Abstract
The accumulation of neutrophils and proinflammatory mediators, such as leukotriene B4 (LTB4), is a classic marker of inflammatory disease. The clearance of apoptotic neutrophils, inhibition of proinflammatory signaling, and production of proresolving lipids (including lipoxins, such as lipoxin A4 [LXA4]) are imperative for resolving inflammation. Tulathromycin (TUL), a macrolide used to treat bovine respiratory disease, confers immunomodulatory benefits via mechanisms that remain unclear. We recently reported the anti-inflammatory properties of TUL in bovine phagocytes in vitro and in Mannheimia haemolytica-challenged calves. The findings demonstrated that this system offers a powerful model for investigating novel mechanisms of pharmacological immunomodulation. In the present study, we examined the effects of TUL in a nonbacterial model of pulmonary inflammation in vivo and characterized its effects on lipid signaling. In bronchoalveolar lavage (BAL) fluid samples from calves challenged with zymosan particles (50 mg), treatment with TUL (2.5 mg/kg of body weight) significantly reduced pulmonary levels of LTB4 and prostaglandin E2 (PGE2). In calcium ionophore (A23187)-stimulated bovine neutrophils, TUL inhibited phospholipase D (PLD), cytosolic phospholipase A2 (PLA2) activity, and the release of LTB4. In contrast, TUL promoted the secretion of LXA4 in resting and A23187-stimulated neutrophils, while levels of its precursor, 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE], were significantly lower. These findings indicate that TUL directly modulates lipid signaling by inhibiting the production of proinflammatory eicosanoids and promoting the production of proresolving lipoxins.
INTRODUCTION
Arachidonic acid is a basic constituent of all cells and is present in membrane phospholipids. The activation of phospholipases (PLs), notably PLA2, by a variety of stimuli liberates arachidonic acid from the membrane phospholipids, which in turn leads to the production of a rich variety of powerful immunomodulating eicosanoids, including prostaglandins (PGs), leukotrienes (LTs), and lipoxins (LXs). Prostaglandin E2 (PGE2) acts as a proinflammatory prostanoid (1, 2), inducing the expression of a number of inflammatory cytokines, including CXCL-8 (3, 4). Proinflammatory leukotriene B4 (LTB4) is best recognized for its potent chemotactic and leukocyte-activating effects (5, 6). In the airway, LTB4 stimulates the secretion of mucus (7), elastase, superoxide radicals (8), and the release of inflammatory cytokines, including LTB4 (9). LTs have been identified as mediators of a variety of inflammatory diseases, including bacterial pneumonia (10, 11).
The resolution of inflammation is critical for restoring homeostasis following infection or injury (12). In addition to having proinflammatory actions, certain lipoxygenase (LO)-derived eicosanoids, like LXs, have immunosuppressive and proresolving benefits. These include the inhibition of neutrophil recruitment, production of proinflammatory cytokines, and bacterial killing in leukocytes (13). Lipoxins also promote the removal of apoptotic cells and stimulate the production of other anti-inflammatory mediators (13).
Disruptions in the resolution phase of inflammation can lead to the development of a severe and chronic inflammatory state. Bacterial pneumonia in humans, cattle, or swine is a prime example in which both bacterial virulence factors and host inflammatory responses participate in disease pathogenesis. This is indeed the case in the bovine respiratory disease (BRD) complex, or shipping fever, which is frequently observed in feedlot cattle. A number of bacterial species have been implicated in BRD, including Mannheimia haemolytica serotype A1 (14). Ultimately, M. haemolytica infection causes a self-amplifying inflammatory milieu within the lungs that is associated with a massive influx of neutrophils and excessive production of proinflammatory mediators, reactive oxygen species (ROS), and proteolytic enzymes within the lower respiratory tract (15, 16). This exaggerated inflammatory response is the cause of the severe tissue damage in BRD. In light of its multifactorial pathogenesis, BRD is an ideal model for studying pulmonary inflammation and mucosal inflammatory disease.
Macrolide antibiotics have gained interest for their ability to confer dual antimicrobial and anti-inflammatory effects. Accordingly, these drugs have proven to be highly effective in treating diseases with significant inflammatory consequences, such as asthma (17), cystic fibrosis (18), and pneumococcal pneumonia (19). Macrolides accumulate within host cells, particularly in phagocytes, serving as a vehicle for the transport of the drug to the sites of infection (20, 21). High concentrations of pharmacological compounds within the lysosomes may inhibit phospholipases and promote the accumulation of intracellular phospholipids within myelin-like lamellar bodies, a phenomenon known as phospholipidosis (22). Azithromycin was the first macrolide reported to cause phospholipidosis in cultured fibroblasts (23), an effect that was reversible following the release of the drug from the cells (24). To date, only a few macrolides have been shown to induce phospholipidosis (23–25). Interestingly, a recent report demonstrated that the inherent anti-inflammatory activities of certain macrolides correlated with their degree of intracellular accumulation and phospholipidosis, but the mechanisms for this remain unclear (24).
Tulathromycin is a semisynthetic 15-membered ring macrolide derivative of erythromycin. It represents the first member of a subclass of macrolides known as triamilides, and it has shown superior clinical efficacy against respiratory diseases in swine (26) and cattle (27). Tulathromycin has a high affinity for uptake within bovine neutrophils (26). The antimicrobial properties of tulathromycin alone cannot fully explain its effectiveness in clearing the infection and inflammation associated with BRD, and recent observations support the hypothesis that the drug may promote the resolution of inflammation (28, 29) via mechanisms that are not fully understood.
The present study examined the effects of tulathromycin on lipid signaling in bovine neutrophils. Specifically, the effects on arachidonic acid signaling and the generation of proinflammatory and proresolving eicosanoids were assessed. The findings illustrate how the inhibition of PLA2, LTB4, and PGE2 synthesis and the concurrent promotion of LXA4 release may confer direct proresolution properties to an antibiotic, independent of its antimicrobial effects.
MATERIALS AND METHODS
Animals.
A first set of experiments assessed the effects of TUL on inflammation in vivo in the absence of confounding bacterial parameters, using zymosan. Healthy male Holstein calves (2 to 3 weeks old, 50 kg ± 5 kg) were used in all in vivo experiments. After 7 days of acclimation, the calves were randomly assigned to 1 of 3 groups: (i) control calves given 10 ml endotoxin-free Hanks' balanced salt solution (HBSS) vehicle with NaHCO3, without phenol red, calcium chloride, or magnesium sulfate (Sigma-Aldrich, Oakville, Ontario, Canada), (ii) calves challenged intratracheally with 50 mg of sonicated zymosan A particles from Saccharomyces cerevisiae (β-glucan of yeast cell wall; Sigma) in HBSS in combination with a subcutaneous (s.c.) injection of 25% propylene glycol vehicle, or (iii) calves challenged with zymosan in HBSS in combination with an s.c. injection of 2.5 mg/kg tulathromycin (Draxxin; Pfizer Animal Health, Kalamazoo, MI). The calves were housed at the University of Calgary's Veterinary Sciences Research Station (VSRS) (Calgary, Alberta, Canada), fed antibiotic-free milk replaced 2 times a day, and given access to water ad libitum. The care of the animals and experimental practices were conducted according to the standards of the Canadian Council on Animal Care and approved by the University of Calgary Life and Environmental Sciences Animal Care Committee.
Inflammatory challenge in vivo.
Following local lidocaine anesthesia (lidocaine HCl 2% and epinephrine injection; Bimeda-MTC, Animal Health, Inc.), a sterile trocar was inserted through a percutaneous incision into the trachea of each calf. A sterile 1.7-mm-diameter catheter was threaded through the trocar, with the catheter tip extending to the tracheal bifurcation, where zymosan or HBSS was injected into the lungs, as previously described (30). Rectal temperatures, respiratory rates, and heart rates were measured to ensure all calves were healthy prior to and throughout the experimental period.
Bronchoalveolar lavage fluid.
At 3 h and 24 h postchallenge, bronchoalveolar lavage (BAL) fluid samples were collected from the calves by 3 sequential washings with 20 ml HBSS, as previously described (30). CytoSpin cytocentrifuge (Thermo Scientific) preparations of BAL fluid were fixed with Diff-Quik stain (Baxter Healthcare Corp., Miami, FL) in phosphate-buffered saline (PBS) solution (pH 7.2) (0.15 M NaCl; Sigma). The infiltration of neutrophils was calculated for each sample as the percentage of neutrophils of the total leukocytes. The absence of bacteria in the BAL fluid samples of the experimental calves was verified by plating on Columbia blood agar. Aliquots of the BAL fluid supernatants were snap-frozen in liquid nitrogen and stored at −80°C until analysis.
Neutrophil purification.
Purified neutrophil preparations were obtained from peripheral whole blood samples drawn from the jugular veins of healthy blood donor calves into Vacutainers containing 1.5 ml Anticoagulant acid-citrate-dextrose (ACD solution A) (Becton, Dickinson Vacutainer Systems, Franklin Lakes, NJ). The blood was centrifuged at 1,200 × g for 20 min in a Beckman J-6B centrifuge (Beckman Instruments, Palo Alto, CA) at 4°C without braking. The plasma and buffy coat were removed, and contaminating erythrocytes were removed with 20 ml of cold filter-sterilized hypotonic lysis solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4) for 1 min. Isotonicity was restored with 10 ml of cold 3× hypertonic restoring solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4, 462 mM NaCl). The cell pellet was resuspended in warm (37°C) HBSS containing 10% heat-inactivated fetal bovine serum (HI-FBS) (Sigma). Neutrophil viability was assessed using trypan blue (0.1%) exclusion. Differential cell counts were performed on Cytospin preparations stained with Diff-Quik. The cell populations were >90% neutrophils and >90% viable for all experiments.
Reagents, inhibitors, and antibodies for in vitro studies.
For certain experiments, the cells were exposed to 1 μg/ml lipopolysaccharide (LPS) from Escherichia coli O111:B4 (Sigma), 3 μM calcium ionophore A23187 (Sigma), 1 μg/ml phorbol myristate acetate (PMA) (Sigma), or 1 μM arachidonic acid (Cayman Chemical Co., Ann Arbor, MI). Treatment with 1 μM staurosporine from Streptomyces sp. (Sigma) served as a positive proapoptotic control.
Leukotriene B4 assay.
LTB4 in BAL fluid or supernatants was measured at 405 nm using a competitive enzyme immunometric assay kit (Leukotriene B4 EIA kit; Cayman Chemical), according to the manufacturer's instructions. Colorimetric changes were measured using a SpectraMax M2e microplate reader (Molecular Devices).
Reverse-phase high-performance liquid chromatography for lipid detection.
Supernatants were collected from bovine neutrophils incubated with HBSS or tulathromycin in the presence or absence of 3 μM A23187. For reverse-phase high-performance liquid chromatography (RP-HPLC) analysis to detect lipids, ice-cold 95% ethanol was added to the cell supernatants at a ratio of 4:1 for protein precipitation. The samples were vortexed, incubated at −20°C for 5 min, and centrifuged at 3,000 × g for 10 min. The supernatants were collected and the ethanol was evaporated under a gentle stream of nitrogen, loaded onto a C18 column, and eluted using a gradient of 25% acetonitrile–0.1% trifluoroacetic acid (TFA)–H2O to 65% acetonitrile–0.1% TFA–H2O (flow rate of 1 ml/min). Elution of the LXA4 peak was determined by UV profiling of a commercial LXA4 standard (Cayman Chemicals).
Detection of phospholipase A2 activity.
The fluorescent probe acrylodan-labeled intestinal fatty acid binding protein (ADIFAB2) (FFA Sciences, San Diego, CA) was used to measure PLA2 activity. Neutrophils were resuspended in the ADIFAB2 measuring buffer (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM Na2HPO4 [pH 7.4]), treated with HBSS (control) or tulathromycin (2 mg/ml), and loaded into a black clear-bottom 96-well plate (0.2 ml/well) in the absence or presence of melittin (10 μM). ADIFAB2 was added to the wells at a final concentration of 1.0 μM. Readings were then taken every 5 min over a period of 1.0 h at 37°C. Fluorescence was measured using a SpectraMax M2e microplate reader (Molecular Devices) at an excitation wavelength of 386 nm and emission wavelengths of 432 and 505 nm, representing the unbound and bound states of ADIFAB, respectively. The data were expressed as the fluorescence intensity ratios measured at 505 and 432 nm (F505/F432).
Detection of phospholipase D activity.
Phospholipase D (PLD) activity was measured in bovine neutrophils using the Amplex Red phospholipase D assay kit (Molecular Probes). Briefly, bovine neutrophils were treated with HBSS (control) or 2 mg/ml tulathromycin in the absence or presence of 1 μg/ml phorbol myristate acetate (PMA) for 0.5 h. Following incubation, the cells were centrifuged, washed, and resuspended in a working solution of reaction buffer. The cells were lysed by sonication. The assay was carried out on lysate samples as per the manufacturer's instructions. Fluorescence was measured with a fluorescence microplate reader at an excitation at 530 nm and emission at 590 nm using a SpectraMax M2e microplate reader (Molecular Devices).
Detection of phospholipidosis.
The extent of accumulation of phospholipids in the neutrophils was determined using the HCS LipidTOX phospholipidosis and steatosis detection kit (Molecular Probes), as per the manufacturer's instructions. Briefly, bovine neutrophils were treated with HBSS (control) or 2 mg/ml tulathromycin for 0.5 h in the presence of the LipidTOX red phospholipid stain. Following treatment, the cells were centrifuged at 500 × g, washed with HBSS, and resuspended in 4% paraformaldehyde fixative solution containing a 1× Hoechst stain. The cells were incubated in the dark for 0.5 h at room temperature, washed two times with HBSS, and centrifuged for 10 min at 113 × g onto a microscope slide using a Shandon Cytospin 4 cytocentrifuge (Thermo Electron Corporation). Phospholipids appeared red (excitation at 535 nm, emission at 550 nm), and nuclei appeared blue (excitation at 350 nm, emission at 461 nm). Images were taken using a Leica DMR fluorescence microscope and a Retiga 2000x (Q Imaging) camera and were analyzed with Volocity (Improvision).
Statistical analysis.
The numeric values for statistical analysis are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using the Prism 5 software. Comparisons were made using one-way analysis of variance (ANOVA). Multicomparison post hoc analysis for parametric or nonparametric data was performed with Tukey's or Kruskal-Wallis tests. Statistical significance was established at a P value of <0.05.
RESULTS
Effects of tulathromycin in calves challenged intratracheally with zymosan.
To investigate the direct anti-inflammatory actions of tulathromycin in the absence of a bacterial stimulus, calves were challenged intratracheally with zymosan, a known Toll-like receptor 2 (TLR2) ligand that has been used as an inflammatory stimulus in vivo (31). There were no significant differences in the rectal temperatures between the groups at the time of zymosan challenge (0 h) or at 3 h and 24 h postchallenge (Table 1). Inoculation with zymosan significantly increased neutrophil numbers in BAL fluid samples 3 h postchallenge versus the controls; zymosan-induced neutrophil infiltration was not altered by tulathromycin treatment (Fig. 1A).
TABLE 1.
Rectal temperatures of control, sham-treated, and tulathromycin-treated calves challenged intratracheally with zymosan
| Group | Rectal temperature (mean ± SEM) (°C) at indicated time postchallengea: |
||
|---|---|---|---|
| 0 h | 3 h | 24 h | |
| Control | 39.1 ± 0.28 | 39.2 ± 0.36 | 39.1 ± 0.34 |
| Zymb | 38.9 ± 0.16 | 38.7 ± 0.39 | 39.0 ± 0.12 |
| Zym plus TULc | 38.6 ± 0.14 | 39.2 ± 0.28 | 38.7 ± 0.24 |
Rectal temperatures of each calf were taken at 0 h, 3 h, and 24 h after zymosan (50 mg) challenge as a measure of health status. Tulathromycin was administered s.c. at 2.5 mg/kg of body weight. The values were not significantly different between any of the groups. n = 5 to 7/group.
Zym, sham-treated calves.
Zym plus TUL, tulathromycin-treated calves challenged intratracheally with zymosan.
FIG 1.
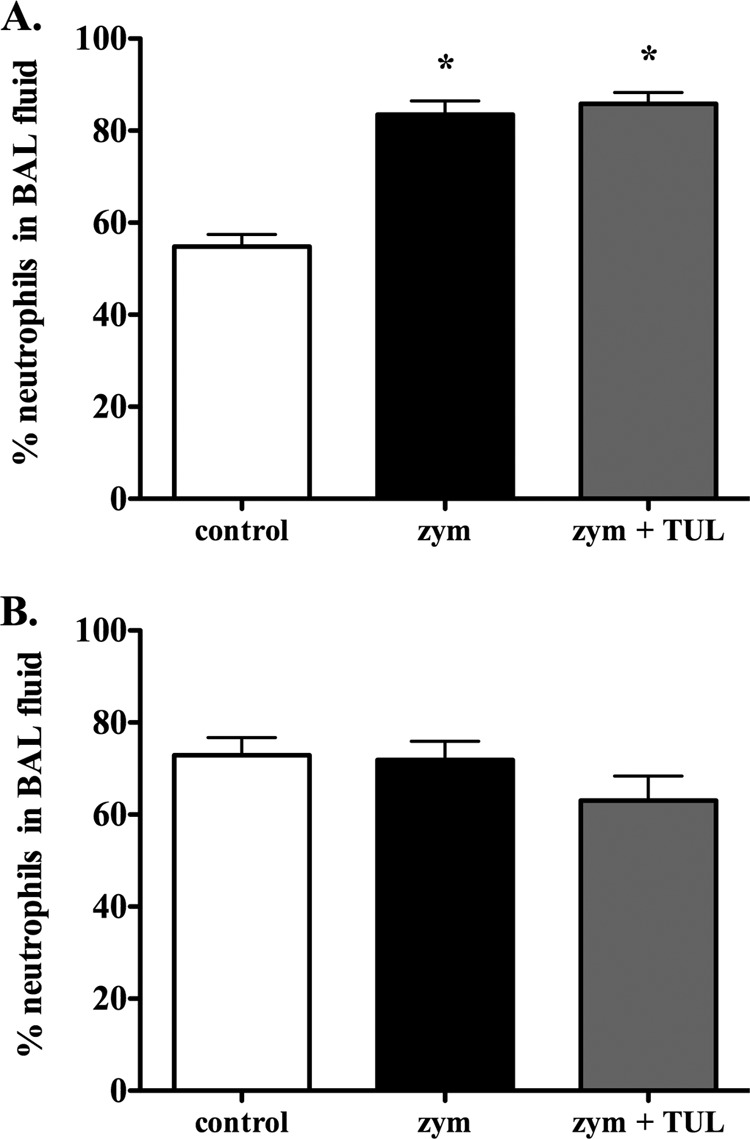
Tulathromycin does not alter the percentage of neutrophils recruited to the lower airways of zymosan-challenged calves. The percentages of neutrophils in the BAL fluid of control calves, sham-treated calves challenged with 50 mg zymosan (zym), and tulathromycin-treated (2.5 mg/kg s.c.) calves challenged with zymosan (zym + TUL) were enumerated at 3 h (A) and 24 h (B) using Diff-Quik staining and light microscopy. The values were not significantly different between any of the groups at 24 h postchallenge. Data are expressed as the group mean ± standard error of the mean (SEM) of the percent neutrophils of the total leukocyte population in the BAL fluid (n = 5 to 7/group). *, P < 0.05 versus the control group.
Tulathromycin treatment inhibits zymosan-induced LTB4 and PGE2 production.
Three hours postchallenge, BAL fluid LTB4 levels were significantly higher in zymosan-challenged sham-treated calves than in control calves (mean ± standard deviation, 209.9 ± 60.5 pg/ml and 51.1 ± 13.1 pg/ml, respectively); tulathromycin abolished this increase in BAL fluid LTB4 levels (63.9 ± 16.0 pg/ml) (Fig. 2A). At 24 h postchallenge, there were no significant differences in the levels of LTB4 between any of the groups (Fig. 2B).
FIG 2.
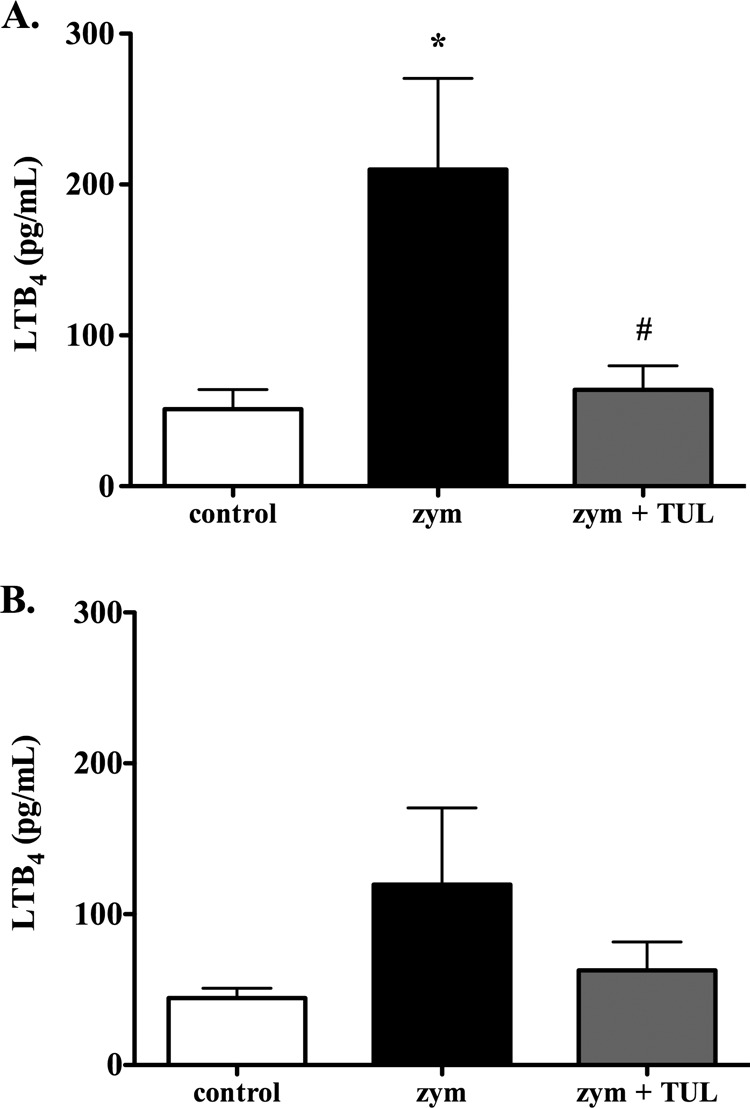
Tulathromycin reduces levels of leukotriene B4 in calves challenged intratracheally with zymosan. The figure shows the LTB4 levels in the BAL fluid samples isolated from control calves, sham-treated calves challenged with 50 mg zymosan (zym), and tulathromycin-treated (2.5 mg/kg s.c.) calves challenged with 50 mg zymosan (zym + TUL) 3 h (A) and 24 h (B) postchallenge. There was no significant difference between any of the groups at 24 h. The values are expressed as the mean ± SEM (n = 6 to 8/group). *, P < 0.05 versus the control group; #, P < 0.05 versus the zym group.
Twenty-four hours postchallenge, the BAL fluid levels of PGE2 were significantly higher in calves challenged with zymosan versus in the controls (594.6 ± 134.5 pg/ml and 142.5 ± 59.2 pg/ml, respectively); tulathromycin treatment inhibited the increase in BAL fluid PGE2 levels caused by zymosan (148.3 ± 34.7 pg/ml) (Fig. 3B). At 3 h postchallenge, the differences in the PGE2 levels between the groups failed to reach statistical significance (Fig. 3A).
FIG 3.
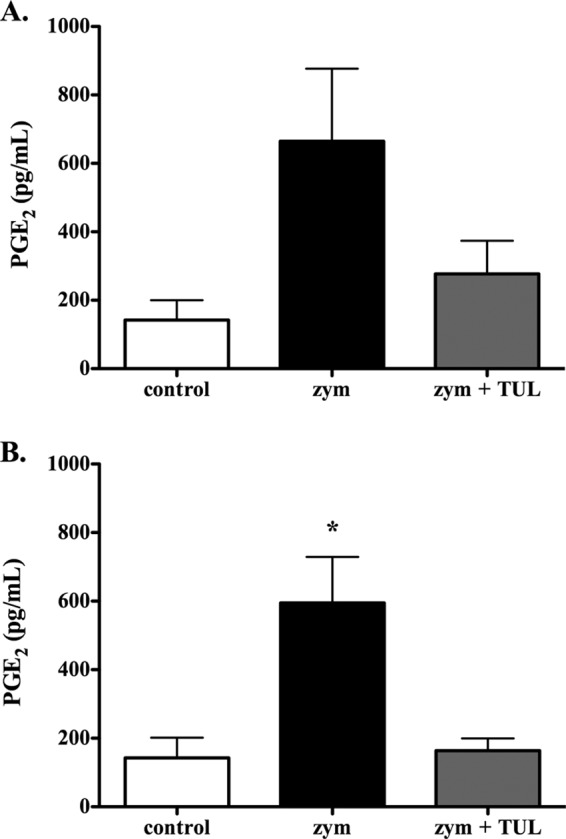
Tulathromycin reduces levels of prostaglandin E2 in calves challenged intratracheally with zymosan. The PGE2 levels in the BAL fluid samples isolated from control calves, sham-treated calves challenged with 50 mg zymosan (zym), and tulathromycin-treated calves challenged with 50 mg zymosan (zym + TUL) were measured at 3 h (A) and 24 h (B) postchallenge. There was no significant difference between any of the groups at 3 h postchallenge (P = 0.0543 between the control and zym groups). Tulathromycin was administered s.c. at 2.5 mg/kg. The values are the mean ± SEM (n = 5 to 7/group). *, P < 0.05 versus the control group.
Tulathromycin inhibits LTB4 secretion in bovine neutrophils, at least in part, via the inhibition of PLA2 activity.
The immunomodulating mechanisms of the TUL activities were further analyzed in bovine cells in vitro. Tulathromycin inhibited the release of free fatty acids in bovine neutrophils stimulated with 10 μM melittin, an activator of PLA2 (32) (Fig. 4). Furthermore, in both resting neutrophils and in those stimulated with 3 μM A23187, tulathromycin significantly reduced secreted levels of LTB4 (Fig. 5). The addition of 3 μM exogenous arachidonic acid partially restored the levels of LTB4 in tulathromycin-treated neutrophils (Fig. 5). The levels of PGE2 in resting neutrophils and in those stimulated with 1 μg/ml LPS or 3 μM A23187 were not detected in this in vitro system using enzyme-linked immunosorbent assay (ELISA) or reverse-phase HPLC methods (data not shown). The direct effects of A23187 on cellular uptake were not examined.
FIG 4.
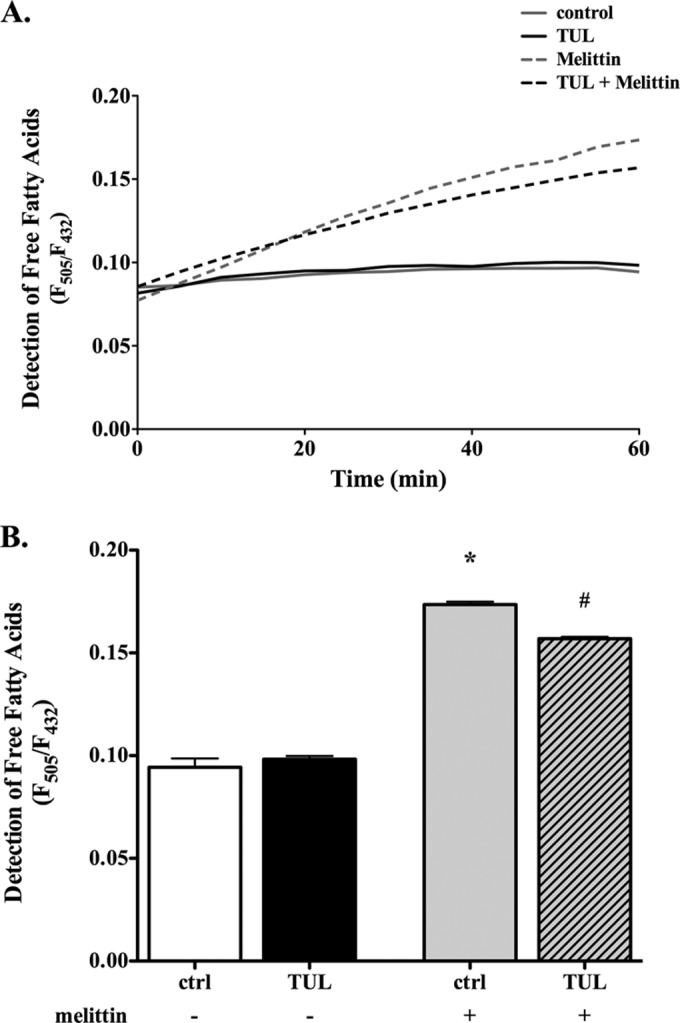
Tulathromycin reduces PLA2 activity in melittin-stimulated bovine neutrophils in vitro. (A) Fluorescent intensity ratio units (F) detecting free fatty acids released from neutrophils treated with HBSS (ctrl) or 2 mg/ml tulathromycin (TUL) in the absence (−) or presence (+) of 10 μM melittin (PLA2 activator) over 60 min at 37°C. The data are expressed as the ratio of fluorescence of the Acrylodan-labeled intestinal fatty acid binding protein (ADIFAB2) probe (1 μM) at 505 nm and 432 nm (F505/F432), which represent the bound and unbound states of ADIFAB2, respectively. (B) Detection of free fatty acids at 1.0 h. Values are the mean ± SEM (n = 3 to 6/group). *, P < 0.0001 versus unstimulated control; #, P < 0.001 versus melittin-stimulated control.
FIG 5.
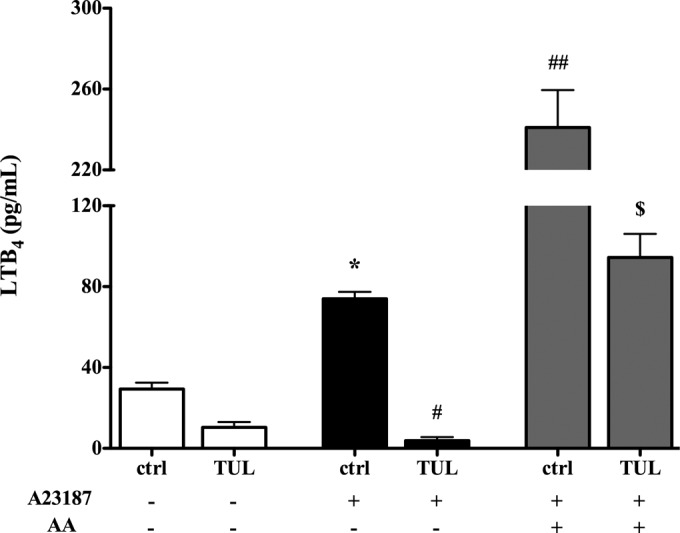
Tulathromycin inhibits LTB4 production in bovine neutrophils, at least in part, via inhibition of PLA2 activity in vitro. Secreted levels of LTB4 from bovine neutrophils treated with HBSS (ctrl) or 2 mg/ml tulathromycin (TUL) for 1.0 h at 37°C in the presence (+) or absence (−) of a 3 μM concentration of the calcium ionophore A23187 and 3 μM exogenous arachidonic acid (AA) are indicated. Values are the mean ± SEM (n = 3/group). *, P < 0.05 versus unstimulated control; #, P < 0.01, and ##, P < 0.001, versus A23187-stimulated control; $, P < 0.05 versus A23187- and AA-stimulated control.
Tulathromycin stimulates LXA4 production in bovine neutrophils.
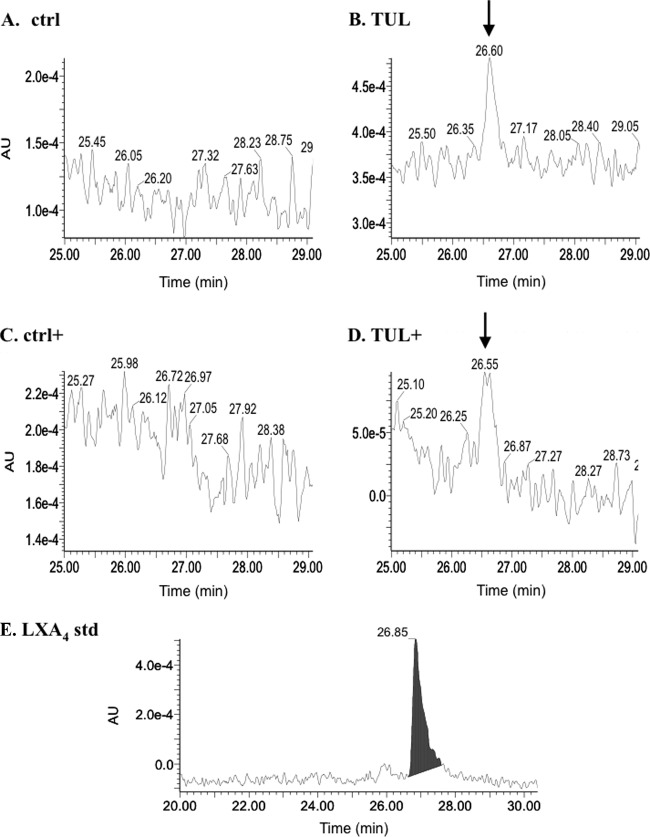
Using reverse-phase HPLC, we found that in the supernatants of tulathromycin-treated bovine neutrophils, there was a prominent lipid peak corresponding to LXA4, which was not observed in the supernatants from HBSS (control)-treated cells following 0.5 h of incubation (Fig. 6A). This peak was detected in both resting and A23187-activated neutrophils treated with tulathromycin (Fig. 6B). Furthermore, secreted levels of a precursor of LXA4, 15(S)-eicosatetraenoic acid [15(S)-HETE], were significantly reduced in tulathromycin-treated neutrophils with or without calcium ionophore activation versus the respective controls (Fig. 7).
FIG 6.
Tulathromycin stimulates the production of LXA4 in resting and activated bovine neutrophils in vitro. Shown are representative reverse-phage HPLC chromatograms of secreted lipids detected in the supernatants from HBSS (ctrl)-treated bovine neutrophils (A) and 2 mg/ml tulathromycin-treated neutrophils (B) after 0.5 h 37°C, LXA4 detected in neutrophils stimulated with 3 μM A23187 and treated with HBSS (ctrl+) (C), bovine neutrophils stimulated with 3 μM A23187 and treated with 2 mg/ml tulathromycin (D) for 0.5 h at 37°C, and a LXA4 standard (1 ng) (E). Following protein precipitation (95% ethyl alcohol [EtOH]), lipids were eluted from a C18 column using a gradient of 25% acetonitrile–0.1% TFA–H2O to 65% acetonitrile–0.1% TFA–H2O over 50 min. Lipoxin A4 (LXA4), which elutes between 26 and 27 min, was detected at 302 nm. The LXA4 peaks (arrows) were clearly detected in tulathromycin-treated cells. AU, absorbance units. Chromatograms are representative of 6 independent experiments.
FIG 7.
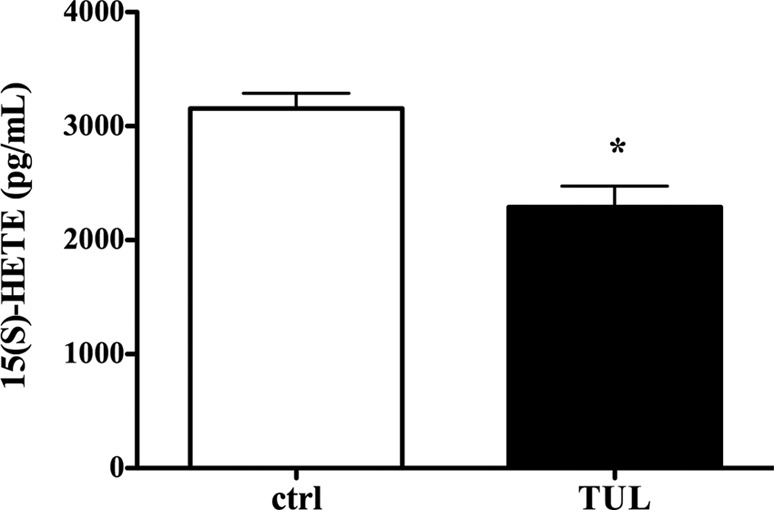
Tulathromycin reduces levels of 15(S)-HETE, a precursor of LXA4, in bovine neutrophils in vitro. Shown are the secreted levels of 15(S)-hydroxyeicosatetraenoic acid [15(S)-HETE] in resting bovine neutrophils treated with HBSS (ctrl) or 2 mg/ml tulathromycin (TUL) for 0.5 h at 37°C. The values are the mean ± SEM (n = 3/group). *, P < 0.05 versus control.
Tulathromycin reduces antiapoptotic PLD activity in bovine neutrophils.
In view of the inhibitory effects of tulathromycin on PLA2 activity, and since caspases can inhibit PLD during apoptosis (33), we investigated whether this drug affects PLD activity in bovine neutrophils. We observed a small but significant reduction in PLD activity in the cells treated with tulathromycin for 0.5 h in the presence or absence of 3 μg/ml phorbol myristate acetate (PMA) (Fig. 8).
FIG 8.
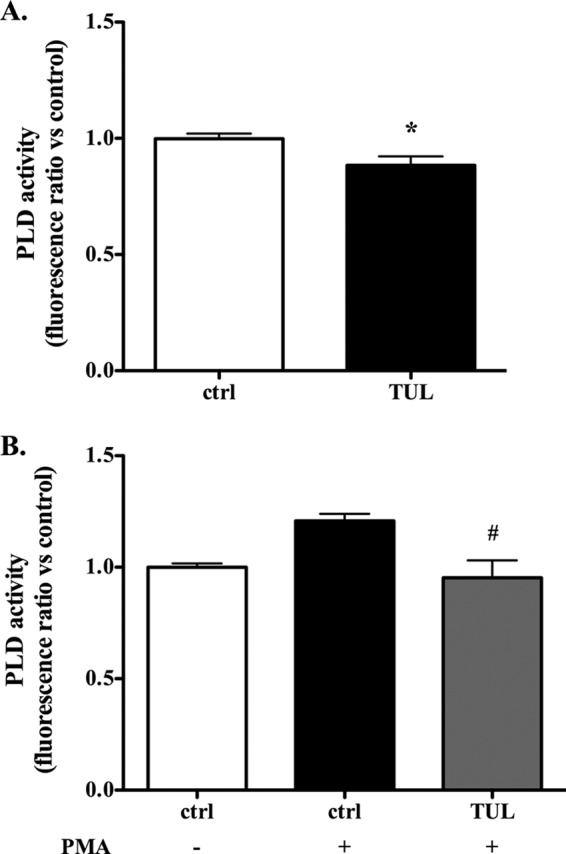
Tulathromycin reduces activity of antiapoptotic phospholipase D in bovine neutrophils in vitro. Shown is PLD activity in bovine neutrophils treated with HBSS (ctrl) or 2 mg/ml tulathromycin for 0.5 h at 37°C (A) and bovine neutrophils exposed to 1 μg/ml phorbol myristate acetate (PMA) and treated with HBSS or 2 mg/ml TUL for 0.5 h at 37°C (B). Exposure to PMA did not significantly increase PLD activity over that with the resting control cells. The data are represented as fluorescence ratios versus values measured in control neutrophils arbitrarily set to 1.0. Values are mean ± SEM (n = 3/group). *, P < 0.05 versus unstimulated control; #, P < 0.05 versus PMA-stimulated control.
Tulathromycin treatment causes an accumulation of intracellular phospholipids.
Some macrolides have been shown to cause intracellular accumulation of phospholipids, a phenomenon referred to as drug-induced phospholipidosis (24). Using a fluorescent Nile Red phospholipid stain, we observed an increase in intracellular phospholipids in bovine neutrophils treated with tulathromycin compared to in HBSS (control)-treated neutrophils following 0.5 h of incubation (Fig. 9).
FIG 9.
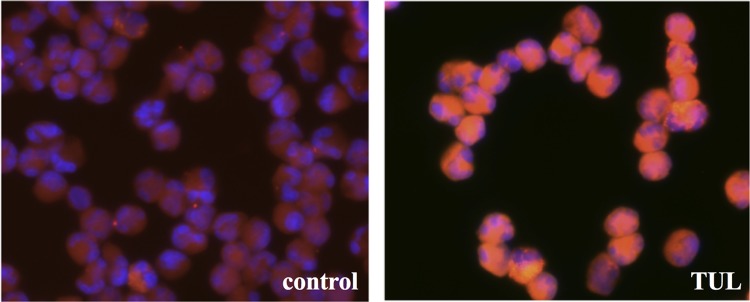
Tulathromycin causes an accumulation of intracellular phospholipids in bovine neutrophils. Shown is representative fluorescent staining of phospholipids in bovine neutrophils treated with HBSS (control) or 2 mg/ml tulathromycin (TUL) in the presence of the LipidTOX red phospholipid stain for 0.5 h at 37°C. Following incubation, cells were fixed with a 4% formaldehyde solution containing a 1× Hoechst nuclei stain (blue). Magnification, ×400.
DISCUSSION
The recruitment of neutrophils into the tissues represents a pivotal mechanism for innate immune protection against foreign pathogens and particulates (34). When neutrophil responses are not resolved, inflammatory tissue destruction ensues, as illustrated in a variety of inflammatory diseases, including pneumonia, arthritis, and inflammatory bowel diseases. We hypothesized that at least part of the recently demonstrated proresolving properties of the antibiotic tulathromycin represents a direct immunomodulating effect of the drug, independent of its antimicrobial effects, and it involves alterations in phospholipase-dependent lipid signaling. Using a clinically relevant model in calves, the data indicate that tulathromycin directly inhibits zymosan-induced production of bronchoalveolar LTB4 and PGE2. Mechanistic studies using bovine neutrophils in vitro established that the LTB4 modulation seen in vivo was indeed reproducible in vitro and was associated with an inhibition of melittin-induced PLA2 activation. Tulathromycin also inhibited calcium-induced LTB4 production, an effect that was partially restored by adding exogenous arachidonic acid. Concurrent with its inhibitory effects on LTB4, tulathromycin stimulated the production of anti-inflammatory LXA4 in neutrophils and reduced the cellular contents of its precursor 15(S)-HETE. Moreover, tulathromycin also inhibited PLD. Finally, the effects of the drug were associated with neutrophil phospholipidosis.
Leukotriene B4 is a potent proinflammatory arachidonic acid metabolite synthesized via the 5-LO pathway. It serves as a strong chemoattractant and activator of neutrophils, and it stimulates the release of neutrophil elastase and the generation of superoxide radicals (35). As a result, it is often used as a biomarker for severe inflammatory disease. The reduction in pulmonary LTB4 levels induced by tulathromycin in zymosan-challenged calves suggests direct anti-inflammatory effects of an antibiotic in vivo that are likely independent of its antimicrobial properties. Extending the length of the study may help illustrate whether this effect indeed leads to reduced neutrophil accumulation in the lungs of treated animals. In neutrophils, type IV cytosolic PLA2 (cPLA2) has been identified as the predominant isoform responsible for the mobilization of arachidonic acid and generation of LTs and PGs. This supports our observations in vitro that tulathromycin inhibits PLA2 activity and that levels of LTB4 in tulathromycin-treated neutrophils are partially restored in the presence of exogenous arachidonic acid (36, 37). Interestingly, LTB4 also has an antiapoptotic effect in neutrophils (38). Future studies will assess whether the proapoptotic effects and modulation of lipid signaling by tulathromycin are linked.
PGE2 has both pro- and anti-inflammatory actions that are tissue dependent. This cyclooxygenase (COX)-derived eicosanoid enhances or inhibits important leukocyte functions, including chemotaxis, aggregation, superoxide generation, lysosomal enzyme release, and LTB4 synthesis (39, 40). Although PGE2 exerts beneficial functions in the lung (41), there is evidence to suggest that PGE2 can dampen antimicrobial responses and heighten the risk of secondary infections. Indeed, PGE2 produced from alveolar macrophages following the ingestion of apoptotic neutrophils suppressed host defenses against a subsequent bacterial infection (42). In view of its multifaceted roles in airway inflammation, future studies will examine the impact of tulathromycin on PGE2 production and the consequence of this effect on airway inflammation. Using the experimental protocol described here, zymosan was found to significantly increase PGE2 production 24 h postchallenge, consistent with previous studies using other models in vivo (43–45) or in vitro (46), an effect that was blocked by tulathromycin. Another immunomodulating antibiotic for cattle and pigs, tilmicosin, also blocks PGE2 production in LPS-stimulated bovine alveolar macrophages via a decrease in COX-2 expression and/or partial inhibition of secretory phospholipase A2 activity (47, 48). Given that the restoration in LTB4 levels in tulathromycin-treated neutrophils exposed to exogenous arachidonic acid was only partial, other inhibitory mechanisms may be involved. One hypothesis that has yet to be explored is that treatment with tulathromycin may reduce expression or activity of 5-LO, COX-2, or LTA4 hydrolase.
In addition to proinflammatory eicosanoids, the arachidonic acid pathway is responsible for the synthesis of lipid mediators that play a pertinent role in the resolution of inflammation. Lipoxins (LXs) are part of a new class of lipid mediators that have gained a significant amount of attention for their potent anti-inflammatory and proresolving actions (13). Indeed, LXs, resolvins, and cyclopentenones have been shown to promote macrophage efferocytosis of apoptotic neutrophils, inhibit the production of proinflammatory mediators, and stop inflammatory cell infiltration (13), all effects which we showed were induced by tulathromycin via unclear mechanisms (28, 29). Using reverse-phase HPLC methods, we discovered that resting and activated neutrophils secrete LXA4 upon tulathromycin treatment. These observations demonstrate, for the first time, that a macrolide antibiotic is capable of stimulating the production of a potent proresolving lipid mediator. A recent study demonstrated that in an acute self-resolving model of pleurisy, different isoforms of PLA2 are responsible for the production of either proinflammatory or proresolving arachidonic acid-derived lipid mediators (49). Indeed, they demonstrated that type IIa and V secretory PLA2 were maximally expressed within inflammatory cells during the resolution phase and were responsible for the production of LXA4 (49). The effects of tulathromycin on the activity of various PLA2 isoforms have yet to be investigated. Traditionally, the synthesis of LXs was thought to involve the interaction between two distinct cell types (13); however, it was recently demonstrated that mediators, such as PGE2, increase the expression of 15-LO in neutrophils, allowing these cells to synthesize LXs on their own (50), which is consistent with increased LXA4 production by purified neutrophil populations. Our findings also indicate that tulathromycin drives the synthesis of LXA4, thereby lowering the total levels of 15(S)-HETE that are secreted and presented within the cell. Little is known about the actions of 15(S)-HETE itself; however, there is evidence to suggest that it exerts both anti- and proinflammatory actions (51–53).
Phospholipase D has a variety of roles in inflammation and cell survival. In addition to its physiological functions (membrane trafficking), PLD is involved in microbial killing (54), neutrophil degranulation (55), and migration in leukocytes (56). In neutrophils, PLD also has an antiapoptotic role (33). Moreover, activated caspases were shown to reduce the expression and activity of PLD in human neutrophils (33). In view of our previous observations that tulathromycin was able to promote neutrophil apoptosis (28), we investigated the effect of tulathromycin on PLD activity. Similar to its effects on PLA2 activity, tulathromycin reduced the activity of PLD in resting neutrophils and in those stimulated with phorbol myristate acetate (PMA). In contrast with its effects on human neutrophils, PMA stimulation did not significantly increase PLD activity in bovine neutrophils. This is consistent with the previously observed species-dependent effects of PMA (57, 58). Having reported that tulathromycin activates caspase-3 in bovine neutrophils, future studies should elucidate whether the effect of tulathromycin on PLD activity is caspase dependent or whether it occurs via a separate mechanism. Interestingly, other erythromycin-derived macrolides, such as roxithromycin, have been shown to activate PLD in nonstimulated neutrophils and inhibit PLD activity in PMA-stimulated human neutrophils (59). LXA4 also inhibits PLD activity (60), which may be another indirect mechanism through which tulathromycin treatment reduces PLD activity in bovine neutrophils. In the context of an M. haemolytica infection, the inhibition of PLD by tulathromycin may offer beneficial therapeutic effects in view of its stimulatory effects on PLA2-mediated LTB4 production in neutrophils (61).
Macrolides have a unique ability to accumulate within phagocytes (62) and, consequently, inhibit phospholipase activity (24, 63). Consistent with the present findings, reports have suggested that the anti-inflammatory actions of macrolides, such as azithromycin, were positively correlated with their intracellular concentrations and degree of phospholipidosis (24, 64). Furthermore, drug-induced phospholipidosis has resulted in caspase-3-mediated cell death (65). Based on its cationic lipophilic structure, we hypothesized that tulathromycin accumulates within the lysosomes, causing phospholipidosis in bovine neutrophils, which in turn may contribute to its immunomodulatory and anti-inflammatory actions, including apoptosis. Indeed, our findings reveal that tulathromycin appears to increase intracellular accumulation of phospholipids in bovine neutrophils.
The present observations offer new opportunities to investigate the mechanisms by which macrolides deliver anti-inflammatory benefits in a target species. In summary, in zymosan-challenged calves, tulathromycin treatment inhibits the production of leukotriene B4 (LTB4) and prostaglandin E2 (PGE2). In bovine neutrophils, tulathromycin inhibits PLD and cytosolic PLA2 activity, with cytosolic PLA2 activity contributing to the inhibitory effects on LTB4 and possibly PGE2 synthesis. Furthermore, tulathromycin induces phospholipidosis in these cells. Lastly, treatment with tulathromycin in bovine neutrophils stimulated the production of the potent proresolving lipid mediator LXA4. Together, the findings illustrate new mechanisms by which a macrolide may offer direct anti-inflammatory and proresolving actions, likely independent of its antimicrobial properties. Challenging calves with zymosan proved to be a powerful model for investigating the direct anti-inflammatory effects of tulathromycin or any macrolide. Future studies may also include tulathromycin-resistant bacterial strains to further characterize the clinically relevant immunomodulating actions of tulathromycin.
ACKNOWLEDGMENTS
We thank members of the Buret laboratory and Inflammation Research Network, along with the veterinarians and staff at the Veterinary Sciences Research Station at the University of Calgary for all of their assistance and guidance with this project.
This research was supported by the National Sciences and Engineering Research Council of Canada, Pfizer Animal Health, and the Margaret Gunn Endowment for Animal Research, University of Calgary.
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Kaley G, Hintze TH, Panzenbeck M, Messina EJ. 1985. Role of prostaglandins in microcirculatory function. Adv. Prostaglandin Thromboxane Leukot. Res. 13:27–35 [PubMed] [Google Scholar]
- 2.Williams TJ, Morley J. 1973. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature 246:215–217. 10.1038/246215a0 [DOI] [PubMed] [Google Scholar]
- 3.Caristi S, Piraino G, Cucinotta M, Valenti A, Loddo S, Teti D. 2005. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J. Biol. Chem. 280:14433–14442. 10.1074/jbc.M410725200 [DOI] [PubMed] [Google Scholar]
- 4.Tavakoli S, Cowan MJ, Benfield T, Logun C, Shelhamer JH. 2001. Prostaglandin E(2)-induced interleukin-6 release by a human airway epithelial cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L127–L133 [DOI] [PubMed] [Google Scholar]
- 5.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. 2003. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 4:982–990. 10.1038/ni970 [DOI] [PubMed] [Google Scholar]
- 6.Miyahara N, Ohnishi H, Matsuda H, Miyahara S, Takeda K, Koya T, Matsubara S, Okamoto M, Dakhama A, Haribabu B, Gelfand EW. 2008. Leukotriene B4 receptor 1 expression on dendritic cells is required for the development of Th2 responses and allergen-induced airway hyperresponsiveness. J. Immunol. 181:1170–1178. 10.4049/jimmunol.181.2.1170 [DOI] [PubMed] [Google Scholar]
- 7.Marom Z, Shelhamer JH, Bach MK, Morton DR, Kaliner M. 1982. Slow-reacting substances, leukotrienes C4 and D4, increase the release of mucus from human airways in vitro. Am. Rev. Respir. Dis. 126:449–451 [DOI] [PubMed] [Google Scholar]
- 8.Canetti C, Silva JS, Ferreira SH, Cunha FQ. 2001. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br. J. Pharmacol. 134:1619–1628. 10.1038/sj.bjp.0704403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crooks SW, Stockley RA. 1998. Leukotriene B4. Int. J. Biochem. Cell Biol. 30:173–178. 10.1016/S1357-2725(97)00123-4 [DOI] [PubMed] [Google Scholar]
- 10.Lewis RA, Austen KF, Soberman RJ. 1990. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 323:645–655 [DOI] [PubMed] [Google Scholar]
- 11.Hopkins H, Stull T, Von Essen SG, Robbins RA, Rennard SI. 1989. Neutrophil chemotactic factors in bacterial pneumonia. Chest 95:1021–1027. 10.1378/chest.95.5.1021 [DOI] [PubMed] [Google Scholar]
- 12.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. 2014. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14:166–180. 10.1038/nri3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. 2011. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 11:629–647. 10.2174/1568026611109060629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteley LO, Maheswaran SK, Weiss DJ, Ames TR, Kannan MS. 1992. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J. Vet. Intern. Med. 6:11–22. 10.1111/j.1939-1676.1992.tb00980.x [DOI] [PubMed] [Google Scholar]
- 15.Hsuan SL, Kannan MS, Jeyaseelan S, Prakash YS, Malazdrewich C, Abrahamsen MS, Sieck GC, Maheswaran SK. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Microb. Pathog. 26:263–273. 10.1006/mpat.1998.0271 [DOI] [PubMed] [Google Scholar]
- 16.Buret AG. 2010. Immuno-modulation and anti-inflammatory benefits of antibiotics: the example of tilmicosin. Can. J. Vet. Res. 74:1–10 [PMC free article] [PubMed] [Google Scholar]
- 17.Good JT, Jr, Rollins DR, Martin RJ. 2012. Macrolides in the treatment of asthma. Curr. Opin. Pulm. Med. 18:76–84. 10.1097/MCP.0b013e32834daff8 [DOI] [PubMed] [Google Scholar]
- 18.McArdle JR, Talwalkar JS. 2007. Macrolides in cystic fibrosis. Clin. Chest Med. 28:347–360. 10.1016/j.ccm.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Falcó V, Sánchez A, Pahissa A, Rello J. 2011. Emerging drugs for pneumococcal pneumonia. Expert Opin. Emerg. Drugs 16:459–477. 10.1517/14728214.2011.576669 [DOI] [PubMed] [Google Scholar]
- 20.Frank MO, Sullivan GW, Carper HT, Mandell GL. 1992. In vitro demonstration of transport and delivery of antibiotics by polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 36:2584–2588. 10.1128/AAC.36.12.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladue RP, Bright GM, Isaacson RE, Newborg MF. 1989. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277–282. 10.1128/AAC.33.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson N, Borlak J. 2006. Drug-induced phospholipidosis. FEBS Lett. 580:5533–5540. 10.1016/j.febslet.2006.08.061 [DOI] [PubMed] [Google Scholar]
- 23.Van Bambeke F, Gerbaux C, Michot JM, d'Yvoire MB, Montenez JP, Tulkens PM. 1998. Lysosomal alterations induced in cultured rat fibroblasts by long-term exposure to low concentrations of azithromycin. J. Antimicrob. Chemother. 42:761–767. 10.1093/jac/42.6.761 [DOI] [PubMed] [Google Scholar]
- 24.Munic V, Banjanac M, Koštrun S, Nujić K, Bosnar M, Marjanović N, Ralić J, Matijašić M, Hlevnjak M, Eraković Haber V. 2011. Intensity of macrolide anti-inflammatory activity in J774A.1 cells positively correlates with cellular accumulation and phospholipidosis. Pharmacol. Res. 64:298–307. 10.1016/j.phrs.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 25.Montenez JP, Van Bambeke F, Piret J, Brasseur R, Tulkens PM, Mingeot-Leclercq MP. 1999. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol. Appl. Pharmacol. 156:129–140. 10.1006/taap.1999.8632 [DOI] [PubMed] [Google Scholar]
- 26.Evans NA. 2005. Tulathromycin: an overview of a new triamilide antibiotic for livestock respiratory disease. Vet. Ther. 6:83–95 [PubMed] [Google Scholar]
- 27.Rooney KA, Nutsch RG, Skogerboe TL, Weigel DJ, Gajewski K, Kilgore WR. 2005. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet. Ther. 6:154–166 [PubMed] [Google Scholar]
- 28.Fischer CD, Beatty JK, Zvaigzne CG, Morck DW, Lucas MJ, Buret AG. 2011. Anti-inflammatory benefits of antibiotic-induced neutrophil apoptosis: tulathromycin induces caspase-3-dependent neutrophil programmed cell death and inhibits NF-kappaB signaling and CXCL8 transcription. Antimicrob. Agents Chemother. 55:338–348. 10.1128/AAC.01052-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer CD, Beatty JK, Duquette SC, Morck DW, Lucas MJ, Buret AG. 2013. Direct and indirect anti-inflammatory effects of tulathromycin in bovine macrophages: inhibition of CXCL-8 secretion, induction of apoptosis, and promotion of efferocytosis. Antimicrob. Agents Chemother. 57:1385–1393. 10.1128/AAC.01598-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin AC, Morck DW, Merrill JK, Ceri H, Olson ME, Read RR, Dick P, Buret AG. 1998. Anti-inflammatory benefits of tilmicosin in calves with Pasteurella haemolytica-infected lungs. Am. J. Vet. Res. 59:765–771 [PubMed] [Google Scholar]
- 31.Takeuchi K, Umeki Y, Matsumoto N, Yamamoto K, Yoshida M, Suzuki K, Aratani Y. 2012. Severe neutrophil-mediated lung inflammation in myeloperoxidase-deficient mice exposed to zymosan. Inflamm. Res. 61:197–205. 10.1007/s00011-011-0401-y [DOI] [PubMed] [Google Scholar]
- 32.Mollay C, Kreil G. 1974. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. 46:141–144. 10.1016/0014-5793(74)80354-6 [DOI] [PubMed] [Google Scholar]
- 33.Lee SY, Oh JY, Lee MJ, Jang MJ, Park HY, Kim JW, Min DS, Park YM, Chang YC, Bae YS, Kwak JY. 2004. Anti-apoptotic mechanism and reduced expression of phospholipase D in spontaneous and Fas-stimulated apoptosis of human neutrophils. Eur. J. Immunol. 34:2760–2770. 10.1002/eji.200425117 [DOI] [PubMed] [Google Scholar]
- 34.Doerschuk CM, Tasaka S, Wang Q. 2000. CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am. J. Respir. Cell Mol. Biol. 23:133–136. 10.1165/ajrcmb.23.2.f193 [DOI] [PubMed] [Google Scholar]
- 35.Claesson HE, Odlander B, Jakobsson PJ. 1992. Leukotriene B4 in the immune system. Int. J. Immunopharmacol. 14:441–449. 10.1016/0192-0561(92)90174-J [DOI] [PubMed] [Google Scholar]
- 36.Marshall J, Krump E, Lindsay T, Downey G, Ford DA, Zhu P, Walker P, Rubin B. 2000. Involvement of cytosolic phospholipase A2 and secretory phospholipase A2 in arachidonic acid release from human neutrophils. J. Immunol. 164:2084–2091. 10.4049/jimmunol.164.4.2084 [DOI] [PubMed] [Google Scholar]
- 37.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. 2002. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and Gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 277:5061–5073. 10.1074/jbc.M109083200 [DOI] [PubMed] [Google Scholar]
- 38.Petrin D, Turcotte S, Gilbert AK, Rola-Pleszczynski M, Stankova J. 2006. The anti-apoptotic effect of leukotriene B4 in neutrophils: a role for phosphatidylinositol 3-kinase, extracellular signal-regulated kinase and Mcl-1. Cell. Signal. 18:479–487. 10.1016/j.cellsig.2005.05.021 [DOI] [PubMed] [Google Scholar]
- 39.O'Flaherty JT, Kreutzer DL, Ward PA. 1979. Effect of prostaglandins E1, E2 and F2alpha on neutrophil aggregation. Prostaglandins 17:201–210 [DOI] [PubMed] [Google Scholar]
- 40.Pouliot M, Fiset ME, Massé M, Naccache PH, Borgeat P. 2002. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J. Immunol. 169:5279–5286. 10.4049/jimmunol.169.9.5279 [DOI] [PubMed] [Google Scholar]
- 41.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. 2004. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 25:40–46. 10.1016/j.it.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 42.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. 2009. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J. Exp. Med. 206:61–68. 10.1084/jem.20082058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hung ND, Kim MR, Sok DE. 2011. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation 34:147–160. 10.1007/s10753-010-9218-z [DOI] [PubMed] [Google Scholar]
- 44.Griffiths RJ, Li SW, Wood BE, Blackham A. 1991. A comparison of the anti-inflammatory activity of selective 5-lipoxygenase inhibitors with dexamethasone and colchicine in a model of zymosan induced inflammation in the rat knee joint and peritoneal cavity. Agents Actions 32:312–320. 10.1007/BF01980892 [DOI] [PubMed] [Google Scholar]
- 45.Leypoldt JK, Kamerath CD, Gilson JF. 2007. Acute peritonitis in a C57BL/6 mouse model of peritoneal dialysis. Adv. Perit. Dial. 23:66–70 [PubMed] [Google Scholar]
- 46.Tolone G, Bonasera L, Brai M, Tolone C. 1977. Prostaglandin production by human polymorphonuclear leucocytes during phagocytosis in vitro. Experientia 33:961–962. 10.1007/BF01951305 [DOI] [PubMed] [Google Scholar]
- 47.Lakritz J, Tyler JW, Marsh AE, Romesburg-Cockrell M, Smith K, Holle JM. 2002. Tilmicosin reduces lipopolysaccharide-stimulated bovine alveolar macrophage prostaglandin E(2) production via a mechanism involving phospholipases. Vet. Ther. 3:7–21 [PubMed] [Google Scholar]
- 48.Cao XY, Dong M, Shen JZ, Wu BB, Wu CM, Du XD, Wang Z, Qi YT, Li BY. 2006. Tilmicosin and tylosin have anti-inflammatory properties via modulation of COX-2 and iNOS gene expression and production of cytokines in LPS-induced macrophages and monocytes. Int. J. Antimicrob. Agents 27:431–438. 10.1016/j.ijantimicag.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 49.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. 2004. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 18:489–498. 10.1096/fj.03-0837com [DOI] [PubMed] [Google Scholar]
- 50.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. 2001. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2:612–619. 10.1038/89759 [DOI] [PubMed] [Google Scholar]
- 51.Takata S, Papayianni A, Matsubara M, Jimenez W, Pronovost PH, Brady HR. 1994. 15-Hydroxyeicosatetraenoic acid inhibits neutrophil migration across cytokine-activated endothelium. Am. J. Pathol. 145:541–549 [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RJ, Justen JM, Nidy EG, Sam LM, Bleasdale JE. 1993. Transmembrane signaling in human polymorphonuclear neutrophils: 15(S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid modulates receptor agonist-triggered cell activation. Proc. Natl. Acad. Sci. U. S. A. 90:7270–7274. 10.1073/pnas.90.15.7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson HG, McNee ML, Sun FF. 1985. 15-Hydroxyeicosatetraenoic acid is a potent inflammatory mediator and agonist of canine tracheal mucus secretion. Am. Rev. Respir. Dis. 131:917–922 [DOI] [PubMed] [Google Scholar]
- 54.Melendez AJ, Bruetschy L, Floto RA, Harnett MM, Allen JM. 2001. Functional coupling of FcgammaRI to nicotinamide adenine dinucleotide phosphate (reduced form) oxidative burst and immune complex trafficking requires the activation of phospholipase D1. Blood 98:3421–3428. 10.1182/blood.V98.12.3421 [DOI] [PubMed] [Google Scholar]
- 55.Hitomi T, Zhang J, Nicoletti LM, Grodzki AC, Jamur MC, Oliver C, Siraganian RP. 2004. Phospholipase D1 regulates high-affinity IgE receptor-induced mast cell degranulation. Blood 104:4122–4128. 10.1182/blood-2004-06-2091 [DOI] [PubMed] [Google Scholar]
- 56.Lehman N, Di Fulvio M, McCray N, Campos I, Tabatabaian F, Gomez-Cambronero J. 2006. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood 108:3564–3572. 10.1182/blood-2006-02-005959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown GB, Roth JA. 1991. Comparison of the response of bovine and human neutrophils to various stimuli. Vet. Immunol. Immunopathol. 28:201–218. 10.1016/0165-2427(91)90115-S [DOI] [PubMed] [Google Scholar]
- 58.Ahmed M, Kimura K, Soliman M, Yamaji D, Okamatsu-Ogura Y, Makondo K, Inanami O, Saito M. 2007. Effects of leptin and tumor necrosis factor-alpha on degranulation and superoxide production of polymorphonuclear neutrophils from Holstein cows. J. Vet. Med. Sci. 69:125–131. 10.1292/jvms.69.125 [DOI] [PubMed] [Google Scholar]
- 59.Abdelghaffar H, Vazifeh D, Labro MT. 1997. Erythromycin A-derived macrolides modify the functional activities of human neutrophils by altering the phospholipase d-phosphatidate phosphohydrolase transduction pathway: l-cladinose is involved both in alterations of neutrophil functions and modulation of this transductional pathway. J. Immunol. 159:3995–4005 [PubMed] [Google Scholar]
- 60.Levy BD, Hickey L, Morris AJ, Larvie M, Keledjian R, Petasis NA, Bannenberg G, Serhan CN. 2005. Novel polyisoprenyl phosphates block phospholipase D and human neutrophil activation in vitro and murine peritoneal inflammation in vivo. Br. J. Pharmacol. 146:344–351. 10.1038/sj.bjp.0706338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Clarke CR, Clinkenbeard KD. 1999. Role of phospholipase D in Pasteurella haemolytica leukotoxin-induced increase in phospholipase A(2) activity in bovine neutrophils. Infect. Immun. 67:3768–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bosnar M, Kelnerić Z, Munić V, Eraković V, Parnham MJ. 2005. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 49:2372–2377. 10.1128/AAC.49.6.2372-2377.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Bambeke F, Montenez JP, Piret J, Tulkens PM, Courtoy PJ, Mingeot-Leclercq MP. 1996. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. Eur. J. Pharmacol. 314:203–214 [DOI] [PubMed] [Google Scholar]
- 64.Banjanac M, Munić Kos V, Nujić K, Vrančić M, Belamarić D, Crnković S, Hlevnjak M, Eraković Haber V. 2012. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 66:357–362. 10.1016/j.phrs.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 65.Piccotti JR, LaGattuta MS, Knight SA, Gonzales AJ, Bleavins MR. 2005. Induction of apoptosis by cationic amphiphilic drugs amiodarone and imipramine. Drug Chem. Toxicol. 28:117–133. 10.1081/DCT-39743 [DOI] [PubMed] [Google Scholar]