Abstract
Burn wound infections are often difficult to treat due to the presence of multidrug-resistant bacterial strains and biofilms. Currently, mupirocin is used to eradicate methicillin-resistant Staphylococcus aureus (MRSA) from colonized persons; however, mupirocin resistance is also emerging. Since we consider antimicrobial peptides to be promising candidates for the development of novel anti-infective agents, we studied the antibacterial activities of a set of synthetic peptides against different strains of S. aureus, including mupirocin-resistant MRSA strains. The peptides were derived from P60.4Ac, a peptide based on the human cathelicidin LL-37. The results showed that peptide 10 (P10) was the only peptide more efficient than P60.4Ac, which is better than LL-37, in killing MRSA strain LUH14616. All three peptides displayed good antibiofilm activities. However, both P10 and P60.4Ac were more efficient than LL-37 in eliminating biofilm-associated bacteria. No toxic effects of these three peptides on human epidermal models were detected, as observed morphologically and by staining for mitochondrial activity. In addition, P60.4Ac and P10, but not LL-37, eradicated MRSA LUH14616 and the mupirocin-resistant MRSA strain LUH15051 from thermally wounded human skin equivalents (HSE). Interestingly, P60.4Ac and P10, but not mupirocin, eradicated LUH15051 from the HSEs. None of the peptides affected the excretion of interleukin 8 (IL-8) by thermally wounded HSEs upon MRSA exposure. In conclusion, the synthetic peptides P60.4Ac and P10 appear to be attractive candidates for the development of novel local therapies to treat patients with burn wounds infected with multidrug-resistant bacteria.
INTRODUCTION
Each year, about 11 million people worldwide seek medical treatment for severe burns, of whom 300,000 will die (1). In the past few decades, great progress has been made in the care and treatment of burn wound victims, but infections with, e.g., Staphylococcus aureus or Pseudomonas aeruginosa still cause serious morbidity and mortality (2–4). Uncontrolled wound infections may result in delayed wound healing and/or cause severe systemic infection in burn patients (5).
The emergence of antimicrobial resistance hampers both current strategies to prevent bacterial colonization of the wound bed and the treatment of infections that arise from colonization. For example, topical antibiotics, like mupirocin (6) and Neosporin (7), are ineffective when resistant bacteria colonize the wound (8, 9). Furthermore, these antimicrobials are not effective because bacteria in biofilms can be up to 1,000-fold less sensitive to antibiotics than their planktonic counterparts (10–12). Other topical disinfectants, such as chlorhexidine (13), silver sulfadiazine (14), and iodine preparations (15), can be very painful when applied to open wounds (16), and the scientific evidence for the efficacy of these agents in wounds is scarce. Clearly, there is an urgent need for novel antimicrobial agents that can be applied topically to (i) prevent colonization and (ii) eliminate infectious agents in burn wounds (17).
We consider antimicrobial peptides (AMPs) to be potential therapeutic compounds. AMPs are essential components of the human innate immune system and as such contribute to the first line of defense against infections (18, 19). The human cathelicidin hCAP-18 releases the active cationic peptide LL-37, which displays bactericidal and antibiofilm properties against Gram-positive S. aureus (20–22) and P. aeruginosa (23) and lipopolysaccharide (LPS) neutralization (24) and aids in wound healing (15, 25). The synthetic LL-37-derived peptide 60.4Ac (P60.4Ac) retains the α-helical structure of the parent peptide (26) and displays enhanced antimicrobial properties against Gram-negative bacteria and fungi compared to those of LL-37. Moreover, P60.4Ac retains the LPS-neutralizing activities of LL-37 (26) and has proven to be beneficial in patients with otitis media (27, 28). LL-37 has been successful in enhancing wound healing in patients with chronic venous leg ulcers (in a clinical phase I/II study conducted by Pergamum) and in diabetic patients suffering from infected wounds (29).
We recently developed a thermally wounded skin infection model and demonstrated that methicillin-resistant S. aureus (MRSA)-infected human skin equivalents (HSEs) can be successfully treated using mupirocin (30). HSEs are air-exposed three-dimensional human skin models that mimic the native skin to a high degree. They can be used to study many properties of the human skin, including barrier properties (16) and wound healing (31). Furthermore, HSEs have been used to study skin colonization with MRSA (32, 33). In the present study, we exploited the thermally wounded HSE infection model to investigate the antimicrobial activities of a new set of synthetic peptides based on the sequence of P60.4Ac. The aim of this study was to compare the antibacterial activities of these novel synthetic AMPs against MRSA to those of the parent peptides P60.4Ac and LL-37, as well as to mupirocin.
MATERIALS AND METHODS
Antimicrobial agents.
All peptides (N-terminal acetylated and C-terminal amidated) were synthesized by solid-phase strategies on an automated multiple peptide synthesizer (Syro II; MultiSyn Tech, Witten, Germany), as described previously (26). A set of 14 peptides was designed by substituting one or more amino acids in the sequence of P60.4Ac in such a way that the α-helix was predicted to be retained. The sequences of the peptides in this study are shown in Table 1. The molecular masses of the peptides were confirmed by mass spectrometry. The purity of the peptides was >90%, as determined by reverse-phase high-performance liquid chromatography; peptides LL-37, P60.4Ac, and peptide 10 (P10) in the experiments were >95% pure. The lyophilized peptides were stored at −20°C until use. For the experiments, the peptides were dissolved in H2O with 0.01% (vol/vol) acetic acid to a stock of 10 mM, and aliquots were made and stored at −20°C. Prior to the experiments, the peptide stocks were further diluted in phosphate-buffered saline (PBS) (pH 7.4). Mupirocin was obtained from Sigma-Aldrich (Zwijndrecht, The Netherlands) and dissolved in PBS.
TABLE 1.
Peptide sequences and in vitro killing of MRSA LUH14616 by a set of synthetic peptides in PBS
| Peptide | Sequence | IC99 (median [range]) (μM)a |
|---|---|---|
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | >16 |
| P60.4Ac | IGKEFKRIVERIKRFLRELVRPLR | 1.3 (1.1–1.4) |
| P1 | IAKEFKRIVERIKRFLRELVRPLR | 1.4 (1.2–1.5) |
| P2 | LARDYKRLVERLKRWLRELVRPLK | 1.7 (1.5–1.8) |
| P3 | IAKEFKRILERIKRFIREITRPIR | 2.1 (2.0–4.0) |
| P4 | TAKEYKRILDRIKRYLRELVRAIK | 1.8 (1.8–2.2) |
| P5 | VAKDYRKVVDRIKRFLRYLLRPVR | 1.2 (1.1–1.4) |
| P6 | LAKDYKKIVERLRKWLREVLRPVK | 1.9 (1.5–2.3) |
| P7 | LAKEYRKIFDRLKKWLRQIVRPSK | 1.5 (1.0–1.6) |
| P8 | LAKEWRKIVDRLKRWLRDILKATK | 1.2 (1.2–1.5) |
| P9 | VAREWRKIVDRIKRYLRDISKATK | 4.3 (3.7–4.8) |
| P10 | LAREYKKIVEKLKRWLRQVLRTLR | 1.1 (0.8–1.3)b |
| P11 | TAREWKRILEKIRKYLRDVSRVTR | 5.4 (5.3–5.5) |
| P12 | TAREWKRILEKIKKYLRDVSRVTR | 5.1 (2.7–7.5) |
| P13 | VAKDWKRIVDKVRRYLREVTKILK | 3.2 (2.9–3.4) |
| P14 | TAKDYRKIFEKIKKYLKDLTRILK | 1.9 (1.6–3.2) |
Log-phase bacteria were exposed to increasing concentrations of the various peptides (range, 0 to 16 μM) for 1 h in PBS, after which the number of viable bacteria was determined microbiologically. The results are expressed as the 99% inhibitory concentration (IC99), which is the lowest concentration of the peptide that results in a 99% reduction in the number of viable bacteria. IC99 values were calculated using linear regression analysis. The values are the median and range from ≥2 experiments, with the exception of P10 and P60.4Ac, which are from ≥9 experiments.
Indicates value significantly different compared to that for P60.4Ac.
Bacteria.
The following S. aureus strains were used in this study: LUH14616 (sequence type 247), LUH15051 (sequence type 239 [ST239]), Saco042, a USA300 strain (ST8), Imp126 (ST121), and NTCT 8325-4 (ST8). LUH14616, a clinical MRSA isolate, was kindly provided by S. Croes (34), and LUH15051, a mupirocin-resistant clinical isolate, was kindly provided by M. Heck (Laboratory of Infectious Diseases and Screening, National Institute for Public Health and Environment, Bilthoven, The Netherlands). The other strains were a kind gift from W. J. B. van Wamel (Department of Medical Microbiology and Infection, Erasmus Medical Center, Rotterdam, The Netherlands). All strains were typed using multilocus sequence typing (35). The bacteria were stored in glycerol at −80°C until use. Prior to the experiments, the bacteria were subcultured on sheep blood agar plates (bioMérieux, Marcy l'Etoile, France).
In vitro killing assay.
The bacteria were cultured to mid-log phase in tryptic soy broth (TSB) (Oxoid Limited, Basingstoke, United Kingdom) for 3 h at 37°C with continuous rotation (200 rpm). Next, the bacteria were centrifuged at 1,000 × g for 10 min and, after removal of the broth, were resuspended in PBS to a concentration of 2 × 106 CFU/ml, as calculated from the optical density at 600 nm. Subsequently, 50 μl of the bacterial suspension was mixed with 50 μl of PBS containing increasing peptide concentrations, from 0.13 μM to 16 μM, and 1% TSB. Next, the bacteria-peptide suspensions were incubated for 1 h, 2 h, or 24 h at 37°C. To determine the number of viable bacteria, 10-fold serial dilutions were made and plated onto diagnostic sensitivity test (DST) plates (Oxoid, Ltd.). Next, the 99% inhibitory concentration (IC99) values were calculated using linear regression analysis. For the killing of biofilm-associated LUH14616, the bacteria were mechanically removed from 24-h-matured biofilms by scraping with pipette tips and vigorous vortexing. Subsequently, the same procedure was followed as that described above (concentrations, 0.5 to 32 μM) in PBS for 1 h or 4 h.
Biofilm assay.
Log-phase bacteria were diluted to 108 CFU/ml in biofilm-adjusted medium containing 62 mM potassium phosphate buffer (pH 7), 7 mM (NH4)2SO4, 2 mM MgSO4, 10 μM FeSO4, 0.4% (wt/vol) glucose, and 0.5% (wt/vol) Casamino Acids, further referred to as BM2 (36). The bacteria were cultured for 24 h in a flat-bottom polypropylene microtiter plate (MaxiSorp; Greiner, Nürtingen, Germany) at 37°C, with or without antimicrobial peptides (concentrations, 0.5 to 32 μM). Thereafter, the biofilms were washed and stained using 1% (wt/vol) crystal violet (Sigma-Aldrich). Crystal violet was eluted in 96% ethanol, and the optical density (OD) values at 595 nm were measured to quantitate the biofilm mass. The results are expressed as the 50% effective concentration (EC50).
Cell cultures.
Cell cultures of normal human keratinocytes (NHK) and fibroblasts (NHF) were established from fresh human mamma or abdominal surplus skin, as described earlier (37, 38). In short, fat tissue was removed, and the skin was incubated with 2.4 U/ml dispase II (Roche, Woerden, The Netherlands), after which the dermis and epidermis were mechanically separated. NHK were isolated from the epidermis after treatment with 0.05% (wt/vol) trypsin (BD Falcon; Breda, The Netherlands) and cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Breda, The Netherlands) diluted 3:1 in Ham's F-12 medium (Gibco, Bleiswijk, The Netherlands) supplemented with 5% (vol/vol) fetal calf serum (FCS) (HyClone Greiner), 100 U/ml penicillin-100 μg/ml streptomycin (Invitrogen), 1 μM hydrocortisone, 1 μM isoproterenol, and 0.1 μM insulin (all Sigma-Aldrich), further referred to as keratinocyte medium. NHF were isolated after incubation in a 3:1 mixture of collagenase (Gibco) and dispase (Roche) for 2 h at 37°C and cultured in DMEM supplemented with 5% (vol/vol) FCS and penicillin-streptomycin, here called fibroblast medium.
Generation of human skin equivalents.
Human skin equivalents (HSEs) were generated as described earlier (31). In short, 3 ml of rat tail collagen (4 mg/ml) was mixed with 1.25 × 105 NHF. Next, the cells were pipetted onto a filter insert (6 wells, 0.4-μm filter; Corning, Amsterdam, The Netherlands) to allow for polymerization of the collagen. The fibroblasts in collagen were cultured under submerged conditions in fibroblast medium for 2 days, after which 5 × 105 NHK/filter were seeded on top of the collagen layer. The cells were cultured under submerged conditions using keratinocyte medium supplemented with 1% (vol/vol) FCS, 1 μM selenous acid, 10 μM l-carnitine, 1 mM l-serine, and a lipid mixture containing 25 μM palmitic acid, 15 μM linoleic acid, 7 μM arachidonic acid, and 24 μM bovine serum albumin. After 2 days, the HSEs were lifted to the air-liquid interface and cultured for an additional 10 days in serum-free keratinocyte medium containing 30 μM linoleic acid and supplements as described above. All medium supplements were purchased from Sigma-Aldrich. A representation of the HSEs is presented in Fig. 1A and B.
FIG 1.
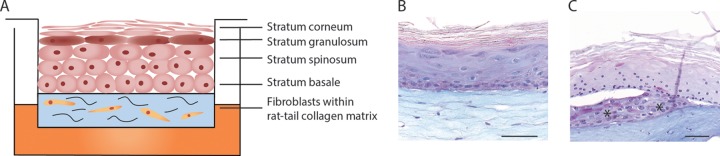
Schematic overview of HSE and thermal wounding of HSEs. (A) Schematic representation of HSEs showing the different layers of the epidermis and fibroblast-populated collagen layer on top of the filter insert. PAS-alcian blue staining of intact HSE (B) and HSE 24 h after wounding with liquid nitrogen (C). The asterisks indicate keratinocytes migrating over fibroblast-populated collagen layer, underneath the dead keratinocyte layer. Scale bars, 50 μm.
Thermal wounding.
HSEs were thermally wounded, as described earlier (30, 31). In brief, wounds were created on 10-day air-exposed models using a 2 by 10 mm blunt metal bar, which was placed in liquid nitrogen for 2 min. Immediately thereafter, the metal bar was applied onto the HSEs for 15 s without any pressure. Fig. 1C demonstrates a periodic acid-Schiff (PAS)-alcian blue staining of the wounded HSEs.
Wound infection and treatment.
One hundred microliters of log-phase bacteria at a concentration of 1 × 106 CFU/ml in PBS was added to the thermally wounded HSEs. After 1 h, the nonadherent and loosely adherent MRSA cells were removed by aspiration, and the adherent bacteria were exposed to 100 μl peptide/antibiotic (1 mg/ml in PBS). At 4 and 24 h thereafter, the numbers of viable detachable and adherent bacteria on the models were determined microbiologically using DST plates. The number of detachable bacteria was determined by washing one HSE, by pipetting 1 ml PBS on top of the model, and pipetting this up and down 3 times. Next, serial dilutions of the peptides were made as described above and plated onto DST plates. To assess the number of viable adherent bacteria, two punch biopsy specimens (4 mm) were taken from each washed HSE, homogenized in 1 ml of PBS, and serially diluted for CFU counting. The number of adherent bacteria per HSE (113 mm2) was calculated by multiplying the number of adherent bacteria in two biopsy specimens, 25.2 mm2 (12.6 mm2 per biopsy specimen) by 4.5.
MTT assay.
Epidermal skin equivalents, prepared as previously described (39), were exposed for 24 h to different peptide concentrations (50, 100, or 200 μg of peptide/skin equivalent) at 37°C and 7.3% CO2. Next, the models were washed two times with PBS and then transferred to a fresh 12-well plate containing 600 μl of keratinocyte medium (prepared with transparent DMEM) containing 1 mg/ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma-Aldrich). After 3 h incubation at 37°C and 7% CO2, the MTT solution was removed and the models were washed twice with PBS. After air-drying for ≥2 h, 1 ml of isopropanol was pipetted on top of each insert and 1 ml under each insert. Next, the 12-well plates were sealed with parafilm, stored overnight at room temperature, and then shaken for ≥30 min on a plate shaker to extract all color. Thereafter, the extracts were transferred to a 96-well plate to measure the OD at 570 nm using a Tecan reader (Infinite F200; Männedorf, Germany). As a control, 100 μl of 100 mM SDS was applied to the models.
Enzyme-linked immunosorbent assay.
The protein level of IL-8 was measured (enzyme-linked immunosorbent assay [ELISA]) in the culture medium that was collected from HSEs (Invitrogen, Bleiswijk, The Netherlands). The ELISA procedures were carried out according to supplier's instructions. The lower limit of detection was 15 pg/ml.
Hematoxylin and eosin staining.
Histochemical staining was performed on paraffin-embedded HSE sections. The slides (5 μm) were cut, deparaffinized, rehydrated, and washed with PBS. Subsequently sections were stained with hematoxylin and eosin (Klinipath, Duiven, The Netherlands) and embedded in Depex (Serva Biophoretics, Truckee, CA, USA). Their morphologies were assessed using a light microscope.
Statistical analysis.
The data were analyzed by unpaired t test or for the experiments using HSEs with the Wilcoxon signed-rank test. Differences between P values of ≤0.05 were considered significant. The IC99 values were calculated using linear regression analysis on the killing curves.
RESULTS
Effects of LL-37, P60.4Ac, and peptides derived thereof on log-phase MRSA LUH14616.
To assess whether substituting one or more amino acids in the sequence of P60.4Ac improves or decreases the antibacterial activity of this peptide, we compared the bactericidal activities of a set of 14 peptides derived from P60.4Ac toward MRSA LUH14616 in an in vitro killing assay. The peptides were incubated for 2 h with bacteria in PBS. The results revealed that P60.4Ac was more effective than LL-37 in killing LUH14616 in PBS (Table 1). Interestingly, only P10 killed >99% of the bacteria at a significantly (P = 0.02) lower concentration than did P60.4Ac, with a median (interquartile range) concentration of 1.1 (0.8 to 1.3) μM compared to 1.3 (1.1 to 1.4) μM, respectively. Based on these data, we selected P10 for further studies.
Effects of LL-37, P60.4Ac, and P10 on different S. aureus strains.
Next, we investigated the antibacterial activities of LL-37, P60.4Ac, and P10 toward four different S. aureus strains. For comparison, we included mupirocin, an antibiotic commonly used for topical eradication of (methicillin-resistant) S. aureus (40) in these experiments. We used both short (1-h) and long (24-h) incubation periods, and since S. aureus does not survive for 24 h in PBS, we supplemented the PBS with 1% TSB for these experiments. Already after 1 h, the synthetic peptides had killed >99% of the viable bacteria at concentrations ranging from 1.7 μM to 2.7 μM for P60.4Ac and 2.0 μM to 3.3 μM for P10 (Table 2). After 24 h, the IC99 values for the different strains ranged from 1.2 μM to 5.0 μM for P60.4Ac and 1.2 μM to 3.3 μM for P10 (Table 2). Interestingly, P60.4Ac and P10 were as effective as mupirocin against the different strains; moreover, P60.4Ac and P10 were also effective against the mupirocin-resistant strain LUH15051, with IC99s of 2.7 μM and 3.3 μM, respectively, after 24 h of incubation.
TABLE 2.
IC99 concentrations of LL-37, P60.4Ac, P10, and mupirocin on methicillin-resistant S. aureus strains
| S. aureus strain | IC99 (μM) for peptides ata: |
MIC (μg/ml)b | ||||||
|---|---|---|---|---|---|---|---|---|
| 1h |
24 h |
|||||||
| LL-37 | P60.4Ac | P10 | LL-37 | P60.4Ac | P10 | mup | ||
| LUH14616 | >16 | 2.0 (1.5–2.3) | 2.0 (1.6–2.2) | >16 | 1.2 (1.1–1.3) | 1.2 (0.3–1.4) | 0.5 (0.4–0.6) | 0.4 |
| LUH15051 | >16 | 2.7 (1.7–4.5) | 2.9 (2.8–3.2) | >16 | 2.7 (1.7–4.5) | 3.3 (0.8–5.3) | >64 | >1,024 |
| Saco042 | >16 | 2.4 (1.7–5.8) | 3.3 (1.9–3.4) | >16 | 3.6 (2.6–5.4) | 3.1 (2.3–6.2) | 2.5 (0.4–7.7) | 0.2 |
| Imp-126 | >16 | 2.2 (1.7–3.0) | 2.0 (1.6–4.6) | >16 | 2.6 (2.0–6.5) | 2.3 (1.1–2.7) | 0.5 (0.4–0.5) | 0.1 |
| 8325-4 | >16 | 1.7 (0.7–2.2) | 2.5 (0.8–3.3) | 9.9 (7.7–10.1) | 1.8 (1.7–2.0) | 1.6 (1.1–2.2) | 4.3 (3.6–4.8) | 0.1 |
Bacteria were incubated with peptides or mupirocin for 1 h or 24 h in PBS supplemented with 1% TSB. The results are expressed as the median (range) IC99 from 3 to 5 experiments. mup, mupirocin.
MICs were assessed by Etest (bioMérieux).
Effects of LL-37, P60.4Ac, and P10 on biofilm formation.
As biofilms can play a major role in infections, we further investigated the antibiofilm activities of these peptides. For this purpose, we first evaluated the abilities of LL-37, P60.4Ac, and P10 to inhibit the formation of biofilms on plastic. The results revealed that LUH14616 formed a large biofilm on plastic, while the other strains developed small/moderate biofilms (Fig. 2A). Therefore, we selected LUH14616 to test the effects of the peptides on biofilm formation. The results revealed a dose-dependent inhibition of biofilm formation by all three peptides; the maximum biofilm inhibition rates were 83% for LL-37, 85% for P10, and 86% P60.4Ac (Fig. 2B). P10 proved to be the most potent peptide for inhibiting biofilm formation, with a 50% reduction in biofilm formation (EC50) at a median (interquartile range) of 2.0 μM (1.7 to 2.0 μM). This concentration was statistically significant (P = 0.002) compared to that of P60.4Ac, which had an EC50 of 2.8 μM (2.6 to 3.0 μM), and LL-37, which had an EC50 of 3.4 μM (2.6 to 3.7 μM). In addition, none of the peptides was bactericidal in the biofilm medium at the concentrations evaluated (data not shown), indicating that the inhibition of biofilm formation was not the result of killing the bacteria by the peptides.
FIG 2.
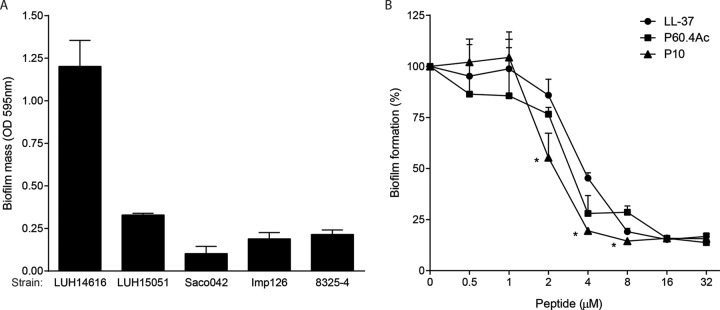
Biofilm formation by different S. aureus strains and inhibition of biofilm formation with LL-37, P60.4Ac, and P10. (A) Biofilm formation by 5 S. aureus strains in BM2 medium for 24 h at 37°C was assessed using crystal violet staining. (B) Effect of peptides on biofilm formation by LUH14616 for 24 h. The results are shown as the percent biofilm formation compared to biofilm formation in the absence of peptides. The results are the medians and interquartile ranges of six independent experiments (P < 0.05). *, biofilm formation significantly different between P10 and the other peptides.
Effects of LL-37, P60.4Ac, and P10 on established biofilm and biofilm-associated bacteria.
Next, we tested the capabilities of the peptides to degrade established biofilms. For this purpose, we added peptides in PBS to 24-h-matured biofilms of MRSA LUH14616. After 4 h of exposure to the peptide, the number of viable bacteria was assessed. LL-37, P60.4Ac, and P10 degraded biofilms in a concentration-dependent manner. Concentrations of >12.5 μM resulted in an approximately 25% reduction in the number of viable bacteria (Fig. 3A). As bacteria within biofilms are less sensitive to antibiotics than are log-phase bacteria, we determined the effects of the different peptides on biofilm-associated bacteria obtained by mechanical disruption of the biofilm. These biofilm-associated bacteria were treated with the peptides in PBS for 1 or 4 h, after which the number of viable bacteria was determined. Up to 32 μM, LL-37 did not reduce the number of viable biofilm-associated bacteria. Treatment for 1 h with P60.4Ac and P10, however, resulted in a >90% reduction in the number of viable bacteria at concentrations of ≥3.2 μM, while 4-h treatment resulted in a >2-log reduction in the number of viable bacteria with concentrations of ≥1.6 μM; moreover, P10 was more effective than P60.4Ac (Fig. 2B and C).
FIG 3.
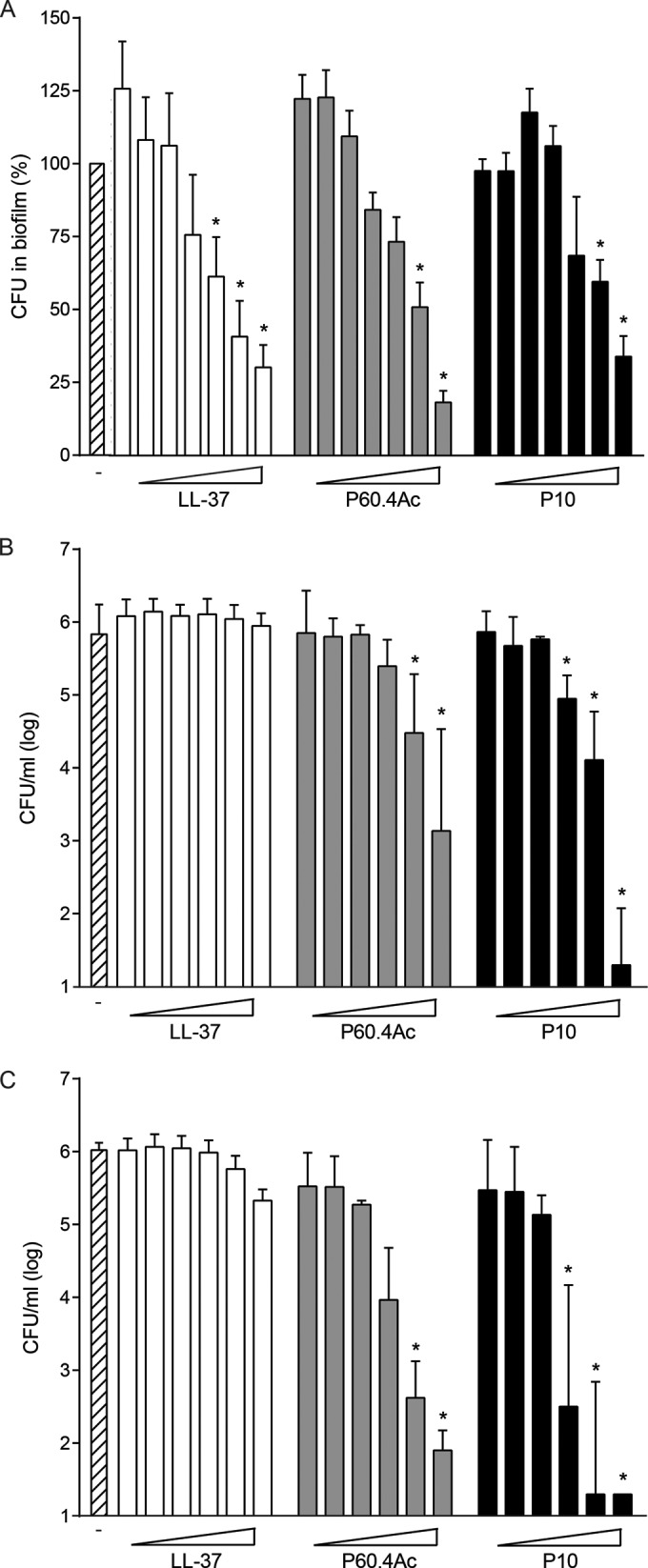
Effects of LL-37, P60.4Ac, and P10 on biofilms and biofilm-associated bacteria. (A) Breakdown of existing biofilms. Biofilms of LUH14616 were grown for 24 h and subsequently incubated for 4 h with peptides (concentrations, 1.5, 3, 6, 12, 25, 50, and 100 μM). Next, bacteria were scraped from the wells, and the number of viable bacteria was assessed microbiologically. The data are expressed as the percentage of surviving bacteria compared to the control biofilms. (B and C) Effects of peptides on biofilm-associated bacteria from 24-h-matured biofilms. The bacteria were scraped from the wells, diluted to 1 × 106 CFU/ml, and incubated for 1 h (B) and 4 h (C) with peptides (0.2, 0.4, 0.8, 1.6, 3.2, and 32 μM). White bars, LL-37; gray bars, P60.4Ac; black bars, P10; striped bar, control (no peptide present). The results are displayed as medians and interquartile ranges of 4 to 5 independent experiments. * indicates CFU/ml significantly less than that in the absence of peptide.
Antimicrobial peptides do not affect epidermal cell viability.
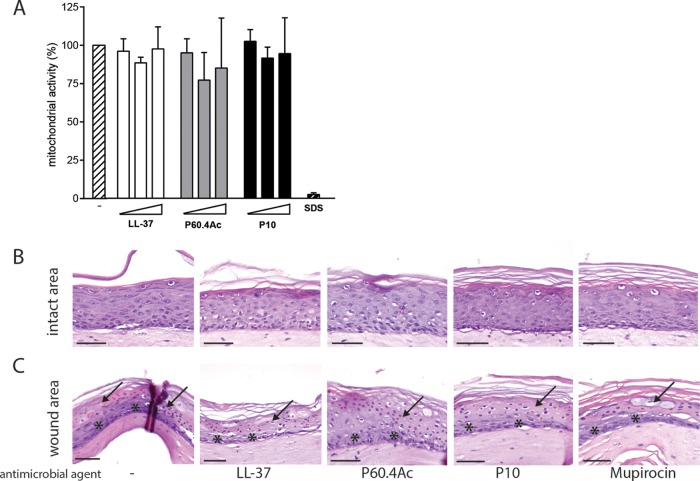
To exclude the possible toxic effects of the peptides on HSEs, we determined the cell viability in the HSEs after exposure to increasing concentrations of the peptides by the MTT assay. Since the collagen layer of the HSE retains compounds, like MTT, we used an epidermal skin model to assess the effect of the AMPs on mitochondrial activity. The application of LL-37, P60.4Ac, and P10 at concentrations up to 200 μg/model onto the stratum corneum for 24 h did not affect mitochondrial activity (Fig. 4A). Mupirocin also did not affect the epidermal viability (data not shown).
FIG 4.
Effects of LL-37, P60.4Ac, and P10 on mitochondrial activity and morphology. (A) MTT assay in epidermal skin models. Peptides LL-37, P60.4Ac, and P10 (50, 100, or 200 μg/model) or 100 mM SDS was applied for 24 h onto the epidermal models. The results are shown as the percent cell viability compared to the untreated models and are presented as the medians and interquartile ranges of 4 independent experiments. White bars, LL-37; gray bars, P60.4Ac; black bars, P10; striped bar, control (no peptide present). (B to C) Hematoxylin and eosin staining of thermally wounded and LUH14616-infected HSEs exposed for 24 h to 100 μg/HSE of the various peptides and mupirocin, showing the intact area (B) and the wound area (C). The arrows mark the dead keratinocyte layer after thermal wounding, and the asterisks indicate keratinocytes migrating over the wound bed. Scale bars, 50 μm.
Next, we assessed the effects of the peptides on a thermally wounded HSE infection model (30). HSEs were thermally wounded using liquid nitrogen, as shown in Fig. 1, and subsequently infected with 1 × 105 CFU/HSE. After topical application of 100 μg peptide in 100 μl PBS per thermally wounded and infected HSE, we observed no change in cell morphology, both in the wounded and unwounded parts of the model (Fig. 4B). Moreover, we observed similar wound healing between the treated and untreated models, as observed by keratinocytes migrating over the wound bed (Fig. 4C, asterisks), underneath the dead keratinocyte layer (Fig. 4D, arrows).
Effects of LL-37, P60.4Ac, and P10 on bacteria on thermally wounded HSEs.
Based on these results, we concluded that the peptides are suitable to use for topical application. Next, we determined the effects of LL-37, P60.4Ac, and P10 on thermally wounded HSEs infected with MRSA LUH14616. The infected HSEs were exposed to a single dose of 100 μg in 100 μl per HSE of LL-37, P60.4Ac, P10, or 100 μM (100 μl) mupirocin in PBS for 24 h. The preliminary experiments showed that 25 and 50 μg of the peptides per HSE were not effective (data not shown). Applications of P60.4Ac, P10, and mupirocin, but not LL-37, were highly effective in reducing the number of viable LUH14616 bacteria on the wounded HSEs. While P60.4Ac reduced the CFU from 1.1 × 107 on the untreated models to a median of 7.0 × 104 CFU/model (87% reduction), P10 reduced the number of viable bacteria to 2.3 × 103 CFU/model (>99.9% reduction); this difference was not significant.
To test whether the peptides were also effective against mupirocin-resistant MRSA bacteria, we performed the same experiments using mupirocin-resistant MRSA LUH15051. P60.4Ac and P10, but not LL-37 or mupirocin, were effective in eliminating LUH15051 for thermally wounded and infected HSEs, giving 96% and 99% reductions, respectively, in the number of viable bacteria (Fig. 5B). To determine the effects of the peptides on the inflammatory response induced by MRSA in the HSE, we measured the levels of excreted IL-8 in the supernatant. Exposure of the skin to MRSA LUH14616 resulted in increased IL-8 production of 15.6 ng/HSE, compared to 8.1 ng/HSE in the uninfected HSEs. Interestingly, we observed no effect of the various peptides on IL-8 secretion in HSEs (Fig. 5C), indicating that these peptides do not affect the inflammatory response in these models. Similar results were seen for mupirocin (data not shown).
FIG 5.
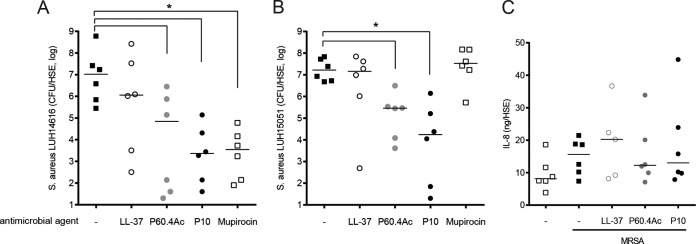
Effects of LL-37, P60.4Ac, and P10 on MRSA and mupirocin-resistant MRSA-infected thermally wounded HSEs. Thermally wounded HSEs were inoculated with LUH14616 (A) or LUH15051 (B) and after 1 h were subsequently exposed to 100 μg of the various peptides or mupirocin per model. After 24 h, the number of viable bacteria was determined microbiologically. (C) IL-8 in the culture supernatants of the HSEs wounded and infected with LUH14616 after 24 h of exposure to the peptides. ○, LL-37; gray circles, P60.4Ac; ●, P10. Horizontal lines represent the median CFU counts. *, CFU/HSE significantly less than in colonized thermally wounded HSEs without antimicrobial agent (◼).
DISCUSSION
The aim of the present study was to develop new synthetic AMPs that may be used to reduce the bacterial burden of wounds, thus decreasing the risks of systemic infections and improving wound healing in, for example, burn patients (5). For this purpose, we designed a set of 14 synthetic peptides with a predicted intact α-helical structure and net positive charge based on the amino acid sequence of the LL-37-derived peptide P60.4Ac (26). Our main finding is that only one of these peptides, i.e., P10, was more efficient than P60.4Ac in killing MRSA LUH14616. In agreement with this finding, we observed that P60.4Ac and P10, but not LL-37, were highly effective against five (methicillin-resistant) S. aureus strains. Interestingly, P60.4Ac and P10 retained the antibiofilm activities of LL-37 (22). Moreover, P60.4Ac and P10 were more effective than LL-37 in eradicating MRSA and mupirocin-resistant MRSA from infected wounded HSEs. Importantly, all three antibacterial peptides did not affect the viability of the keratinocytes in these models or trigger an inflammatory reaction. Based on these data, we conclude that P60.4Ac and P10 are interesting candidates for topical application to treat infected wounds.
LL-37 has many functions in the human immune defense systems, such as its roles in inflammation (41), wound healing (15, 25), LPS neutralization (24), and its antimicrobial activity against a variety of microorganisms (42, 43). Because of its immune-regulating and wound-healing properties, we aimed to develop synthetic LL-37-derived peptides that retain these beneficial properties while displaying enhanced bactericidal activities compared to that of LL-37. In line with this, it has been reported that the LL-37-derived peptide P60.4Ac was more effective against Gram-negative bacteria and fungi than LL-37 while retaining its LPS-neutralizing capabilities (26). In this study, we demonstrate that P60.4Ac and its derivative P10 are highly bactericidal toward (methicillin-resistant) S. aureus, an important pathogen in burn wound infections, whereas LL-37 is not. In addition, the two synthetic peptides were more effective than LL-37 against biofilm-associated bacteria derived from mechanically disrupted biofilms, reaching IC99 values after 4 h of incubation similar to the concentrations needed to kill log-phase bacteria. However, P60.4Ac and P10 were as effective as LL-37 in eradicating MRSA organisms residing within a 24-h biofilm. Of note, the effective concentrations of the various peptides when directly applied onto biofilms were much higher than those needed to kill planktonic bacteria, as has been extensively described for conventional antibiotics (11).
It has been established that LL-37 can prevent biofilm formation and attachment of bacteria to surfaces (20, 22). Other synthetic cationic peptides, named antimicrobial peptidomimetics, have been shown to degrade existing S. aureus biofilms (44). Here, we demonstrate that the synthetic peptides P10 and P60.4Ac can prevent biofilm formation as well as LL-37 can. All these results together suggest that synthetic peptides can degrade biofilms and subsequently eliminate bacteria in biofilms.
One of the aims of our study was to develop synthetic AMPs that can be used to reduce the bacterial burden of wounds. To investigate this, we used an in vitro infection model in which we inoculated thermally wounded HSEs with MRSA (45). Topical application of P60.4Ac and P10 significantly reduced the numbers of viable MRSA and mupirocin-resistant MRSA organisms from these surfaces, indicating that these peptides are suitable for topical application. However, it should be realized that these HSEs lack various characteristics of human skin, such as sweat glands, hair follicles, immune cells, and blood circulation. Because of these limitations, the results obtained in the described in vitro wound infection model may not fully reflect the effects of the peptides on (wounded) human skin. Nevertheless, we hypothesize that the results obtained using this model are more relevant for human wound infection treatment than those from testing the peptides in buffers or body fluids. However, we are aware that body fluids, such as plasma, may affect the effectiveness of the antimicrobial activities of the peptides, for example by binding to plasma components, such as albumin (46).
It has long been established that LL-37 can enhance wound healing (reviewed by Steinstraesser et al. [47]), and recently, a phase II study demonstrating enhanced wound healing in patients with chronic wounds was successfully completed (see above). In agreement with these findings, others have demonstrated that a bovine cathelicidin-derived peptide, IDR1018, can aid in wound healing in S. aureus-infected murine wounds (48). In addition, another LL-37-derived synthetic peptide, named IG-19, decreased disease severity and significantly reduced the serum levels of antibodies against collagen type II in a collagen-induced arthritis model (49). Besides allowing for an assessment of the antibacterial activities of peptides on infected tissues, the advantage of using an in vitro infection model that mimics the human skin is that the possible effects of the peptides on inflammation (as exemplified by IL-8 release), cell viability, and wound healing can be monitored in the same model. This is of particular importance when studying the effects of agents that affect various processes that are interlinked, such as the inflammatory reaction and wound healing. In line with this, we observed no effects of P60.4Ac and P10 on IL-8 production by wounded HSEs in response to MRSA. Furthermore, no effects of these peptides on mitochondrial function or epidermal morphology in these models have been observed. It should also be noted that P60.4Ac has already been successfully applied to patients suffering from otitis media, and no negative side effects of this peptide were reported (27). Moreover, a dose up to 1.5 mg/kg of body weight/day of P10 has been administered intravenously to rats and miniature pigs without a negative impact on the animals (P. H. Nibbering, unpublished data).
Together, our results demonstrate the potential of the synthetic antibacterial peptides P60.4Ac and P10 as novel therapeutic agents for the treatment of wound infections. Moreover, these peptides may have additional beneficial effects, such as anti-inflammatory activities and enhancement of wound healing. This makes these synthetic antimicrobial peptides promising candidates, either alone or in combination with other antimicrobials (50), for the treatment of wound infections caused by (multidrug-resistant) bacteria.
ACKNOWLEDGMENTS
This study was financially supported by the Dutch Burns Foundation (new strategies for the prevention and treatment of burn wound infections; project 10.106) and ZonMW (topical treatment with a cathelicidin-based antimicrobial peptide as a novel approach to eradicate methicillin-resistant Staphylococcus aureus carriage; project 40.41200-98-90).
We declare no conflicts of interest.
Footnotes
Published ahead of print 19 May 2014
REFERENCES
- 1.Peck MD. 2011. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 37:1087–1100. 10.1016/j.burns.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. 2008. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 16:23–29. 10.1111/j.1524-475X.2007.00303.x [DOI] [PubMed] [Google Scholar]
- 3.Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. 2004. Changes of microbial flora and wound colonization in burned patients. Burns 30:357–361. 10.1016/j.burns.2003.12.013 [DOI] [PubMed] [Google Scholar]
- 4.Kooistra-Smid M, Nieuwenhuis M, van Belkum A, Verbrugh H. 2009. The role of nasal carriage in Staphylococcus aureus burn wound colonization. FEMS Immunol. Med. Microbiol. 57:1–13. 10.1111/j.1574-695X.2009.00565.x [DOI] [PubMed] [Google Scholar]
- 5.Church D, Elsayed S, Reid O, Winston B, Lindsay R. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403–434. 10.1128/CMR.19.2.403-434.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rode H, Hanslo D, de Wet PM, Millar AJ, Cywes S. 1989. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob. Agents Chemother. 33:1358–1361. 10.1128/AAC.33.8.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R, Agarwal RK, Agarwal M. 1997. Povidone iodine plus neosporin in superficial burns–a continuing study. Burns 23:626–628. 10.1016/S0305-4179(97)00069-7 [DOI] [PubMed] [Google Scholar]
- 8.Cookson BD. 1998. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J. Antimicrob. Chemother. 41:11–18 [DOI] [PubMed] [Google Scholar]
- 9.Hetem DJ, Bonten MJ. 2013. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J. Hosp. Infect. 85:249–256. 10.1016/j.jhin.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35:322–332. 10.1016/j.ijantimicag.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 11.Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138. 10.1016/s0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- 12.Anderl JN, Franklin MJ, Stewart PS. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818–1824. 10.1128/AAC.44.7.1818-1824.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. 2010. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch. Surg. 145:240–246. 10.1001/archsurg.2010.5 [DOI] [PubMed] [Google Scholar]
- 14.Selcuk CT, Durgun M, Ozalp B, Tekin A, Tekin R, Akçay C, Alabalik U. 2012. Comparison of the antibacterial effect of silver sulfadiazine 1%, mupirocin 2%, Acticoat and octenidine dihydrochloride in a full-thickness rat burn model contaminated with multi drug resistant Acinetobacter baumannii. Burns 38:1204–1209. 10.1016/j.burns.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 15.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sørensen O, Borregaard N, Ståhle-Bäckdahl M. 2003. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 120:379–389. 10.1046/j.1523-1747.2003.12069.x [DOI] [PubMed] [Google Scholar]
- 16.Thakoersing VS, Gooris GS, Mulder A, Rietveld M, El Ghalbzouri A, Bouwstra JA. 2012. Unraveling barrier properties of three different in-house human skin equivalents. Tissue Eng. Part C Methods 18:1–11. 10.1089/ten.TEC.2011.0175 [DOI] [PubMed] [Google Scholar]
- 17.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. 2010. Topical antimicrobials for burn wound infections. Recent Pat. Antiinfect. Drug Discov. 5:124–151. 10.2174/157489110791233522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. 10.1038/415389a [DOI] [PubMed] [Google Scholar]
- 19.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457. 10.1038/35106587 [DOI] [PubMed] [Google Scholar]
- 20.Dean SN, Bishop BM, van Hoek ML. 2011. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 11:114. 10.1186/1471-2180-11-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaiou M, Nizet V, Gallo RL. 2003. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Invest. Dermatol. 120:810–816. 10.1046/j.1523-1747.2003.12132.x [DOI] [PubMed] [Google Scholar]
- 22.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 76:4176–4182. 10.1128/IAI.00318-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Jr, Lehrer RI, Welsh MJ, Tack BF. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect. Immun. 68:2748–2755. 10.1128/IAI.68.5.2748-2755.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. 2005. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 175:4662–4668. 10.4049/jimmunol.175.7.4662 [DOI] [PubMed] [Google Scholar]
- 26.Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, Drijfhout JW, Grote JJ. 2006. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 27:649–660. 10.1016/j.peptides.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 27.Peek FAW, Nell MJ, Brand R, Jansen-Werkhoven TM, van Hoogdalem EJ, Frijns JHM. 2009. Double-blind placebo-controlled study of the novel peptide drug P60.4AC in chronic middle ear infection, presentation L1-337. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 28.Fox JL. 2013. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 31:379–382. 10.1038/nbt.2572 [DOI] [PubMed] [Google Scholar]
- 29.Duplantier AJ, van Hoek ML. 2013. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 4:143. 10.3389/fimmu.2013.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haisma EM, Rietveld M, de Breij A, van Dissel JT, El Ghalbzouri A, Nibbering PH. 2013. Inflammatory and antimicrobial responses to methicillin-resistant Staphylococcus aureus in an in vitro wound infection model. PLoS One 8:e82800. 10.1371/journal.pone.0082800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Ghalbzouri A, Hensbergen P, Gibbs S, Kempenaar J, van der Schors R, Ponec M. 2004. Fibroblasts facilitate re-epithelialization in wounded human skin equivalents. Lab. Invest. 84:102–112. 10.1038/labinvest.3700014 [DOI] [PubMed] [Google Scholar]
- 32.Holland DB, Bojar RA, Farrar MD, Holland KT. 2009. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 290:149–155. 10.1111/j.1574-6968.2008.01402.x [DOI] [PubMed] [Google Scholar]
- 33.Holland DB, Bojar RA, Jeremy AH, Ingham E, Holland KT. 2008. Microbial colonization of an in vitro model of a tissue engineered human skin equivalent–a novel approach. FEMS Microbiol. Lett. 279:110–115. 10.1111/j.1574-6968.2007.01021.x [DOI] [PubMed] [Google Scholar]
- 34.Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE. 2009. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 9:229. 10.1186/1471-2180-9-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Fuente-Nuñez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 56:2696–2704. 10.1128/AAC.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.el-Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. 2002. Effect of fibroblasts on epidermal regeneration. Br. J. Dermatol. 147:230–243. 10.1046/j.1365-2133.2002.04871.x [DOI] [PubMed] [Google Scholar]
- 38.Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra J, Mommaas AM. 1997. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J. Invest. Dermatol. 109:348–355. 10.1111/1523-1747.ep12336024 [DOI] [PubMed] [Google Scholar]
- 39.El Ghalbzouri A, Siamari R, Willemze R, Ponec M. 2008. Leiden reconstructed human epidermal model as a tool for the evaluation of the skin corrosion and irritation potential according to the ECVAM guidelines. Toxicol. In Vitro 22:1311–1320. 10.1016/j.tiv.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 40.van Rijen M, Bonten M, Wenzel R, Kluytmans J. 2008. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst. Rev. (4):CD006216. 10.1002/14651858.CD006216.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahlenberg JM, Kaplan MJ. 2013. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J. Immunol. 191:4895–4901. 10.4049/jimmunol.1302005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanetti M, Gennaro R, Romeo D. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1–5 [DOI] [PubMed] [Google Scholar]
- 43.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. 2005. Impact of LL-37 on anti-infective immunity. J. Leukoc. Biol. 77:451–459. 10.1189/jlb.0704380 [DOI] [PubMed] [Google Scholar]
- 44.Flemming K, Klingenberg C, Cavanagh JP, Sletteng M, Stensen W, Svendsen JS, Flaegstad T. 2009. High in vitro antimicrobial activity of synthetic antimicrobial peptidomimetics against staphylococcal biofilms. J. Antimicrob. Chemother. 63:136–145. 10.1093/jac/dkn464 [DOI] [PubMed] [Google Scholar]
- 45.Haisma EM, Rietveld MH, de Breij A, van Dissel JT, El Ghalbzouri A, Nibbering PH. 2013. Inflammatory and antimicrobial responses to methicillin-resistant Staphylococcus aureus in an in vitro wound infection model. PLoS One 8:e82800. 10.1371/journal.pone.0082800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svenson J, Brandsdal BO, Stensen W, Svendsen JS. 2007. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J. Med. Chem. 50:3334–3339. 10.1021/jm0703542 [DOI] [PubMed] [Google Scholar]
- 47.Steinstraesser L, Koehler T, Jacobsen F, Daigeler A, Goertz O, Langer S, Kesting M, Steinau H, Eriksson E, Hirsch T. 2008. Host defense peptides in wound healing. Mol. Med. 14:528–537. 10.2119/2008-00002.Steinstraesser [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinstraesser L, Hirsch T, Schulte M, Kueckelhaus M, Jacobsen F, Mersch EA, Stricker I, Afacan N, Jenssen H, Hancock RE, Kindrachuk J. 2012. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS One 7:e39373. 10.1371/journal.pone.0039373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow LN, Choi KY, Piyadasa H, Bossert M, Uzonna J, Klonisch T, Mookherjee N. 2014. Human cathelicidin LL-37-derived peptide IG-19 confers protection in a murine model of collagen-induced arthritis. Mol. Immunol. 57:86–92. 10.1016/j.molimm.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 50.Mataraci E, Dosler S. 2012. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 56:6366–6371. 10.1128/AAC.01180-12 [DOI] [PMC free article] [PubMed] [Google Scholar]