Abstract
The genus Tenacibaculum, a member of the family Flavobacteriaceae, is an abundant component of marine bacterial ecosystems that also hosts several fish pathogens, some of which are of serious concern for marine aquaculture. Here, we applied multilocus sequence analysis (MLSA) to 114 representatives of most known species in the genus and of the worldwide diversity of the major fish pathogen Tenacibaculum maritimum. Recombination hampers precise phylogenetic reconstruction, but the data indicate intertwined environmental and pathogenic lineages, which suggests that pathogenicity evolved independently in several species. At lower phylogenetic levels recombination is also important, and the species T. maritimum constitutes a cohesive group of isolates. Importantly, the data reveal no trace of long-distance dissemination that could be linked to international fish movements. Instead, the high number of distinct genotypes suggests an endemic distribution of strains. The MLSA scheme and the data described in this study will help in monitoring Tenacibaculum infections in marine aquaculture; we show, for instance, that isolates from tenacibaculosis outbreaks in Norwegian salmon farms are related to T. dicentrarchi, a recently described species.
INTRODUCTION
The fast development of aquaculture (1) faces an array of sanitary issues, causing important economic losses and with an impact on the environment and animal welfare. As a result, there is growing interest in the analysis of the pathogenic bacteria infecting cultured aquatic organisms. In 2001, the genus Tenacibaculum (a member of the family Flavobacteriaceae, phylum Bacteroidetes) was proposed to reclassify T. maritimum and T. ovolyticum, two species of marine fish-pathogenic bacteria formerly included in the genus Flexibacter (2). The number of described species in the genus Tenacibaculum has since grown rapidly; it currently contains a total of 21 fish-pathogenic and environmental species (http://www.bacterio.net/tenacibaculum.html).
The best known of the pathogens in this genus, T. maritimum (3), has been repeatedly identified as a cause of high levels of mortality and economic losses in many cultured marine fish species worldwide (4). The disease, often referred to as tenacibaculosis, typically consists of external lesions and necrosis that can affect virtually all areas of the body surface (5). T. ovolyticum has been described as a bacterium attacking Atlantic halibut (Hippoglossus hippoglossus) eggs and larvae (6, 7). The type strains of T. discolor, T. gallaicum, T. soleae, and T. dicentrarchi (8–10) were isolated from different species of cultured marine fish or their close environment; evidence of pathogenicity resulted from the isolation source (external lesions) and from the results of experimental infection trials (10–12), but general data on the distribution, degree of pathogenicity, and impact on fish farming are lacking for these species.
Tenacibaculum strains not apparently associated with fish diseases also are widespread in marine environments, where they may decompose organic matter, as suggested by their ability to degrade a variety of biopolymers, such as various cellulose derivatives, xylan, agar, and chitin (2, 13–16). T. mesophilum, T. amylolyticum, T. aiptasiae, T. adriaticum, T. crassostreae, and “T. halocynthiae” (quotation marks denote names that have not been validly published) were isolated from marine organisms (2, 15, 17–19). Also belonging to this category is T. litopenaei, a chitinolytic bacterium isolated from the water of a shrimp mariculture pond (20). Other Tenacibaculum species were retrieved from inorganic substrates; T. litoreum, T. lutimaris, T. aestuarii, and T. caenipelagi were isolated from tidal flat sediments (14, 21–23), while T. skagerrakense, T. jejuense, T. geojense, and T. xiamenense were isolated from seawater at various distances from the shore and positions in the water column (13, 16, 24, 25).
Clarification of the taxonomy of the family Flavobacteriaceae (26–28) and description of the genus Tenacibaculum (2) represented key steps toward a more rational description of the relationships between members of the phylum Bacteroidetes. However, our knowledge of the evolutionary relationships between members of the genus Tenacibaculum remains very scarce, and no practical molecular technique is available to monitor the diversity and incidence of Tenacibaculum infections in marine aquaculture systems worldwide. To bridge this gap, in this work we conducted a multilocus sequence analysis (MLSA) (29, 30) encompassing representatives of the 18 Tenacibaculum species published by March 2013 (excluding the environmental species T. caenipelagi, T. halocynthiae, and T. xiamenense) as well as a collection of T. maritimum isolates representative of the worldwide diversity of this fish pathogen. In contrast to DNA-DNA hybridization (30), randomly amplified polymorphic DNA (RAPD), and serotyping schemes (31, 32) that already have been used to assess genetic diversity within the genus, MLSA has the advantage of relying on sequence data directly amenable to evolutionary analysis. Furthermore, the data can easily be stored in databases, compared across experiments, and progressively enriched by the addition of new isolates. The generality of MLSA also makes it complementary to more rapid and less expensive detection methods, such as PCR or immunohistochemical assays already proposed for T. maritimum (33–36) and T. soleae (37). To illustrate the applicability of our approach at the genus level, we include in this report the results of MLSA on a selection of isolates responsible for recent tenacibaculosis outbreaks in Norwegian salmon farms (38) and in Italian sea bass and sea bream farms.
MATERIALS AND METHODS
Loci, strains, and experimental protocol.
The 11 loci are located within single-copy protein-coding genes conserved across the family Flavobacteriaceae for which it was possible to design generic degenerated PCR primers for the genus Tenacibaculum. Data on the genetic polymorphism in the populations of Tenacibaculum strains was not used to select these loci, except that we required enough conservation of the 21-bp sequences recognized by the primers. These loci can be considered typical core genome genes whose polymorphism is more likely relatively neutral (i.e., not experiencing frequent adaptive selection). Individually, each of these genes had already been used in MLST studies of other bacterial species and genera. The 16S rRNA sequences of Tenacibaculum type strains were retrieved from complete genomes (unpublished data); the sequences of the strains used as outgroups were obtained from GenBank.
Tenacibaculum strains were grown in marine 2216E broth (Difco) for 24 h at 28°C and 70 rpm, and the genomic DNA was extracted from the pellet using the Wizard genomic DNA purification kit (Promega). PCR amplification was performed in a 20-μl reaction volume using GoTaq polymerase (Promega) and the following touchdown protocol: 94°C for 5 min, 24 cycles at 94°C for 0.5 min, 55°C for 0.5 min (−0.4°C/cycle), and 72°C for 1 min (+2 s/cycle); 12 cycles at 94°C for 0.5 min, 45°C for 0.5 min, and 72°C for 2 min (+3 s/cycle); and a final extension step at 72°C for 10 min. The sequences of the primers are listed in Table 1. Five microliters of the PCR products was resolved in a 1% agarose–Tris-borate-EDTA (TBE) gel to check amplification. For sequencing, one microliter of the PCR products was purified by using exonuclease I (Biolabs)-alkaline phosphatase (USB) for 1 h at 37°C, followed by enzyme inactivation for 5 min at 94°C. One-tenth of the purified PCR products was sequenced on both strands using the sequencing primers, the BigDye Terminator version 3.1 sequencing kit (Applied Biosystems), and an Applied Biosystems 3730 automated sequencer.
TABLE 1.
Primers used for PCR and sequencing of the 11 loci
| Locus | Step | Primer sequence (5′–3′) |
|
|---|---|---|---|
| Forward | Reverse | ||
| atpA | PCR | ATTGGWGAYCGTCAAACWGG | CCAAAYTTAGCRAAHGCTTC |
| dnaK | PCR | GGWACYACNAAYTCDTGTGT | TCWATCTTMGCTTTYTCAGC |
| glyA | PCR | CAYTTAACWCAYGGWTCDCC | ACCATRTTTTTRTTTACHGT |
| gyrB | PCR | AGTATYCARGCRCTRGAAGG | GTWCCTCCTTCRTGYGTRTT |
| ileS | PCR | CCWACHTTTGGWGCHGAYGA | GAATCRAACCAWACATCAAT |
| infB | PCR | ATGCCDCAAACWAAAGARGC | GTAATHGCTCCAACYCCTTT |
| rlmN | PCR | GCKTGTGTDTCDAGYCARGT | CCRCADGCDGCATCWATRTC |
| tgt | PCR | GAAACWCCWATWTTYATGCC | TAYAWYTCTTCNGCWGGTTC |
| trpB | PCR | GTWGCNCGWATGAAAATGYT | CCWGGRTARTCYAATCCTGC |
| tuf | PCR | AGAGAWTTATTRTCTTTCTA | GTTACCTGACCWGCWCCWAC |
| yqfO | PCR | GCBGAARRTTTTGAYAAYGT | AYTTCRTARGCDACYTCTTC |
| All | Sequencing | CAGGAAACAGCTATGACC | TGTAAAACGACGGCCAGT |
The 114 Tenacibaculum isolates included in this study are listed in Table 2, along with their origins; duplicated stocks of all strains were stored in glycerol at −80°C. This collection encompasses strains originating from five continents since 1976, including the type strains of all Tenacibaculum species available by March 2013. European isolates account for 77 strains. A total of 18 host fish species are represented: black sea bream (Acanthopagrus schlegeli), white sea bass (Atractoscion nobilis), European sea bass (Dicentrarchus labrax), sharpsnouted bream (Diplogus sargus), northern anchovy (Engraulis mordax), cod (Gadus morhua), striped trumpeter (Latris lineata), Coho salmon (Oncorhynchus kisutch), rainbow trout (Oncorhynchus mykiss), Japanese red sea bream (Pagrus major), European red sea bream (Pagellus bogaraveo), Japanese flounder (Paralichthys olivaceus), Atlantic salmon (Salmo salar), turbot (Scophthalmus maximus), Japanese amberjack or yellowtail (Seriola quinqueradiata), gilthead sea bream (Sparus aurata), Senegalese sole (Solea senegalensis), and Dover sole (Solea solea). Most strains were retrieved from external (i.e., skin, mouth, eye, head, and tail) lesions, but some were collected from the kidney.
TABLE 2.
List of the 114 Tenacibaculum isolates included in this study
| Strain no. in this study | Strain identifier as received | Country/state | Origin | Tissue | Yr | ST | Bacterial species | Contributora |
|---|---|---|---|---|---|---|---|---|
| 1 | NCIMB 2154T | Japan | Pagrus major | Kidney | 1977 | 1 | T. maritimum | NCIMB |
| 2 | ACC13.1 | Portugal | Solea senegalensis | Kidney | 2004 | 2 | T. maritimum | AET |
| 3 | ACR485.1 | Spain | Solea senegalensis | Kidney | 2011 | 3 | T. maritimum | AET |
| 4 | ACR488.1 | Spain | Solea senegalensis | Kidney | 2011 | 3 | T. maritimum | AET |
| 5 | ACR491.1 | Spain | Solea senegalensis | Kidney | 2011 | 3 | T. maritimum | AET |
| 6 | AF37.1 | Spain | Pagellus bogaraveo | Kidney | 2006 | 4 | T. maritimum | AET |
| 7 | AF39.1 | Spain | Pagellus bogaraveo | Tail | 2006 | 5 | T. maritimum | AET |
| 8 | CA42.1 | Spain | Solea senegalensis | NAb | 2006 | 6 | T. maritimum | AET |
| 9 | CA43.1 | Spain | Solea senegalensis | NA | 2006 | 6 | T. maritimum | AET |
| 10 | COS2.1 | Spain | Solea senegalensis | Kidney | 2011 | 7 | T. maritimum | AET |
| 11 | COS3.1 | Spain | Solea senegalensis | Tail | 2011 | 7 | T. maritimum | AET |
| 12 | FS08(1) | Italy | Sparus aurata | Skin | 2006 | 8 | T. maritimum | FS |
| 13 | NCIMB 2153 | Japan | Acanthopagrus schlegeli | Kidney | 1976 | 9 | T. maritimum | NCIMB |
| 14 | NCIMB 2158 | Scotland | Solea solea | Skin | 1981 | 10 | T. maritimum | NCIMB |
| 15 | PC1012.1 | Spain | Scophthalmus maximus | Head | 2008 | 11 | T. maritimum | AET |
| 16 | PC424.1 | Spain | Scophthalmus maximus | Kidney | 2000 | 12 | T. maritimum | AET |
| 17 | PC503.1 | Spain | Solea senegalensis | Skin | 2001 | 13 | T. maritimum | AET |
| 18 | PC538.1 | Spain | Sparus aurata | Tail | 2002 | 14 | T. maritimum | AET |
| 19 | PC824.1 | Spain | Sparus aurata | Kidney | 2003 | 4 | T. maritimum | AET |
| 20 | PC834.1 | Spain | Sparus aurata | Kidney | 2003 | 5 | T. maritimum | AET |
| 21 | RI93.1 | Spain | Scophthalmus maximus | Head | 2002 | 12 | T. maritimum | AET |
| 22 | RIM70.1 | Spain | Scophthalmus maximus | Mouth | 2009 | 15 | T. maritimum | AET |
| 23 | USC RP67.1 | Spain | Scophthalmus maximus | Mouth | 1993 | 16 | T. maritimum | AET |
| 24 | USC RPM539.1 | Spain | Scophthalmus maximus | Mouth | 1993 | 17 | T. maritimum | AET |
| 25 | USC SE30.1 | Spain | Oncorhynchus kisutch | Mouth | 1993 | 18 | T. maritimum | AET |
| 26 | DPIF 90/1445 | Tasmania | Salmo salar | Skin | 1990 | 19 | T. maritimum | JC |
| 27 | DPIF 89/0239-1 | Tasmania | Salmo salar | Skin | 1989 | 20 | T. maritimum | JC |
| 28 | DPIF 89/0235-3 | Tasmania | Oncorhynchus mykiss | Skin | 1989 | 21 | T. maritimum | JC |
| 29 | DPIF 89/0329-11 | Tasmania | Salmo salar | Skin | 1989 | 22 | T. maritimum | JC |
| 30 | DPIF 89/0329-5 | Tasmania | Salmo salar | Skin | 1989 | 22 | T. maritimum | JC |
| 31 | DPIF 89/0578-4 | Tasmania | Salmo salar | Skin | 1989 | 23 | T. maritimum | JC |
| 32 | DPIF 89/0699 | Tasmania | Salmo salar | Skin | 1989 | 22 | T. maritimum | JC |
| 33 | DPIF 89/1288-8 | Tasmania | Oncorhynchus mykiss | Skin | 1989 | 22 | T. maritimum | JC |
| 34 | DPIF 89/3001-6.2 | Tasmania | Latris lineata | Skin | 1989 | 24 | T. maritimum | JC |
| 35 | DPIF 89/0528-1 | Tasmania | Salmo salar | Skin | 1989 | 21 | T. maritimum | JC |
| 36 | Baxa 1y 1-1 | Japan | Acanthopagrus schlegeli | Skin | 1985 | 25 | T. maritimum | RPB |
| 37 | JIP 46/00 | France | Scophthalmus maximus | Skin | 2000 | 26 | T. maritimum | GG |
| 38 | CVI10001048 | Holland | Solea solea | Skin | 2010 | 27 | T. maritimum | OH |
| 39 | Baxa DBA-4a | Japan | Seriola quinqueradiata | Skin | 1986 | 28 | T. maritimum | RPB |
| 40 | FC | Chile | Scophthalmus maximus | Eye | 1998 | 29 | T. maritimum | JM |
| 41 | FM1068 | France | Dicentrarchus labrax | Skin | 1993 | 30 | T. maritimum | JFP |
| 42 | FPC371 | Japan | Pagrus major | Skin | 1977 | 31 | T. maritimum | HW |
| 43 | FPC386 | Japan | Pagrus major | Skin | 1978 | 32 | T. maritimum | HW |
| 44 | FPC394 | Japan | Pagrus major | Skin | 1982 | 32 | T. maritimum | HW |
| 45 | FPC454 | Japan | Pagrus major | Skin | 1983 | 33 | T. maritimum | HW |
| 46 | Baxa GBF-8601 | Japan | Paralichthys olivaceus | Skin | 1986 | 34 | T. maritimum | RPB |
| 47 | JIP 05/00(1) | France | Scophthalmus maximus | Skin | 2000 | 11 | T. maritimum | FL |
| 48 | JIP 10/97 | France | Scophthalmus maximus | Skin | 1997 | 12 | T. maritimum | FL |
| 49 | JIP 21/91-1 | France | Dicentrarchus labrax | Skin | 1991 | 3 | T. maritimum | JFB |
| 50 | JIP 21/91-2 | France | Dicentrarchus labrax | Skin | 1991 | 3 | T. maritimum | JFB |
| 51 | JIP21/91-3 | France | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 52 | JIP 24/99 | France | Scophthalmus maximus | Skin | 1999 | 12 | T. maritimum | FL |
| 53 | JIP 31/99 | France | Scophthalmus maximus | Skin | 1999 | 12 | T. maritimum | FL |
| 54 | JIP 32/91-1 | Corsica | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 55 | JIP 32/91-3 | Corsica | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 56 | JIP 32/91-4 | Corsica | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 57 | JIP 32/91-5 | Corsica | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 58 | JIP 32/91-6 | Corsica | Dicentrarchus labrax | Skin | 1991 | 35 | T. maritimum | JFB |
| 59 | JIP 32/99 | France | Dicentrarchus labrax | Skin | 1991 | 27 | T. maritimum | CS |
| 60 | LVDH 1577.01 | France | Dicentrarchus labrax | Skin | 2001 | 36 | T. maritimum | NK |
| 61 | USC RPM522.1 | Spain | Scophthalmus maximus | Mouth | 1992 | 37 | T. maritimum | AET |
| 62 | UCD SB2 | California | Atractoscion nobilis | NA | 1995 | 38 | T. maritimum | RH |
| 63 | UCD SD26 | California | Atractoscion nobilis | NA | 1995 | 39 | T. maritimum | RH |
| 64 | NAC SLCC 101 | Malta | Dicentrarchus labrax | Skin | 1995 | 40 | T. maritimum | JT |
| 65 | NAC SLCC 105 | Malta | Dicentrarchus labrax | Skin | 1995 | 41 | T. maritimum | JT |
| 66 | NAC SLCC 109 | Malta | Dicentrarchus labrax | Skin | 1995 | 42 | T. maritimum | JT |
| 67 | NAC SLCC 115 | Malta | Dicentrarchus labrax | Skin | 1996 | 42 | T. maritimum | JT |
| 68 | NAC SLCC 120 | Malta | Dicentrarchus labrax | Skin | 1996 | 42 | T. maritimum | JT |
| 69 | NAC SLCC MFF | Malta | Dicentrarchus labrax | Skin | NA | 43 | T. maritimum | ALB |
| 70 | USC SP9.1 | Spain | Salmo salar | Skin | 1993 | 44 | T. maritimum | AET |
| 71 | UCD V2b | California | Atractoscion nobilis | NA | 1993 | 45 | T. maritimum | RH |
| 72 | UCD V6f | California | Engraulis mordax | Skin | 1994 | 46 | T. maritimum | RH |
| 73 | UCD WSB-1b | California | Atractoscion nobilis | Skin | 1994 | 47 | T. maritimum | RH |
| 74 | 147/ITT | Italy | Dicentrarchus labrax | Kidney | 1989 | NA | T. discolor | AM |
| 75 | 253/ITT-1 | Italy | Sparus aurata | Kidney | 2004 | NA | T. mesophilum | AM |
| 76 | 269/ITT | Italy | Dicentrarchus labrax | Skin | 2010 | NA | T. discolor | AM |
| 77 | 43/ITT | Italy | Dicentrarchus labrax | Kidney | 2010 | NA | T. discolor | AM |
| 78 | FSIXSp1 | Italy | Dicentrarchus labrax | Eye | 1998 | NA | T. discolor | FS |
| 79 | TNO001 | Norway | Salmo salar | Skin | 2011 | NA | Tenacibaculum sp. | ABO |
| 80 | TNO002 | Norway | Salmo salar | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 81 | TNO003 | Norway | Salmo salar | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 82 | TNO004 | Norway | Salmo salar | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 83 | TNO005 | Norway | Salmo salar | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 84 | TNO006 | Norway | Salmo salar | Skin | 2011 | NA | Tenacibaculum sp. | ABO |
| 85 | TNO007 | Norway | Salmo salar | Skin | 2011 | NA | Tenacibaculum sp. | ABO |
| 86 | TNO008 | Norway | Salmo salar | Kidney | 2011 | NA | Tenacibaculum sp. | ABO |
| 87 | TNO009 | Norway | Salmo salar | Skin | 1996 | NA | Tenacibaculum sp. | ABO |
| 88 | TNO010 | Norway | Salmo salar | Skin | 1998 | NA | Tenacibaculum sp. | ABO |
| 89 | TNO011 | Norway | Salmo salar | Skin | 1998 | NA | Tenacibaculum sp. | ABO |
| 90 | TNO012 | Norway | Gadus morhua | Skin | 2009 | NA | Tenacibaculum sp. | ABO |
| 91 | TNO013 | Norway | Gadus morhua | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 92 | TNO014 | Norway | Gadus morhua | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 93 | TNO015 | Norway | Gadus morhua | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 94 | TNO018 | Norway | Gadus morhua | Skin | 2010 | NA | Tenacibaculum sp. | ABO |
| 95 | TNO019 | Norway | Salmo salar | Kidney | 1998 | NA | Tenacibaculum sp. | ABO |
| 96 | TNO020 | Norway | Salmo salar | Skin | 1998 | NA | Tenacibaculum sp. | ABO |
| 97 | LL04 12.1.7T | Spain | Solea senegalensis | NA | 2004 | NA | T. soleae | YSR |
| 98 | DSM 18961T | Croatia | Schizobrachiella sanguinea | NA | 2008 | NA | T. adriaticum | DSMZ |
| 99 | JCM 13491T | South Korea | Tidal flat sediment | NA | 2006 | NA | T. aestuarii | JCM |
| 100 | LMG 24004T | Taiwan | Aiptasia pulchella | NA | 2008 | NA | T. aiptasiae | BCCM/LMG |
| 101 | CIP 107214T | Philippines | Avrainvilla riukiuensis | NA | 2001 | NA | T. amylolyticum | CIP |
| 102 | JCM 15428T | South Korea | Crassostea gigas | NA | 2009 | NA | T. crassostreae | JCM |
| 103 | USC 35/09T | Spain | Dicentrarchus labrax | Skin | 2012 | NA | T. dicentrarchi | YSR |
| 104 | YSR-01 | Spain | Solea senegalensis | NA | 2010 | NA | T. discolor | YSR |
| 105 | LL04 11.1.1T | Spain | Solea senegalensis | Kidney | 2008 | NA | T. discolor | YSR |
| 106 | A37.1T | Spain | Seawater from a tank containing turbot | NA | 2008 | NA | T. gallaicum | YSR |
| 107 | LMG 23706T | Taiwan | Litopenaeus vannamei | NA | 2007 | NA | T. litopenaei | BCCM/LMG |
| 108 | JCM 13039T | South Korea | Tidal flat sediment | NA | 2006 | NA | T. litoreum | JCM |
| 109 | DSM 16505T | South Korea | Tidal flat sediment | NA | 2005 | NA | T. lutimaris | DSMZ |
| 110 | CIP 107215T | Japan | Halichondria okadai | NA | 2001 | NA | T. mesophilum | CIP |
| 111 | EKD 002T | Norway | Hippoglossus hippoglossus | Egg | 1992 | NA | T. ovolyticum | GHH |
| 112 | DSM 14836T | Denmark | Seawater | NA | 2004 | NA | T. skagerrakense | DSMZ |
| 113 | KCTC 23423T | South Korea | Seawater | NA | 2012 | NA | T. geojense | KCTC |
| 114 | KCTC 22618T | South Korea | Seawater | NA | 2012 | NA | T. jejuense | KCTC |
NCIMB, National Collection of Industrial and Marine Bacteria Ltd. (Aberdeen, Scotland); AET, A. Estévez Toranzo (Universidad de Santiago de Compostela, Spain); FS, F. Salati (State Veterinary Institute, Oristano, Italy); JC, J. Carson (Department of Primary Industry and Fisheries, Kings Meadows, Tasmania, Australia); RPB, R. P. Burchard (then at the University of Maryland, Baltimore); GG, G. Gauthier (N.A.T.A., France Turbot, l'Epine, France); OH, O. Haenen (Central Veterinary Institute, Lelystad, the Netherlands); JM, J. Montaña (then at Fundación Chile, Puerto Montt, Chile); JFP, J.-F. Pépin (then at IFREMER, Palavas-les-Flots, France); HW, H. Wakabayashi (then at the University of Tokyo, Japan); FL, F. Leveau (N.A.T.A., France Turbot, l'Epine, France); JFB, J.-F. Bernardet (Institut National de la Recherche Agronomique, Jouy-en-Josas, France); CS, C. Sauvegrain (Aquanord France, Gravelines, France); NK, N. Keck (Laboratoire Départemental Vétérinaire de l'Hérault, Montpellier, France); RH, R. Hedrick (then at the University of California, Davis); JT, J. Tabone (National Aquaculture Center, Marsaxlokk, Malta); ALB, A. Le Breton (VET'EAU Selarl, Grenade-sur-Garonne, France); AM, A. Manfrin (Istituto Zooprofilattico Sperimentale delle Venezie, Adria, Italy); ABO, A.-B. Olsen (National Veterinary Institute, Bergen, Norway); YSR, Y. Santos Rodríguez (Universidad de Santiago de Compostela, Spain); DSMZ, Leibniz Institut Deutsche Sammlung von Mikro-organismen und Zellkulturen GmbH (Braunschweig, Germany); JCM, Japan Collection of Microorganisms (Tsukuba, Japan); BCCM/LMG, Belgian Coordinated Collections of Microorganisms/Laboratory of Microbiology Gent (Ghent, Belgium); CIP, Collection de l'Institut Pasteur (Paris, France); GHH, G. H. Hansen (University of Bergen, Norway); KCTC, Korean Collection for Type Cultures (Daejeon, South Korea).
NA, no data available.
Data analysis.
The sequences were assembled using Phred/Phrap/Consed (39) and verified manually to ensure high quality. Alignments were generated with a two-step Biopython (40) wrapper: nucleotide sequences first were translated and aligned at the protein level with MUSCLE 5V3.8.31 (41); protein alignment then was back converted into a nucleotide alignment that served for all of the analyses. According to MLST standards (42), arbitrary numbers were used for unambiguous identification of the allele types (ATs; particular alleles at particular loci) and sequence types (STs; unique combinations of ATs at the different loci). Maximum likelihood trees were obtained with PhyML v3.0 (43) using the substitution model selected with Modelgenerator v0.85 (44), which corresponded to GTR+I+G. The gamma distribution was approximated with 4 categories of sites. At the T. maritimum species level, recombination challenges exact phylogenetic reconstruction. Nevertheless, a tentative tree was constructed by neighbor joining to graphically represent the sequence divergence between isolates. For this purpose, we used a simple Jukes-Cantor substitution model and the dnadist and neighbor programs included in Phylip (45) on concatenated nucleotide sequences. All phylogenetic trees were drawn in R using the ape package (46).
The detection of recombination within and between loci involved the computation of h and Rmin. The minimal number of apparent homoplasies (47), designated h, was computed on the most parsimonious tree found with the dnapars program included in Phylip (45). The Hudson and Kaplan lower bound on the minimal number of recombination events in an infinite site model (48), designated Rmin, was computed on biallelic sites by using LDhat (49). A quantitative estimate of the contribution of recombination versus that of mutation in short-term nucleotide divergence between strains was obtained using ClonalFrame (50). A total of 150,000 MCMC iterations (including 50,000 for burn-in) were performed for this analysis, and we checked that results from independent runs were comparable. The parameters θ (rate of mutation on the branches of the genealogy) and ν (rate of nucleotide differences in the recombination tracts) were fixed to the average level of pairwise nucleotide diversity of the sequences (π). The ratio of per-nucleotide changes that could be attributed to recombination to those that could be attributed to mutation (r/m ratio) (51) was computed from the parameter estimates provided by ClonalFrame as (R × ν × δ)/θ (as described in reference 52), where R is the rate of recombination, δ is the average length of a recombination tract, ν is the amount of nucleotide divergence between the two sequences that recombine, and θ is the mutation rate.
Association between genotypes and isolation sources in the T. maritimum species were investigated using analysis of molecular variance (AMOVA) (53) based on simple Euclidean distances (d) between STs (d = , where n is the number of differences between two nucleotide sequences). A nonparametric estimate of the statistical significance was obtained using random permutations of the genotypes with respect to isolation sources. These analyses were conducted in R with the pegas package (54).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the course of this work were deposited in GenBank under accession numbers KJ402457 to KJ403732 .
RESULTS
Evolutionary relationships within the genus Tenacibaculum.
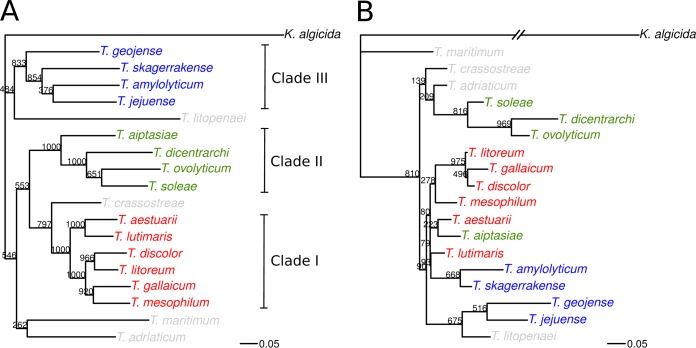
The PCR and sequencing protocols proposed in this study allowed sequencing of 11 loci (total length of 5,811 bp) across the whole diversity of the genus Tenacibaculum, represented here by 114 isolates. Figure 1A shows an MLSA phylogenetic tree reconstructed by maximum likelihood on the concatenated nucleotide sequences of the 18 Tenacibaculum type strains included in our collection and using Kordia algicida as an outgroup. The internal nodes of this phylogeny come with much higher bootstrap supports than those in the tree reconstructed on the 16S rRNA locus shown in Fig. 1B: out of 17 internal nodes, 10 reach a bootstrap support of at least 80% in the concatenated MLSA tree, whereas only 3 meet this criterion in the 16S rRNA tree.
FIG 1.
Comparison between the maximum-likelihood phylogenetic trees reconstructed on the 11 loci and on the 16S rRNA locus for the 18 Tenacibaculum type strains. (A) Concatenated MLSA tree; (B) 16S rRNA tree. The Kordia algicida type strain was included as an outgroup. Bootstrap supports estimated on 1,000 replicate data sets are reported above each internal node. Colors indicate the three clades identified based on high bootstrap support in the concatenated MLSA tree (I in red, II in green, and III in blue); the four isolated lineages that branch more deeply in the three are represented in gray. The same branch-length scale (measured in expected number of nucleotide substitutions per site) is used in both trees. The branch leading to K. algicida in the 16S rRNA tree has been shortened by a factor of 3 for the sake of representation.
On the basis of the most ancestral nodes with bootstrap support above 80%, the 18 Tenacibaculum species could be divided into three distinct clades plus four more-distant lineages that root deeper in the tree (T. adriaticum, T. crassostreae, T. litopenaei, and T. maritimum). The three clades contain 6, 4, and 4 species, respectively. They will be referred to here as clade I for the group T. aestuarii, T. discolor, T. gallaicum, T. litoreum, T. lutimaris, and T. mesophilum; clade II for T. aiptasiae, T. dicentrarchi, T. ovolyticum, and T. soleae; and clade III for T. amylolyticum, T. geojense, T. jejuense, and T. skagerrakense. In trees reconstructed on the basis of 16S rRNA (Fig. 1B) and the 11 individual loci (see Fig. S1 in the supplemental material), this three-clade distribution is not visible but is not strongly contradicted, as conflicting nodes never receive bootstrap support above 80%. In contrast, the history of the individual loci often seems to conflict with more recent nodes of the MLSA tree, suggesting recombination, at least between closely related species. For instance, the grouping T. litoreum, T. discolor, and T. gallaicum in the 16S rRNA tree (98% bootstrap support) is incompatible with the grouping T. gallaicum-T. mesophilum (92% bootstrap support) in the concatenated MLSA tree.
The 18 Tenacibaculum type strains included in our study can be divided into four categories according to their origins: 3 were isolated from seawater, 3 from sediments, 5 from diseased fish, and 6 from other marine organisms. The analysis of the distribution of these four categories in the seven different lineages delineated by our examination of the genus Tenacibaculum (clades I, II, and III and the four isolated lineages) reveals a statistically significant correlation (P = 0.045 by Fisher exact test), suggesting some degree of linkage between the position in the phylogeny of the genus and the ecological niche. In summary, clade I contains a balanced mix of strains isolated from sediments and marine organisms (including two fish pathogens), clade II is exclusively composed of strains isolated from marine organisms (primarily from diseased fish), and clade III contains a majority of strains isolated from seawater (all those included in our sample) and none of the fish-pathogenic species. The four species whose lineages root deeper in the genus, including the important fish pathogen T. maritimum, all were isolated from marine organisms (fish, oyster, crustacean, or bryozoan). Figure 2 shows the phylogenetic position of the other isolates included in our study. Importantly, all Tenacibaculum isolates retrieved from fish that are not T. maritimum belong to clades I and II, which corroborates the hypothesis of a nonrandom association between the clades I, II, and III and the ecological niches.
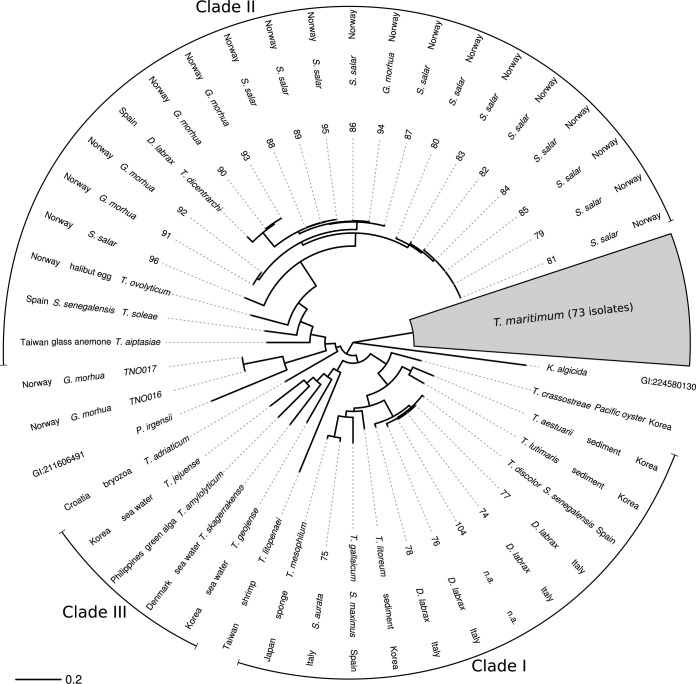
FIG 2.
Concatenated MLSA tree reconstructed on the 11 loci by maximum likelihood for the 114 Tenacibaculum isolates included in this study. A condensed representation (gray area) is used for the 73 isolates that group with the type strain of T. maritimum. For each other Tenacibaculum strain, the following information is reported: isolate identifier or bacterial species for type strains, isolation source (binomial names for fish species), and country of origin. The type strains of Polaribacter irgensii and Kordia algicida (accession numbers NZ_CH724148.1 and NZ_DS544873.1, respectively) were included to help rooting, but only K. algicida could easily be used as an outgroup. Branch length is measured as expected number of nucleotide substitutions per site.
Patterns of polymorphism in the fish-pathogenic species T. maritimum.
The concatenated MLSA tree of our 114 isolates allowed unambiguous classification of 73 of them as T. maritimum (Fig. 2). These isolates encompass 16 species of host fish, 5 continents (Europe, Australia, Asia, North America, and South America), and over 30 years of sampling (from 1976 to 2011). Thus, the data provide a broad overview of the genetic diversity in this important fish-pathogenic species.
Sequence comparisons revealed 168 single-nucleotide polymorphisms (SNPs) across the 5,811 bp surveyed in the 73 T. maritimum isolates. A summary of the main characteristics of the polymorphisms and their distribution across the 11 loci is presented in Table 3. Overall, 2.9% of the positions showed variations, and the pairs of sequences differed (pairwise nucleotide diversity, π) at 0.44% of the sites on average. The number of SNPs and the π differed between loci, from 6 SNPs and 0.16% nucleotide diversity at locus rlmN to 25 SNPs and 0.82% nucleotide diversity at locus atpA. As expected given the low level of divergence between the sequences, the vast majority of the SNPs were biallelic; only four were triallelic, and one was quadriallelic. Out of the 168 SNPs, 138 corresponded to synonymous variations, suggesting that most of the polymorphisms examined here are selectively neutral or near neutral, which is a desired property for unbiased analysis of population structure inside species by MLSA.
TABLE 3.
Summary of statistics on nucleotide polymorphism in T. maritimum
| Locus | Length (bp) | No. of ATsa | Snucb (no.) | Sprotc (no.) | πd (bp−1) | Rmine | hf (no.) |
|---|---|---|---|---|---|---|---|
| atpA | 567 | 20 | 25 (0/1) | 2 | 0.0082 | 2 | 7 |
| dnaK | 573 | 9 | 9 | 0 | 0.0021 | 2 | 3 |
| glyA | 558 | 13 | 13 (1/0) | 4 | 0.0035 | 2 | 4 |
| gyrB | 597 | 11 | 15 (2/0) | 3 | 0.0038 | 1 | 1 |
| ileS | 546 | 7 | 10 | 2 | 0.0020 | 0 | 0 |
| infB | 564 | 16 | 18 | 1 | 0.0037 | 1 | 3 |
| rlmN | 549 | 7 | 6 | 0 | 0.0016 | 1 | 1 |
| tgt | 486 | 16 | 15 | 4 | 0.0044 | 2 | 5 |
| trpB | 369 | 11 | 15 | 4 | 0.0070 | 0 | 0 |
| tuf | 555 | 16 | 21 | 1 | 0.0077 | 4 | 9 |
| yqfO | 447 | 16 | 16 (1/0) | 9 | 0.0060 | 2 | 4 |
| Sumg | 5,811 | 163 (4/1) | 30 | 17 | 37 | ||
| Concatenationh | 5,811 | 47 STs | 163 (4/1) | 30 | 0.0044 | 160 |
Number of allele types (sequence types for the concatenation).
Number of nucleotide polymorphisms, including triallelic and quadriallelic polymorphisms (indicated in parentheses).
Number of amino acid polymorphisms.
Average pairwise nucleotide diversity.
Hudson and Kaplan lower bound on the number of recombination events.
Number of apparent homoplasies.
Sum of the summary statistics over the 11 loci.
Concatenated sequences of the 11 loci.
The presence of intraspecies recombination in the genealogy of the T. maritimum sequences was detected by means of two summary statistics, Rmin and h (Table 3). Rmin is a lower bound on the minimal number of recombination events when each polymorphism arises from a single mutation, which is a reasonable assumption for most sites given the low divergence between the sequences (48). Rmin was greater than 0 for 9 loci and summed to 17 over the 11 loci; the 2 loci where recombination could not be detected (ileS and trpB) also were among the least polymorphic, making recombination more difficult to detect. The second statistic, h, is the minimal number of apparent homoplasies (47). It is obtained as the difference between the number of observed polymorphisms and the minimal number of mutations to obtain the sequences, assuming evolution along the branches of the same tree for all of the polymorphic sites. The value of h is 0 in the absence of recurrent mutations and recombinations. Here, h was 160 for the concatenated sequences of the 11 loci, which is similar to the number of polymorphic sites. The values of h for the loci analyzed separately were consistent with the Rmin values obtained at the same loci.
The r/m ratio was estimated to be 2.7:1 (95% credibility, 1.7 to 4.0) for T. maritimum based on the posterior distribution of the evolutionary parameters obtained from our data set with ClonalFrame (50). We also used the available data for a second group of closely related strains (the 19 T. dicentrarchi or T. dicentrarchi-like strains shown in Fig. 2) to examine how the values of the r/m ratio could differ across species of the genus Tenacibaculum. Our estimate of the r/m ratio for the T. dicentrarchi or T. dicentrarchi-like strains was 3.4:1 (95% credibility, 2.2 to 4.7), which is quite similar to the value obtained for T. maritimum.
Population structure of T. maritimum.
The number of distinct alleles at a particular locus ranged from 7 for rlmN and ileS to 20 for atpA among the 73 T. maritimum isolates (Table 3). The combination of the allele types (ATs) at the 11 loci allowed distinguishing 47 distinct sequence types (STs), which corresponds to an average of 1.6 isolates per ST. None of the ST contained more than 6 isolates, and only three clusters of STs (i.e., clonal complexes) could be identified on the basis of single-locus variation (SLV) links: ST4-ST5, ST16-ST18-ST44, and ST3-ST40. Individually, none of these three clusters accounted for more than 5 isolates. Importantly, strains with the same ST or for which STs are connected by SLV links always originated from the same geographical area. For instance, the small clonal complex ST4-ST5 is composed exclusively of isolates from Spain, and ST3 is composed of isolates sampled 20 years apart in the neighboring countries France and Spain.
For each strain, the ST, ATs, and information on sampling origin are reported in Fig. 3, along with its position in a tentative phylogenetic tree based on concatenated nucleotide sequences. According to this tree, it is tempting to describe our collection of T. maritimum isolates as composed of three subgroups, here designated A, B, and C. Subgroup A contains only 9 strains distributed into 6 distinct STs. All of these strains come from south European countries (Spain, Malta, and Italy), and 6 of 9 were retrieved from host fish of the family Sparidae. In particular, all gilthead sea bream (Sparus aurata) isolates in our collection belong to subgroup A. Subgroup B contains 59 strains; thus, it accounts for the majority of the T. maritimum isolates in our collection. Interestingly, the relative positions of the isolates in subgroup B seemed correlated with fish host and geographical origin (which are highly correlated to each other). Subgroup C consists of only 5 strains, but it is indeed far more heterogeneous than the two other subgroups and may justify further delineation in future studies.
FIG 3.
Genotype and background information for the 73 Tenacibaculum maritimum isolates. From left to right: tentative phylogenetic tree, isolate identification numbers (see Table S2 in the supplemental material), sequence types, allele types at the 11 loci, and information on the isolation source (year, tissue, host fish, and country; n.a., not available). The tree was obtained by neighbor joining with a simple Jukes-Cantor substitution model on concatenated nucleotide sequences. Branch length is measured in expected number of nucleotide substitutions per site. Bootstrap support was estimated on 1,000 replicate data sets, and only values greater than 500 are shown. The three subgroups of isolates designated A, B, and C are labeled and delineated by vertical bars.
The association between the isolation sources and genotypes was statistically confirmed and quantitatively assessed by AMOVA (53). The results are presented in Table 4. The total molecular variance explained by taking each type of information individually was 33.92% for the host fish, 33.52% for the year, and 18.27% for the country; all of these associations were statistically significant at the 5% level. The fraction of variance explained by the tissue also was statistically significant but accounted for only 4.31% of the total variance. Because these values could partly reflect sampling biases and correlations between the three types of information, we also applied AMOVA after discarding 15 isolates, including identical genotypes from the same host fish species, year, and country. As expected, removing replicates decreased the fraction of variance explained by each of these three factors. By far, the most important decrease concerned the fraction of variance explained by the year that diminished by 35.7%, whereas the fraction explained by host and country diminished by only 12.6% and 13.7%, respectively. In parallel, removing replicates slightly raised the amount of variance explained by the tissue that reached 10.41%. Importantly, the divergence between the three subgroups of isolates (A, B, and C) was not responsible for these results, since globally similar estimates of the fraction of explained variance were obtained within subgroup B (Table 4), except for tissue that did not seem to correlate with genotype in this subgroup.
TABLE 4.
Analysis of molecular variance on 11 loci in T. maritimumd
| % molecular variance | |||
|---|---|---|---|
| Information type | Alla | Uniqueb | Subgroup Bc |
| Host fish | 33.924* | 29.635* | 27.396* |
| Country | 18.272* | 15.763* | 17.731* |
| Yr | 33.522* | 21.561* | 25.943* |
| Tissue | 4.310* | 10.415* | −1.850 |
The whole collection of 73 T. maritimum isolates.
The 59 unique isolates obtained by removing replicate genotypes with the same host fish, country, and year.
The unique isolates belonging to subgroup B only.
The fraction of the total variance explained by each individual type of information (host fish, country, year, and tissue) is reported for three sets of isolates. *, permutation-based π value of ≤0.05.
MLSA as a tool for monitoring Tenacibaculum infections worldwide.
MLSA, also known as MLST when focused on a single species, currently is recognized as a reference method for the genotyping of isolates in many bacterial species. In particular, it proved useful to monitor the emergence and prevalence of different strains and to back-trace the contamination routes in a large number of pathogenic species (55). As the result of a balance between cost and resolving power, most MLST schemes rely on seven loci. Thus, for MLST to be effective, the sequences of a few loci have to provide enough information to discriminate a large number of STs. The pattern of nucleotide polymorphism reported in this study shows that this is indeed the case for T. maritimum. Our data also allow selecting the most informative loci for this genotyping purpose. We evaluated all of the combinations of loci and propose that future MLSA surveys of Tenacibaculum strains should use the 6 loci atpA, dnaK, glyA, infB, rlmN, and tgt, which allow distinguishing all 47 STs identified in the species T. maritimum based on the 11 loci, plus the gyrB locus, as this gene historically was used to define the genus Tenacibaculum. Of note, our gyrB sequence lies entirely within the 1,422 bp considered by Suzuki et al. (2). In the supplemental material, we provide additional versions of Fig. 2 and 3 based on the selected 7 loci (see Fig. S2 and S3). It can be seen that these 7 loci capture not only the whole diversity of STs in T. maritimum but also the important features of the phylogenetic trees, such as the division into three subgroups in the T. maritimum species and the existence of three clades plus four more distant lineages in the genus Tenacibaculum. The genotype data of the 114 isolates at these 7 loci has been deposited in a dedicated BIGSdb database (56) available at http://pubmlst.org/tenacibaculum/, which will be enriched progressively with new genotypes.
As a case study, we used the MLSA approach to characterize a number of suspected Tenacibaculum sp. isolates retrieved from marine fish. Our data set included strains isolated during recent outbreaks of tenacibaculosis in Norway (38) and Italy. Our MLSA data indicate that 18 Norwegian isolates actually represent T. dicentrarchi or T. dicentrarchi-like strains, while the two remaining strains were allocated to the genus Polaribacter (Fig. 2). The Italian isolates were identified as 5 T. discolor strains and 1 T. mesophilum strain.
DISCUSSION
Distribution and evolution of fish pathogenicity in the genus Tenacibaculum.
In light of our MLSA data, the genus appears structured in distinct clades that cannot be observed from the tree reconstructed from the sequence of the 16S rRNA locus. More generally, the lack of resolution and the discrepancies found when analyzing trees based on the individual loci suggest that homologous recombination between species occurred and clearly argue for grounding evolutionary analyses on multilocus data.
The fish-pathogenic strains are distributed into several well-delineated clades, and fish-pathogenic lineages are intertwined with the lineages of strains isolated from other marine organisms and even from sediments. This observation strongly suggests parallel evolution of fish pathogenicity in several lineages of the genus Tenacibaculum. An alternative hypothesis is that fish pathogenicity is an ancestral characteristic, but this seems very unlikely given the number of lineages of isolates from a diversity of other sources that root deeply in the genus. Importantly, sampling biases most certainly contribute to the underrepresentation of environmental strains among the described Tenacibaculum species, which strengthens our line of reasoning.
Of note, two isolates collected from diseased fish (Gadus morhua) included in our collection (TNO016 and TNO017) were found to cluster with the Polaribacter representative in the phylogenetic reconstruction (Fig. 2). Therefore, it is tempting to speculate that virulent lineages infecting fish also have evolved in this sister genus, which is currently thought of as grouping with environmental, nonpathogenic bacteria (see reference 57 and references therein). Interestingly, our data also indicate that the Polaribacter clade is not clearly distinct from the Tenacibaculum clade. Indeed, trees reconstructed from concatenated loci (Fig. 2 shows 11 loci; see Fig. S2 in the supplemental material for 7-locus trees) and from loci taken separately (data not shown) often differ with respect to the position of Polaribacter species relative to Tenacibaculum. More systematic analyses using complete genome data may shed light on the relationships between the genera Tenacibaculum and Polaribacter and the genealogical discrepancies between loci.
In this context of parallel evolution of fish pathogenicity, it seems likely that the census of the pathogenic Tenacibaculum species is still incomplete. New pathogenic species probably will be described in the future and may be recognized as being responsible for economically important problems as marine aquaculture grows and involves a greater variety of cultured organisms. As an illustration, one of the strains from Italy (isolate 75) that we identified as T. mesophilum was retrieved from the kidney of a sea bream (Sparus aurata), suggesting that this bacterial species infects fish, although the type strain was isolated from a sponge.
Genetic diversity of T. maritimum colonizing aquaculture systems and comparison to other fish-pathogenic bacteria.
Our analysis examined polymorphism within 73 isolates of the fish-pathogenic species T. maritimum. With average pairwise nucleotide diversity (π) estimated to be 0.44% and an r/m ratio estimated to be 2.7 (95% credibility, 1.7 to 4.0), the species can be described as exhibiting moderate levels of nucleotide diversity and recombination (58). Despite recombination, we showed that our collection of T. maritimum strains is composed of three subgroups. We also found a statistically significant association between the genotypes and the background information on the isolation source (host fish, year, and geographical origin), but that could account for only a limited amount of the total genetic variance.
After Flavobacterium psychrophilum ( 59), Yersinia ruckeri (60), and Renibacterium salmoninarum (61), T. maritimum becomes the fourth species of fish-pathogenic bacteria for which sequence data are available for a significant number of strains. The niche of T. maritimum differs from that of the other three species, as it is the only marine bacterium with broad host range. The three other species have been reported as primarily infecting salmonids, and only Renibacterium salmoninarum is regularly isolated from marine fish. Out of the four species, T. maritimum is also the one whose attacks have the most marked localization toward fish body surfaces. It is worth attempting a comparison of the patterns of polymorphism and population structures, but we need to have in mind the limited number of loci and the differences between strain sampling schemes.
It is interesting that despite its broader host range and worldwide geographical distribution, T. maritimum exhibits a level of diversity comparable to that of F. psychrophilum (∼0.4%) and Y. ruckeri (∼0.7%), suggesting that population sizes are of the same order of magnitude, although other factors, such as mutation rate and selective sweeps, also can contribute to shape the level of nucleotide diversity. However, nucleotide diversity is much lower in R. salmoninarium (<0.08%). In terms of per-nucleotide r/m ratio, the rate of recombination in T. maritimum may be slightly lower than that in Y. ruckeri (∼7:1) and is clearly lower than that in the highly recombinogenic bacterium F. psychrophilum (∼26:1) (see references 58 and 59 for even higher estimates). In contrast, a near absence of recombination was reported for R. salmoninarum (61).
The features by which the T. maritimum data really stand out are the small number of representatives collected for each ST, the lack of large clonal complexes, and the absence of any trace of transcontinental dissemination. This situation is in sharp contrast to that in F. psychrophilum (59, 62, 63), R. salmoninarium (61), and Y. ruckeri (60), for which the sequence data unambiguously revealed transcontinental dissemination linked to the international trade of broodfish and eggs. Furthermore, as a result of preferential dissemination routes or of adaptive niche specificity, the large clonal complexes detected in F. psychrophilum tended to be strongly associated with particular host fish species (59, 62, 64).
Taken together, the population structure described here for T. maritimum strongly suggests the endemic colonization of fish farms by local strains with little or no contribution of long-distance contamination linked to fish movements. As most of the marine fish farmers usually buy fry from geographically distant hatcheries, this population structure was not necessarily anticipated. Furthermore, our data indicate that the same ST often is found to infect multiple species of host fish in the same geographical area, which points to the possibility of cross-species contaminations in fish farms by the same bacterial lineage. Interestingly, our results on T. maritimum population structure echo the empirical observations that environmental conditions and fish health status are major factors for tenacibaculosis outbreaks (4). Indeed, outbreaks often might correspond to new contaminations from the local environment when conditions are favorable to the pathogen.
Supplementary Material
ACKNOWLEDGMENTS
We thank the individuals who kindly provided the Tenacibaculum isolates included in this study (see Table S2 in the supplemental material). We thank Tatiana Vallaeys for critical reading of the manuscript. We also are grateful to Keith Jolley at the University of Oxford for hosting the Tenacibaculum MLSA website, with funding from the Wellcome Trust.
C.H., A.H., A.L., J.-F.B., P.N., and E.D. received support from AIP INRA Bio-Resources 2010 (Tenacibaculum genomics), FUI-11 (PathotrackFish), and EU EMIDA ERA-NET (ANR 2010-EMID-006-01 Pathofish); A.B.O. and H.N. received support from the EU EMIDA ERA-NET (RCN 202834/E40 PathoFish) project; and A.E.T. and N.C. received support from the European Project Maximus (FP7-SME-2011-286200).
Footnotes
Published ahead of print 27 June 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01177-14.
REFERENCES
- 1.Food and Agriculture Organization of the United Nations Fisheries and Aquaculture Department. 2012. The state of world fisheries and aquaculture 2012. Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 2.Suzuki M, Nakagawa Y, Harayama S, Yamamoto S. 2001. Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov., and description of Tenacibaculum mesophilum sp. nov. and Tenacibaculum amylolyticum sp. nov. Int. J. Syst. Evol. Microbiol. 51:1639–1652. 10.1099/00207713-51-5-1639 [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi H, Hikida M, Masumura K. 1986. Flexibacter maritimus sp. nov., a pathogen of marine fishes. Int. J. Syst. Bacteriol. 36:396–398. 10.1099/00207713-36-3-396 [DOI] [Google Scholar]
- 4.Avendaño-Herrera R, Toranzo AE, Magariños B. 2006. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis. Aquat. Organ. 71:255–266. 10.3354/dao071255 [DOI] [PubMed] [Google Scholar]
- 5.Van Gelderen R, Carson J, Nowak B. 2011. Experimentally induced marine flexibacteriosis in Atlantic salmon smolts Salmo salar. II. Pathology. Dis. Aquat. Organ. 95:125–135. 10.3354/dao02329 [DOI] [PubMed] [Google Scholar]
- 6.Hansen GH, Bergh Ø, Michaelsen J, Knappskog D. 1992. Flexibacter ovolyticus sp. nov., a pathogen of eggs and larvae of Atlantic halibut, Hippoglossus hippoglossus L. Int. J. Syst. Bacteriol. 42:451–458. 10.1099/00207713-42-3-451 [DOI] [PubMed] [Google Scholar]
- 7.Bergh O, Nilsen F, Samuelsen OB. 2001. Diseases, prophylaxis and treatment of the Atlantic halibut Hippoglossus hippoglossus: a review. Dis. Aquat. Organ. 48:57–74. 10.3354/dao048057 [DOI] [PubMed] [Google Scholar]
- 8.Piñeiro-Vidal M, Riaza A, Santos Y. 2008. Tenacibaculum discolor sp. nov. and Tenacibaculum gallaicum sp. nov., isolated from sole (Solea senegalensis) and turbot (Psetta maxima) culture systems. Int. J. Syst. Evol. Microbiol. 58:21–25. 10.1099/ijs.0.65397-0 [DOI] [PubMed] [Google Scholar]
- 9.Piñeiro-Vidal M, Carballas CG, Gomez-Barreiro O, Riaza A, Santos Y. 2008. Tenacibaculum soleae sp. nov., isolated from diseased sole (Solea senegalensis Kaup). Int. J. Syst. Evol. Microbiol. 58:881–885. 10.1099/ijs.0.65539-0 [DOI] [PubMed] [Google Scholar]
- 10.Piñeiro-Vidal M, Gijón D, Zarza C, Santos Y. 2012. Tenacibaculum dicentrarchi sp. nov., a marine bacterium of the family Flavobacteriaceae isolated from European sea bass. Int. J. Syst. Evol. Microbiol. 62:425–429. 10.1099/ijs.0.025122-0 [DOI] [PubMed] [Google Scholar]
- 11.Piñeiro-Vidal M, Centeno-Sestelo G, Santos Y. 2007. Isolation of pathogenic Tenacibaculum maritimum-related organisms from diseased turbot and sole cultured in the northwest of Spain. Bull. Eur. Fish Pathol. 27:29–35 [Google Scholar]
- 12.López JR, Piñeiro-Vidal M, García-Lamas N, De La Herran R, Navas JI, Hachero-Cruzado I, Santos Y. 2010. First isolation of Tenacibaculum soleae from diseased cultured wedge sole, Dicologoglossa cuneata (Moreau), and brill, Scophthalmus rhombus (L.). J. Fish Dis. 33:273–278. 10.1111/j.1365-2761.2009.01105.x [DOI] [PubMed] [Google Scholar]
- 13.Frette L, Jørgensen Irming NOH, Kroer N. 2004. Tenacibaculum skagerrakense sp. nov., a marine bacterium isolated from the pelagic zone in Skagerrak, Denmark. Int. J. Syst. Evol. Microbiol. 54:519–524. 10.1099/ijs.0.02398-0 [DOI] [PubMed] [Google Scholar]
- 14.Choi DH, Kim YG, Hwang CY, Yi H, Chun J, Cho BC. 2006. Tenacibaculum litoreum sp. nov., isolated from tidal flat sediment. Int. J. Syst. Evol. Microbiol. 56:635–640. 10.1099/ijs.0.64044-0 [DOI] [PubMed] [Google Scholar]
- 15.Heindl H, Wiese J, Imhoff JF. 2008. Tenacibaculum adriaticum sp. nov., from a bryozoan in the Adriatic Sea. Int. J. Syst. Evol. Microbiol. 58:542–547. 10.1099/ijs.0.65383-0 [DOI] [PubMed] [Google Scholar]
- 16.Oh YS, Kahng HY, Lee DH, Lee SB. 2012. Tenacibaculum jejuense sp. nov., isolated from coastal seawater. Int. J. Syst. Evol. Microbiol. 62:414–419. 10.1099/ijs.0.030114-0 [DOI] [PubMed] [Google Scholar]
- 17.Wang JT, Chou YJ, Chou JH, Chen CA, Chen WM. 2008. Tenacibaculum aiptasiae sp. nov., isolated from a sea anemone Aiptasia pulchella. Int. J. Syst. Evol. Microbiol. 58:761–766. 10.1099/ijs.0.65437-0 [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Baik KS, Park SY, Kim EM, Lee DH, Kahng HY, Jeon CO, Jung JS. 2009. Tenacibaculum crassostreae sp. nov., isolated from the Pacific oyster, Crassostrea gigas. Int. J. Syst. Evol. Microbiol. 59:1609–1614. 10.1099/ijs.0.006866-0 [DOI] [PubMed] [Google Scholar]
- 19.Kim YO, Park S, Nam BH, Jung YT, Kim DG, Jee YJ, Yoon JH. 2013. Tenacibaculum halocynthiae sp. nov., a member of the family Flavobacteriaceae isolated from sea squirt Halocynthia roretzi. Antonie Van Leeuwenhoek 103:1321–1327. 10.1007/s10482-013-9913-5 [DOI] [PubMed] [Google Scholar]
- 20.Sheu SY, Lin KY, Chou JH, Chang PS, Arun AB, Young CC, Chen WM. 2007. Tenacibaculum litopenaei sp. nov., isolated from a shrimp mariculture pond. Int. J. Syst. Evol. Microbiol. 57:1148–1153. 10.1099/ijs.0.64920-0 [DOI] [PubMed] [Google Scholar]
- 21.Yoon JH, Kang SJ, Oh TK. 2005. Tenacibaculum lutimaris sp. nov., isolated from a tidal flat in the Yellow Sea, Korea. Int. J. Syst. Evol. Microbiol. 55:793–798. 10.1099/ijs.0.63416-0 [DOI] [PubMed] [Google Scholar]
- 22.Jung SY, Oh TK, Yoon JH. 2006. Tenacibaculum aestuarii sp. nov., isolated from a tidal flat sediment in Korea. Int. J. Syst. Evol. Microbiol. 56:1577–1581. 10.1099/ijs.0.64302-0 [DOI] [PubMed] [Google Scholar]
- 23.Park S, Yoon JH. 2013. Tenacibaculum caenipelagi sp. nov., a member of the family Flavobacteriaceae isolated from tidal flat sediment. Antonie Van Leeuwenhoek 104:225–231. 10.1007/s10482-013-9941-1 [DOI] [PubMed] [Google Scholar]
- 24.Kang SJ, Lee SY, Lee MH, Oh TK, Yoon JH. 2012. Tenacibaculum geojense sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 62:18–22. 10.1099/ijs.0.029702-0 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wei J, Yang C, Lai Q, Chen Z, Li D, Zhang H, Tian Y, Zheng T. 2013. Tenacibaculum xiamenense sp. nov., an algicidal species isolated from coastal seawater. Int. J. Syst. Evol. Microbiol. 63:3481–3486. 10.1099/ijs.0.050765-0 [DOI] [PubMed] [Google Scholar]
- 26.Bernardet JF, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. 1996. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (Basonym, Cytophaga aquatilis Strohl and Tait 1978). Int. J. Syst. Bacteriol. 46:128–148. 10.1099/00207713-46-1-128 [DOI] [Google Scholar]
- 27.Bernardet J-F, Nakagawa Y, Holmes B. 2002. Proposed minimal standards for describing new taxa of the family Flavobacteriaceae and emended description of the family. Int. J. Syst. Evol. Microbiol. 52:1049–1070. 10.1099/ijs.0.02136-0 [DOI] [PubMed] [Google Scholar]
- 28.Bernardet J-F. 2011. Family I. Flavobacteriaceae Reichenbach 1992, p 106–111 In Krieg NR, Ludwig W, Whitman WB, Hedlund BP, Paster BJ, Staley JT, Ward NL, Brown DR, Parte AC. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 4 Springer, New York, NY [Google Scholar]
- 29.Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145. 10.1073/pnas.95.6.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gevers D, Cohan FM, Lawrence JG, Spratt BG, Coenye T, Feil EJ, Stackebrandt E, Van de Peer Y, Vandamme P, Thompson FL, Swings J. 2005. Reevaluating prokaryotic species. Nat. Rev. Microbiol. 3:733–739. 10.1038/nrmicro1236 [DOI] [PubMed] [Google Scholar]
- 31.Avendaño-Herrera R, Rodriguez J, Magariños B, Romalde JL, Toranzo AE. 2004. Intraspecific diversity of the marine fish pathogen Tenacibaculum maritimum as determined by randomly amplified polymorphic DNA-PCR. J. Appl. Microbiol. 96:871–877. 10.1111/j.1365-2672.2004.02217.x [DOI] [PubMed] [Google Scholar]
- 32.Avendaño-Herrera R, Magariños B, López-Romalde S, Romalde JL, Toranzo AE. 2004. Phenotypic characterization and description of two major O-serotypes in Tenacibaculum maritimum strains from marine fishes. Dis. Aquat. Organ. 58:1–8. 10.3354/dao058001 [DOI] [PubMed] [Google Scholar]
- 33.Wilson T, Carson J. 2003. Development of sensitive, high-throughput one-tube RT-PCR-enzyme hybridisation assay to detect selected bacterial fish pathogens. Dis. Aquat. Organ. 54:127–134. 10.3354/dao054127 [DOI] [PubMed] [Google Scholar]
- 34.Avendaño-Herrera R, Magariños B, Toranzo AE, Beaz R, Romalde JL. 2004. Species-specific polymerase chain reaction primer sets for the diagnosis of Tenacibaculum maritimum infection. Dis. Aquat. Organ. 62:75–83. 10.3354/dao062075 [DOI] [PubMed] [Google Scholar]
- 35.Fringuelli E, Savage PD, Gordon A, Baxter EJ, Rodger HD, Graham DA. 2012. Development of a quantitative real-time PCR for the detection of Tenacibaculum maritimum and its application to field samples. J. Fish Dis. 35:579–590. 10.1111/j.1365-2761.2012.01377.x [DOI] [PubMed] [Google Scholar]
- 36.Faílde LD, Bermúdez R, Losada AP, Riaza A, Santos Y, Quiroga MI. 26 November 2013. Immunohistochemical diagnosis of tenacibaculosis in paraffin-embedded tissues of Senegalese sole Solea senegalensis Kaup, 1858. J. Fish Dis. 10.1111/jfd.12199 [DOI] [PubMed] [Google Scholar]
- 37.López JR, Hamman-Khalifa AM, Navas JI, de la Herran R. 2011. Characterization of ISR region and development of a PCR assay for rapid detection of the fish pathogen Tenacibaculum soleae. FEMS Microbiol. Lett. 324:181–188. 10.1111/j.1574-6968.2011.02404.x [DOI] [PubMed] [Google Scholar]
- 38.Olsen AB, Nilsen H, Sandlund N, Mikkelsen H, Sørum H, Colquhoun DJ. 2011. Tenacibaculum sp. associated with winter ulcers in sea-reared Atlantic salmon Salmo salar. Dis. Aquat. Organ. 94:189–199. 10.3354/dao02324 [DOI] [PubMed] [Google Scholar]
- 39.Ewing B, Hillier L, Wendl MC, Green P. 1998. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 8:175–185 [DOI] [PubMed] [Google Scholar]
- 40.Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, de Hoon MJL. 2009. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25:1422–1423. 10.1093/bioinformatics/btp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enright MC, Spratt BG. 1999. Multilocus sequence typing. Trends Microbiol. 7:482–487. 10.1016/S0966-842X(99)01609-1 [DOI] [PubMed] [Google Scholar]
- 43.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 44.Keane TM, Creevey CJ, Pentony MM, Naughton TJ, Mclnerney JO. 2006. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 6:29. 10.1186/1471-2148-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felsenstein J. 1989. PHYLIP–phylogeny inference package (version 3.2). Cladistics 5:164–166 [Google Scholar]
- 46.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- 47.Maynard Smith J, Smith NH. 1998. Detecting recombination from gene trees. Mol. Biol. Evol. 15:590–599. 10.1093/oxfordjournals.molbev.a025960 [DOI] [PubMed] [Google Scholar]
- 48.Hudson RR, Kaplan NL. 1985. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111:147–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auton A, McVean G. 2007. Recombination rate estimation in the presence of hotspots. Genome Res. 17:1219–1227. 10.1101/gr.6386707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Didelot X, Falush D. 2007. Inference of bacterial microevolution using multilocus sequence data. Genetics 175:1251–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guttman DS, Dykhuizen DE. 1994. Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science 266:1380–1383. 10.1126/science.7973728 [DOI] [PubMed] [Google Scholar]
- 52.Dalmasso M, Nicolas P, Falentin H, Valence F, Tanskanen J, Jatila H, Salusjärvi T, Thierry A. 2011. Multilocus sequence typing of Propionibacterium freudenreichii. Int. J. Food Microbiol. 145:113–120. 10.1016/j.ijfoodmicro.2010.11.037 [DOI] [PubMed] [Google Scholar]
- 53.Excoffier L, Smouse PE, Quattro JM. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paradis E. 2010. pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics 26:419–420. 10.1093/bioinformatics/btp696 [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Losada M, Cabezas P, Castro-Nallar E, Crandall KA. 2013. Pathogen typing in the genomics era: MLST and the future of molecular epidemiology. Infect. Genet. Evol. 16:38–53. 10.1016/j.meegid.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 56.Jolley KA, Maiden MCJ. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim BC, Oh HW, Kim H, Park D-S, Hong SG, Lee HK, Bae KS. 2013. Polaribacter sejongensis sp. nov., isolated from Antarctic soil, and emended descriptions of the genus Polaribacter, Polaribacter butkevichii and Polaribacter irgensii. Int. J. Syst. Evol. Microbiol. 63(Part 11):4000–4005. 10.1099/ijs.0.047100-0 [DOI] [PubMed] [Google Scholar]
- 58.Vos M, Didelot X. 2009. A comparison of homologous recombination rates in bacteria and archaea. ISME J. 3:199–208. 10.1038/ismej.2008.93 [DOI] [PubMed] [Google Scholar]
- 59.Nicolas P, Mondot S, Achaz G, Bouchenot C, Bernardet J-F, Duchaud E. 2008. Population structure of the fish-pathogenic bacterium Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:3702–3709. 10.1128/AEM.00244-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastardo A, Ravelo C, Romalde JL. 2012. Multilocus sequence typing reveals high genetic diversity and epidemic population structure for the fish pathogen Yersinia ruckeri: MLST of Yersinia ruckeri. Environ. Microbiol. 14:1888–1897. 10.1111/j.1462-2920.2012.02735.x [DOI] [PubMed] [Google Scholar]
- 61.Brynildsrud O, Feil EJ, Bohlin J, Castillo-Ramirez S, Colquhoun D, McCarthy U, Matejusova IM, Rhodes LD, Wiens GD, Verner-Jeffreys DW. 2014. Microevolution of Renibacterium salmoninarum: evidence for intercontinental dissemination associated with fish movements. ISME J. 8:746–756. 10.1038/ismej.2013.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujiwara-Nagata E, Chantry-Darmon C, Bernardet J-F, Eguchi M, Duchaud E, Nicolas P. 2013. Population structure of the fish pathogen Flavobacterium psychrophilum at whole-country and model river levels in Japan. Vet. Res. 44:34. 10.1186/1297-9716-44-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avendaño-Herrera R, Balboa S, Castro N, Contreras-González A, Magariños B, Fernández J, Toranzo AE, Romalde JL. 26 February 2014. Comparative polyphasic characterization of Streptococcus phocae strains with different host origin and description of the new subspecies Streptococcus phocae subsp. salmonis subsp. nov. Int. J. Syst. Evol. Microbiol. 10.1099/ijs.0.056978-0 [DOI] [PubMed] [Google Scholar]
- 64.Siekoula-Nguedia C, Blanc G, Duchaud E, Calvez S. 2012. Genetic diversity of Flavobacterium psychrophilum isolated from rainbow trout in France: predominance of a clonal complex. Vet. Microbiol. 161:169–178. 10.1016/j.vetmic.2012.07.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.