequent cultures were not collected to determine if the population susceptibility had shifted, and therefore, it is unknown if further-reduced vancomycin susceptibility might have increased, especially for patients with persistent bacteremia. Third, PAP MIC may not be clinically practical until current methods evolve to become more rapid, less expensive, and less labor intensive. Fourth, molecular typing (e.g., spa typing or pulsed-field gel electrophoresis) was not performed for this study; therefore, it is unknown if the clonal relatedness of the isolates had a direct influence on the clinical outcomes. However, we did perform SCCmec typing and agr genotyping, which may suggest a hospital or community origin and some genotypic diversity (37). Finally, selection bias is a potential concern with outcomes in retrospective studies, especially when vancomycin susceptibility has been identified (e.g., automated vancomycin MIC); however, clinicians were unaware of the modified population analysis profile vancomycin susceptibility and, thus, did not alter therapy or management during hospitalization.
In conclusion, the observations made in this study support the concept that a PAP MIC of ≥4 mg/liter is unfavorable to outcomes in patients with MRSA IE. This study demonstrated that the vancomycin susceptibility obtained by using modified population analysis profiles provides an alternative technique in susceptibility testing and displays a stronger association with clinical outcome than Etest and BMD. Further studies are warranted to determine if this finding may be useful for other populations with MRSA BSI, especially concomitant sites of high-inoculum infections. In addition, a more rapid process for determining the subpopulation MIC for screening patient isolates may be necessary for the practicality of this patient outcome predictor. Our data would support that vancomycin heteroresistance, specifically subpopulations of PAP MIC of ≥4 mg/liter, is important based on the overall impact on patient outcomes, including the association of 30-day attributable mortality and persistent bacteremia.
ACKNOWLEDGMENTS
A.M.C. received grant support from Cubist, Forest, and the Michigan Department of Community Health. S.L.D. received grant support from Cubist and Forest and served as a consultant for Forest, Durata, Premier, and Pfizer. R.K. is an employee of Cubist Pharmaceuticals. D.P.L. received grant support from Cubist, Cerexa, and AstraZeneca, served as a consultant for Forest, Cubist, Cerexa, Theravance, R-Pharm, and Rib-X, and served on a speaker's bureau for Cubist and Forest. M.J.R. received grant support from Cubist, Forest, Cerexa, Trius, the National Institutes of Health, and the Michigan Department of Community Health, served as a consultant for Durata, Trius, Cubist, Forest, Cepheid, and Theravance, and served on a speaker's bureau for Cubist, Forest, and Novartis. All other authors report no potential conflict of interests.
No financial support was received for the conduct of this study.
Footnotes
Published ahead of print 2 June 2014
REFERENCES
- 1.Furuno JP, Johnson JK, Schweizer ML, Uche A, Stine OC, Shurland SM, Forrest GN. 2011. Community-associated methicillin-resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect. Dis. 11:298. 10.1186/1471-2334-11-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landrum ML, Neumann C, Cook C, Chukwuma U, Ellis MW, Hospenthal DR, Murray CK. 2012. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA 308:50–59. 10.1001/jama.2012.7139 [DOI] [PubMed] [Google Scholar]
- 3.Laupland KB, Lyytikäinen O, Søgaard M, Kennedy KJ, Knudsen JD, Ostergaard C, Galbraith JC, Valiquette L, Jacobsson G, Collignon P, Schønheyder HC, International Bacteremia Surveillance Collaborative 2013. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin. Microbiol. Infect. 19:465–471. 10.1111/j.1469-0691.2012.03903.x [DOI] [PubMed] [Google Scholar]
- 4.Benito N, Miró JM, de Lazzari E, Cabell CH, del Río A, Altclas J, Commerford P, Delahaye F, Dragulescu S, Giamarellou H, Habib G, Kamarulzaman A, Kumar AS, Nacinovich FM, Suter F, Tribouilloy C, Venugopal K, Moreno A, Fowler VG, Jr, ICE-PCS (International Collaboration on Endocarditis Prospective Cohort Study) Investigators 2009. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann. Intern. Med. 150:586–594. 10.7326/0003-4819-150-9-200905050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS, ICE Investigators 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 6.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38:521–528. 10.1086/381202 [DOI] [PubMed] [Google Scholar]
- 7.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG, Jr, International Collaboration on Endocarditis Merged Database Study Group 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. 41:507–514. 10.1086/431979 [DOI] [PubMed] [Google Scholar]
- 8.Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. 2013. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One 8:e60033. 10.1371/journal.pone.0060033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae IG, Federspiel JJ, Miró JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG, Jr, International Collaboration on Endocarditis-Microbiology Investigator 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis. 200:1355–1366. 10.1086/606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 11.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin. Infect. Dis. 54:755–771. 10.1093/cid/cir935 [DOI] [PubMed] [Google Scholar]
- 12.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52:975–981. 10.1093/cid/cir124 [DOI] [PubMed] [Google Scholar]
- 13.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320. 10.1128/AAC.00113-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalueza A, Chaves F, San Juan R, Daskalaki M, Otero JR, Aguado JM. 2010. Is high vancomycin minimum inhibitory concentration a good marker to predict the outcome of methicillin-resistant Staphylococcus aureus bacteremia? J. Infect. Dis. 201:311–312. 10.1086/649572 [DOI] [PubMed] [Google Scholar]
- 15.Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 65:1015–1018. 10.1093/jac/dkq050 [DOI] [PubMed] [Google Scholar]
- 16.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 50:3039–3047. 10.1128/AAC.00422-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399–403. 10.1093/jac/47.4.399 [DOI] [PubMed] [Google Scholar]
- 18.van Hal SJ, Paterson DL. 2011. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:405–410. 10.1128/AAC.01133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, Bashore T, Corey GR. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633–638. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128–140. 10.1016/0196-6553(88)90053-3 [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institutes. 2013. Performance standards for antimicrobial susceptibility testing, 23rd informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22.Milheiriço C, Oliveira DC, de Lencastre H. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3374–3377. 10.1128/AAC.00275-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilot P, Lina G, Cochard T, Poutrel B. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40:4060–4067. 10.1128/JCM.40.11.4060-4067.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. 2008. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and Panton-Valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 46:1118–1122. 10.1128/JCM.01309-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traber K, Novick R. 2006. A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate delta- and alpha-haemolysins. Mol. Microbiol. 59:1519–1530. 10.1111/j.1365-2958.2006.04986.x [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42:377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenover FC, Moellering RC., Jr 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44:1208–1215. 10.1086/513203 [DOI] [PubMed] [Google Scholar]
- 30.Tenover FC, Sinner SW, Segal RE, Huang V, Alexandre SS, McGowan JE, Jr, Weinstein MP. 2009. Characterisation of a Staphylococcus aureus strain with progressive loss of susceptibility to vancomycin and daptomycin during therapy. Int. J. Antimicrob. Agents 33:564–568. 10.1016/j.ijantimicag.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob. Agents Chemother. 57:4252–4259. 10.1128/AAC.00380-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh TR, Bolmström A, Qwörnström A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 39:2439–2444. 10.1128/JCM.39.7.2439-2444.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takesue Y, Nakajima K, Takahashi Y, Ichiki K, Ishihara M, Wada Y, Tsuchida T, Uchino M, Ikeuchi H. 2011. Clinical characteristics of vancomycin minimum inhibitory concentration of 2 μg/ml methicillin-resistant Staphylococcus aureus strains isolated from patients with bacteremia. J. Infect. Chemother. 17:52–57. 10.1007/s10156-010-0086-0 [DOI] [PubMed] [Google Scholar]
- 34.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. 2006. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch. Intern. Med. 166:2138–2144. 10.1001/archinte.166.19.2138 [DOI] [PubMed] [Google Scholar]
- 35.Soriano A, Marco F, Martínez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200. 10.1086/524667 [DOI] [PubMed] [Google Scholar]
- 36.Jang HC, Kang SJ, Choi SM, Park KH, Shin JH, Choy HE, Jung SI, Kim HB. 2012. Difference in agr dysfunction and reduced vancomycin susceptibility between MRSA bacteremia involving SCCmec types IV/IVa and I-III. PLoS One 7:e49136. 10.1371/journal.pone.0049136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiramatsu K, Cui L, Kuroda M, Ito T. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9:486–493. 10.1016/S0966-842X(01)02175-8 [DOI] [PubMed] [Google Scholar]
- 38.Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J. Antimicrob. Chemother. 64:441–446. 10.1093/jac/dkp241 [DOI] [PubMed] [Google Scholar]
- 39.Chua T, Moore CL, Perri MB, Donabedian SM, Masch W, Vager D, Davis SL, Lulek K, Zimnicki B, Zervos MJ. 2008. Molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J. Clin. Microbiol. 46:2345–2352. 10.1128/JCM.00154-08 [DOI] [PMC free article] [PubMed] [Google Scholar]