Abstract
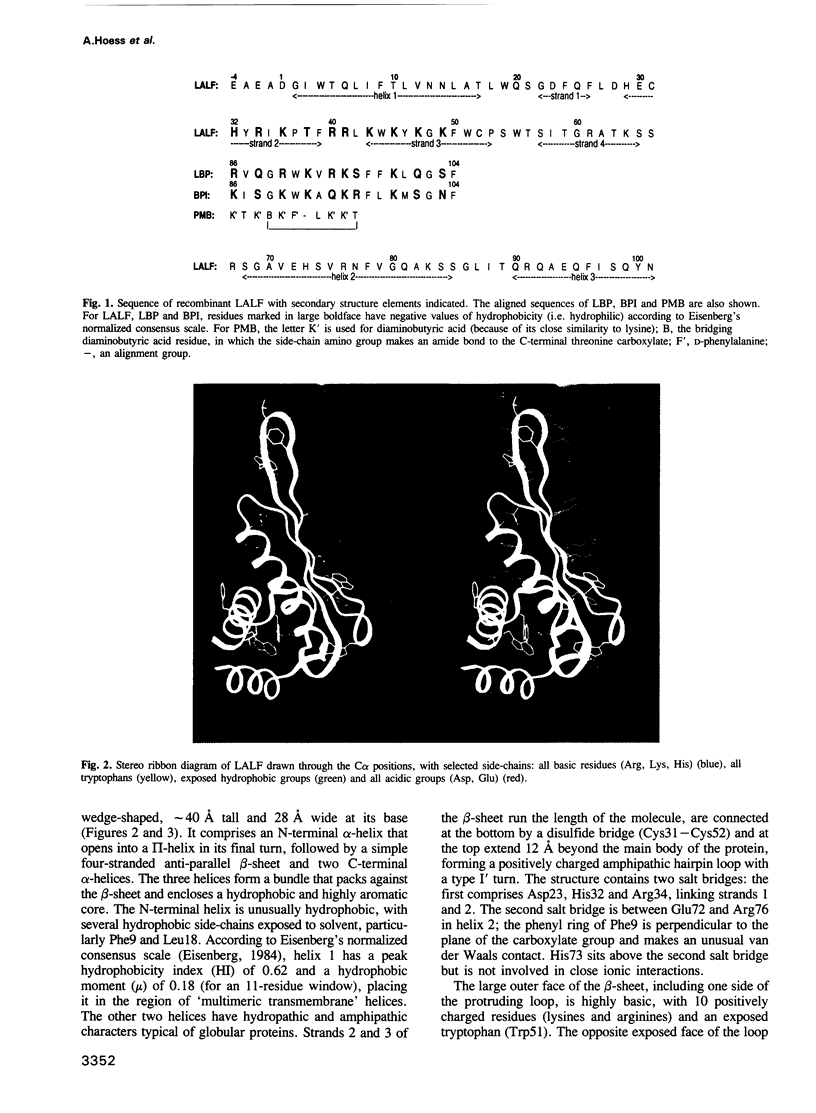
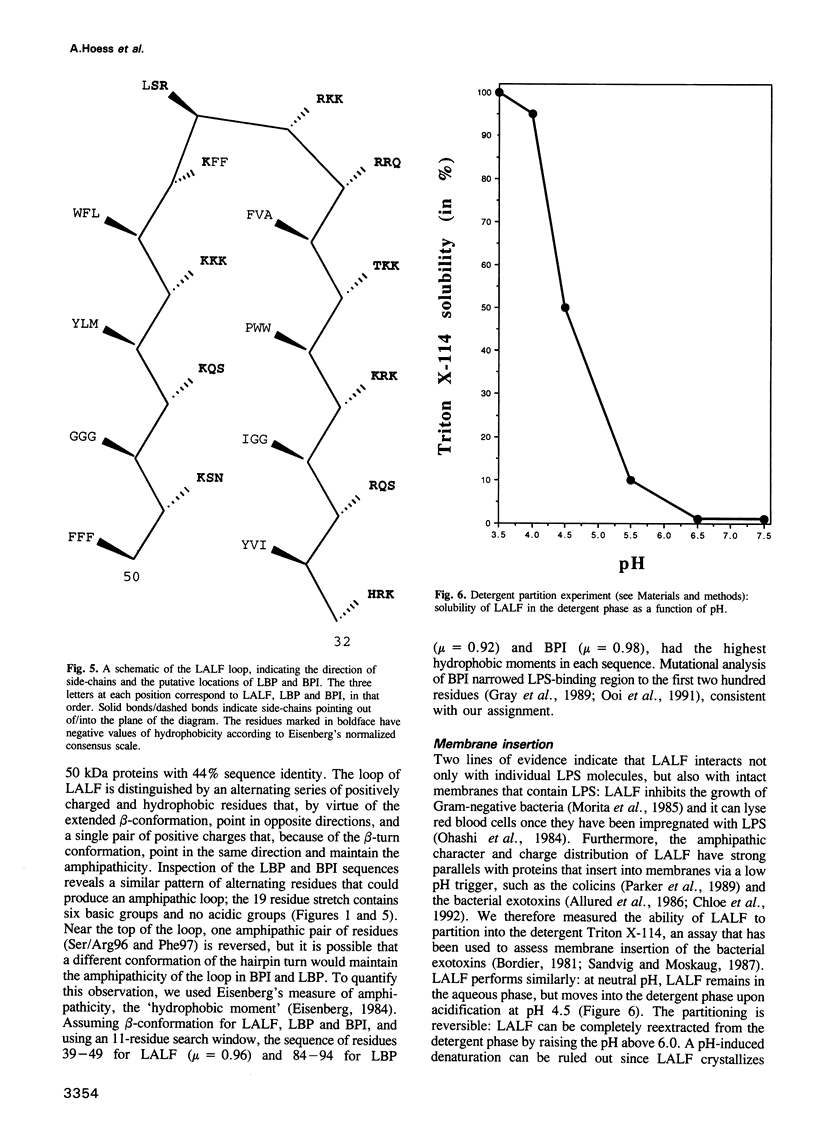
Lipopolysaccharide (LPS), or endotoxin, is the major mediator of septic shock, a serious complication of Gram-negative bacterial infections in humans. Molecules that bind LPS and neutralize its biological effects or enhance its clearance could have important clinical applications. Limulus anti-LPS factor (LALF) binds LPS tightly, and, in animal models, reduces mortality when administered before or after LPS challenge or bacterial infection. Here we present the high resolution structure of a recombinant LALF. It has a single domain consisting of three alpha-helices packed against a four-stranded beta-sheet. The wedge-shaped molecule has a striking charge distribution and amphipathicity that suggest how it can insert into membranes. The binding site for LPS probably involves an extended amphipathic loop, and we propose that two mammalian LPS-binding proteins will have a similar loop. The amphipathic loop structure may be used in the design of molecules with therapeutic properties against septic shock.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aketagawa J., Miyata T., Ohtsubo S., Nakamura T., Morita T., Hayashida H., Miyata T., Iwanaga S., Takao T., Shimonishi Y. Primary structure of limulus anticoagulant anti-lipopolysaccharide factor. J Biol Chem. 1986 Jun 5;261(16):7357–7365. [PubMed] [Google Scholar]
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert G., Baldwin G., Thompson C., Wainwright N., Novitsky T. J., Gillis Z., Parsonnet J., Fleisher G. R., Siber G. R. Limulus antilipopolysaccharide factor protects rabbits from meningococcal endotoxin shock. J Infect Dis. 1992 Mar;165(3):494–500. doi: 10.1093/infdis/165.3.494. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Choe S., Bennett M. J., Fujii G., Curmi P. M., Kantardjieff K. A., Collier R. J., Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992 May 21;357(6375):216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- Cohen J., Glauser M. P. Septic shock: treatment. Lancet. 1991 Sep 21;338(8769):736–739. doi: 10.1016/0140-6736(91)91453-2. [DOI] [PubMed] [Google Scholar]
- Couturier C., Haeffner-Cavaillon N., Caroff M., Kazatchkine M. D. Binding sites for endotoxins (lipopolysaccharides) on human monocytes. J Immunol. 1991 Sep 15;147(6):1899–1904. [PubMed] [Google Scholar]
- Craig W. A., Turner J. H., Kunin C. M. Prevention of the generalized Shwartzman reaction and endotoxin lethality by polymyxin B localized in tissues. Infect Immun. 1974 Aug;10(2):287–292. doi: 10.1128/iai.10.2.287-292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desch C. E., O'Hara P., Harlan J. M. Antilipopolysaccharide factor from horseshoe crab, Tachypleus tridentatus, inhibits lipopolysaccharide activation of cultured human endothelial cells. Infect Immun. 1989 May;57(5):1612–1614. doi: 10.1128/iai.57.5.1612-1614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma R. J. Gram-negative bacillary infections. Pathogenic and pathophysiologic correlates. Am J Med. 1985 Jun 7;78(6A):154–164. doi: 10.1016/0002-9343(85)90119-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Flaggs G., Leong S. R., Gumina R. J., Weiss J., Ooi C. E., Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989 Jun 5;264(16):9505–9509. [PubMed] [Google Scholar]
- Kastowsky M., Gutberlet T., Bradaczek H. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol. 1992 Jul;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Collier R. J. Effects of eliminating a disulfide bridge within domain II of Pseudomonas aeruginosa exotoxin A. Infect Immun. 1989 Jul;57(7):1873–1878. doi: 10.1128/iai.57.7.1873-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Collins M. S., Snable J. L., Thornton M. B., Scott R. W. The role of bactericidal/permeability-increasing protein as a natural inhibitor of bacterial endotoxin. J Immunol. 1992 Jan 15;148(2):532–537. [PubMed] [Google Scholar]
- Morita T., Ohtsubo S., Nakamura T., Tanaka S., Iwanaga S., Ohashi K., Niwa M. Isolation and biological activities of limulus anticoagulant (anti-LPS factor) which interacts with lipopolysaccharide (LPS). J Biochem. 1985 Jun;97(6):1611–1620. doi: 10.1093/oxfordjournals.jbchem.a135218. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Muta T., Miyata T., Tokunaga F., Nakamura T., Iwanaga S. Primary structure of anti-lipopolysaccharide factor from American horseshoe crab, Limulus polyphemus. J Biochem. 1987 Jun;101(6):1321–1330. doi: 10.1093/oxfordjournals.jbchem.a121999. [DOI] [PubMed] [Google Scholar]
- Nachum R., Watson S. W., Sullivan J. D., Jr, Siegel S. E. Antimicrobial defense mechanisms in the horseshoe crab, Limulus polyphemus: preliminary observations with heat-derived extracts of Limulus amoebocyte lysate. J Invertebr Pathol. 1979 May;33(3):290–299. doi: 10.1016/0022-2011(79)90029-6. [DOI] [PubMed] [Google Scholar]
- Ohashi K., Niwa M., Nakamura T., Morita T., Iwanaga S. Anti-LPS factor in the horseshoe crab, Tachypleus tridentatus. Its hemolytic activity on the red blood cell sensitized with lipopolysaccharide. FEBS Lett. 1984 Oct 15;176(1):207–210. doi: 10.1016/0014-5793(84)80942-4. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Doerfler M. E., Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55-60 kD bactericidal/permeability-increasing protein of human neutrophils. J Exp Med. 1991 Sep 1;174(3):649–655. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Pattus F., Tucker A. D., Tsernoglou D. Structure of the membrane-pore-forming fragment of colicin A. Nature. 1989 Jan 5;337(6202):93–96. doi: 10.1038/337093a0. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Parker M. M., Natanson C., Suffredini A. F., Danner R. L., Cunnion R. E., Ognibene F. P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990 Aug 1;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Rustici A., Velucchi M., Faggioni R., Sironi M., Ghezzi P., Quataert S., Green B., Porro M. Molecular mapping and detoxification of the lipid A binding site by synthetic peptides. Science. 1993 Jan 15;259(5093):361–365. doi: 10.1126/science.8420003. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Moskaug J. O. Pseudomonas toxin binds triton X-114 at low pH. Biochem J. 1987 Aug 1;245(3):899–901. doi: 10.1042/bj2450899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Glennon M. L., Wainwright N., Amato S. F., Black K. M., Kirsch S. J., Riveau G. R., Whyte R. I., Zapol W. M., Novitsky T. J. Binding and neutralization of endotoxin by Limulus antilipopolysaccharide factor. Infect Immun. 1992 Jun;60(6):2506–2513. doi: 10.1128/iai.60.6.2506-2513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Patel M., Miller D. S. Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992 Sep 1;176(3):719–727. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. A 'molten-globule' membrane-insertion intermediate of the pore-forming domain of colicin A. Nature. 1991 Dec 5;354(6352):408–410. doi: 10.1038/354408a0. [DOI] [PubMed] [Google Scholar]





