Abstract
Tuberculosis is characterized by extensive destruction and remodelling of the pulmonary extracellular matrix. Stromal cell-derived matrix metalloproteinases (MMPs) are implicated in this process and may be a target for adjunctive immunotherapy. We hypothesized that MMPs are elevated in bronchoalveolar lavage fluid of tuberculosis patients and that antimycobacterial agents may have a modulatory effect on MMP secretion. Concentrations of MMP-1, -2, -3, -7, -8, and -9 were elevated in the bronchoalveolar lavage fluid from tuberculosis patients compared to those in bronchoalveolar lavage fluid from patients with other pulmonary conditions. There was a positive correlation between MMP-3, MMP-7, and MMP-8 and a chest radiological score of cavitation and parenchymal damage. Respiratory epithelial cell-derived MMP-3 was suppressed by moxifloxacin, rifampicin, and azithromycin in a dose-dependent manner. Respiratory epithelial cell-derived MMP-1 was suppressed by moxifloxacin and azithromycin, whereas MMP-9 secretion was only decreased by moxifloxacin. In contrast, moxifloxacin and azithromycin both increased MMP-1 and -3 secretion from MRC-5 fibroblasts, demonstrating that the effects of these drugs are cell specific. Isoniazid did not affect MMP secretion. In conclusion, MMPs are elevated in bronchoalveolar lavage fluid from tuberculosis patients and correlate with parameters of tissue destruction. Antimycobacterial agents have a hitherto-undescribed immunomodulatory effect on MMP release by stromal cells.
INTRODUCTION
Tuberculosis (TB) is a formidable challenge globally and remains a leading cause of death due to an infectious agent. TB is characterized by extensive extracellular matrix (ECM) degradation and cavitation, which allow the mycobacterium to evade the host response and which may also hamper drug penetration to the site of infection (1, 2). Much of the pathogenesis in TB is due to the host immune response. Inflammatory cytokines and chemokines upregulated in TB do not have the capacity to degrade the lung ECM, and host-derived proteases are important effector molecules. These include the matrix metalloproteinases (MMPs), which are a family of 24 mammalian proteases collectively capable of degrading all components of the ECM at neutral pH (3, 4). MMPs have been implicated in several inflammatory and infectious conditions of the lung, such as chronic obstructive pulmonary disease (COPD), lung cancer, and pulmonary fibrosis (5–8). MMP-1 (interstitial collagenase) has been found to be elevated in type II pneumocytes in emphysema subjects (9) and is associated with a poor prognosis of adenocarcinoma of the lung (10).
Our group developed the concept of a matrix degrading phenotype in TB, in which MMP activity is increased and not countered by the specific tissue inhibitors of metalloproteinases (TIMPs) (11, 12). MMPs are emerging as critical regulators of tissue destruction and cellular recruitment to the granuloma in TB (13–16). In the zebrafish model of TB, ESAT-6 was shown to drive MMP-9 (gelatinase B) secretion from epithelial cells, resulting in monocyte recruitment to the granuloma (16). In the initial response to Mycobacterium tuberculosis infection, respiratory epithelial cells mount an important response via inflammatory mediators (17, 18). Several clinical and immunohistochemical studies have identified that inside the TB granuloma, stromal cells, such as epithelial cells and fibroblasts, express high levels of MMP-1, MMP-3 (a stromelysin), and MMP-9 in response to intercellular networking effects (19–21).
Conventional first-line treatment for TB has remained unchanged for approximately 40 years and comprises quadruple therapy with rifampin, isoniazid, pyrazinamide and ethambutol. More recently, moxifloxacin, a fluoroquinolone, has shown unusual potency against M. tuberculosis and is becoming established as an anti-TB drug (22, 23). Fluoroquinolones are known to modulate bacterial adherence, release of inflammatory products, and phagocytosis (24). The possibility that quinolones may alter matrix degradation is suggested by the fact that these drugs occasionally cause rupture of the Achilles tendon, which is a dramatic example of matrix remodelling (25). Macrolides, in particular azithromycin, which is a first-line drug for nontuberculous mycobacterial (NTM) infections, have a well-established immunomodulatory role and regulate the secretion of several proinflammatory mediators from phagocytes and epithelial cells (26, 27). Inhibition of prostaglandins and other arachidonic acid products by rifampin has been postulated to contribute to its efficacy in TB (28). Our group has previously shown that p-amino salicylic acid (PAS), a drug developed more than 60 years ago that is now being used to treat TB resistant to first-line drugs, inhibits M. tuberculosis-driven prostaglandin E2 (PGE2) accumulation and MMP-1 secretion, without increasing M. tuberculosis killing, suggesting that immunotherapy targeting MMP activity has been in use, unbeknownst to us, for many years (29).
Here, we hypothesized that MMPs are elevated in the lung in TB subjects and that antimycobacterial drugs have the potential to reduce tissue destruction by modulating MMP activity. We showed for the first time in a study focusing exclusively on bronchoalveolar lavage fluid (BALF) from TB patients, which more closely reflects the pulmonary microenvironment, that MMP-1, -2, -3, -7, -8, and -9 concentrations are elevated in TB. The study was conducted in India, which, according to the 2011 WHO report (30), has the largest population of TB in the world. BALF MMP-3, -8, and -9 concentrations positively correlated with a radiological tissue destruction score. Next, we demonstrate that the antimycobacterial drugs rifampin, moxifloxacin, and azithromycin but not isoniazid modulate MMP gene expression and secretion from human airway epithelial cells and fibroblasts in M. tuberculosis-dependent networks, thereby identifying an immunomodulatory effect.
MATERIALS AND METHODS
Clinical study.
BALF samples were collected from patients being routinely investigated for respiratory symptoms at Nalanda University Hospitals, Patna, India. The study was approved by the ethics review board at Nalanda Medical College and University Hospitals (reference number SS/0810/TB). To limit user and procedure variability and ensure consistency of sample collection between donors, bronchoscopy was performed by one of two bronchoscopists using flexible bronchoscopes and an identical bronchial wash protocol. Samples were stored at −20°C. Demographic, symptomatic, hematologic, and radiologic data were collected on a standardized proforma. Exclusion criteria were a history of TB (to decrease the likelihood of multidrug-resistant [MDR]/extensively drug-resistant [XDR] cases), age of <18 years, severe chronic lung disease, malignancy, positive HIV status, exposure to corticosteroids or immunosuppressive drugs, or inability to consent. Heavily blood-stained samples were excluded from the study. A chest X-ray (CXR) scoring system was devised, derived from published scoring systems (31–35). Zero to three points were scored reflecting the absence of cavities, presence of cavities of <2 cm, cavities of 2 to 4 cm, and cavities of >4 cm in size respectively. Next, one extra point was scored for consolidation in each of the zones of West (i.e., right upper/middle/lower or left upper/middle/lower), and a final point was scored if the lesions were present bilaterally.
Samples were centrifuged to remove cellular debris and then sterile filtered through a 0.2-μm Durapore membrane, (Millipore, United Kingdom) to remove M. tuberculosis from the samples. This does not interfere with detection of MMPs and cytokines (36). Concentrations of MMP-1, -2, -3, -7, -8, -9, -12, and -13 (R&D Systems, Europe) and gamma interferon (IFN-γ), interleukin 1 beta (IL-1β), IL-2, IL-4, IL-6, IL-10, IL-13, tumor necrosis factor alpha (TNF-α), IL-17, and IL-23 (Invitrogen, Paisley, United Kingdom) were measured using a Bioplex Luminex 200 instrument (Bio-Rad, United Kingdom).
Reagents.
General laboratory reagents were purchased from Sigma (Poole, United Kingdom) and Invitrogen (Paisley, United Kingdom). M. tuberculosis culture reagents were from BD Biosciences (Oxford, United Kingdom). Moxifloxacin, levofloxacin, rifampin, azithromycin, and isoniazid were from Tocris (Bristol, United Kingdom).
M. tuberculosis culture and generation of TB medium.
M. tuberculosis H37Rv was cultured in Middlebrook 7H9 medium with 10% albumin, dextrose-catalase enrichment medium, 0.2% glycerol, 0.02% Tween 80, and 2.5 μg/ml amphotericin with agitation. Culture growth was monitored using a Biowave cell density meter (Walker Precision Instruments), and M. tuberculosis was subcultured when the optical density exceeded 1.0. For infection experiments, culture at mid-log growth at an optical density of 0.60 was used. This corresponded to 1 × 108 CFU per ml. Optical density was correlated with CFU by performing colony counts in triplicate on Middlebrook 7H11 agar. Endotoxin contamination was excluded by the amebocyte lysate assay (Associates of Cape Cod, Massachusetts, USA). TB medium was generated by centrifuging M. tuberculosis cultures at 14,000 × g for 5 min and sterile filtering the supernatant through an Anopore 0.2-μm-filter membrane (Whatman, Maidstone, United Kingdom).
Monocyte purification and infection.
Primary blood mononuclear cells (PBMCs) were isolated from single-donor buffy coats (National Blood Transfusion Service, United Kingdom) by density centrifugation through Ficoll-Paque (Amersham Biosciences, Amersham, United Kingdom). Monocytes were infected with M. tuberculosis at a multiplicity of infection (MOI) of 1 in RPMI with 2 μm glutamine and 10 mg/ml ampicillin. Cell culture medium was harvested at 24 h. Medium was spun at 13,000 relative centrifugal force (RCF) to remove cellular debris and then sterile filtered through a 0.2-μM Anopore membrane. Medium from infected monocytes was termed conditioned medium from monocytes infected with M. tuberculosis, or CoMTb. Control medium (CoMCont) was generated in a similar manner without addition of M. tuberculosis to cell cultures. To control for variability, the same batch of CoMTb was used for all experiments in this study.
Cell culture.
Primary normal human bronchial epithelial cells (NHBE) were cultured in bronchial epithelial growth medium according to the supplier's instructions (Lonza, Basel, Switzerland). All experiments were performed between passages 4 and 5. Cell viability at the end of experiments was analyzed by trypan blue exclusion. Subculture was performed when cells were 70 to 80% confluent. The human MRC-5 fibroblast line was grown in Eagle's medium with 10% fetal calf serum (FCS) and subcultured when cells were 70 to 80% confluent. Adherent cells were washed with phosphate-buffered saline (PBS) and then detached from the surface with 0.25% trypsin-EDTA solution. Cells were resuspended in fresh medium at a seeding density of 2 × 104 to 4 × 104 cells/cm2. For experiments, 1 × 104 to 2 × 104 cells/cm2 were seeded in a 24-well plate in fresh medium with 1% FCS and stimulated at 70 to 80% confluence. Cells were then stimulated with a 1-in-5 dilution of CoMTb. For specific experiments, cells were pretreated for 2 h with antimycobacterial drugs. Supernatants were harvested after 72 h for MMP analysis.
Gelatin zymography.
MMP-9 gelatinolytic activity was detected by zymography using standard methodology (37). In brief, standards and cell culture supernatants were loaded with 5× loading buffer (0.25 M Tris [pH 6.8], 50% glycerol, 5% SDS, and bromphenol blue) and run on 11% acrylamide gels impregnated with 0.1% gelatin as a substrate. After 3.5 h at 180 V (buffer, 25 mM Tris, 190 mM glycine, and 0.1% SDS), the gel was renatured in 2.5% Triton X-100 for 1 h with agitation. After two washes in collagenase buffer (55 mM Tris base, 200 mM sodium chloride, 5 mM calcium chloride, and 0.02% Brij, pH 7.6), gels were incubated overnight in fresh collagenase buffer at 37°C. Gelatinolytic activity was detected using 0.02% Coomassie blue in 1:3:6 acetic acid/methanol/water (vol/vol/vol). All experimental samples were run in parallel with 2 ng recombinant MMP-9 standard (Merck, Chemicals Ltd, Nottingham, United Kingdom). Digital image acquisition (UV Products) of the bands was followed by densitometric analysis of bands using the software program Scion Image, with normalization to the MMP-9 standard to control for gel-to-gel variability.
Measurement of MMP-1, MMP-3, and MMP-9 concentrations.
MMP-1, MMP-3, and MMP-9 concentrations in cell culture medium were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Europe) according to the manufacturer's instructions. The lower level of detection was 30 pg/ml. MMP-1, -3, and -9 concentrations were also analyzed by using a Fluorokine multianalyte profiling kit according to the manufacturer's protocol (R&D Systems, Europe) on the Luminex 200 platform (Bio-Rad, Hemel Hempstead, United Kingdom). The minimum level of detection for MMPs was 10 pg/ml.
RNA extraction, cDNA synthesis and real-time RT-PCR.
RNA extraction was performed using the Qiagen RNeasy minikit according to the manufacturer's instructions (Qiagen, Manchester, United Kingdom). RNA was eluted with RNase-free water and stored at −80°C. cDNA synthesis was performed using the Quantitect reverse transcription (RT) kit. RNA was quantified on a NanoDrop machine (NanoDrop Technologies Inc). A volume of RNA equivalent to 1 μg was diluted to 12 μl with RNase-free water. Two microliters of gDNA wipeout buffer was added to each sample. Samples were then heated at 42°C for 2 min. Six microliters of the reverse transcription master mix was added to each sample and then heated again at 42°C for 15 min followed by 95°C for 3 min. cDNA was stored at −20°C. Real-time quantitative RT-PCR was performed with Brilliant II QPCR master mix (Stratagene, Cambridge, United Kingdom) on a Stratagene Mx3000P platform. MMP primers and probes have been described previously (38). The threshold cycle (CT) at which amplification entered the exponential phase was determined. A lower CT indicates a higher quantity of starting RNA. To determine the relative RNA levels within samples, standard curves were prepared by making 5-fold serial dilutions of one sample. Standard curves for CT versus input RNA were prepared, and relative quantities of starting RNA in each sample were determined. Experimental MMP data were normalized to the 3 reference genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 18S rRNA, and cyclophilin A, whose CT values remained stable under different experimental conditions. Analysis of MMP mRNA expression was first undertaken by the standard curve method, and results were corroborated by using the CT values to assess levels of gene expression.
Statistical analysis.
Data are presented as means (± standard deviations [SD]) and represent experiments performed in triplicate on at least two occasions, unless otherwise stated. Paired groups were compared using the Student t test. Multiple intervention experiments were compared by one-way analysis of variance (ANOVA) and Tukey's correction for multiple pairwise comparisons. BALF MMP concentrations were compared using the Mann-Whitney U test. Clinical and demographic data between patients and controls were compared using Fisher's exact test. P values of <0.05 were taken as significant. Spearman's rank test was used to investigate correlations that were taken to be significant with an r value of >0.5 and a P value of <0.05. In figures, a P value of <0.05 is illustrated with one asterisk (*), a P value of <0.01 with two (**), a P value of <0.001 with three (***), and a P value of <0.0001 with four (****).
RESULTS
Demographics of the study population.
First, we characterized the demographics of our study population at a university hospital in India. The population was comprised of 17 subjects with confirmed pulmonary TB and 18 well-matched respiratory patients undergoing bronchoscopy. Controls had a diverse range of pulmonary diagnoses, including smoking-related bronchitis (n = 3), pneumonia (n = 4), carcinoma (n = 3), sarcoidosis (n = 2), foreign body aspiration (n = 3), and pulmonary vasculitis (n = 2). Demographic, clinical, and radiological data are presented in Table 1. Bronchoalveolar lavage was performed as part of the patients' diagnostic work-up, so no patients were on TB treatment at the time the specimens were taken. There was no significant difference between TB cases and symptomatic respiratory patients with regard to age, gender, smoking patterns, respiratory examination, and hospitalization rate. No subjects had a history of TB. The majority of patients in the study were men, which may reflect the fact that women in the lower socioeconomic strata in India tend not to seek medical help promptly. All TB patients had respiratory symptoms, and 8 reported hemoptysis. TB patients reported constitutional symptoms such as fever, night sweats, and weight loss more frequently than control patients.
TABLE 1.
Demographic, clinical, and radiological data for TB patients and symptomatic controls undergoing fiberoptic bronchoscopy
| Characteristic | Value for group (na) |
P valueb | |
|---|---|---|---|
| Tuberculosis (17) | Controls (18) | ||
| Mean age, yr (range) | 34 (25.5–46.5) | 43.5 (34.25–50.25) | NS |
| No. (%) of patients | |||
| Male | 12 (70) | 14 (77) | NS |
| Smoker, current or former | 10 (58) | 12 (65) | NS |
| Clinical features | |||
| Hemoptysis | 8 (47) | 5 (27) | NS |
| Dyspnea | 17 (100) | 12 (67) | NS |
| Cough | 13 (76) | 8 (44) | <0.05 |
| Weight Loss | 10 (59) | 3 (17) | <0.05 |
| Fever | 14 (78) | 5 (28) | <0.05 |
| Abnormal respiratory examination | 8 (47) | 12 (67) | NS |
| Hospitalization | 5 (29) | 4 (22) | NS |
| Previous TB | 0 | 0 | NS |
| TB household contacts | 10 (59) | None known | |
| Mean CXR score [0–10] (range) | 5 (3.5–6.5) | 1 (0–4.5) | 0.0082 |
n, no. of patients.
Comparison between groups was performed by Fisher's exact test. NS, not significant.
MMPs are upregulated in TB BALF and positively correlate with CXR score.
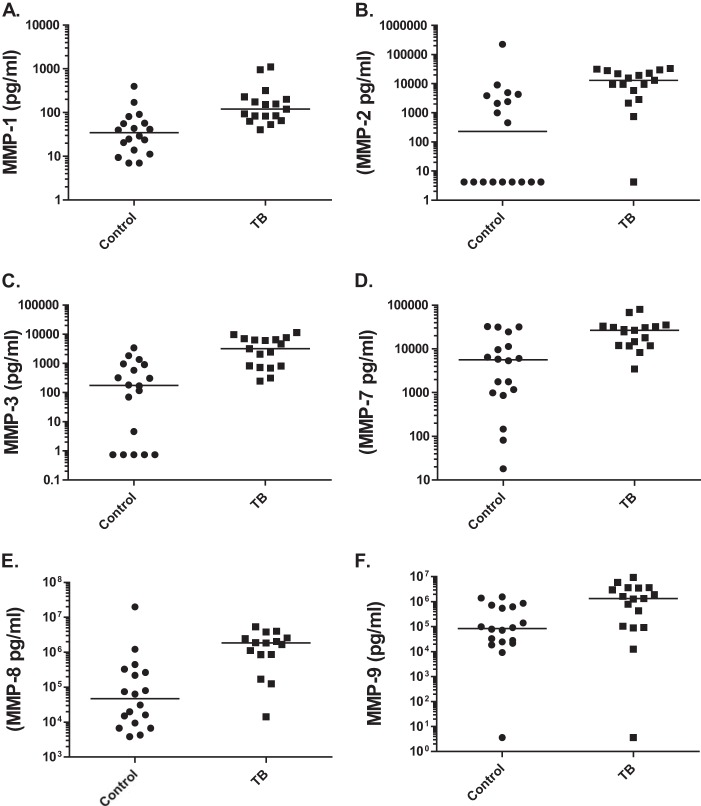
Next, we measured MMP concentrations in BALF from TB and control subjects by using a Luminex array. Median MMP-1, -2, -3, -7, -8, and -9 concentrations in BALF were significantly upregulated in patients with TB compared to those in respiratory control subjects (Fig. 1; all P values ≤ 0.001). High CXR scores reflect greater tissue damage. BALF MMP-3, MMP-7, and MMP-8 concentrations correlated with CXR scores, with r values of 0.62, 0.69, and 0.55, respectively (all P values < 0.003). All patients went on to successfully complete treatment using standard WHO drug protocols.
FIG 1.
MMP concentrations in bronchoalveolar lavage fluid (BALF). In BALF from TB patients, MMP-1 (A), MMP-2 (B), MMP-3 (C), MMP-7 (D), MMP-8 (E), and MMP-9 (F) are upregulated (all P values ≤ 0.001 by Mann-Whitney U test), compared to levels for respiratory controls with diverse pulmonary diseases.
Quinolones downregulate MMP-1, -3, and -9 from CoMTb-stimulated NHBE cells.
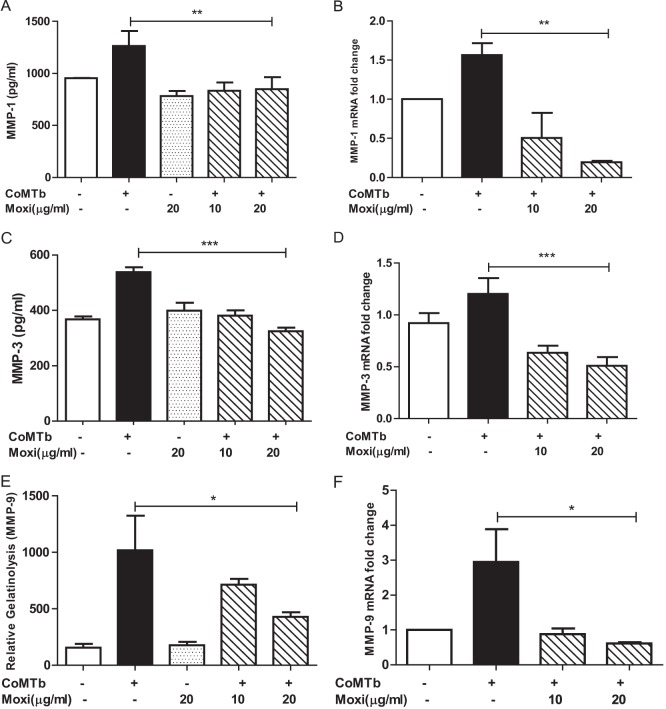
Next, we examined the modulation of MMP-1, -3, and -9 secretion from NHBEs stimulated with CoMTb and exposed to moxifloxacin. Of the MMPs that were elevated in BALF, only these 3 MMPs are secreted by NHBEs. Moxifloxacin suppressed MMP-1 secretion to baseline levels, and mRNA accumulation decreased below baseline (Fig. 2A and B; both P values were <0.01). Maximal decrease in gene expression was achieved with 20 μg/ml moxifloxacin, which is comparable to the concentrations achieved in epithelial lining fluid and bronchial mucosa (39, 40). Similarly, MMP-3 secretion and mRNA accumulation were suppressed maximally by 20 μg/ml moxifloxacin (Fig. 2C and D; both P values were <0.001). Moxifloxacin also decreased MMP-9-mediated gelatinolysis and gene expression (Fig. 2E and F; P < 0.01 and P < 0.05, respectively). Moxifloxacin on its own did not have a significant effect on epithelial MMP-1, -3, or -9 secretion. These experiments were repeated with levofloxacin to determine whether the observations were a class effect of quinolones, and similar suppression was observed (data not shown). No antimycobacterial agent altered NHBE survival in culture as analyzed by trypan blue analysis.
FIG 2.
Moxifloxacin suppresses CoMTb-driven epithelial MMP-1, -3, and -9. MMP-1 secretion (A) and MMP-1 gene expression (B) from NHBEs are suppressed by costimulation with CoMTb and moxifloxacin (P < 0.01 for both). This effect occurred at both 10 μg/ml and 20 μg/ml for secretion and was maximal at 20 μg/ml for gene expression. MMP-3 secretion (C) and MMP-3 gene expression (D) from NHBEs were also suppressed (P < 0.001 for both), with maximal suppression at 20 μg/ml of the drug. TB-driven gelatinolyic activity (E) and MMP-9 mRNA accumulation (F) from NHBEs were suppressed to baseline levels by preincubation with moxifloxacin (P < 0.01 and P < 0.05, respectively). Cells stimulated with 20 μg/ml of moxifloxacin alone did not exhibit an alteration in MMP secretion.
Rifampin but not isoniazid downregulates MMP-3 but not MMP-1 or -9.
Pretreatment of NHBE cells with rifampin decreased CoMTb-driven MMP-3 secretion from 538 ± 17 pg/ml to a baseline value of 271 ± 20 pg/ml, (Fig. 3A) (P < 0.001). MMP-3 gene expression was suppressed to below baseline (Fig. 3B, P < 0.01). However, MMP-1 and MMP-9 mRNA accumulation and secretion were not altered by pretreatment with rifampin (data not shown), nor was MMP-3 secretion altered significantly by rifampin alone. The other major bactericidal anti-tuberculous drug, isoniazid, did not alter MMP gene expression or secretion in any way (data not shown).
FIG 3.
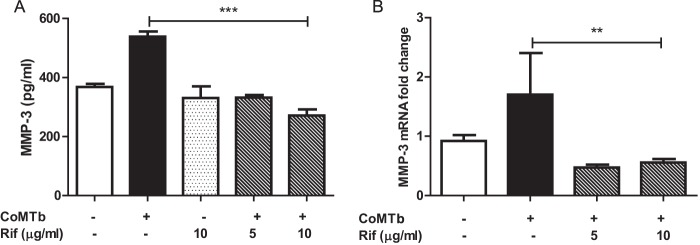
Rifampin suppresses CoMTb-driven epithelial MMP-3. Rifampin had an inhibitory effect on CoMTb-driven MMP-3 secretion (A) (P < 0.001) and MMP-3 mRNA accumulation (B) (P < 0.01) from NHBEs. The suppression was maximal at 10 μg/ml in cells that had been costimulated with CoMTb.
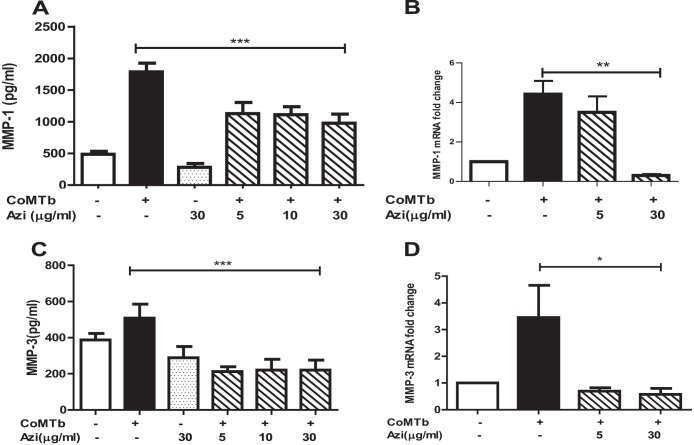
Azithromycin downregulates MMP-1 and -3.
Next, we investigated the modulation of MMPs by azithromycin. Azithromycin is an established immunomodulator and, although rarely used in the treatment of TB, is employed in the therapy of nontuberculous mycobacterial infections. CoMTb-driven MMP-1 secretion from NHBEs was suppressed by 50% from a peak of 1,786 ± 137 pg/ml to 975 ± 143 pg/ml (Fig. 4A) (P < 0.001). MMP-1 mRNA expression was suppressed to below the baseline level by azithromycin (Fig. 4B) (P < 0.01). In addition, azithromycin suppressed CoMTb-driven MMP-3 secretion to 220 ± 54 pg/ml (Fig. 4C) (P < 0.001) and MMP-3 mRNA accumulation to the baseline level (Fig. 4D) (P < 0.05). Maximal suppression was observed using 30 μg/ml of azithromycin, but this concentration did not significantly affect baseline secretion of MMP-1 or -3. In contrast, MMP-9 secretion and gene expression from NHBEs were not altered by azithromycin (data not shown).
FIG 4.
Azithromycin suppresses CoMTb-driven epithelial MMP-1 and -3. Azithromycin suppressed CoMTb-driven NHBE-derived MMP-1 secretion (A) from 1,786 pg/ml to 975 pg/ml (P < 0.001). MMP-1 mRNA gene expression (B) was further suppressed to the baseline level (P < 0.05). Azithromycin also suppressed CoMTb-driven MMP-3 secretion (C) (P < 0.001) and MMP-3 mRNA accumulation (D) (P < 0.05). Maximal suppression was observed with 30 μg/ml of azithromycin, but no change in MMP-1 or -3 secretion was observed when the cells were treated with azithromycin alone.
Moxifloxacin and azithromycin upregulate MMP-1 and -3 from CoMTb-stimulated MRC-5 fibroblasts.
Finally, we investigated the effect of antimycobacterial agents on human MRC-5 fibroblasts stimulated by CoMTb. In contrast to results with NHBE cells, both moxifloxacin and azithromycin increased fibroblast MMP-1 and MMP-3 secretion (Fig. 5). Preincubation with moxifloxacin resulted in a 1.8-fold increase in TB-dependent MMP-1 and -3 secretion from MRC-5 cells (Fig. 5A) (P < 0.05). A similar 2.5-fold increase in MMP-1 and -3 was observed upon preincubation with azithromycin (Fig. 5B) (P < 0.05). MMP-9 secretion was not analyzed because it is not secreted by MRC-5 cells. Drug concentrations greater than10 μg/ml for either antibiotic decreased fibroblast cell viability.
FIG 5.
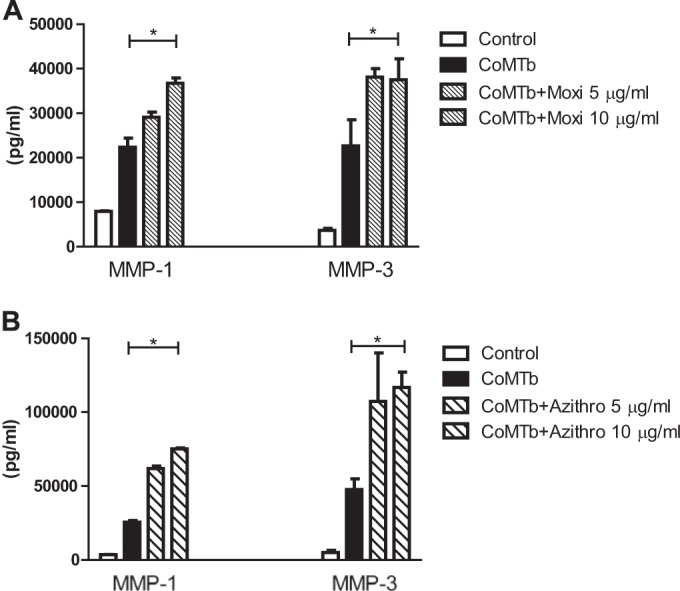
Moxifloxacin and azithromycin increase fibroblast MMP-1 and -3 secretion. Moxifloxacin upregulated both MMP-1 and MMP-3 secretion (A) from MRC-5 fibroblast cells stimulated with CoMTb (P < 0.05). The response was observed maximally at drug concentrations of 10 μg/ml, above which there was decreased cell viability. Azithromycin increased MMP-1 and MMP-3 secretion (B) from MRC-5 fibroblasts. The increment was maximal at 10 μg/ml (P < 0.05).
DISCUSSION
First, we showed that MMP-1, -2, -3, -7, -8, and -9 are elevated in BALF from 17 patients with pulmonary TB compared to levels for 18 well-matched symptomatic controls. Controls were patients with other respiratory conditions. Some of these diseases, such as carcinoma, pneumonia, and smoking-related bronchitis, are associated with MMP upregulation in their own right, suggesting that the increase we identified in TB patients could have been more marked had the controls been healthy individuals (41). The study was conducted in collaboration with a university hospital in eastern India. According to the 2011 WHO report, India has the highest TB burden in the world, with an annual incidence of 2.2 million cases (42–45). In response, India runs the world's largest health care program for the eradication of TB under its revised national TB control program (RNTCP) (46), but the problem is compounded by poverty (47), poor compliance, and more recently the surge in drug resistance (48–50).
In the study population, there was a positive correlation between CXR scores and concentrations of MMPs-3, -7, and -8, which further implicates these proteases as key in tissue damage in TB, consistent with our previous data (14). In this patient cohort, we did not find that MMP-9 was associated with disease severity or extent of radiological damage (51). In contrast, we have previously shown that cerebrospinal fluid leukocyte-derived MMP-9 concentrations were associated with neurological damage and death (11) and that MMP-9 concentrations in pleural fluid were elevated in the presence of granulomas in TB (52). MMP-12 and MMP-13 levels were undetectable in BALF. This was our first study investigating MMP concentrations exclusively in BALF as opposed to induced sputum (15); the data from both studies are consistent. MMP-1 did not significantly correlate with chest X-ray score in the present study and similarly was not elevated in an investigation of patients with pleural disease (52). However, this contrasts with findings in induced sputum (14) and in central nervous system disease (53) and may reflect the fact that this patient group was relatively small.
The second and key observation from this study is that we have identified for the first time that the antimycobacterial drugs moxifloxacin, rifampin, and azithromycin may modulate the gene expression and secretion of the MMPs elevated in the BALF of TB patients and secreted by stromal cells. Such effects were MMP and cell specific. In a previous study, we had shown that doxycycline, a licensed MMP inhibitor, suppressed TB-dependent MMP-1 and MMP-9 from human macrophages and epithelial cells (15) and that PAS suppresses macrophage MMP-1 production by inhibiting cyclooxygenase (29). In the current study, focusing on stromal cells and first-line antimycobacterial agents, we demonstrated that moxifloxacin suppressed MMP-1, -3, and -9 secretion and gene expression in human airway epithelial cells but augmented MMP-1 and -3 secretion in MRC-5 fibroblasts. This is a class effect of quinolones, since similar data were obtained with levofloxacin. Fluoroquinolones are known to have immunomodulatory properties. Moxifloxacin significantly inhibited proinflammatory cytokine secretion and mitogen-activated protein kinase activity, as well as NF-κB activation in human monocytes (54) and a cystic fibrosis epithelial cell line (55). The effects on respiratory epithelial cell MMP gene expression and secretion may reduce innate immune inflammatory damage, thus providing additional benefit beyond the drug's direct antimycobacterial activity. It is reasonable to speculate that the increased MMP-1 and -3 secretion from fibroblasts may be at least part of the reason why use of this drug may be associated with tendon rupture. We did not investigate macrophages, another key source of MMPs in TB (56), and consequently study of antimycobacterial immunomodulatory effects in other cell types is required, since MMP regulation may be cell specific.
Pharmacodynamic studies of fluoroquinolones and macrolides have previously indicated that attainment of higher ratios of free area under the concentration-time curve (AUC) to MIC of the drug is a predictor of microbiologic eradication (57–59). Both fluoroquinolones and macrolides are known to penetrate well into infectious foci, such as bronchial mucosa (BM), epithelial lining fluid (ELF), and alveolar macrophages (AM), with significantly higher target site concentrations than the peak plasma concentration (39, 40, 60). We chose a dose range for moxifloxacin and azithromycin in our experiments to try to model these relatively elevated intrapulmonary concentrations, and the highest concentrations we used were therefore higher than the peak plasma concentrations.
Rifampin was not predicted to affect extracellular matrix turnover but does suppress epithelial MMP-3 production. We have not yet established whether or not this is a consequence of rifampin's actions on the prostaglandin pathway, analogous to published data on PAS (29). In contrast, isoniazid did not alter MMP gene expression or secretion from epithelial cells. Azithromycin suppressed respiratory epithelial cell MMP-1 and MMP-3 mRNA accumulation and secretion but had no effect on MMP-9. Similar to moxifloxacin, azithromycin increased MMP-1 and MMP-3 secretion from MRC-5 cells. Immunomodulatory effects of azithromycin include inhibition of IL-8 secretion and NF-κB/AP-1 binding activities in lung epithelial cells (26). Azithromycin also suppresses TNF-α in cystic fibrosis airway epithelial cell lines via effects on NF-κB and Sp1 DNA binding (61). The mechanisms of MMP inhibition in respiratory epithelial cells are not yet dissected, but since they are regulated by NF-κB and AP-1 (3, 62, 63), modulation of promoter binding is likely. Our data are consistent with decreased MMP-9 secretion observed in airway epithelial cells treated with azithromycin and costimulated by inflammatory soluble factors from cystic fibrosis airways (64).
In summary, here we report for the first time that MMP-1, -2, -3, -7, -8, and -9 are upregulated in the BALF of TB patients and that the key anti-TB drugs, rifampin and moxifloxacin, decrease epithelial cell gene expression and secretion of MMPs. Azithromycin is also similarly immunomodulatory. Each drug had MMP- and cell-specific effects. It is noteworthy that all drugs inhibited gene expression of MMP-3 in the epithelium, which was strongly associated with tissue damage in the clinical part of the study. MMP-1 and -3 secretion was increased in fibroblasts treated with moxifloxacin and azithromycin. These data suggest that current TB therapies may inadvertently be modulating extracellular matrix turnover, and more targeted modulation merits investigation with animal models and with humans. Modulation of MMP activity may have diverse consequences, including improved drug penetration, reduced morbidity, and reduced transmission to a new host (65). TB treatment switches the sputum MMP profile from a proinflammatory to a resolving phenotype soon after treatment is commenced (66), suggesting that the use of immunomodulators could be effective early in the course of therapy. Comprehensive analysis of MMP modulation by antituberculous agents will require study using the appropriate model system, including caseous foci, which are typical of human TB, to dissect its effects on matrix turnover.
ACKNOWLEDGMENTS
This work was funded by Medical Research Council (United Kingdom) and Scadding Moriston-Davies Fellowship grants to Shivani Singh. Jon S. Friedland and Paul T. Elkington acknowledge the support of the NIHR BRC funding scheme at Imperial College London.
We thank Ramji Prasad and Sandeep Sen for help with the clinical study in India.
Footnotes
Published ahead of print 2 June 2014
REFERENCES
- 1.Elkington PT, Friedland JS. 2006. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61:259–266. 10.1136/thx.2005.051979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell DG. 2007. Who puts the tubercle in tuberculosis? Nat. Rev. Microbiol. 5:39–47. 10.1038/nrmicro1538 [DOI] [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Matrisian LM. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 3:207–214. 10.1038/nrm763 [DOI] [PubMed] [Google Scholar]
- 4.Parks WC, Wilson CL, Lopez-Boado YS. 2004. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4:617–629. 10.1038/nri1418 [DOI] [PubMed] [Google Scholar]
- 5.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277:2002–2004. 10.1126/science.277.5334.2002 [DOI] [PubMed] [Google Scholar]
- 6.Finlay GA, Russell KJ, McMahon KJ, D'Arcy E, Masterson MJB, FitzGerald MX, O'Connor CM. 1997. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax 52:502–506. 10.1136/thx.52.6.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manoury B, Nenan S, Guenon I, Lagente V, Boichot E. 2007. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int. Immunopharmacol. 7:900–911. 10.1016/j.intimp.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Jumper C, Cobos E, Lox C. 2004. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Respir. Med. 98:173–177. 10.1016/j.rmed.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. 2001. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am. J. Respir. Crit. Care Med. 163:786–791. 10.1164/ajrccm.163.3.2001073 [DOI] [PubMed] [Google Scholar]
- 10.Kodate M, Kasai T, Hashimoto H, Yasumoto K, Iwata Y, Manabe H. 1997. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol. Int. 47:461–469. 10.1111/j.1440-1827.1997.tb04525.x [DOI] [PubMed] [Google Scholar]
- 11.Price NM, Farrar J, Tran TT, Nguyen TH, Tran TH, Friedland JS. 2001. Identification of a matrix-degrading phenotype in human tuberculosis in vitro and in vivo. J. Immunol. 166:4223–4230. 10.4049/jimmunol.166.6.4223 [DOI] [PubMed] [Google Scholar]
- 12.Elkington PT, D'Armiento JM, Friedland JS. 2011. Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci. Transl. Med. 3:71ps6. 10.1126/scitranslmed.3001847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salgame P. 2011. MMPs in tuberculosis: granuloma creators and tissue destroyers. J. Clin. Invest. 121:1686–1688. 10.1172/JCI57423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D'Armiento J, Friedland JS. 2011. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Invest. 121:1827–1833. 10.1172/JCI45666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker NF, Clark SO, Oni T, Andreu N, Tezera L, Singh S, Saraiva L, Pedersen B, Kelly DL, Tree JA, D'Armiento JM, Meintjes G, Mauri FA, Williams A, Wilkinson RJ, Friedland JS, Elkington PT. 2012. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 185:989–997. 10.1164/rccm.201110-1769OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. 2010. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327:466–469. 10.1126/science.1179663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickremasinghe MI, Thomas LH, Friedland JS. 1999. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network. J. Immunol. 163:3936–3947 [PubMed] [Google Scholar]
- 18.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. 1999. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 162:3549–3558 [PubMed] [Google Scholar]
- 19.Elkington PT, Emerson JE, Lopez-Pascua LD, O'Kane CM, Horncastle DE, Boyle JJ, Friedland JS. 2005. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J. Immunol. 175:5333–5340. 10.4049/jimmunol.175.8.5333 [DOI] [PubMed] [Google Scholar]
- 20.Elkington PT, Green JA, Emerson JE, Lopez-Pascua LD, Boyle JJ, O'Kane CM, Friedland JS. 2007. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am. J. Respir. Cell Mol. Biol. 37:431–437. 10.1165/rcmb.2007-0011OC [DOI] [PubMed] [Google Scholar]
- 21.O'Kane CM, Elkington PT, Friedland JS. 2008. Monocyte-dependent oncostatin M and TNF-alpha synergize to stimulate unopposed matrix metalloproteinase-1/3 secretion from human lung fibroblasts in tuberculosis. Eur. J. Immunol. 38:1321–1330. 10.1002/eji.200737855 [DOI] [PubMed] [Google Scholar]
- 22.Conde MB, Efron A, Loredo C, De Souza GR, Graca NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, Chaisson RE. 2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373:1183–1189. 10.1016/S0140-6736(09)60333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, Heilig CM, Bernardo J, Choudhri S, Grosset JH, Guy E, Guyadeen P, Leus MC, Maltas G, Menzies D, Nuermberger EL, Villarino M, Vernon A, Chaisson RE. 2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 180:273–280. 10.1164/rccm.200901-0078OC [DOI] [PubMed] [Google Scholar]
- 24.Dalhoff A, Shalit I. 2003. Immunomodulatory effects of quinolones. Lancet Infect. Dis. 3:359–371. 10.1016/S1473-3099(03)00658-3 [DOI] [PubMed] [Google Scholar]
- 25.Stephenson AL, Wu W, Cortes D, Rochon PA. 2013. Tendon injury and fluoroquinolone use: a systematic review. Drug Saf. 36:709–721. 10.1007/s40264-013-0089-8 [DOI] [PubMed] [Google Scholar]
- 26.Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. 2006. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun. 350:977–982. 10.1016/j.bbrc.2006.09.132 [DOI] [PubMed] [Google Scholar]
- 27.Ishizawa K, Suzuki T, Yamaya M, Jia YX, Kobayashi S, Ida S, Kubo H, Sekizawa K, Sasaki H. 2005. Erythromycin increases bactericidal activity of surface liquid in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L565–L573. 10.1152/ajplung.00316.2004 [DOI] [PubMed] [Google Scholar]
- 28.Yuhas Y, Azoulay-Alfaguter I, Berent E, Ashkenazi S. 2007. Rifampin inhibits prostaglandin E2 production and arachidonic acid release in human alveolar epithelial cells. Antimicrob. Agents Chemother. 51:4225–4230. 10.1128/AAC.00985-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rand L, Green JA, Saraiva L, Friedland JS, Elkington PT. 2009. Matrix metalloproteinase-1 is regulated in tuberculosis by a p38 MAPK-dependent, p-aminosalicylic acid-sensitive signaling cascade. J. Immunol. 182:5865–5872. 10.4049/jimmunol.0801935 [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 2011. Global tuberculosis control 2011. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/2011/en/ [Google Scholar]
- 31.Lawson L, Yassin MA, Thacher TD, Olatunji OO, Lawson JO, Akingbogun TI, Bello CS, Cuevas LE, Davies PD. 2008. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand. J. Infect. Dis. 40:30–35. 10.1080/00365540701509899 [DOI] [PubMed] [Google Scholar]
- 32.Den Boon S, Bateman ED, Enarson DA, Borgdorff MW, Verver S, Lombard CJ, Irusen E, Beyers N, White NW. 2005. Development and evaluation of a new chest radiograph reading and recording system for epidemiological surveys of tuberculosis and lung disease. Int. J. Tuberc. Lung Dis. 9:1088–1096 [PubMed] [Google Scholar]
- 33.Dawson R, Masuka P, Edwards DJ, Bateman ED, Bekker LG, Wood R, Lawn SD. 2010. Chest radiograph reading and recording system: evaluation for tuberculosis screening in patients with advanced HIV. Int. J. Tuberc. Lung Dis. 14:52–58 [PMC free article] [PubMed] [Google Scholar]
- 34.Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, Mc Neeley DF. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405. 10.1056/NEJMoa0808427 [DOI] [PubMed] [Google Scholar]
- 35.Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, Wilks MJ, Waramori G, Tjitra E, Sandjaja Kenagalem E, Pontororing GJ, Anstey NM, Kelly PM. 2010. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax 65:863–869. 10.1136/thx.2010.136242 [DOI] [PubMed] [Google Scholar]
- 36.Elkington PT, Green JA, Friedland JS. 2006. Filter sterilization of highly infectious samples to prevent false negative analysis of matrix metalloproteinase activity. J. Immunol. Methods 309:115–119. 10.1016/j.jim.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 37.Leber TM, Balkwill FR. 1997. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal. Biochem. 249:24–28. 10.1006/abio.1997.2170 [DOI] [PubMed] [Google Scholar]
- 38.Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. 2003. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol. Cancer Res. 1:333–345 [PubMed] [Google Scholar]
- 39.Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, Nicolau DP. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965–973. 10.1378/chest.125.3.965 [DOI] [PubMed] [Google Scholar]
- 40.Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. 1999. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 44:835–838. 10.1093/jac/44.6.835 [DOI] [PubMed] [Google Scholar]
- 41.Greenlee KJ, Werb Z, Kheradmand F. 2007. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 87:69–98. 10.1152/physrev.00022.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chadha VK, Sarin R, Narang P, John KR, Chopra KK, Jitendra R, Mendiratta DK, Vohra V, Shashidhara AN, Muniraj G, Gopi PG, Kumar P. 2013. Trends in the annual risk of tuberculous infection in India. Int. J. Tuberc. Lung Dis. 17:312–319. 10.5588/ijtld.12.0330 [DOI] [PubMed] [Google Scholar]
- 43.Akachi Y, Zumla A, Atun R. 2012. Investing in improved performance of national tuberculosis programs reduces the tuberculosis burden: analysis of 22 high-burden countries, 2002–2009. J. Infect. Dis. 205(Suppl 2):S284–S292. 10.1093/infdis/jis189 [DOI] [PubMed] [Google Scholar]
- 44.Sisodia RS, Jain DK, Agarwal SS, Gupta A. 2011. TB control in India—efforts, challenges and priorities. J. Indian Med. Assoc. 109:921–924, 928 [PubMed] [Google Scholar]
- 45.Jain RC. 2011. Tuberculosis—challenges and opportunities. Indian J. Tuberc. 58:148–154 [PubMed] [Google Scholar]
- 46.Kumar A. 2012. Status report on RNTCP. Indian J. Tuberc. 59:107–111 [PubMed] [Google Scholar]
- 47.Oxlade O, Murray M. 2012. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One 7:e47533. 10.1371/journal.pone.0047533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behera D. 2012. Totally drug resistant tuberculosis—a fact or myth? Indian J. Tuberc. 59:190–193 [PubMed] [Google Scholar]
- 49.Behera D. 2012. New strategies of TB control in India: are we on the right track? Indian J. Tuberc. 59:130–134 [PubMed] [Google Scholar]
- 50.Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, Bhatnagar AK, Behera D, Pande JN, Singh UB. 2013. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS One 8:e55299. 10.1371/journal.pone.0055299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hrabec E, Strek M, Zieba M, Kwiatkowska S, Hrabec Z. 2002. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int. J. Tuberc. Lung Dis. 6:713–719 [PubMed] [Google Scholar]
- 52.Sheen P, O'Kane CM, Chaudhary K, Tovar M, Santillan C, Sosa J, Caviedes L, Gilman RH, Stamp G, Friedland JS. 2009. High MMP-9 activity characterises pleural tuberculosis correlating with granuloma formation. Eur. Respir. J. 33:134–141. 10.1183/09031936.00127807 [DOI] [PubMed] [Google Scholar]
- 53.Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, Friedland JS. 2010. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and activator protein-1-dependent monocyte networks. J. Immunol. 184:6492–6503. 10.4049/jimmunol.0903811 [DOI] [PubMed] [Google Scholar]
- 54.Shalit I, Halperin D, Haite D, Levitov A, Romano J, Osherov N, Fabian I. 2006. Anti-inflammatory effects of moxifloxacin on IL-8, IL-1beta and TNF-alpha secretion and NFkappaB and MAP-kinase activation in human monocytes stimulated with Aspergillus fumigatus. J. Antimicrob. Chemother. 57:230–235. 10.1093/jac/dki441 [DOI] [PubMed] [Google Scholar]
- 55.Blau H, Klein K, Shalit I, Halperin D, Fabian I. 2007. Moxifloxacin but not ciprofloxacin or azithromycin selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-kappaB activation in a cystic fibrosis epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:L343–L352. 10.1152/ajplung.00030.2006 [DOI] [PubMed] [Google Scholar]
- 56.Elkington PT, Nuttall RK, Boyle JJ, O'Kane CM, Horncastle DE, Edwards DR, Friedland JS. 2005. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am. J. Respir. Crit. Care Med. 172:1596–1604. 10.1164/rccm.200505-753OC [DOI] [PubMed] [Google Scholar]
- 57.Zhanel GG, Noreddin AM. 2001. Pharmacokinetics and pharmacodynamics of the new fluoroquinolones: focus on respiratory infections. Curr. Opin. Pharmacol. 1:459–463. 10.1016/S1471-4892(01)00080-7 [DOI] [PubMed] [Google Scholar]
- 58.Craig WA. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl 3):S233–S237. 10.1086/321854 [DOI] [PubMed] [Google Scholar]
- 59.Andes D. 2001. Pharmacokinetic and pharmacodynamic properties of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 14:165–172. 10.1097/00001432-200104000-00010 [DOI] [PubMed] [Google Scholar]
- 60.Ulrich M, Berger J, Moller JG, Doring G. 2005. Moxifloxacin and ciprofloxacin protect human respiratory epithelial cells against Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, and Haemophilus influenzae in vitro. Infection 33(Suppl 2):50–54. 10.1007/s15010-005-8208-9 [DOI] [PubMed] [Google Scholar]
- 61.Cigana C, Assael BM, Melotti P. 2007. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob. Agents Chemother. 51:975–981. 10.1128/AAC.01142-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bond M, Fabunmi RP, Baker AH, Newby AC. 1998. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 435:29–34. 10.1016/S0014-5793(98)01034-5 [DOI] [PubMed] [Google Scholar]
- 63.Barchowsky A, Frleta D, Vincenti MP. 2000. Integration of the NF-kappaB and mitogen-activated protein kinase/AP-1 pathways at the collagenase-1 promoter: divergence of IL-1 and TNF-dependent signal transduction in rabbit primary synovial fibroblasts. Cytokine 12:1469–1479. 10.1006/cyto.2000.0743 [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, Boucher RC, O'Neal WK. 2009. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS One 4:e5806. 10.1371/journal.pone.0005806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedland JS. 2008. Tackling tissue destruction in tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 102:953–954. 10.1016/j.trstmh.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 66.Ugarte-Gil CA, Elkington P, Gilman RH, Coronel J, Tezera LB, Bernabe-Ortiz A, Gotuzzo E, Friedland JS, Moore DA. 2013. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One 8:e61333. 10.1371/journal.pone.0061333 [DOI] [PMC free article] [PubMed] [Google Scholar]