Abstract
A series of colistin-resistant Klebsiella pneumoniae isolates recovered from different countries was investigated in order to evaluate the involvement of the PmrA/PmrB two-component system in this resistance. Six isolates possessed a mutated PmrB protein, which is encoded by the pmrB gene, part of the pmrCAB operon involved in lipopolysaccharide modification. The same amino acid substitution (Thr157Pro) in PmrB was identified in the six isolates. The six isolates belonged to four distinct clonal groups, recovered in South Africa (sequence type 14 [ST14]), Turkey (ST101), and Colombia (ST258 and ST15). Three out of the four clones produced a carbapenemase, OXA-181, OXA-48, or KPC-3, while a single isolate did not produce any carbapenemase. Expression assays revealed an overexpression of the pmrA (70-fold), pmrB (70-fold), pmrC (170-fold), and pmrK (40-fold) genes in the pmrB-mutated isolate compared to expression of the pmrB wild-type isogenic K. pneumoniae isolate, confirming that the PmrB substitution was responsible for increased expression levels of those genes. Complementation assays leading to the expression of a wild-type PmrB protein restored the susceptibility to colistin in all isolates, confirming that the substitution in PmrB was responsible for the resistance phenotype. This study identified a key amino acid located in the PmrB protein as being responsible for the overexpression of pmrCAB and pmrHFIJKLM operons, leading to resistance to colistin.
INTRODUCTION
Klebsiella pneumoniae is a Gram-negative pathogen often associated with nosocomial infections, including pneumonia, bacteremia, urinary tract infection, and sometimes even life-threatening septic shock (1). Infection caused by multidrug-resistant (MDR) K. pneumoniae is now a worldwide issue, considering the wide dissemination of MDR clones. In particular, the recent worldwide emergence of carbapenemase-producing isolates has often resulted in very limited therapeutic options. As a consequence, polymyxin antibiotics are now considered the last resort in the armamentarium for treatment of infections caused by MDR Gram-negative pathogens.
Polymyxins are derivatives of the Bacillus polymyxa subspecies colistinus that are active only against Gram-negative bacteria. Structurally, they are decapeptides bound to a fatty acid chain (2). The clinically available forms, polymyxin B and colistin (also known as polymyxin E), are administered intravenously or by inhalation. Like other antimicrobial peptides (APs), colistin interacts with the lipid A moiety of the Gram-negative bacterial lipopolysaccharide (LPS). The polycationic peptide ring competes for and substitutes the calcium and magnesium bridges stabilizing the LPS, promoting membrane permeability and thus disrupting the integrity of the outer membrane of Gram-negative bacteria, thus leading to bacterial death (2).
Nevertheless, resistance to polymyxins has been reported in K. pneumoniae, but little is known about the molecular mechanisms sustaining this resistance trait. The two-component regulatory systems (TCS) PmrA/PmrB and PhoP/PhoQ have been identified as regulatory systems involved in the resistance to polymyxin B (3). The insertional inactivation of the PhoQ/PhoP mgrB-encoding regulator has also been recently associated with colistin resistance (4, 5).
The main mechanisms leading to resistance to polymyxins are modifications of the bacterial outer membrane, mainly through the covalent addition of both phosphoethanolamine (pEtN) and 4-deoxyaminoarabinose (Lara4N) to the LPS (6–8). The pmrCAB operon encodes the PmrC phosphoethanolamine phosphotransferase, the PmrA response regulator (also named BasR), and the PmrB sensor kinase (also named BasS) (6). In Escherichia coli and Salmonella enterica species, PmrB acts as a sensor cytoplasmic membrane-bound kinase activated by high concentrations of iron (Fe3+) and an acidic pH (pH 5.5). Upon activation, it activates PmrA by phosphorylation (2). In vivo, PmrAB is involved in sensing the environment and is required for intramacrophage survival and virulence (6). Mutations in the pmrA or pmrB genes usually result in constitutive activation of PmrA, which upregulates in turn three loci, namely, pmrC encoding an aminotransferase involved in the decoration of the LPS with pEtN, pmrE (previously identified as pagA or ugd) encoding a UDP-glucose dehydrogenase, which is the first enzyme in the Ara4N biosynthetic pathway, and the pmrHFIJKLM operon (also called pmrF, pbg, or arn operon) encoding enzymes responsible for the synthesis and transfer of the Lara4N to lipid A (6). Overall, this constitutive activation of PmrA leads to a more positively charged LPS, thus reducing the affinity of positively charged polymyxins.
Using a collection of K. pneumoniae isolates recovered worldwide, in this study we aimed to investigate whether the mechanism leading to resistance to colistin might be related to the PmrAB two-component system.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Thirty-five colistin-resistant K. pneumoniae clinical isolates were included in this study. They had been collected in France, Turkey, Colombia, and South Africa. Isolates were identified by using the API20E system (bioMérieux, Marcy l'Etoile, France). E. coli TOP10 (Invitrogen, Illkirch, France) was used as the host strain for cloning experiments with selection based on kanamycin (50 μg/ml). Colistin-resistant K. pneumoniae clinical isolates were also used for transformation assays and selection based on zeocin (a formulation of phleomycin D1, a glycopeptide antibiotic produced by Streptomyces verticillus) (100 μg/ml).
Antimicrobial susceptibility assays.
The antibiotic susceptibility testing was performed using Etest strips (AB bioMérieux, La Balme-les-Grottes, France) on Mueller-Hinton agar plates (Bio-Rad, Marnes-la-Coquette, France) with 0.5 McFarland standard inoculum. MICs were also determined by broth microdilution in cation-adjusted Mueller-Hinton broth (MHB-CA) according to CLSI guidelines (9–11). Polymyxin B and colistin (Sigma-Aldrich, Saint-Quentin Fallavier, France) were tested with Tween 80 (a surfactant preventing binding of colistin to drug panels) over a range of dilution from 0.125 to 64 μg/ml (9, 12). Briefly, MHB-CA with a final concentration of 0.002% Tween 80 and 5 × 105 CFU/ml in each well was used. Following the EUCAST breakpoints (http://www.eucast.org/), isolates with a colistin MIC of ≤2 μg/ml were categorized as susceptible, although those with MICs of >2 μg/ml were resistant.
PCR amplification and sequencing.
The chromosomal DNA was isolated using the commercially available QIAquick kit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. The pmrA, pmrB, phoP, phoQ, and mgrB genes possibly involved in resistance to colistin were amplified using specific oligonucleotides (Table 1). The PCR amplification for detection of the narrow- and expanded-spectrum β-lactamase genes blaCTX-M, blaSHV, blaTEM, blaOXA, blaOXA-48-like, and blaKPC was carried out as described previously (13, 14). The amplified DNA fragments were purified with the QIAquick PCR purification kit (Qiagen). Both strands of the amplification products obtained were sequenced with an ABI 3100 sequencer (Applied Biosystems, Foster City, CA). The nucleotide and deduced protein sequences were analyzed at the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov) by the Basic Local Alignment Search Tool (BLAST) program.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′) | Gene | Reference or source |
|---|---|---|---|
| pmrA ext F | CAT TTC CGC GCA CTG TCT GC | pmrA | This study |
| pmrA ext R | CAG CTT TCA GTT GCA AAC AG | pmrA | This study |
| pmrB ext F | ACC TAC GCG AAA AGA TTG GC | pmrB | This study |
| pmrB ext R | GAT GAG GAT AGC GCC CAT GC | pmrB | This study |
| phoP ext F | GAG CTT CAG ACT ACT ATC GA | phoP | This study |
| phoP ext R | GGG AAG ATA TGC CGC AAC AG | phoP | This study |
| phoQ ext F | ATA CCC ACA GGA CGT CAT CA | phoQ | This study |
| phoQ ext R | CAG GTG TCT GAC AGG GAT TA | phoQ | This study |
| mgrB ext F | TTA AGA AGG CCG TGC TAT CC | mgrB | 4 |
| mgrB ext R | AAG GCG TTC ATT CTA CCA CC | mgrB | 4 |
| mdh ext F | CCC AAC TCG CTT CAG GTT CAG | mdh | 15 |
| mdh ext R | CCG TTT TTC CCC AGC AGC AG | mdh | 15 |
| pmrC int F | GCG TGA TGA ATA TCC TCA CCA | pmrC | This study |
| pmrC int R | CAC GCC AAA GTT CCA GAT GA | pmrC | This study |
| pmrA int F | GAT GAA GAC GGG CTG CAT TT | pmrA | This study |
| pmrA int R | ACC GCT AAT GCG ATC CTC AA | pmrA | This study |
| pmrB int F | TGC CAG CTG ATA AGC GTC TT | pmrB | This study |
| pmrB int R | TTC TGG TTG TTG TGC CCT TC | pmrB | This study |
| pmrD int F | GAT CGC AGA GAT TGA AGC CT | pmrD | This study |
| pmrD int R | GCG TTG CGG ATC TTC AAA GT | pmrD | This study |
| pmrE int F | GCA TAC CGT AAT GCC GAC TA | pmrE | This study |
| pmrE int R | GGG TTG ATC TCT GTG ACA TC | pmrE | This study |
| pmrK int F | AGT ATC GGT CAG TGG CTG TT | pmrK | This study |
| pmrK int R | CCG CTT ATC ACG AAA GAT CC | pmrK | This study |
| rpsL int F | CCG TGG CGG TCG TGT TAA AGA | rpsL | 4 |
| rpsL int R | GCC GTA CTT GGA GCG AGC CTG | rpsL | 4 |
Analysis of the primary and secondary structures of the PmrB protein.
The primary structure of the PmrB protein was analyzed using the Ensembl Bacteria database (http://bacteria.ensembl.org/index.html). The predicted secondary structures of the two PmrB proteins (wild-type and mutated) were obtained using the GOR method with EMBOSS 6.3.1 software, available on the Mobyle@Pasteur portal (http://mobyle.pasteur.fr/cgi-bin/portal.py).
Complementation assays.
The pmrB gene from the K. pneumoniae reference strain ATCC 53153 (colistin MIC of 0.125 μg/ml) was amplified by PCR using 2× Phusion HF master mix (Finnzymes; Life Technologies, Illkirch, France) and primers pmrB ext F and pmrB ext R. The partial and therefore noncoding mdh sequence (supposed to encode a malate dehydrogenase [15]) was PCR amplified with primers mdh ext F and mdh ext R (Table 1). Using the Zero Blunt TOPO PCR cloning kit (Invitrogen), the amplified fragments were cloned into the high-copy-number plasmid pCR-BluntII-TOPO encoding resistance to kanamycin and zeocin. The resulting plasmids pTOPO-pmrB and pTOPO-mdh were separately transformed into electrocompetent E. coli TOP10 strains by electroporation. Transformants were selected by overnight incubation at 37°C on Mueller-Hinton agar supplemented with kanamycin (50 μg/ml). Plasmids were then isolated using the QIAprep spin miniprep column kit (Qiagen) and transformed into colistin-resistant K. pneumoniae clinical isolates. Transformants were selected by overnight incubation at 37°C on Mueller-Hinton agar supplemented with zeocin (100 μg/ml), and the presence of the cloned gene was checked by PCR. The MICs of transformants for colistin and polymyxin were determined as described above.
Strain genotyping by PFGE and MLST.
The genetic relatedness of the isolates was assessed by pulsed-field gel electrophoresis (PFGE) analysis with XbaI-digested genomic DNA as described previously (16). Multilocus sequence typing (MLST) was carried out with seven standard housekeeping loci (rpoB, gapA, mdh, pgi, phoE, infB, and tonB) according to Diancourt et al. (15). Sequence types were analyzed by using the Institut Pasteur database (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html).
Growth curves.
In order to evaluate the putative impact of modified LPS, growth curves were determined. Briefly, 200 ml Luria broth was inoculated independently with 108 CFU of each strain, and cultures were grown for 24 h at 37°C under gentle shaking. Absorbance at a wavelength of 600 nm was measured during 24 h. The colony counting was performed by serial dilution and final plating on solid medium. Experiments were repeated three times. Zeocin (100 μg/ml) was added to the growth medium in order to select the recombinant plasmids when K. pneumoniae strain AF1b complemented with pTOPO-pmrB or pTOPO-mdh was used.
Transcriptional analysis by quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) was used to measure the expression of the pmrC, pmrA, pmrB, pmrD, pmrE, and pmrK genes, using the primers listed in Table 1. RNA preparations were obtained using the RNeasy minikit (Qiagen), according to the manufacturer's instructions. qRT-PCR was carried out using a Rotor-Gene Q instrument (Qiagen). Expression of the rpsL gene encoding a ribosomal protein was evaluated with the primers rpsL int F and rpsL int R (Table 1), and this expression rate was used as a signal reporter (4). QuantiFast SYBR green (Qiagen) was used as an internal standard (4). Data were compared to those obtained with the rpsL gene using the threshold cycle (ΔΔCT) method (relative), and the obtained values were then normalized against the values obtained for the susceptible isolate. Experiments were repeated three times.
Nucleotide sequence accession number.
The nucleotide and protein sequences of the mutated PmrB protein were registered in GenBank under accession no. KJ626267.
RESULTS
A point mutation in the pmrB gene of colistin-resistant K. pneumoniae isolates.
Sequence analysis of the pmrB genes of all the colistin-resistant clinical isolates from our collection revealed the same single-nucleotide substitution (A to C) in six isolates (AF1b, C3, C4, C19, T2, and T3) compared to those in the pmrB gene sequences from K. pneumoniae available in GenBank (Table 2). No amino acid substitutions were identified in the PmrA, PhoP, PhoQ, and MgrB proteins in those isolates compared with sequences obtained from wild-type isolates (data not shown). This mutated PmrB protein differed from the wild type by a single threonine to proline amino acid substitution at position 157. Since a colistin-susceptible isolate, AF1a (colistin MIC of 0.125 μg/ml), recovered from the same patient from whom the colistin-resistant isolate AF1b had been recovered was available (17), its pmrB gene was also sequenced. A Thr residue was identified at position 157 (as for the other wild-type strains), thus reinforcing the hypothesis that Thr157Pro might play a key role in acquired resistance to colistin (Table 2).
TABLE 2.
MICs, molecular features, and genotyping analysis of K. pneumoniae isolates carrying the pmrB gene mutation and of isolate AF1a, the isogenic colistin-susceptible counterpart of AF1b
| Isolate | Country of isolation | MIC (μg/ml) fora: |
Colistin profileb | PmrB amino acid change | Carbapenemase | ESBLc | Additional β-lactamases | MLSTd | PFGEe pulsotype | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERT | IPM | MEM | DOR | CST | |||||||||
| AF1a | South Africa | >32 | >32 | >32 | >32 | 0.125 | S | WTf | OXA-181 | CTX-M-15 | TEM-1, SHV-1 | ST14 | 1 |
| AF1b | South Africa | >32 | >32 | >32 | >32 | 3 | R | T157P | OXA-181 | CTX-M-15 | TEM-1, SHV-1 | ST14 | 1 |
| C3 | Colombia | >32 | >32 | >32 | >32 | 4 | R | T157P | KPC-3 | None | TEM-1, SHV-1 | ST258 | 2 |
| C4 | Colombia | >32 | >32 | >32 | >32 | 4 | R | T157P | KPC-3 | None | TEM-1, SHV-1 | ST258 | 2 |
| C19 | Colombia | 0.25 | 0.19 | 0.094 | 0.064 | 4 | R | T157P | None | None | TEM-1, SHV-1 | ST15 | 3 |
| T2 | Turkey | 16 | >32 | >32 | 24 | 6 | R | T157P | OXA-48 | CTX-M-15 | TEM-1, SHV-1, OXA-1 | ST101 | 4 |
| T3 | Turkey | 16 | >32 | >32 | 24 | 6 | R | T157P | OXA-48 | CTX-M-15 | TEM-1, SHV-1, OXA-1 | ST101 | 4 |
ERT, ertapenem; IPM, imipenem; MEM, meropenem; DOR, doripenem; CST, colistin.
R, resistant; S, susceptible.
ESBL, extended-spectrum β-lactamase.
MLST, multilocus sequence type; ST, sequence type.
PFGE, pulsed-field gel electrophoresis.
WT, wild type.
Impact of amino acid 157 substitution on the structure of the PmrB protein.
By analyzing the primary structure of the PmrB protein, amino acid 157 was identified in the PmrB domain, which is involved in the dimerization of the protein. We might speculate that the replacement of a polar by a nonpolar amino acid in this domain may have a significant impact in the dimerization process of PmrB and therefore induce constitutive activation of PmrA.
The predicted secondary structures of the two PmrB proteins (wild type and mutated) revealed that the Thr157Pro amino acid substitution significantly modified the secondary structure of the mutated protein, with an interruption of the alpha-helix (see Fig. S1 in the supplemental material).
Thr157Pro substitution in protein PmrB identified among clonally unrelated colistin-resistant K. pneumoniae isolates.
The genetic relationship of the six colistin-resistant isolates exhibiting the same Thr157Pro substitution, together with the colistin-susceptible isolate AF1a, was evaluated. PFGE analysis confirmed that isolates AF1a and AF1b recovered from a single patient were clonally related (17) (data not shown). In addition, isolates C3 and C4, recovered from two patients from the same hospital, were also clonally related, and isolates T2 and T3, again from two patients from the same hospital, were clonally related (data not shown). Overall, four colistin-resistant clones were identified (Table 2). Further data obtained by MLST were in accordance with the PFGE results (Table 2).
Identification of additional resistance mechanisms to broad-spectrum β-lactams.
The clonally related isolates AF1a (colistin-susceptible) and AF1b (colistin-resistant) recovered from South Africa were found to produce the OXA-181 (OXA-48-like) carbapenemase (Table 2). The clonally related C3 and C4 isolates from Colombia produced carbapenemase KPC-3. Isolate C19 from Colombia, clonally unrelated compared to other isolates in this study, did not produce any carbapenemase. Finally, clonally related isolates T2 and T3 recovered from Turkey produced carbapenemase OXA-48.
Complementation experiments.
To determine whether resistance to colistin might be related to the PmrB mutation, complementation experiments were performed for each clonally unrelated strain (AF1b, C3, C19, and T2). The wild-type pmrB gene was cloned into the high-copy-number plasmid pCR-BluntII-TOPO, which was then transformed into the colistin-resistant clinical isolates. The results of complementation showed a complete reversion to susceptibility to colistin when we transformed each of those resistant isolates with plasmid pTOPO-pmrB (Table 3). As expected, transformation with plasmid pTOPO-mdh used as a negative control did not restore the susceptibility to colistin.
TABLE 3.
MIC results for colistin and polymyxin B determined by broth microdilution before and after complementation with plasmids pTOPO-mdh (negative control) and pTOPO-pmrB for the four clonally unrelated and colistin-resistant K. pneumoniae isolates
| Isolate | MIC (μg/ml) of colistin: |
MIC (μg/ml) of polymyxin B: |
||||
|---|---|---|---|---|---|---|
| Before complementation | After complementation with pTOPO-mdh | After complementation with pTOPO-pmrB | Before complementation | After complementation with pTOPO-mdh | After complementation with pTOPO-pmrB | |
| AF1b | 16 | 16 | 1 | 8 | 8 | 1 |
| C3 | 16 | 16 | 2 | 8 | 8 | 1 |
| C19 | 16 | 16 | 1 | 8 | 8 | 2 |
| T2 | 16 | 16 | 1 | 8 | 8 | 2 |
Additionally, three other clonally unrelated and colistin-resistant K. pneumoniae for which no substitution was identified in PmrB were transformed with plasmid pTOPO-pmrB encoding the wild-type PmrB. MICs of colistin remained unchanged, thus showing that restoration of the susceptibility to colistin by complementation with a wild-type PmrB protein was not due to PmrB overproduction. This was also a direct proof of the involvement of the protein PmrB in the resistance pattern to colistin.
Growth rates of colistin-susceptible and colistin-resistant strains.
No difference was observed between the growth of isogenic K. pneumoniae AF1a (colistin-susceptible) and AF1b (colistin-resistant) isolates, thus suggesting that the PmrB substitution and the subsequent modification of the LPS did not modify the growth properties of resistant isolates (data not shown). This observation was further confirmed by the lack of difference between the growth curves obtained for K. pneumoniae AF1b complemented with wild-type PmrB protein (plasmid pTOPO-pmrB) (colistin-susceptible) or not complemented with PmrB but with plasmid pTOPO (pTOPO-mdh) (colistin-resistant) (data not shown).
Thr157Pro substitution in PmrB mutation associated with overexpression of pmrCAB and pmrHFIJKLM operons.
Expression levels of the pmr genes were measured in order to evaluate the impact of the pmrB mutation. RT-PCR assays were performed with isolates AF1a and AF1b, since they corresponded to likely isogenic strains. Upregulation of the expression levels of the pmrC, pmrA, pmrB, and pmrK genes was observed for AF1b compared to that for the isogenic colistin-susceptible isolate AF1a. The relative mean increases were estimated to be 170-fold for pmrC, 70-fold for pmrA, 70-fold for pmrB, and 40-fold for pmrK, whereas no significant differences in expression levels were observed with the pmrD and pmrE genes (increases of 1.9- and 1.4-fold, respectively) (Fig. 1).
FIG 1.
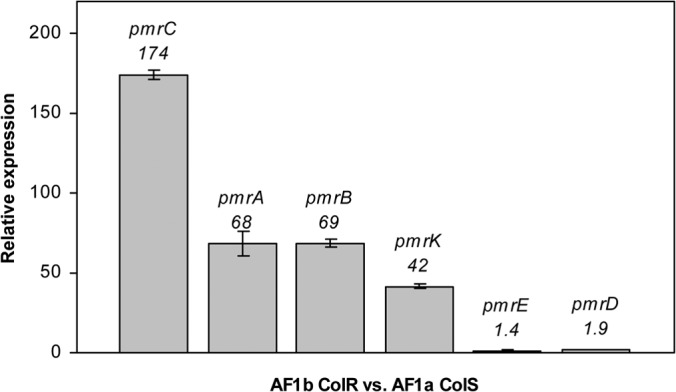
Relative expression levels of the pmrC, pmrA, pmrB, pmrK, pmrE, and pmrD genes in the AF1b colistin-resistant strain (ColR) compared with those in the AF1a colistin-susceptible strain (ColS). Values are the means and the standard deviations from three independent experiments.
DISCUSSION
Since data on the molecular bases of colistin resistance in K. pneumoniae are scarce, we analyzed a collection of colistin-resistant isolates of worldwide origin. Interestingly, we found six isolates harboring an unique and identical amino acid substitution in the protein PmrB. Complementation assays performed with a wild-type PmrB protein generated a complete reversion of the colistin-resistant trait. This result suggested that the PmrB substitution identified (Thr157Pro) was involved in the resistance of the clinical isolates. Of note, while this work was in progress, Choi and Ko (18) identified the same mutation in a colistin-resistant K. pneumoniae isolate and suggested that it might be involved in colistin resistance, but no further demonstration was provided to confirm this hypothesis. The involvement of the protein PmrB in resistance to colistin correlates results obtained with colistin-resistant Pseudomonas aeruginosa isolates (19) or colistin-resistant Acinetobacter baumannii isolates (20, 21).
Furthermore, since we had two very likely isogenic isolates (one susceptible and one resistant) recovered from the same patient, this hypothesis was confirmed by the identification of this same Thr to Pro substitution at position 157 in the colistin-resistant isolate. Of note, the colistin-resistant isolate had been recovered after a selective digestive tract decontamination process based on oral colistin administration (17). Considering that a single amino acid substitution in a single protein (here PmrB) may generate resistance to colistin, we suggest that colistin-based oral decontamination has to be used with caution.
The identification of four clonally unrelated K. pneumoniae clinical isolates harboring the same Thr157Pro substitution in PmrB is surprising considering that they have been recovered worldwide and do not correspond to the spread of a single clone. It strongly suggests that position 157 in protein PmrB is a key position for colistin resistance. It also suggests that the Pro residue might be a key residue for resistance to colistin in K. pneumoniae, in contrast to recent observations made in P. aeruginosa or A. baumannii isolates from which many different amino acid substitutions occurring in PmrB have been shown to be involved in colistin resistance (18–20). Further studies, using site-directed mutagenesis in PmrB, will be performed to evaluate our hypotheses, targeting different positions, including amino acids at position 157.
Analysis of the pmrC, pmrA, and pmrB transcription levels by qRT-PCR in isolate AF1b carrying a pmrB mutation compared to those in the susceptible and isogenic AF1a indicated that this mutation leads to an overexpression of the entire pmrCAB operon. This result suggests that the modified PmrB induces the constitutive activation of PmrA, which in turn autoregulates (activates) the pmrCAB promoter, as previously demonstrated in Salmonella spp. (6). Expression of the pmrC gene, being the first gene in the pmrCAB operon, has been shown to lead to phosphoethanolamine addition to lipid A in Salmonella spp. Moreover, analysis of the level of transcription of the pmrK gene, which belongs to the pmrHFIJKLM operon, confirmed that this operon was also activated by PmrA as described previously (8). The products of this operon are responsible for 4-deoxyaminoarabinose addition and therefore modification of the LPS target. Addition of both pEtN and Ara4N to the LPS creates a more positively charged LPS molecule and thus reduces the affinity of positively charged polymyxins (6–8).
The expression of the pmrE gene was not significantly modified here in the studied colistin-resistant K. pneumoniae isolates. Therefore, in contrast to what has been observed for S. enterica and E. coli (22), PmrA may upregulate pmrE expression in K. pneumoniae. Also, the expression of the pmrD gene was not significantly modified, which is different from what has been observed in S. enterica (23), for which the transcription of the pmrD gene was repressed by the PmrA protein. Those findings are in accordance with data obtained by Cheng et al. (3) showing that the pmrD expression is independent of PmrA/PmrB in Klebsiella.
In conclusion, we showed here that a specific point mutation in the PmrB protein is responsible for an upregulation of the pmrCAB and pmrHFIJKLM operons that confers resistance to colistin in K. pneumoniae. The identification of six clinical isolates of worldwide origin belonging to four distinct clones with this same substitution in PmrB suggests that this constitutes a clinically relevant mechanism of resistance in K. pneumoniae. In addition, it confirms that the PmrAB two-component system plays a major regulatory role in polymyxin B resistance in that species.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the INSERM, France, by the University of Fribourg, Switzerland, and by grants from the European Community (R-GNOSIS, FP7/HEALTH-F3-2011−282512, and MagicBullet, FP7/HEALTH-F3-2001-278232).
Footnotes
Published ahead of print 9 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00084-14.
REFERENCES
- 1.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas ME, Rafailidis PI, Matthaiou DK. 2010. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist. Updat. 13:132–138. 10.1016/j.drup.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Cheng HY, Chen YF, Peng HL. 2010. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 17:60. 10.1186/1423-0127-17-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannatelli A, D'Andrea MM, Giani T, Di Pilato V, Arena F, Ambretti S, Gaibani P, Rossolini GM. 2013. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemase mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 57:5521–5526. 10.1128/AAC.01480-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Camacho E, Gómez-Gil R, Tobes R, Manrique M, Lorenzo M, Galván B, Salvarelli E, Moatassim Y, Salanueva IJ, Pareja E, Codoñer FM, Alvarez-Tejado M, Garcillán-Barcia MP, De la Cruz F, Mingorance J. 2014. Genomic analysis of the emergence and evolution of multidrug resistance during a Klebsiella pneumoniae outbreak including carbapenem and colistin resistance. J. Antimicrob. Chemother. 69:632–636. 10.1093/jac/dkt419 [DOI] [PubMed] [Google Scholar]
- 6.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 16:284–290. 10.1016/j.tim.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 7.Breazeale SD, Ribeiro AA, McClerren AL, Raetz CR. 2005. A formyltransferase required for polymyxin resistance in Escherichia coli and the modification of lipid A with 4-amino-4-deoxy-l-arabinose. Identification and function of UDP-4-deoxy-4-formamido-l-arabinose. J. Biol. Chem. 280:14154–14167. 10.1074/jbc.M414265200 [DOI] [PubMed] [Google Scholar]
- 8.Gunn JS, Ryan SS, Van Velkinburgh JC, Ernst RK, Miller SI. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139–6146. 10.1128/IAI.68.11.6139-6146.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J. Clin. Microbiol. 51:1678–1684. 10.1128/JCM.03385-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2012. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. CLSI document M07-A9 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn. Microbiol. Infect. Dis. 74:412–414. 10.1016/j.diagmicrobio.2012.08.025 [DOI] [PubMed] [Google Scholar]
- 13.Dortet L, Cuzon G, Nordmann P. 2014. Dissemination of carbapenemase-producing Enterobacteriaceae in France, 2012. J. Antimicrob. Chemother. 69:623–627. 10.1093/jac/dkt433 [DOI] [PubMed] [Google Scholar]
- 14.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrër A, Lassel L, Fortineau N, Mansouri M, Anguel N, Richard C, Nordmann P. 2009. Outbreak of CTX-M-15-producing Klebsiella pneumoniae in the intensive care unit of a French hospital. Microb. Drug Resist. 15:47–54. 10.1089/mdr.2009.0868 [DOI] [PubMed] [Google Scholar]
- 17.Brink AJ, Coetzee J, Corcoran C, Clay CG, Hari-Makkan D, Jacobson RK, Richards GA, Feldman C, Nutt L, van Greune J, Deetlefs JD, Swart K, Devenish L, Poirel L, Nordmann P. 2013. Emergence of OXA-48 and OXA-181 carbapenemases among Enterobacteriaceae in South Africa and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastrointestinal tract. J. Clin. Microbiol. 51:369–372. 10.1128/JCM.02234-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi MJ, Ko KS. 2014. Mutant prevention concentrations of colistin for Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae clinical isolates. J. Antimicrob. Chemother. 69:275–277. 10.1093/jac/dkt315 [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz SM, Brannon MK, Dasgupta N, Pier M, Sgambati N, Miller AK, Selgrade SE, Miller SI, Denton M, Conway SP, Johansen HK, Hoiby N. 2012. PmrB mutations promote polymyxin resistance of Pseudomonas aeruginosa isolated from colistin-treated cystic fibrosis patients. Antimicrob. Agents Chemother. 56:1019–1030. 10.1128/AAC.05829-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53:3628–3634. 10.1128/AAC.00284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55:3370–3379. 10.1128/AAC.00079-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breazeale SD, Ribeiro AA, Raetz CR. 2002. Oxidative decarboxylation of UDP-glucuronic acid in extracts of polymyxin-resistant Escherichia coli. Origin of lipid a species modified with 4-amino-4-deoxy-l-arabinose. J. Biol. Chem. 277:2886–2896. 10.1074/jbc.M109377200 [DOI] [PubMed] [Google Scholar]
- 23.Mitrophanov AY, Jewett MW, Hadley TJ, Groisman EA. 2008. Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 4:e1000233. 10.1371/journal.pgen.1000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


