Abstract
Bartonella spp. are worldwide-distributed facultative intracellular bacteria that exhibit an immense genomic diversity across mammal and arthropod hosts. The occurrence of cattle-associated Bartonella species was investigated in the cattle tail louse Haematopinus quadripertusus and in dairy cattle blood from Israel. Lice were collected from cattle from two dairy farms during summer 2011, and both lice and cow blood samples were collected from additional seven farms during the successive winter. The lice were identified morphologically and molecularly using 18S rRNA sequencing. Thereafter, they were screened for Bartonella DNA by conventional and real-time PCR assays using four partial genetic loci (gltA, rpoB, ssrA, and internal transcribed spacer [ITS]). A potentially novel Bartonella variant, closely related to other ruminant bartonellae, was identified in 11 of 13 louse pools collected in summer. In the cattle blood, the prevalence of Bartonella infection was 38%, identified as B. bovis and B. henselae (24 and 12%, respectively). A third genotype, closely related to Bartonella melophagi and Bartonella chomelii (based on the ssrA gene) and to B. bovis (based on the ITS sequence) was identified in a single cow. The relatively high prevalence of these Bartonella species in cattle and the occurrence of phylogenetically diverse Bartonella variants in both cattle and their lice suggest the potential role of this animal system in the generation of Bartonella species diversity.
INTRODUCTION
The Bartonella genus represents a fascinating example of diverse bacteria, formed by more than 30 species and subspecies, that can be found in a wide range of mammalian and arthropod hosts (e.g., fleas, lice, sandflies, and ticks) and exhibits a great genetic variation (1). In mammalian hosts, Bartonella species produce long-persistent infections, characterized by an apparent subclinical and cyclic bacteremia, allowing the bacteria to establish and be transmitted from one animal to another through bloodsucking arthropods (i.e., vectors) (2). Thereby, the maintenance and circulation of bartonellae in nature within arthropod-mammal systems is reflected by their diversity within a particular niche (e.g., feline, human, rodent, and ruminant bartonellae) (3). However, the incidental occurrence of Bartonella infection in nonadapted hosts may lead to the manifestation of disease; such is the case of cat-scratch disease in humans caused by the feline-associated Bartonella henselae (4, 5). Thus, the elucidation of the Bartonella cycle in nature, including the identification of the arthropod vectors and the Bartonella species distributed in a particular host and geographical area, is of great relevance in trimming the risk of their zoonotic potential, especially on those close and common human-animal interactions.
Bartonella species were previously detected in cattle, and three species have been identified: Bartonella bovis, Bartonella schoenbuchensis, and Bartonella chomelii (6–8). Interestingly, phylogenetic analyses of the ruminant-associated Bartonella species have clustered them as a separated lineage from the other Bartonella species (9, 10). Bartonella bovis is the most common reported species in cattle, while the other two have only been occasionally reported in these animals. Moreover, B. bovis has been associated with cases of bovine endocarditis (11, 12), and its zoonotic potential is still unknown. The prevalence of Bartonella in cattle varied across the studies and geographical areas, ranging from apparent uninfected-cattle regions such as Kenya and Japan to up to 90% infection rates in regions such as Marneuli District, Georgia (13).
Cattle are commonly infested by many ectoparasites, resulting in a constant menace for animal health either directly through blood consumption and physical burden, or indirectly through the transmission of pathogens (14, 15). Among these ectoparasites, lice (Phthiraptera) are important pests in cattle worldwide. Five louse species have been identified in cattle, including four sucking louse species and one biting louse species. They are characterized by a site affinity, each localized on a particular body region of the cow (15). Accordingly, the cattle tail sucking louse, Haematopinus quadripertusus, is commonly found infesting the distal area of the tail and is prevalent in tropical and subtropical areas such as Israel (15). Sucking lice are known vectors of human pathogens such as Borrelia recurrentis, Rickettsia prowazekii, and Bartonella quintana (3, 16, 17). Moreover, recent studies have identified Bartonella DNA in several species of lice collected from wild rodents (18, 19), suggesting that these arthropods have a role in the active maintenance of Bartonella in other mammals. Bartonella DNA has been also detected in cattle-associated hematophagous arthropods, such as Rhipicephalus (Boophilus) microplus ticks collected from Taiwanese cattle (20), biting flies such as Haematobia species, and Stomoxys species from Californian cattle (21) and Hippobosca equina from Europe (22). Nevertheless, the vector involved in Bartonella transmission to cattle has not been elucidated to date. Thus, along with these blood sucking parasites, sucking lice in cattle could represent a possible candidate for the active maintenance and transmission of cattle-associated Bartonella species.
The accelerated origination of Bartonella species from a common ancestor in different ecological niches (“adaptive radiation”) is especially evidenced in Bartonella species from ruminants (10). This, together with the unclarified ecological Bartonella cycle in ruminants, makes this system an interesting model to study the bacterium-vector-host interaction in nature. Thus, in the present study the occurrence of Bartonella species in cattle and their cattle tail lice was investigated. The potential role of cattle and the cattle tail lice in maintenance and transmission of bartonellae in ruminants is discussed.
MATERIALS AND METHODS
Collection of lice from cattle.
Lice were collected in two periods from dairy cattle farms. The first collection period (summer) took place on September 2011 in two dairy cattle farms from Mevo Horon (31°50′57.04″N, 35°2′9.34″E) and Galon (31°37′58.07″N, 34°50′51.72″ E). Lice were manually collected from the tail of each cow. Totals of 510 and 600 specimens were collected from 17 and 20 cows (∼30 lice per cow) from the Galon and Mevo Horon farms, respectively. The specimens were placed in microtubes containing 1 ml of 70% ethanol, transported to the laboratory, and kept at room temperature. They were then classified to the genus and species level by morphological characteristics (23) and by molecular characterization of the 18S rRNA gene (described below). The second sample collection occurred on December 2011 (winter) in nine dairy cattle farms: Mevo Horon, Galon, Givat Hashlosha (32°5′53.88″N, 34°55′15.59″E), Ahisamakh (31°56′5.63″N, 34°54′26.27″), Matzliah (31°54′28.08″N, 34°52′26.4″E), Nehalim (32°3′30.6″N, 34°54′49.31″E), Yarhiv (32°9′8.27″N, 34°58′4.07″E), Nahshonim (32°3′36.35″N, 34°56′51.71″E), and Gat (31°37′37.91″N, 34°47′38.76″E) (Fig. 1). During the second collection, lice were collected from 50 cows. Approximately 10 to 20 lice were collected from each cow and transported in 1 ml of 70% ethanol.
FIG 1.
Map of Israel indicating the locations of the cattle dairy farms where lice and cattle blood samples were collected. The map was constructed using ArcMap 10.0 software (Esri, Redlands, CA).
Collection of cattle blood.
During winter collection, cow blood samples were collected from the above-mentioned 50 cows (from which lice were also collected). Accordingly, blood samples were drawn in EDTA tubes, chilled in cool-boxes, and transported to the laboratory, where they were kept at −80°C until further analyzed.
DNA extraction from lice and cattle blood.
DNA was extracted from louse pools (2 to 20 lice per pool according to the farms and/or season of collection) and from individual lice (from winter collection), as follows. First, the lice were washed once in 1 ml of ethanol 70% for 10 min and twice in 1 ml of sterile phosphate-buffered saline for 10 min. Thereafter, they were transferred to a new DNA-free vial and homogenized with a sterile pestle until a clear solution was obtained. Finally, the DNA was extracted using a DNA extraction kit (Illustra Tissue & Cells GenomicPrep Mini Spin kit; GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer's instructions.
DNA was extracted from 50 μl of EDTA-blood of each cow using a DNA extraction kit (BiOstic bacteremia DNA isolation kit; MO BIO Laboratories, Inc., Carlsbad, CA) according to the manufacturer's instructions. The DNA was obtained in 50 μl of elution buffer. For quality assurance, a Bartonella-free blood sample was used as an extraction control.
Molecular identification of the cattle tail louse.
The 18S rRNA gene (∼2,970 bp) was amplified by conventional PCR from louse pools and single louse, using primers EUKA (AACCTGGTTGATCCTGCCAGT) and EUKB (GATCCTTCTGCAGGTTCACCTAC) (24). In order to clarify the targeted sequence of the 18S rRNA gene, additional internal primers were required. Accordingly, the primer pairs HF18S (CGACGAAACTTACCGTCGGA)/HR18S (ATTAAGCCGCAAGCTCCACT) and 454F (AAGCTCGAAAGGAATCCGCA)/1620R (TGTTGAGATCGCGTCGGAAA), which targeted 1,188- and 1,200-bp fragments, respectively, were designed. All PCRs were performed under the following thermal cycling conditions: an initial step of 94°C for 5 min, followed by 30 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 90 s, with a final step of 72°C for 10 min. Amplified fragments were sequenced in an MJ Research PTC-225 Peltier thermal cycler using an ABI Prism BigDye Terminator cycle sequencing kit with AmpliTaq DNA polymerase (FS enzyme; Applied Biosystems, Carlsbad, CA) according to the protocols supplied by the manufacturer. Further analyses of sequences were done with MEGA alignment software version 5.05 (The Biodesign Institute, Tempe, AZ), and the final sequence was obtained by the assembly of all of the sequenced 18S rRNA gene fragments.
Molecular screening of Bartonella DNA from lice and cattle blood DNA.
The molecular screening for Bartonella DNA on lice was assessed by conventional PCR and high-resolution melt (HRM) real-time PCR assays. Conventional PCR assays were performed (i) targeting 379- and 800-bp fragments of the citrate synthase (gltA) gene using the primers Bhcs.781p and Bhcs.1137n (25) and the primers CS443f and CS1210r (26, 27), respectively, (ii) targeting a 795-bp fragment of the RNA polymerase (rpoB) gene using the primers 1400F and 2300R (28), and (iii) targeting a 602-bp fragment of the 16S-23S internal transcribed spacer (ITS) using the primers 321s and 983as (29). The PCRs were carried out in a 25-μl final volume using PCR-Ready high-specificity ready mix (Syntezza Bioscience, Ltd., Jerusalem, Israel) containing 1 μl of a 10 μM solution of each primer, 21 μl of double-distilled water (DDW), and 2 μl of each extracted DNA sample. Thermal conditions were performed according to the authors' recommendations. In order to increase the sensitivity of the Bartonella screening, HRM real-time PCR assays were performed for the amplification of partial fragments for the ITS (190 bp) and the transfer-mRNA (ssrA) gene (301 bp) using primer sets and protocols as previously described (29–31). The real-time PCRs were carried out in a 20-μl final volume containing 1 μl of 0.5 μM solution of each primer, 0.6 μl of 1.5 μM solution of Syto9 (Invitrogen, CA), 3.4 μl of DDW, 10 μl of MAXIMA Hot-Start PCR master mix 2X (Thermo Scientific, Surrey, United Kingdom), and 4 μl of each genomic DNA. All HRM real-time reactions were carried out in the Rotor Gene 6000 cycler (Corbett Research, Sydney, Australia). Molecular screening of cattle blood for Bartonella species DNA was assessed by using HRM real-time PCR assays targeting the ITS and ssrA fragments, as described above.
All Bartonella-positive real-time and conventional PCR products were purified with a PCR purification kit (Exo-SAP; New England BioLabs, Inc., Ipswich, MA) and subsequently sequenced by using BigDye Terminator cycle sequencing chemistry from an Applied Biosystems ABI 3700 DNA analyzer and the ABI's data collection and sequence analysis software (ABI, Carlsbad, CA). Further analysis was done with MEGA alignment software (version 5.05; The Biodesign Institute).
Nucleotide sequence accession numbers.
Newly determined sequence data were deposited in GenBank under accession numbers KJ522491, KJ522487, KJ522489, KJ522488, and KJ522490.
RESULTS
Identification of cattle tail lice.
Louse specimens were identified as Haematopinus quadripertusus by morphological characteristics. The characterization of the 18S rRNA gene sequence resulted in a partial fragment of 2,835 bp. In all 18S fragments sequenced, a common region of ambiguous nucleotides was noticed (from the nucleotide positions 992 to 1387); thus, this area was manually annotated and clarified from 13 louse 18S rRNA gene amplicon chromatograms. The consensus 18S rRNA gene sequence was deposited in GenBank under accession number KJ522491. The obtained 18S rRNA sequence was 91.2% similar to the Haematopinus sp. strain NKU-011 18S rRNA gene (JQ309927.1) collected from cattle from Sichuan Province, China (32; Qiang Xie, personal communication), the longest available Haematopinus sp. 18S rRNA partial gene available in the GenBank database.
Bartonella DNA detection in Haematopinus quadripertusus.
Eleven of 13 tested louse pools (median of 10 lice) collected from two farms during summer (8 from Galon and 5 from Mevo Horon) (Fig. 1) were determined to be positive for Bartonella DNA through the PCR screening methods. The sequences obtained by the different genetic target loci (gltA, rpoB, ITS, and ssrA) showed 100% identity between the louse pool samples amplified from both farms. Those Bartonella sequences were deposited in GenBank under the following accession numbers: gltA (KJ522487, 748 bp), rpoB (KJ522489, 852 bp), ITS (KJ522488, 424 bp), and ssrA (KJ522490, 253 bp). Phylogenetic analyses of the Bartonella species detected from H. quadripertusus lice demonstrate that this species is closely related to other ruminant bartonellae (Table 1 and Fig. 2). During the winter collection period, none of the louse pools (from 39 cows) or the single louse (from 29 cows) tested was determined to be positive for Bartonella DNA by any conventional or real-time PCR assay.
TABLE 1.
Pairwise distance analysis between uncultured Bartonella sp. clone Hq (from Haematopinus quadripertusus) versus ruminant bartonellae and Bartonella grahamii (outgroup) sequences from the GenBank database
| Matched Bartonella sp. | % similarity between sequences |
|||
|---|---|---|---|---|
| gltA (354 bp) | rpoB (852 bp) | ITS (424 bp) | ssrA (250 bp) | |
| B. chomelii | 95.1 | 95.6 | 93.3 | 98.8 |
| B. schoenbuchensis | 94.8 | 95.4 | 93.0 | 98.4 |
| B. bovis | 94.8 | 94.1 | 95.2 | 96.7 |
| B. capreoli | 95.1 | 95.2 | 92.6 | 98.8 |
| B. melophagi | 95.8 | 95.1 | 91.1 | 98.8 |
| B. grahamii | 88.7 | 82.3 | 41.9 | 92.4 |
FIG 2.
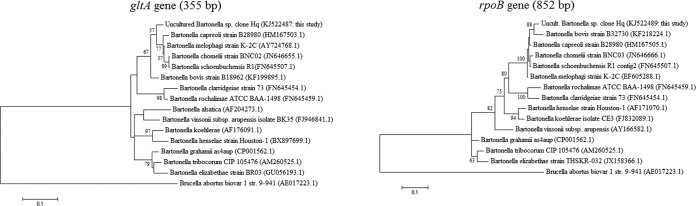
Maximum-likelihood phylogenetic trees based on partial rpoB (825-bp) and gltA (355-bp) genes. Bootstrap values higher than 50% are indicated. Phylogenetic trees were obtained from the uncultured Bartonella sp. clone Hq sequences detected from Haematopinus quadripertusus lice and common Bartonella species sequences, including ruminant-associated Bartonella species (sequences were obtained from the GenBank database, and accession numbers are indicated in parentheses). The Brucella abortus biovar 1, strain 9-941, sequence was used as an outgroup.
Bartonella DNA detection in cattle blood.
Bartonella DNA was detected in 38% (19/50) of all cattle blood samples by real-time PCR assays. Both ssrA and ITS real-time PCR assays were found positive in 10 cow blood samples, and yet in 9 additional cases only one of the target regions was successfully amplified (Table 2). The Bartonella DNAs identified from the cow blood samples were closely related to B. bovis or B. henselae DNA sequences. In addition, one cow presented an ssrA genotype closely related to B. melophagi and B. chomelii DNA sequences (both with 98% similarities), and the ITS sequence detected was 100% similar to the B. bovis ITS sequence. The ssrA sequence was deposited in GenBank under the accession number KJ540110. Accordingly, B. bovis showed an infection rate of 24% (12/50), and B. henselae showed an infection rate of 12% (6/50). Seven dairy farms presented at least one cow infected with Bartonella DNA. The only locations where no Bartonella infections were detected in the cattle samples were Gat and Mevo Horon. Furthermore, most positive cases were identified in Givat Hashlosha (7/19), followed by the Ahisamakh dairy farm (6/19), which are located ∼20 km apart.
TABLE 2.
Molecular detection of Bartonella DNA from cattle blood samples collected from dairy farms in Israel (winter collection)
| Cow | Farm | Real-time PCR |
Bartonella sp. (% identity)a | |
|---|---|---|---|---|
| ITS | ssrA | |||
| 1 | Ahisamakh | + | 0 | B. henselae (100)* |
| 2 | Ahisamakh | 0 | + | B. bovis (100)† |
| 3 | Ahisamakh | + | + | B. bovis (100)‡ |
| 4 | Ahisamakh | + | + | B. bovis (99)* |
| 5 | Ahisamakh | + | + | B. bovis (100)‡ |
| 6 | Ahisamakh | + | + | B. henselae (100)* |
| 7 | Galon | 0 | + | B. bovis (100)† |
| 8 | Givat Hashlosha | + | + | B. bovis (100)† |
| 9 | Givat Hashlosha | + | 0 | B. henselae (100)* |
| 10 | Givat Hashlosha | 0 | + | B. bovis (100)† |
| 11 | Givat Hashlosha | + | 0 | B. henselae (100)* |
| 12 | Givat Hashlosha | + | + | B. melophagi-B. chomelii/B. bovisb |
| 13 | Givat Hashlosha | + | 0 | B. henselae (100)* |
| 14 | Givat Hashlosha | + | + | B. bovis (100)† |
| 15 | Matzliah | 0 | + | B. bovis (100)† |
| 16 | Nachshonim | + | + | B. bovis (100)† |
| 17 | Nehalim | + | + | B. bovis (100)‡ |
| 18 | Yarhiv | + | 0 | B. henselae (100)* |
| 19 | Yarhiv | + | + | B. bovis (100)† |
*, identity based on the ITS sequence; †, identity based on the ssrA sequence; ‡, identity based on both the ITS and the ssrA sequences.
The Bartonella genotype was 98% similar to those of B. melophagi and B. chomelii based on the ssrA fragment, and the Bartonella genotype was 100% similar to that of B. bovis based on the ITS fragment.
DISCUSSION
This study demonstrated the occurrence of Bartonella DNA sequences closely related to ruminant Bartonella species in the cattle tail louse H. quadripertusus. In addition, cattle and feline associated Bartonella DNA were detected in the blood of dairy cattle from seven Israeli dairy farms.
Previous studies that screened Bartonella DNA on Haematopinus species lice collected from pigs and cattle reported negative results (33, 34). Therefore, to the best of our knowledge, this is the first report of Bartonella detection in this louse genus. The current molecular characterization of the Bartonella DNA found in the H. quadripertusus lice exhibits certain features suggesting that it may represent a single bacterial clone or variant. First, the positive lice were collected from two geographically distant cattle farms (Mevo Horon and Galon, ∼40 km apart). Second, the Bartonella genetic targets showed 100% identity between the louse pool samples amplified from both farms. Interestingly, the sequence analyses of these amplicons revealed a close but distinguishable phylogenetic relationship between this Bartonella with the other known ruminant-associated Bartonella species, suggesting a new Bartonella species according to the La Scola taxonomic classification (35). Despite this evidence, we are aware that, in order to confirm the single bacterial origin of these DNA products, bacterium isolations were required (attempts to isolate the Bartonella from lice and cattle blood were assessed without success [data not shown]). Moreover, Bartonella DNA was only detected in specimens from the summer collection and not from those collected during winter. This phenomenon can be related to the higher relative abundance and activity of H. quadripertusus during the summer season (15), suggesting a potential seasonal effect on Bartonella species acquisition by these lice.
Bartonella infection in cattle has been extensively reported worldwide (6, 13, 31, 36, 37). Bartonella bovis has been the most common species identified in these animals. Accordingly, in the present study, Bartonella DNA was detected in a relatively high percentage of the animals (38% of the cattle tested), with B. bovis DNA being the most common Bartonella sequence identified (24%). Interestingly, the global distribution of B. bovis has shown a great inconsistency in the infection rates across and within geographical areas. For instance, Bai et al. (13) investigated the Bartonella infection in cattle from Thailand, Kenya, Japan, Georgia, and Guatemala and reported overall prevalence that varied from 0% (Japan and Kenya) to 57% (Georgia). Similarly, B. bovis infection rates from cattle from the United States varied across the regions studied, being as high as 82.4% in North Carolina (38) and California (81 to 96%) (6) and less pronounced in Georgia (47%) (31). In Europe, the prevalence of B. bovis in cattle has shown similar variability, with reports from France (59%) (39), Italy (24.2%) (40), and Poland (6.8%) (37). It should be noted that B. schoenbuchensis and B. chomelii, the other two Bartonella species detected in cattle, were initially isolated from cows in France (7, 8). In the present study, DNA sequences of two additional Bartonella species were identified. First, DNA of B. henselae, a feline-associated and zoonotic Bartonella species, was detected in six cows in our study. This finding represents the second report of this Bartonella species in cattle worldwide since its first detection in beef cattle from North Carolina (38). The occurrence of B. henselae in cattle may reflect the frequent spread of this species in Israel. Recently, the prevalence of this species was determined in cats from this country and shown to be evenly distributed in stray and domestic cats (30). Thus, despite their acknowledged feline association, B. henselae seems to have a permissive cycle in nature since it has been detected in several ecological niches (hosts and vectors) (18, 41). Finally, a third Bartonella genotype was detected in one cow blood sample, which was closely related to B. chomelii and B. melophagi, two ruminant-associated Bartonella species, according to the ssrA sequence, and to B. bovis according to the ITS sequence. The uncertainty in species identification could be explained by coinfection with two Bartonella variants (B. bovis and a variant related to B. chomelii or B. melophagi) or by infection with a variant that contained recombinant sequences. Nevertheless, both possible scenarios illustrate the challenge of Bartonella identification using direct detection of housekeeping genes and the variation of these genes that complicate the taxonomic classification (as in the case of the ssrA sequence). These phenomena have been extensively observed in wild rodent bartonellae (1, 42, 43).
Lice are considered one of the most abundant ectoparasites infesting dairy cattle in Israel. Since all known Bartonella species are associated with a vector-borne life cycle (1), lice were proposed as potential vehicles for cattle-associated Bartonella in this country. It should be noted that all cows included in the study were found to be highly infested with these arthropods, especially in summer. However, the incrimination of these lice as active Bartonella vectors was challenged since the screening of Bartonella DNA showed no detection of Bartonella during winter, although the cattle blood samples were positive during this season. Moreover, the Bartonella variants identified in either the louse pools or cattle blood were different. A potential explanation for the latter finding may suggest that these species/variants coexist in cattle, but a selective pressure may occur in the lice toward the acquisition of the detected Bartonella variant and only the dominant ones could be detected in the cattle hosts. Coinfection with multiple Bartonella variants in a single carrier and an apparent selective distribution of those variants have been revealed in other ecological niches, such as wild rodents and their fleas (44). Furthermore, the Bartonella variant found in lice could represent a unique bacterium from this niche, without strict association with the cattle hosts. Lice are known to have symbiotic association with bacteria, which become essential to their growth and reproduction (15, 45). For instance, B. melophagi has been suggested as an endosymbiont of Melophagus ovinus sheep keds (22). Thus, a similar evolutionary process between H. quadripertusus and the Bartonella detected during the summer might be taking place. On the other hand, the role of other bloodsucking arthropods (e.g., biting fleas or ticks) in this cattle system may be more relevant ecologically, especially for the B. bovis and B. henselae found in the cattle. Overall, our results suggest that H. quadripertusus lice probably do not play an important role in the transmission of these cattle-associated Bartonella species.
In conclusion, our findings demonstrated that Bartonella species are prevalent in dairy cattle from Israel and that H. quadripertusus lice can host a novel Bartonella genotype/species, which was not found in the tested cattle. The infection rates of B. bovis in cattle blood were higher than for other related species and much higher than those reported from other geographical areas worldwide. In addition, the wide spread of B. bovis and the presence of feline-zoonotic B. henselae in cattle must be highlighted since these organisms represent a veterinary and zoonotic health hazard. The identification of potentially novel Bartonella variants in both cattle and their lice suggest that this system plays a role in the generation of Bartonella diversity. This system adds evidence for a complex maintenance of Bartonella in nature, as was observed in other mammals such as wild rodents. Finally, further studies should target other blood-sucking arthropods in dairy farms in order to elucidate the potential role of other vectors in the life cycle of these cattle-associated Bartonella species and to determine the ecological factors affecting bartonella occurrence in each of the players in this important agriculture system.
ACKNOWLEDGMENTS
We thank Maor Kedmi, Tamir Goshen, and Ori Koren (Hachaklait Veterinary Services, Ltd., Israel) for their assistance in the collection of samples. We also thank Etai Kahana for assistance in the production of Fig. 1.
This research was supported by the Ministerio de Ciencia y Tecnología and the Consejo Nacional para Investigaciones Científicas y Tecnológicas, Costa Rica.
Footnotes
Published ahead of print 27 June 2014
REFERENCES
- 1.Kosoy M, Hayman DT, Chan KS. 2012. Bartonella bacteria in nature: where does population variability end and a species start? Infect. Genet. Evol. 12:894–904. 10.1016/j.meegid.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 2.Harms A, Dehio C. 2012. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 25:42–78. 10.1128/CMR.05009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 40:29. 10.1051/vetres/2009011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mainardi JL, Figliolini C, Goldstein FW, Blanche P, Baret-Rigoulet M, Galezowski N, Fournier PE, Raoult D. 1998. Cat scratch disease due to Bartonella henselae serotype Marseille (Swiss cat) in a seronegative patient. J. Clin. Microbiol. 36:2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitschwerdt EB. 2008. Feline bartonellosis and cat scratch disease. Vet. Immunol. Immunopathol. 123:167–171. 10.1016/j.vetimm.2008.01.025 [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Chomel BB, Kasten RW, Heller RM, Ueno H, Yamamoto K, Bleich VC, Pierce BM, Gonzales BJ, Swift PK, Boyce WM, Jang SS, Boulouis HJ, Piemont Y, Rossolini GM, Riccio ML, Cornaglia G, Pagani L, Lagatolla C, Selan L, Fontana R. 2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg. Infect. Dis. 6:306–311. 10.3201/eid0603.000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maillard R, Riegel P, Barrat F, Bouillin C, Thibault D, Gandoin C, Halos L, Demanche C, Alliot A, Guillot J, Piemont Y, Boulouis HJ, Vayssier-Taussat M. 2004. Bartonella chomelii sp. nov., isolated from French domestic cattle (Bos taurus). Int. J. Syst. Evol. Microbiol. 54:215-220. 10.1099/ijs.0.02770-0 [DOI] [PubMed] [Google Scholar]
- 8.Rolain JM, Rousset E, La Scola B, Duquesnel R, Raoult D. 2003. Bartonella schoenbuchensis isolated from the blood of a French cow. Ann. N. Y. Acad. Sci. 990:236–238. 10.1111/j.1749-6632.2003.tb07370.x [DOI] [PubMed] [Google Scholar]
- 9.Guy L, Nystedt B, Toft C, Zaremba-Niedzwiedzka K, Berglund EC, Granberg F, Naslund K, Eriksson AS, Andersson SG. 2013. A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella. PLoS Genet. 9:e1003393. 10.1371/journal.pgen.1003393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel P, Salzburger W, Liesch M, Chang CC, Maruyama S, Lanz C, Calteau A, Lajus A, Medigue C, Schuster SC, Dehio C. 2011. Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella. PLoS Genet. 7:e1001296. 10.1371/journal.pgen.1001296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maillard R, Petit E, Chomel B, Lacroux C, Schelcher F, Vayssier-Taussat M, Haddad N, Boulouis HJ. 2007. Endocarditis in cattle caused by Bartonella bovis. Emerg. Infect. Dis. 13:1383–1385. 10.3201/eid1309.070236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erol E, Jackson C, Bai Y, Sells S, Locke S, Kosoy M. 2013. Bartonella bovis isolated from a cow with endocarditis. J. Vet. Diagn. Invest. 25:288–290. 10.1177/1040638713477408 [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Malania L, Alvarez Castillo D, Moran D, Boonmar S, Chanlun A, Suksawat F, Maruyama S, Knobel D, Kosoy M. 2013. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multilocus sequence typing. PLoS One 8:e80894. 10.1371/journal.pone.0080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byford RL, Craig ME, Crosby BL. 1992. A review of ectoparasites and their effect on cattle production. J. Anim. Sci. 70:597–602 [DOI] [PubMed] [Google Scholar]
- 15.Durden LA, Loyd JE. 2009. Lice (Phthiraptera), p 59–82 In Mullen GR, Durden LA. (ed), Medical and veterinary entomology, 2nd ed. Academic Press, Ltd, London, United Kingdom [Google Scholar]
- 16.Azad AF, Beard CB. 1998. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 4:179–186. 10.3201/eid0402.980205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouqui P, Raoult D. 2006. Arthropod-borne diseases in homeless. Ann. N. Y. Acad. Sci. 1078:223–235. 10.1196/annals.1374.041 [DOI] [PubMed] [Google Scholar]
- 18.Durden LA, Ellis BA, Banks CW, Crowe JD, Oliver JH., Jr 2004. Ectoparasites of gray squirrels in two different habitats and screening of selected ectoparasites for bartonellae. J. Parasitol. 90:485–489. 10.1645/GE-3299 [DOI] [PubMed] [Google Scholar]
- 19.Nelder MP, Reeves WK, Adler PH, Wozniak A, Wills W. 2009. Ectoparasites and associated pathogens of free-roaming and captive animals in zoos of South Carolina. Vector-Borne Zoonotic Dis. 9:469–477. 10.1089/vbz.2008.0008 [DOI] [PubMed] [Google Scholar]
- 20.Tsai YL, Chomel BB, Chang CC, Kass PH, Conrad PA, Chuang ST. 2011. Bartonella and Babesia infections in cattle and their ticks in Taiwan. Comp. Immunol. Microbiol. Infect. Dis. 34:179–187. 10.1016/j.cimid.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Chung CY, Kasten RW, Paff SM, Van Horn BA, Vayssier-Taussat M, Boulouis HJ, Chomel BB. 2004. Bartonella spp. DNA associated with biting flies from California. Emerg. Infect. Dis. 10:1311–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halos L, Jamal T, Maillard R, Girard B, Guillot J, Chomel B, Vayssier-Taussat M, Boulouis HJ. 2004. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Appl. Environ. Microbiol. 70:6302–6305. 10.1128/AEM.70.10.6302-6305.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KC, Pratt HD, Stojanovich CJ. 1986. The sucking lice of North America: an illustrated manual for identification. Pennsylvania State University Press, University Park, PA [Google Scholar]
- 24.Medlin L, Elwood HJ, Stickel S, Sogin ML. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499. 10.1016/0378-1119(88)90066-2 [DOI] [PubMed] [Google Scholar]
- 25.Norman AF, Regnery R, Jameson P, Greene C, Krause DC. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birtles RJ, Raoult D. 1996. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int. J. Syst. Bacteriol. 46:891–897. 10.1099/00207713-46-4-891 [DOI] [PubMed] [Google Scholar]
- 27.Billeter SA, Gundi VA, Rood MP, Kosoy MY. 2011. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvegicus rats in Los Angeles, California. Appl. Environ. Microbiol. 77:7850–7852. 10.1128/AEM.06012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renesto P, Gouvernet J, Drancourt M, Roux V, Raoult D. 2001. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 39:430–437. 10.1128/JCM.39.2.430-437.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi RG, Breitschwerdt EB. 2005. Potential limitations of the 16S-23S rRNA intergenic region for molecular detection of Bartonella species. J. Clin. Microbiol. 43:1171–1176. 10.1128/JCM.43.3.1171-1176.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutiérrez R, Morick D, Gross I, Winkler R, Abdeen Z, Harrus S. 2013. Bartonellae in domestic and stray cats from Israel: comparison of bacterial cultures and high-resolution melt real-time PCR as diagnostic methods. Vector-Borne Zoonotic Dis. 13:857–864. 10.1089/vbz.2013.1308 [DOI] [PubMed] [Google Scholar]
- 31.Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY. 2012. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. J. Clin. Microbiol. 50:1645–1649. 10.1128/JCM.06621-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Engel MS, Rafael JA, Dang K, Wu H, Xie Q, Bu W. 2013. A unique box in 28S rRNA is shared by the enigmatic insect orders Zoraptera and Dictyoptera. PLoS One 8:e53679. 10.1371/journal.pone.0053679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeves WK, Szumlas DE, Moriarity JR, Loftis AD, Abbassy MM, Helmy IM, Dasch GA. 2006. Louse-borne bacterial pathogens in lice (Phthiraptera) of rodents and cattle from Egypt. J. Parasitol. 92:313–318. 10.1645/GE-717R.1 [DOI] [PubMed] [Google Scholar]
- 34.Kernif T, Socolovschi C, Wells K, Lakim MB, Inthalad S, Slesak G, Boudebouch N, Beaucournu JC, Newton PN, Raoult D, Parola P. 2012. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp. Immunol. Microbiol. Infect. Dis. 35:51–57. 10.1016/j.cimid.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Scola B, Zeaiter Z, Khamis A, Raoult D. 2003. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 11:318–321. 10.1016/S0966-842X(03)00143-4 [DOI] [PubMed] [Google Scholar]
- 36.Breitschwerdt EB, Sontakke S, Cannedy A, Hancock SI, Bradley JM. 2001. Infection with Bartonella weissii and detection of Nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39:879–882. 10.1128/JCM.39.3.879-882.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welc-Faleciak R, Grono K. 2013. The first cases of Bartonella bovis infection in cattle from Central Europe. Vet. Microbiol. 162:954–956. 10.1016/j.vetmic.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 38.Cherry NA, Maggi RG, Cannedy AL, Breitschwerdt EB. 2009. PCR detection of Bartonella bovis and Bartonella henselae in the blood of beef cattle. Vet. Microbiol. 135:308–312. 10.1016/j.vetmic.2008.09.063 [DOI] [PubMed] [Google Scholar]
- 39.Maillard R, Grimard B, Chastant-Maillard S, Chomel B, Delcroix T, Gandoin C, Bouillin C, Halos L, Vayssier-Taussat M, Boulouis HJ. 2006. Effects of cow age and pregnancy on Bartonella infection in a herd of dairy cattle. J. Clin. Microbiol. 44:42-46. 10.1128/JCM.44.1.42-46.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martini M, Menandro ML, Mondin A, Pasotto D, Mazzariol S, Lauzi S, Stelletta C. 2008. Detection of Bartonella bovis in a cattle herd in Italy. Vet. Rec. 162:58–59. 10.1136/vr.162.2.58 [DOI] [PubMed] [Google Scholar]
- 41.Morick D, Krasnov BR, Khokhlova IS, Shenbrot GI, Kosoy MY, Harrus S. 2010. Bartonella genotypes in fleas (Insecta: Siphonaptera) collected from rodents in the Negev desert, Israel. Appl. Environ. Microbiol. 76:6864–6869. 10.1128/AEM.00879-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue K, Maruyama S, Kabeya H, Hagiya K, Izumi Y, Une Y, Yoshikawa Y. 2009. Exotic small mammals as potential reservoirs of zoonotic Bartonella spp. Emerg. Infect. Dis. 15:526–532. 10.3201/eid1504.081223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrus S, Bar-Gal GK, Golan A, Elazari-Volcani R, Kosoy MY, Morick D, Avidor B, Baneth G. 2009. Isolation and genetic characterization of a Bartonella strain closely related to Bartonella tribocorum and Bartonella elizabethae in Israeli commensal rats. Am. J. Trop. Med. Hyg. 81:55–58 [PubMed] [Google Scholar]
- 44.Gutiérrez R, Morick D, Cohen C, Hawlena H, Harrus S. 27 February 2014. The effect of ecological and temporal factors on the composition of Bartonella infection in rodents and their fleas. ISME J. 10.1038/ismej.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hypsa V, Krizek J. 2007. Molecular evidence for polyphyletic origin of the primary symbionts of sucking lice (Phthiraptera, Anoplura). Microb. Ecol. 54:242–251. 10.1007/s00248-006-9194-x [DOI] [PubMed] [Google Scholar]