Abstract
Enterococci are the third most frequent cause of infective endocarditis. A high-inoculum stationary-phase in vitro pharmacodynamic model with simulated endocardial vegetations was used to simulate the human pharmacokinetics of daptomycin at 6 or 10 mg/kg of body weight/day or linezolid at 600 mg every 12 h (q12h), alone or in combination with gentamicin at 1.3 mg/kg q12h or rifampin at 300 mg q8h or 900 mg q24h. Biofilm-forming, vancomycin-susceptible Enterococcus faecalis and vancomycin-resistant Enterococcus faecium (vancomycin-resistant enterococcus [VRE]) strains were tested. At 24, 48, and 72 h, all daptomycin-containing regimens demonstrated significantly more activity (decline in CFU/g) than any linezolid-containing regimen against biofilm-forming E. faecalis. The addition of gentamicin to daptomycin (at 6 or 10 mg/kg) in the first 24 h significantly improved bactericidal activity. In contrast, the addition of rifampin delayed the bactericidal activity of daptomycin against E. faecalis, and the addition of rifampin antagonized the activities of all regimens against VRE at 24 h. Also, against VRE, the addition of gentamicin to linezolid at 72 h improved activity and was bactericidal. Rifampin significantly antagonized the activity of linezolid against VRE at 72 h. In in vivo Galleria mellonella survival assays, linezolid and daptomycin improved survival. Daptomycin at 10 mg/kg improved survival significantly over that with linezolid against E. faecalis. The addition of gentamicin improved the efficacy of daptomycin against E. faecalis and those of linezolid and daptomycin against VRE. We conclude that in enterococcal infection models, daptomycin has more activity than linezolid alone. Against biofilm-forming E. faecalis, the addition of gentamicin in the first 24 h causes the most rapid decline in CFU/g. Of interest, the addition of rifampin decreased the activity of daptomycin against both E. faecalis and VRE.
INTRODUCTION
Despite major advances in medicine and surgery, infective endocarditis (IE) remains a concerning disease associated with considerable morbidity and mortality (1). Bacterial causes of IE and bacteremia have changed over the past few decades, and now streptococci, staphylococci, and enterococci have emerged as the major pathogens (2). Among these, Enterococcus spp. have become the most challenging to treat. Barriers in treating these infections include the need for multiple agents to demonstrate bactericidal activity and microbiological cure (1), biofilm production by these bacteria (3, 4), and resistance to the mainstays of therapy (i.e., ampicillin, penicillin, and vancomycin) (5). Biofilm production is common in Enterococcus faecalis, with worldwide rates between 26 and 100% reported, and 93% reported in the United States (3). The 2005 recommendations of the American Heart Association for drug-resistant enterococcal IE include linezolid and quinupristin-dalfopristin, both of which are bacteriostatic against enterococci (1).
Daptomycin, at high doses, demonstrates bactericidal activity against enterococci in other types of infections and against Staphylococcus aureus in endocarditis (6, 7). This is due to the mechanism of action of daptomycin: it disrupts the cell membrane potential and is growth phase independent (8). There are promising data demonstrating in vitro synergy for gentamicin-and-daptomycin combination therapy against vancomycin-resistant enterococci (VRE) (9–13), and case reports also support these findings (11, 14, 15). Therefore, the addition of gentamicin, a ribosomal active agent, may provide a synergistic approach to VRE IE infections. Additionally, since E. faecalis often produces biofilms (3), it is of interest to evaluate the activity of daptomycin in combination with rifampin (16–18). Finally, since daptomycin demonstrates concentration-dependent killing, evaluation of approved doses (6 mg/kg of body weight) and higher doses (10 mg/kg) may show increased activity and resistance prevention with the latter (19), since efficacy has been established in other types of infection (20) with appropriate safety data (21).
We therefore evaluated the in vitro activities of daptomycin and linezolid alone and in combination with gentamicin or rifampin against enterococci in an in vitro model with sequestered high-inoculum stationary-phase infection using simulated endocardial vegetations (SEVs) (20, 22, 23). We also tested these regimens in an in vivo survival assay using Galleria mellonella larvae. We used a vancomycin-susceptible, biofilm-producing E. faecalis strain and a vancomycin-resistant E. faecium strain. We also evaluated biofilm production by these isolates.
MATERIALS AND METHODS
Bacterial strains.
We evaluated a vancomycin-susceptible, ampicillin-susceptible E. faecalis strain, ATCC 29212 (which is also susceptible to gentamicin and rifampin), and a vancomycin-resistant Enterococcus faecium clinical isolate (vancomycin-resistant enterococcus [VRE]; this isolate is also resistant to penicillin and rifampin and susceptible to gentamicin) from the Providence Veterans Affairs Medical Center. Both isolates were susceptible to linezolid and daptomycin.
Antimicrobial agents.
Linezolid (lots 11C03U04 and 10H10Z16; Pfizer, Inc., NY) was obtained commercially, and daptomycin was obtained from Cubist Pharmaceuticals, Inc. (Lexington, MA). Rifampin (lot 085K1929) and gentamicin (lots 050K03421 and 097K06887V) were purchased from Sigma Chemical Company (St. Louis, MO). Stock solutions of each antibiotic were freshly prepared at the beginning of each week and were kept frozen at −4°C.
Medium.
As described previously, Mueller-Hinton broth (Becton Dickinson, Sparks, MD) supplemented with calcium and adjusted to physiological conditions of 50 mg/liter calcium chloride (ionized Ca; 1.03 to 1.23 mmol/liter) and 12.5 mg/liter magnesium was used for all susceptibility analyses and in vitro pharmacodynamic (PD) analyses (24). Bacto tryptic soy broth (TSB; Becton, Dickinson) supplemented with 1% glucose and 50 mg/liter calcium chloride was used to optimize biofilm production in the biofilm assay (25, 26). Colony counts were determined using tryptic soy agar (TSA; Difco, Becton Dickinson). For the in vivo study, strains were grown overnight at 30°C in brain heart infusion (BHI) with agitation. The inoculum was confirmed by plating serial dilutions on BHI agar.
Susceptibility.
MICs and minimum bactericidal concentrations (MBCs) were determined in triplicate at both the standard inoculum (∼106 CFU/ml) and a high inoculum (∼109 CFU/ml) by using broth microdilution according to CLSI methods (27). All samples were incubated at 35°C for 24 h prior to the interpretation of results.
Biofilm formation.
Under growth conditions (see “Medium” above) that optimize biofilm production in Enterococcus species, biofilm formation was quantified using the microtiter plate assay first described by Christensen et al. (28) and modified as follows. Briefly, stationary-phase cultures of the enterococcal strains grown overnight (1%, vol/vol) were diluted into fresh cation- and glucose-supplemented TSB. The inoculated medium was dispensed into wells of sterile flat-bottom 96-well polystyrene tissue culture plates (Costar; catalog no. 3596; Corning Inc., Corning, NY, USA). We examined two sets of plates that had been incubated at 35°C for 24 h and 48 h, respectively, based on previous studies (29). The attached bacteria were then fixed and were stained with crystal violet. After drying, the optical densities (OD) of stained adherent bacterial films were read using a μQuant Microplate Spectrophotometer microtiter plate reader (BioTek Instruments, Inc. Winooski, VT, USA). The OD of bacterial films were assigned to the following categories: no biofilm production or weakly (+), moderately (++), or strongly (+++) adherent biofilms (30). The test was carried out in triplicate. The results were averaged.
Preparation of SEVs for in vitro pharmacodynamic infection model.
As described previously, organism stocks containing approximately 1010 CFU/ml were prepared by inoculating 5-ml test tubes of normal saline with colonies harvested from fresh overnight growth on TSA (20, 22, 24, 31, 32). Simulated endocardial vegetations (SEVs) containing 109 CFU/g were prepared by combining 0.05 ml of the organism suspension with 0.4 ml of human cryoprecipitated antihemolytic factor (AHF) from volunteer donors (Rhode Island Blood Bank, Providence, RI), 0.05 ml of an aprotinin suspension, and 0.025 ml of a platelet suspension (platelets mixed with normal saline; 250,000 to 500,000 platelets per clot) in 1.5-ml Eppendorf tubes. Bovine thrombin (5,000 U/ml; 50 μl) was added to each tube after the insertion of a sterile monofilament line into the mixture. The resultant SEVs were removed from the Eppendorf tubes with a sterile 21-gauge needle and were introduced into the model. This methodology results in SEVs containing approximately 3 to 3.5 g/dl of albumin and 6.8 to 7.4 g/dl of total protein (22).
In vitro pharmacodynamic infection model.
An in vitro infection model consisting of a 250-ml one-compartment glass apparatus with ports where the SEVs are suspended was utilized for all simulations. The apparatus was prefilled with the medium, and antibiotics were administered as boluses into the central compartment via an injection port over a 72-h period. The models were placed in a 35°C water bath throughout the procedure with a magnetic stir bar for thorough mixing of the drug in the model. Fresh medium was continuously supplied and removed from the model via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL, USA) set to simulate the half-lives of the antibiotics. Two SEVs were removed from each model at 0, 4, 8, 24, 32, 48, 56, and 72 h. Once removed, SEVs were immediately homogenized in trypsin, plated onto TSA, and incubated at 35°C for 24 h before enumeration by colony counts. This method results in a limit of detection of 2.0 log10 CFU/g (23). Antimicrobial carryover was minimized by serial dilution (1:10 to 1:10,000) of plated samples in conjunction with vacuum filtration, when necessary, where samples were washed through a 0.22-μm filter with sterile water. These filters were then plated onto TSA and were incubated at 35°C for 24 h. Colonies were counted on filter paper; the limit of detection is 1.0 log10 CFU/g.
Daptomycin was administered to simulate a 6-mg/kg dose (peak concentration, 98.6 μg/ml) and a 10-mg/kg dose (peak concentration, 141 μg/ml) every 24 h (q24h), with the pump rate set to achieve a half-life of 8 h (21, 33). Linezolid was administered to simulate 600 mg q12h, with a half-life of 6 h and a peak concentration of 21 μg/ml (27). Gentamicin was administered to simulate 1.3 mg/kg q12h (approximate peak concentration, 6 μg/ml; approximate trough concentration, 0.4 μg/ml), with a half-life of 2 h (24). Rifampin was administered to simulate a dose of 300 mg q8h (approximate peak concentration,14.5 μg/ml) and a half-life of 4 h (24). Additionally, a regimen simulating 900 mg rifampin once daily in combination with linezolid or with 6 mg/kg daptomycin was performed in duplicate to assess the effects of the rifampin dosage schedule and concentration.
For combination regimen experiments, the elimination rate was set for the drug with the shortest half-life; the drug with the longer half-life was supplemented. Unless otherwise noted, all model experiments were performed in triplicate so as to ensure reproducibility. In addition, simulations in the absence of antibiotics were performed at the shortest half-life so as to ensure adequate growth of the organisms in the model.
Pharmacodynamic analysis.
Reductions in log10 CFU/g over 72 h were determined by plotting time-kill curves and were compared between regimens. Bactericidal activity (99.9% killing) was defined as a ≥3-log10 CFU/g reduction in the colony count from that in the initial inoculum. Bacteriostatic activity was defined as a <3-log10 CFU/g reduction in the colony count from that in the initial inoculum, while inactivity was defined as no observed reductions from the initial inoculum. The time to achieve 99.9% killing was determined either by nonlinear regression (using a minimum of 4 data points) if r2 was ≥0.95 or by visual inspection. Enhancement of activity was defined as a ≥2-log10 CFU/g increase in killing by use of a combination of antimicrobials over the level with the most active single agent of the combination. Improvement was defined as a 1- to 2-log10 CFU/g increase in killing over the level with the most active single agent, while a ≥1-log10 increase in bacterial growth with a combination over the level with the most active single agent was considered to represent antagonism. The terms “improvement” and “enhancement” were used because our simulations involve therapeutically obtained concentrations in serum, which do not permit the mathematical modeling necessary to consider the standard terms “additivity” and “synergy” (34). Indifference was defined as a <1-log10 CFU/g change in activity.
Resistance.
The development of resistance was evaluated for each monotherapy and combination model at 24, 48, and 72 h. MIC testing (using the Etest) of daptomycin, linezolid, gentamicin, and rifampin was conducted with isolates obtained from the 24-, 48-, and 72-h time points to identify any MIC shifts. Plates were examined for growth after 24 h of incubation at 35°C.
Pharmacokinetic analysis.
Samples for pharmacokinetic (PK) analyses were obtained through the injection port at 0.5, 1, 2, 4, 6, 8, and 24 h in order to verify target antibiotic concentrations. All samples were stored at −80°C until analysis. Daptomycin concentrations were determined by a previously described and validated high-performance liquid chromatography (HPLC) method (Center for Anti-Infective Research and Development, Hartford, CT) (20). Gentamicin concentrations were determined by a homogeneous particle-enhanced turbidimetric immunoassay (PETIA) (Architect system; Multigent assay; Abbott Diagnostics, Abbott Park, IL, USA) at the Providence Veteran Affairs Medical Center. The gentamicin assay was known to have a range of detection of 0.3 to 10.0 μg/ml, a between-day sample precision of 1.35%, and a coefficient of variation, expressed as a percentage (CV%), of <2.75%. The linezolid and rifampin concentrations were evaluated using HPLC (University of Florida, Gainesville, FL) as described previously (23, 24). Only single drug concentrations were evaluated, all in duplicate. The half-lives, maximum concentrations (Cmax), and minimum concentrations (Cmin) of the antibiotics were determined by the trapezoidal method utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
In vivo Galleria mellonella survival assay.
The efficacy of daptomycin or linezolid in enterococcal infection was tested using a Galleria mellonella survival assay. Galleria mellonella caterpillars at the final-instar stage of development were acquired from the vendor (Vanderhorst Wholesale Inc., St. Marys, OH) and were used within 7 days of shipment. All experiments were performed according to previously described protocols with minor modifications (35, 36). Sixteen larvae of appropriate weight (0.25 to 0.35 g) were randomly selected to constitute each group. Larvae were inoculated with either ∼4 × 106 CFU of E. faecalis or 7 × 106 to 9 × 106 CFU of E. faecium, followed by the test drug or by phosphate-buffered saline (PBS) as a control ∼1 h after inoculation. These inocula were chosen after an initial virulence pilot study of these strains, because they were able to kill at least 90% of the larvae within 72 h. One group that was injected twice with PBS and one untouched group were used as controls in each experiment. All injections were performed with a volume of 10 μl by using a Hamilton syringe. After injection, G. mellonella larvae were incubated at 37°C, and survival was measured daily. Each experiment was repeated at least twice, and the results of representative experiments are presented. The results of any experiment with more than two dead larvae in any control group were discarded. The doses simulated free peak concentrations seen in humans treated with 6 mg/kg daptomycin, 10 mg/kg daptomycin, or 600 mg linezolid (Table 1). Gentamicin (1.3 mg/kg) and rifampin (300 mg) were also tested in combination with either linezolid or 6 mg/kg daptomycin.
TABLE 1.
Targeted peak concentrations versus concentrations administered in G. mellonella models
| Antimicrobial (human dose) | Targeted free peak concn (mg/liter) | Concn (mg/liter) administered in G. mellonella model |
|---|---|---|
| Daptomycin (6 mg/kg) | 9.8 | 9.15 |
| Daptomycin (10 mg/kg) | 14.0 | 13.07 |
| Linezolid (600 mg) | 14.0 | 8.00a |
| Gentamicin (1.3 mg/kg) | 6.0 | 5.60 |
| Rifampin (300 mg) | 2.6 | 2.50 |
Linezolid concentrations were lower than targeted due to limits on the available pharmaceutical concentrations.
Statistical analysis.
For the in vitro model, changes in CFU/g at 8, 24, 48, and 72 h and the time to 99.9% killing were compared by two-way analysis of variance with Tukey's post hoc test. Statistical analyses were performed using SPSS statistical software (release 20; SPSS, Inc., Chicago, IL). Survival in the G. mellonella model was plotted using Kaplan-Meier curves, and groups were compared using the log rank test (GraphPad Prism software, version 5). For all experiments, a P value of ≤0.05 was considered significant.
RESULTS
Susceptibility testing.
Daptomycin, linezolid, gentamicin, and rifampin MICs for the two strains of enterococci are shown in Table 2. In the presence of high E. faecalis inocula, there were minimal increases (1 and 2 dilutions, respectively) in the MICs of daptomycin and linezolid. Against a high inoculum of VRE, the MICs of daptomycin and linezolid increased by 3 dilutions and 2 dilutions, respectively. There were minimal increases (0 to 2 dilutions) in the gentamicin and rifampin MICs when the isolates were evaluated at high inocula. These findings are consistent with those of published studies (10, 23).
TABLE 2.
MICs of antimicrobial agents against enterococcal isolates at standard and high inocula
| Antimicrobial | MIC (mg/liter) for the standard (high) inoculuma |
|
|---|---|---|
| E. faecalis ATCC 29212 | E. faecium L2001 | |
| Daptomycin | 2 (4) | 1 (8) |
| Linezolid | 1 (4) | 1 (4) |
| Gentamicin | 16 (32) | 16 (32) |
| Rifampin | 0.5 (0.5) | 4 (16) |
| Vancomycin | 2 | >256 |
The standard inoculum was 5 × 105 CFU/ml, and the high inoculum was 5 × 109 CFU/ml.
In vitro pharmacokinetics and pharmacodynamics.
The pharmacokinetic parameters of the antimicrobial agents were within the targeted ranges (Table 3). All Cmax values obtained were within 5% of the targeted Cmax. The areas under the concentration-time curve (AUC) (averages ± standard deviations) were 1,028 ± 36 for 6 mg/kg daptomycin, 1,430 ± 47 for 10 mg/kg daptomycin, and 348 ± 16 for linezolid.
TABLE 3.
Valuesa for targeted and obtained pharmacokinetic parameters in SEV infection models
| Regimenb | Peak concn (mg/liter) |
Half-life (h) |
||
|---|---|---|---|---|
| Targeted | Obtained | Targeted | Obtained | |
| Daptomycin, 6 mg/kg q24h | 98.6 | 102.5 ± 1.96 | 8 | 7.92 ± 0.18 |
| Daptomycin, 10 mg/kg q24h | 140.0 | 143.2 ± 1.94 | 8 | 7.87 ± 0.21 |
| Linezolid, 600 mg q12h | 21.0 | 21.9 ± 0.86 | 6 | 6.52 ± 0.87 |
| Gentamicin, 1.3 mg/kg q12h | 6.0 | 5.7 ± 0.51 | 2 | 2.08 ± 0.17 |
| Rifampin, 300 mg q8h | 10.5 | 11.0 ± 1.23 | 4 | 3.60 ± 0.50 |
Means ± standard deviations.
Based on a 75-kg patient.
Biofilm production.
The E. faecalis isolate is a biofilm-positive control and consistently produced biofilms (++) at 24 and 48 h. The E. faecium isolate did not produce biofilms (0) at 24 h and was weakly adherent (+) at 48 h.
In vitro pharmacodynamic infection model with simulated endocardial vegetations (SEVs).
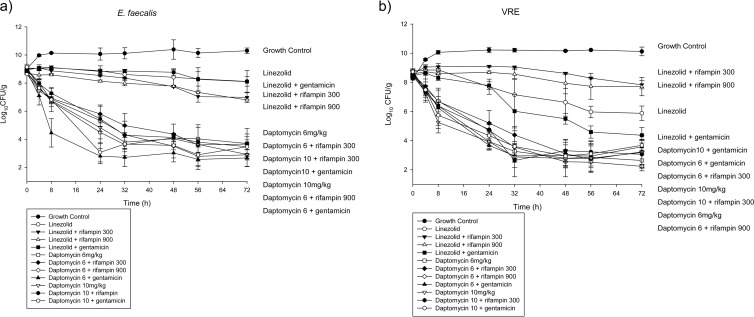
The antimicrobial activities of daptomycin and linezolid were evaluated alone and in combination with gentamicin or rifampin against enterococci at a high inoculum (109 CFU/g) in a simulated IE vegetation model (Fig. 1). Bactericidal activity (≥3-log10 decrease in CFU/g) was achieved by 6 or 10 mg/kg daptomycin against E. faecalis at 24 h and by 10 mg/kg daptomycin against E. faecium at 8 h. Linezolid monotherapy did not achieve bactericidal activity against either isolate tested at any time point. The AUC/MIC ratios were 514 to 1,028 (MIC range, 1 to 2 μg/ml) for 6 mg/kg daptomycin, 715 to 1,430 (MIC range, 1 to 2 μg/ml) for 10 mg/kg daptomycin, and 348 (MIC, 1 μg/ml) for linezolid. The percentage of time that the drug concentration exceeded the MIC (%TMIC) was 100% for the daptomycin and linezolid regimens.
FIG 1.
Activities (changes in log10 CFU/g) of daptomycin- or linezolid-containing regimens against Enterococcus faecalis (susceptible to vancomycin, gentamicin, rifampin, daptomycin, and linezolid) (a) and Enterococcus faecium (resistant to vancomycin and rifampin; susceptible to gentamicin, daptomycin, and linezolid) (b).
Against the biofilm-forming E. faecalis strain, daptomycin-containing regimens demonstrated significantly more activity (as measured by a decline in the mean CFU/g) than linezolid-containing regimens from 8 h through the end of the experiment (P, ≤0.005) (Fig. 1a; Table 4). The addition of gentamicin significantly increased activity for 10 mg/kg daptomycin at 24 h (95% confidence interval [95% CI], 0.954 to 3.4029; P, 0.033). The combination of 6 mg/kg daptomycin with gentamicin was significantly more active than any other regimen tested at 8 h (P, ≤0.001). At 24 h, there was a 3-log10 CFU/g difference in activity between the combination of 6 mg/kg daptomycin with gentamicin and the combination of 6 mg/kg daptomycin with rifampin (P, 0.010), although the difference was no longer significant at 48 h. There was no significant difference between linezolid monotherapy and combinations of linezolid with rifampin or gentamicin at any time point during the 72-h experiment, although the addition of rifampin to linezolid met the definition for improvement at 72 h. Changing the schedule of rifampin dosing from 300 mg three times daily to 900 mg once daily had no effect on either regimen.
TABLE 4.
Change in bacterial density from that of the starting inoculum in the SEV model at 8, 24, and 72 h
| Antimicrobial regimen | Mean change in bacterial density (log10 CFU/g) from that of the starting inoculuma |
|||||
|---|---|---|---|---|---|---|
|
E. faecalis |
E. faecium |
|||||
| 8 h | 24 h | 72 h | 8 h | 24 h | 72 h | |
| Growth control | +1.13 | +1.06 | +1.29 | +1.82 | +1.93 | +1.86 |
| Daptomycin, 6 mg/kg | −2.07b | −4.28b | −5.07b | −2.11b | −4.56b | −5.86b |
| Daptomycin, 6 mg/kg, + rifampin | −1.88b | −2.99b | −5.13b | −1.84b | −3.33b | −5.30b |
| Daptomycin, 6 mg/kg, + gentamicin | −4.36b | −6.02b | −6.15b | −2.38b | −4.96b | −5.05b |
| Daptomycin, 10 mg/kg | −2.23b | −4.17b | −6.07b | −3.57b | −4.90b | −5.63b |
| Daptomycin, 10 mg/kg, + rifampin | −1.65b | −3.48b | −5.46b | −2.09b | −3.71b | −5.41b |
| Daptomycin, 10 mg/kg, + gentamicin | −2.32b | −6.07b | −5.67b | −2.99b | −4.08b | −5.04b |
| Linezolid | +0.02 | −0.19 | −0.95 | +0.07 | −1.08b | −2.90b |
| Linezolid + rifampin | −0.07 | −0.40 | −1.96b | +0.45 | +0.48 | −0.79 |
| Linezolid + gentamicin | +0.13 | −0.15 | −0.88b | −0.14 | −0.67b | −4.08b |
The starting inoculum was 5 × 109 CFU/g. Positive values indicate growth.
Statistically significantly different from the result with the growth control.
Against the VRE isolate, daptomycin-containing regimens had significantly (P, ≤0.005) more activity than any of the linezolid-containing regimens at 24 and 48 h (Fig. 1b). The addition of gentamicin improved linezolid activity, such that at 72 h, linezolid plus gentamicin was significantly different only from 6 mg/kg daptomycin (the most active regimen) (95% CI, 0.0144 to 3.4556; P, 0.047) among the daptomycin-containing regimens. Linezolid plus gentamicin was not, however, significantly more active than linezolid monotherapy. The combination of gentamicin with 6 mg/kg daptomycin was significantly more active than the combination of rifampin with 6 mg/kg daptomycin at 24 h (95% CI, 0.2349 to 2.9984; P, 0.013). Rifampin antagonized all regimens at 24 h. The addition of rifampin also significantly antagonized linezolid activity at 48 h (95% CI, 0.0546 to 3.9921; P, 0.040) and 72 h (95% CI, 0.0595 to 4.1772; P, 0.040). At 72 h, the log10 CFU/g with linezolid plus rifampin was not significantly different from that with the growth control. Changing rifampin dosing from three times daily to once daily did not significantly increase activity; however, the combination of linezolid and rifampin once daily was significantly more active than the growth control at 72 h (95% CI, 0.1546 to 4.6654; P, 0.028).
Neither gentamicin monotherapy nor rifampin monotherapy demonstrated any significant activity against either isolate during the study. Resistance occurred in the rifampin and gentamicin monotherapy models by 24 h. The linezolid and daptomycin MICs differed at each time point but never exceeded 4 μg/ml. The MICs of rifampin in combination with either daptomycin or linezolid increased throughout the 72-h experiments against the VRE isolate, from 4 to >32 μg/ml. Gentamicin MICs remained constant throughout the combination regimen experiments.
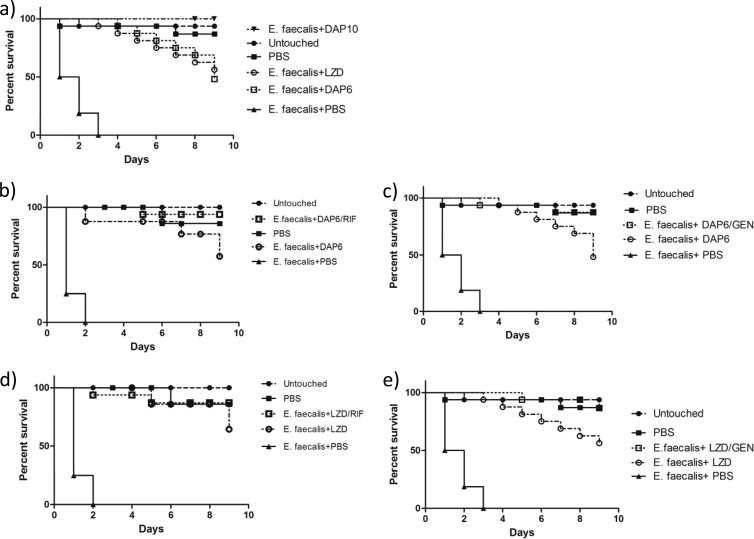
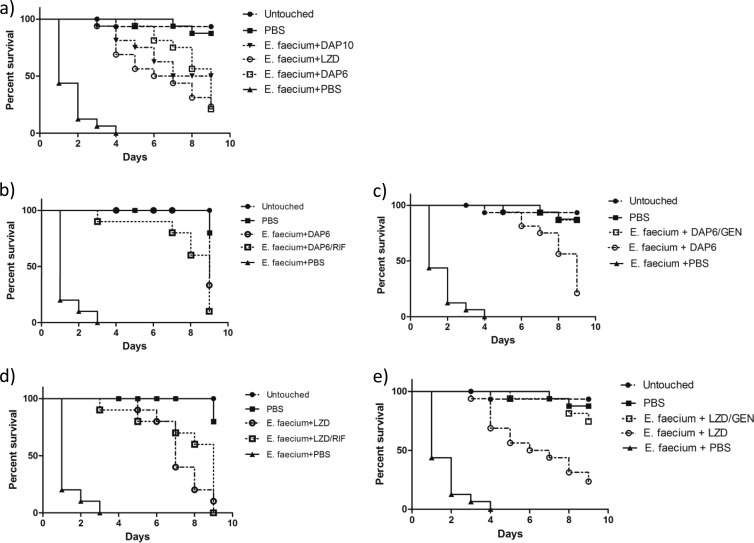
In vivo Galleria mellonella survival assay.
All antimicrobial regimens tested improved survival in all Galleria mellonella assays (P, <0.0001) (Fig. 2 and 3). Against E. faecalis, monotherapy with 10 mg/kg daptomycin improved survival significantly over that with linezolid alone (P, 0.0032) (Fig. 2a). Gentamicin improved the efficacy of 6 mg/kg daptomycin (P, 0.0361) but not that of linezolid (Fig. 2c and e), as observed in the in vitro model. Against E. faecium, gentamicin improved the efficacy of both the 6-mg/kg daptomycin regimen and the linezolid regimen (P, 0.0009 and 0.0015, respectively) (Fig. 3c and e). The addition of rifampin was not significant for daptomycin or linezolid against either strain (Fig. 2b and d and 3b and d). Although no antagonism was observed for rifampin, the other results concur with our in vitro pharmacodynamic findings.
FIG 2.
Efficacies of compounds against E. faecalis in a G. mellonella infection model. Aside from those representing controls (Untouched and PBS), each line on each graph represents the survival of a group of 16 larvae injected first with E. faecalis and then with the indicated drug(s). Shown are the percentages of survival for larvae receiving monotherapy with 6 mg/kg daptomycin (DAP6), 10 mg/kg daptomycin (DAP10), or linezolid (LZD) (a), 6 mg/kg daptomycin alone or in combination with rifampin (RIF) (b), 6 mg/kg daptomycin alone or in combination with gentamicin (GEN) (c), linezolid alone or in combination with rifampin (d), or linezolid alone or in combination with gentamicin (e) in comparison with those for controls.
FIG 3.
Efficacies of compounds against E. faecium in a G. mellonella infection model. Aside from those representing controls (Untouched and PBS), each line on each graph represents the survival of a group of 16 larvae injected first with E. faecium and then with the indicated drug(s). Shown are the percentages of survival for larvae receiving monotherapy with 6 mg/kg daptomycin, 10 mg/kg daptomycin, or linezolid (a), 6 mg/kg daptomycin alone or in combination with rifampin (b), 6 mg/kg daptomycin alone or in combination with gentamicin (c), linezolid alone or in combination with rifampin (d), or linezolid alone or in combination with gentamicin (e) in comparison with those for controls.
DISCUSSION
Infective endocarditis vegetations often carry a high bacterial burden (108 to 1010 organisms per g of tissue) (37). This high bacterial density and the limited blood supply to this area allow for a diminished immune response and limited antimicrobial drug access. The location of the vegetation (right-sided versus left-sided endocarditis), patient comorbidities, and surgical interventions determine the success of treatment (38, 39). The ability of bacteria to form biofilms may contribute to treatment failure, since biofilm-forming bacteria are inherently less susceptible to antibiotics due to decreased growth rates, nutrient restriction, and adaptive stress responses (40–43).
Endocarditis caused by enterococci requires treatment with synergistic antimicrobials; traditionally, a cell wall-active agent (a beta-lactam or vancomycin) and an aminoglycoside. High-level resistance to vancomycin eliminates the main therapeutic options in the management of serious enterococcal infections. Currently, the options for resistant E. faecalis IE include ampicillin in combination with either imipenem-cilastatin or ceftriaxone (1). While treatment with ampicillin in combination with ceftriaxone against high-level aminoglycoside-resistant (HLAR) E. faecalis is becoming more common, further investigations into PK/PD activity and dosage are needed. The 2005 American Heart Association guidelines for the treatment of IE recommend ≥8 weeks of linezolid or quinupristin-dalfopristin monotherapy for the treatment of “native or prosthetic valve enterococcal endocarditis caused by strains resistant to penicillin, aminoglycoside, and vancomycin” (1). In many cases, these treatments are not ideal. Linezolid has inherent bacteriostatic activity (6, 44) and can cause myelosuppression (45, 46), and failure in bacteremia and IE has been documented in animal studies and human case reports (47–50). The use of quinupristin-dalfopristin is also limited, because it demonstrates inherent bacteriostatic activity against VRE (51), lack of activity against E. faecalis (6), and musculoskeletal toxicities in approximately 50% of the population, and because it requires the use of a central line for administration (52). Daptomycin is commonly used for the treatment of VRE infections (53), although the optimal dose and combinations are unknown.
Studies have shown that daptomycin demonstrates activity in enterococcal infections and may provide an option for patients with allergies or contraindications to other therapies. In a retrospective cohort study of VRE bloodstream infections, treatment with daptomycin or linezolid demonstrated no difference in mortality; however, infection with E. faecium and concurrent treatment with rifampin or gentamicin were independent risk factors for mortality (54). Antagonistic activity is often observed when rifampin is added to bactericidal agents in high-inoculum infections, due to high rates of mutations conferring resistance (∼1 in 106) (31, 55, 56). The in vitro model demonstrated antagonism with rifampin. The in vivo model used a lower bacterial burden, so antagonism from rifampin resistance may not have been as evident. In contrast, previous in vitro studies have shown synergy between daptomycin and rifampin and nonantagonism between daptomycin and gentamicin (6).
G. mellonella is an invertebrate model host that shares many of the advantages of mammalian models and is free of the ethical and logistical constraints that accompany their use (57). Specifically, G. mellonella larvae can grow at 37°C, thus effectively simulating human temperatures, and can be directly injected with the inoculum and compounds to be tested, thus allowing for exact quantification of the experimental concentrations (58). As a result, this model host is well established in the screening of the efficacy and safety of antimicrobial compounds against a variety of infections (59) and has also been used effectively to test antibiotics against Enterococcus spp. in the past (60). G. mellonella possesses both cellular and humoral defenses and has extensive structural and functional similarities to vertebrate immune systems (61). Finally, G. mellonella larvae have also been proven effective for the identification of the immunomodulatory properties of several compounds that would have gone unnoticed in in vitro experiments (62). Our in vivo model demonstrated improvement with the addition of gentamicin to 6 mg/kg daptomycin. It is possible that this improvement would not be seen with higher daptomycin doses, since survival was 100% at 9 days with the 10-mg/kg dose.
Another in vitro model with simulated endocardial vegetations, used by Hall et al., successfully demonstrated the concentration-dependent activity of daptomycin against VRE, supporting doses of >6 mg/kg/day, as well as demonstrating that daptomycin activity was superior to linezolid activity (32). A recent meta-analysis of VRE bacteremia demonstrated a trend toward higher survival with linezolid treatment than with daptomycin treatment (63). These differences, however, were not statistically significant, and the studies used suffered from problems of different definitions of mortality, low doses of daptomycin (average dose, ∼6 mg/kg), and a possible treatment selection bias in the cohorts (64). A recent cohort study of patients with Gram-positive infective endocarditis demonstrated no significant difference in mortality between standard-of-care antibiotics and daptomycin given at an average dose of ∼8 mg/kg in the E. faecalis group (65). The E. faecalis group treated with daptomycin had a significantly shorter average length of stay than patients treated with standard antibiotics (17.5 [range, 13.5 to 19.5] versus 31 [range, 19.0 to 50.0] days; P, 0.02) (65). Although small, this study also demonstrated no significant increase in adverse events with higher-dose daptomycin. Our work demonstrates no statistically significant differences between any of the daptomycin regimens at 72 h. There is some in vitro evidence to support the use of high-dose daptomycin in complicated enterococcal bacteremia and IE: 10 mg/kg, but not 6 mg/kg, can prevent increases in MICs for daptomycin-nonsusceptible S. aureus isolates (66). The addition of gentamicin for the first 24 h decreased the bacterial burden faster than daptomycin alone but provided no benefit after 24 h.
In conclusion, daptomycin-containing regimens generally were more active against enterococcal isolates than linezolid throughout the experiments. The addition of rifampin to either linezolid or daptomycin did not significantly increase antibacterial activity in an in vitro sequestered high-inoculum model of enterococcal endocarditis at 72 h, and rifampin delayed the bactericidal activity of daptomycin during the first 24 h. The inhibition of bacterial RNA synthesis may be responsible for delaying the killing activities of cell wall-active agents (67). The addition of gentamicin improved the bactericidal activity of daptomycin against E. faecalis most in the first 24 h and increased the activity of linezolid against vancomycin-resistant E. faecium at 72 h. It is currently unclear how linezolid, a protein synthesis inhibitor, demonstrates improved activity in the presence of gentamicin. This improved activity has also been observed against S. aureus and a vancomycin-resistant E. faecalis strain (67–69). We believe that our work supports the use of 6 or 10 mg/kg daptomycin, with the addition of 24 h of gentamicin for E. faecalis, as the most active therapy for enterococcal endocarditis. Other clinical studies have demonstrated worse outcomes with rifampin, while gentamicin adds activity only in the first 24 h, and its use should be limited due to concerns about nephrotoxicity.
A limitation of this study is the use of only two isolates. In addition, we cannot conclude that our in vitro results will hold true with treatment durations longer than 72 h. Our findings on daptomycin and linezolid monotherapy are consistent with those published for clinical, in vitro, and animal models (7, 32, 70). The linezolid concentration in G. mellonella larvae, while active, was lower than desired due to limits on available pharmaceutical concentrations. It is possible that the differences seen would not be significant if a higher concentration were used. While G. mellonella larvae received doses targeting the free peak concentration achieved in humans, each drug was dosed only once, survival was measured over 9 days, and pharmacokinetic information, including metabolism and excretion, is unknown.
The results support the use of 6 or 10 mg/kg daptomycin against VRE and 6 or 10 mg/kg daptomycin plus 24 h of gentamicin against E. faecalis in simulated endocardial vegetations. Nonetheless, our results should be applied to clinical practice with caution. Confirmation of these results in clinical studies is needed before these regimens can be adopted for use in the care of patients.
ACKNOWLEDGMENTS
We thank Kayla Babcock for laboratory assistance. We gratefully acknowledge Christine Long, Core Laboratory Supervisor, and Clyde Belgrave, Chief of Laboratory Services, at the Veterans Affairs Medical Center in Providence, RI, for analysis of the gentamicin samples. We also gratefully acknowledge David P. Nicolau and Christina Sutherland of the Center for Anti-Infective Research and Development at Hartford Hospital (Hartford, CT) for HPLC analysis of daptomycin concentrations and Charles Peloquin of the University of Florida (Gainesville, FL) for HPLC analysis of the linezolid and rifampin samples.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs. This material is the result of work supported with resources and the use of facilities at the Providence VA Medical Center.
This research was funded in part by Cubist Pharmaceuticals. M.K.L. received research funding from Cubist and Pfizer. K.L.L. received research funding and compensation for services as an advisor and/or consultant from Cubist, Astellas, Theravance, Forest, Davol, Marvao, and Pfizer.
Footnotes
Published ahead of print 27 May 2014
REFERENCES
- 1.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394–e434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Marcos FJ, Lomas-Cabezas JM, Hidalgo-Tenorio C, de la Torre-Lima J, Plata-Ciezar A, Reguera-Iglesias JM, Ruiz-Morales J, Marquez-Solero M, Galvez-Acebal J, de Alarcon-Gonzalez A. 2009. Enterococcal endocarditis: a multicenter study of 76 cases. Enferm. Infecc. Microbiol. Clin. 27:571–579 (In Spanish.) 10.1016/j.eimc.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Mohamed JA, Huang DB. 2007. Biofilm formation by enterococci. J. Med. Microbiol. 56:1581–1588. 10.1099/jmm.0.47331-0 [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 5.Biedenbach DJ, Moet GJ, Jones RN. 2004. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002). Diagn. Microbiol. Infect. Dis. 50:59–69. 10.1016/j.diagmicrobio.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Canton R, Ruiz-Garbajosa P, Chaves RL, Johnson AP. 2010. A potential role for daptomycin in enterococcal infections: what is the evidence? J. Antimicrob. Chemother. 65:1126–1136. 10.1093/jac/dkq087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervera C, Castaneda X, Pericas JM, Del Rio A, de la Maria CG, Mestres C, Falces C, Marco F, Moreno A, Miro JM. 2011. Clinical utility of daptomycin in infective endocarditis caused by Gram-positive cocci. Int. J. Antimicrob. Agents 38:365–370. 10.1016/j.ijantimicag.2010.11.038 [DOI] [PubMed] [Google Scholar]
- 8.Steenbergen JN, Alder J, Thorne GM, Tally FP. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 55:283–288. 10.1093/jac/dkh546 [DOI] [PubMed] [Google Scholar]
- 9.Caron F, Kitzis MD, Gutmann L, Cremieux AC, Maziere B, Vallois JM, Saleh-Mghir A, Lemeland JF, Carbon C. 1992. Daptomycin or teicoplanin in combination with gentamicin for treatment of experimental endocarditis due to a highly glycopeptide-resistant isolate of Enterococcus faecium. Antimicrob. Agents Chemother. 36:2611–2616. 10.1128/AAC.36.12.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRyke CA, Sutherland C, Zhang B, Nicolau DP, Kuti JL. 2006. Serum bactericidal activities of high-dose daptomycin with and without coadministration of gentamicin against isolates of Staphylococcus aureus and Enterococcus species. Antimicrob. Agents Chemother. 50:3529–3534. 10.1128/AAC.00290-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanafani ZA, Federspiel JJ, Fowler VG., Jr 2007. Infective endocarditis caused by daptomycin-resistant Enterococcus faecalis: a case report. Scand. J. Infect. Dis. 39:75–77. 10.1080/00365540600786465 [DOI] [PubMed] [Google Scholar]
- 12.Ramos MC, Grayson ML, Eliopoulos GM, Bayer AS. 1992. Comparison of daptomycin, vancomycin, and ampicillin-gentamicin for treatment of experimental endocarditis caused by penicillin-resistant enterococci. Antimicrob. Agents Chemother. 36:1864–1869. 10.1128/AAC.36.9.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snydman DR, McDermott LA, Jacobus NV. 2005. Evaluation of in vitro interaction of daptomycin with gentamicin or beta-lactam antibiotics against Staphylococcus aureus and enterococci by FIC index and timed-kill curves. J. Chemother. 17:614–621. 10.1179/joc.2005.17.6.614 [DOI] [PubMed] [Google Scholar]
- 14.Barber GR, Lauretta J, Saez R. 2007. A febrile neutropenic patient with Enterococcus gallinarum sepsis treated with daptomycin and gentamicin. Pharmacotherapy 27:927–932. 10.1592/phco.27.6.927 [DOI] [PubMed] [Google Scholar]
- 15.Das SS, Anderson JR, Macdonald AA, Somerville KW. 1994. Endocarditis due to high level gentamicin resistant Enterococcus faecium. J. Infect. 28:185–191. 10.1016/S0163-4453(94)95680-4 [DOI] [PubMed] [Google Scholar]
- 16.Rand KH, Houck HJ, Silverman JA. 2007. Daptomycin-reversible rifampicin resistance in vancomycin-resistant Enterococcus faecium. J. Antimicrob. Chemother. 59:1017–1020. 10.1093/jac/dkm045 [DOI] [PubMed] [Google Scholar]
- 17.Cilli F, Aydemir S, Tunger A. 2006. In vitro activity of daptomycin alone and in combination with various antimicrobials against Gram-positive cocci. J. Chemother. 18:27–32. 10.1179/joc.2006.18.1.27 [DOI] [PubMed] [Google Scholar]
- 18.Rand KH, Houck H. 2004. Daptomycin synergy with rifampicin and ampicillin against vancomycin-resistant enterococci. J. Antimicrob. Chemother. 53:530–532. 10.1093/jac/dkh104 [DOI] [PubMed] [Google Scholar]
- 19.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. 2009. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219. 10.1345/aph.1M085 [DOI] [PubMed] [Google Scholar]
- 20.Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459. 10.1128/AAC.45.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249. 10.1128/AAC.00247-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershberger E, Coyle EA, Kaatz GW, Zervos MJ, Rybak MJ. 2000. Comparison of a rabbit model of bacterial endocarditis and an in vitro infection model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 44:1921–1924. 10.1128/AAC.44.7.1921-1924.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672. 10.1128/AAC.48.12.4665-4672.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaPlante KL, Woodmansee S. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 53:3880–3886. 10.1128/AAC.00134-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaPlante KL, Mermel LA. 2007. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol. Dial. Transplant. 22:2239–2246. 10.1093/ndt/gfm141 [DOI] [PubMed] [Google Scholar]
- 26.Pillai SK, Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Murray BE, Inouye RT. 2004. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J. Infect. Dis. 190:967–970. 10.1086/423139 [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition, M07-A9.. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 28.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin X, Singh KV, Weinstock GM, Murray BE. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J. Bacteriol. 183:3372–3382. 10.1128/JB.183.11.3372-3382.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40:175–179. 10.1016/S0167-7012(00)00122-6 [DOI] [PubMed] [Google Scholar]
- 31.Rose WE, Leonard SN, Rybak MJ. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061–3067. 10.1128/AAC.00102-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. 2012. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 56:3174–3180. 10.1128/AAC.06439-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vouillamoz J, Moreillon P, Giddey M, Entenza JM. 2006. Efficacy of daptomycin in the treatment of experimental endocarditis due to susceptible and multidrug-resistant enterococci. J. Antimicrob. Chemother. 58:1208–1214. 10.1093/jac/dkl406 [DOI] [PubMed] [Google Scholar]
- 34.Allen GP, Cha R, Rybak MJ. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606–2612. 10.1128/AAC.46.8.2606-2612.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Jr, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 53:2605–2609. 10.1128/AAC.01533-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. 2010. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1:475–482. 10.4161/viru.1.6.12985 [DOI] [PubMed] [Google Scholar]
- 37.Bayer AS, Norman DC. 1990. Valve site-specific pathogenetic differences between right-sided and left-sided bacterial endocarditis. Chest 98:200–205. 10.1378/chest.98.1.200 [DOI] [PubMed] [Google Scholar]
- 38.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JT, Elliott TS, Levine DP, Bayer AS. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 39.Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665. 10.1056/NEJMoa053783 [DOI] [PubMed] [Google Scholar]
- 40.Gotz F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367–1378. 10.1046/j.1365-2958.2002.02827.x [DOI] [PubMed] [Google Scholar]
- 41.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown MR, Allison DG, Gilbert P. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777–780. 10.1093/jac/22.6.777 [DOI] [PubMed] [Google Scholar]
- 43.Xu KD, McFeters GA, Stewart PS. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146(Part 3):547–549 [DOI] [PubMed] [Google Scholar]
- 44.Irinoda K, Nomura S, Hashimoto M. 2002. Antimicrobial and clinical effect of linezolid (ZYVOX), new class of synthetic antibacterial drug. Nippon Yakurigaku Zasshi 120:245–252 (In Japanese.) 10.1254/fpj.120.245 [DOI] [PubMed] [Google Scholar]
- 45.French G. 2003. Safety and tolerability of linezolid. J. Antimicrob. Chemother. 51(Suppl 2):ii45–ii53. 10.1093/jac/dkg253 [DOI] [PubMed] [Google Scholar]
- 46.Green SL, Maddox JC, Huttenbach ED. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291. 10.1001/jama.285.10.1291 [DOI] [PubMed] [Google Scholar]
- 47.Jacqueline C, Caillon J, Le Mabecque V, Miegeville AF, Hamel A, Bugnon D, Ge JY, Potel G. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397–3400. 10.1128/AAC.01242-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rouse MS, Steckelberg JM, Patel R. 2007. In vitro activity of ceftobiprole, daptomycin, linezolid, and vancomycin against methicillin-resistant staphylococci associated with endocarditis and bone and joint infection. Diagn. Microbiol. Infect. Dis. 58:363–365. 10.1016/j.diagmicrobio.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 49.Ruiz ME, Guerrero IC, Tuazon CU. 2002. Endocarditis caused by methicillin-resistant Staphylococcus aureus: treatment failure with linezolid. Clin. Infect. Dis. 35:1018–1020. 10.1086/342698 [DOI] [PubMed] [Google Scholar]
- 50.Zimmer SM, Caliendo AM, Thigpen MC, Somani J. 2003. Failure of linezolid treatment for enterococcal endocarditis. Clin. Infect. Dis. 37:e29–e30. 10.1086/375877 [DOI] [PubMed] [Google Scholar]
- 51.Linden PK. 2002. Treatment options for vancomycin-resistant enterococcal infections. Drugs 62:425–441. 10.2165/00003495-200262030-00002 [DOI] [PubMed] [Google Scholar]
- 52.Linden PK, Moellering RC, Jr, Wood CA, Rehm SJ, Flaherty J, Bompart F, Talbot GH. 2001. Treatment of vancomycin-resistant Enterococcus faecium infections with quinupristin/dalfopristin. Clin. Infect. Dis. 33:1816–1823. 10.1086/323899 [DOI] [PubMed] [Google Scholar]
- 53.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10:266–278. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, Segreti J. 2010. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin. Ther. 32:1713–1719. 10.1016/j.clinthera.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 55.Murillo O, Pachon ME, Euba G, Verdaguer R, Tubau F, Cabellos C, Cabo J, Gudiol F, Ariza J. 2008. Antagonistic effect of rifampin on the efficacy of high-dose levofloxacin in staphylococcal experimental foreign-body infection. Antimicrob. Agents Chemother. 52:3681–3686. 10.1128/AAC.00458-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. 2008. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch. Intern. Med. 168:805–819. 10.1001/archinte.168.8.805 [DOI] [PubMed] [Google Scholar]
- 57.Arvanitis M, Glavis-Bloom J, Mylonakis E. 2013. Invertebrate models of fungal infection. Biochim. Biophys. Acta 1832:1378–1383. 10.1016/j.bbadis.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 58.Glavis-Bloom J, Muhammed M, Mylonakis E. 2012. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv. Exp. Med. Biol. 710:11–17. 10.1007/978-1-4419-5638-5_2 [DOI] [PubMed] [Google Scholar]
- 59.Desbois AP, Coote PJ. 2012. Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv. Appl. Microbiol. 78:25–53. 10.1016/B978-0-12-394805-2.00002-6 [DOI] [PubMed] [Google Scholar]
- 60.Chibebe Junior J, Fuchs BB, Sabino CP, Junqueira JC, Jorge AO, Ribeiro MS, Gilmore MS, Rice LB, Tegos GP, Hamblin MR, Mylonakis E. 2013. Photodynamic and antibiotic therapy impair the pathogenesis of Enterococcus faecium in a whole animal insect model. PLoS One 8:e55926. 10.1371/journal.pone.0055926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook SM, McArthur JD. 2013. Developing Galleria mellonella as a model host for human pathogens. Virulence 4:350–353. 10.4161/viru.25240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly J, Kavanagh K. 2011. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J. Med. Microbiol. 60:189–196. 10.1099/jmm.0.025494-0 [DOI] [PubMed] [Google Scholar]
- 63.Whang DW, Miller LG, Partain NM, McKinnell JA. 2013. Systematic review and meta-analysis of linezolid and daptomycin for treatment of vancomycin-resistant enterococcal bloodstream infections. Antimicrob. Agents Chemother. 57:5013–5018. 10.1128/AAC.00714-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla BS, Gauthier TP, Correa R, Smith L, Abbo L. 2013. Treatment considerations in vancomycin-resistant enterococcal bacteremia: daptomycin or linezolid? A review. Int. J. Clin. Pharm. 35:697–703. 10.1007/s11096-013-9825-5 [DOI] [PubMed] [Google Scholar]
- 65.Carugati M, Bayer AS, Miro JM, Park LP, Guimaraes AC, Skoutelis A, Fortes CQ, Durante-Mangoni E, Hannan MM, Nacinovich F, Fernandez-Hidalgo N, Grossi P, Tan RS, Holland T, Fowler VG, Jr, Corey RG, Chu VH. 2013. High-dose daptomycin therapy for left-sided infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Antimicrob. Agents Chemother. 57:6213–6222. 10.1128/AAC.01563-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gould IM, Miro JM, Rybak MJ. 2013. Daptomycin: the role of high-dose and combination therapy for Gram-positive infections. Int. J. Antimicrob. Agents 42:202–210. 10.1016/j.ijantimicag.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 67.Grohs P, Kitzis MD, Gutmann L. 2003. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:418–420. 10.1128/AAC.47.1.418-420.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wareham DW, Abbas H, Karcher AM, Das SS. 2006. Treatment of prosthetic valve infective endocarditis due to multi-resistant Gram-positive bacteria with linezolid. J. Infect. 52:300–304. 10.1016/j.jinf.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 69.Jacqueline C, Asseray N, Batard E, Le Mabecque V, Kergueris MF, Dube L, Bugnon D, Potel G, Caillon J. 2004. In vivo efficacy of linezolid in combination with gentamicin for the treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 24:393–396. 10.1016/j.ijantimicag.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 70.Levine DP. 2008. Clinical experience with daptomycin: bacteraemia and endocarditis. J. Antimicrob. Chemother. 62(Suppl 3):iii35–iii39. 10.1093/jac/dkn369 [DOI] [PubMed] [Google Scholar]